Borate Anion Dopant Inducing Oxygen Vacancies over Co3O4 Nanocages for Enhanced Oxygen Evolution
Abstract
1. Introduction
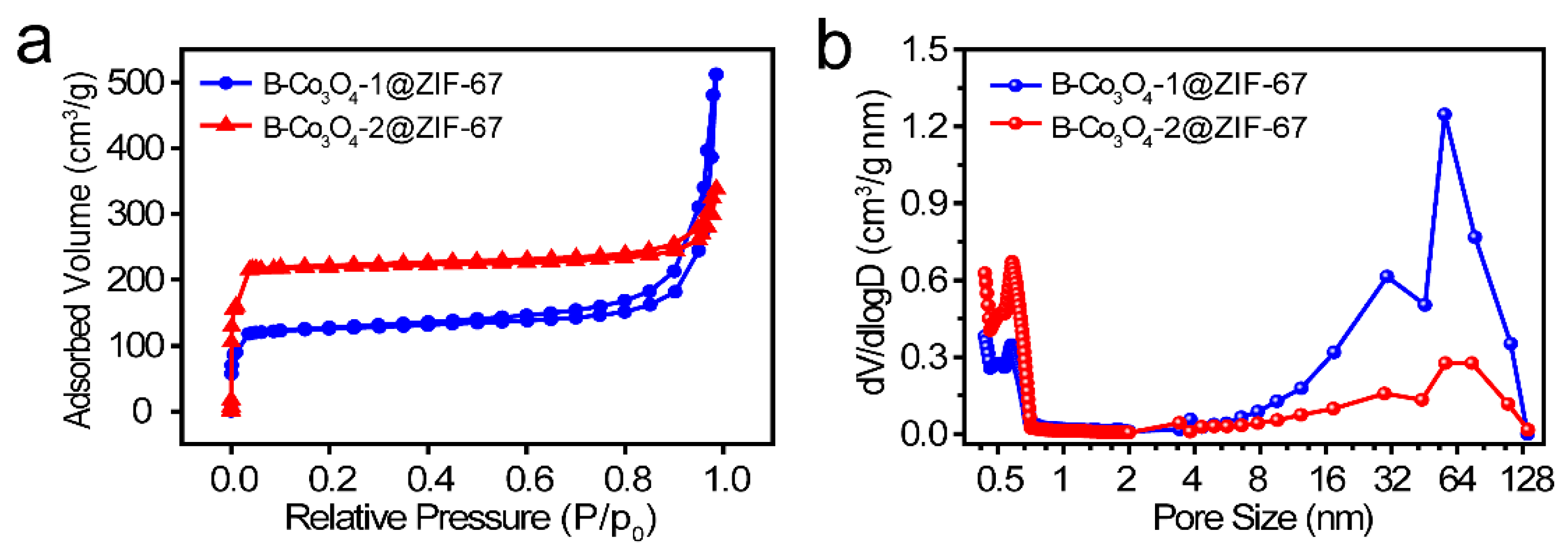
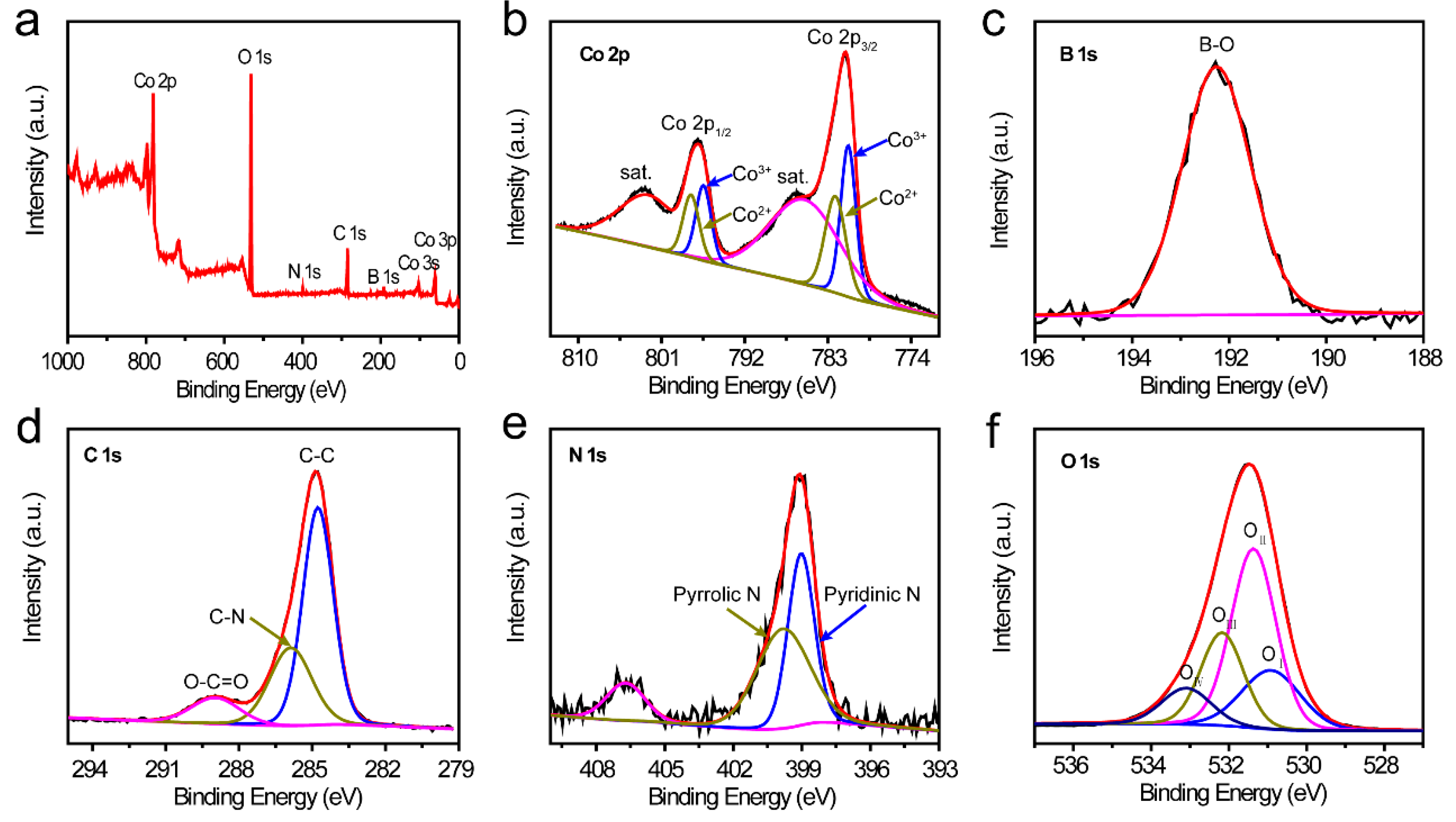
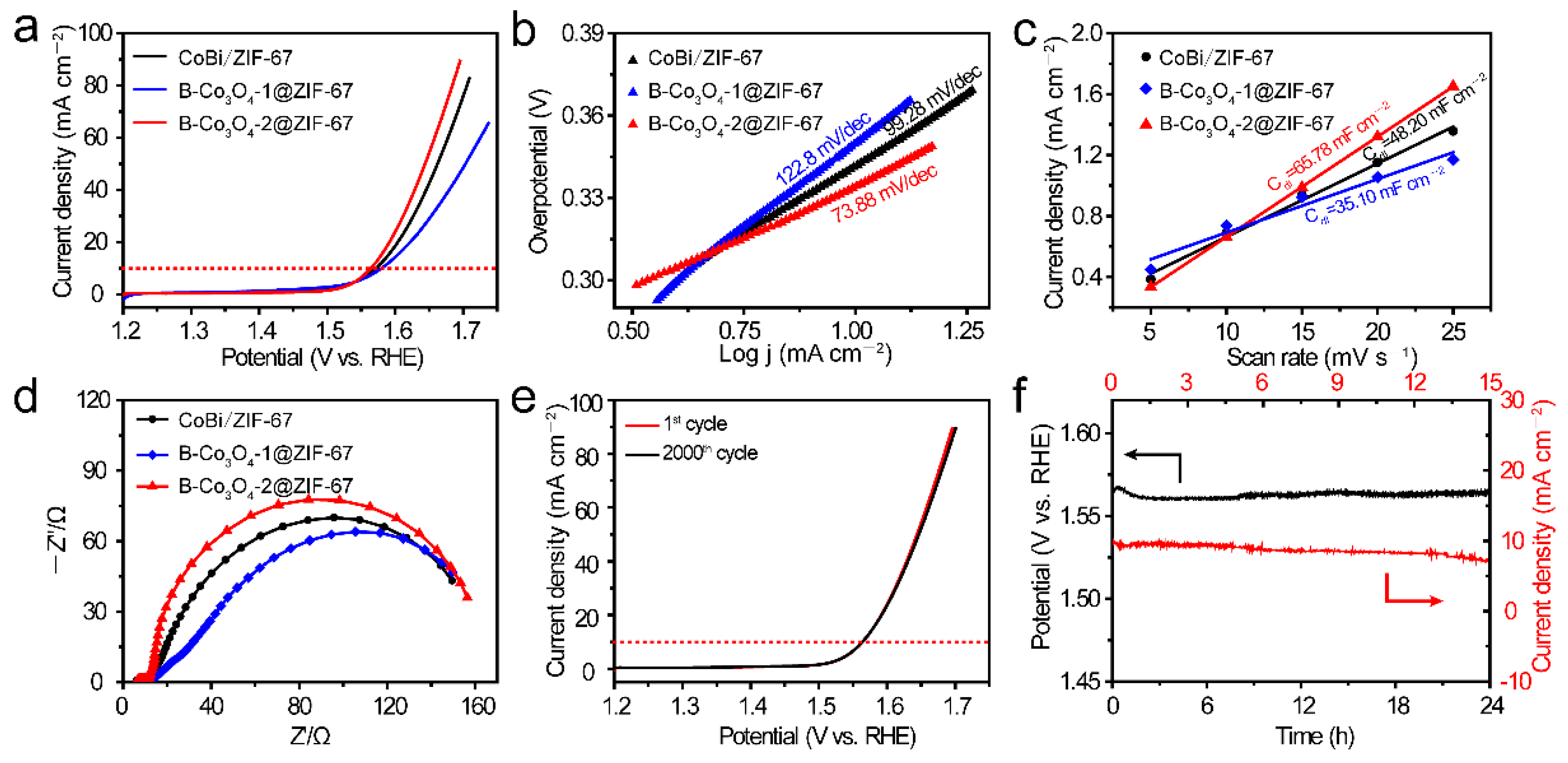
2. Results and Discussion
3. Experimental Section
3.1. Materials
3.2. Materials Synthesis
3.3. Physicochemical Characterization
3.4. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, D.; Su, Z.; Chen, Y.; Srinivas, K.; Gao, J.; Zhang, W.; Wang, Z.; Lin, H. Electronic modulation of hierarchical spongy nanosheets toward efficient and stable water electrolysis. Small 2021, 17, 2006881. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, T.; Lee, J.Y. 110th Anniversary: A total water splitting electrocatalyst based on borate/Fe Co-doping of nickel sulfide. Ind. Eng. Chem. Res. 2019, 58, 13053–13063. [Google Scholar] [CrossRef]
- Zang, W.; Sun, T.; Yang, T.; Xi, S.; Waqar, M.; Kou, Z.; Lyu, Z.; Feng, Y.P.; Wang, J.; Pennycook, S.J. Efficient hydrogen evolution of oxidized Ni-N3 defective sites for alkaline freshwater and seawater electrolysis. Adv. Mater. 2021, 33, 2003846. [Google Scholar] [CrossRef]
- Yu, J.; Wang, J.; Long, X.; Chen, L.; Cao, Q.; Wang, J.; Qiu, C.; Lim, J.; Yang, S. Formation of FeOOH nanosheets induces substitutional doping of CeO2−x with high-valence Ni for efficient water oxidation. Adv. Energy Mater. 2020, 11, 2002731. [Google Scholar] [CrossRef]
- Zhu, J.; Wei, M.; Meng, Q.; Chen, Z.; Fan, Y.; Hasan, S.W.; Zhang, X.; Lyu, D.; Tian, Z.Q.; Shen, P.K. Ultrathin-shell IrCo hollow nanospheres as highly efficient electrocatalysts towards the oxygen evolution reaction in acidic media. Nanoscale 2020, 12, 24070–24078. [Google Scholar] [CrossRef]
- Quang, N.D.; Majumder, S.; Van, P.C.; Jeong, J.-R.; Kim, C.; Kim, D. Co3O4/reduced graphene oxide/BiVO4 nanorod as high performance photoanode for water oxidation. Electrochim. Acta 2020, 364, 137283–137293. [Google Scholar] [CrossRef]
- Lei, Y.; Xu, T.; Ye, S.; Zheng, L.; Liao, P.; Xiong, W.; Hu, J.; Wang, Y.; Wang, J.; Ren, X.; et al. Engineering defect-rich Fe-doped NiO coupled Ni cluster nanotube arrays with excellent oxygen evolution activity. Appl. Catal. B Environ. 2021, 285, 119809–119818. [Google Scholar] [CrossRef]
- Zhang, S.L.; Guan, B.Y.; Lu, X.F.; Xi, S.; Du, Y.; Lou, X.W.D. Metal atom-doped Co3O4 hierarchical nanoplates for electrocatalytic oxygen evolution. Adv. Mater. 2020, 32, 2002235. [Google Scholar] [CrossRef]
