Advanced Synthesis and Characterization of Vanadia/Titania Catalysts through a Molecular Approach
Abstract
1. Introduction
2. Results and Discussion
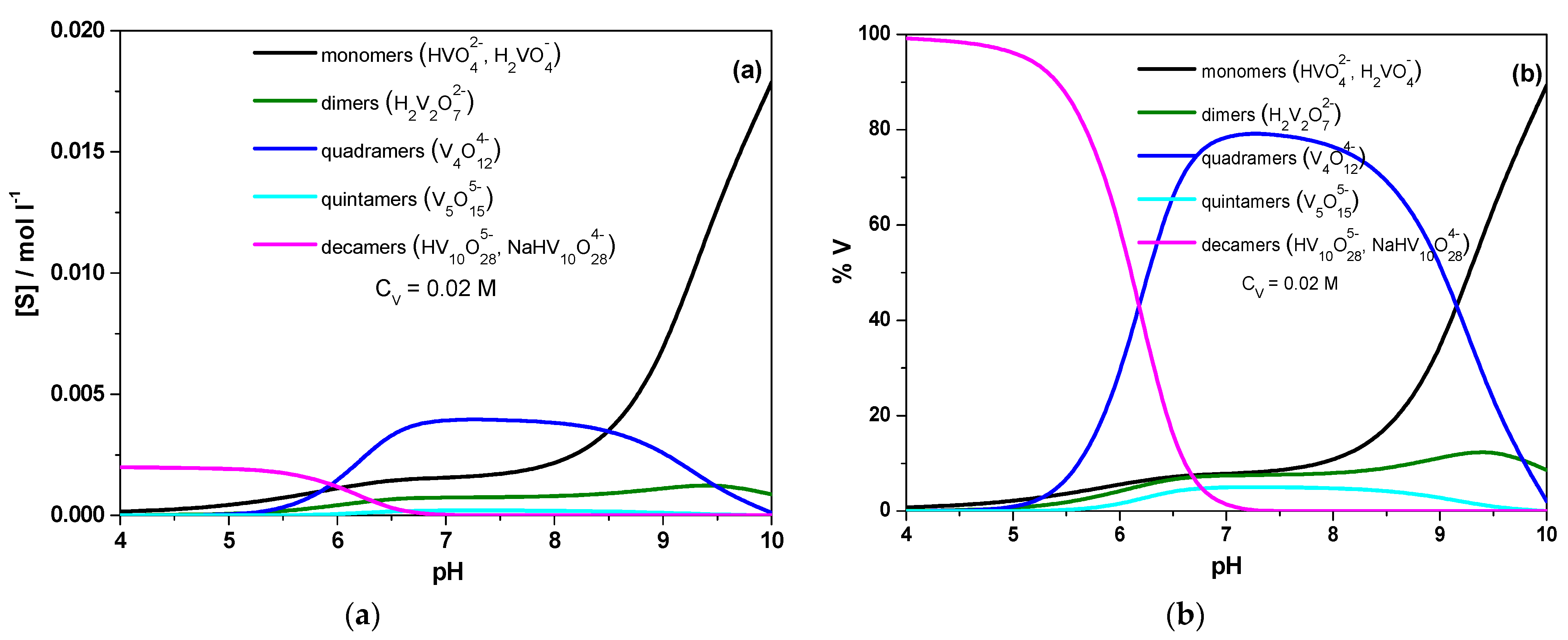
2.1. Solution Speciation of Vanadates
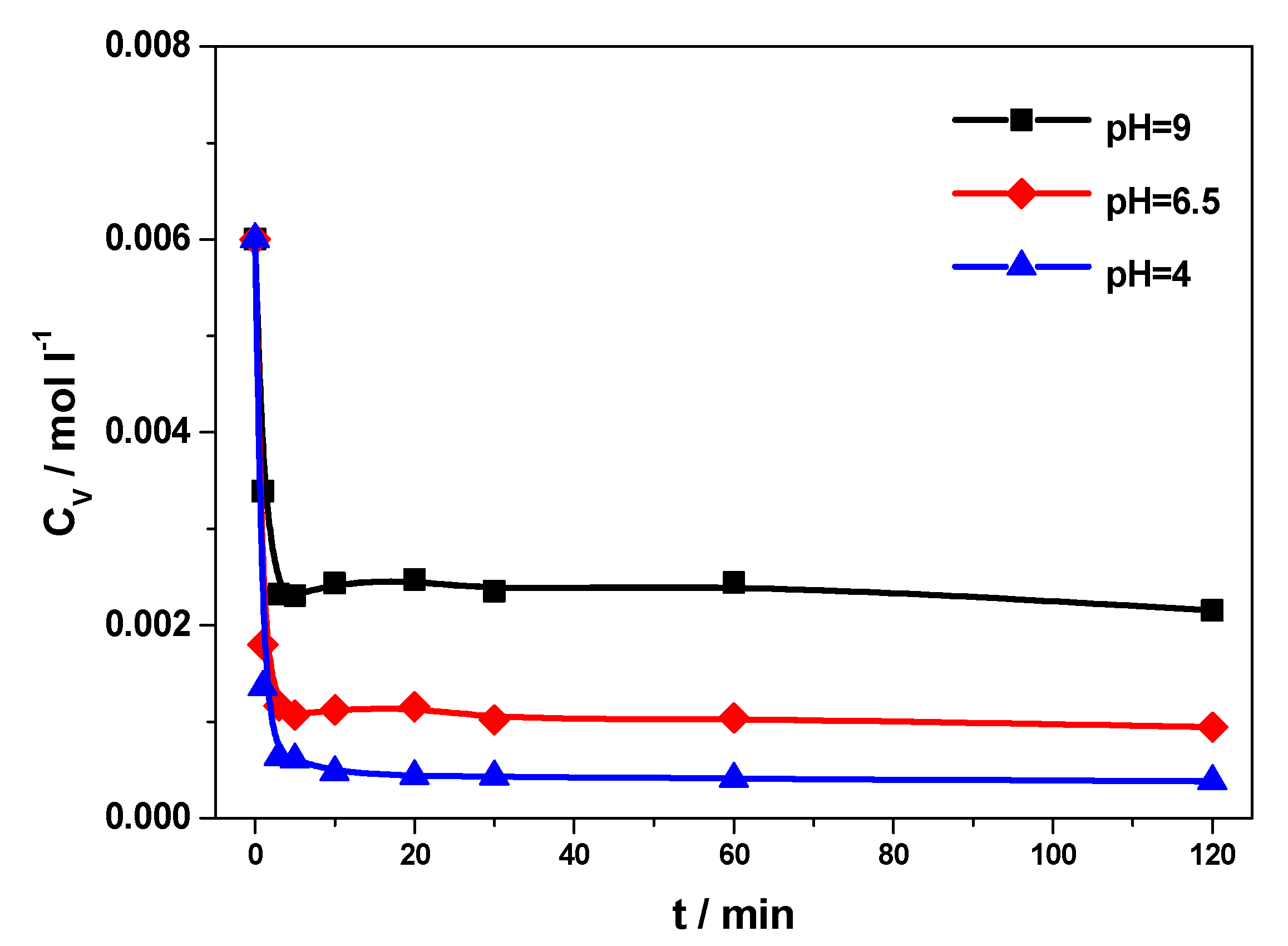
2.2. Kinetic Results
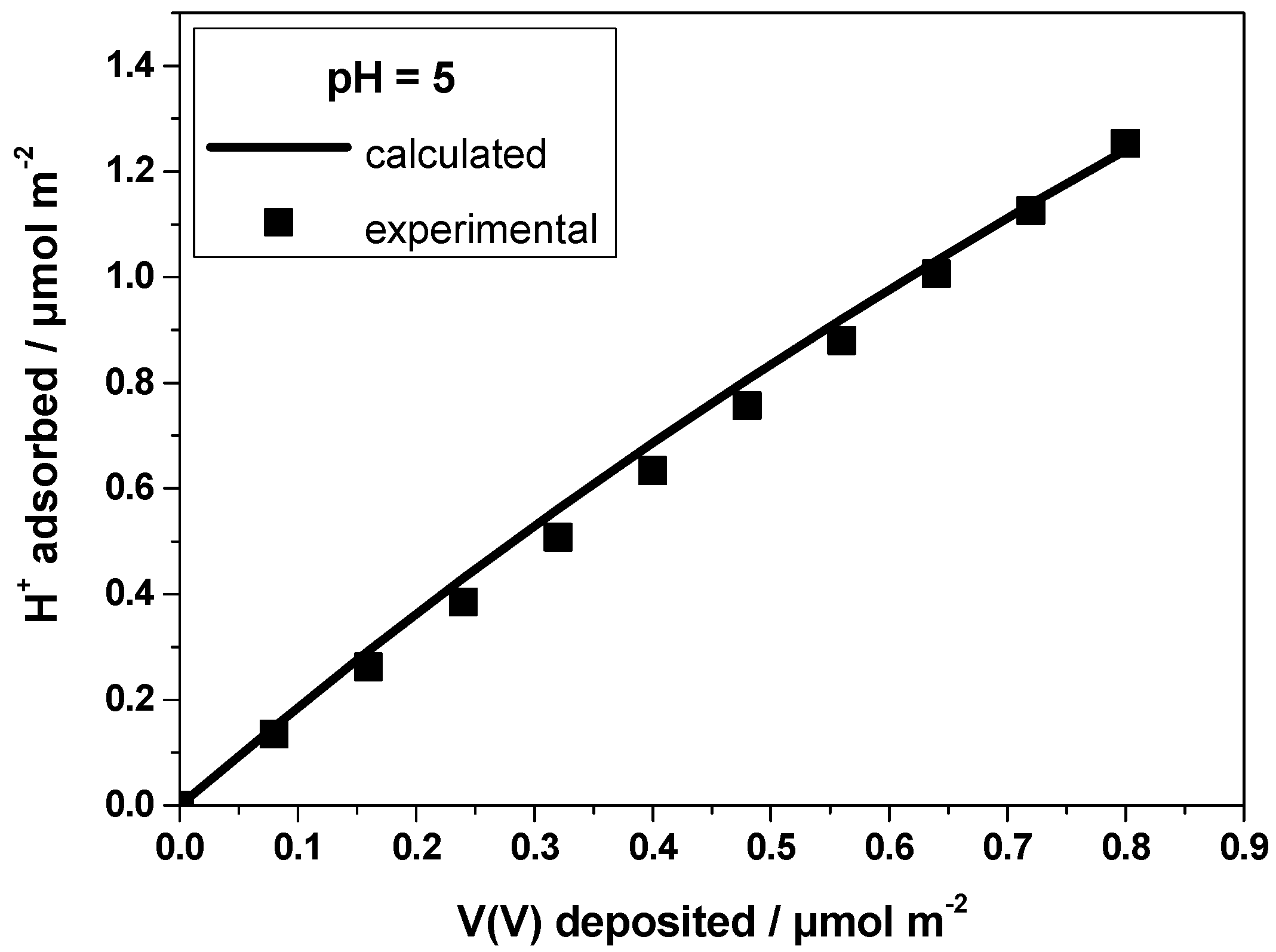
2.3. Modeling the Deposition of Vanadates on Titania Surface
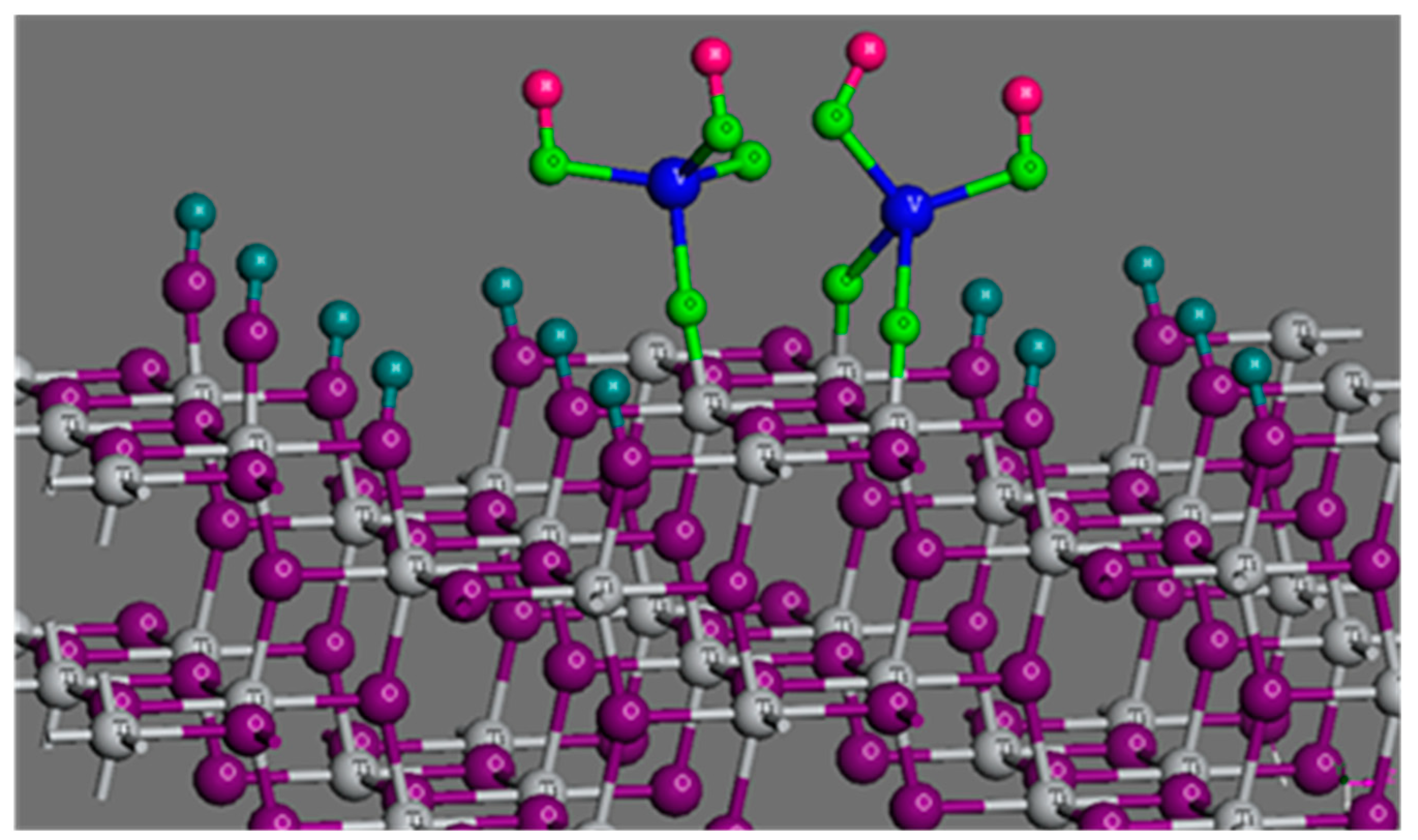
2.4. Determination of the Interfacial Speciation of Vanadates Adsorbed on Titania Surface
2.5. Raman Spectroscopy
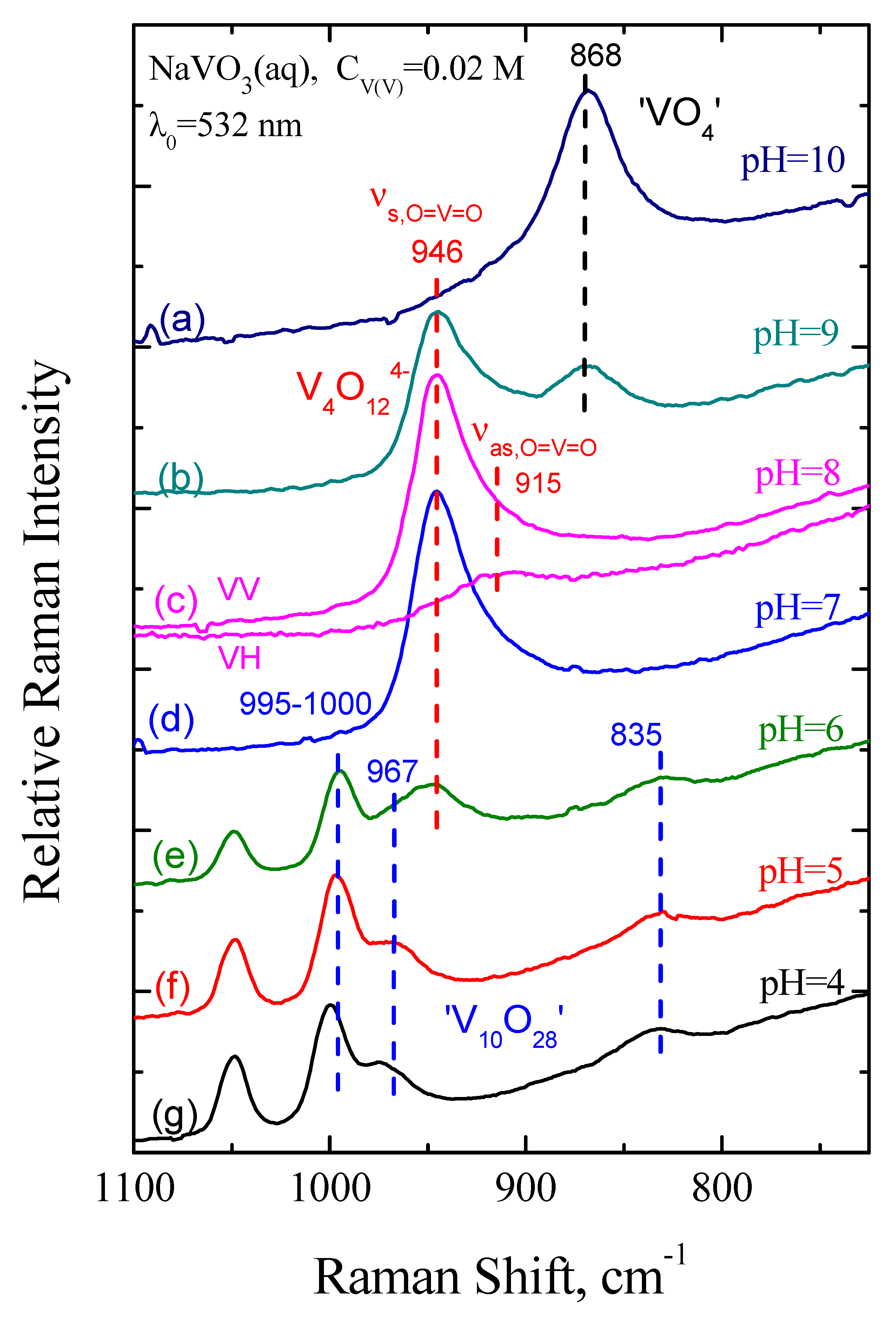
2.5.1. Solution Speciation of Vanadates
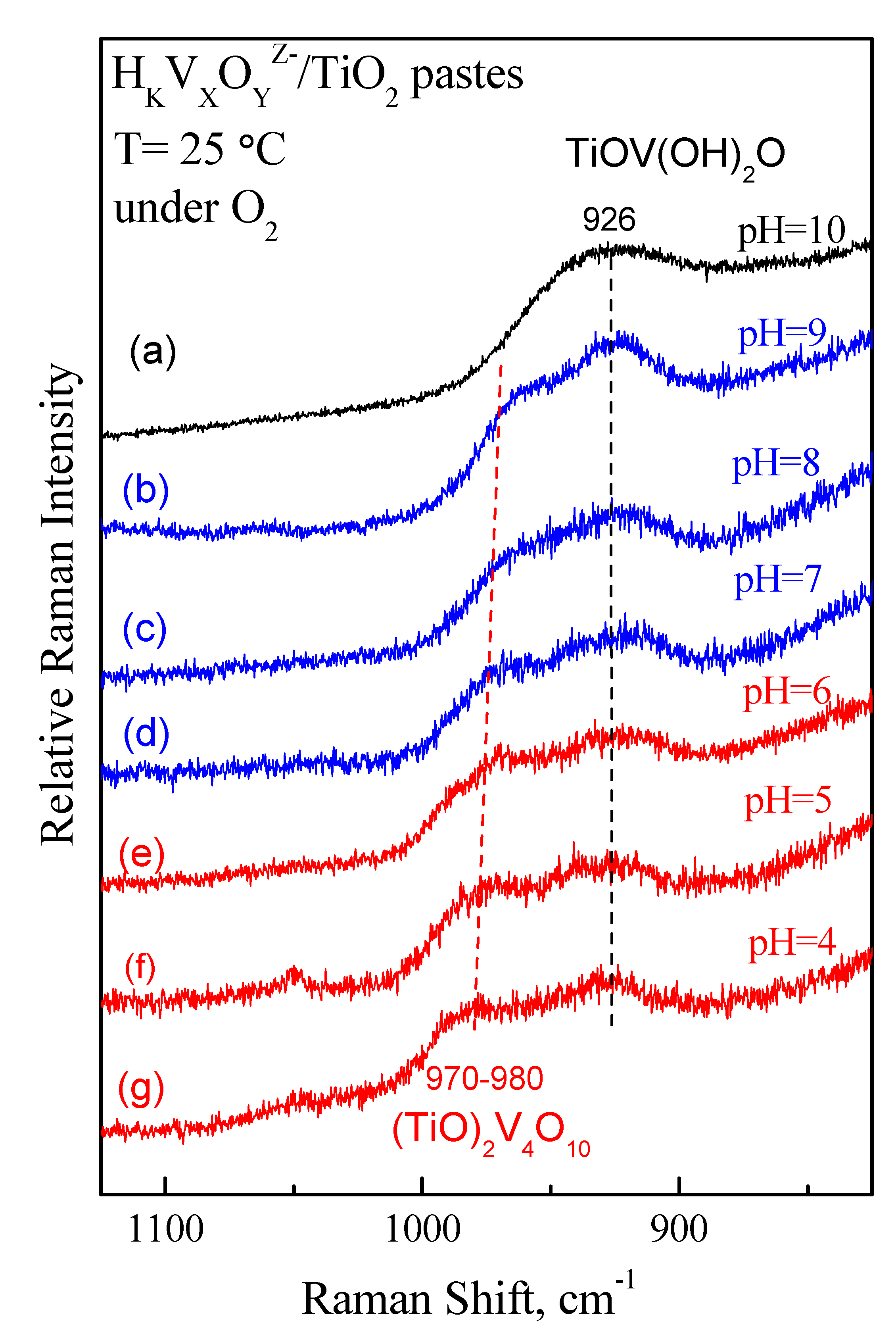
2.5.2. Raman Spectra of Wet ΗKVXOYZ−/TiO2 Pastes. Verification of the Interfacial Speciation of Vanadates by In Situ Raman Spectroscopy
2.5.3. Temperature-Dependent Evolution of the Molecular Structure of Vanadates Deposited on Titania
3. Materials and Methods
3.1. Substances
3.2. Proton-ion Titrations and Adsorption Experiments
3.3. Kinetic Experiments
3.4. Simulations
3.5. Synthesis of Vanadia/Titania Samples
3.6. Raman Spectroscopy
3.6.1. Raman Spectra of Precursor Solutions
3.6.2. In situ Raman Spectra of Wet ΗKVXOYZ−/TiO2 Pastes and Calcined Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Surface Species | Dissolved Components | Surface Component | Electrostatic Components | Intrinsic Formation Constant (logK) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H+ | Na+ | NO3− | HVO42− | TiO0.35− | Ti2O0.57− | |||||
| Ti2O0.57− | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | - |
| TiO0.35− | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | - |
| Ti2OH0.43+ | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | logK1 = 7.8 |
| TiOH0.65+ | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | logK2 = 4.6 |
| Ti2O0.57−-Na+ | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0.7 | 0.3 | logKNa = −1.7 |
| TiO0.35−-Na+ | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0.7 | 0.3 | logKNa = −1.7 |
| Ti2OH0.43+-NO3− | 1 | 0 | 1 | 0 | 0 | 1 | 1 | −0.7 | −0.3 | logK1 + logKNO3 = 5.6 |
| TiOH0.65+-NO3− | 1 | 0 | 1 | 0 | 1 | 0 | 1 | −0.7 | −0.3 | logK2 + logKNO3 = 2.4 |
| TiOV(OH)2O 0.65+ | 1 | 0 | 0 | 1 | 0 | 1 | 0.8 | −1.8 | 0 | logKT1 = 30.3 ± 0.5 |
| (TiO)2V(OH)2 2.3+ | 2 | 0 | 0 | 1 | 1 | 0 | 1.5 | −1.5 | 0 | logKT2 = 45.3 ± 0.5 |
| (TiO)2V4O10 0.7− | 18 | 0 | 0 | 7 | 5 | 5 | 10 | −2 | −4 | logKT3 = 88 ± 0.5 |
| Sum | Σ1 | Σ2 | Σ3 | Σ4 | Σ5 | Σ6 | Σ7 | Σ8 | Σ9 | - |
References
- Guerrero-Pérez, M.O. Supported, bulk and bulk-supported vanadium oxide catalysts: A short review with an historical perspective. Catal. Today 2017, 285, 226–233. [Google Scholar] [CrossRef]
- Kaichev, V.V.; Chesalov, Υ.Α.; Saraev, Α.Α.; Tsapina, Α.Μ. A Mechanistic Study of Dehydrogenation of Propane over Vanadia−Titania Catalysts. J. Phys. Chem. C 2019, 123, 19668–19680. [Google Scholar] [CrossRef]
