Abstract
Herein, we report a facile synthetic methodology for the preparation of 2,3-dialkylquinolines from anilines and propionaldehydes. This cyclization involved environmentally friendly Nafion® NR50 as an acidic catalyst with microwave irradiation as the heating source. A series of substituted 2-ethyl-3-methylquinolines were prepared from various anilines and propionaldehyde derivatives through this protocol with good to excellent yields. Some new chemical structures were confirmed by X-ray single-crystal diffraction analysis and the related data were provided. The plausible reaction mechanism studies are also discussed.
1. Introduction
A quinoline scaffold is a versatile synthetic building block in various natural products [1,2,3,4,5], exceptional pharmaceuticals [6,7,8], physical materials [9,10,11,12,13], and is an important intermediate for asymmetric synthesis [14,15,16,17,18]. Functionalized quinolines are broadly used in agrochemicals [19,20], dyes [21,22], and some biologically active molecules for antimalarial [23,24,25], anticancer [26,27,28,29,30], antiviral [31], antifungal [32], anti-bacterial [33], and anti-inflammatory functions [34,35]. In particular, since the 17th century, the quinoline alkaloid quinine has been viewed historically as the first cure for treating or preventing malaria, [36,37]. Recently, some reports indicated that some quinoline-containing compounds are potentially active SARS-CoV-2 inhibitors [38,39,40], and some quinoline-derived drugs, as shown in Scheme 1, are currently being investigated as a possible cure for COVID-19 infection [41,42]. Numerous classical methodologies for the construction of the quinoline skeleton, such as the Combes reaction, the Conrad–Limpach–Knorr reaction, the Doebner–Von Miller reaction, the Friedlander reaction, the Povaror reaction, the Pfitzinger reaction, and the Skraup reaction have been well documented [43,44,45,46,47].
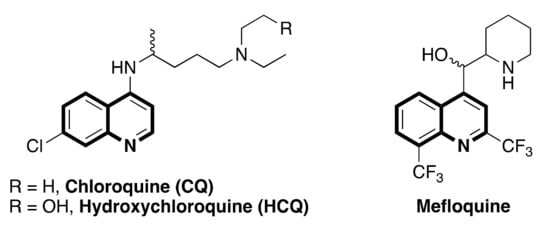
Scheme 1.
Some quinoline-based antimalarial drugs.
Alkylated quinolines display some meaningful activity against cancer, inflammation, malaria, and tuberculosis [48,49,50,51,52]. Among them, 2-alkylquinolines show better activities compared to the other alkyl-substituted quinolines. The alkyl groups at the C-2 position are adjacent to the quinoline nitrogen atom, which increases the lipophilicity and promotes cell permeability [53]. Although the synthesis of 2,3-dialkylated quinolines involving readily available starting materials such as aldehydes, ketones, alkenes, alkynes, cyclobutanes, or allyl alcohols is broadly reported [54,55,56,57,58,59,60,61,62,63,64,65,66,67], the protocol to synthesize alkylated quinolines poses a challenge to medicinal chemists.
In continuation of our investigations toward the synthesis of nitrogen-containing heterocyclic compounds [68,69,70,71], we recently provided a synthetic route for the Friedländer quinoline synthesis from 2-aminobenzophenone and acetylacetone catalyzed by Nafion® NR50 particles under microwave irradiation ((1) in Scheme 2) [69]. Nafion® is a commercially available synthetic polymer particle which possesses ionic properties [72]. Its unique ionic properties result from the copolymerization of incorporating perfluorovinyl ether groups terminated with sulfonate groups onto a tetrafluoroethylene (PTFE) skeleton [73]. The chemical structure of Nafion® NR50 is shown in Scheme 2. Considering the importance of alkylated quinolines in medicinal chemistry, the efficiency of microwave irradiation and recyclable features of Nafion® NR50 in green chemistry, the development of a facile synthetic protocol is of great interest. Herein, we provide an efficient synthetic protocol for the preparation of a 2-ethyl-3-methylquinoline skeleton from aniline and propionaldehyde using Nafion® NR50 with good to excellent yields under microwave irradiation ((2) in Scheme 2). Four chemical structures are confirmed by X-ray single-crystal diffraction analysis.
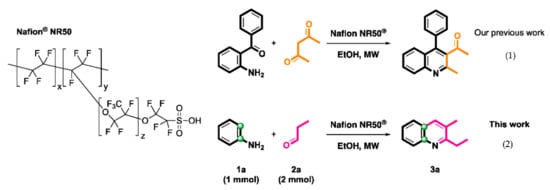
Scheme 2.
Nafion® NR50-mediated synthesis of quinolines.
2. Results and Discussion
Initially, we conducted the investigation with the cyclization of readily available aniline 1a and propionaldehyde 2a as model substrates in the solvent under microwave irradiation (T = 150 °C), and the related results are summarized in Table 1. By using liquid acids, such as AcOH (acetic acid), TFA (trifluoroacetic acid), and TfOH (triflic acid), quinoline 1a was obtained at 15% to 40% yield (entries 1–3). The involvement of substituted sulfonic acids, including MsOH (methansulfonic acid), BsOH (benzenesulfonic acid), and p-TsOH.H2O (p-toluenesulfonic acid) provided product 3a in similar yields (entries 4–6). Other metal triflates such as AgOTf, Bi(OTf)3, Fe(OTf)2, Fe(OTf)3, and Sn(OTf)2 were also examined in this reaction, as shown in entries 7–11, and the isolated yields of 3a were lower than that obtained after using sulfonic acids. Other Lewis acids including BF3.OEt2, InCl3, and AlCl3 promoted the reactions, and the isolated yields are shown in entries 12–14. Among these entries, BF3.OEt2 showed the best performance. According to our previous work [69], we further examined the environmentally friendly solid acid Nafion® NR50, and the desired product 3a was obtained at 93% yield (entry 15). However, only a 30% yield of 3a was observed when liquid Nafion® NR117 was used as an acid catalyst (entry 16). No better yields were observed by changing the reaction solvents (entries 17–20). Changing the heating source from microwave irradiation to normal hot plate, the yield was decreased to 40% yield. It is possible that the boiling point of propionaldehyde is lower than the reaction temperature, and the reaction was conducted in the open system (entry 21). Considering the green chemistry concept and environmentally friendly nature, we selected Nafion® NR50 as acid and ethanol as a solvent under microwave irradiation as the best reaction condition for this synthetic protocol. Microwave irradiation has attracted considerable attention in the past decade for increasing reaction efficiency [74,75]. Therefore, we confirmed that the condition of entry 15 is the most suitable condition in this double intermolecular cyclization for the construction of the corresponding 2-ethyl-3-methylquinolines.

Table 1.
Optimization of the reaction conditions a.
After obtaining the optimal reaction conditions, the scope of the reaction with respect to various anilines was evaluated. As shown in Scheme 3, a variety of commercially available anilines, 1b–1p, were investigated with propionaldehyde 2a in this intermolecular reaction. The anilines containing both electron-withdrawing groups (EWGs) and electron-donating groups (EDGs) on different positions successfully gave good to excellent yields of the corresponding quinolines 3b–3p. The structure of 3p was confirmed by single-crystal X-ray crystallography [76].
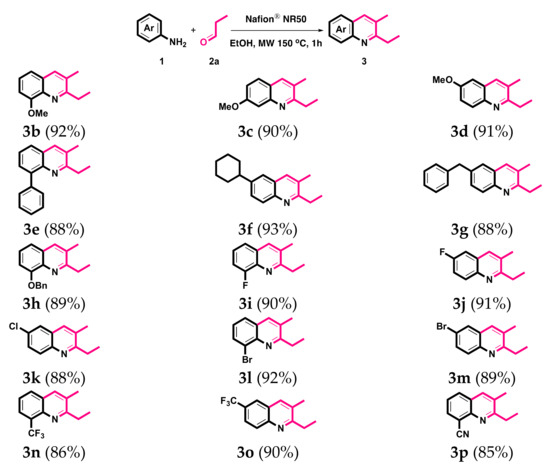
Scheme 3.
Synthesis of 3. Reaction conditions: 1 (1.0 mmol), 2a (2.1 mmol), Nafion® NR50 (0.1 mmol), EtOH (10 mL). Isolated yields.
Quinolinyl ketones are regarded as a versatile directing group by the cooperation of transition metal catalysts for activating the organic transformation [77,78,79,80,81,82]. A variety of 2-aminobenzophenone 4 were subjected to react with propionaldehyde 2a in the optimized reaction condition. As shown in Scheme 4, a wide range of 2-aminobenzophenones could be used in this cyclization. Various substituted groups at different positions of the Ar1 and Ar2 rings smoothly delivered good to excellent yields of the desired 8-substituted quinolinyl ketones 5 in 2 h. Not only non-substituted 2-aminobenzophenone 4a, but some functional groups such as chloro- (4b), bromo- (4c), methyl- (4d), dimethoxy- (4e), phenyl- (4f), p-methoxyphenyl- (4g) and p-fluorophenyl- (4h) on the Ar1 ring; fluoro- (4i), chloro- (4j), bromo- (4k), methyl- (4l), methoxy- (4m), trimethoxy- (4n) on Ar2, and both EWGs dichloro- (4o) and both EWGs multimethoxy- (4p-4s) on the Ar1 and Ar2 rings were well tolerated in this transformation. The related 8-substituted quinolinyl ketones 5 are illustrated in Scheme 4. The structures of 5a, 5b, and 5d were also confirmed by single-crystal X-ray crystallography [76].
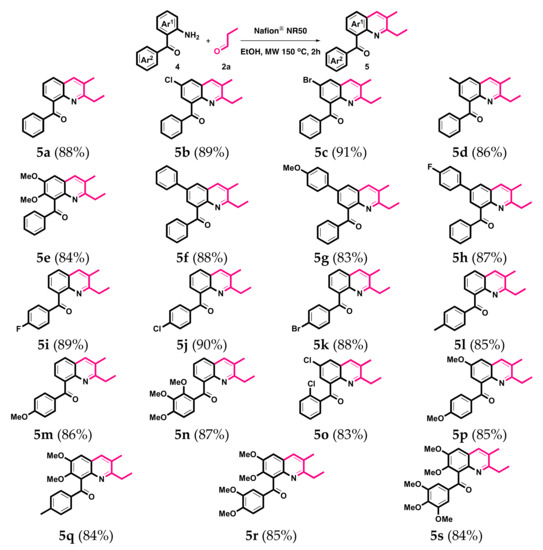
Scheme 4.
The synthesis of 5. Reaction conditions: 4 (1.0 mmol), 2a (2.1 mmol), Nafion® NR50 (0.1 mmol), and EtOH (10 mL). Isolated yields.
We next investigated the scope of substituents on the terminal of propionaldehyde by using aniline 1a and 2-aminobenzophenone 4a as the model aminobenzene substrate. Four propionaldehyde analogues were investigated, including valeraldehyde 2b, isovaleraldehyde 2c, 3-phenylpropionaldehyde 2d and 4,4,4-trifluorobutyraldehyde 2e. As illustrated in Scheme 5, changing the substituted group on propionaldehydes 2 did not influence the preparation of corresponding quinolines 6a–6h and gave modest to good yields. Based on our previous experience for the synthesis of quinolines by Friedländer reaction from 2-aminobenzophenone 4a with monocarbonyl synthons [69], it is possible that the electron-withdrawing CF3 group on 2e change in α-methylene reactivity could also access the Friedländer-type protocol to prepare the desired quinoline 7 by decreasing to one equivalent (Scheme 6). Friedländer quinoline synthesis is the reaction of 2-aminobenzaldehyde with acetylaldehyde to generate quinoline skeleton. In 2020, we developed a Nafion® NR50-catalyzed Friedländer quinoline synthesis; the related mechanism was also reported [69].
