The Effects of Promoter Cs Loading on the Hydrogen Production from Ammonia Decomposition Using Ru/C Catalyst in a Fixed-Bed Reactor
Abstract
1. Introduction
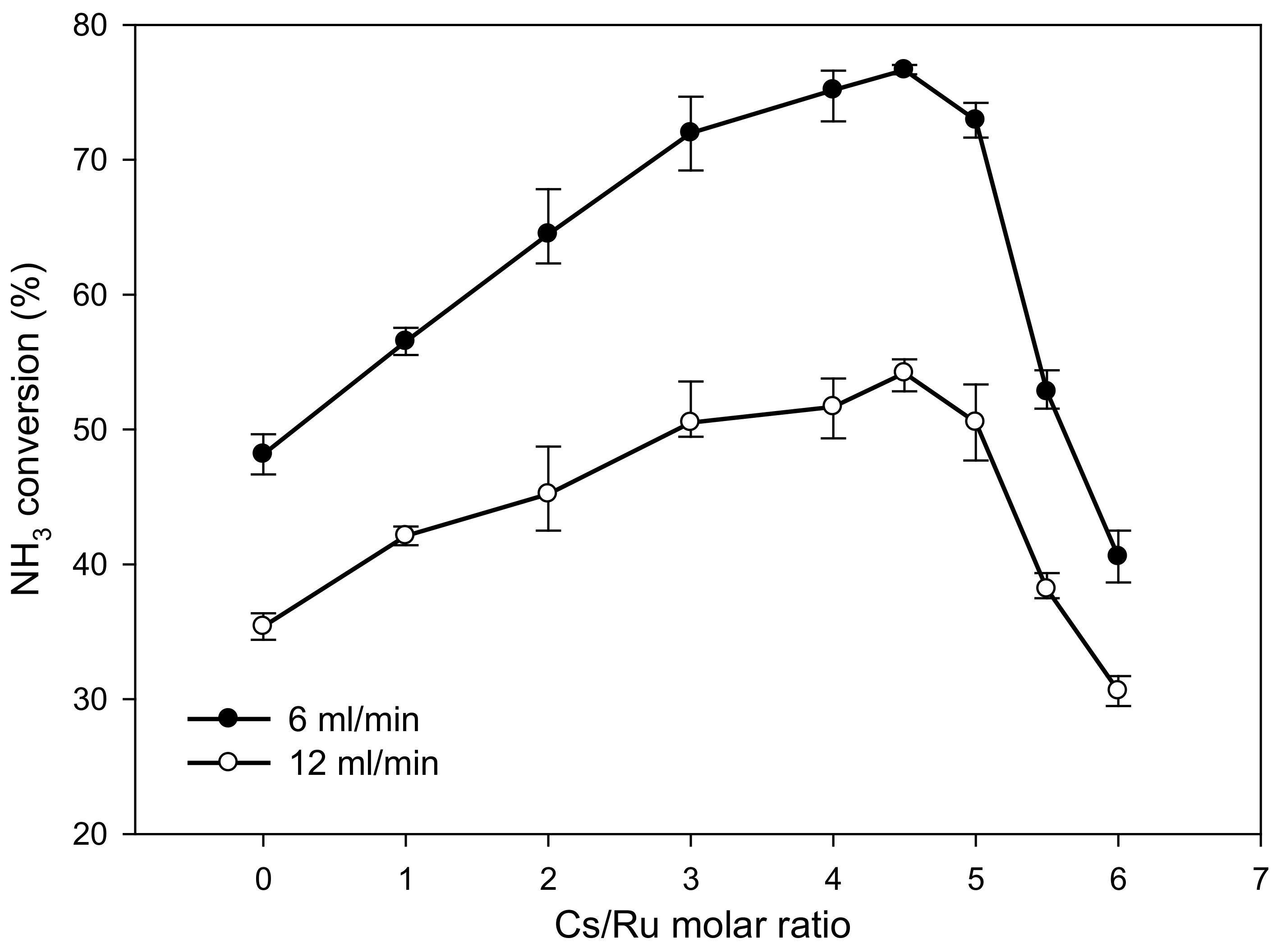
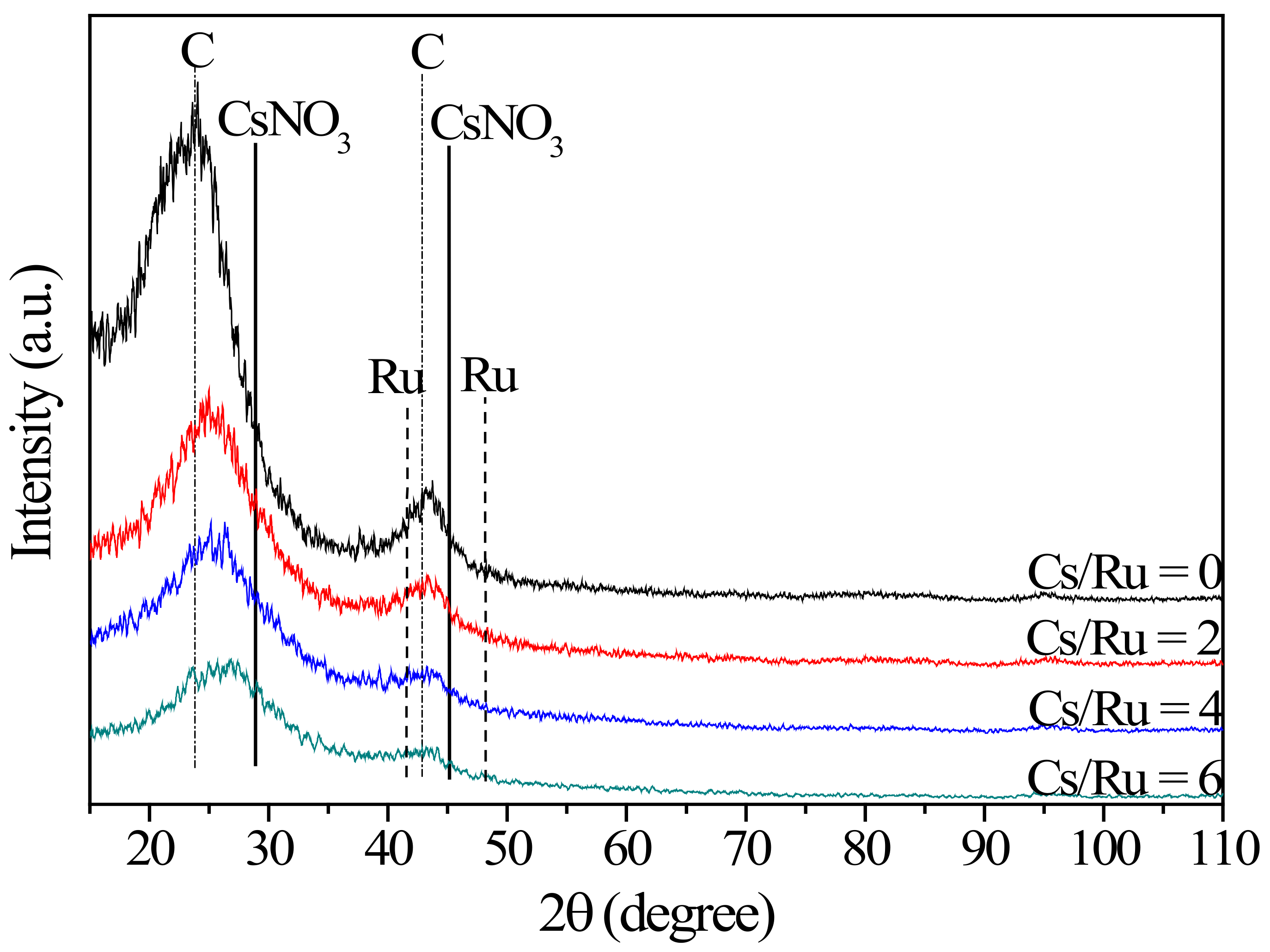
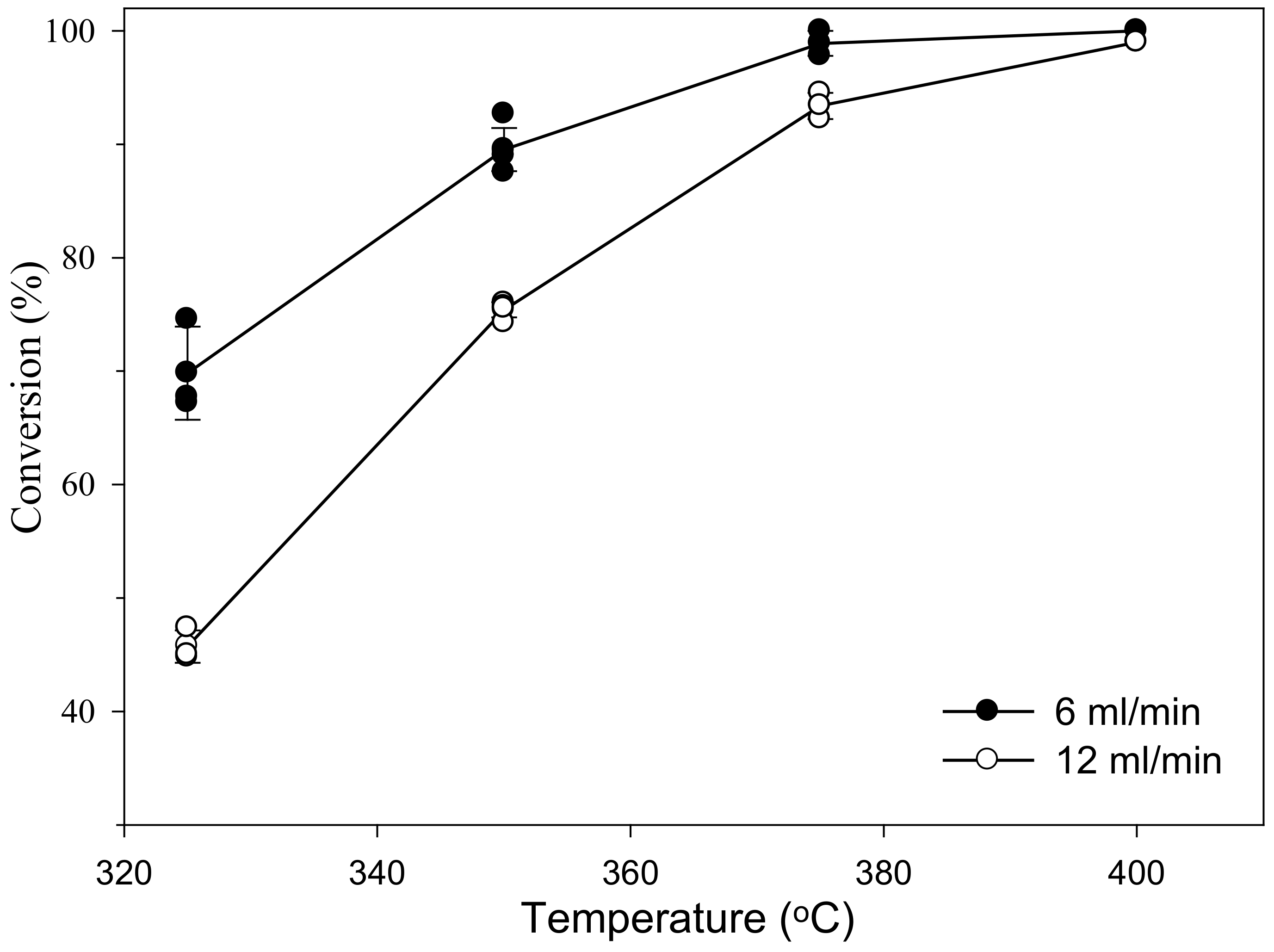
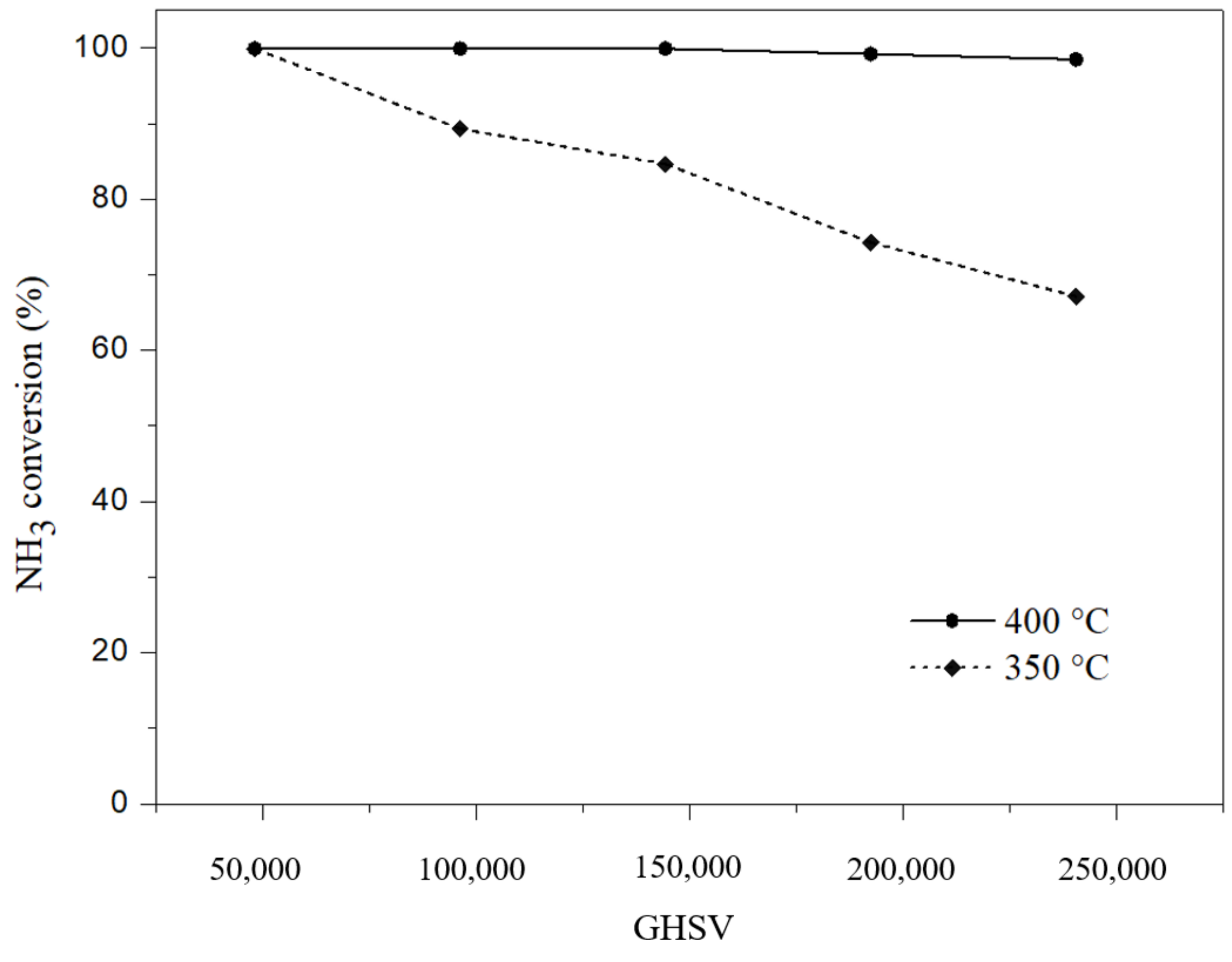
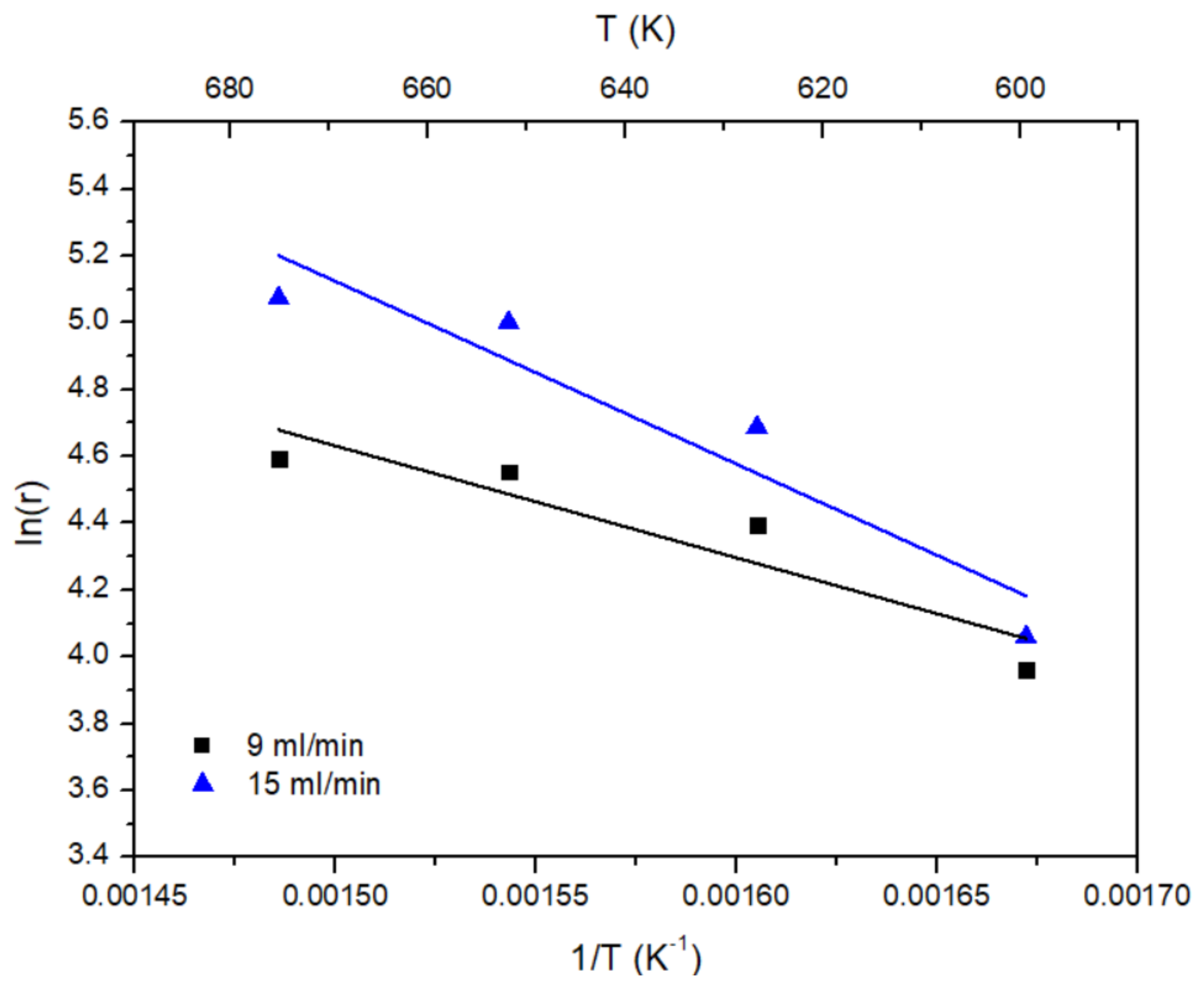
2. Results and Discussion
3. Materials and Methods
3.1. Catalyst Preparation
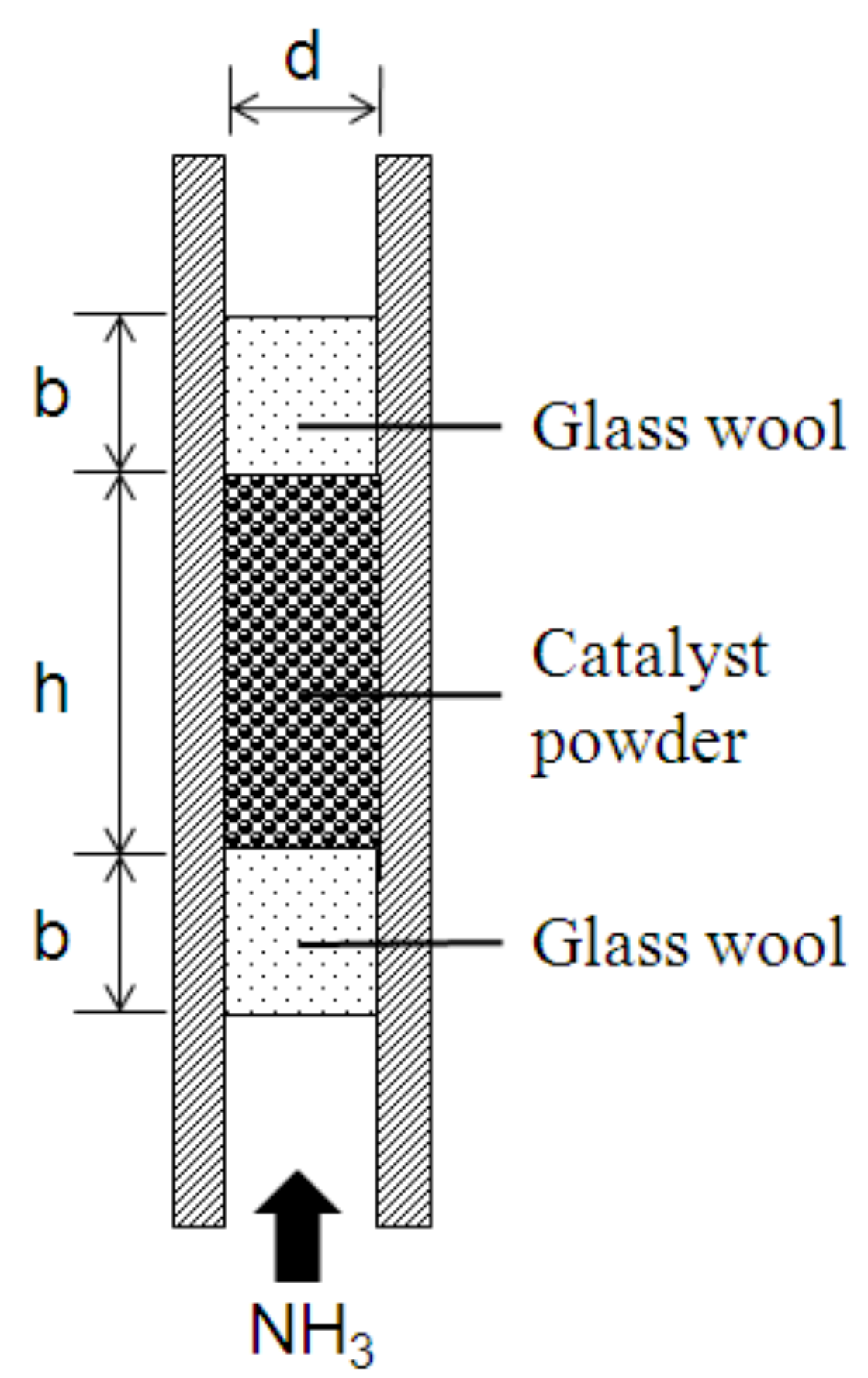
3.2. Fixed-Bed Reactor
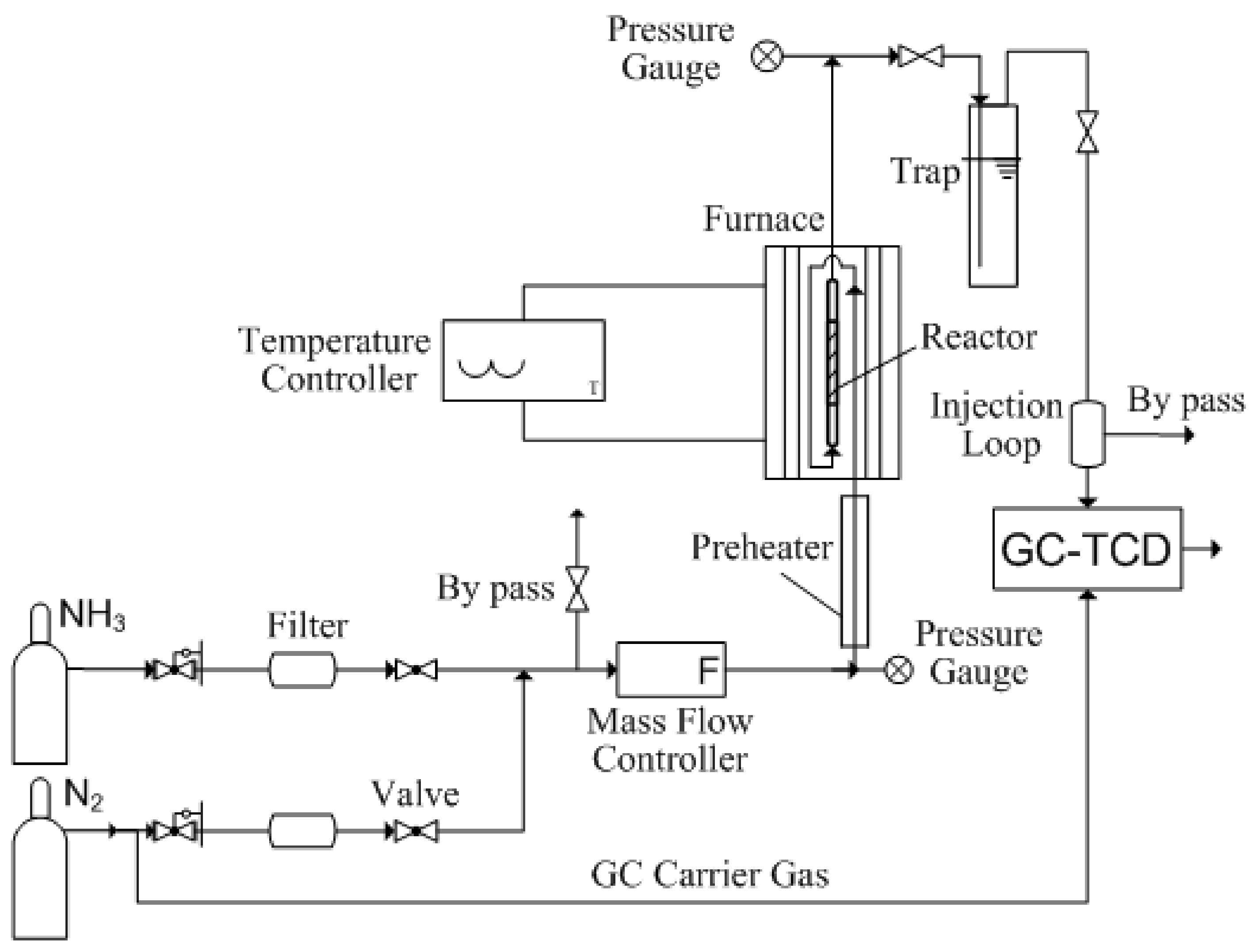
3.3. Experimental Setup and Performance Measurement System
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xue, M.; Wang, Q.; Lin, B.L.; Tsunemi, K. Assessment of Ammonia as an Energy Carrier from the Perspective of Carbon and Nitrogen Footprints. ACS Sustain. Chem. Eng. 2019, 7, 12494–12500. [Google Scholar] [CrossRef]
- Giddey, S.; Badwal, S.P.S.; Munnings, C.; Dolan, M. Ammonia as a Renewable Energy Transportation Media. ACS Sustain. Chem. Eng. 2017, 5, 10231–10239. [Google Scholar] [CrossRef]
- Zamfirescu, C.; Dincer, I. Ammonia as a Green Fuel and Hydrogen Source for Vehicular Applications. Fuel Process. Technol. 2009, 90, 729–737. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A Comparative Overview of Hydrogen Production Processes. Renew. Sust. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Chen, L.; Qi, Z.; Zhang, S.; Su, J.; Somorjai, G.A. Catalytic Hydrogen Production from Methane: A Review on Recent Progress and Prospect. Catalysts 2020, 10, 858. [Google Scholar] [CrossRef]
- Park, H.G.; Han, S.Y.; Jun, K.W.; Woo, Y.; Park, M.J.; Kim, S.K. Bench-scale Steam Reforming of Methane for Hydrogen Production. Catalysts 2019, 9, 615. [Google Scholar] [CrossRef]
