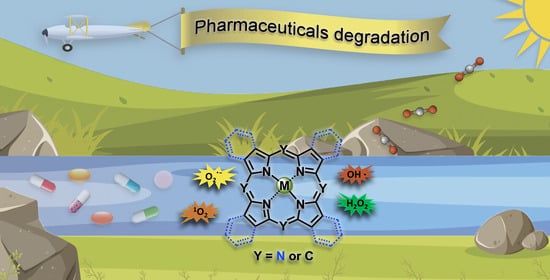
Oxidative Degradation of Pharmaceuticals: The Role of Tetrapyrrole-Based Catalysts
Abstract
:1. Introduction
Oxidation Mechanisms Using Tetrapyrrolic Macrocycle-Based Catalysts
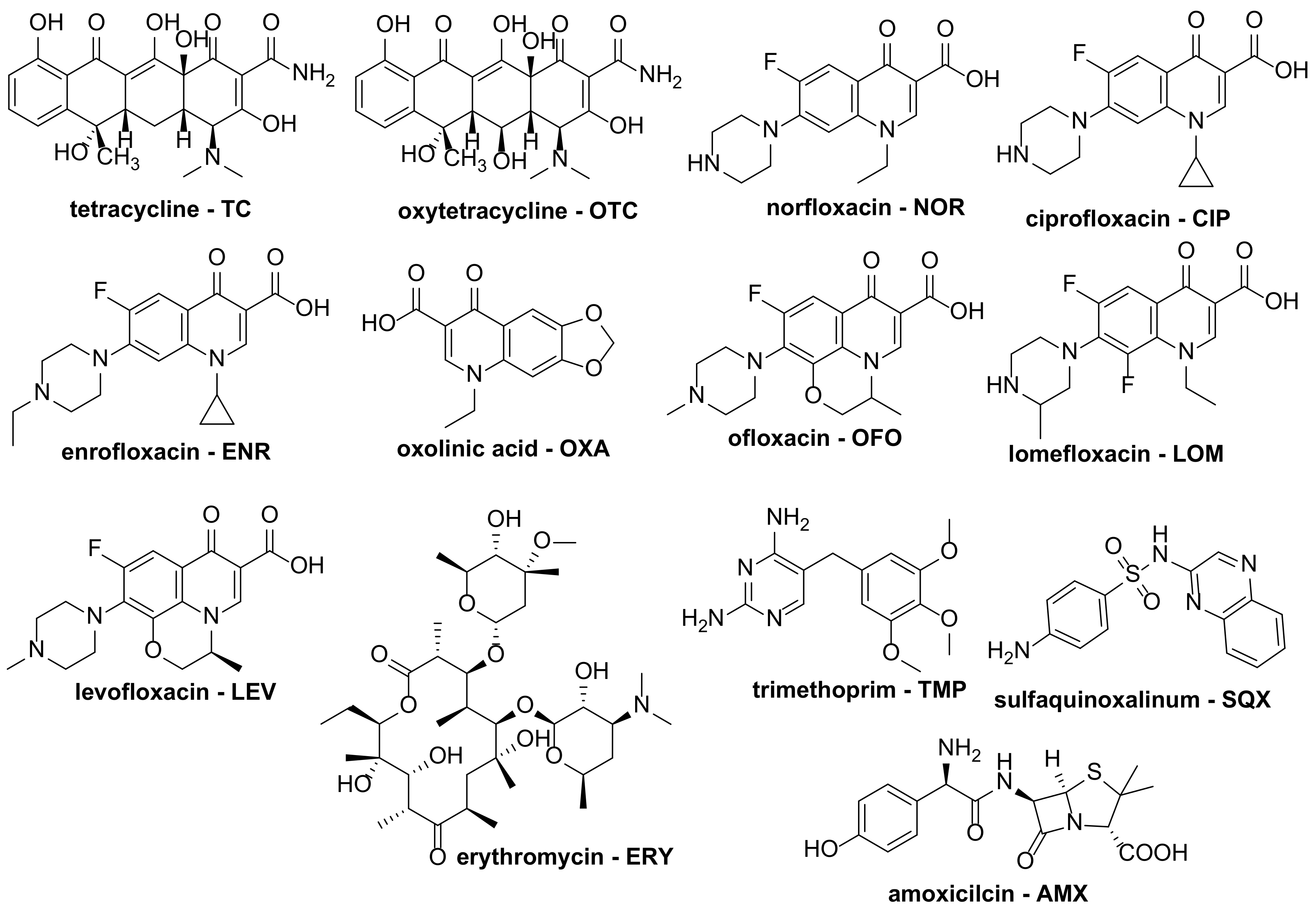
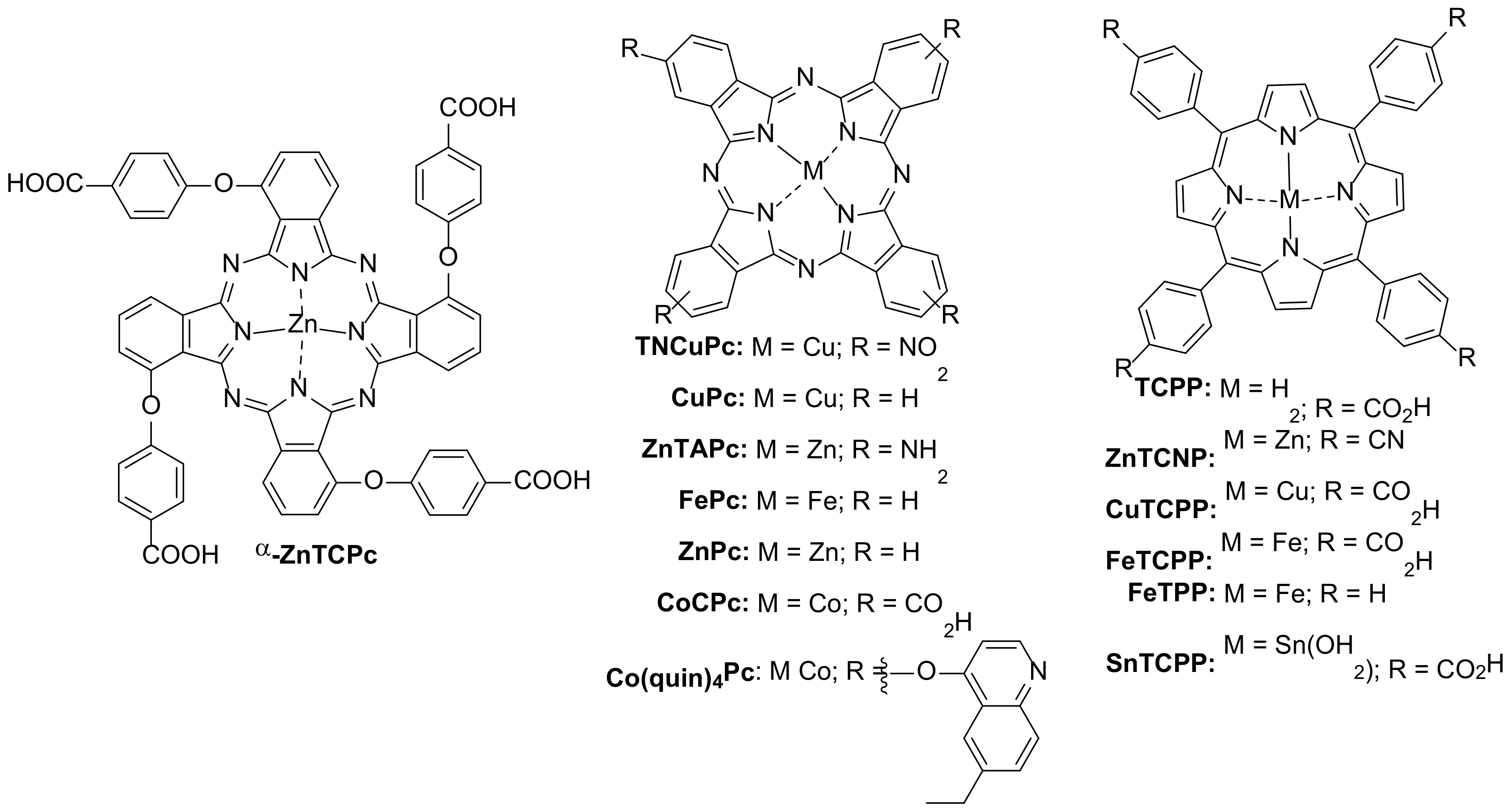
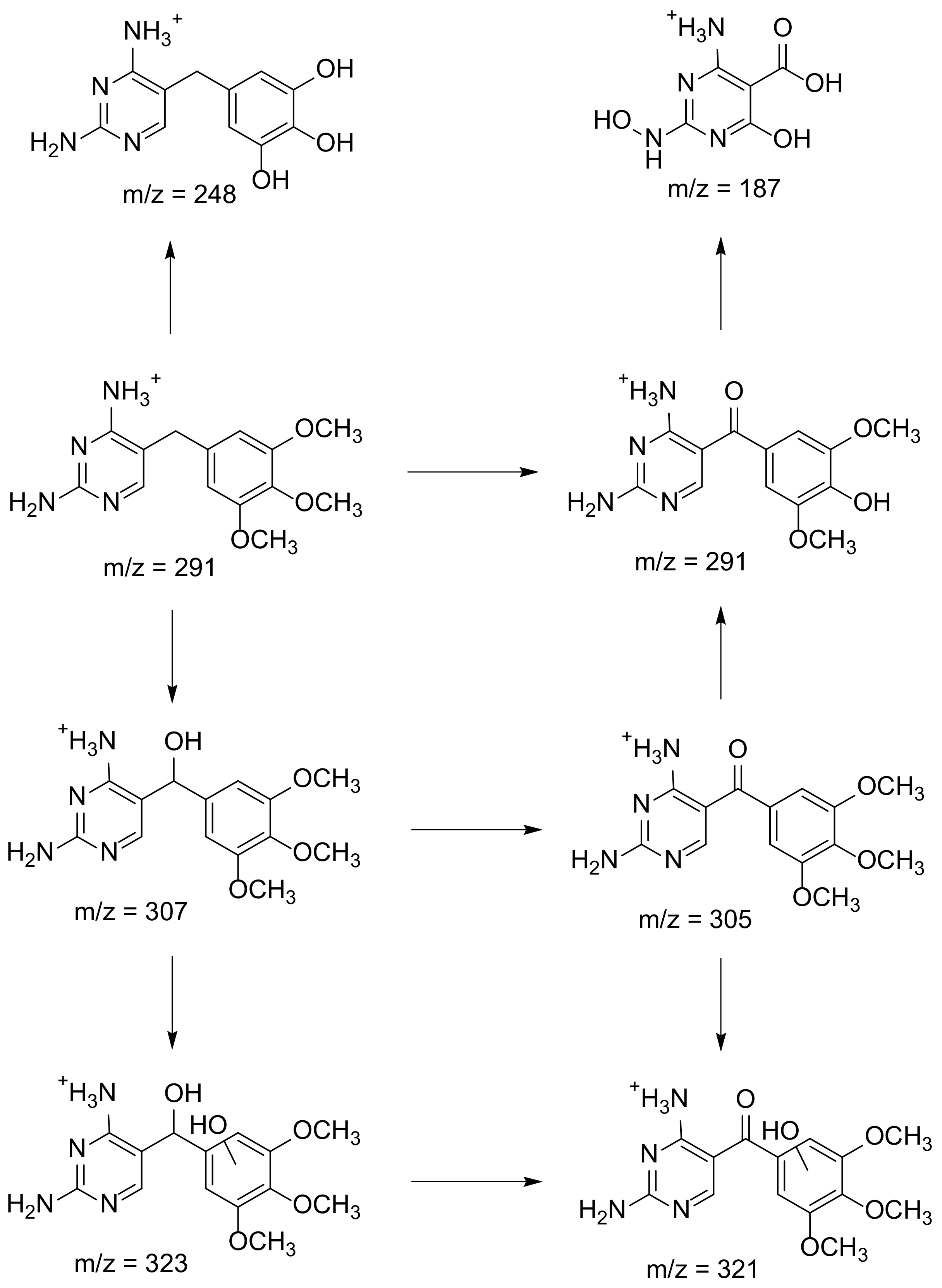
2. Degradation of Antibiotics
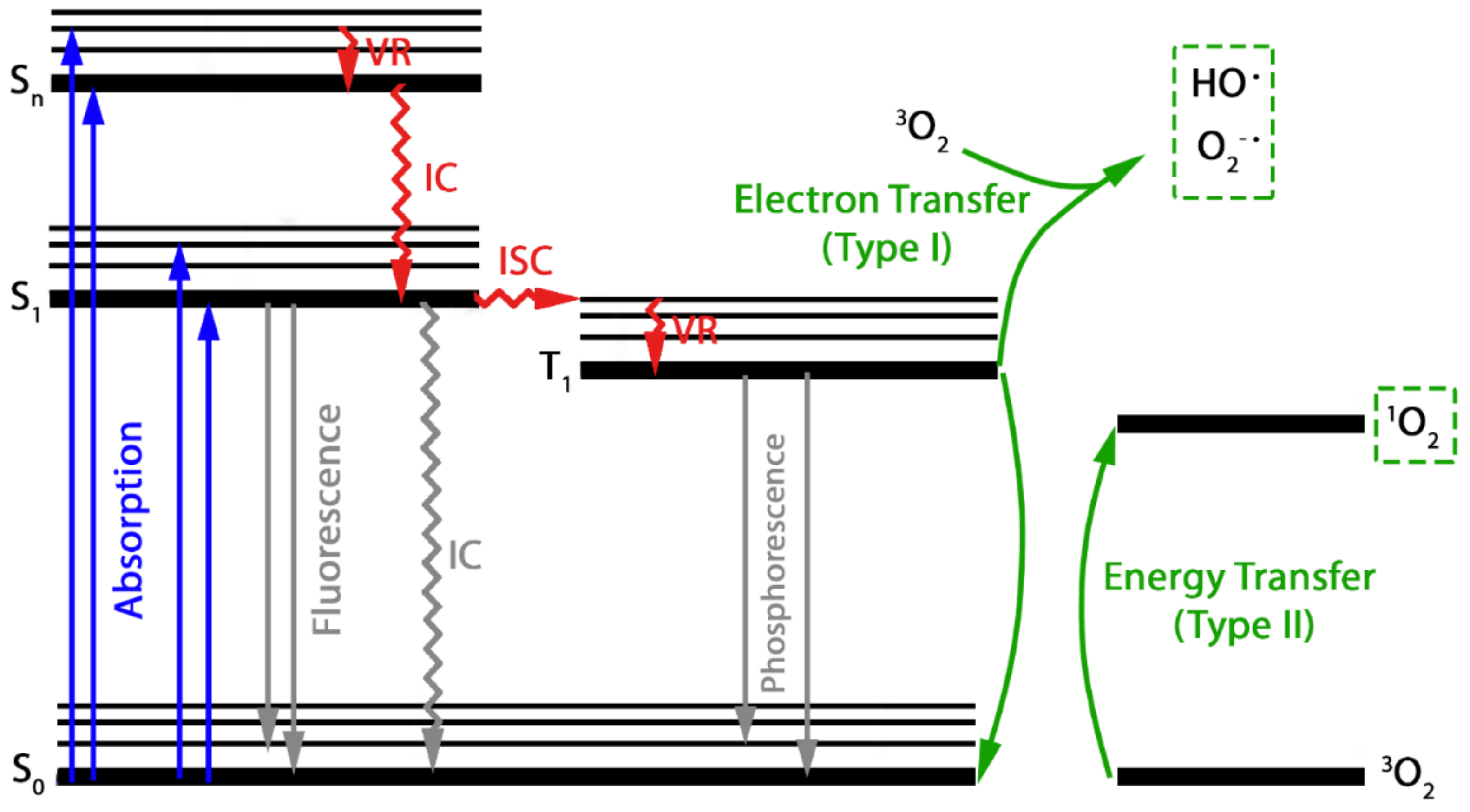
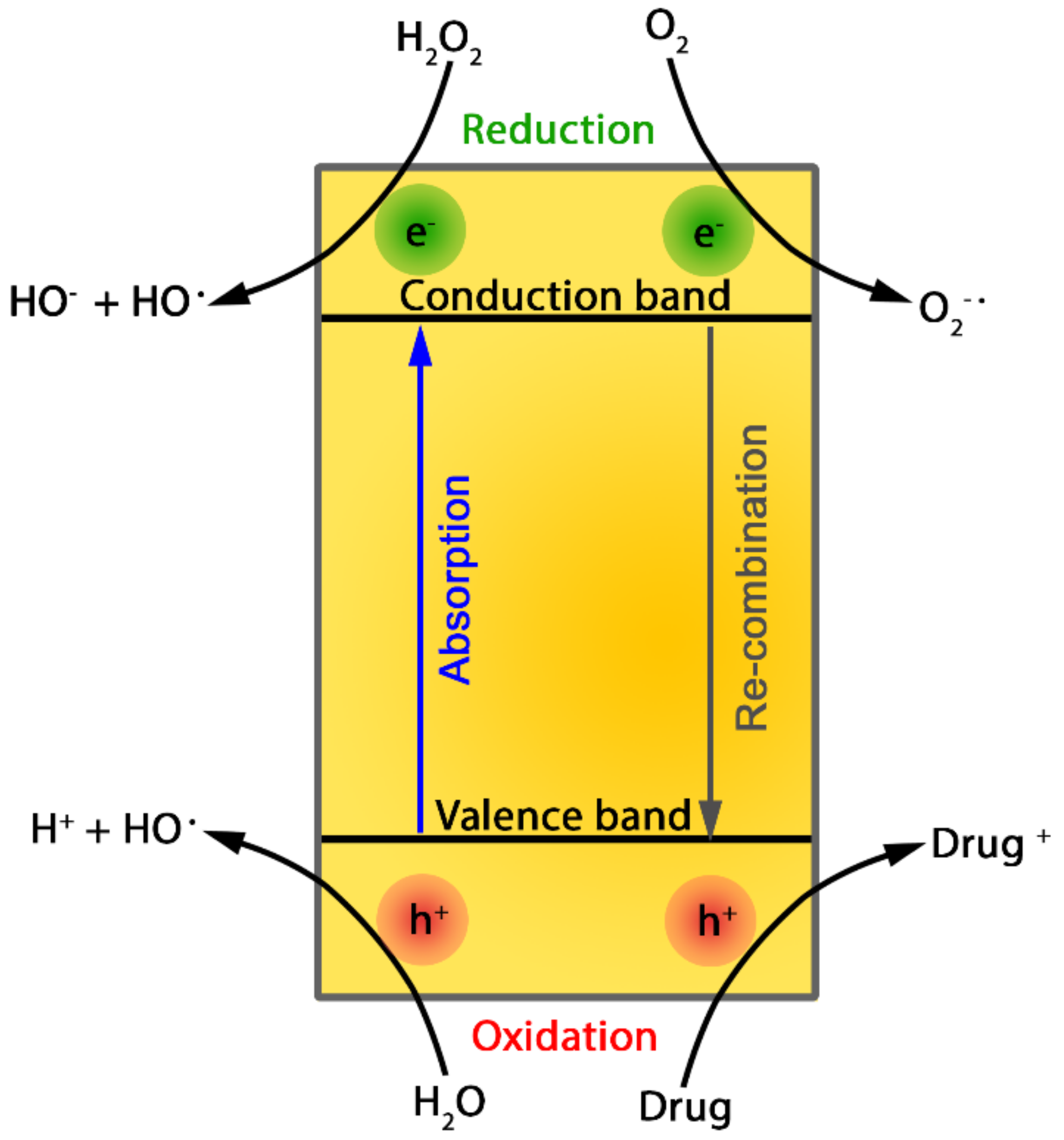
2.1. Photochemical Degradation
| #/Ref | Catalyst | Drug | Experimental Conditions | Comments |
|---|---|---|---|---|
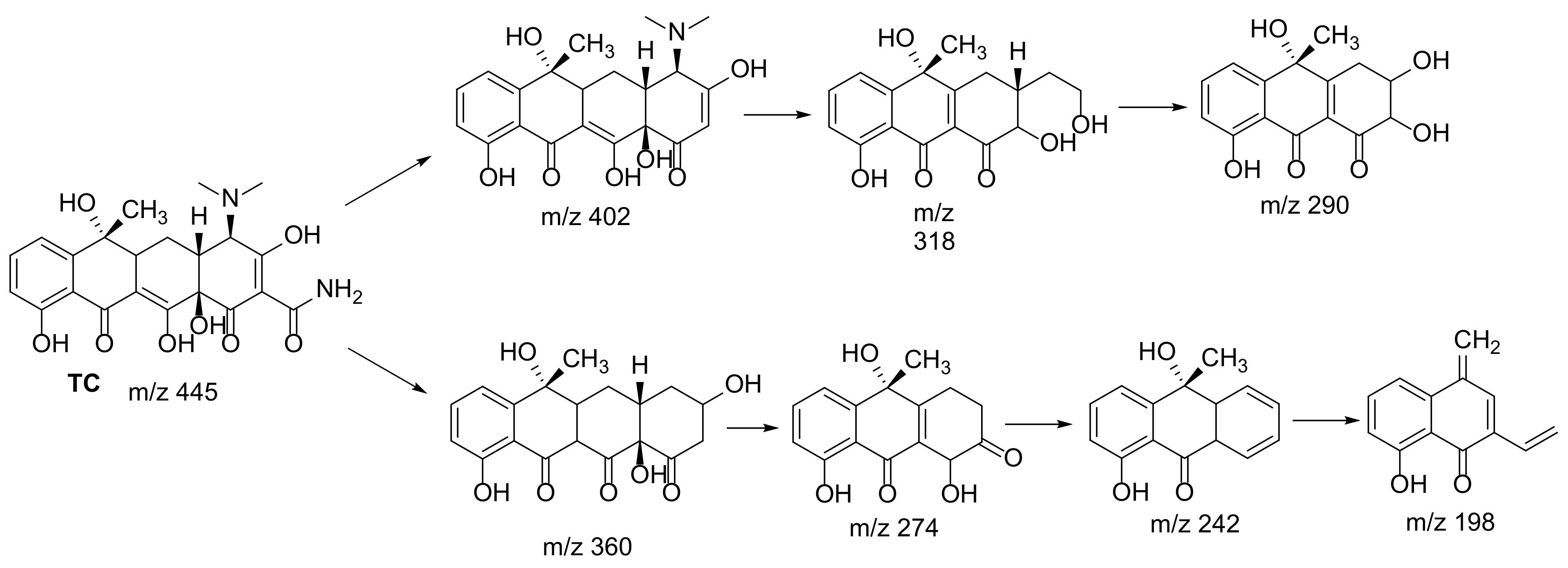
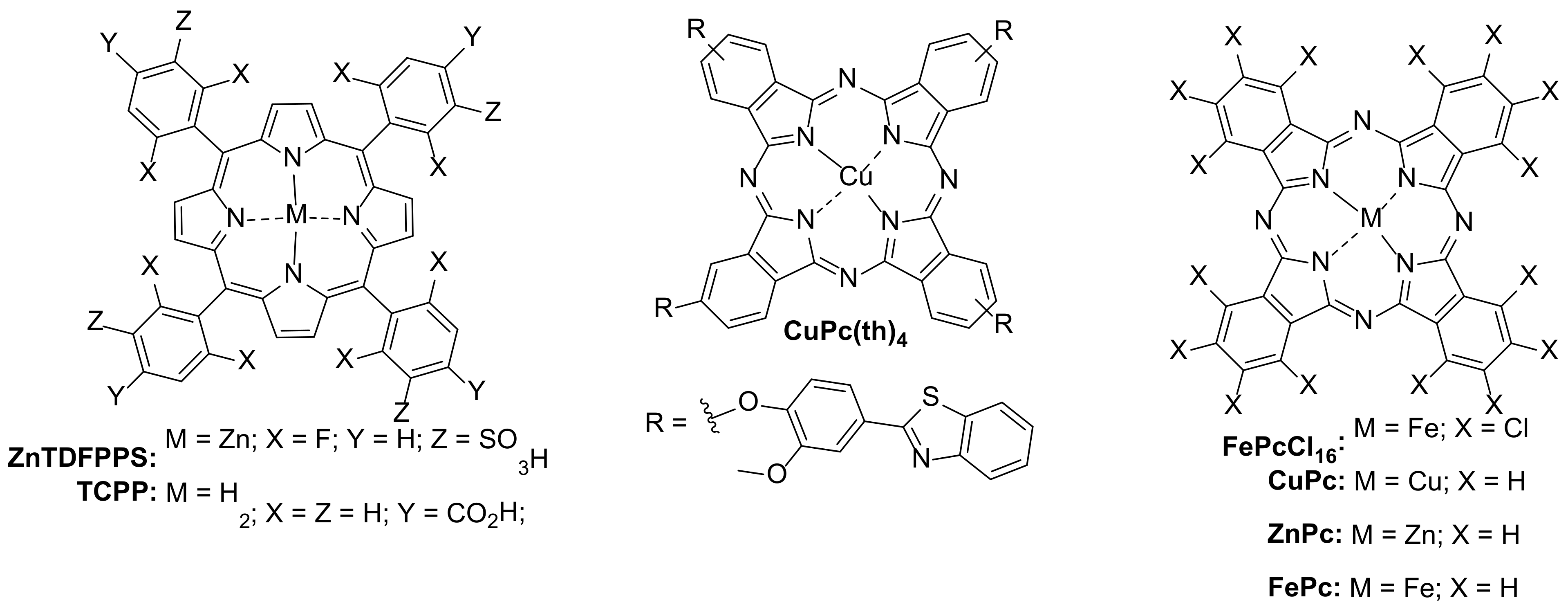
| 1 [31] | α-ZnTCPc@g-C3N4 | [TC] = 30 mg/L |
|
|
| 2 [21] | TNCuPc@CeO2/Bi2MoO6 | [TC] = 50 mg/L |
|
|
| 3 [32] | CuPc@AgHPMo12 | [TC∙HCl] = 20 mg/L |
|
|
| 4 [33] | ZnTAPc@Cu2O-TiO2 | [TC∙HCl] = 20 mg/L |
|
|
| 5 [18] | TCPP@rGO-Bi2WO6 | [TC] = 15 mg/L |
|
|
