Oxy-Steam Reforming of Liquefied Natural Gas (LNG) on Mono- and Bimetallic (Ag, Pt, Pd or Ru)/Ni Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Activity Tests
2.2. Temperature Programmed Reduction (TPR-H2) Measurements
2.3. Temperature Programmed Desorption (TPD-NH3) Analysis
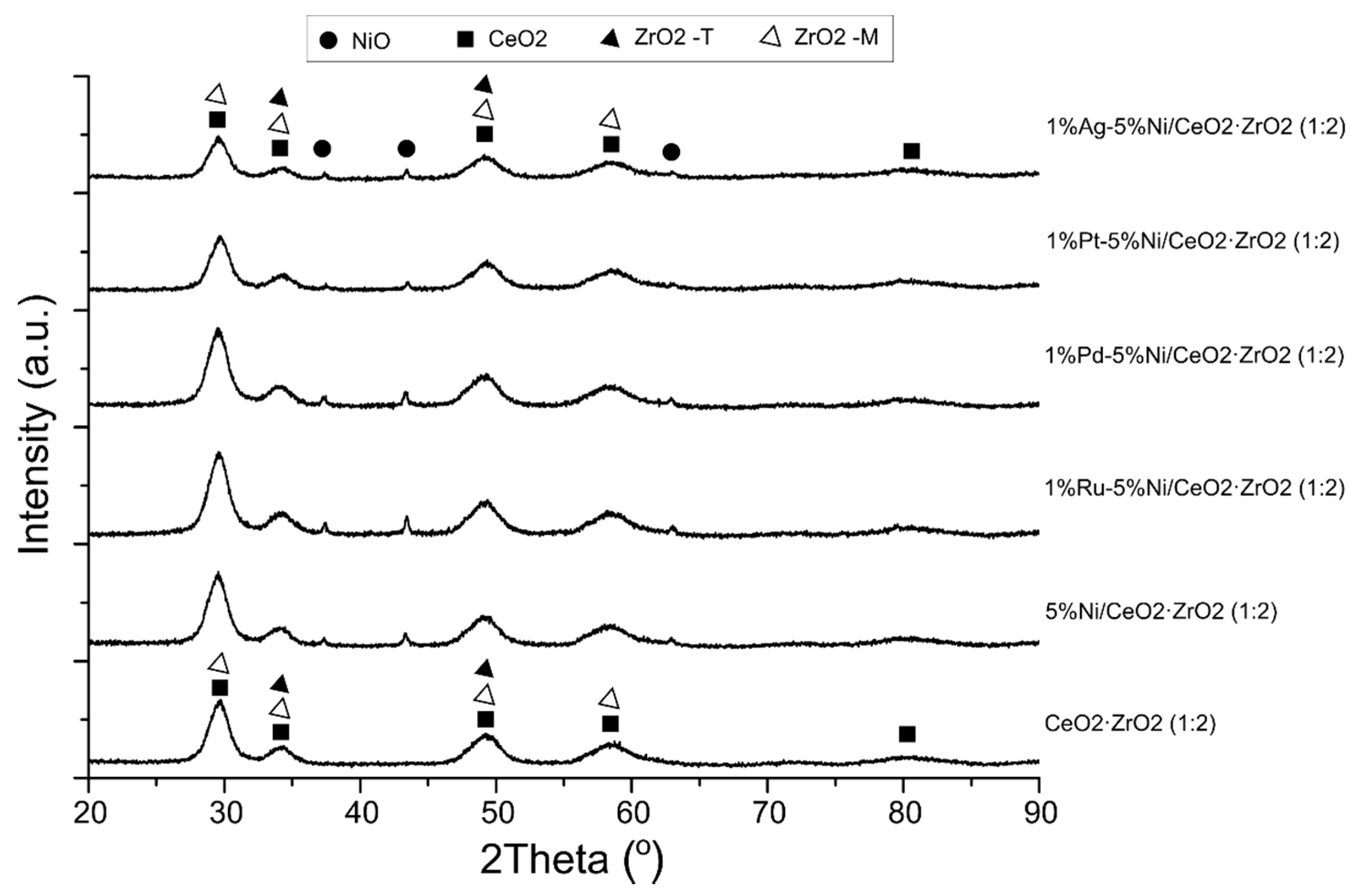
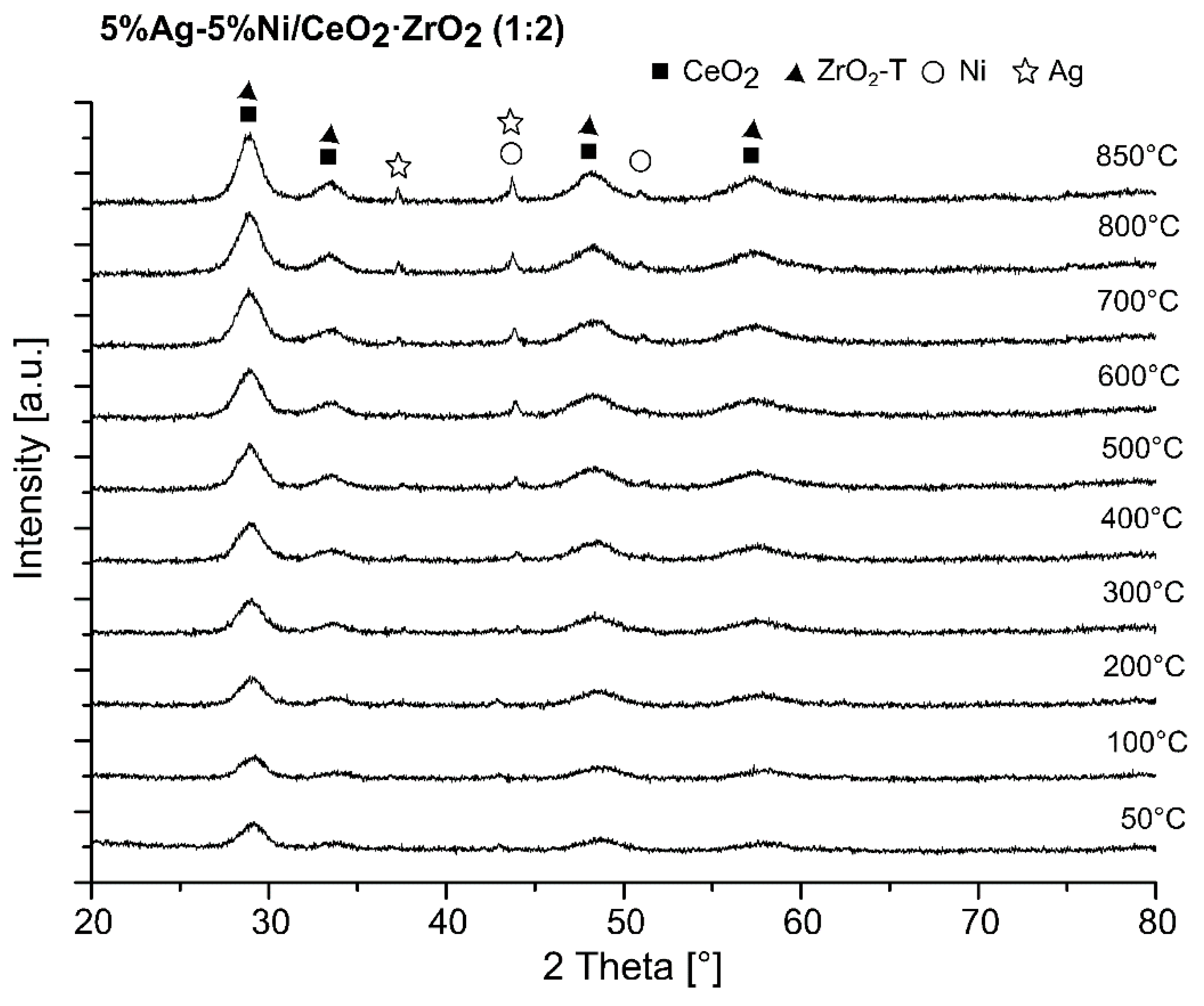
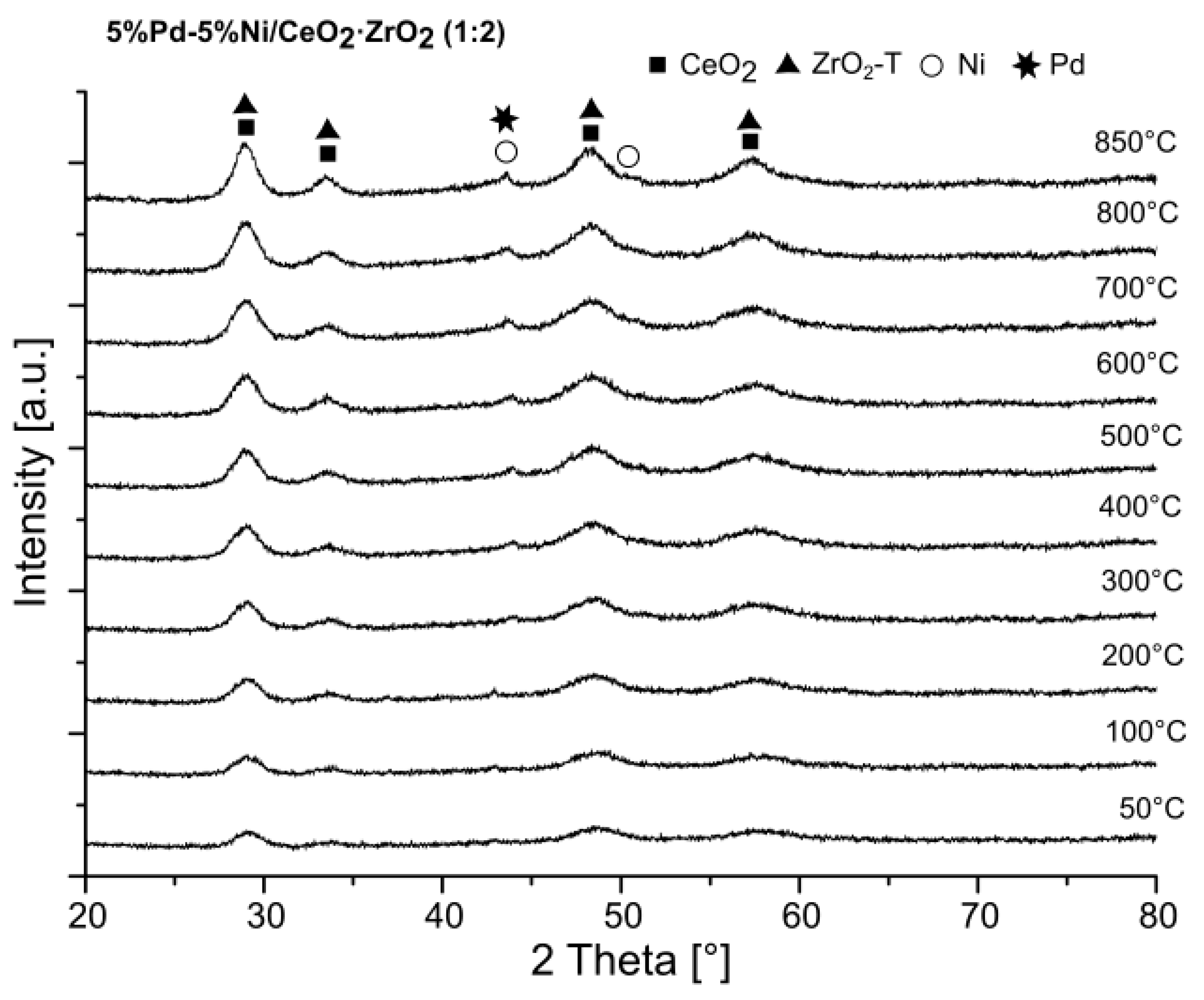
2.4. X-ray Diffraction (XRD) Studies
2.5. SEM-EDS Measurements
2.6. XPS Surface Analysis of the Mono- and Bimetallic Ni Catalysts Supported on CeO2·ZrO2 (1:2) System
3. Materials and Methods
3.1. Catalytic Material Preparation
3.2. Characterization Techniques
3.3. Catalytic Activity Tests in Oxy-Steam Reforming of Liquified Natural Gas (OSR-LNG)
- —the moles of hydrocarbon (methane, ethane, propane, and butane) at the reactor inlet;
- —the moles of hydrocarbon (methane, ethane, propane, and butane) at the reactor outlet;
- —the moles of the CO at the reactor outlet;
- —the moles of the CO2 at the reactor outlet;
- —the moles of the H2 at the reactor outlet;
- —the sum of the moles of the hydrocarbons (methane, ethane, propane, and butane) at the reactor inlet;
- —the sum of the moles of the hydrocarbons (methane, ethane, propane, and butane) at the reactor outlet.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mierczynski, P.; Mierczynska, A.; Maniukiewicz, W.; Maniecki, T.P.; Vasilev, K. MWCNTs as a catalyst in oxy-steam reforming of methanol. RSC Adv. 2016, 6, 81408–81413. [Google Scholar] [CrossRef]
- Gupta, R.B. Hydrogen Fuel: Production, Transport, and Storage; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Ji, M.; Wang, J. Review and comparison of various hydrogenproduction methods based on costs and life cycleimpact assessment indicators. Int. J. Hydrogen Energy 2021, 46, 38612–38635. [Google Scholar] [CrossRef]
- Mierczynski, P.; Mosinska, M.; Maniukiewicz, W.; Nowosielska, M.; Czylkowska, A.; Szynkowska, M.I. Oxy-steam reforming of methanol on copper catalysts. React. Kinet. Mech. Catal. 2019, 127, 857–874. [Google Scholar] [CrossRef] [Green Version]
