Environmentally Friendly Route for Fabricating Conductive Agent for Lithium-Ion Batteries: Carbon Nanoparticles Derived from Polyethylene
Abstract
1. Introduction
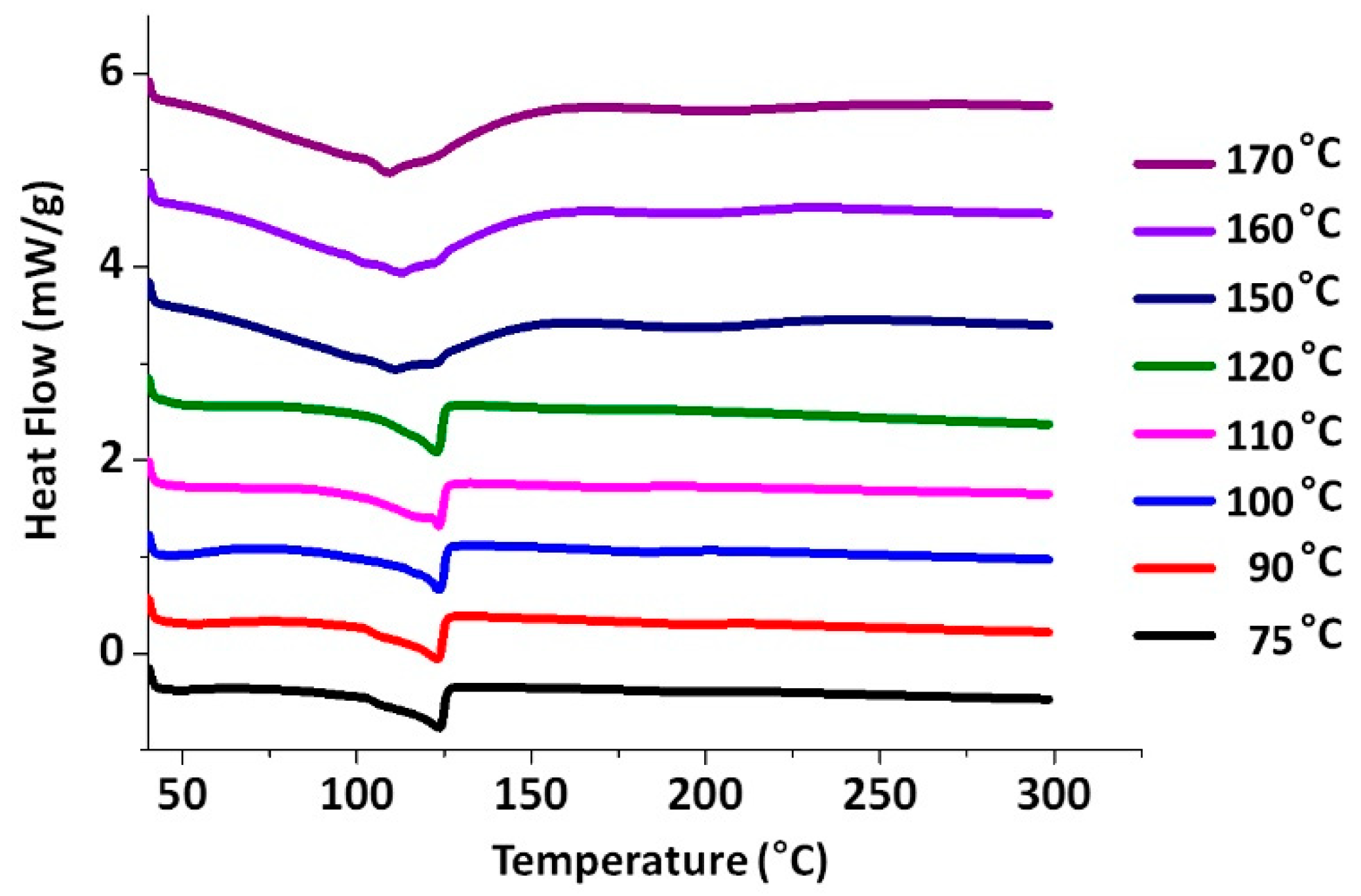
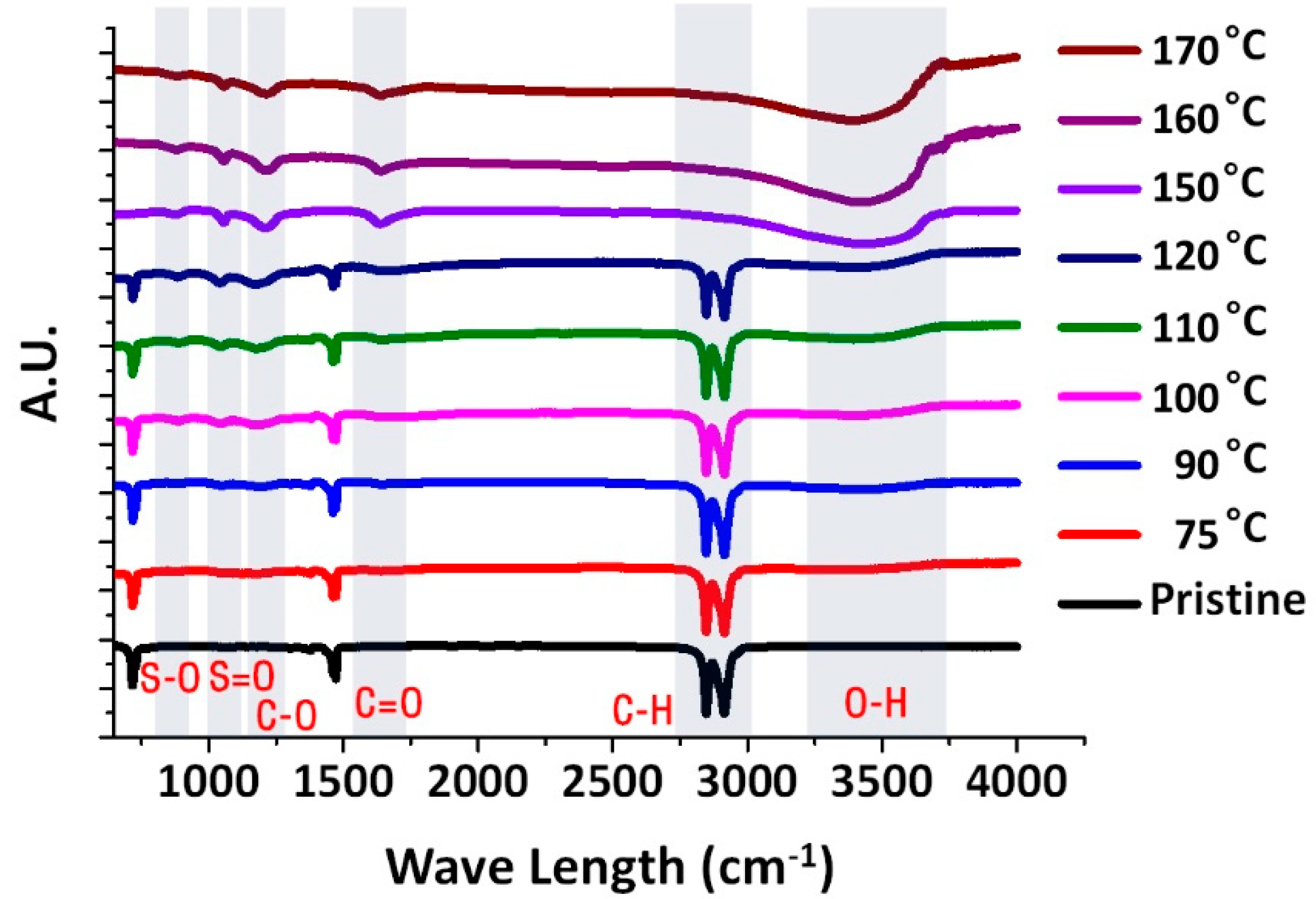
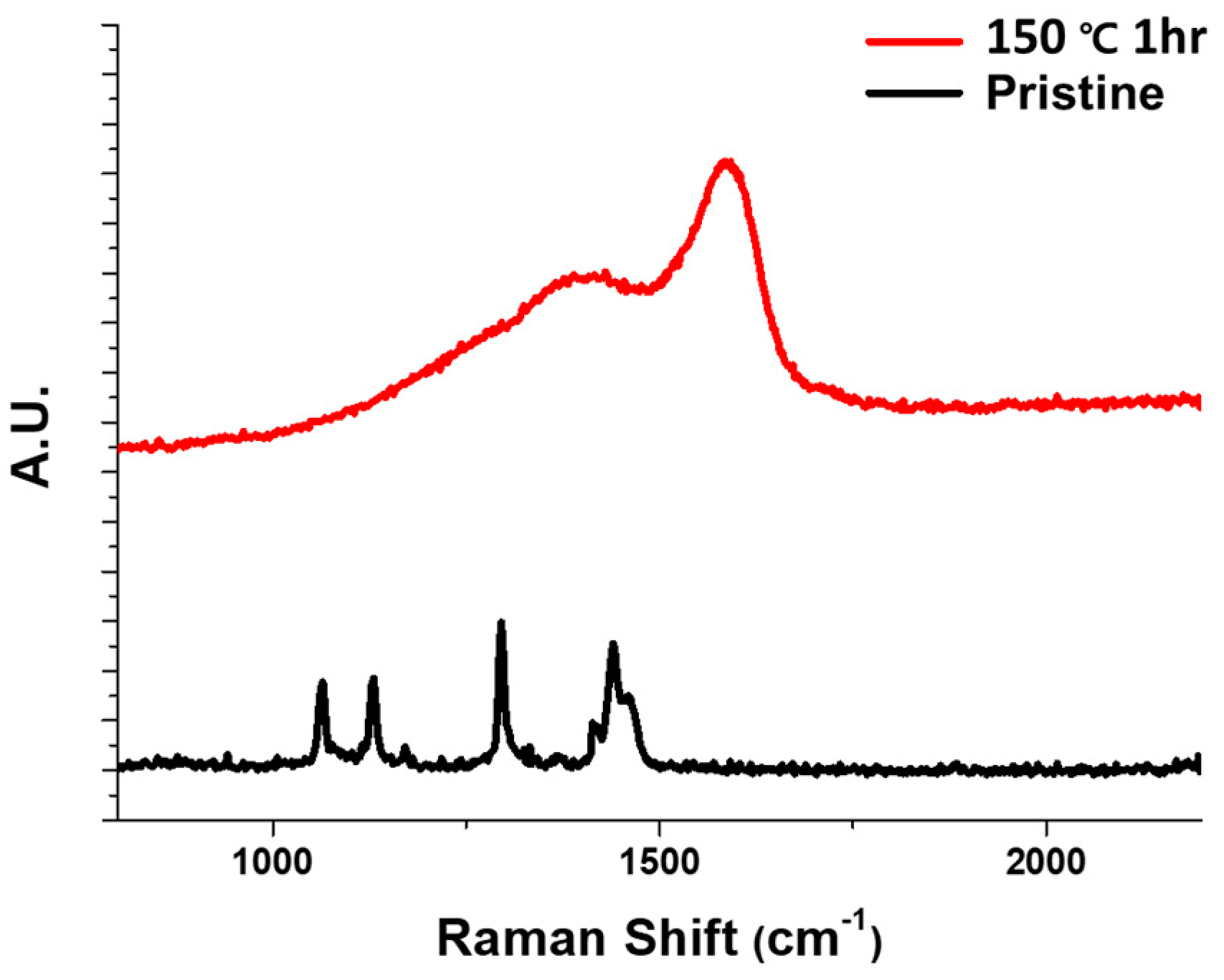
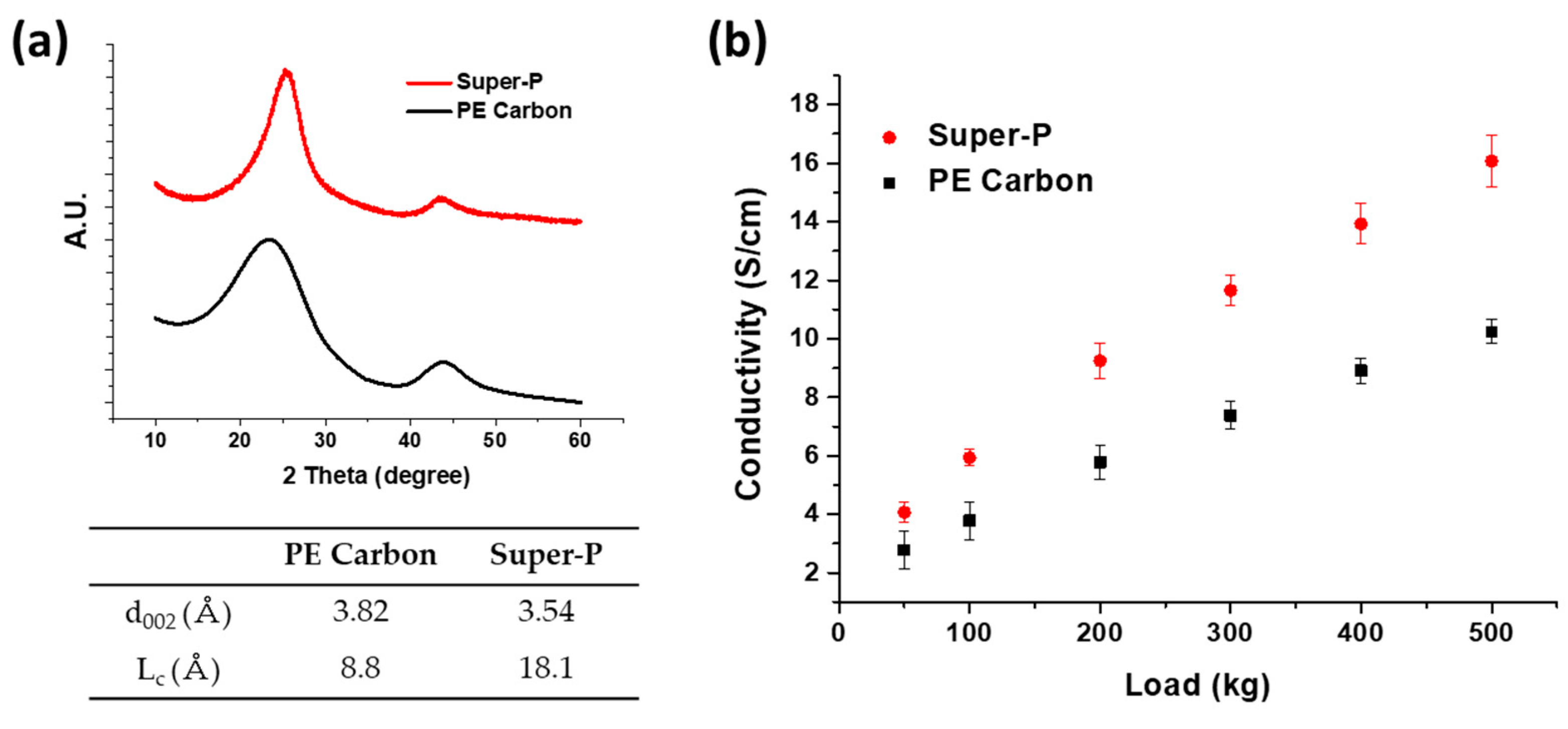
