2D-Layered Non-Precious Electrocatalysts for Hydrogen Evolution Reaction: Fundamentals to Applications
Abstract
1. Introduction
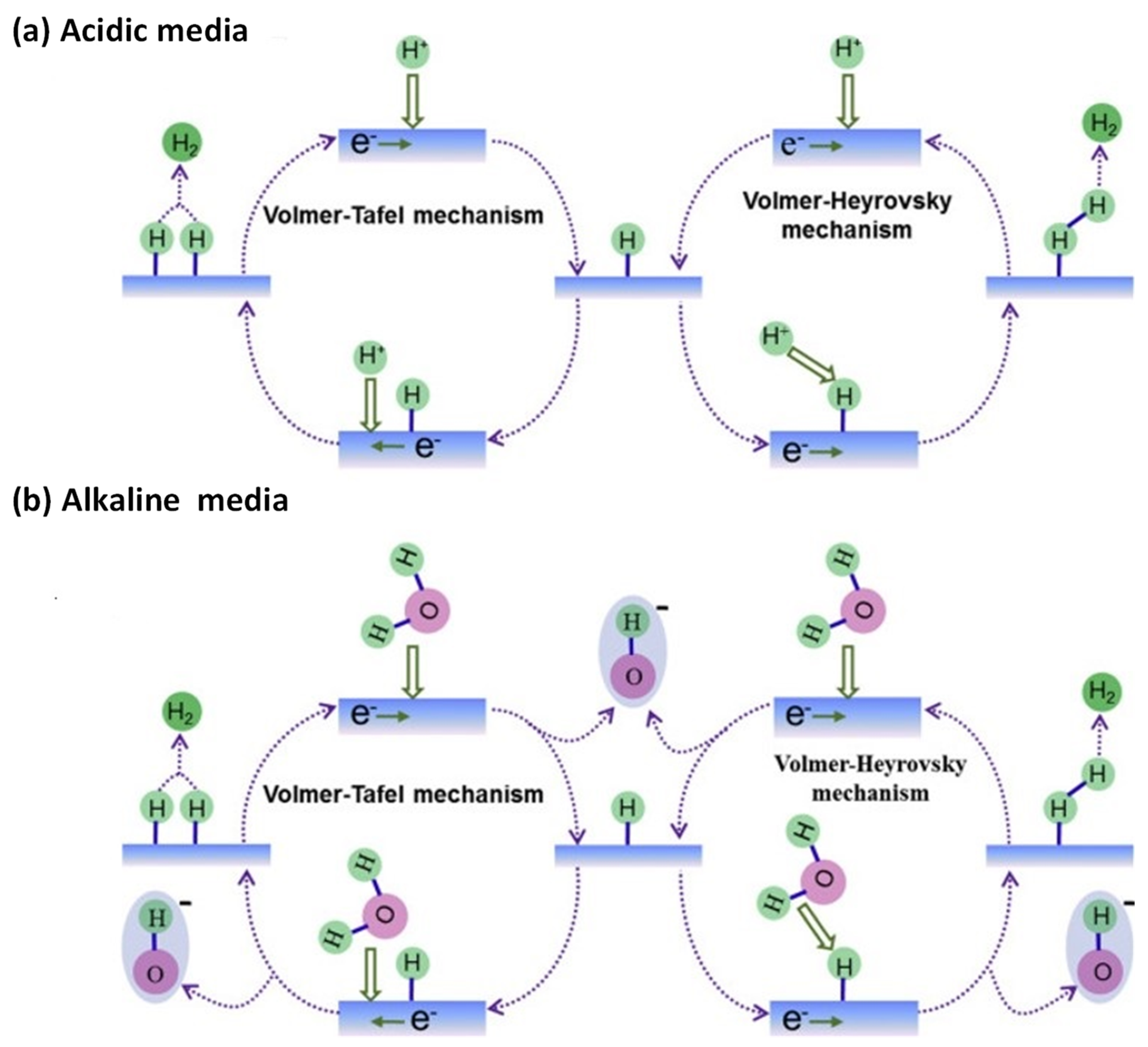
1.1. Fundamentals to Electrocatalytic Hydrogen Evolution Reaction
1.2. Parameters Using in the Electrochemical Hydrogen Evolution Process
1.2.1. Overpotential
1.2.2. Tafel Plot
1.2.3. Faradic Efficiency
1.2.4. Turnover Frequency (TOF)
1.2.5. Gibbs Free Energy
1.2.6. Electrochemical Active Surface Area
2. 2D Materials Used for Electrocatalytic HER
2.1. Synthesis Techniques for 2D Materials
2.1.1. Mechanical and Liquid Exfoliation Method
2.1.2. Chemical Vapor Deposition (CVD) Method
2.1.3. Hydrothermal Method
2.1.4. Solvothermal Method
2.1.5. Electrodeposition Method
2.2. Applications of 2D Materials on HER
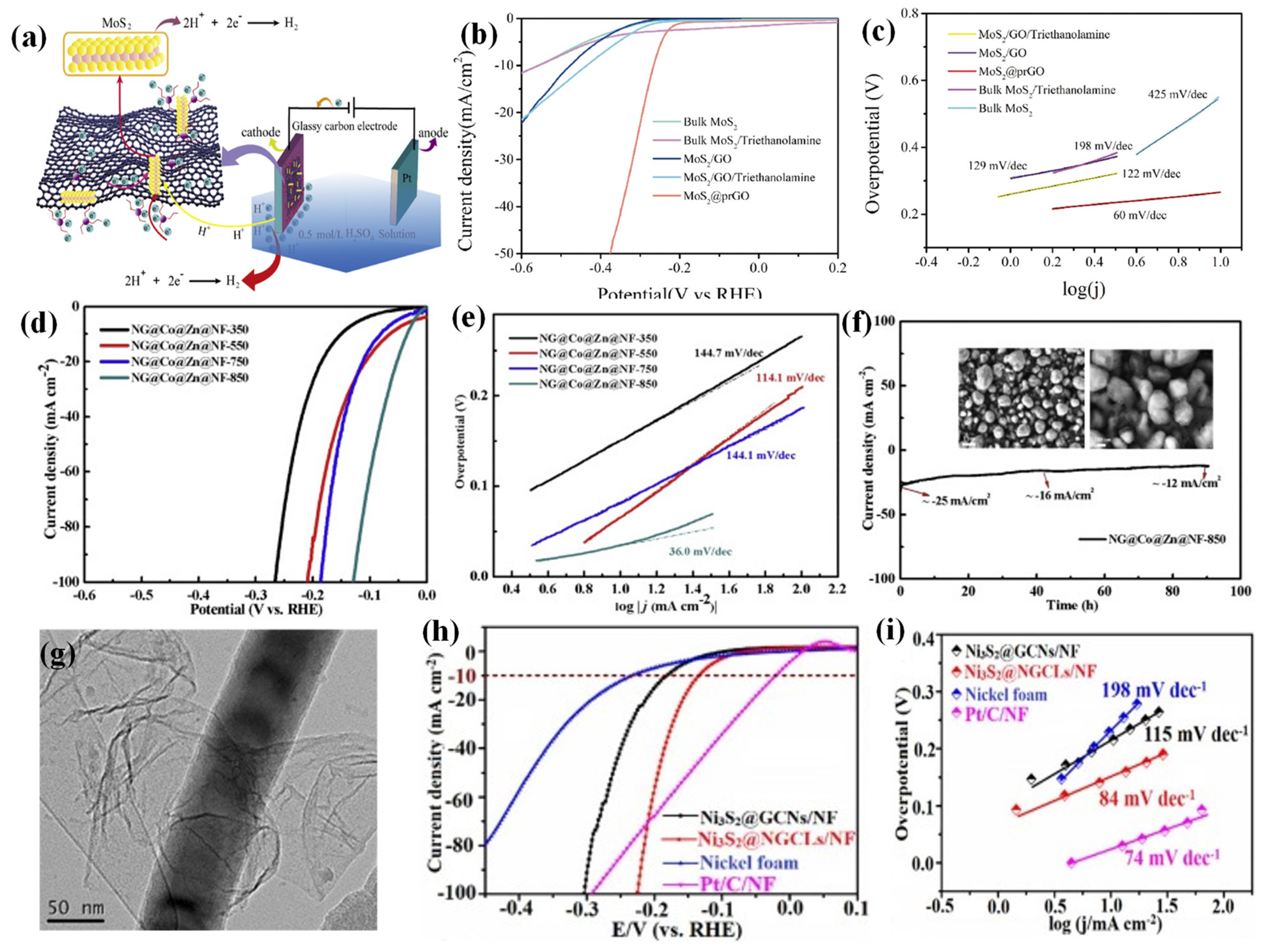
2.2.1. Graphene
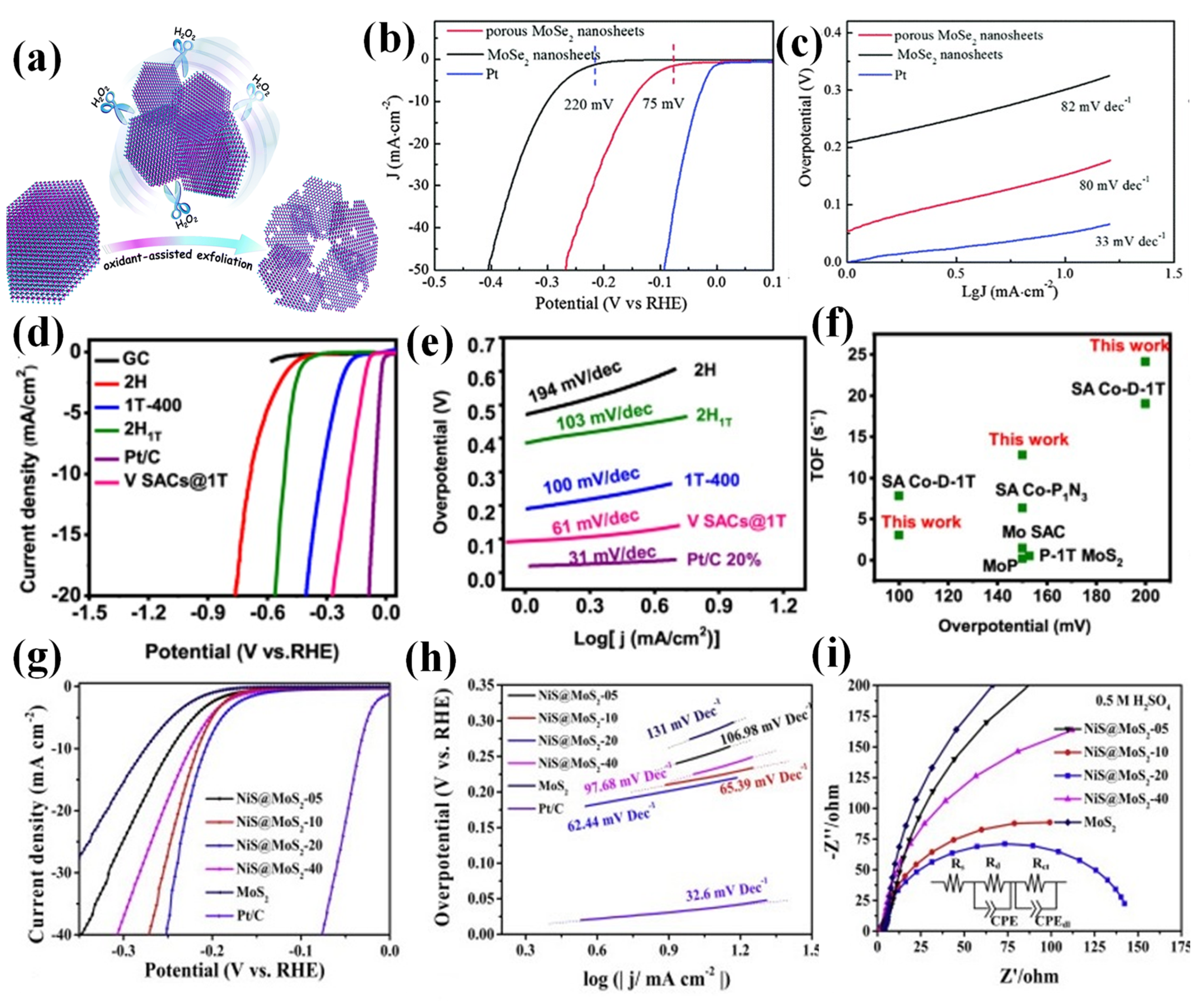
2.2.2. Transition Metal Dichalcogenides (TMDs)
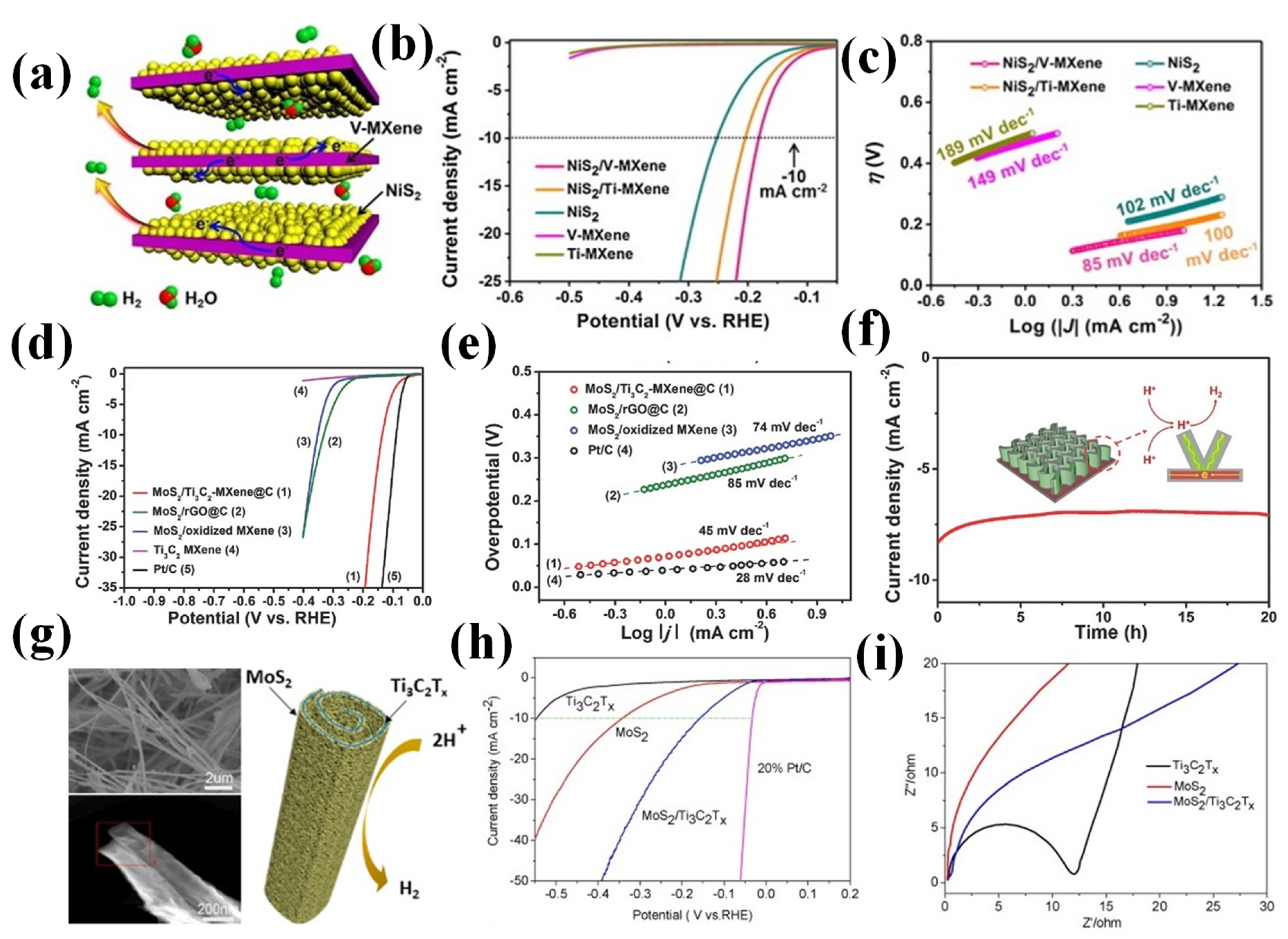
2.2.3. MXenes
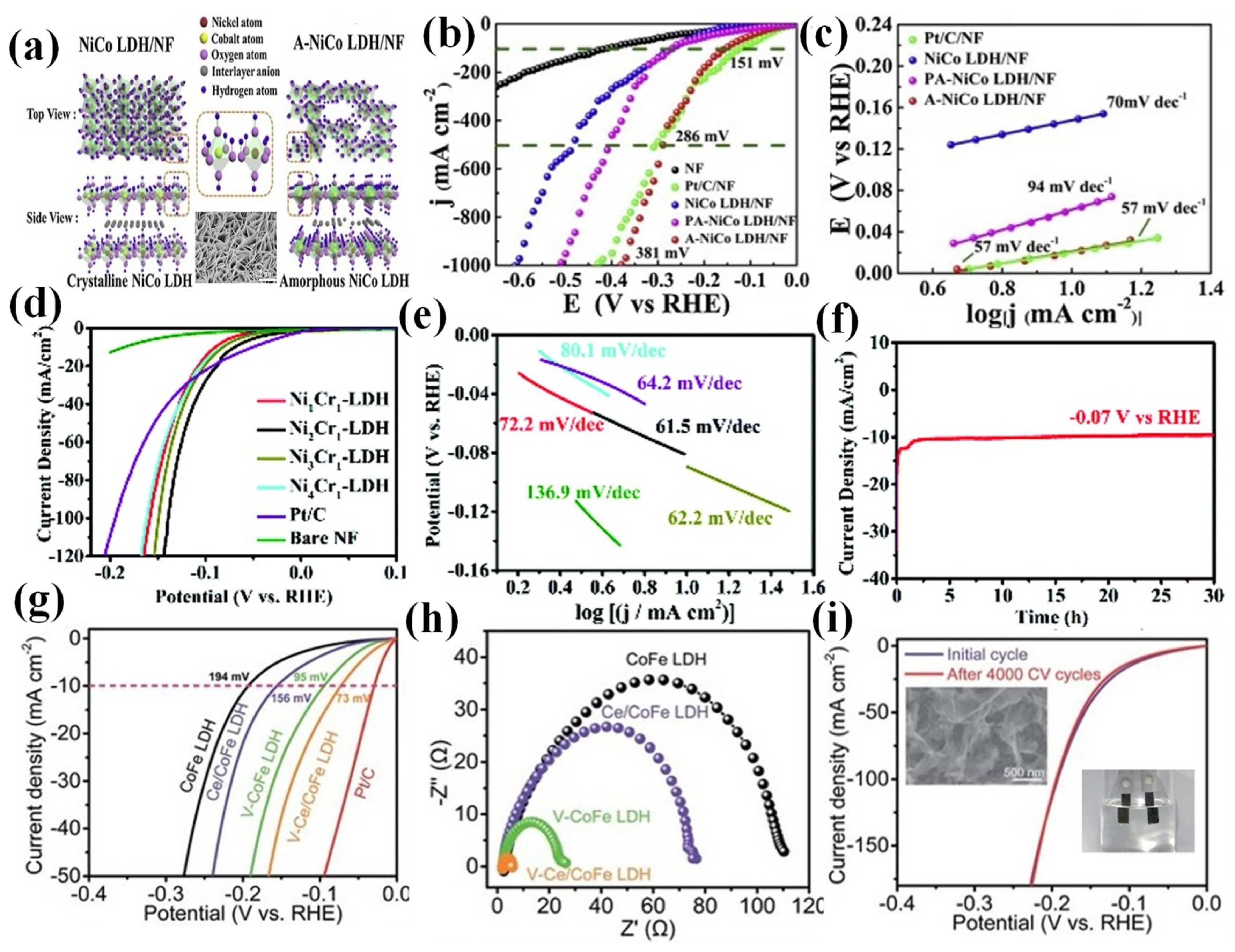
2.2.4. Layered Doubled Hydroxide (LDH)
2.2.5. Graphitic Carbon Nitride (g-C3N4)
3. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fujishima, A.; Honda, K.J.N. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Peng, J.; Dong, W.; Wang, Z.; Meng, Y.; Liu, W.; Song, P.; Liu, Z. Recent advances in 2D transition metal compounds for electrocatalytic full water splitting in neutral media. Mater. Today Adv. 2020, 8, 100081–100097. [Google Scholar] [CrossRef]
- Pan, H. Principles on design and fabrication of nanomaterials as photocatalysts for water-splitting. Renew. Sustain. Energy Rev. 2016, 57, 584–601. [Google Scholar] [CrossRef]
- Luo, Y.; Ren, K.; Wang, S.; Chou, J.-P.; Yu, J.; Sun, Z.; Sun, M. First-principles study on transition-metal dichalcogenide/BSe van der waals heterostructures: A promising water-splitting photocatalyst. J. Phys. Chem. C 2019, 123, 22742–22751. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, S.; Ren, K.; Chou, J.-P.; Yu, J.; Sun, Z.; Sun, M. Transition-metal dichalcogenides/Mg(OH)2 van der waals heterostructures as promising water-splitting photocatalysts: A first-principles study. Phys. Chem. Chem. Phys. 2019, 21, 1791–1796. [Google Scholar] [CrossRef]
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Turner, J.; Sverdrup, G.; Mann, M.K.; Maness, P.-C.; Kroposki, B.; Ghirardi, M.; Evans, R.J.; Blake, D. Renewable hydrogen production. Int. J. Energy Res. 2008, 32, 379–407. [Google Scholar] [CrossRef]
- Grigoriev, S.A.; Porembsky, V.I.; Fateev, V.N. Pure hydrogen production by PEM electrolysis for hydrogen energy. Int. J. Hydrog. Energy 2006, 31, 171–175. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, T. Manufacture of hydrogen. Catal. Today 2005, 106, 293–296. [Google Scholar] [CrossRef]
- Dicks, A.L. Hydrogen generation from natural gas for the fuel cell systems of tomorrow. J. Power Sources 1996, 61, 113–124. [Google Scholar] [CrossRef]
