Isolation and Identification of an Efficient Aerobic Denitrifying Pseudomonas stutzeri Strain and Characterization of Its Nitrite Degradation
Abstract
:1. Introduction
2. Results and Discussion
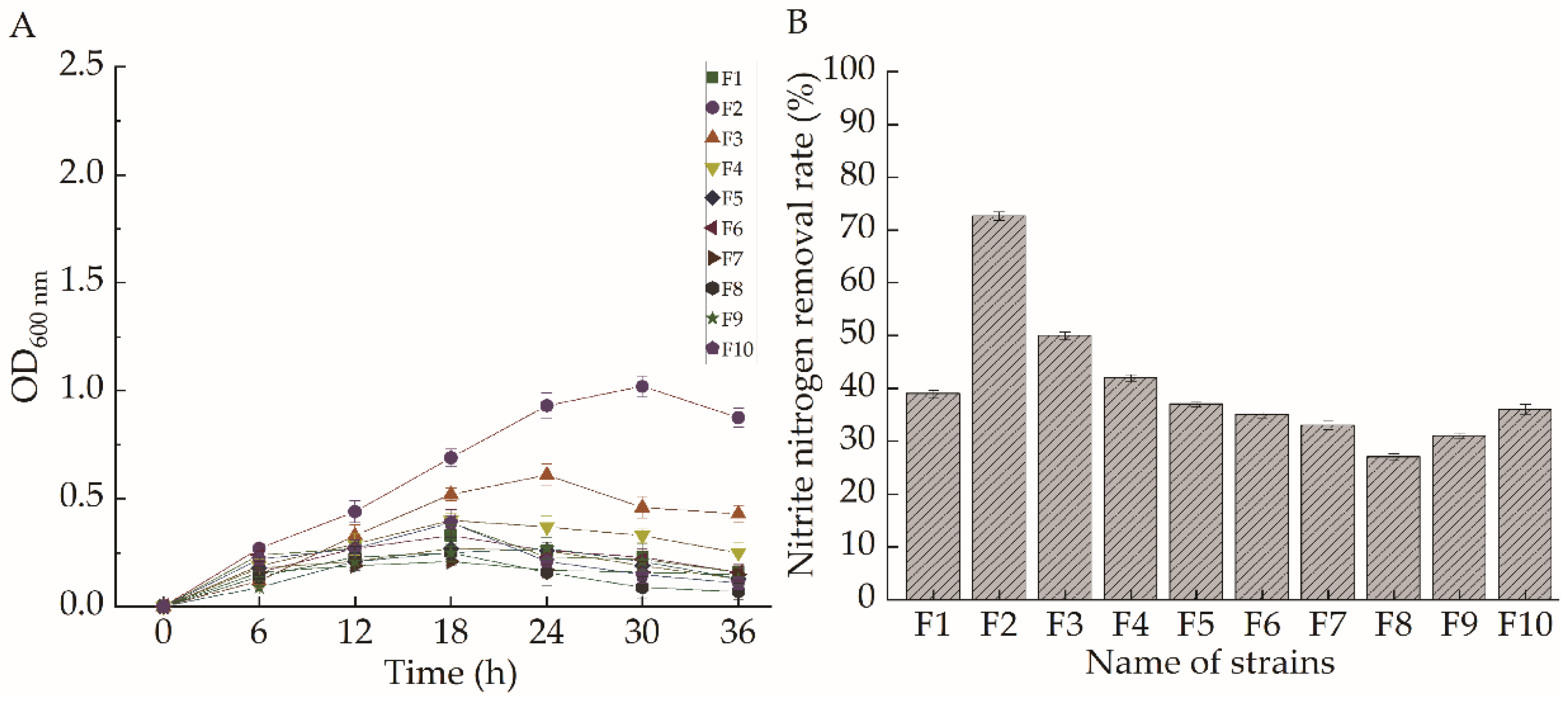
2.1. Strain Selection
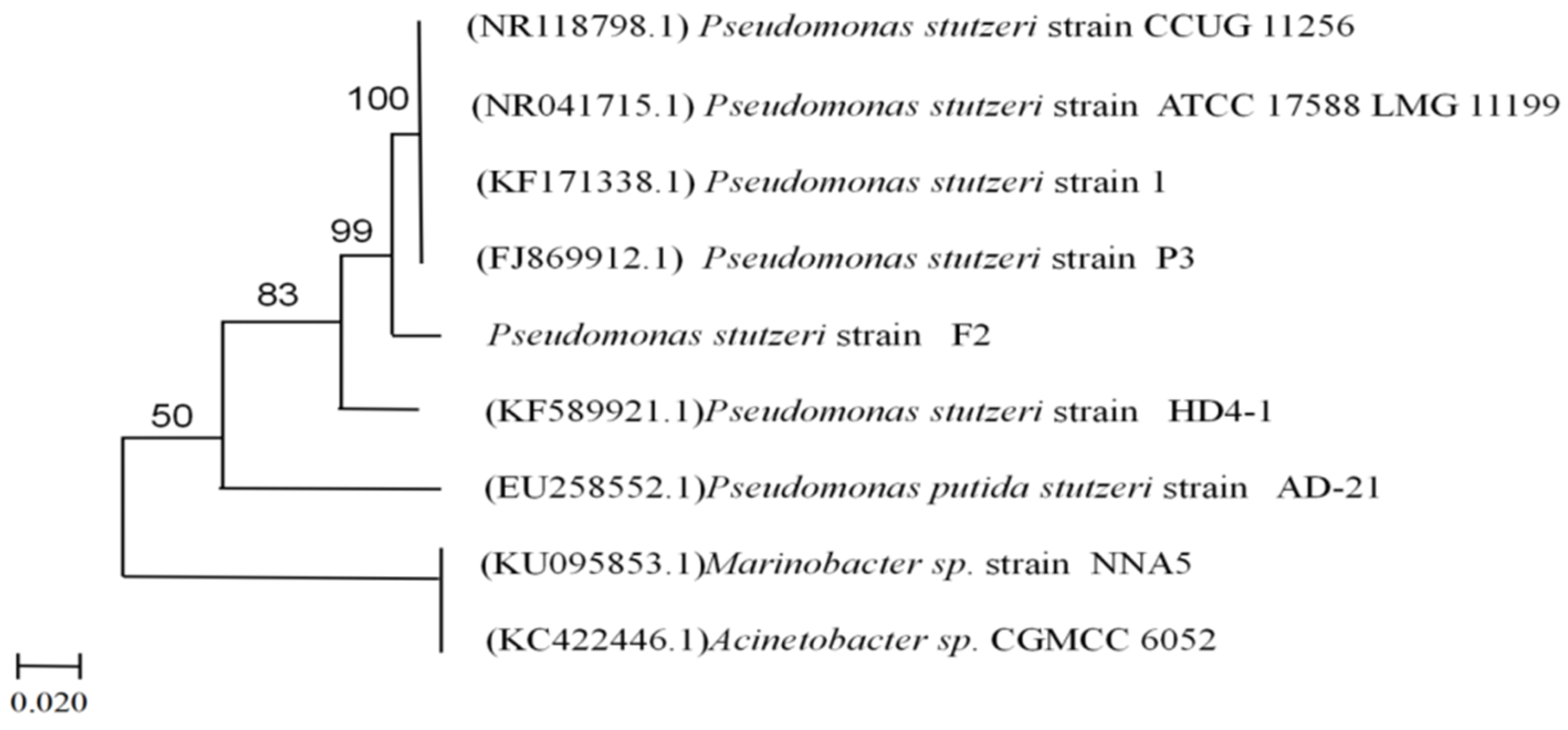
2.2. Identification of Strain F2
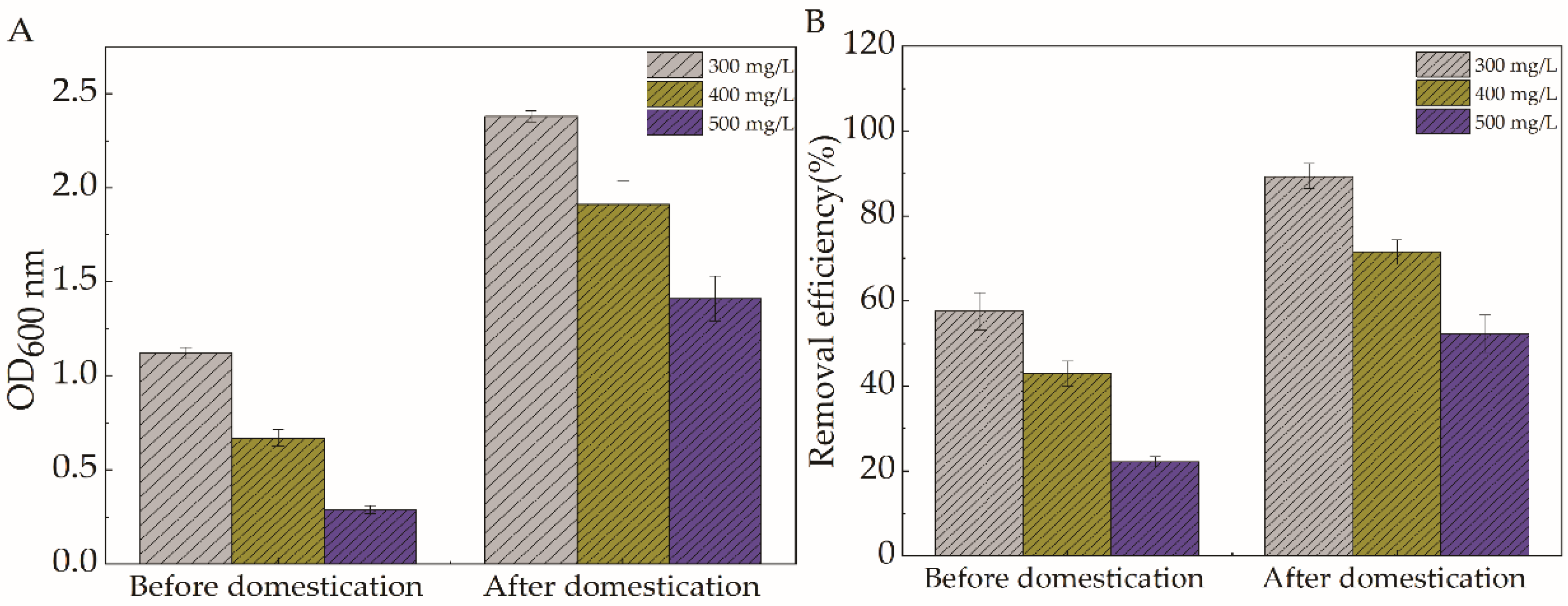
2.3. Analysis of the Results of Targeted Domestication
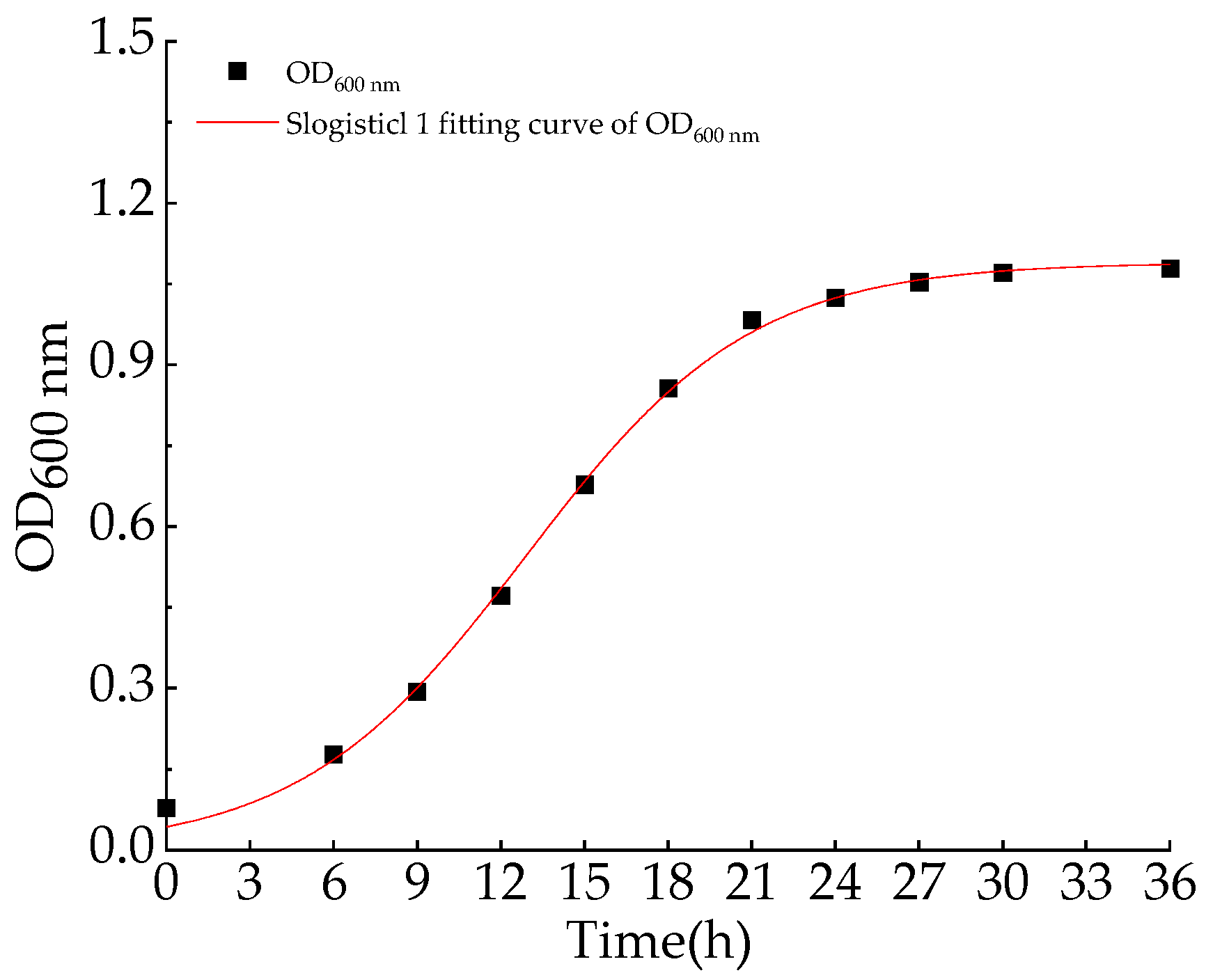
2.4. Aerobic Denitrification
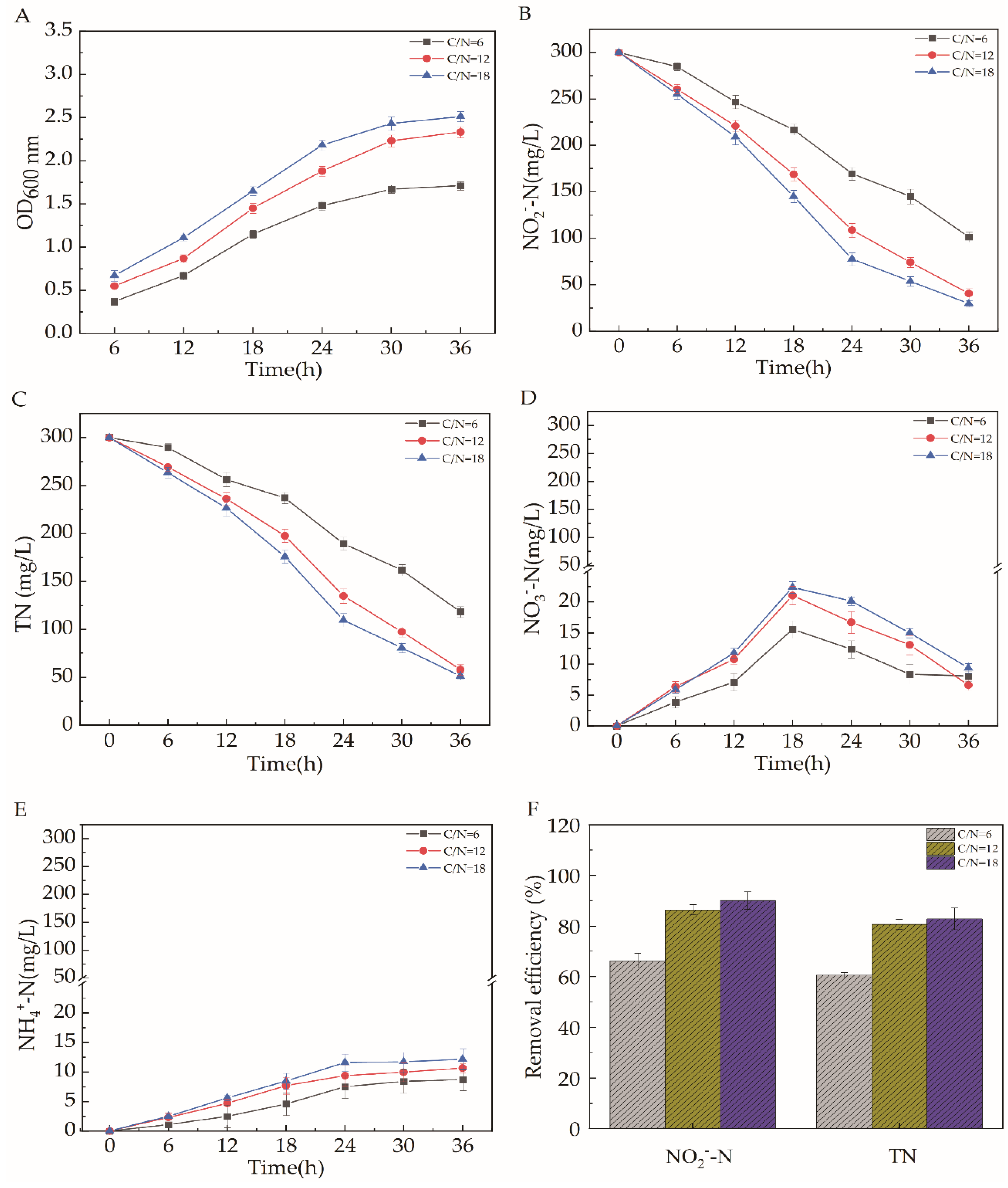
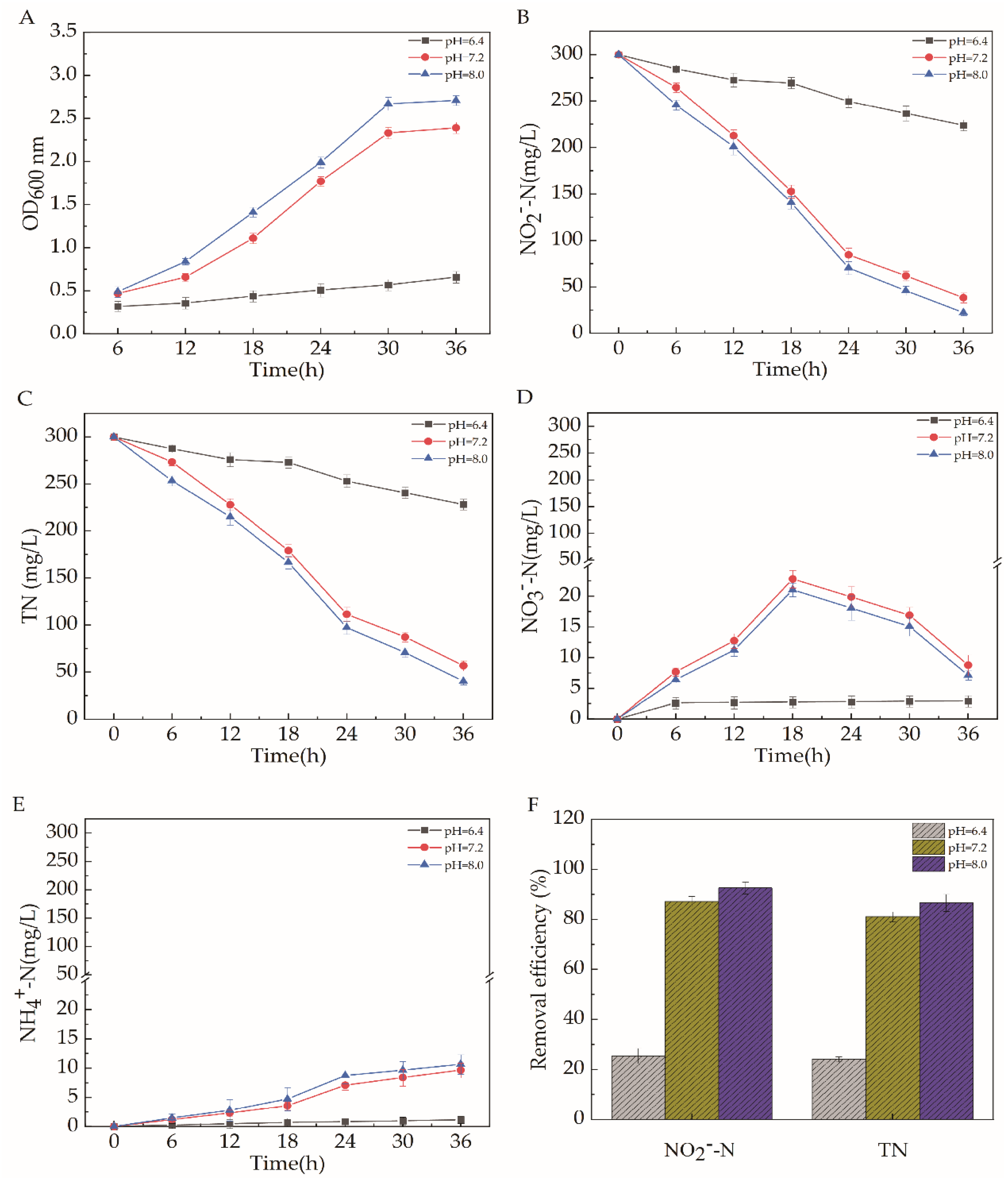
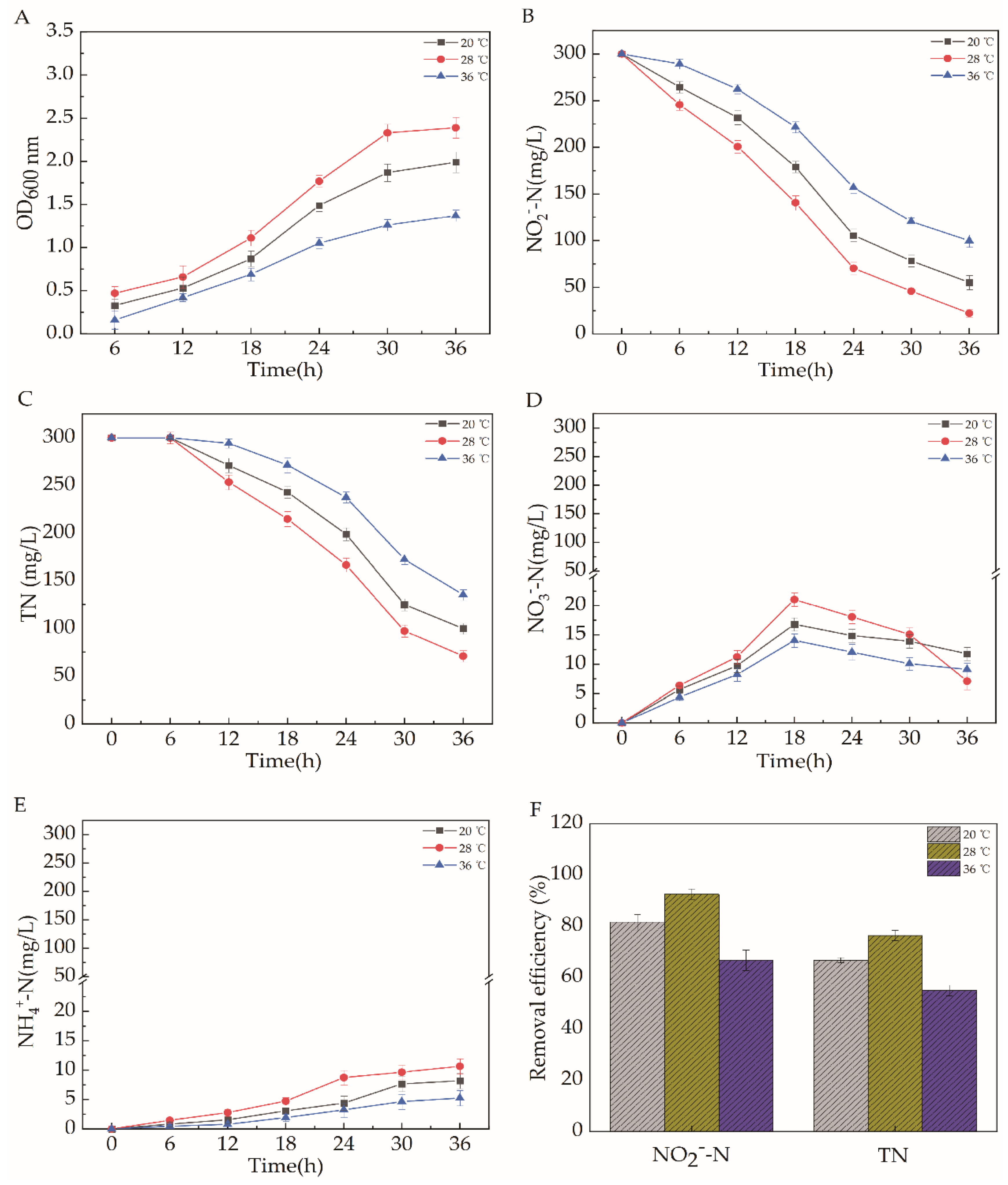
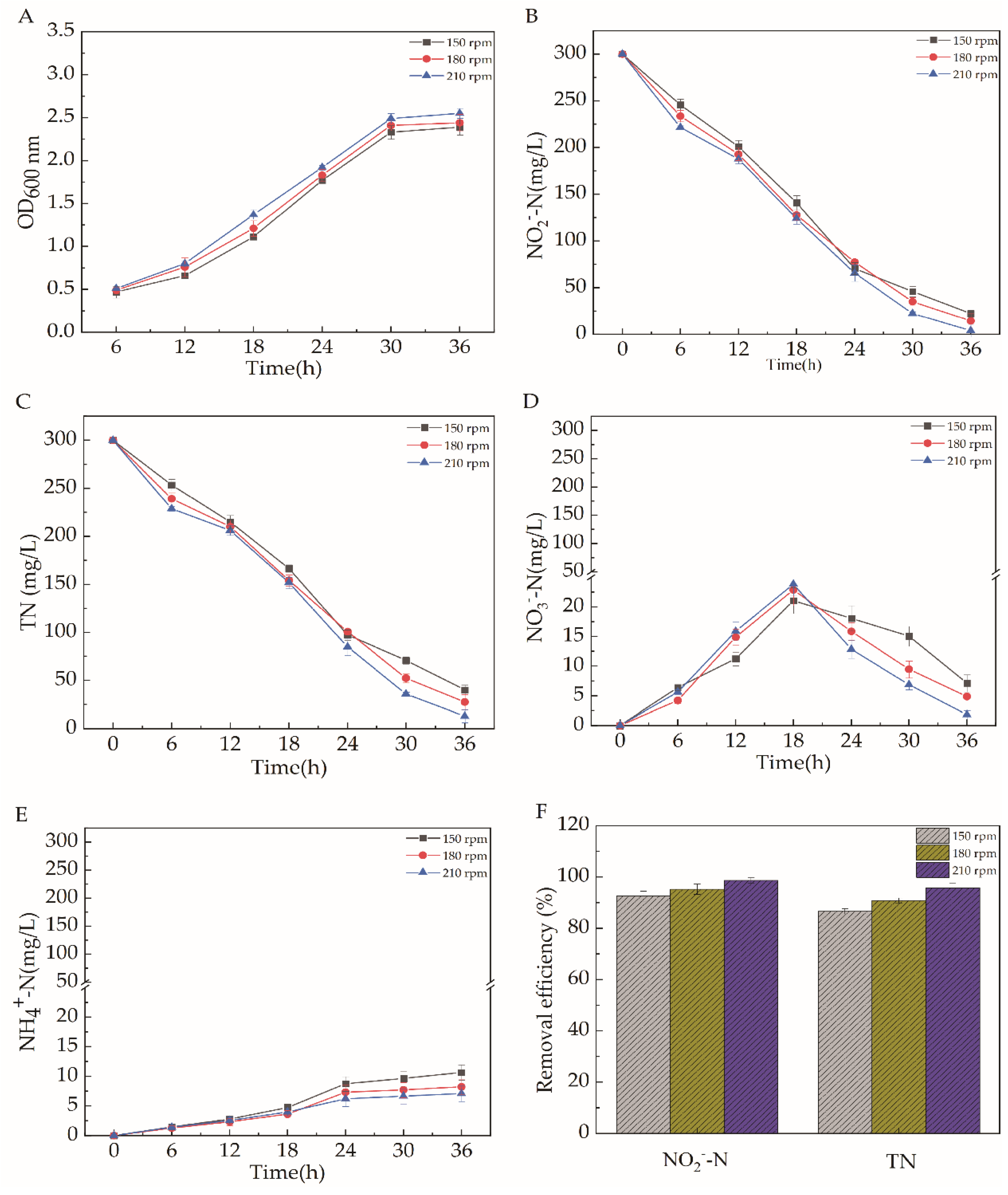
2.5. Effects of Different Carbon Source Types, C/N, pH, Temperature, and Dissolved Oxygen on the Efficiency of Aerobic Denitrification
2.5.1. Different Carbon Source Types
2.5.2. C/N
2.5.3. pH
2.5.4. Temperature
2.5.5. Dissolved Oxygen (DO)
3. Materials and Methods
3.1. Media
3.2. Strain Screening and Identification
3.3. Targeted Domestication of Strains and Detection of Nitrogen Reduction
3.4. Single Factor Affecting Aerobic Denitrification
3.5. Analytical Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Emparanza, E. Problems affecting nitrification in commercial RAS with fixed-bed biofilters for salmonids in Chile. Aquac. Eng. 2009, 41, 91–96. [Google Scholar] [CrossRef]