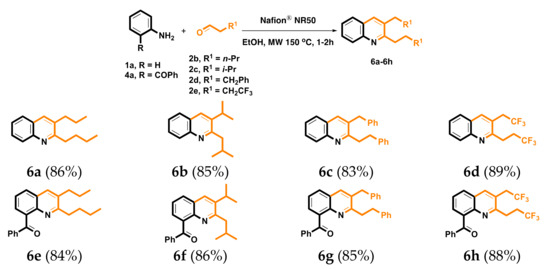
Scheme 5.
The synthesis of 6. Reaction conditions: 1a or 4a (1.0 mmol), 2a (2.1 mmol), Nafion® NR50 (0.1 mmol), and EtOH (10 mL). Reaction time: 1 h for 6a–6d and 2 h for 6e–6h. Isolated yields.
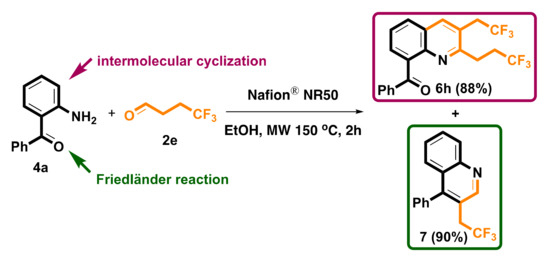
Scheme 6.
The Friedländer quinoline synthesis of 7.
Based on the results of current assays, compounds 1a and 2a are selected as model substrates to speculate the possible mechanism for the desired quinoline 3a synthesis, as shown in Scheme 7. To start with, the first 1.0 equiv. of propionaldehyde 2a was protonated by Nafion® NR50 and reacted with 1a to produce intermediate I, then proton transfer occurred to form intermediate II. The keto-enol tautomerization of the second 1.0 equivalent of 2a conducted with intermediate II to generate intermediate III via a consecutive intramolecular cyclization. Dehydration was then performed in acidic conditions to give an intermediate IV. The aromatic cyclization of intermediate IV was performed under microwave irradiation to produce the desired quinoline 3a.
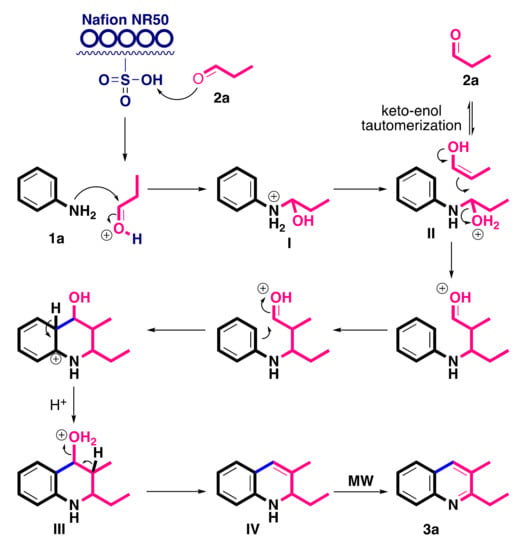
Scheme 7.
A plausible mechanism for the synthesis of quinoline 3a.
To observe the efficiency of the environmentally friendly Nafion® NR50 particles, the recycling experiments were conducted at least 10 times, and the corresponding yields are shown in Figure 1. Photos of the physical states for every experiment are provided in Table S1 in the Supporting Information. The recovered Nafion® NR50 particles was reused 10 times in the reaction of 1a and 2a. Although the shapes of particles became different, no obvious yield change was observed.
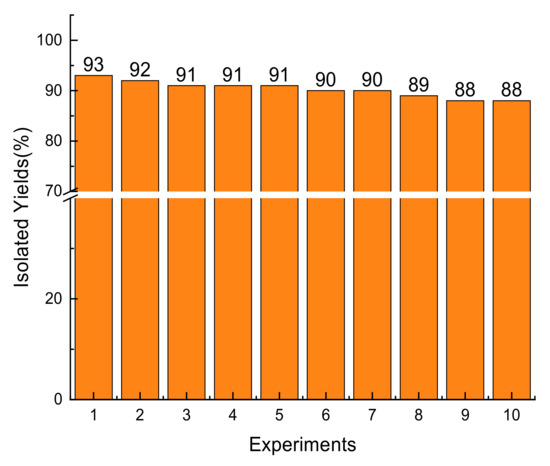
Figure 1.
Recycling experiments.
On the basis of the abovementioned results, four new chemical structures are confirmed by single-crystal X-ray crystallography. The corresponding crystal structures of 3p, 5a, 5b and 5d, and related data are described in Table 2. Furthermore, no alert A and B are presented in the checkcif output, as reported in the Supplementary Materials file.

Table 2.
Crystal data for compounds 3p, 5a, 5b and 5d.
3. Experimental Section
3.1. General Information
All reagents and solvents were commercially available (Sigma-Aldrich, St. Louis, MO, USA) and used without further purification. Reactions were routinely performed using the Discover SP system (2010 version, CEM Corporation, Matthews, NC, USA) in the sealed reaction vessels in standard mode with the temperature monitored using a vertically focused IR sensor. All reactions were monitored by TLC on silica gel 60 F254 (Merck) with detection by UV light. Column chromatography was performed using silica gel (200–300 mesh). Products in organic solvents were dried with anhydrous magnesium sulfate before concentration in vacuo. Melting points were determined with a MP-2D (Mandarin In Scientific, New Taipei, Taiwan) melting apparatus. 1H and 13C NMR spectra were recorded on a Bruker AVIII 500 MHz instruments operating at 500 and at 125 MHz, respectively. Data are reported as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, m = multiplet, br = broad), coupling constants (Hz) and integration. HRMS were obtained on a Waters LCT Premier XE (Waters Corp., Manchester, UK) instrument equipped with an electrospray source. The X-ray intensity data were measured at low temperature 100 K using Mo Kα radiation diffractometer equipped with a kappa geometry goniometer and corrected for absorption effects using the numerical method (SADABS). The yields are provided in mol% and the corresponding product weights are also shown.
3.2. General Procedure for the Synthesis of Skeletons 3, 5, 6 and 7
A mixture of anilines 1 or 4 (1.0 mmol), substituted propionaldehydes 2 (2.1 mmol), Nafion® NR50 (0.1 mmol) in ethanol (10 mL), in a dried 35 mL microwave vial at 25 °C. The mixture was subjected to a microwave irradiation instrument and stirred at 150 °C for skeleton between 1 and 2 h. The consumption of the starting materials were confirmed by TLC. The reaction was cooled to 25 °C, the mixture of crude product was transferred to a 100 mL round bottom flask, and the solvent was concentrated to afford crude product under reduced pressure. Purification on silica gel (hexanes/EtOAc = 4/1–1/1) afforded compounds 3a–3p, 5a–5s, 6a–6h and 7.
4. Data
4.1. 2-Ethyl-3-methylquinoline (3a)
Yield = 93% (159 mg); colorless solid; mp = 63–64 °C; HRMS (ESI, M+ + H) calcd for C12H14N 172.1126, found 172.1125; 1H NMR (500 MHz, CDCl3): δ 8.02 (d, J = 8.5 Hz, 1H), 7.79 (s, 1H), 7.67 (d, J = 8.0 Hz, 1H), 7.60 (t, J = 8.0 Hz, 1H), 7.42 (t, J = 8.0 Hz, 1H), 2.98 (q, J = 8.5 Hz, 2H), 2.45 (s, 3H), 1.37 (t, J = 7.5 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 163.20, 146.59, 135.62, 129.30, 128.44, 128.18, 127.24, 126.59, 125.49, 29.42, 19.01, 12.76. The NMR spectroscopic data of this compound are consistent with reported literature [83].
4.2. 2-Ethyl-8-methoxy-3-methylquinoline (3b)
Yield = 92% (185 mg); colorless solid; mp = 84–85 °C; HRMS (ESI, M+ + H) calcd for C13H16NO 202.1232, found 202.1229; 1H NMR (500 MHz, CDCl3): δ 7.80 (s, 1H), 7.35 (t, J = 8.0 Hz, 1H), 7.27 (d, J = 7.5 Hz, 1H), 6.96 (d, J = 7.5 Hz, 1H), 4.07 (s, 3H), 3.06 (q, J = 7.5 Hz, 2H), 2.49 (s, 3H), 1.37 (t, J = 7.5 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 162.39, 154.97, 138.47, 135.90, 129.94, 128.50, 125.64, 118.71, 106.80, 56.04, 29.82, 19.06, 13.13. The NMR spectroscopic data of this compound are consistent with reported literature [84].
4.3. 2-Ethyl-7-methoxy-3-methylquinoline (3c)
Yield = 90% (181 mg); brown gum; HRMS (ESI, M+ + H) calcd for C13H16NO 202.1226, found 202.1232; 1H NMR (500 MHz, CDCl3): δ 7.74 (s, 1H), 7.57 (d, J = 9.0 Hz, 1H), 7.36 (d, J = 2.5 Hz, 1H), 7.10 (dd, J = 2.5, 9.0 Hz, 1H), 3.93 (s, 3H), 2.96 (q, J = 7.5 Hz, 2H), 2.44 (s, 3H), 1.36 (t, J = 7.5 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 163.46, 159.90, 148.15, 135.64, 127.66, 126.90, 122.39, 118.60, 106.73, 55.42, 29.52, 18.83, 12.95. The NMR spectroscopic data of this compound are consistent with reported literature [59].
4.4. 2-Ethyl-6-methoxy-3-methylquinoline (3d)
Yield = 91% (183 mg); brown gum; HRMS (ESI, M+ + H) calcd for C13H16NO 202.1226, found 202.1222; 1H NMR (500 MHz, CDCl3): δ 7.91 (d, J = 9.5 Hz, 1H), 7.70 (s, 1H), 7.25 (dd, J = 2.5, 9.5 Hz, 1H), 6.95 (d, J = 3.0 Hz, 1H), 3.88 (s, 3H), 2.94 (q, J = 8.0 Hz, 2H), 2.44 (s, 3H), 1.34 (t, J = 7.5 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 160.62, 157.07, 142.60, 134.72, 129.88, 129.58, 128.07, 120.59, 104.43, 55.36, 29.18, 19.04, 12.87. The NMR spectroscopic data of this compound are consistent with reported literature [67].
4.5. 2-Ethyl-3-methyl-8-phenylquinoline (3e)
Yield = 88% (217 mg); yellow gum; HRMS (ESI, M+ + H) calcd for C18H18N 248.1434, found 248.1430; 1H NMR (500 MHz, CDCl3): δ 7.91 (d, J = 8.0 Hz, 2H), 7.87 (s, 1H), 7.76–7.70 (m, 2H), 7.57–7.51 (m, 3H), 7.44 (t, J = 7.0 Hz, 1H), 2.97 (q, J = 7.5 Hz, 2H), 2.49 (s, 3H), 1.40 (t, J = 7.5 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 162.09, 143.86, 139.67, 139.63, 135.34, 131.08 (2×), 129.32, 128.90, 127.64, 127.44 (2×), 126.86, 126.37, 125.34, 28.99, 18.90, 11.59.
4.6. 6-Cyclohexyl-2-ethyl-3-methylquinoline (3f)
Yield = 93% (235 mg); yellow gum; HRMS (ESI, M+ + H) calcd for C18H24N 254.1903, found 254.1904; 1H NMR (500 MHz, CDCl3): δ 7.95 (d, J = 8.5 Hz, 1H), 7.74 (s, 1H), 7.49 (dd, J = 1.5, 8.5 Hz, 1H), 7.46 (s, 1H), 2.96 (q, J = 7.5 Hz, 2H), 2.68–2.59 (m, 1H), 2.43 (s, 3H), 1.94 (d, J = 12.5 Hz, 2H), 1.87 (d, J = 12.5 Hz, 2H), 1.77 (d, J = 12.5 Hz, 1H), 1.55–1.23 (m, 8H); 13C NMR (125 MHz, CDCl3): δ 162.27, 145.46, 145.22, 135.43, 129.02, 128.43, 128.13, 127.24, 123.11, 44.32, 34.32 (2×), 29.33, 26.79 (2×), 26.08, 18.98, 12.91. The NMR spectroscopic data of this compound are consistent with reported literature [85].
