Characterization of an Immobilized Amino Acid Racemase for Potential Application in Enantioselective Chromatographic Resolution Processes
Abstract
1. Introduction
2. Theory
2.1. Kinetics of Racemization
2.2. Mass Balance and Productivity of the Enzymatic Fixed Bed Reactor
3. Results
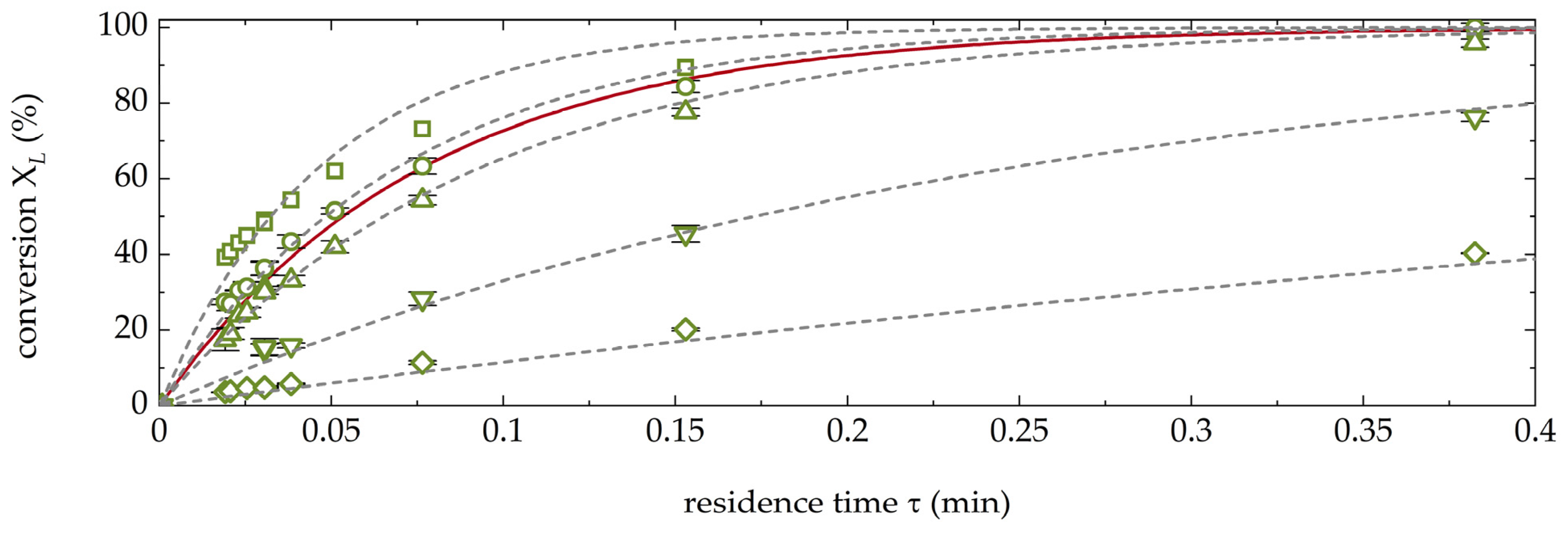
3.1. Kinetics of the AAR
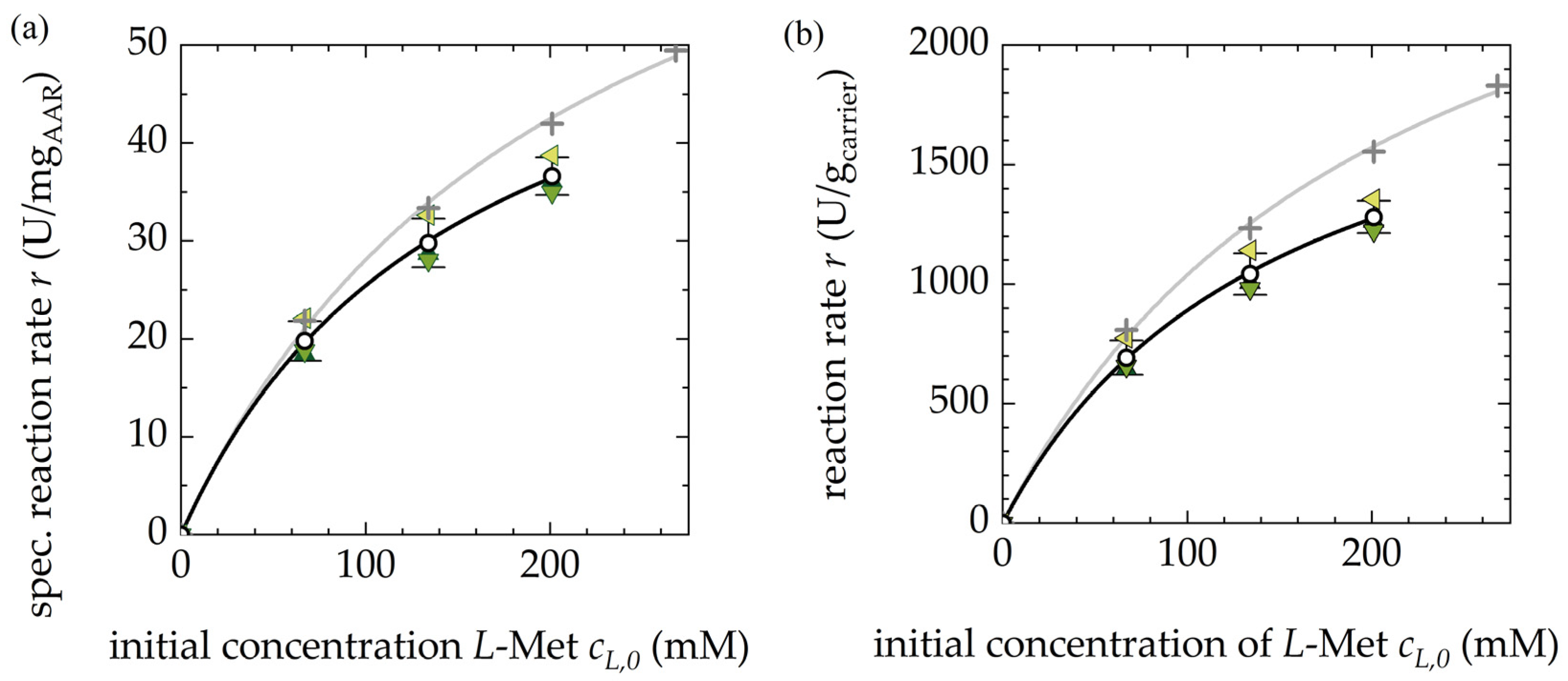
3.1.1. Activity of the Free and Immobilized AAR
3.1.2. Reproducibility of Different Fermentation Batches of AAR
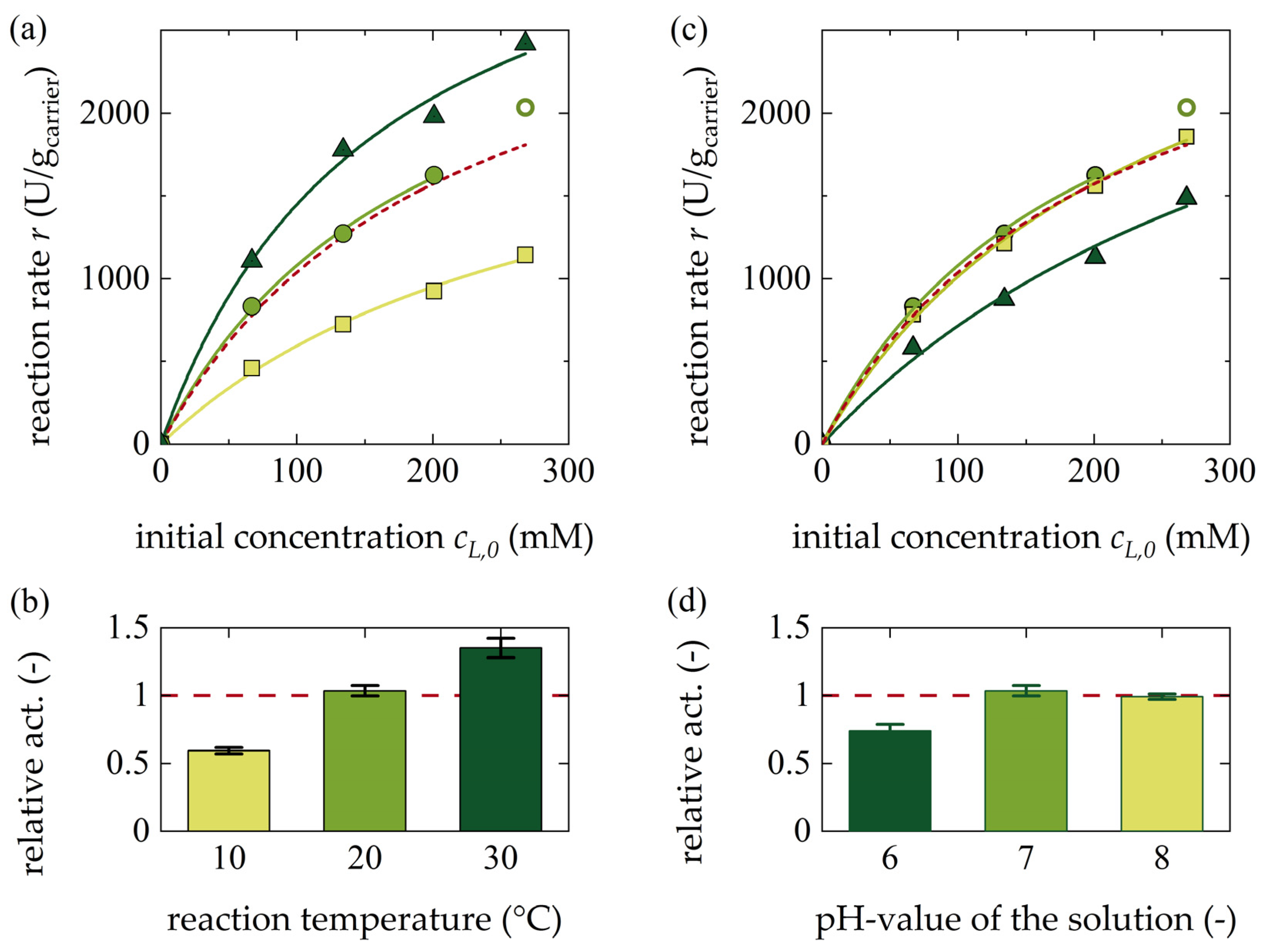
3.1.3. Influence of Temperature and pH Value
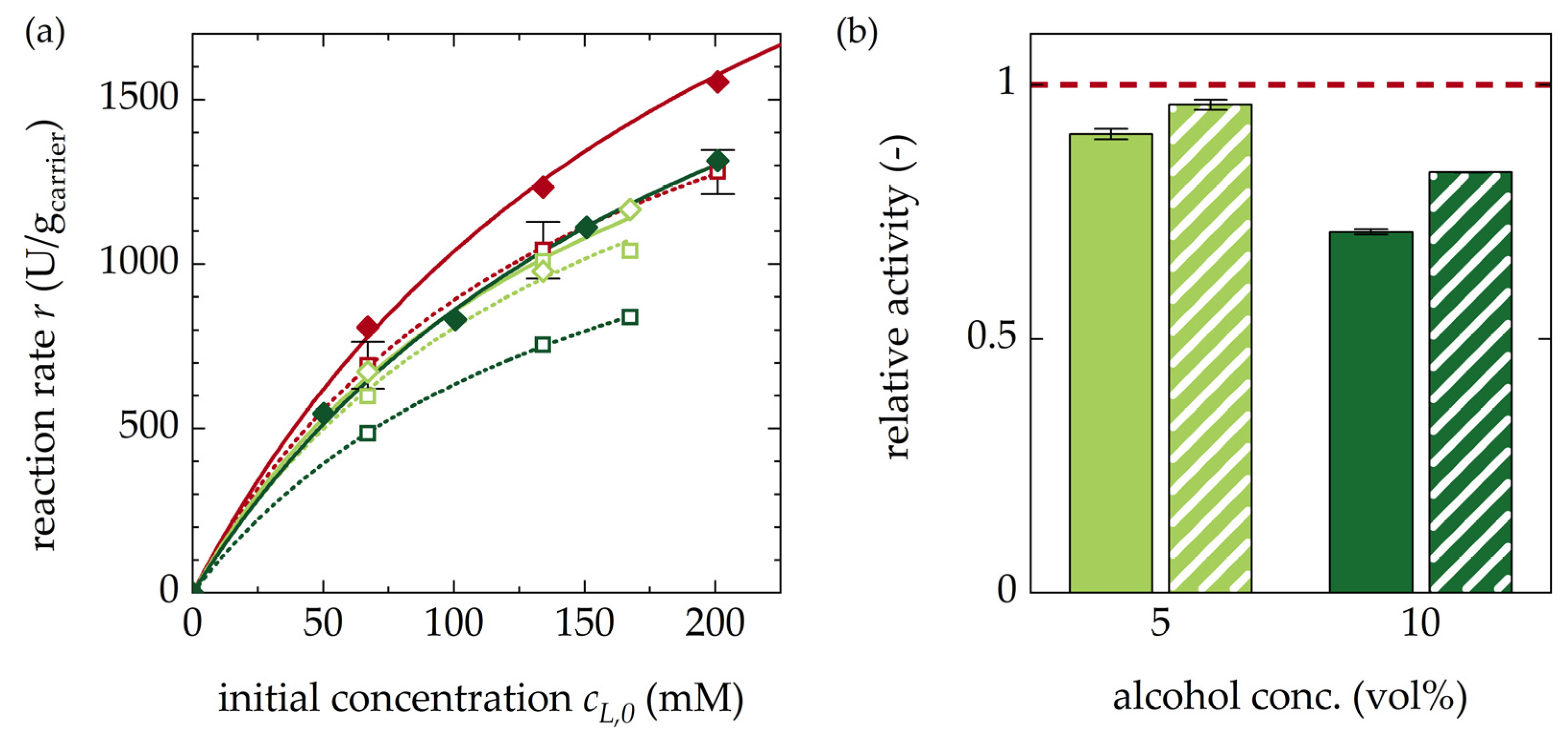
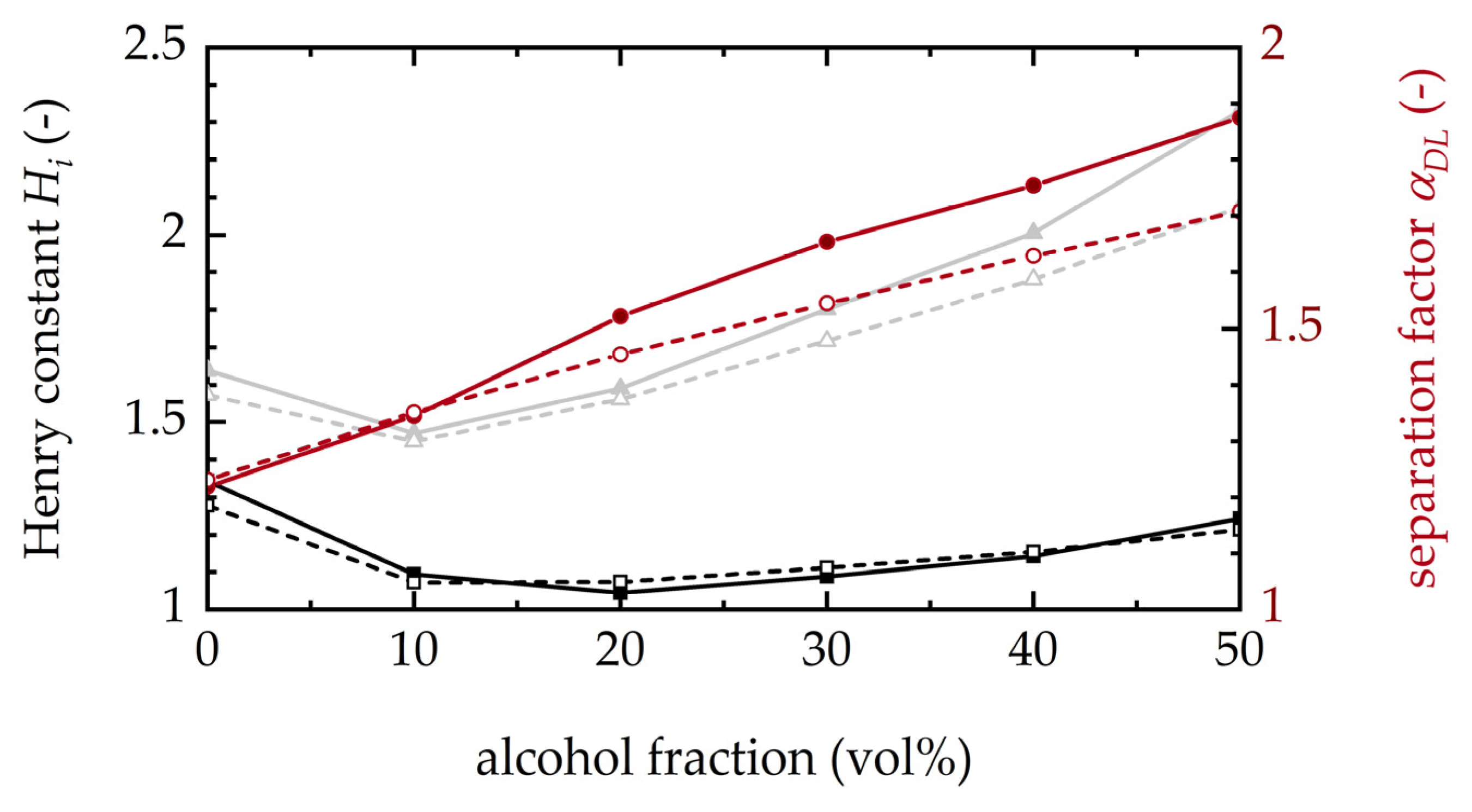
3.1.4. Impact of Alcohols on Racemization Rates
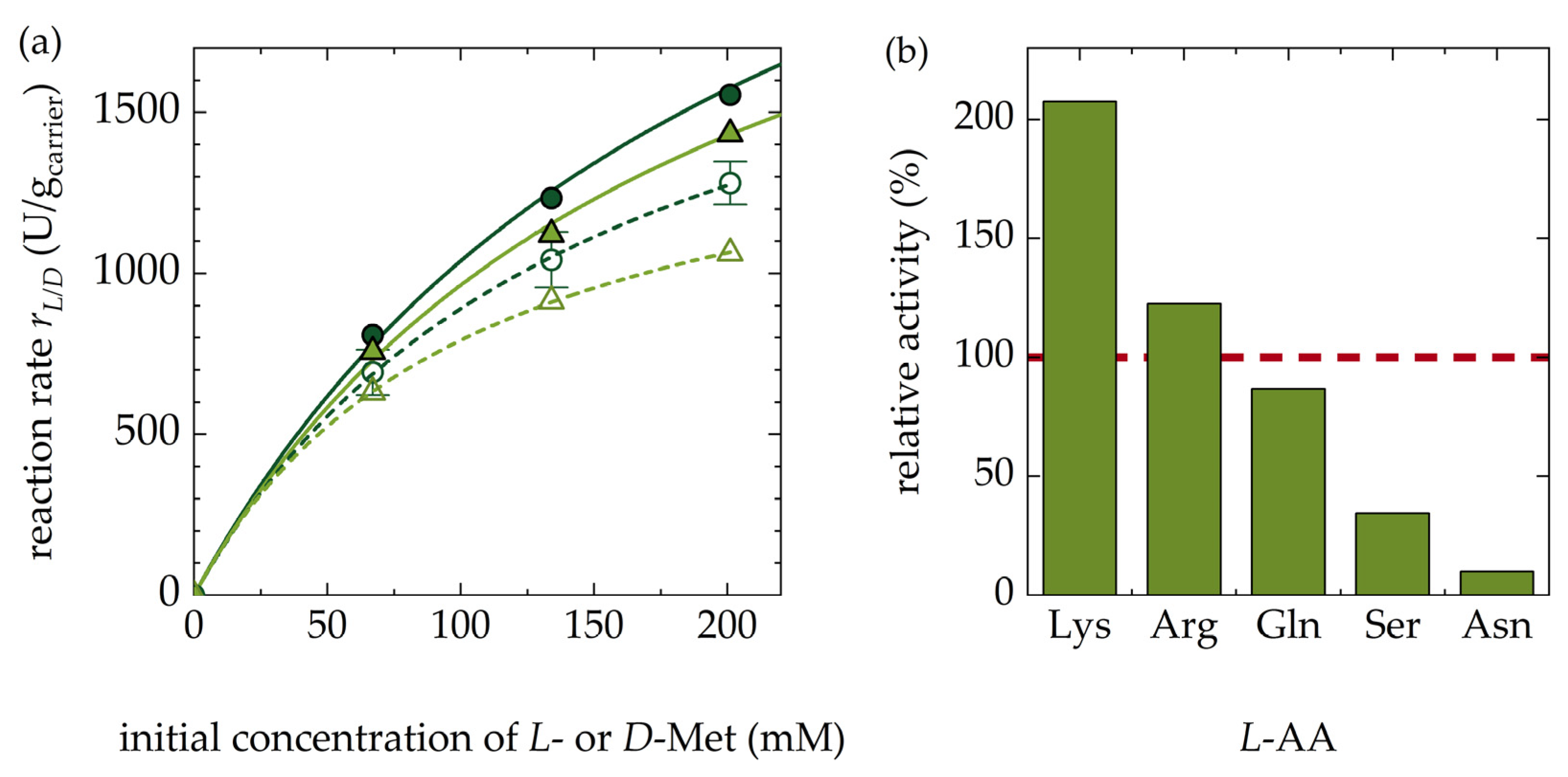
3.1.5. Kinetics of Racemization for D-Met and Other Amino Acids (AA)
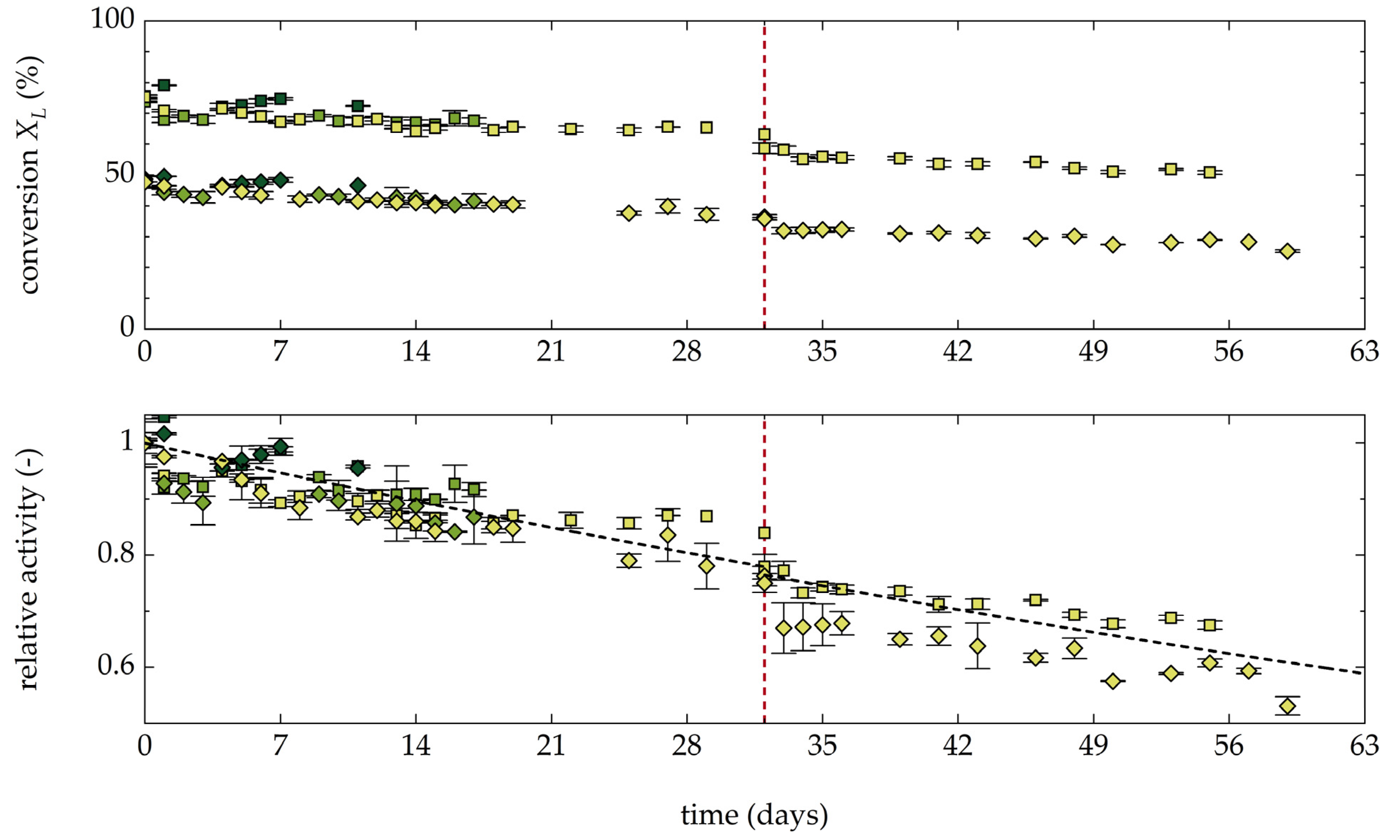
3.1.6. Stability and Productivity of the AAR under Operating Conditions
3.2. Preliminary Experiments for the Chromatographic Resolution of D-/L-Met
4. Discussion: Potential Process Configurations
4.1. Selection of Eluent and Reaction Temperature for the EFBR
4.2. Design Aspects for Scaling the EFBR
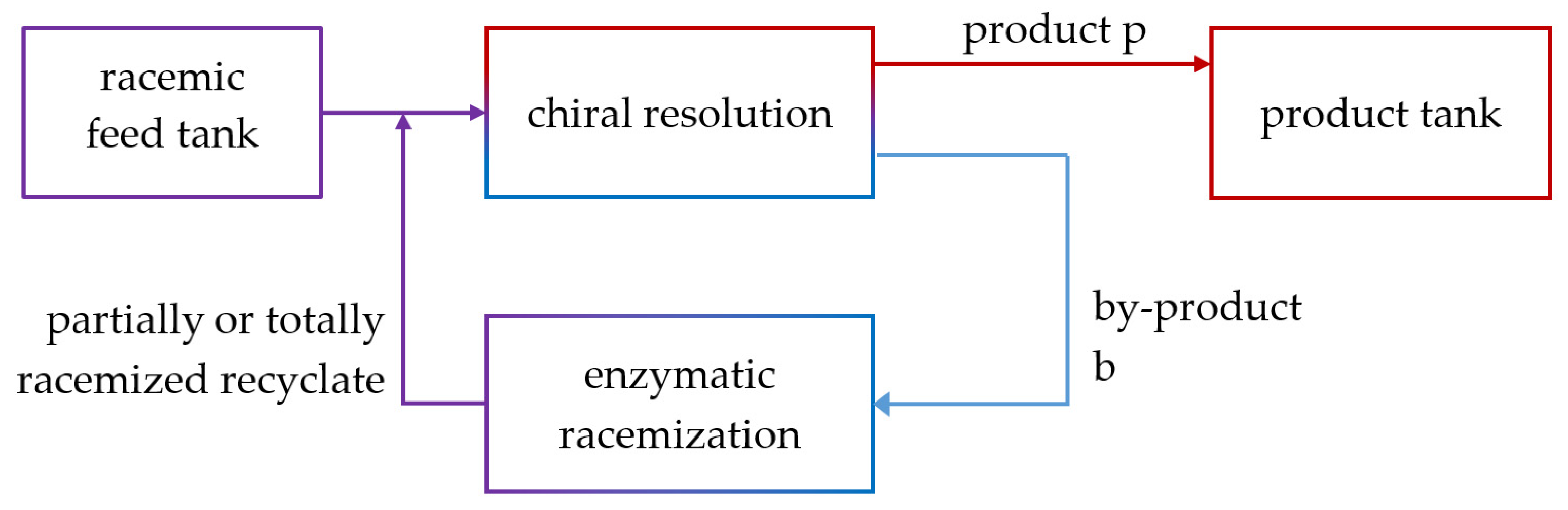
4.3. Integrated Process: Enantioselective Chromatography and Racemization
4.3.1. Constraints for Process Coupling
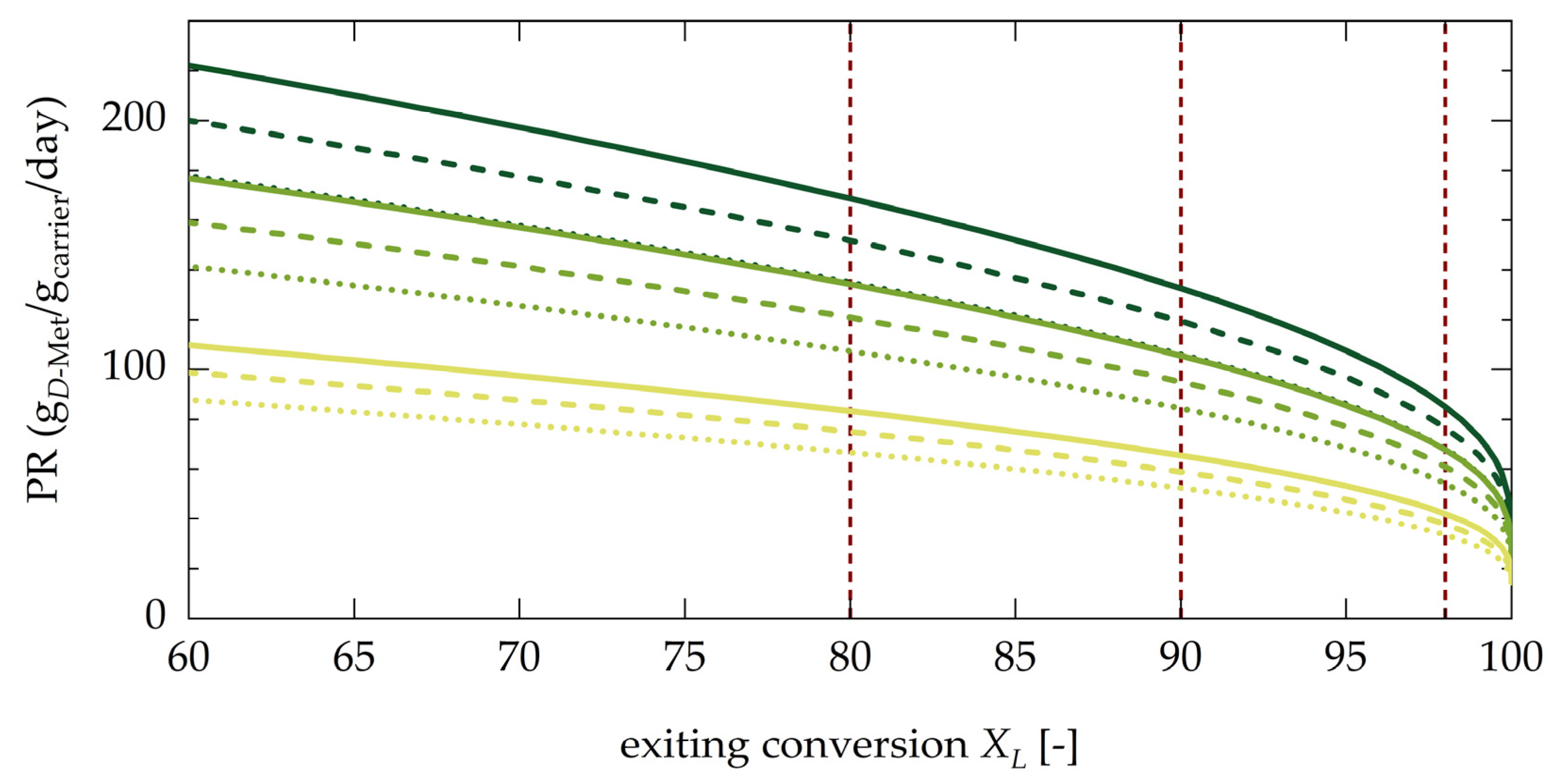
4.3.2. Evaluation of Productivity of the Coupled Process
5. Materials and Methods
5.1. Racemase, Immobilization Supports, and Substrates
5.2. Enzymatic Fixed Bed Reactor (EFBR)
