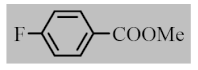
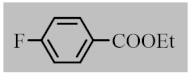
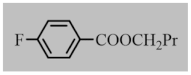
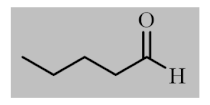
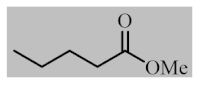
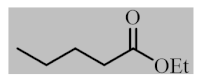
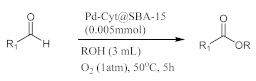Abstract
The synthesis of esters is one of the most fundamental and significant subjects in organic chemistry and chemical industry because they are used in high-value products such as cosmetics, biofuel, pharmaceuticals, surfactants, and food ingredients. In this study, an efficient, economic, sustainable, and green protocol for oxidative esterification reaction has been developed. A one-pot direct transformation of aliphatic, aromatic, and unsaturated aldehydes into esters in the presence of oxygen has been carried out over mesoporous organosilica-supported palladium nanocatalyst (Pd-Cyt@SBA-15) under ambient conditions. Pd-Cyt@SBA-15 efficiently catalyzed selectively large-scale conversion of aldehydes into esters in high yields and large turnover numbers (TON = 98,000). Pd-Cyt@SBA-15 nanocatalyst demonstrated excellent reusability and stability and could be recycled up to ten times without loss of significant reactivity. ICP-AES analysis showed that no leaching of active palladium species occurred during the recycling process of the heterogeneous Pd-Cyt@SBA-15 nanocatalyst.
1. Introduction
In the past few decades, sustainable and green processes have urged scientific research to shift towards greener and more efficient catalytic systems for chemical transformations [1,2,3]. In this context, direct oxidative synthesis of esters from a wide range of aldehydes has emerged as a green chemistry method and is considered good for atom utilization efficiency due to the incorporation of two separate reaction steps, oxidation of aldehydes to carboxylic acids and C–O bond formation with alcohols, into a single one-pot reaction. Over the last decade, numerous homogeneous [4,5,6] and heterogeneous catalysts including transition metal (-free) for direct esterification of aldehydes have been developed by using several organic and inorganic oxidants such as sodium hypochlorite, pyridinium hydrobromide perbromide, hydrogen peroxide, iodine-based oxidants, and oxone. However, most of these methods outlined above suffer from drawbacks due to steric attributes of aldehydes and/or the alcohols, limited substrate scope, using expensive and noble metals such as gold and palladium, toxic reagents, harsh operational conditions, ow efficiency, and environmental impacts. In particular, for industrial implications of one-pot esterification of aldehydes, homogeneous catalysts are mainly restricted by difficulties and catalyst separation from the reaction media and recovery of the catalyst. Recoverable heterogeneous catalysts have advantages for commercially sustainable industrialization and have attracted more attention during recent years. By far, heterogenization of homogeneous catalysts on various organic and inorganic materials has been supported and applied for the one-pot oxidative conversion of aldehydes to ester products. Supported gold and palladium catalysts have been reported to exhibit good performance in oxidative esterification of aldehydes with alcohols. For instance, Tan et al. used Zn-Al-hydrotalcites (HTs) and recently supported atomically precise Au25 nanoclusters (Au25/ZnxAl-400) for the aerobic oxidative esterification of aldehydes (no reusability study) [7]. Patel et al. found that a bi-functional catalyst consisting of nickel and supported 12-tungstophosphoric acid (Ni-TPA/ZrO2) showed a synergic effect in oxidative esterification of aldehydes, with methanol in the presence of hydrogen peroxide as an oxidant [8]. Leadbeater and coworkers reported hexafluoroisopropyl and aldehydes by oxoammonium tetrafluoroborate salt as an oxidant in the presence of pyridine. However, the heterogeneous oxoammonium tetrafluoroborate salt could be only reused for one oxidative esterification reaction cycle [9]. The Aerobic oxidative esterification of aldehydes with methanol was also reported using gold–nickel oxide nanoparticle catalysts with a core–shell