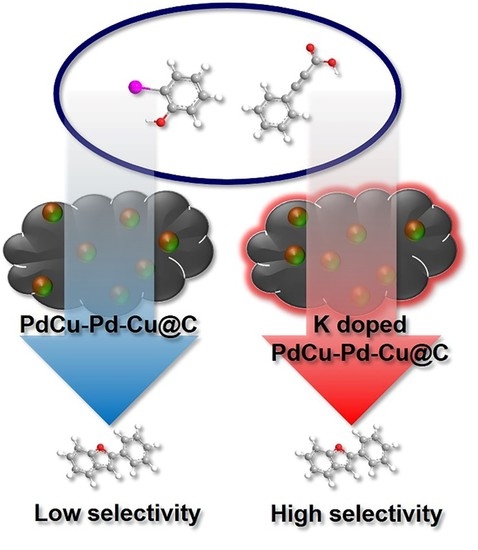
Non-Solvent Synthesis of a Robust Potassium-Doped PdCu-Pd-Cu@C Nanocatalyst for High Selectively Tandem Reactions
Abstract
:1. Introduction
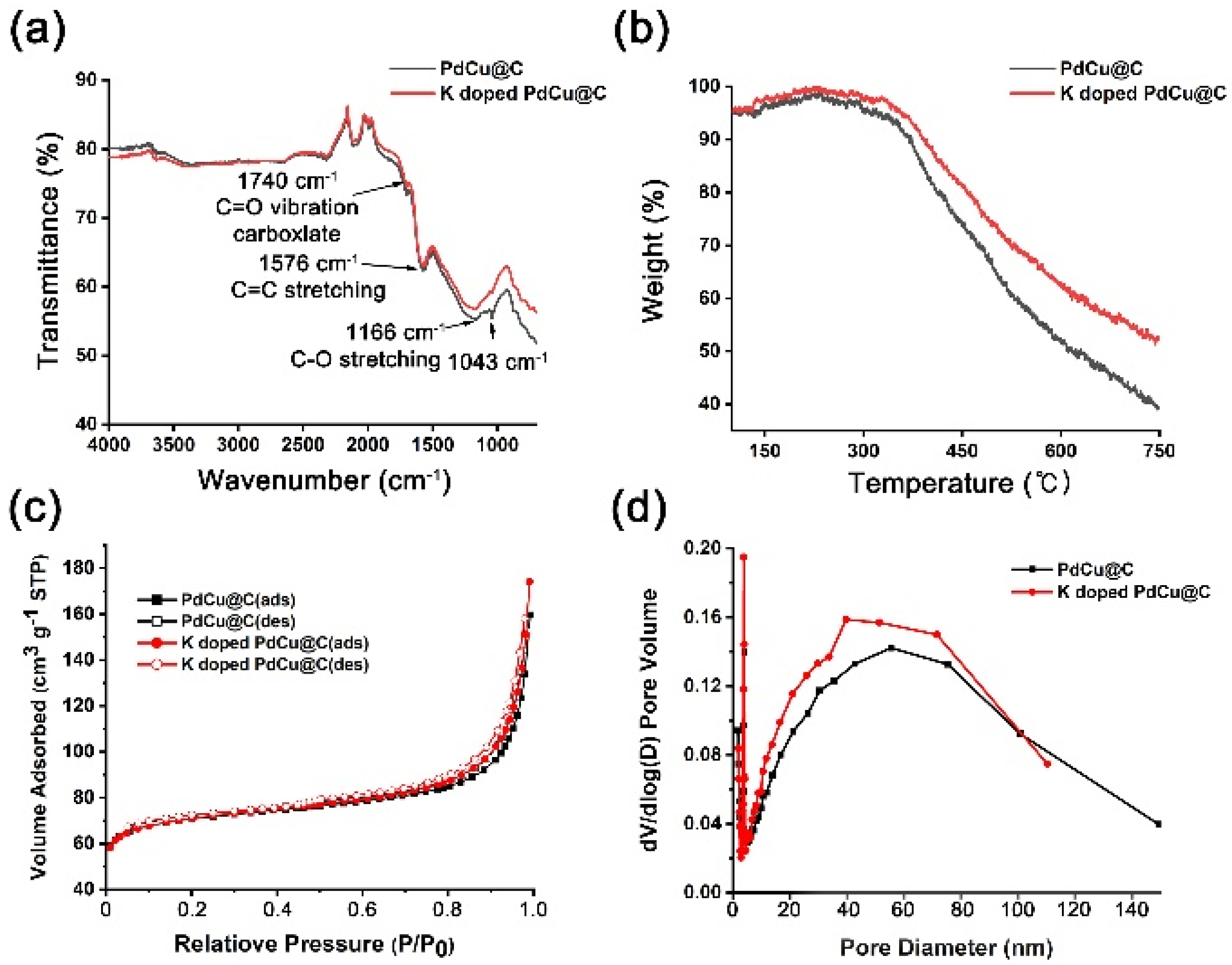
2. Results and Discussion
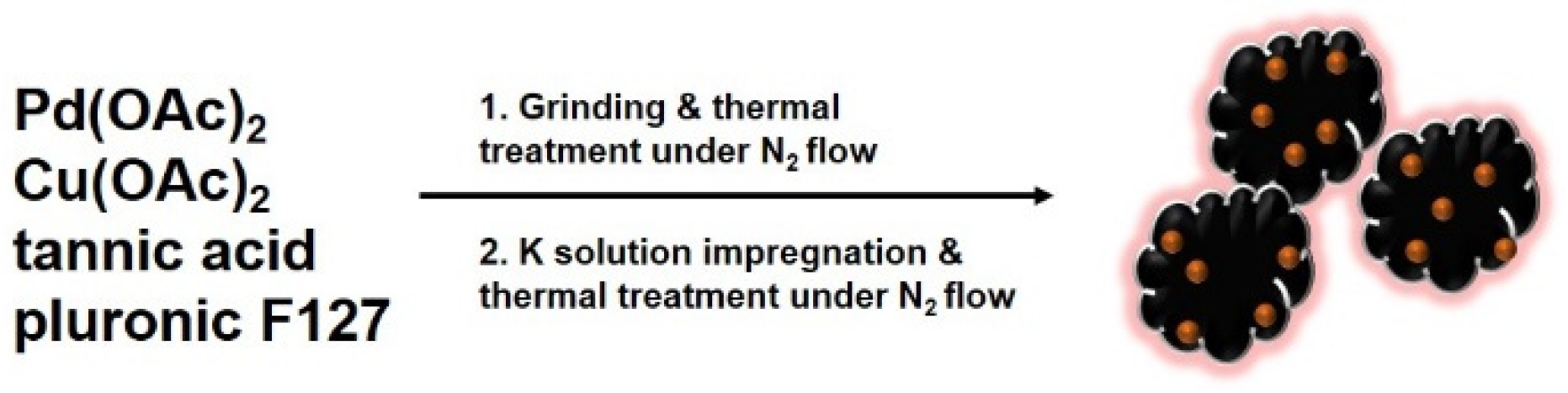
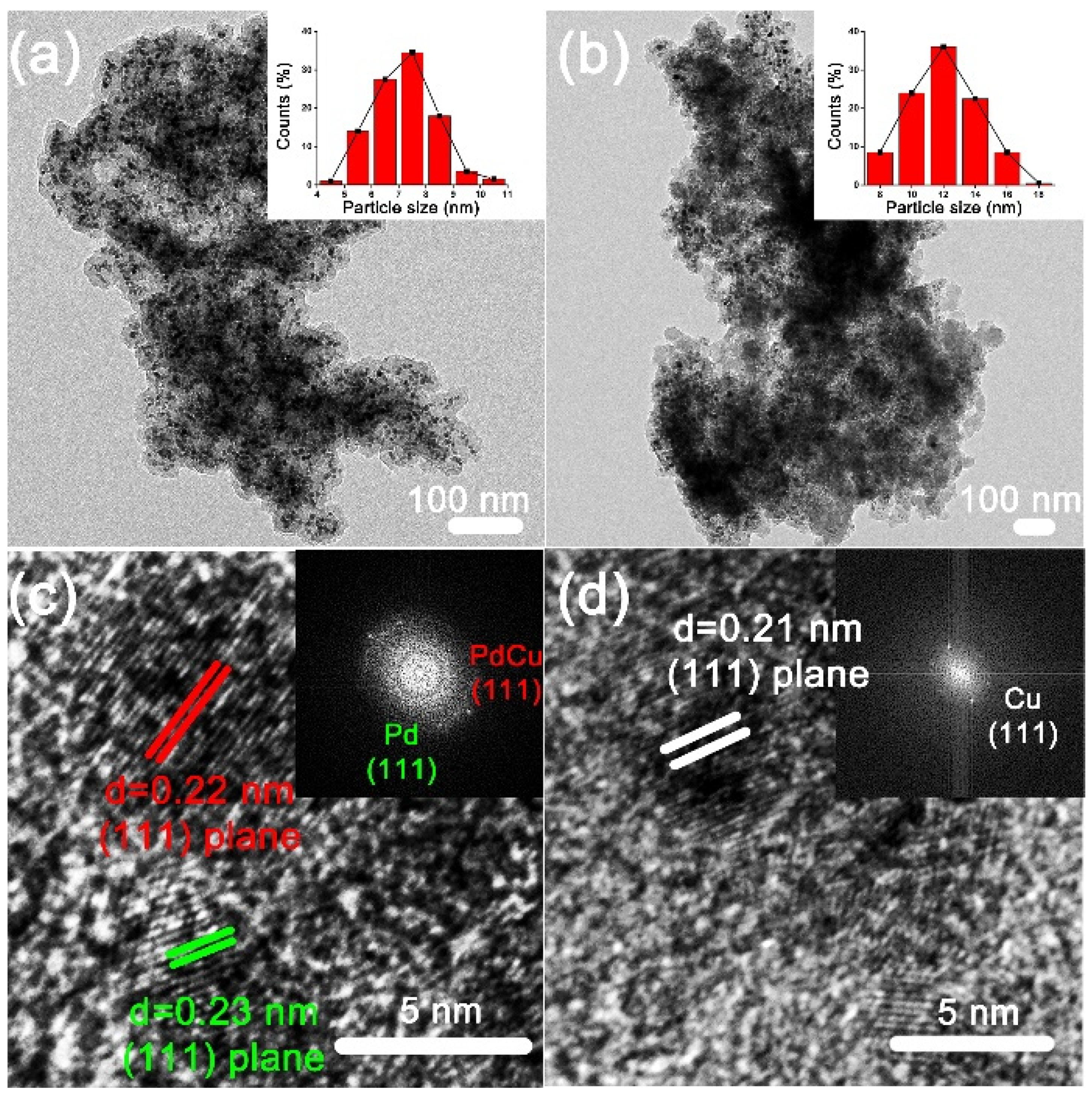
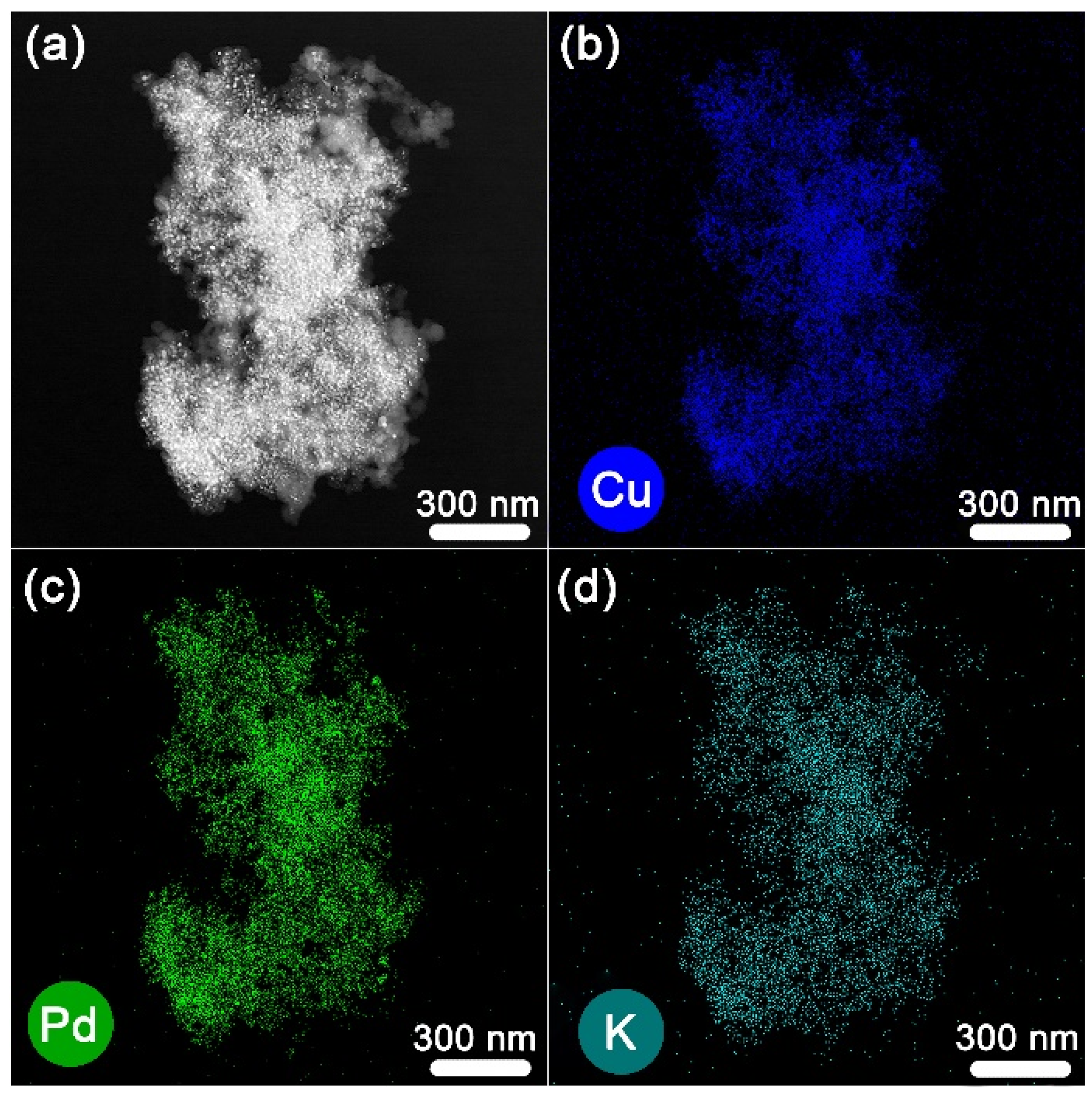
2.1. Preparation of K-Doped PdCu-Pd-Cu @C Nanocatalyst
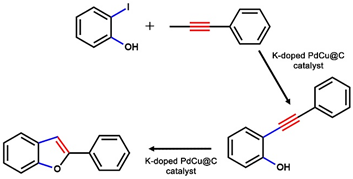
2.2. The Catalytic Activity of Nanocatalysts toward the Tandem Reaction of 2-Benzofuran
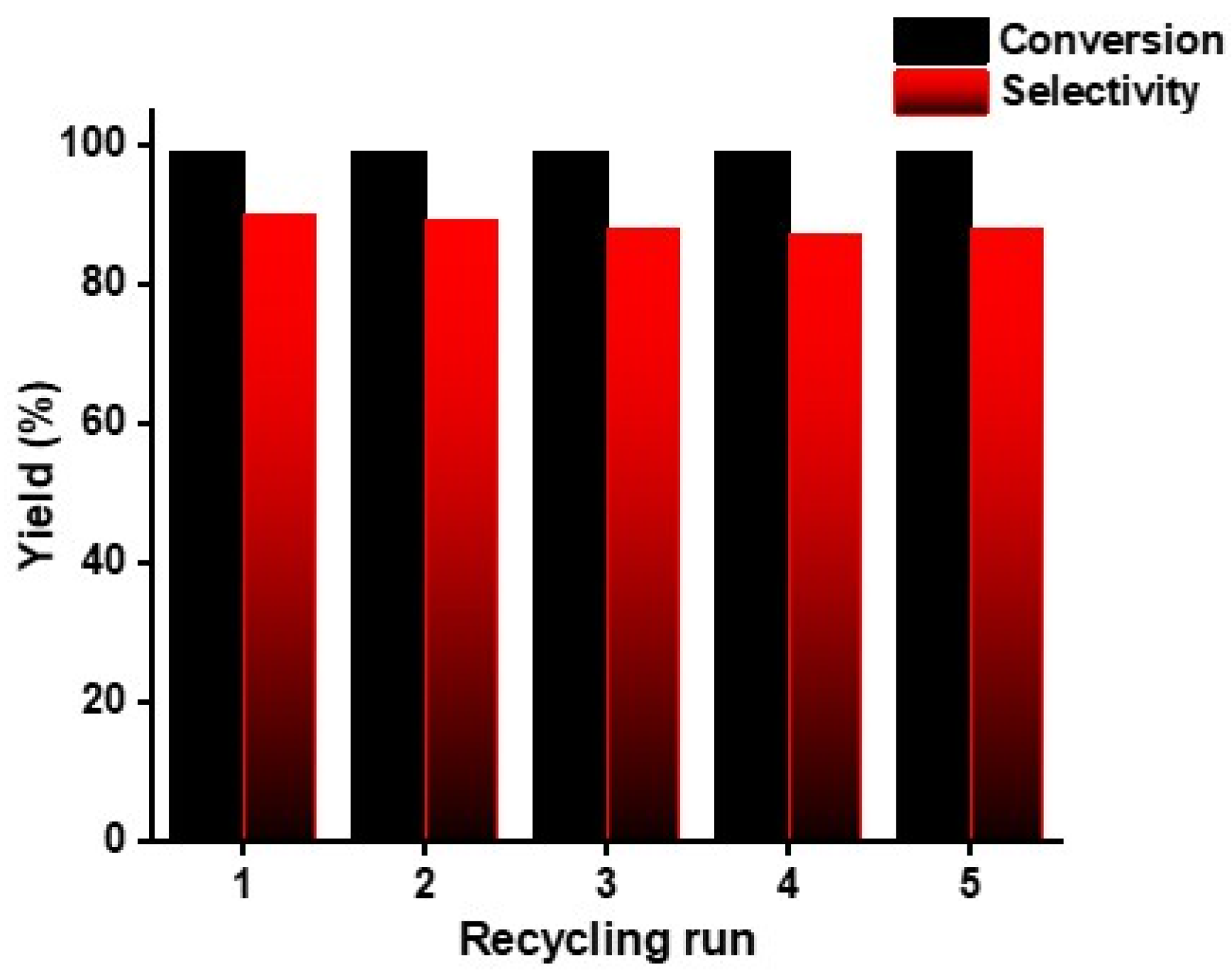
2.3. Recyclability Test
3. Materials and Methods
3.1. Chemicals and Characterization
3.2. Synthesis of PdCu-Pd-Cu@C and K-Doped PdCu-Pd-Cu@C Nanocatalysts
