1H NMR Analysis of the Metathesis Reaction between 1-Hexene and (E)-Anethole Using Grubbs 2nd Generation Catalyst: Effect of Reaction Conditions on (E)-1-(4-Methoxyphenyl)-1-hexene Formation and Decomposition
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Spectroscopic Characterisation Techniques
3.2.1. 1H NMR Spectroscopy
3.2.2. Monitoring of the Metathesis Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bruneau, C.; Fischmeister, C.; Miao, X.; Malacea, R.; Dixneuf, P.H. Cross-Metathesis with Acrylonitrile and Applications to Fatty Acid Derivatives. Eur. J. Lipid Sci. Technol. 2010, 112, 3–9. [Google Scholar] [CrossRef]
- Connon, S.J.; Blechert, S. Recent Developments in Olefin Cross-Metathesis. Angew. Chem. Int. Ed. 2003, 42, 1900–1923. [Google Scholar] [CrossRef]
- Morzycki, J.W. Application of Olefin Metathesis in the Synthesis of Steroids. Steroids 2011, 76, 949–966. [Google Scholar] [CrossRef]
- Schuster, M.; Blechert, S. Olefin in Metathesis in Organic Chemistry. Angew. Chem. Ed. Engl. 1997, 36, 2036–2056. [Google Scholar] [CrossRef]
- Herndon, J.W. The Chemistry of the Carbon-Transition Metal Double and Triple Bond: Annual Survey Covering the Year 2018. Coord. Chem. Rev. 2019, 401, 213051. [Google Scholar] [CrossRef]
- Ogba, O.M.; Warner, N.C.; O’Leary, D.J.; Grubbs, R.H. Recent Advances in Ruthenium-Based Olefin Metathesis. Chem. Soc. Rev. 2018, 47, 4510–4544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, D.S.; Baslé, O.; Mauduit, M. A Tutorial Review of Stereoretentive Olefin Metathesis Based on Ruthenium Dithiolate Catalysts. Beilstein J. Org. Chem. 2018, 14, 2999–3010. [Google Scholar] [CrossRef]
- Dong, Y.; Matson, J.B.; Edgar, K.J. Olefin Cross-Metathesis in Polymer and Polysaccharide Chemistry: A Review. Biomacromolecules 2017, 18, 1661−1676. [Google Scholar] [CrossRef]
