Methanol to Formaldehyde: An Overview of Surface Studies and Performance of an Iron Molybdate Catalyst
Abstract
:1. Introduction
Iron Molybdate an Active Catalyst for Methanol to Formaldehyde Production
2. Synthesis of Iron Molybdate
Role of pH
3. Role of Fe2(MoO4)3 as an Active Phase in Partial Oxidation of Methanol
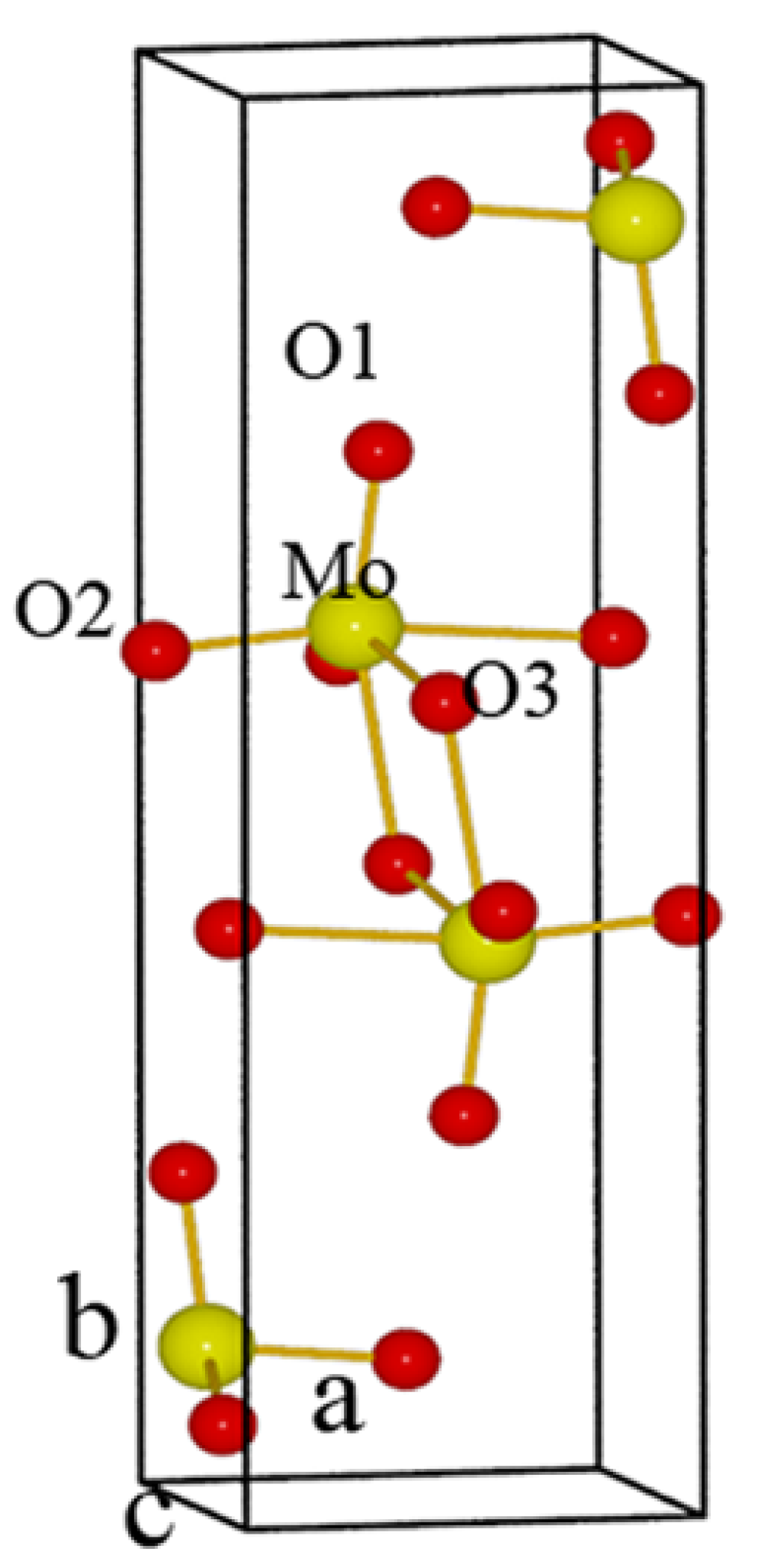
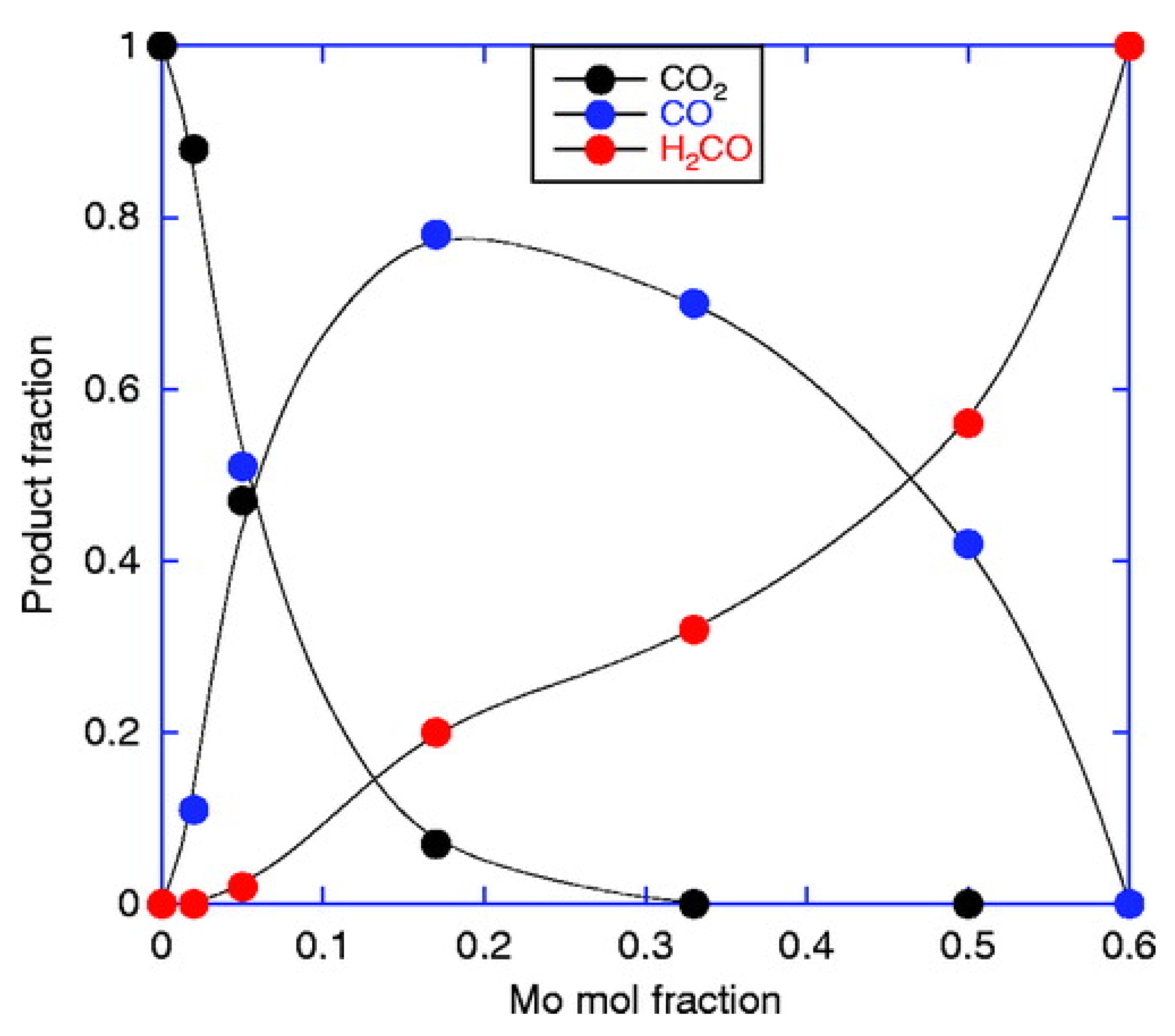
4. Role of Excess MoO3 in Iron Molybdate Catalyst
5. Role of Catalyst Support
6. Promoters
Role of Fe
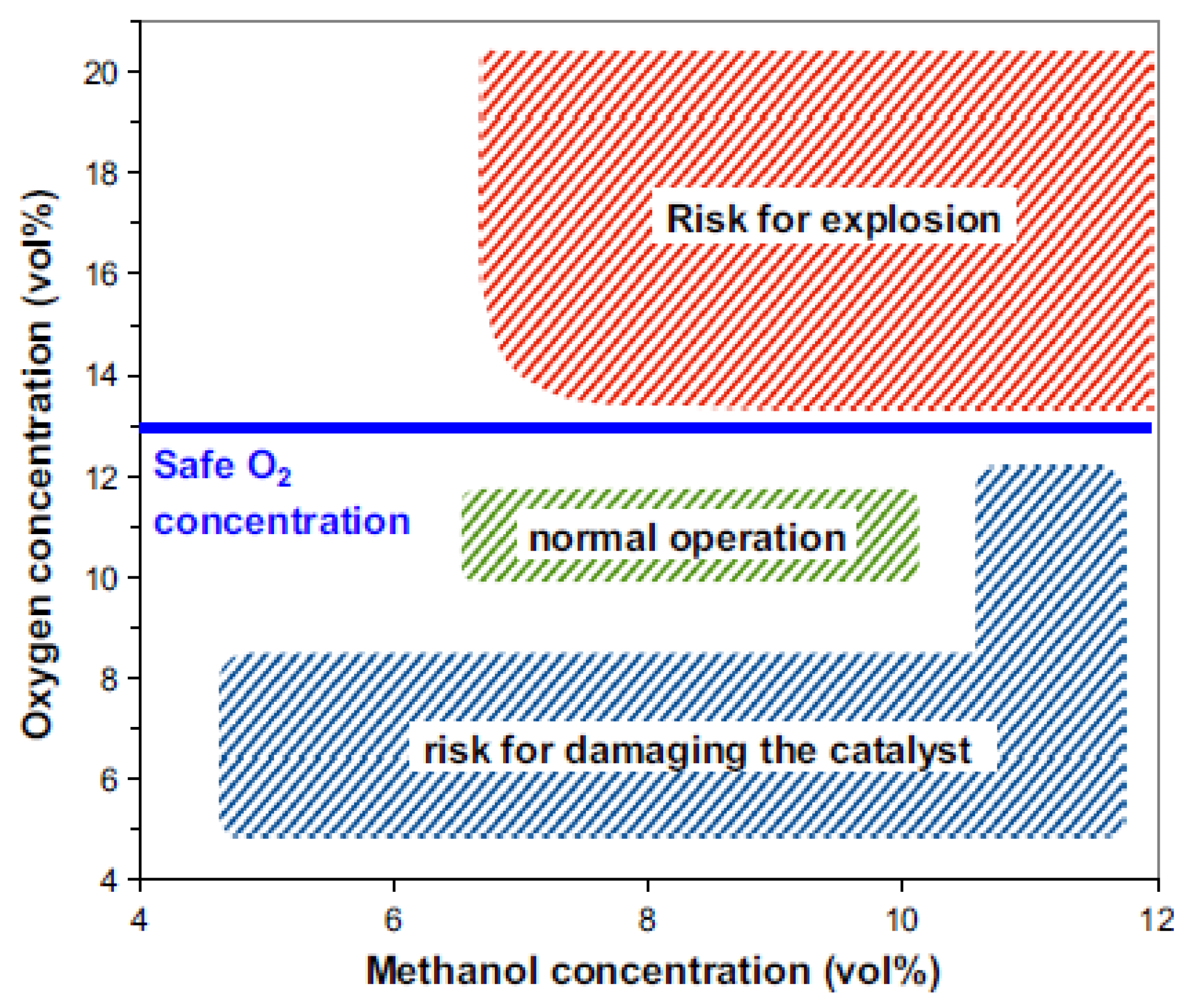
7. Role of Oxygen
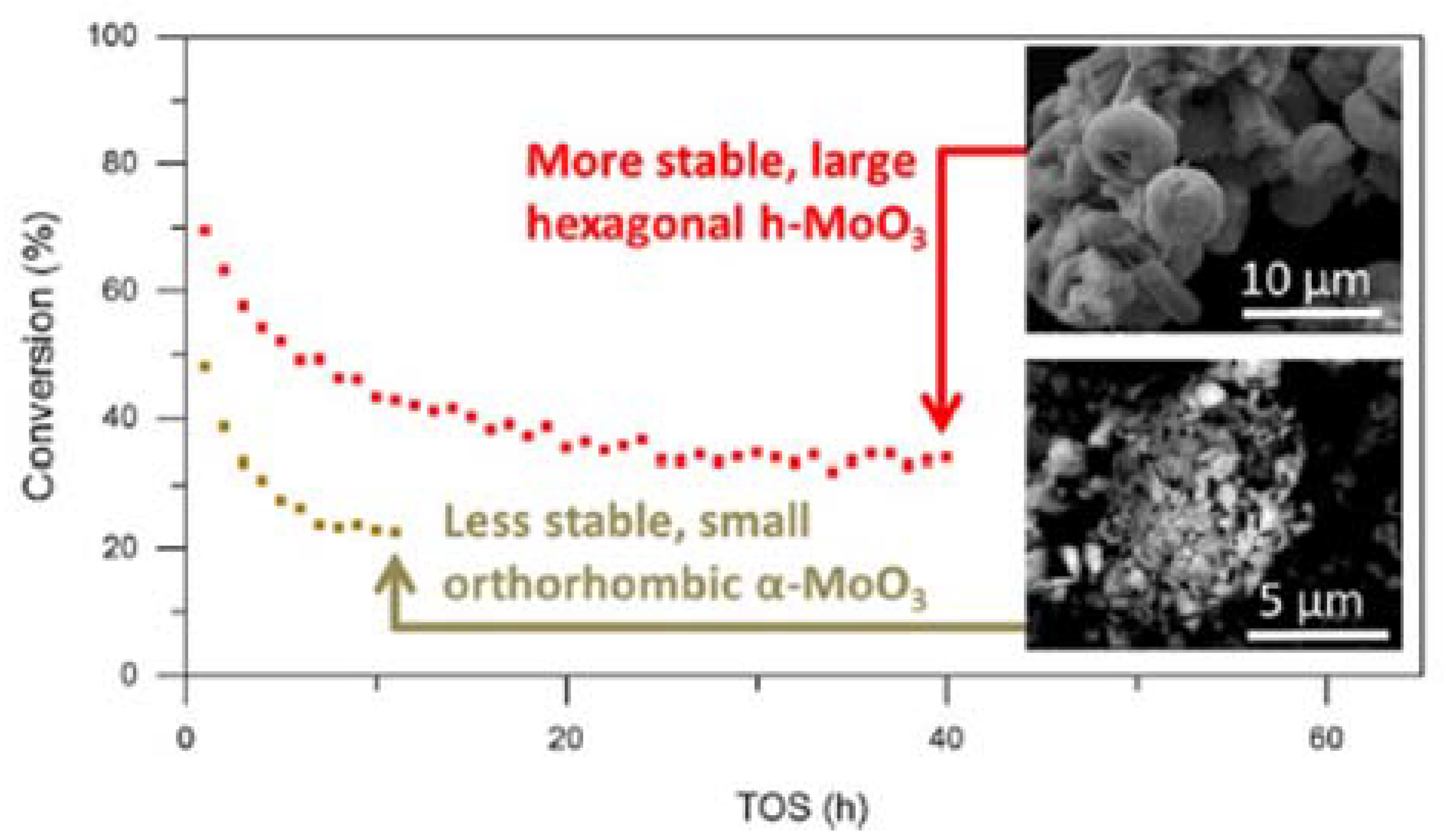
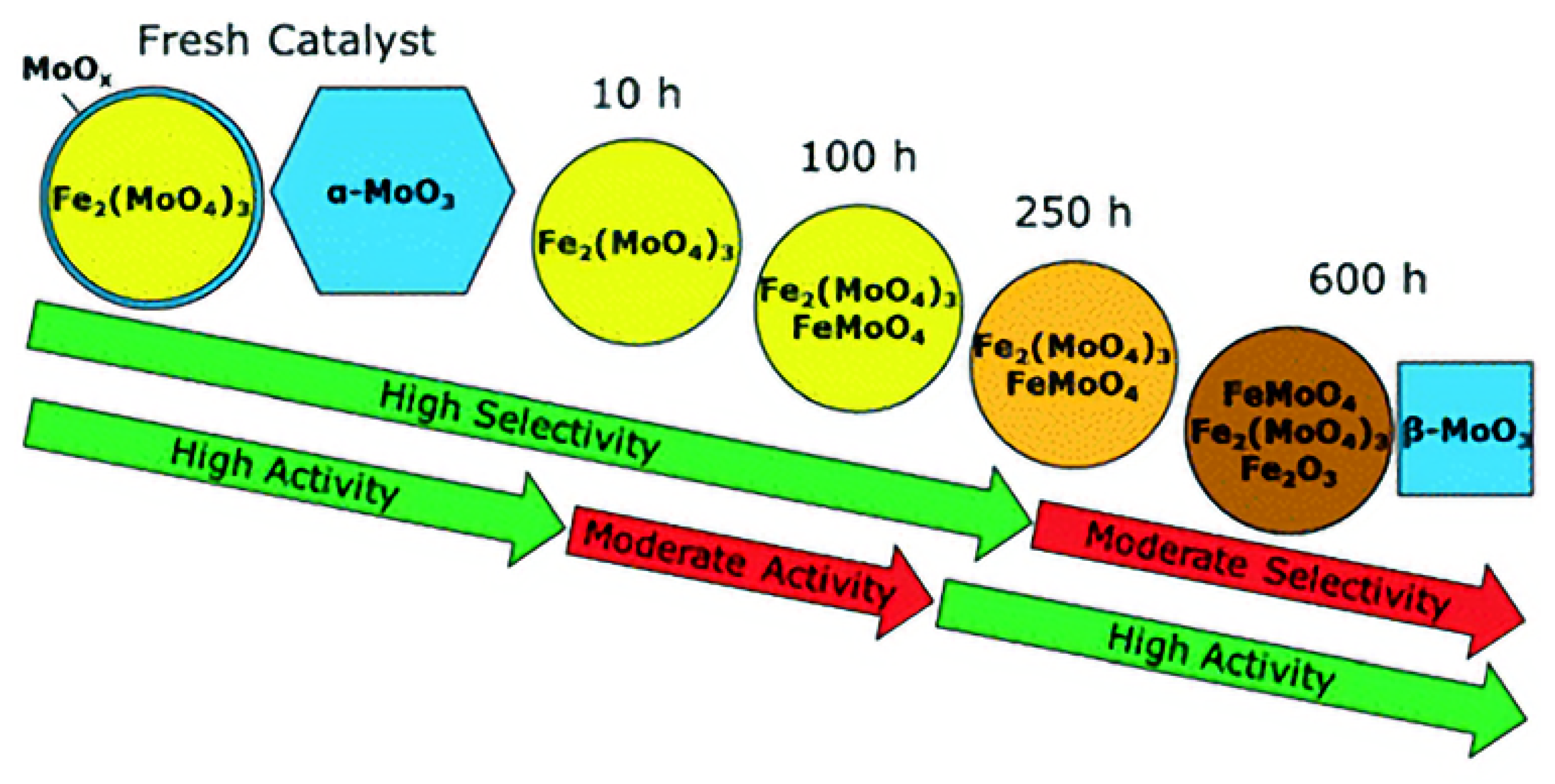
8. Deactivation Studies
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tatibouët, J.M. Methanol oxidation as a catalytic surface probe. Appl. Catal. A Gen. 1997, 148, 213–252. [Google Scholar] [CrossRef]
- Araya, S.S.; Liso, V.; Cui, X.; Li, N.; Zhu, J.; Sahlin, S.L.; Jensen, S.H.; Nielsen, M.P.; Kær, S.K. A Review of The Methanol Economy: The Fuel Cell Route. Energies 2020, 13, 596. [Google Scholar] [CrossRef] [Green Version]
- Dalena, F.; Senatore, A.; Marino, A.; Gordano, A.; Basile, M.; Basile, A. Methanol Production and Applications: An Overview. In Methanol; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–28. ISBN 9780444640109. [Google Scholar]
- Kajaste, R.; Hurme, M.; Oinas, P.; Kajaste, R.; Hurme, M.; Oinas, P. Methanol-Managing greenhouse gas emissions in the production chain by optimizing the resource base. AIMS Energy 2018, 6, 1074–1102. [Google Scholar] [CrossRef]
- Hovda, K.E.; McMartin, K.; Jacobsen, D. Methanol and Formaldehyde Poisoning. In Critical Care Toxicology; Springer International Publishing: Cham, The Netherlands, 2017; pp. 1–18. [Google Scholar]
- Shakeel, K.; Javaid, M.; Muazzam, Y.; Naqvi, S.R.; Taqvi, S.A.A.; Uddin, F.; Mehran, M.T.; Sikander, U.; Niazi, M.B.K. Performance Comparison of Industrially Produced Formaldehyde Using Two Different Catalysts. Processes 2020, 8, 571. [Google Scholar] [CrossRef]
- Zhang, L. Introduction to Formaldehyde. In Issues in Toxicology; Royal Society of Chemistry: London, UK, 2018; Volume 2018, pp. 1–19. [Google Scholar]
- Bahmanpour, A.M.; Hoadley, A.; Tanksale, A. Critical review and exergy analysis of formaldehyde production processes. Rev. Chem. Eng. 2014, 30, 583–604. [Google Scholar] [CrossRef]
- Solt, P.; Konnerth, J.; Gindl-Altmutter, W.; Kantner, W.; Moser, J.; Mitter, R.; van Herwijnen, H.W.G. Technological performance of formaldehyde-free adhesive alternatives for particleboard industry. Int. J. Adhes. Adhes. 2019, 94, 99–131. [Google Scholar] [CrossRef]
