Abstract
The heptyl butanoate ester was synthesized from butanoic acid and heptanol in a heterogeneous medium in the presence of sulfonated activated carbon (AC-SO3H) catalyst particles subjected to microwave irradiation, which led to higher conversion yields (greater product yields) than conventional heating with an oil bath. The advantage of the microwaves appeared only when the moisture content in the butanoic acid batch(es) was high, suggesting that, unlike conventional heating, the reverse reaction caused by the moisture content and/or by the byproduct water was suppressed by the microwaves. This contrasted with the results that were found when carrying out the reaction in a homogeneous medium in the presence of the 2,4,6-trimethylpyridinium-p-toluene sulfonate (TMP-PTS) catalyst, as product yields were not improved by microwave heating relative to conventional heating. The removal of moisture/water content in the reaction solution was more pronounced when the reactor was cooled, as the reaction yields were enhanced via selective heating of the heterogeneous catalyst. A coupled electromagnetic field/heat transfer analysis gave credence to the selective heating of the AC-SO3H catalyst, which was further enhanced by cooling the reactor. It was deduced that unforeseen impurities and local high-temperature fields generated on the surface of small fine catalyst particles may have had an effect on the microwave chemistry such that the associated phenomena could be mistaken as originating from a nonthermal effect of the microwaves. Accordingly, it is highly recommended that impurities and selective heating be taken into consideration when examining and concluding the occurrence of a microwave nonthermal effect.
1. Introduction
Early cases in microwave chemistry that used microwaves to carry out chemical reactions were reported as long ago as 1986 by Giguere et al. [1], who demonstrated that microwaves could promote reactions such as oxidation by permanganic acid, esterification of sebostic acid, and ether synthesis via an SN2-type process. Since then, many reviews [2,3,4,5] and specialized books [6,7,8,9,10] have been published on microwave chemistry. The appeal is that microwaves can lead to faster reactions than conventional heating, as evidenced by three principal features of microwave chemistry. First, microwave heating typically results in high heating efficiency, whereas with conventional heating, the transfer of heat to the solution reactants is limited by the surface area of the reaction vessel. In contrast, to the extent that microwaves can heat the reactants directly, there is no limitation with regard to the area of heat transfer such that microwave energy can be provided directly to the reacting substrates, thereby enhancing the heating rate. The second feature is selective heating. Microwaves consist of an alternating electric field and an alternating magnetic field; therefore, polar substances, magnetic substances, and substances with moderate conductivity absorb the microwave energy to generate heat, which can be delivered selectively to the reaction components even when the reaction systems consist of multiple mixed components. The third feature, and the focus of this article, is often referred to as the microwave effect, that is, a nonthermal effect of microwaves. The latter is a promoting effect on reactions that finds no parallel in conventional heating.
Although often invoked, the main cause of this microwave effect nonetheless remains elusive, although various studies and articles have attempted to describe this feature of microwaves. For instance, Rosana and coworkers [11] reported that the rate of the Friedel–Crafts alkylation process under microwave irradiation was significantly improved and speculated that the cause was due to an increase in the effective collision frequency amongst the reaction components. However, Kappe and coworkers [12] pointed out that Rosana et al. [11] measured the reaction temperature using an infrared sensor, which measured a temperature that was different from the internal temperature of the reactants in solution, and thus, countered that the reaction rate was improved because the actual temperature was higher than the measured temperature of the external reactor walls. As a basis for their different interpretation, they showed [12] that the reaction rate did not improve when the actual reaction temperature was measured using an optical fiber-type fluorescence thermometer, which is unaffected by the microwaves, and that the reaction rate of the Friedel–Crafts alkylation did not accelerate, even under conditions of microwave chemistry. However, later on, Dudley’s group [13] performed a detailed kinetic analysis of the reaction with the internal temperature being measured by the fluorescence thermometer and did demonstrate that, indeed, the reaction rate of the Friedel–Crafts alkylation at 100 °C under microwave irradiation was nearly eightfold faster than under conventional heating (oil bath). Consequently, they again argued for the reaction acceleration effect to be a nonthermal microwave effect. Nonetheless, to this day this microwave effect remains controversial and its presence in microwave chemistry is being examined with the use of various microwave devices and processes.
The results of reactions aided by microwaves may also be affected by the characteristics of microwave oscillators. For instance, Horikoshi and coworkers [14] noted that the degree of reaction evolution changes depending on the type of microwave generator. These authors used a traditional magnetron generator and a semiconductor generator to examine a microwave-induced Suzuki–Miyaura coupling reaction catalyzed by a palladium catalyst supported on activated carbon particulates. This coupling reaction was either accelerated or otherwise slowed down depending on the specific type of magnetron oscillator used. Measurements with an electrical sensor found that the microwave output from one of the magnetron oscillators consisted of 5 ms pulsed waves and that the peak value of the incident power was nearly fourfold higher than for the two other oscillators, even though the same output power was used. As a consequence, the device characteristics and trace impurity components can greatly affect the results in microwave chemistry, thus making it difficult to expose and substantiate the nonthermal microwave effect.
Esters are one of the most important compounds in the chemical industry, as they are used in fragrances, pharmaceuticals, functional chemicals, plasticizers, and chemical fibers, among others [15,16]. Several studies have reported on the use and importance of microwave radiation in esterification reactions [17,18,19]. In particular, the compatible combination of microwaves and the sulfonated activated carbon solid catalyst might promote a reaction via selective heating of the catalyst, and consequently, could be applied to carry out valuable reactions, such as the synthesis of methylated esters of fatty acids (FAMEs) [20,21]. However, in many cases, previous use of sulfonated activated carbon and microwaves has resulted in reaction systems that contained an excess of highly polar substrates such that preferential absorption of the microwaves’ energy by these substrates over the catalysts cannot be precluded.