structure at high pressures of O2 (3MPa) [10]. Ni-TPA/ZrO2 could be recycled three times. Recently, Au nanoparticles bound to crystalline CeO2 dispersed on magnesium hydroxide (Au/CeO2-Mg(OH)2) were found to be efficient multifunctional heterogeneous catalysts for aerobic oxidation of aldehydes to esters using oxygen (9 bar) with recyclability of up to eight reaction runs [11]. However, in various cases, the effectiveness of these supported catalysts is not optimum due to its low activity, mass transfer, high oxygen pressure, low ester selectivity, and substrate limitation of immobilized homogeneous catalysts as well as the aforementioned issues of catalyst reusability [12,13]. Accordingly, it is necessary to develop new heterogeneous catalysts that can be efficiently recovered while retaining the original activity and selectivity of the active sites. Among different routes with respect to the heterogenization of homogeneous catalysts, the most common route is covalent immobilization of homogeneous catalysts on solid supports. In this regard, several attempts have been successfully developed for the immobilization of homogeneous catalysts on solid supports, including organic and inorganic polymers. For these reasons and following recent efforts from the group in the design of supported nanocatalysts for green and sustainable chemical transformations [14,15,16], we have studied the possibility of developing a green and versatile alternative protocol for one-pot conversions of various aliphatic and aromatic aldehydes with common alcohols using a palladium bearing a functionalized cytosine on the surface of mesoporous silica materials (Pd-Cyt@SMA-15) as an efficient and selective reusable nanocatalyst (Scheme 1). In this regard, we have investigated the possibility of developing a more general method that is effective for converting a wide range of aldehydes to corresponding esters and report herein the catalytic activity of recycling-supported cytosine–palladium complex nanomaterial in the direct esterification of aldehydes with alcohols under aerobic conditions. This study demonstrated an excellent recoverable nanocatalytic system for the selective esterification of aldehydes using benign oxygen as terminal oxidant under extremely mild conditions, which is an industrially important aspect for developing practical and cost-effective catalytic oxidative esterification processes with a broad scope of aldehydes and alcohols.
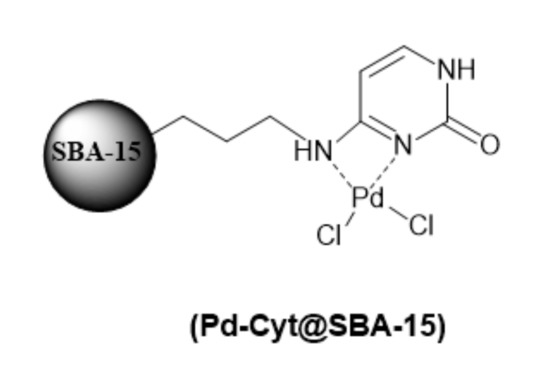
Scheme 1.
Chemical structure of Pd-Cyt@SBA-15.
2. Experimental
2.1. Synthesis of Pd-Cyt@SBA-15 Nanomaterial
The preparation and surface textural properties of Pd-Cyt@SBA-15 nanomaterial are already conducted and reported according to a previously established protocol [16]. Palladium chloride (0.4 mmol, 0.071 g) was added to a suspension of Cyt@SBA-15 (1 g) and dry acetone (20 mL). The reaction mixture was stirred at room temperature overnight, and all volatiles were removed under vacuum, and the residue was washed with ether. Finally, it was dried in an oven at 50 °C overnight in order to obtain the corresponding catalyst PdCyt@SBA-15 at a loading ca. 0.35 ± 0.001 mmol g−1 measured by ICP-MS analysis.
2.2. One-Pot Oxidative Esterification of Aldehydes Catalyzed by Pd-Cyt@SBA-15 Nanocatalyst
In a two necked flask equipped with a condenser, Pd-Cyt@SBA-15 (0.005 mmol, 14 mg) was added to a solution of aldehyde (5 mmol) in alcohol (3 mL). The resulting mixture was then stirred at 50 °C under an oxygen atmosphere (2.5 L balloon) for 5 h. The reaction progress was monitored by thin layer chromatography (TLC, mixture of hexane: ethyl acetate = 5:1). After completion of the reaction, the supported palladium nanocatalyst was filtered from the reaction mixture, rinsed with ethyl acetate, and dried before its reuse in another reaction run. Reuse reactions were carried out under similarly optimized reaction conditions. The combined filtrate was eventually dried over sodium sulfate and purified by pad of silica gel. The solvent was evaporated under reduced pressure to afford the corresponding ester products. Products were characterized according to their 1H and 13CNMR spectra.
