Effect of the Ni/Al Ratio on the Performance of NiAl2O4 Spinel-Based Catalysts for Supercritical Methylcyclohexane Catalytic Cracking
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
2.1.1. XRD Analysis
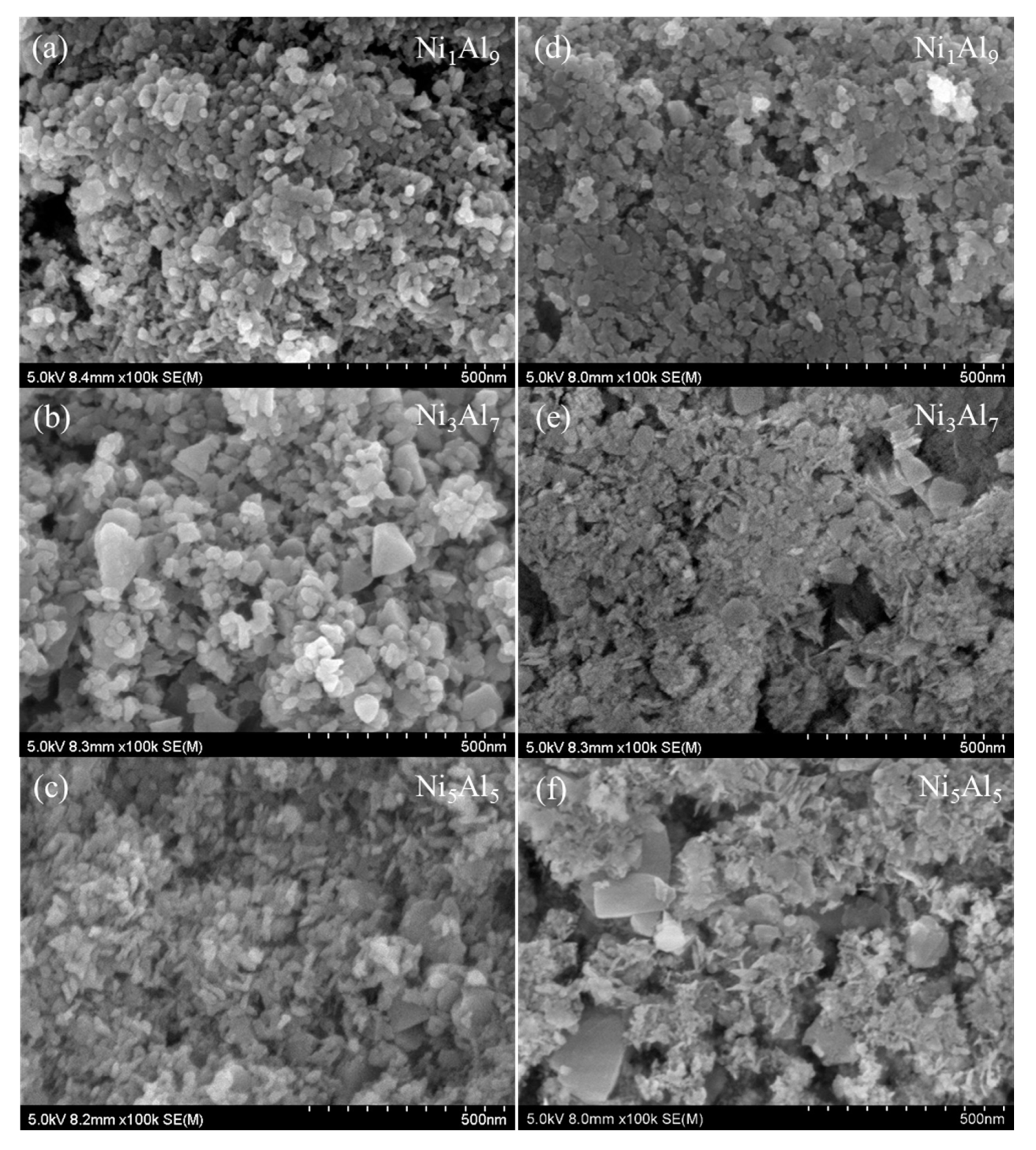
2.1.2. SEM Analysis
2.1.3. TEM Analysis
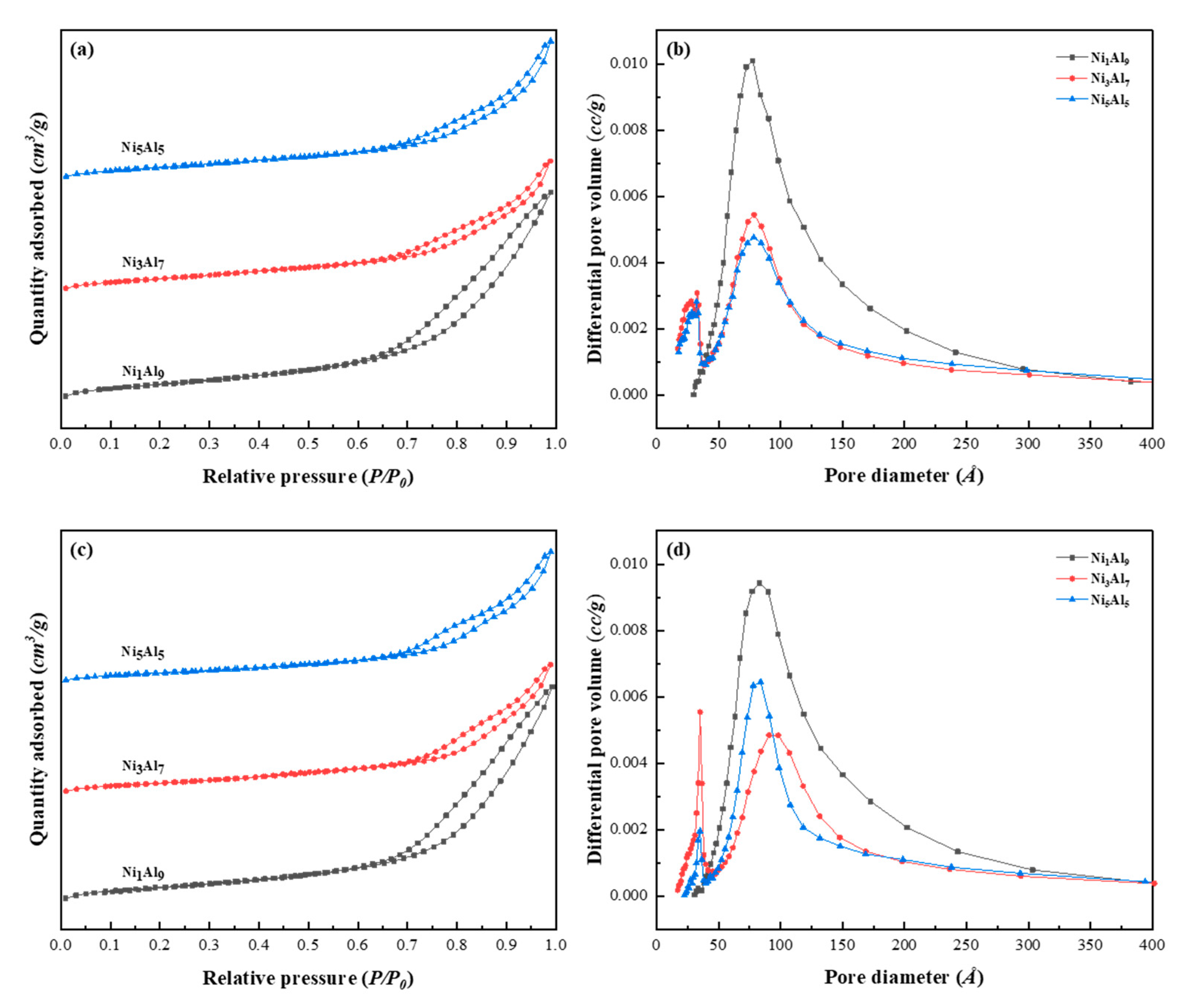
2.1.4. Physicochemical Properties
2.1.5. Acidity
2.2. Catalyst Activity
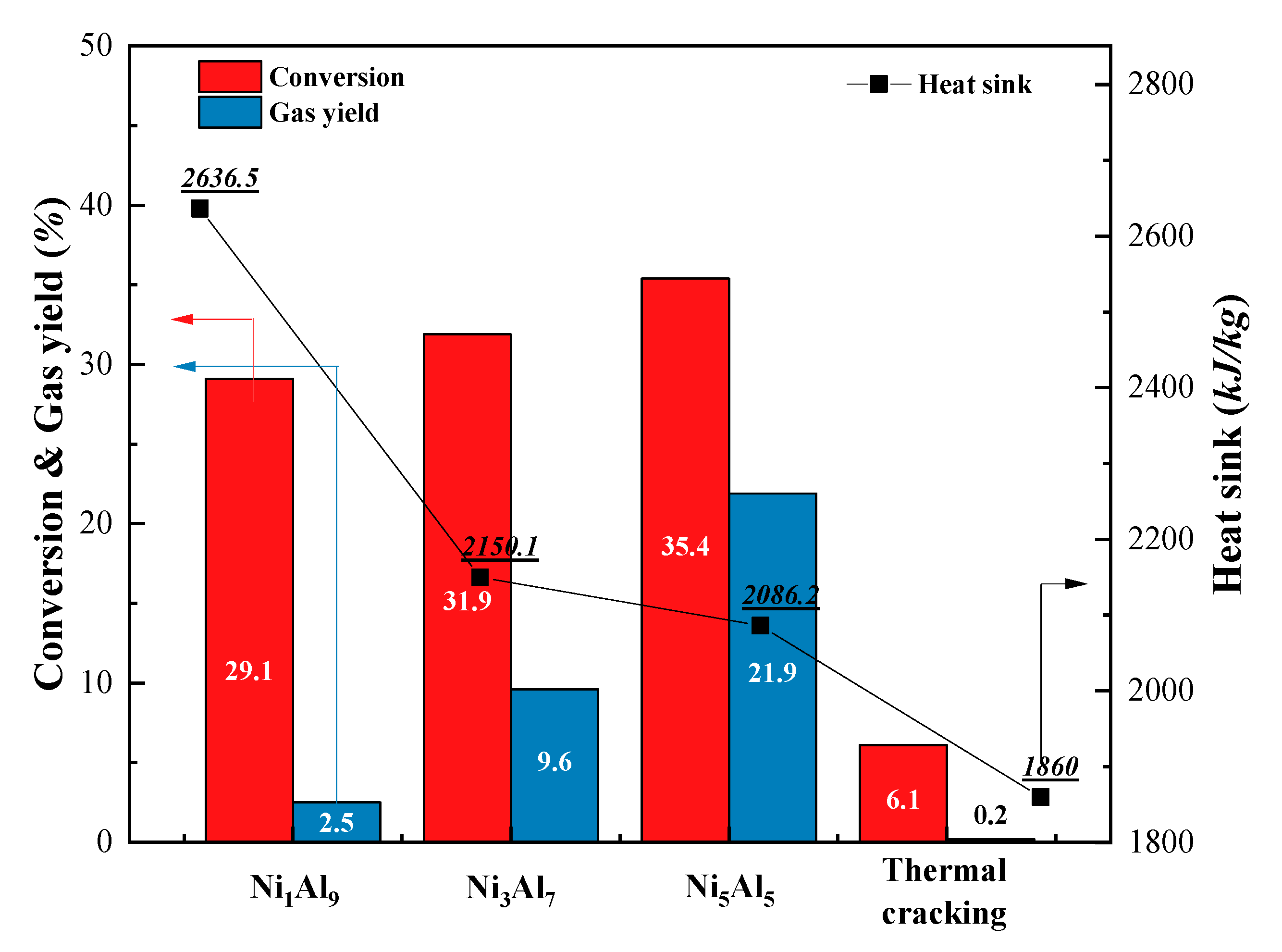
2.2.1. Cracking Evaluation
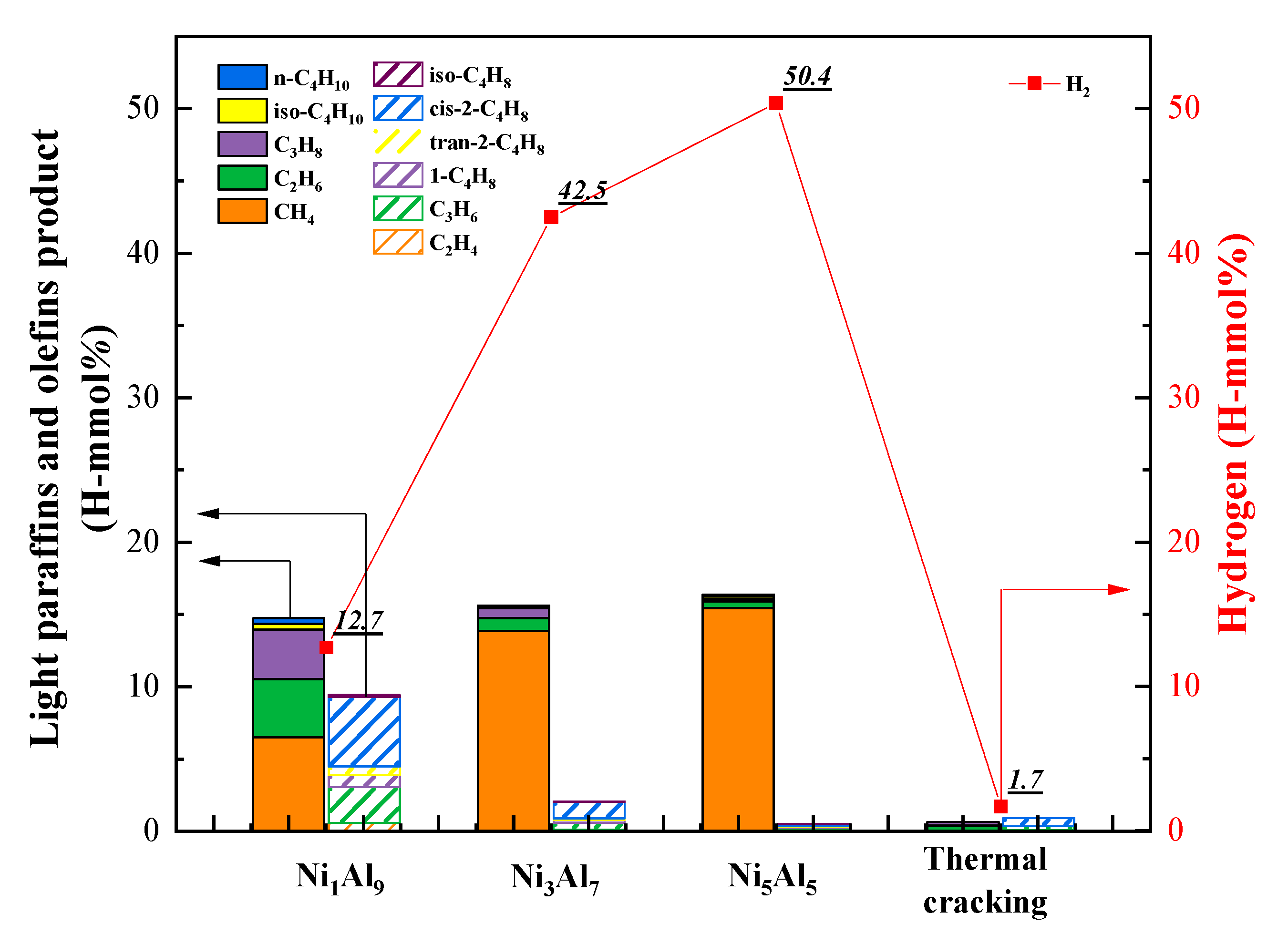
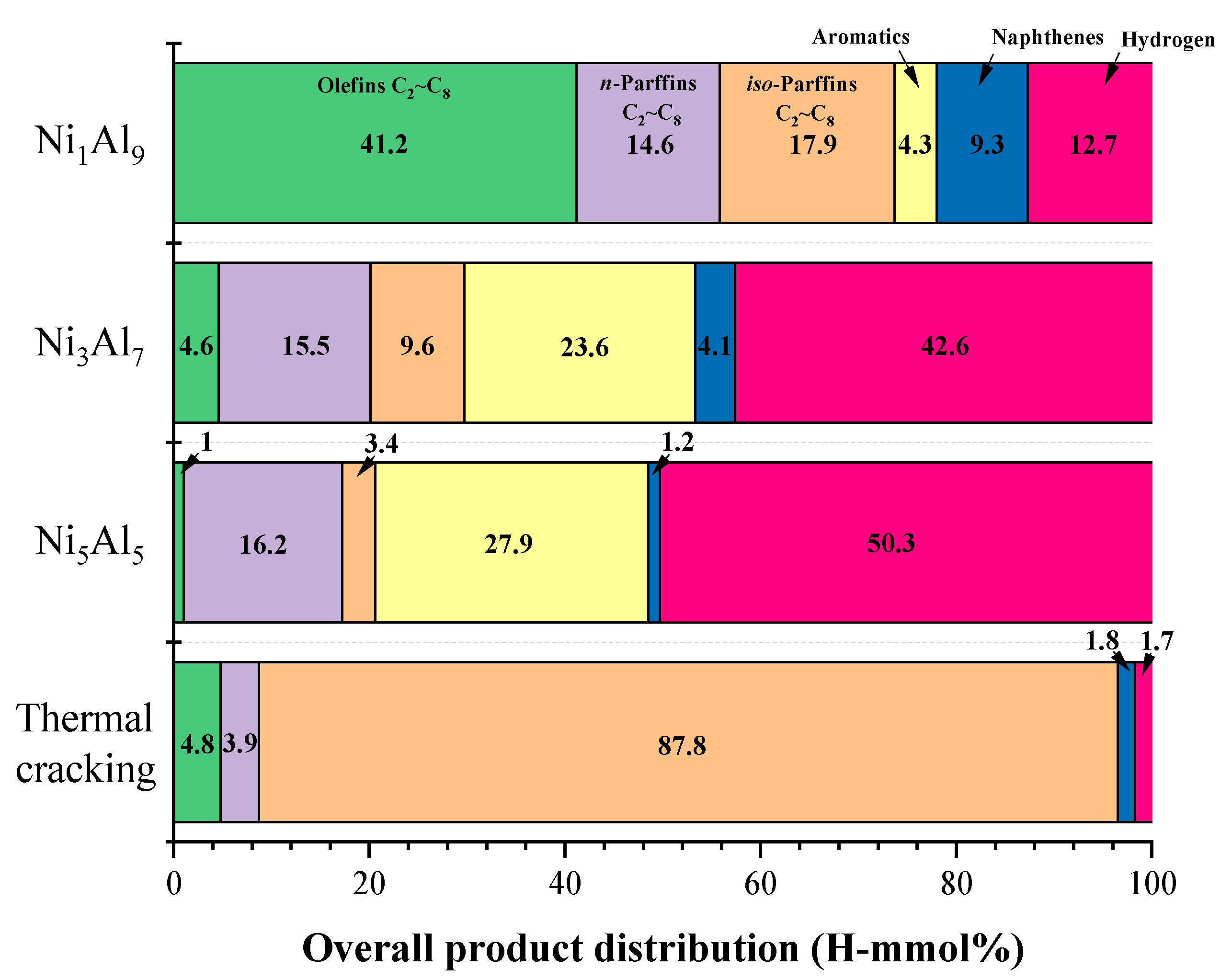
2.2.2. Product Distribution
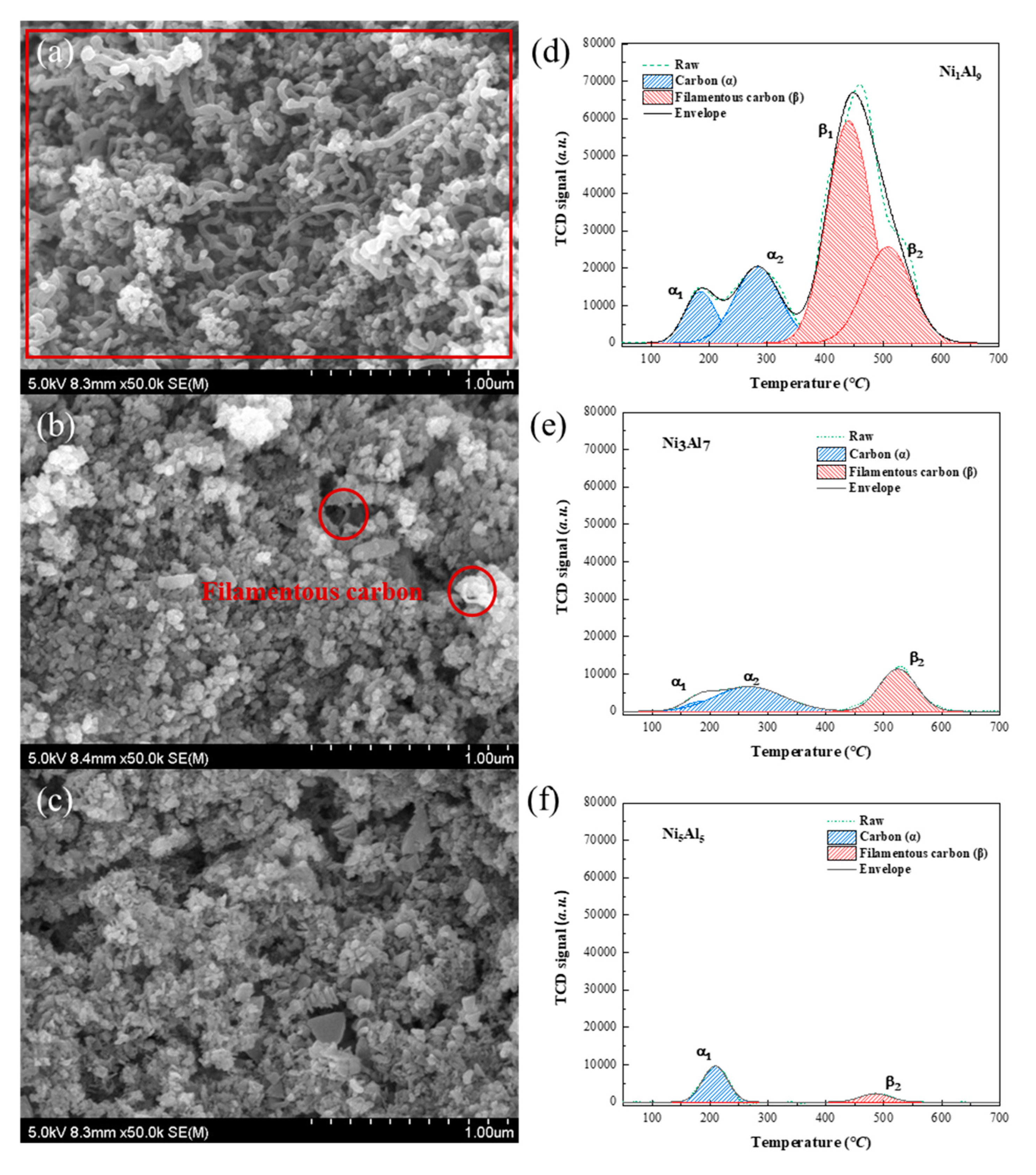
2.3. Carbon Formation
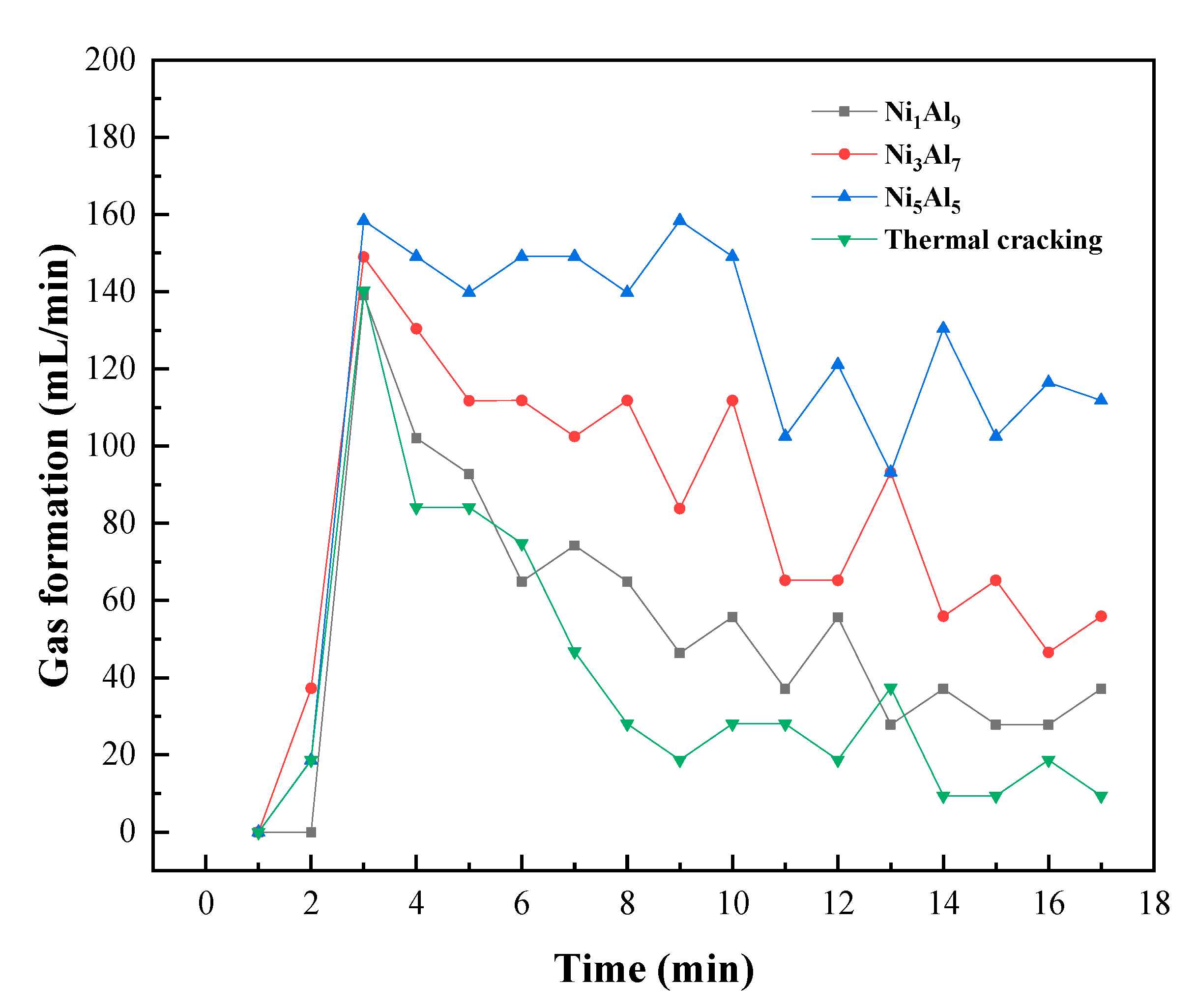
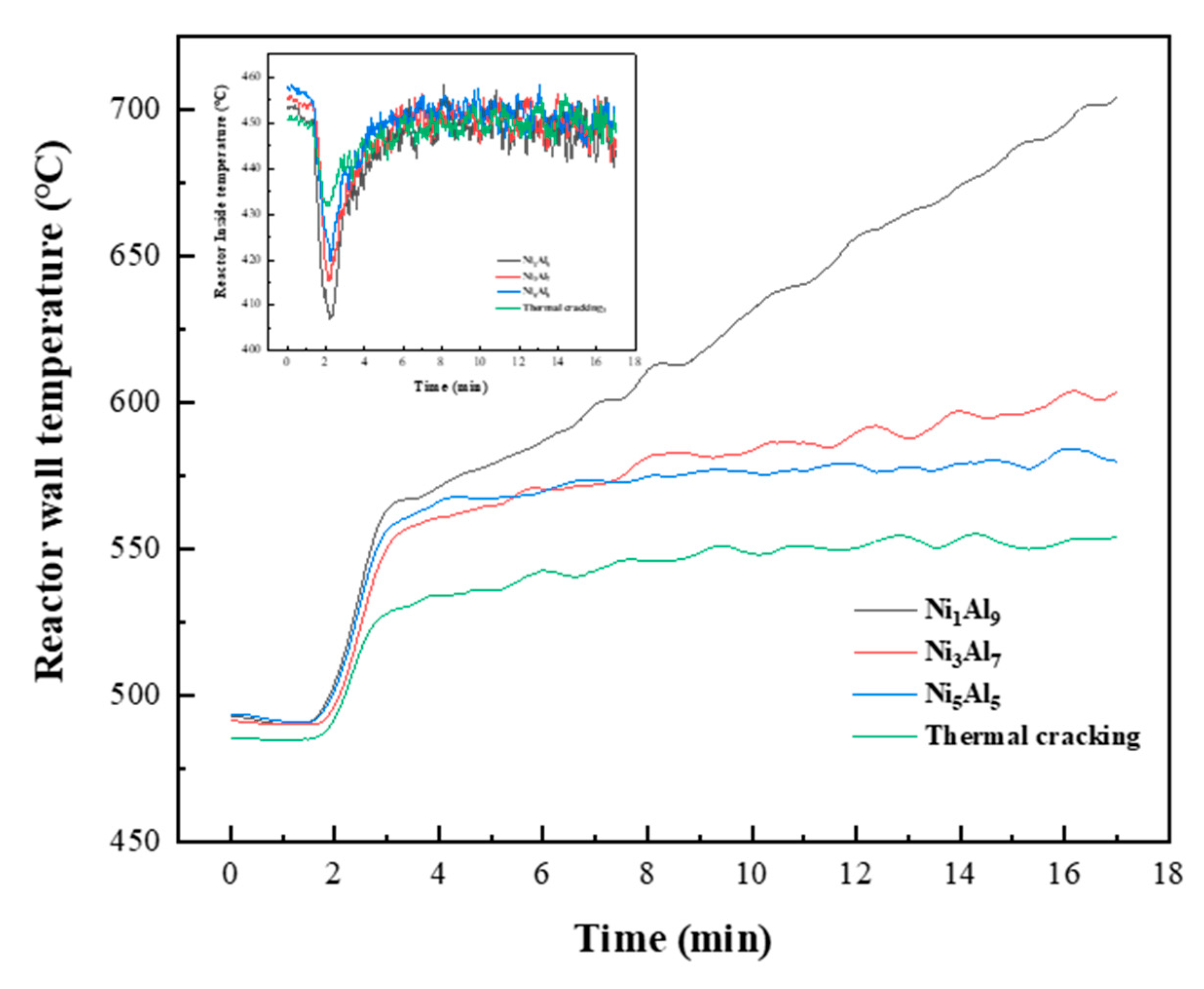
2.4. Gas Formation and Reactor Temperature Behavior
