Influence of NiO/La2O3 Catalyst Preparation Method on Its Reactivity in the Oxy-Steam Reforming of LNG Process
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalytic Activity Tests
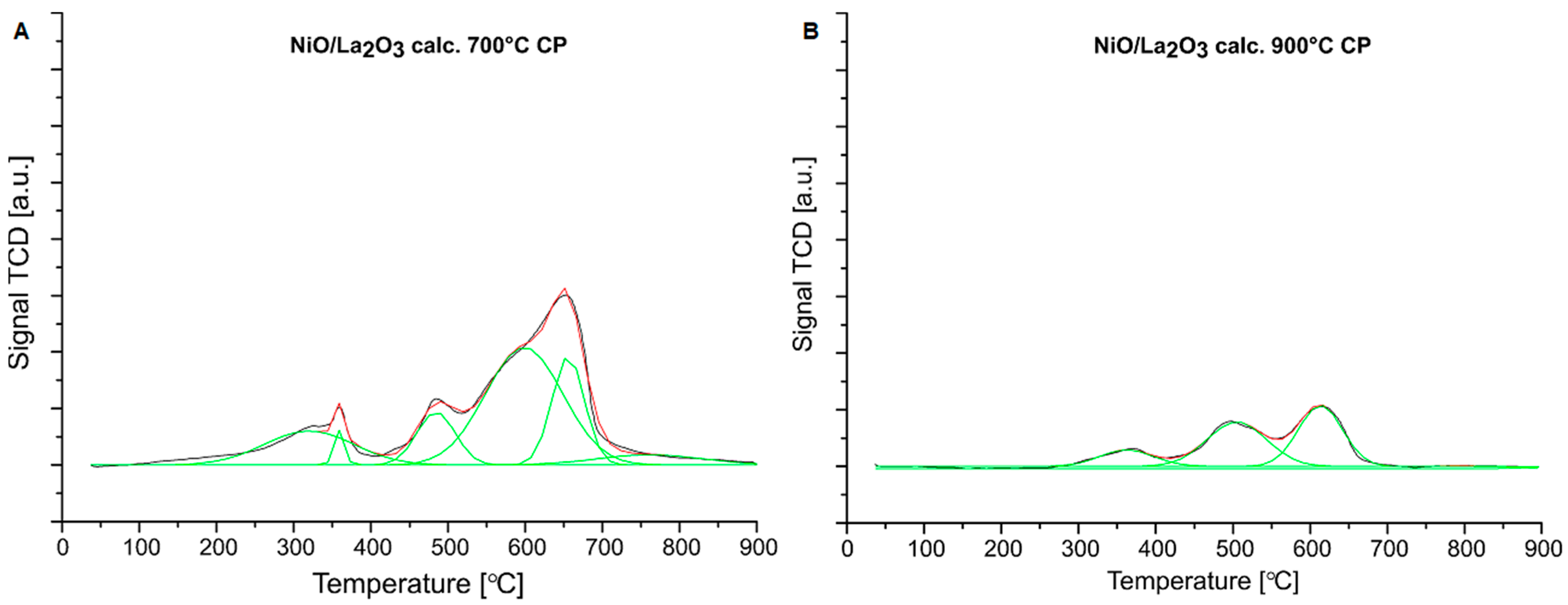
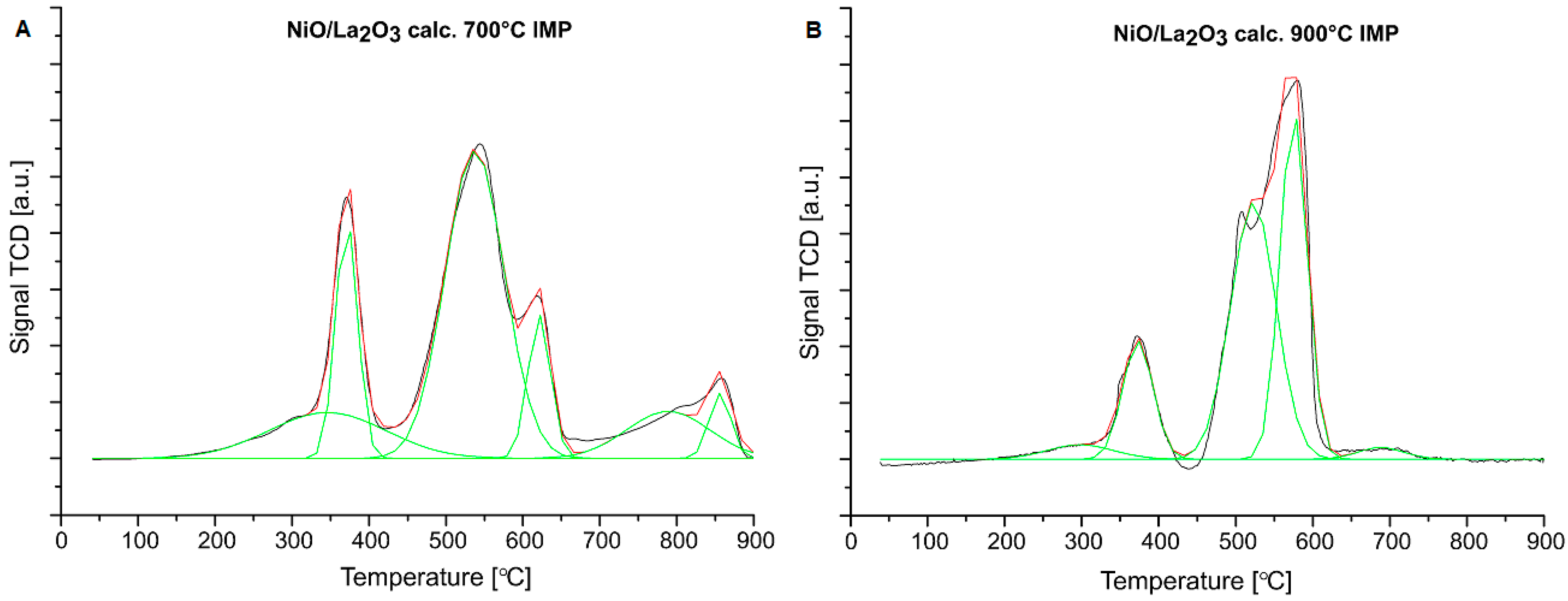
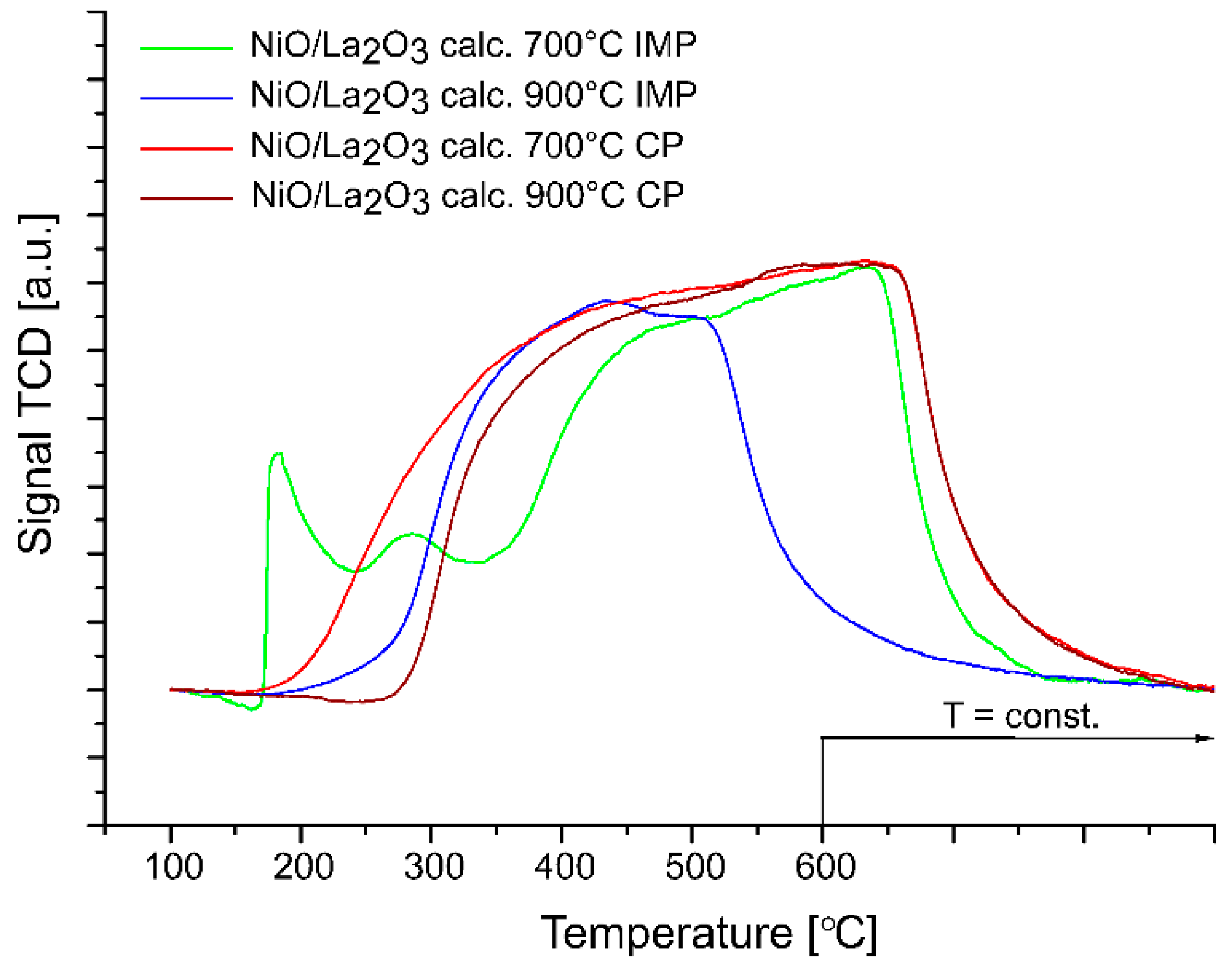
2.2. Reduction Behavior of the Investigated Catalysts
2.3. Specific Surface Area Measurements of Investigated Catalysts
2.4. Acidity Measurements of Investigated Catalysts
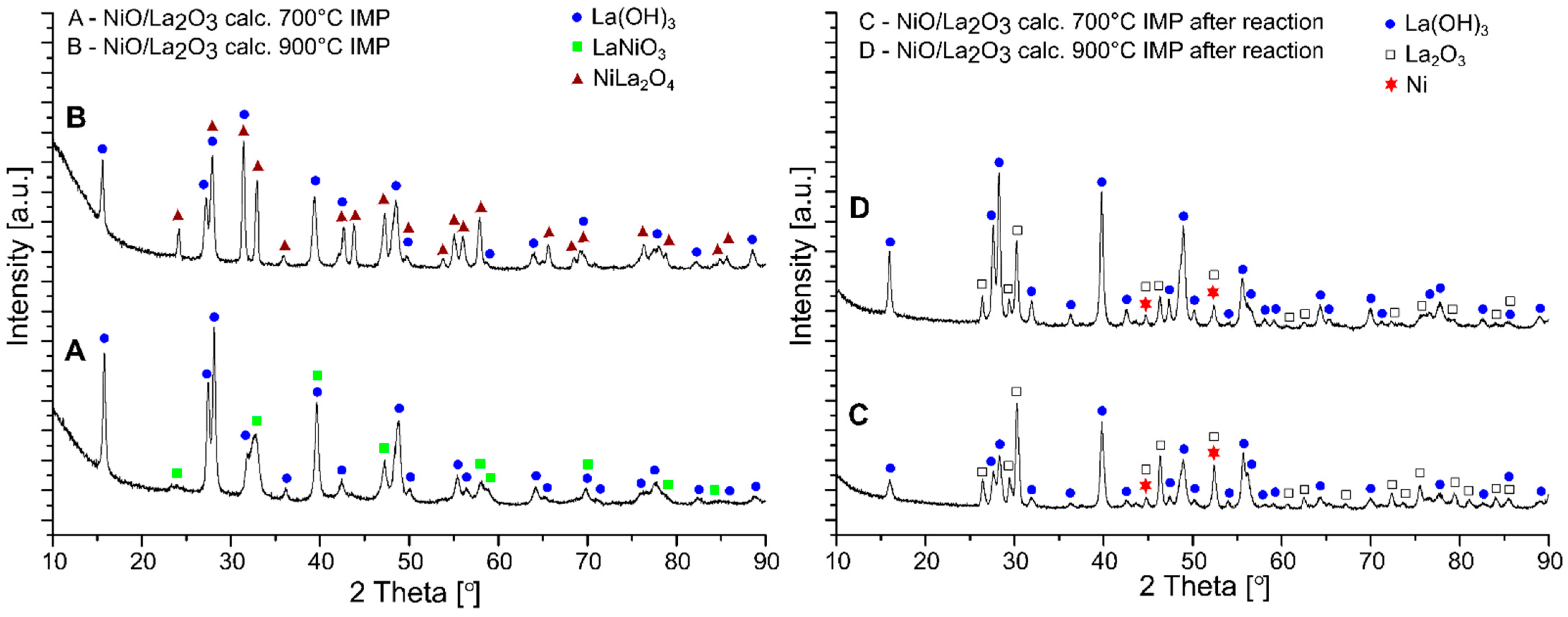
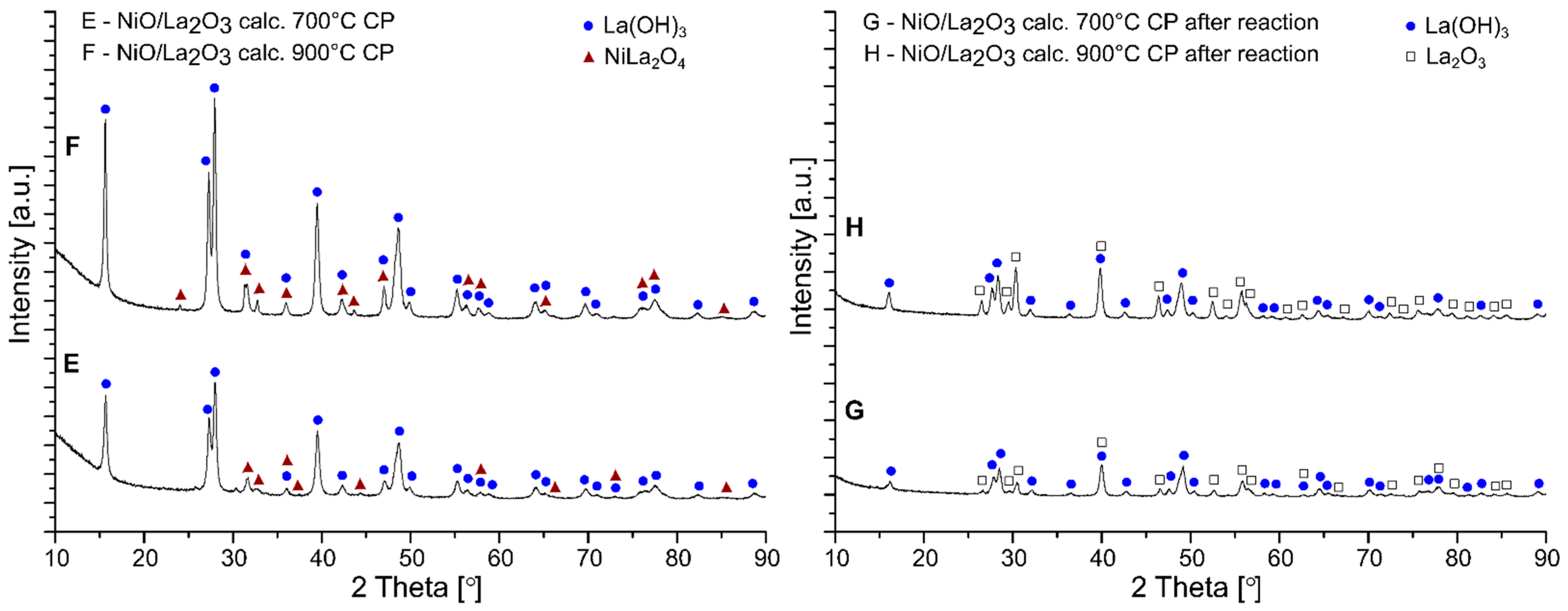
2.5. Phase Composition Studies of Investigated Catalysts
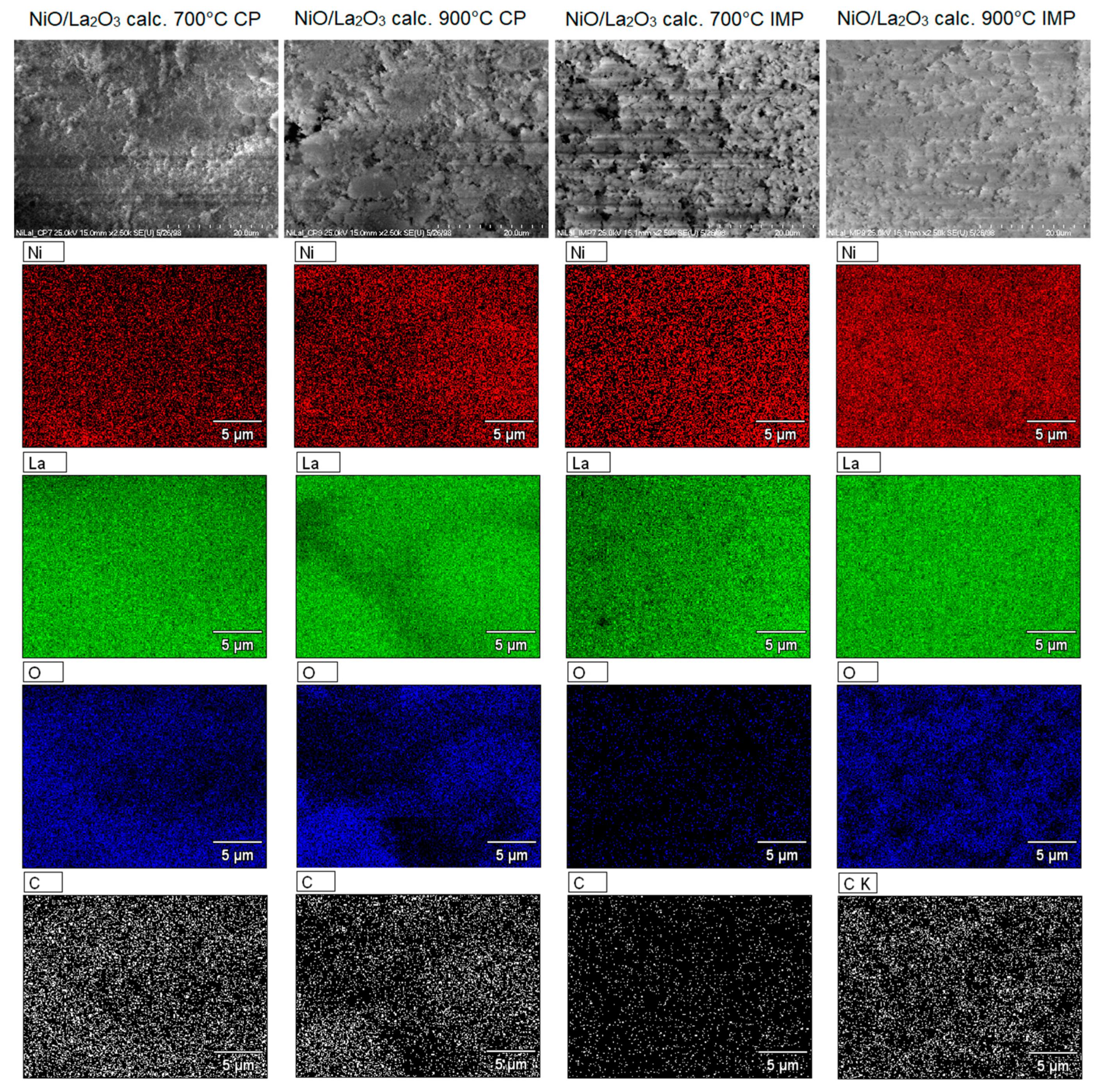
2.6. SEM-EDS Measurements of Investigated Catalysts
3. Materials and Methods
3.1. Supports and Catalysts Preparation
3.2. Characterization of the Catalytic Materials
3.3. Catalytic Activity Measurements in Oxy-Steam Reforming of the Liqufied Natural Gas Reaction (OSR-LNG)
- —the moles of hydrocarbon at the reactor inlet;
- —the moles of hydrocarbon at the reactor outlet;
- —the moles of the CO at the reactor outlet;
- —the moles of the CO2 at the reactor outlet;
- —the moles of the H2 at the reactor outlet;
- —the sum of the moles of the hydrocarbons the reactor inlet;
- —the sum of the moles of the hydrocarbons at the reactor outlet.
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schwarz, J.A.; Contescu, C.; Contescu, A. Methods for Preparation of Catalytic Materials. Chem. Rev. 1995, 95, 477–510. [Google Scholar] [CrossRef]
