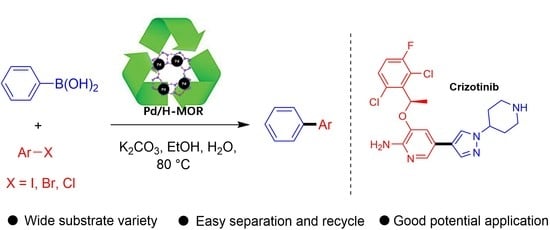
Novel Recyclable Pd/H-MOR Catalyst for Suzuki-Miyaura Coupling and Application in the Synthesis of Crizotinib
Abstract
:1. Introduction
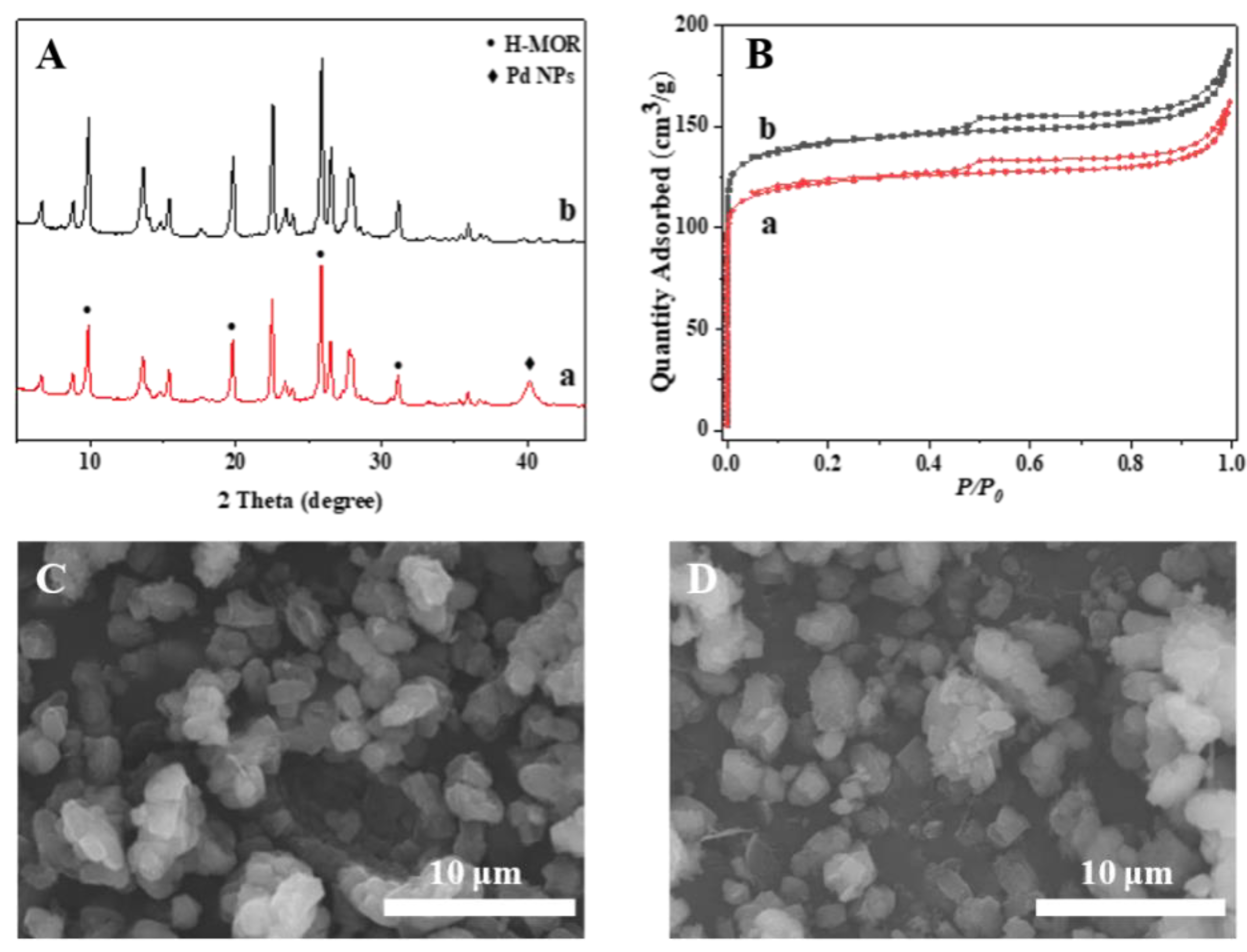
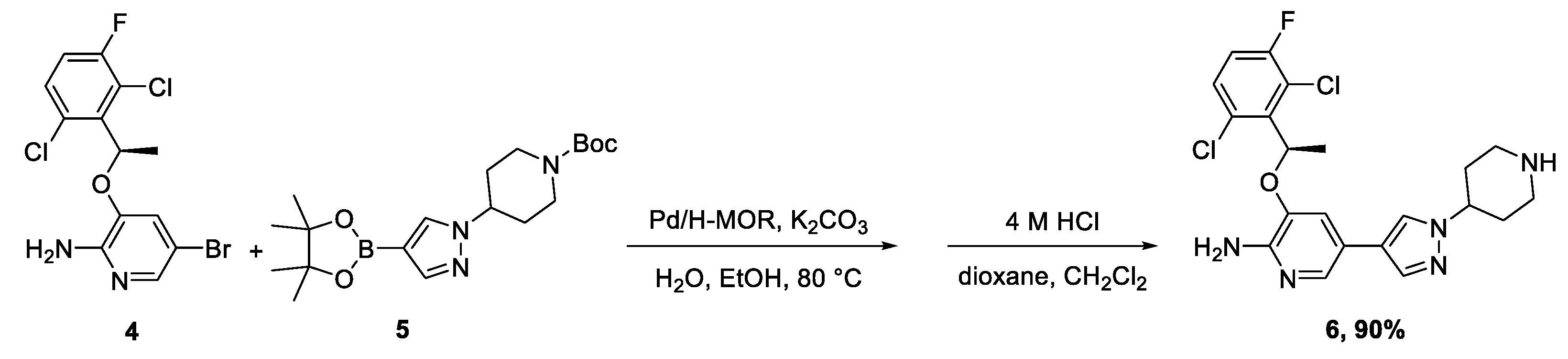
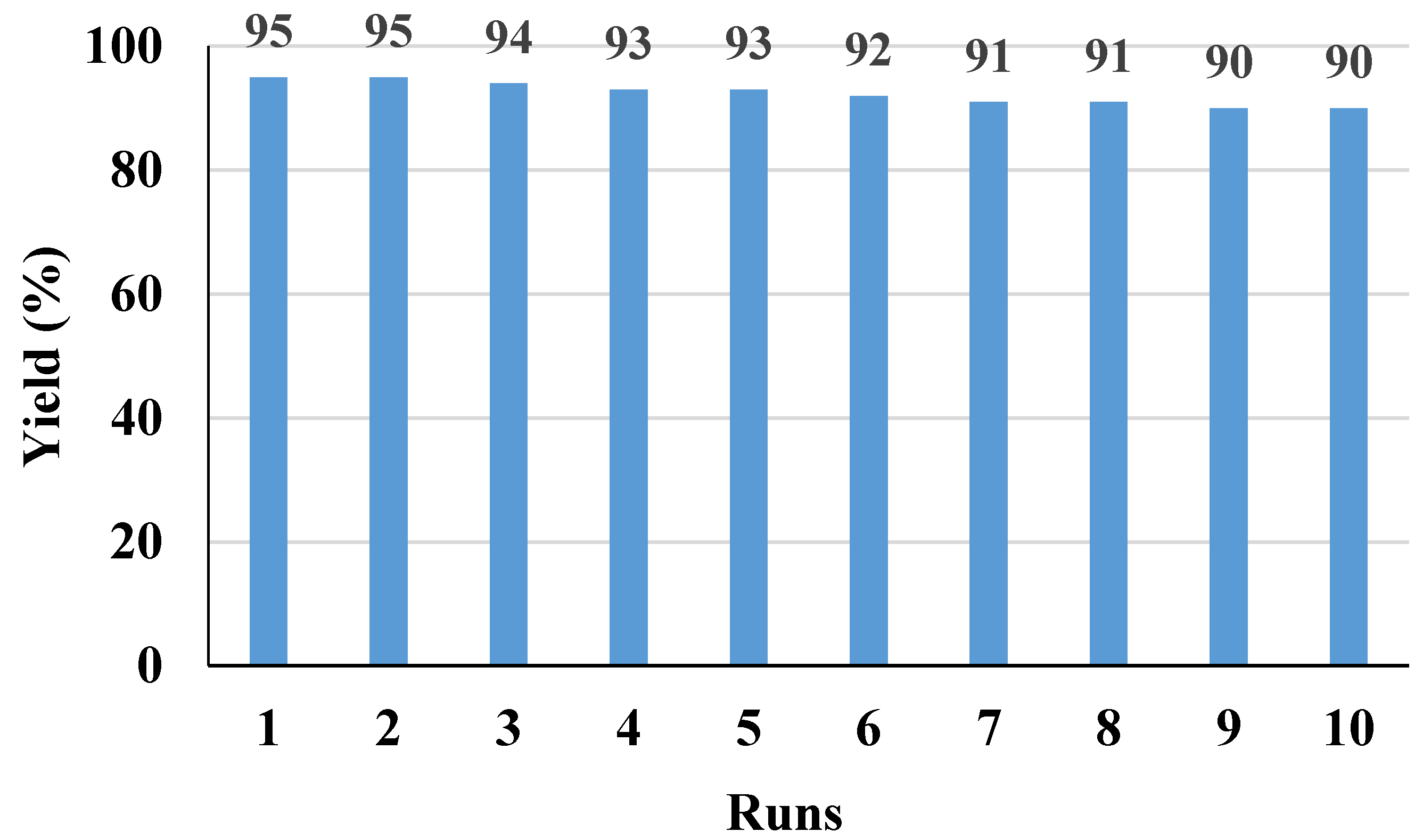
2. Results and Discussion
3. Experimental Materials
3.1. Characterization
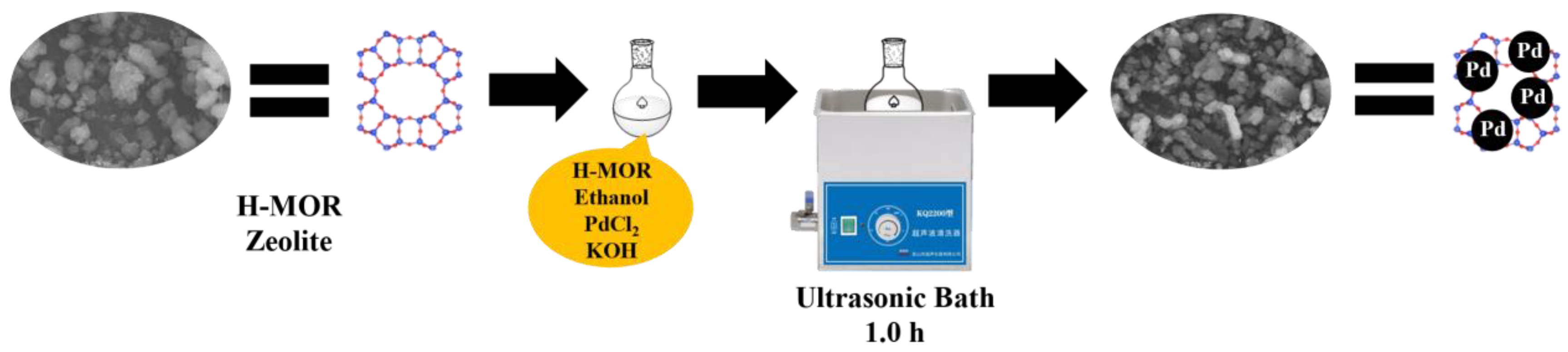
3.2. Preparation of Pd/H-MOR Catalyst
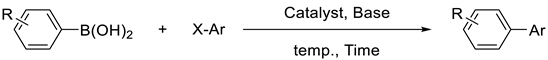
3.3. General Procedure for the Suzuki Coupling Reactions
3.4. General Procedure for Catalyst Recovery
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sen, T.; Jana, S.; Koner, S.; Patra, A. Efficient energy transfer between confined dye and Y-zeolite functionalized Au nanoparticles. J. Phys. Chem. C 2010, 114, 19667–19672. [Google Scholar] [CrossRef]
- Kumari, S.; Das, B.; Ray, S. An insight into the catalytic activity of palladium schiff-base complexes towards the Heck coupling reaction: Routes via encapsulation in zeolite-Y. Dalton Trans. 2019, 48, 15942–15954. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhu, J.; Qiao, J.; Yu, X.; Sun, N.; Bian, C.; Li, J.; Zhu, L. Solvent-free synthesis of Aluminosilicate SSZ-39 zeolite. Micropor. Mesopor. Mat. 2021, 312, 110736. [Google Scholar] [CrossRef]
- Cui, T.; Ke, W.; Zhang, W.; Wang, H.; Li, X.; Chen, J. Encapsulating palladium nanoparticles inside mesoporous MFI zeolite nanocrystals for shape-selective catalysis. Angew. Chem. Int. Ed. 2016, 55, 9178–9182. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Huang, B.; Liu, H.; Cai, M. A phosphine-free, atom-efficient cross-coupling reaction of triorganoindiums with acyl chlorides catalyzed by immobilization of palladium (0) in MCM-41. RSC Adv. 2017, 7, 42570–42578. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liao, J.; Xie, Z.; Zhang, K.; Wu, Y.; Zuo, P.; Zhang, W.; Li, J.; Gao, Z. Zeolite-enhanced sustainable Pd-catalyzed C-C cross-coupling reaction: Controlled release and capture of palladium. ACS Appl. Mater. Interfaces 2020, 12, 11419–11427. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fu, W.; Zhang, L.; Shen, R.; Cai, G.; Chen, Q.; Tang, T. Basic Cu/ETS-10-N catalyst induced the chemisorption and alkyl radical formation of the substrates enhanced the cinnamic acid decarboxylative cross-coupling reaction activity. Ind. Eng. Chem. Res. 2019, 58, 7014–7024. [Google Scholar] [CrossRef]
- Garniera, T.; Saklya, R.; Danela, M.; Chassaing, S.; Pale, P. Chan-Lam-Type C-N cross-coupling reactions under base- and ligand-free CuI-zeolite catalysis. Synthesis 2017, 49, 1223–1230. [Google Scholar]
- Moglie, Y.; Buxaderas, E.; Mancini, A.; Alonso, F.; Radivoy, G. Amide bond formation catalyzed by recyclable copper nanoparticles supported on zeolite Y under mild conditions. ChemCatChem 2019, 11, 1487–1494. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Cao, Y.; Fang, G.; Ruan, F.; Ke, Q. Synergistic effect between cerium species and micropores in CeAlPO-5 molecular sieves. ChemCatChem 2019, 11, 3178–3181. [Google Scholar] [CrossRef]
- Mitrofanov, A.Y.; Murashkina, A.V.; Martín-García, I.; Alonso, F.; Beletskaya, I.P. Formation of C-C, C-S and C-N bonds catalysed by supported copper nanoparticles. Catal. Sci. Technol. 2017, 7, 4401–4412. [Google Scholar] [CrossRef] [Green Version]