- Chen, W.H.; Lin, S.C. Characterization of Catalytic Partial Oxidation of Methane with Carbon Dioxide Utilization and Excess Enthalpy Recovery. Appl. Energy 2016, 162, 1141–1152. [Google Scholar] [CrossRef]
- Fasolini, A.; Ruggieri, S.; Femoni, C.; Basile, F. Highly Active Catalysts Based on the Rh4(CO)12 Cluster Supported on Ce0.5Zr0.5 and Zr Oxides for Low-Temperature Methane Steam Reforming. Catalysts 2019, 9, 800. [Google Scholar] [CrossRef]
- Karadeniz, H.; Karakaya, C.; Tischer, S.; Deutschmann, O. Numerical Simulation of Methane and Propane Reforming over a Porous Rh/Al2O3 Catalyst in Stagnation-flows: Impact of Internal and External Mass Transfer Limitations on Species Profiles. Catalysts 2020, 10, 915. [Google Scholar] [CrossRef]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An Overview of Hydrogen Production Technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Chen, W.H.; Cheng, T.C.; Hung, C.I. Numerical Predictions on Thermal Characteristic and Performance of Methanol Steam Reforming with Microwave-assisted Heating. Int. J. Hydrog. Energy 2011, 36, 8279–8291. [Google Scholar] [CrossRef]
- Baschuk, J.J.; Li, X. Carbon Monoxide Poisoning of Proton Exchange Membrane Fuel Cells. Int. J. Energy Res. 2001, 25, 695–713. [Google Scholar] [CrossRef]
- Valdés-López, V.F.; Mason, T.; Shearing, P.R.; Brett, D.J.L. Carbon Monoxide Poisoning and Mitigation Strategies for Polymer Electrolyte Membrane Fuel Cells—A Review. Prog. Energy Combus. Sci. 2020, 79, 100842. [Google Scholar] [CrossRef]
- Soltani, R.; Rosen, M.; Dincer, I. Assessment of CO2 Capture Options from Various Points in Steam Methane Reforming for Hydrogen Production. Int. J. Hydrog. Energy 2014, 39, 20266–20275. [Google Scholar] [CrossRef]