- Irandoust, A.; Haghtalab, A. A Hybrid Reduction–Impregnation Method in Preparation of Co–Ru/γ-Al2O3 Catalyst for Fischer–Tropsch Synthesis. Catal. Lett. 2017, 147, 2967–2981. [Google Scholar] [CrossRef]
- Sietsma, J.R.A.; Jos van Dillen, A.; de Jongh, P.E.; de Jong, K.P. Application of ordered mesoporous materials as model supports to study catalyst preparation by impregnation and drying. In Studies in Surface Science and Catalysis; Gaigneaux, E.M., Devillers, M., De Vos, D.E., Hermans, S., Jacobs, P.A., Martens, J.A., Ruiz, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 162, pp. 95–102. [Google Scholar]
- Yamada, Y.; Akita, T.; Ueda, A.; Shioyama, H.; Kobayashi, T. Instruments for preparation of heterogeneous catalysts by an impregnation method. Rev. Sci. Instrum. 2005, 76, 62226. [Google Scholar] [CrossRef]
- Li, G.; Hu, L.; Hill, J.M. Comparison of reducibility and stability of alumina-supported Ni catalysts prepared by impregnation and co-precipitation. Appl. Catal. A Gen. 2006, 301, 16–24. [Google Scholar] [CrossRef]
- Liu, F.; Xu, S.; Cao, L.; Chi, Y.; Zhang, T.; Xue, D. A Comparison of NiMo/Al2O3 Catalysts Prepared by Impregnation and Coprecipitation Methods for Hydrodesulfurization of Dibenzothiophene. J. Phys. Chem. C 2007, 111, 7396–7402. [Google Scholar] [CrossRef]
- Pereira, A.L.C.; Berrocal, G.J.P.; Marchetti, S.G.; Albornoz, A.; de Souza, A.O.; Rangel, M.d.C. A comparison between the precipitation and impregnation methods for water gas shift catalysts. J. Mol. Catal. A Chem. 2008, 281, 66–72. [Google Scholar] [CrossRef]