4.7. 6-Benzyl-2-ethyl-3-methylquinoline (3g)
Yield = 88% (230 mg); brown gum; HRMS (ESI, M+ + H) calcd for C19H20N 262.1590, found 252.1589; 1H NMR (500 MHz, CDCl3): δ 8.01 (d, J = 9.0 Hz, 1H), 7.73 (s, 1H), 7.51–7.45 (m, 2H), 7.37–7.30 (m, 2H), 7.28–7.21 (m, 3H), 4.15 (s, 2H), 3.00 (q, J = 7.5 Hz, 2H), 2.46 (s, 3H), 1.41 (t, J = 7.5 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 162.55, 145.43, 140.65, 138.29, 135.27, 129.90, 129.27, 128.86 (2×), 128.48, 128.38 (2×), 127.19, 126.06, 125.69, 41.69, 29.29, 18.93, 12.78.
4.8. 8-(Benzyloxy)-2-ethyl-3-methylquinoline (3h)
Yield = 89% (247 mg); brown gum; HRMS (ESI, M+ + H) calcd for C19H20NO 278.1539, found 278.1545; 1H NMR (500 MHz, CDCl3): δ 7.79 (s, 1H), 7.57 (d, J = 7.5 Hz, 2H), 7.38 (t, J = 8.0 Hz, 2H), 7.33–7.27 (m, 3H), 7.03–6.95 (m, 1H), 5.46 (s, 2H), 3.07 (q, J = 7.5 Hz, 2H), 2.48 (s, 3H), 1.44 (t, J = 8.0 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 162.05, 153.97, 138.88, 137.49, 135.56, 129.84, 128.57, 128.37 (2×), 127.46, 126.85 (2×), 125.47, 119.26, 110.07, 70.93, 29.43, 18.99, 12.69.
4.9. 2-Ethyl-8-fluoro-3-methylquinoline (3i)
Yield = 90% (170 mg); colorless solid; mp = 65–66 °C; HRMS (ESI, M+ + H) calcd for C12H13FN 190.1027, found 190.1022; 1H NMR (500 MHz, CDCl3): δ 7.76 (s, 1H), 7.41 (d, J = 8.0 Hz, 1H), 7.35–7.19 (m, 2H), 2.99 (q, J = 7.5 Hz, 2H), 2.43 (s, 3H), 1.35 (t, J = 8.0 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 163.62, 157.60 (d, J = 253.625 Hz), 136.64 (d, J = 11.125 Hz), 135.21 (d, J = 2.25 Hz), 130.51, 128.94 (d, J = 1.375 Hz), 125.12 (d, J = 8.0 Hz), 122.19 (d, J = 4.25 Hz), 122.22 (d, J = 19.125 Hz), 29.45, 18.98, 12.66. The NMR spectroscopic data of this compound are consistent with reported literature [59].
4.10. 2-Ethyl-6-fluoro-3-methylquinoline (3j)
Yield = 91% (172 mg); brown gum; HRMS (ESI, M+ + H) calcd for C12H13FN 190.1027, found 190.1022; 1H NMR (500 MHz, CDCl3): δ 8.02–7.94 (m, 1H), 7.70 (s, 1H), 7.37–7.31 (m, 1H), 7.27–7.23 (m, 1H), 2.94 (q, J = 7.5 Hz, 2H), 2.43 (s, 3H), 1.35 (t, J = 8.0 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 162.42 (d, J = 2.0 Hz), 159.92 (d, J = 244.5 Hz), 143.61, 134.89 (d, J = 4.875 Hz), 130.82 (d, J = 9.0 Hz), 130.28, 127.69 (d, J = 10.0 Hz), 118.10 (d, J = 25.375 Hz), 109.54 (d, J = 21.5 Hz), 29.21, 18.97, 12.56. The NMR spectroscopic data of this compound are consistent with reported literature [59].
4.11. 6-Chloro-2-ethyl-3-methylquinoline (3k)
Yield = 88% (180 mg); brown gum; HRMS (ESI, M+ + H) calcd for C12H13ClN 206.0731, found 206.0726; 1H NMR (500 MHz, CDCl3): δ 7.89 (d, J = 9.0 Hz, 1H), 7.57 (s, 1H), 7.54 (s, 1H), 7.46 (dd, J = 1.5, 8.5 Hz, 1H), 2.90 (q, J = 7.5 Hz, 2H), 2.37 (s, 3H), 1.32 (t, J = 8.0 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 163.35, 144.79, 134.41, 130.91, 130.34, 130.02, 128.86, 127.69, 125.16, 29.20, 18.91, 12.40. The NMR spectroscopic data of this compound are consistent with reported literature [59].
4.12. 8-Bromo-2-ethyl-3-methylquinoline (3l)
Yield = 92% (229 mg); colorless solid; mp = 50–51 °C; HRMS (ESI, M+ + H) calcd for C12H13BrN 250.0226, found 250.0221; 1H NMR (500 MHz, CDCl3): δ 7.91 (d, J = 7.5 Hz, 1H), 7.74 (s, 1H), 7.62 (d, J = 8.0 Hz, 1H), 7.25 (t, J = 8.0 Hz, 1H), 3.00 (q, J = 7.5 Hz, 2H), 2.44 (s, 3H), 1.46 (t, J = 7.5 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 163.85, 143.34, 135.49, 131.64, 130.48, 128.29, 126.49, 125.80, 124.41, 29.16, 18.74, 11.89.
4.13. 6-Bromo-2-ethyl-3-methylquinoline (3m)
Yield = 89% (222 mg); colorless solid; mp = 53–54 °C; HRMS (ESI, M+ + H) calcd for C12H13BrN 250.0226, found 250.0220; 1H NMR (500 MHz, CDCl3): δ 7.83 (d, J = 9.0 Hz, 1H), 7.75 (s, 1H), 7.62 (s, 1H), 7.60 (s, 1H), 2.91 (q, J = 7.5 Hz, 2H), 2.40 (s, 3H), 1.33 (t, J = 8.0 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 163.57, 145.02, 134.36, 131.45, 130.37, 130.20, 128.54, 128.28, 119.08, 29.28, 18.98, 12.42. The NMR spectroscopic data of this compound are consistent with reported literature [84].
4.14. 2-Ethyl-3-methyl-8-(trifluoromethyl)quinoline (3n)
Yield = 86% (206 mg); white solid; mp = 68–69 °C; HRMS (ESI, M+ + H) calcd for C13H13F3N 240.1000, found 240.0997; 1H NMR (500 MHz, CDCl3): δ 7.95 (d, J = 7.0 Hz, 1H), 7.87 (d, J = 8.0 Hz, 1H), 7.84 (s, 1H), 7.46 (t, J = 7.5 Hz, 1H), 2.90 (q, J = 7.0 Hz, 2H), 2.47 (s, 3H), 1.45 (t, J = 7.0 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 163.62, 142.95, 134.90, 131.07, 130.65, 127.57, 127.20 (q, J = 28.875 Hz), 126.44 (q, J = 5.5 Hz), 124.36 (q, J = 271.875 Hz), 124.05, 29.11, 18.98, 11.30.
4.15. 2-Ethyl-3-methyl-6-(trifluoromethyl)quinoline (3o)
Yield = 90% (215 mg); yellow gum; HRMS (ESI, M+ + H) calcd for C13H13F3N 240.0995, found 240.0992; 1H NMR (500 MHz, CDCl3): δ 8.09 (d, J = 9.0 Hz, 1H), 7.96 (s, 1H), 7.83 (s, 1H), 7.75 (dd, J = 1.5, 8.5 Hz, 1H), 2.98 (q, J = 7.5 Hz, 2H), 2.46 (s, 3H), 1.37 (t, J = 7.5 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 165.65, 147.53, 136.06, 130.93, 129.61, 127.36 (q, J = 32.25 Hz), 126.15, 124.58 (q, J = 4.25 Hz), 124.20 (q, J = 270.5 Hz), 123.85 (q, J = 2.625 Hz), 29.47, 19.00, 12.38. The NMR spectroscopic data of this compound are consistent with reported literature [86].
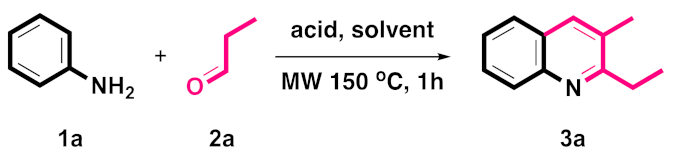
4.16. 2-Ethyl-3-methylquinoline-8-carbonitrile (3p)
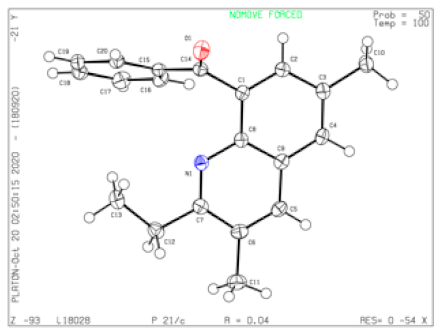
Yield = 85% (167 mg); colorless solid; mp = 121–122 °C; HRMS (ESI, M+ + H) calcd for C13H13N2 197.1073, found 197.1073; 1H NMR (500 MHz, CDCl3): δ 7.92 (d, J = 7.0 Hz, 1H), 7.87 (d, J = 7.0 Hz, 1H), 7.79 (s, 1H), 7.43 (t, J = 7.0 Hz, 1H), 2.96 (q, J = 7.0 Hz, 2H), 2.44 (s, 3H), 1.41 (t, J = 7.0 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 165.38, 145.62, 135.13, 133.94, 131.58, 126.91, 124.60, 117.58, 112.02, 29.03, 18.90, 11.52. Single-crystal X-ray diagram: crystal of 3p was grown by slow diffusion of EtOAc into a solution of 3p in CH2Cl2 to yield colorless prisms. The compound crystallizes in the monoclinic crystal system, space group C 2/c, a = 20.8096(12) Å, α = 90°; b = 8.4535(5) Å, β = 128.6800(10)°; c = 15.3108(16) Å, γ = 90°. V = 2102.6(3) Å3, Z = 8, dcalcd = 1.240 Mg/m3, F(000) = 832, 2θ range 2.687–28.345, R indices (all data) R1 = 0.0971, wR2 = 0.1285. CCDC number is 2016728.
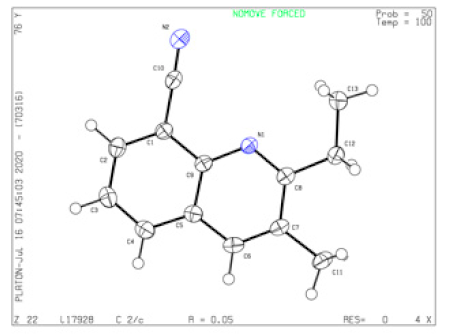
4.17. (2-Ethyl-3-methylquinolin-8-yl)(phenyl)methanone (5a)
Yield = 88% (242 mg); colorless solid; mp = 129–130 °C; HRMS (ESI, M+ + H) calcd for C19H18NO 276.1383, found 276.1381; 1H NMR (500 MHz, CDCl3): δ 7.84 (d, J = 8.0 Hz, 1H), 7.83 (s, 1H), 7.76 (d, J = 8.0 Hz, 2H), 7.74 (dd, J = 1.5, 7.5 Hz, 1H), 7.53 (t, J = 7.5 Hz, 1H), 7.50 (t, J = 7.5 Hz, 1H), 7.36 (t, J = 7.5 Hz, 2H), 2.71 (q, J = 7.5 Hz, 2H), 2.41 (s, 3H), 0.90 (t, J = 7.5 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 199.14, 162.68, 144.27, 139.13, 138.62, 134.63, 132.35, 130.21, 129.76 (2×), 128.89, 127.91 (2×), 127.55, 126.98, 125.05, 28.62, 19.01, 10.64. Single-crystal X-ray diagram: crystal of 5a was grown by slow diffusion of EtOAc into a solution of 5a in CH2Cl2 to yield colorless prisms. The compound crystallizes in the triclinic crystal system, space group P -1, a = 8.4108(3) Å, α = 103.454(2)°; b = 8.9126(3) Å, β = 97.603(2)°; c = 10.9019(3) Å, γ = 111.8150(10)°. V = 715.90(4) Å3, Z = 2, dcalcd = 1.277 Mg/m3, F(000) = 292, 2θ range 2.586–27.103, R indices (all data) R1 = 0.0607, wR2 = 0.1047. CCDC number is 2002378.