3. Results and Discussion
The preparation and surface textural properties of Pd-Cyt@SBA-15 nanomaterial was already reported according to a previously established protocol [16]. Pd-Cyt@SBA-15 was characterized by XRD (Bruker-AXS diffractometer using Cu Kα radiation (λ = 1.5409 Å), Rheinstetten, Germany), BET (Micromeritics ASAP 2000 instrument, Norcross, GA, USA), N2 adsorption, and desorption analysis and TG (NETZSCH STA 409 PC/PG Instrument, Selb, Germany). The characteristics of the Pd-Cyt@SBA-15 nanomaterial used herein are also found to be the same as the previous study; thus, it will not be re-reported. Palladium loading was around 0.35 mmolg−1 and was determined by ICP-AES analysis (Philips PU 70000 sequential spectrometer, Philips, Almelo, The Netherlands). Further characterization results of the Pd-Cyt@SBA-15 nanomaterial indicated a surface area of 367 m2g−1, with an average pore size of 3.55 nm and mesoporous pore volume of 0.76 cm3g−1, with an excellent homogeneous dispersion of palladium species on the 2D-hexagonal ordered structure of nanomaterial support.
In order to optimize various parameters such as catalyst loading, oxidant, reaction time, and temperature, a preliminary screening of the catalytic activity of Pd-Cyt@SBA-15 nanomaterial in the model oxidative esterification reaction of benzaldehyde and methanol was conducted under different conditions included in Table 1 (Scheme 2). The one-pot esterification reaction was initially performed at 60 °C using 30% aqueous hydrogen peroxide. The blank run (in the absence of catalyst) produced no yield to the ester product even after 10 h of reaction time (Table 1, entry 1). When a similar oxidative esterification reaction was conducted in the presence of 0.002 mmol of Pd-Cyt@SBA-15 as catalyst, methyl benzoate was obtained at 48% yield (entry 2). The oxidative esterification of the same reaction with atmospheric air afforded ester products at low yields (entry 3). Then, the aerobic oxidative esterification reaction was investigated by using an oxygen balloon. Molecular oxygen is a cheap, unlimited, eco-friendly, and ideal oxidant from the viewpoint of green and sustainable chemistry. Remarkably, moderate yield was obtained (entry 4) when oxygen was used as oxidant in the model reaction. Interestingly, decreasing the reaction temperature from 60 to 50 °C produced the same reaction yield (entry 5). Increasing the amount of catalyst up to 0.5 mol% provided improved results, and methyl benzoate was obtained in good to excellent yield (entries 5–8). Entries 8–11 display the effect of reaction time from 10 to 5 h. Interestingly, the reaction could still afford an excellent yield of ester product after 5 h (entry 10). However, the oxidative esterification reaction yield decreased if the reaction temperature decreased to 40 °C.

Table 1.
Screening of reaction conditions in the oxidative esterification of benzaldehyde and methanol.
Scheme 2.
Esterification reaction of benzaldehyde and methanol over Pd-Cyt@SBA-15 nano catalyst (model reaction).
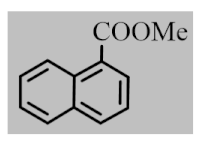
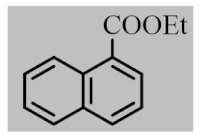
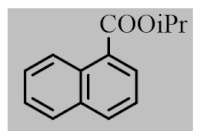
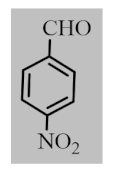
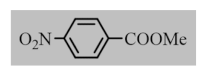
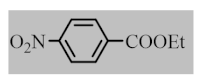
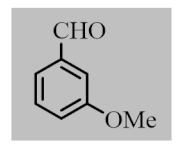
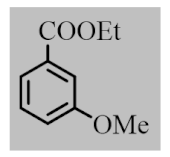
After the optimization of reaction conditions, the scope and limitation of the catalytic system were investigated for a range of aliphatic and aromatic aldehydes with short chain primary and secondary alcohols (Table 2). The substituted aromatic aldehydes with electron-withdrawing group as well as electron-donating group at different positions of the aromatic rings showed excellent reactivity during the oxidative esterification reaction and underwent oxidative esterification transformation to ester products (entries 2–17). 1-naphthaldehyde relatively showed lower reactivity than compared to other aromatic aldehydes (entries 12–14), indicating a possible steric hindrance. Remarkably, the oxidative esterification reaction of aliphatic aldehydes presented similar reaction yields compare with aromatic aldehydes (entries 18–19). These results showed that the Pd-Cyt@SBA-15 nanomaterial has excellent activity and selectivity for structurally varied aldehydes and alcohols. Neither steric nor electronic factors of aldehydes and alcohols had significant influence on the yield of ester products. Pd-Cyt@SBA-15 nanocatalyst exhibited excellent catalytic performance in terms of both selectivity and substrate scope for one-pot esterification of aldehydes with short chain alcohols under green conditions.

Table 2.