- Kazerooni, H.; Darian, J.T.; Mortazavi, Y.; Khadadadi, A.A.; Asadi, R. Titania-Supported Vanadium Oxide Synthesis by Atomic Layer Deposition and Its Application for Low-Temperature Oxidative Dehydrogenation of Propane. Catal. Lett. 2020, 150, 2807–2822. [Google Scholar] [CrossRef]
- Kraemer, S.; Rondinone, A.J.; Tsai, Y.-T.; Schwartz, V.; Overbury, S.H.; Idrobo, J.-C.; Wu, Z. Oxidative dehydrogenation of isobutane over vanadia catalysts supported by titania nanoshapes. Catal. Today 2016, 263, 84–90. [Google Scholar] [CrossRef]
- Zhang, W.; Innocenti, G.; Ferbinteanu, M.; Ramos-Fernandez, E.V.; Sepulveda-Escribano, A.; Wu, H.; Cavani, F.; Rothenberg, G.; Shiju, N.R. Understanding the oxidative dehydrogenation of ethyl lactate to ethyl pyruvate over vanadia/titania. Catal. Sci. Technol. 2018, 8, 3737–3747. [Google Scholar] [CrossRef]
- Yun, D.; Herrera, J.E. A novel methodology for in situ redox active site titration of TiO2-supported vanadia during ethanol partial oxidation catalysis. J. Catal. 2017, 350, 72–85. [Google Scholar] [CrossRef]
- Koivikko, N.; Laitinen, T.; Mouammine, A.; Ojala, S.; Keiski, R.L. Catalytic Activity Studies of Vanadia/Silica–Titania Catalysts in SVOC Partial Oxidation to Formaldehyde: Focus on the Catalyst Composition. Catalysts 2018, 8, 56. [Google Scholar] [CrossRef]
- Liu, Ζ.; Zhang, R.; Wang, S.; Li, N.; Sima, R.; Liu, G.; Wu, P.; Zeng, G.; Li, S.; Sun, Y. Highly Efficient and Stable Vanadia−Titania−Sulfate Catalysts for Methanol Oxidation to Methyl Formate: Synthesis and Mechanistic Study. J. Phys. Chem. C 2016, 120, 6591–6600. [Google Scholar] [CrossRef]
- Zhang, W.; Innocenti, G.; Oulego, P.; Gitis, V.; Wu, H.; Ensing, B.; Cavani, F.; Rothenberg, G.; Shiju, N.R. Highly Selective Oxidation of Ethyl Lactate to Ethyl Pyruvate Catalyzed by Mesoporous Vanadia−Titania. ACS Catal. 2018, 8, 2365–2374. [Google Scholar] [CrossRef]
- Andrushkevich, T.V.; Kaichev, V.V.; Chesalov, Y.A.; Saraev, A.A.; Buktiyarov, V.I. Selective oxidation of ethanol over vanadia-based catalysts: The influence of support material and reaction mechanism. Catal. Today 2017, 279, 95–106. [Google Scholar] [CrossRef]
- Ferretti, F.; Ribeiro, A.P.C.; Alegria, E.C.B.A.; Ferraria, A.M.; Kopylovich, M.N.; Guedes da Silva, M.F.C.; Marchetti, F.; Pombeiro, A.J.L. Synergistic catalytic action of vanadia–titania composites towards the microwave-assisted benzoin oxidation. Dalton Trans. 2019, 48, 3198–3203. [Google Scholar] [CrossRef]
- Sethi, D.; Jada, N.; Tiwari, A.; Ramasamy, S.; Dash, T.; Pandey, S. Photocatalytic destruction of Escherichia coli in water by V2O5/TiO2. J. Photochem. Photobiol. B 2015, 144, 68–74. [Google Scholar] [CrossRef]