- Thangavel, M.; Chin, S.Y. Cross-Metathesis of Plant Oil: A Mini Review on Reaction Condition and Catalysis. IOP Conf. Ser. Mater. Sci. Eng. 2020, 991, 012073. [Google Scholar] [CrossRef]
- Sinclair, F.; Alkattan, M.; Prunet, J.; Shaver, M.P. Olefin Cross-Metathesis and Ring-Closing Metathesis in Polymer Chemistry. Polym. Chem. 2017, 8, 3385–3398. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.A.; Chalker, J.M.; Davis, B.G. Olefin Cross-Metathesis on Proteins: Investigation of Allylic Chalcogen Effects and Guiding Principles in Metathesis Partner Selection. J. Am. Chem. Soc. 2010, 132, 16805–16811. [Google Scholar] [CrossRef]
- Ritter, T.; Hejl, A.; Wenzel, A.G.; Funk, R.W.; Grubbs, R.H. A Standard System of Characterization for Olefin Metathesis. Organometallics 2006, 25, 5740–5745. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, A.K.; Sanders, D.P.; Grubbs, R.H. Synthesis of Symmetrical Trisubstituted Olefins by Cross Metathesis. Org. Lett. 2002, 4, 1939–1942. [Google Scholar] [CrossRef]
- Vummaleti, S.V.C.; Cavallo, L.; Poater, A. The Driving Force Role of Ruthenacyclobutanes. Theor. Chem. Acc. 2015, 134, 22. [Google Scholar] [CrossRef]
- Chatterjee, A.K.; Choi, T.-L.; Sanders, D.P.; Grubbs, R.H. A General Model for Selectivity in Olefin Cross Metathesis. J. Am. Chem. Soc. 2003, 125, 11360–11370. [Google Scholar] [CrossRef] [Green Version]
- Allaert, B.; Ledoux, N.; Dieltiens, N.; Vander Mierde, H.; Stevens, C.V.; Van Der Voort, P.; Verpoort, F. Secondary Metathesis with Grubbs Catalysts in the 1,4-Polybutadiene System. Catal. Commun. 2008, 9, 1054–1059. [Google Scholar] [CrossRef]