- Dong, F.; Li, L.; Kong, Z.; Xu, X.; Zhang, Y.; Gao, Z.; Dongyang, B.; Ni, M.; Liu, Q.; Lin, Z. Materials engineering in perovskite for optimized oxygen evolution electrocatalysis in alkaline condition. Small 2021, 17, 2006638. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wei, G.; He, J.; An, C.; Hu, M.; Shu, M.; Zhu, J.; Yao, S.; Xi, W.; Si, R.; et al. Interfacial electronic interaction of atomically dispersed IrClx on ultrathin Co(OH)2/CNTs for efficient electrocatalytic water oxidation. Appl. Catal. B Environ. 2020, 279, 119398–119405. [Google Scholar] [CrossRef]
- Srinivas, K.; Chen, Y.; Wang, B.; Yu, B.; Lu, Y.; Su, Z.; Zhang, W.; Yang, D. Metal-organic framework-derived Fe-doped Ni3Fe/NiFe2O4 heteronanoparticle-decorated carbon nanotube network as a highly efficient and durable bifunctional electrocatalyst. ACS Appl. Mater. Interfaces 2020, 12, 55782–55794. [Google Scholar] [CrossRef]
- Moschkowitsch, W.; Dhaka, K.; Gonen, S.; Attias, R.; Tsur, Y.; Caspary Toroker, M.; Elbaz, L. Ternary NiFeTiOOH catalyst for the oxygen evolution reaction: Study of the effect of the addition of Ti at different loadings. ACS Catal. 2020, 10, 4879–4887. [Google Scholar] [CrossRef]
- Choi, J.; Kim, D.; Zheng, W.; Yan, B.; Li, Y.; Lee, L.Y.S.; Piao, Y. Interface engineered NiFe2O4−x/NiMoO4 nanowire arrays for electrochemical oxygen evolution. Appl. Catal. B Environ. 2021, 286, 119857–119865. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, X.; Xue, H.; Feng, L. A nitrogen-doped CoP nanoarray over 3D porous Co foam as an efficient bifunctional electrocatalyst for overall water splitting. J. Mater. Chem. A 2019, 7, 13242–13248. [Google Scholar] [CrossRef]
- Yuan, G.; Bai, J.; Zhang, L.; Chen, X.; Ren, L. The effect of P vacancies on the activity of cobalt phosphide nanorods as oxygen evolution electrocatalyst in alkali. Appl. Catal. B Environ. 2021, 284, 119693–119701. [Google Scholar] [CrossRef]
- Naderi, A.; Yong, X.; Karamad, M.; Cai, J.; Zang, Y.; Gates, I.; Siahrostami, S.; Wang, G. Ternary cobalt–iron sulfide as a robust electrocatalyst for water oxidation: A dual effect from surface evolution and metal doping. Appl. Sur. Sci. 2021, 542, 148681–148687. [Google Scholar] [CrossRef]
- Masa, J.; Weide, P.; Peeters, D.; Sinev, I.; Xia, W.; Sun, Z.; Somsen, C.; Muhler, M.; Schuhmann, W. Amorphous cobalt boride (Co2B) as a highly efficient nonprecious catalyst for electrochemical water splitting: Oxygen and hydrogen evolution. Adv. Energy Mater. 2016, 6, 1502313. [Google Scholar] [CrossRef]
- Yi, L.; Niu, Y.; Feng, B.; Zhao, M.; Hu, W. Simultaneous phase transformation and doping via a unique photochemical–electrochemical strategy to achieve a highly active Fe-doped Ni oxyhydroxide oxygen evolution catalyst. J. Mater. Chem. A 2021, 9, 4213–4220. [Google Scholar] [CrossRef]