- Huang, W.J.; Yu, C.T.; Sheu, W.J.; Chen, Y.C. The Effect of Non-uniform Temperature on the Sorption-enhanced Steam Methane Reforming in a Tubular Fixed-bed Reactor. Int. J. Hydrog. Energy 2020. [Google Scholar] [CrossRef]
- Chen, W.H.; Chen, C.Y. Water Gas Shift Reaction for Hydrogen Production and Carbon Dioxide Capture: A Review. Appl. Energy 2020, 258, 114078. [Google Scholar] [CrossRef]
- Choudhary, T.V.; Svadinaragana, C.; Goodman, D.W. Catalytic Ammonia Decomposition: COx-free Hydrogen Production for Fuel Cell Applications. Catal. Lett. 2001, 72, 197–201. [Google Scholar] [CrossRef]
- Yin, S.F.; Zhang, Q.H.; Xu, B.Q.; Zhu, W.X.; Ng, C.F.; Au, C.T. Investigation on the Catalysis of COx-free Hydrogen Generation from Ammonia. J. Catal. 2004, 224, 384–396. [Google Scholar] [CrossRef]
- Ganley, J.C.; Seebauer, E.G.; Masel, R.I. Development of a Micro-reactor for the Production of Hydrogen from Ammonia. J. Power Sources 2004, 137, 53–61. [Google Scholar] [CrossRef]
- Li, L.; Zhu, Z.H.; Yan, Z.F.; Lu, G.Q.; Rintoul, L. Catalytic Ammonia Decomposition Over Ru-carbon Catalysts: The Importance of the Structure of Carbon Support. Appl. Catal. A Gen. 2007, 320, 166–172. [Google Scholar] [CrossRef]
- Carcia-Garcia, F.R.; Ma, Y.H.; Rodriguez-Ramos, I.; Guerrero-Ruiz, A. High Purity Hydrogen Production by Low Temperature Catalytic Ammonia Decomposition in a Multifunctional Membrane Reactor. Catal. Commun. 2008, 9, 482–486. [Google Scholar] [CrossRef]
- Li, G.; Kanezashi, M.; Lee, H.R.; Maeda, M.; Yoshioka, T.; Tsuru, T. Preparation of a Novel Bimodal Catalytic Membrane Reactor and Its Application to Ammonia Decomposition for COx-free Hydrogen Production. Int. J. Hydrog. Energy 2012, 37, 12105–12113. [Google Scholar] [CrossRef]
