A Novel Route of Mixed Catalysis for Production of Fatty Acid Methyl Esters from Potential Seed Oil Sources
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Catalyst on Yield of Biodiesel
2.2. Physiochemical Properties of Biodiesel
2.3. Fatty Acid Profile of Biodiesel
3. Materials and Methods
3.1. Material
3.2. Transesterification
3.3. Immobilization of Lipase
3.4. Determination of Physiochemical Characteristics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Amani, M.A.; Davoudi, M.S.; Tahvildari, K.; Nabavi, S.M.; Davoudi, M.S. Biodiesel production from Phoenix dactylifera as a new feedstock. Ind. Crop. Prod. 2013, 43, 40–43. [Google Scholar] [CrossRef]
- Hoang, A.T.; Tabatabaei, M.; Aghbashlo, M.; Carlucci, A.P.; Ölçer, A.I.; Le, A.T.; Ghassemi, A. Rice bran oil-based biodiesel as a promising renewable fuel alternative to petrodiesel: A review. Renew. Sustain. Energy Rev. 2021, 135, 110204. [Google Scholar] [CrossRef]
- Mumtaz, M.W.; Adnan, A.; Anwar, F.; Mukhtar, H.; Raza, M.A.; Ahmad, F.; Rashid, U. Response surface methodology: An emphatic tool for optimized biodiesel production using rice bran and sunflower oils. Energies 2012, 5, 3307–3328. [Google Scholar] [CrossRef] [Green Version]
- Konur, O. Biodiesel and Petrodiesel Fuels: Science, Technology, Health, and the Environment, Biodiesel Fuels; CRC Press: Boca Raton, FL, USA, 2021; pp. 3–36. [Google Scholar]
- Demirbas, A. Progress and recent trends in biodiesel fuels. Energy Convers. Manag. 2009, 50, 14–34. [Google Scholar] [CrossRef]
- Abomohra, A.E.-F.; Zheng, X.; Wang, Q.; Huang, J.; Ebaid, R. Enhancement of biodiesel yield and characteristics through in-situ solvo-thermal cotransesterification of wet microalgae with spent coffee grounds. Bioresour. Technol. 2021, 323, 124640. [Google Scholar] [CrossRef]