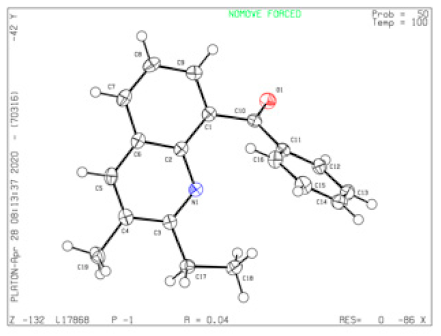
4.18. (6-Chloro-2-ethyl-3-methylquinolin-8-yl)(phenyl)methanone (5b)
Yield = 89% (275 mg); white solid; mp = 117–118 °C; HRMS (ESI, M+ + H) calcd for C19H17ClNO 310.0993, found 310.0993; 1H NMR (500 MHz, CDCl3): δ 7.76 (d, J = 2.5 Hz, 1H), 7.74 (d, J = 7.0 Hz, 1H), 7.73 (s, 1H), 7.70 (s, 1H), 7.65 (d, J = 2.0 Hz, 1H), 7.51 (t, J = 7.0 Hz, 1H), 7.37 (t, J = 8.0 Hz, 2H), 2.68 (q, J = 7.5 Hz, 2H), 2.37 (s, 3H), 0.87 (t, J = 7.5 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 197.30, 162.98, 142.55, 140.17, 138.43, 133.63, 132.67, 131.30, 130.62, 129.66 (2×), 127.98 (2×), 127.87, 127.71, 127.23, 28.50, 18.93, 10.43. Single-crystal X-ray diagram: crystal of 5b was grown by slow diffusion of EtOAc into a solution of 5b in CH2Cl2 to yield colorless prisms. The compound crystallizes in the triclinic crystal system, space group P -1, a = 7.4420(3) Å, α = 103.4120(10)°; b = 10.3406(3) Å, β = 100.533(2)°; c = 10.7949(4) Å, γ = 90.7170(10)°. V = 793.14(5) Å3, Z = 2, dcalcd = 1.297 Mg/m3, F(000) = 324, 2θ range 2.028–33.138, R indices (all data) R1 = 0.0482, wR2 = 0.1123. CCDC number is 2013112.
4.19. (6-Bromo-2-ethyl-3-methylquinolin-8-yl)(phenyl)methanone (5c)
Yield = 91% (321 mg); yellow solid; mp = 141–142 °C; HRMS (ESI, M+ + H) calcd for C19H17BrNO 354.0488, found 354.0489; 1H NMR (500 MHz, CDCl3): δ 7.96 (d, J = 2.0 Hz, 1H), 7.77 (d, J = 2.0 Hz, 1H), 7.75–7.71 (m, 3H), 7.51 (t, J = 7.5 Hz, 1H), 7.37 (t, J = 8.0 Hz, 2H), 2.68 (q, J = 7.5 Hz, 2H), 2.39 (s, 3H), 0.88 (t, J = 7.5 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 197.22, 163.18, 142.83, 140.32, 138.47, 133.55, 132.71, 131.32, 130.60, 130.39, 129.72 (2×), 128.25, 128.02 (2×), 118.58, 28.59, 18.99, 10.45.
4.20. (2-Ethyl-3,6-dimethylquinolin-8-yl)(phenyl)methanone (5d)
Yield = 86% (249 mg); yellow solid; mp = 132–133 °C; HRMS (ESI, M+ + H) calcd for C20H20NO 290.1539, found 290.1547; 1H NMR (500 MHz, CDCl3): δ 7.76 (d, J = 8.0 Hz, 2H), 7.72 (s, 1H), 7.58 (d, J = 8.5 Hz, 2H), 7.49 (t, J = 7.5 Hz, 1H), 7.36 (t, J = 7.5 Hz, 2H), 2.68 (q, J = 7.5 Hz, 2H), 2.54 (s, 3H), 2.37 (s, 3H), 0.88 (t, J = 7.5 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 199.23, 161.61, 142.84, 139.12, 138.30, 134.75, 134.03, 132.25, 130.04, 129.71 (2×), 129.57, 127.87, 127.83 (2×), 127.01, 28.43, 21.39, 18.95, 10.62. Single-crystal X-ray diagram: crystal of 5d was grown by slow diffusion of EtOAc into a solution of 5d in CH2Cl2 to yield colorless prisms. The compound crystallizes in the monoclinic crystal system, space group P 21/c, a = 13.2934(5) Å, α = 90°; b = 14.2302(5) Å, β = 108.5200(10)°; c = 8.6189(3) Å, γ = 90°. V = 1545.98(10) Å3, Z = 4, dcalcd = 1.243 Mg/m3, F(000) = 616, 2θ range 2.158–27.098, R indices (all data) R1 = 0.0479, wR2 = 0.0968. CCDC number is 2039753.
4.21. (2-Ethyl-6,7-dimethoxy-3-methylquinolin-8-yl)(phenyl)methanone (5e)
Yield = 84% (282 mg); white solid; mp = 130–131 °C; HRMS (ESI, M+ + H) calcd for C21H22NO3 336.1594, found 336.1596; 1H NMR (500 MHz, CDCl3): δ 8.09 (s, 1H), 7.73 (d, J = 8.0 Hz, 2H), 7.59 (s, 1H), 7.49 (t, J = 7.0 Hz, 1H), 7.35 (t, J = 8.0 Hz, 2H), 4.05 (s, 3H), 4.01 (s, 3H), 2.65 (q, J = 7.5 Hz, 2H), 2.39 (s, 3H), 0.81 (t, J = 7.0 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 198.42, 160.70, 147.30, 143.70, 140.30, 139.45, 134.28, 132.18, 130.11, 129.74 (2×), 128.61, 127.80 (2×), 122.41, 116.58, 61.31, 56.79, 28.30, 19.19, 10.41.
4.22. (2-Ethyl-3-methyl-6-phenylquinolin-8-yl)(phenyl)methanone (5f)
Yield = 88% (309 mg); yellow solid; mp = 140–141 °C; HRMS (ESI, M+ + H) calcd for C25H22NO 352.1696, found 352.1694; 1H NMR (500 MHz, CDCl3): δ 8.03 (d, J = 1.5 Hz, 1H), 8.00 (d, J = 2.0 Hz, 1H), 7.87 (s, 1H), 7.82 (d, J = 7.5 Hz, 2H), 7.74 (d, J = 7.5 Hz, 2H), 7.53–7.47 (m, 3H), 7.42–7.38 (m, 3H), 2.73 (q, J = 7.0 Hz, 2H), 2.42 (s, 3H), 0.93 (t, J = 7.5 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 198.94, 162.73, 143.67, 139.99, 139.03, 138.99, 137.82, 134.84, 132.45, 130.57, 129.81 (2×), 128.92 (2×), 127.94 (2×), 127.68, 127.31 (2×), 127.26, 127.10, 126.43, 28.62, 19.03, 10.69.
4.23. (2-Ethyl-6-(4-methoxyphenyl)-3-methylquinolin-8-yl)(phenyl)methanone (5g)
Yield = 83% (316 mg); yellow solid; mp = 96–97 °C; HRMS (ESI, M+ + H) calcd for C26H24NO2 382.1802, found 382.1805; 1H NMR (500 MHz, CDCl3): δ 7.96 (dd, J = 2.0, 6.0 Hz, 2H), 7.86–7.78 (m, 3H), 7.66 (d, J = 8.5 Hz, 2H), 7.54–7.48 (m, 1H), 7.38 (t, J = 8.0 Hz, 2H), 7.02 (d, J = 9.0 Hz, 2H), 3.86 (s, 3H), 2.72 (q, J = 7.5 Hz, 2H), 2.40 (s, 3H), 0.92 (t, J = 7.5 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 199.03, 162.36, 159.44, 143.38, 139.02, 138.82, 137.40, 134.70, 132.40, 130.47, 129.79 (2×), 128.33 (2×), 127.91 (2×), 127.30, 126.88, 125.62, 114.36 (2×), 55.31, 28.56, 19.00, 10.68.
4.24. (2-Ethyl-6-(4-fluorophenyl)-3-methylquinolin-8-yl)(phenyl)methanone (5h)
Yield = 87% (321 mg); yellow solid; mp = 146–147 °C; HRMS (ESI, M+ + H) calcd for C25H21FNO 370.1602, found 370.1602; 1H NMR (500 MHz, CDCl3): δ 7.96 (d, J = 2.0 Hz, 1H), 7.93 (d, J = 2.0 Hz, 1H), 7.85 (s, 1H), 7.81 (s, 1H), 7.80 (s, 1H), 7.69–7.66 (m, 2H), 7.52 (t, J = 7.5 Hz, 1H), 7.38 (t, J = 7.5 Hz, 2H), 7.17 (t, J = 8.5 Hz, 2H), 2.72 (q, J = 7.0 Hz, 2H), 2.41 (s, 3H), 0.91 (t, J = 7.0 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 198.87, 162.82, 162.66 (d, J = 245.875 Hz), 143.58, 139.05 (d, J = 26.375 Hz), 136.85, 136.14 (d, J = 3.125 Hz), 134.76, 132.50, 130.71, 129.79 (2×), 128.92 (d, J = 8.125 Hz, 2×), 127.96 (2×), 127.24, 126.89, 126.27, 115.83 (d, J = 21.5 Hz, 2×), 28.62, 19.03, 10.65.
4.25. (2-Ethyl-3-methylquinolin-8-yl)(4-fluorophenyl)methanone (5i)
Yield = 89% (261 mg); yellow solid; mp = 124–125 °C; HRMS (ESI, M+ + H) calcd for C19H17FNO 294.1289, found 294.1288; 1H NMR (500 MHz, CDCl3): δ 7.86–7.81 (m, 2H), 7.80–7.75 (m, 2H), 7.71 (d, J = 7.0 Hz, 1H), 7.52 (t, J = 7.5 Hz, 1H), 7.03 (t, J = 8.5 Hz, 2H), 2.72 (q, J = 7.0 Hz, 2H), 2.40 (s, 3H), 0.92 (t, J = 7.0 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 197.52, 165.33 (d, J = 252.125 Hz), 162.73, 144.09, 138.23, 135.54 (d, J = 2.625 Hz), 134.66, 132.30 (d, J = 9.25 Hz, 2×, 130.29, 129.05, 127.57, 126.96, 125.08, 114.95 (d, J = 21.75 Hz, 2×), 28.58, 18.96, 10.63.
4.26. (4-Chlorophenyl)(2-ethyl-3-methylquinolin-8-yl)methanone (5j)
Yield = 90% (278 mg); White solid; mp = 127–128 °C; HRMS (ESI, M+ + H) calcd for C19H17ClNO 310.0993, found 310.0998; 1H NMR (500 MHz, CDCl3): δ 7.84 (d, J = 8.5 Hz, 1H), 7.82 (s, 1H), 7.72 (d, J = 7.0 Hz, 1H), 7.68 (d, J = 8.5 Hz, 2H), 7.53 (t, J = 7.5 Hz, 1H), 7.33 (d, J = 8.5 Hz, 2H), 2.72 (q, J = 7.0 Hz, 2H), 2.40 (s, 3H), 0.91 (t, J = 7.0 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 197.90, 162.76, 144.10, 138.58, 137.98, 137.58, 134.66, 131.08 (2×), 130.33, 129.21, 128.18 (2×), 127.71, 126.96, 125.10, 28.59, 18.97, 10.59.