- Rarog, W.; Szmigiel, D.; Kowalczyk, Z.; Jodzis, S.; Zielinski, J. Ammonia Decomposition over the Carbon-based Ruthenium Catalyst Promoted with Barium or Cesium. J. Catal. 2003, 218, 465–469. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, J.; Xu, H.; Li, W. NH3 Decomposition Kinetics on Supported Ru Clusters: Morphology and Particle Size Effect. Catal. Lett. 2007, 119, 311–318. [Google Scholar] [CrossRef]
- Sorensen, R.Z.; Klerke, A.; Quaade, U.; Jensen, S.; Hansen, O.; Christensen, C.H. Promoted Ru on High-surface Area Graphite for Efficient Miniaturized Production of Hydrogen from Ammonia. Catal. Lett. 2006, 112, 77–81. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Li, J.; Lu, Y.; Wu, H.; Xue, Q.; Chen, L. Monolithic Microfibrous Nickel Catalyst Co-modified with Ceria and Alumina for Miniature Hydrogen Production via Ammonia Decomposition. Appl. Catal. A Gen. 2007, 328, 77–82. [Google Scholar] [CrossRef]
- Chellappa, A.S.; Fischer, C.M.; Thomson, W.J. Ammonia Decomposition Kinetics over Ni-Pt/Al2O3 for PEM Fuel Cell Applications. Appl. Catal. A 2002, 227, 231–240. [Google Scholar] [CrossRef]
- Wang, M.; Li, J.; Chen, L.; Lu, Y. Miniature NH3 Cracker Based on Microfibrous Entrapped Ni-CeO2/Al2O3 Catalyst Monolith for Portable Fuel Cell Power Supplies. Int. J. Hydrog. Energy 2009, 34, 1710–1716. [Google Scholar] [CrossRef]
- Plana, C.; Armenise, S.; Monzón, A.; García-Bordejé, E. Ni on Alumina-coated Cordierite Monoliths for in situ Generation of CO-free H2 from Ammonia. J. Catal. 2010, 275, 228–235. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, H.; Jin, X.; Ge, O.; Li, W. Characterizations and Activities of the Nano-sized Ni/Al2O3 and Ni/La–Al2O3 Catalysts for NH3 Decomposition. Appl. Catal. A Gen. 2005, 290, 87–96. [Google Scholar] [CrossRef]