- Mierczynski, P.; Mosinska, M.; Maniukiewicz, W.; Vasilev, K.; Szynkowska, M.I. Novel Rh(Pd)-Cu(Ni) supported catalysts for oxy-steam reforming of methanol. Arab. J. Chem. 2018, 13, 3183–3195. [Google Scholar] [CrossRef]
- Mierczynski, P.; Mosinska, M.; Zakrzewski, M.; Dawid, B.; Ciesielski, R.; Maniecki, T.; Maniukiewicz, W. Influence of the Zn–Al binary oxide composition on the physicochemical and catalytic properties of Ni catalysts in the oxy-steam reforming of methanol. React. Kinet. Mech. Catal. 2017, 121, 453–472. [Google Scholar] [CrossRef] [Green Version]
- Mierczynski, P.; Mierczynska, A.; Ciesielski, R.; Mosinska, M.; Nowosielska, M.; Czylkowska, A.; Maniukiewicz, W.; Szynkowska, M.I.; Vasilev, K. High Active and Selective Ni/CeO2–Al2O3 and Pd–Ni/CeO2–Al2O3 Catalysts for Oxy-Steam Reforming of Methanol. Catalysts 2018, 8, 380. [Google Scholar] [CrossRef] [Green Version]
- Gil Seo, J.; Youn, M.H.; Park, S.; Lee, J.; Lee, S.H.; Lee, H.; Song, I.K. Hydrogen production by steam reforming of LNG over Ni/Al2O3-ZrO2 catalysts: Effect of ZrO2 and preparation method of Al2O3-ZrO2. Korean J. Chem. Eng. 2008, 25, 95–98. [Google Scholar] [CrossRef]
- Mierczynski, P.; Mosinska, M.; Stepinska, N.; Chalupka, K.; Nowosielska, M.; Maniukiewicz, W.; Rogowski, J.; Goswami, N.; Vasilev, K.; Szynkowska, M.I. Effect of the support composition on catalytic and physicochemical properties of Ni catalysts in oxy-steam reforming of methane. Catal. Today 2020, 364, 46–60. [Google Scholar] [CrossRef]
- Mosinska, M.; Stepinska, N.; Chalupka, K.; Maniukiewicz, W.; Szynkowska, M.I.; Mierczynski, P. Effect of Ag-Addition on the Catalytic and Physicochemical Properties of Ni/ZrO2 Catalyst in Oxy-Steam Reforming of CH4 and LNG Processes. Catalysts 2020, 10, 855. [Google Scholar] [CrossRef]
- Mosinska, M.; Szynkowska, M.I.; Mierczynski, P. Oxy-Steam Reforming of Natural Gas on Ni Catalysts—A Minireview. Catalysts 2020, 10, 896. [Google Scholar] [CrossRef]
- Park, S.; Yoo, J.; Han, S.J.; Song, J.H.; Lee, E.J.; Song, I.K. Steam reforming of liquefied natural gas (LNG) for hydrogen production over nickel–boron–alumina xerogel catalyst. Int. J. Hydrogen Energy 2017, 42, 15096–15106. [Google Scholar] [CrossRef]