3.3. Tandem Reaction of 2-Phenylbenzofurans
3.4. Recyclability Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moussa, S.; Siamaki, A.R.; Gupton, B.F.; El-Shall, M.S. Pd-Partially Reduced Graphene Oxide Catalysts (Pd/PRGO): Laser Synthesis of Pd Nanoparticles Supported on PRGO Nanosheets for Carbon-Carbon Cross Coupling Reactions. ACS Catal. 2012, 2, 145–154. [Google Scholar] [CrossRef]
- Tan, L.; Wu, X.; Chen, D.; Liu, H.; Meng, X.; Tang, F. Confining Alloy or Core–Shell Au–Pd Bimetallic Nanocrystals in Silica Nanorattles for Enhanced Catalytic Performance. J. Mater. Chem. A 2013, 1, 10382–10388. [Google Scholar] [CrossRef]
- Veisi, H.; Sedrpoushan, A.; Maleki, B.; Hekmati, M.; Heidari, M.; Hemmati, S. Palladium Immobilized on Amidoxime-Functionalized Magnetic Fe3O4 Nanoparticles: A Highly Stable and Efficient Magnetically Recoverable Nanocatalyst for Sonogashira Coupling Reaction. Appl. Organomet. Chem. 2015, 29, 834–839. [Google Scholar] [CrossRef]
- Jang, S.; Hira, S.A.; Annas, D.; Song, S.; Yusuf, M.; Park, J.C.; Park, S.; Park, K.H. Recent Novel Hybrid Pd-Fe3O4 Nanoparticles as Catalysts for Various C-C Coupling Reactions. Processes 2019, 7, 422. [Google Scholar] [CrossRef] [Green Version]
- Woo, H.; Lee, K.; Park, J.C.; Park, K.H. Facile Synthesis of Pd/Fe3O4/Charcoal Bifunctional Catalysts with High Metal Loading for High Product Yields in Suzuki-Miyaura Coupling Reactions. New J. Chem. 2014, 38, 5626–5632. [Google Scholar] [CrossRef]
