Advances in Visible-Light-Mediated Carbonylative Reactions via Carbon Monoxide (CO) Incorporation
Abstract
:1. Introduction
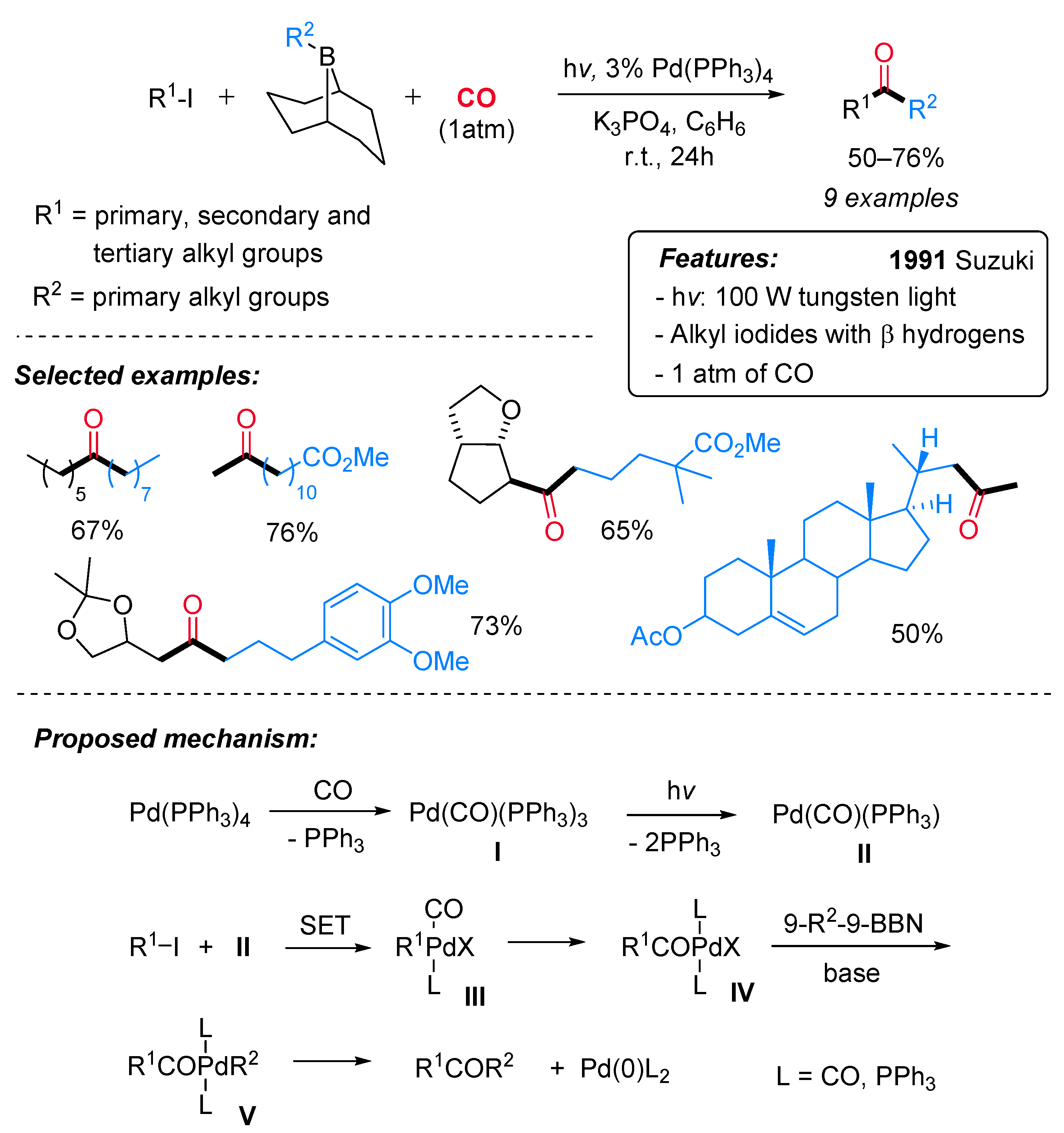
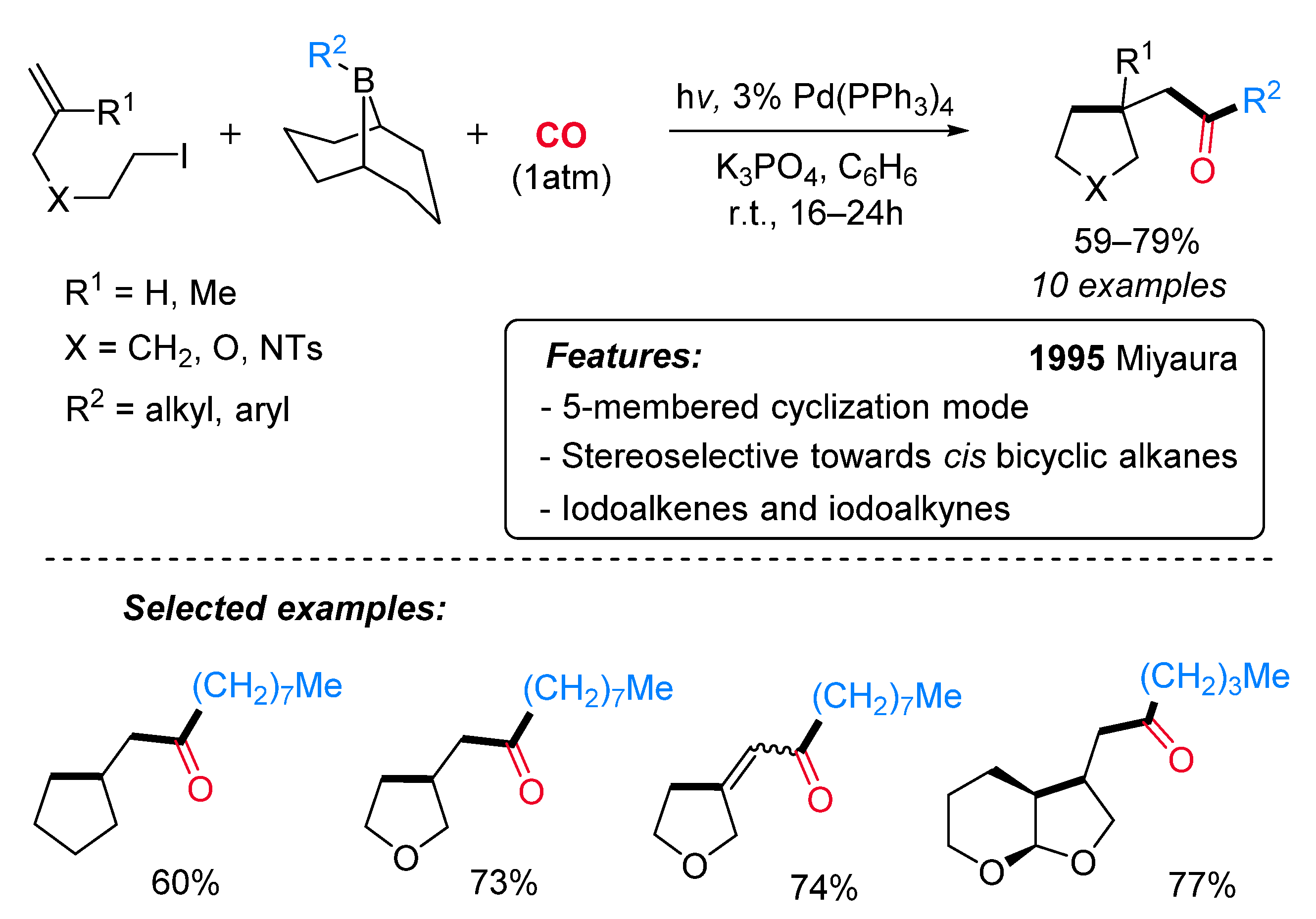
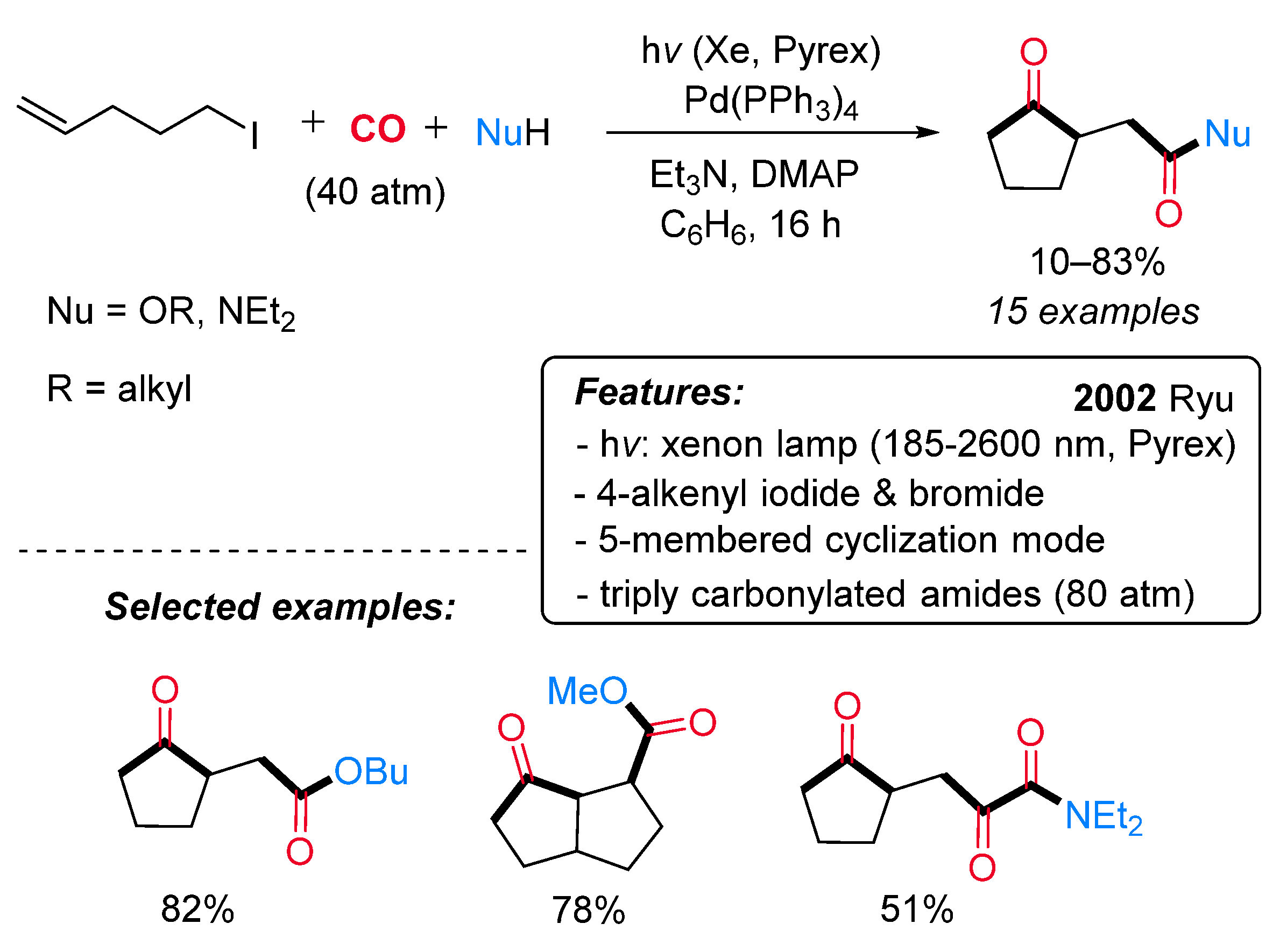
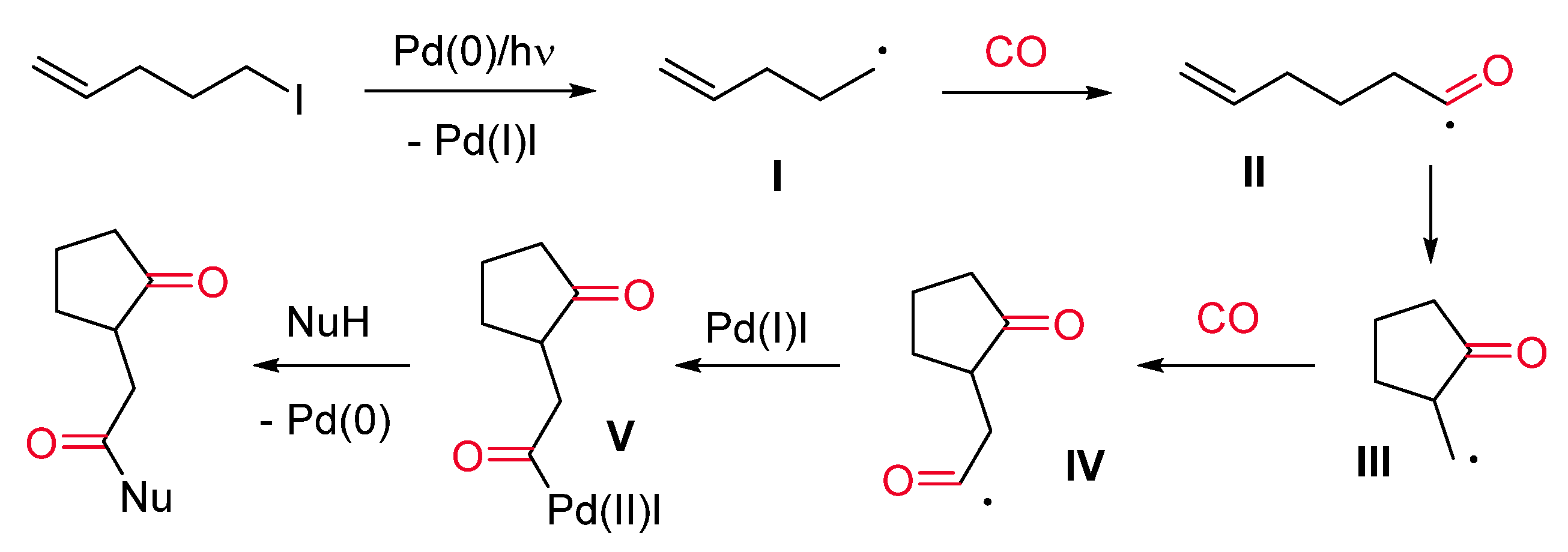
1.1. Visible-Light-Promoted Palladium-Catalyzed Carbonylations
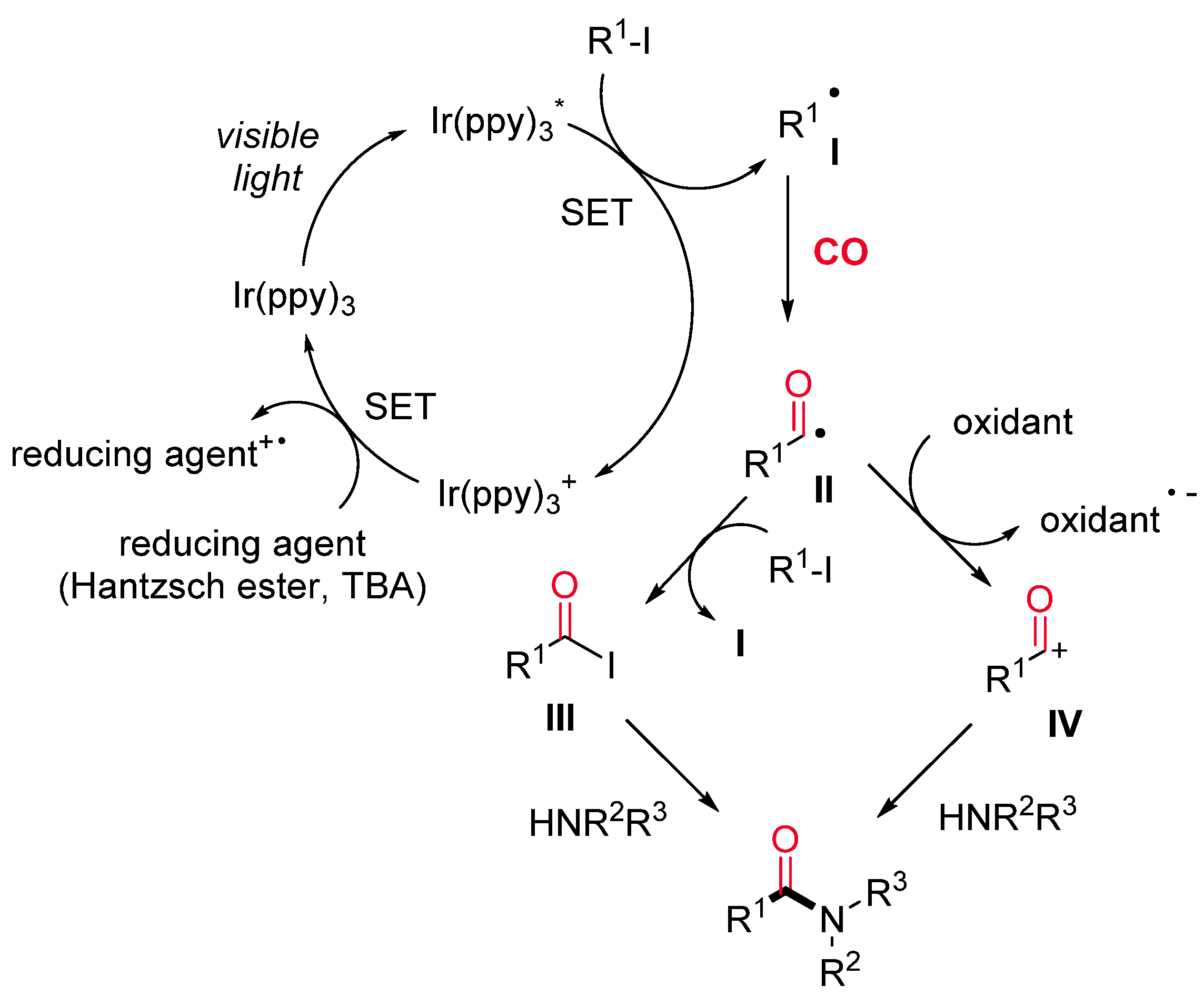
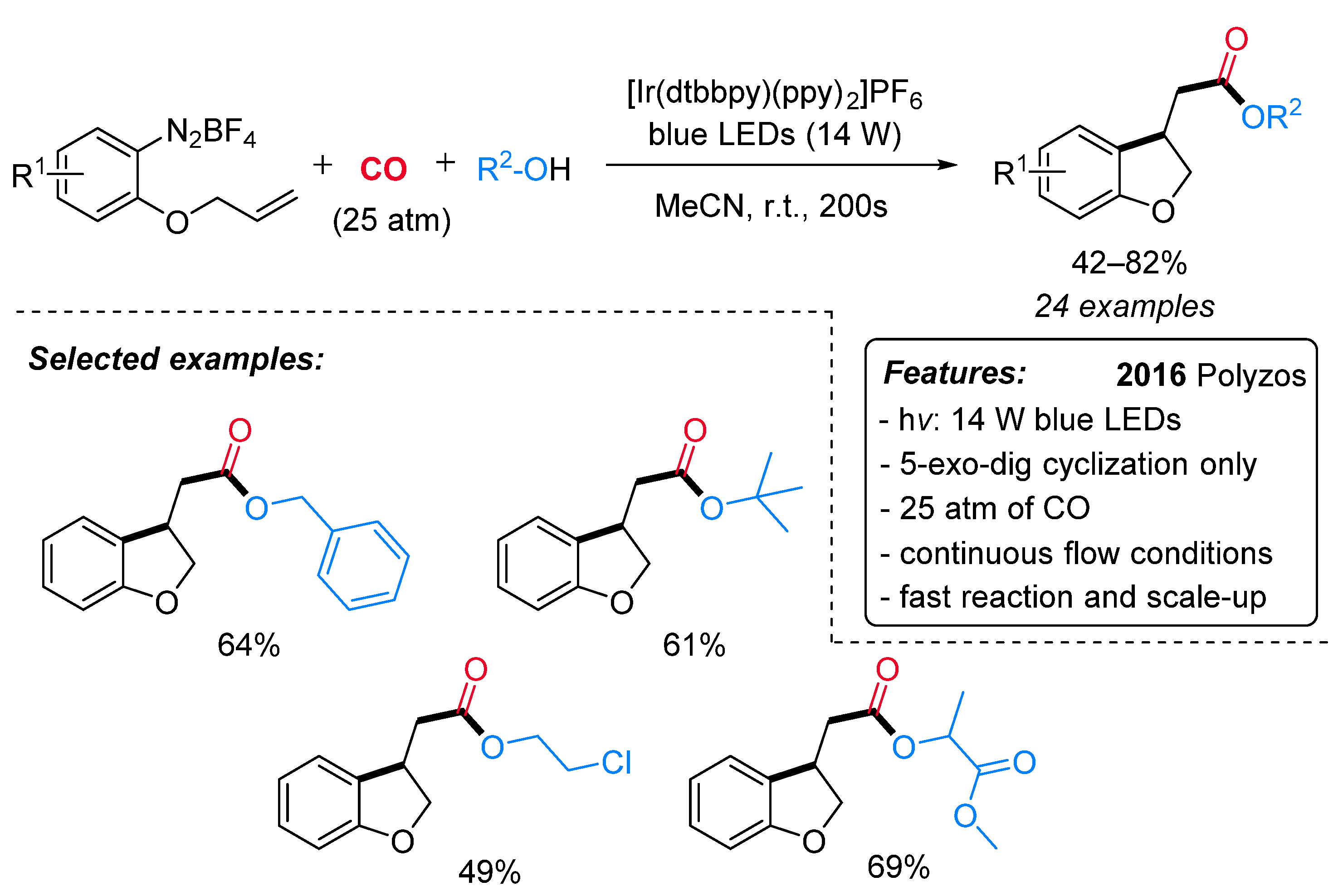
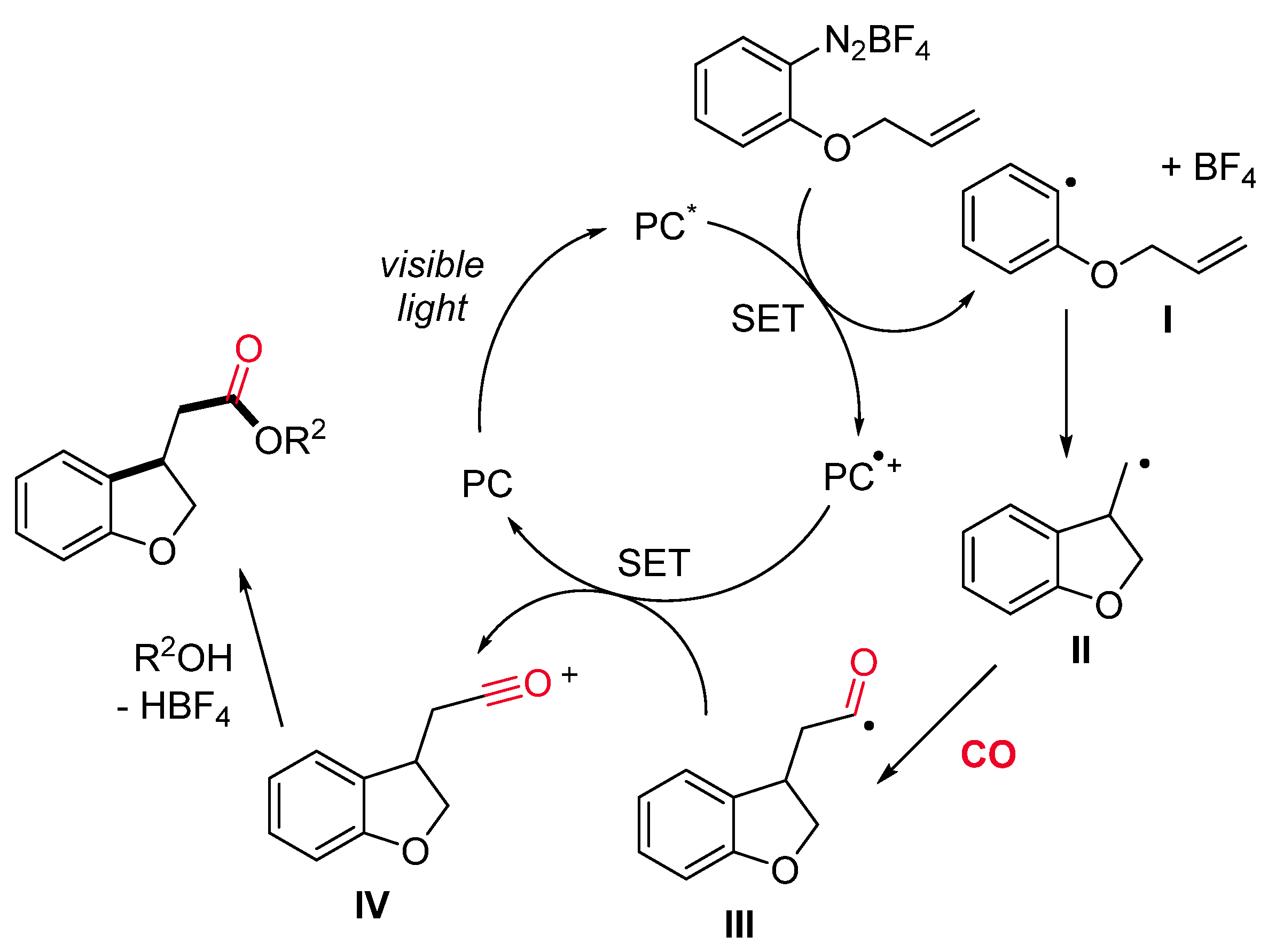
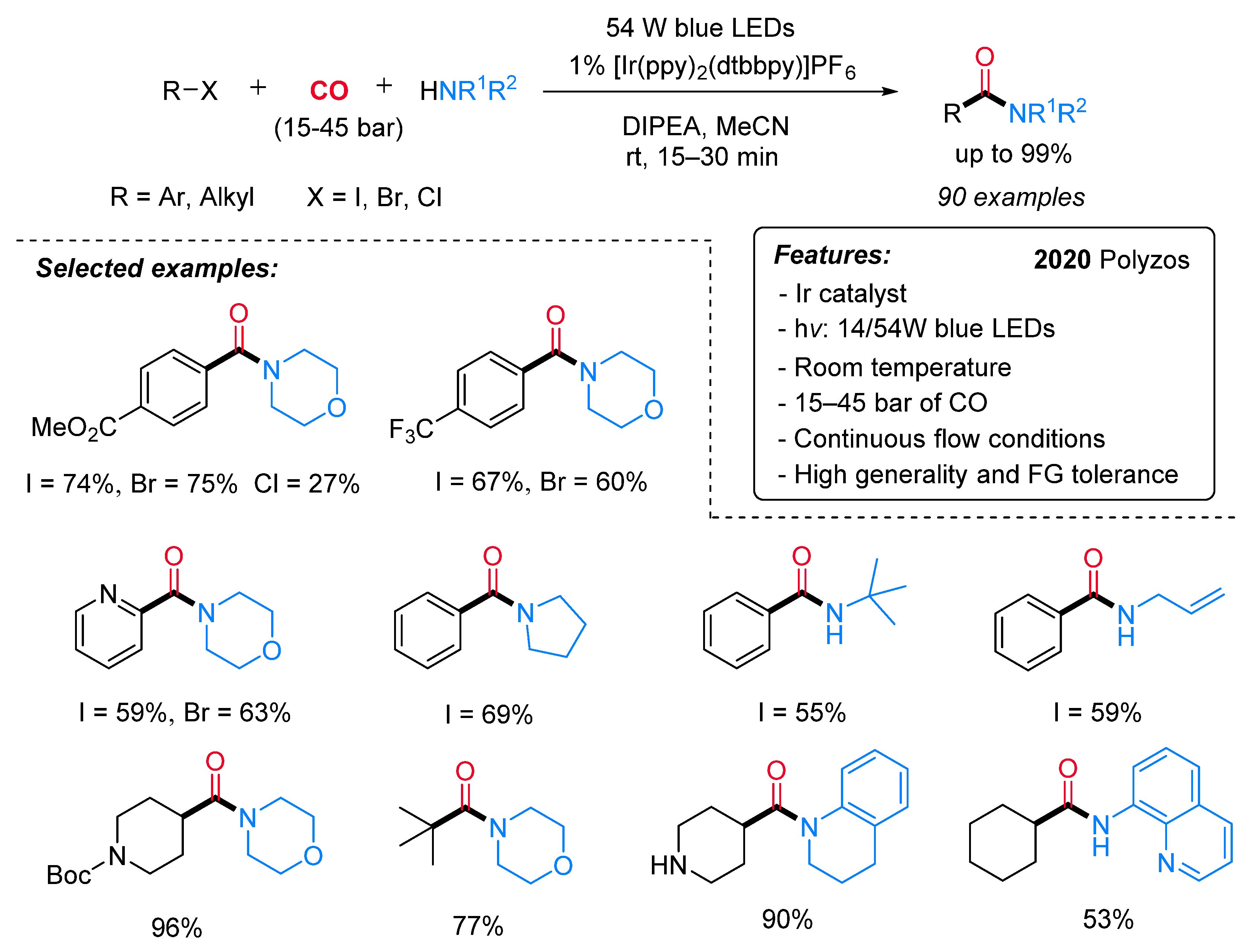
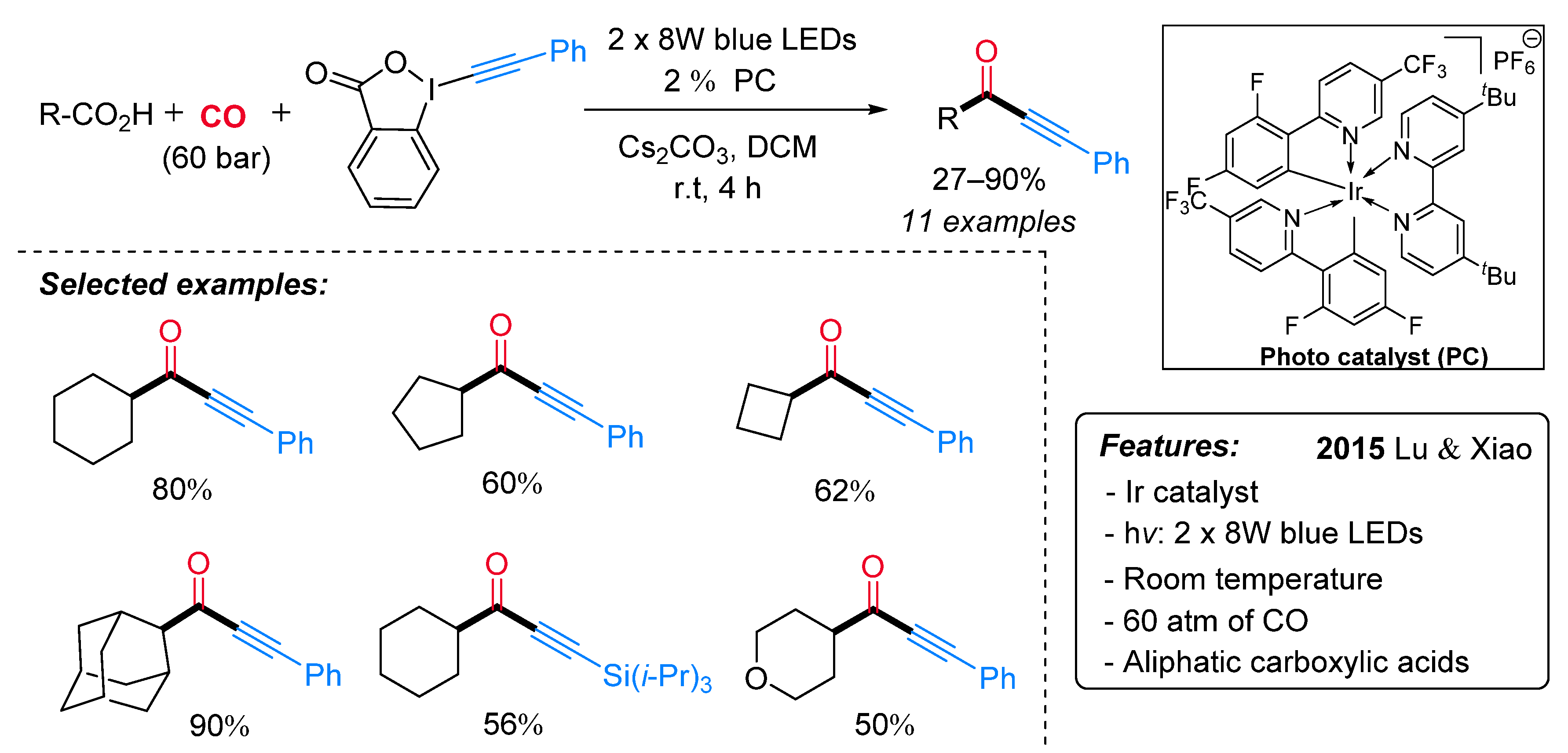
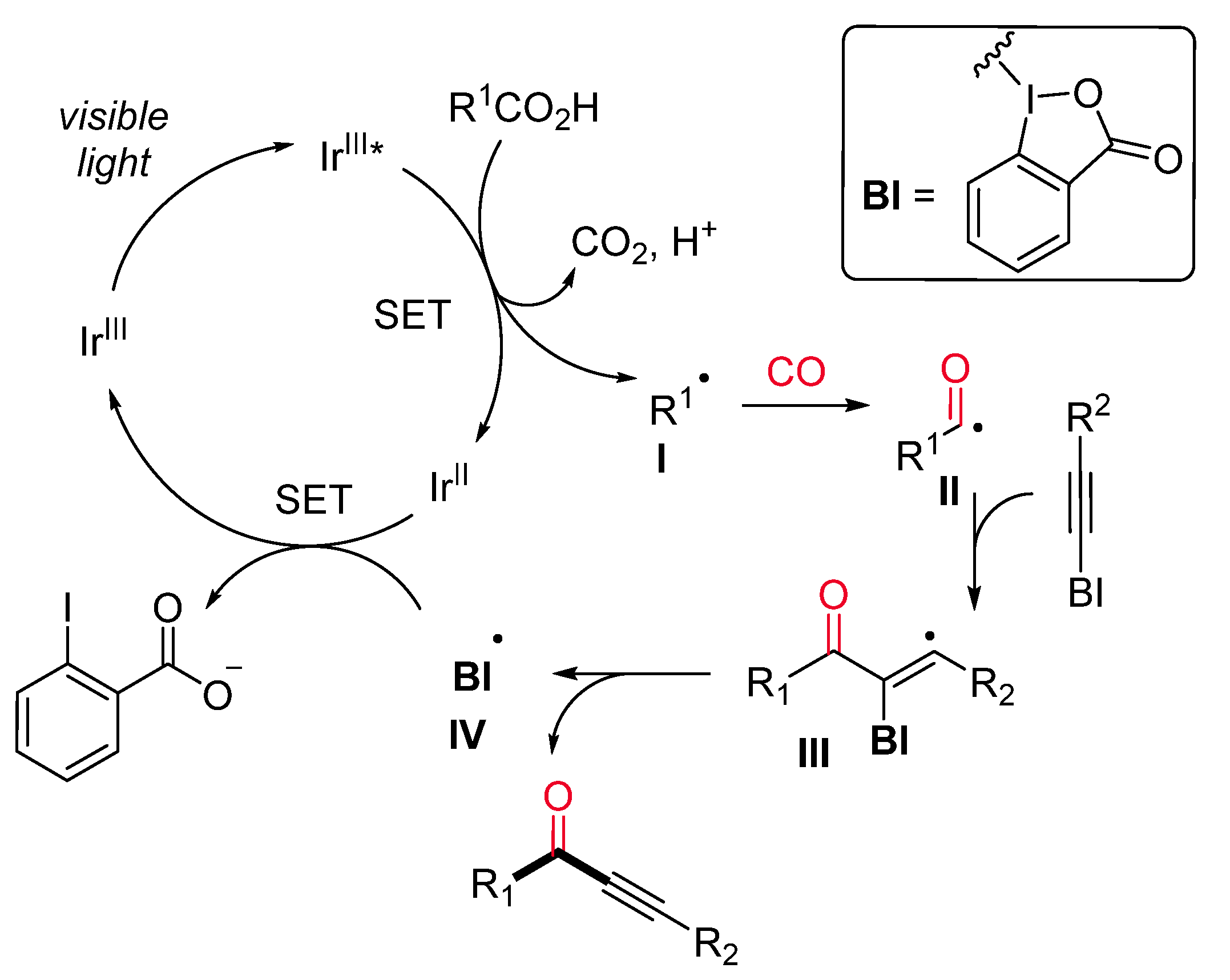
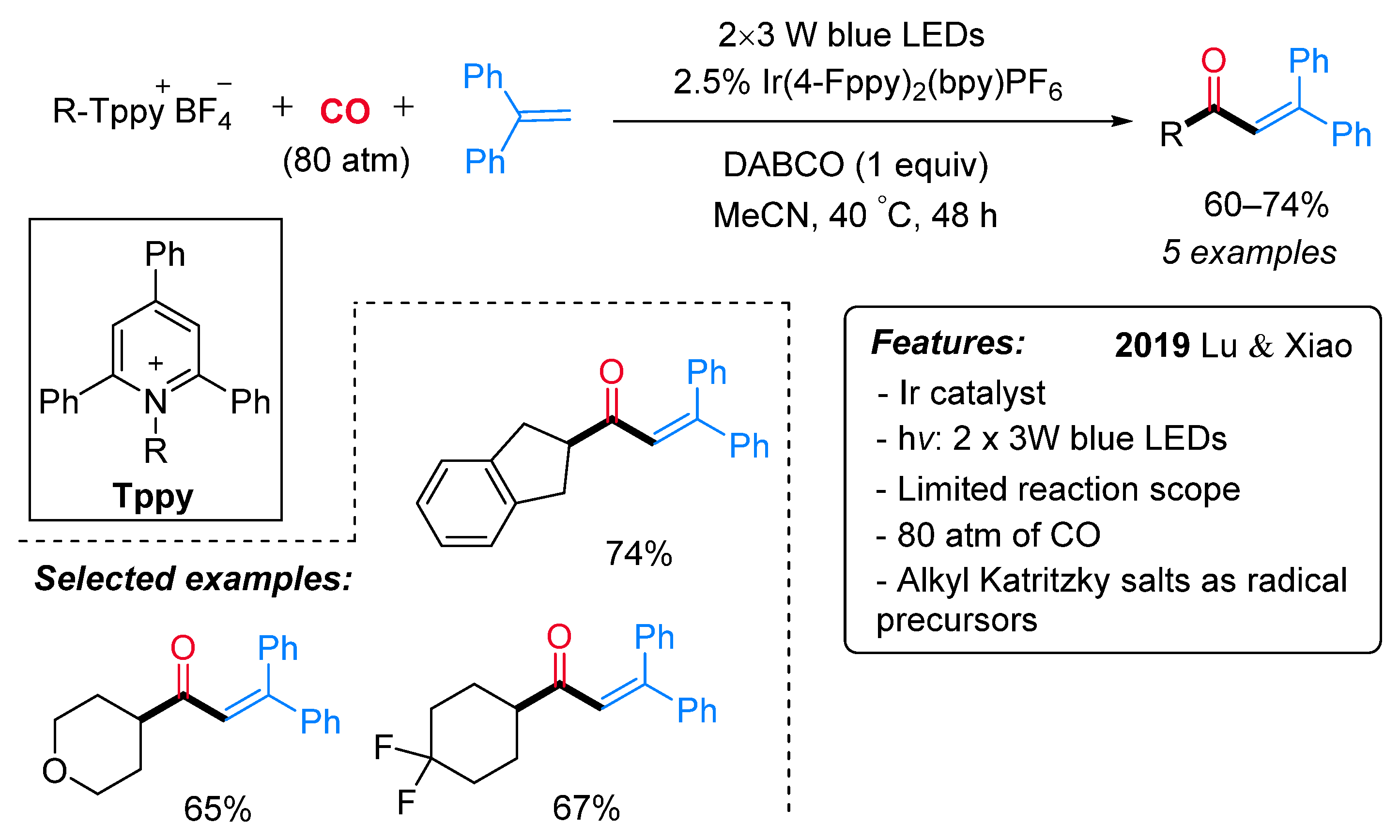
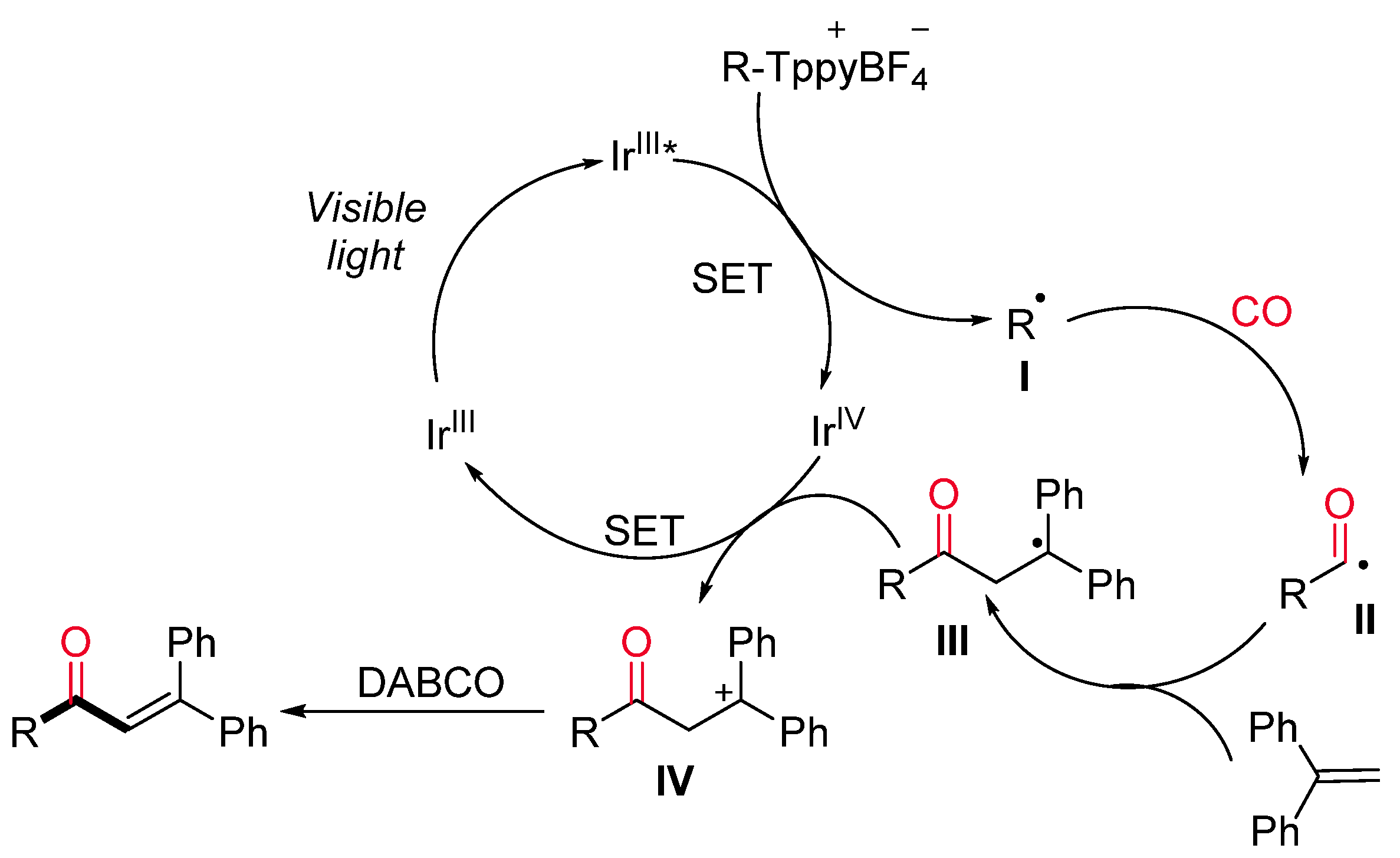
1.2. Visible-Light-Promoted Iridium-Catalyzed Carbonylations
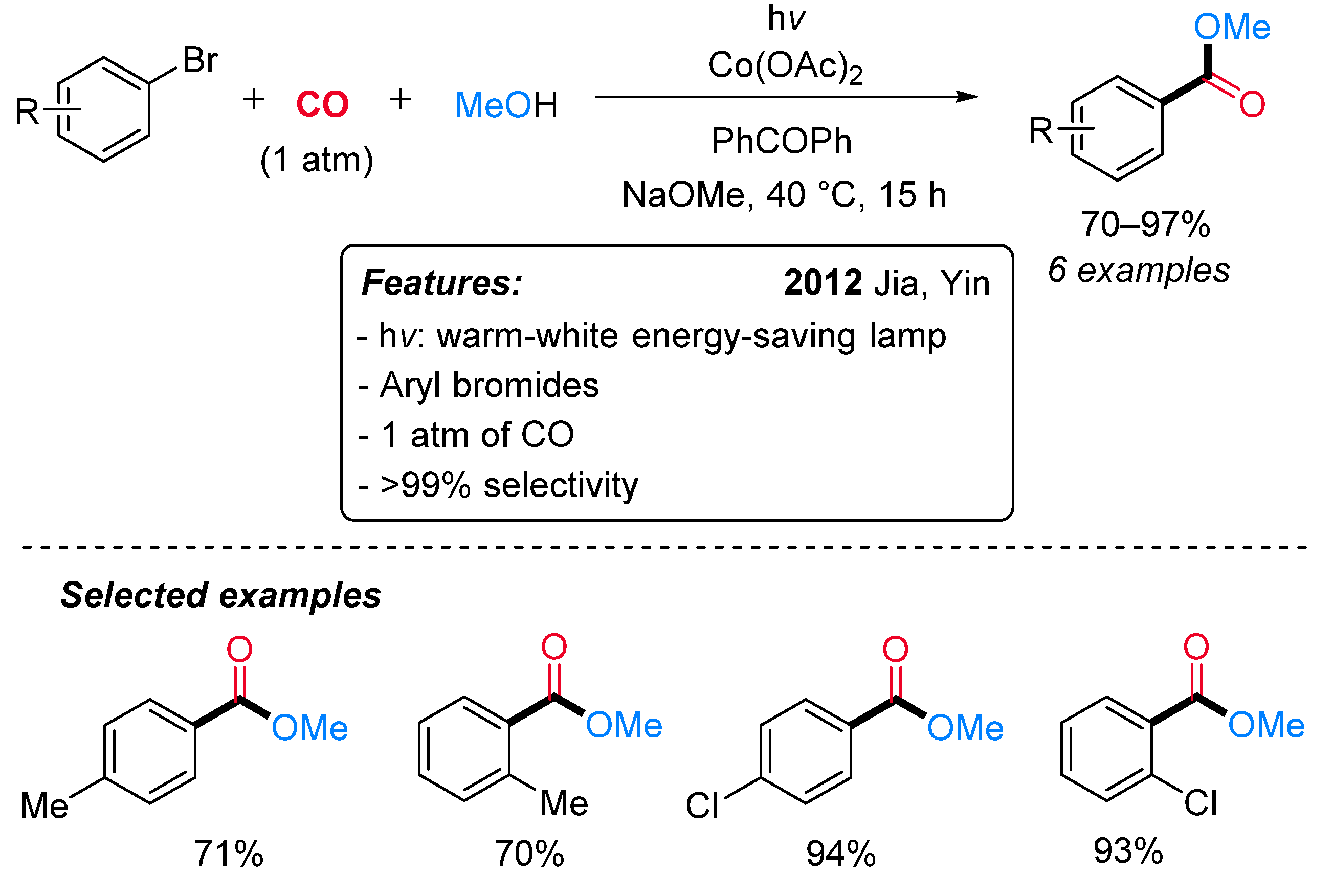
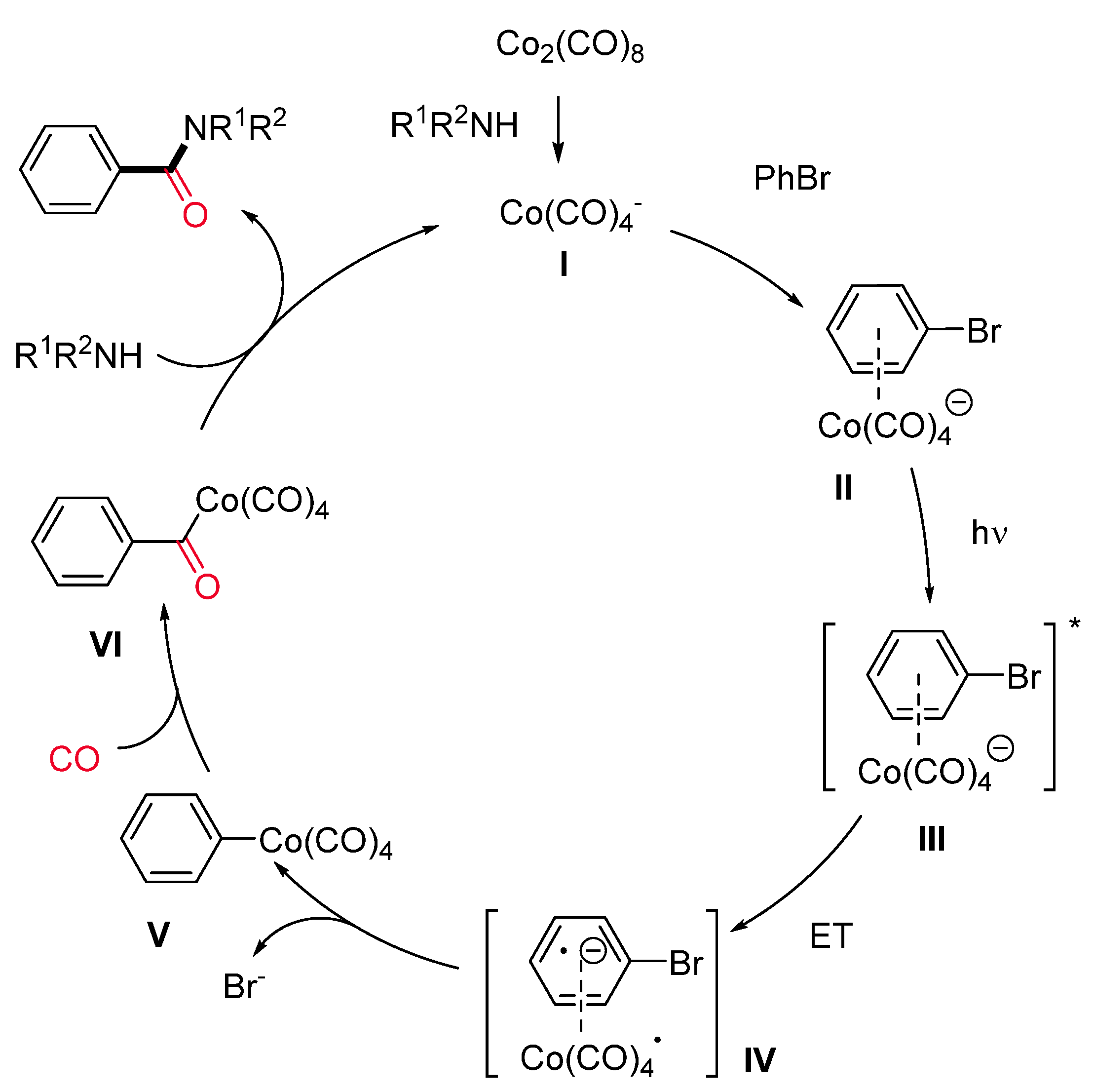
1.3. Visible-Light-Promoted Cobalt-Catalyzed Carbonylations
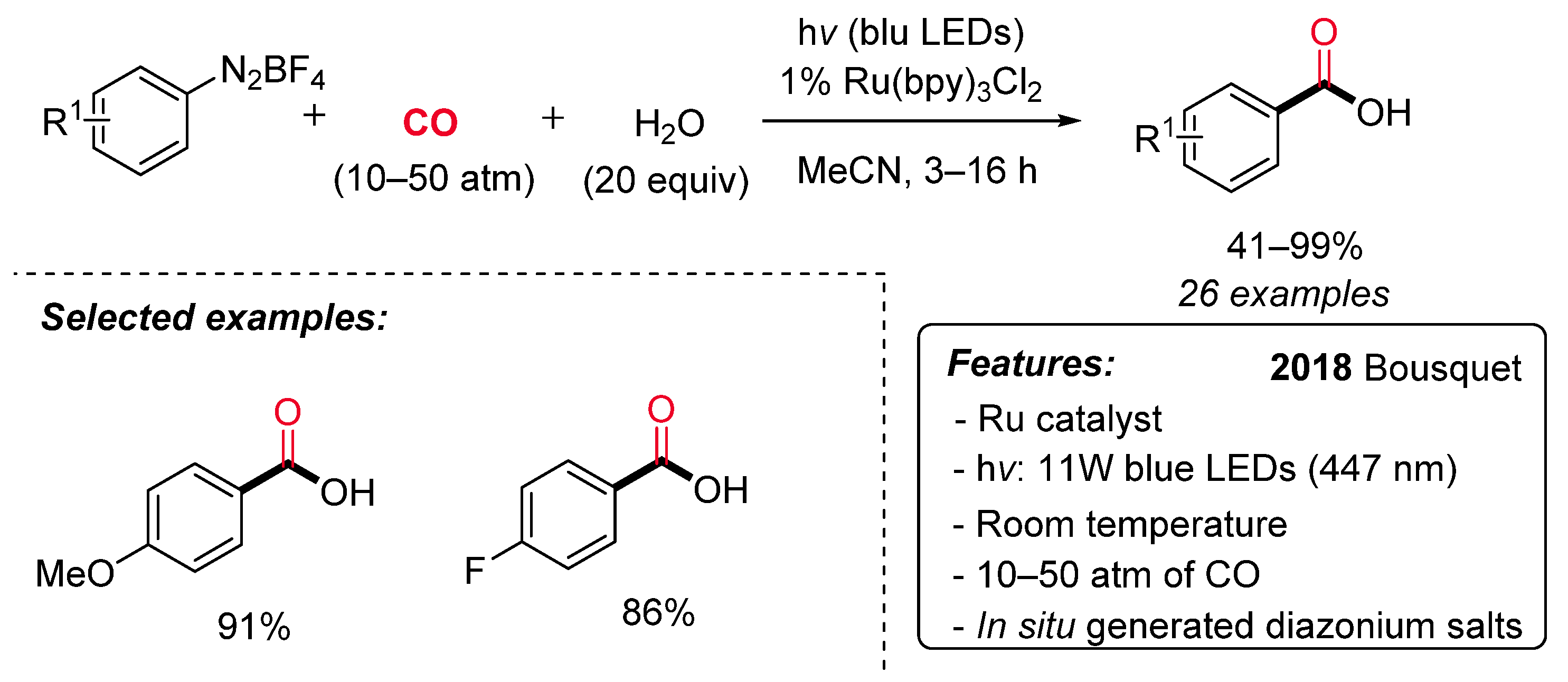
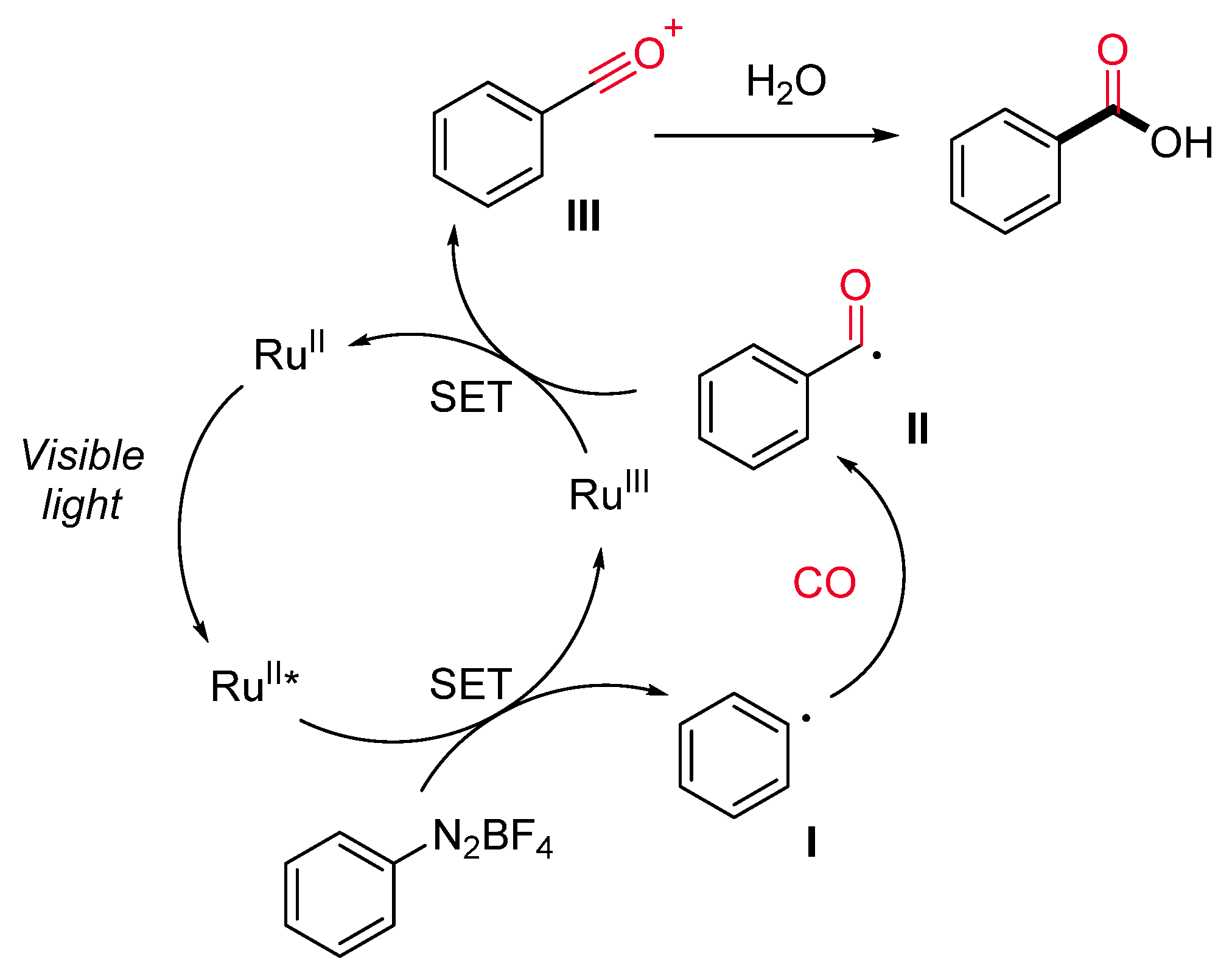
1.4. Visible-Light-Promoted Rutenium-Catalyzed Carbonylations
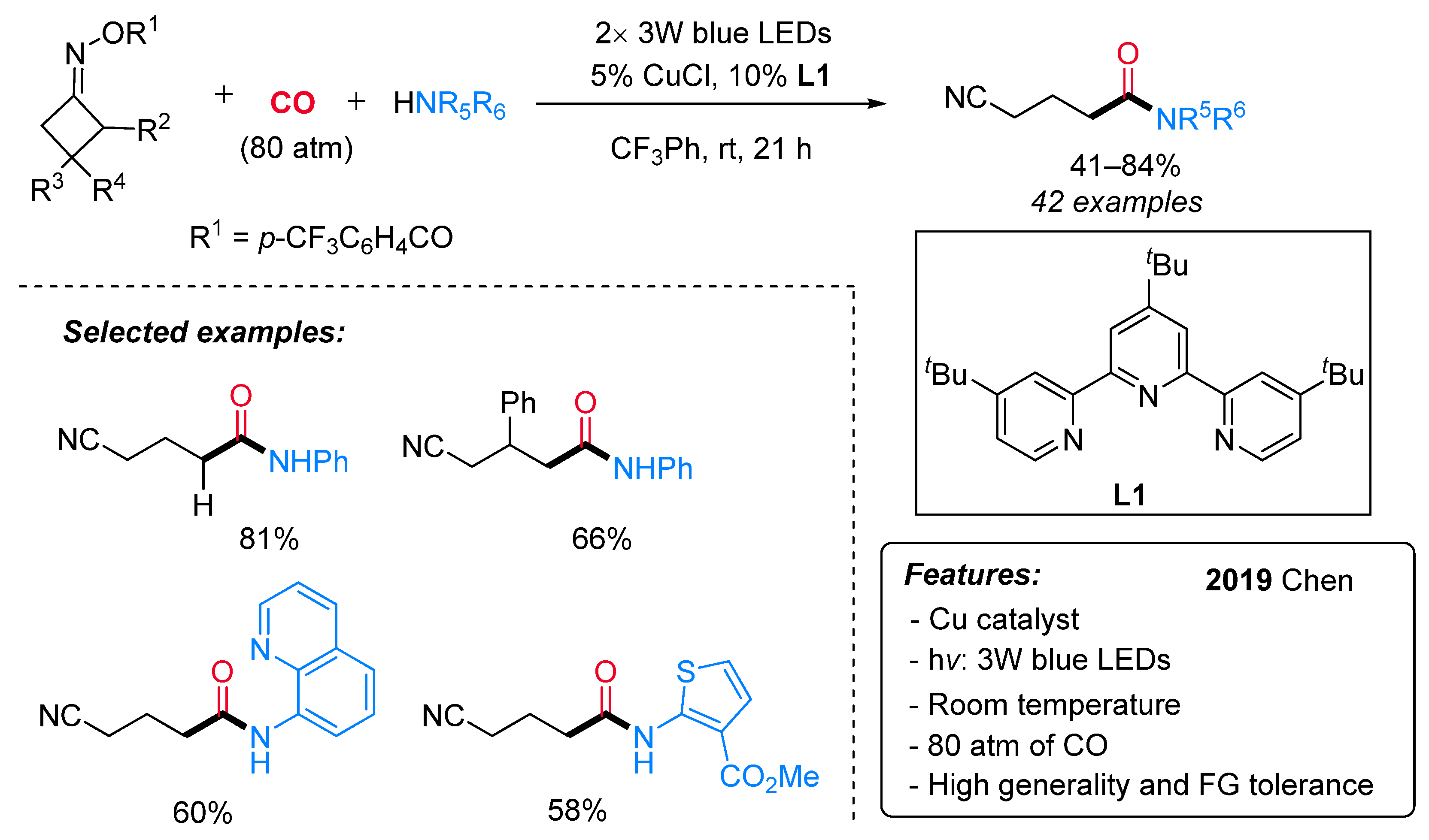
1.5. Visible-Light-Promoted Copper-Catalyzed Carbonylations
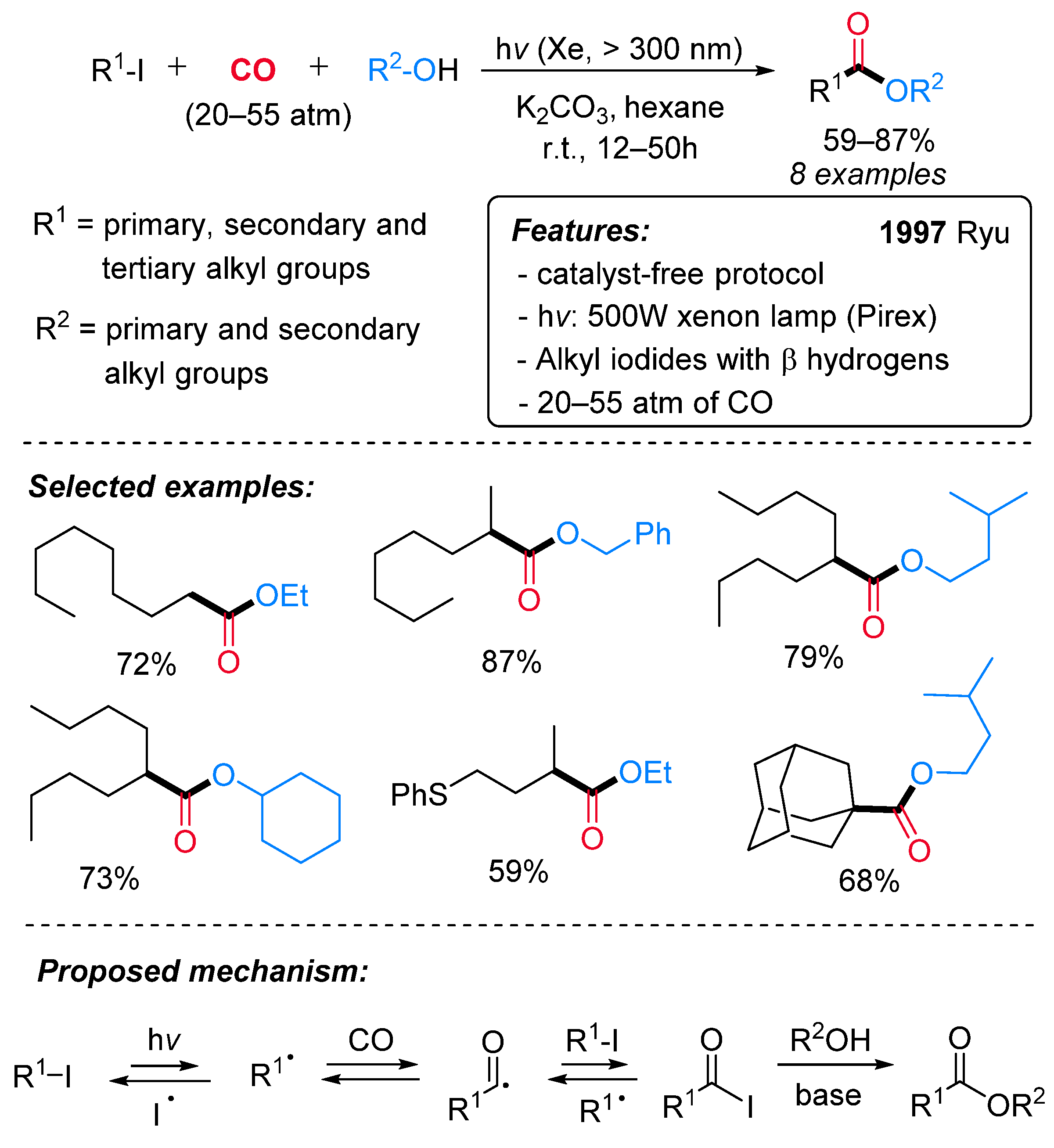
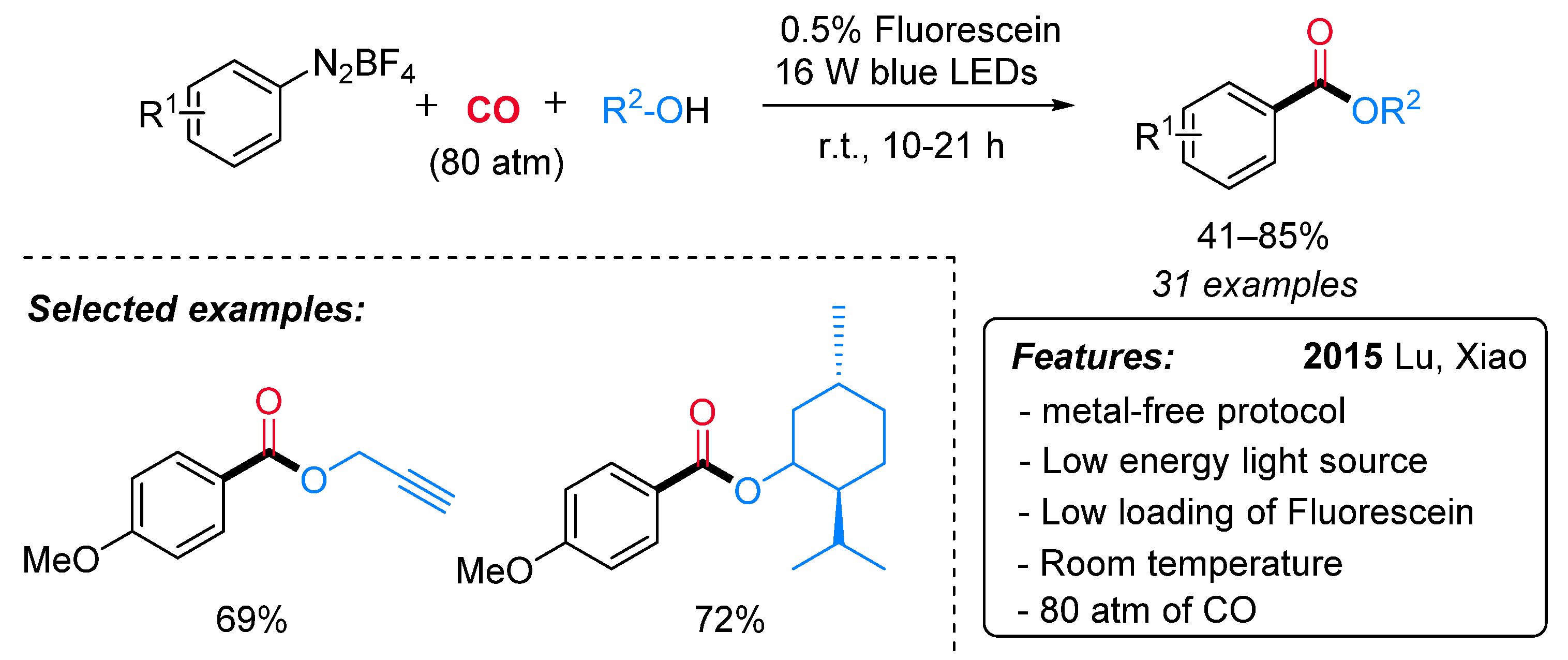
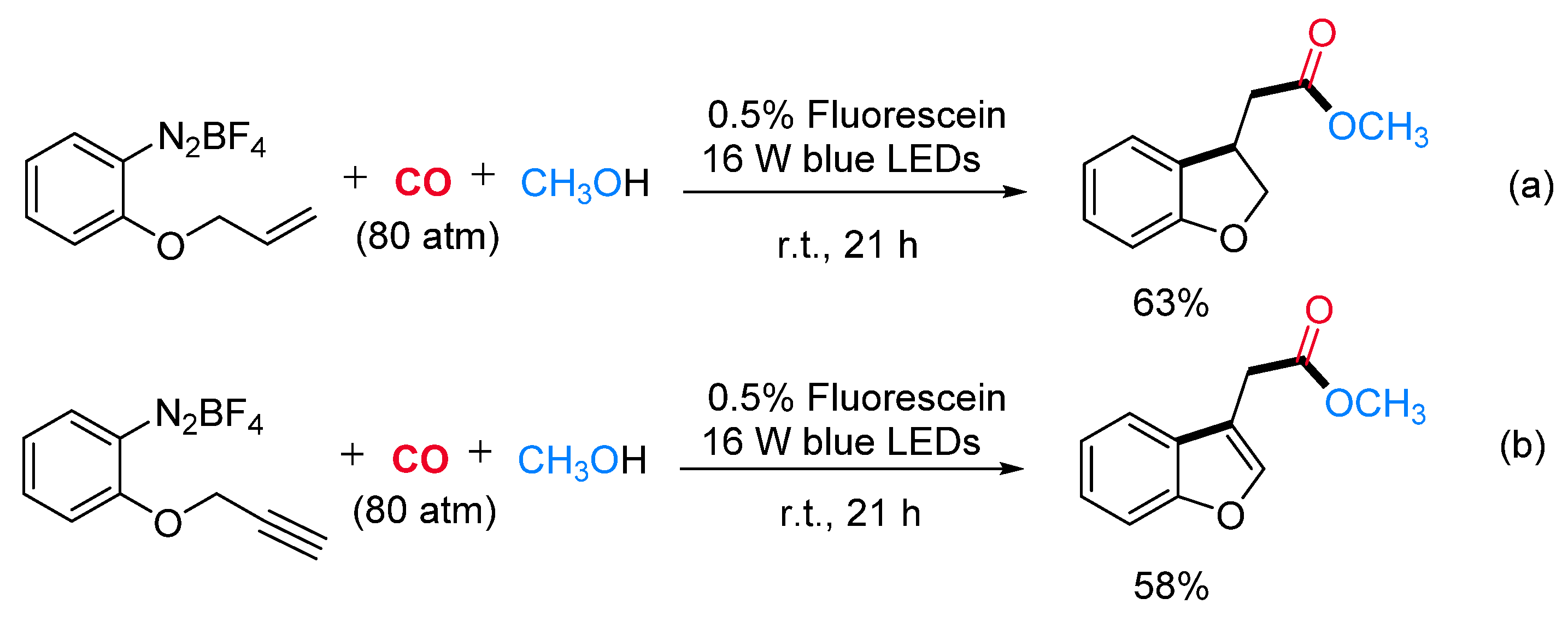
1.6. Visible-Light-Promoted Metal-Free Carbonylations
2. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, C.; Liu, J.; Li, M.-B.; Bäckvall, J.-E. Palladium-Catalyzed Oxidative Dehydrogenative Carbonylation Reactions Using Carbon Monoxide and Mechanistic Overviews. Chem. Soc. Rev. 2020, 49, 341–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Z.; Xu, J.-X.; Wu, X.-F. No Making without Breaking: Nitrogen-Centered Carbonylation Reactions. ACS Catal. 2020, 10, 6510–6531. [Google Scholar] [CrossRef]
- Zhang, S.; Neumann, H.; Beller, M. Synthesis of α,β-Unsaturated Carbonyl Compounds by Carbonylation Reactions. Chem. Soc. Rev. 2020, 49, 3187–3210. [Google Scholar] [CrossRef]
- Peng, J.-B.; Geng, H.-Q.; Wu, X.-F. The Chemistry of CO: Carbonylation. Chemistry 2019, 5, 526–552. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.-B.; Wu, F.-P.; Wu, X.-F. First-Row Transition-Metal-Catalyzed Carbonylative Transformations of Carbon Electrophiles. Chem. Rev. 2019, 119, 2090–2127. [Google Scholar] [CrossRef]
- Mancuso, R.; Della Ca’, N.; Veltri, L.; Ziccarelli, I.; Gabriele, B. PdI2-Based Catalysis for Carbonylation Reactions: A Personal Account. Catalysts 2019, 9, 610. [Google Scholar] [CrossRef] [Green Version]
- Romero, N.A.; Nicewicz, D.A. Organic Photoredox Catalysis. Chem. Rev. 2016, 116, 10075–10166. [Google Scholar] [CrossRef] [PubMed]