- Zhang, L. Formaldehyde: Exposure, Toxicity and Health Effects; Royal Society of Chemistry: London, UK, 2018; ISBN 9781788014519. [Google Scholar]
- Partopour, B.; Dixon, A.G. Effect of particle shape on methanol partial oxidation in a fixed bed using CFD reactor modeling. AICHE J. 2020, 66, e16904. [Google Scholar] [CrossRef]
- Heim, L.E.; Konnerth, H.; Prechtl, M.H.G. Future perspectives for formaldehyde: Pathways for reductive synthesis and energy storage. Green Chem. 2017, 19, 2347–2355. [Google Scholar] [CrossRef] [Green Version]
- Alarcón, E. World Formaldehyde Production to Exceed 52 Mln Tonnes in 2017; Merchant Research & Consulting Ltd.: Birmingham, UK, 2014. [Google Scholar]
- Millar, G.J.; Collins, M. Industrial Production of Formaldehyde Using Polycrystalline Silver Catalyst. Ind. Eng. Chem. Res. 2017, 56, 9247–9265. [Google Scholar] [CrossRef] [Green Version]
- Goon, S.; Bipasha, M.; Islam, M.S.; Hossain, M.B. Fish Marketing Status with Formalin Treatment in Bangladesh. Int. J. Public Heal. Sci. 2014, 3, 95. [Google Scholar] [CrossRef]
- Liu, X.; Kong, L.; Liu, C.; Xu, S.; Zhang, D.; Ma, F.; Lu, Z.; Sun, J.; Chen, J. Study on the formation process of MoO3/Fe2(MoO4)3 by mechanochemical synthesis and their catalytic performance in methanol to formaldehyde. J. Therm. Anal. Calorim. 2020, 142, 1363–1376. [Google Scholar] [CrossRef]
- Abdollahi, M.; Hosseini, A. Formaldehyde. In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 653–656. [Google Scholar]
- Subasi, N.T. Formaldehyde Advantages and Disadvantages: Usage Areas and Harmful Effects on Human Beings. In Biochemical Toxicology—Heavy Metals and Nanomaterials; IntechOpen: London, UK, 2020. [Google Scholar]
- Jokar, S.M.; Keshavarz, M.R.; Zhubin, M.; Parvasi, P.; Basile, A. A novel tubular membrane reactor for pure hydrogen production in the synthesis of formaldehyde by the silver catalyst process. Int. J. Hydrogen Energy 2021, 46, 21953–21964. [Google Scholar] [CrossRef]
- Maldonado, C.; Fierro, J.L.; Birke, G.; Martinez, E.; Reyes, P. Conversion of methanol to formaldehyde on TiO2 supported Ag nanoparticles. J. Chil. Chem. Soc. 2010, 55, 506–510. [Google Scholar] [CrossRef]
- Mursics, J.; Urbancl, D.; Goricanec, D. Process of Formaldehyde and Volatile Organic Compounds’ Removal fromWaste Gases. Appl. Sci. 2020, 10, 4702. [Google Scholar] [CrossRef]
- Taylor, S. Reflections on Catalytic Selective Oxidation: Opportunities and Challenges. Catalysts 2017, 7, 34. [Google Scholar] [CrossRef] [Green Version]
- Lloyd, L. Industrial Catalysts; Springer: Heidelberg, Germany, 2008; ISBN 978-0-387-24682-6. [Google Scholar]