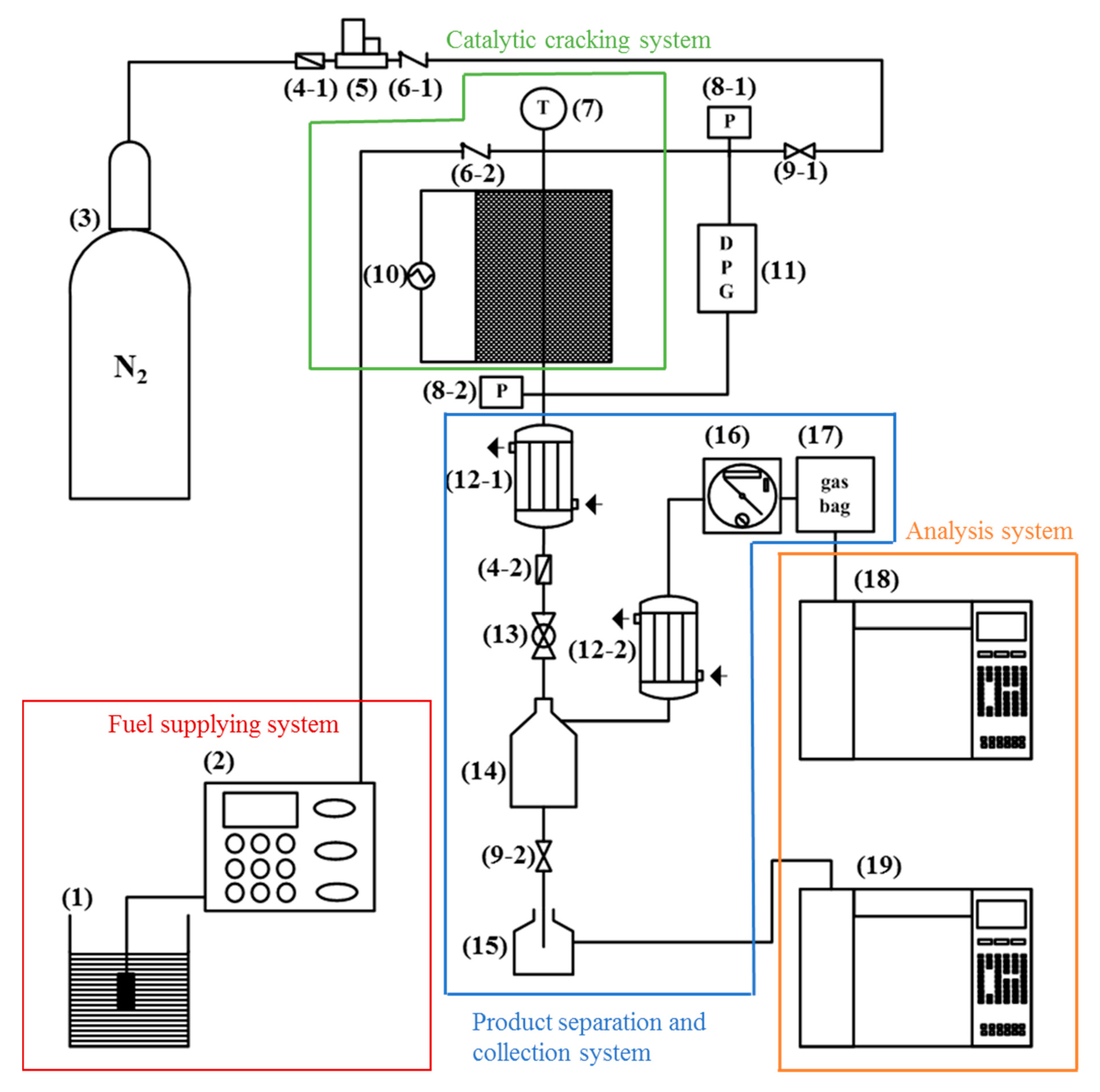
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalytic Activity Evaluation
3.4. Catalyst Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Edwards, T. Liquid Fuels and Propellants for Aerospace Propulsion: 1903–2003. J. Propuls. Power 2003, 19. [Google Scholar] [CrossRef]
- Kay, I.W.; Peschke, W.T.; Guilet, R.N. Hydrocarbon-Fueled Scramjet Combustor Investigation. J. Propuls. Power 1992, 8, 507–512. [Google Scholar] [CrossRef]
- Puri, P.; Ma, F.; Choi, J.Y.; Yang, V. Ignition characteristics of cracked JP-7 fuel. Combust. Flame 2005, 142, 454–457. [Google Scholar] [CrossRef]
- Heneghan, S.P.; Martel, C.R.; Williams, T.F.; Ballal, D.R. Studies of jet thermal stability in a flowing system. In Proceedings of the ASME International Gas Turbine and