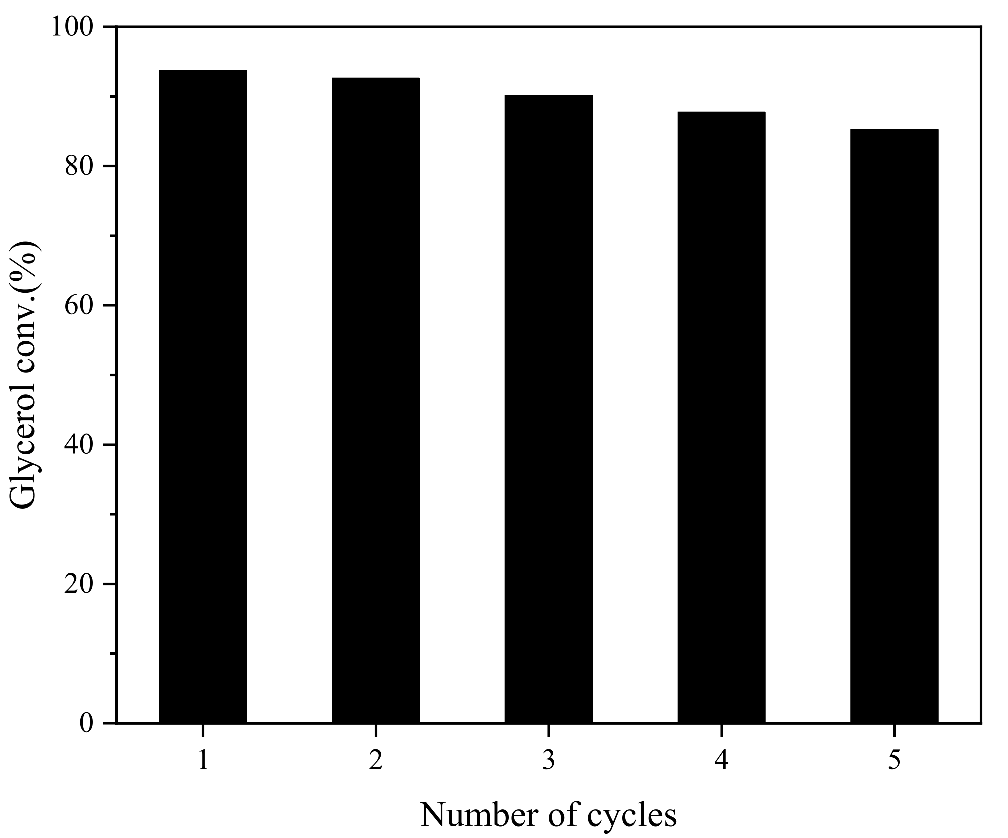1. Introduction
Cyclodextrin (CD) usually contains several D-glucopyranose units connected by α-glycosidic bonds as the degradation product of amylose [
1,
2]. The outer side of truncated conical cyclodextrin is hydrophilic due to the ports with rich hydroxyl while the inner cavity is hydrophobic owing to the shielding effect of carbon chains [
3]. This special structure enables different organic compounds embedded in the inner cavity of cyclodextrin to exist in hydrophilic solvents, which is necessary for the formation of inclusion complexes and molecular assembly systems [
4,
5,
6].
A-Cyclodextrin, β-Cyclodextrins and γ-Cyclodextrin containing 6, 7 and 8 glucopyranosyl units respectively were three kinds of common cyclodextrins [
7]. β-CD was widely used and studied because of its moderate cavity size, excellent physicochemical properties and relatively low price [
8,
9]. Cyclodextrin derivatives and their composite with different materials greatly expanded the application of cyclodextrin in different fields [
10].
Chen et al. [
11] synthesized water-soluble complexes by nestification of β-cyclodextrin and a variety of hydrophobic monomers. The even dispersion of hydrophobic monomers in aqueous solution realized the aqueous dispersion polymerization by induced self-assembly of monomers. Prochowicz et al. [
12] considered that the coordination flexibility of β-cyclodextrin and metal ions enabled the properties of β-cyclodextrin derivatives controllable in aqueous environment. Trans et al. [
13] used β-Cyclodextrin derivatives as ligands of rhodium ions to catalyze the aqueous hydroformylation of water-soluble olefins. The structure of β-cyclodextrin derivatives played an important role on the reaction regioselectivity.
Hydrotalcites has developed rapidly in recent years as columnar compounds composed of positively charged brucite layers and negatively charged anions filled between layers [
14,
15,
16,
17]. Takagaki et al. [
18] considered hydrotalcites as an efficient solid base catalyst for glycerol carbonate formation from glycerol with dialkyl carbonates under mild reaction conditions. Szabados et al. [
19] synthesized different hydrotalcites using different metal ions and verified their catalytic activity in the transesterification of glycerol and dimethyl carbonate. Zheng et al. [
20] and Granados-Reyes et al. [
21] used Mg-Al hydrotalcites and Ca-Al hydrotalcites as catalyst for the transesterification of glycerol and dimethyl carbonate. The selectivity towards GC was 97.1% catalyzed by Mg-Al hydrotalcites while the selectivity catalyzed by Ca-Al hydrotalcites depended on the content of calcium in the catalyst.
Different kinds of ion groups such as organic groups, inorganic groups, isopoly anions and heteropoly anions can be introduced into hydrotalcites to change the layer spacing or enhance the active centers in hydrotalcites to obtain various functional composites through the design and modification of interlayer exchangeable anions of hydrotalcites [
22,
23,
24,
25,
26,
27].
Wang et al. [
28] introduced sulfonated β-cyclodextrin into the interlayer of Mg-Al hydrotalcites by ion exchange. XRD analysis proved that β-cyclodextrin were arranged with single layer in hydrotalcite layers and the intercalation of β-cyclodextrin increased the interlayer spacing of hydrotalcites. Mohanambe et al. [
29] synthesized hydrophobic nano inclusion complexes by introducing β-Cyclodextrin into the interlayer of hydrotalcites. Anthracene molecules of polar solvent can be effectively immobilized in the nano inclusion complexes.