- Brookes, C. New Insights on the Selective Oxidation of Methanol to Formaldehyde on FeMo Based Catalysts. PhD Thesis, Cardiff University, Cardiff, UK, 2015. [Google Scholar]
- Raun, K.V. Understanding the Deactivation of the Iron Molybdate Catalyst and its Influence on the Formox Process; Technical University of Denmark: Lyngby, Denmark, 2018. [Google Scholar]
- Adkins, H.; Peterson, W.R. The oxidation of methanol with air over iron, molybdenum, and iron-molybdenum oxides. J. Am. Chem. Soc. 1931, 53, 1512–1520. [Google Scholar] [CrossRef]
- Brookes, C.; Bowker, M.; Wells, P. Catalysts for the selective oxidation of methanol. Catalysts 2016, 6, 92. [Google Scholar] [CrossRef] [Green Version]
- Günther, R.; Disteldorf, W.; Gamer, A.O.; Reuss, G.; Hilt, A. Formaldehyde. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2000; Volume 40, p. 34. ISBN 9783527303854. [Google Scholar]
- Routray, K.; Zhou, W.; Kiely, C.J.; Grünert, W.; Wachs, I.E. Origin of the synergistic interaction between MoO3 and iron molybdate for the selective oxidation of methanol to formaldehyde. J. Catal. 2010, 275, 84–98. [Google Scholar] [CrossRef]
- Dehnamaki, H.; Iranshahi, D. Simultaneous Synthesis and Oxidation of Methanol to Formaldehyde, Thermally Coupled with Cyclohexane Dehydrogenation in a Trifunctional Reactor. Energy Fuels 2019, 33, 4487–4498. [Google Scholar] [CrossRef]
- Reuss, G.; Disteldorf, W.; Gamer, A.H. Formaldehyde Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2012; Volume 15. [Google Scholar]
- Yeo, B.R.; Pudge, G.J.F.; Bugler, K.G.; Rushby, A.V.; Kondrat, S.; Bartley, J.; Golunski, S.; Taylor, S.H.; Gibson, E.; Wells, P.P.; et al. The surface of iron molybdate catalysts used for the selective oxidation of methanol. Surf. Sci. 2016, 648, 163–169. [Google Scholar] [CrossRef] [Green Version]
- Raun, K.V.; Lundegaard, L.F.; Chevallier, J.; Beato, P.; Appel, C.C.; Nielsen, K.; Thorhauge, M.; Jensen, A.D.; Høj, M. Deactivation behavior of an iron-molybdate catalyst during selective oxidation of methanol to formaldehyde. Catal. Sci. Technol. 2018, 8, 4626–4637. [Google Scholar] [CrossRef] [Green Version]
- Soares, A.P.V.; Portela, M.F.; Kiennemann, A. Methanol selective oxidation to formaldehyde over Iron-Molybdate catalysts. Catal. Rev. 2005, 47, 125–174. [Google Scholar] [CrossRef]
- Liu, X.; Kong, L.; Xu, S.; Liu, C.; Ma, F. Modified iron-molybdate catalysts with various metal oxides by a mechanochemical method: Enhanced formaldehyde yield in methanol partial oxidation. Front. Chem. Sci. Eng. 2021, 1–12. [Google Scholar] [CrossRef]
- Matthey, J. A Formaldehyde Magazine from Johnson Matthey—The FORMOX Process. Informal Speaking. Available online: https://matthey.com/en (accessed on 22 July 2016).