- Talati, A.; Haghighi, M.; Rahmani, F. Impregnation vs. coprecipitation dispersion of Cr over TiO2 and ZrO2 used as active and stable nanocatalysts in oxidative dehydrogenation of ethane to ethylene by carbon dioxide. RSC Adv. 2016, 6, 44195–44204. [Google Scholar] [CrossRef]
- Oemar, U.; Ang, M.L.; Hidajat, K.; Kawi, S. Enhancing performance of Ni/La2O3 catalyst by Sr-modification for steam reforming of toluene as model compound of biomass tar. RSC Adv. 2015, 5, 17834–17842. [Google Scholar] [CrossRef]
- Oemar, U.; Hidajat, K.; Kawi, S. Role of catalyst support over PdO–NiO catalysts on catalyst activity and stability for oxy-CO2 reforming of methane. Appl. Catal. A Gen. 2011, 402, 176–187. [Google Scholar] [CrossRef]
- Yu, Y.; Gan, Y.-M.; Huang, C.; Lu, Z.-H.; Wang, X.; Zhang, R.; Feng, G. Ni/La2O3 and Ni/MgO–La2O3 catalysts for the decomposition of NH3 into hydrogen. Int. J. Hydrog. Energy 2020, 45, 16528–16539. [Google Scholar] [CrossRef]
- Kathiraser, Y.; Thitsartarn, W.; Sutthiumporn, K.; Kawi, S. Inverse NiAl2O4 on LaAlO3–Al2O3: Unique Catalytic Structure for Stable CO2 Reforming of Methane. J. Phys. Chem. C 2013, 117, 8120–8130. [Google Scholar] [CrossRef]
- Barbero, J.; Peña, M.A.; Campos-Martin, J.M.; Fierro, J.L.G.; Arias, P.L. Support Effect in Supported Ni Catalysts on Their Performance for Methane Partial Oxidation. Catal. Lett. 2003, 87, 211–218. [Google Scholar] [CrossRef]
- Requies, J.; Cabrero, M.A.; Barrio, V.L.; Güemez, M.B.; Cambra, J.; Arias, P.L.; Pérez-Alonso, F.J.; Ojeda, M.; Peña, M.A.; Fierro, J.L.G. Partial oxidation of methane to syngas over Ni/MgO and Ni/La2O3 catalysts. Appl. Catal. A Gen. 2005, 289, 214–223. [Google Scholar] [CrossRef]