- Kellogg, W.W.; Cadle, R.D.; Allen, E.R.; Lazrus, A.L.; Martell, E.A. The sulfur cycle. Science 1972, 175, 587. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H.; Sumathy, K. An overview of hydrogen production from biomass. Fuel Process. Technol. 2006, 87, 461–472. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Thomas, I.L. Alternative energy technologies. Nature 2001, 414, 332–337. [Google Scholar] [CrossRef]
- Morales-Guio, C.G.; Stern, L.-A.; Hu, X. Nanostructured hydrotreating catalysts for electrochemical hydrogen evolution. Chem. Soc. Rev. 2014, 43, 6555–6569. [Google Scholar] [CrossRef] [PubMed]
- McCrory, C.C.L.; Jung, S.; Ferrer, I.M.; Chatman, S.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water splitting devices. J. Am. Chem. Soc. 2015, 137, 4347–4357. [Google Scholar] [CrossRef] [PubMed]
- Stephens, I.E.L.; Chorkendorff, I. Minimizing the use of platinum in hydrogen-evolving electrodes. Angew. Chem. Int. Ed. 2011, 50, 1476–1477. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, X.; Zhang, J.; Wan, S.; Guo, S.; Lu, G.; Yao, J.; Huang, X. Precise tuning in platinumnickel/nickel sulfide interface nanowires for synergistic hydrogen evolution catalysis. Nat. Commun. 2017, 8, 14580. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Sultan, S.; Myung, C.W.; Yoon, T.; Li, N.; Ha, M.; Harzandi, A.M.; Park, H.J.; Kim, D.Y.; Chandrasekaran, S.S.; et al. Multicomponent electrocatalyst with ultralow Pt loading and high hydrogen evolution activity. Nat. Energy 2018, 3, 773–782. [Google Scholar] [CrossRef]
- Popczun, E.J.; McKone, J.R.; Read, C.G.; Biacchi, A.J.; Wiltrout, A.M.; Lewis, N.S.; Schaak, R.E. Nanostructured nickel phosphide as an electrocatalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2013, 135, 9267–9270. [Google Scholar] [CrossRef]
- Gong, M.; Zhou, W.; Tsai, M.-C.; Zhou, J.; Guan, M.; Lin, M.-C.; Zhang, B.; Hu, Y.; Wang, D.-Y.; Yang, J.; et al. Nanoscale nickel oxide/nickel heterostructures for active hydrogen evolution electrocatalysis. Nat. Commun. 2014, 5, 4695. [Google Scholar] [CrossRef]
- Vrubel, H.; Hu, X. Molybdenum boride and carbide catalyze hydrogen evolution in both acidic and basic solutions. Angew. Chem. Int. Ed. 2012, 51, 12703–12706. [Google Scholar] [CrossRef] [PubMed]
- Conway, B.E.; Tilak, B.V. Interfacial processes involving electrocatalytic evolution and oxidation of H2, and the role of chemisorbed H. Electrochim. Acta 2002, 47, 3571–3594. [Google Scholar] [CrossRef]
- Chen, Z.; Qing, H.; Zhou, K.; Sun, D.; Wu, R. Metal-organic framework-derived nanocomposites for electrocatalytic hydrogen evolution reaction. Prog. Mater. Sci. 2020, 108, 100618. [Google Scholar] [CrossRef]
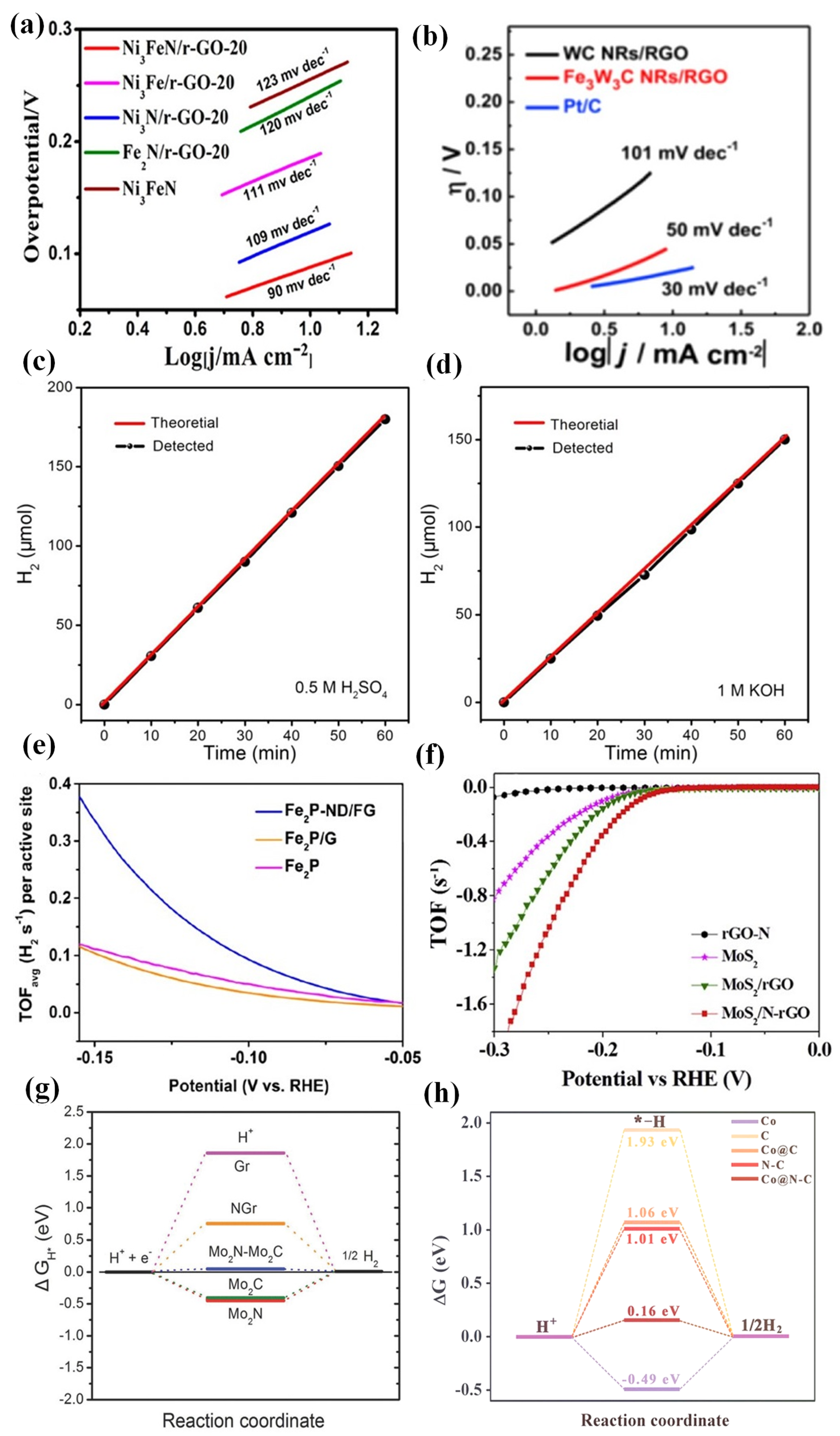
- Gu, Y.; Chen, S.; Ren, J.; Chen, C.; Komarneni, S.; Yang, D.; Yao, X. Electronic structure tuning in Ni3FeN/r-GO aerogel toward bifunctional electrocatalyst for overall water splitting. ACS Nano 2018, 12, 245–253. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Bo, T.; Wang, B.; Tao, J. RGO induced one-dimensional bimetallic carbide nanorods: An efficient and pH-universal hydrogen evolution reaction electrocatalyst. Nano Energy 2019, 62, 85–93. [Google Scholar] [CrossRef]