- Alagharu, V.; Palanki, S.; West, K.N. Analysis of Ammonia Decomposition Reactor to Generate Hydrogen for Fuel Cell Applications. J. Power Sources 2010, 195, 829–833. [Google Scholar] [CrossRef]
- Chein, R.Y.; Chen, Y.C.; Chang, C.S.; Chung, J.N. Numerical Modeling of Hydrogen Production from Ammonia Decomposition for Fuel Cell Applications. Int. J. Hydrog. Energy 2010, 35, 589–597. [Google Scholar] [CrossRef]
- Hill, A.K.; Torrente-Murciano, L. Low Temperature H2 Production from Ammonia Using Ruthenium-based Catalysts: Synergetic Effect of Promoter and Support. Appl. Catal. B. Environ. 2015, 172, 129–135. [Google Scholar] [CrossRef]
- Hu, Z.; Mahin, J.; Torrente-Murciano, L. A MOF-templated Approach for Designing Ruthenium-cesium Catalysts for Hydrogen Generation from Ammonia. Int. J. Hydrog. Energy 2019, 44, 30108–30118. [Google Scholar] [CrossRef]
- Parker, L.A.; Carter, J.H.; Dummer, N.F.; Richards, N.; Morgan, D.J.; Golunski, S.E.; Hutchings, G.J. Ammonia Decomposition Enhancement by Cs-promoted Fe/Al2O3 Catalysts. Catal. Lett. 2020, 150, 3369–3376. [Google Scholar] [CrossRef]
- Xie, P.; Yao, Y.; Huang, Z.; Liu, Z.; Zhang, J.; Li, T.; Wang, G.; Shahbazian-Yassar, R.; Hu, L.; Wang, C. Highly Efficient Decomposition of Ammonia Using High-entropy Alloy Catalysts. Nat. Commu. 2019, 10, 4011. [Google Scholar] [CrossRef]
- Li, G.; Kanezashi, M.; Tsuru, T. Catalytic Ammonia Decomposition Over High-performance Ru/graphene Nanocomposites for Efficient COx-free Hydrogen Production. Catalysts 2017, 7, 23. [Google Scholar] [CrossRef]
- Ju, X.; Liu, L.; Yu, P.; Guo, J.; Zhang, X.; He, T.; Wu, G.; Chen, P. Mesoporous Ru/MgO Prepared by a Deposition-precipitation Method as Highly Active Catalyst for Producing COx-free Hydrogen from Ammonia Decomposition. Appl. Catal. B Environ. 2017, 211, 167–175. [Google Scholar] [CrossRef]