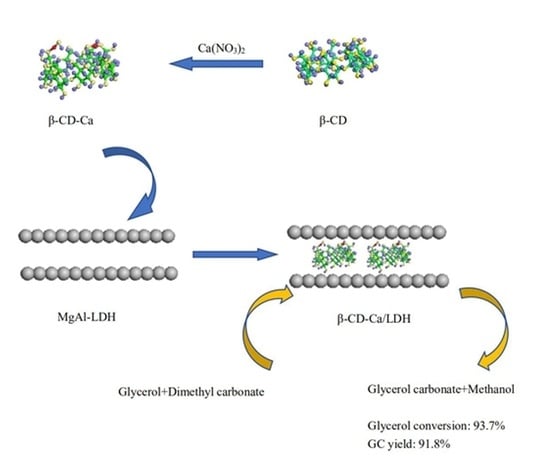
β-cyclodextrin metal complexes (β-CD-M) with alkaline centers were prepared and intercalated into MgAl-LDH in this thesis as heterogeneous catalyst for GC synthesis by transesterification between glycerol and dimethyl carbonate. The structural changes of hydrotalcites before and after intercalation with β-CD-Ca were analyzed by FT-IR, XRD, SEM and nitrogen adsorption-desorption. The optimal reaction conditions were determined by single variable method. The catalytic performance and reuse ability of β-CD-Ca/LDH in transesterification was investigated.
2. Results and Discussion
2.1. Characterization of Synthetic β-Cyclodextrin Derivatives
β-cyclodextrin-metal complexes were characterized by FT-IR, XRD, XPS and alkalinity determination respectively as shown in
Figure 1 and
Figure 2 and
Table 1.
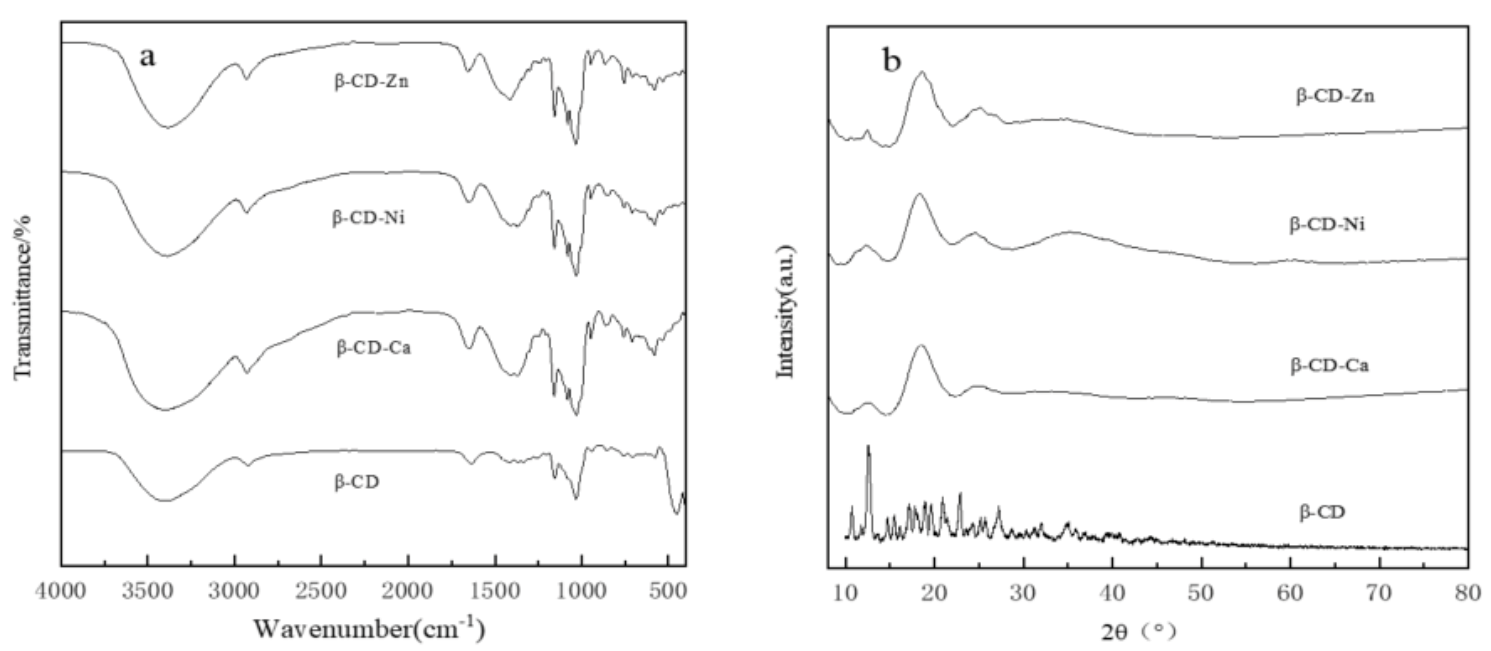
The FT-IR spectra of β-CD, β-CD-Zn, β-CD-Ni and β-CD-Ca were shown in
Figure 1a. The absorption peak of β-CD and β-CD-M at 3450 cm
−1 was attributed to the stretching vibration of hydroxyl in glucopyranose units. The stretching vibration peak of C-O bond in glucopyranose units appeared at 1460 cm
−1. The absorption peak at 1590 cm
−1 corresponded to the stretching vibration peak of carbon skeleton. The characteristic peak of β-CD-Ca at 550 cm
−1 can be ascribed to the stretching vibration of metal-OH(M-OH) bond, which was absent in the FT-IR spectrum of β-CD. Everything above indicated that β-CD-Zn, β-CD-Ni and β-CD-Ca with M-OH group was synthesized by the reaction of β-cyclodextrin with zinc nitrate, nickel nitrate and calcium nitrate.
