Lignocellulosic Waste Pretreatment Solely via Biocatalysis as a Partial Simultaneous Lignino-Holocellulolysis Process
Abstract
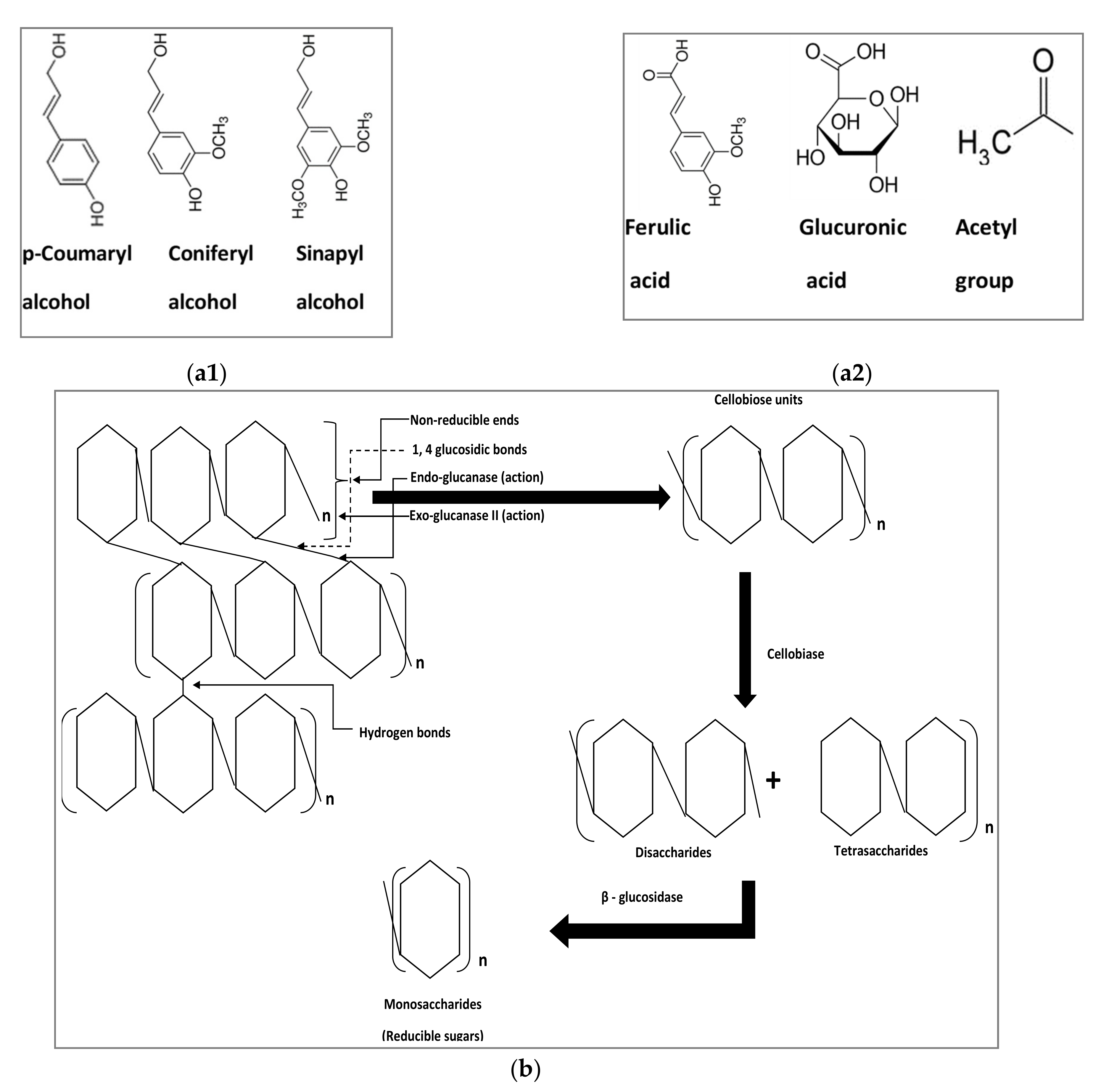
1. Introduction
| Components in Lignocellulosic Waste | Process | Enzymes | Source of Enzymes in Plants and/or Organisms | Reference | |
|---|---|---|---|---|---|
| Lignin | Delignification (ligninolysis) | Lignin peroxidase, Manganese peroxidase, Laccase | Phanerochaete chrysosporium, Phaseolus vulgaris, Ganoderma lucidum IBL-05, Trametes villosa | [16,33,34,35] | |
| Cellulose | Cellulolysis | (holocellulolysis) | β-glucosidases Cellulases Endo-glucanase Exo β-1.3-β-1.4-glucanase Acetyl xylan esterase Cellulase-free xylanase Arabinofuranosidase α-arabinofuranosidase, feruloyl esterase | Bacillus sonorensis BD92 N. mirabilis Bacillus subtilis CBS31 Thielavia terrestris Co3Bag1 Alkalibacillus favidus Paenibacillus sp. N1 Streptomyces lividus Aspergillus hortai CRM1919, Lactobacillus crispatus | [36,37,38,39,40,41,42,43,44,45] |
| Hemicellulose | Hemicellulolysis | ||||
| Lignocellulose Waste Component | Concentration in Untreated Waste (%) | Concentration in Pretreated Waste (%) |
|---|---|---|
| Cellulose | 43.4–35.9 | 42.8–32.4 |
| Hemicellulose | 29.1–18.7 | 23.1–10.7 |
| Lignin | 30.1–29.0 | 25.8–4.1 |
| Solution/Extract (Characteristics) | Oxidation Reduction Potential (mV) | pH | Acid Strength |
|---|---|---|---|
| N. mirabilis digestives pod extracts (contains symbionts and a cocktail of enzymes known for lignino-holocellulolysis) | 510–526 | 1.8–2.2 | Strong |