- Messaoudi, H.; Thomas, S.; Slyemi, S.; Djaidja, A.; Barama, A. Syngas Production via Methane Dry Reforming over La-Ni-Co and La-Ni-Cu Catalysts with Spinel and Perovskite Structures. Bull. Chem. React. Eng. Catal. 2020, 15, 885–897. [Google Scholar] [CrossRef]
- Messaoudi, H.; Thomas, S.; Djaidja, A.; Slyemi, S.; Barama, A. Study of LaxNiOy and LaxNiOy/MgAl2O4 catalysts in dry reforming of methane. J. CO2 Util. 2018, 24, 40–49. [Google Scholar] [CrossRef]
- Arandia, A.; Remiro, A.; García, V.; Castaño, P.; Bilbao, J.; Gayubo, A.G. Oxidative Steam Reforming of Raw Bio-Oil over Supported and Bulk Ni Catalysts for Hydrogen Production. Catalysts 2018, 8, 322. [Google Scholar] [CrossRef] [Green Version]
- Maneerung, T.; Hidajat, K.; Kawi, S. LaNiO3 perovskite catalyst precursor for rapid decomposition of methane: Influence of temperature and presence of H2 in feed stream. Catal. Today 2011, 171, 24–35. [Google Scholar] [CrossRef]
- Lin, K.-H.; Wang, C.-B.; Chien, S.-H. Catalytic performance of steam reforming of ethanol at low temperature over LaNiO3 perovskite. Int. J. Hydrog. Energy 2013, 38, 3226–3232. [Google Scholar] [CrossRef]
- Moradi, G.R.; Rahmanzadeh, M.; Khosravian, F. The effects of partial substitution of Ni by Zn in LaNiO3 perovskite catalyst for methane dry reforming. J. CO2 Util. 2014, 6, 7–11. [Google Scholar] [CrossRef]
- Wei, T.; Liu, B.; Jia, L.; Li, R. Perovskite materials for highly efficient catalytic CH4 fuel reforming in solid oxide fuel cell. Int. J. Hydrog. Energy 2021, 46, 24441–24460. [Google Scholar] [CrossRef]