Oxidative esterification reaction of aldehydes with alcohols catalyzed by Pd-Cyt@SBA-15 nanocatalyst a.
A hot filtration test evidenced that the removal of the solid catalyst by hot filtration of the heterogeneous catalyst from the original reaction mixture completely stopped the reaction, running the reaction mixture under catalyst free conditions for an additional 10 h after the catalyst filtration had not affected the increase in reaction yield. Furthermore, ICP-AES analysis of the filtrate solution showed no detectable amounts of palladium, indicating no leaching of palladium from the support. All findings strongly supported an active and genuine heterogeneous nature of catalysis.
For practical applications of heterogeneous catalysis systems, in addition to the ease of separation, the lifetime of the catalyst and its recovery and extension of reusability are very important factors. We, therefore, devised a set of experiments to recover and reutilize the Pd-Cyt@SBA-15 nanocatalyst in the model oxidative esterification reaction. All reactions were conducted under similar conditions (Figure 1). The leaching of palladium in the solution was tested by ICP-AES analysis of the reaction mixture, and palladium content was found below detectable level (0.01 ppm), which confirms no significant leaching of palladium catalyst and the high stability of the supported palladium complex in the last reuse. XRD analyses of Pd-Cyt@SBA-15 nanocatalyst conducted before and after recycling are shown in Figure 2. The patterns are identical, and no obvious change was observed, which could be another evidence of the strong stability of the Pd-Cyt@SBA-15 nanocatalyst.
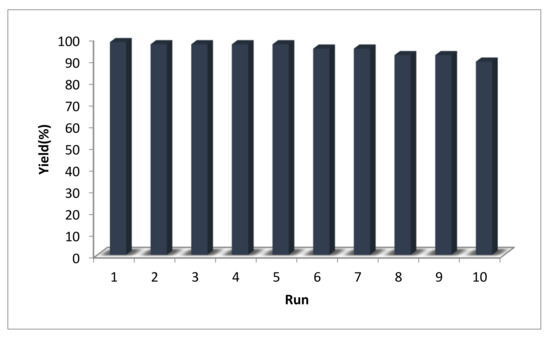
Figure 1.
Recycling activities obtained for the nanocatalyst after 10 runs. Reaction conditions: benzaldehyde (5 mmol), MeOH (3 mL), Pd-Cyt@SBA-15 (14 mg, 0.005 mmol), 50 °C, O2 (filled balloon), and 5 h.
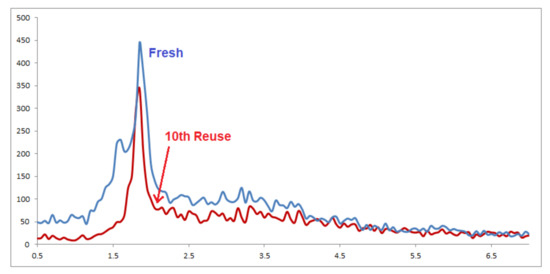
Figure 2.
XRD pattern of fresh and recycled Pd-Cyt@SBA-15 nanocatalyst.
After achieving these promising results and using Pd-Cyt@SBA-15 for industrial applications, the large-scale possibility was examined by using 100 mmol of benzaldehyde in 60 mL of methanol and 280 mg of Pd-Cyt@SBA-15 under aerobic conditions. What is noteworthy that the excellent yield (98%) of methyl benzoate was obtained with high TON (98000) under optimized conditions. Compared with reported procedures in the literature using other catalytic aerobic oxidative esterification systems [7,8,9,10,11], the results obtained using the method described herein provide more environmentally benign and economically attractive systems (Table 3).

Table 3.
Comparison of catalytic activities in aerobic oxidation of benzaldehyde using heterogeneous catalyst.
4. Conclusions
The Pd-Cyt@SBA-15 nanocatalyst was found to be efficient for one-pot esterification of aldehydes and alcohols with superior characteristics such as activity, selectivity, durability, and reusability, which are important factors for sustainable industrial applications. A broad range of short chain alcohols with aldehydes including aromatic and aliphatic aldehydes was selectively large-scale converted to the corresponding ester products in excellent yield and TON. The Pd-Cyt@SBA-15 nanocatalyst showed high stability and reusability over ten reaction runs under mild aerobic conditions. Overall, a highly efficient, selective, and green approach was developed along with the characteristics of easy catalyst recovery, low catalyst loading, atom economic, and green oxidant.
Author Contributions
F.R. and R.L. conceived the study, contributed with characterization and writing of the manuscript; F.R. performed the experiments; C.H.C., M.S. and L.G.V. contributed with the useful advice and editing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This publication has been supported by RUDN University Strategic Academic Leadership Program (L. Voskressensky).