4.27. (4-Bromophenyl)(2-ethyl-3-methylquinolin-8-yl)methanone (5k)
Yield = 88% (311 mg); yellow solid; mp = 117–118 °C; HRMS (ESI, M+ + H) calcd for C19H17BrNO 354.0488, found 354.0496; 1H NMR (500 MHz, CDCl3): δ 7.85 (d, J = 8.5 Hz, 1H), 7.83 (s, 1H), 7.72 (dd, J = 1.0, 7.0 Hz, 1H), 7.60 (d, J = 8.5 Hz, 2H), 7.53 (t, J = 8.0 Hz, 1H), 7.50 (d, J = 8.5 Hz, 2H), 2.72 (q, J = 7.0 Hz, 2H), 2.41 (s, 3H), 0.91 (t, J = 7.0 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 198.13, 162.80, 144.12, 138.02, 137.94, 134.67, 131.22 (2×), 131.18 (2×), 130.36, 129.25, 127.76, 127.33, 126.98, 125.12, 28.62, 19.00, 10.60.
4.28. (2-Ethyl-3-methylquinolin-8-yl)(p-tolyl)methanone (5l)
Yield = 85% (246 mg); yellow solid; mp = 104–105 °C; HRMS (ESI, M+ + H) calcd for C20H20BrNO 290.1539, found 290.1543; 1H NMR (500 MHz, CDCl3): δ 7.83 (s, 1H), 7.82 (d, J = 7.5 Hz, 1H), 7.68–7.65 (m, 3H), 7.51 (t, J = 7.0 Hz, 1H), 7.16 (d, J = 8.0 Hz, 2H), 2.74 (q, J = 7.0 Hz, 2H), 2.41 (s, 3H), 2.39 (s, 3H), 0.95 (t, J = 7.5 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 198.69, 162.72, 144.26, 143.16, 138.91, 136.46, 134.65, 130.15, 130.00 (2×), 128.64 (2×), 128.59, 127.23, 126.99, 124.98, 28.69, 21.65, 19.01, 10.83.
4.29. (2-Ethyl-3-methylquinolin-8-yl)(4-methoxyphenyl)methanone (5m)
Yield = 86% (262 mg); yellow solid; mp = 95–96 °C; HRMS (ESI, M+ + H) calcd for C20H20BrNO2 306.1489, found 306.1485; 1H NMR (500 MHz, CDCl3): δ 7.82 (s, 1H), 7.81 (d, J = 8.5 Hz, 1H), 7.75 (d, J = 8.5 Hz, 2H), 7.66 (d, J = 7.0 Hz, 1H), 7.50 (t, J = 7.5 Hz, 1H), 6.85 (d, J = 8.5 Hz, 2H), 3.84 (s, 3H), 2.75 (q, J = 7.5 Hz, 2H), 2.41 (s, 3H), 0.99 (t, J = 7.5 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 197.54, 163.13, 162.75, 144.19, 139.00, 134.68, 132.22 (2×), 131.97, 130.12, 128.45, 127.11, 126.98, 124.99, 113.15 (2×), 55.37, 28.69, 19.00, 10.96.
4.30. (2-Ethyl-3-methylquinolin-8-yl)(2,3,4-trimethoxyphenyl)methanone (5n)
Yield = 87% (318 mg); yellow gum; HRMS (ESI, M+ + H) calcd for C22H24NO4 366.1700, found 366.1696; 1H NMR (500 MHz, CDCl3): δ 7.79 (s, 1H), 7.78 (d, J = 7.5 Hz, 2H), 7.50 (t, J = 7.5 Hz, 1H), 7.45 (d, J = 9.0 Hz, 1H), 6.68 (d, J = 9.0 Hz, 1H), 3.89 (s, 3H), 3.75 (s, 3H), 3.27 (s, 3H), 2.69 (q, J = 7.5 Hz, 2H), 2.37 (s, 3H), 0.90 (t, J = 7.5 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 197.01, 162.14, 156.84, 153.74, 144.06, 141.70, 140.50, 134.72, 129.84, 128.83, 128.79, 127.39, 126.73, 126.19, 125.07, 106.46, 60.98, 60.69, 56.04, 28.49, 18.95, 10.62.
4.31. (6-Chloro-2-ethyl-3-methylquinolin-8-yl)(2-chlorophenyl)methanone (5o)
Yield = 83% (285 mg); yellow solid; mp = 123–124 °C; HRMS (ESI, M+ + H) calcd for C19H16Cl2NO 344.0604, found 344.0606; 1H NMR (500 MHz, CDCl3): δ 7.93 (d, J = 2.5 Hz, 1H), 7.83 (d, J = 2.0 Hz, 1H), 7.70 (s, 1H), 7.54 (d, J = 8.0 Hz, 1H), 7.38–7.33 (m, 2H), 7.30–7.25 (m, 1H), 2.62 (q, J = 7.5 Hz, 2H), 2.36 (s, 3H), 0.77 (t, J = 7.5 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 196.28, 163.07, 142.55, 140.59, 139.30, 133.78, 132.05, 131.41, 131.25, 130.94, 130.49, 130.15, 130.05, 129.09, 127.82, 126.45, 28.61, 19.01, 10.24.
4.32. (2-Ethyl-6-methoxy-3-methylquinolin-8-yl)(4-methoxyphenyl)methanone (5p)
Yield = 85% (285 mg); yellow solid; mp = 176–177 °C; HRMS (ESI, M+ + H) calcd for C21H22NO3 336.1594, found 336.1591; 1H NMR (500 MHz, CDCl3): δ 8.22 (s, 1H), 7.73 (d, J = 9.0 Hz, 2H), 7.67 (d, J = 8.0 Hz, 1H), 6.84 (d, J = 8.5 Hz, 3H), 4.03 (s, 3H), 3.84 (s, 3H), 2.72 (q, J = 7.0 Hz, 2H), 2.41 (s, 3H), 0.93 (t, J = 7.0 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 197.19, 162.83, 162.77, 156.42, 145.28, 132.75, 132.19 (2×), 131.40, 129.47, 129.08, 128.71, 118.83, 112.98 (2×), 102.91, 55.79, 55.36, 28.61, 19.09, 10.80.
4.33. (2-Ethyl-6,7-dimethoxy-3-methylquinolin-8-yl)(p-tolyl)methanone (5q)
Yield = 84% (293 mg); yellow solid; mp = 92–93 °C; HRMS (ESI, M+ + H) calcd for C22H24NO3 350.1751, found 350.1749; 1H NMR (500 MHz, CDCl3): δ 8.09 (s, 1H), 7.65 (d, J = 8.0 Hz, 2H), 7.53 (s, 1H), 7.16 (d, J = 8.0 Hz, 2H), 4.04 (s, 3H), 4.00 (s, 3H), 2.67 (q, J = 7.0 Hz, 2H), 2.40 (s, 3H), 2.38 (s, 3H), 0.87 (t, J = 7.0 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 197.95, 160.71, 147.24, 143.38, 142.99, 140.27, 136.69, 134.68, 130.06, 130.00 (2×), 128.60, 128.52 (2×), 122.41, 116.23, 61.29, 56.77, 28.36, 21.59, 19.18, 10.59.
4.34. (3,4-Dimethoxyphenyl)(2-ethyl-6,7-dimethoxy-3-methylquinolin-8-yl)methanone (5r)
Yield = 85% (336 mg); yellow solid; mp = 141–142 °C; HRMS (ESI, M+ + H) calcd for C23H26NO5 396.1806, found 396.1805; 1H NMR (500 MHz, CDCl3): δ 8.08 (s, 1H), 7.59 (d, J = 1.5 Hz, 1H), 7.48 (s, 1H), 7.13 (dd, J = 1.5, 8.5 Hz, 1H), 6.72 (d, J = 8.5 Hz, 1H), 4.03 (s, 3H), 3.98 (s, 3H), 3.89 (s, 3H), 3.88 (s, 3H), 2.70 (q, J = 7.5 Hz, 2H), 2.40 (s, 3H), 0.94 (t, J = 7.5 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 196.81, 160.85, 152.85, 148.59, 147.16, 143.15, 140.27, 134.71, 132.12, 130.05, 128.64, 126.07, 122.43, 115.98, 111.14, 109.50, 61.28, 56.76, 55.95, 55.92, 28.42, 19.20, 10.98.
4.35. (2-Ethyl-6,7-dimethoxy-3-methylquinolin-8-yl)(3,4,5-trimethoxyphenyl)methanone (5s)
Yield = 84% (357 mg); yellow solid; mp = 121–122 °C; HRMS (ESI, M+ + H) calcd for C24H28NO6 426.1911, found 426.1912; 1H NMR (500 MHz, CDCl3): δ 8.08 (s, 1H), 7.52 (s, 1H), 7.02 (s, 2H), 4.03 (s, 3H), 3.99 (s, 3H), 3.88 (s, 3H), 3.72 (s, 6H), 2.69 (q, J = 7.0 Hz, 2H), 2.39 (s, 3H), 0.92 (t, J = 7.0 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 196.95, 160.88, 152.54 (2×), 147.10, 143.52, 142.11, 140.24, 134.43, 134.02, 130.04, 128.69, 122.40, 116.43, 107.69 (2×), 61.25, 60.79, 56.74, 56.17 (2×), 28.31, 19.17, 10.86.
4.36. 2-Butyl-3-propylquinoline (6a)
Yield = 86% (195 mg); yellow gum; HRMS (ESI, M+ + H) calcd for C16H22N 228.1747, found 228.1741; 1H NMR (500 MHz, CDCl3): δ 8.02 (d, J = 8.5 Hz, 1H), 7.83 (s, 1H), 7.70 (d, J = 8.0 Hz, 1H), 7.60 (t, J = 8.0 Hz, 1H), 7.43 (t, J = 8.0 Hz, 1H), 3.01–2.95 (m, 2H), 2.80–2.73 (m, 2H), 1.83–1.68 (m, 4H), 1.55–1.46 (m, 2H), 1.04 (t, J = 7.5 Hz, 3H), 0.99 (t, J = 7.5 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 162.23, 146.47, 134.77, 133.79, 128.41, 128.24, 127.15, 126.81, 125.44, 35.59, 34.36, 31.82, 23.53, 23.00, 14.02, 13.99. The NMR spectroscopic data of this compound are consistent with reported literature [60].
4.37. 2-Isobutyl-3-isopropylquinoline (6b)
Yield = 85% (193 mg); yellow gum; HRMS (ESI, M+ + H) calcd for C16H22N 228.1747, found 228.1746; 1H NMR (500 MHz, CDCl3): δ 8.03 (d, J = 8.5 Hz, 1H), 7.94 (s, 1H), 7.73 (d, J = 8.0 Hz, 1H), 7.62–7.59 (m, 1H), 7.45–7.42 (m, 1H), 3.36–3.30 (m, 1H), 2.93 (d, J = 7.5 Hz, 2H), 2.31–2.25 (m, 1H), 1.33 (d, J = 7.0 Hz, 6H), 1.01 (d, J = 6.5 Hz, 6H); 13C NMR (125 MHz, CDCl3): δ 160.68, 146.12, 140.67, 131.44, 128.46, 128.25, 127.21, 126.96, 125.42, 44.05, 29.31, 28.73, 23.79 (2×), 22.57 (2×). The NMR spectroscopic data of this compound are consistent with reported literature [87].