The crystal structures of β-CD, β-CD-Zn, β-CD-Ni and β-CD-Ca were analyzed by XRD as shown in
Figure 1b. The XRD pattern of β-CD exhibited sharp characteristic diffraction peaks while almost no obvious diffraction peaks can be observed in the XRD pattern of β-CD-Zn, β-CD-Ni and β-CD-Ca, which suggested that the cyclodextrin metal complexes presented poor crystallinity and amorphous state. This was mainly because the introduction of metal ions changed the original crystal phase of β-cyclodextrin.
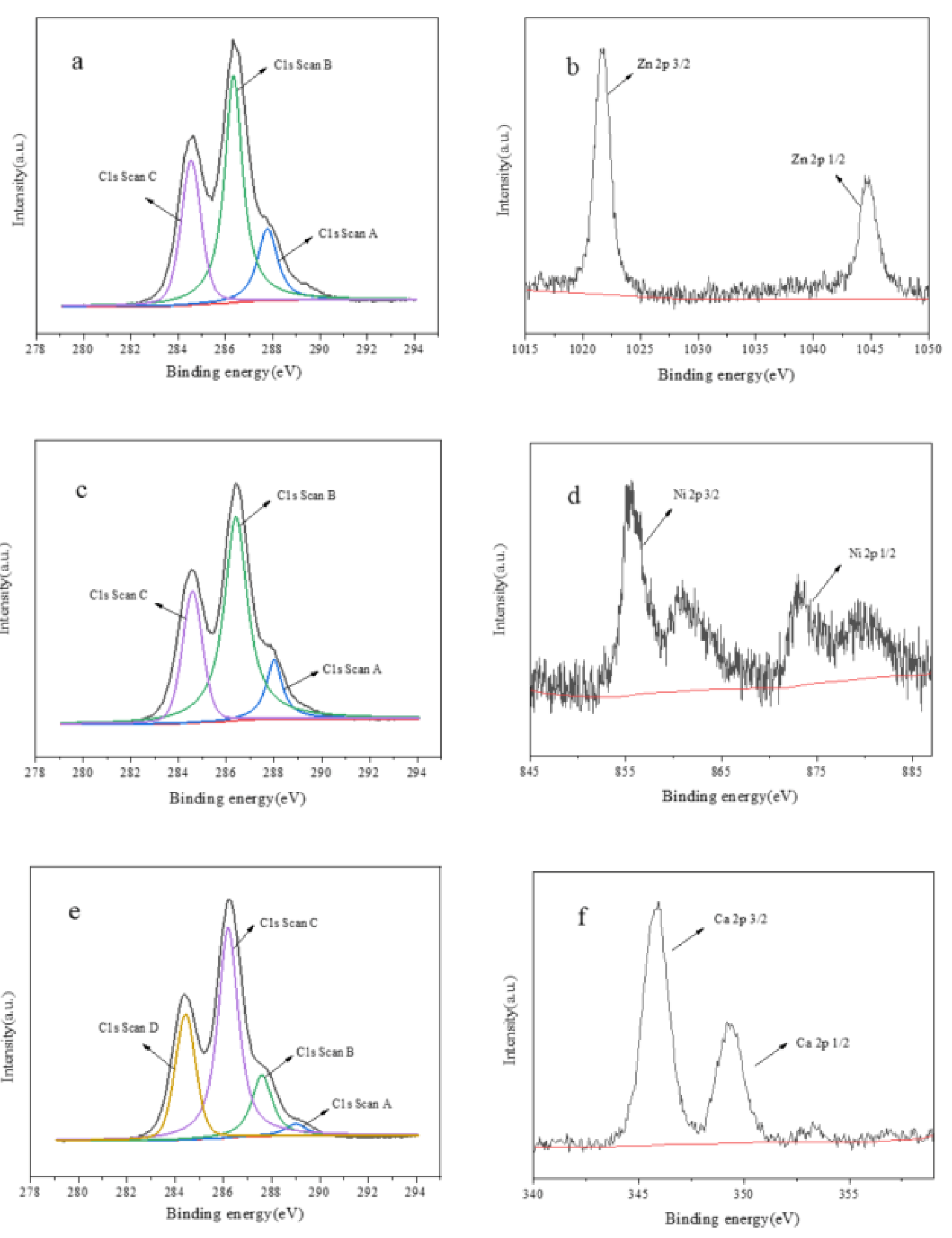
XPS can be used to determine the bonding forms between metal and β-cyclodextrin in β-CD-Zn, β-CD-Ni and β-CD-Ca. The blinding energies of C 1s and Zn 2p in β-CD-Ni were displayed in
Figure 2a,b. Three types of C species, C=O, C-O, C-C were observed where binding energies were 284.4 eV, 286.5 eV and 288.1 eV. The binding energies of Zn 2p were 1022 eV and 1045 eV, indicating the existence of Zn-O bond. It can be inferred that C-O-Zn-OH had been formed according to the groups contained in β-cyclodextrin and the synthesis method of complexes. The C 1s spectrum of β-CD-Ni was similar to that of β-CD-Zn in
Figure 2c. The binding energies of Ni 2p were 855.5 eV and 874.0 eV in
Figure 2d, which also suggested the existence of C-O-Ni-OH.
The blinding energies of C 1s and Ca 2p in β-CD-Ca were displayed in
Figure 2e,f. The C 1s spectrum revealed the presence of four types of C species, C=O, C-O, C-C and O-C=O at which the binding energies were 284.4 eV, 286.5 eV, 288.1 eV and 289.2 eV, respectively. The binding energies of Ca 2p were 346.5 eV and 348.7 eV, which proved the formation of C-O-Ca-OH and O=C-O-Ca when Ca
2+ interacted with β-CD according to the XPS spectra of β-CD-Ca. The interaction between metal ions and β-cyclodextrin increased the alkalinity of β-cyclodextrin-metal complexes at different levels. The data in
Table 1 proved that β-CD-Ca presented stronger alkalinity than β-CD-Zn, β-CD-Ni and β-CD.