- Xu, Z.; Ying, Y.; Zhang, G.; Li, K.; Liu, Y.; Fu, N.; Guo, X.; Yu, F.; Huang, H. Engineering NiFe layered double hydroxide by valence control and intermediate stabilization toward the oxygen evolution reaction. J. Mater. Chem. A 2020, 8, 26130–26138. [Google Scholar] [CrossRef]
- He, J.; Zhou, X.; Xu, P.; Sun, J. Promoting electrocatalytic water oxidation through tungsten-modulated oxygen vacancies on hierarchical FeNi-layered double hydroxide. Nano Energy 2021, 80, 105540–105548. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Z.; Wang, J.; Xiao, Z.; Huang, X.; Liu, Z.; Wang, S. N-doped nanoporous Co3O4 nanosheets with oxygen vacancies as oxygen evolving electrocatalysts. Nanotechnology 2017, 28, 165402–165409. [Google Scholar] [CrossRef]
- Zhang, J.; Qian, J.; Ran, J.; Xi, P.; Yang, L.; Gao, D. Engineering lower coordination atoms onto NiO/Co3O4 heterointerfaces for boosting oxygen evolution reactions. ACS Catal. 2020, 10, 12376–12384. [Google Scholar] [CrossRef]
- Zeng, H.; Oubla, M.H.; Zhong, X.; Alonso-Vante, N.; Du, F.; Xie, Y.; Huang, Y.; Ma, J. Rational defect and anion chemistries in Co3O4 for enhanced oxygen evolution reaction. Appl. Catal. B Environ. 2021, 281, 119535–119543. [Google Scholar] [CrossRef]
- Zou, L.; Xu, Q. Synthesis of a hierarchically porous C/Co3O4 nanostructure with boron doping for oxygen evolution reaction. Chem. Asian J. 2020, 15, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Huang, Z.; Lv, C.; Zhang, C. A self-supported porous hierarchical core–shell nanostructure of cobalt oxide for efficient oxygen evolution reaction. ACS Catal. 2017, 7, 8205–8213. [Google Scholar] [CrossRef]
- Zhang, B.; Jiang, K.; Wang, H.; Hu, S. Fluoride-induced dynamic surface self-reconstruction produces unexpectedly efficient oxygen-evolution catalyst. Nano Lett. 2019, 19, 530–537. [Google Scholar] [CrossRef]
- Wang, Q.; Xue, X.; Lei, Y.; Wang, Y.; Feng, Y.; Xiong, X.; Wang, D.; Li, Y. Engineering of electronic states on Co3O4 ultrathin nanosheets by cation substitution and anion vacancies for oxygen evolution reaction. Small 2020, 16, 2001571. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yang, Z.; Liang, W.; Yan, H.; Tuo, Y.; Li, Y.; Zhou, Y.; Zhang, J. Ultra-small Co/CoO nanoparticles dispersed on N-doped carbon nanosheets for highly efficient electrocatalytic oxygen evolution reaction. J. Energy Chem. 2021, 55, 345–354. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; You, W.; Yu, J. Core-shell nitrogen-doped carbon hollow spheres/Co3O4 nanosheets as advanced electrode for high-performance supercapacitor. Small 2018, 14, 1702407–1702414. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tan, M.; Huang, C.; Luo, W.; Yin, S.-F.; Yang, W. Co2(OH)3Cl and MOF mediated synthesis of porous Co3O4/NC nanosheets for efficient OER catalysis. Appl. Sur. Sci. 2021, 542, 148739–148747. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, S.; Ma, Z.; Kundu, M.; Tang, B.; Li, J.; Wang, X. Oxygen vacancies engineered self-supported B doped Co3O4 nanowires as an efficient multifunctional catalyst for electrochemical water splitting and hydrolysis of sodium borohydride. Chem. Eng. J. 2021, 404, 126474–126486. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, W.; Chen, X.; Peng, Y.; Song, Y.; Lv, C.; Liu, H.; Sun, J.; Yuan, D.; Li, X.; et al. Defect-rich nitrogen doped Co3O4/C porous nanocubes enable high-efficiency bifunctional oxygen electrocatalysis. Adv. Funct. Mater. 2019, 29, 1902875. [Google Scholar] [CrossRef]
- Yang, H.; Chen, Z.; Guo, P.; Fei, B.; Wu, R. B-doping-induced amorphization of LDH for large-current-density hydrogen evolution reaction. Appl. Catal. B Environ. 2020, 261, 118240–118248. [Google Scholar] [CrossRef]
- Lai, Y.; Xiao, L.; Tao, Y.; Gao, Z.; Zhang, L.; Su, X.; Dai, Y. Enhancing one-dimensional charge transport in metal-organic framework hexagonal nanorods for electrocatalytic oxygen evolution. ChemSusChem 2021, 14, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhang, M.; Zhao, L.; Wang, L.; Cao, D.; Gong, Y. Ru doped bimetallic phosphide derived from 2D metal organic framework as active and robust electrocatalyst for water splitting. Appl. Sur. Sci. 2021, 536, 147952–147964. [Google Scholar] [CrossRef]
- Weng, Y.G.; Yin, W.Y.; Jiang, M.; Hou, J.L.; Shao, J.; Zhu, Q.Y.; Dai, J. Tetrathiafulvalene-based metal-organic framework as a high-performance anode for lithium-ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 52615–52623. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Wang, N.; Ren, L.; Casillas-Garcia, G.; Liu, N.; Liu, Y.; Xu, X.; Hao, W.; Dou, S.X.; Du, Y. In-situ grafting of N-doped carbon nanotubes with Ni encapsulation onto MOF-derived hierarchical hybrids for efficient electrocatalytic hydrogen evolution. Carbon 2020, 163, 178–185. [Google Scholar] [CrossRef]
- Marks, S.D.; Riascos-Rodriguez, K.; Arrieta-Pérez, R.R.; Yakovenko, A.A.; Exley, J.; Evans, P.G.; Hernández-Maldonado, A.J. Lattice expansion and ligand twist during CO2 adsorption in flexible Cu bipyridine metal–organic frameworks. J. Mater. Chem. A 2020, 8, 18903–18915. [Google Scholar] [CrossRef]
