Abstract
Titanium dioxide (TiO2) is a commonly used wide bandgap semiconductor material for energy and environmental applications. Although it is a promising candidate for photovoltaic and photocatalytic applications, its overall performance is still limited due to low mobility of porous TiO2 and its limited spectral response. This limitation can be overcome by several ways, one of which is doping that could be used to improve the light harvesting properties of TiO2 by tuning its bandgap. TiO2 doped with elements, such as alkali-earth metals, transition metals, rare-earth elements, and nonmetals, were found to improve its performance in the photovoltaic and photocatalytic applications. Among the doped TiO2 nanomaterials, transition metal doped TiO2 nanomaterials perform efficiently by suppressing the relaxation and recombination of charge carriers and improving the absorption of light in the visible region. This work reports the possibility of enhancing the performance of TiO2 towards Dye Sensitised Solar Cells (DSSCs) and photocatalytic degradation of methylene blue (MB) by employing Zn doping on TiO2 nanomaterials. Zn doping was carried out by varying the mole percentage of Zn on TiO2 by a facile solvothermal method and the synthesized nanomaterials were characterised. The XRD (X-Ray Diffraction) studies confirmed the presence of anatase phase of TiO2 in the synthesized nanomaterials, unaffected by Zn doping. The UV-Visible spectrum of Zn-doped TiO2 showed a red shift which could be attributed to the reduced bandgap resulted by Zn doping. Significant enhancement in Power Conversion Efficiency (PCE) was observed with 1.0 mol% Zn-doped TiO2 based DSSC, which was 35% greater than that of the control device. In addition, it showed complete degradation of MB within 3 h of light illumination and rate constant of resembling zeroth order reaction. These improvements are attributed to the reduced bandgap energy and the reduced charge recombination by Zn doping on TiO2.
1. Introduction
The global population is increasing rapidly, and the limited supply of nonrenewable energy leads to rising energy demands, especially in developing countries. This risks the depletion of cheap fossil energy sources and an increase in environmental pollution as well as climate change [1]. Hence, researchers around the world were constantly inventing solutions to expand the energy sources and reduce greenhouse gas emissions. This took renewable energy sources into the spotlight [2]. Renewable energy sources such as solar, wind, geothermal, hydropower, biofuel, and biomass are considered to be cleaner and more environmentally friendly sources of power. However, energy production from these alternative energy sources is yet restricted because of high production costs and poor energy conversion efficiency. Nanotechnology is increasingly playing an active role in overcoming the above limitations [3,4].
Titanium dioxide (TiO2) was widely used as a golden standard nanomaterial for sustainable energy generation and the removal of environmental pollutants [5,6] due to its efficient photocatalytic activity, low cost, nontoxic nature and high stability [7]. Although TiO2 exhibits the desired performance in ultraviolet light, its overall performance is still limited because of low mobility of porous TiO2 [8] and its limited spectral response wide bandgap (3.0–3.2 eV) that cannot make use of visible light [9]. Several strategies were investigated and reported to overcome this limitation, which include preparation of nanocomposites [10,11,12], doping/codoping [5,13,14], and synthesis of particles with different nanostructures [15,16], such as nanorods, nanowires, nanotubes, etc. Among these strategies, doping/co-doping displays major impacts on the band structure and trap states of TiO2, and hence, alters its properties such as conduction band energy, charge transport, recombination, and collection significantly [17]. Even though nonmetals, alkali-earth metals, and rare-earth elements are being used as dopants, transition metal doped TiO2 nanomaterials are notable candidates for photovoltaic and photocatalytic degradation applications as they improve absorption in the visible region and suppress relaxation and recombination of charge carriers [13].
Zinc (Zn) is one of the promising n-type transition metals; it improves the photocurrent of Dye Sensitized Solar Cell (DSSC) when incorporated with TiO2 [18]. It also enhances the photocatalytic activity in visible region [19] by reducing the bandgap of TiO2 [20,21]. Zhu et al. demonstrated the photovoltaic analysis of hydrothermally synthesized Zn-doped TiO2 photoanode, and their findings reveal that incorporation of Zn2+ ions into an anatase lattice of TiO2 elevates the edge of conduction band (CB) of the photoanode and the Fermi level is shifted towards the CB edge, which contributes to the improvement in open-circuit voltage (VOC), and thus, enhanced photovoltaic performance [22]. Huang et al. reported that the device fabricated with Zn-doped TiO2 nanomaterials, synthesized by agarose gel method, shows better photovoltaic performance due to the improved short circuit current density (JSC) [18]. Zn-doped TiO2 is widely used in photocatalysis as well. Zhao et al. analyzed the photocatalytic degradation of Rhodamine B dye using Zn-doped TiO2, synthesized by hydrogen–oxygen diffusion flame method, where Zn doping creates appropriate energetic position between ZnO and the excited state of dye molecule, which enhances the electron injection into the conduction band of TiO2 by capturing electrons, and subsequently promotes the formation of reactive oxygen species which enhances the degradation process [20]. Chen et al. demonstrated the degradation of Methyl orange by Zn-doped TiO2 synthesized by stearic acid gel method, and higher photocatalytic activity was achieved due to greater BET surface area by Zn doping [23]. Zn-doped TiO2 was synthesized using facile sol-gel reflux method by Tariq et al., and the synthesized nanomaterials were used to degrade methylene blue (MB) and methyl orange dyes. Here, Zn acted as a reductant to facilitate the Ti3+ formation and a stabilizer for the oxygen vacancies [21]. Hence, Zn effectively modified the properties of TiO2 and improved the degradation of dye molecules.
The doped TiO2 nanomaterials can be synthesized by different methods including hydrothermal synthesis [22], solvothermal synthesis [24], spray pyrolysis [25], microwave synthesis [26], and spin coating [27]. However, hydrothermal and solvothermal methods are preferable, as high crystalline nanomaterials are formed in these syntheses which leads to uniform particle distribution [28]. Although several studies reported on photovoltaic and photocatalytic activities of Zn-doped TiO2, the present study reports a simple solvothermal synthesis of Zn-doped TiO2 nanomaterials utilizing cost effective reaction bottles instead of autoclave followed by characterization of the same, and investigates their efficiency on photovoltaic and photocatalytic degradation applications.