| 6 [34] | ZnTCNP MOC | [TC] = 5 mg/L |
|
|
| 7 [35] | CuTCPP MOF | [TC] = 40 mg/L [NOR] = 20 mg/L |
|
|
| 8 [36] | FeTCPP@TDI–TiO2 | [TC∙HCl] = 25 mg/L [NOR] = 25 mg/L |
|
|
| 9 [37] | FePc@BiOBr | [TC] = 20 mg/L [CIP] = 10 mg/L |
|
|
| 10 [19] | TCPP@BiOCl | [TC] = 20 mg/L [CIP] = 10 mg/L [ENR] = 10 mg/L |
|
|
| 11 [38] | FePc@N-PR | [OTC∙HCl] = 100 mg/L |
|
|
| 12 [17] | MTCPP@TiO2M = H2, Zn and Cu | [OTC] = ~8 mg/L [OXA] = ~8 mg/L |
|
|
| 13 [39] | FeTPP@Cr-TiO2 | [NOR] = 25 mg/L [OFO] = 25 mg/L [LOM] = 25 mg/L |
|
|
| 14 [40] | PCN-222@g-C3N4(PCN-222 = FeTCPP Zr-MOF) | [OFO] = 20 mg/L |
|
|
| 15 [41] | PCN-222@PW12/TiO2(PCN-222 =FeTCPP Zr-MOF) | [OFO] = 20 mg/L |
|
|
| 16 [42] | SnTCPP@g-C3N4/Bi2WO6 | [LEV] = 10 mg/L |
|
|
| 17 [43] | ZnPc@TiO2 | [ERY] = 7.5 mg/L |
|
|
| 18 [44] | FePc@P4VP/PET | [SQX] = 6 mg/L |
|
|
| 19 [16] | CoCPc@K-TiO2 | [TMP] = 25 mg/L |
|
|
| 20 [45] | Co(quin)4Pc@TiO2 | [AMX] = 20 mg/L |
|
|