- Mohan, M.; Essalhi, M.; Durette, D.; Rana, L.K.; Ayevide, F.K.; Maris, T.; Duong, A. A rational design of microporous nitrogen-rich lanthanide metal-organic frameworks for CO2/CH4 separation. ACS Appl. Mater. Interfaces 2020, 12, 50619–50627. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, C.; Sun, J.; Xing, Y.; Quan, B.; Li, D.; Jiang, D. Interfacial engineering of Co3FeN embedded N-doped carbon nanoarray derived from metal–organic frameworks for enhanced oxygen evolution reaction. Electrochim. Acta 2020, 354, 136629–136636. [Google Scholar] [CrossRef]
- Wang, C.; Chen, W.; Yuan, D.; Qian, S.; Cai, D.; Jiang, J.; Zhang, S. Tailoring the nanostructure and electronic configuration of metal phosphides for efficient electrocatalytic oxygen evolution reactions. Nano Energy 2020, 69, 104453–104460. [Google Scholar] [CrossRef]
- Wu, H.B.; Guan, B.Y.; He, P.; Yu, X.-Y. Synthesis of ZIF-67 nanocubes with complex structures co-mediated by dopamine and polyoxometalate. J. Mater. Chem. A 2018, 6, 19338–19341. [Google Scholar] [CrossRef]
- Lv, Z.; Zhong, Q.; Bu, Y. Controllable synthesis of Ni-Co nanosheets covered hollow box via altering the concentration of nitrate for high performance supercapacitor. Electrochim. Acta 2016, 215, 500–505. [Google Scholar] [CrossRef]
- Chen, W.; Han, B.; Tian, C.; Liu, X.; Liang, S.; Deng, H.; Lin, Z. MOFs-derived ultrathin holey Co3O4 nanosheets for enhanced visible light CO2 reduction. Appl. Catal. B Environ. 2019, 244, 996–1003. [Google Scholar] [CrossRef]
- Silva, F.L.G.; Veiga, A.G.; Carvalho, N.M.F. Manganese oxides treated with organic compounds as catalysts for water oxidation reaction. Int. J. Hydrog. Energy 2021, 46, 11677–11687. [Google Scholar] [CrossRef]
- Wang, H.; Fu, W.; Chen, Y.; Xue, F.; Shan, G. ZIF-67-derived Co3O4 hollow nanocage with efficient peroxidase mimicking characteristic for sensitive colorimetric biosensing of dopamine. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 246, 119006–119015. [Google Scholar] [CrossRef]
- Garg, N.; Kumar, M.; Kumari, N.; Deep, A.; Sharma, A.L. Chemoresistive room-temperature sensing of ammonia using zeolite imidazole framework and reduced graphene oxide (ZIF-67/rGO) composite. ACS Omega 2020, 5, 27492–27501. [Google Scholar] [CrossRef]
- Pei, W.; Zhang, J.; Tong, H.; Ding, M.; Shi, F.; Wang, R.; Huo, Y.; Li, H. Removal and reutilization of metal ions on ZIF-67/GO membrane via synergistic photocatalytic-photothermal route. Appl. Catal. B Environ. 2021, 282, 119575–119584. [Google Scholar] [CrossRef]