- Wang, Z.; Cai, Z.; Wei, Z. Highly Active Ruthenium Catalyst Supported on Barium Hexaaluminate for Ammonia Decomposition to COx-free Hydrogen. ACS Sustain. Chem. Eng. 2019, 7, 8226–8235. [Google Scholar] [CrossRef]
- Hu, Z.; Mahin, J.; Datta, S.; Bell, T.E.; Torrente-Murciano, L. Ru-based Catalysts for H2 Production from Ammonia: Effect of 1D Support. Top. Catal. 2019, 62, 1169–1177. [Google Scholar] [CrossRef]
- Hu, X.C.; Fu, X.P.; Wang, W.W.; Wang, X.; Wu, K.; Si, R.; Ma, C.; Jia, C.J.; Yan, C.H. Ceria-supported Ruthenium Clusters Transforming from Isolated Single Atoms for Hydrogen Production via Decomposition of Ammonia. Appl. Catal. B Environ. 2020, 268, 118424. [Google Scholar] [CrossRef]
- Pinzón, M.; Romero, A.; Consuegra, A.D.L.; Osa, A.R.D.L.; Sánchez, P. Hydrogen Production by Ammonia Decomposition over Ruthenium Supported on SiC Catalyst. J. Indus. Eng. Chem. 2021, 94, 326–335. [Google Scholar] [CrossRef]
- Im, Y.; Muroyama, H.; Matsui, T.; Eguchi, K. Ammonia Decomposition over Nickel Catalysts Supported on Alkaline Earth Metal Aluminate for H2 Production. Int. J. Hydrog. Energy 2020, 45, 26979–26988. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Chang, Y.C.; Hung, W.Y.; Lin, H.P.; Shih, H.Y.; Xie, W.A.; Li, S.N.; Hsu, C.H. Green Synthesis of Porous Ni-silicate Catalyst for Hydrogen Generation via Ammonia Decomposition. Int. J. Energy Res. 2020, 44, 9748–9756. [Google Scholar] [CrossRef]
- Yu, Y.; Gan, Y.M.; Huang, C.; Lu, Z.H.; Wang, X.; Zhang, R.; Feng, G. Ni/La2O3 and Ni/MgO-La2O3 Catalysts for the Decomposition of NH3 into Hydrogen. Int. J. Hydrog. Energy 2020, 45, 16528–16539. [Google Scholar] [CrossRef]