| Sulphuric acid (1% v/v, free of symbionts and enzymes) | 354.2 | 0.7 | Strong |
2. Current Beneficiation of Lignocellulosic Waste Using Different Pretreatment Techniques
3. Ligninolysis of Lignocellulosic Waste: Physico-Chemical and Biological Methods
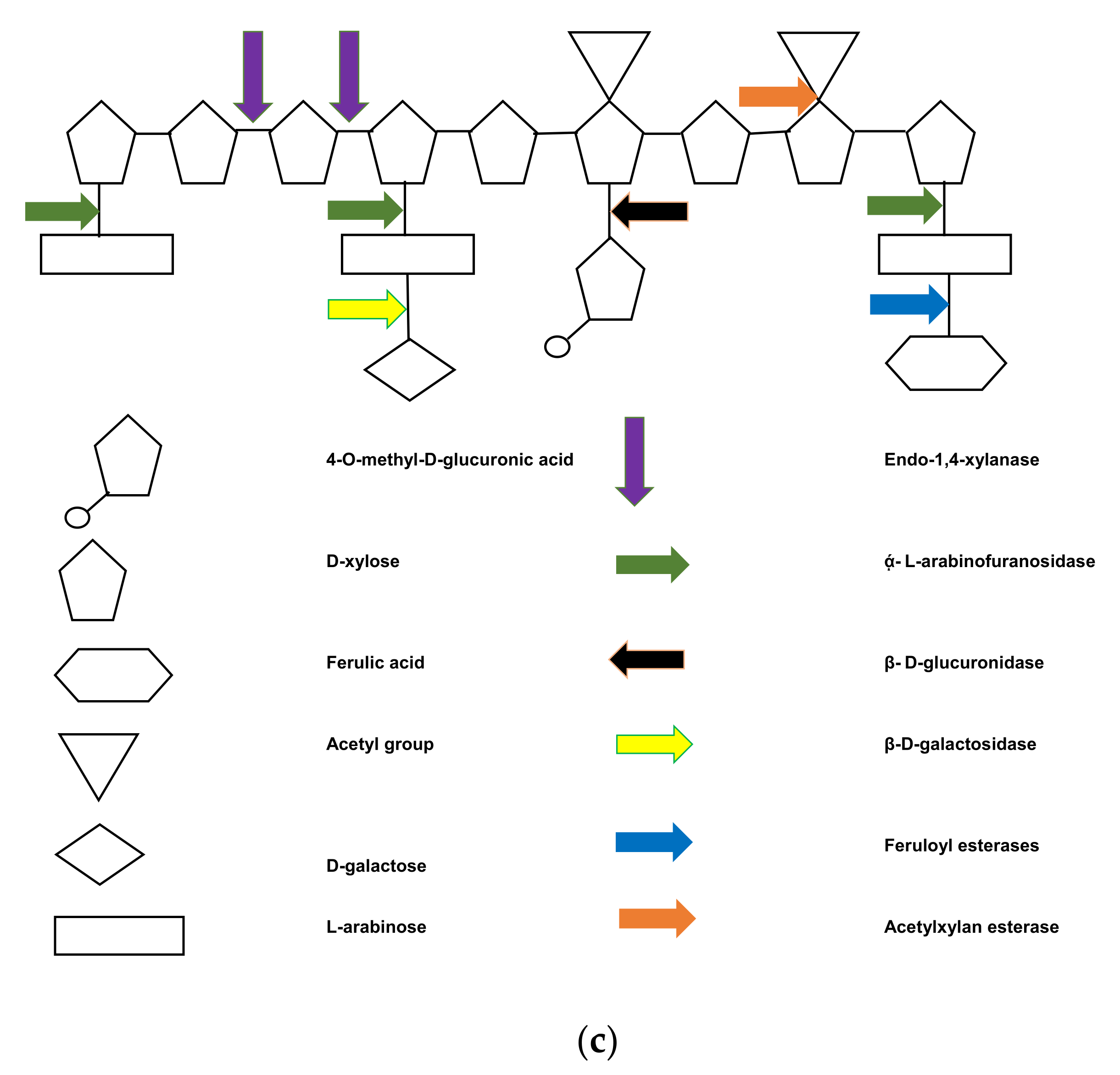
4. Holocellulolysis of Lignocellulosic Waste
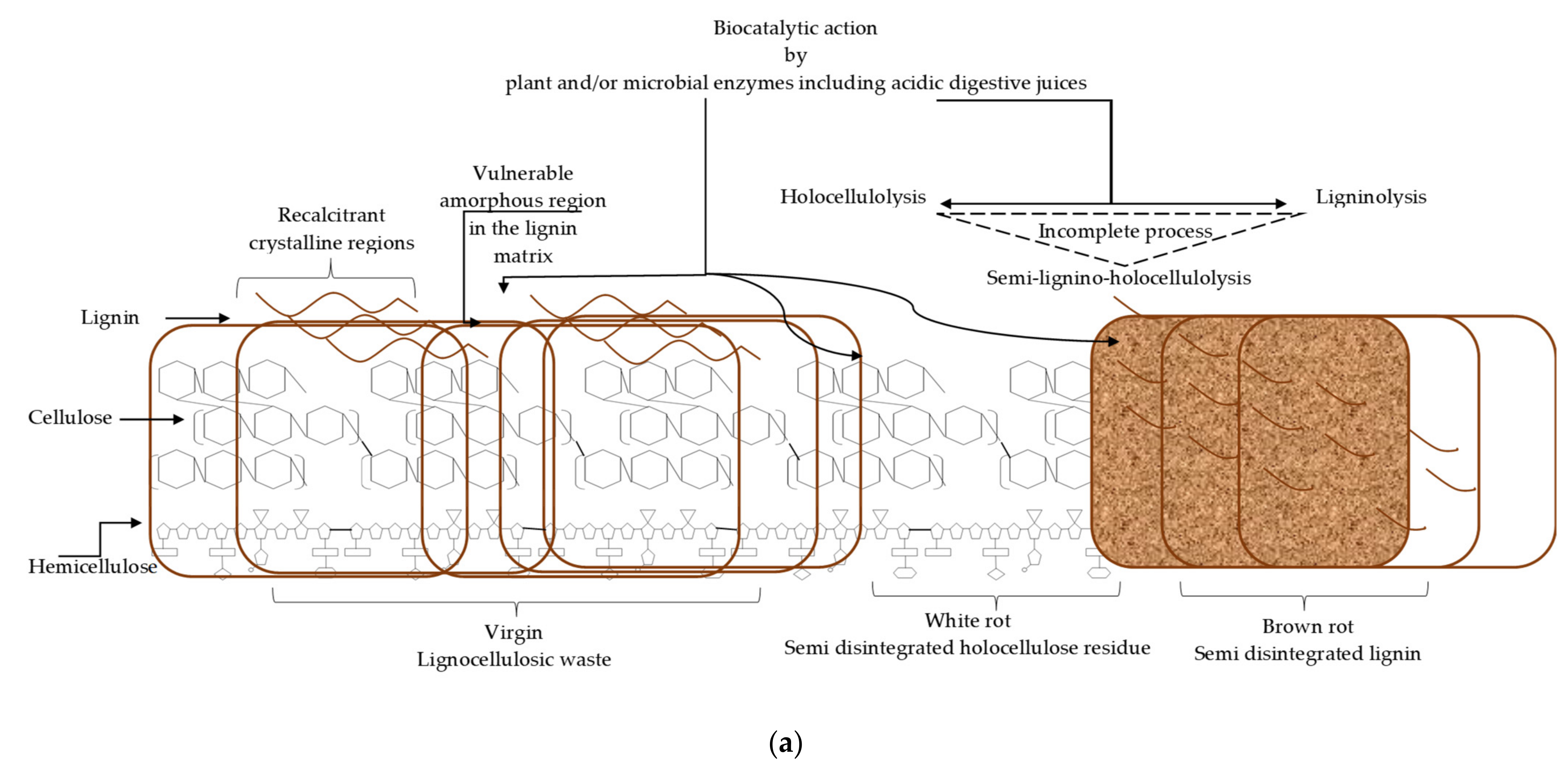
5. Simultaneous Partial Biological Ligninolysis and Holocellulolysis
5.1. Perspective on Semi-Delignino-Holocellulolysis
5.2. Plant Exudates and Enzyme Cocktails for Semi-Lignino-Holocellulolysis of Lignocellulosic Waste
6. Future Perspective, Mitigation of Limitations, and Economic Impact
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rex, J.; Dubé, S.; Krauskopf, P.; Berch, S. Investigating Potential Toxicity of Leachate from Wood Chip Piles Generated by Roadside Biomass Operations. Forests 2016, 7, 40. [Google Scholar] [CrossRef]
- Svensson, H. Characterization, Toxicity and Treatment of Wood Leachate Generated Outdoors by The Wood-Based Industry. In Biology and Environmental Science; Linnaeus University Press: Småland, Sweden, 2014; Available online: http://lnu.diva-portal.org/smash/record.jsf?pid=diva2%3A1089621&dswid=1842 (accessed on 6 November 2020)ISBN 978-91-87427-86-2.
- Taylor, B.R.; Goudey, J.S.; Carmichael, N.B. Toxicity of aspen wood leachate to aquatic life: Laboratory studies. Environ. Toxicol. Chem. 1996, 15, 150–159. [Google Scholar] [CrossRef]