- Ali, A.; Shen, P.K. Nonprecious metal’s graphene-supported electrocatalysts for hydrogen evolution reaction: Fundamentals to applications. Carbon Energy 2020, 2, 99–121. [Google Scholar] [CrossRef]
- Yan, H.; Xie, Y.; Jiao, Y.; Wu, A.; Tian, C.; Zhang, X.; Wang, L.; Fu, H. Holey reduced graphene oxide coupled with an Mo2N-Mo2C heterojunction for efficient hydrogen evolution. Adv. Mater. 2018, 30, 1704156. [Google Scholar] [CrossRef]
- Huang, H.; Yu, C.; Yang, J.; Han, X.; Zhao, C.; Li, S.; Liu, Z.; Qiu, J. Ultrasmall diiron phosphide nanodots anchored on graphene sheets with enhanced electrocatalytic activity for hydrogen production via high efficiency water splitting. J. Mater. Chem. A 2016, 4, 16028–16035. [Google Scholar] [CrossRef]
- Zhao, L.; Hong, C.; Lin, L.; Wu, H.; Su, Y.; Zhang, X.; Liu, A. Controllable nanoscale engineering of vertically aligned MoS2 ultrathin nanosheets by nitrogen doping of 3D graphene hydrogel for improved electrocatalytic hydrogen evolution. Carbon 2017, 116, 223–231. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, R.; Liu, Y.; Ha, Y.; Guo, Y.; Sun, D.; Liu, M.; Fang, F. Ultrafine Co nanoparticles encapsulated in carbon-nanotubes-grafted graphene sheets as advanced electrocatalysts for the hydrogen evolution reaction. Adv. Mater. 2018, 30, 1802011. [Google Scholar] [CrossRef]
- Sabatier, P. Hydrogenations et dehydrogenations par catalyse. Eur. J. Inorg. Chem. 1911, 44, 1984–2001. [Google Scholar]
- Wang, Y.; Wu, Q.; Zhang, B.; Tian, L.; Li, K.; Zhang, X. Recent Advances in Transition Metal Carbide Electrocatalysts for Oxygen Evolution Reaction. Catalysts 2020, 10, 1164. [Google Scholar] [CrossRef]
- Cai, Z.; Bu, X.; Wang, P.; Ho, J.C.; Yang, J.; Wang, X. Recent advances in layered double hydroxide electrocatalysts for the oxygen evolution reaction. J. Mater. Chem. A 2019, 7, 5069–5089. [Google Scholar] [CrossRef]
- Xiong, L.; Wang, K.; Li, D.; Luo, X.; Weng, J.; Liu, Z.; Zhang, H. Research progress on the preparations, characterizations and applications of large scale 2D transition metal dichalcogenides films. FlatChem 2020, 21, 100161. [Google Scholar] [CrossRef]
- Song, F.; Hu, X. Exfoliation of layered double hydroxides for enhanced oxygen evolution catalysis. Nat. Commun. 2014, 5, 4477. [Google Scholar] [CrossRef]
- Xiong, D.; Li, X.; Bai, Z.; Lu, S. Recent advances in layered Ti3C2Tx MXene for electrochemical energy storage. Small 2018, 14, 1703419. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, S.; Ma, D.; Ge, Y.; Deng, L.; Bowen, C.; Roscow, J.; Zhang, Y.; Lin, Z.; Misra, R.D.K.; Li, J.; et al. Electronic structure engineering on two-dimensional (2D) electrocatalytic materials for oxygen reduction, oxygen evolution, and hydrogen evolution reactions. Nano Energy 2020, 77, 105080. [Google Scholar] [CrossRef]