- Anderson, D.R.; Ung, T.; Mkrtumyan, G.; Bertrand, G.; Grubbs, R.H.; Schrodi, Y. Kinetic Selectivity of Olefin Metathesis Catalysts Bearing Cyclic (Alkyl)(Amino)Carbenes. Organometallics 2008, 27, 563–566. [Google Scholar] [CrossRef] [Green Version]
- Montgomery, T.P.; Johns, A.M.; Grubbs, R.H. Recent Advancements in Stereoselective Olefin Metathesis Using Ruthenium Catalysts. Catalysts 2016, 6, 87. [Google Scholar] [CrossRef] [Green Version]
- Shahane, S.; Bruneau, C.; Fischmeister, C. Z Selectivity: Recent Advances in One of the Current Major Challenges of Olefin Metathesis. ChemCatChem 2013, 5, 3436–3459. [Google Scholar] [CrossRef]
- Gottumukkala, A.L.; Madduri, A.V.R.; Minnaard, A.J. Z-Selectivity: A Novel Facet of Metathesis. ChemCatChem 2012, 4, 462–467. [Google Scholar] [CrossRef]
- Zhu, W.; Gunnoe, T.B. Advances in Rhodium-Catalyzed Oxidative Arene Alkylation. Acc. Chem. Res. 2020, 53, 920–936. [Google Scholar] [CrossRef]
- Webster-Gardiner, M.S.; Chen, J.; Vaughan, B.A.; McKeown, B.A.; Schinski, W.; Gunnoe, T.B. Catalytic Synthesis of “Super” Linear Alkenyl Arenes Using an Easily Prepared Rh(I) Catalyst. J. Am. Chem. Soc. 2017, 139, 5474–5480. [Google Scholar] [CrossRef] [PubMed]
- Vasseur, A.; Bruffaerts, J.; Marek, I. Remote Functionalization Through Alkene Isomerization. Nature Chem. 2016, 8, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Sarkic, A.; Stappen, I. Essential Oils and Their Single Compounds in Cosmetics—A Critical Review. Cosmetics 2018, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Diep, P.T.M.; Pawlowska, A.M.; Cioni, P.L.; Nghi, D.H.; Huong, L.M.; Minh, C.V.; Braca, A. Chemical and Biological Studies of the Essential Oils of Micromelum hirsutum. Nat. Prod. Commun. 2007, 2, 691–694. [Google Scholar] [CrossRef] [Green Version]
