Magnetically Reusable Fe3O4@NC@Pt Catalyst for Selective Reduction of Nitroarenes
Abstract
:1. Introduction
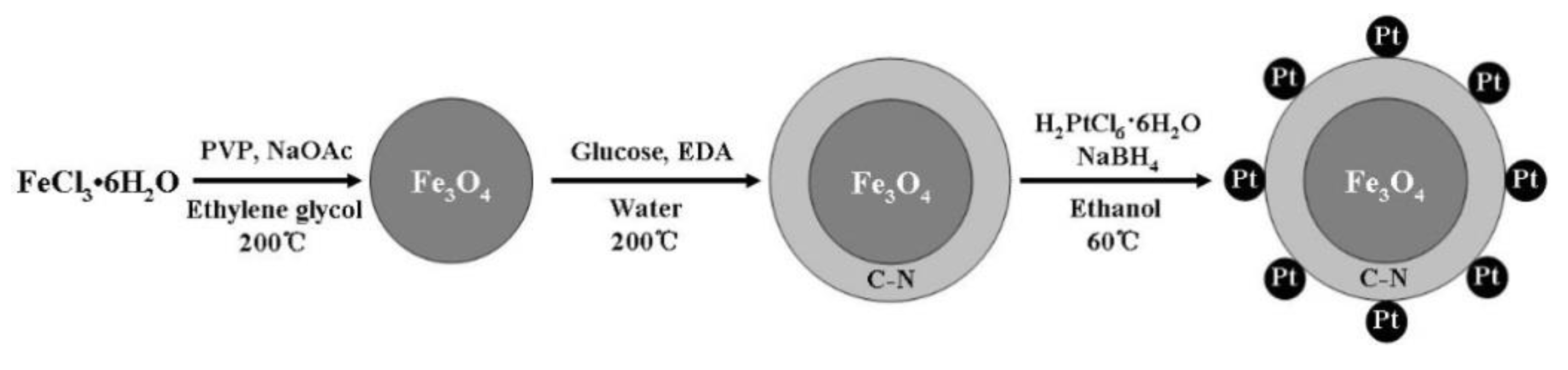
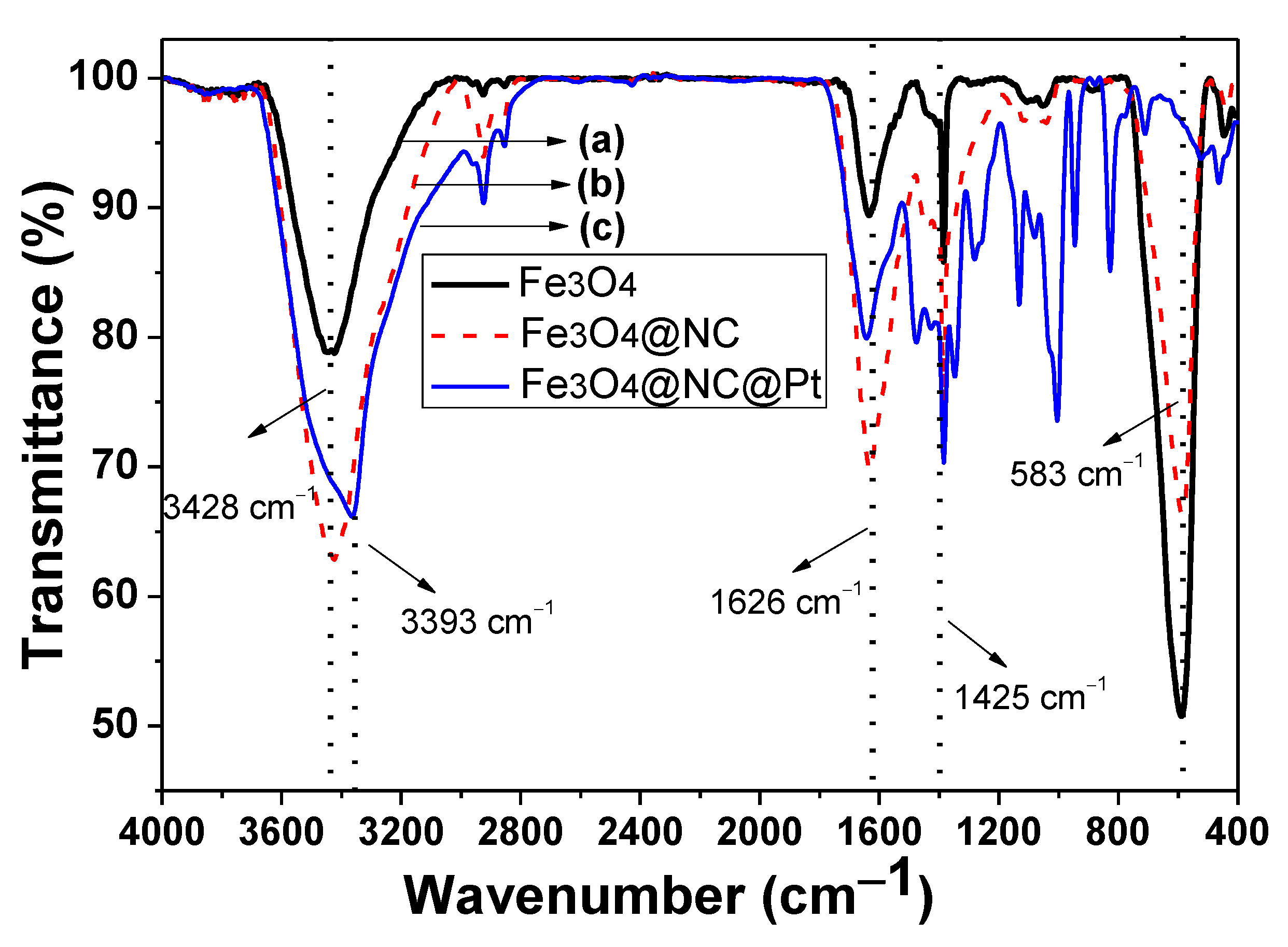
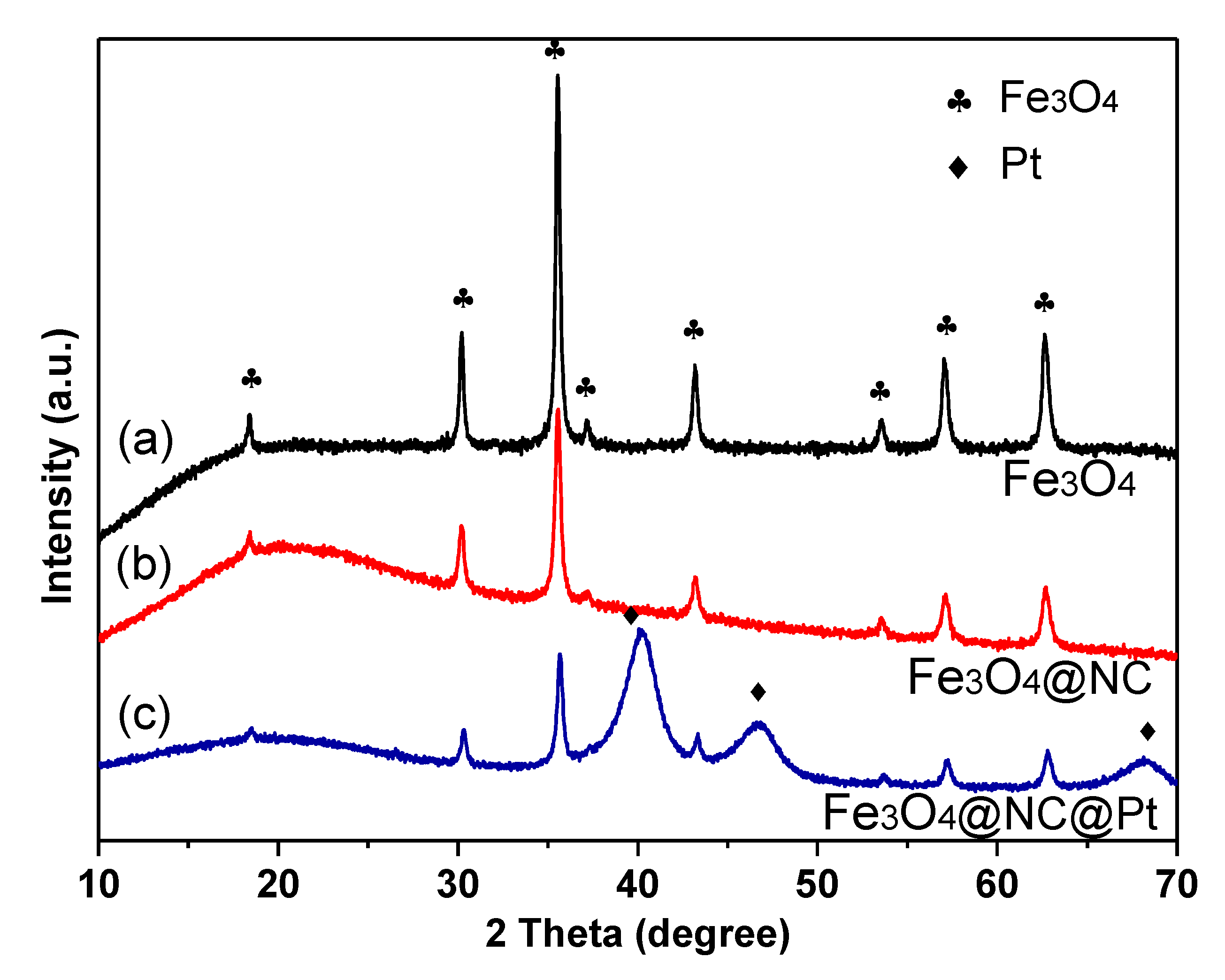
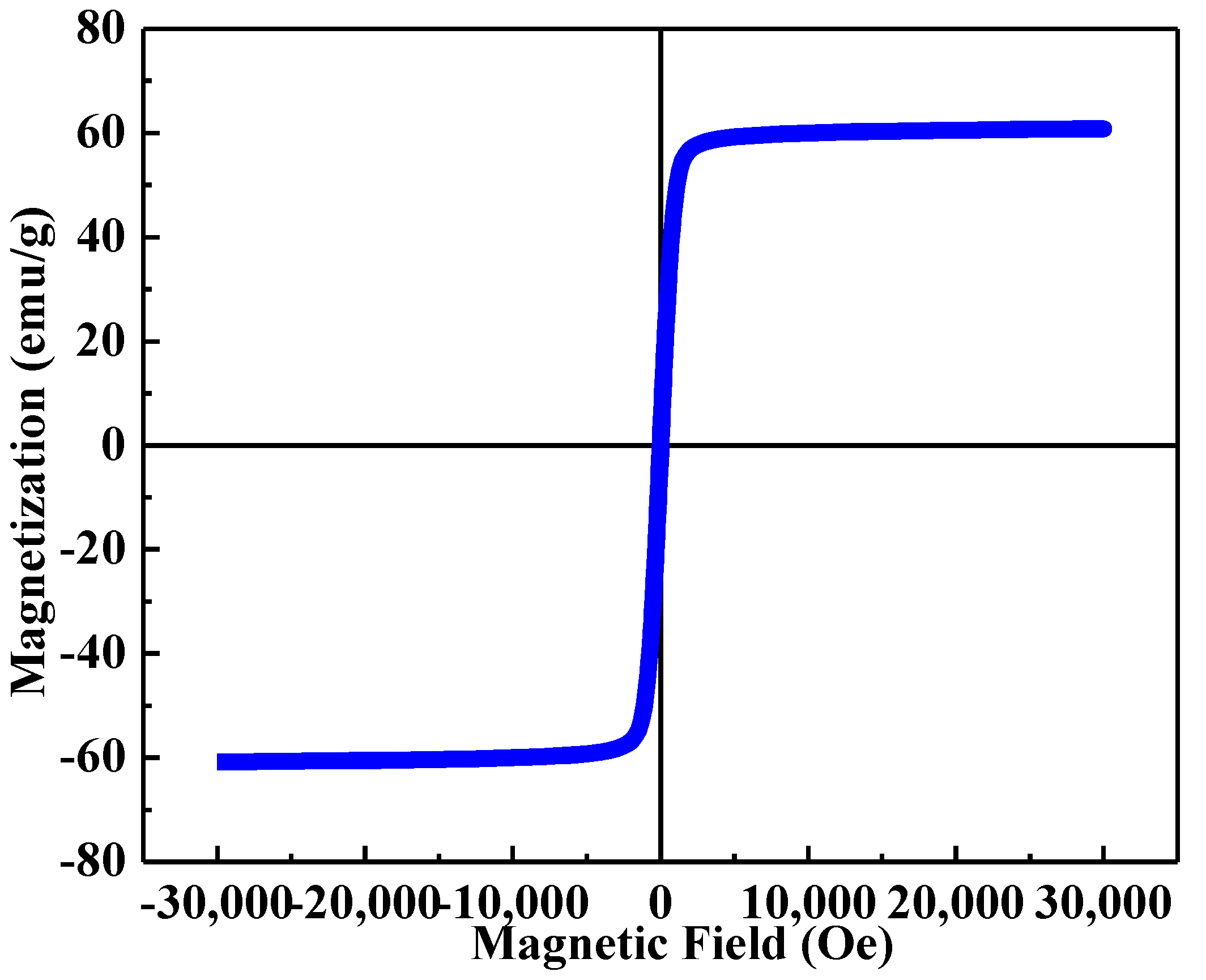
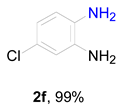
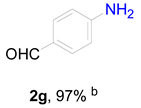
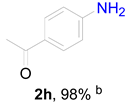
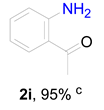
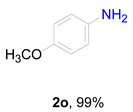
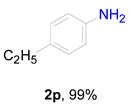
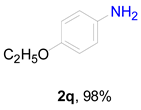
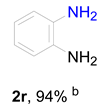
2. Results and Discussion
3. Experimental Materials
3.1. Characterization
3.2. Preparation of Fe3O4 Microspheres
3.3. Preparation of Fe3O4@C Microspheres
3.4. Preparation of Fe3O4@NC Microspheres
3.5. Preparation of the Fe3O4@C@Pt and Fe3O4@NC@Pt Catalyst
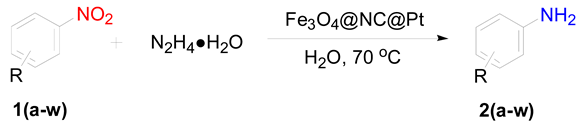
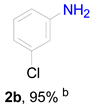
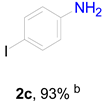
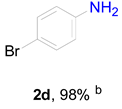
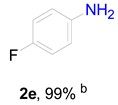
3.6. Procedure for the Selective Hydrogenation of Nitroarenes Reactions
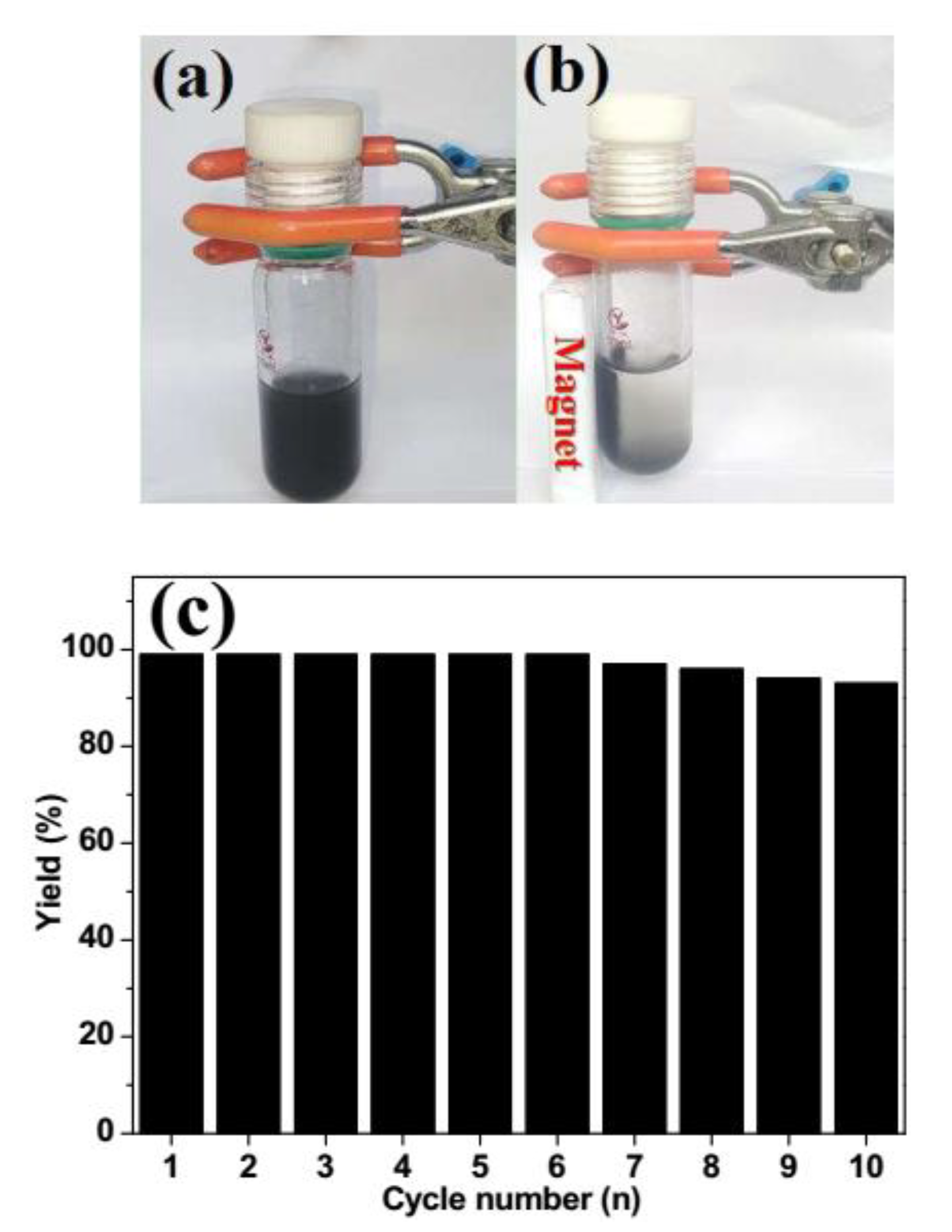
3.7. Procedure for Catalyst Reused
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jagadeesh, R.V.; Surkus, A.E.; Junge, H.; Pohl, M.M.; Radnik, J.; Rabeah, J.; Huan, H.M.; Schunemann, V.; Brückner, A.; Beller, M. Nanoscale Fe2O3-based catalysts for selective hydrogenation of nitroarenes to anilines. Science 2013, 342, 1073–1076. [Google Scholar] [CrossRef]
- Pang, H.B.; Gallou, F.; Sohn, H.; Camacho-Bunquin, J.; Delferro, M.; Lipshutz, B.H. Synergistic effects in Fe nanoparticles doped with ppm levels of (Pd+Ni). A new catalyst for sustainable nitro group reductions. Green Chem. 2018, 20, 130–135. [Google Scholar] [CrossRef]