5.2.1. AAR Production, Purification, and Immobilization
5.2.2. Packing of the EFBR
5.3. Determining the Rate of Racemization for the AAR
5.3.1. Free AAR Activity Assay
5.3.2. Setup for the Characterization of Immobilized AAR in an EFBR
5.4. Storage and Stability Tests of the AAR
5.5. Enantioselective Analytical HPLC Method
5.6. Methods and Parameters to Quantify Enantioselective Chromatographic Resolution
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jacques, J.; Collet, A.; Wilen, S.H. Enantiomers, Racemates, and Resolutions; Wiley: Hoboken, NJ, USA, 1981. [Google Scholar]
- Lorenz, H.; Seidel-Morgenstern, A. Processes to separate enantiomers. Angew. Chem. Int. Ed. 2014, 53, 1218–1250. [Google Scholar] [CrossRef]
- Kaspereit, M.; Sainio, T. Simplified design of steady-state recycling chromatography under ideal and nonideal conditions. Chem. Eng. Sci. 2011, 66, 5428–5438. [Google Scholar] [CrossRef]
- Swernath, S.; Kaspereit, M.; Kienle, A. Coupled continuous chromatography and racemization processes for the production of pure enantiomers. Chem. Eng. Technol. 2014, 37, 643–651. [Google Scholar] [CrossRef]
- Huerta, F.F.; Minidis, A.B.E.; Bäckvall, J.-E. Racemisation in asymmetric synthesis. Dynamic kinetic resolution and related processes in enzyme and metal catalysis. Chem. Soc. Rev. 2001, 30, 321–331. [Google Scholar] [CrossRef]