- Libralato, G.; Losso, C.; Ghirardini, A.V. Toxicity of untreated wood leachates towards two saltwater organisms (Crassostrea gigas and Artemia franciscana). J. Hazard. Mater. 2007, 144, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Dubey, S.K.; Kumar, V. Kinetic study for aerobic treatment of phenolic wastewater. Water Resour. Ind. 2015, 11, 81–90. [Google Scholar] [CrossRef]
- Santos, B.A.Q. Continuous Bioremediation of Electroplating Effluent. In Chemical Engineering; Cape Peninsula University of Technology: Cape Town, South Africa, 2014; Available online: http://etd.cput.ac.za/handle/20.500.11838/865 (accessed on 6 November 2020).
- Szymańska-Chargot, M.; Chylińska, M.; Gdula, K.; Kozioł, A.; Zdunek, A. Isolation and characterization of cellulose from different fruit and vegetable pomaces. Polymers 2017, 9, 495. [Google Scholar] [CrossRef]
- Bensah, E.C.; Mensah, M. Chemical Pretreatment Methods for the Production of Cellulosic Ethanol: Technologies and Innovations. Int. J. Chem. Eng. 2013, 2013, 719607. [Google Scholar] [CrossRef]
- Barana, D.; Salanti, A.; Orlandi, M.; Ali, D.S.; Zoia, L. Biorefinery process for the simultaneous recovery of lignin, hemicelluloses, cellulose nanocrystals and silica from rice husk and Arundo donax. Ind. Crops Prod. 2016, 86, 31–39. [Google Scholar] [CrossRef]
- Pielhop, T.; Amgarten, J.; von Rohr, P.R.; Studer, M.H. Steam explosion pretreatment of softwood: The effect of the explosive decompression on enzymatic digestibility. Biotechnol. Biofuels 2016, 9, 152. [Google Scholar] [CrossRef]