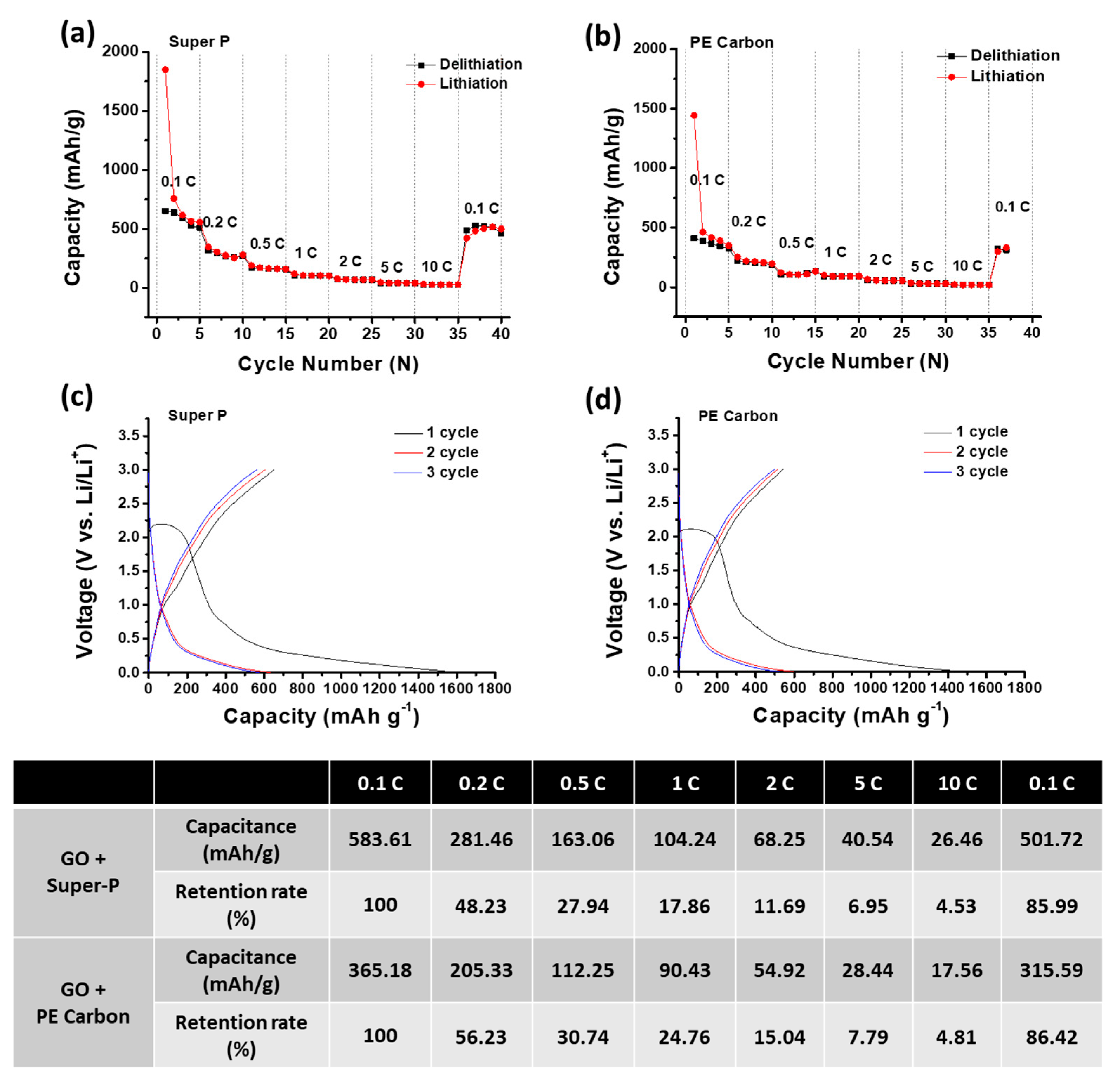
2. Results
3. Materials and Methods
3.1. Experimental Details
3.2. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, H.; Wang, Z.; Chen, L.; Huang, X. Research on Advanced Materials for Li-ion Batteries. Adv. Mater. 2009, 21, 4593–4607. [Google Scholar] [CrossRef]