2. Results and Discussion
The Zn-doped and undoped TiO2 nanomaterials, synthesized by a simple solvothermal method, were structurally and optically characterized utilizing XRD, EDX (Energy Dispersive X-ray), and UV-Visible spectroscopies, and their photovoltaic and photocatalytic degradation activity were studied.
2.1. X-ray Diffraction (XRD) Spectroscopy
The diffraction pattern and crystal structure of the synthesized, undoped, and 0.5, 1.0, 1.5, and 2.0 mol% Zn-doped TiO2 nanomaterials were examined by XRD, as illustrated in Figure 1. For XRD analysis, the powder sample was placed on a microscopic glass plate and pressed by another glass plate to obtain thin layer of the sample. The peaks attained at the 2θ values of 25.2°, 37.6°, 48.2°, 53.7°, 55.0°, 62.5°, 68.5°, 70.2°, 74.8°, and 82.5° correspond to the reflection planes of (101), (004), (200), (105), (211), (204), (116), (220), (215), and (224), respectively. The observed peaks, corresponding to only anatase phase of TiO2, for all undoped and Zn-doped TiO2 nanomaterials indicate that incorporation of Zn on TiO2 did not influence phase transformation. This may be attributed to the similar ionic radii of Ti4+ (0.74 Å) and Zn2+ (0.605 Å) ions [29] which allow Zn2+ ions to be easily accommodated on to the TiO2 lattice without altering their crystal structure.
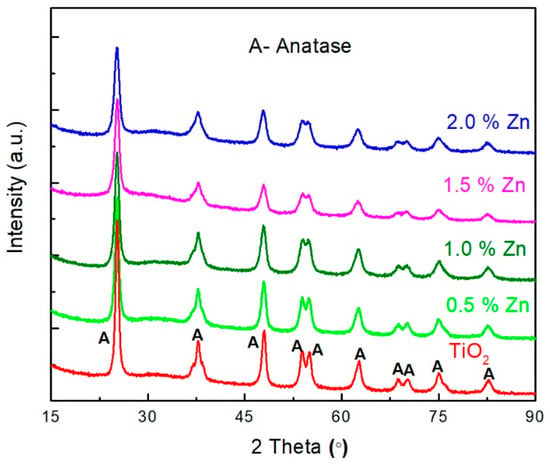
Figure 1.
X-Ray Diffraction (XRD) patterns of the undoped and Zn-doped TiO2 nanomaterials.
The average crystalline size of the synthesized nanomaterials was estimated by Debye–Scherrer equation using the predominant anatase (101) plane.
where, d is the average crystalline size of the particles, k is the dimensionless shape factor which has a typical value of about 0.89, λ is the wavelength of the X-ray beam (0.5406 nm), θ is the Bragg angle, and β is the full width at half maxima (FWHM), and is calculated from the predominant anatase (101) plane. The estimated crystalline sizes were about 12, 10, 9, 8, and 7 nm for undoped, 0.5, 1.0, 1.5, and 2.0 mol% Zn-doped TiO2 nanomaterials, respectively. These results demonstrate that Zn doping reduces the crystalline size of nanomaterials. Huang et al. supported this observation by reporting that Zn–O–Ti bonds are formed during the doping of Zn on TiO2, which inhibit the growth of crystal grains of TiO2 and subsequently reduce the size of particles [18]. The lattice strain of the nanomaterials was calculated using Williamson–Hall (W–H) plot method.
where, λ is the wavelength of X-ray radiation, β is FWHM, and θ is the Bragg angle of the diffraction peaks, is the crystallite size with lattice strain, and ε is the effective value of the lattice strain. βCosθ is plotted against 4Sinθ, and after linear fitting, the slope gives the value of lattice strain [30,31]. The values of the lattice strain are 7.53 × 10−5, 1.16 × 10−4, 5.38 × 10−4, −0.00499, and −0.00296 for the undoped, and 0.5, 1.0, 1.5, and 2.0 for the mol% Zn-doped TiO2 nanomaterials, respectively. Positive lattice strain value indicates that the system is under tensile strain, and negative value indicates that the system is under compressive strain [32]. Lattice strain of the 1.0 and 0.5 mol% of Zn-doping is higher than undoped TiO2 due to the reduced crystalline size, and the values are negative for the 1.5 and 2.0 mol% of Zn doping [32,33]. The formation of ZnO in the higher Zn doping may cause distortion, which might be the reason for the negative value. Further, the absence of peaks for Zn in the XRD of Zn-doped TiO2 nanomaterials may be attributed to the very small quantity of Zn doped on TiO2 and homogeneous dispersion of Zn on TiO2; the same was reported by Yanqi et al. [34] and Feng et al. [22].
2.2. Energy Dispersive X-ray (EDX) Spectroscopy
The existence of Zn in the Zn-doped TiO2 nanomaterials was confirmed by EDX analysis, as shown in Figure 2. The summary of EDX results, presented in Table 1, confirms that the amount of Zn doped in TiO2 rises with the increase in Zn dopant used for the synthesis of Zn-doped TiO2 nanomaterials, and the distribution of Zn dopant in TiO2 is found to be uniform.
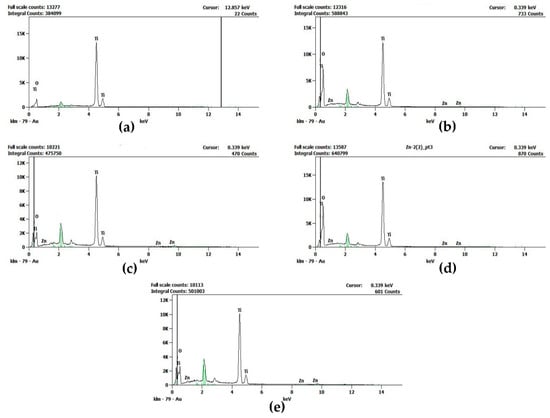
Figure 2.