- Gil Seo, J.; Youn, M.H.; Park, S.; Chung, J.S.; Song, I.K. Hydrogen production by steam reforming of liquefied natural gas (LNG) over Ni/Al2O3–ZrO2 xerogel catalysts: Effect of calcination temperature of Al2O3–ZrO2 xerogel supports. Int. J. Hydrogen Energy 2009, 34, 3755–3763. [Google Scholar] [CrossRef]
- Bang, Y.; Park, S.; Han, S.J.; Yoo, J.; Song, J.H.; Choi, J.H.; Kang, K.H.; Song, I.K. Hydrogen production by steam reforming of liquefied natural gas (LNG) over mesoporous Ni/Al2O3 catalyst prepared by an EDTA-assisted impregnation method. Appl. Catal. B Environ. 2015, 180, 179–188. [Google Scholar] [CrossRef]
- Bang, Y.; Han, S.J.; Yoo, J.; Choi, J.H.; Lee, J.K.; Song, J.H.; Lee, J.; Song, I.K. Hydrogen production by steam reforming of simulated liquefied natural gas (LNG) over nickel catalyst supported on mesoporous phosphorus-modified alumina xerogel. Appl. Catal. B Environ. 2013, 148–149, 269–280. [Google Scholar] [CrossRef]
- Bang, Y.; Gil Seo, J.; Song, I.K. Hydrogen production by steam reforming of liquefied natural gas (LNG) over mesoporous Ni–La–Al2O3 aerogel catalysts: Effect of La content. Int. J. Hydrogen Energy 2011, 36, 8307–8315. [Google Scholar] [CrossRef]
- Gil Seo, J.; Youn, M.H.; Song, I.K. Hydrogen production by steam reforming of LNG over Ni/Al2O3–ZrO2 catalysts: Effect of Al2O3–ZrO2 supports prepared by a grafting method. J. Mol. Catal. A Chem. 2007, 268, 9–14. [Google Scholar] [CrossRef]
- Bang, Y.; Han, S.J.; Gil Seo, J.; Youn, M.H.; Song, J.H.; Song, I.K. Hydrogen production by steam reforming of liquefied natural gas (LNG) over ordered mesoporous nickel–alumina catalyst. Int. J. Hydrogen Energy 2012, 37, 17967–17977. [Google Scholar] [CrossRef]
- Armor, J. The multiple roles for catalysis in the production of H2. Appl. Catal. A Gen. 1999, 176, 159–176. [Google Scholar] [CrossRef]
- Matsumura, Y.; Nakamori, T. Steam reforming of methane over nickel catalysts at low reaction temperature. Appl. Catal. A Gen. 2004, 258, 107–114. [Google Scholar] [CrossRef]