- Li, P.; Yu, B.; Li, J.; Yao, X.; Zhao, Y.; Li, Y. A single layer solid oxide fuel cell composed of La2NiO4 and doped ceria-carbonate with H2 and methanol as fuels. Int. J. Hydrog. Energy 2016, 41, 9059–9065. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Zhang, J.; Zhang, X. Comparative study of LaNiO3 and La2NiO4 catalysts for partial oxidation of methane. React. Kinet. Catal. Lett. 2008, 95, 89. [Google Scholar] [CrossRef]
- Fleys, M.; Simon, Y.; Swierczynski, D.; Kiennemann, A.; Marquaire, P.-M. Investigation of the Reaction of Partial Oxidation of Methane over Ni/La2O3 Catalyst. Energy Fuels 2006, 20, 2321–2329. [Google Scholar] [CrossRef]
- Khalfaoui, M.; Knani, S.; Hachicha, M.A.; Lamine, A.B. New theoretical expressions for the five adsorption type isotherms classified by BET based on statistical physics treatment. J. Colloid Interface Sci. 2003, 263, 350–356. [Google Scholar] [CrossRef]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface area and pore texture of catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Muroyama, H.; Saburi, C.; Matsui, T.; Eguchi, K. Ammonia decomposition over Ni/La2O3 catalyst for on-site generation of hydrogen. Appl. Catal. A Gen. 2012, 443, 119–124. [Google Scholar] [CrossRef]



| Catalysts | Temp [°C] | Methane Conversion [%] | Ethane Conversion [%] | Propane Conversion [%] | Butane Conversion [%] |
|---|---|---|---|---|---|
| NiO/La2O3 calc. 700 °C CP | 400 | 31 | 34 | 37 | 44 |
| 500 | 37 | 60 | 78 | 91 | |
| 600 | 43 | 68 | 85 | 95 | |
| 700 | 45 | 70 | 88 | 100 | |
| 900 | 60 | 93 | 100 | 100 | |