2.2. Characterization of β-CD-Ca Intercalated MgAl Hydrotalcites
The composition and crystal structures of β-CD-Ca/LDH were analyzed by XRD, FT-IR, SEM and N
2 adsorption-desorption as shown in
Figure 3 and
Table 2 and
Table 3.
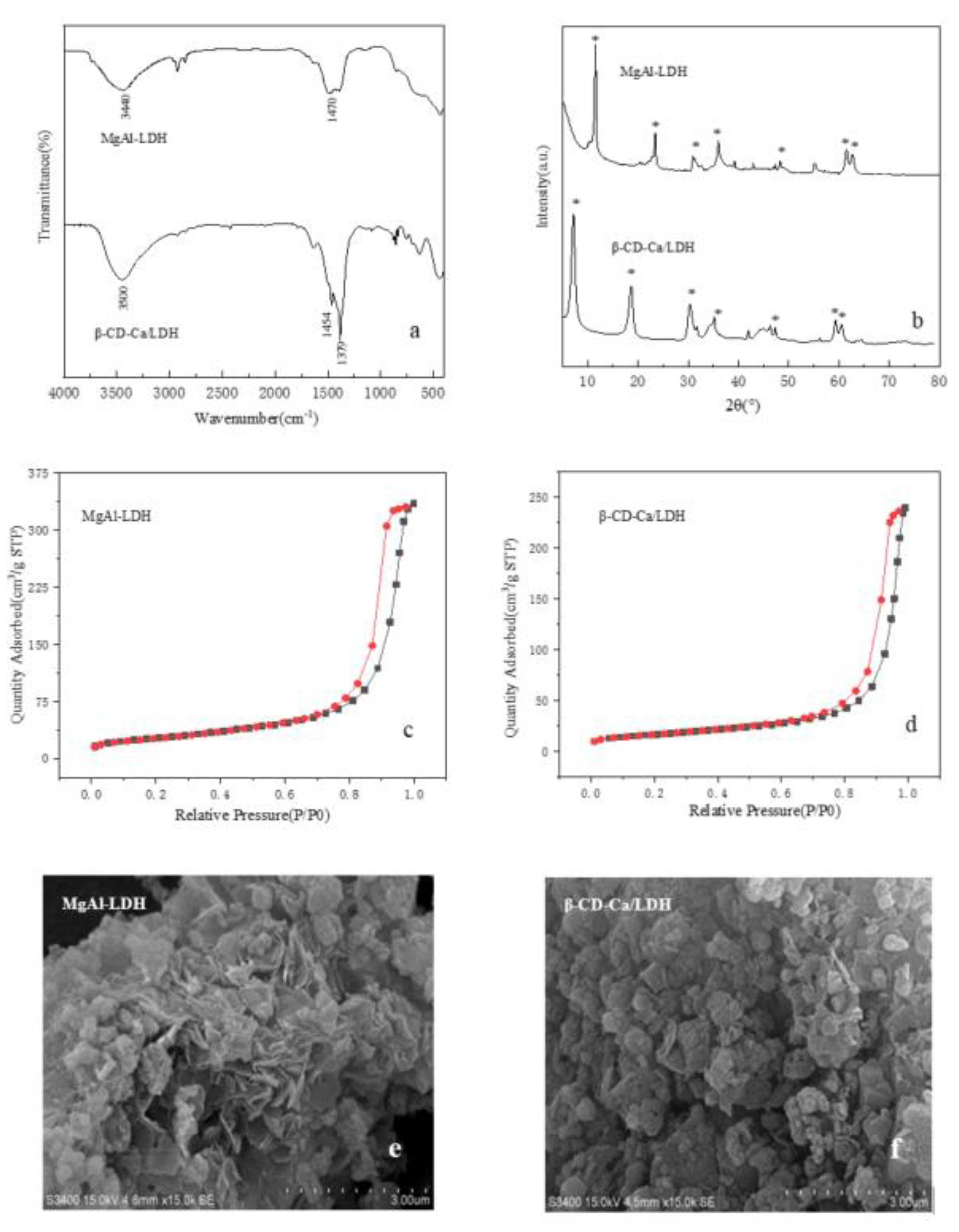
Figure 3a was the FT-IR spectra of MgAl-LDH and β-CD-Ca/LDH. The wide absorption peak at 3440 cm
−1 in MgAl-LDH was caused by the stretching vibration of hydroxyl in hydrotalcite laminates. The absorption peak at 1470 cm
−1 corresponded to C-O bond in the carbonate of hydrotalcites. The peak intensity of hydroxyl at about 3500 cm
−1 was enhanced in the spectrum of β-CD-Ca/LDH because of rich hydroxyl in β-cyclodextrin molecules. The absorption peaks at 1454 cm
−1 and 1379 cm
−1 were ascribed to the stretching vibration of C-O and C-O-C in glucopyranose units of β-cyclodextrin. The characteristic peak of β-cyclodextrin can be observed in the spectrum of β-CD-Ca/LDH, which means that β-CD-Ca had been successfully inserted into the interlayer of MgAl hydrotalcites.
Figure 3b exhibited XRD spectra of MgAl hydrotalcites before and after intercalation by β-CD-Ca. The characteristic diffraction peaks of hydrotalcites (003), (006), (009), (015), (018), (110) and (113) can be clearly observed when 2θ is 10.14°, 21.70°, 33.36°, 38.18°, 46.40°, 59.42° and 60.68°. The insertion of β-cyclodextrin metal complexes did not destroy the crystal structures of hydrotalcites. The narrow and sharp diffraction peaks with high intensity indicated that the synthesized β-CD-Ca/LDH presented high crystallinity and regular structures.