- Dantas, S.C.; Escritori, J.C.; Soares, R.R.; Hori, C.E. Effect of different promoters on Ni/CeZrO2 catalyst for autothermal reforming and partial oxidation of methane. Chem. Eng. J. 2010, 156, 380–387. [Google Scholar] [CrossRef]
- Wu, H.; Pantaleo, G.; La Parola, V.; Venezia, A.M.; Collard, X.; Aprile, C.; Liotta, L.F. Bi- and trimetallic Ni catalysts over Al2O3 and Al2O3-MO (M = Ce or Mg) oxides for methane dry reforming: Au and Pt additive effects. Appl. Catal. B Environ. 2014, 156–157, 350–361. [Google Scholar] [CrossRef]
- Takeguchi, T.; Furukawa, S.-N.; Inoue, M.; Eguchi, K. Autothermal reforming of methane over Ni catalysts supported over CaO–CeO2–ZrO2 solid solution. Appl. Catal. A Gen. 2003, 240, 223–233. [Google Scholar] [CrossRef]
- Mierczynski, P. Comparative Studies of Bimetallic Ru–Cu, Rh–Cu, Ag–Cu, Ir–Cu Catalysts Supported on ZnO–Al2O3, ZrO2–Al2O3 Systems. Catal. Lett. 2016, 146, 1825–1837. [Google Scholar] [CrossRef]
- Mierczynski, P.; Vasilev, K.; Mierczynska, A.; Maniukiewicz, W.; Maniecki, T. Highly selective Pd–Cu/ZnAl2O4 catalyst for hydrogen production. Appl. Catal. A Gen. 2014, 479, 26–34. [Google Scholar] [CrossRef]
- Ashok, J.; Ang, M.; Kawi, S. Enhanced activity of CO2 methanation over Ni/CeO2-ZrO2 catalysts: Influence of preparation methods. Catal. Today 2016, 281, 304–311. [Google Scholar] [CrossRef]
- Mierczynski, P.; Ciesielski, R.; Kedziora, A.; Shtyka, O.; Maniecki, T. Methanol Synthesis Using Copper Catalysts Supported on CeO2−Al2O3 Mixed Oxide. Fibre Chem. 2016, 48, 271–275. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, K.; Wang, H.; Wang, Y.; Tian, D.; Wei, Y.; Zhu, X.; Zeng, C.; Luo, Y. Structure dependence and reaction mechanism of CO oxidation: A model study on macroporous CeO2 and CeO2-ZrO2 catalysts. J. Catal. 2016, 344, 365–377. [Google Scholar] [CrossRef]
- Pérez-Hernández, R.; Gutiérrez-Martínez, A.; Palacios, J.; Vega-Hernández, M.; Rodríguez-Lugo, V. Hydrogen production by oxidative steam reforming of methanol over Ni/CeO2–ZrO2 catalysts. Int. J. Hydrogen Energy 2011, 36, 6601–6608. [Google Scholar] [CrossRef]