- Ilijeva, R.; Buchbauer, G. Biological Properties of Some Volatile Phenylpropanoids. Nat. Prod. Commun. 2016, 11, 1616–1629. [Google Scholar] [CrossRef] [Green Version]
- Carrasco, M.R.; Nikitine, C.; Hamou, M.; de Bellefon, C.; Thieuleux, C.; Meille, V. Self-Metathesis of Methyl Oleate Using Ru-NHC Complexes: A Kinetic Study. Catalysts 2020, 10, 435. [Google Scholar] [CrossRef] [Green Version]
- Samojłowicz, C.; Bieniek, M.; Grela, K. Ruthenium-Based Olefin Metathesis Catalysts Bearing N-Heterocyclic Carbene Ligands. Chem. Rev. 2009, 109, 3708–3742. [Google Scholar] [CrossRef]
- Tsedalu, A.A. A Review on Olefin Metathesis Reactions as a Green Method for the Synthesis of Organic Compounds. J. Chem. 2021, 2021, 3590613. [Google Scholar]
- Van der Gryp, P.; Marx, S.; Vosloo, H.C.M. Experimental, DFT and Kinetic Study of 1-Octene Metathesis with Hoveyda-Grubbs Second Generation Precatalyst. J. Mol. Catal. A Chem. 2012, 355, 85–95. [Google Scholar] [CrossRef]
- Moussaoui, Y.; Saïd, K.; Salem, R.B. Anionic Activation of the Wittig Reaction Using a Solid-Liquid Phase Transfer: Examination of the Medium-, Temperature-, Base- and Phase-Transfer Catalyst Effects. ARKIVOC 2006, 12, 1–22. [Google Scholar] [CrossRef]
- Guerrini, A.; Sacchetti, G.; Muzzoli, M.; Rueda, G.M.; Medici, A.; Besco, E.; Bruni, R. Composition of the Volatile Fraction of Ocotea bofo Kunth (Lauraceae) Calyces by GC-MS and NMR Fingerprinting and Its Antimicrobal and Antioxidant Activity. J. Agric. Food Chem. 2006, 54, 7778–7788. [Google Scholar] [CrossRef]
- Hanna, S.; Buthcher, T.W.; Hartwig, J.F. Contra-thermodynamic Olefin Iomerization by Chain-Walking Hydrofunctionalization and Formal Retro-hydrofunctionalization. Org. Lett. 2019, 21, 7129–7133. [Google Scholar] [CrossRef] [Green Version]