- Vercammen, J.; Bocus, M.; Neale, S.; Bugaev, A.; Tomkins, P.; Hajek, J.; Van Minnebruggen, S.; Soldatov, A.; Krajnc, A.; Mali, G.; et al. Shape-selective C-H activation of aromatics to biarylic compounds using molecular palladium in zeolites. Nat. Catal. 2020, 3, 1002–1009. [Google Scholar] [CrossRef]
- Li, L.; Wang, Q.; Liu, H.; Sun, T.; Fan, D.; Yang, M.; Tian, P.; Liu, Z. Preparation of spherical mordenite zeolite assemblies with excellent catalytic performance for dimethyl ether carbonylation. ACS Appl. Mater. Interfaces 2018, 10, 32239–32246. [Google Scholar] [CrossRef] [PubMed]
- Thokchom, B.; Qiu, P.; Cui, M.; Park, B.; Pandit, A.B.; Khim, J. Magnetic Pd@Fe3O4 composite nanostructure as recoverable catalyst for sonoelectrohybrid degradation of ibuprofen. Ultrason. Sonochem. 2017, 34, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Padilla, R.H.; Priecel, P.; Lin, M.; Lopez-Sanchez, J.A.; Zhong, Z. A versatile sonication-assisted deposition-reduction method for preparing supported metal catalysts for catalytic applications. Ultrason. Sonochem. 2017, 35, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Tadjarodi, A.; Dehghani, M.; Imani, M. Green Synthesis and characterization of palladium nanoparticles supported on zeolite Y by sonochemical method, powerful and efficient catalyst for Suzuki-Miyaura coupling of aryl halides with phenylboronic acid. Appl. Organomet. Chem. 2018, 32, e4594. [Google Scholar] [CrossRef]
- Ezoddin, M.; Majidi, B.; Abdi, K. Ultrasound-assisted supramolecular dispersive liquid-liquid microextraction based on solidification of floating organic drops for preconcentration of palladium in water and road dust samples. J. Mol. Liq. 2015, 209, 515–519. [Google Scholar] [CrossRef]