- Tan, T.; Jiang, X.; Wang, C.; Yao, B.; Zhang, H. 2D material optoelectronics for information functional device applications: Status and challenges. Adv. Sci. 2020, 7, 2000058. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, X.; Wu, W.; Liu, T.; Liu, Y.; Guo, B.; Zhang, R. One-step chemical exfoliation of graphite to ∼100% few-layer graphene with high quality and large size at ambient temperature. Chem. Eng. Technol. 2019, 355, 181–185. [Google Scholar] [CrossRef]
- Wei, J.; Vo, T.; Inam, F. Epoxy/graphene nanocomposites-processing and properties: A review. RSC Adv. 2015, 5, 73510–73524. [Google Scholar] [CrossRef]
- Yan, Z.; He, X.; She, L.; Sun, J.; Jiang, R.; Xu, H.; Shi, F.; Lei, Z.; Liu, Z.H. Solvothermal-assisted liquid-phase exfoliation of large size and high-quality black phosphorus. J. Mater. 2018, 4, 129–134. [Google Scholar] [CrossRef]
- Ma, H.; Shen, Z.; Ben, S. Understanding the exfoliation and dispersion of MoS2 nanosheets in pure water. J. Colloid Interface Sci. 2018, 517, 204–212. [Google Scholar] [CrossRef]
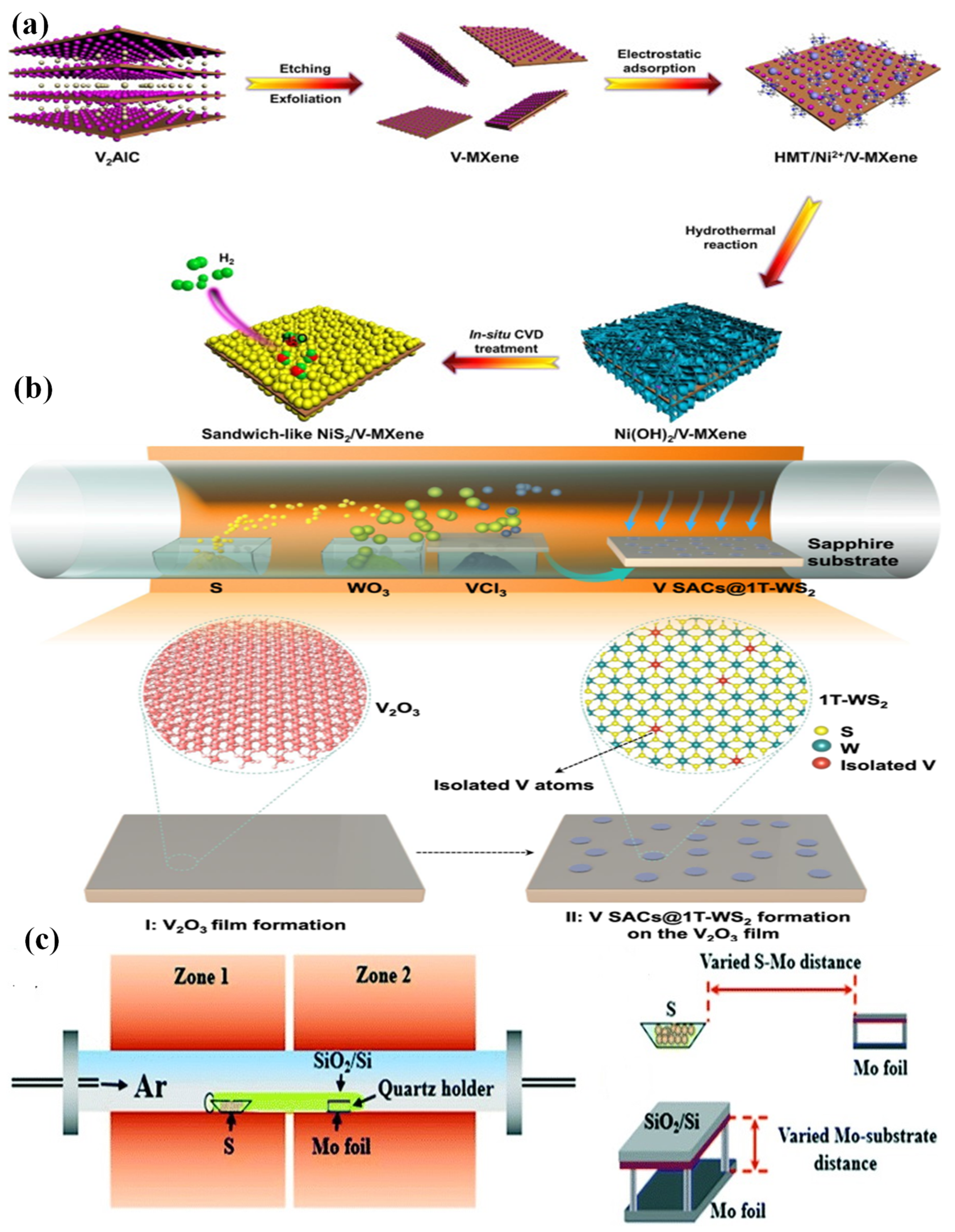
- Kuang, P.; He, M.; Zhu, B.; Yu, J.; Fan, K.; Jaroniec, M. 0D/2D NiS2/V-MXene composite for electrocatalytic H2 evolution. J. Catal. 2019, 375, 8–20. [Google Scholar] [CrossRef]
- Pacile, D.; Meyer, J.C.; Girit, Ç.Ö.; Zettl, A. The two-dimensional phase of boron nitride: Few-atomic-layer sheets and suspended membranes. Appl. Phys. Lett. 2008, 92, 133107. [Google Scholar] [CrossRef]
- Liu, J.; Zeng, Z.; Cao, X.; Lu, G.; Wang, L.H.; Fan, Q.L.; Huang, W.; Zhang, H. Preparation of MoS2-polyvinylpyrrolidone nanocomposites for flexible nonvolatile rewritable memory devices with reduced graphene oxide electrodes. Small 2012, 8, 3517–3522. [Google Scholar] [CrossRef] [PubMed]
- Han, A.; Zhou, X.; Wang, X.; Liu, S.; Xiong, Q.; Zhang, Q.; Gu, L.; Zhuang, Z.; Zhang, W.; Li, F.; et al. One-step synthesis of single-site vanadium substitution in 1T-WS2 monolayers for enhanced hydrogen evolution catalysis. Nat. Commun. 2021, 12, 709. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Jiang, X.; Shao, J.; Lu, B.; Fu, L.; Zhao, S.; Suc, W. Space-confined CVD growth of 2D-MoS2 crystals with tunable dimensionality via adjusting growth conditions. CrystEngComm 2021, 23, 1345–1351. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, F.; Shenoy, V.B.; Tang, M.; Lou, J. Towards controlled synthesis of 2D crystals by chemical vapor deposition (CVD). Mater. Today 2020, 40, 132–139. [Google Scholar] [CrossRef]
- Kim, K.K.; Lee, H.S.; Lee, Y.H. Synthesis of hexagonal boron nitride heterostructures for 2D van der Waals electronics. Chem. Soc. Rev. 2018, 47, 6342–6369. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, X.; Tan, S.J.R.; Xu, H.; Wu, B.; Liu, B.; Fu, D.; Fu, W.; Geng, D.; Liu, Y.; et al. Chemical vapor deposition of large-size monolayer MoSe2 crystals on molten glass. J. Am. Chem. Soc. 2017, 139, 1073–1076. [Google Scholar] [CrossRef]