- Xie, J.; Sun, X.; Barrett, L.; Walker, B.R.; Karote, D.R.; Langemeier, J.M.; Leaym, X.; Kroh, F.; Traylor, W.; Feng, J.; et al. Autothermal reforming and partial oxidation of n-hexadecane via Pt/Ni bimetallic catalysts on ceria-based supports. Int. J. Hydrogen Energy 2015, 40, 8510–8521. [Google Scholar] [CrossRef]
- Mierczynski, P.; Ciesielski, R.; Kedziora, A.; Zaborowski, M.; Maniukiewicz, W.; Nowosielska, M.; Szynkowska, M.I.; Maniecki, T.P. Novel Pd-Cu/ZnAl2O4-ZrO2 Catalysts for Methanol Synthesis. Catal. Lett. 2014, 144, 723–735. [Google Scholar] [CrossRef]
- Mierczynski, P.; Maniukiewicz, W.; Maniecki, T.P. Comparative studies of Pd, Ru, Ni, Cu/ZnAl2O4 catalysts for the water gas shift reaction. Open Chem. 2013, 11, 912–919. [Google Scholar] [CrossRef]
- Mierczynski, P.; Stępińska, N.; Mosinska, M.; Chalupka, K.; Albinska, J.; Maniukiewicz, W.; Rogowski, J.; Nowosielska, M.; Szynkowska, M.I. Hydrogen Production via the Oxy-Steam Reforming of LNG or Methane on Ni Catalysts. Catalysts 2020, 10, 346. [Google Scholar] [CrossRef] [Green Version]
- Mierczynski, P.; Vasilev, K.; Mierczynska, A.; Maniukiewicz, W.; Szynkowska, M.I.; Maniecki, T.P. Bimetallic Au–Cu, Au–Ni catalysts supported on MWCNTs for oxy-steam reforming of methanol. Appl. Catal. B Environ. 2016, 185, 281–294. [Google Scholar] [CrossRef]
- Chen, Y.S.; Kang, J.F.; Chen, B.; Gao, B.; Liu, L.F.; Liu, X.Y.; Wang, Y.Y.; Wu, L.; Yu, H.Y.; Wang, J.Y.; et al. Microscopic mechanism for unipolar resistive switching behaviour of nickel oxides. J. Phys. D Appl. Phys. 2012, 45, 65303. [Google Scholar] [CrossRef]
- Jin, Q.; He, Y.; Miao, M.; Guan, C.; Du, Y.; Feng, J.; Li, D. Highly selective and stable PdNi catalyst derived from layered double hydroxides for partial hydrogenation of acetylene. Appl. Catal. A Gen. 2015, 500, 3–11. [Google Scholar] [CrossRef]