- Prier, C.K.; Rankic, D.A.; Mac-Millan, D.W. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef] [Green Version]
- Xuan, J.; Xiao, W.-J. Visible-Light Photoredox Catalysis. Angew. Chem. Int. Ed. 2012, 51, 6828–6838. [Google Scholar] [CrossRef] [PubMed]
- Narayanam, J.M.R.; Stephenson, C.R.J. Visible Light Photoredox Catalysis: Applications in Organic Synthesis. Chem. Soc. Rev. 2011, 40, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Sharma, S.; Sharma, A. Photocatalytic Carbonylation Strategies: A Recent Trend in Organic Synthesis. J. Org. Chem. 2021, 86, 24–48. [Google Scholar] [CrossRef]
- Hu, X.-Q.; Liu, Z.-K.; Xiao, W.-J. Radical Carbonylative Synthesis of Heterocycles by Visible Light Photoredox Catalysis. Catalysts 2020, 10, 1054. [Google Scholar] [CrossRef]
- Zhao, S.; Mankad, N.P. Metal-Catalysed Radical Carbonylation Reactions. Catal. Sci. Technol. 2019, 9, 3603–3613. [Google Scholar] [CrossRef]
- Peng, J.-B.; Qi, X.; Wu, X.-F. Visible Light-Induced Carbonylation Reactions with Organic Dyes as the Photosensitizers. ChemSusChem 2016, 9, 2279–2283. [Google Scholar] [CrossRef]
- Sumino, S.; Fusano, A.; Fukuyama, T.; Ryu, I. Carbonylation Reactions of Alkyl Iodides through the Interplay of Carbon Radicals and Pd Catalysts. Acc. Chem. Res. 2014, 47, 1563–1574. [Google Scholar] [CrossRef] [PubMed]
- Kunin, A.J.; Eisenberg, R. Photochemical Carbonylation of Benzene by Iridium(I) and Rhodium(I) Square-Planar Complexes. Organometallics 1988, 7, 2124–2129. [Google Scholar] [CrossRef]
- Ferguson, R.R.; Crabtree, R.H. Mercury-Photosensitized Sulfination, Hydrosulfination, and Carbonylation of Hydrocarbons: Alkane and Alkene Conversion to Sulfonic Acids, Ketones, and Aldehydes. J. Org. Chem. 1991, 56, 5503–5510. [Google Scholar] [CrossRef]
- Sakakura, T.; Sodeyama, T.; Sasaki, K.; Wada, K.; Tanaka, M. Carbonylation of Hydrocarbons via Carbon-Hydrogen Activation Catalyzed by RhCl(CO)(PMe3)2 under Irradiation. J. Am. Chem. Soc. 1990, 112, 7221–7229. [Google Scholar] [CrossRef]
- Jaynes, B.S.; Hill, C.L. Radical Carbonylation of Alkanes via Polyoxotungstate Photocatalysis. J. Am. Chem. Soc. 1995, 117, 4704–4705. [Google Scholar] [CrossRef]
- Ryu, I.; Muraoka, H.; Kambe, N.; Komatsu, M.; Sonoda, N. Group Transfer Carbonylations: Photoinduced Alkylative Carbonylation of Alkenes Accompanied by Phenylselenenyl Transfer. J. Org. Chem. 1996, 61, 6396–6403. [Google Scholar] [CrossRef] [PubMed]