- Saliling, W.; Westerman, P.W.; Losordo, T.M. Wood chips and wheat straw as alternative biofilter media for denitrification reactors treating aquaculture and other wastewaters with high nitrate concentrations. Aquac. Eng. 2007, 37, 222–233. [Google Scholar] [CrossRef]
- Manju, N.J.; Deepesh, V.; Achuthan, C.; Rosamma, P.; Singh, I. Immobilization of nitrifying bacterial consortia on wood particles for bioaugmenting nitrification in shrimp culture systems. Aquaculture 2009, 294, 65–75. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Thomaidis, N.S.; Xu, J. Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: A critical review. J. Hazard. Mater. 2016, 323, 274–298. [Google Scholar] [CrossRef]
- Zhu, R.; Wang, S.; Li, J.; Wang, K.; Miao, L.; Ma, B.; Peng, Y. Biological nitrogen removal from landfill leachate using anaerobic–aerobic process: Denitritation via organics in raw leachate and intracellular storage polymers of microorganisms. Bioresour. Technol. 2013, 128, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Robertson, L.A.; Kuenen, J.G. Thiosphaera pantotropha gen. nov. sp. nov., a Facultatively Anaerobic, Facultatively Autotrophic Sulphur Bacterium. Microbiology 1983, 129, 2847–2855. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Cruz, M.; García-Galán, M.; Guerra, P.; Jelic, A.; Postigo, C.; Eljarrat, E.; Farré, M.; Alda, M.; Petrovic, M.; Barceló, D. The Handbook of Environmental Chemistry. TrAC Trends Anal. Chem. 2009, 28, 1263–1275. [Google Scholar] [CrossRef]
- Pai, S.L.; Chong, N.M.; Chen, C.H. Potential applications of aerobic denitrifying bacteria as bioagents in wastewater treatment. Bioresour. Technol. 1999, 68, 179–185. [Google Scholar] [CrossRef]
- Diekmann, T.L.H. Aerobic denitrification by a newly isolated heterotrophic bacterium strain TL1. Biotechnol. Lett. 1997, 19, 1157–1159. [Google Scholar]
- Chen, F.; Xia, Q.; Ju, L.K. Aerobic Denitrification of Pseudomonas aeruginosa Monitored by Online NAD(P)H Fluorescence. Appl. Environ. Microbiol. 2003, 69, 6715–6722. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.K.; Park, K.J.; Cho, K.S.; Nam, S.W.; Park, T.J.; Bajpai, R. Aerobic nitrification-denitrification by heterotrophic Bacillus strains. Bioresour. Technol. 2005, 96, 1897–1906. [Google Scholar] [CrossRef]
- Wang, H.Y.; Fang, M.A.; Zhou, D.D. The Mechanism and Research Progress of Synchronous Nitrification and Aerobic Denitrification in Biological Nitrogen Removal. Sichuan Environ. 2004, 36, 11–14. [Google Scholar]