- Andersson, A.; Hernelind, M.; Augustsson, O. A study of the ageing and deactivation phenomena occurring during operation of an iron molybdate catalyst in formaldehyde production. Catal. Today 2006, 112, 40–44. [Google Scholar] [CrossRef]
- Bahmanpour, A.M.; Hoadley, A.; Mushrif, S.H.; Tanksale, A. Hydrogenation of carbon monoxide into formaldehyde in liquid media. ACS Sustain. Chem. Eng. 2016, 4, 3970–3977. [Google Scholar] [CrossRef]
- Braz, C.G.; Mendes, A.; Rocha, J.; Alvim, R.; Matos, H.A. Model of an industrial multitubular reactor for methanol to formaldehyde oxidation in the presence of catalyst deactivation. Chem. Eng. Sci. 2019, 195, 347–355. [Google Scholar] [CrossRef]
- Zhang, S.; Han, M. Effect of Mo Dispersion on the Catalytic Properties and Stability of Mo–Fe Catalysts for the Partial Oxidation of Methanol. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Gaur, A.; Schumann, M.; Raun, K.V.; Stehle, M.; Beato, P.; Jensen, A.D.; Grunwaldt, J.-D.; Høj, M. Operando XAS/XRD and Raman Spectroscopic Study of Structural Changes of the Iron Molybdate Catalyst during Selective Oxidation of Methanol. ChemCatChem 2019, 11, 4871–4883. [Google Scholar] [CrossRef]
- Thrane, J.; Lundegaard, L.F.; Beato, P.; Mentzel, U.V.; Thorhauge, M.; Jensen, A.D.; Høj, M. Alkali Earth Metal Molybdates as Catalysts for the Selective Oxidation of Methanol to Formaldehyde—Selectivity, Activity, and Stability. Catalysts 2020, 10, 82. [Google Scholar] [CrossRef] [Green Version]
- Brookes, C.; Bowker, M.; Gibson, E.K.; Gianolio, D.; Mohammed, K.M.H.; Parry, S.; Rogers, S.M.; Silverwood, I.P.; Wells, P.P. In situ spectroscopic investigations of MoOx/Fe2O3 catalysts for the selective oxidation of methanol. Catal. Sci. Technol. 2016, 6, 722–730. [Google Scholar] [CrossRef] [Green Version]
- House, M.P.; Carley, A.F.; Echeverria-Valda, R.; Bowker, M. Effect of varying the cation ratio within iron molybdate catalysts for the selectivev oxidation of methanol. J. Phys. Chem. C 2008, 112, 4333–4341. [Google Scholar] [CrossRef]
- Kim, T.H.; Ramachandra, B.; Choi, J.S.; Saidutta, M.B.; Choo, K.Y.; Song, S.D.; Rhee, Y.W. Selective oxidation of methanol to formaldehyde using modified iron-molybdate catalysts. Catal. Lett. 2004, 98, 161–165. [Google Scholar] [CrossRef]
- Pernicone, N. Deactivation of Fe-Mo oxide catalyst in industrial plant and simulation tests on laboratory scale. Catal. Today 1991, 11, 85–91. [Google Scholar] [CrossRef]
- Brookes, C.; Wells, P.P.; Dimitratos, N.; Jones, W.; Gibson, E.K.; Morgan, D.J.; Cibin, G.; Nicklin, C.; Mora-Fonz, D.; Scanlon, D.O.; et al. The Nature of the Molybdenum Surface in Iron Molybdate. The Active Phase in Selective Methanol Oxidation. J. Phys. Chem. C 2014, 118, 26155–26161. [Google Scholar] [CrossRef]
- Gaur, A.; Stehle, M.; Raun, K.V.; Thrane, J.; Jensen, A.D.; Grunwaldt, J.-D.; Høj, M. Structural dynamics of an iron molybdate catalyst under redox cycling conditions studied with in situ multi edge XAS and XRD. Phys. Chem. Chem. Phys. 2020, 22, 11713. [Google Scholar] [CrossRef] [PubMed]
- Nikolenko, N.V.; Kosynyuk, A.O.; Kalashnikov, Y.V.; Cheremis, E.A. The calculation of the thermodynamic equilibrium in Fe3+/MoO42−/H+(OH−)/H2O system and determination of reasonable conditions for iron molybdate deposition. Russ. J. Appl. Chem. 2012, 85, 1814–1819. [Google Scholar] [CrossRef]
- Nikolenko, N.V.; Kozhevnikov, I.V.; Kostyniuk, A.O.; Bayahia, H.; Kalashnykov, Y.V. Preparation of iron molybdate catalysts for methanol to formaldehyde oxidation based on ammonium molybdoferrate(II) precursor. J. Saudi Chem. Soc. 2018, 22, 372–379. [Google Scholar] [CrossRef]
- Raun, K.V.; Lundegaard, L.F.; Beato, P.; Appel, C.C.; Nielsen, K.; Thorhauge, M.; Schumann, M.; Jensen, A.D.; Grunwaldt, J.D.; Høj, M. Stability of Iron-Molybdate catalysts for selective oxidation of methanol to formaldehyde: Influence of preparation method. Catal. Lett. 2020, 150. [Google Scholar] [CrossRef]
- Raun, K.V.; Johannessen, J.; McCormack, K.; Appel, C.C.; Baier, S.; Thorhauge, M.; Høj, M.; Jensen, A.D. Modeling of the molybdenum loss in iron molybdate catalyst pellets for selective oxidation of methanol to formaldehyde. Chem. Eng. J. 2019, 361, 1285–1295. [Google Scholar] [CrossRef]
- Deshmukh, S.A.R.K.; Van Sint Annaland, M.; Kuipers, J.A.M. Kinetics of the partial oxidation of methanol over a Fe-Mo catalyst. Appl. Catal. A Gen. 2005, 289, 240–255. [Google Scholar] [CrossRef]
- Raun, K.V.; Thorhauge, M.; Høj, M.; Jensen, A.D. Modeling of molybdenum transport and pressure drop increase in fixed bed reactors used for selective oxidation of methanol to formaldehyde using iron molybdate catalysts. Chem. Eng. Sci. 2019, 202, 347–356. [Google Scholar] [CrossRef]
- House, M.P.; Carley, A.F.; Bowker, M. Selective oxidation of methanol on iron molybdate catalysts and the effects of surface reduction. J. Catal. 2007, 252, 88–96. [Google Scholar] [CrossRef]
- Uhlrich, J.J.; Sainio, J.; Lei, Y.; Edwards, D.; Davies, R.; Bowker, M.; Shaikhutdinov, S.; Freund, H.J. Preparation and characterization of iron-molybdate thin films. Surf. Sci. 2011, 605, 1550–1555. [Google Scholar] [CrossRef]
- Okamoto, Y.; Morikawa, F.; Oh-Hiraki, K.; Imanaka, T.; Teranishi, S. Role of excess of MoO3 in Fe2O3-MoO3 methanol oxidation catalysts studied by X-ray photoelectron spectroscopy. J. Chem. Soc. Chem. Commun. 1981, 1018–1019. [Google Scholar] [CrossRef]
- Sun-Kou, M.R.; Mendioroz, S.; Fierro, J.L.G.; Palacios, J.M.; Guerrero-Ruiz, A. Influence of the preparation method on the behaviour of Fe-Mo catalysts for the oxidation of methanol. J. Mater. Sci. 1995, 30, 496–503. [Google Scholar] [CrossRef]
- Bowker, M.; Holroyd, R.; Elliott, A.; Morrall, P.; Alouche, A.; Entwistle, C.; Toerncrona, A. The selective oxidation of methanol to formaldehyde on iron molybdate catalysts and on component oxides. Catal. Lett. 2002, 83, 165–176. [Google Scholar] [CrossRef]
- Rellán-Piñeiro, M.; López, N. The Active molybdenum oxide phase in the methanol oxidation to formaldehyde (formox process): A DFT study. ChemSusChem 2015, 8, 2231–2239. [Google Scholar] [CrossRef] [PubMed]
- Oudghiri-Hassani, H. Synthesis, characterization and catalytic performance of iron molybdate Fe2(MoO4)3 nanoparticles. Catal. Commun. 2015, 60, 19–22. [Google Scholar] [CrossRef]
- Thavornprasert, K.; Capron, M.; Jalowiecki-Duhamel, L.; Gardoll, O.; Trentesaux, M.; Mamede, A.S.; Fang, G.; Faye, J.; Touati, N.; Vezin, H.; et al. Highly productive iron molybdate mixed oxides and their relevant catalytic properties for direct synthesis of 1,1-dimethoxymethane from methanol. Appl. Catal. B Environ. 2014, 145, 126–135. [Google Scholar] [CrossRef]
- Pradhan, S.; Bartley, J.K.; Bethell, D.; Carley, A.F.; Conte, M.; Golunski, S.; House, M.P.; Jenkins, R.L.; Lloyd, R.; Hutchings, G.J. Non-lattice surface oxygen species implicated in the catalytic partial oxidation of decane to oxygenated aromatics. Nat. Chem. 2012, 4, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Nisar, J.; Hassan, S.; Khan, M.I.; Iqbal, M.; Nazir, A.; Sharif, A.; Ahmed, E. Hetero-structured Iron Molybdate Nanoparticles: Synthesis, Characterization and Photocatalytic Application. Int. J. Chem. React. Eng. 2020, 18. [Google Scholar] [CrossRef]
- Wang, Y.; He, P.; Lei, W.; Dong, F.; Zhang, T. Novel FeMoO4/graphene composites based electrode materials for supercapacitors. Compos. Sci. Technol. 2014, 103, 16–21. [Google Scholar] [CrossRef]
- Barik, R.; Mukherjee, P.; Sahu, K.K.; Sanjay, K.; Ghosh, M.K.; Mohapatra, M. Synthesis of iron molybdate from molybdenum spent catalyst and evaluation of its electrochemical properties. Environ. Prog. Sustain. Energy 2021, 40, e13560. [Google Scholar] [CrossRef]