Accordingly, the present study was designed to use a model reaction that involved a long carbon chain alcohol with low polarity to facilitate the absorption of the microwaves by the catalyst in order to be able to discriminate between thermal and nonthermal microwave effects. In the process of verifying the effect of microwaves using the synthesis of the heptyl butanoate ester from butanoic acid and heptanol as our model reaction, we found that the microwave effect remained elusive when the reaction was catalyzed by the solid sulfonated activated carbon catalyst particulates and was affected by the moisture content present in one of the reaction components, namely, butanoic acid. It appeared that microwaves suppressed the reverse reaction (Scheme 1) because the moisture in the butanoic acid batch(es) and the byproduct water were removed under microwave conditions in the presence of the solid catalyst AC-SO3H, and consequently, this biased the reaction equilibrium toward the product. This is contrary to the product yields that resulted in the presence of the homogeneous TMP-PTS catalytic system. Additionally, the microwaves appeared more effective than conventional heating at higher moisture contents, while microwave heating displayed little advantage vis-à-vis conventional heating at lower moisture contents.
Scheme 1.
Reaction between an organic acid and an alcohol to give the corresponding ester.
An important point raised in this article is the consequence that unforeseen impurities and local high-temperature fields, i.e., hot spots, generated on the solid catalyst surface may have on microwave chemistry. To be precise, overlooking these phenomena could lead to a misunderstanding of the nonthermal effect. Accordingly, the need to consider selective heating and the presence of impurities is emphasized when carrying out microwave chemistry prior to concluding the occurrence of a nonthermal microwave effect.
2. Results and Discussion
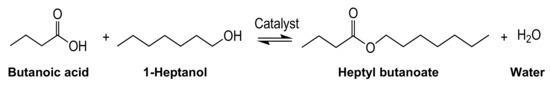
The yields of the heptyl butanoate ester produced from the reaction in Scheme 1 catalyzed by AC-SO3H (first experimental run) are reported in Figure 1a. Unless otherwise noted, experiments were conducted without air cooling. The synthetic yield under conventional heating (CH) was only 53.0%, while the yield under microwave heating (MW) was 80.3%, which represents a significant 1.5-fold increase relative to conventional heating with an oil bath. In both cases, the internal temperature measured with an optical fiber thermometer reached 130 °C within ≈5 min and was otherwise constant throughout the reaction period (Figure 1b). Consequently, the difference in conversion yield could not be ascribed to differences in temperature within the bulk of the reaction. The relative dielectric losses (εr”) of butanoic acid and heptanol (0.36 and 1.04, respectively, at 29.9 °C) were significantly smaller than that of water (8.29 at 29 °C); therefore, the microwaves heated the sulfonated activated carbon particulates selectively. Our previous studies [22] showed that, in fact, under microwave heating, the temperature of activated carbon particulates was higher than the bulk solution temperature. There is no doubt then that the temperature at each AC-SO3H particle’s surface was also greater than that of the solution bulk (see below).
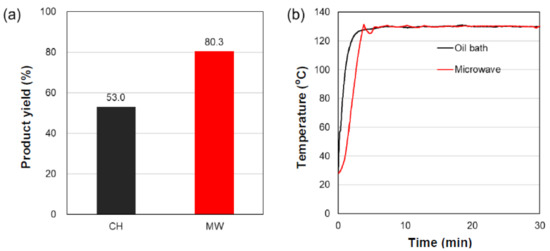
Figure 1.
(a) Product yields and (b) internal temperature profiles in the synthesis of the heptyl butanoate ester catalyzed by AC-SO3H particulates under oil-bath heating (CH) and microwave heating (MW)—first experimental run (moisture content determined in pre-distilled butanoic acid batch: 0.052%).
Even though the microwave irradiation in the experiments that employed a single-mode applicator and a semiconductor generator was carried out at a precise microwave frequency of 2.45 GHz, results from the second experimental run (Figure 2a) were very different from those of the first run (Figure 1a). That is, the 1.5-fold difference in product yields between the CH method (53.0%) and the MW method (80.3%) reported in Figure 1a was not established in the second run, as the yields were very similar: CH method, 86.5%; MW method, 84.9% (Figure 2a). Even more surprising, the conversion yields of the microwave method were somewhat greater in the second run (84.9% versus 80.3%), but more importantly, the yield from the oil-bath CH method changed substantively from 53.0% to 86.5%. Even more curious, the use of microwaves and conventional heating in the second run displayed nearly identical conversion yields (84.9% and 86.5%, respectively). Note that the internal reaction temperatures of the solution in the first and second runs were also nearly the same between the microwave heating and conventional heating cases (compare Figure 1b versus Figure 2b). In studies carried out to date, such observations may have been overlooked and taken as an experimental error where a multimode microwave device was used.
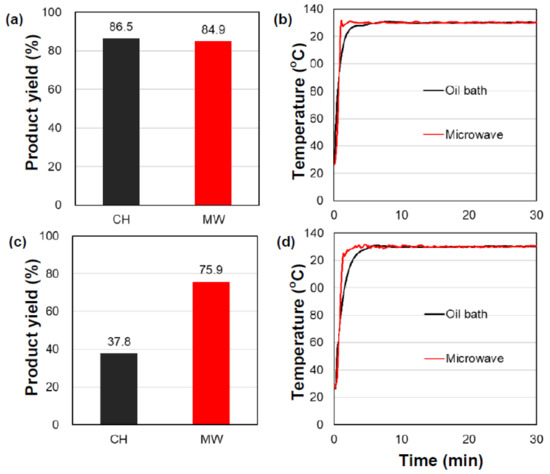
Figure 2.