- Odoom-Wubah, T.; Rubio, S.; Tirado, J.L.; Ortiz, G.F.; Akoi, B.J.; Huang, J.; Li, Q. Waste Pd/Fish-Collagen as anode for energy storage. Renew. Sustain. Energy Rev. 2020, 131, 109968. [Google Scholar] [CrossRef]
- Hou, H.; Qiu, X.; Wei, W.; Zhang, Y.; Ji, X. Carbon Anode Materials for Advanced Sodium-Ion Batteries. Adv. Energy Mater. 2017, 7, 1602898. [Google Scholar] [CrossRef]
- Azuma, H.; Imoto, H.; Yamada, S.N.I.; Sekai, K. Advanced carbon anode materials for lithium ion cells. J. Power Sour. 1999, 81–82, 1–7. [Google Scholar] [CrossRef]
- Kaskhedikar, N.A.; Maier, J. Lithium Storage in Carbon Nanostructures. Adv. Mater. 2009, 21, 2664–2680. [Google Scholar] [CrossRef]
- De Palmenaer, A. Polyethylene as a suitable precursor material for future carbon fibre production. In Proceedings of the Carbon Fibre Future Directions Conference 2017, Geelong, Australia, 1–3 March 2017. [Google Scholar]
- Baker, F.S. Low Cost Carbon Fiber from Renewable Resources; U.S. Department of Energy: Washington, DC, USA, 2010.