- Pagenkopf, B.L.; Livinghouse, T. Photochemical Promotion of the Intramolecular Pauson−Khand Reaction. A New Experimental Protocol for Cobalt-Catalyzed [2 + 2 + 1] Cycloadditions. J. Am. Chem. Soc. 1996, 118, 2285–2286. [Google Scholar] [CrossRef]
- Ogawa, A.; Sumino, Y.; Nanke, T.; Ohya, S.; Sonoda, N.; Hirao, T. Photoinduced Reduction and Carbonylation of Organic Chlorides with Samarium Diiodide. J. Am. Chem. Soc. 1997, 119, 2745–2746. [Google Scholar] [CrossRef]
- Sigman, M.S.; Eaton, B.E. The First Iron-Mediated Catalytic Carbon-Nitrogen Bond Formation: [4 + 1] Cycloaddition of Allenyl Imines and Carbon Monoxide. J. Org. Chem. 1994, 59, 7488–7491. [Google Scholar] [CrossRef]
- Ishiyama, T.; Miyaura, N.; Suzuki, A. Palladium-Catalyzed Carbonylative Cross-Coupling Reaction of Iodoalkanes with 9-Alkyl-9-BBN Derivatives. A Direct and Selective Synthesis of Ketones. Tetrahedron Lett. 1991, 32, 6923–6926. [Google Scholar] [CrossRef]
- Kramer, A.V.; Osborn, J.A. Mechanistic Studies of Oxidative Addition to Low Valent Metal Complexes. IV. CIDNP Effects in Platinum(0) and Palladium(0) Reactions. J. Am. Chem. Soc. 1974, 96, 7832–7833. [Google Scholar] [CrossRef]
- Ishiyama, T.; Murata, M.; Suzuki, A.; Miyaura, N. Synthesis of Ketones from Iodoalkenes, Carbon Monoxide and 9-Alkyl-9-Borabicyclo[3.3.1]Nonane Derivatives via a Radical Cyclization and Palladium-Catalysed Carbonylative Cross-Coupling Sequence. J. Chem. Soc. Chem. Commun. 1995, 295–296. [Google Scholar] [CrossRef]
- Matsubara, H.; Kawamoto, T.; Fukuyama, T.; Ryu, I. Applications of Radical Carbonylation and Amine Addition Chemistry: 1,4-Hydrogen Transfer of 1-Hydroxylallyl Radicals. Acc. Chem. Res. 2018, 51, 2023–2035. [Google Scholar] [CrossRef] [PubMed]
- Ryu, I. Radical Carboxylations of Iodoalkanes and Saturated Alcohols Using Carbon Monoxide. Chem. Soc. Rev. 2001, 30, 16–25. [Google Scholar] [CrossRef]
- Nagahara, K.; Ryu, I.; Komatsu, M.; Sonoda, N. Radical Carboxylation: Ester Synthesis from Alkyl Iodides, Carbon Monoxide, and Alcohols under Irradiation Conditions. J. Am. Chem. Soc. 1997, 119, 5465–5466. [Google Scholar] [CrossRef]
- Ryu, I.; Kreimerman, S.; Araki, F.; Nishitani, S.; Oderaotoshi, Y.; Minakata, S.; Komatsu, M. Cascade Radical Reactions Catalyzed by a Pd/Light System: Cyclizative Multiple Carbonylation of 4-Alkenyl Iodides. J. Am. Chem. Soc. 2002, 124, 3812–3813. [Google Scholar] [CrossRef] [PubMed]
- Brennführer, A.; Neumann, H.; Beller, M. Palladium-Catalyzed Carbonylation Reactions of Aryl Halides and Related Compounds. Angew. Chem. Int. Ed. 2009, 48, 4114–4133. [Google Scholar] [CrossRef]
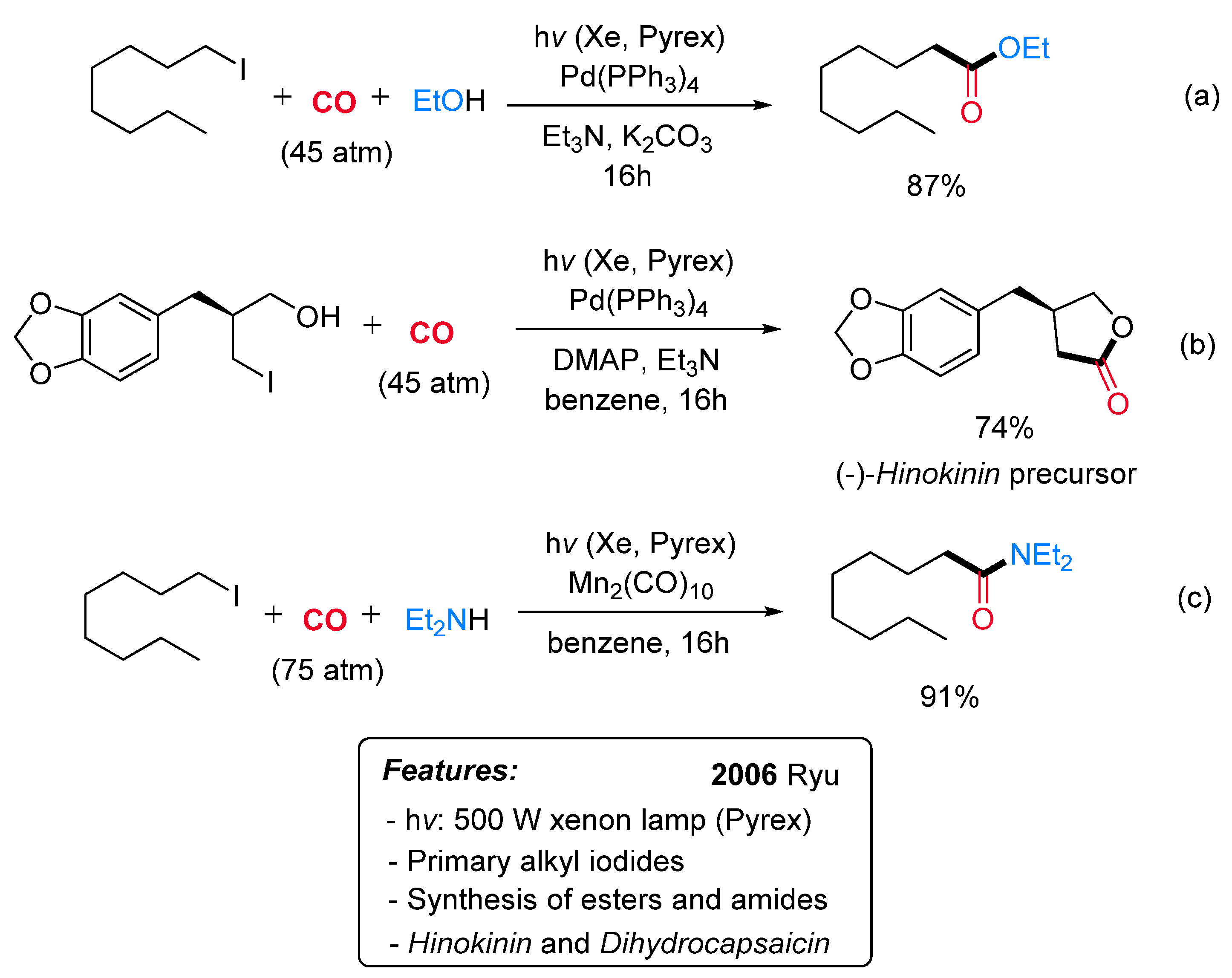