- Liu, Z.; Gong, Y.; Zhou, W.; Ma, L.; Yu, J.; Idrobo, J.C.; Jung, J.; MacDonald, A.H.; Vajtai, R.; Lou, J.; et al. Ultrathin high-temperature oxidation-resistant coatings of hexagonal boron nitride. Nat. Commun. 2013, 4, 2541. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Zhou, J.; Gao, H.; Wang, J.; Wang, H.; Su, L.; Wan, P. Emerging one-/two-dimensional hetero nanostructure integrating SiC nanowires with MoS2 nanosheets for efficient electrocatalytic hydrogen evolution. ACS Appl. Mater. Interfaces 2020, 12, 19519–19529. [Google Scholar] [CrossRef] [PubMed]
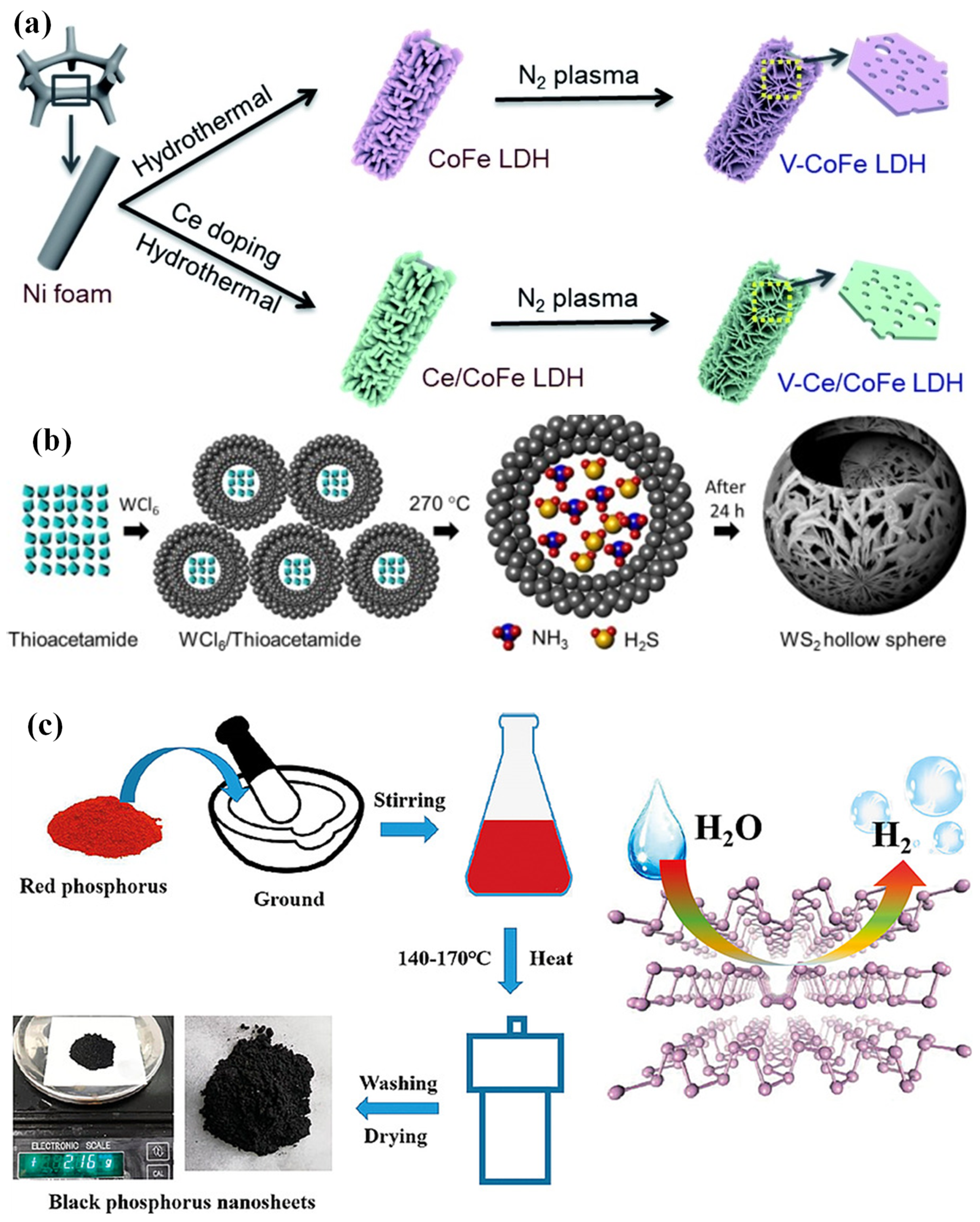
- Liu, S.; Zhu, J.; Sun, M.; Ma, Z.; Hu, K.; Tomohiko, N.; Liu, X.; Schmuki Patrik, S.; Wang, L. Promoting the hydrogen evolution reaction through oxygen vacancies and phase transformation engineering on layered double hydroxide nanosheets. J. Mater. Chem. A 2020, 8, 2490–2497. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Nguyen, D.L.T.; Nguyen, V.H.; Le, T.H.; Ly, Q.V.; Vo, D.V.N.; Van Le, Q. Facile synthesis of WS2 hollow spheres and their hydrogen evolution reaction performance. Appl. Surf. Sci. 2020, 505, 144574. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, B.; Hu, X.; Xie, Z. Surfactant-assisted hydrothermal synthesis of nitrogen doped Mo2C@ C composites as highly efficient electrocatalysts for hydrogen evolution reaction. Int. J. Hydrog. Energy 2019, 44, 3702–3710. [Google Scholar] [CrossRef]
- Wang, C.; Wang, A.W.; Feng, J.; Li, Z.; Chen, B.; Wu, Q.H.; Jiang, J.; Lu, J.; Li, Y.Y. Hydrothermal preparation of hierarchical MoS2-reduced graphene oxide nanocomposites towards remarkable enhanced visible-light photocatalytic activity. Ceram. Int. 2017, 43, 2384–2388. [Google Scholar] [CrossRef]
- Wang, H.; Peng, R.; Hood, Z.D.; Naguib, M.; Adhikari, S.P.; Wu, Z. Titania Composites with 2D Transition Metal Carbides as Photocatalysts for Hydrogen Production under Visible-Light Irradiation. ChemSusChem 2016, 9, 1490–1497. [Google Scholar] [CrossRef]
- Zhu, S.; Liang, Q.; Xu, Y.; Fu, H.; Xiao, X. Facile Solvothermal synthesis of black phosphorus nanosheets from red phosphorus for efficient photocatalytic hydrogen evolution. Eur. J. Inorg. Chem. 2020, 2020, 773–779. [Google Scholar] [CrossRef]
- Govindaraju, V.R.; Sreeramareddygari, M.; Hanumantharayudu, N.D.; Devaramani, S.; Thippeswamy, R.; Surareungchai, W. Solvothermal decoration of Cu3SnS4 on reduced graphene oxide for enhanced electrocatalytic hydrogen evolution reaction. Environ. Prog. Sustain. Energy 2020, 40, e13558. [Google Scholar]
- Chen, W.; Sun, L.; Li, Q.; Huo, L.; Zhao, H. Defect-rich MoS2/r-GO hybrid via microwave-assisted solvothermal process for efficient electrocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2020, 45, 22459–22468. [Google Scholar] [CrossRef]
- Cai, X.; Shen, X.; Ma, L.; Ji, Z.; Xu, C.; Yuan, A. Solvothermal synthesis of NiCo-layered double hydroxide nanosheets decorated on RGO sheets for high performance supercapacitor. Chem. Eng. J. 2015, 268, 251–259. [Google Scholar] [CrossRef]
- Feng, X.; Tang, Q.; Zhou, J.; Fang, J.; Ding, P.; Sun, L.; Shi, L. Novel mixed–solvothermal synthesis of MoS2 nanosheets with controllable morphologies. Cryst. Res. Technol. 2013, 48, 363–368. [Google Scholar] [CrossRef]