- MacDonald, L.; Rausch, B.; Symes, M.D.; Cronin, L. Selective hydrogenation of nitroarenes using an electrogenerated polyoxometalate redox mediator. Chem. Commun. 2018, 54, 1093–1096. [Google Scholar] [CrossRef] [Green Version]
- Tian, M.; Cui, X.L.; Yuan, M.; Yang, J.; Ma, J.T.; Dong, Z.P. Efficient chemoselective hydrogenation of halogenated nitrobenzenes over an easily prepared γ-Fe2O3-modified mesoporous carbon catalyst. Green Chem. 2017, 19, 1548–1554. [Google Scholar] [CrossRef]
- Lian, C.; Liu, H.Q.; Xiao, C.; Yang, W.; Zhang, K.; Liu, Y.; Wang, Y. Solvent-free selective hydrogenation of chloronitrobenzene to chloroaniline over a robust Pt/Fe3O4 catalyst. Chem. Commun. 2012, 48, 3124–3126. [Google Scholar] [CrossRef]
- Wei, H.S.; Liu, X.Y.; Wang, A.Q.; Zhang, L.L.; Qiao, B.T.; Yang, X.F.; Huang, Y.Q.; Miao, S.; Liu, J.Y.; Zhang, T. FeOx-supported platinum single-atom and pseudo-single-atom catalysts for chemoselective hydrogenation of functionalized nitroarenes. Nat. Commun. 2014, 5, 5634–5641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, S.M.; Lipshutz, B.H. Chemoselective reductions of nitroaromatics in water at room temperature. Org. Lett. 2014, 16, 98–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junge, K.; Wendt, B.; Shaikh, N.; Beller, M. Iron-catalyzed selective reduction of nitroarenes to anilines using organosilanes. Chem. Commun. 2010, 46, 1769–1771. [Google Scholar] [CrossRef]
- Liu, L.C.; Corma, A. Metal catalysts for heterogeneous catalysis: From single atoms to nanoclusters and nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Ji, S.F.; Liu, Y.W.; Cao, X.; Tian, S.B.; Chen, Y.J.; Niu, Z.Q.; Li, Y.D. Well-defined materials for heterogeneous catalysis: From nanoparticles to isolated single-atom sites. Chem. Rev. 2020, 120, 623–682. [Google Scholar] [CrossRef] [PubMed]