- Belhekar, A.A.; Ayyappan, S.; Ramaswamy, A.V. FT-IR studies on the evolution of different phases and their interaction in ferric molybdate—Molybdenum trioxide catalysts. J. Chem. Technol. Biotechnol. 1994, 59, 395–402. [Google Scholar] [CrossRef]
- Machiels, C.J.; Sleight, A.W. Kinetic isotope effect in the selective oxidation of methanol to formaldehyde over some molybdate catalysts. J. Catal. 1982, 76, 238–239. [Google Scholar] [CrossRef]
- Söderhjelm, E.; House, M.P.; Cruise, N.; Holmberg, J.; Bowker, M.; Bovin, J.-O.; Andersson, A. On the Synergy Effect in MoO3-Fe2(MoO4)3 Catalysts for Methanol Oxidation to Formaldehyde. Top. Catal. 2008, 50, 145–155. [Google Scholar] [CrossRef]
- Brookes, C.; Wells, P.P.; Cibin, G.; Dimitratos, N.; Jones, W.; Morgan, D.J.; Bowker, M. Molybdenum oxide on Fe2O3 core-shell catalysts: Probing the nature of the structural motifs responsible for methanol oxidation catalysis. ACS Catal. 2014, 4, 243–250. [Google Scholar] [CrossRef]
- Wang, C.T.; Willey, R.J. Oxidation of methanol over iron oxide based aerogels in supercritical CO2. J. Non. Cryst. Solids 1998, 225, 173–177. [Google Scholar] [CrossRef]
- Garba Wawata, I. Methanol Oxidation on Molybdenum Oxide Catalysts; Cardiff University: Cardiff, UK, 2015. [Google Scholar]
- Bowker, M.; Carley, A.F.; House, M. Contrasting the behaviour of MoO3 and MoO2 for the oxidation of methanol. Catal. Letters 2008, 120, 34–39. [Google Scholar] [CrossRef]
- Carbucicchio, M.; Trifirò, F. Surface and bulk redox processes in iron-molybdate-based catalysts. J. Catal. 1976, 45, 77–85. [Google Scholar] [CrossRef]
- Price, S. Catalytic Oxidation of Methanol to Formaldehyde by Mass-Selected Vanadium Oxide Clusters Supported on a TiO2 (110) Surface. J. Phys. Chem. 2014, 118, 8309. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Davarpanah, A.; Mokhtarian, N.; Farahbod, F. Integrated feasibility experimental investigation of hydrodynamic, geometrical and, operational characterization of methanol conversion to formaldehyde. Int. J. Ambient. Energy 2018, 42, 89–103. [Google Scholar] [CrossRef]
- Inokawa, H.; Zaman, S.F.; Driss, H.; Daous, M.; Al-Zahrani, A.; Miyaoka, H.; Ichikawa, T.; Kojima, Y.; Petrov, L.A. Formaldehyde production via partial oxidation of methanol over oxides of Cr, Mo and W supported on ceria-zirconia. IOP Conf. Ser. Mater. Sci. Eng. 2018, 458, 012018. [Google Scholar] [CrossRef]
- Hou, X.X.; Xu, C.H.; Liu, Y.L.; Li, J.J.; Hu, X.D.; Liu, J.; Liu, J.Y.; Xu, Q. Improved methanol synthesis from CO2 hydrogenation over CuZnAlZr catalysts with precursor pre-activation by formaldehyde. J. Catal. 2019, 379, 147–153. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Y.; Varma, A. Low-temperature selective oxidation of methanol over Pt-Bi bimetallic catalysts. J. Catal. 2018, 363, 144–153. [Google Scholar] [CrossRef]
- Chen, D.; Qu, Z.; Shen, S.; Li, X.; Shi, Y.; Wang, Y.; Fu, Q.; Wu, J. Comparative studies of silver based catalysts supported on different supports for the oxidation of formaldehyde. Catal. Today 2011, 175, 338–345. [Google Scholar] [CrossRef]
- Cortés Ortiz, W.G.; Delgado, D.; Guerrero Fajardo, C.A.; Agouram, S.; Sanchís, R.; Solsona, B.; López Nieto, J.M. Partial oxidation of methane and methanol on FeOx-, MoOx- and FeMoOx-SiO2 catalysts prepared by sol-gel method: A comparative study. Mol. Catal. 2020, 491, 110982. [Google Scholar] [CrossRef]
- Marcinkowski, M.D.; Yuk, S.F.; Doudin, N.; Smith, R.S.; Nguyen, M.T.; Kay, B.D.; Glezakou, V.A.; Rousseau, R.; Dohnálek, Z. Low-Temperature Oxidation of Methanol to Formaldehyde on a Model Single-Atom Catalyst: Pd Atoms on Fe3O4(001). ACS Catal. 2019, 10977–10982. [Google Scholar] [CrossRef]
- Matthey, J. A Formaldehyde Magazine from Johnson Matthey—The FORMOX Process. Informal Speaking. Available online: https://matthey.com/en (accessed on 22 July 2020).
- Häggblad, R.; Wagner, J.B.; Hansen, S.; Andersson, A. Oxidation of methanol to formaldehyde over a series of Fe1−xAlx-V-oxide catalysts. J. Catal. 2008, 258, 345–355. [Google Scholar] [CrossRef]
- Grasselli, R.K.; Lugmair, C.G.; Volpe, A.F. Towards an Understanding of the Reaction Pathways in Propane Ammoxidation Based on the Distribution of Elements at the Active Centers of the M1 Phase of the MoV(Nb,Ta)TeO System. Top. Catal. 2011, 54, 595–604. [Google Scholar] [CrossRef]
- Duprez, D.; Cavani, F. Handbook of Advanced Methods and Processes in Oxidation Catalysis: From Laboratory to Industry; Imperial College Press: London, UK, 2014; ISBN 9781848167513. [Google Scholar]
- Lintz, H.-G.; Müller, S.P. The partial oxidation of propane on mixed metal oxides—A short overview. Appl. Catal. A Gen. 2009, 357, 178–183. [Google Scholar] [CrossRef]
- Liu, Y.M.; Feng, W.L.; Li, T.C.; He, H.Y.; Dai, W.L.; Huang, W.; Cao, Y.; Fan, K.N. Structure and catalytic properties of vanadium oxide supported on mesocellulous silica foams (MCF) for the oxidative dehydrogenation of propane to propylene. J. Catal. 2006, 239, 125–136. [Google Scholar] [CrossRef]
- Rosowski, F.; Altwasser, S.; Dobner, C.K.; Storck, S.; Zühlke, J.; Hibst, H. New silver- and vanadium-containing multimetal oxides for oxidation of aromatic hydrocarbons. In Proceedings of the Catalysis Today; Elsevier: Amsterdam, The Netherlands, 2010; Volume 157, pp. 339–344. [Google Scholar]
- Rebsdat, S.; Mayer, D. Ethylene Oxide. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2001; p. 26. [Google Scholar]
- Grasselli, R.K.; Buttrey, D.J.; DeSanto, P.; Burrington, J.D.; Lugmair, C.G.; Volpe, A.F.; Weingand, T. Active centers in Mo–V–Nb–Te–O (amm)oxidation catalysts. In Proceedings of the Catalysis Today; Elsevier: Amsterdam, The Netherlands, 2004; Volume 91–92, pp. 251–258. [Google Scholar]
- Mistry, C.R.; Mewada, R.K.; Srivastava, V.; Jasra, R. Characteristics of Oxidation and Oxidative Dehydrogenation Catalysts for Gas Phase Reactions: A Review. In Proceedings of the Nirma University International Conference on Engineering (Nuicone), Ahmedabad, India, 8–10 December 2011. [Google Scholar]
- Riittonen, T.; Toukoniitty, E.; Madnani, D.K.; Leino, A.R.; Kordas, K.; Szabo, M.; Sapi, A.; Arve, K.; Wärnå, J.; Mikkola, J.P. One-pot liquid-phase catalytic conversion of ethanol to 1-butanol over aluminium oxide-the effect of the active metal on the selectivity. Catalysts 2012, 2, 68–84. [Google Scholar] [CrossRef] [Green Version]
- Védrine, J. Heterogeneous Partial (amm)Oxidation and Oxidative Dehydrogenation Catalysis on Mixed Metal Oxides. Catalysts 2016, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Arntz, D.; Fischer, A.; Höpp, M.; Jacobi, S.; Sauer, J.; Ohara, T.; Sato, T.; Shimizu, N.; Schwind, H. Acrolein and Methacrolein. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012. [Google Scholar]
- Cavani, F. Catalytic selective oxidation: The forefront in the challenge for a more sustainable chemical industry. Catal. Today 2010, 157, 8–15. [Google Scholar] [CrossRef]
- Whiting, G.T.; Bartley, J.K.; Dummer, N.F.; Hutchings, G.J.; Taylor, S.H. Vanadium promoted molybdenum phosphate catalysts for the vapour phase partial oxidation of methanol to formaldehyde. Appl. Catal. A Gen. 2014, 485, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Laitinen, T.; Ojala, S.; Cousin, R.; Koivikko, N.; Poupin, C.; El Assal, Z.; Aho, A.; Keiski, R.L. Activity, selectivity, and stability of vanadium catalysts in formaldehyde production from emissionsof volatile organic compounds. J. Ind. Eng. Chem. 2020, 83, 375–386. [Google Scholar] [CrossRef]
- Andersson, A.; Holmberg, J.; Häggblad, R. Process improvements in methanol oxidation to formaldehyde: Application and catalyst development. Top. Catal. 2016, 59, 1589–1599. [Google Scholar] [CrossRef]
- Matthey, J. A Formaldehyde Magazine from Johnson Matthey—The FORMOX Process. Informal Speaking. Available online: https://matthey.com/en (accessed on 22 July 2019).