- O’Dwyer, J.; Walshe, D.; Byrne, K.A. Wood waste decomposition in landfills: An assessment of current knowledge and implications for emissions reporting. Waste Manag. 2018, 73, 181–188. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. A comprehensive review on pre-treatment strategy for lignocellulosic food industry waste: Challenges and opportunities. Bioresour. Technol. 2016, 199, 92–102. [Google Scholar] [CrossRef]
- Zabed, H.; Sahu, J.N.; Boyce, A.N.; Faruq, G. Fuel ethanol production from lignocellulosic biomass: An overview on feedstocks and technological approaches. Renew. Sustain. Energy Rev. 2016, 66, 751–774. [Google Scholar] [CrossRef]
- Passos, D.d.F.; Pereira, N.; Castro, A.M.d. A comparative review of recent advances in cellulases production by Aspergillus, Penicillium and Trichoderma strains and their use for lignocellulose deconstruction. Curr. Opin. Green Sustain. Chem. 2018, 14, 60–66. [Google Scholar] [CrossRef]
- Leonowicz, A.; Matuszewska, A.; Luterek, J.; Ziegenhagen, D.; Wojtaś-Wasilewska, M.; Cho, N.S.; Hofrichter, M.; Rogalski, J. Biodegradation of lignin by white rot fungi. Fungal Genet. Biol. 1999, 27, 175–185. [Google Scholar] [CrossRef]
- Angadam, J.O. Tertiary Biovalorisation of Grape Pomace; Cape Peninsula University of Technology: Cape Town, South Africa, 2018; Available online: https://etd.cput.ac.za/handle/20.500.11838/2836 (accessed on 6 November 2020).