- Lin, K.-S.; Lin, Y.-G.; Cheng, H.-W.; Haung, Y.-H. Preparation and characterization of V-Loaded titania nanotubes for adsorption/photocatalysis of basic dye and environmental hormone contaminated wastewaters. Catal. Today 2018, 307, 119–130. [Google Scholar] [CrossRef]
- Chang, J.-H.; Wang, Y.-L.; Dong, C.-D.; Shen, S.-Y. Electrocatalytic Degradation of Azo Dye by Vanadium-Doped TiO2 Nanocatalyst. Catalysts 2020, 10, 482. [Google Scholar] [CrossRef]
- Guan, B.; Zhan, R.; Lin, H.; Huang, Z. Review of state of the art technologies of selective catalytic reduction of NOx from diesel engine exhaust. Appl. Therm. Eng. 2014, 66, 395–414. [Google Scholar] [CrossRef]
- Forzatti, P. Present status and perspectives in de-NOx SCR catalysis. Appl. Catal. A 2001, 222, 221–236. [Google Scholar] [CrossRef]
- Han, L.; Cai, S.; Gao, M.; Hasegawa, J.; Wang, P.; Zhang, J.; Shi, L.; Zhang, D. Selective Catalytic Reduction of NOx with NH3 by Using Novel Catalysts: State of the Art and Future Prospects. Chem. Rev. 2019, 119, 10916–10976. [Google Scholar] [CrossRef]
- Lai, J.-K.; Wachs, I.E. A Perspective on the Selective Catalytic Reduction (SCR) of NO with NH3 by Supported V2O5−WO3/TiO2 Catalysts. ACS Catal. 2018, 8, 6537–6551. [Google Scholar] [CrossRef]
- Gan, L.; Li, K.; Niu, H.; Peng, Y.; Chen, J.; Huang, Y.; Li, J. Simultaneous removal of NOx and chlorobenzene on V2O5/TiO2 granular catalyst: Kinetic study and performance prediction. Front. Environ. Sci. Eng. 2021, 15, 70. [Google Scholar] [CrossRef]
- Gallastegi-Villa, M.; Aranzabal, A.; Boukha, Z.; González-Marcos, J.A.; González-Velasco, J.R.; Martínez-Huerta, M.V.; Banares, M.A. Role of surface vanadium oxide coverage support on titania for the simultaneous removal of o-dichlorobenzene and NOx from waste incinerator flue gas. Catal. Today 2015, 254, 2–11. [Google Scholar] [CrossRef]
- Xu, J.; Chen, G.; Guo, F.; Xie, J. Development of wide-temperature vanadium-based catalysts for selective catalytic reducing of NOx with ammonia: Review. Chem. Eng. J. 2018, 353, 507–518. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Chen, P.; Zhu, B. Research Status and Prospect on Vanadium-Based Catalysts for NH3-SCR Denitration. Materials 2018, 11, 1632. [Google Scholar] [CrossRef]
- Weckhuysen, B.M.; Keller, D.E. Chemistry, spectroscopy and the role of supported vanadium oxides in heterogeneous catalysis. Catal. Today 2003, 78, 25–46. [Google Scholar] [CrossRef]
- Kompio, P.G.W.A.; Brückner, A.; Hipler, F.; Manoylova, O.; Auer, G.; Mestl, G.; Grünert, W. V2O5-WO3/TiO2 catalysts under thermal stress: Responses of structure and catalytic behavior in the selective catalytic reduction of NO by NH3. Appl. Catal. B 2017, 217, 365–377. [Google Scholar] [CrossRef]
- Arnarson, L.; Falsig, H.; Rasmussen, S.B.; Lauritsen, J.V.; Moses, P.G. A complete reaction mechanism for standard and fast selective catalytic reduction of nitrogen oxides on low coverage VOx/TiO2(001) catalysts. J. Catal. 2017, 346, 188–197. [Google Scholar] [CrossRef]