Aeroengine Congress and Exposition: Coal, Biomass and Alternative Fuels; Combustion and Fuels; Oil and Gas Applications; Cycle Innovations, Cologne, Germany, 1–4 June 1992; Volume 3, pp. 1–7. [Google Scholar] [CrossRef]
- Balster, L.M.; Corporan, E.; DeWitt, M.J.; Edwards, J.T.; Ervin, J.S.; Graham, J.L.; Lee, S.Y.; Pal, S.; Phelps, D.K.; Rudnick, L.R.; et al. Development of an advanced, thermally stable, coal-based jet fuel. Fuel Process. Technol. 2008, 89, 364–378. [Google Scholar] [CrossRef]
- Klavetter, E.A.; Martin, S.J.; Wessendorf, K.O. Monitoring Jet Fuel Thermal Stability Using a Quartz Crystal Microbalance. Energy Fuels 1993, 7, 582–588. [Google Scholar] [CrossRef]
- Kim, J.; Hee, S.; Chun, B.; Hun, B.; Sik, J.; Hyun, S. Improvement of the heats of reaction in endothermic reactions of methylcyclohexane with zeolites. Catal. Today 2012, 185, 47–53. [Google Scholar] [CrossRef]
- Ho, T.C.; Thilakaratne, R.; Gardner, D.W.; Brown, R.C.; Tessonnier, J. Thermal Stability of Aluminum-Rich ZSM-5 Zeolites and Consequences on Aromatization Reactions. J. Phys. Chem. C 2016, 120, 20103–20113. [Google Scholar]
- Mihindou-koumba, P.C.; Cerqueira, H.S.; Magnoux, P.; Guisnet, M. Methylcyclohexane Transformation over HFAU, HBEA, and HMFI Zeolites: II. Deactivation and Coke Formation. Ind. Eng. Chem. Res. 2001, 40, 1042–1051. [Google Scholar] [CrossRef]
- Wojciechowski, B.W. The Reaction Mechanism of Catalytic Cracking: Quantifying Activity, Selectivity, and Catalyst Decay. Catal. Rev. 1998, 40, 209–328. [Google Scholar] [CrossRef]
- Regali, F.; Francesca, L.; Maria, A.; Boutonnet, M.; Järås, S. Applied Catalysis A: General Hydroconversion of n-hexadecane on Pt/silica-alumina catalysts: Effect of metal loading and support acidity on bifunctional and hydrogenolytic activity. Appl. Catal. A Gen. 2014, 469, 328–339. [Google Scholar] [CrossRef]
- Yue, L.; Lu, X.; Chi, H.; Guo, Y.; Xu, L.; Fang, W.; Li, Y.; Hu, S. Heat-sink enhancement of decalin and aviation kerosene prepared as nanofluids with palladium nanoparticles. Fuel 2014, 121, 149–156. [Google Scholar] [CrossRef]
- Nixon, A.C.; Ackerman, G.H.; Hawthorme, R.D.; Ritchie, A.W.; Henderson, H.T.; Bjorklund, I.S. Vaporization and Endothermic Fuels for Advanced Engine Applications: AFAPL TDR 64-117 Parts I, II, and III; Defense Technical Information Center: Fort Belvoir, VA, USA, 1964.