- Ntwampe, S.K.O. Multicapillary Membrane Bioreactor Design; Cape Peninsula University of Technology: Cape Town, South Africa, 2005; Available online: http://etd.cput.ac.za/handle/20.500.11838/897 (accessed on 6 November 2020).
- Al-Kharousi, M.M.; Sivakumar, N.; Elshafie, A. Characterization of cellulase enzyme produced by Chaetomium sp. isolated from books and archives. EurAsian J. Biosci. 2015, 9, 52–60. [Google Scholar]
- Jaeger, M. Study on Enzyme Activity of Nepenthesins in Carnivorous Nepenthes Alata; Max Planck Institute for Chemical Ecology: Thuringia, Germany, 2016. [Google Scholar]
- Adibah, W.N.; Zakaria, W.; MohdAizat, W.; Goh, H.-H.; Noor, M.H. Proteomic analysis of pitcher fluid from Nepenthes ventrata. Data Brief 2018, 17, 517–519. [Google Scholar]
- Moran, J.A.; Clarke, C.M.; Hawkins, B.J. From Carnivore to Detritivore? Isotopic Evidence for Leaf Litter Utilization by the Tropical Pitcher Plant Nepenthes ampullaria. Int. J. Plant Sci. 2003, 164, 635–639. [Google Scholar] [CrossRef]
- Dlangamandla, N.; Ntwampe, S.K.O.; Angadam, J.O.; Itoba-Tombo, E.F.; Chidi, B.S.; Mekuto, L. Integrated hydrolysis of mixed agro-waste for a second generation biorefinery using Nepenthes mirabilis pod digestive fluids. Processes 2019, 7, 64. [Google Scholar] [CrossRef]
- Rottloff, S.; Miguel, S.; Biteau, F.; Nisse, E.; Hammann, P.; Kuhn, L.; Chicher, J.; Bazile, V.; Gaume, L.; Mignard, B.; et al. Proteome analysis of digestive fluids in Nepenthes pitchers. Ann. Bot. 2016, 117, 479–495. [Google Scholar] [CrossRef]
- Growing Nepenthes Hydroponically: Preliminary Results 2016. 2021. Available online: https://pitcherplants.proboards.com/thread/13543/growing-nepenthes-hydroponically-preliminary-results (accessed on 6 November 2020).
- Tayeb, A.H.; Amini, E.; Ghasemi, S.; Tajvidi, M. Cellulose nanomaterials—Binding properties and applications: A review. Molecules 2018, 23, 2684. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, B.; Ji, Y.; Li, H. Hydrolysis of hemicellulose to produce fermentable monosaccharides by plasma acid. Carbohydr. Polym. 2013, 97, 518–522. [Google Scholar] [CrossRef]
- Manavalan, T.; Liu, R.; Zhou, Z.; Zou, G. Optimization of acetyl xylan esterase gene expression in Trichoderma reesei and its application to improve the saccharification efficiency on different biomasses. Process Biochem. 2017, 58, 160–166. [Google Scholar] [CrossRef]
- Yang, T.; Kumaran, J.; Li, X.; Amartey, S.A.; Maki, M.; Lu, F.; Qin, W. Chapter 5—Biofuels and Bioproducts Produced through Microbial Conversion of Biomass. In Bioenergy Research: Advances and Applications; Gupta, V.K., Tuohy, M.G., Kubicek, C.P., Saddler, J., Xu, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 71–93. [Google Scholar]
- Balat, M. Production of bioethanol from lignocellulosic materials via the biochemical pathway: A review. Energy Convers. Manag. 2011, 52, 858–875. [Google Scholar] [CrossRef]
- Hasan, F.; Shah, A.A.; Hameed, A. Industrial applications of microbial lipases. Enzym. Microb. Technol. 2006, 39, 235–251. [Google Scholar] [CrossRef]
- Cheng, C.L.; Che, P.Y.; Chen, B.Y.; Lee, W.J.; YueLin, C.; Chang, J.S. Biobutanol production from agricultural waste by an acclimated mixed bacterial microflora. Appl. Energy 2012, 100, 3–9. [Google Scholar] [CrossRef]
- Panagiotou, G.; Olsson, L. Effect of compounds released during pretreatment of wheat straw on microbial growth and enzymatic hydrolysis rates. Biotechnol. Bioeng. 2007, 96, 250–258. [Google Scholar] [CrossRef]