- Jin, Q.; Lin, C.; Chang, Y.; Yang, C.; Yeh, C. Roles of textural and surface properties of nanoparticles in ultrasound-responsive systems. Langmuir 2018, 34, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Miyaura, N.; Yamada, K.; Suzuki, A. A new stereospecific cross-coupling by the palladium-catalyzed reaction of 1-alkenylboranes with 1-alkenyl or 1-alkynyl halides. Tetrahedron Lett. 1979, 20, 3437–3440. [Google Scholar] [CrossRef] [Green Version]
- Miyaura, N.; Yamada, K.; Suginome, H.; Suzuki, A. Novel and convenient method for the stereo- and regiospecific synthesis of conjugated alkadienes and alkenynes via the palladium-catalyzed cross-coupling reaction of 1-alkenylboranes with bromoalkenes and bromoalkynes. J. Am. Chem. Soc. 1985, 107, 972–980. [Google Scholar] [CrossRef]
- Ke, W.; Cui, T.; Yu, Q.; Wang, M.; Lv, L.; Wang, H.; Jiang, Z.; Li, X.; Chen, J. Mesoporous H-ZSM-5 nanocrystals with programmable number of acid sites as “Solid Ligands” to activate Pd nanoparticles for C-C coupling reactions. Nano Res. 2017, 11, 874–881. [Google Scholar] [CrossRef]
- Dehghani, M.; Tadjarodi, A.; Chamani, S. Synthesis and characterization of magnetic zeolite Y-palladium-nickel ferrite by ultrasonic irradiation and investigating its catalytic activity in Suzuki-Miyaura cross-coupling reactions. ACS Omega. 2019, 4, 10640–10648. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Pang, W. Chemistry-Zeolites and Porous Materials; Science Press: Beijing, China, 2004; p. 44. [Google Scholar]
- Shao, Y.; Zeng, H. Nanowire networks of metal-organosilicates as reversible Pd(II) reservoirs for Suzuki coupling reactions. ACS Appl. Nano Mater. 2021. [Google Scholar] [CrossRef]
- Magano, J.; Dunetz, J.R. Large-Scale applications of transition metal-catalyzed couplings for the synthesis of pharmaceuticals. Chem. Rev. 2011, 111, 2177–2250. [Google Scholar] [CrossRef] [PubMed]