- Bing, L.I.; Lin, W.T. Study on Aerobic Denitrification Characteristics of Bacillus Strain D5. J. Hydroecology 2009, 3, 48–52. [Google Scholar]
- Zufarzaana, Z.; Zaharin, A.A.; Shamsuddin, Z.H.; Kamil, Y.M. Cation Dependence, pH Tolerance, and Dosage Requirement of a Bioflocculant Produced by Bacillus spp. UPMB13: Flocculation Performance Optimization through Kaolin Assays. Sci. World J. 2012, 2012, 495659. [Google Scholar]
- Zhao, B.; Cheng, D.Y.; Tan, P.; An, Q.; Guo, J.S. Characterization of an aerobic denitrifier Pseudomonas stutzeri strain XL-2 to achieve efficient nitrate removal. Bioresour. Technol. 2017, 250, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Li, W.F.; Fu, L.Q.; Deng, B.; Chen, N.N.; Zhou, X.X. Identification and denitrification characteristics of an aerobic denitrifier. Huan Jing Ke Xue = Huanjing Kexue 2011, 32, 2403–2408. [Google Scholar]
- Tian, X.; Cheng, Y.; Zhang, Y.; Shi, X. Isolation, identification and nitrogen removal characteristics of a heterotrophic nitrification-aerobic denitrification bacterium. Chin. J. Environ. Eng. 2017. [Google Scholar]