- Martín-Matute, B.; Bäckvall, J.-E. Dynamic kinetic resolution catalyzed by enzymes and metals. Curr. Opin. Chem. Biol. 2007, 11, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Von Langermann, J.; Kaspereit, M.; Shakeri, M.; Lorenz, H.; Hedberg, M.; Jones, M.J.; Larson, K.; Herschend, B.; Arnell, R.; Temmel, E.; et al. Design of an integrated process of chromatography, crystallization and racemization for the resolution of 2, 6-pipecoloxylidide (PPX). Org. Process Res. Dev. 2012, 16, 343–352. [Google Scholar] [CrossRef]
- Fuereder, M.; Femmer, C.; Storti, G.; Panke, S.; Bechtold, M. Integration of simulated moving bed chromatography and enzymatic racemization for the production of single enantiomers. Chem. Eng. Sci. 2016, 152, 649–662. [Google Scholar] [CrossRef]
- Femmer, C.; Bechtold, M.; Roberts, T.M.; Panke, S. Exploiting racemases. Appl. Microbiol. Biotechnol. 2016, 100, 7423–7436. [Google Scholar] [CrossRef]
- Hashimoto, K.; Adachi, S.; Noujima, H.; Ueda, Y. A new process combining adsorption and enzyme reaction for producing higher-fructose syrup. Biotechnol. Bioeng. 1983, 25, 2371–2393. [Google Scholar] [CrossRef]
- Panke, S.; Held, M.; Wubbolts, M. Trends and innovations in industrial biocatalysis for the production of fine chemicals. Curr. Opin. Biotechnol. 2004, 15, 272–279. [Google Scholar] [CrossRef]
- Schmid, A.; Dordick, J.S.; Hauer, B.; Kiener, A. Industrial biocatalysis today and tomorrow. Nature 2001, 409, 258. [Google Scholar] [CrossRef]
- Xu, X.; Balchen, S.; Høy, C.-E.; Adler-Nissen, J. Production of specific-structured lipids by enzymatic interesterification in a pilot continuous enzyme bed reactor. J. Am. Oil Chem. Soc. 1998, 75, 1573–1579. [Google Scholar] [CrossRef]
- Klibanov, A.M. Enzyme stabilization by immobilization. Anal. Biochem. 1979, 93, 1–25. [Google Scholar] [CrossRef]