- Cai, Z.X.; Song, X.H.; Wang, Y.R.; Chen, X. Electrodeposition-Assisted Synthesis of Ni2P Nanosheets on 3D Graphene/Ni Foam Electrode and Its Performance for Electrocatalytic Hydrogen Production. ChemElectroChem 2015, 2, 1665–1671. [Google Scholar] [CrossRef]
- Babar, P.; Lokhande, A.; Karade, V.; Pawar, B.; Gang, M.G.; Pawar, S.; Kim, J.H. Bifunctional 2D electrocatalysts of transition metal hydroxide nanosheet arrays for water splitting and urea electrolysis. ACS Sustain. Chem. Eng. 2019, 7, 10035–10043. [Google Scholar] [CrossRef]
- Li, C.; Bo, X.; Li, M.; Guo, L. Facile electrodeposition fabrication of molybdenum-tungsten sulfide on carbon cloth for electrocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2017, 42, 15479–15488. [Google Scholar] [CrossRef]
- Jia, L.; Sun, X.; Jiang, Y.; Yu, S.; Wang, C. A novel MoSe2–reduced graphene oxide/polyimide composite film for applications in electrocatalysis and photoelectrocatalysis hydrogen evolution. Adv. Funct. Mater. 2015, 25, 1814–1820. [Google Scholar] [CrossRef]
- Huang, H.; Yan, M.; Yang, C.; He, H.; Jiang, Q.; Yang, L.; Yamauchi, Y. Graphene nanoarchitectonics: Recent advances in graphene-based electrocatalysts for hydrogen evolution reaction. Adv. Mater. 2019, 31, 1903415. [Google Scholar] [CrossRef]
- Ali, A.; Shen, P.K. Recent progress in graphene-based nanostructured electrocatalysts for overall water splitting. Electrochem. Energy Rev. 2020, 3, 370–394. [Google Scholar] [CrossRef]
- Yu, C.; Cao, Z.F.; Yang, F.; Wang, S.; Zhong, H. MoS2 confined on graphene by triethanolamine for enhancing electrocatalytic hydrogen evolution performance. Int. J. Hydrogen Energy 2019, 44, 28151–28162. [Google Scholar] [CrossRef]
- Yan, H.; Tian, C.; Wang, L.; Wu, A.; Meng, M.; Zhao, L.; Fu, H. Phosphorus-modified tungsten nitride/reduced graphene oxide as a high-performance, non-noble-metal electrocatalyst for the hydrogen evolution reaction. Angew. Chem. 2015, 127, 6423–6427. [Google Scholar] [CrossRef]
- Jiao, M.; Chen, Z.; Zhang, X.; Mou, K.; Liu, L. Multicomponent N doped graphene coating Co@Zn heterostructures electrocatalysts as high efficiency HER electrocatalyst in alkaline electrolyte. Int. J. Hydrogen Energy 2020, 45, 16326–16336. [Google Scholar] [CrossRef]
- Li, B.; Li, Z.; Pang, Q.; Zhang, J.Z. Core/shell cable-like Ni3S2 nanowires/N-doped graphene-like carbon layers as composite electrocatalyst for overall electrocatalytic water splitting. Chem. Eng. J. 2020, 401, 126045. [Google Scholar] [CrossRef]
- Lin, L.; Sherrell, P.; Liu, Y.; Lei, W.; Zhang, S.; Zhang, H.; Chen, J. Engineered 2D transition metal dichalcogenides-A vision of viable hydrogen evolution reaction catalysis. Adv. Energy Mater. 2020, 10, 1903870. [Google Scholar] [CrossRef]
- Yuan, S.; Pang, S.Y.; Hao, J. 2D transition metal dichalcogenides, carbides, nitrides, and their applications in supercapacitors and electrocatalytic hydrogen evolution reaction. Appl. Phys. Rev. 2020, 7, 021304. [Google Scholar] [CrossRef]