Energy Dispersive X-ray (EDX) spectroscopic images of (a) undoped, (b) 0.5 mol% Zn-doped, (c) 1.0 mol% Zn-doped, (d) 1.5 mol % Zn-doped, and (e) 2.0 mol% Zn-doped TiO2 nanomaterials.

Table 1.
Mole percentage of Zn in Zn-doped TiO2 nanomaterials.
2.3. UV-Visible Spectroscopy
To study the optical properties of the Zn-doped and undoped TiO2, the powder samples were coated on the microscopic glass plate by doctor-blade method and analyzed by UV-visible spectroscopy, as shown in Figure 3. Zn doping in TiO2 led to a red-shift in the UV-visible light absorption of TiO2 and the shift moved towards longer wavelength when the Zn content increased in the Zn-doped TiO2 nanomaterials.
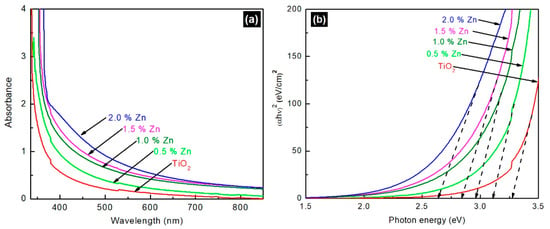
Figure 3.
(a) UV-visible absorption spectra of undoped and Zn-doped TiO2 nanomaterials; (b) Tauc-plot on estimated bandgap values of undoped and Zn-doped TiO2 nanomaterials.
The Tauc’s formula, given below, was used to estimate the bandgap of the films from UV-visible Spectra:
where is the absorbance coefficient, is the photon energy, A is a constant, is the bandgap energy, and n is the exponential constant index which depends on the nature of transition (n = ½ and 2 for indirectly and directly allowed transitions, respectively). The estimated bandgap values obtained from Tauc-plot are 3.3, 3.1, 3.0, 2.9, and 2.7 eV for the undoped, 0.5, 1.0, 1.5, and 2.0 mol% Zn-doped TiO2, respectively. The above bandgap values, which reveal Zn-doping in TiO2 reduces the bandgap of TiO2, are in good agreement with the theoretical values reported by the Wang et al. [35] and experimental trends reported by Arunachalam et al. [36] and Aware et al. [37]. It is noteworthy that observations contradictory to the present study were also reported in literature [22].
2.4. Photovoltaic Measurement
2.4.1. J-V Measurement
The photovoltaic performance of the fabricated DSSCs with undoped and Zn-doped TiO2 photoanodes was tested under simulated irradiation of intensity 100 mW/cm2 with Air Mass (AM) 1.5 filter. The results are illustrated in Figure 4, and the related photovoltaic parameters are summarized in Table 2.
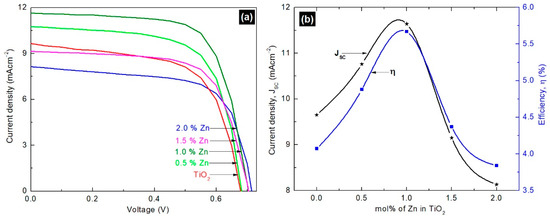
Figure 4.
(a) Photovoltaic performances of Dye Sensitized Solar Cells (DSSCs) with undoped and Zn-doped TiO2 photoanodes; (b) variations in short circuit current density (Jsc) and efficiency (η) with different mol% Zn dopants under illumination of intensity 100 mWcm−2 with Air Mass (AM) 1.5 filter.

Table 2.
Photovoltaic parameters of DSSCs with undoped and Zn-doped TiO2 photoanodes under illumination of intensity 100 mWcm−2 with AM 1.5 filter.
The results reveal improved JSC, open circuit voltage (VOC), and fill factor (FF) values for DSSCs fabricated with 0.5 and 1.0 mol% Zn-doped TiO2 compared to that of the control device. The FF value increased up to 0.69 for the device made with 1.0 mol% Zn-doped TiO2, followed by a reduction to 0.66 for the one made with 2.0 mol% Zn dopant. The VOC sequentially increased from 0.67 to 0.71 V with increase in Zn content in the Zn-doped TiO2. Nevertheless, the performance of the device, η, was mainly dominated by its JSC value (as illustrated in Figure 4 (b)). The observed highest JSC value of 11.64 mAcm−2 corresponds to the device with 1.0 mol% Zn-doped TiO2 photoanode. Zn is a n-type dopant, and hence, Zn doping increases the electron density in the conduction band of TiO2, and thereby improves charge transport in the device [38]. A similar trend was observed in the present study up to 1.0 mol% of Zn content, but the charge transport was reduced with 1.5 and 2.0 mol% of dopant concentrations, maybe due to the recombination of electron-hole pairs. Jianping et al. reported on the formation of a shallow trapping centre of photo-generated carriers at low amounts of Zn dopant, which results in efficient separation of electron-hole pairs and long carrier lifetime and subsequent reduction in carrier recombination followed by rise in photocurrent. However, at higher amounts of Zn dopant, ZnO clusters onto the surface of TiO2 by forming surface states. Hence, ZnO acts as a defect site and captures the electrons, which results in an increase in the recombination rate and causes a decrease in JSC [38]. The present study exhibited an improved PCE of 5.67% for 1.0 mol% Zn-doped TiO2 photoanode-based device, which was 35% higher than that of the control device fabricated with undoped TiO2. This improvement in PCE is mainly attributed to the enhanced JSC value; this observation was further confirmed by the EIS measurements.