- Chen, L.; Bai, J.; Zhao, Y.; Tian, W.; Zhang, Y.; Dang, J.; Li, K. Identification and denitrification characteristics of an aerobic denitrifier in estuary phragmites wetland. Acta Microbiol. Sin. 2016, 56, 1314–1325. [Google Scholar]
- Xiu, H.; Zhu, Z.; Ding, A.; Zheng, L. Isolation and identification of the aerobic denitrifying strain DF2 and its physiological and biochemical analysis. Ecol. Environ. Sci. 2011, 20, 1307–1314. [Google Scholar]
- Huang, H.K.; Tseng, S.K. Nitrate reduction by Citrobacter diversus under aerobic environment. Appl. Microbiol. Biotechnol. 2001, 55, 90–94. [Google Scholar] [CrossRef]
- Patureau, D.; Bernet, N.; Moletta, R. Effect of oxygen on denitrification in continuous chemostat culture with Comamonas sp. SGLY2. J. Ind. Microbiol. 1996, 16, 124–128. [Google Scholar] [CrossRef]
- Gupta, A.B. Thiosphaera pantotropha: A sulphur bacterium capable of simultaneous heterotrophic nitrification and aerobic denitrification. Enzym. Microb. Technol. 1997, 21, 589–595. [Google Scholar] [CrossRef]
- Timmermans, P.; Haute, A.V. Denitrification with methanol-fundamental study of the growth and denitrification capacity of Hyphomicrobium spp. Water Res. 1983, 17, 1249–1255. [Google Scholar] [CrossRef]