| NiO/La2O3 calc. 900 °C CP | 400 | 34 | 36 | 38 | 36 |
| 500 | 31 | 38 | 44 | 48 | |
| 600 | 38 | 64 | 79 | 91 | |
| 700 | 46 | 69 | 86 | 93 | |
| 900 | 57 | 86 | 99 | 100 | |
| NiO/La2O3 calc. 700 °C IMP | 400 | 33 | 34 | 40 | 44 |
| 500 | 35 | 43 | 55 | 68 | |
| 600 | 38 | 57 | 73 | 87 | |
| 700 | 64 | 87 | 96 | 100 | |
| 900 | 100 | 100 | 100 | 100 | |
| NiO/La2O3 calc. 900 °C IMP | 400 | 33 | 36 | 39 | 46 |
| 500 | 35 | 44 | 54 | 68 | |
| 600 | 38 | 60 | 77 | 83 | |
| 700 | 39 | 62 | 81 | 100 | |
| 900 | 100 | 100 | 100 | 100 |
| Catalysts | Temp [°C] | CO Selectivity [%] | CO2 Selectivity [%] | H2 Yield [%] |
|---|---|---|---|---|
| NiO/La2O3 calc. 700 °C CP | 400 | 0 | 100 | 0 |
| 500 | 0 | 100 | 4 | |
| 600 | 0 | 100 | 4 | |
| 700 | 0 | 100 | 11 | |
| 900 | 71 | 29 | 46 | |
| NiO/La2O3 calc. 900 °C CP | 400 | 0 | 100 | 0 |
| 500 | 0 | 100 | 0 | |
| 600 | 0 | 100 | 3 | |
| 700 | 0 | 100 | 2 | |
| 900 | 74 | 26 | 39 | |
| NiO/La2O3 calc. 700 °C IMP | 400 | 0 | 100 | 0 |
| 500 | 0 | 100 | 0 | |
| 600 | 0 | 100 | 0 | |
| 700 | 65 | 35 | 29 | |
| 900 | 90 | 10 | 56 | |
| NiO/La2O3 calc. 900 °C IMP | 400 | 0 | 100 | 0 |
| 500 | 0 | 100 | 0 | |
| 600 | 0 | 100 | 1 | |
| 700 | 0 | 100 | 20 | |
| 900 | 98 | 2 | 54 |
| Catalysts | Peak Contribution to the Overall TPR Peak Area (%) | |||||
|---|---|---|---|---|---|---|
| I-Peak (Tmax) | II-Peak (Tmax) | III-Peak (Tmax) | IV-Peak (Tmax) | V-Peak (Tmax) | VI-Peak (Tmax) | |
| NiO/La2O3 calc. 700 °C CP | 0.16 (325 °C) | 0.02 (360 °C) | 0.11 (487 °C) | 0.47 (585 °C) | 0.18 (656 °C) | 0.06 (807 °C) |
| NiO/La2O3 calc. 900 °C CP | - | 0.14 (371 °C) | 0.43 (506 °C) | 0.43 (614 °C) | - | - |