Table 2 displayed the crystal structure parameters of MgAl-LDH and β-CD-Ca/LDH. The spacing D of each characteristic crystal plane can be obtained by Bragg equation. D (003) was the interlayer spacing of hydrotalcites. The calculation results showed that the interlayer spacing of MgAl-LDH and β-CD-Ca/LDH was 0.7655 nm and 1.238 nm, which indicated the intercalation of β-CD-Ca increased the interlayer spacing of hydrotalcites effectively. Moreover, the intercalation by β-CD-Ca in Mg-Al hydrotalcites not only expanded the pore size of catalyst but also enhanced the basic strength of catalyst as shown in
Table 3, which was beneficial for the formation of glycerol anions.
Figure 3c,d showed the adsorption isotherms of hydrotalcites before and after intercalation by β-CD-Ca. Both adsorption isotherms with obvious hysteresis loop belonged to Type IV isotherm in IUPAC classification, indicating MgAl-LDH and β-CD-Ca/LDH were mesoporous materials. The hysteresis loops with saturated adsorption platform in the adsorption isotherm suggested the regular crystal structures and the uniform pore size distribution of MgAl-LDH and β-CD-Ca/LDH.
The specific surface area and pore size of MgAl-LDH and β-CD-Ca/LDH were shown in
Table 4. The pore size of hydrotalcites increased from 17.78 nm to 19.22 nm due to the intercalation of β-CD-Ca. The layer expansion of hydrotalcites was beneficial for the reduction of diffusion resistance and the collision between reactants and active sites in the catalytic reaction, thus increasing the reaction efficiency. However, some layers of hydrotalcites were squeezed due to the insertion of β-CD-Ca, which resulted in the disappearance of some pore structures and the decrease of specific surface area.
The morphological characteristics of hydrotalcites before and after intercalation by β-CD-Ca were observed via SEM. The petal-like lamellar structures of hydrotalcites can be seen in
Figure 3e,f. There were visible pore structures on the rough surfaces of MgAl-LDH and β-CD-Ca/LDH, which was conducive to the diffusion and circulation of reactants in the transesterification system. The lamellae of hydrotalcites was distorted in some way after intercalation by β-CD-Ca. The intercalation by β-CD-Ca in Mg-Al hydrotalcites not only expanded the pore size of catalyst but also enhanced the basic strength of catalyst as shown in
Table 4.
2.3. Effect of Different β-CD-M on Transesterification
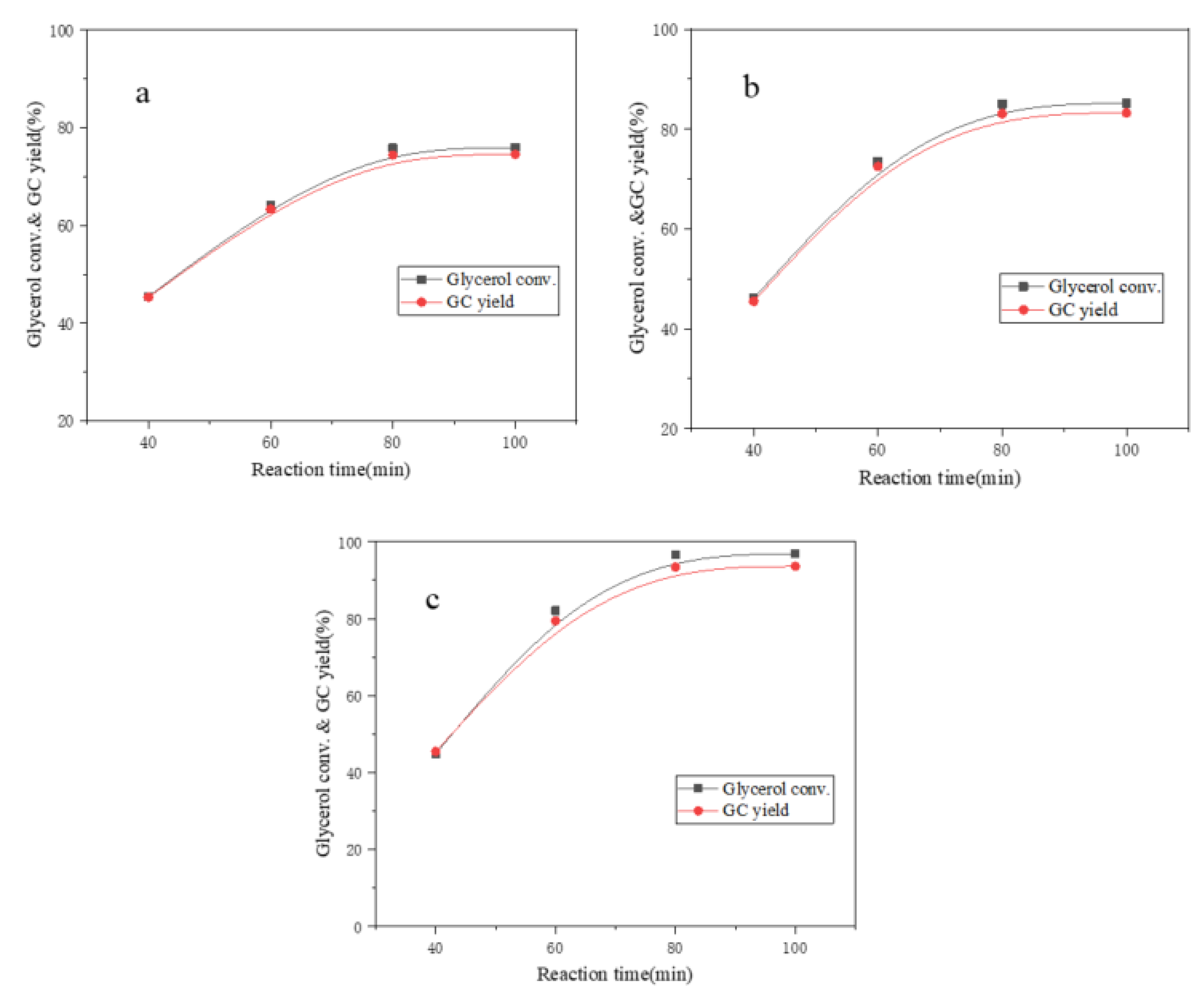
The effect of different β-cyclodextrin-metal complexes on the transesterification of DMC and glycerol was investigated under the conditions of 3:1 molar ratio of DMC and glycerol, 1 wt.% catalyst dosage, 75 °C reaction temperature and 90 min reaction time.