2.4.2. Electrochemical Impedance Spectroscopy (EIS)
The EIS measurements were performed on the fabricated DSSCs by two electrode method at frequencies from 10−2 to 106 Hz in the dark, with a bias applied voltage of 10 mV. Figure 5 shows the Nyquist plot of the electrochemical impedance spectra of DSSCs fabricated with undoped and Zn-doped TiO2 photoanodes.
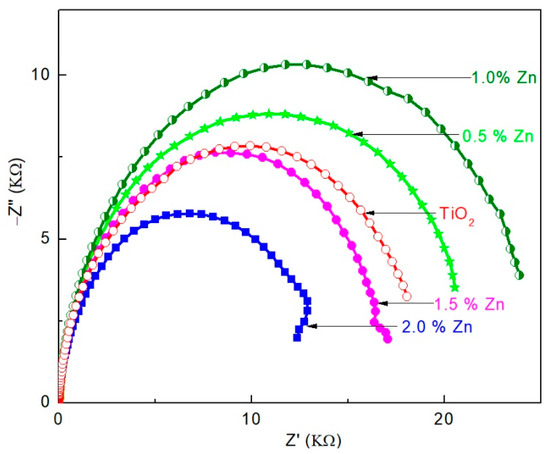
Figure 5.
Nyquist plot of DSSCs with undoped, 0.5, 1.0, 1.5, and 2.0 mol% Zn-doped TiO2 in dark.
As shown in the Figure 5, these Nyquist plots are related to the charge recombination resistance (R2) across the TiO2/electrolyte interface, with a partial contribution from electron transport and accumulation in TiO2 photoanode [39]. R2 value of the undoped and Zn-doped TiO2 photoanodes-based DSSCs can be estimated from the diameter of the respective semicircles [13]; a high R2 value indicates low charge recombination rate [13,40]. In the present study, the R2 value of undoped TiO2 is lower than those of 0.5 and 1.0 mol% Zn-doped TiO2 based DSSCs and higher than that of 1.5 and 2.0 mol% Zn-doped TiO2 based device. Hence, the lowest charge recombination rate is attributed to the 1.0 mol% Zn-doped TiO2 based device. These results are in good agreement with the JSC values and the corresponding η values of the fabricated devices.
2.5. Photocatalytic Degradation Measurement
Photocatalytic degradation of MB using Zn-doped and undoped TiO2 nanomaterials was performed under direct sunlight. Prior to the light exposure, the reaction suspension was allowed to establish adsorption-desorption equilibrium by stirring the suspension in dark for 30 min. Subsequently, the suspension was exposed to direct sunlight for a stipulated period (noon daytime) during which the MB retained in the suspension was measured at regular time intervals. The concentration of MB decreased with time, and the absorption peak corresponding to MB (λ = 663 nm) was found to disappear after 2.5 h. The concentration vs. time plots obtained for different Zn-doped TiO2 (0.5, 1.0, 1.5, and 2.0 mol%) and undoped TiO2 samples are depicted in Figure 6.
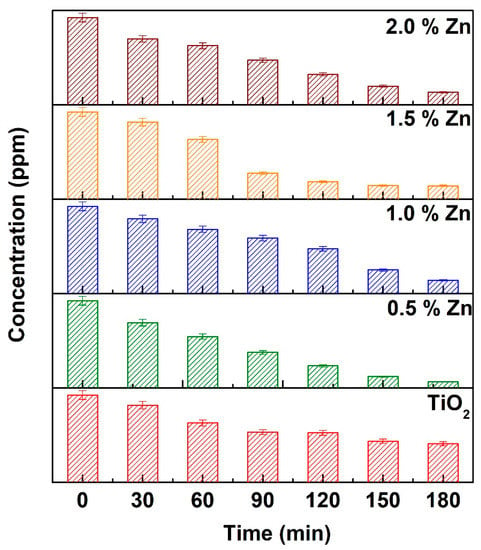
Figure 6.
Concentration vs. time plot for degradation of methylene blue (MB) by undoped, 0.5, 1.0, 1.5, and 2.0 mol% Zn-doped TiO2 as photocatalysts under direct sunlight.
The MB dye was used as probing molecule to explore the photocatalytic degradation property of the synthesized nanomaterials. For the overall reaction, the zeroth order kinetic model with the optimum rate constant of 1.5466 ± 0.0873 × 10−4 s−1 for TiO2 doped 1.0% Zn doping (Table 3). There was a minimum 41% of increase in the activity while Zn was doped in 0.5% over bare TiO2. Moreover, the dark adsorption was carried out for 30 min to let the adsorption-desorption equilibrium be achieved, and during this process, the dye molecules are merely transferred from liquid stage to the solid state. With the absorbance vs. wavelength plot (as illustrated in Figure S1a) of dark adsorption studies, once the adsorption-desorption equilibrium is achieved, there is not much change in the concentration of dye with time. However, the degradation occurs under the light exposure in the presence of catalyst (as illustrated in Figure S1b), and these results validate the contribution of catalyst in the dye degradation process. Further, the study without catalyst confirms that there is no self-degradation of dyes (as illustrated in Figure S1c). In summary, the catalysts used in this study are very effective against dye degradation in the presence of sunlight. Further, recycling study was carried out with 1% Zn-doped TiO2 nanomaterial to examine its reusability. After the photocatalytic experiment, the catalyst was separated from the reaction mixture by centrifugation and used for 2nd cycle by following the same experimental conditions.

Table 3.
Overall rate constants for photocatalytic degradation of MB under direct sunlight with undoped, 0.5, 1.0, 1.5, and 2.0 mol% Zn-doped TiO2 as photocatalysts.
As shown in Figure 7, the photocatalyst did not exhibit any significant loss in the 2nd cycle of degradation of MB under the same conditions. The calculated degradation percentage were found to be 99% and 82% for 1st and 2nd use, respectively. The results confirm that the synthesized Zn-doped TiO2 photocatalyst shows good stability and sustainability. Moreover, the rate of the photocatalytic degradation results obtained in this study is compared with that of the literature and summarized in the Table 4.