- Nelson, D.J.; Manzini, S.; Urbina-Blanco, C.A.; Nolan, S.P. Key Processes in Ruthenium-Catalysed Olefin Metathesis. Chem. Commun. 2014, 50, 10355–10375. [Google Scholar] [CrossRef] [Green Version]
- Grela, K. Olefin Metathesis Theory and Practice; Wiley: Hoboken, NJ, USA, 2014; p. 40. [Google Scholar]
- Yang, G.; Lee, J.K. Curing Kinetics and Mechanical Properties of endo-Dicyclopentadiene Synthesized Using Different Grubbs’ Catalysts. Ind. Eng. Chem. Res. 2014, 53, 3001–3011. [Google Scholar] [CrossRef]
- Thiel, V.; Hendann, M.; Wannowius, K.-J.; Plenio, H. On the Mechanism of the Initiation Reaction in Grubbs–Hoveyda Complexes. J. Am. Chem. Soc. 2011, 134, 1104–1114. [Google Scholar] [CrossRef]
- Sanford, M.S.; Love, J.A.; Grubbs, R.H. Mechanism and Activity of Ruthenium Olefin Metathesis Catalysts. J. Am. Chem. Soc. 2001, 123, 6543–6554. [Google Scholar] [CrossRef] [Green Version]
- Forcina, V.; Garcia-Dominguez, A.; Lloyd-Jones, G.C. Kinetics of Initiation of the Third Generation Grubbs Metathesis Catalyst: Convergent Associative and Dissociative Pathways. Faraday Discuss. 2019, 220, 179–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyatt, M.G.; Walsh, D.J.; Lord, R.L.; Andino Martinez, J.G.; Guironnet, D. Mechanistic and Kinetic Studies of the Ring Opening Metathesis Polymerization of Norbornenyl Monomers by a Grubbs Third Generation Catalyst. J. Am. Chem. Soc. 2019, 141, 17918–17925. [Google Scholar] [CrossRef]
- Dinger, M.B.; Mol, J.C. High Turnover Numbers with Ruthenium-Based Metathesis Catalysts. Adv. Synth. Catal. 2002, 344, 671–677. [Google Scholar] [CrossRef]
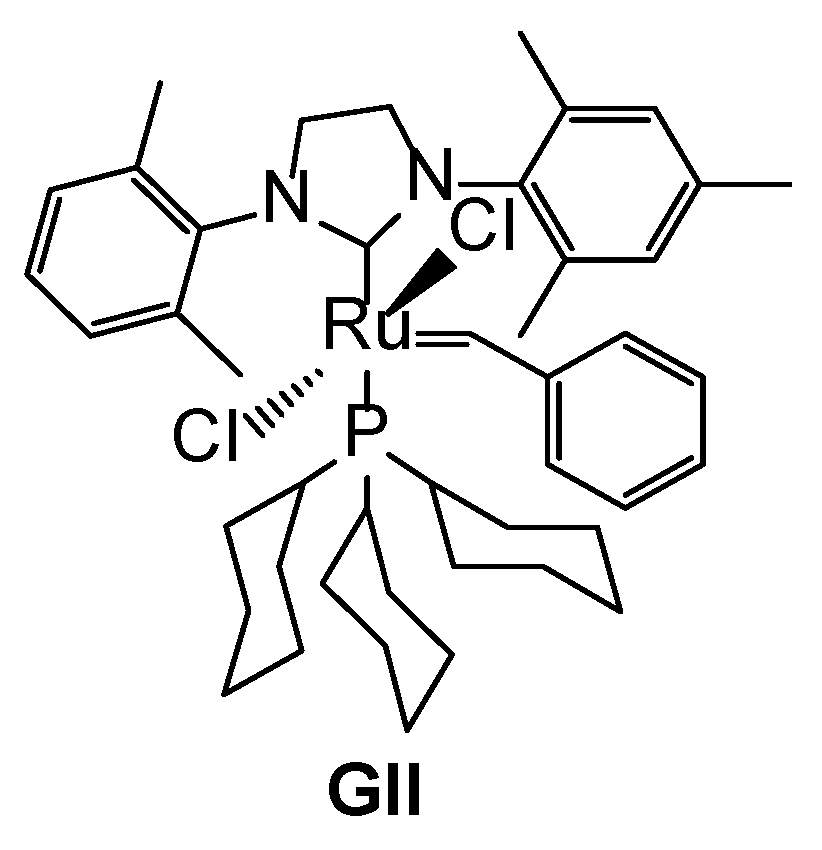
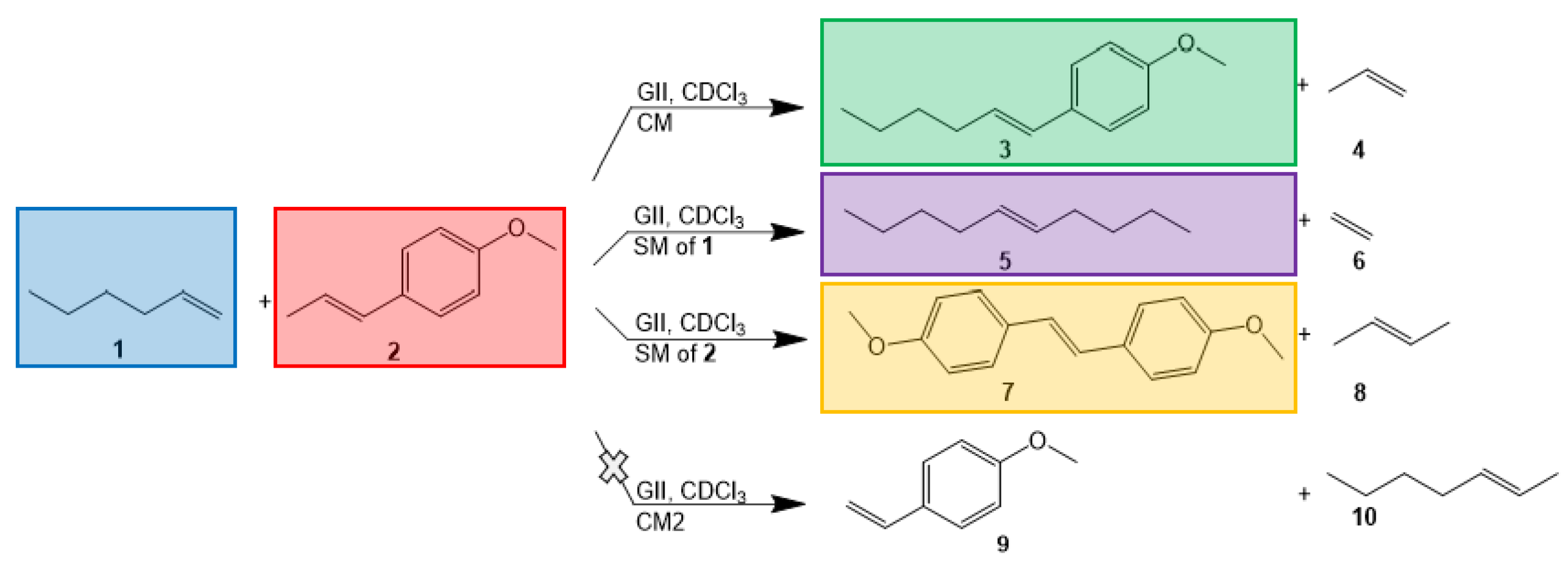
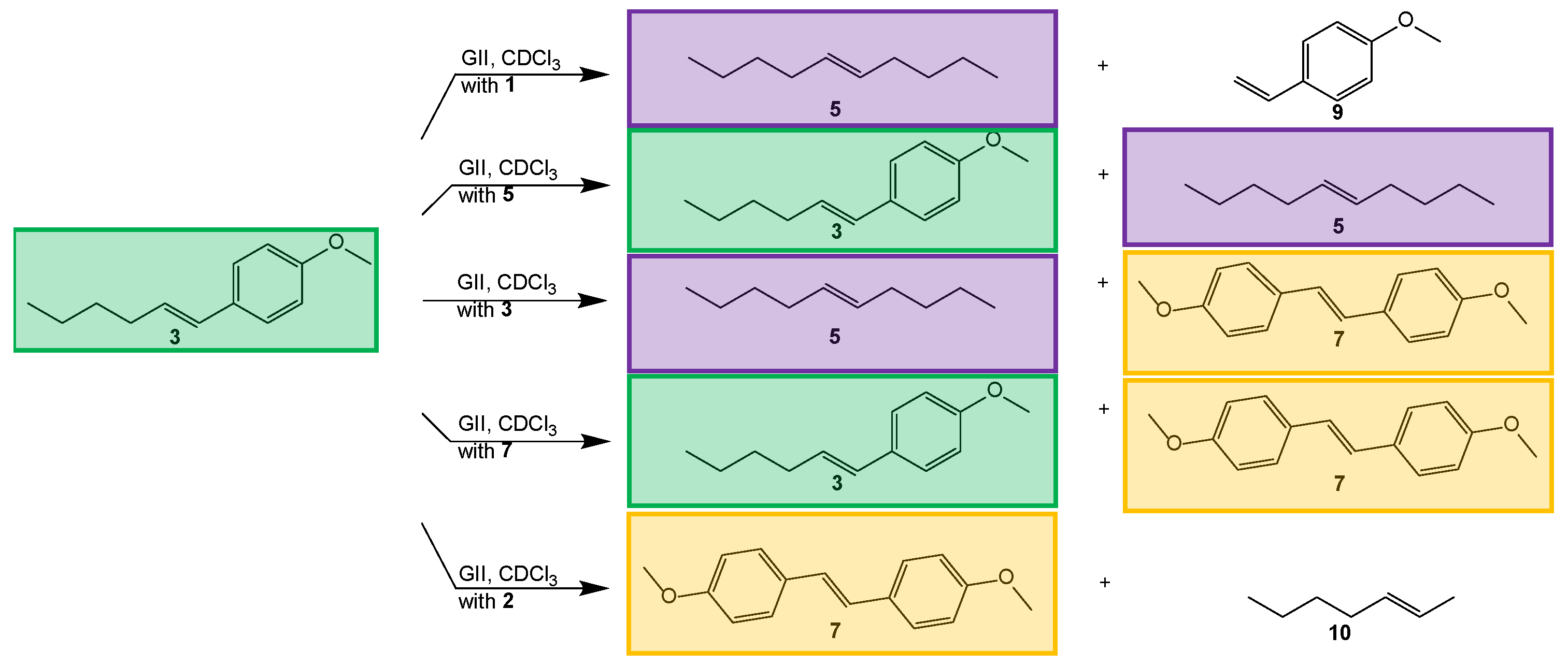




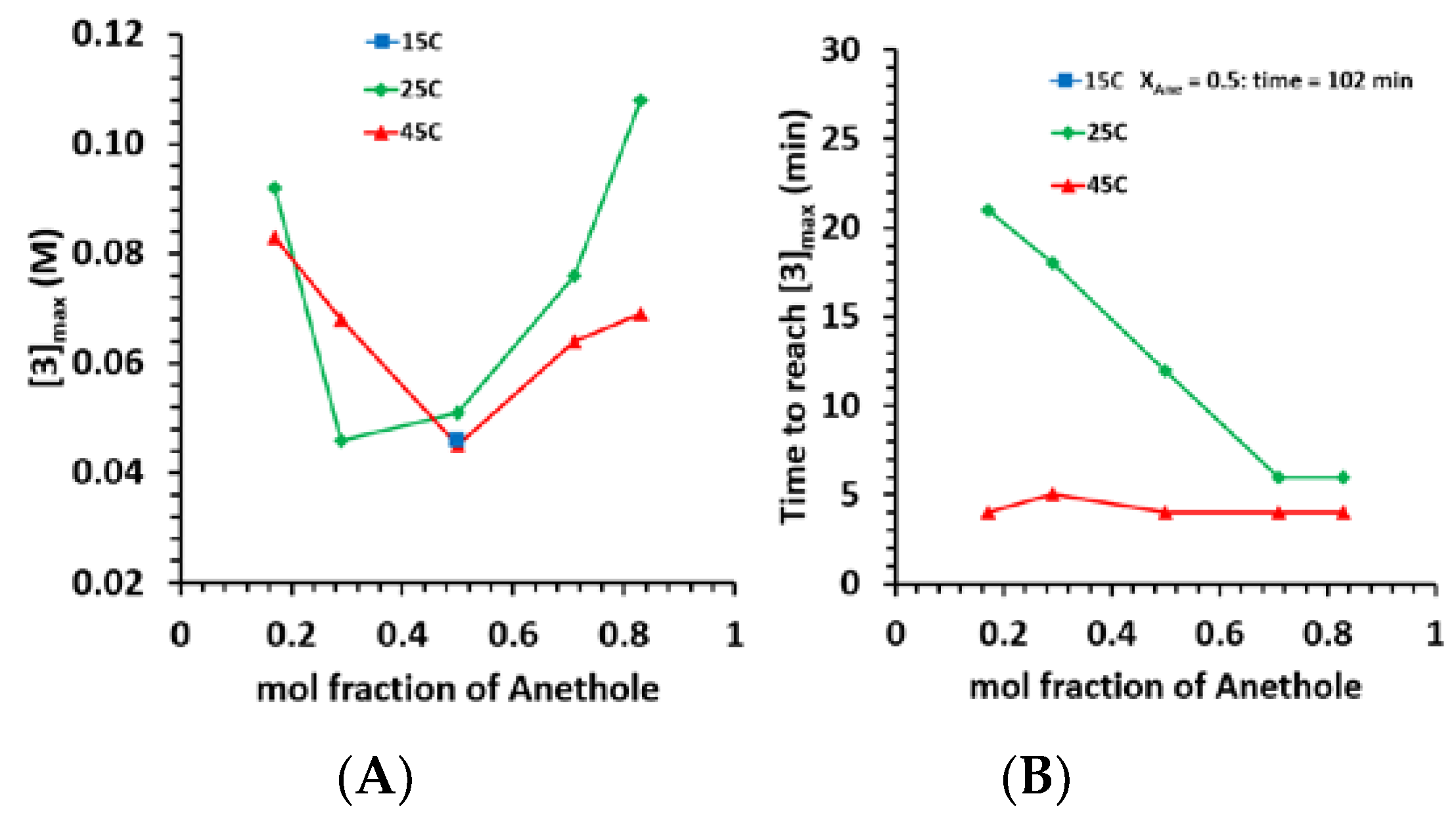