- Fukuyama, T.; Nishitani, S.; Inouye, T.; Morimoto, K.; Ryu, I. Effective Acceleration of Atom Transfer Carbonylation of Alkyl Iodides by Metal Complexes. Application to the Synthesis of the Hinokinin Precursor and Dihydrocapsaicin. Org. Lett. 2006, 8, 1383–1386. [Google Scholar] [CrossRef]
- Fusano, A.; Sumino, S.; Nishitani, S.; Inouye, T.; Morimoto, K.; Fukuyama, T.; Ryu, I. Pd/Light-Accelerated Atom-Transfer Carbonylation of Alkyl Iodides: Applications in Multicomponent Coupling Processes Leading to Functionalized Carboxylic Acid Derivatives. Chem. Eur. J. 2012, 18, 9415–9422. [Google Scholar] [CrossRef] [PubMed]
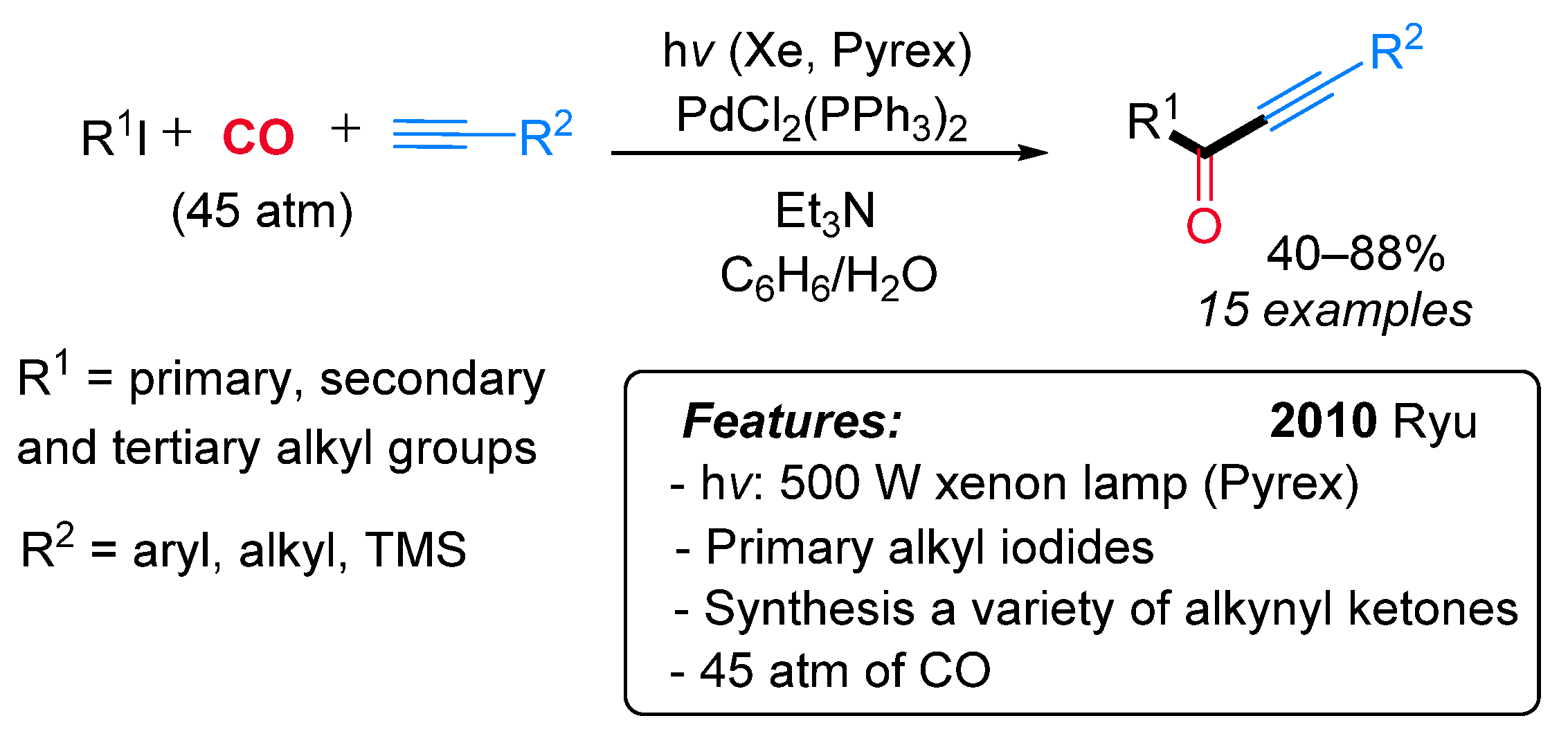
- Fusano, A.; Fukuyama, T.; Nishitani, S.; Inouye, T.; Ryu, I. Synthesis of Alkyl Alkynyl Ketones by Pd/Light-Induced Three-Component Coupling Reactions of Iodoalkanes, CO, and 1-Alkynes. Org. Lett. 2010, 12, 2410–2413. [Google Scholar] [CrossRef]
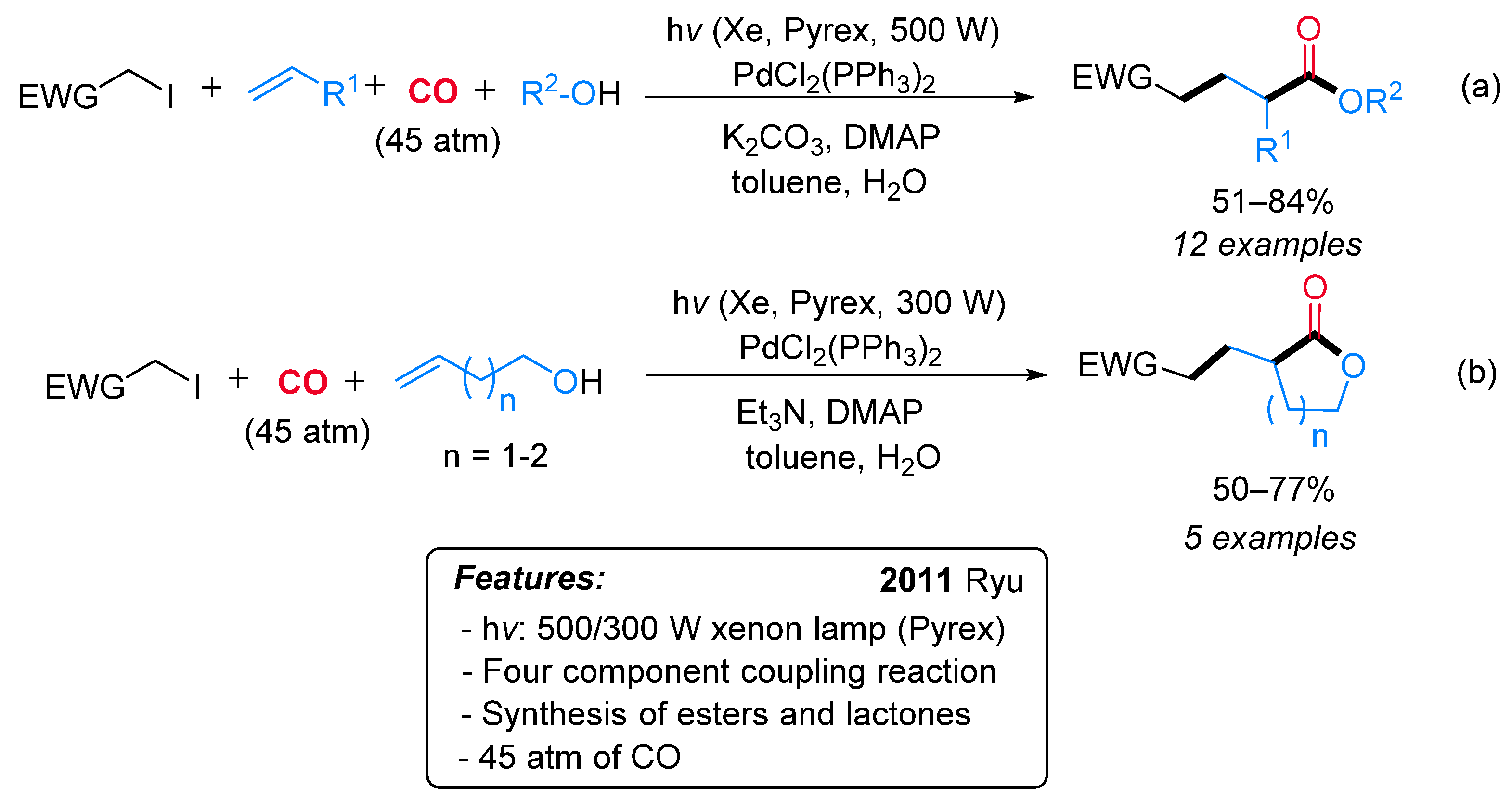
- Fusano, A.; Sumino, S.; Fukuyama, T.; Ryu, I. Vicinal C-Functionalization of Alkenes. Pd/Light-Induced Multicomponent Coupling Reactions Leading to Functionalized Esters and Lactones. Org. Lett. 2011, 13, 2114–2117. [Google Scholar] [CrossRef]
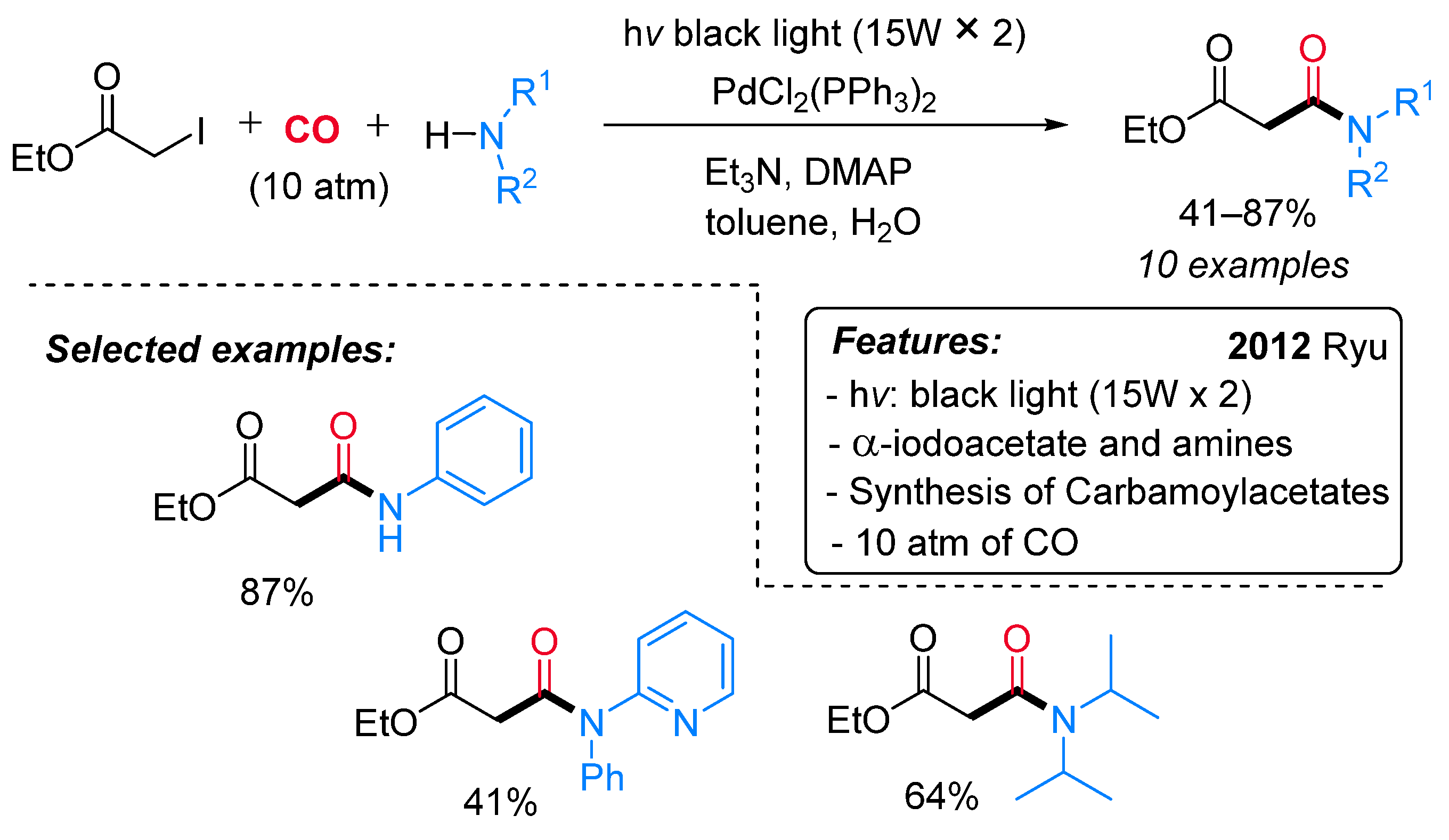
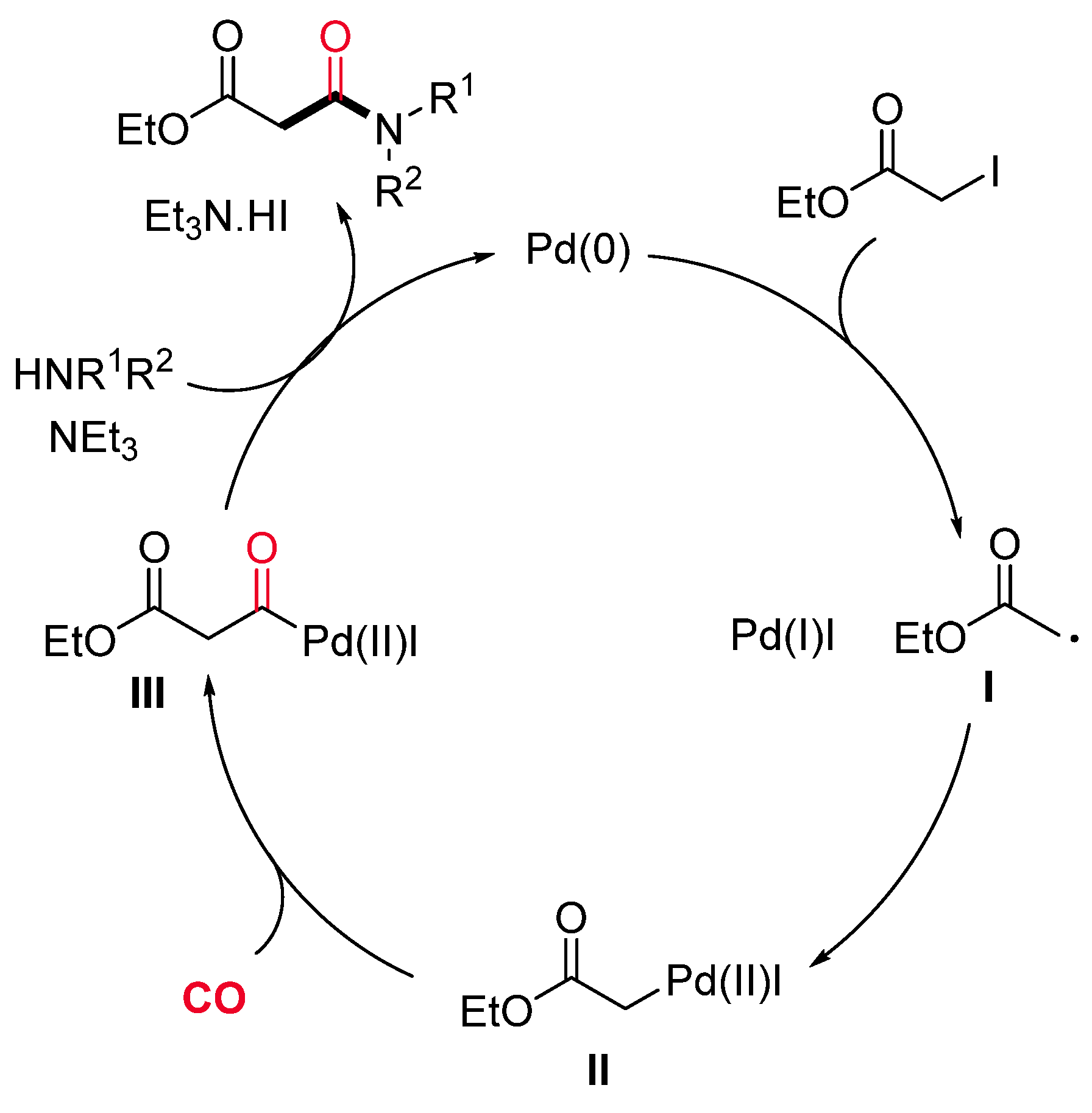
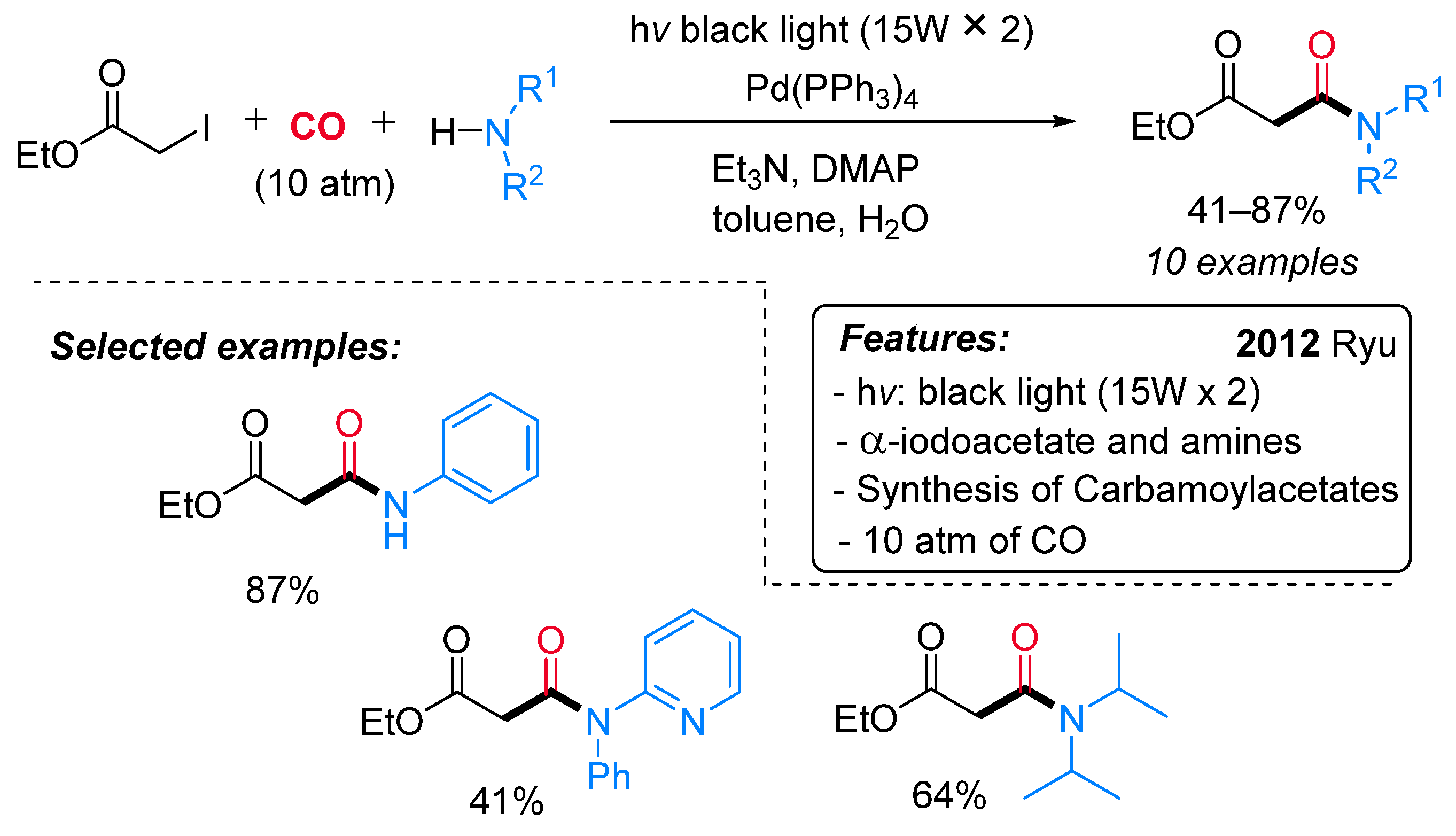
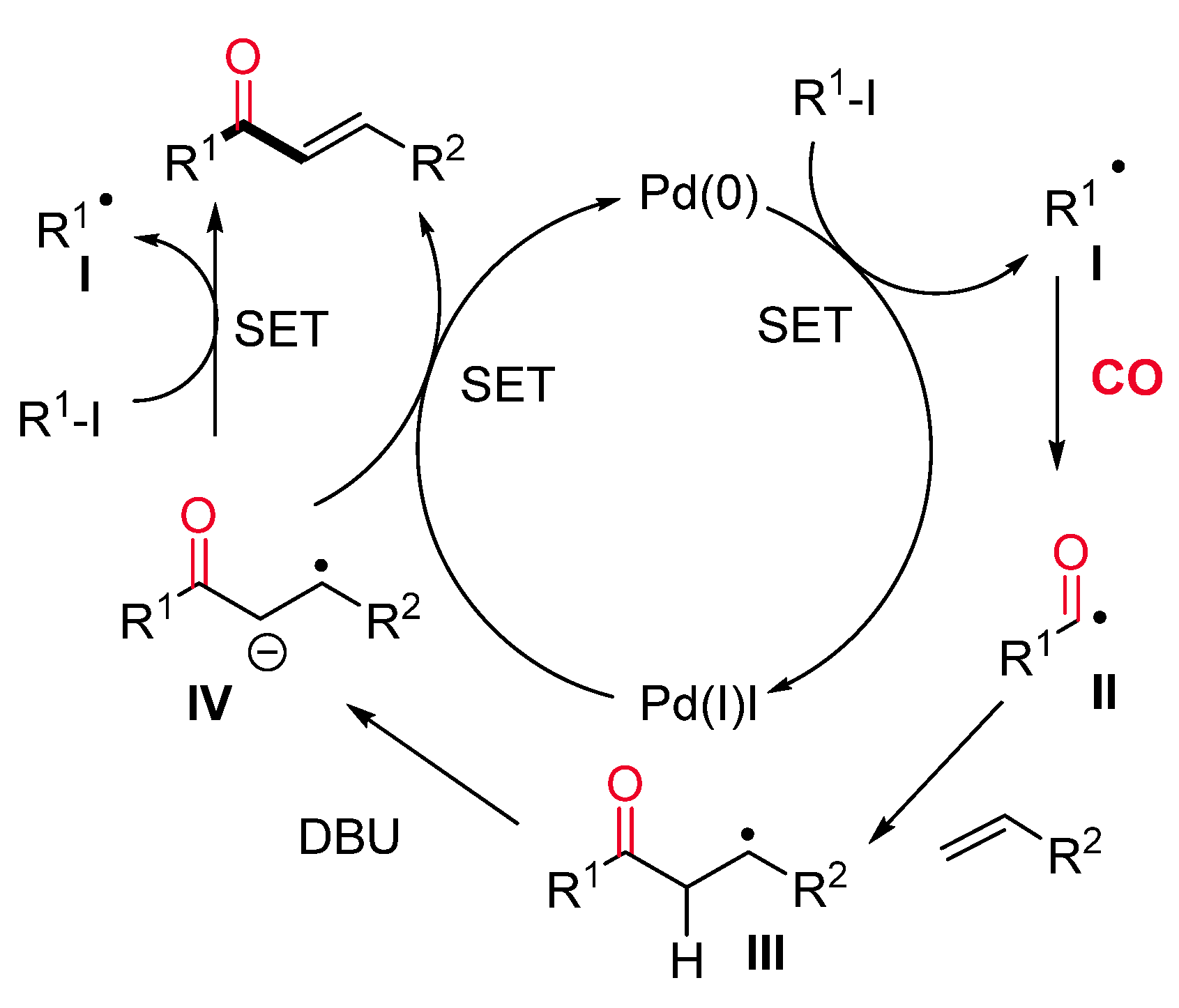
- Sumino, S.; Fusano, A.; Fukuyama, T.; Ryu, I. Synthesis of Carbamoylacetates from α-Iodoacetate, CO, and Amines under Pd/Light Combined Conditions. Synlett 2012, 23, 1331–1334. [Google Scholar] [CrossRef]
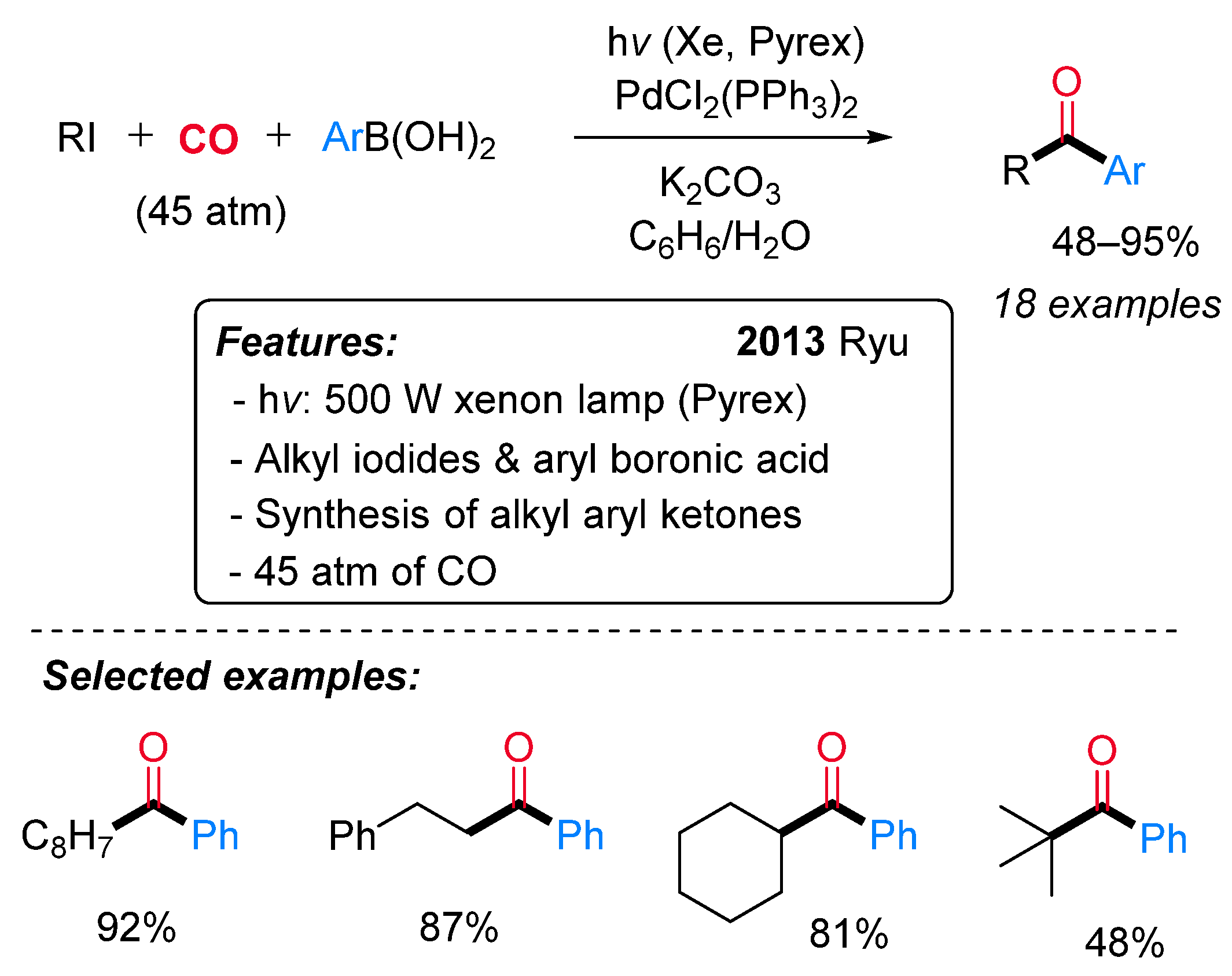
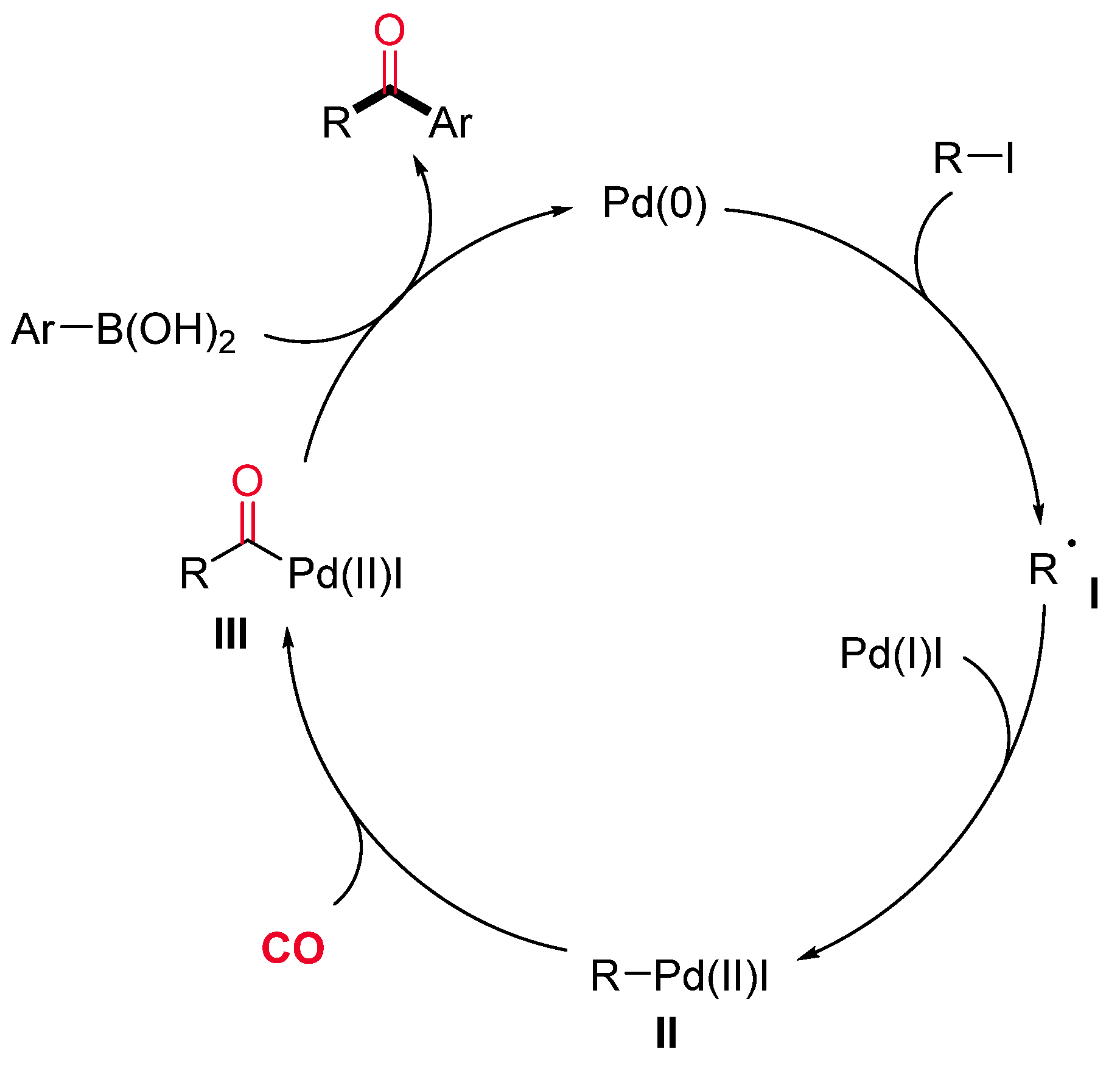
- Sumino, S.; Ui, T.; Ryu, I. Synthesis of Alkyl Aryl Ketones by Pd/Light Induced Carbonylative Cross-Coupling of Alkyl Iodides and Arylboronic Acids. Org. Lett. 2013, 15, 3142–3145. [Google Scholar] [CrossRef]
- Sumino, S.; Ui, T.; Ryu, I. Synthesis of Aromatic β-Keto Esters via a Carbonylative Suzuki–Miyaura Coupling Reaction of α-Iodo Esters with Arylboronic Acids. Org. Chem. Front. 2015, 2, 1085–1087. [Google Scholar] [CrossRef]
- Feng, X.; Li, Z. Photocatalytic Promoting Dimethylformamide (DMF) Decomposition to In-Situ Generation of Self-Supplied CO for Carbonylative Suzuki Reaction. J. Photochem. Photobiol. 2017, 337, 19–24. [Google Scholar] [CrossRef]
- Sumino, S.; Ui, T.; Hamada, Y.; Fukuyama, T.; Ryu, I. Carbonylative Mizoroki–Heck Reaction of Alkyl Iodides with Arylalkenes Using a Pd/Photoirradiation System. Org. Lett. 2015, 17, 4952–4955. [Google Scholar] [CrossRef]