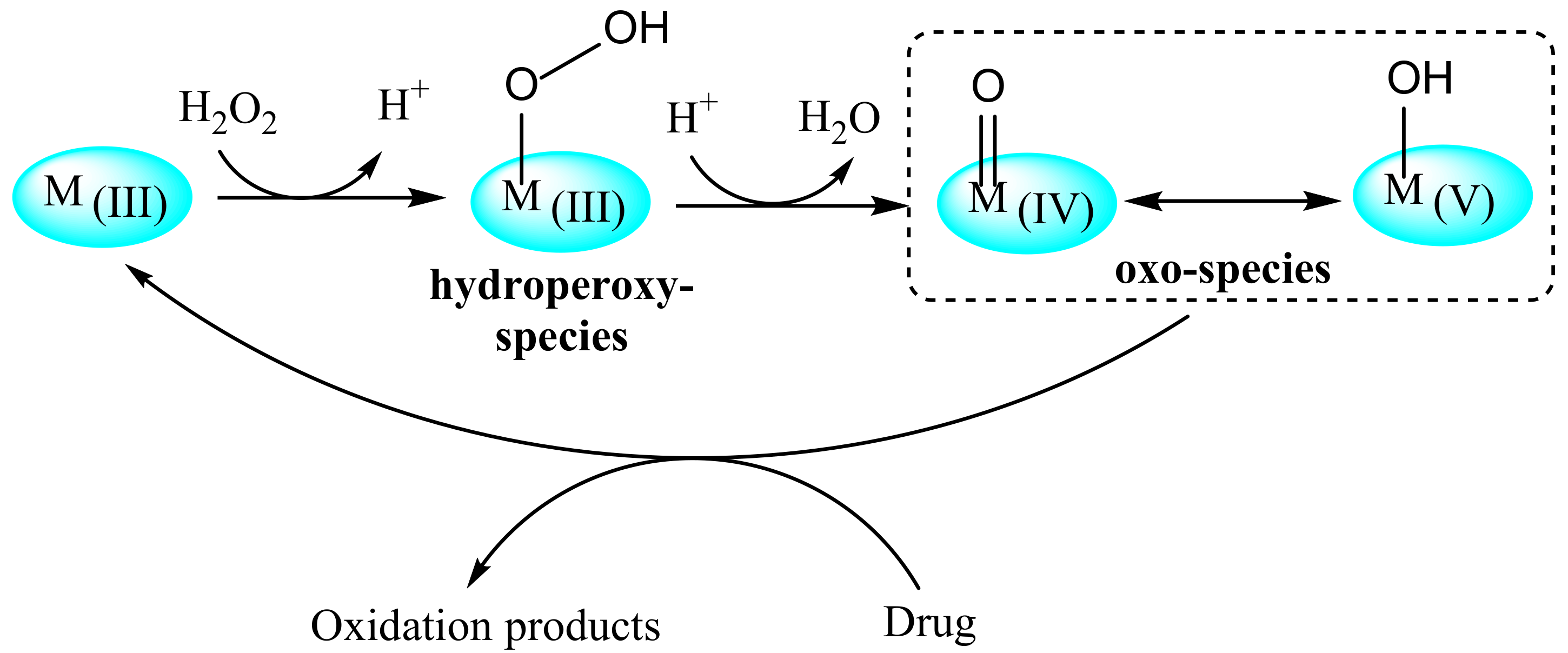
2.2. Oxidative Chemical Degradation
| #/Ref | Catalyst | Drug | Experimental Conditions | Comments |
|---|---|---|---|---|
| 1 [47] | MnIII(TPP)Cl, MnIII(TCPP)Cl, MnIII(Br8TCPP)Cl | [CIP] = 10 mg/L |
|
|
| 2 [48,50] | MnIII(TCPP)Cl, MnIII(Br8TCPP)Cl | [CIP] = 10 mg/L [LEV] = 10 mg/L [NOR] = 10 mg/L |
|
|
| 3 [49] | MnIII(TPP)Cl, MnIII(T2,3DCPP)Cl, MnIII(T2,6CFPP)Cl | [NOR] = 14 mg/L (stock) |
|
|
| 4 [51] | CoPc@GO | [NOR] = 10 mg/L |
|
|
| 5 [52] | FePc@N-PR | [TC∙HCl] = 100 mg/L |
|
|
| 6 [53] | FePc@P4VP/PAN | [SQX] = 6 mg/L |
|
|
| 7 [54] | MnTDCPPS@N-SiO2 | [TMP] = 130 mg/L |
|
|
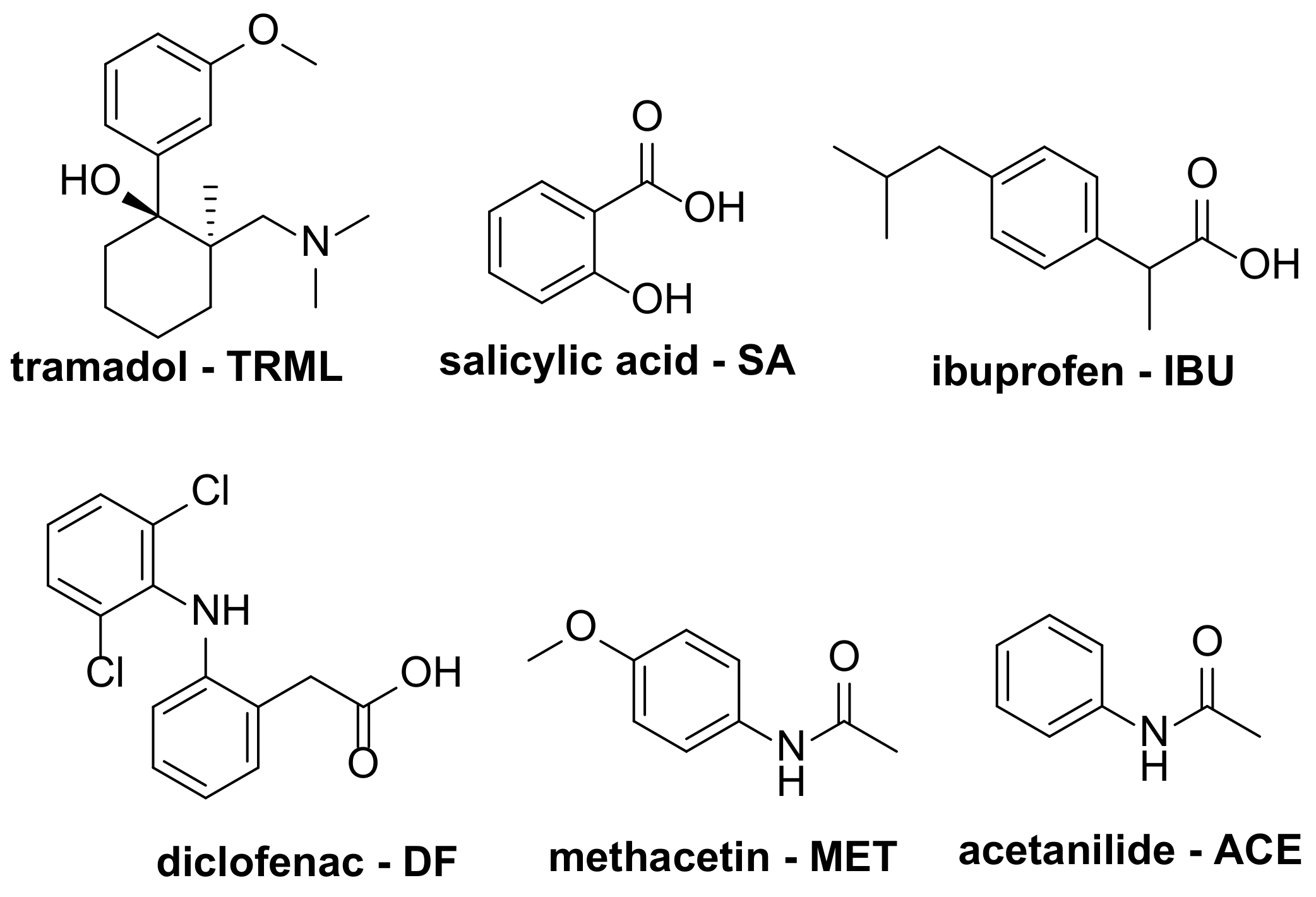
3. Degradation of Analgesic Drugs
3.1. Photochemical Degradation
| #/Ref | Catalyst | Drug | Experimental Conditions | Comments |
|---|---|---|---|---|
| 1 [57] | ZnTDFPPS@TiO2 | [TRML] = 10 mg/L |
|
|
| 2 [58] | FePcCl16 | [SA] = 70 mg/L |
|
|