- Arnarson, L.; Rasmussen, S.B.; Falsig, H.; Lauritsen, J.V.; Moses, P.G. Coexistence of Square Pyramidal Structures of Oxo Vanadium (+5) and (+4) Species Over Low-Coverage VOX/TiO2 (101) and (001) Anatase Catalysts. J. Phys. Chem. C 2015, 119, 23445–23452. [Google Scholar] [CrossRef]
- Koust, S.; Arnarson, L.; Moses, P.G.; Li, Z.; Beinik, I.; Lauritsen, J.V.; Wendt, S. Facile embedding of single vanadium atoms at the anatase TiO2(101) surface. Phys. Chem. Chem. Phys. 2017, 19, 9424–9431. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-Z.; Gao, F.; Li, Y.; Walter, E.D.; Liu, J.; Peden, C.H.F.; Wang, Y. Nanocrystalline Anatase Titania-Supported Vanadia Catalysts: Facet-Dependent Structure of Vanadia. J. Phys. Chem. C 2015, 119, 15094–15102. [Google Scholar] [CrossRef]
- Marberger, A.; Ferri, D.; Elsener, M.; Krocher, O. The Significance of Lewis Acid Sites for the Selective Catalytic Reduction of Nitric Oxide on Vanadium-Based Catalysts. Angew. Chem. Int. Ed. 2016, 55, 11989–11994. [Google Scholar] [CrossRef]
- Kwon, D.W.; Park, K.H.; Hong, S.C. Influence of VOx surface density and vanadyl species on the selective catalytic reduction of NO by NH3 over VOx/TiO2 for superior catalytic activity. Appl. Catal. A 2015, 499, 1–12. [Google Scholar] [CrossRef]
- Murkute, A.D.; Vanderwiel, D. Active Sites Evaluation of Vanadia Based Powdered and Extruded SCR Catalysts Prepared on Commercial Titania. Catal. Lett. 2015, 145, 1224–1236. [Google Scholar] [CrossRef]
- Marberger, A.; Ferri, D.; Rentsch, D.; Krumeich, F.; Elsener, M.; Kröcher, O. Effect of SiO2 on co-impregnated V2O5/WO3/TiO2 catalysts for the selective catalytic reduction of NO with NH3. Catal. Today 2019, 320, 123–132. [Google Scholar] [CrossRef]
- Liu, X.; Chen, H.; Wu, X.; Cao, L.; Jiang, P.; Yu, Q.; Ma, Y. Effects of SiO2 modification on the hydrothermal stability of the V2O5/WO3–TiO2 NH3-SCR catalyst: TiO2 structure and vanadia species. Catal. Sci. Technol. 2019, 9, 3711–3720. [Google Scholar] [CrossRef]
- Shutilov, R.A.; Shutilov, A.A.; Zenkovets, G.A. Nanocrystalline V2O5,WO3/(CeO2-TiO2) and V2O5,WO3/(Y2O3-TiO2) catalysts with enhance thermal stability and activity in the reduction of NO with NH3 into N2. Mater. Today Proc. 2017, 4, 11490–11494. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, S.; Hu, W.; Zhu, X.; Qu, R.; Wu, W.; Zheng, C.; Gao, X. New insight into alkali resistance and low temperature activation on vanadia-titania catalysts for selective catalytic reduction of NO. Appl. Surf. Sci. 2019, 466, 99–109. [Google Scholar] [CrossRef]
- Marberger, A.; Ferri, D.; Elsener, M.; Sagar, A.; Artner, C.; Schermanz, K.; Kröcher, O. Relationship between structures and activities of supported metal vanadates for the selective catalytic reduction of NO by NH3. Appl. Catal. B 2017, 218, 731–742. [Google Scholar] [CrossRef]