4.38. 3-Benzyl-2-phenethylquinoline (6c)
Yield = 83% (268 mg); white solid; mp = 102–103 °C; HRMS (ESI, M+ + H) calcd for C24H22N 324.1747, found 324.1749; 1H NMR (500 MHz, CDCl3): δ 8.10 (d, J = 8.5 Hz, 1H), 7.77 (s, 1H), 7.72 (d, J = 8.0 Hz, 1H), 7.68 (t, J = 8.0 Hz, 1H), 7.48 (t, J = 8.0 Hz, 1H), 7.34–7.15 (m, 8H), 7.12 (d, J = 7.5 Hz, 2H), 4.09 (s, 2H), 3.29–3.19 (m, 2H), 3.16–3.04 (m, 2H); 13C NMR (125 MHz, CDCl3): δ 161.20, 146.85, 141.93, 139.26, 136.35, 132.43, 128.88 (2×), 128.78, 128.66 (2×), 128.53 (3×), 128.32 (2×), 127.12, 127.10, 126.48, 125.89, 125.86, 38.68, 37.69, 35.28. The NMR spectroscopic data of this compound are consistent with reported literature [85].
4.39. 3-(2,2,2-Trifluoroethyl)-2-(3,3,3-trifluoropropyl)quinoline (6d)
Yield = 89% (273 mg); white solid; mp = 92–93 °C; HRMS (ESI, M+ + H) calcd for C14H12F6N 308.0869, found 308.0870; 1H NMR (500 MHz, CDCl3): δ 8.07 (s, 1H), 8.03 (d, J = 8.5 Hz, 1H), 7.80 (d, J = 8.0 Hz, 1H), 7.76–7.70 (m, 1H), 7.57–7.52 (m, 1H), 3.62 (q, J = 10.0 Hz, 2H), 3.30–3.22 (m, 2H), 2.94–281 (m, 2H); 13C NMR (125 MHz, CDCl3): δ 157.16, 147.30, 138.82, 130.09, 128.82, 127.41 (q, J = 274.625 Hz), 127.31, 126.73, 126.65, 125.58 (q, J = 272.0 Hz), 121.97, 36.47 (q, J = 90 Hz), 31.87 (q, J = 28.75 Hz), 27.40.
4.40. (2-Butyl-3-propylquinolin-8-yl)(phenyl)methanone (6e)
Yield = 84% (278 mg); yellow solid; mp = 68–69 °C; HRMS (ESI, M+ + H) calcd for C23H26NO 332.2009, found 332.2005; 1H NMR (500 MHz, CDCl3): δ 7.85 (dd, J = 1.5, 8.0 Hz, 1H), 7.83 (s, 1H), 7.79–7.74 (m, 2H), 7.71 (dd, J = 1.5, 7.0 Hz, 1H), 7.55–7.48 (m, 2H), 7.36 (t, J = 7.5 Hz, 2H), 2.71 (q, J = 7.0 Hz, 4H), 1.75–1.64 (m, 2H), 1.39–1.31 (m, 2H), 1.17–1.08 (m, 2H), 1.03 (t, J = 7.0 Hz, 3H), 0.72 (t, J = 7.0 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 199.18, 161.84, 144.12, 139.06, 138.58, 134.54, 133.84, 132.36, 129.73 (2×), 128.98, 127.93 (2×), 127.42, 126.83, 124.95, 34.46, 34.30, 29.33, 23.08, 22.34, 14.02, 13.94.
4.41. (2-Isobutyl-3-isopropylquinolin-8-yl)(phenyl)methanone (6f)
Yield = 86% (285 mg); yellow gum; HRMS (ESI, M+ + H) calcd for C23H26NO 332.2009, found 332.2003; 1H NMR (500 MHz, CDCl3): δ 7.95 (s, 1H), 7.87 (dd, J = 1.5, 8.5 Hz, 1H), 7.80–7.73 (m, 2H), 7.70 (dd, J = 1.5, 7.0 Hz, 1H), 7.54–7.47 (m, 2H), 7.36 (t, J = 8.0 Hz, 2H), 3.29–3.20 (m, 1H), 2.66 (d, J = 7.0 Hz, 2H), 1.91–1.83 (m, 1H), 1.31 (d, J = 6.5 Hz, 6H), 0.68 (d, J = 6.5 Hz, 6H); 13C NMR (125 MHz, CDCl3): δ 199.26, 160.62, 143.87, 141.17, 138.96, 138.67, 132.33, 130.74, 129.67 (2×), 129.03, 127.94 (2×), 127.22, 126.92, 124.90, 43.54, 28.56, 27.28, 23.50 (2×), 22.47 (2×).
4.42. (3-Benzyl-2-phenethylquinolin-8-yl)(phenyl)methanone (6g)
Yield = 85% (363 mg); yellow solid; mp = 137–138 °C; HRMS (ESI, M+ + H) calcd for C31H26NO 428.2009, found 428.2009; 1H NMR (500 MHz, CDCl3): δ 7.90–7.77 (m, 4H), 7.76 (s, 1H), 7.57 (t, J = 8.0 Hz, 1H), 7.54 (t, J = 7.5 Hz, 1H), 7.42 (t, J = 8.0 Hz, 2H), 7.36–7.30 (m, 2H), 7.29–7.24 (m, 1H), 7.21–7.16 (m, 2H), 7.15–7.09 (m, 3H), 6.94 (d, J = 7.5 Hz, 2H), 4.04 (s, 2H), 3.01 (t, J = 8.0 Hz, 2H), 2.62 (t, J = 8.0 Hz, 2H); 13C NMR (125 MHz, CDCl3): δ 198.93, 160.78, 144.30, 142.26, 139.14, 138.71, 138.49, 135.32, 133.10, 132.49, 129.73 (2×), 129.30, 128.91 (2×), 128.64 (2×), 128.38 (2×), 128.09 (2×), 128.04 (3×), 126.82, 126.49, 125.54, 125.32, 38.44, 36.85, 32.88.
4.43. Phenyl(3-(2,2,2-trifluoroethyl)-2-(3,3,3-trifluoropropyl)quinolin-8-yl)methanone (6h)
Yield = 88% (362 mg); yellow gum; HRMS (ESI, M+ + H) calcd for C21H16F6NO 412.1131, found 412.1123; 1H NMR (500 MHz, CDCl3): δ 8.10 (s, 1H), 7.96 (dd, J = 2.0, 8.5 Hz, 1H), 7.91 (dd, J = 2.0, 8.5 Hz, 1H), 7.75–7.69 (m, 2H), 7.68–7.63 (m, 1H), 7.56–7.52 (m, 1H), 7.40 (t, J = 8.0 Hz, 2H), 3.56 (q, J = 10.0 Hz, 2H), 3.03–2.97 (m, 2H), 2.11–1.99 (m, 2H); 13C NMR (125 MHz, CDCl3): δ 198.32, 157.04, 144.56, 139.20, 138.53, 138.42, 132.69, 129.95, 129.79, 129.43 (2×), 128.18 (2×), 127.43 (q, J = 274.375 Hz), 126.36, 125.44 (q, J = 275.75 Hz), 122.65, 122.63, 36.21 (q, J = 30.25 Hz), 30.07 (q, J = 28.625 Hz), 27.17.
4.44. 4-Phenyl-3-(2,2,2-trifluoroethyl)quinoline (7)
Yield = 90% (258 mg); yellow gum; HRMS (ESI, M+ + H) calcd for C17H13F3N 288.0995, found 288.0992; 1H NMR (500 MHz, CDCl3): δ 8.98 (s, 1H), 8.16 (d, J = 8.0 Hz, 1H), 7.74–7.68 (m, 1H), 7.57–7.49 (m, 3H), 7.47–7.37 (m, 2H), 7.27 (dd, J = 2.0, 8.0 Hz, 2H), 3.42 (q, J = 10.5 Hz, 2H); 13C NMR (125 MHz, CDCl3): δ 151.67, 149.24, 147.58, 135.35, 129.51 (d, J = 20.25 Hz), 129.37 (2×), 129.19, 128.76, 128.64 (2×), 128.47, 127.59, 126.82 (d, J = 35.5 Hz), 125.45 (q, J = 275.875 Hz), 120.97 (d, J = 2.125 Hz), 35.23 (q, J = 30.25 Hz).
5. Conclusions
In summary, an environmentally friendly and atom-economical synthetic route is reported for the preparation of functionalized 2,3-dialkylquinolines from substituted anilines and functionalized propionaldehydes using Nafion® NR50 as an acidic catalyst. The reaction worked well with various substituted anilines and propionaldehydes and provided the corresponding quinolines in good to excellent yields. A series of quinolinyl ketones were also synthesized, indicating that this reaction features good functional group tolerance. Moreover, the Nafion® NR50 particles could be repeatedly used and exhibit good efficiency, demonstrating their environmentally friendly properties. Four structures were confirmed by single-crystal X-ray crystallography. Further investigations of Nafion® NR50 particles are currently underway in our group.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/catal11080877/s1. Detailed experimental procedures and spectroscopic data for all compounds and X-ray analysis data for 3p, 5a, 5b and 5d (PDF).
Author Contributions
Conceptualization, C.-K.C.; methodology, C.-K.C. and C.-Y.L.; investigation, C.-K.C. and C.-Y.L.; data curation, C.-K.C. and C.-Y.L.; writing—original draft preparation, C.-K.C.; writing—review and editing, C.-K.C. and C.-C.W.; supervision, C.-K.C. and C.-C.W.; funding acquisition, C.-C.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Academia Sinica (MOST 108-3114-Y-001-002; AS-SUMMIT-109) and the Ministry of Science and Technology, Taiwan (MOST 108-2113-M-001-019-; MOST 109-2113-M-001-005).
Data Availability Statement
All required data are presented in this file.
Acknowledgments
We would like to thank the Academia Sinica and Ministry of Science and Technology, Taiwan for financial supports. Chieh-Kai Chan thanks Academia Sinica for financial support through a Postdoctoral Scholar Program award.
Conflicts of Interest
The authors declare no competing financial interests.