| 3 [15] | CuPc(th)4@TiO2/ZnO | [IBU] = 5 mg/L |
|
|
| 4 [20] | CuPc@TiO2 ZnPc@TiO2 | [IBU] = 10 mg/L |
|
|
| 5 [59] | FePc@ZnO | [IBU] = 20 mg/L |
|
|
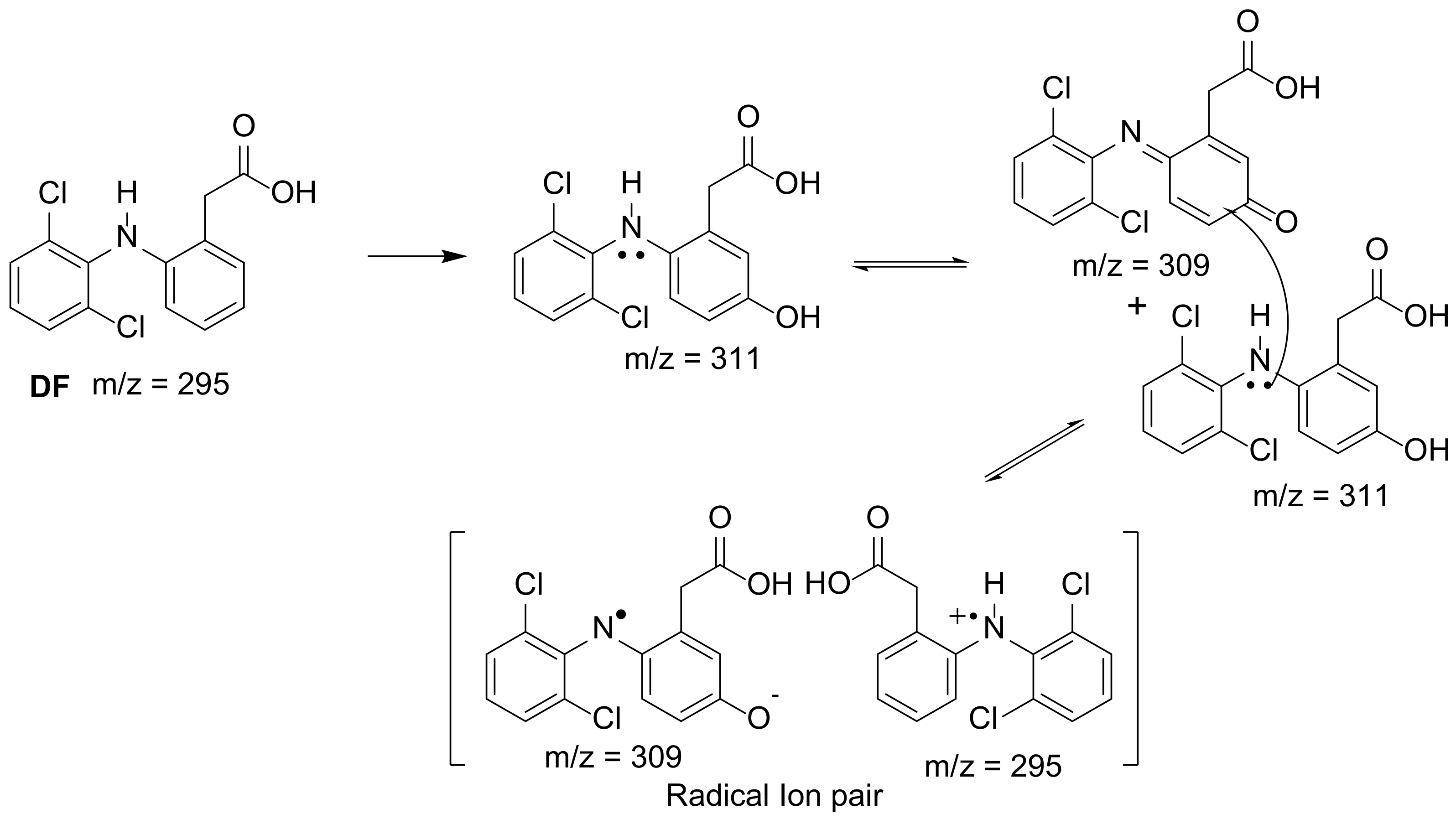
| 6 [60] | PCN-134 (TCPP@Zr-BTB MOF) | [DF] = 30 mg/L |
|
|
| 7 [61] | TCPP@UiO-66 MOF | [DF] = 30 mg/L |
|
|
3.2. Oxidative Chemical Degradation
| #/Ref | Catalyst | Drug | Experimental Conditions | Comments |
|---|---|---|---|---|
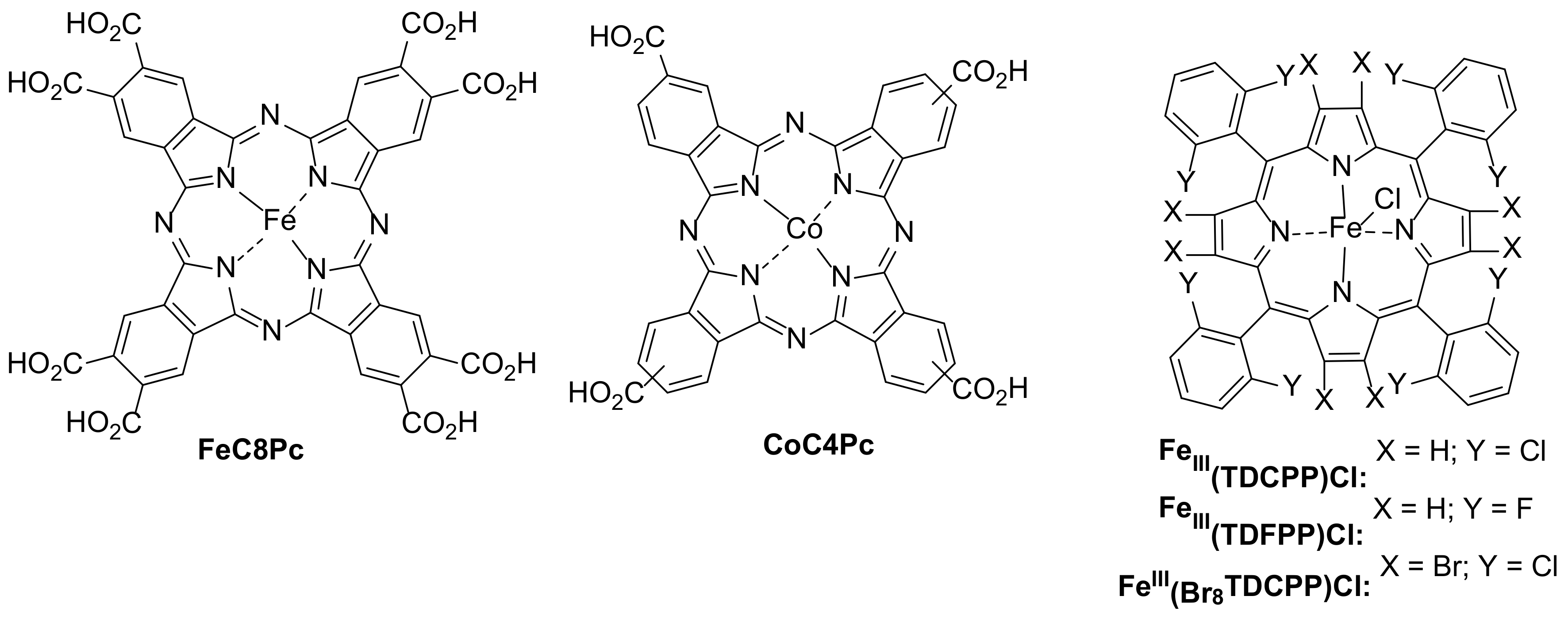
| 1 [55] | FeC8Pc | [DF] = 6 mg/L |
|
|
| 2 [62] | CoC4Pc@CNOMS | [DF] = 10 mg/L |
|
|
| 3 [63] | FeIII(TDCPP)Cl FeIII(TDFPP)Cl FeIII(Br8TDCPP)Cl | [MET] = 60 g/L [ACE] = 60 g/L |
|
|
4. Degradation of Neurological Pharmaceuticals
4.1. Photochemical Degradation
| #/Ref | Catalyst | Drug | Experimental Conditions | Comments |
|---|---|---|---|---|
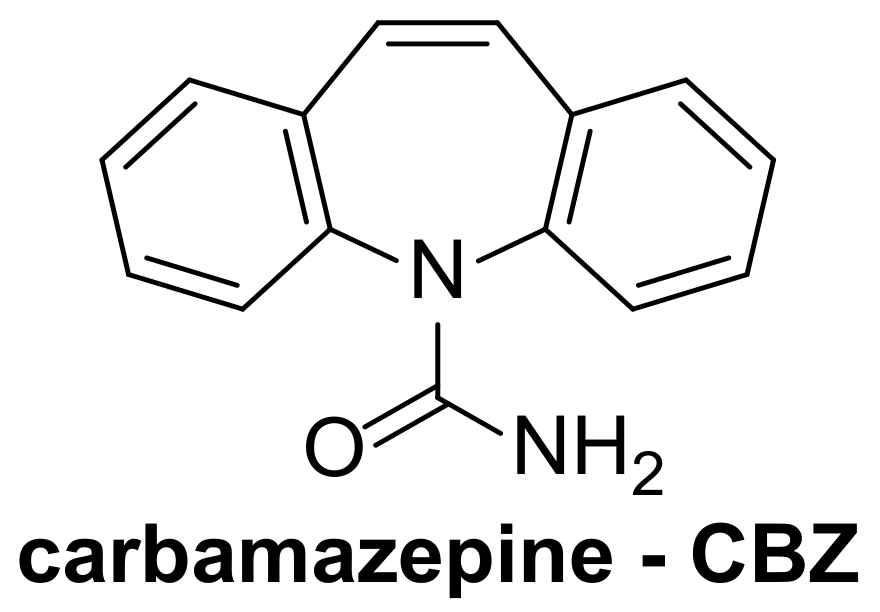
| 1 [65] | ZnTCPc@g-C3N4/PAN nanofibers | [CBZ] = 2.5 mg/L |
|
|