- Wesenberg, D.; Kyriakides, I.; Agathos, S.N. White-rot fungi and their enzymes for the treatment of industrial dye effluents. Biotechnol. Adv. 2003, 22, 161–187. [Google Scholar] [CrossRef]
- Carneiro, R.T.d.; Lopes, M.A.; Silva, M.L.C.; Santos, V.d.; de Souza, V.B.; de Sousa, A.O.; Pirovani, C.P.; Koblitz, M.G.B.; Benevides, R.G.; Góes-Neto, A. Trametes villosa lignin peroxidase (TvLiP): Genetic and molecular characterization. J. Microbiol. Biotechnol. 2017, 27, 179–188. [Google Scholar] [CrossRef]
- Shaheen, R.; Asgher, M.; Hussain, F.; Bhattib, H.N. Immobilized lignin peroxidase from Ganoderma lucidum IBL-05 with improved dye decolorization and cytotoxicity reduction properties. Int. J. Biol. Macromol. 2017, 103, 57–64. [Google Scholar] [CrossRef]
- Zhang, Y.-H.P.; Lynd, L.R. Toward an aggregated understanding of enzymatic hydrolysis of cellulose: Noncomplexed cellulase systems. Biotechnol. Bioeng. 2004, 88, 797–824. [Google Scholar] [CrossRef] [PubMed]
- Danna, K. Controlled Production of Cellulases in Plants for Biomass Conversion. Annual Report, 11 March 1997–14 March 1998; Colorado University: Boulder, CO, USA, 1998. [Google Scholar]
- Ali, S.; Mahmood, S. Mutagenesis of a thermophilic Alkalibacillus flavidus for anhanced production of an extracellular acetyl xylan esterase in semi-solid culture of linseed meal. Waste Biomass Valorization 2019, 11, 3327–3335. [Google Scholar] [CrossRef]
- Olajide, A.; Adesina, F.C.; Onilude, A.A. A thermostable and alkalitolerant arabinofuranosidase by Streptomyces lividus. Biotechnol. J. Int. 2020, 24, 35–47. [Google Scholar] [CrossRef]
- Pathania, S.; Sharma, N.; Verma, A.S.K. Optimization of cellulase-free xylanase produced by a potential thermoalkalophilic Paenibacillus sp. N1 isolated from hot springs of Northern Himalayas in India. J. Microbiol. Biotechnol. Food Sci. 2012, 2, 1–24. [Google Scholar]
- Raza, A.; Bashir, S.; Tabassum, R. Statistical based experimental optimization for co-production of endo-glucanase and xylanase from Bacillus sonorensis BD92 with their application in biomass saccharification. Folia Microbiol. 2019, 64, 295–305. [Google Scholar] [CrossRef]
- Regmi, S.; Choi, Y.S.; Kim, Y.K.; Khan, M.M.; Lee, S.H.; Cho, S.S.; Jin, Y.Y.; Lee, D.Y.; Yoo, J.C.; Suh, J.W. Endoglucanase produced by Bacillus subtilis strain CBS31: Biochemical characterization, thermodynamic study, enzymatic hydrolysis, and bio-industrial applications. Biotechnol. Bioprocess Eng. 2020, 25, 104–116. [Google Scholar] [CrossRef]
- Mendoza, J.R.; Hernández, A.S.; Zúñiga, M.T.A.; Antón, M.G.; Osorio, G.A.; Lara, M.E.H. Purification and biochemical characterization of a novel thermophilic exo-β-1, 3-glucanase from the thermophile biomass-degrading fungus Thielavia terrestris Co3Bag1. Electron. J. Biotechnol. 2019, 41, 60–71. [Google Scholar] [CrossRef]
- Terrone, C.C.; Nascimento, J.M.d.; Terrasan, C.R.F.; Brienzo, M.; Carmonab, E.C. Salt-tolerant α-arabinofuranosidase from a new specie Aspergillus hortai CRM1919: Production in acid conditions, purification, characterization and application on xylan hydrolysis. Biocatal. Agric. Biotechnol. 2020, 23, 101460. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, T.; Zhang, S. Extracellular secretion of feruloyl esterase derived from Lactobacillus crispatus in Escherichia coli and its application for ferulic acid production. Bioresour. Technol. 2019, 288, 121526. [Google Scholar] [CrossRef]