- Jafarinasab, M.; Akbari, A.; Omidkhah, M.; Shakeri, M. An efficient Co-based metal–organic framework nanocrystal (Co-ZIF-67) for adsorptive desulfurization of dibenzothiophene: Impact of the preparation approach on structure tuning. Energy Fuels 2020, 34, 12779–12791. [Google Scholar] [CrossRef]
- Chameh, B.; Moradi, M.; Hajati, S.; Hessari, F.A. Design and construction of ZIF(8 and 67) supported Fe3O4 composite as advanced materials of high performance supercapacitor. Phys. E Low Dimens. Syst. Nanostruct. 2021, 126, 114442–114452. [Google Scholar] [CrossRef]
- Modi, A.; Bellare, J. Zeolitic imidazolate framework-67/carboxylated graphene oxide nanosheets incorporated polyethersulfone hollow fiber membranes for removal of toxic heavy metals from contaminated water. Sep. Purif. Technol. 2020, 249, 117160–117169. [Google Scholar] [CrossRef]
- Qin, J.; Wang, S.; Wang, X. Visible-light reduction CO2 with dodecahedral zeolitic imidazolate framework ZIF-67 as an efficient co-catalyst. Appl. Catal. B Environ. 2017, 209, 476–482. [Google Scholar] [CrossRef]
- Xu, W.; Wang, X.; Wu, Y.; Li, W.; Chen, C. Functionalized graphene with Co-ZIF adsorbed borate ions as an effective flame retardant and smoke suppression agent for epoxy resin. J. Hazard Mater. 2019, 363, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Cao, D.; Yang, F.; Xu, Y.; Sun, G.; Ye, K.; Yin, J.; Wang, G. Facile synthesis of morphology-controlled Co3O4 nanostructures through solvothermal method with enhanced catalytic activity for H2O2 electroreduction. J. Power Sources 2014, 253, 214–223. [Google Scholar] [CrossRef]
- Zhou, T.; Lu, P.; Zhang, Z.; Wang, Q.; Umar, A. Perforated Co3O4 nanoneedles assembled in chrysanthemum-like Co3O4 structures for ultra-high sensitive hydrazine chemical sensor. Sens. Actuators B Chem. 2016, 235, 457–465. [Google Scholar] [CrossRef]
- Zhang, R.; Ke, W.; Chen, S.; Yue, X.; Hu, Z.; Ning, T. Phase evolution of vulcanized Co3O4 catalysts during oxygen evolution reaction. Appl. Sur. Sci. 2021, 546, 148819. [Google Scholar] [CrossRef]
- Yan, W.; Shen, Y.; An, C.; Li, L.; Si, R.; An, C. FeOx clusters decorated hcp Ni nanosheets as inverse electrocatalyst to stimulate excellent oxygen evolution performance. Appl. Catal. B Environ. 2021, 284, 119687–119695. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Z.; Xia, Z.; Dai, L. A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Nat. Nanotechnol. 2015, 10, 444–452. [Google Scholar] [CrossRef]