- Prabhu, P.; Jose, V.; Lee, J.M. Design strategies for development of TMD-based heterostructures in electrochemical energy systems. Matter 2020, 2, 526–553. [Google Scholar] [CrossRef]
- Lei, Z.; Xu, S.; Wu, P. Ultra-thin and porous MoSe2 nanosheets: Facile preparation and enhanced electrocatalytic activity towards the hydrogen evolution reaction. Phys. Chem. Chem. Phys. 2016, 18, 70–74. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, X.; Xin, P.; Wang, H.; Wu, Y.; Gao, C.; He, Q.; Jiang, Y.; Hu, Z.; Huang, S. Interface engineering of NiS@MoS2 core-shell microspheres as an efficient catalyst for hydrogen evolution reaction in both acidic and alkaline medium. J. Alloys Compd. 2021, 853, 157352. [Google Scholar] [CrossRef]
- Kumar, S.; Sahoo, P.K.; Satpati, A.K. Electrochemical and SECM investigation of MoS2/GO and MoS2/rGO nanocomposite materials for HER electrocatalysis. ACS Omega 2017, 2, 7532–7545. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Nguyen, D.M.T.; Le, H.K.; Vo, D.V.N.; Lam, S.S.; Varma, R.S.; Shokouhimehr, M.; Nguyen, C.C.; Le, Q.V. MXenes: Applications in electrocatalytic, photocatalytic hydrogen evolution reaction and CO2 reduction. Mol. Catal. 2020, 486, 110850. [Google Scholar] [CrossRef]
- Yoon, Y.; Tiwari, A.P.; Lee, M.; Choi, M.; Song, W.; Im, J.; An, K.S. Enhanced electrocatalytic activity by chemical nitridation of two-dimensional titanium carbide MXene for hydrogen evolution. J. Mater. Chem. A 2018, 6, 20869–20877. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Z.; Yu, M.; Xiu, L.; Qiu, J. Stabilizing the MXenes by carbon nano plating for developing hierarchical nanohybrids with efficient lithium storage and hydrogen evolution capability. Adv. Mater. 2017, 29, 1607017. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Xu, D.; Zhu, Y.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. Hierarchical “nanoroll” like MoS2/Ti3C2Tx hybrid with high electrocatalytic hydrogen evolution activity. Appl. Catal. B Environ. 2019, 241, 89–94. [Google Scholar] [CrossRef]
- Laipan, M.; Yu, J.; Zhu, R.; Zhu, J.; Smith, A.T.; He, H.; O’Hare, D.; Sun, L. Functionalized layered double hydroxides for innovative applications. Mater. Horiz. 2020, 7, 715–745. [Google Scholar]
- Yang, H.; Chen, Z.; Guo, P.; Fei, B.; Wu, R. B-doping-induced amorphization of LDH for large-current-density hydrogen evolution reaction. Appl. Catal. B Environ. 2020, 261, 118240. [Google Scholar] [CrossRef]
- Ye, W.; Fang, X.; Chen, X.; Yan, D. A three-dimensional nickel-chromium layered double hydroxide micro/nanosheet array as an efficient and stable bifunctional electrocatalyst for overall water splitting. Nanoscale 2018, 10, 19484–19491. [Google Scholar] [CrossRef]
- Li, K.; Guo, D.; Kang, J.; Wei, B.; Zhang, X.; Chen, Y. Hierarchical hollow spheres assembled with ultrathin CoMn double hydroxide nanosheets as trifunctional electrocatalyst for overall water splitting and Zn air battery. ACS Sustain. Chem. Eng. 2018, 6, 14641–14651. [Google Scholar] [CrossRef]