- Yin, S.; Chin, Y.; Amiridis, M.D. Hydrogen production via the catalytic cracking of ethane over Ni/SiO2 catalysts. Appl. Catal. A Gen. 2006, 300, 8–13. [Google Scholar] [CrossRef]
- He, Z.; Jiao, Y.; Wang, J.; Chen, Y. Journal of Analytical and Applied Pyrolysis Effects of M(Zr, Nb, Y) modifiers on the catalytic performance of Ni/Ce-Al2O3 bimetallic catalyst in steam reforming of n-ecane. J. Anal. Appl. Pyrolysis 2016, 122, 142–150. [Google Scholar] [CrossRef]
- Taherian, Z.; Khataee, A.; Orooji, Y. Facile synthesis of yttria-promoted nickel catalysts supported on MgO-MCM-41 for syngas production from greenhouse gases. Renew. Sustain. Energy Rev. 2020, 134, 110130. [Google Scholar] [CrossRef]
- Taherian, Z.; Shahed Gharahshiran, V.; Khataee, A.; Meshkani, F.; Orooji, Y. Comparative study of modified Ni catalysts over mesoporous CaO-Al2O3 support for CO2/methane reforming. Catal. Commun. 2020, 145, 106100. [Google Scholar] [CrossRef]
- Jime, C.; Boukha, Z.; Rivas, B.D.; Gonza, J.R.; Gutie, J.I. Behavior of Coprecipitated NiAl2O4/Al2O3 Catalysts for Low-Temperature Methane Steam Reforming. Energy Fuels 2014, 28, 7109–7121. [Google Scholar] [CrossRef]
- Enger, B.C.; Lødeng, R.; Walmsley, J.; Holmen, A. Inactive aluminate spinels as precursors for design of CPO and reforming catalysts. Appl. Catal. A Gen. 2010, 383, 119–127. [Google Scholar] [CrossRef]
- Ross, J.R.H.; Steel, M.C.F.; Zeini-Isfahani, A. Evidence for the participation of surface nickel aluminate sites in the steam reforming of methane over nickel/alumina catalysts. J. Catal. 1978, 52, 280–290. [Google Scholar] [CrossRef]
- Christensen, K.O.; Chen, D.; Lødeng, R.; Holmen, A. Effect of supports and Ni crystal size on carbon formation and sintering during steam methane reforming. Appl. Catal. A Gen. 2006, 314, 9–22. [Google Scholar] [CrossRef]
- Diarra, M.; Amara, H.; Ducastelle, F.; Bichara, C. Carbon solubility in nickel nanoparticles: A grand canonical Monte Carlo study. Phys. Status Solidi Basic Res. 2012, 249, 2629–2634. [Google Scholar] [CrossRef]
- Jenness, G.R.; Christiansen, M.A.; Caratzoulas, S.; Vlachos, D.G.; Gorte, R.J. Site-dependent Lewis acidity of γ-Al2O3 and its impact on ethanol dehydration and etherification. J. Phys. Chem. C 2014, 118, 12899–12907. [Google Scholar] [CrossRef]
- Stytsenko, V.D. Surface modified bimetallic catalysts: Preparation, characterization, and applications. Appl. Catal. A Gen. 1995, 126, 1–26. [Google Scholar] [CrossRef]
- Sahli, N.; Petit, C.; Roger, A.C.; Kiennemann, A.; Libs, S.; Bettahar, M.M. Ni catalysts from NiAl2O4 spinel for CO2 reforming of methane. Catal. Today 2006, 113, 187–193. [Google Scholar] [CrossRef]
- Otero Areán, C.; Pearroya Mentruit, M.; López López, A.J.; Parra, J.B. High surface area nickel aluminate spinels prepared by a sol-gel method. Coll. Surfaces A Physicochem. Eng. Asp. 2001, 180, 253–258. [Google Scholar] [CrossRef]
- Deraz, N.M. Synthesis and characterization of nano-sized nickel aluminate spinel crystals. Int. J. Electrochem. Sci. 2013, 8, 5203–5212. [Google Scholar]
- Rahbar Shamskar, F.; Rezaei, M.; Meshkani, F. The influence of Ni loading on the activity and coke formation of ultrasound-assisted co-precipitated Ni–Al2O3 nanocatalyst in dry reforming of methane. Int. J. Hydrogen Energy 2017, 42, 4155–4164. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, E.; Xu, X.; Wang, J.; Li, Z. Supplied Oxygen Properties of NiO/NiAl2O4 in Chemical Looping Re-Forming of Biomass Pyrolysis Gas: The Influence of Synthesis Method. ACS Sustain. Chem. Eng. 2018, 6, 14660–14668. [Google Scholar] [CrossRef]
- Millet, M.M.; Frei, E.; Algara-Siller, G.; Schlögl, R.; Tarasov, A. Surface titration of supported Ni catalysts by O2-pulse thermal analysis. Appl. Catal. A Gen. 2018, 566, 155–163. [Google Scholar] [CrossRef]
- Choong, C.K.S.; Huang, L.; Zhong, Z.; Lin, J.; Hong, L.; Chen, L. Effect of calcium addition on catalytic ethanol steam reforming of Ni/Al2O3: II. Acidity/basicity, water adsorption and catalytic activity. Appl. Catal. A Gen. 2011, 407, 155–162. [Google Scholar] [CrossRef]
- Wang, Y.; Shah, N.; Huffman, G.P. Pure hydrogen production by partial dehydrogenation of cyclohexane and methylcyclohexane over nanotube-supported Pt and Pd catalysts. Energy Fuels 2004, 18, 1429–1433. [Google Scholar] [CrossRef]
- Iwasa, N.; Takezawa, N. New supported Pd and Pt alloy catalysts for steam reforming and dehydrogenation of methanol. Top. Catal. 2003, 22, 215–224. [Google Scholar] [CrossRef]
- Luo, Q.; Feng, G.; Beller, M.; Jiao, H. Formic acid dehydrogenation on Ni(111) and comparison with Pd(111) and Pt(111). J. Phys. Chem. C 2012, 116, 4149–4156. [Google Scholar] [CrossRef]
- Sobel, D.R.; Spadaccini, L.J. Hydrocarbon Fuel Cooling Technologies for Advanced Propulsion. J. Eng. Gas Turbines Power. 1997, 119, 344–351. [Google Scholar] [CrossRef]
- Korus, A.; Samson, A.; Szle, A.; Katelbach-woz, A. Pyrolytic toluene conversion to benzene and coke over activated carbon in a fixed-bed reactor. Fuel 2017, 207, 283–292. [Google Scholar] [CrossRef]
- Song, Y.; Lin, W.; Guo, X.; Dong, L.; Mu, X.; Tian, H.; Wang, L. Aromatization and isomerization of methylcyclohexane over Ni catalysts supported on different supports. Green Energy Environ. 2019, 4, 75–82. [Google Scholar] [CrossRef]
- Castaño, P. Role of oxygenates and effect of operating conditions in the deactivation of a Ni supported catalyst during the steam reforming of bio-oil. Green Chem. 2017, 19, 4315–4333. [Google Scholar] [CrossRef]
- Montero, C.; Ochoa, A.; Castaño, P.; Bilbao, J.; Gayubo, A.G. Monitoring Ni0 and coke evolution during the deactivation of a Ni/La2O3– αAl2O3 catalyst in ethanol steam reforming in a fluidized bed. J. Catal. 2015, 331, 181–192. [Google Scholar] [CrossRef]
- Wang, S.; Lu, G.Q.M. A Comprehensive Study on Carbon Dioxide Reforming of Methane over Ni/γ-Al2O3 Catalysts. Ind. Eng. Chem. Res. 1999, 2615–2625. [Google Scholar] [CrossRef]
- Zaikovskii, V.I.; Chesnokov, V.V.; Buyanov, R.A. The Relationship between the State of Active Species in a Ni/Al2O3 Catalyst and the Mechanism of Growth of Filamentous Carbon 1. Kinet. Catal. 2001, 42, 813–820. [Google Scholar] [CrossRef]
- Bokx, D.E. The Formation of Filamentous Carbon on Iron and Nickel Catalysts. J. Catal. 1985, 480, 468–480. [Google Scholar] [CrossRef][Green Version]
- Choi, E.G.; Song, K.H.; An, S.R.; Lee, K.Y.; Youn, M.H.; Park, K.T.; Jeong, S.K.; Kim, H.J. Cu/ZnO/AlOOH catalyst for methanol synthesis through CO2 hydrogenation. Korean J. Chem. Eng. 2018, 35, 73–81. [Google Scholar] [CrossRef]
- ASTM D6730-01: Standard Test Method for Determination of Individual Components in Spark Ignition Engine Fuels by 100–Metre Capillary (with Precolumn) High-Resolution Gas Chromatography; ASTM International: West Conshohocken, PA, USA, 2011.