- Robert, A.W.; Johnstone, A.H.; Wilby, A.H.; Entwistle, I.D. Heterogeneous catalytic transfer hydrogenation and its relation to other methods for reduction of organic compounds. Chem. Rev. 1985, 85, 129–170. [Google Scholar]
- Sharma, R.K.; Monga, Y.; Puri, A. Magnetically separable silica@Fe3O4 core-shell supported nano-structured copper(II) composites as a versatile catalyst for the reduction of nitroarenes in aqueous medium at room temperature. J. Mol. Catal. A-Chem. 2014, 393, 84–95. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, X.W.; Liang, H.J.; Ge, H.B.; Gu, X.M.; Chen, S.; Yang, H.M.; Qin, Y. Tailoring Pt-Fe2O3 interfaces for selective reductive coupling reaction to synthesize imine. ACS Catal. 2016, 6, 6560–6566. [Google Scholar] [CrossRef]
- Byun, S.; Song, Y.M.; Kim, B.M. Heterogenized bimetallic Pd–Pt–Fe3O4 nanoflakes as extremely robust, magnetically recyclable catalysts for chemoselective nitroarene reduction. ACS Appl. Mater. Interfaces 2016, 8, 14637–14647. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chung, J.; Jang, Y.; Mao, S.; Kim, B.M.; Wang, Y.; Guo, X. Magnetically recoverable nanoflake-shaped iron oxide/Pt heterogeneous catalysts and their excellent catalytic performance in the hydrogenation reaction. ACS Appl. Mater. Interfaces 2014, 6, 1887–1892. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Kim, T.; Jun, S.W.; Shin, K.; Jang, Y.J.; Kim, B.H.; Kim, J.; Hyeon, T. Magnetically separable carbon nanocomposite catalysts for efficient nitroarene reduction and Suzuki reactions. Appl. Catal. A-Gen. 2014, 476, 133–139. [Google Scholar] [CrossRef]