- Reddy, G.K.; Ling, C.; Peck, T.C.; Jia, H. Understanding the chemical state of palladium during the direct NO decomposition—Influence of pretreatment environment and reaction temperature. RSC Adv. 2017, 7, 19645–19655. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Li, Y.; Chai, R.; Zhao, G.; Liu, Y.; Lu, Y. Low-temperature active, oscillation-free PdNi(alloy)/Ni-foam catalyst with enhanced heat transfer for coalbed methane deoxygenation via catalytic combustion. Appl. Catal. B Environ. 2016, 187, 238–248. [Google Scholar] [CrossRef]
- Khalakhan, I.; Vega, L.; Vorokhta, M.; Skála, T.; Viñes, F.; Yakovlev, Y.; Neyman, K.M.; Matolínová, I. Irreversible structural dynamics on the surface of bimetallic PtNi alloy catalyst under alternating oxidizing and reducing environments. Appl. Catal. B Environ. 2019, 264, 118476. [Google Scholar] [CrossRef]
- Mazzotta, E.; Rella, S.; Turco, A.; Malitesta, C. XPS in development of chemical sensors. RSC Adv. 2015, 5, 83164–83186. [Google Scholar] [CrossRef]
- Yurderi, M.; Bulut, A.; Zahmakiran, M.; Kaya, M. Carbon supported trimetallic PdNiAg nanoparticles as highly active, selective and reusable catalyst in the formic acid decomposition. Appl. Catal. B Environ. 2014, 160–161, 514–524. [Google Scholar] [CrossRef]
- Firet, N.J.; Blommaert, M.A.; Burdyny, T.; Venugopal, A.; Bohra, D.; Longo, A.; Smith, W.A. Operando EXAFS study reveals presence of oxygen in oxide-derived silver catalysts for electrochemical CO2 reduction. J. Mater. Chem. A 2019, 7, 2597–2607. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Deka, S. Multiply Twinned AgNi Alloy Nanoparticles as Highly Active Catalyst for Multiple Reduction and Degradation Reactions. ACS Appl. Mater. Interfaces 2014, 6, 16071–16081. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Sun, H.; Zheng, J.; Yu, C.; Zhang, N.; Shu, Q.; Chen, B.H. Combining Ru, Ni and Ni(OH)2 active sites for improving catalytic performance in benzene hydrogenation. Mater. Chem. Phys. 2017, 192, 8–16. [Google Scholar] [CrossRef]

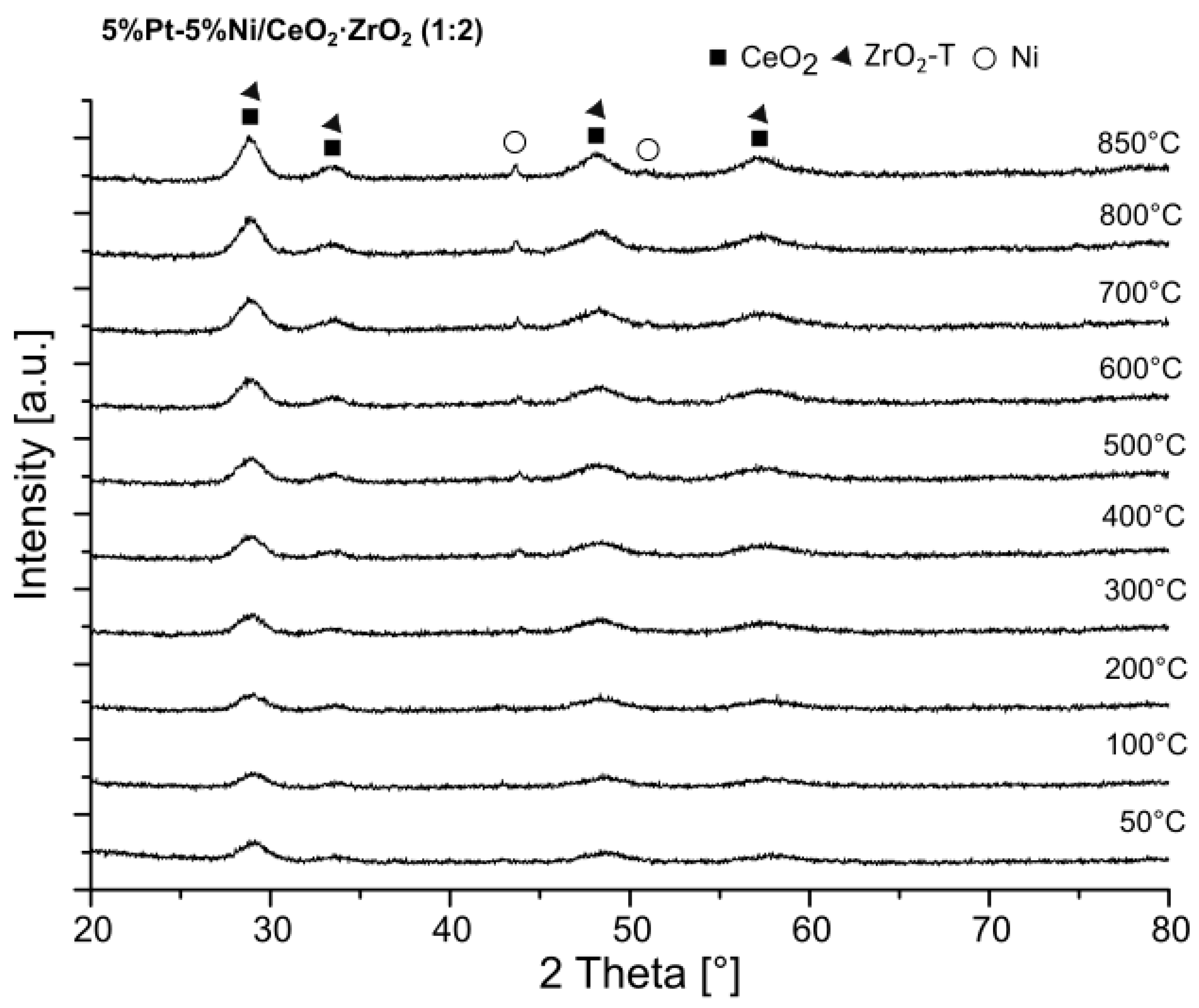
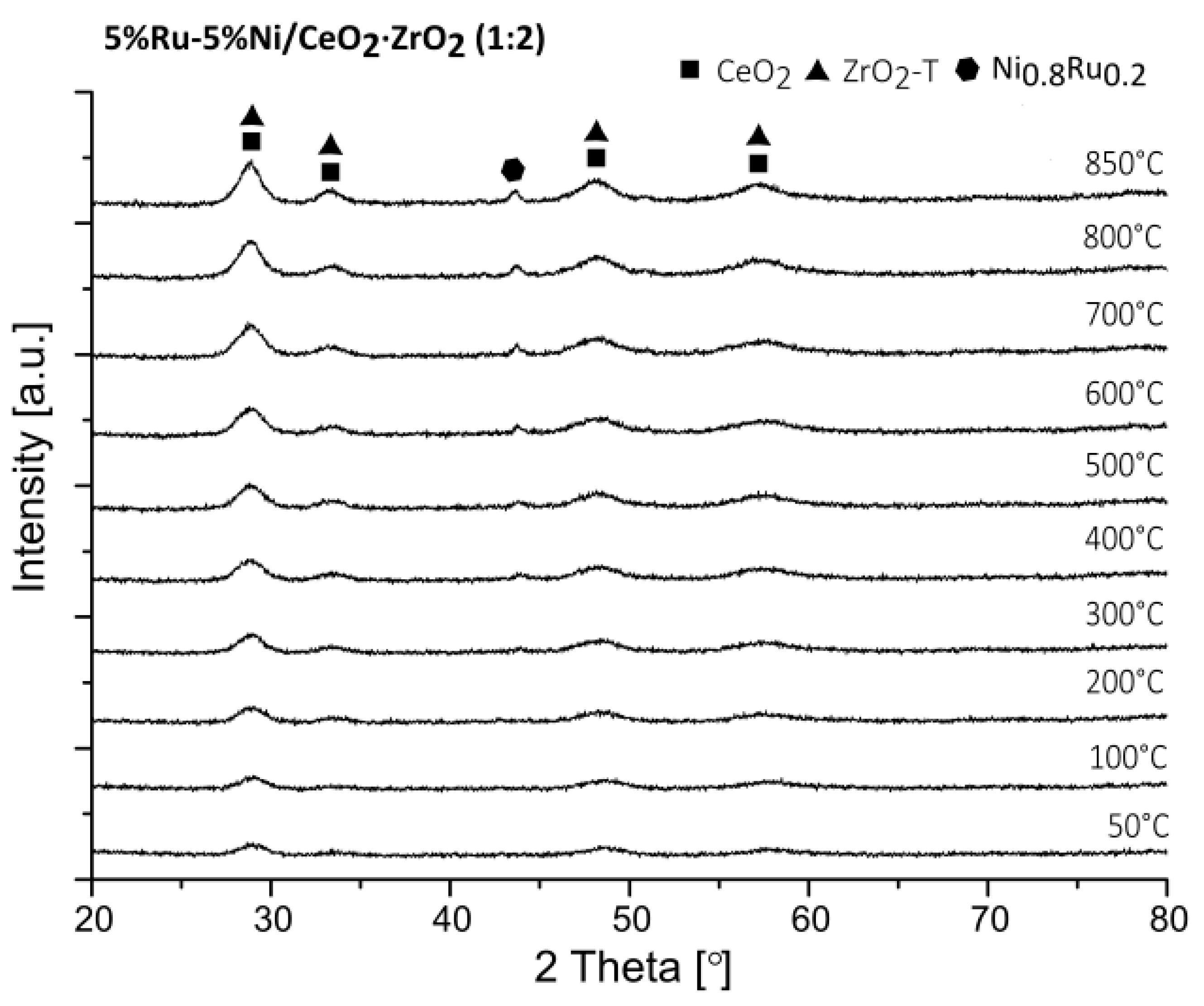

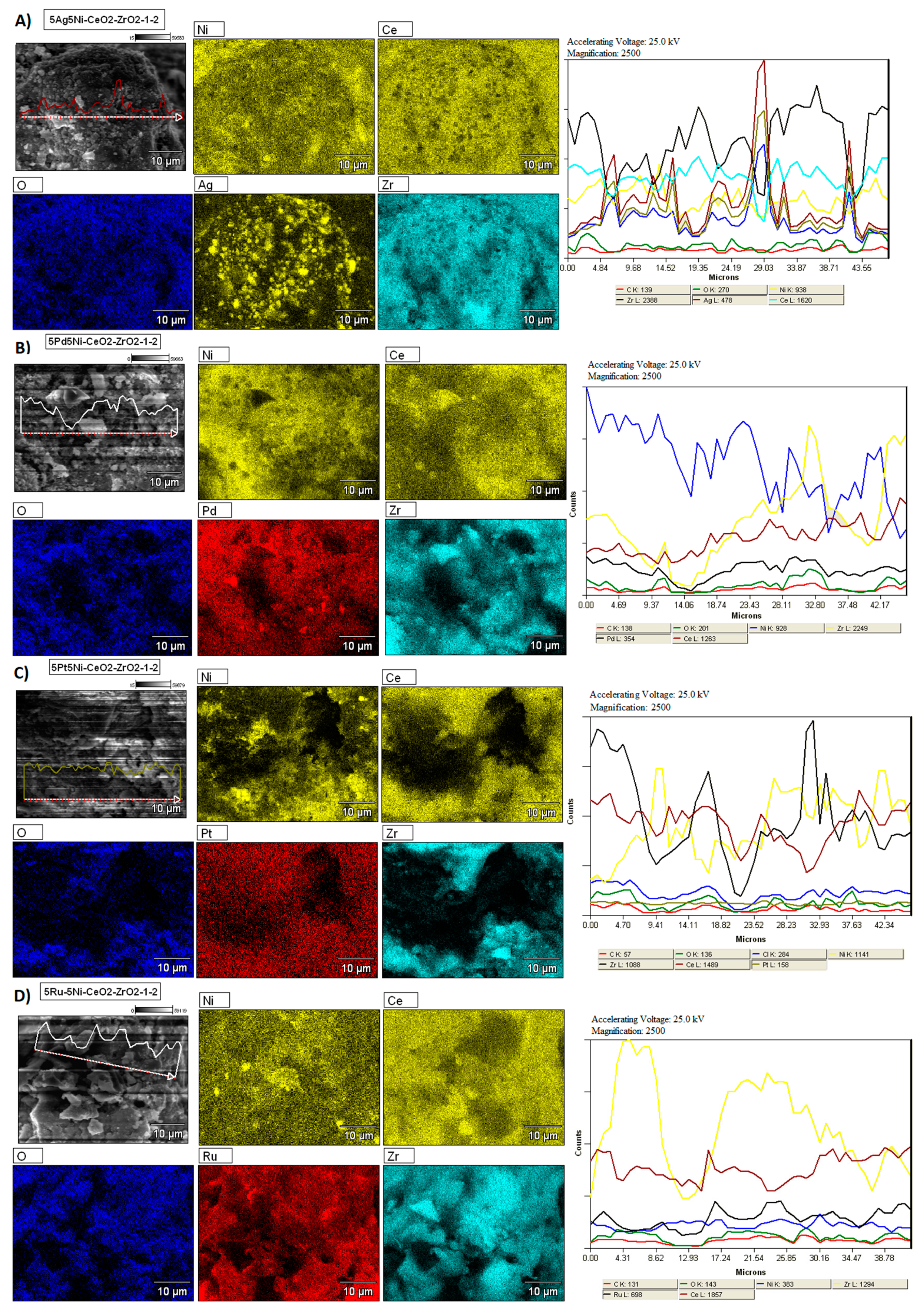
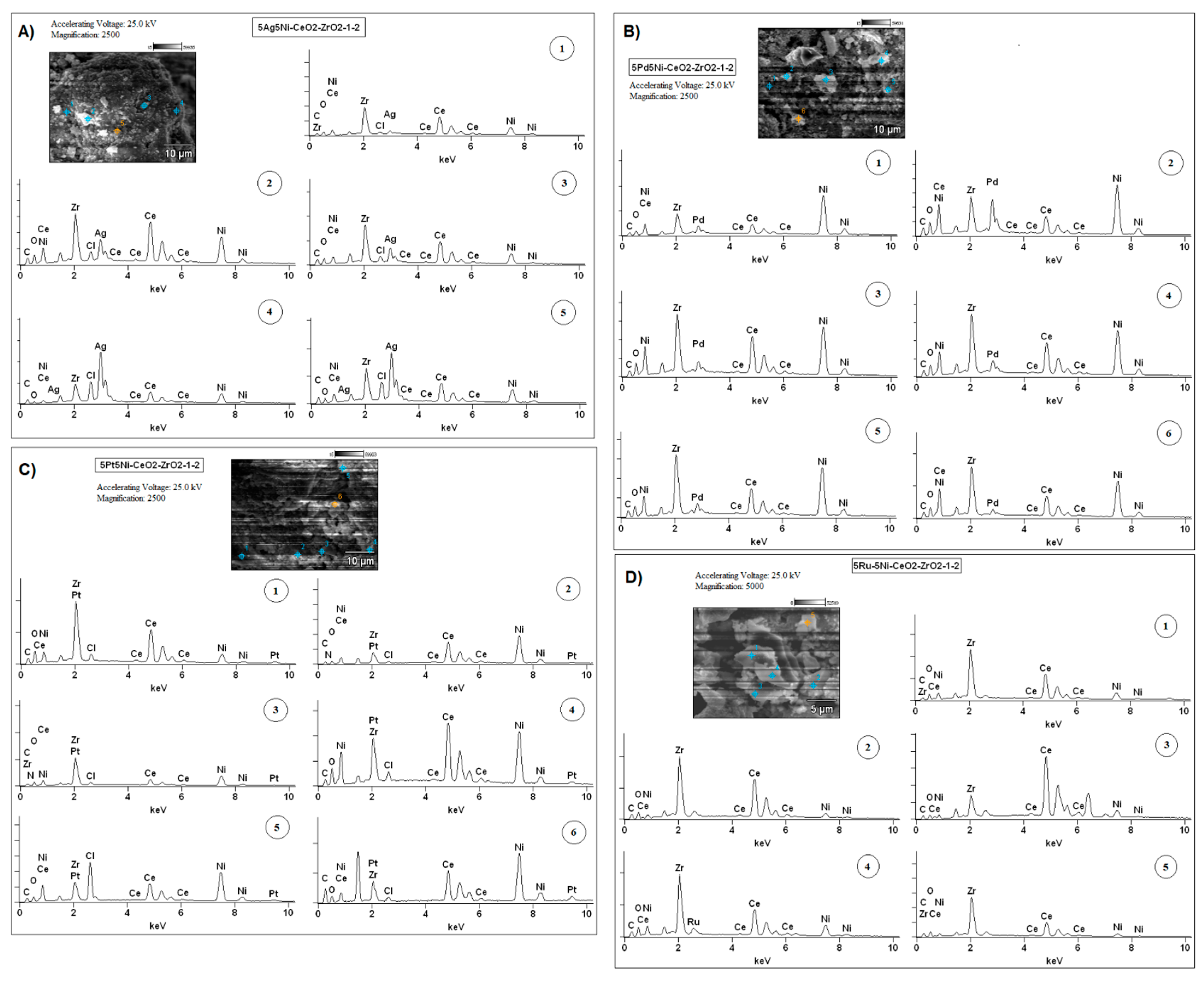

| Catalysts | Temperature (°C) | Methane Conversion (%) | Ethane Conversion (%) | Propane Conversion (%) | Butane Conversion (%) |