Figure 4 showed the experimental results of β-cyclodextrin-metal complexes as catalyst for transesterification. The glycerol conversion catalyzed by β-CD-Zn, β-CD-Ni and β-CD-Ca increased from 45.4%, 46.1% and 46.9% to 75.8%, 85.0% and 96.6% respectively when the reaction time increased from 40 min to 80 min. The GC yield increased from 45.1%, 45.5% and 46.2% to 74.4%, 83.1% and 93.4% respectively. The basic groups C-O-Ca-OH, C-O-Zn-OH and C-O-Ni-OH were formed by the coordination of Zn
2+, Ni
2+ and Ca
2+ with port hydroxyl of β-cyclodextrin. In addition, the enrichment of reactants in the lipophilic cavity of β-cyclodextrin increased the collision probability of reactants. The glycerol molecules were activated by the interaction with basic hydroxyl when entering the cavity of β-cyclodextrin. The generated glycerol anions attacked the carbonyl in dimethyl carbonate molecules, thus initiating the transesterification.
The experimental results showed the synthesized β-cyclodextrin-metal complexes presented different catalytic ability on the transesterification. Nickel ions and zinc ions tended to form the cap-structure due to the existence of d orbit, thus hindering the entry and exit of substances at the port of β-cyclodextrin, which led to weaker catalytic effect than β-CD-Ca. Calcium ions were not easy to form metal bridging complexes compared with transition metal ions nickel and zinc, which was conducive to the contact of reactants. Therefore, β-CD-Ca was used as a research object in the further experiment.
2.4. Effect of Molar Ratio of β-Cyclodextrin and Metal Ions in β-CD-Ca on Transesterification
The effect of molar ratio of β-cyclodextrin and metal ions in β-CD-Ca on catalytic performance was investigated under the conditions of 3:1 molar ratio of DMC and glycerol, 1 wt.% catalyst dosage, 75 °C reaction temperature and 90 min reaction time.
The glycerol conversion rate was 81.2%, and the GC yield was 80.3% when the molar ratio of β-cyclodextrin and calcium ion was 1:1 in
Figure 5. The glycerol conversion rate increased to 96.6% and the GC yield reached 93.4% when the molar ratio of β-cyclodextrin and calcium ion was 1:2. However, excessive metal ions formed coordination bonds at the ring port of β-cyclodextrin and prevented reactant molecules from entering the β-cyclodextrin cavity. Therefore, the glycerol conversion and GC yield decreased slightly when the content of Ca
2+ in β-CD-Ca continued to mount up.
2.5. Effect of β-CD-Ca Dosage in β-CD-Ca/LDH on Transesterification
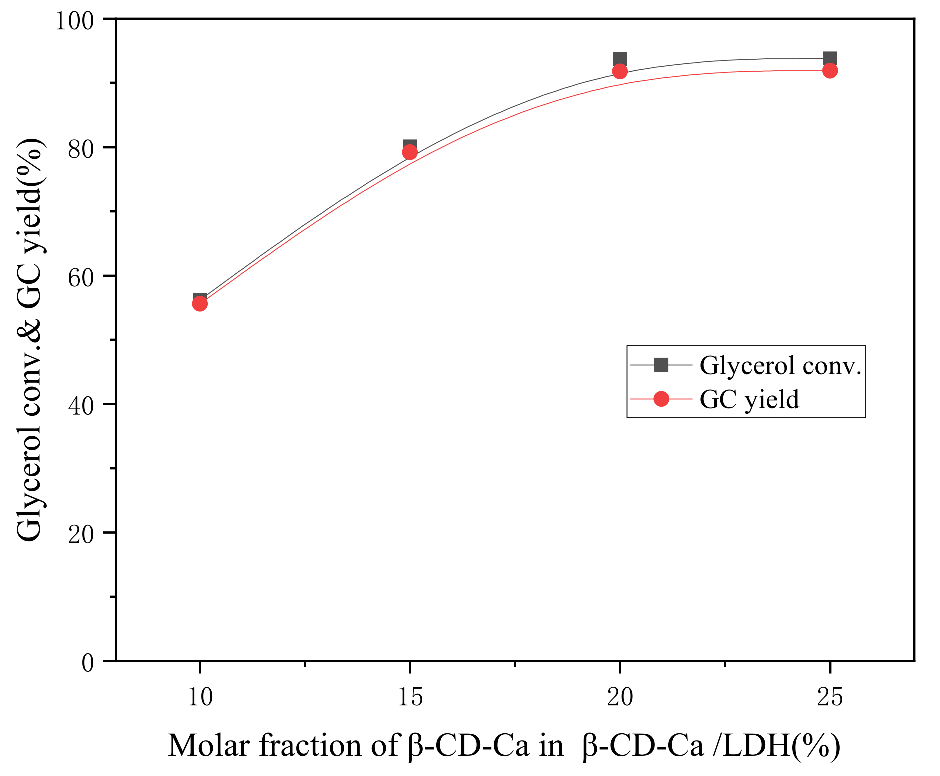
The effect of β-CD-Ca dosage in β-CD-Ca/LDH on the catalytic performance was investigated under the conditions of 3:1 molar ratio of DMC and glycerol, 4 wt.% catalyst dosage, 75 °C reaction temperature and 100 min reaction time.