 | |||||
| Entry | Catalyst | Base | Solvent | Temp. (°C) | Yield b (%) |
|---|---|---|---|---|---|
| 1 | Pd/H-MOR | K2CO3 | EtOH | 80 | 92 |
| 2 | PdCl2 | K2CO3 | EtOH | 80 | 94 c |
| 3 | MOR | K2CO3 | EtOH | 80 | NR |
| 4 | H-MOR | K2CO3 | EtOH | 80 | NR |
| 5 | Pd/H-MOR | Na2CO3 | EtOH | 80 | 92 |
| 6 | Pd/H-MOR | NaHCO3 | EtOH | 80 | 27 |
| 7 | Pd/H-MOR | KOH | EtOH | 80 | 90 |
| 8 | Pd/H-MOR | CsCO3 | EtOH | 80 | 92 |
| 9 | Pd/H-MOR | K2CO3 | H2O | 80 | 78 |
| 10 | Pd/H-MOR | K2CO3 | H2O:EtOH (1:1) | 80 | 93 |
| 11 | Pd/H-MOR | K2CO3 | H2O:EtOH (3:2) | 80 | 94 |
| 12 | Pd/H-MOR | K2CO3 | H2O:EtOH (4:1) | 80 | 95 |
| 13 | Pd/H-MOR | K2CO3 | H2O:EtOH (4:1) | 60 | NR |
| 14 | Pd/H-MOR | K2CO3 | H2O:EtOH (4:1) | 70 | NR |
| 15 | Pd/H-MOR | K2CO3 | H2O:EtOH (4:1) | 90 | 95 |
| 16 | Pd/H-MOR | K2CO3 | H2O:EtOH (4:1) | 80 | 95 d |
| 17 | Pd/H-MOR | K2CO3 | H2O:EtOH (4:1) | 80 | 95 e |
 | |||||||
| Number | R | X | Ar | Catalyst | Reaction Conditions | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | H | p-I | C6H4-CH3 | Pd/H-MOR | H2O:EtOH(4:1)/K2CO3/80 °C/0.5 h | 98 | This work |
| 2 | H | p-I | C6H4-OCH3 | Pd@mnc-S1 | MeOH/K2CO3/80 °C/0.5 h | 93 | [4] |
| 3 | H | p-I | C6H4-CH3 | Pd-OS | EtOH/K2CO3/80 °C/0.5 h | 93 | [24] |
| 4 | H | p-I | Ph | Z-Y-Pd NPs | H2O:EtOH(1:1)/K2CO3/Reflux/1.5 h | 88 | [16] |
 | ||||
| Entry | R | X | Ar | Yield b (%) |
| 1 | H | p-I | C6H4-OCH3 | 95 (3a) |
| 2 | H | p-I | C6H4-NH2 | 95 (3b) |
| 3 | H | p-I | C6H4-OH | 96 (3c) |
| 4 | H | p-I | C6H4-CH3 | 98 (3d) |
| 5 | H | p-I | C6H4-NO2 | 98 (3e) |
| 6 | H | p-I | C6H4-CHO | 97 (3f) |
| 7 | H | p-I | C6H4-COCH3 | 95 (3g) |
| 8 | H | p-I | C6H4-Cl | 95 (3h) |