(a,c) Product yields and (b,d) internal temperature profiles in the synthesis of the heptyl butanoate ester in the presence of the AC-SO3H catalyst subjected to oil-bath heating (CH) and microwave heating (MW): (a,b) the moisture content in the pre-distilled second butanoic acid batch was 0.022% (second experimental run) and (c,d) the moisture content in the post vacuum-distilled butanoic acid was 0.058% (third experimental run).
To resolve this puzzling finding, it happened that the lot number of the butanoic acid batch used in the second experimental run was different from the first, as there was little of the first butanoic acid batch left after the first experimental run. The fresh butanoic acid batch used in the second experimental run led to the decreased advantage of the superior microwave heating vis-à-vis conventional heating (Figure 2a,b), which led us to suspect the possible presence of extraneous impurities that might have affected such an outcome. Accordingly, a quantity of this second batch was vacuum distilled to be used for the third experimental run, which, just like the first experimental run (Figure 1a), showed once again a higher conversion yield under microwave heating (75.9%) than under conventional oil-bath heating (37.8%) (see Figure 2c). There were no significant differences in the results of the component analysis of butanoic acid using the GC/MS technique before and after vacuum distillation, which left only the moisture content as the most plausible factor that affected the product yields (compare Figure 1a with Figure 2a,b).
The moisture content in the butanoic acid batch used in the first experiment was 0.052%, while the moisture content was 0.022% (Figure 2a,b) for the as-received new batch used in the second experimental run; for the third run, a quantity of the second butanoic acid batch was vacuum-distilled, which upon measurement, yielded a moisture content of 0.058% (Table 1). The latter greater moisture content may have resulted from the vacuum distillation, whereas the higher moisture content of the first butanoic acid batch (0.052%) used in the first experimental run was likely due to moisture pickup during the long-term storage. Evidently and contrary to expectations, the lower moisture content in the second run had a dramatic effect on the product yields of the heptyl butanoate ester from the oil-bath method (Figure 2a,b). In other words, the dependence of the conversion yield on the moisture content of the butanoic acid showed a negative correlation, with the trend in the case of oil-bath heating being more obvious than for microwave heating. Thus, confirmation that the moisture content was indeed a significant factor impacting the reaction efficiency was demonstrated for the microwave-induced reaction (Scheme 1), a factor that particularly affected the yields from the conventional oil-bath heating method.

Table 1.
Moisture content in the starting butanoic acid samples and conversion yields with microwave and conventional heating methods using both the heterogeneous catalyst (AC-SO3H) and the homogeneous catalyst (TMP-PTS).
Clearly, the advantages of the microwaves appeared more pronounced at the higher moisture content in the acid batches, and that the microwave method somehow inhibited the reverse of the reaction in Scheme 1 as it necessitated the presence of water for de-esterification to occur. To the best of our knowledge, there are no studies that report similar findings. This process is more likely to occur under conventional heating, as the water is not sufficiently removed from the solution bulk, in contrast to microwave heating, wherein the moisture presence and the byproduct water are adsorbed on the selectively heated AC-SO3H catalyst particulates and subsequently eliminated through their evaporation and adsorption onto the cotton swab. Additionally, inasmuch as the product ester was of low polarity and a weak hydrogen bonding aspect, this would make it difficult to interact with the acidic function on the surface of the activated carbon such that the product easily desorbed from the catalyst via the thermal convection generated by the temperature gradient between the catalyst surface and the bulk solution [23]. Thus, to the extent that both heptanol and butyric acid are polar substrates with hydrogen-bonding properties as compared to the produced ester, the reactants can approach the heated catalyst and cause the reaction to proceed. Note that the water interacted more strongly with the catalyst surface than the substrates present in the solution such that the produced ester desorbed rapidly from the catalyst surface, and thus, precluded the reverse reaction from occurring. Accordingly, the de-esterification process via hydrolysis was minimized, if not altogether suppressed from taking place, in the heterogeneous medium under microwave irradiation.
In order to substantiate the inferences stating that the activated carbon under microwave irradiation can eliminate the water byproduct and trace amounts of moisture, experiments were also undertaken with the homogeneous catalyst 2,4,6-trimethylpyridinium-p-toluene sulfonate (TMP-PTS) and the butanoic acid batch from the second run, together with the predistilled butanoic acid used for the third experimental run, wherein the moisture contents were 0.022% and 0.058%, respectively (Table 1). Unlike the activated carbon particulates that adsorbed the moisture in the dispersion, the homogeneous catalyst could not duplicate this event, which could seriously affect the reverse reaction, and concomitantly, the product yields. The data reported in Figure 3 and summarized in Table 1 show that there was no significant difference between the microwave and oil-bath heating but do show a significant difference in the product yields when the moisture contents differed (compare 0.022% in Figure 3a,b versus 0.058% in Figure 3c,d). In other words, there was no dissipation of the moisture when the homogeneous catalyst was used, and thus, at least in this case, there was no advantage in using the microwaves vis-à-vis conventional heating to drive the reaction.
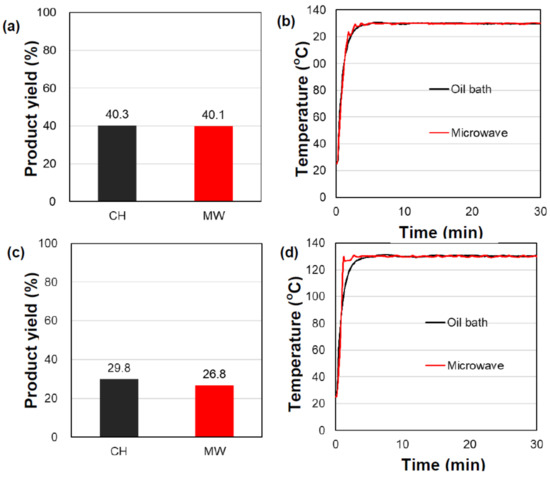
Figure 3.