- Song, K.H.; Jeong, S.K.; Jeong, B.H.; Lee, K.Y.; Kim, H.J. Acid/base-treated activated carbon catalysts for the low-temperature endothermic cracking of n-dodecane with applications in hypersonic vehicle heat management systems. Catalysts 2020, 10, 1149. [Google Scholar] [CrossRef]
- Song, K.H.; Jeong, S.K.; Park, K.T.; Lee, K.Y.; Kim, H.J. Supercritical catalytic cracking of n-dodecane over air-oxidized activated charcoal. Fuel 2020, 276, 118010. [Google Scholar] [CrossRef]
- Anjaneyulu, C.; Naveen Kumar, S.; Vijay Kumar, V.; Naresh, G.; Bhargava, S.K.; Chary, K.V.R.; Venugopal, A. Influence of la on reduction behaviour and Ni metal surface area of Ni-Al2O3 catalysts for COx free H2 by catalytic decomposition of methane. Int. J. Hydrog. Energy 2015, 40, 3633–3641. [Google Scholar] [CrossRef]
- Jiménez-González, C.; Boukha, Z.; De Rivas, B.; Delgado, J.J.; Cauqui, M.Á.; González-Velasco, J.R.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Structural characterisation of Ni/alumina reforming catalysts activated at high temperatures. Appl. Catal. A Gen. 2013, 466, 9–20. [Google Scholar] [CrossRef]

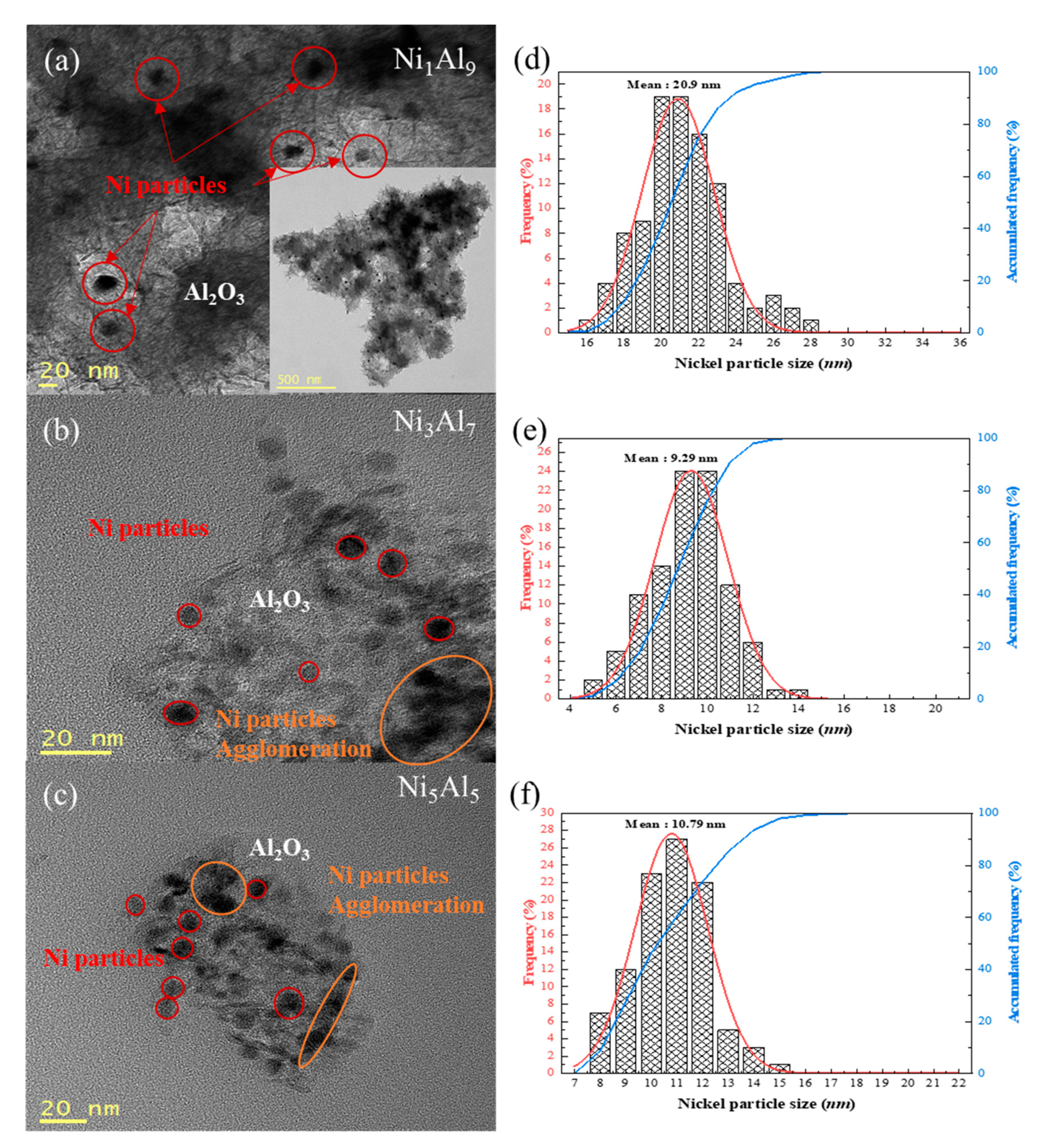


| Sample | dNi, nm (XRD) | dNi, nm (TEM) | dNi, nm (O2 Chemisorption) |
|---|---|---|---|
| Ni1Al9 | 20.8 | 20.9 | 20.7 |
| Ni3Al7 | 9.5 | 9.3 | 9.6 |
| Ni5Al5 | 10.4 | 10.8 | 10.7 |
| Sample | Ni Metal Surface Area, m2/g Ni Content | Ni Dispersion, % | Calcined Samples | Reduced Samples | ||||
|---|---|---|---|---|---|---|---|---|
| SBET, m2/g | Vp, cm3/g | dp, nm | SBET, m2/g | Vp, cm3/g | dp, nm | |||
| Ni1/Al9 | 27.4 | 4.1 | 316.1 | 1.10 | 13.5 | 291.8 | 1.10 | 15.1 |
| Ni3/Al7 | 65.1 | 9.4 | 254 | 0.68 | 13.9 | 210.3 | 0.66 | 15.7 |
| Ni5/Al5 | 63.2 | 10 | 251.7 | 0.66 | 11.5 | 190.0 | 0.65 | 14.1 |
| Sample | XRF (Bulk Composition) | ||
|---|---|---|---|
| Ni, wt. % | Al, wt. % | Ni/Al | |
| Ni1/Al9 | 9.3 | 90.7 | 0.10 |
| Ni3/Al7 | 27.2 | 72.8 | 0.35 |
| Ni5/Al5 | 52.6 | 47.4 | 1.20 |
| Catalyst | Weak Acid Sites (α) (μmol-NH3/g-cat) | Medium Acid Sites (β) (μmol-NH3/g-cat) | Strong Acid Sites (γ) (μmol-NH3/g-cat) | Total Acid Sites (μmol-NH3/g-cat) |
|---|---|---|---|---|
| Ni1Al9 | 1.7 | 34.3 | 27.1 | 63 |
| Ni3Al7 | 13.7 | 23.8 | ∙ | 37.6 |
| Ni5Al5 | 9.1 | 12.1 | ∙ | 21.2 |
| Ni1Al9 | Ni3Al7 | Ni5Al5 | Thermal Cracking | ||
|---|---|---|---|---|---|
| Gas Products | |||||
| H2 | Hydrogen | 12.72 | 42.52 | 50.4 | 1.66 |
| CH4 | Methane | 6.01 | 13.86 | 15.44 | 0.4 |
| C2H6 | Ethane | 4.00 | 0.88 | 0.49 | 0.07 |
| C2H4 | Ethylene | 0.57 | 0.12 | 0.19 | 0.25 |
| C3H8 | Propane | 3.15 | 0.7 | 0.19 | 0.29 |
| C3H6 | Propylene | 2.42 | 0.48 | 0.06 | |
| C4H10 | n-Butane | 0.41 | 0.08 | 0.19 | |
| iso-Butane | 0.38 | 0.1 | 0.09 | ||
| C4H8 | 1-Butene | 0.84 | 0.18 | 0.1 | |
| tran-2-Butene | 0.61 | 0.12 | 0.01 | ||
| cis-2-Butene | 4.80 | 1.15 | 0.11 | 0.55 | |
| iso-Butene | 0.14 | 0.03 | 0.02 | ||
| C4H6 | 1,3-butadiene | 3.26 | |||
| Alkanes | 13.95 | 15.62 | 16.40 | 0.76 | |
| Alkenes | 9.38 | 2.08 | 0.49 | 4.06 | |
| Alkenes/alkanes | 0.67 | 0.13 | 0.03 | 5.34 | |
| (hydrogen + alkenes)/alkanes | 1.58 | 2.86 | 3.10 | 7.53 | |