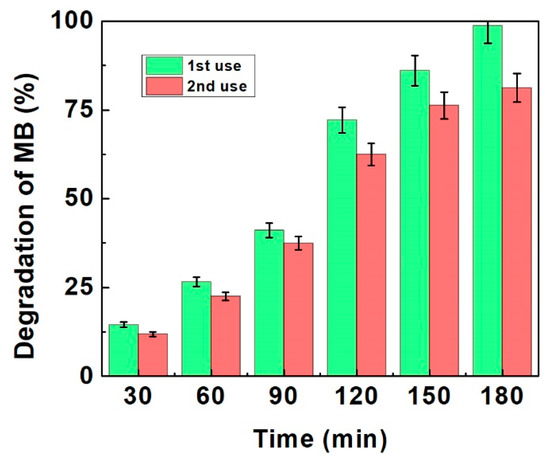
Figure 7.
Reusability of optimized 1% Zn-doped TiO2 nanomaterial.

Table 4.
A comparison of photocatalytic degradation of MB by different doped/codoped TiO2 nanomaterials.
Although the experimental conditions and other factors vary, based on the summary of the table, our proposed synthesised nanomaterials effectively degrade the MB under the sunlight compared to that of the other reports. Therefore, this cost effective solvothermal method is preferable for the synthesis of nanomaterials for energy and environmental applications.
3. Materials and Methods
3.1. Materials
Absolute ethanol (>99%), Triton X-100, Zinc acetate dihydrate (≥98%), Titanium tetraisopropoxide (TTIP, >98%), Di-tetrabutylammonium cis-bis (isothiocyanato)bis(2,2′-bipyridyl-4,4′-dicarboxylato) ruthenium (II) dye (95%), Acetonitrile, Tert-butyl alcohol (≥99.7%), and MB (95%) were purchased from Sigma–Aldrich, Oslo, Norway AS. Acetylacetone (≥99.5%) was purchased from Fluka Analytical, Munich, Germany. All the materials were used without further purification.
3.2. Methodology
3.2.1. Synthesis of Zn-Doped and Undoped TiO2 Nanomaterials
A 3 mL mixture of TTIP and ethanol in 1:16 volume ratio was vigorously stirred in a 50 mL reaction bottle. Appropriate amounts of Zn(CH3COO)2.2H2O were then added separately to the above solution to prepare different photocatalysts with 0.5, 1.0, 1.5, and 2.0 mol% Zn, and the resultant mixtures were stirred well for one hour. Subsequently, a solution containing ethanol and deionized water in 1:1 volume ratio was added separately to the above mixtures. A white color precipitate was attained after addition of ethanol-water mixture. Then, the products were sintered at 90 °C for 6 h, dried at 100 °C to get powder samples, and subsequently calcinated at 500 °C for 2 h to obtain 0.5, 1.0, 1.5, and 2.0 mol% Zn-doped TiO2 nanomaterials [46]. The undoped TiO2 was synthesized by adopting the same procedure without employing Zn(CH3COO)2.2H2O.
3.2.2. Device Fabrication
The Fluorine doped Tin Oxide (FTO) coated glass substrates (Sigma–Aldrich surface resistivity 7.5 Ω/cm2) were cleaned in ultrasonic bath for 10 min with soap water, distilled water and ethanol, successively. The synthesized undoped and Zn-doped TiO2 nanomaterials were coated separately on cleaned FTO substrates by doctor-blade method. Then, the resultant films were calcinated at 500 °C for 30 min and soaked in 0.3 mM solution of N719 dye and prepared in a mixture of acetonitrile and tert-butyl alcohol [46,47] for 12 h. Afterwards, the dye coated films were rinsed in acetonitrile and subsequently dried. The platinum (Pt) coated glass substrate [48] was assembled with each dye-coated photoanode as counter electrode. Finally, a small amount of / electrolyte was injected in between the dye coated photoanode and Pt counter electrode to complete the fabrication of DSSC.
3.2.3. Photocatalytic Degradation
Photocatalytic degradation of MB solution was performed using undoped and 0.5, 1.0, 1.5, and 2.0 mol% Zn-doped TiO2 nanomaterials. As reported in our previous study [49], 25.0 mg of the photocatalyst was suspended in 50 mL of MB solution (initial concentration was 10 ppm) under direct sunlight (intensity of ~100 mWcm−2). Prior to irradiation, the MB solution with photocatalyst was stirred in the dark for 30 min to ensure the establishment of adsorption or desorption equilibrium. Periodically, 3 mL of suspension was withdrawn and centrifuged, and its absorbance was obtained using UV-visible spectrophotometer (JENWAY 6800, OSA, UK).
3.3. Characterization
The structural properties of the synthesized nanomaterials were studied by the X-ray diffraction method (PANalytical-AERIS, Almelo, The Netherland) using scan range (2θ) between 20–90° with step size of 0.02° and scan speed of 1°/min. The optical absorbance spectra were recorded using Shimadzu 1800 Scanning Double Beam UV-visible spectrophotometer. The elemental composition of the synthesized nanomaterials was analyzed by the energy-dispersive X-ray spectroscopy technique. The photovoltaic performance of the cells was studied using Keithley-2400 source measurement unit (SMU) under simulated irradiation by 150 W Xe lamp of intensity 100 mWcm−2 with AM 1.5 filter (Peccell-PEC-L12, Kanagawa, Japan), and the effective area of the devices is 0.25 cm2. Electrochemical impedance spectroscopy (EIS) measurements were carried out on the DSSCs using Metrohm Autolab potentiostat/galvanostat (PGSTAT 128N, Utrecht, Netherlands) with a frequency response analyzer (FRA 32M).