- Li, Y.; Li, N.; Jiang, W.; Ma, G.Y.; Zangene, M.M. In situ decorated Au NPs on pectin-modified Fe3O4 NPs as a novel magnetic nanocomposite (Fe3O4/Pectin/Au) for catalytic reduction of nitroarenes and investigation of its anti-human lung cancer activitiesInternational. Int. J. Biol. Macromol. 2020, 163, 2162–2171. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, J.; Taheri-Ledari, R.; Niksefat, M.; Maleki, A. Enhanced reduction of nitrobenzene derivatives: Effective strategy executed by Fe3O4/PVA-10%Ag as a versatile hybrid nanocatalyst. Catal. Commun. 2020, 134, 105850. [Google Scholar] [CrossRef]
- Dayan, S.; Arslan, F.; Ozpozan, N.K. Ru(II) impregnated Al2O3, Fe3O4, SiO2 and N-coordinate ruthenium(II) arene complexes: Multifunctional catalysts in the hydrogenation of nitroarenes and the transfer hydrogenation of aryl ketones. Appl. Catal. B Environ. 2015, 164, 305–315. [Google Scholar] [CrossRef]
- Hu, Z.N.; Zhou, J.J.; Ai, Y.J.; Liu, L.; Qi, L.; Jiang, R.H.; Bao, H.J.; Wang, J.T.; Hu, J.S.; Sun, H.B.; et al. Two dimensional Rh/Fe3O4/g-C3N4-N enabled hydrazine mediated catalytic transfer hydrogenation of nitroaromatics: A predictable catalyst model with adjoining Rh. J. Catal. 2018, 368, 20–30. [Google Scholar] [CrossRef]
- Sheng, Y.; Wang, X.G.; Xing, Z.K.; Chen, X.B.; Zou, X.J.; Lu, X.G. Highly active and chemoselective reduction of halogenated nitroarenes catalysed by ordered mesoporous carbon supported platinum nanoparticles. ACS Sustain. Chem. Eng. 2019, 7, 8908–8916. [Google Scholar] [CrossRef]
- Antony, R.; Marimuthu, R.; Murugavel, R. Bimetallic nanoparticles anchored on core-shell support as an easily recoverable and reusable catalytic system for efficient nitroarene reduction. ACS Omega 2019, 4, 9241–9250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.; Bae, H.S.; Seo, E.Y.; Jang, S.W.; Park, K.H.; Kim, B.S. Hybrid gold nanoparticle-reduced graphene oxide nanosheets as active catalysts for highly efficient reduction of nitroarenes. J. Mater. Chem. 2011, 21, 15431. [Google Scholar] [CrossRef]