- Khan, F.M. Ethno-veterinary medicinal usage of flora of greater Cholistan desert (Pakistan). Pak. Vet. J. 2009, 29, 75–80. [Google Scholar]
- Inam, S.; Khan, S.; Nadeem, F. Impacts of derivatization on physiochemical fuel quality parameters of fatty acid methyl esters (FAME)-a comprehensive review. Int. J. Chem. Biochem. Sci. 2019, 15, 42–49. [Google Scholar]
- Iqra, Y.; Umer, R.; Farwa, N. Alumina supported catalytic materials for biodiesel production-a detailed review. Int. J. Chem. Biochem. Sci. 2019, 16, 41–53. [Google Scholar]
- Kalsoom, M.; El Zerey-Belaskri, A.; Nadeem, F.; Shehzad, M.R. Fatty acid chain length optimization for biodiesel production using different chemical and biochemical approaches–a comprehensive. Int. J. Chem. Biochem. Sci. 2017, 11, 75–94. [Google Scholar]
- Mehboob, A.; Nisar, S.; Rashid, U.; Choong, T.S.Y.; Khalid, T.; Qadeer, H.A. Reactor designs for the production of biodiesel. Int. J. Chem. Biochem. Sci. 2016, 10, 87–94. [Google Scholar]
- Nadeem, F.; Shahzadi, A.; El Zerey-Belaskri, A.; Abbas, Z. Conventional and advanced purification techniques for crude biodiesel—A critical review. Int. J. Chem. Biochem. Sci. 2017, 12, 113–121. [Google Scholar]
- Shahzadi, A.; Grondahl, L.; Nadeem, F. Development of effective composite supports for production of biodiesela detailed review. Int. J. Chem. Biochem. Sci. 2019, 16, 76–86. [Google Scholar]
- Waseem, H.H.; El Zerey-Belaskri, A.; Nadeem, F.; Yaqoob, I. The downside of biodiesel fuel—A review. Int. J. Chem. Biochem. Sci. 2016, 9, 97–106. [Google Scholar]