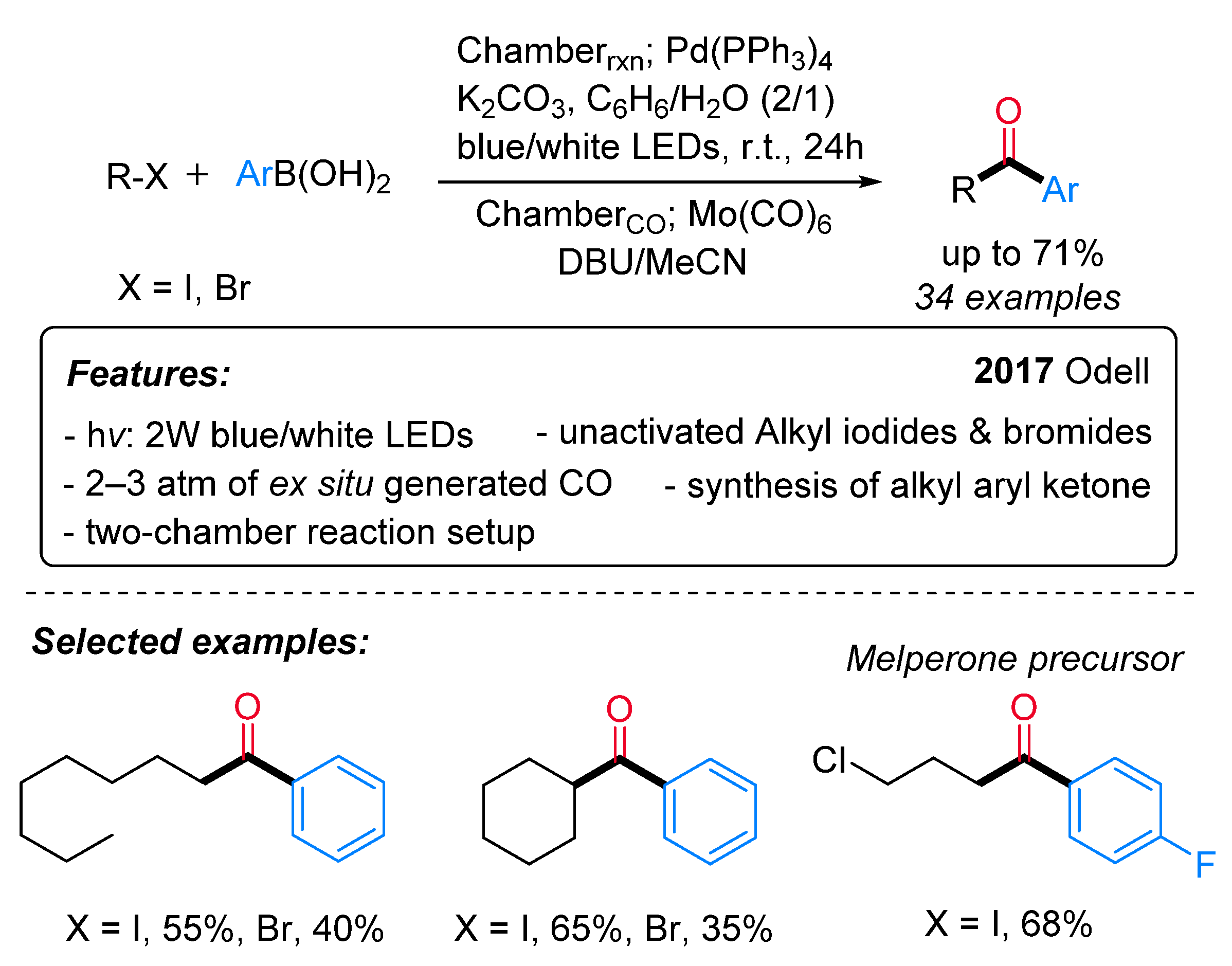
- Roslin, S.; Odell, L.R. Palladium and Visible-Light Mediated Carbonylative Suzuki–Miyaura Coupling of Unactivated Alkyl Halides and Aryl Boronic Acids. Chem. Commun. 2017, 53, 6895–6898. [Google Scholar] [CrossRef] [Green Version]
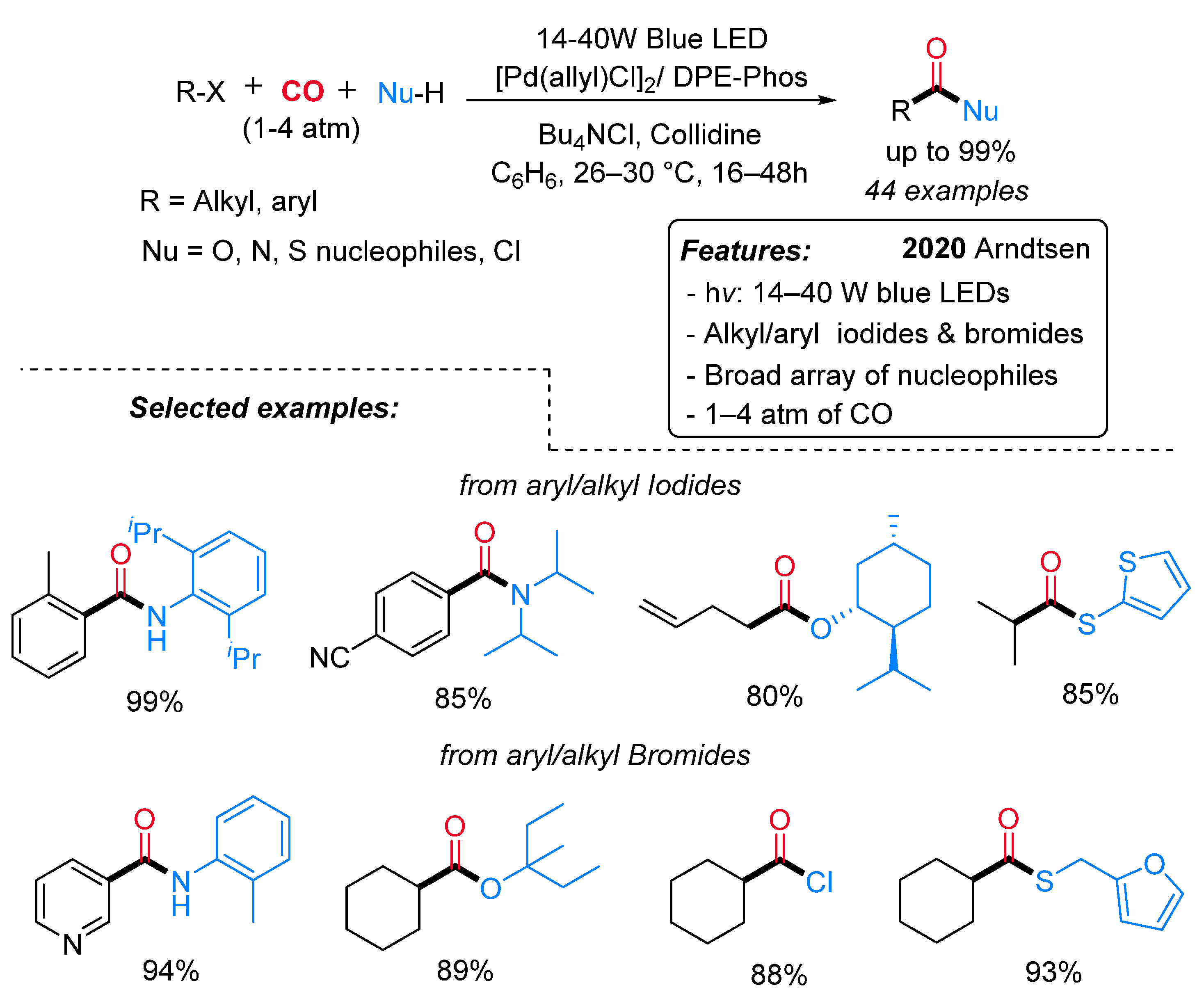
- Torres, G.M.; Liu, Y.; Arndtsen, B.A. A Dual Light-Driven Palladium Catalyst: Breaking the Barriers in Carbonylation Reactions. Science 2020, 368, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Sardana, M.; Bergman, J.; Ericsson, C.; Kingston, L.P.; Schou, M.; Dugave, C.; Audisio, D.; Elmore, C.S. Visible-Light-Enabled Aminocarbonylation of Unactivated Alkyl Iodides with Stoichiometric Carbon Monoxide for Application on Late-Stage Carbon Isotope Labeling. J. Org. Chem. 2019, 84, 16076–16085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curreli, F.; Choudhury, S.; Pyatkin, I.; Zagorodnikov, V.P.; Bulay, A.K.; Altieri, A.; Kwon, Y.D.; Kwong, P.D.; Debnath, A.K. Design, Synthesis, and Antiviral Activity of Entry Inhibitors That Target the CD4-Binding Site of HIV-1. J. Med. Chem. 2012, 55, 4764–4775. [Google Scholar] [CrossRef] [PubMed]
- Schneekönig, J.; Junge, K.; Beller, M. Manganese Catalyzed Asymmetric Transfer Hydrogenation of Ketones Using Chiral Oxamide Ligands. Synlett 2019, 30, 503–507. [Google Scholar] [CrossRef] [Green Version]
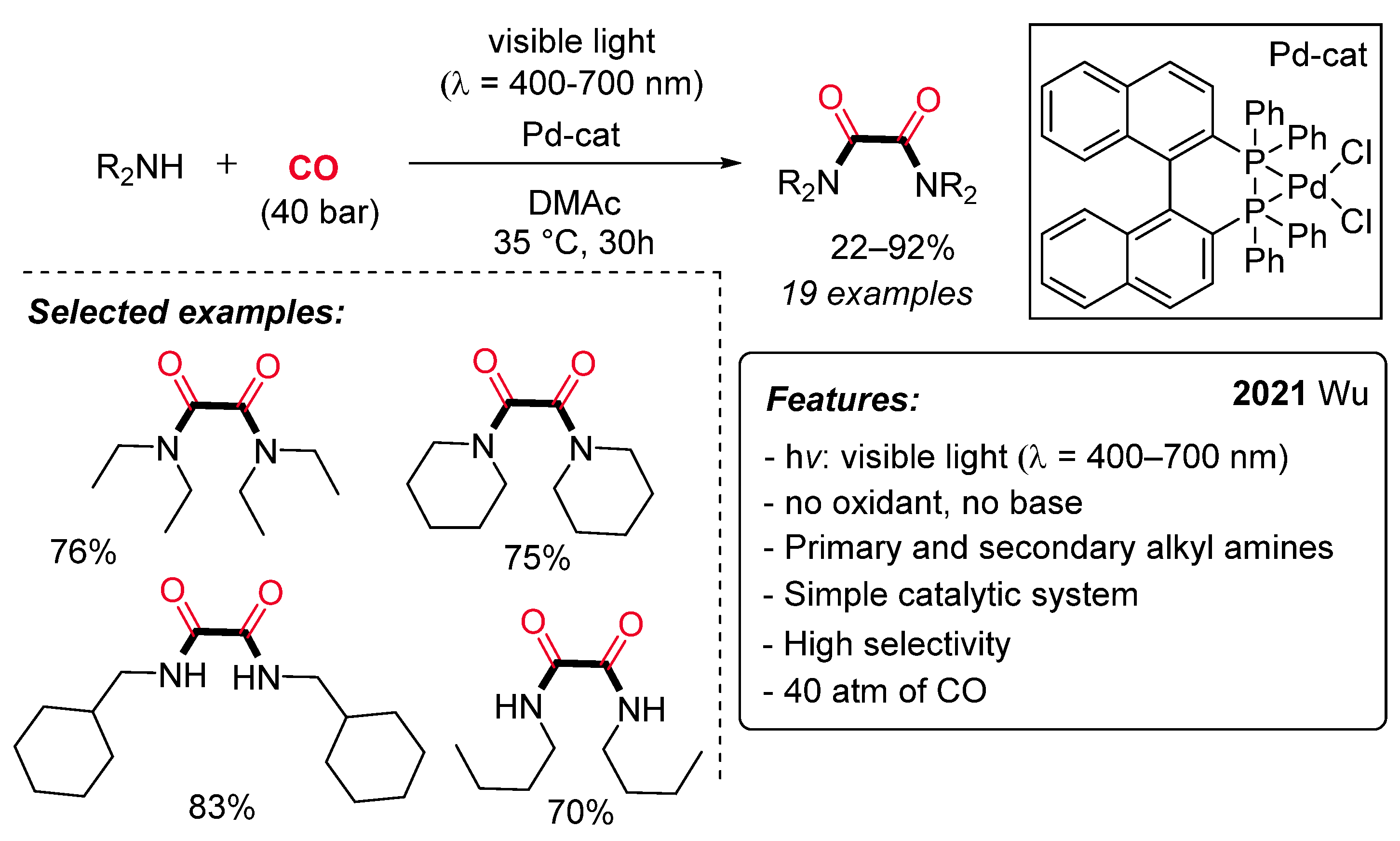
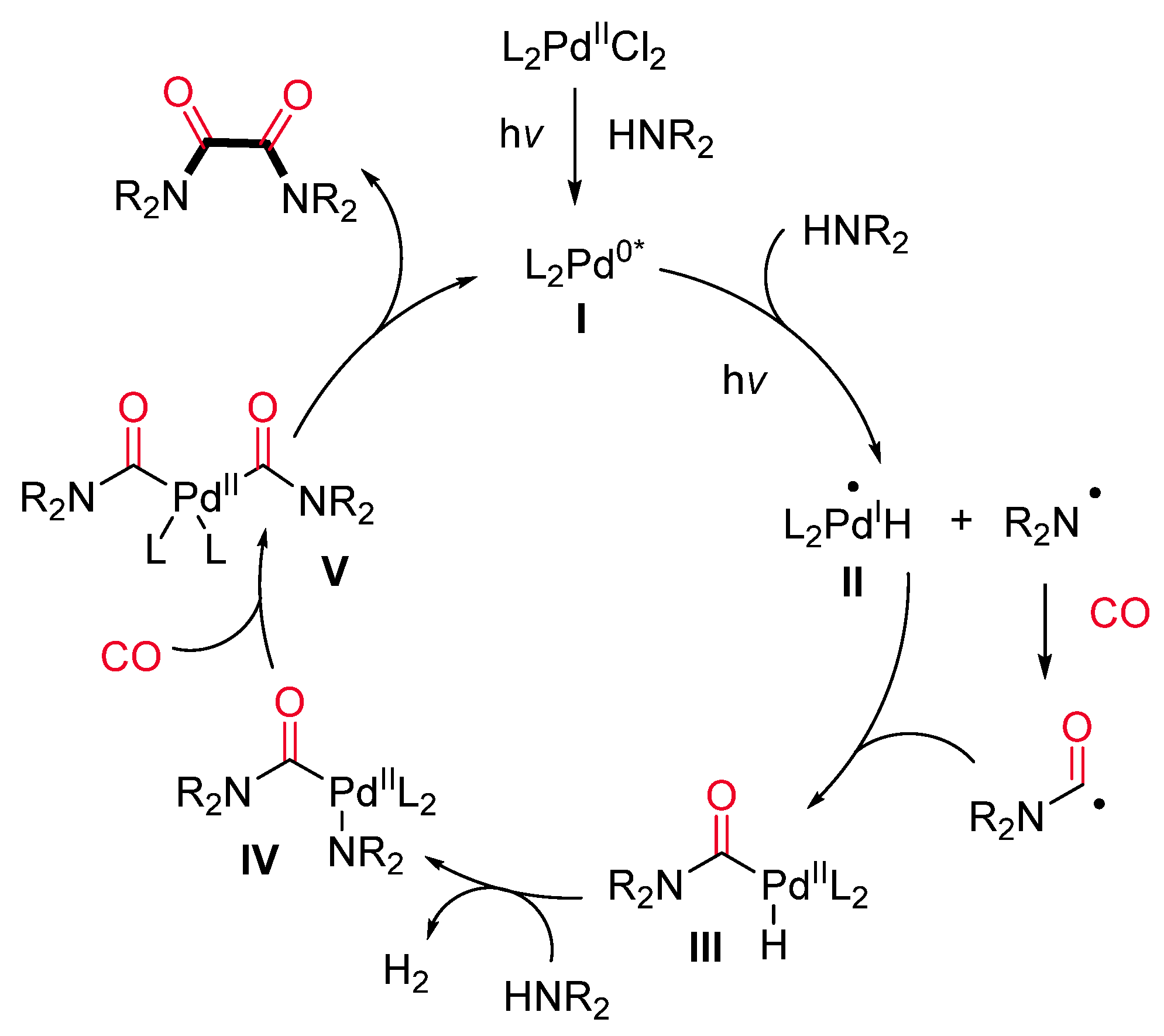
- Meyer, T.; Rabeah, J.; Brückner, A.; Wu, X. Visible-Light-Induced Palladium-Catalyzed Dehydrogenative Carbonylation of Amines to Oxalamides. Chem. Eur. J. 2021, 27, 5642–5647. [Google Scholar] [CrossRef] [PubMed]
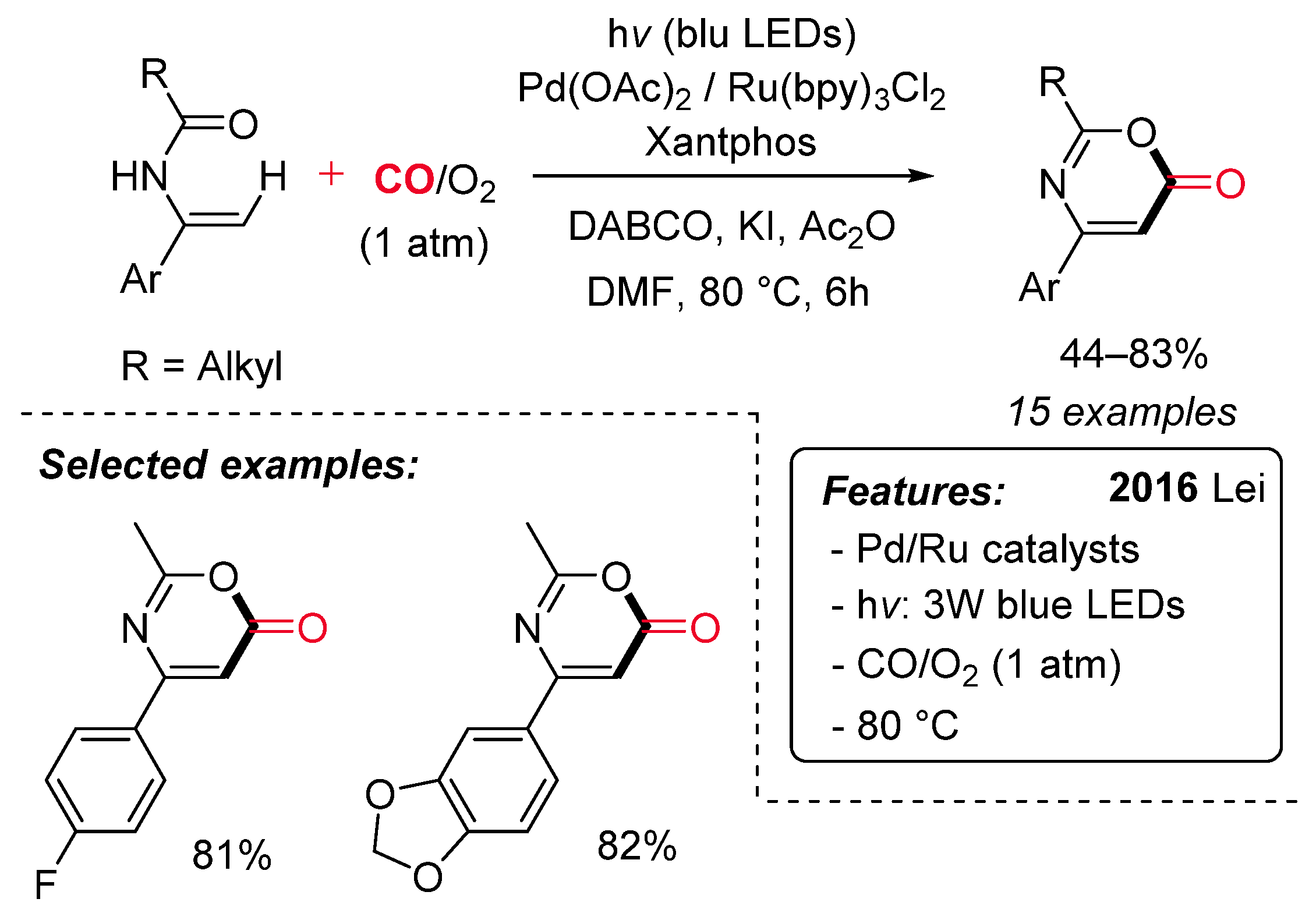
- Liu, K.; Zou, M.; Lei, A. Aerobic Oxidative Carbonylation of Enamides by Merging Palladium with Photoredox Catalysis. J. Org. Chem. 2016, 81, 7088–7092. [Google Scholar] [CrossRef] [PubMed]
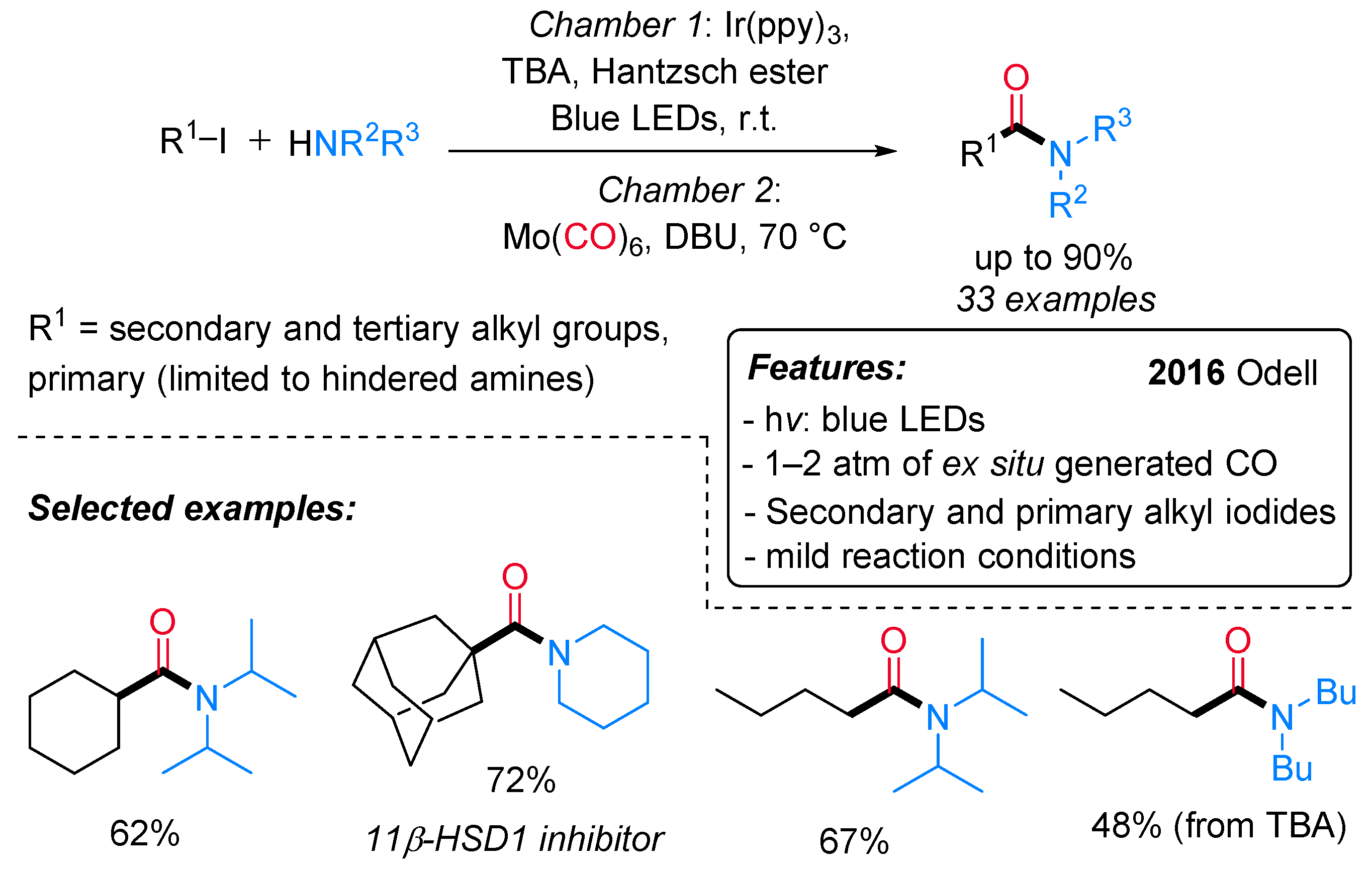
- Chow, S.Y.; Stevens, M.Y.; Åkerbladh, L.; Bergman, S.; Odell, L.R. Mild and Low-Pressure fac-Ir(Ppy)3 -Mediated Radical Aminocarbonylation of Unactivated Alkyl Iodides through Visible-Light Photoredox Catalysis. Chem. Eur. J. 2016, 22, 9155–9161. [Google Scholar] [CrossRef] [PubMed]
- Micic, N.; Polyzos, A. Radical Carbonylation Mediated by Continuous-Flow Visible-Light Photocatalysis: Access to 2,3-Dihydrobenzofurans. Org. Lett. 2018, 20, 4663–4666. [Google Scholar] [CrossRef] [PubMed]