(a,c) Product yields and (b,d) internal temperature profiles in the synthesis of the heptyl butanote ester using oil-bath heating (CH) and microwave heating (MW) in a homogeneous phase with the catalyst TMP-PTS: (a,b) the moisture content in the predistilled butanoic acid batch was 0.022% (second experiment run) and (c,d) the moisture content in the butanoic acid batch after the vacuum distillation was 0.058% (third experiment run).
As a further substantiation of the above inferences, additional experiments were conducted in which microwave heating alone was compared with microwave heating under cooling conditions; in the latter case, the temperature of the bulk solution decreased, which allowed for the use of microwaves at greater microwave output power. This led to higher temperatures that could selectively heat the heterogeneous activated carbon catalytic particles. The butanoic acid batch of the first experimental run was used for these experiments. With cooling, the microwave output was increased from ≈2.90 W to ≈5.60 W; the temperature of the solution with the 2.90 W microwaves remained unchanged, even though the 5.60 W microwaves delivered nearly twice as much microwave energy to the reactor. As a result, the yield of the heptyl butanoate ester with cooling (MW-cooling) conditions improved significantly to 92.9%, which was a 1.7-fold increase compared to the yields from the conventional CH method (see Figure 4a) and higher than simply using the 2.90 W microwave heating with no cooling (80.3%; see Figure 1a and Table 1). Evidently, the increase in the local temperature at the catalyst’s surface was also an important factor for improving product yields.
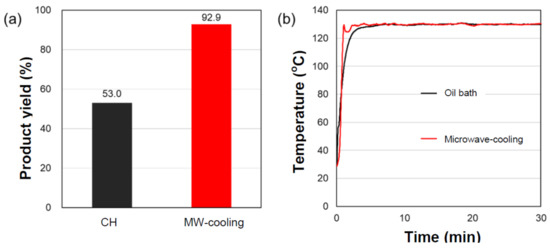
Figure 4.
(a) Product yields and (b) internal temperature profiles in the synthesis of the butanoate ester using oil-bath heating (CH) and microwave heating with air cooling (MW-cooling) in the presence of the heterogeneous AC-SO3H catalytic particles. The moisture content in the predistilled butanoic acid batch was 0.052%.
The cause(s) for the enhanced product yields resulting from the effect of selective heating of the activated carbon catalyst by microwaves with air-cooling was clarified by measuring the temperature of the activated carbon catalyst inside the reactor subjected to this heating method using a coupled electromagnetic/thermal analysis with and without air cooling. Figure 5a,c display images of the temperature distribution in the reactor irradiated with microwaves and with microwaves under cooling conditions, respectively, whereas Figure 5b,d show the simulated images of the heterogeneous sulfonated activated carbon particulates heated by 2.90 W microwaves and by 5.60 W microwaves with the reactor being air-cooled (MW-cooling), respectively. Note the 38.5 °C difference in temperature of the particulates under both conditions of microwave heating (178.6 °C versus 217.1 °C) and the difference in the yields (80.3% versus 92.9%; compare Figure 1a versus Figure 4a).
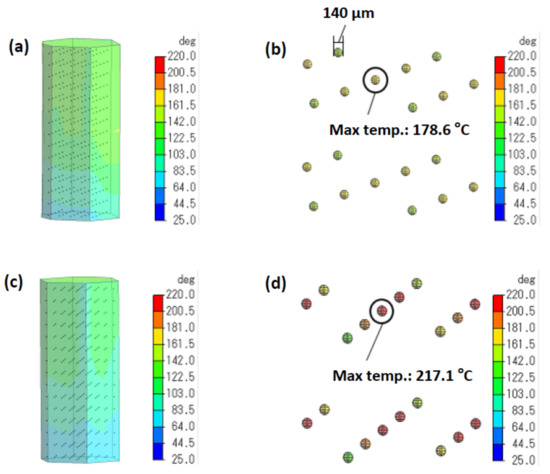
Figure 5.
(a,c) Colored images displaying the results of the simulated temperature distribution while irradiating the reactor with the 2.90 W microwaves and with the 5.60 W microwaves with air cooling, respectively. (b,d) Heterogeneous sulfonated activated carbon particulates heated using the 2.90 W microwaves and the 5.60 W microwaves with air cooling, respectively. The red-colored particulates in (d) reflect the higher temperature distribution on the particle surface.
The stepwise changes of the average temperature of the substrate solution and the maximal temperature at the activated carbon surface are displayed in Figure 6a,b, which show a temperature gap of 47 °C (Figure 6a) between the activated carbon particle surface and the solution under a no-air-cooling condition, whereas, under microwave-air-cooling conditions, the difference was 86 °C (Figure 6b). It is important to emphasize that the temperatures of the solutions were the same with and without air cooling in both cases, while the temperatures at the particulate surface were quite different. This explains the difference in product yields between those from the MW-cooling method (Figure 4a) and those from the MW method (Figure 1a) owing to a nearly twofold increase in temperature difference in the former.
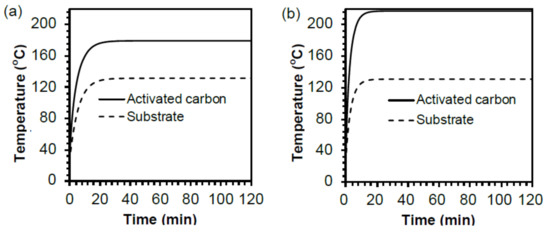
Figure 6.