| 2 [66] | ZnTCPc@g-C3N4/GQD | [CBZ] = 6 mg/L |
|
|
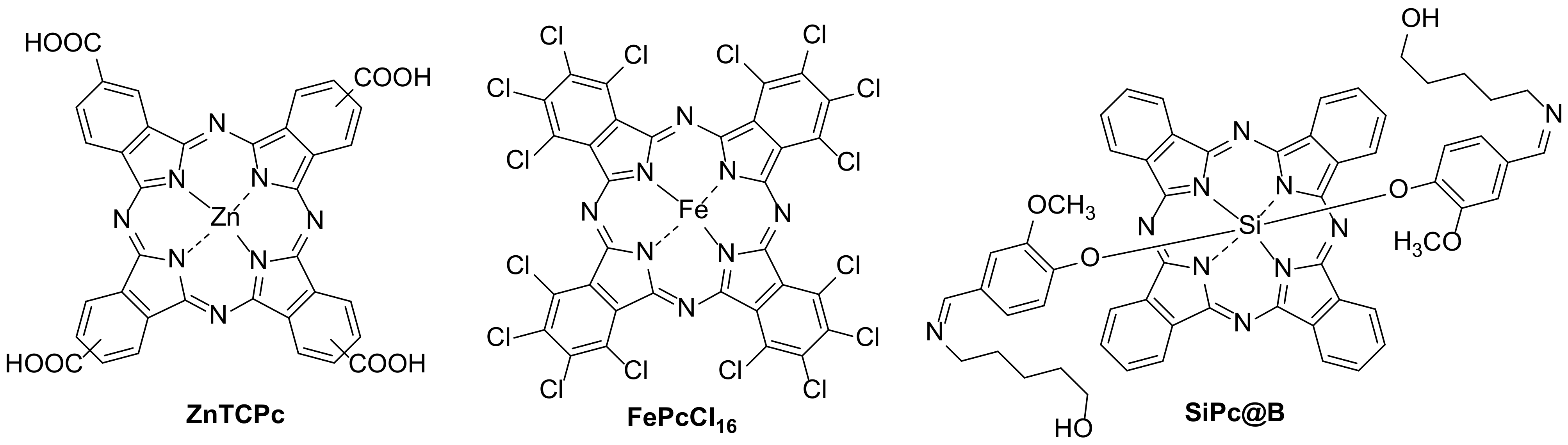
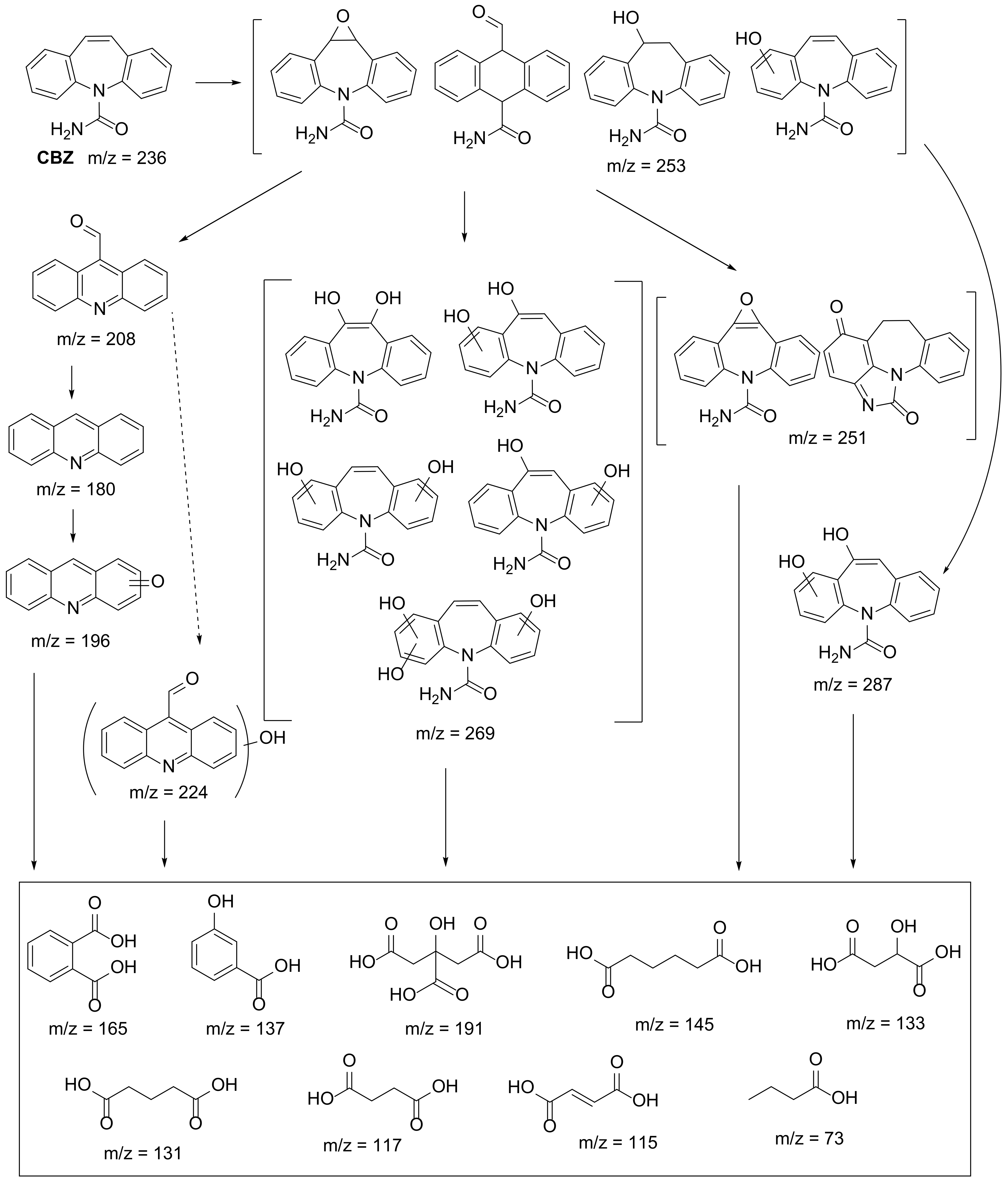
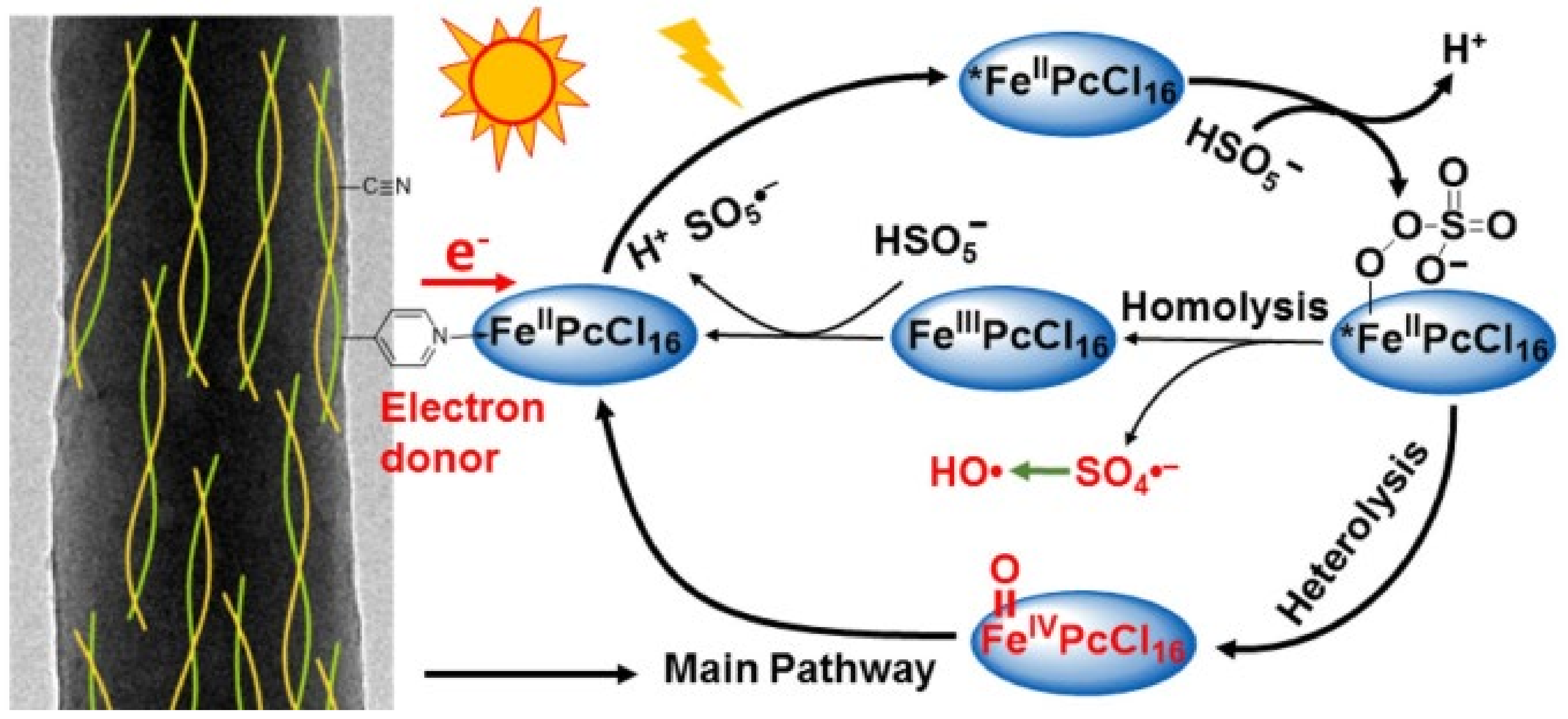
| 3 [64] | FePcCl16 | [CBZ] = 6 mg/L |
|
|
| 4 [67,68,69] | FePcCl16@g-C3N4-IMA FePcCl16@g-C3N4-INA FePcCl16@P4VP/PAN | [CBZ] = 6 mg/L |
|
|
| 5 [14] | SiPc@B/NaF-TiO2 | [CBZ] = 10 mg/L |
|
|
4.2. Oxidative Chemical Degradation
| #/Ref | Catalyst | Drug | Experimental Conditions | Comments |
|---|---|---|---|---|
| 1 [70] | FePc@PAN | [CBZ] = 6 mg/L |
|
|
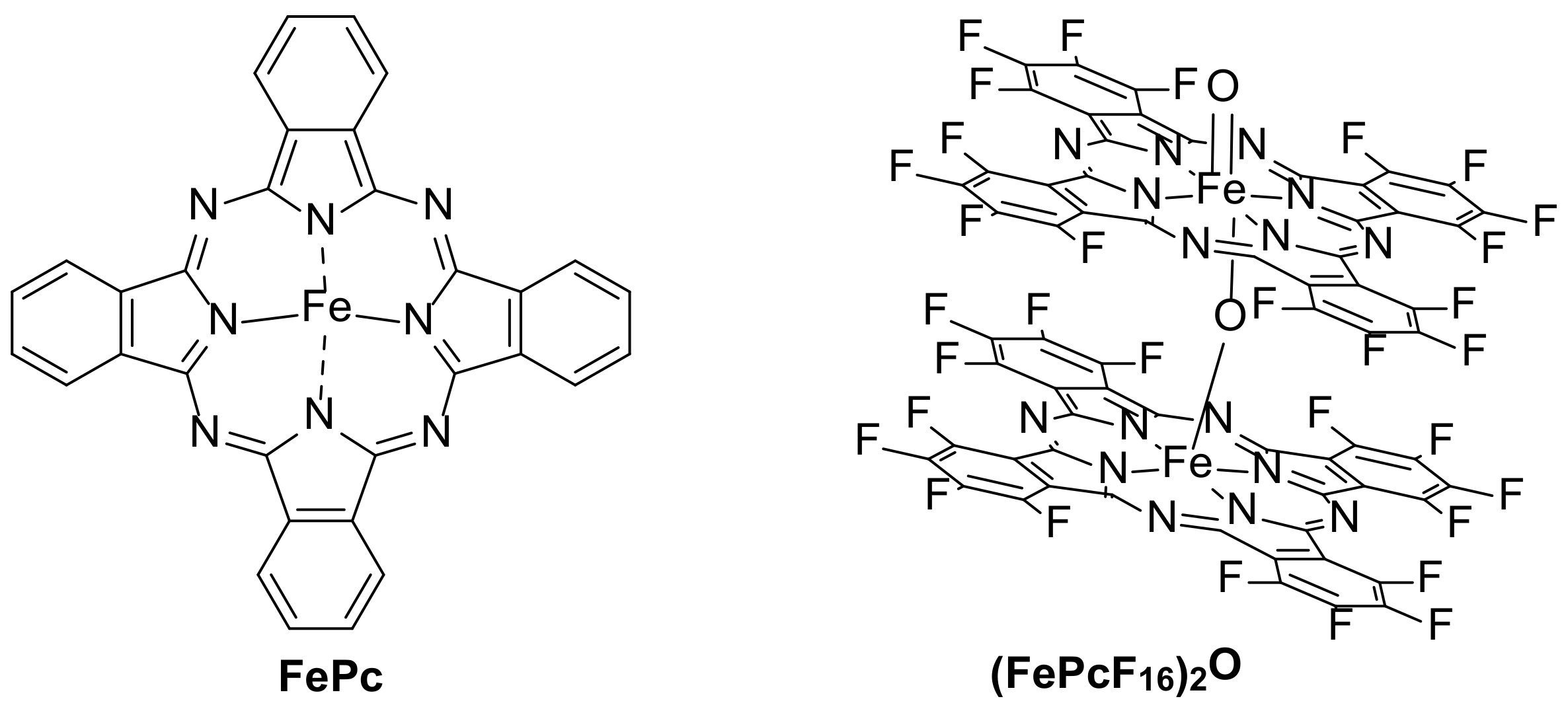
| 2 [71] | (FePcF16)2O | [CBZ] = 6 mg/L |
|
|
| 3 [72] | (FePcF16)2O@MWCNT | [CBZ] = 6 mg/L |
|
|
5. Degradation of Miscellaneous Pharmaceuticals
| #/Ref | Catalyst | Drug | Experimental Conditions | Comments |
|---|---|---|---|---|
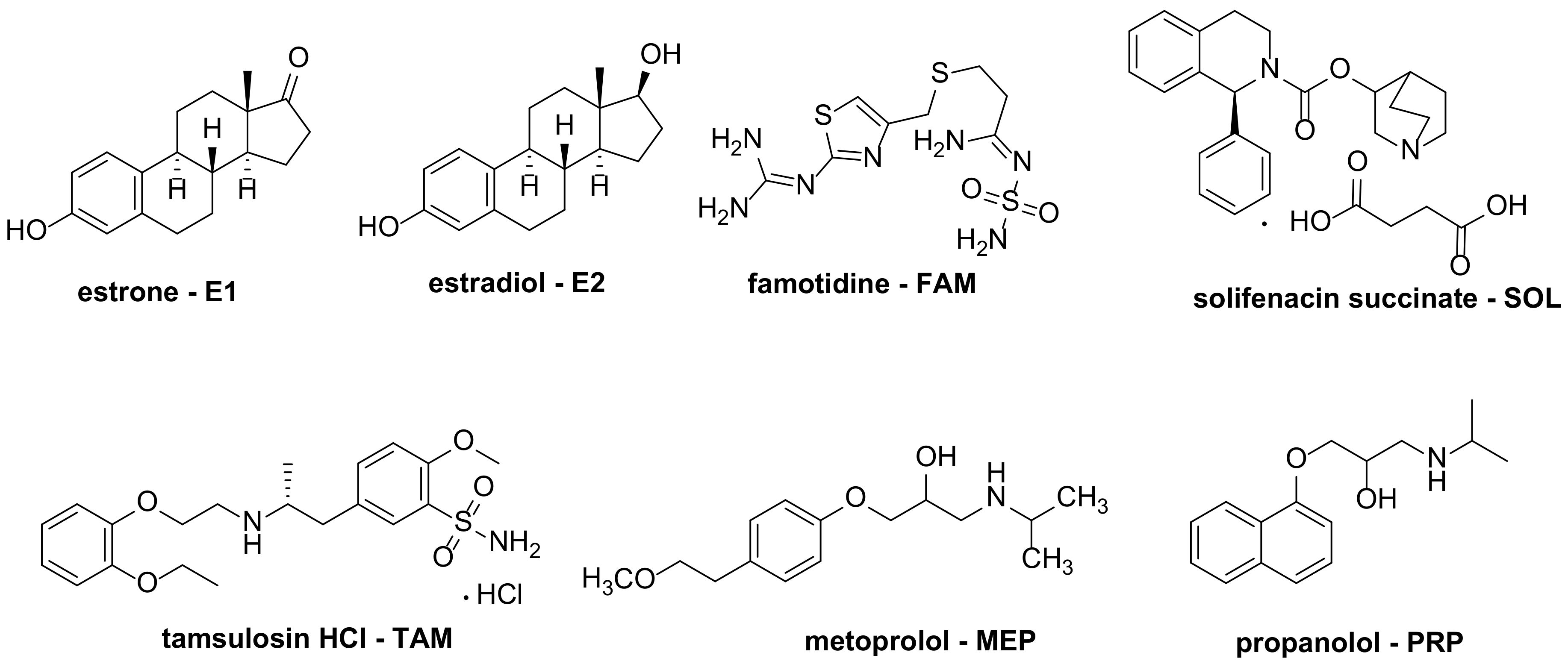
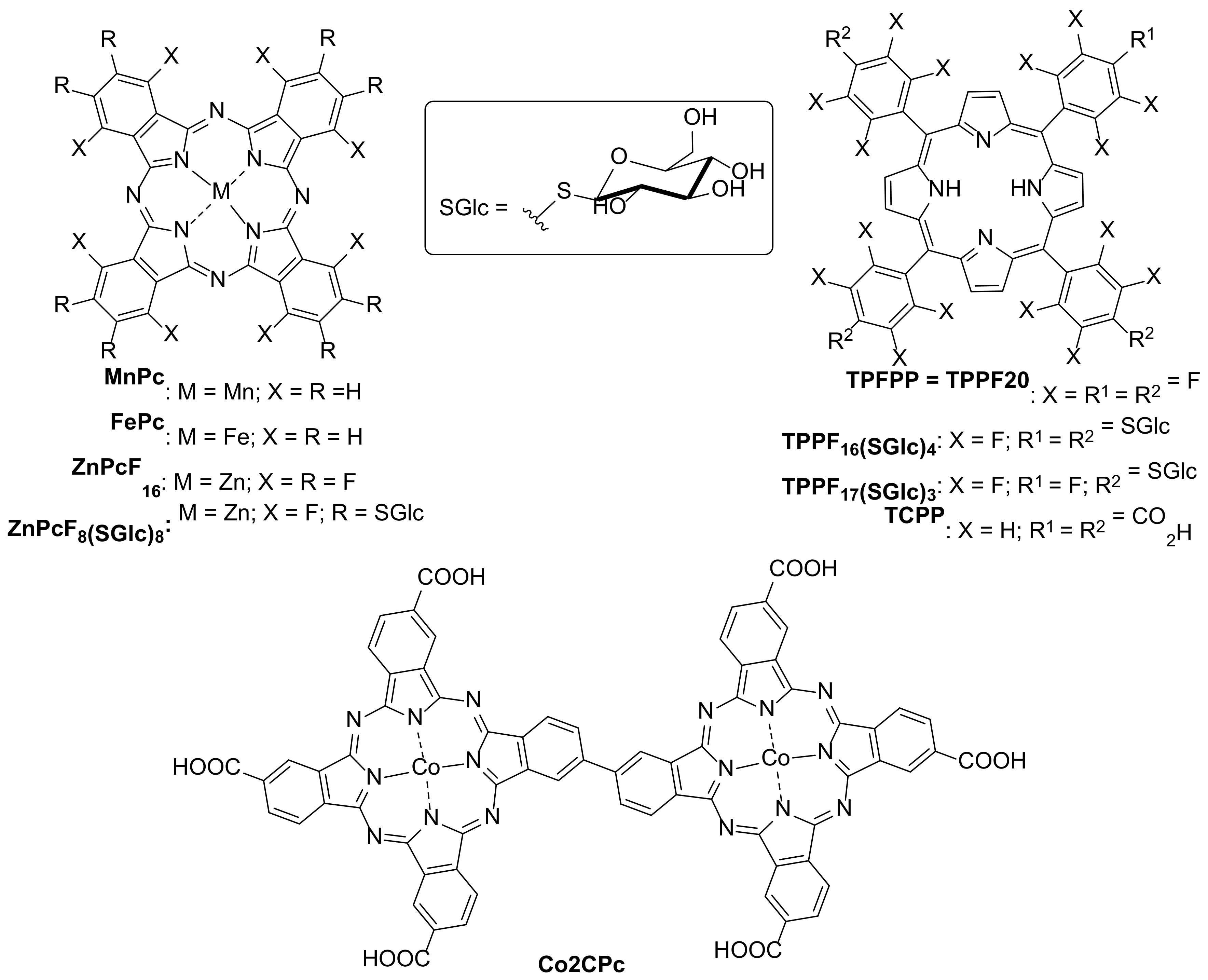
| 1 [73] | FePc MnPc | [E1] = 8 mg/L [E2] = 8 mg/L |