- Guo, M.; Qu, Y.; Yuan, C.; Chen, S. Electrochemical exfoliation of hierarchical Co3O4 microflowers and their conversion into CoP as high-efficiency hydrogen evolution electrocatalyst. Electrochim. Acta 2019, 322, 134768–134777. [Google Scholar] [CrossRef]
- Vikraman, D.; Hussain, S.; Karuppasamy, K.; Feroze, A.; Kathalingam, A.; Sanmugam, A.; Chun, S.-H.; Jung, J.; Kim, H.-S. Engineering the novel MoSe2-Mo2C hybrid nanoarray electrodes for energy storage and water splitting applications. Appl. Catal. B Environ. 2020, 264, 118531–118543. [Google Scholar] [CrossRef]
- Li, Y.; Li, F.-M.; Meng, X.-Y.; Li, S.-N.; Zeng, J.-H.; Chen, Y. Ultrathin Co3O4 nanomeshes for the oxygen evolution reaction. ACS Catal. 2018, 8, 1913–1920. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, T.; Lee, J.Y. Enhancement effect of borate doping on the oxygen evolution activity of α-nickel hydroxide. ACS Appl. Nano Mater. 2018, 1, 751–758. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, L.; Yang, T.; Gao, J.; He, P.; Jia, L.; Dong, F.; Jia, B.; Zhang, H. Facile one-step synthesis of tunable nanochain-like Fe–Mo–B: A highly efficient and stable catalyst for oxygen evolution reaction. J. Alloys Compd. 2020, 822, 153517–153524. [Google Scholar] [CrossRef]
- Yang, Y.X.; Li, B.L.; Zhang, Q.; Guo, W.H.; Wang, X.H.; Li, L.J.; Luo, H.Q.; Li, N.B. Prussian blue analogues–derived CoFe–B nanocubes with increased specific surface area and modulated electronic structure as enhanced oxygen evolution electrocatalysts. Energy Technol. 2020, 9, 2000178. [Google Scholar] [CrossRef]
- Xiao, Z.; Wang, Y.; Huang, Y.-C.; Wei, Z.; Dong, C.-L.; Ma, J.; Shen, S.; Li, Y.; Wang, S. Filling the oxygen vacancies in Co3O4 with phosphorus: An ultra-efficient electrocatalyst for overall water splitting. Energy Environ. Sci. 2017, 10, 2563–2569. [Google Scholar] [CrossRef]
- Ji, L.; Wang, J.; Teng, X.; Meyer, T.J.; Chen, Z. CoP nanoframes as bifunctional electrocatalysts for efficient overall water splitting. ACS Catal. 2019, 10, 412–419. [Google Scholar] [CrossRef]
- Yang, X.; Chen, J.; Chen, Y.; Feng, P.; Lai, H.; Li, J.; Luo, X. Novel Co3O4 nanoparticles/nitrogen-doped carbon composites with extraordinary catalytic activity for oxygen evolution reaction (OER). Nano-Micro Lett. 2018, 10, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Lian, Y.; Sun, H.; Wang, X.; Qi, P.; Mu, Q.; Chen, Y.; Ye, J.; Zhao, X.; Deng, Z.; Peng, Y. Carved nanoframes of cobalt-iron bimetal phosphide as a bifunctional electrocatalyst for efficient overall water splitting. Chem. Sci. 2019, 10, 464–474. [Google Scholar] [CrossRef]