- Ombaka, L.M.; Ndungu, P.G.; Kibet, J.; Nyamori, V.O. The effect of pyridinic- and pyrrolic-nitrogen in nitrogen-doped carbon nanotubes used as support for Pd-catalyzed nitroarene reduction: An experimental and theoretical study. J. Mater. Sci. 2017, 52, 10751–10765. [Google Scholar] [CrossRef]
- Nie, R.F.; Wang, J.H.; Wang, L.N.; Qin, Y.; Chen, P.; Hou, Z.Y. Platinum supported on reduced graphene oxide as a catalyst for hydrogenation of nitroarenes. Carbon 2012, 50, 586–596. [Google Scholar] [CrossRef]
- Shen, C.; Xu, J.; Yu, W.; Zhang, P. ChemInform abstract: A highly active and easily recoverable Chitosan@Copper catalyst for the C-S coupling and its application in the synthesis of zolimidine. Green Chem. 2014, 16, 3007–3012. [Google Scholar] [CrossRef]
- Shen, C.; Shen, H.; Yang, M.; Xia, C.; Zhang, P. Novel D-glucosamine-derived pyridyl-triazole@palladium catalyst for solvent-free Mizoroki-Heck reactions and its application in the synthesis of Axitinib. Green Chem. 2014, 17, 225–230. [Google Scholar] [CrossRef]
- Shen, H.; Shen, C.; Chen, C.; Wang, A.; Zhang, P. Novel glycosyl pyridyl-triazole@palladium nanoparticles: Efficient and recoverable catalysts for C–C cross-coupling reactions. Catal. Sci. Technol. 2015, 5, 2065–2071. [Google Scholar] [CrossRef]
- Shen, C.; Zhang, P.; Sun, Q.; Bai, S.; Andy Hor, T.A.; Liu, X. Recent advances in C-S bond formation via C-H bond functionalization and decarboxylation. Chem. Soc. Rev. 2015, 46, 291–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.W.; Zheng, K.; Tong, J.Y.; Jin, J.Z.; Shen, C. Novel biomass-derived Fe3O4@Pd NPs as efcient and sustainablenanocatalyst for nitroarene reduction in aqueous media. Catal. Lett. 2019, 149, 2607–2613. [Google Scholar] [CrossRef]