Differences in temperatures between the activated carbon particles and the temperatures in the solution bulk: (a) under normal heating with 2.90 W microwaves and (b) under heating with 5.60 W microwaves with air-cooling.
3. Materials and Methods
3.1. Microwave Device Setup
The interaction(s) between microwaves and the reaction components was examined using a microwave heating device associated with a semiconductor generator, which afforded control of the microwave radiation at a precise fixed frequency of 2.45 GHz. The microwave setup, which is schematically illustrated in Figure 7a,b, included a single-mode TE103 (transverse electric 103) mode cavity, a short plunger, an iris, and an E/H-tuner (E, electric field; H, magnetic field). Continuous microwaves were generated from the 2.45-GHz semiconductor generator equipped with an isolator (GEAP-A003A01, LeanFa Srl, Ruvo di Puglia, Bari, Italy; maximum power, 250 W). The resonance of the microwaves was adjusted with the iris and the plunger at 1.5 cycles. Heating of the reaction components was achieved by positioning the quartz tube reactor in the single-mode microwave apparatus of Figure 7a,b at position (i), which had the maximal electric field density within the waveguide. The wavelength of propagation of the microwaves in the TE103 mode within the waveguide was ≈14.78 cm, as estimated from Equation (1):
where λ is the wavelength in the waveguide; λ0 = 12.24 cm is the wavelength in a vacuum given by c/f, with c being the speed of light (2.9979 × 1010 cm s−1), and f is the microwave frequency (2.45 GHz or 2.45 × 109 s−1); b is the height of the waveguide (10.92 cm).
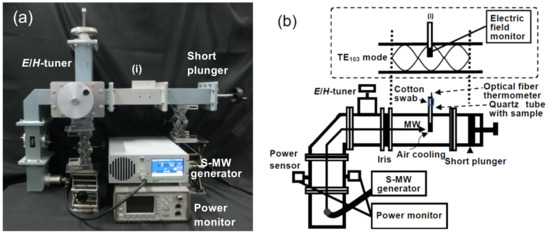
Figure 7.
Photograph (a) and schematic (b) of the microwave irradiation system consisting of a 2.45 GHz S-MW generator (semiconductor microwave generator), an optical fiber thermometer, a power sensor, a power monitor, and a single-mode applicator. Inset in (b): distribution of the (i) electric field inside the single-mode applicator and location of the sample. E, electric field; H, magnetic field; TE, transverse electric.
The maximal position of the E-field from the iris was located at three-quarters of the wavelength of the standing wave in the waveguide, namely, 11.09 cm. The maximal position of the H-field from the iris was at half the wavelength of the standing wave in the waveguide, namely, 7.39 cm [24]. The incident and reflected waves of the microwave were measured precisely with a power meter (E4419B EPM series dual-channel power meter, Keysight Technologies, Santa Rosa, CA, USA) and a power sensor (E9300A E-series average power sensor, Keysight Technologies, Santa Rosa, CA, USA), respectively. The temperatures of the solutions were measured at 1 s intervals with an optical fiber thermometer (FL-2000, Anritsu Meter, Co., Ltd., Meguro-ku, Japan). The tip of the optical fiber thermometer was set 1 cm above the bottom of the reaction vessel. The temperature measurement of a water sample with this thermometer was no different than the temperature measured using an alcohol-based thermometer. Microwave heating with air cooling was achieved using an air blower (air speed = 1000 m min−1) that was installed a distance of 5 cm from the sample insertion opening.
3.2. Synthesis of the Heptyl Butanoate Ester
Butanoic acid (Tokyo Chemical Industry Co., Ltd., Chuo-ku, Tokyo, Japan), 1-heptanol (Kanto Chemical Co. Inc., Tokyo, Japan), and the catalysts were added into a quartz tube reactor (outer diameter = 1.4 mm, height = 120.0 mm) that displayed very low microwave absorption; the reaction was performed at 130 °C. The reaction was carried out for 30 min from the time of immersion in the oil bath or the time when microwave irradiation was started. In preliminary experiments, the tip of the optical fiber thermometer was moved from the top to the bottom of the solution; the temperature difference was less than 1 °C. The heterogeneous catalyst was sulfonated activated carbon particulates (AC-SO3H; ZP150H, Futamura Chemical Co., Ltd., Nakamura-ku. Nagoya, Aichi, Japan; particle size ≈ 140 μm), while 2,4,6-trimethyl-pyridinium-p-toluene sulfonate (TMP-PTS; Fujifilm Wako Pure Chem. Co., Chuo-ku, Osaka, Japan) was the homogeneous phase catalyst. The initial concentrations of the reaction components and the catalysts in the synthesis of the ester are summarized in Table 2. In this experiment, dehydration with a Dean–Stark tube could not be performed because of the small amount of reaction solution. Therefore, it was necessary to use a water-absorbing material; however, general water-absorbing materials, such as molecular sieves and magnesium sulfate, were avoided as they could contaminate the reaction solution. Hence, a cotton swab (rolled cotton into a round shape) was packed at 100 mm from the bottom of the quartz tube reactor and used as a sink for the evaporated byproduct water to suppress/minimize the reverse reaction (Scheme 1). In a preliminary experiment, we confirmed that the cotton swab had sufficient water retention capacity. Conventional heating was achieved by immersing the reaction vessel into an oil bath that was preheated to 132.8 °C.

Table 2.
Experimental parameters used in the synthesis of the heptyl butanoate ester: type of catalyst, type of phase, and quantities of the substrates and catalyst used.