- Ryu, I.; Niguma, T.; Minakata, S.; Komatsu, M.; Hadida, S.; Curran, D.P. Hydroxymethylation of Organic Halides. Evaluation of a Catalytic System Involving a Fluorous Tin Hydride Reagent for Radical Carbonylation. Tetrahedron Lett. 1997, 38, 7883–7886. [Google Scholar] [CrossRef]
- Forni, J.A.; Micic, N.; Connell, T.U.; Weragoda, G.; Polyzos, A. Tandem Photoredox Catalysis: Enabling Carbonylative Amidation of Aryl and Alkylhalides. Angew. Chem. Int. Ed. 2020, 59, 18646–18654. [Google Scholar] [CrossRef]
- Wang, Z.; Li, L.; Huang, Y. A General Synthesis of Ynones from Aldehydes via Oxidative C–C Bond Cleavage under Aerobic Conditions. J. Am. Chem. Soc. 2014, 136, 12233–12236. [Google Scholar] [CrossRef]
- Zhou, Q.-Q.; Guo, W.; Ding, W.; Wu, X.; Chen, X.; Lu, L.-Q.; Xiao, W.-J. Decarboxylative Alkynylation and Carbonylative Alkynylation of Carboxylic Acids Enabled by Visible-Light Photoredox Catalysis. Angew. Chem. Int. Ed. 2015, 54, 11196–11199. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhang, M.; Xiong, W.; Lu, L.; Xiao, W. Deaminative (Carbonylative) Alkyl-Heck-type Reactions Enabled by Photocatalytic C−N Bond Activation. Angew. Chem. Int. Ed. 2019, 58, 2402–2406. [Google Scholar] [CrossRef]
- Brunet, J.-J.; Sidot, C.; Caubere, P. Cobalt Carbonyl Catalyzed SRN1 Carbonylation of Aryl and Vinyl Halides by Phase Transfer Catalysis. Tetrahedron Lett. 1981, 22, 1013–1016. [Google Scholar] [CrossRef]
- Brunet, J.J.; Sidot, C.; Caubere, P. Sunlamp-Irradiated Phase-Transfer Catalysis. 1. Cobalt Carbonyl Catalyzed SRN1 Carbonylations of Aryl and Vinyl Halides. J. Org. Chem. 1983, 48, 1166–1171. [Google Scholar] [CrossRef]
- Dragojlovic, V.; Gao, D.B.; Chow, Y.L. Multigram Scale Cobalt Catalyzed Photochemical Methoxycarbonylation of Alkenes. J. Mol. Catal. Chem. 2001, 171, 43–51. [Google Scholar] [CrossRef]
- Cash, D.; Combs, A.; Dragojlovic, V. Cobalt-Catalyzed Photolytic Methoxycarbonylation of Bromoalkanes in the Presence of a Lewis Acid. Tetrahedron Lett. 2004, 45, 1143–1145. [Google Scholar] [CrossRef]
- Jia, Y.P.; Cui, Y.N.; Yin, J.M.; Zhou, G.Y.; Li, S.M.; Gao, D.B.; Wang, X.S. Cobalt-Catalyzed Photopromoted Carbonylation of Chloroalkanes in the Presence of KI. Chin. Chem. Lett. 2010, 21, 1033–1036. [Google Scholar] [CrossRef]
- Mu, L.Z.; Jia, Y.P.; Yin, J.M.; Zhou, G.Y.; Cui, Y.N.; Gao, D.B. Photopromoted Carbonylation of Bromobenzene under Ambient Conditions. Chin. Chem. Lett. 2009, 20, 531–534. [Google Scholar] [CrossRef]
- Zhong, W.H.; Cui, Y.N.; Li, S.M.; Jia, Y.P.; Yin, J.M. The Carbonylation of Phenyl Bromide and Its Derivatives under Visible Light Irradiation. Chin. Chem. Lett. 2012, 23, 29–32. [Google Scholar] [CrossRef]