- Zhu, M.Y.; Diao, G.W. Magnetically recyclable Pd nanoparticles immobilized on magnetic Fe3O4@C nanocomposites: Preparation, characterization, and their catalytic activity toward Suzuki and heck coupling reactions. J. Phys. Chem. C 2011, 115, 24743–24749. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Liu, R.J. Superparamagnetic α-Fe2O3/Fe3O4 heterogeneous nanoparticles with enhanced biocompatibility. Nanomaterials 2021, 11, 834. [Google Scholar] [CrossRef]
- Serga, V.; Burve, R.; Maiorov, M.; Krumina, A.; Skaudžius, R.; Zarkov, A.; Kareiva, A.; Popov, A.I. Impact of Gadolinium on the structure and magnetic properties of nanocrystalline powders of iron oxides produced by the extraction-pyrolytic method. Materials 2020, 13, 4147. [Google Scholar] [CrossRef]
- Alzahrani, F.M.; Alsaiari, N.S.; Katubi, K.M.; Amari, A.; Elkhaleefa, A.M.; Rebah, F.B.; Tahoon, M.A. Magnetic nitrogen-doped porous carbon nanocomposite for Pb(II) adsorption from aqueous solution. Molecules 2021, 26, 4809. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halend, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reino, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Chew, L.M.; Kostka, A.; Muhlera, M.; Xia, W. The structural and electronic promoting effect of nitrogendoped carbon nanotubes on supported Pd nanoparticles for selective olefin hydrogenation. Catal. Sci. Technol. 2013, 3, 1964–1971. [Google Scholar] [CrossRef]