- Marberger, A.; Elsener, M.; Nuguid, R.J.G.; Ferri, D.; Kröcher, O. Thermal activation and aging of a V2O5/WO3-TiO2 catalyst for the selective catalytic reduction of NO with NH3. Appl. Catal. A 2019, 573, 64–72. [Google Scholar] [CrossRef]
- Hess, C.; Waleska, P.; Ratzka, M.; Janssens, T.V.W.; Rasmussen, S.B.; Beato, P. Hierarchical Vanadia Model Catalysts for Ammonia Selective Catalytic Reduction. Top. Catal. 2017, 60, 1631–1640. [Google Scholar] [CrossRef]
- Shen, J.; Hess, C. High Surface Area VOx/TiO2/SBA-15 Model Catalysts for Ammonia SCR Prepared by Atomic Layer Deposition. Catalysts 2020, 10, 1386. [Google Scholar] [CrossRef]
- He, Y.; Ford, M.E.; Zhu, M.; Liu, Q.; Tumuluri, U.; Wu, Z.; Wachs, I.E. Influence of catalyst synthesis method on selective catalytic reduction (SCR) of NO by NH3 with V2O5-WO3/TiO2 catalysts. Appl. Catal. B 2016, 193, 141–150. [Google Scholar] [CrossRef]
- Bourikas, K.; Kordulis, C.; Lycourghiotis, A. The role of the liquid—Solid interface in the preparation of supported catalysts. Catal. Rev. Sci. Eng. 2006, 48, 363–444. [Google Scholar] [CrossRef]
- Bourikas, K.; Kordulis, C.; Lycourghiotis, A. Titanium Dioxide (Anatase and Rutile): Surface Chemistry, Liquid−Solid Interface Chemistry, and Scientific Synthesis of Supported Catalysts. Chem. Rev. 2014, 114, 9754–9823. [Google Scholar] [CrossRef]
- Munnik, P.; de Jongh, P.E.; de Jong, K.P. Recent Developments in the Synthesis of Supported Catalysts. Chem. Rev. 2015, 115, 6687–6718. [Google Scholar] [CrossRef]
- Carrier, X.; Royer, S.; Marceau, E. Synthesis of metal oxide catalysts. In Metal Oxides in Heterogeneous Catalysis; Védrine, J.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 43–103. [Google Scholar] [CrossRef]
- Carrier, X.; Marceau, E.; Che, M. Physical techniques and catalyst preparation: Determining the interactions of transition-metal complexes with oxide surfaces. Pure Appl. Chem. 2006, 78, 1039–1055. [Google Scholar] [CrossRef][Green Version]
- de Jong, K.P. Synthesis of Solid Catalysts; Wiley: New York, NY, USA, 2008. [Google Scholar] [CrossRef]
- Mehrabadi, B.A.T.; Eskandari, S.; Khan, U.; White, R.D.; Regalbuto, J.R. A Review of Preparation Methods for Supported Metal Catalysts. Adv. Catal. 2017, 61, 1–35. [Google Scholar] [CrossRef]
- Marceau, E.; Bonneviot, L.; Dzwigaj, S.; Lambert, J.-F.; Louis, C.; Carrier, X. Interfacial Coordination Chemistry for Catalyst Preparation. J. Catal. 2021, in press. [Google Scholar] [CrossRef]
- Panagiotou, G.D.; Petsi, T.; Bourikas, K.; Kalampounias, A.G.; Boghosian, S.; Kordulis, C.; Lycourghiotis, A. Interfacial Impregnation Chemistry in the Synthesis of Molybdenum Catalysts supported on Titania. J. Phys. Chem. C 2010, 114, 11868–11879. [Google Scholar] [CrossRef]