|---|---|---|---|---|---|
| 5% Ni/CeO2·ZrO2 (1:2) | 400 | 3 | 7 | 19 | 64 |
| 500 | 11 | 55 | 89 | 100 | |
| 600 | 79 | 100 | 100 | 100 | |
| 700 | 97 | 100 | 100 | 100 | |
| 800 | 90 | 100 | 100 | 100 | |
| 900 | 100 | 100 | 100 | 100 | |
| 1%Ru-5%Ni/CeO2·ZrO2 (1:2) | 400 | 2 | 49 | 98 | 100 |
| 500 | 6 | 54 | 91 | 100 | |
| 600 | 76 | 100 | 100 | 100 | |
| 700 | 98 | 100 | 100 | 100 | |
| 800 | 100 | 100 | 100 | 100 | |
| 900 | 100 | 100 | 100 | 100 | |
| 1%Pd-5% Ni/CeO2·ZrO2 (1:2) | 400 | 6 | 10 | 17 | 100 |
| 500 | 4 | 13 | 26 | 100 | |
| 600 | 38 | 99 | 100 | 100 | |
| 700 | 62 | 100 | 100 | 100 | |
| 800 | 89 | 100 | 100 | 100 | |
| 900 | 97 | 100 | 100 | 100 | |
| 1%Pt-5%Ni/CeO2·ZrO2 (1:2) | 400 | 8 | 70 | 96 | 100 |
| 500 | 26 | 100 | 100 | 100 | |
| 600 | 71 | 100 | 100 | 100 | |
| 700 | 96 | 100 | 100 | 100 | |
| 800 | 100 | 100 | 100 | 100 | |
| 900 | 100 | 100 | 100 | 100 | |
| 1%Ag-5% Ni/CeO2·ZrO2 (1:2) | 400 | 5 | 11 | 23 | 100 |
| 500 | 10 | 39 | 76 | 100 | |
| 600 | 13 | 37 | 63 | 100 | |
| 700 | 18 | 48 | 73 | 100 | |
| 800 | 29 | 72 | 91 | 100 | |
| 900 | 64 | 97 | 100 | 100 |
| Catalysts | Temperature (°C) | CO Selectivity (%) | CO2 Selectivity (%) | H2 Yield (%) |
|---|---|---|---|---|
| 5% Ni/CeO2·ZrO2 (1:2) | 400 | 0 | 100 | 0 |
| 500 | 0 | 100 | 0 | |
| 600 | 66 | 34 | 59 | |
| 700 | 82 | 18 | 60 | |
| 800 | 78 | 22 | 61 | |
| 900 | 86 | 14 | 58 | |
| 1%Ru-5%Ni/CeO2·ZrO2 (1:2) | 400 | 0 | 100 | 0 |
| 500 | 0 | 100 | 0 | |
| 600 | 50 | 50 | 60 | |
| 700 | 79 | 21 | 50 | |
| 800 | 87 | 13 | 57 | |
| 900 | 87 | 13 | 48 | |
| 1%Pd-5% Ni/CeO2·ZrO2 (1:2) | 400 | 0 | 100 | 0 |
| 500 | 0 | 100 | 0 | |
| 600 | 0 | 100 | 35 | |
| 700 | 29 | 71 | 47 | |
| 800 | 62 | 38 | 59 | |
| 900 | 71 | 29 | 57 | |
| 1%Pt-5%Ni/CeO2·ZrO2 (1:2) | 400 | 0 | 100 | 0 |
| 500 | 0 | 100 | 44 | |
| 600 | 52 | 48 | 60 | |
| 700 | 72 | 28 | 55 | |
| 800 | 84 | 16 | 52 | |
| 900 | 85 | 15 | 54 | |
| 1%Ag-5% Ni/CeO2·ZrO2 (1:2) | 400 | 0 | 100 | 0 |
| 500 | 0 | 100 | 0 | |
| 600 | 0 | 100 | 0 | |
| 700 | 3 | 97 | 57 | |
| 800 | 14 | 86 | 45 | |
| 900 | 73 | 27 | 56 |