- Sheldon, R.A. Enzyme immobilization: The quest for optimum performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A general overview of support materials for enzyme immobilization: Characteristics, properties, practical utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Bechtold, M.; Makart, S.; Reiss, R.; Alder, P.; Panke, S. Model-based characterization of an amino acid racemase from Pseudomonas putida DSM 3263 for application in medium-constrained continuous processes. Biotechnol. Bioeng. 2007, 98, 812–824. [Google Scholar] [CrossRef] [PubMed]
- Radkov, A.D.; Moe, L.A. Amino acid racemization in Pseudomonas putida KT2440. J. Bacteriol. 2013, 195, 5016–5024. [Google Scholar] [CrossRef]
- Würges, K.; Petrusevska, K.; Serci, S.; Wilhelm, S.; Wandrey, C.; Seidel-Morgenstern, A.; Elsner, M.P.; Lütz, S. Enzyme-assisted physicochemical enantioseparation processes: Part I. Production and characterization of a recombinant amino acid racemase. J. Mol. Catal. B Enzym. 2009, 58, 10–16. [Google Scholar] [CrossRef]
- Carneiro, T.; Wrzosek, K.; Bettenbrock, K.; Lorenz, H.; Seidel-Morgenstern, A. Immobilization of an amino acid racemase for application in crystallization-based chiral resolutions of asparagine monohydrate. Eng. Life Sci. 2020, 20, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Intaraboonrod, K.; Harriehausen, I.; Carneiro, T.; Seidel-Morgenstern, A.; Lorenz, H.; Flood, A. Temperature Cycling Induced Deracemization of DL-Asparagine with Immobilized Amino Acid Racemase. Cryst. Growth Des. 2020, 2020. [Google Scholar] [CrossRef]