- Peterson, C.; Taberski, J.; Thompson, J.; Chase, C. The effect of biodiesel feedstock on regulated emissions in chassis dynamometer tests of a pickup truck. Trans. ASAE 2000, 43, 1371–1381. [Google Scholar] [CrossRef] [Green Version]
- Pedras, M.C.; Zaharia, I.L. Sinalbins A and B, phytoalexins from Sinapis alba: Elicitation, isolation, and synthesis. Phytochemistry 2000, 55, 213–216. [Google Scholar] [CrossRef]
- Christov, M. Contribution of interspecific hybridization to sunflower breeding. Helia 2012, 35, 37–46. [Google Scholar] [CrossRef]
- Zabaniotou, A.; Kantarelis, E.; Theodoropoulos, D. Sunflower shells utilization for energetic purposes in an integrated approach of energy crops: Laboratory study pyrolysis and kinetics. Bioresour. Technol. 2008, 99, 3174–3181. [Google Scholar] [CrossRef]
- Slama, A.; Cherif, A.; Sakouhi, F.; Boukhchina, S.; Radhouane, L. Fatty acids, phytochemical composition and antioxidant potential of pearl millet oil. J. Consum. Prot. Food Saf. 2020, 15, 145–151. [Google Scholar] [CrossRef]
- Azeem, M.W.; Hanif, M.A.; Al-Sabahi, J.N.; Khan, A.A.; Naz, S.; Ijaz, A. Production of biodiesel from low priced, renewable and abundant date seed oil. Renew. Energy 2016, 86, 124–132. [Google Scholar] [CrossRef]
- Bhatti, H.; Hanif, M.; Faruq, U.; Sheikh, M. Acid and base catalyzed transesterification of animal fats to biodiesel. Iran J. Chem. Chem. Eng. 2008, 27, 41–48. [Google Scholar]
- Atadashi, I.; Aroua, M.K.; Aziz, A.A.; Sulaiman, N. Production of biodiesel using high free fatty acid feedstocks. Renew. Sustain. Energy Rev. 2012, 16, 3275–3285. [Google Scholar] [CrossRef]
- Akhtar, N.; Adnan, Q.; Ahmad, M.; Mehmood, A.; Farzana, K. Rheological studies and characterization of different oils. J. Chem. Soc. Pak. 2009, 31, 201–206. [Google Scholar]