The glycerol conversion rate increased from 56.2% to 93.7% and the GC yield increased from 54.6% to 91.8% when the mass fraction of β-CD-Ca in β-CD-Ca/LDH was risen from 10% to 20% as can be seen from
Figure 6. There was no significant change in glycerol conversion and GC yield when the content of β-CD-Ca continued to mount up. The active centers and layer spacing of hydrotalcites increased with the increase of β-CD-Ca amount, thus improving the catalytic performance on transesterification. However, the space between laminates becomes crowded with the increase of interlayer substances of hydrotalcites. Partial pores of catalyst that were blocked although active sites were ascended. Therefore, β-CD-Ca/LDH presented the best catalytic performance on transesterification between glycerol and DMC when the molar ratio of β-CD and Ca
2+ was 1:2 and the mass fraction of β-CD-Ca was 20% in β-CD-Ca/LDH.
2.6. Catalytic Performance of β-CD-Ca/LDH and MgAl-LDH on Transesterification
The catalytic performance of MgAl-LDH and β-CD-Ca/LDH on transesterification of dimethyl carbonate and glycerol was compared to investigate the effect of β-CD-Ca under the condition of 4 wt.% catalyst dosage, 3:1 molar ratio of DMC to glycerol at 75 °C.
The glycerol conversion increased from 42.0% to 93.7% and the GC yield increased from 41.2% to 91.8% catalyzed by β-CD-Ca/LDH when the reaction time rose from 60 min to 100 min in
Figure 7a. The glycerol conversion ascended from 35.0% to 49.4% and the GC yield ascended from 34.5% to 48.6% catalyzed by MgAl-LDH within the same time slot in
Figure 7b. The glycerol conversion and GC yield only rose to about 26% at 100 min without any catalyst in
Figure 7c. β-CD-Ca met the basicity requirement for transesterification reaction and the intercalation in hydrotalcites expanded the pore size of catalyst. In addition, the enrichment of organic reactants in β-cyclodextrin cavity was also beneficial to the catalytic reaction. Therefore, the catalytic performance of β-CD-Ca/LDH for transesterification was much better than that of MgAl-LDH.
2.7. Effect of Different Reaction Conditions on Transesterification
The reaction conditions were optimized since the glycerol conversion and GC yield depended strongly on the catalyst dosage, molar ratio of reactants, reaction temperature and reaction time.
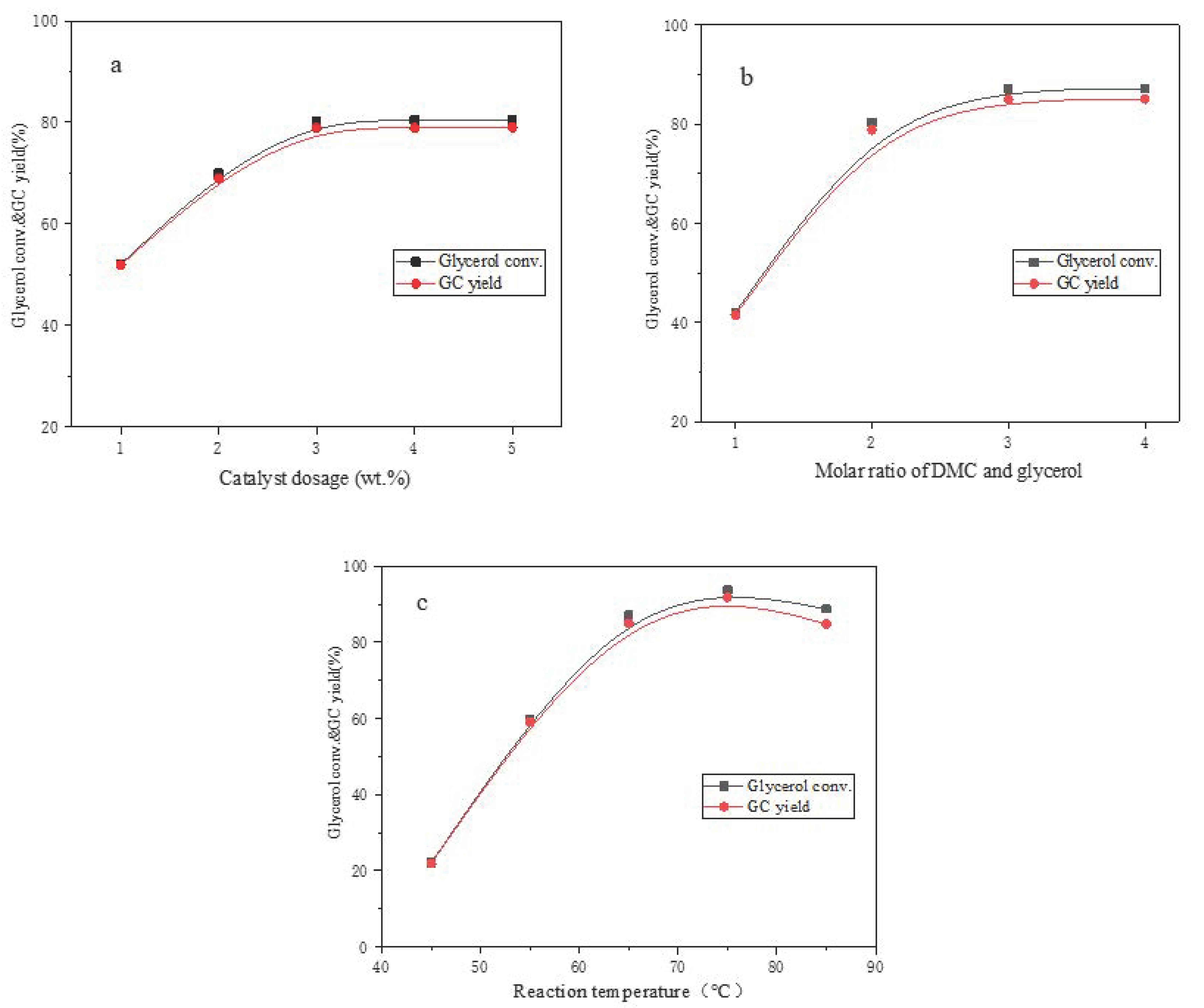
The effect of catalyst dosage on the catalytic performance was investigated as shown in
Figure 8a when the reaction temperature was 65 °C, the reaction time was 80 min and the molar ratio of dimethyl carbonate to glycerol was 2:1. The glycerol conversion and glycerol carbonate yield increased sharply to 80.3% and 78.9% when the amount of β-CD-Ca/LDH rose from 1 wt.% to 3 wt.% due to the increase of active centers. There was no obvious upward tendency in glycerol conversion and GC yield with the further increase of catalyst dosage, which was mainly because the mass transfer resistance caused by the agglomeration solid catalyst particles reduced the utilization rate of β-CD-Ca/LDH. Therefore, 3 wt.% can be considered as the optimal dosage of β-CD-Ca/LDH in the reaction.