- Palacios, J.G.; Kaspereit, M.; Kienle, A. Conceptual Design of Integrated Chromatographic Processes for the Production of Single (Stereo-) Isomers. Chem. Eng. Technol. Ind. Chem. Plant Equip. Process Eng. Biotechnol. 2009, 32, 1392–1402. [Google Scholar] [CrossRef]
- Schmidt-Traub, H.; Schulte, M.; Seidel-Morgenstern, A. Preparative Chromatography: Of Fine Chemicals and Pharmaceutical Agents, 3rd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2020. [Google Scholar]
- Harriehausen, I.; Wrzosek, K.; Lorenz, H.; Seidel-Morgenstern, A. Assessment of process configurations to combine enantioselective chromatography with enzymatic racemization. Adsorption 2020, 26, 1199–1213. [Google Scholar] [CrossRef]
- Zhang, L.; Gedicke, K.; Kuznetsov, M.A.; Staroverov, S.M.; Seidel-Morgenstern, A. Application of an eremomycin-chiral stationary phase for the separation of dl-methionine using simulated moving bed technology. J. Chromatogr. A 2007, 1162, 90–96. [Google Scholar] [CrossRef]
- Fuereder, M.; Panke, S.; Bechtold, M. Simulated moving bed enantioseparation of amino acids employing memory effect-constrained chromatography columns. J. Chromatogr. A 2012, 1236, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Briggs, G.E.; Haldane, J.B.S. A Note on the Kinetics of Enzyme Action. Biochem. J. 1925, 19, 338–339. [Google Scholar] [CrossRef]
- Moulijn, J.A.; van Diepen, A.E.; Kapteijn, F. Catalyst deactivation: Is it predictable?: What to do? Appl. Catal. General 2001, 212, 3–16. [Google Scholar] [CrossRef]