Data Availability Statement
Data is contained within the article.
Acknowledgments
F.R. thanks Payame Noor University (PNU) for partial supporting this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hussein, K. Applications of nanotechnology to improve the performance of solar collectors—Recent advances and overview. Renew. Sustain. Energy Rev. 2016, 62, 767. [Google Scholar] [CrossRef]
- Sheldon, R.A. Metrics of green chemistry and sustainability: Past, present, and future. ACS Sustain. Chem. Eng. 2018, 6, 32. [Google Scholar] [CrossRef] [Green Version]
- Rothenberg, G. Catalysis—Concepts and Green Applications; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2008. [Google Scholar]
- Lerebours, R.; Wolf, C. Chemoselective nucleophilic arylation and single-step oxidative esterification of aldehydes using siloxanes and a palladium−phosphinous acid as a reaction switch. J. Am. Chem. Soc. 2006, 128, 13052. [Google Scholar] [CrossRef] [PubMed]
- Murahashi, S.; Naota, T.; Ito, K.; Maeda, Y.; Taki, H. Ruthenium-Catalyzed Oxidative Transformation of Alcohols and Aldehydes to Esters and Lactones. J. Org. Chem. 1987, 52, 4319. [Google Scholar] [CrossRef]
- Tschaen, B.A.; Schmink, J.R.; Molander, G.A. Pd-catalyzed aldehyde to ester conversion: A hydrogen transfer approach. Org. Lett. 2013, 15, 500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Tan, Y.; Chen, X.; Yang, W.; Huang, C.; Li, J.; Ding, Y. Efficient Synthesis of Methyl Methacrylate by One Step Oxidative Esterification over Zn-Al-Mixed Oxides Supported Gold Nanocatalysts. Catalysts 2021, 11, 162. [Google Scholar] [CrossRef]
- Patel, A.; Patel, A. Nickel exchanged supported 12-tungstophosphoric acid: Synthesis, characterization and base free one-pot oxidative esterification of aldehyde and alcohol. RSC Adv. 2019, 9, 1460. [Google Scholar] [CrossRef] [Green Version]
- Kelly, C.B.; Mercadante, M.A.; Wiles, R.J.; Leadbeater, N.E. Oxidative Esterification of Aldehydes Using a Recyclable Oxoammonium Salt. Org. Lett. 2013, 15, 2222. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Yamaguchi, T.; Matsushita, K.; Iitsuka, C.; Miura, J.; Akaogi, T.; Ishida, H. Aerobic Oxidative Esterification of Aldehydes with Alcohols by Gold–Nickel Oxide Nanoparticle Catalysts with a Core–Shell Structure. ACS Catal. 2013, 3, 1845. [Google Scholar] [CrossRef]
- Lim, S.; Kwon, S.; Kim, N.; Na, K. Multifunctional Au/CeO2-Mg(OH)2 Catalyst for One-Pot Aerobic Oxidative Esterification of Aldehydes with Alcohols to Alkyl Esters. Nanomaterials 2021, 11, 1536. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.S.; Ying, X.; Wanping, B.; Fang, G.; Lee, Y. Immobilizing catalysts on porous materials. Mater. Today 2006, 9, 32. [Google Scholar] [CrossRef]
- Farnetti, E.; Monte, R.D.; Kašpar, J. Inorganic and Bio-Inorganic Chemistry; Vol. II—Homogeneous and Heterogeneous Catalysis; Eolss: Oxford, UK, 2009. [Google Scholar]
- Rajabi, F.; Zare Ebrahimi, A.; Rabiee, A.; Pineda, A.; Luque, R. Synthesis and Characterization of Novel Pyridine Periodic Mesoporous Organosilicas and Its Catalytic Activity in the Knoevenagel Condensation Reaction. Materials 2020, 13, 1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajabi, F.; Luque, R. Highly ordered mesoporous functionalized pyridinium protic ionic liquids framework as efficient system in esterification reactions for biofuels production. Mol. Catal. 2020, 498, 111238. [Google Scholar] [CrossRef]
- Rajabi, F.; Fayyaz, F.; Bandyopadhyay, R.; Ivars-Barcelo, F.; Puente-Santiago, A.R.; Luque, R. Cytosine Palladium Hybrid Complex Immobilized on SBA-15 as Efficient Heterogeneous Catalyst for the Aqueous Suzuki-Miyaura Coupling. ChemistrySelect 2018, 3, 6102. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).