- Eevera, T.; Rajendran, K.; Saradha, S. Biodiesel production process optimization and characterization to assess the suitability of the product for varied environmental conditions. Renew. Energy 2009, 34, 762–765. [Google Scholar] [CrossRef]
- Srivastava, A.; Prasad, R. Triglycerides-based diesel fuels. Renew. Sustain. Energy Rev. 2000, 4, 111–133. [Google Scholar] [CrossRef]
- Sanders, T.H. Ground nut oil. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 2967–2974. [Google Scholar]
- Fernando, S.; Karra, P.; Hernandez, R.; Jha, S.K. Effect of incompletely converted soybean oil on bio-diesel quality. Energy 2007, 32, 844–851. [Google Scholar]
- Saloua, F.; Saber, C.; Hedi, Z. Methyl ester of [Maclura pomifera (Rafin.) Schneider] seed oil: Biodiesel production and characterization. Bioresour. Technol. 2010, 101, 3091–3096. [Google Scholar] [CrossRef] [PubMed]
- Gerpen, V.J. Biodiesel processing and production. Fuel Process. Technol. 2005, 86, 1097–1107. [Google Scholar] [CrossRef]
- Canakci, M.; van Gerpen, J. Biodiesel production from oils and fats with high free fatty acids. Trans. ASAE 2001, 44, 1429–1436. [Google Scholar] [CrossRef]
- Goering, C.E.; Schwab, A.W.; Daugherty, M.J.; Pryde, E.H.; Heakin, A.J. Fuel Properties of Eleven Vegetable Oils. Trans. ASAE 1982, 25, 1472–1477. [Google Scholar] [CrossRef]
- Teo, C.L.; Jamaluddin, H.; Zain, N.A.M.; Idris, A. Biodiesel production via lipase catalysed transesterification of microalgae lipids from Tetraselmis sp. Renew. Energy 2014, 68, 1–5. [Google Scholar] [CrossRef]