- Gu, Y.; Ma, Y.; Long, Z.; Zhao, S.; Wang, Y.; Zhang, W. One-pot Synthesis of Supported Ni@Al2O3 Catalysts with Uniform Small-sized Ni for Hydrogen Generation via Ammonia Decomposition. Int. J. Hydrog. Energy 2021, 46, 4045–4054. [Google Scholar] [CrossRef]
- Yin, S.F.; Xu, B.Q.; Zhou, X.P.; Au, C.T. A Mini-review on Ammonia Decomposition Catalysts for On-site Generation of Hydrogen for Fuel Cell Applications. Appl. Catal. A Gen. 2004, 277, 1–9. [Google Scholar] [CrossRef]
- Schuth, F.; Palkovits, R.; Schlogl, R.; Su, D.S. Review: Ammonia as a Possible Element in an Energy Infrastructure: Catalysts for Ammonia Decomposition. Energy Environ. Sci. 2012, 5, 6278–6289. [Google Scholar] [CrossRef]
- Chiuta, S.; Everson, R.C.; Neomagus, H.W.J.P.; Gryp, P.V.D.; Bessarabov, D.G. Reactor Technology Options for Distributed Hydrogen Generation via Ammonia Decomposition: A Review. Int. J. Hydrog. Energy 2013, 38, 14968–14991. [Google Scholar] [CrossRef]
- Mukherjee, S.; Devaguptapu, S.V.; Sviripa, A.; Lund, C.R.F.; Wu, G. Review Low-temperature Ammonia Decomposition Catalysts for Hydrogen Generation. Appl. Catal. B Environ. 2018, 226, 162–181. [Google Scholar] [CrossRef]
- Huang, D.C.; Jiang, C.H.; Liu, F.J.; Cheng, Y.C.; Chen, Y.C.; Hsueh, K.L. Preparation of Ru-Cs Catalyst and Its Application in Hydrogen Production by Ammonia Decomposition, Int. J. Hydrog. Energy 2013, 38, 3233–3240. [Google Scholar] [CrossRef]
- Kowalczyk, Z.; Krukowski, M.; Raróg-Pilecka, W.; Szmigiel, D.; Zielinski, J. Carbon-based Ruthenium Catalyst for Ammonia Synthesis: Role of the Barium and Caesium Promoters and Carbon Support. Appl. Catal. A Gen. 2003, 248, 67–73. [Google Scholar] [CrossRef]
- Tennison, S.R. Catalytic Ammonia Synthesis: Fundamentals and Practice; Jennings, J.R., Ed.; Chapter 9; Plenum Press: New York, NY, USA, 1991. [Google Scholar]