|
|
| 2 [74] | TPPF20@MNP TPPF16(SGlc)4@MNP TPPF17(SGlc)3@MNP ZnPcF16@MNP ZnPcF8(SGlc)8@MNP | [E2] = 5 mg/L |
|
|
| 3 [22] | TCPP@TiO2 | [FAM] = 28 mg/L [SOL] = 30 mg/L [TAM] = 34 mg/L |
|
|
| 4 [75] | TCPP@ATiNT TCPP@Si-ATiNT | [FAM] = 100 mg/L |
|
|
| 5 [76] | TPFPP@NH2-SiO2 | [MEP] = 50 mg/L |
|
|
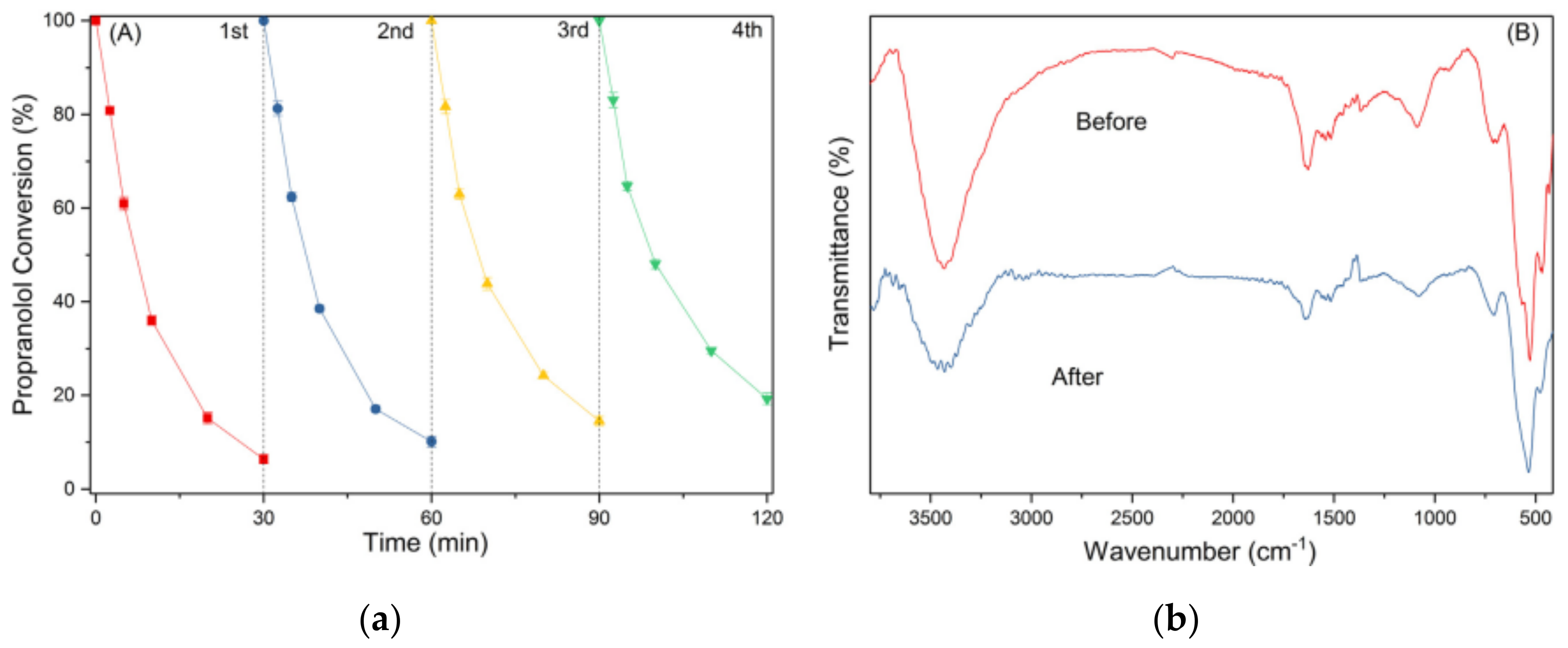
| 6 [77] | Co2CPc@CNOMS | [PRP] = 5 mg/L |
|
|
6. Conclusions and Perspectives
- (i)
- the TPM, considering its modulability and functionality, including substitution patterns for activity/stability;
- (ii)
- the type of support, aiming preferential immobilization at efficient reutilization and/or the holding of suitable semiconducting characteristics;
- (iii)
- the light source, when designing a photocatalytic system, preferentially using visible/solar energy;
- (iv)
- the oxidants, when designing oxidative chemical systems, giving preference to environmentally benign ones.