- Atabani, A.; Silitonga, A.; Badruddin, I.A.; Mahlia, T.M.I.; Masjuki, H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- Nielsen, S.S. Food Analysis; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Ahmed, S.; Hassan, M.H.; Kalam, A.; Rahman, S.A.; Abedin, J.; Shahir, A. An experimental investigation of biodiesel production, characterization, engine performance, emission and noise of Brassica juncea methyl ester and its blends. J. Clean. Prod. 2014, 79, 74–81. [Google Scholar] [CrossRef]
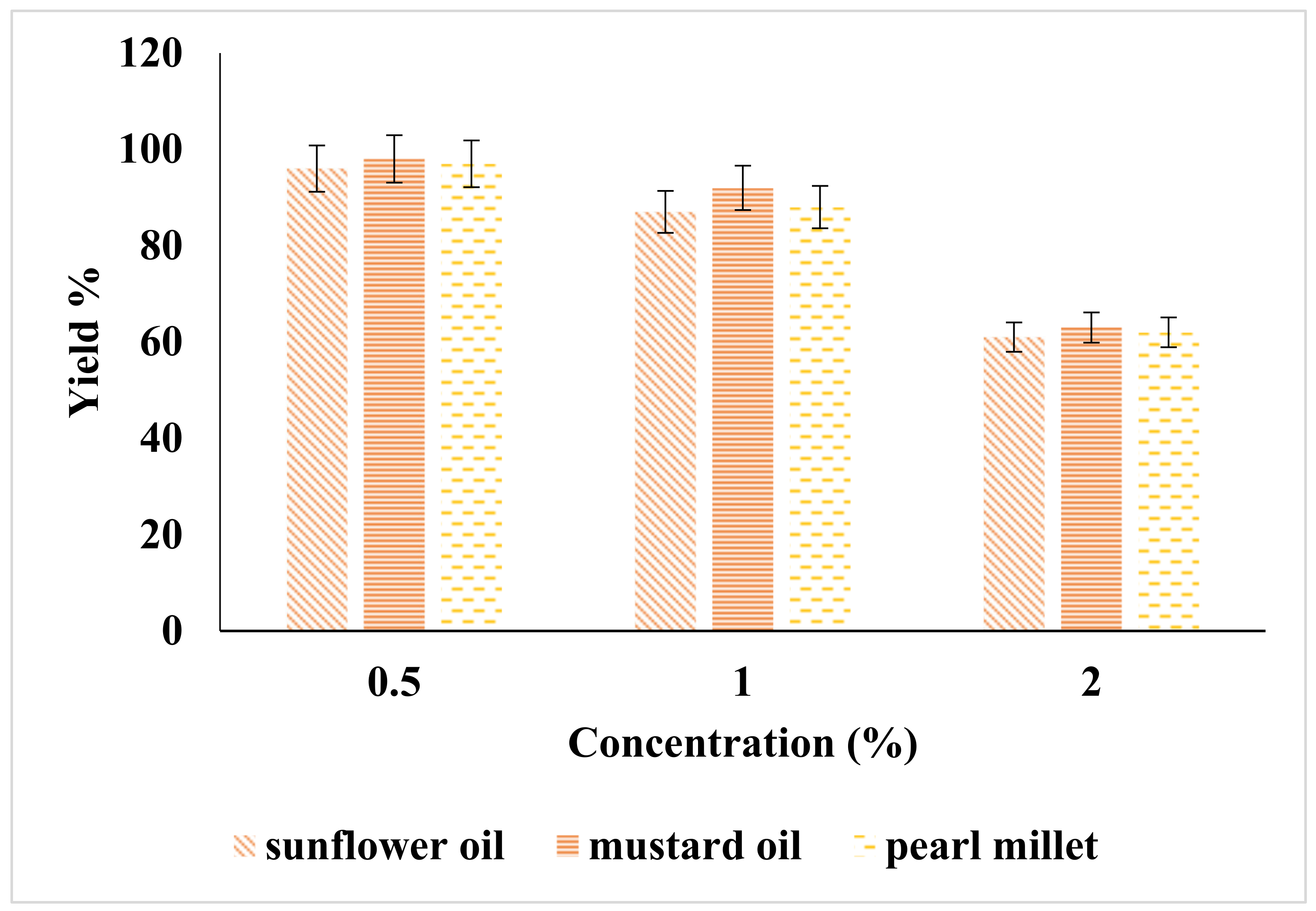
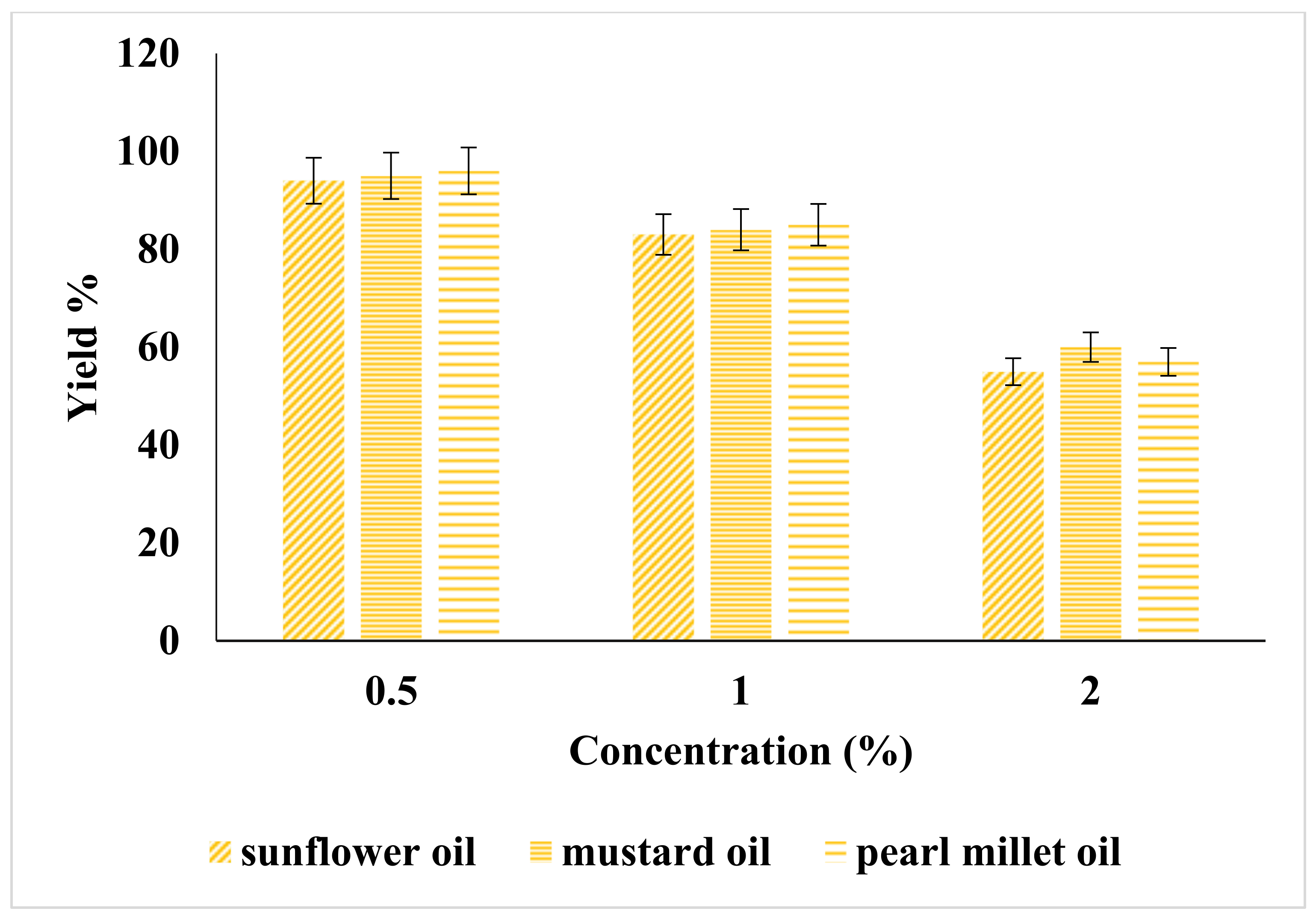
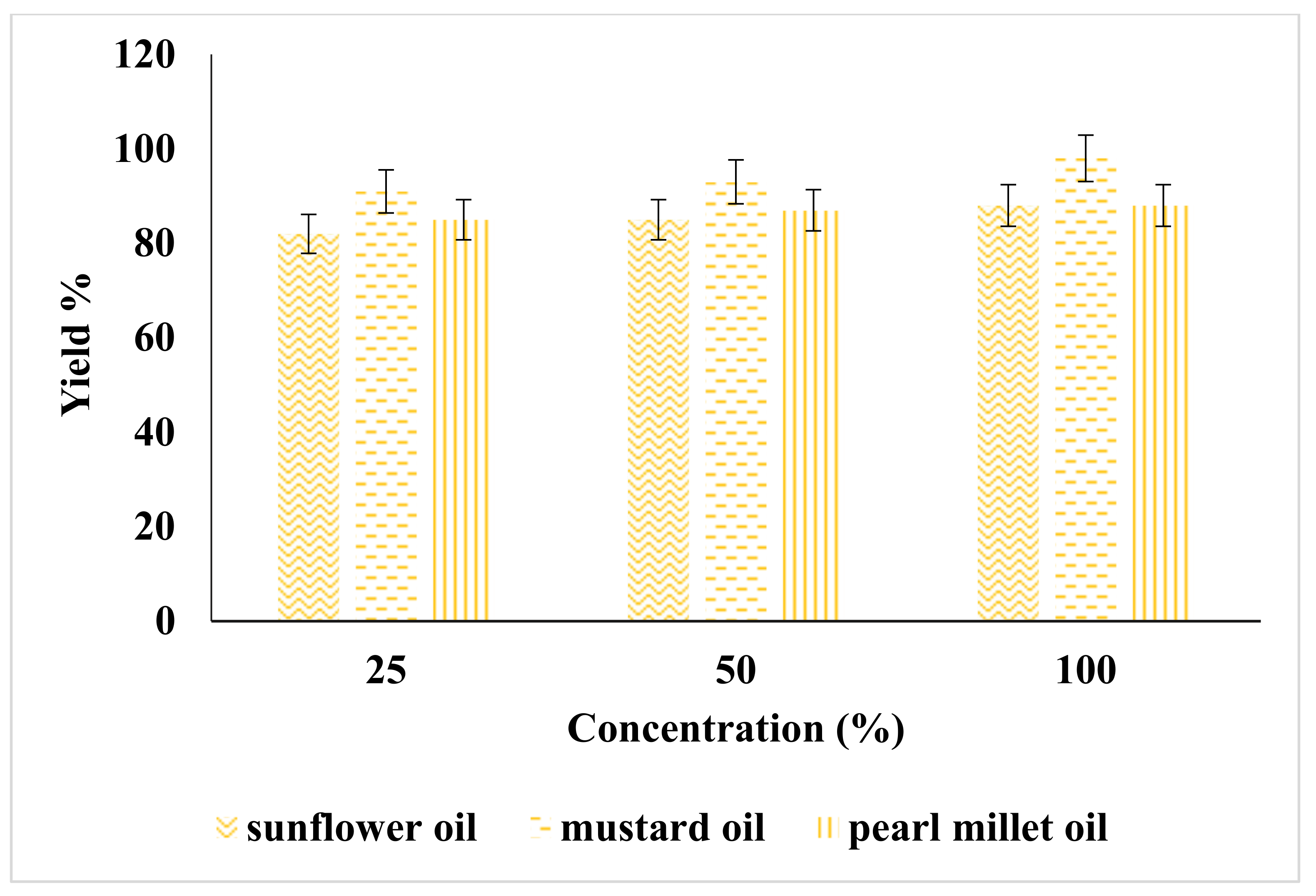

| Catalyst | Catalyst Conc. (%) | Sunflower | Mustard | Pearl Millet | American Standard ASTM | European Standards EN |
|---|---|---|---|---|---|---|
| KOH | 0.5 | 0.85 ± 0.003 | 0.87 ± 0.005 | 0.82 ± 0.004 | Not specified | |
| 1 | 0.83 ± 0.004 | 0.85 ± 0.006 | 0.81 ± 0.003 | 0.86–0.90 | ||
| 2 | 0.81 ± 0.005 | 0.84 ± 0.004 | 0.80 ± 0.005 | |||
| NaOH | 0.5 | 0.85 ± 0.003 | 0.85 ± 0.005 | 0.83 ± 0.005 | ||
| 0.1 | 0.83 ± 0.004 | 0.83 ± 0.003 | 0.81 ± 0.003 | |||
| 2 | 0.82 ± 0.006 | 0.83 ± 0.004 | 0.81 ± 0.006 | |||
| HCl | 25 | 0.81 ± 0.002 | 0.85 ± 0.005 | 0.86 ± 0.004 | ||
| 50 | 0.81 ± 0.007 | 0.86 ± 0.002 | 0.87 ± 0.003 | |||
| 100 | 0.83 ± 0.006 | 0.88 ± 0.006 | 0.89 ± 0.004 | |||
| Immobilized lipase | 3 | 0.81 ± 0.004 | 0.84 ± 0.007 | 0.85 ± 0.007 | ||
| 4 | 0.82 ± 0.003 | 0.85 ± 0.003 | 0.86 ± 0.003 | |||
| 5 | 0.81 ± 0.004 | 0.82 ± 0.004 | 0.84 ± 0.004 |
| Catalyst | Catalyst Conc. (%) | Sunflower | Mustard | Pearl Millet | American Standard ASTM | European Standards EN |
|---|---|---|---|---|---|---|
| KOH | 0.5 | 175 ± 0.5 | 187 ± 0.4 | 190 ± 0.6 | Not specified | Not specified |
| 1 | 177 ± 0.5 | 189 ± 0.5 | 192 ± 0.6 | |||
| 2 | 179 ± 0.6 | 191 ± 0.4 | 193 ± 0.6 | |||
| NaOH | 0.5 | 174 ± 0.6 | 186 ± 0.4 | 190 ± 0.6 | ||
| 0.1 | 176 ± 0.5 | 189 ± 0.4 | 192 ± 0.6 | |||