- Xu, S.L.; Shen, S.C.; Zhao, S.; Ding, Y.W.; Chu, S.Q.; Chen, P.; Lin, Y.; Liang, H.W. Synthesis of carbon-supported sub-2 nanometer bimetallic catalysts by strong metal–sulfur interaction. Chem. Sci. 2020, 11, 7933–7939. [Google Scholar] [CrossRef]
- Zheng, K.; Shen, C.; Qiao, J.; Tong, J.Y.; Jin, J.Z.; Zhang, P.F. Novel magnetically-recyclable, nitrogen-doped Fe3O4@Pd NPs for Suzuki–Miyaura coupling and their application in the synthesis of crizotinib. Catalysts 2018, 8, 443. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.Y.; Zhang, C.L.; Luo, L.; Chang, Z.J.; Sun, X.M. One-pot synthesis and catalyst support application of mesoporous N-doped carbonaceous materials. J. Mater. Chem. 2012, 22, 12149–12154. [Google Scholar] [CrossRef]
- Kumar, B.S.; Amali, A.J.; Pitchumani, K. Mesoporous microcapsules through d-Glucose promoted hydrothermal self-assembly of colloidal silica: Reusable catalytic containers for palladium catalyzed hydrogenation reactions. Appl. Mater. Interfaces 2015, 7, 22907–22917. [Google Scholar]

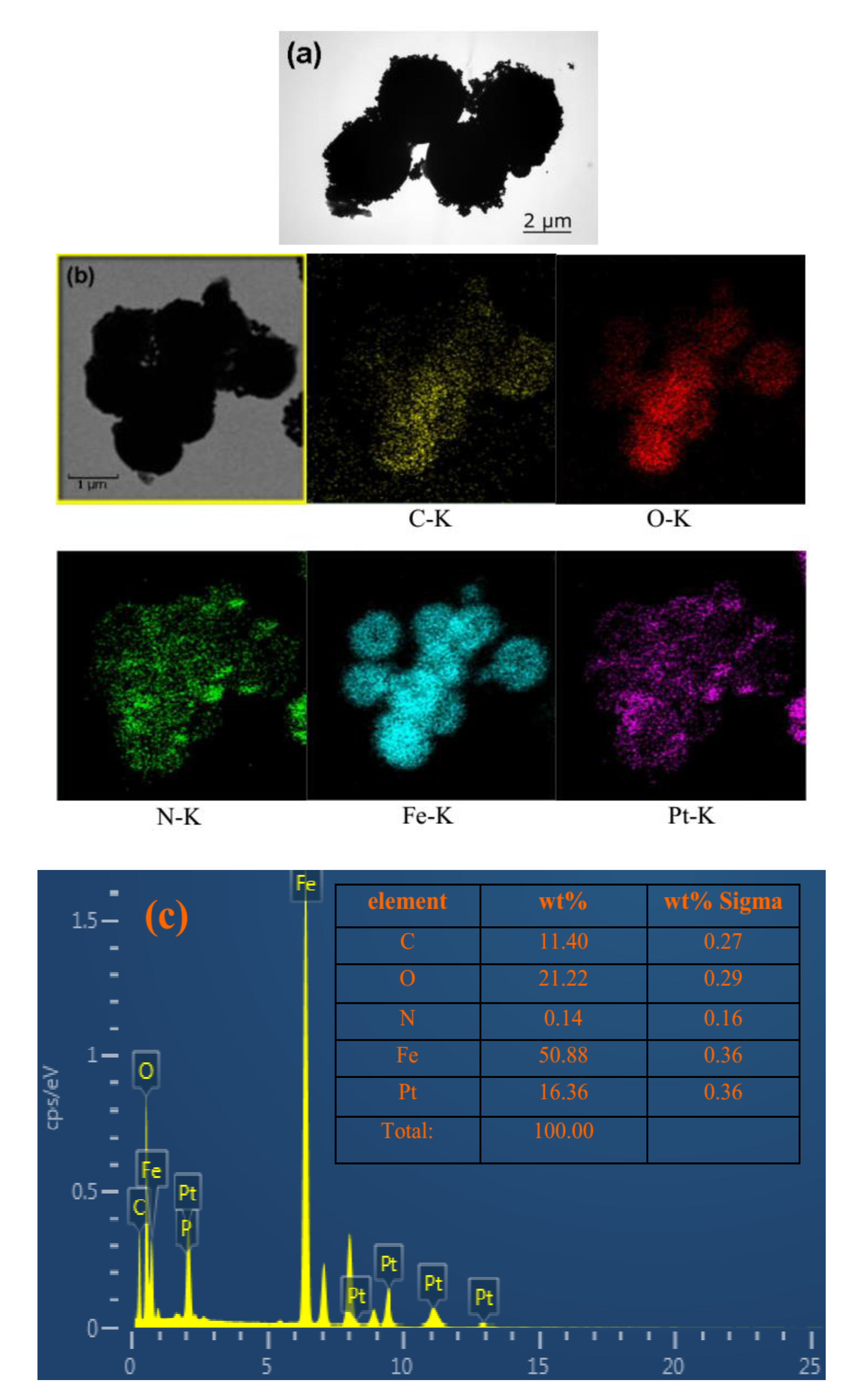
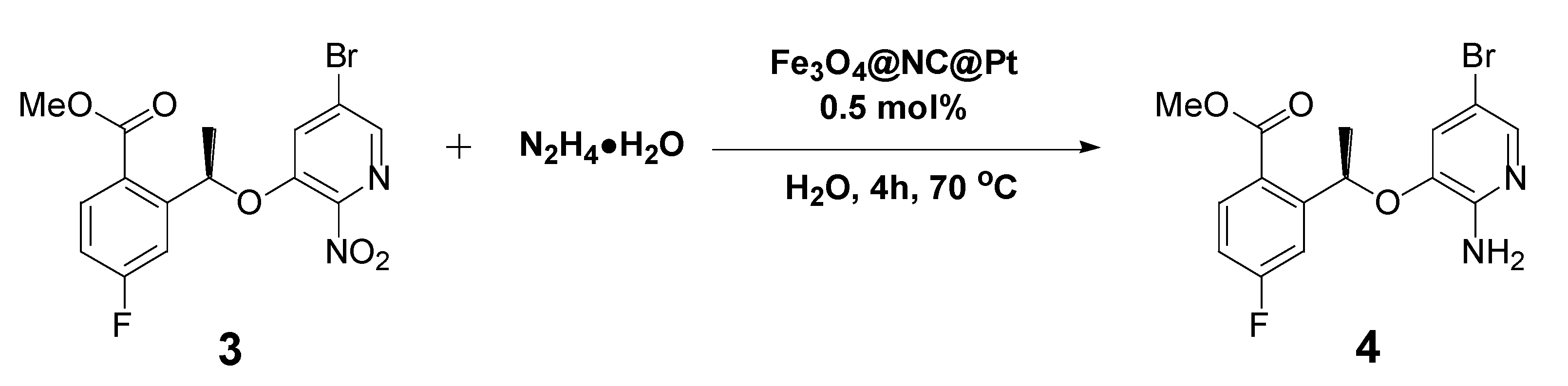
 | ||||||
| Entry | Catalyst | Reducing Agent | Temp (°C) | Time(h) | Yield b (%) | Ref. |
|---|---|---|---|---|---|---|
| 1 | - | hydrazine hydrate | 70 | 4 | - | this work |
| 2 | Fe3O4@NC | hydrazine hydrate | 70 | 4 | - | this work |
| 3 | Fe3O4@C@Pt | hydrazine hydrate | 70 | 4 | 70 | this work |
| 4 | Fe3O4@NC@Pt | hydrazine hydrate | 70 | 4 | 95 | this work |
| 5 | Fe3O4@NC@Pt | Hydrogen | 70 | 4 | 85 | this work |
| 6 | Fe3O4@NC@Pt | Sodium borohydride | 70 | 4 | 70 | this work |
| 7 | Fe3O4@NC@Pt | Ammonium formate | 70 | 4 | 30 | this work |
| 8 | Pt-Co/S-C | 2 bar H2 | 40 | 2 | 99 | [39] |
| 9 | Pd–Pt–Fe3O4 | NH3BH3 | RT | 0.83 | 92 | [14] |
| 10 | Pt/RGO-EG | 1 bar H2 | 40 | 2 | 96 | [25] |
 | |||||
| Entry | Catalyst | Solvent | Temp(°C) | Time(h) | Yield b (%) |