- Zhao, B.; Yi, L.H.; Hughes, J.; Xiao, F.Z. Heterotrophic nitrogen removal by a newly isolated Acinetobacter calcoaceticus HNR. Bioresour. Technol. 2010, 101, 5194–5200. [Google Scholar] [CrossRef]
- Song, Z.F.; An, J.; Fu, G.H.; Yang, X.L. Isolation and characterization of an aerobic denitrifying Bacillus sp. YX-6 from shrimp culture ponds. Aquaculture 2011, 319, 188–193. [Google Scholar] [CrossRef]
- Joo, H.S.; Hirai, M.; Shoda, M. Characteristics of ammonium removal by heterotrophic nitrification-aerobic denitrification by Alcaligenes faecalis No. 4. J. Biosci. Bioeng. 2005, 100, 184–191. [Google Scholar] [CrossRef]
- Huang, T.; Guo, L.; Zhang, H.; Su, J.; Wen, G.; Zhang, K. Nitrogen-removal efficiency of a novel aerobic denitrifying bacterium, Pseudomonas stutzeri strain ZF31, isolated from a drinking-water reservoir. Bioresour. Technol. 2015, 196, 209–216. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, C.; Shen, J.; Wei, R.; Gao, Y.; Miao, A.; Xiao, L.; Yang, L. Characterization of Aerobic Denitrifying Bacterium Pseudomonas mendocina Strain GL6 and Its Potential Application in Wastewater Treatment Plant Effluent. Int. J. Environ. Res. Public Health 2019, 16, 364. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.X.; Lei, Y.; Liang, X. The characteristics of a novel heterotrophic nitrifying and aerobic denitrifying bacterium, Acinetobacter junii YB. Bioresour. Technol. 2014, 171, 1–9. [Google Scholar] [CrossRef]