In the heterogeneous reaction with the AC-SO3H catalyst, filtration of the suspension was achieved using a syringe and a membrane filter (DISMIC 25HP045AN, Advantec Toyo Kaisha, Ltd., Chiyoda-ku, Tokyo, Japan). At the completion of the reaction, the product was extracted from the catalyst with 20 mL of diethyl ether and filtered, after which, the filtrate was concentrated by heating at 60 °C for 10 min. Then, after completion of the homogeneous reaction that was catalyzed by TMP-PTS, the ester was again extracted with 20 mL of diethyl ether and 5 mL of a saturated saline solution, subsequent to which, the organic layer was also concentrated by heating at 60 °C for 10 min. The recovered samples of the ester were then analyzed using GC/MS (GCMS-QP2010, Shimadzu Corporation, Nakagyo-ku, Kyoto, Japan) equipped with a capillary column (Rxi®-5Sil MS, Restek Corporation, Bellefonte, PA, USA). The temperature increase of the GC was programmed to start at 40 °C and end at 250 °C at a heating rate of 20 °C min−1.
The conversion yield Y of butanoic acid to the ester was calculated using Equation (2); the concentration of butanoic acid remaining after the reaction (Ct) was determined from a previously prepared calibration curve (R2 ≥ 0.99):
where C0 was the initial concentration of butanoic acid, while the product yield P was estimated using Equation (3) [25]:
where S is the selectivity of the formation of the heptyl butanoate ester, which was estimated as the magnitude of the peak area of the target ester with respect to the total peak area of all products, which was confirmed to be ≥ 99% complete in all experiments.
3.3. Dielectric Parameter Measurements
The relative dielectric constant (εr’) and the dielectric loss (εr”) of the heptyl butanoate ester and the reaction components were measured at frequencies between 300 MHz and 6.5 GHz using a network analyzer (E5071C ENA Series Network Analyzer, Agilent Technologies, Santa Clara, CA, USA) and a dielectric probe (High-Temperature Dielectric Probe Kit, Agilent Technologies, Santa Clara, CA, USA).
3.4. Moisture Content in Butanoic Acid Batches
The moisture content in the as-received batches of butanoic acid was determined using a Karl Fischer moisture meter (870 KF Titrino plus, Metrohm AG, Herisau, Switzerland for the first and second experimental runs prior to the vacuum distillation. For the third experimental run, the moisture content was determined after a fraction of the butanoic acid batch (from the second run) had been vacuum distilled. Measurements of the moisture content in 1 g of a butanoic acid batch were repeated three times and the results were averaged.
3.5. Computer Simulation of the Coupled Electromagnetic/Thermal Analysis
A coupled electromagnetic–thermal analysis of the esterification reaction was performed using Femtet software version 2019 (Murata Software Co., Ltd., Shibuya-ku, Tokyo, Japan). The reaction solution was contained in a cylindrical reactor (diameter = 14 mm, height of the solution or suspension = 30 mm). In the simulation, the reaction solution was set to contain 375 activated carbon particles with a particle size of 140 μm (Figure 8).
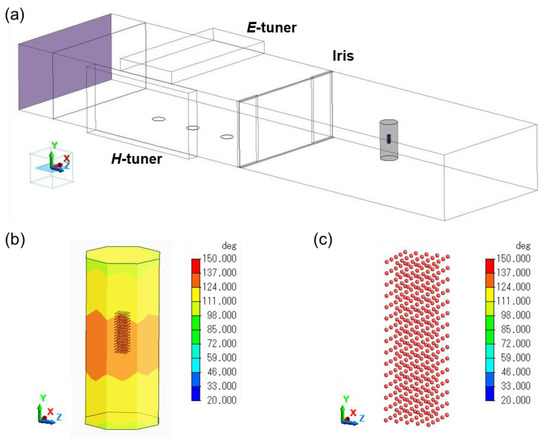
Figure 8.
Cartoon images of (a) the microwave single-mode cavity, (b) the reactor containing activated carbon, and (c) the activated carbon particulates in the reactor used in the simulation.
The relative dielectric constant [26], electrical conductivity [26], thermal conductivity [27], and specific heat [27] of the activated carbon used in the simulation were taken to be 36.26, 44.24 S m−1, 140 mW m−1 K−1, and 1.3 kJ kg−1 K−1, respectively. Except for the activated carbon particulates, actual measured values were used for the relative dielectric constant and loss tangent (tan δ) of the substrate layer that consisted mostly of heptanol (see Table 2; ε’ = 3.64 and tan δ = 0.273 at 302.4 K). In addition, the thermal conductivity (0.151 W m−1 K−1 at 298.06 K) [28] and the specific heat (270.8 J mol−1 K−1 at 298.15 K) [29] of heptanol were also used as additional parameters for the substrate layer. A coupled electromagnetic/thermal analysis was performed until the reaction solution temperature reached the maximal equilibrium (time step = 14.4 s, number of steps = 500). The microwave output power that was actually used was recorded during the synthesis experiment. In the case of air cooling, the air speed was adjusted such that the average temperature of the substrate layer was ≈130 °C, the temperature at which the simulation was performed.
4. Conclusions
This study proposed a novel way of thinking about microwave catalytic chemistry based on both experiments and simulations in order to comprehend the new phenomena exposed by the experimental results. The synthesis of the heptyl butanoate ester from butanoic acid and heptanol in the presence of the heterogeneous sulfonated activated carbon particulate AC-SO3H catalyst was described, with the reaction being driven either by microwave heating or by conventional heating with an oil bath. The moisture content in the butanoic acid batches proved to be an important factor that affected the product yields: the lower the moisture content, the greater the yield. In particular, when the moisture content was at its lowest (0.022%), there were no differences in product yields, independent of whether the reactants were heated by microwaves or conventionally by an oil bath. Specifically, the results may be summarized as follows, noting, however, that the internal temperature measured using an optical thermometer was ≈130 °C in all cases:
- At the moisture content of 0.022%, the product yields of the ester were essentially the same in the presence of the heterogeneous catalyst AC-SO3H, independent of whether heating was supplied by microwaves or conventionally with an oil bath.