| Liquid Products | |||||
| n-paraffins | |||||
| C7H16 | n-heptane | 1.10 | |||
| iso-paraffins | |||||
| C5H12 | i-pentane | 15.78 | 8.55 | 2.66 | 86.89 |
| C7H16 | 2,3-dimethylpentane-2 | 0.90 | |||
| 3-ethylpentane | 0.86 | ||||
| 3,3-dimethylpentane | 0.39 | ||||
| 2-methylhexane | 0.79 | 0.17 | 0.93 | ||
| 3-ethylpentane | 0.18 | ||||
| Olefins | |||||
| C5H10 | t-pentene-2 | 0.86 | |||
| 2-methylbutene-2 | 1.27 | ||||
| c-pentene-2 | 1.02 | ||||
| C6H12 | t-hexene-2 | 0.70 | |||
| 2-methylpentene-2 1 | 0.49 | ||||
| C7H12 | 1,6-heptadiene | 4.42 | 0.99 | 0.24 | 0.89 |
| C7H14 | 4,4-dimethyl-c-pentene-2 | 0.48 | |||
| 2,4-dimethylpentene-1 | 1.49 | 0.18 | |||
| 5-methylhexene-1 | 0.58 | 0.19 | |||
| 5-methyl-t-hexene-2 | 13.16 | 2.06 | |||
| 5-methyl-c-hexene-2 | 4.68 | 1.03 | 0.23 | 0.98 | |
| heptene-1 | 0.52 | ||||
| 3-methyl-c-hexene-3 | 0.68 | ||||
| 3-methyl-t-hexene-3 | 0.18 | ||||
| c-heptene-3 | 0.54 | ||||
| t-heptene-2 | 0.87 | ||||
| Naphthenes | |||||
| C5H8 | Cyclopentene | 0.49 | |||
| C6H10 | Cyclohexene | 3.58 | 3.1 | 0.63 | |
| C6H12 | Ethylcyclopentane | 4.13 | 0.59 | 0.27 | |
| C7H14 | 1t,3-dimethylcyclopentane | 1.08 | 0.46 | 0.26 | 1.77 |
| Aromatics | |||||
| C6H6 | Benzene | 3.1 | 4.45 | ||
| C7H8 | Toluene | 4.27 | 19.94 | 22.6 | |
| C8H10 | 1,3-dimethylbenzene | 0.5 | 0.81 | ||
| Aromatics (total) | 4.27 | 23.54 | 27.86 | 0.00 | |
| Conversion (%) | 29.10 | 31.9 | 35.4 | 6.1 | |
| Gaseous products | 36.05 | 60.22 | 67.29 | 6.48 | |
| Liquid products | 63.95 | 39.78 | 32.71 | 93.52 | |
| Mass of coke (mg, 17 min) | 121.75 | 10.34 | 4.26 | ||
| Carbon balance | Error (%) | 8.59 | 2.46 | 2.45 | 0.82 |
| Hydrogen balance | Error (%) | 3.26 | 5.86 | 6.12 | 0.45 |
| Sample | α (mg) Encapsulated Carbon | β (mg) Filamentous Carbon | Total (mg) | α/β Ratio |
|---|---|---|---|---|
| Ni1Al9 | 33.13 | 88.62 | 121.75 | 0.37 |
| Ni3Al7 | 5.92 | 4.42 | 10.34 | 1.33 |
| Ni5Al5 | 2.75 | 0.75 | 3.50 | 3.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, K.H.; Jeong, S.K.; Jeong, B.H.; Lee, K.-Y.; Kim, H.J. Effect of the Ni/Al Ratio on the Performance of NiAl2O4 Spinel-Based Catalysts for Supercritical Methylcyclohexane Catalytic Cracking. Catalysts 2021, 11, 323. https://doi.org/10.3390/catal11030323
Song KH, Jeong SK, Jeong BH, Lee K-Y, Kim HJ. Effect of the Ni/Al Ratio on the Performance of NiAl2O4 Spinel-Based Catalysts for Supercritical Methylcyclohexane Catalytic Cracking. Catalysts. 2021; 11(3):323. https://doi.org/10.3390/catal11030323
Chicago/Turabian StyleSong, Kyoung Ho, Soon Kwan Jeong, Byung Hun Jeong, Kwan-Young Lee, and Hak Joo Kim. 2021. "Effect of the Ni/Al Ratio on the Performance of NiAl2O4 Spinel-Based Catalysts for Supercritical Methylcyclohexane Catalytic Cracking" Catalysts 11, no. 3: 323. https://doi.org/10.3390/catal11030323
APA StyleSong, K. H., Jeong, S. K., Jeong, B. H., Lee, K.-Y., & Kim, H. J. (2021). Effect of the Ni/Al Ratio on the Performance of NiAl2O4 Spinel-Based Catalysts for Supercritical Methylcyclohexane Catalytic Cracking. Catalysts, 11(3), 323. https://doi.org/10.3390/catal11030323