- Petsi, T.; Panagiotou, G.D.; Bourikas, K.; Kordulis, C.; Voyiatzis, G.A.; Lycourghiotis, A. Interfacial Impregnation Chemistry in the Synthesis of Chromium Catalysts Supported on Titania. ChemCatChem 2011, 3, 1072–1082. [Google Scholar] [CrossRef]
- Bourikas, K.; Stavropoulos, J.; Garoufalis, C.S.; Kordulis, C.; Petsi, T.; Lycourghiotis, A. Interfacial Impregnation Chemistry in the Synthesis of Nickel Catalysts Supported on Titania. Chem. Eur. J. 2011, 17, 1201–1213. [Google Scholar] [CrossRef] [PubMed]
- Petsi, T.; Panagiotou, G.D.; Garoufalis, C.S.; Kordulis, C.; Stathi, P.; Deligiannakis, Y.; Lycourghiotis, A.; Bourikas, K. Interfacial Impregnation Chemistry in the Synthesis of Cobalt Catalysts Supported on Titania. Chem. Eur. J. 2009, 15, 13090–13104. [Google Scholar] [CrossRef]
- Panagiotou, G.D.; Petsi, T.; Bourikas, K.; Kordulis, C.; Lycourghiotis, A. The Interfacial Chemistry of the Impregnation Step Involved in the Preparation of Tungsten (VI) Supported Titania Catalysts. J. Catal. 2009, 262, 266–279. [Google Scholar] [CrossRef]
- Rasmussen, S.B.; Abrams, B.L. Fundamental chemistry of V-SCR catalysts at elevated temperatures. Catal. Today 2017, 297, 60–63. [Google Scholar] [CrossRef]
- Fan, F.; Feng, Z.; Li, C. UV Raman spectroscopic studies on active sites and synthesis mechanisms of transition metal-containing microporous and mesoporous materials. Acc. Chem. Res. 2010, 43, 378–387. [Google Scholar] [CrossRef]
- Giakoumelou, I.; Fountzoula, C.; Kordulis, C.; Boghosian, S. Molecular structure and catalytic activity of V2O5/TiO2 catalysts for the SCR of NO by NH3: In situ Raman spectra in the presence of O2, NH3, NO, H2, H2O and SO2. J. Catal. 2006, 239, 1–12. [Google Scholar] [CrossRef]
- Kryukova, G.N.; Zenkovets, G.A.; Mestl, G.; Schlögl, R. Structural study of titanium-doped vanadia and vanadium-doped titania catalysts. React. Kinet. Catal. Lett. 2003, 80, 161–169. [Google Scholar] [CrossRef]
- Wachs, I.E. Catalysis science of supported vanadium oxide catalysts. Dalton Trans. 2013, 42, 11762–11769. [Google Scholar] [CrossRef]
- Martinez-Huerta, M.V.; Fierro, J.L.G.; Banaraes, M.A. Monitoring the states of vanadium oxide during the transformation of TiO2 anatase-to-rutile under reactive environments: H2 reduction and oxidative dehydrogentation of ethane. Catal. Commun. 2009, 11, 15–19. [Google Scholar] [CrossRef]
- Tribalis, A.; Panagiotou, G.D.; Tsilomelekis, G.; Kalampounias, A.G.; Bourikas, K.; Kordulis, C.; Boghosian, S.; Lycourghiotis, A. Temperature -dependent evolution of the molecular configuration of oxo-tungsten(VI) species deposited on the surface of titania. J. Phys. Chem. C 2014, 118, 11319–11332. [Google Scholar] [CrossRef]
- Tsilomelekis, G.; Panagiotou, G.D.; Stathi, P.; Kalampounias, A.G.; Bourikas, K.; Kordulis, C.; Deligiannakis, Y.; Boghosian, S.; Lycourghiotis, A. Molybdena deposited on titania by equilibrium deposition filtration: Structural evolution of oxo-molybdenum(V)) sites with temperature. Phys. Chem. Chem. Phys. 2016, 18, 23980–23989. [Google Scholar] [CrossRef]