- At the moisture contents of 0.052% and 0.058%, the product yields of the ester in the presence of the heterogeneous catalyst AC-SO3H were substantially greater when heating with microwaves than conventionally with an oil bath.
- At the moisture content of 0.052%, the product yields of the ester were substantively greater under microwave heating with air-cooling than with conventional heating with an oil bath in the presence of the heterogeneous catalyst AC-SO3H.
- Finally, in the presence of the TMP-PTS catalyst in a homogeneous phase, the product yields of the butanoate ester were essentially the same, independent of whether the moisture content was 0.022% or 0.058% and whether heating was supplied by microwaves or an oil bath, although the yields were nearly double when the moisture content was 0.022%.
Thus, the product yields were in general greater when using microwaves because the solid catalyst was selectively heated, which removed any of the adsorbed byproduct water (and pre-existing moisture), thereby minimizing or preventing the reverse reaction from occurring, unlike conventional heating, which failed to remove the byproduct water that led to the hydrolysis of the ester produced (de-esterification). It is important to be aware that extraneous components in a reaction system can influence the conversion efficiencies when carrying out reactions with microwaves. Nonetheless, in the present instance, the use of microwaves was proven to be beneficial to esterification reactions, especially when the reactants contained higher moisture content; a clear advantage of microwaves over conventional heating and, consequently, a clear benefit for the chemical industry was established. For example, for the synthesis of fatty acid methyl esters (FAMEs) by esterification of free fatty acids (FFA) in waste cooking oils (wco) that contain a large amount of water, the use of microwaves would have clear industrial advantages in that the microwaves would prevent the reverse of the reaction shown in Scheme 1 from taking place.
Disappointingly, because of the several factors that impinged on the product yields, the nonthermal microwave effect remains an elusive concept, as unexpected impurities and local high-temperature fields generated on the surfaces of the small fine particles may have had a significant effect on the microwave chemistry such that the resultant phenomena could not be unequivocally attributed to a nonthermal effect of the microwaves. Accordingly, it is highly recommended that impurities and selective heating always be taken into consideration when attempting to interpret results in terms of the nonthermal effect of microwaves.
Author Contributions
Conceptualization, methodology, and validation: S.H.; project supervision and administration: S.H.; investigation and experiments: D.S.; writing the first draft: D.S. and S.H.; writing, reviewing, and editing multiple subsequent drafts, including the final draft and proofs: N.S. and S.H.; funding acquisition: S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Japan Society for the Promotion of Science, grant number C-25420820.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to the Japan Society for the Promotion of Science (JSPS) for financial support to S.H. through a Grant-in-aid for Scientific Research (no. C-25420820). We also wish to thank the Toyo Gosei Kogyo Co., Ltd. (Japan) for their technical support and research foundation. One of us (N.S.) thanks the staff of the PhotoGreen Laboratory of the Dipartimento di Chimica, Università degli Studi di Pavia, Italy, for their continued hospitality.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Giguère, R.J.; Bray, T.L.; Duncan, S.M.; Majetich, G. Application of commercial microwave ovens to organic synthesis. Tetrahedron Lett. 1986, 27, 4945–4948. [Google Scholar] [CrossRef]
- Galema, S.A. Microwave Chemistry. Chem. Soc. Rev. 1997, 26, 233–238. [Google Scholar] [CrossRef]
- Gawande, M.B.; Shelke, S.N.; Zboril, R.; Varma, R.S. Microwave-assisted chemistry: Synthetic applications for rapid assembly of nanomaterials and organics. Acc. Chem. Res. 2014, 47, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Ortiz, Á.; Prieto, P.; de la Hoz, A. A critical overview on the effect of microwave irradiation in organic synthesis. Chem. Rec. 2018, 18, 1–14. [Google Scholar] [CrossRef]
- Nain, S.; Singh, R.; Ravichandran, S. Importance of Microwave Heating in Organic Synthesis. Adv. J. Chem. A 2019, 2, 94–104. [Google Scholar] [CrossRef]
- De la Hoz, A.; Loupy, A. (Eds.) Microwaves in Organic Synthesis, 3rd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012. [Google Scholar]
- Horikoshi, S.; Serpone, N. (Eds.) Microwaves in Catalysis: Methodology and Applications; Wiley-VCH Verlag GmbH & Co. KgaH: Weinheim, Germany, 2016. [Google Scholar]
- Cravotto, G.-C.; Carnaroglio, D. (Eds.) Microwave Chemistry; Walter De Gruyter GmbH: Berlin, Germany, 2017. [Google Scholar]