The effect of reactant ratio on the catalytic performance of β-CD-Ca/LDH was displayed in
Figure 8b when the catalyst dosage was 3 wt.%, the reaction time was 80 min and the reaction temperature was 65 °C. The increased reactant concentration forced the reaction equilibrium moving towards the products as the transesterification of dimethyl carbonate with glycerol was reversible. The glycerol conversion increased from 42.1% to 87.1% and GC yield increased from 41.5% to 85.0% when the molar ratio of dimethyl carbonate to glycerol ascended from 1:1 to 3:1. There was no significant change in glycerol conversion and GC yield when the proportion of dimethyl carbonate continued to mount up. Therefore, 3:1 molar ratio of dimethyl carbonate and glycerol was selected as the optimal reactant ratio for the transesterification.
The glycerol conversion and GC yield under different reaction temperature was exhibited in
Figure 8c when the catalyst dosage was 3 wt.%, the reaction time was 100 min and the molar ratio of dimethyl carbonate and glycerol was 3:1. The temperature rise of reaction system can effectively improve the collision probability of activated reaction molecules as well as increase the reaction rate. The glycerol conversion and the yield of glycerol carbonate turned out to be an ascending trend along with reaction temperature before 75 °C. However, the glycerol conversion and GC yield decreased when the temperature rose to the boiling point of dimethyl carbonate since the serious vaporization of reactant was unfavorable to the transesterification. In summary,
Figure 7 and
Figure 8 indicated that β-CD-Ca/LDH presented best catalytic performance when the catalyst dosage was 3 wt.%, the molar ratio of dimethyl carbonate and glycerol was 3:1, the reaction time was 100 min and the reaction temperature was 75 °C.
2.8. Reuse Ability of β-CD-Ca/LDH
The catalyst reuse experiment was carried out to investigate the stability of active centers in β-CD-Ca/LDH for the transesterification of DMC and glycerol. The reaction conditions were consistent with the above-mentioned conditions.
Figure 9 displayed the change of glycerol conversion during five reaction cycles. The conversion of glycerol can still reach more than 85% catalyzed by β-CD-Ca/LDH in the fifth cycles, which indicated β-CD-Ca as active component was stable in the hydrotalcites. This was mainly because the electrostatic attraction between rich hydroxyl groups on the offside of β-cyclodextrin and metal ions in hydrotalcites enhanced the binding ability between β-CD-Ca and MgAl-LDH. Therefore, β-CD-Ca/LDH still presented excellent catalytic performance after five times reuse. Mg-Al hydrotalcites presented interlayer anion exchangeability as layered materials. The functional guest substances can be introduced into the layer gap and expand interlayer spacing to form pillared compounds. The formation of C-O-Ca-OH after the coordination of β-cyclodextrin and calcium ions increased the negative charges of hydroxyl groups on the outside of β-cyclodextrin. The metal ions constituting the hydrotalcite laminates can be stably combined with the hydroxyl groups of β-cyclodextrin through electrostatic interaction when β-CD-Ca was intercalated into MgAl-LDH, which not only increased the layer spacing of hydrotalcites but also improved the reusability of the catalyst.
β-CD-Ca/LDH presented remarkable catalytic performance on the transesterification of dimethyl carbonate with glycerol. XRD analysis showed that β-CD-Ca increased the pore size of hydrotalcites, which enhanced mass transfer process in the reaction. The basicity determination proved that β-CD-Ca/LDH met the alkalinity requirement of catalysts in the transesterification due to the formation of C-O-Ca-OH observed in the FT-IR spectrum and XPS spectrum. The interaction between β-CD-Ca and MgAl hydrotalcites enabled β-CD-Ca/LDH to keep stable in the reaction system. Hence β-CD-Ca/LDH can be considered as an excellent catalyst for the synthesis of glycerol carbonate by transesterification.
4. Conclusions
β-cyclodextrin derivative β-CD-Ca was introduced into the interlayer of MgAl hydrotalcites by intercalation method to prepare β-CD-Ca/LDH catalyst for the preparation of glycerol carbonate by transesterification of dimethyl carbonate with glycerol. The intercalation of β-CD-Ca not only increased the alkalinity of hydrotalcites but also expanded the interlayer spacing of hydrotalcites, thus increasing the pore size of catalyst and reducing the mass transfer resistance in the transesterification. The enrichment of organic reactants in the hydrophobic cavity of β-cyclodextrin also raised the collision probability of activated molecules. The glycerol conversion and GC yield was 93.7% and 91.8% respectively catalyzed by β-CD-Ca/LDH when the molar ratio of DMC and glycerol was 3:1, the catalyst dosage was 4 wt.%, the reaction temperature was 75 °C and the reaction time was 100 min. Moreover, β-CD-Ca/LDH presented significant reusability. The glycerol conversion can still reach more than 85% in the fifth reaction cycle. Therefore, β-CD-Ca/LDH can be considered as solid base catalyst with potential application value in industry.