- Andriopoulou, C.; Boghosian, S. Tuning the configuration of dispersed oxometallic sites in supported transition metal oxide catalysts: A temperature dependent Raman study. Catal. Today 2019, 336, 74–83. [Google Scholar] [CrossRef]
- Andriopoulou, C.; Boghosian, S. Molecular structure and termination configuration of Oxo-Re(VII) catalyst sites supported on Titania. Catal. Today 2020, 355, 665–677. [Google Scholar] [CrossRef]
- Tella, E.; Panagiotou, G.D.; Petsi, T.; Bourikas, K.; Kordulis, C.; Lycourghiotis, A. The mechanism of retention of vanadium oxo-species at the “titanium oxide/aqueous solution” interface. Global NEST J. 2010, 12, 231–238. [Google Scholar] [CrossRef]
- Gustafsson, J.P. Visual MINTEQ, version 2.40; Department of Land and Water Resources Engineering, Royal Institute of Technology: Stockholm, Sweden, 2005. [Google Scholar]
- Panagiotou, G.D.; Petsi, T.; Bourikas, K.; Garoufalis, C.S.; Tsevis, A.; Spanos, N.; Kordulis, C.; Lycourghiotis, A. Mapping the surface (hydr)oxo–groups of titanium oxide and its interface with an aqueous solution: The state of the art and a new approach. Adv. Colloid Interface Sci. 2008, 142, 20–42. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 6th ed.; Wiley-Interscience: New York, NY, USA, 2009. [Google Scholar]
- Boghosian, S. Vibrational Modes and Structure of Vanadium (V) Complexes in M2SO4-V2O5 (M= K, Cs) Molten Salt Mixtures. J. Chem. Soc. Faraday Trans. 1998, 94, 3463–3469. [Google Scholar] [CrossRef]
- Aureliano, M.; Ohlin, C.A.; Vieira, M.O.; Marques, M.P.M.; Casey, W.H.; Batista de Carvalho, L.A.E. Characterization of decavanadate and decaniobate solutions by Raman spectroscopy. Dalton Trans. 2016, 45, 7391–7399. [Google Scholar] [CrossRef]
- Busca, G. Differentiation of mono-oxo and polyoxo and of monomeric and polymeric vanadate, molybdate and tungstate species in metal oxide catalysts by IR and Raman spectroscopy. J. Raman Spectrosc. 2002, 33, 348–358. [Google Scholar] [CrossRef]
- Went, G.T.; Oyama, S.T.; Bell, A.T. Laser Raman spectroscopy of supported vanadium oxide catalysts. J. Phys. Chem. 1990, 94, 4240–4246. [Google Scholar] [CrossRef]
- Wachs, I.E.; Weckhuysen, B.M. Structure and reactivity of surface vanadium oxide species on oxide supports. Appl. Catal. A 1997, 157, 67–90. [Google Scholar] [CrossRef]
- Christodoulakis, A.; Machli, M.; Lemonidou, A.A.; Boghosian, S. Molecular structure and reactivity of vanadia-based catalysts for propane oxidative dehydrogenation studied by in situ Raman spectroscopy and catalytic activity measurements. J. Catal. 2004, 222, 293–306. [Google Scholar] [CrossRef]