| NiO/La2O3 calc. 700 °C IMP | 0.14 (311 °C) | 0.14 (369 °C) | 0.49 (548 °C) | 0.09 (626 °C) | 0.10 (807 °C) | 0.04 (862 °C) |
| NiO/La2O3 calc. 900 °C IMP | 0.04 (355 °C) | 0.14 (377 °C) | 0.44 (512 °C) | 0.36 (582 °C) | 0.02 (713 °C) | - |
| Materials | BET Surface Area [m2/g] | Monolayer Capacity [cm3/g] | Average Pore Radius [nm] |
|---|---|---|---|
| La2O3 calc. 700 °C | 0.44 | 0.002 | 19.93 |
| NiO/La2O3 calc. 700 °C CP | 24.41 | 0.144 | 11.94 |
| NiO/La2O3 calc. 900 °C CP | 7.89 | 0.034 | 8.43 |
| NiO/La2O3 calc. 700 °C IMP | 4.99 | 0.035 | 16.31 |
| NiO/La2O3 calc. 900 °C IMP | 3.05 | 0.016 | 13.08 |
| Materials | Total Acidity [mmol/g] | Weak Centers [mmol/g] | Medium Centers [mmol/g] | Strong Centers [mmol/g] |
|---|---|---|---|---|
| 180–600 °C | 180–300 °C | 300–450 °C | 450–600 °C | |
| La2O3 calc. 700 °C | 0.15 | 0.02 | 0.11 | 0.02 |
| NiO/La2O3 calc. 700 °C CP | 3.20 | 0.33 | 1.23 | 1.64 |
| NiO/La2O3 calc. 900 °C CP | 3.73 | 0.02 | 1.24 | 2.47 |
| NiO/La2O3 calc. 700 °C IMP | 3.14 | 0.47 | 1.06 | 1.61 |
| NiO/La2O3 calc. 900 °C IMP | 2.12 | 0.16 | 1.28 | 0.68 |
| Materials | Percentage Contribution of the Phases [wt.%] | ||
|---|---|---|---|
| After calcination | NiO/La2O3 calc. 700 °C CP | La(OH)3 | 93 |
| NiLa2O4 | 7 | ||
| NiO/La2O3 calc. 900 °C CP | La(OH)3 | 88 | |
| NiLa2O4 | 12 | ||
| NiO/La2O3 calc. 700 °C IMP | La(OH)3 | 69 | |
| LaNiO3 | 31 | ||
| NiO/La2O3 calc. 900 °C IMP | La(OH)3 | 45 | |
| NiLa2O4 | 55 | ||
| After OSR-LNG reaction | NiO/La2O3 calc. 700 °C CP | La(OH)3 | 60 |
| La2O3 | 40 | ||