- Horikoshi, S.; Schiffmann, R.F.; Fukushima, J.; Serpone, N. Microwave Chemical and Materials Processing—A Tutorial; Springer Nature, Singapore Pte Ltd.: Singapore, 2018. [Google Scholar]
- Banik, B.K.; Bandyopadhyay, D. (Eds.) Advances in Microwave Chemistry; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Rosana, M.R.; Tao, Y.; Stiegman, A.E.; Dudley, G.B. On the rational design of microwave-actuated organic reactions. Chem. Sci. 2012, 3, 1240–1244. [Google Scholar] [CrossRef]
- Kappe, C.O.; Pieber, B.; Dallinger, D. Microwave effects in organic synthesis: Myth or reality? Angew. Chem. Int. Ed. 2013, 52, 1088–1094. [Google Scholar] [CrossRef]
- Michael, R.; Rosana, J.H.; Ferrari, A.; Southworth, T.A.; Tao, Y.; Stiegman, A.E.; Dudley, G.B. Microwave-specific acceleration of a Friedel–Crafts reaction: Evidence for selective heating in homogeneous solution. J. Org. Chem. 2014, 79, 7437–7450. [Google Scholar]
- Horikoshi, S.; Watanabe, T.; Narita, A.; Suzuki, Y.; Serpone, N. The electromagnetic wave energy effect (s) in microwave–assisted organic syntheses (MAOS). Sci. Rep. 2018, 8, 5151. [Google Scholar] [CrossRef]
- Rajabi, F.; Abdollahi, M.; Luque, R. Solvent-free esterification of carboxylic acids using supported iron oxide nanoparticles as an efficient and recoverable catalyst. Materials 2016, 9, 557. [Google Scholar] [CrossRef]
- Dange, P.N.; Rathod, V.K. Equilibrium and thermodynamic parameters for heterogeneous esterification of butyric acid with methanol under microwave irradiation. Resour. Effic. Technol. 2017, 3, 64–70. [Google Scholar] [CrossRef]
- Yadav, G.D.; Mehta, P.H. Heterogeneous catalysis in esterification reactions: Preparation of phenethyl acetate and cyclohexyl acetate by using a variety of solid acidic catalysts. Ind. Eng. Chem. Res. 1994, 33, 2198–2208. [Google Scholar] [CrossRef]
- Ali, S.H.; Tarakmah, A.; Merchant, S.Q.; Al-Sahhaf, T. Synthesis of esters: Development of the rate expression for the Dowex 50 Wx8-400 catalyzed esterification of propionic acid with 1-propanol. Chem. Eng. Sci. 2007, 62, 3197–3217. [Google Scholar] [CrossRef]
- Torres, S.; Baigorí, M.D.; Swathy, S.L.; Pandey, A.; Castro, G.R. Enzymatic synthesis of banana flavour (isoamyl acetate) by Bacillus licheniformis S-86 esterase. Food Res. Int. 2009, 42, 454–460. [Google Scholar] [CrossRef]
- Lokman, I.M.; Rashid, U.; Taufiq-Yap, Y.H. Microwave-Assisted Methyl Ester Production from Palm Fatty Acid Distillate over a Heterogeneous Carbon-Based Solid Acid Catalyst. Chem. Eng. Technol. 2015, 38, 1837–1844. [Google Scholar] [CrossRef]
- Tumkot, L.; Quitain, A.T.; Boonnoun, P.; Laosiripojana, N.; Kida, T.; Shotipruk, A. Synergizing Sulfonated Hydrothermal Carbon and Microwave Irradiation for Intensified Esterification Reaction. ACS Omega 2020, 5, 23542–23548. [Google Scholar] [CrossRef]
- Horikoshi, S.; Osawa, A.; Suttisawat, Y.; Abe, M.; Serpone, N. A novel Dewar-like reactor for maintaining constant heat and enhancing product yields during microwave-assisted organic syntheses. Org. Process Res. Develop. 2010, 14, 1453–1456. [Google Scholar] [CrossRef]
- Suttisawat, Y.; Horikoshi, S.; Sakai, H.; Abe, M. Hydrogen production from tetralin over microwave-accelerated Pt-supported activated carbon. Int. J. Hydrogen Energy 2010, 35, 6179–6183. [Google Scholar] [CrossRef]
- Horikoshi, S.; Matsubara, A.; Takayama, S.; Sato, M.; Sakai, F.; Kajitani, M.; Abe, M.; Serpone, N. Characterization of microwave effects on metal-oxide materials: Zinc oxide and titanium dioxide. Appl. Catal. B Environ. 2009, 91, 362–367. [Google Scholar] [CrossRef]
- Shi, H.; Zhu, W.; Li, H.; Liu, H.; Zhang, M.; Yan, Y.; Wang, Z. Microwave-accelerated esterification of salicylic acid using Brönsted acidic ionic liquids as catalysts. Catal. Commun. 2010, 11, 588–591. [Google Scholar] [CrossRef]
- Horikoshi, S.; Osawa, A.; Sakamoto, S.; Serpone, N. Control of microwave-generated hot spots. Part V. Mechanisms of hot-spot generation and aggregation of catalyst in a microwave-assisted reaction in toluene catalyzed by Pd-loaded AC particulates. Appl. Catal. A General 2013, 460–461, 52–60. [Google Scholar] [CrossRef]
- Horikoshi, S.; Kamata, M.; Sumi, T.; Serpone, N. Selective heating of Pd/AC catalyst in heterogeneous systems for the microwave-assisted continuous hydrogen evolution from organic hydrides: Temperature distribution in the fixed-bed reactor. Int. J. Hydrogen Energy 2016, 41, 12029–12037. [Google Scholar] [CrossRef]
- Ogiwara, K.; Arai, Y.; Saito, S. Thermal conductivities of liquid alcohols and their binary mixtures. J. Chem. Eng. Jpn. 1982, 15, 335–342. [Google Scholar] [CrossRef]
- Vesely, F.; Barcal, P.; Zabransky, M.; Svoboda, V. Heat capacities of 4-methyl-2-pentanone, 2,6-dimethyl-4-heptanone, 1-hexanol, 1-heptanol, and 1-octanol in the temperature range 298–318 K. Collect. Czech. Chem. Commun. 1989, 54, 602–607. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).