- Liu, P.F.; Yang, S.; Zhang, B.; Yang, H.G. Defect-rich ultrathin cobalt-iron layered double hydroxide for electrochemical overall water splitting. ACS Appl. Mater. Interfaces 2016, 8, 34474–34481. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Ge, L.; Yang, Y.; Li, M.; Jia, Y.; Yao, X.; Zhu, Z. Ultrathin iron-cobalt oxide nanosheets with abundant oxygen vacancies for the oxygen evolution reaction. Adv. Mater. 2017, 29, 1606793–1606799. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Ma, Y.; Lin, D.; Xu, C.; Xie, F.; Zeng, W. Bimetal-organic framework MIL-53(Co-Fe): An efficient and robust electrocatalyst for the oxygen evolution reaction. Nanoscale 2020, 12, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, J.; Wang, H.; Asefa, T.; Huang, X. A facile route to efficient water oxidation electrodes via electrochemical activation of iron in nickel sulfate solution. ACS Sustain. Chem. Eng. 2020, 8, 15550–15559. [Google Scholar] [CrossRef]
- Qu, M.; Jiang, Y.; Yang, M.; Liu, S.; Guo, Q.; Shen, W.; Li, M.; He, R. Regulating electron density of NiFe-P nanosheets electrocatalysts by a trifle of Ru for high-efficient overall water splitting. Appl. Catal. B Environ. 2020, 263, 118324–118332. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, X.; Zhu, X.; Liu, Q.; Lu, S.; Asiri, A.M.; Luo, Y.; Sun, X. Hierarchical CuO@ZnCo LDH heterostructured nanowire arrays toward enhanced water oxidation electrocatalysis. Nanoscale 2020, 12, 5359–5362. [Google Scholar] [CrossRef] [PubMed]
- Hoang, V.C.; Dinh, K.N.; Gomes, V.G. Hybrid Ni/NiO composite with N-doped activated carbon from waste cauliflower leaves: A sustainable bifunctional electrocatalyst for efficient water splitting. Carbon 2020, 157, 515–524. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Liu, H.; He, G.; Zhu, Y.; Xiao, J.; Han, L. Borate Anion Dopant Inducing Oxygen Vacancies over Co3O4 Nanocages for Enhanced Oxygen Evolution. Catalysts 2021, 11, 659. https://doi.org/10.3390/catal11060659
Liu X, Liu H, He G, Zhu Y, Xiao J, Han L. Borate Anion Dopant Inducing Oxygen Vacancies over Co3O4 Nanocages for Enhanced Oxygen Evolution. Catalysts. 2021; 11(6):659. https://doi.org/10.3390/catal11060659
Chicago/Turabian StyleLiu, Xuetao, Heng Liu, Guangling He, Yanlin Zhu, Jiamin Xiao, and Lei Han. 2021. "Borate Anion Dopant Inducing Oxygen Vacancies over Co3O4 Nanocages for Enhanced Oxygen Evolution" Catalysts 11, no. 6: 659. https://doi.org/10.3390/catal11060659
APA StyleLiu, X., Liu, H., He, G., Zhu, Y., Xiao, J., & Han, L. (2021). Borate Anion Dopant Inducing Oxygen Vacancies over Co3O4 Nanocages for Enhanced Oxygen Evolution. Catalysts, 11(6), 659. https://doi.org/10.3390/catal11060659