- Veatch, A.M.; Alexanian, E.J. Cobalt-Catalyzed Aminocarbonylation of (Hetero)Aryl Halides Promoted by Visible Light. Chem. Sci. 2020, 11, 7210–7213. [Google Scholar] [CrossRef] [PubMed]
- Gosset, C.; Pellegrini, S.; Jooris, R.; Bousquet, T.; Pelinski, L. Visible-Light-Mediated Hydroxycarbonylation of Diazonium Salts. Adv. Synth. Catal. 2018, 360, 3401–3405. [Google Scholar] [CrossRef]
- Lu, B.; Cheng, Y.; Chen, L.-Y.; Chen, J.-R.; Xiao, W.-J. Photoinduced Copper-Catalyzed Radical Aminocarbonylation of Cycloketone Oxime Esters. ACS Catal. 2019, 9, 8159–8164. [Google Scholar] [CrossRef]
- Urata, H.; Maekawa, H.; Takahashi, S.; Fuchikami, T. Transition Metal Complex-Catalyzed Carbonylation of Organic Halides in N,N,N’,N’-Tetraalkylurea Solution in the Absence of Added Base. J. Org. Chem. 1991, 56, 4320–4322. [Google Scholar] [CrossRef]
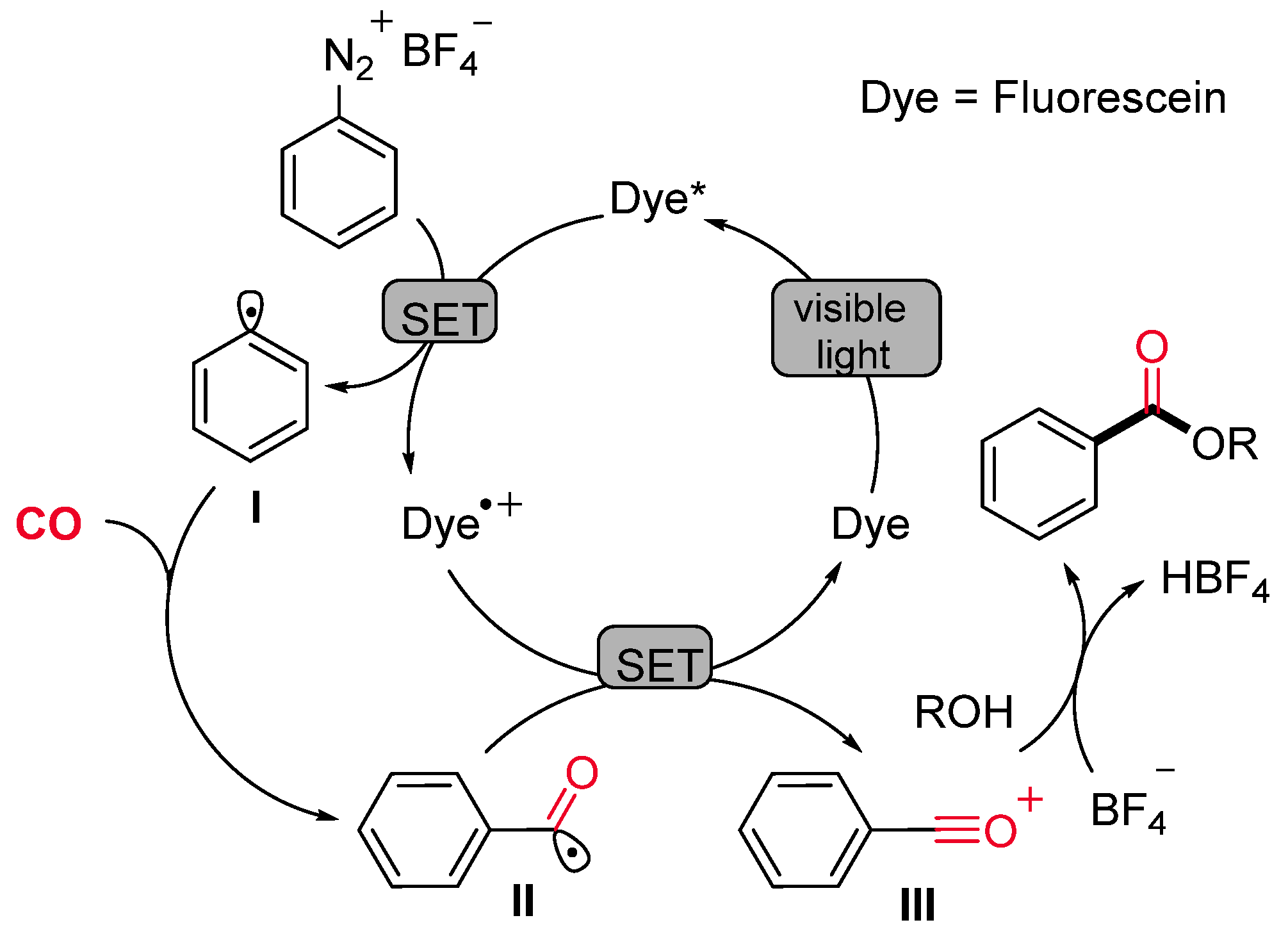
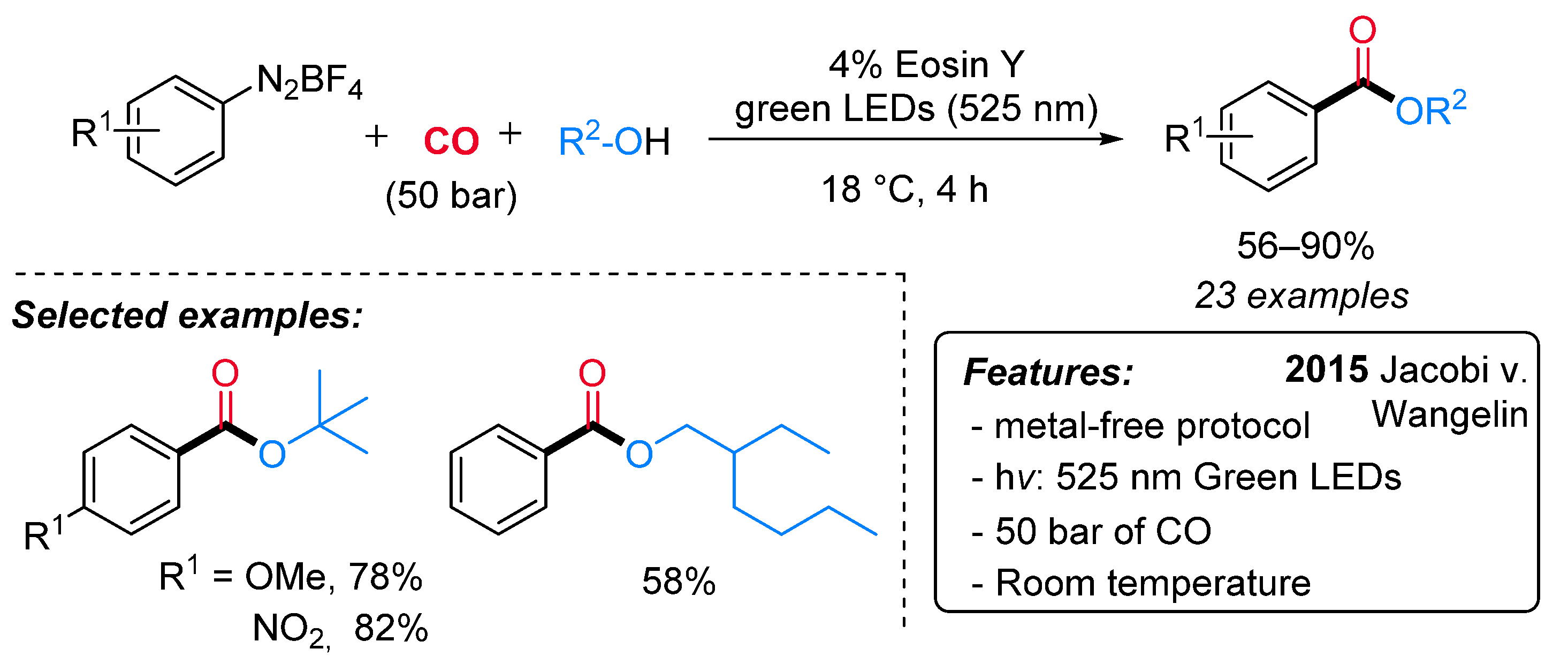
- Guo, W.; Lu, L.-Q.; Wang, Y.; Wang, Y.-N.; Chen, J.-R.; Xiao, W.-J. Metal-Free, Room-Temperature, Radical Alkoxycarbonylation of Aryldiazonium Salts through Visible-Light Photoredox Catalysis. Angew. Chem. Int. Ed. 2015, 54, 2265–2269. [Google Scholar] [CrossRef]
- Majek, M.; Jacobi von Wangelin, A. Metal-Free Carbonylations by Photoredox Catalysis. Angew. Chem. Int. Ed. 2015, 54, 2270–2274. [Google Scholar] [CrossRef]
- Schoenberg, A.; Heck, R.F. Palladium-Catalyzed Amidation of Aryl, Heterocyclic, and Vinylic Halides. J. Org. Chem. 1974, 39, 3327–3331. [Google Scholar] [CrossRef]
- Friis, S.D.; Skrydstrup, T.; Buchwald, S.L. Mild Pd-Catalyzed Aminocarbonylation of (Hetero)Aryl Bromides with a Palladacycle Precatalyst. Org. Lett. 2014, 16, 4296–4299. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Alper, H. Pd-Catalyzed Chemoselective Carbonylation of Aminophenols with Iodoarenes: Alkoxycarbonylation vs Aminocarbonylation. J. Am. Chem. Soc. 2014, 136, 16970–16973. [Google Scholar] [CrossRef]
- Kawamoto, T.; Okada, T.; Curran, D.P.; Ryu, I. Efficient Hydroxymethylation Reactions of Iodoarenes Using CO and 1,3-Dimethylimidazol-2-Ylidene Borane. Org. Lett. 2013, 15, 2144–2147. [Google Scholar] [CrossRef] [PubMed]
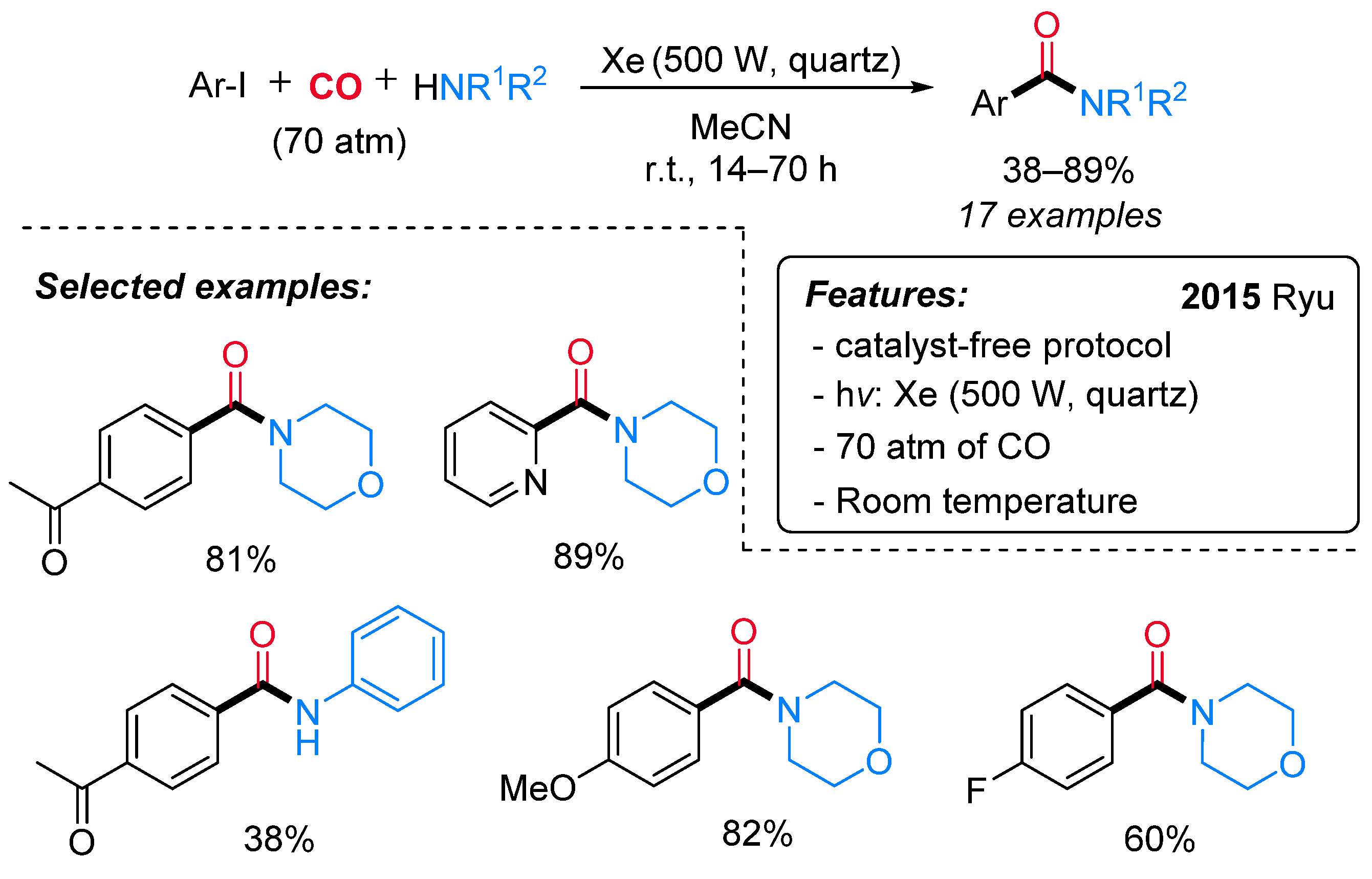
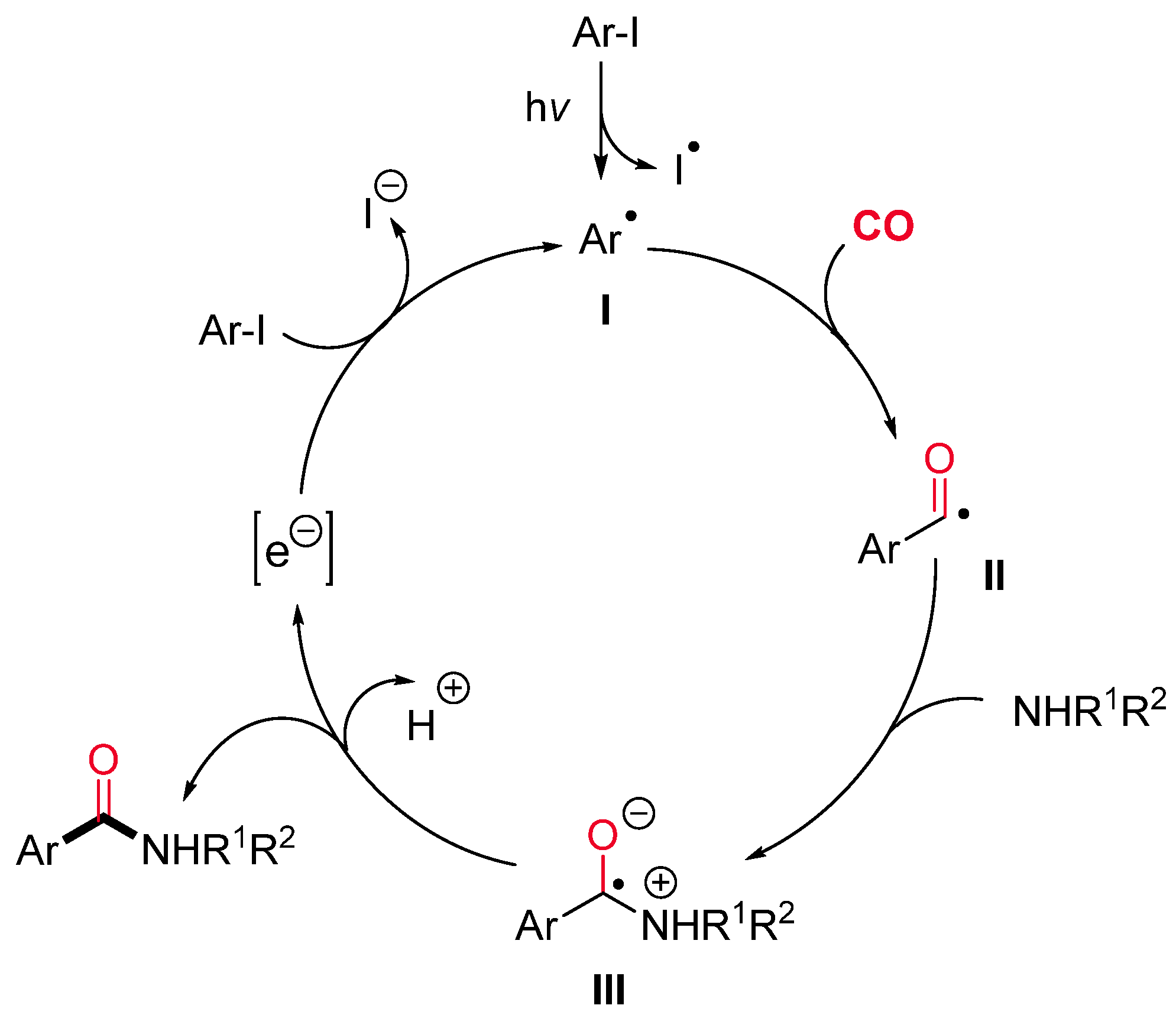
- Kawamoto, T.; Sato, A.; Ryu, I. Photoinduced Aminocarbonylation of Aryl Iodides. Chem. Eur. J. 2015, 21, 14764–14767. [Google Scholar] [CrossRef] [PubMed]
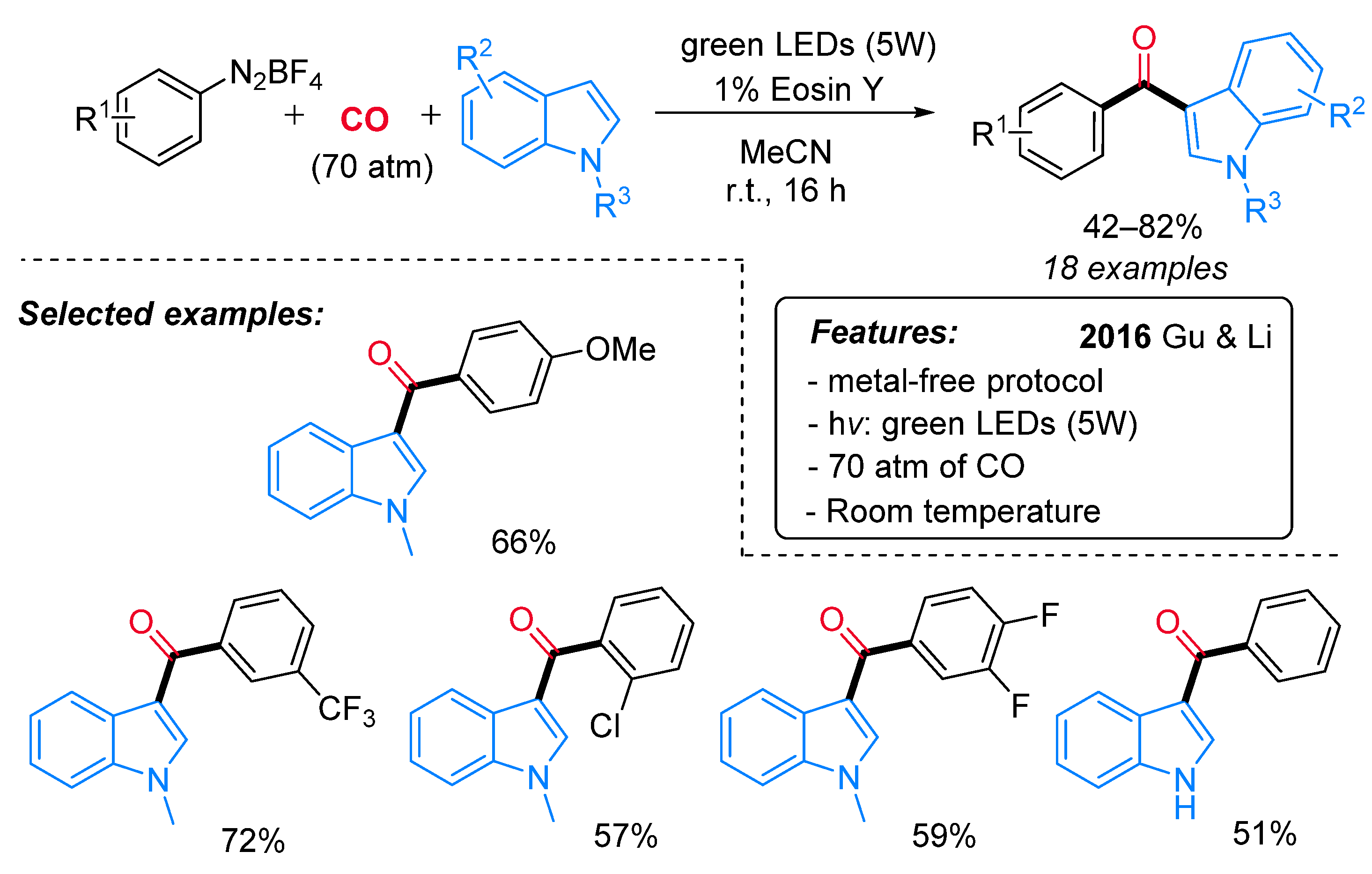
- Zhang, H.-T.; Gu, L.-J.; Huang, X.-Z.; Wang, R.; Jin, C.; Li, G.-P. Synthesis of Indol-3-Yl Aryl Ketones through Visible-Light-Mediated Carbonylation. Chin. Chem. Lett. 2016, 27, 256–260. [Google Scholar] [CrossRef]