| Catalysts | Peak Contribution to the Overall TPR Peak Area (%) | |||||
|---|---|---|---|---|---|---|
| I-Peak | II-Peak | III-Peak | IV-Peak | V-Peak | VI-Peak | |
| CeO2·ZrO2 (1:2) | - | - | - | 39 (350 °C) | 61 (540 °C) | - |
| 5%Ni/CeO2·ZrO2 (1:2) | - | - | 18 (230 °C) | 42 (340 °C) | 35 (435 °C) | 5 (750 °C) |
| 1%Ag-5%Ni/CeO2·ZrO2 (1:2) | 3 (75 °C) | 6 (140 °C) | 21 (180 °C) | 29 (325 °C) | 30 (440 °C) | 11 (725 °C) |
| 1%Ru-5%Ni/CeO2·ZrO2 (1:2) | 13 (115 °C) | 25 (180 °C) | 52 (290 °C) | 10 (435 °C) | - | |
| 1%Pt-5%Ni/CeO2·ZrO2 (1:2) | - | - | 45 (230 °C) | 36 (290 °C) | 19 (470 °C) | - |
| 1%Pd-5%Ni/CeO2·ZrO2 (1:2) | 18 (85 °C) | 16 (120 °C) | 28 (290 °C) | 23 (320 °C) | 15 (450 °C) | - |
| Catalytic Systems | Total Acidity (mmol/g) | Weak Centers (mmol/g) | Medium Centers (mmol/g) | Strong Centers (mmol/g) |
|---|---|---|---|---|
| 180–600 °C | 180–300 °C | 300–450 °C | 450–600 °C | |
| CeO2·ZrO2 (1:2) | 0.93 | 0.26 | 0.31 | 0.36 |
| 5%Ni/CeO2·ZrO2 (1:2) | 0.51 | 0.20 | 0.21 | 0.09 |
| 1%Ru-5%Ni/CeO2·ZrO2 (1:2) | 0.52 | 0.32 | 0.18 | 0.01 |
| 1%Pd-5%Ni/CeO2·ZrO2 (1:2) | 0.42 | 0.22 | 0.19 | 0.02 |
| 1%Pt-5%Ni/CeO2·ZrO2 (1:2) | 0.24 | 0.19 | 0.03 | - |
| 1%Ag-5%Ni/CeO2·ZrO2 (1:2) | 0.36 | 0.32 | 0.04 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mierczynski, P.; Mosinska, M.; Maniukiewicz, W.; Vasilev, K.; Szynkowska-Jozwik, M.I. Oxy-Steam Reforming of Liquefied Natural Gas (LNG) on Mono- and Bimetallic (Ag, Pt, Pd or Ru)/Ni Catalysts. Catalysts 2021, 11, 1401. https://doi.org/10.3390/catal11111401
Mierczynski P, Mosinska M, Maniukiewicz W, Vasilev K, Szynkowska-Jozwik MI. Oxy-Steam Reforming of Liquefied Natural Gas (LNG) on Mono- and Bimetallic (Ag, Pt, Pd or Ru)/Ni Catalysts. Catalysts. 2021; 11(11):1401. https://doi.org/10.3390/catal11111401
Chicago/Turabian StyleMierczynski, Pawel, Magdalena Mosinska, Waldemar Maniukiewicz, Krasimir Vasilev, and Malgorzata Iwona Szynkowska-Jozwik. 2021. "Oxy-Steam Reforming of Liquefied Natural Gas (LNG) on Mono- and Bimetallic (Ag, Pt, Pd or Ru)/Ni Catalysts" Catalysts 11, no. 11: 1401. https://doi.org/10.3390/catal11111401