- Zaak, H.; Fernandez-Lopez, L.; Velasco-Lozano, S.; Alcaraz-Fructuoso, M.T.; Sassi, M.; Lopez-Gallego, F.; Fernandez-Lafuente, R. Effect of high salt concentrations on the stability of immobilized lipases: Dramatic deleterious effects of phosphate anions. Process Biochem. 2017, 62, 128–134. [Google Scholar] [CrossRef]
- Wrzosek, K.; Harriehausen, I.; Seidel-Morgenstern, A. Combination of Enantioselective Preparative Chromatography and Racemization: Experimental Demonstration and Model-Based Process Optimization. Org. Process Res. Dev. 2018, 22, 1761–1771. [Google Scholar] [CrossRef]
- Nelson, K.E. The complete genome sequence of Pseudomonas putida KT2440 is finally available. Environ. Microbiol. 2002, 4, 777–778. [Google Scholar] [CrossRef]
- Eliot, A.C.; Kirsch, J.F. Pyridoxal phosphate enzymes: Mechanistic, structural, and evolutionary considerations. Annu. Rev. Biochem. 2004, 73, 383–415. [Google Scholar] [CrossRef] [PubMed]
- Polenske, D.; Lorenz, H. Solubility and metastable zone width of the methionine enantiomers and their mixtures in water. J. Chem. Eng. Data 2009, 54, 2277–2280. [Google Scholar] [CrossRef]

| Characteristics | Free AAR | Immob. AAR | Immob. AAR |
|---|---|---|---|
| Batch | B2 | B2 | B1 |
| Loading qAAR [mgAAR/gcarrier] | - | 35 | 37 |
| Dosage [mgAAR/mL] | 0.039 | 25.6 | 27.1 |
| Reactor volume [mL] | 3 | 0.092 | 0.092 |
| Temperature [°C] | 30 | 20 | 30 |
| Reaction rate [U/mgAAR] | 139 | 29.8 | 48 |
| Batch | KM [mM] | rmax [U/gcarrier] | rmax [U/mgAAR] | rmax/KM [U/mM/gcarrier] | rmax/KM [U/mM/mgAAR] |