- Tiedje, J.M. Ecology of denitrification and dissimilatory nitrate reduction to ammonium. Biol. Anaerob. Microorg. 1988, 27, 179–244. [Google Scholar]
- Guo, L.J.; Zhao, B.; An, Q.; Tian, M. Characteristics of a Novel Aerobic Denitrifying Bacterium, Enterobacter cloacae Strain HNR. Appl. Biochem. Biotechnol. 2016, 178, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Ni, J.; Tao, M.; Li, C. Heterotrophic nitrification and aerobic denitrification at low temperature by a newly isolated bacterium, Acinetobacter sp. HA2. Bioresour. Technol. 2013, 139, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Zhao, B.; An, Q.; Huang, Y.S. Nitrogen removal by Providencia rettgeri strain YL with heterotrophic nitrification and aerobic denitrification. Environ. Technol. 2016, 37, 2206–2213. [Google Scholar] [CrossRef] [PubMed]

| Initial N (mg/L) | Final N (mg/L) | |||||
|---|---|---|---|---|---|---|
| NO2−-N | NO3−-N | NH4+-N | Organic N | Intracellular N | Gaseous N | |
| 300.0 ± 4.34 | 69.24 ± 4.61 | 5.04 ± 0.12 | 9.13 ± 0.32 | 2.73 ± 0.11 | 18.30 ± 0.22 | 213.86 ± 5.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, W.; Wang, Q.; Chen, S.; Wang, Y.; Wang, Y.; Duan, P.; Yi, G.; Liu, C.; Zhang, X.; Rao, Z. Isolation and Identification of an Efficient Aerobic Denitrifying Pseudomonas stutzeri Strain and Characterization of Its Nitrite Degradation. Catalysts 2021, 11, 1214. https://doi.org/10.3390/catal11101214
Fu W, Wang Q, Chen S, Wang Y, Wang Y, Duan P, Yi G, Liu C, Zhang X, Rao Z. Isolation and Identification of an Efficient Aerobic Denitrifying Pseudomonas stutzeri Strain and Characterization of Its Nitrite Degradation. Catalysts. 2021; 11(10):1214. https://doi.org/10.3390/catal11101214
Chicago/Turabian StyleFu, Weilai, Qiang Wang, Shuhui Chen, Yunshuang Wang, Yaru Wang, Peifeng Duan, Ganfeng Yi, Chao Liu, Xian Zhang, and Zhiming Rao. 2021. "Isolation and Identification of an Efficient Aerobic Denitrifying Pseudomonas stutzeri Strain and Characterization of Its Nitrite Degradation" Catalysts 11, no. 10: 1214. https://doi.org/10.3390/catal11101214
APA StyleFu, W., Wang, Q., Chen, S., Wang, Y., Wang, Y., Duan, P., Yi, G., Liu, C., Zhang, X., & Rao, Z. (2021). Isolation and Identification of an Efficient Aerobic Denitrifying Pseudomonas stutzeri Strain and Characterization of Its Nitrite Degradation. Catalysts, 11(10), 1214. https://doi.org/10.3390/catal11101214