4. Conclusions
In the present study, undoped and Zn-doped TiO2 nanomaterials were synthesized by a simple solvothermal method and characterized. The XRD study revealed the existence of anatase phase without any phase transformation in all the synthesized nanomaterials. The UV-visible spectra of Zn-doped TiO2 nanomaterials demonstrated red shift compared to that of the spectrum of undoped TiO2. The EDX spectroscopy confirmed the inclusion of Zn element in the Zn-doped TiO2 nanomaterials. The photovoltaic performances of DSSCs fabricated with the synthesized undoped and Zn-doped nanomaterials were analyzed; 1.0 mol% Zn-doped TiO2 based device exhibited PCE of 5.67%, which was greater than 35% enhancement compared to that of the control device due to increased JSC. Moreover, 1.0 mol% Zn-doped TiO2 displayed efficient photocatalytic property in the degradation of MB under direct sun light. The improved performance revealed by the 1.0 mol% Zn-doped TiO2 nanomaterials in both photovoltaic and photocatalytic applications could be attributed to its reduced charge recombination rate and enhanced visible light harvesting ability.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/catal11060690/s1, Figure S1. Absorbance vs. wavelength plot for (a) methylene blue with catalyst [1.0 mol% Zn-TiO2] under dark condition (b) methylene blue with catalyst [1.0 mol% Zn-TiO2] in the presence of light (c) photolysis of methylene blue without catalyst in the presence of light.
Author Contributions
Conceptualization, T.R., S.S., V.G and S.Y.; methodology, T.R., S.S., V.G. and S.Y.; software, T.R. and S.S.; validation, T.R., S.S., S.Y., D.V., P.R. and M.S.; formal analysis, T.R., V.G and S.S.; investigation, T.R., S.S. and S.Y.; resources, D.V., P.R.; data curation, T.R., S.S., S.Y., D.V., P.R. and M.S.; writing—original draft preparation, T.R. and S.S.; writing—review and editing, S.Y. and M.S.; supervision, S.Y., D.V., P.R. and M.S.; project administration, D.V. and P.R.; funding acquisition, D.V. and P.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Capacity Building and Establishment of a Research Consortium (CBERC) project, grant number LKA-3182-HRNCET, and Higher Education and Research collaboration on Nanomaterials for Clean Energy Technologies (HRNCET) project, grant number NORPART/2016/10237.
Data Availability Statement
Data available in a publicly accessible repository.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Perera, F. Pollution from fossil-fuel combustion is the leading environmental threat to global pediatric health and equity: Solutions exist. Int. J. Environ. Res. Public Health 2018, 15, 16. [Google Scholar] [CrossRef]
- Owusu, P.A.; Asumadu-Sarkodie, S. A review of renewable energy sources, sustainability issues and climate change mitigation. Cogent Eng. 2016, 3, 1–14. [Google Scholar] [CrossRef]
- Nyankson, E.; Agyei-Tuffour, B.; Asare, J.; Annan, E.; Rwenyagila, E.R.; Konadu, D.S.; Yaya, A.; Dodoo-Arhin, D. Nanostructured TiO2 and their energy applications—A review. ARPN J. Eng. Appl. Sci. 2013, 8, 871–886. [Google Scholar]
- Chen, S.; Li, L.; Sun, H.; Sun, J.; Lu, B. Nanomaterials for renewable energy. J. Nanomater. 2015, 2015, 2–4. [Google Scholar] [CrossRef]
- Shaban, M.; Ahmed, A.M.; Shehata, N.; Betiha, M.A.; Rabie, A.M. Ni-doped and Ni/Cr co-doped TiO2 nanotubes for enhancement of photocatalytic degradation of methylene blue. J. Colloid Interface Sci. 2019, 555, 31–41. [Google Scholar] [CrossRef]
- Lü, X.; Xia, B.; Liu, C.; Yang, Y.; Tang, H. TiO2-Based Nanomaterials for Advanced Environmental and Energy-Related Applications. J. Nanomater. 2016, 2016. [Google Scholar] [CrossRef]
- Moma, J.; Baloyi, J. Modified titanium dioxide for photocatalytic applications. In Photocatalysts—Applications and Attributes; Intechopen: London, UK, 2019; Volume 18. [Google Scholar]
- Aduda, B.O.; Ravirajan, P.; Choy, K.L.; Nelson, J. Effect of morphology on electron drift mobility in porous TiO2. Int. J. Photoenergy 2004, 6, 141–147. [Google Scholar] [CrossRef]
- Kajana, T.; Velauthapillai, D.; Shivatharsiny, Y.; Ravirajan, P.; Yuvapragasam, A.; Senthilnanthanan, M. Structural and photoelectrochemical characterization of heterostructured carbon sheet/Ag2MoO4-SnS/Pt photocapacitor. J. Photochem. Photobiol. A Chem. 2020, 401, 112784. [Google Scholar] [CrossRef]
- Shanmugaratnam, S.; Velauthapillai, D.; Ravirajan, P.; Christy, A.A.; Shivatharsiny, Y. CoS2/TiO2 Nanocomposites for Hydrogen Production under UV Irradiation. Materials 2019, 12, 3882. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qin, M.; Yu, W.; Zhang, Q.; Xie, H.; Sun, Z.; Shao, Q.; Guo, X.; Hao, L.; Zheng, Y. Heterostructured TiO2/WO3 nanocomposites for photocatalytic degradation of toluene under visible light. J. Electrochem. Soc. 2017, 164, H1086. [Google Scholar] [CrossRef]