| NiO/La2O3 calc. 900 °C CP | La(OH)3 | 45 | |
| La2O3 | 55 | ||
| NiO/La2O3 calc. 700 °C IMP | La(OH)3 | 34 | |
| La2O3 | 59 | ||
| Ni | 7 | ||
| NiO/La2O3 calc. 900 °C IMP | La(OH)3 | 55 | |
| La2O3 | 39 | ||
| Ni | 6 | ||
| Catalysts | The Size of Crystallites [nm] | ||
|---|---|---|---|
| Ni | La2O3 | La(OH)3 | |
| NiO/La2O3 calc. 700 °C IMP after OSR-LNG reaction | 22 | 48 | 27 |
| NiO/La2O3 calc. 900 °C IMP after OSR-LNG reaction | 27 | 58 | 26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosinska, M.; Maniukiewicz, W.; Szynkowska-Jozwik, M.I.; Mierczynski, P. Influence of NiO/La2O3 Catalyst Preparation Method on Its Reactivity in the Oxy-Steam Reforming of LNG Process. Catalysts 2021, 11, 1174. https://doi.org/10.3390/catal11101174
Mosinska M, Maniukiewicz W, Szynkowska-Jozwik MI, Mierczynski P. Influence of NiO/La2O3 Catalyst Preparation Method on Its Reactivity in the Oxy-Steam Reforming of LNG Process. Catalysts. 2021; 11(10):1174. https://doi.org/10.3390/catal11101174
Chicago/Turabian StyleMosinska, Magdalena, Waldemar Maniukiewicz, Malgorzata I. Szynkowska-Jozwik, and Pawel Mierczynski. 2021. "Influence of NiO/La2O3 Catalyst Preparation Method on Its Reactivity in the Oxy-Steam Reforming of LNG Process" Catalysts 11, no. 10: 1174. https://doi.org/10.3390/catal11101174
APA StyleMosinska, M., Maniukiewicz, W., Szynkowska-Jozwik, M. I., & Mierczynski, P. (2021). Influence of NiO/La2O3 Catalyst Preparation Method on Its Reactivity in the Oxy-Steam Reforming of LNG Process. Catalysts, 11(10), 1174. https://doi.org/10.3390/catal11101174