|---|---|---|---|---|---|
| B1 | 211 ± 22 | 3236 ± 181 | 87 ± 5 | 15.3 | 0.41 |
| B2 | 151 ± 7 | 2235 ± 54 | 64 ± 2 | 14.8 | 0.42 |
| Buffer | pH [-] | Temp. [°C] | KM [mM] | rmax [U/gcarrier] | rmax/KM [U/mM/gcarrier] |
|---|---|---|---|---|---|
| - | 5.8 2 | 20 | 211 ± 22 | 3236 ± 190 | 15.30 |
| 20 mM 1 | 7 | 10 | 300 ± 56 | 2376 ± 270 | 7.92 |
| 20 mM 1 | 7 | 20 | 190 ± 22 | 3140 ± 200 | 16.50 |
| 20 mM 1 | 7 | 30 | 161 ± 37 | 3775 ± 400 | 23.50 |
| 20 mM 1 | 6 | 20 | 402 ± 165 | 3599 ± 980 | 8.95 |
| 20 mM 1 | 8 | 20 | 250 ± 30 | 3553 ± 240 | 14.20 |
| rmax (T) | KM (T) | |||
|---|---|---|---|---|
| A [U/gcarrier] | EA [kJ/mol] | A0 [mM] | A1 [mM] | A2 [1/K] |
| 2.72 × 106 | 16.6 | 150.6 | 3.7 × 1018 | 7.5 |
| Batch | KM [mM] | rmax [U/gcarrier] | rmax [U/mgAAR] | rmax/KM [U/mM/gcarrier] | rmax/KM [U/mM/mgAAR] |
|---|---|---|---|---|---|
| B1 | 186 ± 19 | 2757 ± 140 | 75 ± 4 | 14.8 | 0.40 |
| B2 | 104 ± 3 | 1617 ± 20 | 46 ± 1 | 15.5 | 0.44 |
| Property | Symbol | Size |
|---|---|---|
| Bed height | 13.0 ± 0.5 mm | |
| Column diameter | 3 mm | |
| Bed volume | 0.092 ± 0.004 mL | |
| Mass of carrier | 56 ± 2 mg | |
| Loading | B1: 37 mgAAR/gcarrier; B2: 35 mgAAR/gcarrier | |
| Porosity | 0.83 ± 0.05 | |
| Dosage | 732 ± 25 mgcarrier/mL |
| Operating Parameter | Range |
|---|---|
| Flow rate | 0.5–3.7 mL/min |
| Temperature | 10–30 °C |
| pH range | 6–8 |
| Buffer and salt | 20 mM NaH2PO4/Na2HPO4 |
| Ethanol | +20 mM NaCl |
| Methanol | 0–10 vol% |
| DL-AA | Column | Eluent | Temp. [°C] | Wavelength [nm] |
|---|---|---|---|---|