- Group, F. Some aspects of a newly developed Fe-Mo/SiO2 oxide for methanol oxidation in a fluidized Bed. Sci. Sin. Chang. Inst. Appl. Chem. 1979, XXII, 777. [Google Scholar]
- Cairati, L.; Di Flore, L.; Forzatti, P.; Pasquen, I.; Trifirò, F. Oxidation of methanol in a fluidized Bed. 1. catalyst attrition resistance and process variable study. Ind. Eng. Chem. Process Des. Dev. 1980, 19, 561–565. [Google Scholar] [CrossRef]
- Pernicone, N. MoO3Fe2(MoO4)3 catalysts for methanol oxidation. J. Less-Common Met. 1974, 36, 289–297. [Google Scholar] [CrossRef]
- Hassan, K.H.; Mitchell, P.C.H. Evaluation of different methods to prepare the Fe2O3/MoO3 catalyst used for selective oxidation of methanol to formaldehyde. Stud. Surf. Sci. Catal. 2010, 175, 475–478. [Google Scholar] [CrossRef]
- Chapman, S.; Brookes, C.; Bowker, M.; Gibson, E.K.; Wells, P.P. Design and stabilisation of a high area iron molybdate surface for the selective oxidation of methanol to formaldehyde. Faraday Discuss. 2016, 188, 115–129. [Google Scholar] [CrossRef] [Green Version]
- Soares, A.P.V.; Portela, M.F.; Kiennemann, A.; Hilaire, L. Mechanism of deactivation of iron-molybdate catalysts prepared by coprecipitation and sol-gel techniques in methanol to formaldehyde oxidation. Chem. Eng. Sci. 2003, 58, 1315–1322. [Google Scholar] [CrossRef]
- Bowker, M.; House, M.; Alshehri, A.; Brookes, C.; Gibson, E.K.; Wells, P.P. Selectivity determinants for dual function catalysts: Applied to methanol selective oxidation on iron molybdate. Catal. Struct. React. 2015, 1. [Google Scholar] [CrossRef]
- Wang, C.-T.; Willey, R.J. Mechanistic aspects of methanol partial oxidation over supported iron oxide aerogels. J. Catal. 2001, 202. [Google Scholar] [CrossRef]
- Bowker, M.; Holroyd, R.; House, M.; Bracey, R.; Bamroongwongdee, C.; Shannon, M.; Carley, A. The Selective Oxidation of Methanol on Iron Molybdate Catalysts. Top. Catal. 2008, 48, 158–165. [Google Scholar] [CrossRef]
- Soares, A.P.V.; Farinha Portela, M.; Kiennemann, A.; Hilaire, L.; Millet, J.M.M. Iron molybdate catalysts for methanol to formaldehyde oxidation: Effects of Mo excess on catalytic behaviour. Appl. Catal. A Gen. 2001, 206, 221–229. [Google Scholar] [CrossRef]
- Beale, A.M.; Jacques, S.D.M.; Sacaliuc-Parvalescu, E.; O’Brien, M.G.; Barnes, P.; Weckhuysen, B.M. An iron molybdate catalyst for methanol to formaldehyde conversion prepared by a hydrothermal method and its characterization. Appl. Catal. A Gen. 2009, 363, 143–152. [Google Scholar] [CrossRef]
- Jin, G.; Weng, W.; Lin, Z.; Dummer, N.F.; Taylor, S.H.; Kiely, C.J.; Bartley, J.K.; Hutchings, G.J. Fe2(MoO4)3/MoO3 nano-structured catalysts for the oxidation of methanol to formaldehyde. J. Catal. 2012, 296, 55–64. [Google Scholar] [CrossRef]
- Soares, A.P.V.; Portela, M.F.; Kiennemann, A. A comparison of iron molybdate catalysts for methanol oxidation prepared by copreciptation and new sol-gel method. Stud. Surf. Sci. Catal. 1997, 110, 807–816. [Google Scholar] [CrossRef]
- Li, J.L.; Zhang, Y.X.; Liu, C.W.; Zhu, Q.M. Improvement in reactivity, reproducibility and stability of Fe-Mo catalysts by wet mixing. Catal. Today 1999, 51, 195–199. [Google Scholar] [CrossRef]
- Popov, B.I.; Shkuratova, L.N.; Skorokhova, N.G. Influence of sodium salts on the catalytic properties of iron-molybdenum oxide catalysts in the oxidation of methanol to formaldehyde. React. Kinet. Catal. Lett. 1975, 3, 463–469. [Google Scholar] [CrossRef]
- Burcham, L.J.; Briand, L.E.; Wachs, I.E. Quantification of active sites for the determination of methanol oxidation turn-over frequencies using methanol chemisorption and in situ infrared techniques. 1: Supported metal oxide catalysts. Langmuir 2001, 17, 6164–6174. [Google Scholar] [CrossRef]
- Demidov, A.; Danilova, I.; Kustova, G.; Plyasova, L.; Skomorokhova, N.; Sedova, L.; Nakrokhin, V.; Popov, B. Effect of anions on the catalytic properties of ferric molybdate in the selective oxidation of methanol. Kinet. Catal. 1992, 33, 910–914. [Google Scholar]
- Kong, L.; Zhang, M.; Liu, X.; Ma, F.; Wei, B.; Wumaier, K.; Zhao, J.; Lu, Z.; Sun, J.; Chen, J.; et al. Green and rapid synthesis of iron molybdate catalyst by mechanochemistry and their catalytic performance for the oxidation of methanol to formaldehyde. Chem. Eng. J. 2019, 364, 390–400. [Google Scholar] [CrossRef]
- Alessandrini, G.; Cairati, L.; Forzatti, P.; Villa, P.L.; Trifirò, F. Chemical, structural and catalytic modifications of pure and doped iron(III) molybdate. J. Less-Common Met. 1977, 54, 373–386. [Google Scholar] [CrossRef]
- Kolovertnov, G.D.; Boreskov, G.K.; Dzisko, V.A.; Popov, B.I.; Tarasova, D.V.; Belugina, G.C. Study on iron-molybdenum oxide catalysts for the oxidation of methanol to formaldehyde. I. Specific activity as a function of the catalyst composition. Kinet. Catal. Engl. Transl. 1965, 6, 950. [Google Scholar]
- Wilson, J.H. Raman Spectroscopic Studies of Iron/Molybdenum Oxide Catalysts for the Oxidation of Methanol to Formaldehyde; University of Wisconsin: Madison, WI, USA, 1986; Volume 1. [Google Scholar]
- Farneth, W.E.; Ohuchi, F.; Staley, R.H.; Chowdhry, U.; Sleight, A.W. Mechanism of partial oxidation of methanol over MoO3 as studied by temperature-programmed desorption. J. Phys. Chem. 1985, 89, 2493–2497. [Google Scholar] [CrossRef]
- Boreskov, G.K.; Kolovertnov, G.D.; Kefeli, L.M.; Plyasova, L.M.; Karakchiev, L.G.; Mastikhin, V.N.; Popov, V.I.; Dzis’Ko, V.A.; Tarasova, D.V. Study of an Iron-Molybdenum Oxide Catalyst for the Oxidation of Methanol to Formaldehyde. II. Phase Composition and Nature of the Catalytically Active Component. Kinet. Catal. Engl.Transl. 1966, 7, 125–130. [Google Scholar]
- Chowdhry, U.; Ferretti, A.; Firment, L.E.; Machiels, C.J.; Ohuchi, F.; Sleight, A.W.; Staley, R.H. Mechanism and surface structural effects in methanol oxidation over molybdates. Appl. Surf. Sci. 1984, 19, 360–372. [Google Scholar] [CrossRef]
- Weckhuysen, B.M.; Wachs, I.E. The Role of Terminal M55O Bonds in Selective Oxidation Reactions Over Metal Oxide Catalysts. In Proceedings of the 11th International Congress on Catalysis—40th Anniversary, Baltimore, MD, USA, 30 June–5 July 1996. [Google Scholar]
- Truong, V.; Nguyen, V.P.L.; Tittarelli, P. Bulk and Surface Characterization of Iron (III) Molybdate Catalysts for the Oxidation of Methanol. Chemistry and Uses of Molybdenum. In Proceedings of the Third International Conference, Ann Arbor, MI, USA, 19–23 August 1979; p. 161. [Google Scholar]
- Wachs, I.E.; Routray, K. Catalysis Science of Bulk Mixed Oxides. ACS Catal. 2012, 2, 1235–1246. [Google Scholar] [CrossRef]