- Muduli, S.; Game, O.; Dhas, V.; Vijayamohanan, K.; Bogle, K.A.; Valanoor, N.; Ogale, S.B. TiO2–Au plasmonic nanocomposite for enhanced dye-sensitized solar cell (DSSC) performance. Sol. Energy 2012, 86, 1428–1434. [Google Scholar] [CrossRef]
- Rajaramanan, T.; Natarajan, M.; Ravirajan, P.; Senthilnanthanan, M.; Velauthapillai, D. Ruthenium (Ru) Doped Titanium Dioxide (P25) electrode for dye sensitized solar cells. Energies 2020, 13, 1532. [Google Scholar] [CrossRef]
- Tanyi, A.R.; Rafieh, A.I.; Ekaneyaka, P.; Tan, A.L.; Young, D.J.; Zheng, Z.; Chellappan, V.; Subramanian, G.S.; Chandrakanthi, R.L.N. Enhanced efficiency of dye-sensitized solar cells based on Mg and la co-doped TiO2 photoanodes. Electrochim. Acta 2015, 178, 240–248. [Google Scholar] [CrossRef]
- Madurai Ramakrishnan, V.; Natarajan, M.; Pitchaiya, S.; Santhanam, A.; Velauthapillai, D.; Pugazhendhi, A. Microwave assisted solvothermal synthesis of quasi cubic F doped TiO2 nanostructures and its performance as dye sensitized solar cell photoanode. Int. J. Energy Res. 2020, 1–10. [Google Scholar] [CrossRef]
- Feng, T.; Feng, G.S.; Yan, L.; Pan, J.H. One-dimensional nanostructured TiO2 for photocatalytic degradation of organic pollutants in wastewater. Int. J. Photoenergy 2014, 2014, 563879. [Google Scholar] [CrossRef]
- Roose, B.; Pathak, S.; Steiner, U. Doping of TiO2 for sensitized solar cells. Chem. Soc. Rev. 2015. [Google Scholar] [CrossRef]
- Huang, F.; Li, Q.; Thorogood, G.J.; Cheng, Y.-B.; Caruso, R.A. Zn-doped TiO2 electrodes in dye-sensitized solar cells for enhanced photocurrent. J. Mater. Chem. 2012, 22, 17128–17132. [Google Scholar] [CrossRef]
- Singla, P.; Sharma, M.; Pandey, O.P.; Singh, K. Photocatalytic degradation of azo dyes using Zn-doped and undoped TiO2 nanoparticles. Appl. Phys. A 2013. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, C.; Liu, X.; Gu, F.; Du, H.L.; Shi, L. Zn-doped TiO2 nanoparticles with high photocatalytic activity synthesized by hydrogen–oxygen diffusion flame. Appl. Catal. B Environ. 2008, 79, 208–215. [Google Scholar] [CrossRef]
- Tariq, M.K.; Riaz, A.; Khan, R.; Wajid, A.; Haq, H.-U.; Javed, S.; Akram, M.A.; Islam, M. Comparative study of Ag, Sn or Zn doped TiO2 thin films for photocatalytic degradation of methylene blue and methyl orange. Mater. Res. Express 2019, 6, 106435. [Google Scholar] [CrossRef]
- Zhu, F.; Zhang, P.; Wu, X.; Fu, L.; Zhang, J.; Xu, D. The Origin of Higher Open-Circuit Voltage in Zn-Doped TiO2 Nanoparticle-Based Dye-Sensitized Solar Cells. ChemPhysChem 2012, 13, 3731–3737. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Z.; Ruan, S.; Zou, B.; Zhao, M.; Wu, F. Photocatalytic degradation of CI Acid Orange 52 in the presence of Zn-doped TiO2 prepared by a stearic acid gel method. Dye. Pigment. 2008, 77, 204–209. [Google Scholar] [CrossRef]
- Yang, G.; Jiang, Z.; Shi, H.; Jones, M.O.; Xiao, T.; Edwards, P.P.; Yan, Z. Study on the photocatalysis of F-S co-doped TiO2 prepared using solvothermal method. Appl. Catal. B Environ. 2010, 96, 458–465. [Google Scholar] [CrossRef]
- Okuya, M.; Nakade, K.; Kaneko, S. Porous TiO2 thin films synthesized by a spray pyrolysis deposition (SPD) technique and their application to dye-sensitized solar cells. Sol. Energy Mater. Sol. Cells 2002, 70, 425–435. [Google Scholar] [CrossRef]
- Ding, K.; Miao, Z.; Liu, Z.; Zhang, Z.; Han, B.; An, G.; Miao, S.; Xie, Y. Facile Synthesis of High Quality TiO2 Nanocrystals in Ionic Liquid via a Microwave-Assisted Process. J. Am. Chem. Soc. 2007, 129, 6362–6363. [Google Scholar] [CrossRef]
- Elfanaoui, A.; Elhamri, E.; Boulkaddat, L.; Ihlal, A.; Bouabid, K.; Laanab, L.; Taleb, A.; Portier, X. Optical and structural properties of TiO2 thin films prepared by sol-gel spin coating. Int. J. Hydrogen Energy 2011, 36, 4130–4133. [Google Scholar] [CrossRef]
- Shahid, M.; McDonagh, A.; Salim, A.P.; Kim, J.H.; Shon, H.K. Magnetised Titanium Dioxide (TiO2) for Water Purification: Preparation, Characterisation, and Application. Desalination Water Treat. 2015, 54, 979–1002. [Google Scholar] [CrossRef]
- Loan, T.T.; Huong, V.H.; Tham, V.T.; Long, N.N. Effect of zinc doping on the bandgap and photoluminescence of Zn2+-doped TiO2 nanowires. Phys. B Condens. Matter 2018, 532, 210–215. [Google Scholar] [CrossRef]
- Kumar, S.; Das, B. Effect of lattice strain on X-ray Diffraction, Raman Spectroscopy and Optical Properties of as Synthesis Nanocomposite ZnO-SnO2—TiO2 Thin Film by Spray Pyrolysis Method. Semant. Sch. 2017, 2017, 5204639. [Google Scholar]
- Alikhanov, N.M.-R.; Rabadanov, M.K.; Orudzhev, F.F.; Gadzhimagomedov, S.K.; Emirov, R.M.; Sadykov, S.A.; Kallaev, S.N.; Ramazanov, S.M.; Abdulvakhidov, K.G.; Sobola, D. Size-dependent structural parameters, optical, and magnetic properties of facile synthesized pure-phase BiFeO3. J. Mater. Sci. Mater. Electron. 2021, 1–13. [Google Scholar] [CrossRef]