Author Contributions
Funding
Conflicts of Interest
References
- Calvete, M.J.F.; Piccirillo, G.; Vinagreiro, C.S.; Pereira, M.M. Hybrid Materials for Heterogeneous Photocatalytic Degradation of Antibiotics. Coord. Chem. Rev. 2019, 395, 63–85. [Google Scholar] [CrossRef]
- Kutuzova, A.; Dontsova, T.; Kwapinski, W. Application of Tio2-Based Photocatalysts to Antibiotics Degradation: Cases of Sulfamethoxazole, Trimethoprim and Ciprofloxacin. Catalysts 2021, 11, 728. [Google Scholar] [CrossRef]
- Ye, S.J.; Zeng, G.M.; Wu, H.P.; Zhang, C.; Liang, J.; Dai, J.; Liu, Z.F.; Xiong, W.P.; Wan, J.; Xu, P.A.; et al. Co-Occurrence and Interactions of Pollutants, and Their Impacts on Soil Remediation—A Review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1528–1553. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.Y.; Ma, Z.B.; Ren, Z.F.; Fan, H.J.; Yang, X.M. Elementary Photocatalytic Chemistry on TiO2 Surfaces. Chem. Soc. Rev. 2016, 45, 3701–3730. [Google Scholar] [CrossRef] [Green Version]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.A.K.; Reddy, P.V.L.; Kwon, E.; Kim, K.-H.; Akter, T.; Kalagara, S. Recent Advances in Photocatalytic Treatment of Pollutants in Aqueous Media. Environ. Int. 2016, 91, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Opoku, F.; Govender, K.K.; van Sittert, C.G.C.E.; Govender, P.P. Recent Progress in the Development of Semiconductor-Based Photocatalyst Materials for Applications in Photocatalytic Water Splitting and Degradation of Pollutants. Adv. Sustain. Syst. 2017, 1, 1700006. [Google Scholar] [CrossRef]
- Fernández, L.; Esteves, V.I.; Cunha, Â.; Schneider, R.J.; Tomé, J.P.C. Photodegradation of Organic Pollutants in Water by Immobilized Porphyrins and Phthalocyanines. J. Porphyr. Phthalocyanines 2016, 20, 150–166. [Google Scholar] [CrossRef]
- Nonell, S.; Flors, C. (Eds.) Singlet Oxygen: Applications in Biosciences and Nanosciences; Comprehensive Series in Photochemical & Photobiological Sciences; RSC Books: Cambridge, UK, 2016; Volume 1. [Google Scholar]
- Wiehe, A.; O’Brien, J.M.; Senge, M.O. Trends and Targets in Antiviral Phototherapy. Photochem. Photobiol. Sci. 2019, 18, 2565–2612. [Google Scholar] [CrossRef]
- Diaz-Uribe, C.E.; Vallejo-L., W.A.; Miranda, J. Photo-Fenton Oxidation of Phenol with Fe(Iii)-Tetra-4-Carboxyphenylporphyrin/Sio2 Assisted with Visible Light. J. Photochem. Photobiol. A 2014, 294, 75–80. [Google Scholar] [CrossRef]
- Guerra-Rodriguez, S.; Rodriguez, E.; Singh, D.N.; Rodriguez-Chueca, J. Assessment of Sulfate Radical-Based Advanced Oxidation Processes for Water and Wastewater Treatment: A Review. Water 2018, 10, 1828. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Tian, J.; Fang, Y.; Liu, T.; Zhang, X.; Xu, X.; Zhang, X. Visible-Light-Driven Photo-Fenton Degradation of Organic Pollutants by a Novel Porphyrin-Based Porous Organic Polymer at Neutral Ph. Chemosphere 2020, 243, 125334. [Google Scholar] [CrossRef]
- Anucha, C.B.; Altin, I.; Fabbri, D.; Degirmencioglu, I.; Calza, P.; Magnacca, G.; Stathopoulos, V.N.; Bacaksiz, E. Synthesis and Characterization of B/Naf and Silicon Phthalocyanine-Modified TiO2 and an Evaluation of Their Photocatalytic Removal of Carbamazepine. Separations 2020, 7, 71. [Google Scholar] [CrossRef]
- Anucha, C.B.; Altin, I.; Bacaksiz, E.; Degirmencioglu, I.; Kucukomeroglu, T.; Yilmaz, S.; Stathopoulos, V.N. Immobilized TiO2/ZnO Sensitized Copper (II) Phthalocyanine Heterostructure for the Degradation of Ibuprofen under Uv Irradiation. Separations 2021, 8, 24. [Google Scholar] [CrossRef]
- Da Silva, T.H.; Ribeiro, A.O.; Nassar, E.J.; Trujillano, R.; Rives, V.; Vicente, M.A.; de Faria, E.H.; Ciuffi, K.J. Kaolinite/TiO2/Cobalt(II) Tetracarboxymetallophthalocyanine Nanocomposites as Heterogeneous Photocatalysts for Decomposition of Organic Pollutants Trimethoprim, Caffeine and Prometryn. J. Braz. Chem. Soc. 2019, 30, 2610–2623. [Google Scholar] [CrossRef]
- Gaeta, M.; Sanfilippo, G.; Fraix, A.; Sortino, G.; Barcellona, M.; Oliveri Conti, G.; Fragalà, M.E.; Ferrante, M.; Purrello, R.; D’Urso, A. Photodegradation of Antibiotics by Noncovalent Porphyrin-Functionalized TiO2 in Water for the Bacterial Antibiotic Resistance Risk Management. Int. J. Mol. Sci. 2020, 21, 3775. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Chen, C.; Zhu, Y.; Zeng, G.; Huang, B.; Chen, W.; Liu, S.; Lei, C.; Li, B.; Yang, Y. Ternary Z-Scheme Heterojunction of Bi2WO6 with Reduced Graphene Oxide (rGO) and Meso-Tetra (4-Carboxyphenyl) Porphyrin (TCPP) for Enhanced Visible-Light Photocatalysis. J. Colloid Interface Sci. 2019, 540, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, P.; Miao, H.; Shao, S.; Wang, L.; Chen, Y.; Jia, C.; Xia, J. Organic-Inorganic Tcpp/Biocl Hybrids with Accelerated Interfacial Charge Separation for Boosted Photocatalytic Performance. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126367. [Google Scholar] [CrossRef]
- Krakowiak, R.; Musial, J.; Frankowski, R.; Spychala, M.; Mielcarek, J.; Dobosz, B.; Krzyminiewski, R.; Sikorski, M.; Bendzinska-Berus, W.; Tykarska, E.; et al. Phthalocyanine-Grafted Titania Nanoparticles for Photodegradation of Ibuprofen. Catalysts 2020, 10, 1328. [Google Scholar] [CrossRef]
- Li, K.; Pang, Y.; Lu, Q. In Situ Growth of Copper(II) Phthalocyanine-Sensitized Electrospun Ceo2/Bi2moo6 Nanofibers: A Highly Efficient Photoelectrocatalyst Towards Degradation of Tetracycline. Inorg. Chem. Front. 2019, 6, 3215–3224. [Google Scholar] [CrossRef]
- Murphy, S.; Saurel, C.; Morrissey, A.; Tobin, J.; Oelgemoller, M.; Nolan, K. Photocatalytic Activity of a Porphyrin/TiO2 Composite in the Degradation of Pharmaceuticals. Appl. Catal. B-Environ. 2012, 119, 156–165. [Google Scholar] [CrossRef]
- Huang, X.; Groves, J.T. Oxygen Activation and Radical Transformations in Heme Proteins and Metalloporphyrins. Chem. Rev. 2018, 118, 2491–2553. [Google Scholar] [CrossRef] [Green Version]
- Pereira, M.M.; Dias, L.D.; Calvete, M.J.F. Metalloporphyrins: Bioinspired Oxidation Catalysts. Acs Catal. 2018, 8, 10784–10808. [Google Scholar] [CrossRef]
- Dubey, K.D.; Shaik, S. Cytochrome P450-the Wonderful Nanomachine Revealed through Dynamic Simulations of the Catalytic Cycle. Acc. Chem. Res. 2019, 52, 389–399. [Google Scholar] [CrossRef]
- Larson, V.A.; Battistella, B.; Ray, K.; Lehnert, N.; Nam, W. Iron and Manganese Oxo Complexes, Oxo Wall and Beyond. Nat. Rev. Chem. 2020, 4, 404–419. [Google Scholar] [CrossRef]
- Chino, M.; Leone, L.; Zambrano, G.; Pirro, F.; D’Alonzo, D.; Firpo, V.; Aref, D.; Lista, L.; Maglio, O.; Nastri, F.; et al. Oxidation Catalysis by Iron and Manganese Porphyrins within Enzyme-Like Cages. Biopolymers 2018, 109, e23107. [Google Scholar] [CrossRef] [PubMed]
- Calvete, M.J.F.; Piñeiro, M.; Dias, L.D.; Pereira, M.M. Hydrogen Peroxide and Metalloporphyrins in Oxidation Catalysis: Old Dogs with Some New Tricks. ChemCatChem 2018, 10, 3615–3635. [Google Scholar] [CrossRef]
- Zanatta, L.; Barbosa, I.; Filho, P.; Zanardi, F.; Bolzon, L.; Serra, O.; Iamamoto, Y. Metalloporphyrins in Drug and Pesticide Catalysis as Powerful Tools to Elucidate Biotransformation Mechanisms. Mini-Rev. Org. Chem. 2016, 13, 281–288. [Google Scholar] [CrossRef] [Green Version]
- Walsh, C. Antibiotics: Actions, Origins, Resistance; ASM Press: Washington, DC, USA, 2003. [Google Scholar]
- He, Y.; Huang, Z.; Ma, Z.; Yao, B.; Liu, H.; Hu, L.; Zhao, Q.; Yang, Q.; Liu, D.; Du, D. Highly Efficient Photocatalytic Performance and Mechanism of A-Zntcpc/G-C3n4 Composites for Methylene Blue and Tetracycline Degradation under Visible Light Irradiation. Appl. Surf. Sci. 2019, 498, 143834. [Google Scholar] [CrossRef]
- Wang, X.; Song, Y.; Li, F.; Xu, W.; Zheng, Y.; Xu, L. A “Concentration-Induced Self-Assembly” Strategy for AGXH3−XPMo12O40 Nanorods: Synthesis, Photoelectric Properties and Photocatalytic Applications. Nanoscale Adv. 2021, 3, 446–454. [Google Scholar] [CrossRef]
- Liu, C.; Cui, X.; Li, Y.; Duan, Q. A Hybrid Hollow Spheres Cu2o@Tio2-G-Zntapc with Spatially Separated Structure as an Efficient and Energy-Saving Day-Night Photocatalyst for Cr(VI) Reduction and Organic Pollutants Removal. Chem. Eng. J. 2020, 399, 125807. [Google Scholar] [CrossRef]
- Jafarizadeh, T.; Hayati, P.; Neyrizi, H.Z.; Mehrabadi, Z.; Farjam, M.H.; Gutiérrez, A.; Adarsh, N.N. Synthesis and Structural Characterization of a Novel Zn(II) Metal Organic Complex (Zn-Moc) and Elimination of Highly Consumed Antibiotic; Tetracycline from Aqueous Solution by Their Nanostructures Photocatalyst under Visible Light. J. Mol. Struct. 2021, 1228, 129448. [Google Scholar] [CrossRef]
- Zhao, S.; Li, S.; Zhao, Z.; Su, Y.; Long, Y.; Zheng, Z.; Cui, D.; Liu, Y.; Wang, C.; Zhang, X.; et al. Microwave-Assisted Hydrothermal Assembly of 2d Copper-Porphyrin Metal-Organic Frameworks for the Removal of Dyes and Antibiotics from Water. Environ. Sci. Pollut. Res. 2020, 27, 39186–39197. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Peng, C.; He, Y.; Zhang, W.; Zhang, Q.; Zhang, T. Conjugated Microspheres FeTCPP–TDI–TiO2 with Enhanced Photocatalytic Performance for Antibiotics Degradation under Visible Light Irradiation. Catal. Lett. 2016, 146, 2543–2554. [Google Scholar] [CrossRef]
- Yin, S.; Chen, Y.; Hu, Q.; Li, M.; Ding, Y.; Shao, Y.; Di, J.; Xia, J.; Li, H. In-Situ Preparation of Iron(II) Phthalocyanine Modified Bismuth Oxybromide with Enhanced Visible-Light Photocatalytic Activity and Mechanism Insight. Colloids Surf. A Physicochem. Eng. Asp. 2019, 575, 336–345. [Google Scholar] [CrossRef]
- Sun, Y.; Feng, X.; Fu, S. Application of Response Surface Methodology for Optimization of Oxytetracycline Hydrochloride Degradation Using Hydrogen Peroxide/Polystyrene-Supported Iron Phthalocyanine Oxidation Process. Water Sci. Technol. 2020, 81, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.H.; Peng, C.; He, Y.Q.; Zhang, W.; Yu, Y.; Zhang, T. Preparation and Visible-Light Photocatalytic Activity of FeTPP-CR-TiO2 Microspheres. J. Chem. Phys. 2016, 29, 717–724. [Google Scholar] [CrossRef]
- Jia, H.; Ma, D.; Zhong, S.; Li, L.; Li, L.; Xu, L.; Li, B. Boosting Photocatalytic Activity under Visible-Light by Creation of PCN-222/g-C3N4 Heterojunctions. Chem. Eng. J. 2019, 368, 165–174. [Google Scholar] [CrossRef]
- Li, L.; Yu, X.; Xu, L.; Zhao, Y. Fabrication of a Novel Type Visible-Light-Driven Heterojunction Photocatalyst: Metal-Porphyrinic Metal Organic Framework Coupled with PW12/TiO2. Chem. Eng. J. 2020, 386, 123955. [Google Scholar] [CrossRef]
- He, Y.; Lv, H.; Daili, Y.; Yang, Q.; Junior, L.B.; Liu, D.; Liu, H.; Ma, Z. Construction of a New Cascade Photogenerated Charge Transfer System for the Efficient Removal of Bio-Toxic Levofloxacin and Rhodamine B from Aqueous Solution: Mechanism, Degradation Pathways and Intermediates Study. Environ. Res. 2020, 187, 109647. [Google Scholar] [CrossRef] [PubMed]