- Chung, J.S.; Miranda, R.; Bennett, C.O. Mechanism of partial oxidation of methanol over MoO3. J. Catal. 1988, 114, 398–410. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, T.; Chen, S.; Zhao, Y.; Ma, X.; Gong, J. Selective oxidation of methanol to dimethoxymethane on V2O5-MoO3/γ-Al2O3 catalysts. Appl. Catal. B Environ. 2014, 160–161, 161–172. [Google Scholar] [CrossRef]
- Huang, P.R.; He, Y.; Cao, C.; Lu, Z.H. Impact of lattice distortion and electron doping on α-MoO3 electronic structure. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowker, M.; Gibson, E.K.; Silverwood, I.P.; Brookes, C. Methanol oxidation on Fe2O3 catalysts and the effects of surface Mo. Faraday Discuss. 2016, 188, 387–398. [Google Scholar] [CrossRef] [Green Version]
- Bowker, M.; Brookes, C.; Carley, A.F.; House, M.P.; Kosif, M.; Sankar, G.; Wawata, I.; Wells, P.P.; Yasenevaz, P. Evolution of active catalysts for the selective oxidative dehydrogenation of methanol on Fe2O3 surface doped with Mo oxide. Phys. Chem. Chem. Phys. 2013, 15, 11988–12003. [Google Scholar] [CrossRef]
- Dias, A.P.S.; Montemor, F.; Portela, M.F.; Kiennemann, A. The role of the suprastoichiometric molybdenum during methanol to formaldehyde oxidation over Mo-Fe mixed oxides. J. Mol. Catal. A Chem. 2015, 397, 93–98. [Google Scholar] [CrossRef]
- Pham, T.T.P.; Nguyen, P.H.D.; Vo, T.T.; Nguyen, H.H.P.; Luu, C.L. Facile method for synthesis of nanosized β-MoO3 and their catalytic behavior for selective oxidation of methanol to formaldehyde. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 045010. [Google Scholar] [CrossRef]
- Machiels, C.J.; Cheng, W.H.; Chowdhry, U.; Farneth, W.E.; Hong, F.; Mc Carron, E.M.; Sleight, A.W. The effect of the structure of molybdenum oxides on the selective oxidation of methanol. Appl. Catal. 1986, 25, 249–256. [Google Scholar] [CrossRef]
- Tatibouet, J.M.; Germain, J.E.; Volta, J.C. Structure-sensitive catalytic oxidation: Alcohols on graphite-supported molybdenum trioxide. J. Catal. 1983, 82, 240–244. [Google Scholar] [CrossRef]
- Castillo, R.; Dewaele, K.; Ruiz, R. Mechanical mixtures of alpha-Sb204 and MoO3 as highly selective catalysts for the oxidation of methanol to formaldehyde. Appl. Catal. A Gen. 1996, 153, 1–8. [Google Scholar] [CrossRef]
- Briand, L.E.; Farneth, W.E.; Wachs, I.E. Quantitative determination of the number of active surface sites and the turnover frequencies for methanol oxidation over metal oxide catalysts. I. Fundamentals of the methanol chemisorption technique and application to monolayer supported molybdenum oxide catalysts. Catal. Today 2000, 62, 219–229. [Google Scholar] [CrossRef]
- Chen, Y.; Wachs, I.E. Tantalum oxide-supported metal oxide (Re2O7, CrO3, MoO3, WO3, V2O5, and Nb2O5) catalysts: Synthesis, Raman characterization and chemically probed by methanol oxidation. J. Catal. 2003, 217, 468–477. [Google Scholar] [CrossRef]
- Cairati, L.; Carbucicchio, M.; Ruggeri, O.; Trifiro, F. Study of the interaction of Fe2O3-MoO3 with several supports. Stud. Surf. Sci. Catal. 1979, 3, 279–292. [Google Scholar] [CrossRef]
- Forzatti, P. On the deactivation of Fe2O3-MoO3/SiO2 catalysts in the oxidation of methanol to formaldehyde. React. Kinet. Catal. Lett. 1982, 20, 213–218. [Google Scholar] [CrossRef]
- Carbucicchio, M.; Forzaiti, P.; Tronconi, E.; Villa, P.L.; Trifiro’, F. Deactivation of silica supported Fe2O3-MoO3 catalyst for the oxidation of methanol. Stud. Surf. Sci. Catal. 1980, 6, 103–113. [Google Scholar] [CrossRef]
- Hill, C.G.; Wilson, J.H. Raman spectroscopy of iron molybdate catalyst systems. Part II. Preparation of supported catalysts. J. Mol. Catal. 1991, 67, 57–77. [Google Scholar] [CrossRef]
- Rozanov, V.V.; Vieira Soares, A.P.; Portela, M.F. Methanol to formal-dehyde oxidation over silica supported Iron-molybdates: Effect of iron precursor. In Proceedings of the Book of Extended Abstracts, 4th ed.; World Congress on Oxidation Catalysis: Berlim/Potsdam, Germany, 2001. [Google Scholar]
- Peyrovi, M.H.; Parsafard, N.; Hasanpour, H. Catalytic Study of the Partial Oxidation Reaction of Methanol to Formaldehyde in the Vapor Phase. Bull. Chem. React. Eng. Catal. 2018, 13, 520–528. [Google Scholar] [CrossRef]
- Nieto, J.; Lopez, F.T. The Role of Promoters in Selective Oxidation with Mixed Oxides Based Catalysts. In Proceedings of the Advances in Catalyst Design, Trieste, Italy, 10–14 November 1992; Volume 2, p. 412. [Google Scholar]
- Klissurski, D.; Rives, V.; Pesheva, Y.; Mitov, I.; Abadzhjieva, N. Iron-chromium-molybdenum oxide catalysts for methanol oxidation. Catal. Lett. 1993, 18, 265–271. [Google Scholar] [CrossRef]
- Estevez Sanchez, A.M.; Fernandez Tena, A.; Marquez Moreno, M.C. Oxidation of Methanol to Formaldehyde on Iron-Molybdenum Oxide Catalysts, with and without Chromium as a promoter. Kinet. Catal. Lett 1989, 38, 193–198. [Google Scholar] [CrossRef]
- Estevez, A.; Marquez, M.; Tena, A.; Del Arco, M.; Martin, C.; Rives, V. Catalytic and selectivity at low temperature of iron-molybdenum oxide catalysts in methanol oxidation to formaldehyde. Chem. Biochem. Eng. Q. 1990, 4, 61–65. [Google Scholar]
- Pesheva, Y.; Mitov, I.; Klissurski, D. Study on the Stability of Molybdate Based Catalysts. In Proceedings of the Book of Abstracts Europacat II Congress; The Maastricht Exhibition & Conference Centre (MECC), Maastricht, The Netherland, 3–8 September 1995. [Google Scholar]
- Ivanov, K.; Mitov, I.; Krustev, S. Selective oxidation of methanol on Fe-Mo-W catalysts. J. Alloys Compd. 2000, 309, 57–60. [Google Scholar] [CrossRef]
- Pernicone, N. Advances and Trends in the Catalysis of Formaldehyde Production Over Fe-Mo Catalysts. In Proceedings of the Meeting on Industrial and Environmental Catalysis, Lisbon, Spain, 12 May 1995; pp. 15–19. [Google Scholar]
- Ding, H.; Lin, H.; Sadigh, B.; Zhou, F.; Ozoliņš, V.; Asta, M. Computational investigation of electron small polarons in α-MoO3. J. Phys. Chem. C 2014, 118, 15565–15572. [Google Scholar] [CrossRef]
- Choksi, T.; Greeley, J. Partial oxidation of methanol on MoO3 (010): A DFT and microkinetic study. ACS Catal. 2016, 6, 7260–7277. [Google Scholar] [CrossRef]
- Ivanov, K.I.; Dimitrov, D.Y. Deactivation of an industrial iron-molybdate catalyst for methanol oxidation. Catal. Today 2010, 154, 250–255. [Google Scholar] [CrossRef]
- Matthey, J. A Formaldehyde Magazine from Johnson Matthey—Safe Operations, the FORMOX Process. Informal Speaking. Available online: https://matthey.com/en (accessed on 22 July 2017).