| 9 | H | I | Ph | 95 (3i) |
| 10 | H | m-I | C6H4-NO2 | 94 (3j) |
| 11 | H | m-I | C6H4-COCH3 | 93 (3k) |
| 12 | H | o-I | C6H4-NH2 | 86 (3l) |
| 13 | H | o-I | C6H4-CH3 | 84 (3m) |
| 14 | H | p-Br | C6H4-CH3 | 79 (3d) |
| 15 | H | p-Br | C6H4-CHO | 92 (3f) |
| 16 | H | Br | Ph | 92 (3i) |
| 17 | H | p-Cl | C6H4-NH2 | 48 c (3b) |
| 18 | H | p-Cl | C6H4-CHO | 50 c (3f) |
| 19 | H | p-Cl | C6H4-COCH3 | 52 c (3g) |
| 20 | H | p-Cl | Ph | 51 c (3i) |
| 21 | 4-CH3O | p-I | C6H4-OCH3 | 96 (3n) |
| 22 | 4-CH3 | p-I | C6H4-OCH3 | 95 (3o) |
| 23 | 4-F | p-I | C6H4-OCH3 | 92 (3p) |
| 24 | 3-NO2 | p-I | C6H4-OCH3 | 86 (3q) |
| 25 | H | o-Br | pyridine | 83 (3r) |
| 26 | H | o-Br | quinoline | 81 (3s) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, E.; Jin, J.; Zheng, K.; Zhang, L.; Xu, H.; Shen, C. Novel Recyclable Pd/H-MOR Catalyst for Suzuki-Miyaura Coupling and Application in the Synthesis of Crizotinib. Catalysts 2021, 11, 1213. https://doi.org/10.3390/catal11101213
Zhou E, Jin J, Zheng K, Zhang L, Xu H, Shen C. Novel Recyclable Pd/H-MOR Catalyst for Suzuki-Miyaura Coupling and Application in the Synthesis of Crizotinib. Catalysts. 2021; 11(10):1213. https://doi.org/10.3390/catal11101213
Chicago/Turabian StyleZhou, Enmu, Jianzhong Jin, Kai Zheng, Letian Zhang, Hao Xu, and Chao Shen. 2021. "Novel Recyclable Pd/H-MOR Catalyst for Suzuki-Miyaura Coupling and Application in the Synthesis of Crizotinib" Catalysts 11, no. 10: 1213. https://doi.org/10.3390/catal11101213
APA StyleZhou, E., Jin, J., Zheng, K., Zhang, L., Xu, H., & Shen, C. (2021). Novel Recyclable Pd/H-MOR Catalyst for Suzuki-Miyaura Coupling and Application in the Synthesis of Crizotinib. Catalysts, 11(10), 1213. https://doi.org/10.3390/catal11101213