| Exp No | Temp (°C) | Mole Substrate | Substrate Concentration | XAnethole | Mole Ratio | Spike after 18 h | |||
|---|---|---|---|---|---|---|---|---|---|
| 1-hexene | (E)-anethole | 1-hexene | (E)-anethole | 1-hexene:(E)-anethole | 1-hexene | (E)-anethole | |||
| (mmol) | (mmol) | (M) | (M) | (mmol) | (mmol) | ||||
| 1 | 15 | 0.119 | 0.114 | 0.20 | 0.19 | 0.49 | 1.00:0.96 | ||
| 2 | Exp No 2 | 0.594 | 0.117 | 0.99 | 0.20 | 0.16 | 5.08:1.00 | ||
| 3 | 0.295 | 0.12 | 0.49 | 0.20 | 0.29 | 2.46:1.00 | |||
| 4 | 0.118 | 0.12 | 0.20 | 0.20 | 0.5 | 1.00:1.02 | |||
| 5 | 0.118 | 0.325 | 0.20 | 0.54 | 0.73 | 1.00:2.75 | |||
| 6 | 0.118 | 0.599 | 0.20 | 1.00 | 0.84 | 1.00:5.08 | |||
| 7 | Exp No 7 | 0.594 | 0.114 | 0.99 | 0.19 | 0.16 | 5.21:1.00 | ||
| 8 | 0.297 | 0.112 | 0.50 | 0.19 | 0.27 | 2.65:1.00 | |||
| 9 | 0.118 | 0.115 | 0.20 | 0.19 | 0.49 | 1.00:0.97 | |||
| 10 | 0.119 | 0.299 | 0.20 | 0.50 | 0.72 | 1.00:2.51 | |||
| 11 | 0.119 | 0.592 | 0.20 | 0.99 | 0.83 | 1.00:4.97 | |||
| 12 | Exp No 12 | 0.118 | 0.12 | 0.20 | 0.20 | 0.5 | 1.00:1.02 | 0.119 | |
| 13 | 0.119 | 0.113 | 0.20 | 0.19 | 0.49 | 1.00:0.95 | 0.113 | ||
| 14 | 0.119 | 0.113 | 0.20 | 0.19 | 0.49 | 1.00:0.95 | 0.117 | 0.113 | |
| Exp No | XAnethole | Temp (°C) | Maximum [3] | TOF t [3]max (min−1) | k’obs 3 Disappearance (min−1) | % Distribution at the Time When [3]max Was Reached | |||
|---|---|---|---|---|---|---|---|---|---|
| Time (min) | [3] (M) | 3 | 5 | 7 | |||||
| 1 | 0.49 | 15 | 102 | 0.046 | 0.08 | 0.0008 | 28.9 | 59.4 | 11.7 |
| 2 | 0.16 | 25 | 21 | 0.092 | 0.64 | 0.0042 | 18.2 | 80.3 | 1.6 |
| 3 | 0.29 | 25 | 18 | 0.046 | 0.43 | 0.0128 | 19.8 | 74.7 | 5.5 |
| 4 | 0.50 | 25 | 12 | 0.051 | 0.71 | 0.0064 | 33.8 | 55.5 | 10.7 |
| 5 | 0.73 | 25 | 6 | 0.076 | 2.11 | 0.0218 | 32.2 | 43.6 | 24.1 |