- Mitov, I.; Asenov, S.; Tomov, T.; Klissurski, D. In situ mössbauer study of the interaction of methanol with an iron-molybdenum oxide catalyst. J. Phys. Chem. C 2007, 111, 5389–5393. [Google Scholar] [CrossRef]
- Jacques, S.D.M.; Leynaud, O.; Strusevich, D.; Beale, A.M.; Sankar, G.; Martin, C.M.; Barnes, P. Redox behavior of Fe-Mo-O catalysts studied by ultrarapid in situ diffraction. Angew. Chemie Int. Ed. 2006, 45, 445–448. [Google Scholar] [CrossRef] [Green Version]
- Jacques, S.D.M.; Leynaud, O.; Strusevich, D.; Stukas, P.; Barnes, P.; Sankar, G.; Sheehy, M.; O’Brien, M.G.; Iglesias-Juez, A.; Beale, A.M. Recent progress in the use of in situ X-ray methods for the study of heterogeneous catalysts in packed-bed capillary reactors. Catal. Today 2009, 145, 204–212. [Google Scholar] [CrossRef]
- Abaulina, L.I.; Kustova, G.N.; Klevtsova, R.F.; Popov, B.I.; Bibin, V.N.; Melekhina, V.A.; Kolomiichuk, V.N.; Boreskov, G.K. Study of an iron-molybdenum oxide catalyst for the oxidation of methanol to formaldehyde. V. The formation of a solid solution of molybdenum trioxide in Iron molybdate and the nature of the catalytically active component. Kinet. Catal. Engl. Transl. 1976, 17, 1126–1132. [Google Scholar]
- Burriesci, N.; Garbassi, F.; Petrera, M.; Petrini, G.; Pernicone, N. Solid state reactions in Fe-Mo oxide catalysts for methanol oxidation during aging in industrial plants. Stud. Surf. Sci. Catal. 1980, 6, 115–126. [Google Scholar] [CrossRef]
- Arruano, J.; Wanke, S. Effect of High Temperature Treatment on the Properties of Fe/Mo Oxide Catalysts. Canad. J. Chem. Eng 1975, 53. [Google Scholar] [CrossRef]
- Aruanno, J.; Wanke, S. Rates of nethanol oxidation over thermally treated Fe-Mo oxide catalysts. Can. J. Chem. Eng. 1977, 55, 93–95. [Google Scholar] [CrossRef]

| Physical Properties [25,28,34,68,69] and Selectivity of Pure Oxide Phases | |||
|---|---|---|---|
| Phases | Mol. Weight [g/mol] | Color | Selectivity Towards |
| Fe2O3 | 159.69 | Red-brown | CO2 [32,70,71] |
| MoO2 | 127.94 | Dark blue-violet | Primarily CO and small amounts of CO2 [32,72] |
| FeMoO4 | 215.78 | Light green | CO [32,37] |
| MoO3 | 143.94 | White-yellow | Formaldehyde [32,47,73] |
| Fe2(MoO4)3 | 591.56 | Brown-yellow-green | Primarily formaldehyde [32,47,74] |
| Process | Catalyst | Selectivity (%) | Conversion (%) | Temperature (°C) |
|---|---|---|---|---|
| Methanol to formaldehyde [84] | Fe-Mo-O | 92–95 | >99 | 250–380 |
| Propene to acrylonitrile [85] | Bi-Mo-O | 80–83 | 98 | 420–450 |
| Propane to acrylonitrile [86] | VSbWOx/SiO2-Al2O3 | 67 | 60 | 500 |
| Propane to acrylic acid [87] | Mo-V-Nb-Te-O | 50–60 | 80 | 350–400 |
| Propane to propene [88] | V-O/MCF | 68 | 41 | 550 |
| Xylene to phthalic anhydride [89] | V-O/TiO2 | 80–82 | 99.9 | 350–450 |
| Ethene to ethylene oxide [90] | Ag/Al2O3 | 80–90 | 7–15 | 200–300 |
| Propane to propylene [86] | V-silicalite | 30 | 70 | 550 |
| Propane to acrylonitrile [91] | Mo-V-Nb-Te-O | 72 | 76 | 420 |
| Ethylene/Acetic acidto Vinyl acetate [92] | Pd-Cu-K on Al2O3 | 92 | 8–12 | - |
| Ethanol to butanol [93] | 20% Ni/Al2O3 | 25 | 80 | 250 |
| Propane to propylene [86] | V-MgO | 38 | 62 | 540 |
| Ethane to ethylene [94] | Ni-NbO | 51 | 90 | 400 |
| Ethane to ethylene [86] | MoVTeNbO | 85 | 88 | 400 |
| Propene to acrolein [95] | Bi-Mo-O | 83–90 | <98 | 300–400 |
| Butane to maleic anhydride [96] | V-P-O | 65–73 | 75–85 | 350–420 |
| Years | Process Development | Results |
|---|---|---|
| 1959 | Dried granules of catalyst were used, and the feed consisted of 6.5 vol% methanol in the air. | First Production Scale |
| 1972 | Gas recirculation, 7.5 vol% methanol, and 10–11 vol% O2 pre-calcined granules. | The gas recirculation and lower oxygen concentration increased productivity and reduced emissions. |
| 1984 | Introduced the ring-shaped catalyst. | Improved gas velocity, lowered the pressure drop and eventually expanded the productivity. |
| 1984 | Introduced the emission control system. | Better environmental impact and steam generation system. |
| 1997 | New loading system for faster and consistent loading with increased pressurization (0.3 bar). | Enhancement in productivity. |
| 2003 | New design standards and introduction of catalyst activity profile (CAP) with higher methanol inlet. | Productivity improvements. |
| 2005 | Pressurization to 0.5 bar g. | Productivity improvements. |
| 2009 | Launched CAP 2.0. | Higher yield, lower pressure drop, less aging. |
| 2011 | CAP 2.0, introduction of turbo charger. | Reduced power consumption, reduced operational cost. |
| 2012 | Launched CAP 3.0. | Yield and lifetime improvement and enabled the operation at even higher methanol inlet (up to 11 vol%). |
| 2016 | Complete loading/reloading service concept. | Reduced the downtime, lowered the risk issues related to unclean tubes. |
| 2018 | Addition of new high pressure plant design features 2.0 bar with a newly developed catalyst system. | Better performance, flexibility and rise in productivity. |
| Mo/Fe Ratio in the Catalyst | Conversion at 180 °C | Selectivity | Surface Area (m2/g) |
|---|---|---|---|
| 0 (Fe2O3) | 2 | 0 (322 °C) | 2.1 |
| 0.2 | 55 | 18 (204 °C) | 55.4 |
| 0.5 | 50 | 27 (210 °C) | 38.7 |
| 1 | 38 | 47 (244 °C) | 16.3 |
| 1.5 | 35 | 73 (249 °C) | 7.8 |
| 2.2 | 29 | 90 (256 °C) | 6.7 |
| Reaction | Oxygen to Methanol Molar Ratio |
|---|---|
| CH3OH + ½ O2 → CO2 + 2H2O | 1.5 |
| CH3OH + 1 O2 → CO + 2H2O | 1 |
| CH3OH + ½ O2 → CH2O + H2O | 0.5 |
| 2 CH3OH → CH3O CH3 + H2O | 0 |
| CH2O+ 2 CH3OH → (CH3O)2 CH2 + H2O | 0 |
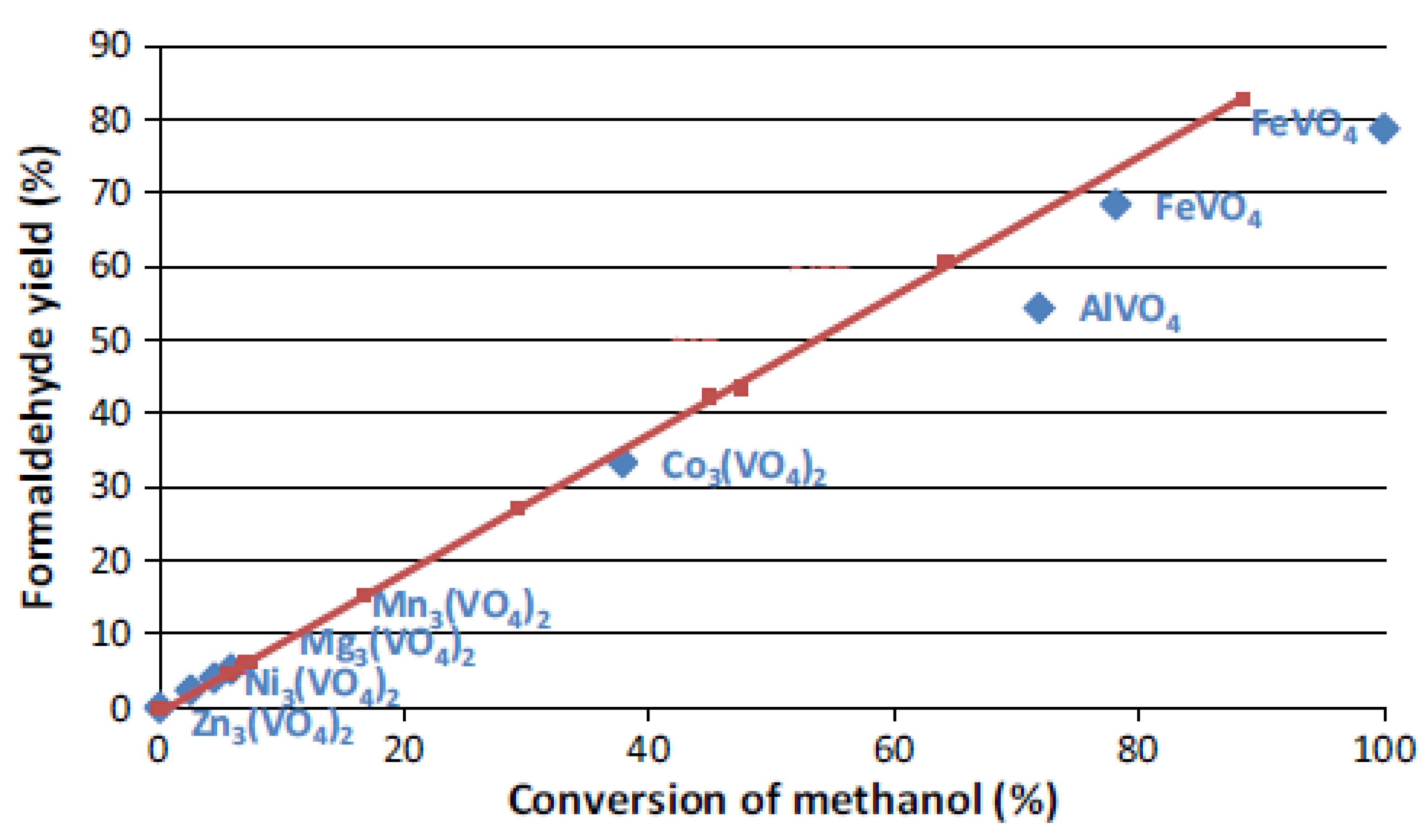
| Catalyst | Mo and V Loss (% per m2) |
|---|---|
| Fe2(MoO4)3 -MoO3 | 9.3 |
| Fe2(MoO4)3 | 2.3 |
| Cr2(MoO4)3 | 6.2 |
| Zr(MoO4)3 | 9.7 |
| FeVO4 | 1.9 |
| AlVO4 | 4.8 |
| Mn3(VO4)2 | 2.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, M.I.; Abatzoglou, N.; Achouri, I.E. Methanol to Formaldehyde: An Overview of Surface Studies and Performance of an Iron Molybdate Catalyst. Catalysts 2021, 11, 893. https://doi.org/10.3390/catal11080893
Malik MI, Abatzoglou N, Achouri IE. Methanol to Formaldehyde: An Overview of Surface Studies and Performance of an Iron Molybdate Catalyst. Catalysts. 2021; 11(8):893. https://doi.org/10.3390/catal11080893
Chicago/Turabian StyleMalik, Muhammad Irfan, Nicolas Abatzoglou, and Inès Esma Achouri. 2021. "Methanol to Formaldehyde: An Overview of Surface Studies and Performance of an Iron Molybdate Catalyst" Catalysts 11, no. 8: 893. https://doi.org/10.3390/catal11080893
APA StyleMalik, M. I., Abatzoglou, N., & Achouri, I. E. (2021). Methanol to Formaldehyde: An Overview of Surface Studies and Performance of an Iron Molybdate Catalyst. Catalysts, 11(8), 893. https://doi.org/10.3390/catal11080893