References
- Michael, J.P. Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep. 2007, 24, 223–246. [Google Scholar] [CrossRef]
- Michael, J.P. Quinoline, quinazoline and acridonealkaloids. Nat. Prod. Rep. 2008, 25, 166–187. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.-F.; Morris-Natschke, S.L.; Liu, Y.-Q.; Guo, X.; Xu, X.-S.; Goto, M.; Li, J.-C.; Yang, G.-Z.; Lee, K.-H. Biologically active quinoline and quinazoline alkaloids part I. Med. Res. Rev. 2018, 38, 775–828. [Google Scholar] [CrossRef] [PubMed]
- Teja, C.; Khan, F.R.N. Radical Tranformations towards the Synthesis of Quinoline: A Review. Chem. Asian J. 2020, 15, 4153–4167. [Google Scholar] [CrossRef] [PubMed]
- Harry, N.A.; Ujwaldev, S.M.; Anikumar, G. Recent Advances and Prospects in the Metal-free Synthesis of Quinolines. Org. Biomol. Chem. 2020, 18, 9775–9790. [Google Scholar] [CrossRef] [PubMed]
- Andries, K.; Verhasselt, P.; Guillemont, J.; Göhlmann, H.W.H.; Neefs, J.-M.; Winkler, H.; Van Gestel, J.; Timmerman, P.; Zhu, M.; Lee, E.; et al. A Diarylquinoline Drug Active on the ATP Synthase of Mycobacterium tuberculosis. Science 2005, 307, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Bax, B.D.; Chan, P.F.; Eggleston, D.S.; Fosberry, A.; Gentry, D.R.; Gorrec, F.; Giordano, I.; Hann, M.M.; Hennessy, A.; Hibbs, M.; et al. Type IIA topoisomerase inhibition by a new class of antibacterial agents. Nature 2010, 466, 935–940. [Google Scholar] [CrossRef]
- Rouffet, M.; de Oliveira, C.A.F.; Udi, Y.; Agrawal, A.; Sagi, I.; McCammon, J.A.; Cohen, S.M. From Sensors to Silencers: Quinoline- and Benzimidazole-Sulfonamides as Inhibitors for Zinc Proteases. J. Am. Chem. Soc. 2010, 132, 8232–8233. [Google Scholar] [CrossRef]
- Zhang, X.; Shetty, A.S.; Jenekhe, S.A. Electroluminescence of multicomponent conjugated polymers. 1. Roles of polymer/polymer interface in emission in semiconducting polymer/polymer heterojunctions. Macromolecules 2000, 33, 2069–2082. [Google Scholar] [CrossRef]
- Jenekhe, S.A.; Lu, L.; Alam, M.M. New conjugated polymers with donor-acceptor architectures: Synthesis and photophysics of carbazole-quinoline and phenothiazine-quinoline copolymers and oligomers exhibiting large intramolecular charge transfer. Macromolecules 2001, 34, 7315–7324. [Google Scholar] [CrossRef]
- Kim, J.I.; Shin, I.-S.; Kim, H.; Lee, J.-K. Efficient Electrogenerated Chemiluminescence from Cyclometalated Iridium(III) Complexes. J. Am. Chem. Soc. 2005, 127, 1614–1615. [Google Scholar] [CrossRef] [PubMed]
- Calus, S.; Gondek, E.; Danel, A.; Jarosz, B.; Pokladko, M.; Kityk, A.V. Electroluminescence of 6-R-1,3-Diphenyl-1H-pyrazolo[3,4-b]-quinoline-Based Organic Light-Emitting Diodes (R = F, Br, Cl, CH3, C2H3 and N(C6H5)2). Mater. Lett. 2007, 61, 3292–3295. [Google Scholar] [CrossRef]
- Li, C.; Shih, H.-H.; Jiang, X.; Sun, P.; Pan, Y.; Cheng, C.-H. Synthesis, Characterization, and Electroluminescent Properties of Iridium Complex Containing 4-Phenylbenzoquinoline Ligand. Synth. Met. 2009, 159, 2070–2074. [Google Scholar] [CrossRef]
- Biddle, M.M.; Lin, M.; Scheidt, K.A. Catalytic Enantioselective Synthesis of Flavanones and Chromanones. J. Am. Chem. Soc. 2007, 129, 3830–3831. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sigman, M.S. Palladium(II)-Catalyzed Enantioselective Aerobic Dialkoxylation of 2-Propenyl Phenols: A Pronounced Effect of Copper Additives on Enantioselectivity. J. Am. Chem. Soc. 2007, 129, 3076–3077. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.-F.; Huang, W.-X.; Chen, Z.-P.; Zhou, Y.-G. Palladium-catalyzed asymmetric hydrogenation of 3-phthalimido substituted quinolines. Chem. Commun. 2014, 50, 9588–9590. [Google Scholar] [CrossRef]
- Wang, W.-B.; Lu, S.-M.; Yang, P.-Y.; Han, X.-W.; Zhou, Y.-G. Highly Enantioselective Iridium-Catalyzed Hydrogenation of Heteroaromatic Compounds, Quinolines. J. Am. Chem. Soc. 2003, 125, 10536–10537. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Cai, C.; Yang, X.; Lv, Z.-C.; Schneider, U. Catalytic Asymmetric Reactions with N,O-Aminals. ACS Catal. 2016, 6, 5747–5763. [Google Scholar] [CrossRef]
- Ayl, M.R.E.; Ibrahim, M.M.; Okael, A.M.; Gherbawy, Y.A.M.H. Synthesis, Insecticidal, and Fungicidal Screening of Some New Quinoline Derivatives. Russ. J. Bioorg. Chem. 2014, 40, 214–227. [Google Scholar]
- Aribi, F.; Panossian, A.; Vors, J.-P.; Pazenok, S.; Leroux, F.R. 2,4-Bis(fluoroalkyl)quinoline-3-carboxylates as Tools for the Development of Potential Agrochemical Ingredients. Eur. J. Org. Chem. 2018, 2018, 3792–3802. [Google Scholar] [CrossRef]
- Malathi, M.; Mohan, P.S.; Butcher, R.J.; Venil, C.K. Benzimidazole quinoline derivatives-An effective green fluorescent dye for bacterial imaging. Can. J. Chem. 2009, 87, 1692–1703. [Google Scholar] [CrossRef]
- Koścień, E.; Gondek, E.; Pokladko, M.; Jarosz, B.; Vlokh, R.O.; Kityk, A.V. Photoluminescence of 1,3-dimethyl pyrazoloquinoline derivatives. Mater. Chem. Phys. 2009, 114, 860–867. [Google Scholar] [CrossRef]
- Kaur, K.; Jain, M.; Reddy, R.P.; Jain, R. Quinolines and structurally related heterocycles as antimalarials. Eur. J. Med. Chem. 2010, 45, 3245–3264. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.W.; Ackermans, K.; Lambregts, J.; Maes, B.U.W.; Orru, R.V.A.; Ruijter, E. Modular Three-Component Synthesis of 4-Aminoquinolines via an Imidoylative Sonogashira/Cyclization Cascade. J. Org. Chem. 2018, 83, 854–861. [Google Scholar] [CrossRef]
- Pinheiro, L.C.S.; Feitosa, L.M.; Gandi, M.O.; Silveira, F.F.; Boechat, N. The Development of Novel Compounds against Malaria: Quinolines, Triazolpyridines, Pyrazolopyridines and Pyrazolopyrimidines. Molecules 2019, 24, 4095. [Google Scholar] [CrossRef] [PubMed]
- Gakhar, G.; Ohira, T.; Shi, A.; Hua, D.H.; Nguyen, T.A. Antitumor effect of substituted quinolines in breast cancer cells. Drug Dev. Res. 2008, 69, 526–534. [Google Scholar] [CrossRef]
- Datta, J.; Ghoshal, K.; Denny, W.A.; Gamage, S.A.; Brooke, D.G.; Phiasivongsa, P.; Redkar, S.; Jacob, S.T. A New Class of Quinoline-Based DNA Hypomethylating Agents Reactivates Tumor Suppressor Genes by Blocking DNA Methyltransferase 1 Activity and Inducing Its Degradation. Cancer Res. 2009, 69, 4277–4285. [Google Scholar] [CrossRef] [PubMed]
- Venditto, V.J.; Simanek, E.E. Cancer Therapies Utilizing the Camptothecins: A Review of in Vivo Literature. Mol. Pharm. 2010, 7, 307–349. [Google Scholar] [CrossRef]
- Ding, Y.; Nguyen, T.D.T.; Hua, D.H.; Nguyen, T.A. The effect of the PQ1 anti-breast cancer agent on normal tissues. Anticancer Drugs 2012, 23, 897–905. [Google Scholar] [CrossRef]
- Marganakop, S.B.; Kamble, R.R.; Hoskeri, J.; Prasad, D.J.; Meti, G.Y. Facile Synthesis of Novel Quinoline Derivatives as Anticancer Agents. Med. Chem. Res. 2014, 23, 2727–2735. [Google Scholar] [CrossRef]
- Chen, S.; Chen, R.; He, M.; Pang, R.; Tan, Z.; Yang, M. Design, synthesis, and biological evaluation of novel quinoline derivatives as HIV-1 Tat-TAR interaction inhibitors. Bioorg. Med. Chem. 2009, 17, 1948–1956. [Google Scholar] [CrossRef]
- Musiol, R.; Serda, M.; Hensel-Bielowka, S.; Polanski, J. Quinoline-Based Antifungals. Curr. Med. Chem. 2010, 17, 1960–1973. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Fang, K.C.; Sheu, J.Y.; Hsu, S.L.; Tzeng, C.C. Synthesis and Antibacterial Evaluation of Certain Quinolone Derivatives. J. Med. Chem. 2001, 44, 2374–2377. [Google Scholar] [CrossRef]
- Roma, G.; Braccio, M.D.; Grossi, G.; Mattioli, F.; Ghia, M. 1,8-Naphthyridines IV. 9-substituted N,N-dialkyl-5-(alkylamino or cycloalkylamino) [1,2,4]-triazolo[4,3-a][1,8]naphthyridine-6-carboxamides, new compounds with anti-aggressive and potent anti-inflammatory activities. Eur. J. Med. Chem. 2000, 35, 1021–1035. [Google Scholar] [CrossRef]
- Tseng, C.-H.; Tung, C.-W.; Peng, S.-I.; Chen, Y.-L.; Tzeng, C.-C.; Cheng, C.-M. Discovery of Pyrazolo[4,3-c]quinoline Derivatives as Potential Anti-Inflammatory Agents through Inhibiting of NO Production. Molecules 2018, 23, 1036. [Google Scholar] [CrossRef]
- De Oliveira, A.R.M.; Szczerbowski, D. Quinina: 470 anos de história, controvérsias e desenvolvimento. Quim. Nova 2009, 32, 1971–1974. [Google Scholar] [CrossRef][Green Version]
- Murie, V.E.; Nishimura, R.H.V.; Rolim, L.A.; Vessecchi, R.; Lopes, N.P.; Clososki, G.C. Base-Controlled Regioselective Functionalization of Chloro-Substituted Quinolines. J. Org. Chem. 2018, 83, 871–880. [Google Scholar] [CrossRef]
- Liu, J.; Cao, R.; Xu, M.; Wang, X.; Zhang, H.; Hu, H.; Li, Y.; Hu, Z.; Zhong, W.; Wang, M. Hydroxychloroquine, a lesstoxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020, 6, 1–4. [Google Scholar] [CrossRef]
- Gao, J.; Tian, Z.; Yang, X. Breakthrough: Chloroquine phosphate has shown apparent efficiency in treatment of COVID-19 associated pneumonia in clinical studies. BioSci. Trends 2020, 14, 72–73. [Google Scholar] [CrossRef]
- Cortegiani, A.; Ingoglia, G.; Ippolito, M.; Giarratano, A.; Einav, S. Asystematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J. Crit. Care 2020, 57, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Bujuq, N.A. Methods of Synthesis of Remdesivir, Favipiravir, Hydroxychloro-quine, and Chloroquine: Four Small Molecules Repurposed for Clinical Trials during the Covid-19 Pandemic. Synthesis 2020, 52, 3735–3750. [Google Scholar] [CrossRef]
- NIH COVID-19 Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov/antiviral-therapy/ (accessed on 20 June 2021).