|---|---|---|---|---|---|
| 1 | Fe3O4@NC@Pt | H2O | 70 | 4 | 99 |
| 2 | Fe3O4@NC@Pt | DMSO | 70 | 4 | 30 |
| 3 | Fe3O4@NC@Pt | Toluene | 70 | 4 | 95 |
| 4 | Fe3O4@NC@Pt | EtOH | 70 | 4 | 40 |
| 5 | Fe3O4@NC@Pt | Isopropanol | 70 | 4 | 50 |
| 6 | Fe3O4@NC@Pt | AcOEt | 70 | 4 | 10 |
| 7 | Fe3O4@NC@Pt | THF | 70 | 4 | 20 |
| 8 | Fe3O4@NC@Pt | Hexane | 70 | 4 | 50 |
| 9 | Fe3O4@NC@Pt | H2O | 50 | 4 | 70 |
| 10 | Fe3O4@NC@Pt | H2O | rt | 4 | 40 |
| 11 | Fe3O4@NC@Pt | H2O | 70 | 2 | 65 |
| 12 | Pt/CMK-3–HQ | EtOH | 80 | 1 | 99 c |
 | ||||
 |  |  |  |  |
 |  |  |  |  |
 |  |  |  |  |
 |  |  | 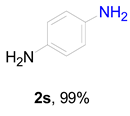 | 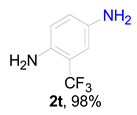 |
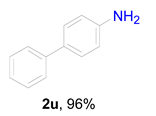 | 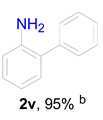 |  | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, J.; Wang, T.; Zheng, K.; Zhou, E.; Shen, C.; Jia, A.; Zhang, Q. Magnetically Reusable Fe3O4@NC@Pt Catalyst for Selective Reduction of Nitroarenes. Catalysts 2021, 11, 1219. https://doi.org/10.3390/catal11101219
Qiao J, Wang T, Zheng K, Zhou E, Shen C, Jia A, Zhang Q. Magnetically Reusable Fe3O4@NC@Pt Catalyst for Selective Reduction of Nitroarenes. Catalysts. 2021; 11(10):1219. https://doi.org/10.3390/catal11101219
Chicago/Turabian StyleQiao, Jun, Tian Wang, Kai Zheng, Enmu Zhou, Chao Shen, Aiquan Jia, and Qianfeng Zhang. 2021. "Magnetically Reusable Fe3O4@NC@Pt Catalyst for Selective Reduction of Nitroarenes" Catalysts 11, no. 10: 1219. https://doi.org/10.3390/catal11101219
APA StyleQiao, J., Wang, T., Zheng, K., Zhou, E., Shen, C., Jia, A., & Zhang, Q. (2021). Magnetically Reusable Fe3O4@NC@Pt Catalyst for Selective Reduction of Nitroarenes. Catalysts, 11(10), 1219. https://doi.org/10.3390/catal11101219