- Ertl, G. Catalytic Ammonia Synthesis: Fundamentals and Practice; Jennings, J.R., Ed.; Chapter 3; Plenum Press: New York, NY, USA, 1991. [Google Scholar]


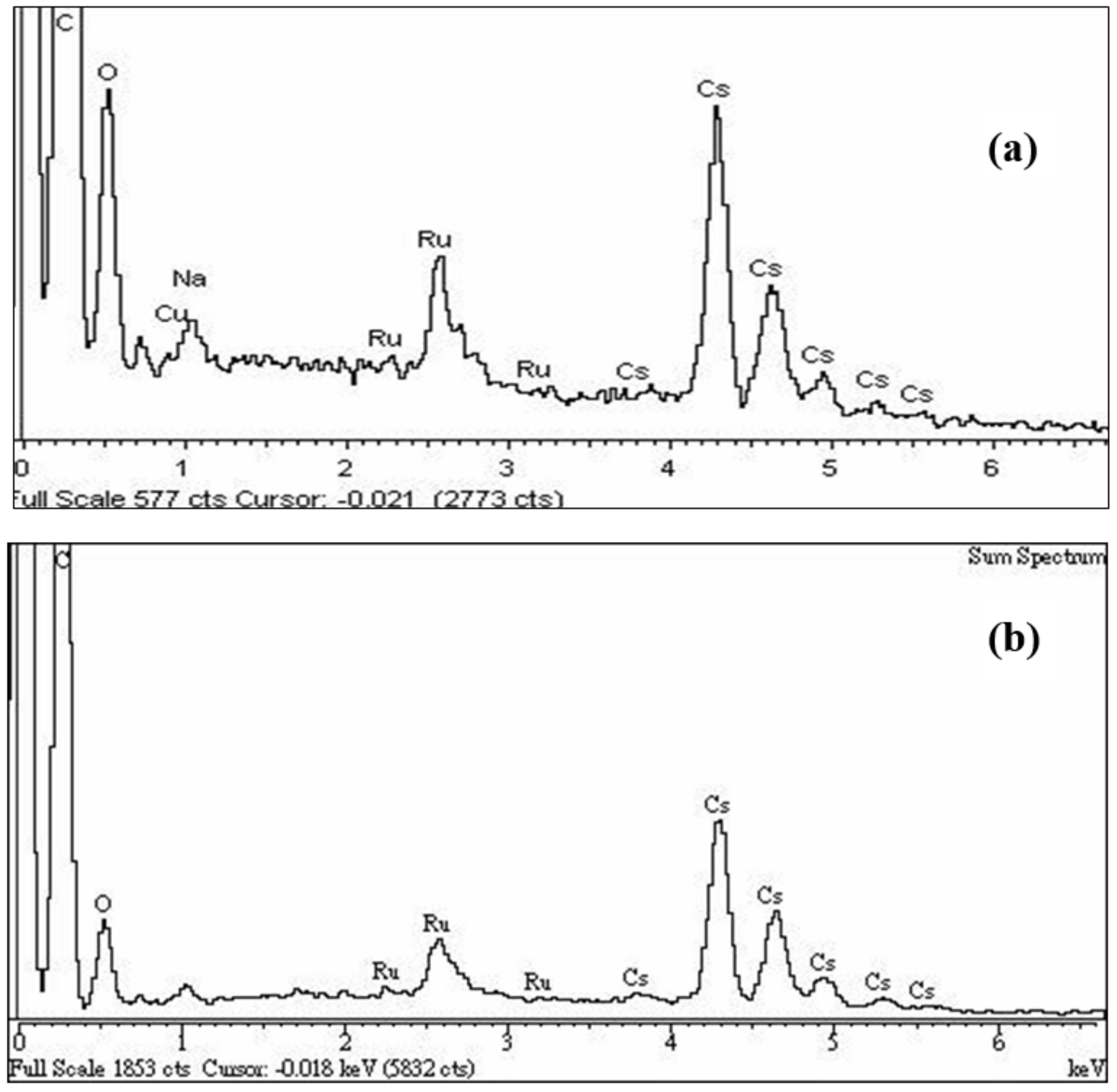
| Percentage of Atom Numbers | 0 | 2 | 4 | 6 | |
|---|---|---|---|---|---|
| Overall Ratio of Cs/Ru | |||||
| C (%) | 99.50 | 97.76 | 96.51 | 94.13 | |
| Ru (%) | 0.50 | 0.73 | 0.67 | 0.82 | |
| Cs (%) | 0 | 1.51 | 2.82 | 5.04 | |
| molar ratio of Cs/Ru in each SEM picture | 0 | 2.07 | 4.21 | 6.15 | |
| Catalyst | Ru/Al2O3 | Ru/C | 5 wt.% Ru/CNT | 7 wt.% Ru/CNT | 5 wt.% Ru/C |
|---|---|---|---|---|---|
| Promoter | Cs | Cs | Cs | Cs | |
| Activation energy (kJ/mole) | 79.4 | 54–72 | 61.9 at 350 °C | 49.5 | 28–46 |
| Reference | 17 | 51 | 34 | 33 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-L.; Juang, C.-F.; Chen, Y.-C. The Effects of Promoter Cs Loading on the Hydrogen Production from Ammonia Decomposition Using Ru/C Catalyst in a Fixed-Bed Reactor. Catalysts 2021, 11, 321. https://doi.org/10.3390/catal11030321
Chen Y-L, Juang C-F, Chen Y-C. The Effects of Promoter Cs Loading on the Hydrogen Production from Ammonia Decomposition Using Ru/C Catalyst in a Fixed-Bed Reactor. Catalysts. 2021; 11(3):321. https://doi.org/10.3390/catal11030321
Chicago/Turabian StyleChen, Yen-Ling, Chin-Fang Juang, and Yen-Cho Chen. 2021. "The Effects of Promoter Cs Loading on the Hydrogen Production from Ammonia Decomposition Using Ru/C Catalyst in a Fixed-Bed Reactor" Catalysts 11, no. 3: 321. https://doi.org/10.3390/catal11030321
APA StyleChen, Y.-L., Juang, C.-F., & Chen, Y.-C. (2021). The Effects of Promoter Cs Loading on the Hydrogen Production from Ammonia Decomposition Using Ru/C Catalyst in a Fixed-Bed Reactor. Catalysts, 11(3), 321. https://doi.org/10.3390/catal11030321