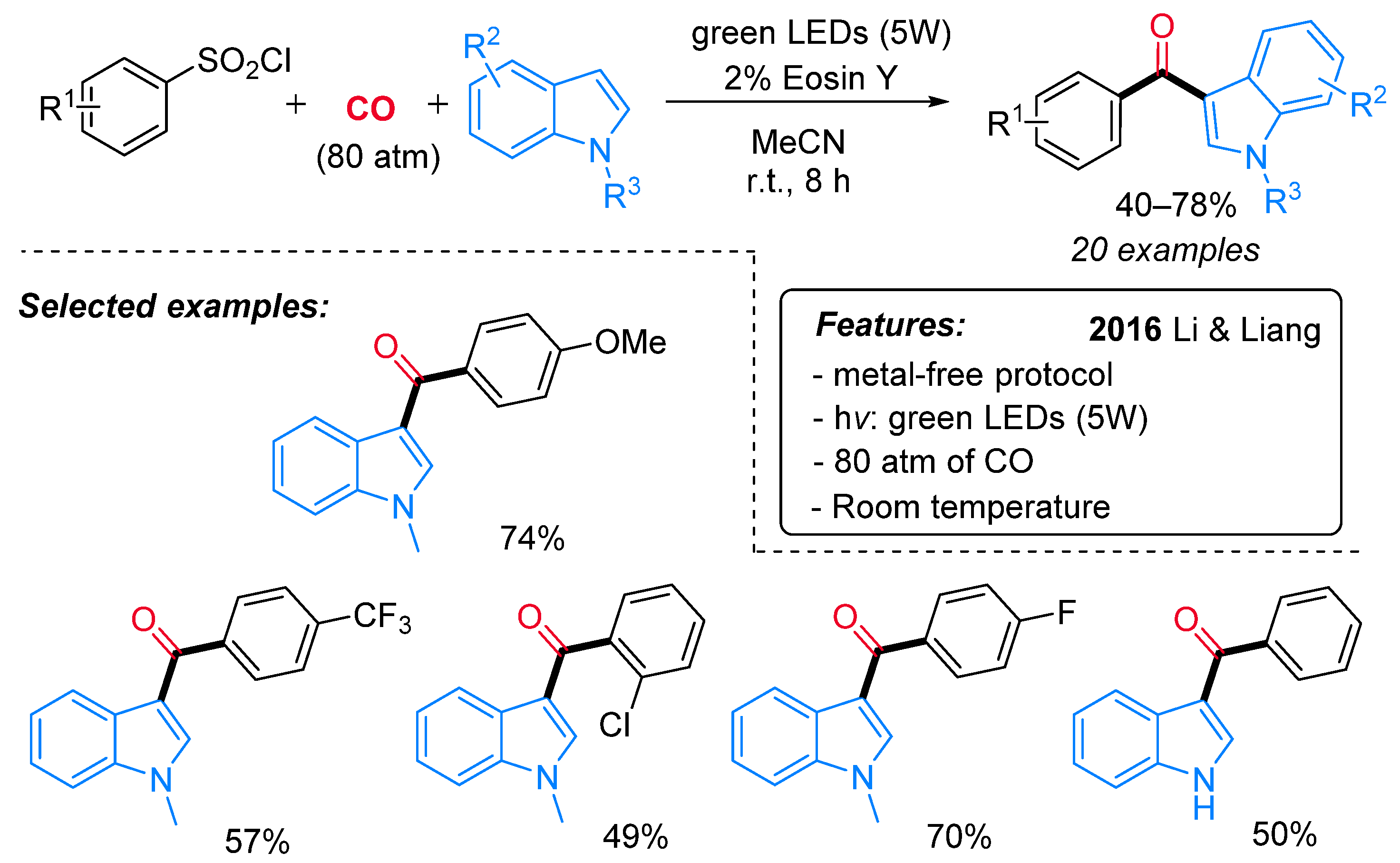
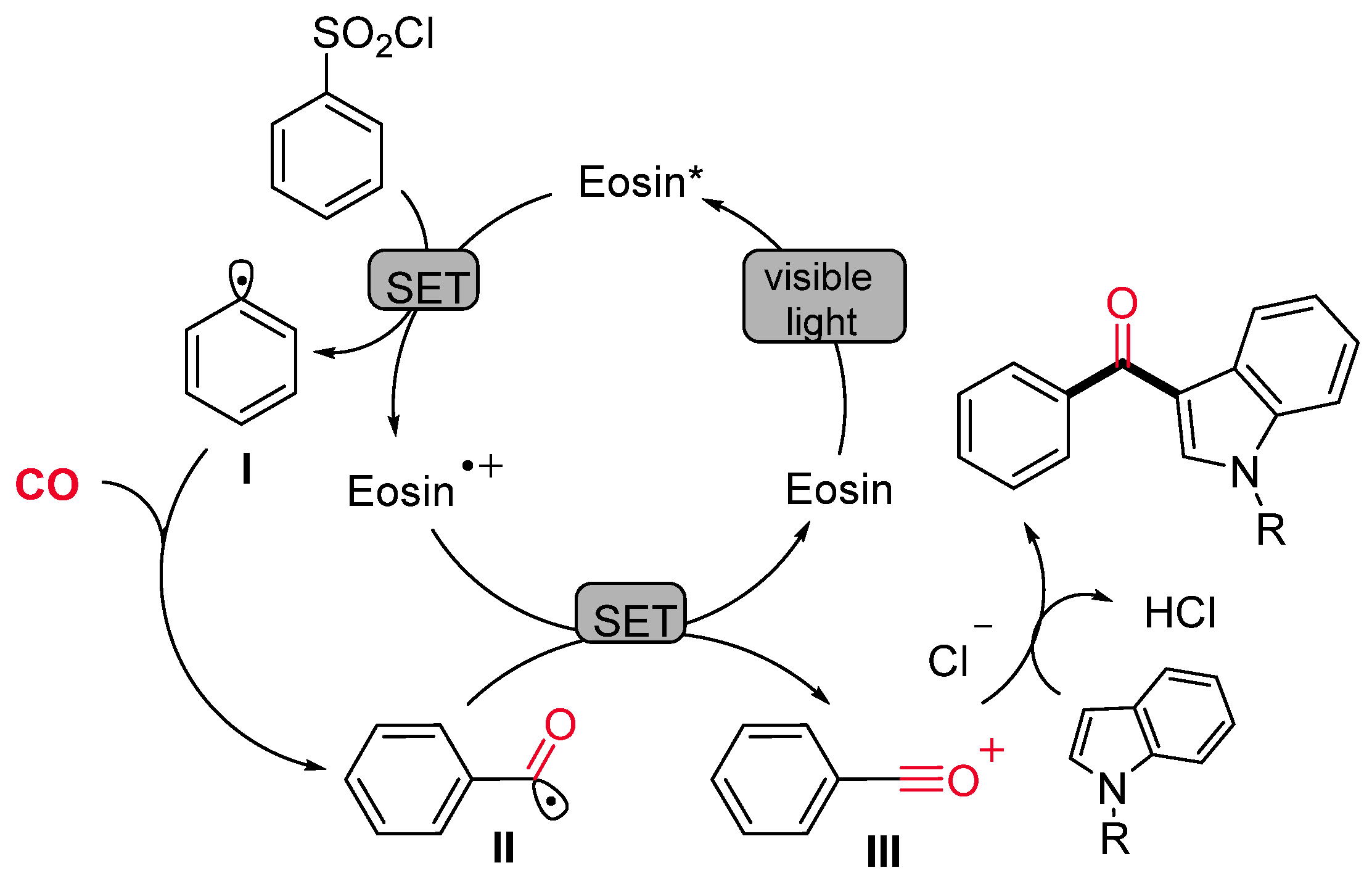
- Li, X.; Liang, D.; Huang, W.; Zhou, H.; Li, Z.; Wang, B.; Ma, Y.; Wang, H. Visible Light-Induced Carbonylation of Indoles with Arylsulfonyl Chlorides and CO. Tetrahedron 2016, 72, 8442–8448. [Google Scholar] [CrossRef]
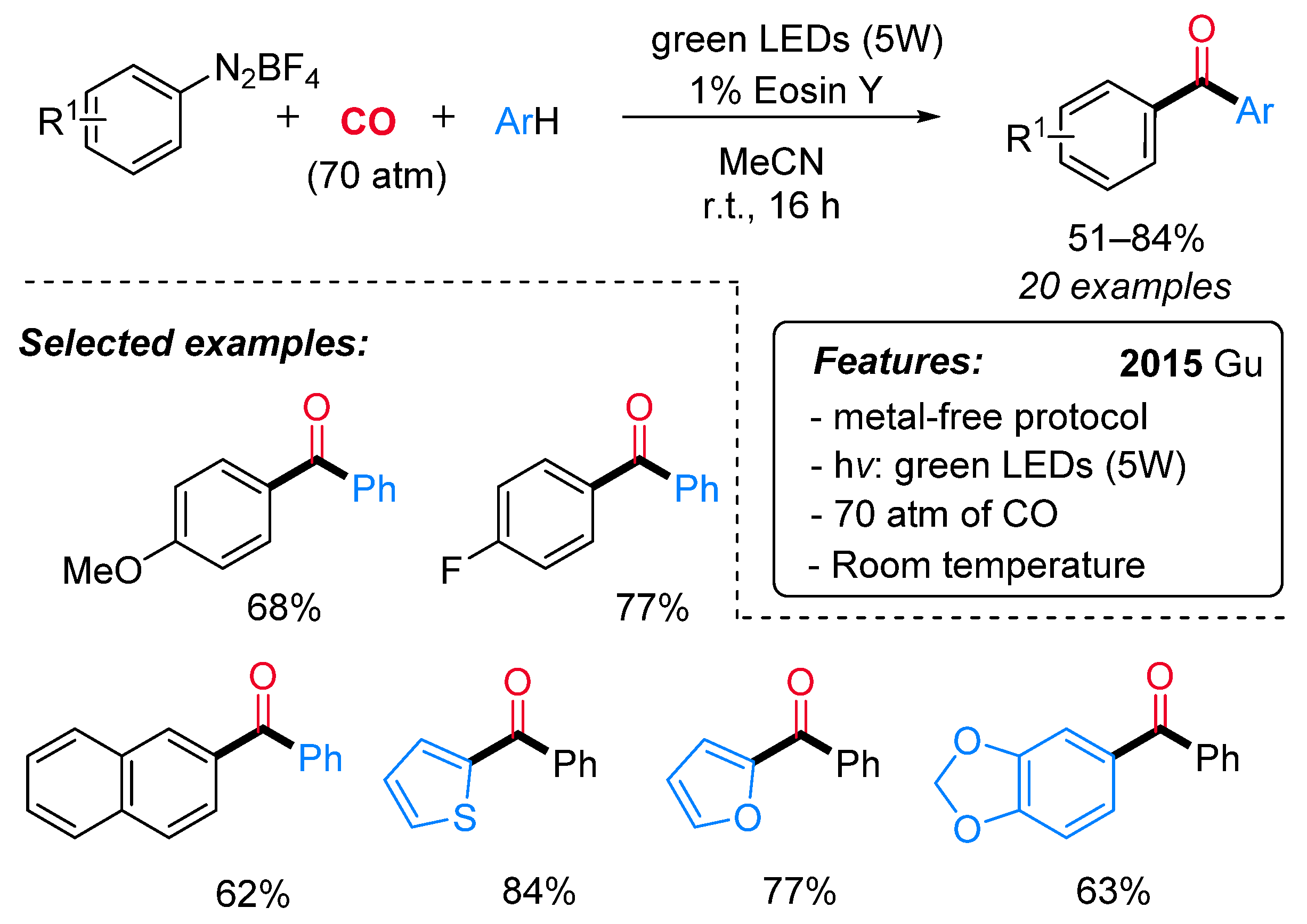
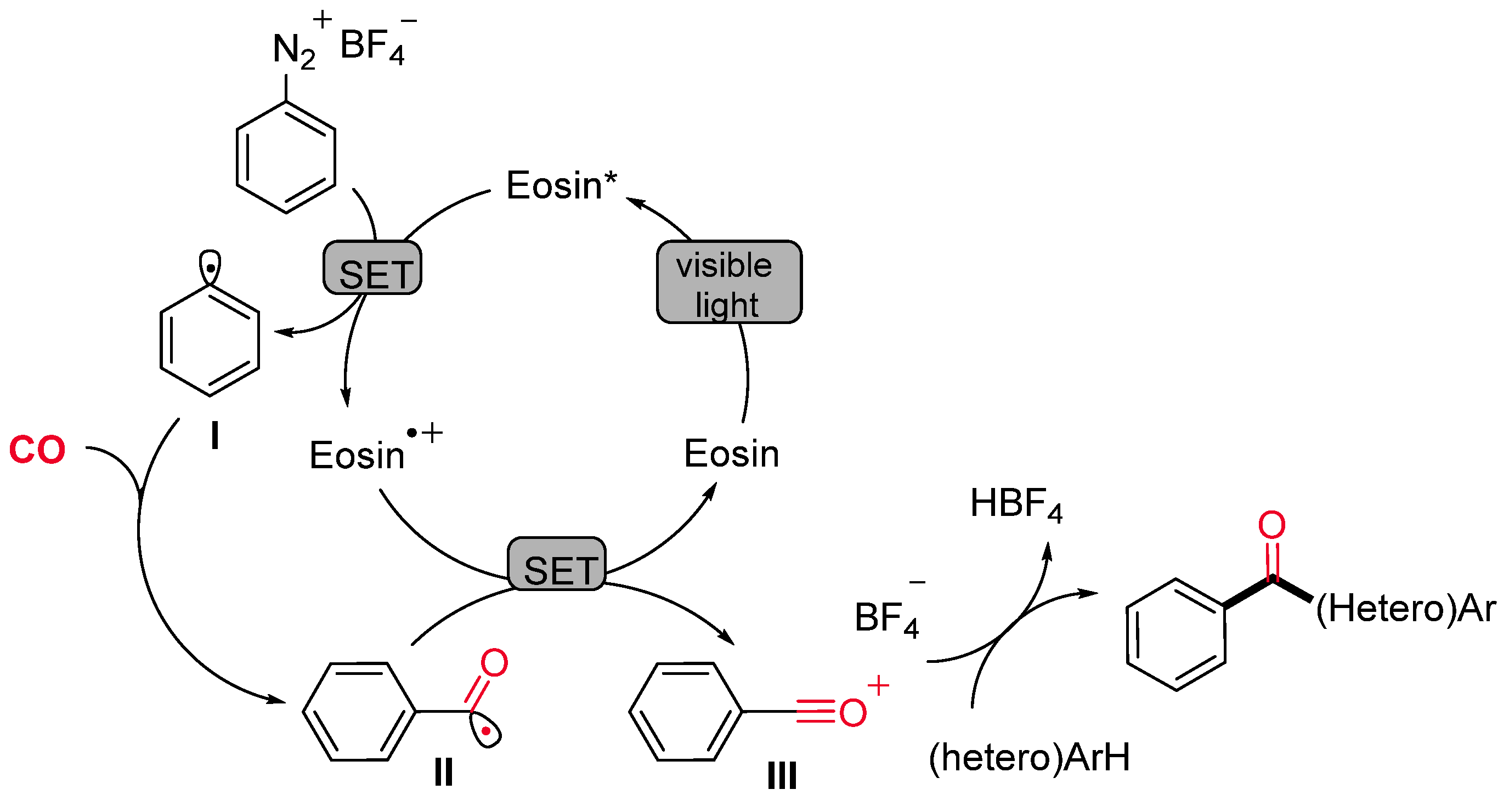
- Gu, L.; Jin, C.; Liu, J. Metal-Free, Visible-Light-Mediated Transformation of Aryl Diazonium Salts and (Hetero)Arenes: An Efficient Route to Aryl Ketones. Green Chem. 2015, 17, 3733–3736. [Google Scholar] [CrossRef]
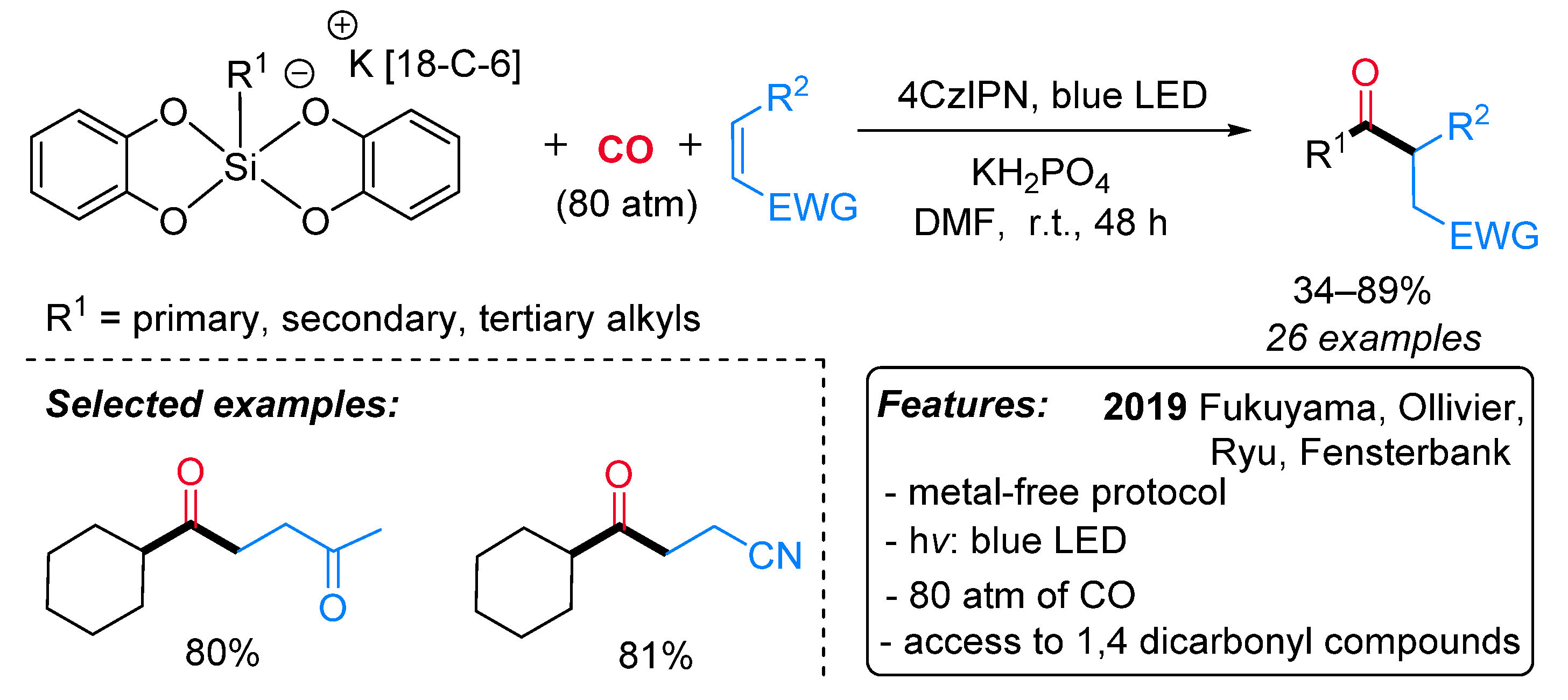
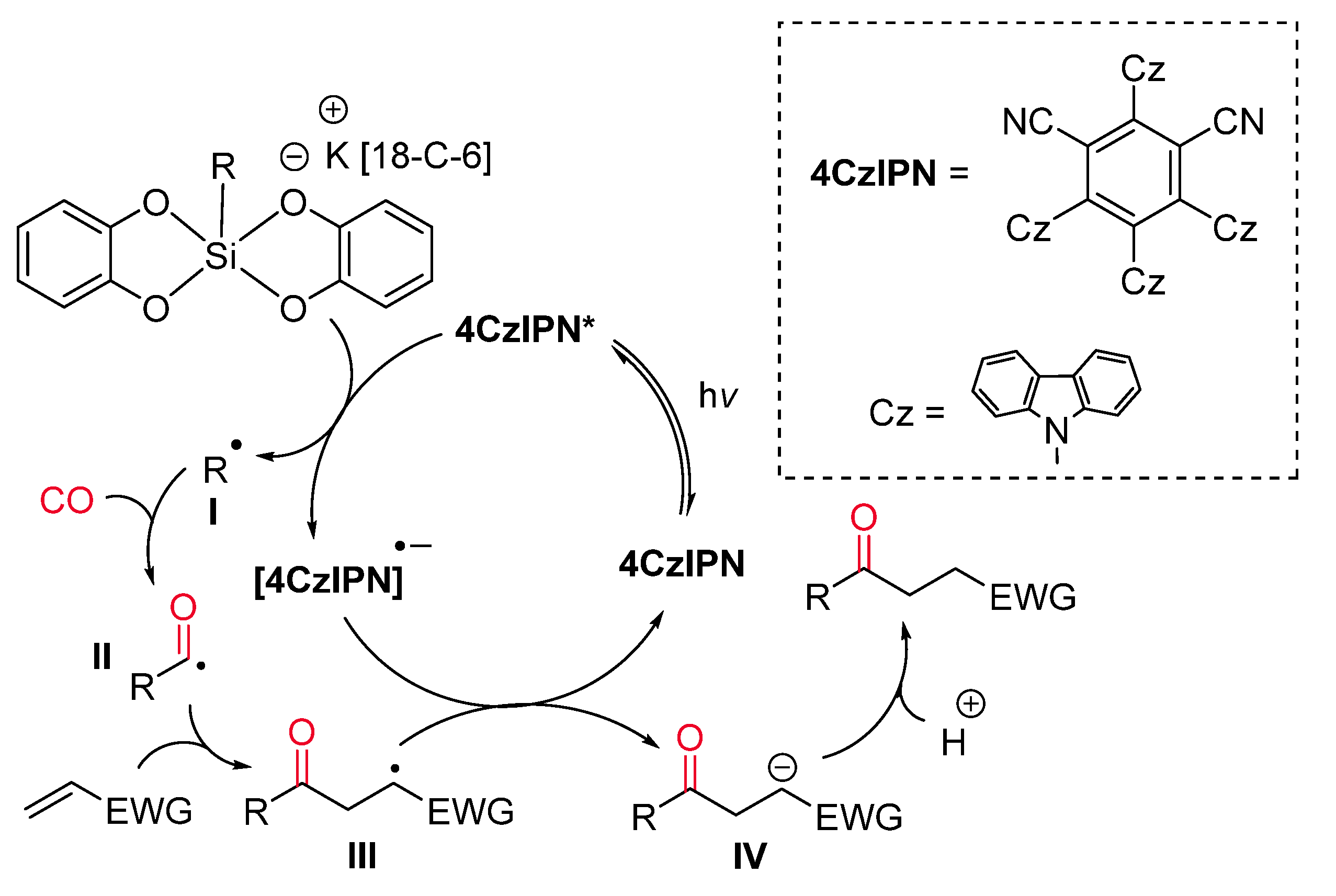
- Cartier, A.; Levernier, E.; Corcé, V.; Fukuyama, T.; Dhimane, A.; Ollivier, C.; Ryu, I.; Fensterbank, L. Carbonylation of Alkyl Radicals Derived from Organosilicates through Visible-Light Photoredox Catalysis. Angew. Chem. Int. Ed. 2019, 58, 1789–1793. [Google Scholar] [CrossRef] [PubMed]
- Cartier, A.; Levernier, E.; Dhimane, A.; Fukuyama, T.; Ollivier, C.; Ryu, I.; Fensterbank, L. Synthesis of Aliphatic Amides through a Photoredox Catalyzed Radical Carbonylation Involving Organosilicates as Alkyl Radical Precursors. Adv. Synth. Catal. 2020, 362, 2254–2259. [Google Scholar] [CrossRef]
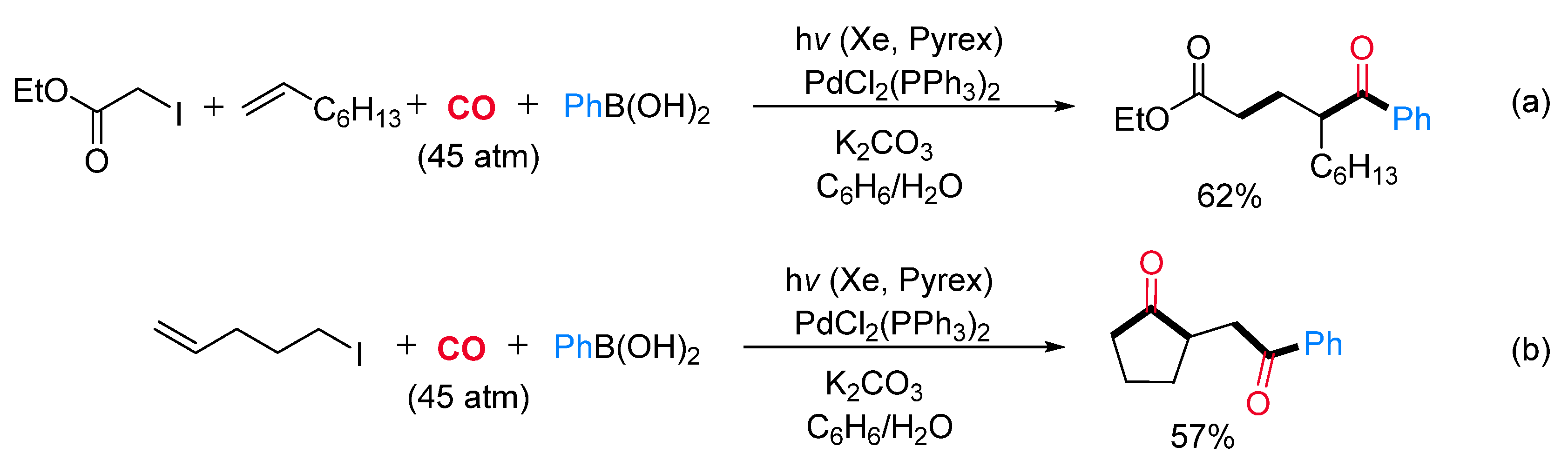



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botla, V.; Voronov, A.; Motti, E.; Carfagna, C.; Mancuso, R.; Gabriele, B.; Della Ca’, N. Advances in Visible-Light-Mediated Carbonylative Reactions via Carbon Monoxide (CO) Incorporation. Catalysts 2021, 11, 918. https://doi.org/10.3390/catal11080918
Botla V, Voronov A, Motti E, Carfagna C, Mancuso R, Gabriele B, Della Ca’ N. Advances in Visible-Light-Mediated Carbonylative Reactions via Carbon Monoxide (CO) Incorporation. Catalysts. 2021; 11(8):918. https://doi.org/10.3390/catal11080918
Chicago/Turabian StyleBotla, Vinayak, Aleksandr Voronov, Elena Motti, Carla Carfagna, Raffaella Mancuso, Bartolo Gabriele, and Nicola Della Ca’. 2021. "Advances in Visible-Light-Mediated Carbonylative Reactions via Carbon Monoxide (CO) Incorporation" Catalysts 11, no. 8: 918. https://doi.org/10.3390/catal11080918
APA StyleBotla, V., Voronov, A., Motti, E., Carfagna, C., Mancuso, R., Gabriele, B., & Della Ca’, N. (2021). Advances in Visible-Light-Mediated Carbonylative Reactions via Carbon Monoxide (CO) Incorporation. Catalysts, 11(8), 918. https://doi.org/10.3390/catal11080918