- Prabu, K.M.; Perumal, S. Micro Strain and Morphological Studies of Anatase and Rutile Phase TiO2 Nanocrystals Prepared via Sol-Gel and Solvothermal Method—A Comparative Study. IJSRSET 2015, 1, 299–304. [Google Scholar]
- Moghaddam, H.M.; Nasirian, S. Dependence of activation energy and lattice strain on TiO2 nanoparticles? Nanosci. Methods 2012, 1, 201–212. [Google Scholar] [CrossRef]
- Lv, Y.; Tong, H.; Cai, W.; Zhang, Z.; Chen, H.; Zhou, X. Boosting the efficiency of commercial available carbon-based perovskite solar cells using Zinc-doped TiO2 nanorod arrays as electron transport layer. J. Alloy. Compd. 2021, 851, 156785. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, R.; Li, J.; Li, L.; Lin, S. First-principles study on transition metal-doped anatase TiO2. Nanoscale Res. Lett. 2014, 9, 46. [Google Scholar] [CrossRef]
- Arunachalam, A.; Dhanapandian, S.; Manoharan, C.; Sivakumar, G. Physical properties of Zn doped TiO2 thin films with spray pyrolysis technique and its effects in antibacterial activity. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2015, 138, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Aware, D.V.; Jadhav, S.S. Synthesis, characterization and photocatalytic applications of Zn-doped TiO2 nanoparticles by sol–gel method. Appl. Nanosci. 2016, 6, 965–972. [Google Scholar] [CrossRef]
- Deng, J.; Wang, M.; Fang, J.; Song, X.; Yang, Z.; Yuan, Z. Synthesis of Zn-doped TiO2 nano-particles using metal Ti and Zn as raw materials and application in quantum dot sensitized solar cells. J. Alloy. Compd. 2019, 791, 371–379. [Google Scholar] [CrossRef]
- Mehnane, H.F.; Wang, C.; Kondamareddy, K.K.; Yu, W.; Sun, W.; Liu, H.; Bai, S.; Liu, W.; Guo, S.; Zhao, X. Hydrothermal synthesis of TiO2 nanoparticles doped with trace amounts of strontium, and their solar cells: Tunable electrical properties & enhanced photo-conversion performance. RSC Adv. 2017, 2358–2364. [Google Scholar] [CrossRef]
- Madurai Ramakrishnan, V.; Muthukumarasamy, N.; Balraju, P.; Pitchaiya, S.; Velauthapillai, D.; Pugazhendhi, A. Transformation of TiO2 nanoparticles to nanotubes by simple solvothermal route and its performance as dye-sensitized solar cell (DSSC) photoanode. Int. J. Hydrogen Energy 2020, 45, 15441–15452. [Google Scholar] [CrossRef]
- Nguyen, C.H.; Fu, C.C.; Juang, R.S. Degradation of methylene blue and methyl orange by palladium-doped TiO2 photocatalysis for water reuse: Efficiency and degradation pathways. J. Clean. Prod. 2018, 202, 413–427. [Google Scholar] [CrossRef]
- Bhosale, R.R.; Pujari, S.R.; Muley, G.G.; Patil, S.H.; Patil, K.R.; Shaikh, M.F.; Gambhire, A.B. Solar photocatalytic degradation of methylene blue using doped TiO2 nanoparticles. Sol. Energy 2014, 103, 473–479. [Google Scholar] [CrossRef]
- Pirbazari, A.E.; Monazzam, P.; Kisomi, B.F. Co/TiO2 nanoparticles: Preparation, characterization and its application for photocatalytic degradation of methylene blue. Desalin. Water Treat. 2017, 63, 283–292. [Google Scholar] [CrossRef]
- Shahmoradi, B.; Negahdary, M.; Maleki, A. Hydrothermal synthesis of surface-modified, manganese-doped TiO2 nanoparticles for photodegradation of methylene blue. Environ. Eng. Sci. 2012, 29, 1032–1037. [Google Scholar] [CrossRef]
- Li, Z.; Shen, W.; He, W.; Zu, X. Effect of Fe-doped TiO2 nanoparticle derived from modified hydrothermal process on the photocatalytic degradation performance on methylene blue. J. Hazard. Mater. 2008, 155, 590–594. [Google Scholar] [CrossRef]
- Madurai Ramakrishnan, V.; Pitchaiya, S.; Muthukumarasamy, N.; Kvamme, K.; Rajesh, G.; Agilan, S.; Pugazhendhi, A.; Velauthapillai, D. Performance of TiO2 nanoparticles synthesized by microwave and solvothermal methods as photoanode in dye-sensitized solar cells (DSSC). Int. J. Hydrogen Energy 2020, 45, 27036–27046. [Google Scholar] [CrossRef]
- Pirashanthan, A.; Murugathas, T.; Robertson, N.; Ravirajan, P.; Velauthapillai, D. A Quarterthiophene-Based Dye as an Efficient Interface Modifier for Hybrid Titanium Dioxide/Poly(3-hexylthiophene)(P3HT) Solar Cells. Polymers 2019, 11, 1752. [Google Scholar] [CrossRef]
- Wijayarathna, T.R.C.K.; Aponsu, G.M.L.P.; Ariyasinghe, Y.P.Y.P.; Premalal, E.V.A.; Kumara, G.K.R.; Tennakone, K. A high efficiency indoline-sensitized solar cell based on a nanocrystalline TiO2surface doped with copper. Nanotechnology 2008, 19, 485703. [Google Scholar] [CrossRef] [PubMed]
- Shanmugaratnam, S.; Selvaratnam, B.; Baride, A.; Koodali, R. SnS2/TiO2 Nanocomposites for Hydrogen Production and Photodegradation Under Extended Solar Irradiation. Catalysts 2021, 11, 589. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).