- Park, Y.C.; Kim, J.S. Comparison of various alkaline pretreatment methods of lignocellulosic biomass. Energy 2012, 47, 31–35. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef]
- Limayem, A.; Ricke, S.C. Lignocellulosic biomass for bioethanol production: Current perspectives, potential issues and future prospects. Prog. Energy Combust. Sci. 2012, 38, 449–467. [Google Scholar] [CrossRef]
- Nitsos, C.K.; Lazaridis, P.A.; Aigner, A.M.; Matis, K.A.; Triantafyllidis, K.S. Enhancing lignocellulosic biomass hydrolysis by hydrothermal pretreatment, extraction of surface lignin, wet milling and production of cellulolytic enzymes. ChemSusChem 2019, 12, 1179–1195. [Google Scholar] [CrossRef] [PubMed]
- Jampatesh, S.; Sawisit, A.; Wong, N.; Jantama, S.S.; Jantama, K. Evaluation of inhibitory effect and feasible utilization of dilute acid-pretreated rice straws on succinate production by metabolically engineered Escherichia coli AS1600a. Bioresour. Technol. 2019, 273, 93–102. [Google Scholar] [CrossRef]
- Van der Pol, E.C.; Bakker, R.R.; Baets, P.; Eggink, G. By-products resulting from lignocellulose pretreatment and their inhibitory effect on fermentations for (bio) chemicals and fuels. Appl. Microbiol. Biotechnol. 2014, 98, 9579–9593. [Google Scholar] [CrossRef]
- Balan, V.; Sousa, L.D.C. De-Esterification of Biomass Prior to Ammonia Pretreatment and Systems and Products Related Thereto. U.S. Patent Application No. 20190010295, 7 June 2018. [Google Scholar]
- Zhang, Y.; Huang, M.; Su, J.; Hu, H.; Yang, M.; Huang, Z.; Chen, D.; Wu, J.; Feng, Z. Overcoming biomass recalcitrance by synergistic pretreatment of mechanical activation and metal salt for enhancing enzymatic conversion of lignocellulose. Biotechnol. Biofuels 2019, 12, 12. [Google Scholar] [CrossRef]
- Dąbkowska, K.; Morales, M.A.; Kuglarz, M.; Angelidakib, I. Miscanthus straw as substrate for biosuccinic acid production: Focusing on pretreatment and downstream processing. Bioresour. Technol. 2019, 278, 82–91. [Google Scholar] [CrossRef]
- Maddox, I.S.; Steiner, E.; Hirsch, S.; Wessner, S.; Gutierrez, N.A.; Gapes, J.R.; Schuster, K.C. The cause of” Acid Crash” and” Acidogenic Fermentations” during the batch acetone-butanol-ethanol (ABE-) fermentation process. J. Mol. Microbiol. Biotechnol. 2000, 2, 95–100. [Google Scholar]
- Menon, V.; Rao, M. Trends in bioconversion of lignocellulose: Biofuels, platform chemicals and biorefinery concept. Prog. Energy Combust. Sci. 2012, 38, 522–550. [Google Scholar] [CrossRef]
- Rawat, R.; Kumbhar, B.; Tewari, L. Optimization of alkali pretreatment for bioconversion of poplar (Populus deltoides) biomass into fermentable sugars using response surface methodology. Ind. Crops Prod. 2013, 44, 220–226. [Google Scholar] [CrossRef]
- Amin, F.R.; Khalid, H.; Zhang, H.; Rahman, S.u.; Zhang, R.; Liu, G.; Chen, C. Pretreatment methods of lignocellulosic biomass for anaerobic digestion. AMB Express 2017, 7, 72. [Google Scholar] [CrossRef]
- Nadir, N.; Ismail, N.L.; Hussain, A. Biomass for Bioenergy. In Fungal Pretreatment of Lignocellulosic Materials; Abomohra, A.E.-F., Ed.; InTech Open: Rijeka, Croatia, 2019. [Google Scholar] [CrossRef]
- Barr, D.P.; Aust, S.D. Mechanisms white rot fungi use to degrade pollutants. Environ. Sci. Technol. 1994, 28, 78A–87A. [Google Scholar] [CrossRef]
- Narayanaswamy, N.; Dheeran, P.; Verma, S.; Kumar, S. Biological Pretreatment of Lignocellulosic Biomass for Enzymatic Saccharification. In Pretreatment Techniques for Biofuels and Biorefineries; Fang, Z., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3–34. [Google Scholar]