| Arg | Chirob. T 1 | 20 mM AAc 3, pH 4.1: MeOH (50:50) | 23 | 205 |
| Asn | Chirex 2 | Perchloric acid, pH 1 | 4 | 210 |
| Gln | Chirob. T 1 | 0.1 mM NaH2PO4: H2O (60:40) | 25 | 220 |
| Met | Chirob. T 1 | MeOH:H2O (60:40) | 25 | 220 |
| Ser | Chirob. T 1 | EtOH:H2O (70:30) | 20 | 210 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harriehausen, I.; Bollmann, J.; Carneiro, T.; Bettenbrock, K.; Seidel-Morgenstern, A. Characterization of an Immobilized Amino Acid Racemase for Potential Application in Enantioselective Chromatographic Resolution Processes. Catalysts 2021, 11, 726. https://doi.org/10.3390/catal11060726
Harriehausen I, Bollmann J, Carneiro T, Bettenbrock K, Seidel-Morgenstern A. Characterization of an Immobilized Amino Acid Racemase for Potential Application in Enantioselective Chromatographic Resolution Processes. Catalysts. 2021; 11(6):726. https://doi.org/10.3390/catal11060726
Chicago/Turabian StyleHarriehausen, Isabel, Jonas Bollmann, Thiane Carneiro, Katja Bettenbrock, and Andreas Seidel-Morgenstern. 2021. "Characterization of an Immobilized Amino Acid Racemase for Potential Application in Enantioselective Chromatographic Resolution Processes" Catalysts 11, no. 6: 726. https://doi.org/10.3390/catal11060726
APA StyleHarriehausen, I., Bollmann, J., Carneiro, T., Bettenbrock, K., & Seidel-Morgenstern, A. (2021). Characterization of an Immobilized Amino Acid Racemase for Potential Application in Enantioselective Chromatographic Resolution Processes. Catalysts, 11(6), 726. https://doi.org/10.3390/catal11060726