- Wortberg, G.; De Palmenaer, A.; Beckers, M.; Seide, G.; Gries, T. Polyethylene-Based Carbon Fibers by the Use of Sulphonation for Stabilization. Fibers 2015, 3, 373–379. [Google Scholar] [CrossRef]
- Hunt, M.A.; Saito, T.; Brown, R.H.; Kumbhar, A.S.; Naskar, A.K. Patterned Functional Carbon Fibers from Polyethylene. Adv. Mater. 2012, 24, 2386–2389. [Google Scholar] [CrossRef]
- Barton, B.E.; Patton, J.; Hukkanen, E.; Behr, M.; Lin, J.-C.; Beyer, S.; Zhang, Y.; Brehm, L.; Haskins, B.; Bell, B.; et al. The chemical transformation of hydrocarbons to carbon using SO3 sources. Carbon 2015, 94, 465–471. [Google Scholar] [CrossRef]
- Choi, D.; Yoo, S.H.; Lee, S. Safer and more effective route for polyethylene-derived carbon fiber fabrication using electron beam irradiation. Carbon N.Y. 2019, 146, 9–16. [Google Scholar] [CrossRef]
- Postema, A.R.; De Groot, H.; Pennings, A.J. Amorphous carbon fibres from linear low density polyethylene. J. Mater. Sci. 1990, 25, 4216–4222. [Google Scholar] [CrossRef]
- Baum, B. The mechanism of polyethylene oxidation. J. Appl. Polym. Sci. 1959, 2, 281–288. [Google Scholar] [CrossRef]
- Gugumus, F. Re-examination of the thermal oxidation reactions of polymers 2. Thermal oxidation of polyethylene. Polym. Degrad. Stab. 2002, 76, 329–340. [Google Scholar] [CrossRef]
- Basfar, A.A.; Idriss Ali, K.M. Natural weathering test for films of various formulations of low density polyethylene (LDPE) and linear low density polyethylene (LLDPE). Polym. Degrad. Stab. 2006, 91, 437–443. [Google Scholar] [CrossRef]
- Saxena, N.; Pal, N.; Ojha, K.; Dey, S.; Mandal, A. Synthesis, characterization, physical and thermodynamic properties of a novel anionic surfactant derived from: Sapindus laurifolius. RSC Adv. 2018, 8, 24485–24499. [Google Scholar] [CrossRef]
- Song, Q.; Cao, S.; Pritchard, R.H.; Ghalei, B.; Al-Muhtaseb, S.A.; Terentjev, E.M.; Cheetham, A.K.; Sivaniah, E. Controlled thermal oxidative crosslinking of polymers of intrinsic microporosity towards tunable molecular sieve membranes. Nat. Commun. 2014, 5, 4813. [Google Scholar] [CrossRef] [PubMed]
- Wind, J.D.; Staudt-Bickel, C.; Paul, D.R.; Koros, W.J. Solid-State Covalent Cross-Linking of Polyimide Membranes for Carbon Dioxide Plasticization Reduction. Macromolecules 2003, 36, 1882–1888. [Google Scholar] [CrossRef]
- Tretinnikov, O.N.; Ogata, S.; Ikada, Y. Surface crosslinking of polyethylene by electron beam irradiation in air. Polymer 1998, 39, 6115–6120. [Google Scholar] [CrossRef]
- Suyama, S.; Ishigaki, H.; Watanabe, Y.; Nakamura, T. Crosslinking of Polyethylene by Dicumyl Peroxide in the Presence of 2,4-Diphenyl-4-methyl-1-pentene. Polym. J. 1995, 27, 371–375. [Google Scholar] [CrossRef]
- Kazimi, M.R.; Shah, T.; Shima Binti Jamari, S.; Ahmed, I.; Ku Mohammad Faizal, C. Sulfonation of low-density polyethylene and its impact on polymer properties. Polym. Eng. Sci. 2014, 54, 2522–2530. [Google Scholar] [CrossRef]
- Karacan, I.; Benli, H. Use of sulfonation procedure for the development of thermally stabilized isotactic polypropylene fibers prior to carbonization. J. Appl. Polym. Sci. 2012, 123, 234–245. [Google Scholar] [CrossRef]
- Kotula, A.P.; Meyer, M.W.; De Vito, F.; Plog, J.; Hight Walker, A.R.; Migler, K.B. The rheo-Raman microscope: Simultaneous chemical, conformational, mechanical, and microstructural measures of soft materials. Rev. Sci. Instrum. 2016, 87, 105105. [Google Scholar] [CrossRef] [PubMed]