| 2 | 177 ± 0.6 | 190 ± 0.5 | 193 ± 0.6 | |||
| HCl | 25 | 177 ± 0.5 | 182 ± 0.4 | 188 ± 0.6 | ||
| 50 | 175 ± 0.5 | 178 ± 0.4 | 187 ± 0.6 | |||
| 100 | 174 ± 0.5 | 177 ± 0.4 | 186 ± 0.6 | |||
| Immobilized lipase | 3 | 176 ± 0.6 | 181 ± 0.4 | 186 ± 0.6 | ||
| 4 | 174 ± 0.5 | 178 ± 0.5 | 184 ± 0.6 | |||
| 5 | 175 ± 0.5 | 180 ± 0.4 | 185 ± 0.6 |
| Catalyst | Catalyst Conc. (%) | Sunflower | Mustard | Pearl Millet | American Standard ASTM | European Standards EN |
|---|---|---|---|---|---|---|
| KOH | 0.5 | 134 ± 0.2 | 106 ± 0.2 | 129 ± 0.3 | Not specified | |
| 1 | 132 ± 0.2 | 105 ± 0.2 | 127 ± 0.3 | ≤120 | ||
| 2 | 131 ± 0.2 | 103 ± 0.2 | 126 ± 0.3 | |||
| NaOH | 0.5 | 133 ± 0.3 | 107 ± 0.2 | 128 ± 0.3 | ||
| 0.1 | 132 ± 0.2 | 105 ± 0.2 | 126 ± 0.3 | |||
| 2 | 130 ± 0.3 | 102 ± 0.3 | 124 ± 0.3 | |||
| HCl | 25 | 134 ± 0.2 | 105 ± 0.2 | 127 ± 0.3 | ||
| 50 | 135 ± 0.2 | 107 ± 0.2 | 128 ± 0.3 | |||
| 100 | 139 ± 0.2 | 108 ± 0.2 | 129 ± 0.3 | |||
| Immobilized lipase | 3 | 134 ± 0.2 | 106 ± 0.3 | 126 ± 0.3 | ||
| 4 | 136 ± 0.2 | 108 ± 0.2 | 128 ± 0.2 | |||
| 5 | 132 ± 0.2 | 105 ± 0.2 | 125 ± 0.5 |
| Catalyst | Catalyst Conc. % | Sunflower | Mustard | Pearl Millet | Biodiesel Standards | ||||
|---|---|---|---|---|---|---|---|---|---|
| Cloud Point (°C) | Pour Point (°C) | Cloud Point (°C) | Pour Point (°C) | Cloud Point (°C) | Pour Point (°C) | Cloud Point | Pour Point | ||
| KOH | 0.5 | 2.4 ± 0.093 | −7.8 ± 0.086 | 4.7 ± 0.084 | −4.3 ± 0.090 | 3.9 ± 0.084 | −4.8 ± 0.088 | Not specific depends on climatic condition | Not specific depends on climatic condition |
| 1 | 2.5 ± 0.089 | −7.7 ± 0.082 | 4.8 ± 0.082 | −4.1 ± 0.082 | 4.4 ± 0.079 | −5.1 ± 0.082 | |||
| 2 | 2.7 ± 0.080 | −7.5 ± 0.090 | 5.0 ± 0.093 | −3.8 ± 0.093 | 4.2 ± 0.076 | −5.0 ± 0.093 | |||