| 6 | 0.84 | 25 | 6 | 0.108 | 3.00 | 0.0352 | 37.9 | 38.6 | 23.5 |
| 7 | 0.16 | 45 | 4 | 0.083 | 3.46 | 0.0515 | 15.9 | 82.5 | 1.6 |
| 8 | 0.27 | 45 | 5 | 0.068 | 2.27 | 0.0447 | 20.0 | 75.9 | 4.1 |
| 9 | 0.49 | 45 | 4 | 0.045 | 1.88 | 0.0681 | 25.2 | 59.5 | 15.3 |
| 10 | 0.72 | 45 | 4 | 0.064 | 2.67 | 0.1278 | 24.3 | 45.4 | 30.3 |
| 11 | 0.83 | 45 | 4 | 0.069 | 2.88 | 0.2892 | 14.9 | 32.9 | 52.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swart, M.R.; Marais, C.; Erasmus, E. 1H NMR Analysis of the Metathesis Reaction between 1-Hexene and (E)-Anethole Using Grubbs 2nd Generation Catalyst: Effect of Reaction Conditions on (E)-1-(4-Methoxyphenyl)-1-hexene Formation and Decomposition. Catalysts 2021, 11, 1483. https://doi.org/10.3390/catal11121483
Swart MR, Marais C, Erasmus E. 1H NMR Analysis of the Metathesis Reaction between 1-Hexene and (E)-Anethole Using Grubbs 2nd Generation Catalyst: Effect of Reaction Conditions on (E)-1-(4-Methoxyphenyl)-1-hexene Formation and Decomposition. Catalysts. 2021; 11(12):1483. https://doi.org/10.3390/catal11121483
Chicago/Turabian StyleSwart, Marthinus Rudi, Charlene Marais, and Elizabeth Erasmus. 2021. "1H NMR Analysis of the Metathesis Reaction between 1-Hexene and (E)-Anethole Using Grubbs 2nd Generation Catalyst: Effect of Reaction Conditions on (E)-1-(4-Methoxyphenyl)-1-hexene Formation and Decomposition" Catalysts 11, no. 12: 1483. https://doi.org/10.3390/catal11121483
APA StyleSwart, M. R., Marais, C., & Erasmus, E. (2021). 1H NMR Analysis of the Metathesis Reaction between 1-Hexene and (E)-Anethole Using Grubbs 2nd Generation Catalyst: Effect of Reaction Conditions on (E)-1-(4-Methoxyphenyl)-1-hexene Formation and Decomposition. Catalysts, 11(12), 1483. https://doi.org/10.3390/catal11121483