- Visentin, M.; Stea, S.; De Clerico, M.; Reggiani, M.; Fagnano, C.; Squarzoni, S.; Toni, A. Determination of crystallinity and crystal structure of hylamerTM polyethylene after in vivo wear. J. Biomater. Appl. 2006, 21, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Tamor, M.A.; Vassell, W.C. Raman ‘“fingerprinting”’ of amorphous carbon films. J. Appl. Phys. 1994, 76, 3823–3830. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Raman spectroscopy of amorphous, nanostructured, diamond–like carbon, and nanodiamond. Philos. Trans. R. Soc. London A Math. Phys. Eng. Sci. 2004, 362, 2477–2512. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef]
- Joh, H.-I.; Lee, S.; Kim, T.-W.; Hwang, S.Y.; Hahn, J.R. Synthesis and properties of an atomically thin carbon nanosheet similar to graphene and its promising use as an organic thin film transistor. Carbon 2013, 55, 299–304. [Google Scholar] [CrossRef]
- Jung, C.-H.; Kim, W.-J.; Jung, C.-H.; Hwang, I.-T.; Khim, D.; Kim, D.-Y.; Lee, J.-S.; Ku, B.-C.; Choi, J.-H. A simple PAN-based fabrication method for microstructured carbon electrodes for organic field-effect transistors. Carbon 2015, 87, 257–268. [Google Scholar] [CrossRef]
- Jurkiewicz, K.; Pawlyta, M.; Burian, A. Structure of Carbon Materials Explored by Local Transmission Electron Microscopy and Global Powder Diffraction Probes. C J. Carbon Res. 2018, 4, 68. [Google Scholar] [CrossRef]
- Ogumi, Z.; Wang, H. Carbon Anode Materials. In Lithium-Ion Batteries; Springer: New York, NY, USA, 2009; pp. 1–25. [Google Scholar]
- Spahr, M.E. Carbon-Conductive Additives for Lithium-Ion Batteries. In Lithium-Ion Batteries; Springer: New York, NY, USA, 2009; pp. 1–38. [Google Scholar]
- Komaba, S.; Murata, W.; Ishikawa, T.; Yabuuchi, N.; Ozeki, T.; Nakayama, T.; Ogata, A.; Gotoh, K.; Fujiwara, K. Electrochemical Na Insertion and Solid Electrolyte Interphase for Hard-Carbon Electrodes and Application to Na-Ion Batteries. Adv. Funct. Mater. 2011, 21, 3859–3867. [Google Scholar] [CrossRef]
- Buiel, E.; Dahn, J.R. Li-insertion in hard carbon anode materials for Li-ion batteries. Electrochim. Acta 1999, 45, 121–130. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mok, J.; Choi, D.; Bhang, S.H. Environmentally Friendly Route for Fabricating Conductive Agent for Lithium-Ion Batteries: Carbon Nanoparticles Derived from Polyethylene. Catalysts 2021, 11, 424. https://doi.org/10.3390/catal11040424
Mok J, Choi D, Bhang SH. Environmentally Friendly Route for Fabricating Conductive Agent for Lithium-Ion Batteries: Carbon Nanoparticles Derived from Polyethylene. Catalysts. 2021; 11(4):424. https://doi.org/10.3390/catal11040424
Chicago/Turabian StyleMok, Jihye, Dalsu Choi, and Suk Ho Bhang. 2021. "Environmentally Friendly Route for Fabricating Conductive Agent for Lithium-Ion Batteries: Carbon Nanoparticles Derived from Polyethylene" Catalysts 11, no. 4: 424. https://doi.org/10.3390/catal11040424
APA StyleMok, J., Choi, D., & Bhang, S. H. (2021). Environmentally Friendly Route for Fabricating Conductive Agent for Lithium-Ion Batteries: Carbon Nanoparticles Derived from Polyethylene. Catalysts, 11(4), 424. https://doi.org/10.3390/catal11040424