- Achinas, S.; Euverink, G.J.W. Theoretical analysis of biogas potential prediction from agricultural waste. Resour. Effic. Technol. 2016, 2, 143–147. [Google Scholar] [CrossRef]
- Mithöfer, A. Carnivorous pitcher plants: Insights in an old topic. Phytochemistry 2011, 72, 1678–1682. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Chaffron, S.; Salcher, M.M.; Inatsugi, R.S.; Kobayashi, M.J.; Diway, B.; Mering, C.; Pernthaler, J.; Shimizu, K.K. Bacterial diversity and composition in the fluid of pitcher plants of the genus Nepenthes. Syst. Appl. Microbiol. 2015, 38, 330–339. [Google Scholar] [CrossRef]
- Stephenson, P.; Hogan, J. Cloning and characterization of a ribonuclease, a cysteine proteinase, and an aspartic proteinase from pitchers of the carnivorous plant Nepenthes ventricosa Blanco. Int. J. Plant. Sci. 2006, 167, 239–248. [Google Scholar] [CrossRef]
- Schulze, W.X.; Sanggaard, K.W.; Kreuzer, I.; Knudsen, A.D.; Bemm, F.; Thøgersen, I.B.; Bräutigam, A.; Thomsen, L.R.; Schliesky, S.; Dyrlund, T.F.; et al. The protein composition of the digestive fluid from the venus flytrap sheds light on prey digestion mechanisms. Mol. Cell. Proteom. 2012, 11, 1306–1319. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Higashi, S.; Nakashima, A.; Ozaki, H.; Abe, M.; Uchiumi, T. Analysis of feeding mechanism in a pitcher of Nepenthes hybrida. J. Plant. Res. 1993, 106, 47–54. [Google Scholar] [CrossRef]
- Bornscheuer, U.T.; Ordoñez, G.R.; Hidalgo, A.; Gollina, A.; Lyon, J.; Hitchman, T.S.; Weiner, D.P. Selectivity of lipases and esterases towards phenol esters. J. Mol. Catal. B Enzym. 2005, 36, 8–13. [Google Scholar] [CrossRef]
- Maroušek, J.; Bartoš, P.; Filip, M.; Kolár, L.; Konvalina, P.; Maroušková, A.; Moudrý, J.; Peterka, J.; Šál, J.; Šoch, M.; et al. Advances in the agrochemical utilization of fermentation residues reduce the cost of purpose-grown phytomass for biogas production. In Energy Sources, Part A: Recovery; Taylor & Francis: Philadelphia, PA, USA, 2020. [Google Scholar]
- Maroušek, J.; Myšková, K.; Žák, J. Managing environmental innovation: Case study on biorefinery concept. Rev. Técnica Fac. Ing. Univ. Zulia 2015, 38, 216–220. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angadam, J.O.; Ntwampe, S.K.O.; Chidi, B.S.; Lim, J.W.; Okudoh, V.I. Lignocellulosic Waste Pretreatment Solely via Biocatalysis as a Partial Simultaneous Lignino-Holocellulolysis Process. Catalysts 2021, 11, 668. https://doi.org/10.3390/catal11060668
Angadam JO, Ntwampe SKO, Chidi BS, Lim JW, Okudoh VI. Lignocellulosic Waste Pretreatment Solely via Biocatalysis as a Partial Simultaneous Lignino-Holocellulolysis Process. Catalysts. 2021; 11(6):668. https://doi.org/10.3390/catal11060668
Chicago/Turabian StyleAngadam, Justine Oma, Seteno Karabo Obed Ntwampe, Boredi Silas Chidi, Jun Wei Lim, and Vincent Ifeanyi Okudoh. 2021. "Lignocellulosic Waste Pretreatment Solely via Biocatalysis as a Partial Simultaneous Lignino-Holocellulolysis Process" Catalysts 11, no. 6: 668. https://doi.org/10.3390/catal11060668
APA StyleAngadam, J. O., Ntwampe, S. K. O., Chidi, B. S., Lim, J. W., & Okudoh, V. I. (2021). Lignocellulosic Waste Pretreatment Solely via Biocatalysis as a Partial Simultaneous Lignino-Holocellulolysis Process. Catalysts, 11(6), 668. https://doi.org/10.3390/catal11060668