| NaOH | 0.5 | 2.2 ± 0.080 | −8.0 ± 0.090 | 4.8 ± 0.089 | −4.3 ± 0.090 | 4.0 ± 0.069 | −4.7 ± 0.090 | ||
| 1 | 2.4 ± 0.079 | −7.9 ± 0.086 | 4.9 ± 0.060 | −4.2 ± 0.060 | 4.3 ± 0.066 | −4.6 ± 0.060 | |||
| 2 | 2.6 ± 0.083 | −7.6 ± 0.087 | 5.1 ± 0.080 | −3.9 ± 0.089 | 4.3 ± 0.082 | −4.9 ± 0.089 | |||
| HCl | 25 | 2.4 ± 0.093 | −7.7 ± 0.071 | 5.0 ± 0.079 | −4.3 ± 0.087 | 4.4 ± 0.087 | −4.8 ± 0.077 | ||
| 50 | 2.5 ± 0.079 | −7.9 ± 0.83 | 5.0 ± 0.83 | −4.2 ± 0.83 | 4.4 ± 0.076 | −5.2 ± 0.83 | |||
| 100 | 2.4 ± 0.082 | −8.1 ± 0.093 | 4.8 ± 0.093 | −4.1 ± 0.093 | 4.3 ± 0.085 | −5.1 ± 0.093 | |||
| Immobilized Lipase | 3 | 2.5 ± 0.081 | −7.8 ± 0.079 | 5.0 ± 0.079 | −4.0 ± 0.079 | 4.5 ± 0.070 | −5.6 ± 0.079 | ||
| 4 | 2.3 ± 0.085 | −8.0 ± 0.082 | 4.8 ± 0.084 | −4.1 ± 0.087 | 4.3 ± 0.082 | −6.1 ± 0.074 | |||
| 5 | 2.3 ± 0.082 | −7.9 ± 0.085 | 4.9 ± 0.088 | −3.7 ± 0.087 | 4.4 ± 0.079 | −6.0 ± 0.085 | |||
| Catalyst | Catalyst Conc. (%) | Sunflower | Mustard | Pearl Millet | American Standard ASTM | European Standards EN |
|---|---|---|---|---|---|---|
| KOH | 0.5 | 47.4 | 51.6 | 46.0 | 47 | 51 |
| 1 | 47.4 | 51.5 | 46.2 | |||
| 2 | 47.3 | 52.5 | 46.2 | |||
| NaOH | 0.5 | 47.7 | 51.6 | 46.2 | ||
| 0.1 | 47.6 | 51.5 | 46.4 | |||
| 2 | 47.9 | 52.1 | 46.7 | |||
| HCl | 25 | 47.0 | 52.7 | 46.8 | ||
| 50 | 47.1 | 52.9 | 46.7 | |||
| 100 | 46.8 | 52.8 | 46.6 | |||
| Immobilized lipase | 3 | 47.2 | 52.6 | 47.3 | ||
| 4 | 47.1 | 52.7 | 47.2 | |||
| 5 | 47.8 | 53.0 | 47.7 |
| Peak No. | Fatty Acid | Relative Contents % | ||
|---|---|---|---|---|
| Sunflower | Mustard | Pearl Millet | ||
| 2 | Palmitoleic acid (C16:1) | - | 0.2 | 0.1 |
| 1 | Palmitic acid (C16:0) | 3.5 | 7.9 | 21.9 |