- Vignesh, K.; Rajarajan, M.; Suganthi, A. Photocatalytic Degradation of Erythromycin under Visible Light by Zinc Phthalocyanine-Modified Titania Nanoparticles. Mater. Sci. Semicond. Process. 2014, 23, 98–103. [Google Scholar] [CrossRef]
- Li, N.; Lu, P.; He, C.; Lu, W.; Chen, W. Catalytic Degradation of Sulfaquinoxalinum by Polyester/Poly-4-Vinylpyridine Nanofibers-Supported Iron Phthalocyanine. Environ. Sci. Pollut. Res. 2018, 25, 5902–5910. [Google Scholar] [CrossRef] [PubMed]
- Keşir, M.K.; Sökmen, M.; Bıyıklıoğlu, Z. Photocatalytic Efficiency of Metallo Phthalocyanine Sensitized TiO2 (MPc/TiO2) Nanocomposites for Cr(VI) and Antibiotic Amoxicillin. Water 2021, 13, 2174. [Google Scholar] [CrossRef]
- Feng, D.; Gu, Z.-Y.; Li, J.-R.; Jiang, H.-L.; Wei, Z.; Zhou, H.-C. Zirconium-Metalloporphyrin PCN-222: Mesoporous Metal–Organic Frameworks with Ultrahigh Stability as Biomimetic Catalysts. Angew. Chem. Int. Ed. 2012, 51, 10307–10310. [Google Scholar] [CrossRef] [PubMed]
- Almeida Lage, A.L.; Moreira Meireles, A.; Capelão Marciano, A.; Martins Ribeiro, J.; de Souza-Fagundes, E.M.; Carvalho da Silva Martins, D. Ciprofloxacin Degradation by First-, Second-, and Third-Generation Manganese Porphyrins. J. Hazard. Mater. 2018, 360, 445–451. [Google Scholar] [CrossRef]
- Lage, A.L.A.; Marciano, A.C.; Venâncio, M.F.; da Silva, M.A.N.; Martins, D.C.d.S. Water-Soluble Manganese Porphyrins as Good Catalysts for Cipro- and Levofloxacin Degradation: Solvent Effect, Degradation Products and Dft Insights. Chemosphere 2021, 268, 129334. [Google Scholar] [CrossRef]
- Meireles, A.M.; Almeida Lage, A.L.; Ribeiro, J.M.; Silva, M.A.N.d.; Souza-Fagundes, E.M.d.; Martins, D.C.d.S. Synthetic Mn(Iii) Porphyrins as Biomimetic Catalysts of Cyp450: Degradation of Antibiotic Norfloxacin in Aqueous Medium. Environ. Res. 2019, 177, 108615. [Google Scholar] [CrossRef] [PubMed]
- De Souza Santos, L.V.; Lebron, Y.A.R.; Moreira, V.R.; Jacob, R.S.; Martins, D.C.d.S.; Lange, L.C. Norfloxacin and Gentamicin Degradation Catalyzed by Manganese Porphyrins under Mild Conditions: The Importance of Toxicity Assessment. Environ. Sci. Pollut. Res. 2021. [CrossRef]
- Zhang, Y.; Li, H.; Huang, H.; Zhang, Q.; Guo, Q. Graphene Oxide Supported Cobalt Phthalocyanine as Heterogeneous Catalyst to Activate Peroxymonosulfate for Efficient Degradation of Norfloxacin Antibiotics. J. Environ. Eng. 2018, 144, 04018052. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, W.; Fu, S.; Singh, R.P. Immobilization of Iron Phthalocyanine on 4-Aminopyridine Grafted Polystyrene Resin as a Catalyst for Peroxymonosulfate Activation in Eliminating Tetracycline Hydrochloride. Chem. Eng. J. 2020, 391, 123611. [Google Scholar] [CrossRef]
- Zhu, Z.; Lu, W.; Li, N.; Xu, T.; Chen, W. Pyridyl-Containing Polymer Blends Stabilized Iron Phthalocyanine to Degrade Sulfonamides by Enzyme-Like Process. Chem. Eng. J. 2017, 321, 58–66. [Google Scholar] [CrossRef]
- Piccirillo, G.; Moreira-Santos, M.; Válega, M.; Eusébio, M.E.S.; Silva, A.M.S.; Ribeiro, R.; Freitas, H.; Pereira, M.M.; Calvete, M.J.F. Supported Metalloporphyrins as Reusable Catalysts for the Degradation of Antibiotics: Synthesis, Characterization, Activity and Ecotoxicity Studies. Appl. Catal. B Environ. 2021, 282, 119556. [Google Scholar] [CrossRef]
- Nackiewicz, J.; Kolodziej, L.; Poliwoda, A.; Broda, M.A. Oxidation of Diclofenac in the Presence of Iron(II) Octacarboxyphthalocyanine. Chemosphere 2021, 265, 129145. [Google Scholar] [CrossRef] [PubMed]
- Barros, W.R.P.; Borges, M.P.; Steter, J.R.; Forti, J.C.; Rocha, R.S.; Lanza, M.R.V. Degradation of Dipyrone by Electrogenerated H2O2 Combined with Fe2+ Using a Modified Gas Diffusion Electrode. J. Electrochem. Soc. 2014, 161, H867–H873. [Google Scholar] [CrossRef] [Green Version]
- Sułek, A.; Pucelik, B.; Kuncewicz, J.; Dubin, G.; Dąbrowski, J.M. Sensitization of Tio2 by Halogenated Porphyrin Derivatives for Visible Light Biomedical and Environmental Photocatalysis. Catal. Today 2019, 335, 538–549. [Google Scholar] [CrossRef]
- Wang, S.L.; Ma, W.H.; Jia, M.K.; Huang, Y.P. Degradation of Pollutants by Hydrophobic FePCCl16 under Ultraviolet and Visible Light. Fresenius Environ. Bull. 2013, 22, 549–555. [Google Scholar]
- Qian, H.; Yu, G.; Hou, Q.; Nie, Y.; Bai, C.; Bai, X.; Wang, H.; Ju, M. Ingenious Control of Adsorbed Oxygen Species to Construct Dual Reaction Centers Zno@Fepc Photo-Fenton Catalyst with High-Speed Electron Transmission Channel for Ppcps Degradation. Appl. Catal. B Environ. 2021, 291, 120064. [Google Scholar] [CrossRef]
- Gao, Y.X.; Xia, J.; Liu, D.C.; Kang, R.X.; Yu, G.; Deng, S.B. Synthesis of Mixed-Linker Zr-Mofs for Emerging Contaminant Adsorption and Photodegradation under Visible Light. Chem. Eng. J. 2019, 378, 122118. [Google Scholar] [CrossRef]
- Gao, Y.X.; Lu, J.; Xia, J.; Yu, G. In Situ Synthesis of Defect-Engineered Mofs as a Photoregenerable Catalytic Adsorbent: Understanding the Effect of Lml, Adsorption Behavior, and Photoreaction Process. ACS Appl. Mater. Interfaces 2020, 12, 12706–12716. [Google Scholar] [CrossRef]
- Wu, M.H.; Fu, K.; Deng, H.P.; Shi, J. Cobalt Tetracarboxyl Phthalocyanine-Manganese Octahedral Molecular Sieve (OMS-2) as a Heterogeneous Catalyst of Peroxymonosulfate for Degradation of Diclofenac. Chemosphere 2019, 219, 756–765. [Google Scholar] [CrossRef]
- Chapman, C.M.; Pruneau, J.M.; Laverack, C.A.; Dutton, A.S.; Jones, G.B. Biomimetic Oxidation of Acetaminophen Prodrugs Catalyzed by Iron Porphyrins: Effect of Nitrogen and Thiolate Axial Ligands on Drug and Metabolite Formation. Appl. Catal. A Gen. 2016, 510, 204–215. [Google Scholar] [CrossRef]
- Wang, L.; Lu, W.; Ni, D.; Xu, T.; Li, N.; Zhu, Z.; Chen, H.; Chen, W. Solar-Initiated Photocatalytic Degradation of Carbamazepine on Excited-State Hexadecachlorophthalocyanine in the Presence of Peroxymonosulfate. Chem. Eng. J. 2017, 330, 625–634. [Google Scholar] [CrossRef]
- Xu, T.; Ni, D.; Chen, X.; Wu, F.; Ge, P.; Lu, W.; Hu, H.; Zhu, Z.; Chen, W. Self-Floating Graphitic Carbon Nitride/Zinc Phthalocyanine Nanofibers for Photocatalytic Degradation of Contaminants. J. Hazard. Mater. 2016, 317, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Wang, D.; Dong, L.; Shen, H.; Lu, W.; Chen, W. Graphitic Carbon Nitride Co-Modified by Zinc Phthalocyanine and Graphene Quantum Dots for the Efficient Photocatalytic Degradation of Refractory Contaminants. Appl. Catal. B Environ. 2019, 244, 96–106. [Google Scholar] [CrossRef]
- Wu, F.; Huang, H.; Xu, T.; Lu, W.; Li, N.; Chen, W. Visible-Light-Assisted Peroxymonosulfate Activation and Mechanism for the Degradation of Pharmaceuticals over Pyridyl-Functionalized Graphitic Carbon Nitride Coordinated with Iron Phthalocyanine. Appl. Catal. B Environ. 2017, 218, 230–239. [Google Scholar] [CrossRef]
- Dong, L.; Xu, T.; Chen, W.; Lu, W. Synergistic Multiple Active Species for the Photocatalytic Degradation of Contaminants by Imidazole-Modified g-C3N4 Coordination with Iron Phthalocyanine in the Presence of Peroxymonosulfate. Chem. Eng. J. 2019, 357, 198–208. [Google Scholar] [CrossRef]
- Zhu, Z.; Lu, W.; Xu, T.; Li, N.; Wang, G.; Chen, W. High-Valent Iron-Oxo Complexes as Dominant Species to Eliminate Pharmaceuticals and Chloride-Containing Intermediates by the Activation of Peroxymonosulfate under Visible Irradiation. Catal. Lett. 2020, 150, 1355–1367. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Chen, Y.; Gu, Y.; Wu, F.; Lu, W.; Xu, T.; Chen, W. Catalytic Degradation of Recalcitrant Pollutants by Fenton-Like Process Using Polyacrylonitrile-Supported Iron (II) Phthalocyanine Nanofibers: Intermediates and Pathway. Water Res. 2016, 93, 296–305. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, F.; Zhu, Z.; Xu, T.; Lu, W. Identification of O-Bridge Iron Perfluorophthalocyanine Dimer and Generation of High-Valent Diiron-Oxo Species for the Oxidation of Organic Pollutants. Chem. Eng. J. 2017, 328, 915–926. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhou, M.; Li, N.; Yao, Y.; Chen, W.; Lu, W. Degradation of Carbamazepine by MWCNTs-Promoted Generation of High-Valent Iron-Oxo Species in a Mild System with O-Bridged Iron Perfluorophthalocyanine Dimers. J. Environ. Sci. 2021, 99, 260–266. [Google Scholar] [CrossRef]
- Kruid, J.; Fogel, R.; Limson, J. Unsubstituted Metallophthalocyanine Catalysts for the Removal of Endocrine Disrupting Compounds Using H2O2 as Oxidant. Environ. Sci. Pollut. Res. 2018, 25, 32346–32357. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, L.; Borzecka, W.; Lin, Z.; Schneider, R.J.; Huvaere, K.; Esteves, V.I.; Cunha, A.; Tome, J.P.C. Nanomagnet-Photosensitizer Hybrid Materials for the Degradation of 17 Beta-Estradiol in Batch and Flow Modes. Dyes Pigm. 2017, 142, 535–543. [Google Scholar] [CrossRef]
- Savitha, R.; Raghunathan, R.; Nolan, K.; Morrissey, A.; Selvam, P.; Chetty, R. Evaluation of Visible-Light Driven Photocatalytic Reaction by Porphyrin Coupled TiO2 Nanotubes Obtained Via Rapid Breakdown Anodization. J. Environ. Chem. Eng. 2020, 8, 104382. [Google Scholar] [CrossRef]
- Neves, C.M.B.; Filipe, O.M.S.; Mota, N.; Santos, S.A.O.; Silvestre, A.J.D.; Santos, E.B.H.; Neves, M.G.P.M.S.; Simões, M.M.Q. Photodegradation of Metoprolol Using a Porphyrin as Photosensitizer under Homogeneous and Heterogeneous Conditions. J. Hazard. Mater. 2019, 370, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Minhui, W.; Jun, S.; Chao, D.; Huiping, D. Binuclear Cobalt Phthalocyanine Supported on Manganese Octahedral Molecular Sieve: High-Efficiency Catalyzer of Peroxymonosulfate Decomposition for Degrading Propranolol. Sci. Total Environ. 2019, 686, 97–106. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccirillo, G.; Aroso, R.T.; Rodrigues, F.M.S.; Carrilho, R.M.B.; Pinto, S.M.A.; Calvete, M.J.F.; Pereira, M.M. Oxidative Degradation of Pharmaceuticals: The Role of Tetrapyrrole-Based Catalysts. Catalysts 2021, 11, 1335. https://doi.org/10.3390/catal11111335
Piccirillo G, Aroso RT, Rodrigues FMS, Carrilho RMB, Pinto SMA, Calvete MJF, Pereira MM. Oxidative Degradation of Pharmaceuticals: The Role of Tetrapyrrole-Based Catalysts. Catalysts. 2021; 11(11):1335. https://doi.org/10.3390/catal11111335
Chicago/Turabian StylePiccirillo, Giusi, Rafael T. Aroso, Fábio M. S. Rodrigues, Rui M. B. Carrilho, Sara M. A. Pinto, Mário J. F. Calvete, and Mariette M. Pereira. 2021. "Oxidative Degradation of Pharmaceuticals: The Role of Tetrapyrrole-Based Catalysts" Catalysts 11, no. 11: 1335. https://doi.org/10.3390/catal11111335
APA StylePiccirillo, G., Aroso, R. T., Rodrigues, F. M. S., Carrilho, R. M. B., Pinto, S. M. A., Calvete, M. J. F., & Pereira, M. M. (2021). Oxidative Degradation of Pharmaceuticals: The Role of Tetrapyrrole-Based Catalysts. Catalysts, 11(11), 1335. https://doi.org/10.3390/catal11111335