- Bulushev, D.; Rainone, F.; Kiwi-Minsker, L.; Renken, A. Influence of potassium doping on the formation of vanadia species in V/Ti oxide catalysts. Langmuir 2001, 17, 5276–5282. [Google Scholar] [CrossRef]
- Due-Hansen, J.; Boghosian, S.; Kustov, A.; Fristrup, P.; Tsilomelekis, G.; Ståhl, K.; Christensen, C.H.; Fehrmann, R. Vanadia-based SCR catalysts supported on tungstated and sulfated zirconia: Influence of doping with potassium. J. Catal. 2007, 251, 459–473. [Google Scholar] [CrossRef]
- Calatayud, M.; Minot, C. Effect of alkali doping on a V2O5/TiO2 catalyst from periodic DFT calculations. J. Phys. Chem. C 2007, 111, 6411–6417. [Google Scholar] [CrossRef]
- Lewandowska, A.E.; Calatayud, M.; Lozano-Diz, E.; Minot, C.; Banares, M.A. Combining theoretical description with experimental in situ studies on the effect of alkali additives on the structure and reactivity of vanadium oxide supported catalysts. Catal. Today 2008, 139, 209–213. [Google Scholar] [CrossRef]
- Tsilomelekis, G.; Boghosian, S. An operando Raman study of molecular structure and reactivity of molybdenum(VI) oxide supported on anatase for the oxidative dehydrogenation of ethane. Phys. Chem. Chem. Phys. 2012, 14, 2216–2228. [Google Scholar] [CrossRef]
- Lian, Z.; Li, Y.; Shan, W.; He, H. Recent Progress on Improving Low-Temperature Activity of Vanadia-Based Catalysts for the Selective Catalytic Reduction of NOx with Ammonia. Catalysts 2020, 10, 1421. [Google Scholar] [CrossRef]
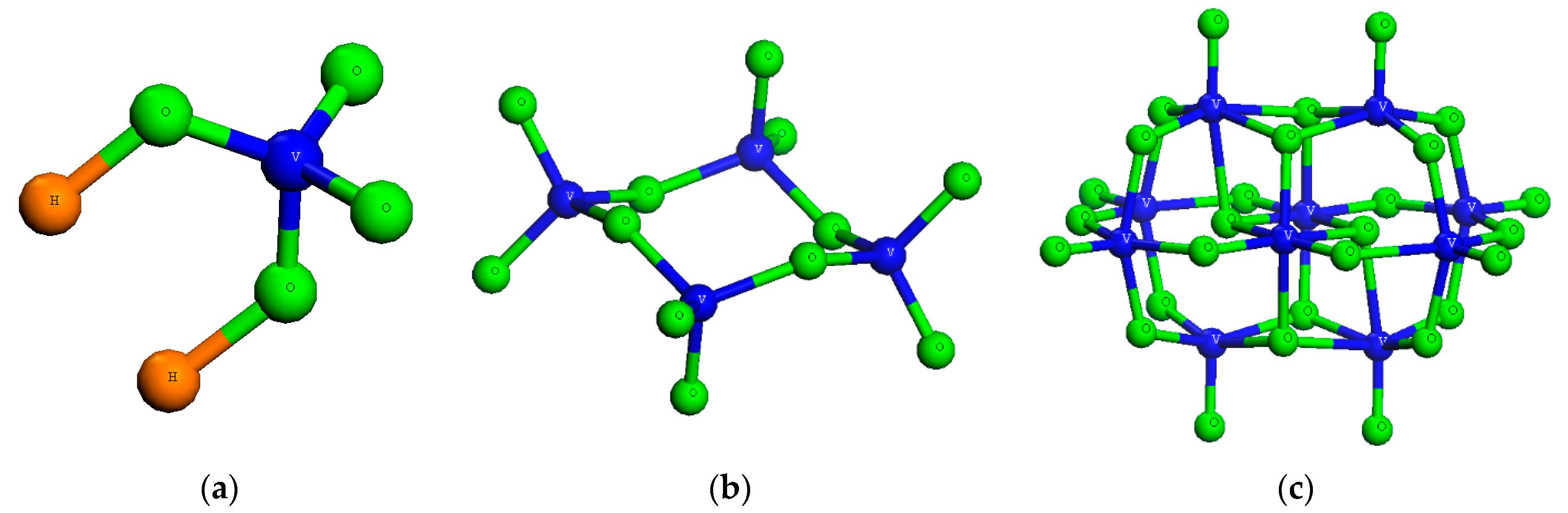





| Vanadium Species | Solution Components | logΚ | |||
|---|---|---|---|---|---|
| - | |||||
| 1 | 1 | 0 | 0 | 8.75 | |
| 2 | 2 | 0 | −1 | 19.8 | |
| 4 | 4 | 0 | −4 | 42.6 | |
| 10 | 15 | 0 | −12 | 141.5 | |
| 10 | 15 | 1 | −12 | 143.68 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tella, E.; Trimpalis, A.; Tsevis, A.; Kordulis, C.; Lycourghiotis, A.; Boghosian, S.; Bourikas, K. Advanced Synthesis and Characterization of Vanadia/Titania Catalysts through a Molecular Approach. Catalysts 2021, 11, 322. https://doi.org/10.3390/catal11030322
Tella E, Trimpalis A, Tsevis A, Kordulis C, Lycourghiotis A, Boghosian S, Bourikas K. Advanced Synthesis and Characterization of Vanadia/Titania Catalysts through a Molecular Approach. Catalysts. 2021; 11(3):322. https://doi.org/10.3390/catal11030322
Chicago/Turabian StyleTella, Eleni, Antonios Trimpalis, Athanasios Tsevis, Christos Kordulis, Alexis Lycourghiotis, Soghomon Boghosian, and Kyriakos Bourikas. 2021. "Advanced Synthesis and Characterization of Vanadia/Titania Catalysts through a Molecular Approach" Catalysts 11, no. 3: 322. https://doi.org/10.3390/catal11030322
APA StyleTella, E., Trimpalis, A., Tsevis, A., Kordulis, C., Lycourghiotis, A., Boghosian, S., & Bourikas, K. (2021). Advanced Synthesis and Characterization of Vanadia/Titania Catalysts through a Molecular Approach. Catalysts, 11(3), 322. https://doi.org/10.3390/catal11030322