| 6 | Linolenic acid (C18:3) | 49.2 | - | - |
| 5 | Linoleic acid (C18:2) | - | - | 73.9 |
| 4 | Oleic acid (C18:1) | - | 82.2 | - |
| 3 | Stearic acid (C18:0) | 1.6 | 3.6 | 3.4 |
| 8 | Gondoic acid (C20:1) | 15.3 | 1.5 | 0.3 |
| 7 | Arachidic acid (C20:0) | 0.6 | 0.4 | 0.3 |
| 9 | Erucic acid (C22:1) | 27.7 | 4.1 | - |
| 11 | Nervonic acid (C24:1) | 1.8 | - | - |
| 10 | Lignoceric acid (C24:0) | 0.2 | - | 0.1 |
| Total | 99.99 | 99.99 | 100.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perveen, S.; Hanif, M.A.; Nadeem, R.; Rashid, U.; Azeem, M.W.; Zubair, M.; Nisar, N.; Alharthi, F.A.; Moser, B.R. A Novel Route of Mixed Catalysis for Production of Fatty Acid Methyl Esters from Potential Seed Oil Sources. Catalysts 2021, 11, 811. https://doi.org/10.3390/catal11070811
Perveen S, Hanif MA, Nadeem R, Rashid U, Azeem MW, Zubair M, Nisar N, Alharthi FA, Moser BR. A Novel Route of Mixed Catalysis for Production of Fatty Acid Methyl Esters from Potential Seed Oil Sources. Catalysts. 2021; 11(7):811. https://doi.org/10.3390/catal11070811
Chicago/Turabian StylePerveen, Shazia, Muhammad Asif Hanif, Razyia Nadeem, Umer Rashid, Muhammad Waqar Azeem, Muhammad Zubair, Numrah Nisar, Fahad A. Alharthi, and Bryan R. Moser. 2021. "A Novel Route of Mixed Catalysis for Production of Fatty Acid Methyl Esters from Potential Seed Oil Sources" Catalysts 11, no. 7: 811. https://doi.org/10.3390/catal11070811
APA StylePerveen, S., Hanif, M. A., Nadeem, R., Rashid, U., Azeem, M. W., Zubair, M., Nisar, N., Alharthi, F. A., & Moser, B. R. (2021). A Novel Route of Mixed Catalysis for Production of Fatty Acid Methyl Esters from Potential Seed Oil Sources. Catalysts, 11(7), 811. https://doi.org/10.3390/catal11070811