- Yousefzadeh, S.; Morovati, N. Modification of the ultrasonication derived-g-C3N4 nanosheets/quantum dots by MoS2 nanostructures to improve electrocatalytic hydrogen evolution reaction. Int. J. Hydrog. Energy 2020, 45, 33512–33520. [Google Scholar] [CrossRef]
- Paul, A.M.; Sajeev, A.; Nivetha, R.; Gothandapani, K.; Bhardwaj, P.; Govardhan, K.; Grace, A.N. Cuprous oxide (Cu2O)/graphitic carbon nitride (g-C3N4) nanocomposites for electrocatalytic hydrogen evolution reaction. Diam. Relat. Mater. 2020, 107, 107899. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, S.; Xu, Y.; Ge, R.; Qu, J.; Zhu, M.; Li, W. FeS2 bridging function to enhance charge transfer between MoS2 and g-C3N4 for efficient hydrogen evolution reaction. Chem. Eng. J. 2020, 127804. [Google Scholar] [CrossRef]

| 2D Materials | 2D Materials-Based Catalysts | Electrolyte | Overpotential (mV) at 10 mA cm−2 | Tafel Slope (mV dec−1) | Ref |
|---|---|---|---|---|---|
| Graphene | MoS2@pr-GO | 0.5 M H2SO4 | 263 | 60 | [70] |
| P-doped WN/r-GO | 0.5M H2SO4 | 85 | 54 | [71] | |
| NG@Co@Zn@NF-850 | 1.0 M KOH | 34 | 36 | [72] | |
| Ni3S2@NGCLs/NF | 1.0 M KOH | 134 | 84 | [73] | |
| TMDs | Porous MoSe2 Nanosheets | 0.5 M H2SO4 | 150 | 80 | [77] |
| V SACs@ 1T-WS2 | 0.5 M H2SO4 | 185 | 61 | [47] | |
| NiS@ MoS2-20 | 1.0 M KOH | 146 | 67 | [78] | |
| MoS2/GO | 0.5 M H2SO4 | 13.1 | 40 | [79] | |
| MXenes | NiS2/V-MXene | 1.0M KOH | 179 | 85 | [44] |
| 11N-Ti2CTx | 0.5 M H2SO4 | 215 | 67 | [81] | |
| MoS2/Ti3C2-MXene@C | 0.5 M H2SO4 | 135 | 45 | [82] | |
| MoS2/TiC2Tx hybrid | 0.5 M H2SO4 | 152 | 70 | [83] | |
| LDHs | A-NiCo LDH/NF | 1 M KOH | 151 | 57 | [85] |
| Ni2Cr1-LDH | 1.0 M KOH | 67 | 62 | [86] | |
| Co2Mn1-DH | 1 M KOH | 187 | 60 | [87] | |
| V-Ce/CoFe LDH | 1 M KOH | 73 | 69 | [54] | |
| g-C3N4 | g-C3N4/FeS2/MoS2 | 0.5 M H2SO4 | 193 | 88 | [90] |
| g-C3N4 NS/QD modified MoS2 | 0.5 M H2SO4 | 280 | 88 | [87] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahoo, P.K.; Bisoi, S.R.; Huang, Y.-J.; Tsai, D.-S.; Lee, C.-P. 2D-Layered Non-Precious Electrocatalysts for Hydrogen Evolution Reaction: Fundamentals to Applications. Catalysts 2021, 11, 689. https://doi.org/10.3390/catal11060689
Sahoo PK, Bisoi SR, Huang Y-J, Tsai D-S, Lee C-P. 2D-Layered Non-Precious Electrocatalysts for Hydrogen Evolution Reaction: Fundamentals to Applications. Catalysts. 2021; 11(6):689. https://doi.org/10.3390/catal11060689
Chicago/Turabian StyleSahoo, Prasanta Kumar, Soubhagya Ranjan Bisoi, Yi-June Huang, Dung-Sheng Tsai, and Chuan-Pei Lee. 2021. "2D-Layered Non-Precious Electrocatalysts for Hydrogen Evolution Reaction: Fundamentals to Applications" Catalysts 11, no. 6: 689. https://doi.org/10.3390/catal11060689
APA StyleSahoo, P. K., Bisoi, S. R., Huang, Y.-J., Tsai, D.-S., & Lee, C.-P. (2021). 2D-Layered Non-Precious Electrocatalysts for Hydrogen Evolution Reaction: Fundamentals to Applications. Catalysts, 11(6), 689. https://doi.org/10.3390/catal11060689