- Marco-Contelles, J.; Pérez-Mayoral, E.; Samadi, A.; do Carmo Carreiras, M.; Soriano, E. Recent Advances in the Friedländer Reaction. Chem. Rev. 2009, 109, 2652–2671. [Google Scholar] [CrossRef]
- Bharate, J.B.; Vishwakarma, R.A.; Bharate, S.B. Metal-free domino one-pot protocols for quinoline synthesis. RSC Adv. 2015, 5, 42020–42053. [Google Scholar] [CrossRef]
- Ramann, G.; Cowen, B. Recent Advances in Metal-Free Quinoline Synthesis. Molecules 2016, 21, 986. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Kour, P.; Kumar, A. A review on transition-metal mediated synthesis of quinolines. J. Chem. Sci. 2018, 130, 73. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, H.; Deng, G.-J.; Huang, H. Copper-Catalyzed Formal [3 + 3] Annulations of Arylketoximes and o-Fluorobenzaldehydes: An Entry to Quinoline Compounds. Org. Lett. 2021, 23, 936–942. [Google Scholar] [CrossRef]
- Jain, R.; Vaitilingam, B.; Nayyar, A.; Palde, P.B. Substituted 4-methylquinolines as a new class of anti-tuberculosis agents. Bioorg. Med. Chem. Lett. 2003, 13, 1051–1054. [Google Scholar] [CrossRef]
- Jain, M.; Vangapanda, S.; Sachdeva, S.; Singh, S.; Singh, P.P.; Jena, G.B.; Tikoo, K.; Ramarao, P.; Kaul, C.L.; Jain, R. Discovery of a Bulky 2-tert-Butyl Group Containing Primaquine Analogue That Exhibits Potent Blood-Schizontocidal Antimalarial Activities and Complete Elimination of Methemoglobin Toxicity. J. Med. Chem. 2004, 47, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Mai, A.; Rotili, D.; Tarantino, D.; Ornaghi, P.; Tosi, F.; Vicidomini, C.; Sbardella, G.; Nebbioso, A.; Miceli, M.; Altucci, L.; et al. Small-Molecule Inhibitors of Histone Acetyltransferase Activity: Identification and Biological Properties. J. Med. Chem. 2006, 49, 6897–6907. [Google Scholar] [CrossRef]
- Solomon, V.R.; Lee, H. Quinoline as a privileged scaffold in cancer drug discovery. Curr. Med. Chem. 2011, 18, 1488–1508. [Google Scholar] [CrossRef]
- Ding, Y.; Nguyen, T.A. PQ1, a quinoline derivative, induces apoptosis in T47D breast cancer cells through activation of caspase-8 and caspase-9. Apoptosis 2013, 18, 1071–1082. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Watanabe, S.; Matsumura, Y.; Tokuoka, Y.; Yokoyama, A. Oxovanadium complexes with quinoline and pyridinone ligands: Syntheses of the complexes and effect of alkyl chains on their apoptosis-inducing activity in leukemia cells. Bioorg. Med. Chem. 2012, 20, 3058–3064. [Google Scholar] [CrossRef]
- Beller, M.; Thiel, O.; Trauthwein, H.; Hartung, C.G. Amination of Aromatic Olefins with Anilines: A New Domino Synthesis of Quinolines. Chem. Eur. J. 2000, 6, 2513–2522. [Google Scholar] [CrossRef]
- McNaughton, B.R.; Miller, B.L. A Mild and Efficient One-Step Synthesis of Quinolines. Org. Lett. 2003, 4, 4257–4259. [Google Scholar] [CrossRef]
- O’Byrne, A.; Evans, P. Rapid synthesis of the tetrahydroquinoline alkaloids: Angustureine, cuspareine and galipinine. Tetrahedron 2008, 64, 8067–8072. [Google Scholar] [CrossRef]
- Shan, G.; Sun, X.; Xia, Q.; Rao, Y. A Facile Synthesis of Substituted 2-Alkylquinolines through [3 + 3] Annulation between 3-Ethoxycyclobutanones and Aromatic Amines at Room Temperature. Org. Lett. 2011, 13, 5770–5773. [Google Scholar] [CrossRef]
- Monrad, R.N.; Madsen, R. Ruthenium-catalysed synthesis of 2- and 3-substituted quinolines from anilines and 1,3-diols. Org. Biomol. Chem. 2011, 9, 610–615. [Google Scholar] [CrossRef]
- Matsubara, Y.; Hirakawa, S.; Yamaguchi, Y.; Yoshida, Z.-I. Assembly of substituted 2-alkylquinolines by a sequential palladium-catalyzed C-N and C-C bond formation. Angew. Chem. Int. Ed. 2011, 50, 7670–7673. [Google Scholar] [CrossRef]
- He, L.; Wang, J.-Q.; Gong, Y.; Liu, Y.-M.; Cao, Y.; He, H.-Y.; Fan, K.-N. Titania-Supported Iridium Subnanoclusters as an Efficient Heterogeneous Catalyst for Direct Synthesis of Quinolines from Nitroarenes and Aliphatic Alcohols. Angew. Chem. Int. Ed. 2011, 50, 10216–10220. [Google Scholar] [CrossRef]
- Ji, X.; Huang, H.; Li, Y.; Chen, H.; Jiang, H. Palladium-Catalyzed Sequential Formation of C-C Bonds: Efficient Assembly of 2-Substituted and 2,3-Disubstituted Quinolines. Angew. Chem. Int. Ed. 2012, 51, 7292–7296. [Google Scholar] [CrossRef]
- Verma, S.; Verma, D.; Jain, S.L. Magnetically separable palladium–graphene nanocomposite as heterogeneous catalyst for the synthesis of 2-alkylquinolines via one pot reaction of anilines with alkenyl ethers. Tetrahedron Lett. 2014, 55, 2406–2409. [Google Scholar] [CrossRef]
- Jin, H.; Tian, B.; Song, X.; Xie, J.; Rudolph, M.; Rominger, F.; Hashmi, A.S.K. Gold-Catalyzed Synthesis of Quinolines from Propargyl Silyl Ethers and Anthranils through the Umpolung of a Gold Carbene Carbon. Angew. Chem. Int. Ed. 2016, 55, 12688–12692. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Yang, H.; Jiang, G. Assembly of Diversely Substituted Quinolines via Aerobic Oxidative Aromatization from Simple Alcohols and Anilines. J. Org. Chem. 2017, 82, 3287–3290. [Google Scholar] [CrossRef]
- Li, Y.; Cao, X.; Liu, Y.; Wan, J.-P. Regioselective three-component synthesis of 2,3-disubstituted quinolines via the enaminon modified Povarov reaction. Org. Biomol. Chem. 2017, 15, 9585–9589. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Xiang, J.; Cui, J.; Hu, B.; Yang, L.; Tang, Y. HOAc-Assisted Synthesis of 2,3-Disubstituted Quinolines from Arylamine and Aliphatic Aldehyde in Water. ChemistrySelect 2019, 4, 9392–9395. [Google Scholar] [CrossRef]
- Ali, S.; Gattu, R.; Singh, V.; Mondal, S.; Khan, A.T.; Dubey, G.; Bharatam, P.V. Reaction behaviour of arylamines with nitroalkenes in the presence of bismuth (III) triflate: An easy access to 2,3-dialkylquinolines. Org. Biomol. Chem. 2020, 18, 1785–1793. [Google Scholar] [CrossRef]
- Chan, C.-K.; Lai, C.-Y.; Lo, W.-C.; Cheng, Y.-T.; Chang, M.-Y.; Wang, C.-C. p-TsOH-mediated synthesis of substituted 2,4-diaryl-3-sulfonylquinolines from functionalized 2-aminobenzophenones and aromatic β-ketosulfones under microwave irradiation. Org. Biomol. Chem. 2020, 18, 305–315. [Google Scholar] [CrossRef]
- Chan, C.-K.; Lai, C.-Y.; Wang, C.-C. Environmentally Friendly Nafion-Mediated Friedländer Quinoline Synthesis under Microwave Irradiation: Application to One-Pot Synthesis of Substituted Quinolinyl Chalcones. Synthesis 2020, 52, 1779–1794. [Google Scholar] [CrossRef]
- Chan, C.-K.; Lai, C.-Y.; Wang, C.-C. TMSOTf-catalyzed synthesis of substituted quinazolines using hexamethyldisilazane as a nitrogen source under neat and microwave irradiation conditions. Org. Biomol. Chem. 2020, 18, 7201–7212. [Google Scholar] [CrossRef]
- Asressu, K.H.; Chan, C.-K.; Wang, C.-C. One-Pot Synthesis of 1,5-Diketones under a Transition-Metal-Free Condition: Application in the Synthesis of 2,4,6-Triaryl Pyridine Derivatives. ACS Omega 2021, 6, 7296–7311. [Google Scholar] [CrossRef]
- Church, S. Del. firm installs fuel cell. The News Journal, 6 January 2007; p. B7. [Google Scholar]
- Mauritz, K.A.; Moore, R.B. State of Understanding of Nafion. Chem. Rev. 2004, 104, 4535–4585. [Google Scholar] [CrossRef]
- Antonetti, C.; Fulignati, S.; Licursi, D.; Galletti, A.M.R. Turning Point toward the Sustainable Production of 5-Hydroxymethyl-2-furaldehyde in Water: Metal Salts for Its Synthesis from Fructose and Inulin. ACS Sustain. Chem. Eng. 2019, 7, 6830–6838. [Google Scholar] [CrossRef]
- Biancalana, L.; Fulignati, S.; Antonetti, C.; Zacchini, S.; Provinciali, G.; Pampaloni, G.; Galletti, A.M.R.; Marchetti, F. Ruthenium p-cymene complexes with α-diimine ligands as catalytic precursors for the transfer hydrogenation of ethyl levulinate to γ-valerolactone. New J. Chem. 2018, 42, 17574–17586. [Google Scholar] [CrossRef]
- CCDC 1940046 (3p), 2002378 (5a), 2013112 (5b) and 2039753 (5d) Contain the Supplementary Crystallographic Data for this Paper. Available online: www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 20 June 2021).
- Ko, S.; Kang, B.; Chang, S. Cooperative Catalysis by Ru and Pd for the Direct Coupling of a Chelating Aldehyde with Iodoarenes or Organostannanes**. Angew. Chem. Int. Ed. 2005, 44, 455–457. [Google Scholar] [CrossRef]
- Rathbun, C.M.; Johnson, J.B. Rhodium-Catalyzed Acylation with Quinolinyl Ketones: Carbon-Carbon Single Bond Activation as the Turnover-Limiting Step of Catalysis. J. Am. Chem. Soc. 2011, 133, 2031–2033. [Google Scholar] [CrossRef]
- Wang, J.; Chen, W.; Zuo, S.; Liu, L.; Zhang, X.; Wang, J. Direct Exchange of a Ketone Methyl or Aryl Group to Another Aryl Group through C-C Bond Activation Assisted by Rhodium Chelation**. Angew. Chem. Int. Ed. 2012, 51, 12334–12338. [Google Scholar] [CrossRef]
- Wang, J.; Zuo, S.; Chen, W.; Zhang, X.; Tan, K.; Tian, Y.; Wang, J. Catalytic Formation of Ketones from Unactivated Esters through Rhodium Chelation-Assisted C−O Bond Activation. J. Org. Chem. 2013, 78, 8217–8231. [Google Scholar] [CrossRef]
- Dennis, J.M.; Compagner, C.T.; Dorn, S.K.; Johnson, J.B. Rhodium-Catalyzed Interconversion of Quinolinyl Ketones with Boronic Acids via C–C Bond Activation. Org. Lett. 2016, 18, 3334–3337. [Google Scholar] [CrossRef]
- Gregerson, C.E.; Trentadue, K.N.; Phipps, E.J.T.; Kirsch, J.K.; Reed, K.M.; Dyke, G.D.; Jansen, J.H.; Otteman, C.B.; Stachowski, J.L.; Johnson, J.B. Oxidative coupling of Michael acceptors with aryl nucleophiles produced through rhodium-catalyzed C–C bond activation†. Org. Biomol. Chem. 2017, 15, 5944–5948. [Google Scholar] [CrossRef]
- Chen, X.; Qiu, S.; Wang, S.; Wang, H.; Zhai, H. Blue-light-promoted carbon-carbon double bond isomerization and its application in the syntheses of quinolones. Org. Biomol. Chem. 2017, 15, 6349–6352. [Google Scholar] [CrossRef]
- Shao, T.; Yin, Y.; Lee, R.; Zhao, X.; Chai, G.; Jiang, Z. Sequential photoredox catalysis for cascade aerobic decarboxylative povarov and oxidative dehydrogenation reactions of N-aryl α-amino acids. Adv. Synth. Catal. 2018, 360, 1754–1760. [Google Scholar] [CrossRef]
- Jacob, J.; Jones, W.D. Selective conversion of diallylanilines and arylimines to quinolones. J. Org. Chem. 2003, 68, 3563–3568. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jones, W.-D. Mechanistic Investigation of the Cobalt-Catalyzed Selective Conversion of Diallylanilines to Quinolines Involving C−N and C−H Activations. J. Am. Chem. Soc. 2007, 129, 10707–10713. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.-S.; Lee, N.-Y.; Kim, T.-J.; Shim, S.-C. Ruthenium-catalyzed formal alkyl group transfer: Synthesis of quinolines from nitroarenes and alkylammonium halides. J. Heterocycl. Chem. 2004, 41, 423–429. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).