- Tan, Q.; Du, C.; Sun, Y.; Yin, G.; Gao, Y. Pd-around-CeO2-x Hybrid Nanostructure Catalyst: Three-Phase-Transfer Synthesis, Electrocatalytic Properties and Dual Promoting Mechanism. J. Mater. Chem. A 2014, 2, 1429–1435. [Google Scholar] [CrossRef]
- Truong, Q.D.; Le, T.S.; Ling, Y.C. Pt Deposited TiO2 Catalyst Fabricated by Thermal Decomposition of Titanium Complex for Solar Hydrogen Production. Solid State Sci. 2014, 38, 18–24. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Mohammad Sajadi, S.; Rostami-Vartooni, A.; Khalaj, M. Green Synthesis of Pd/Fe3O4 Nanoparticles Using Euphorbia Condylocarpa M. Bieb Root Extract and Their Catalytic Applications as Magnetically Recoverable and Stable Recyclable Catalysts for the Phosphine-Free Sonogashira and Suzuki Coupling Reactions. J. Mol. Catal. A Chem. 2015, 396, 31–39. [Google Scholar] [CrossRef]
- Siamaki, A.R.; Khder, A.E.R.S.; Abdelsayed, V.; El-Shall, M.S.; Gupton, B.F. Microwave-Assisted Synthesis of Palladium Nanoparticles Supported on Graphene: A Highly Active and Recyclable Catalyst for Carbon-Carbon Cross-Coupling Reactions. J. Catal. 2011, 279, 1–11. [Google Scholar] [CrossRef]
- Elazab, H.A.; Siamaki, A.R.; Moussa, S.; Gupton, B.F.; El-Shall, M.S. Highly Efficient and Magnetically Recyclable Graphene-Supported Pd/Fe3O4 Nanoparticle Catalysts for Suzuki and Heck Cross-Coupling Reactions. Appl. Catal. A Gen. 2015, 491, 58–69. [Google Scholar] [CrossRef]
- Hosseini, S.G.; Abazari, R.; Ghavi, A. Pure CuCr2O4 Nanoparticles: Synthesis, Characterization and Their Morphological and Size Effects on the Catalytic Thermal Decomposition of Ammonium Perchlorate. Solid State Sci. 2014, 37, 72–79. [Google Scholar] [CrossRef]
- Woo, H.; Kim, D.; Park, J.C.; Kim, J.W.; Park, S.; Lee, J.M.; Park, K.H. A New Hybrid Nanocatalyst Based on Cu-Doped Pd–Fe3O4 for Tandem Synthesis of 2-Phenylbenzofurans. J. Mater. Chem. A 2015, 3, 20992–20998. [Google Scholar] [CrossRef]
- Massart, R. Preparation of Aqueous Magnetic Liquids in Alkaline and Acidic Media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Mazarío, E.; Mayoral, A.; Salas, E.; Menéndez, N.; Herrasti, P.; Sánchez-Marcos, J. Synthesis and Characterization of Manganese Ferrite Nanoparticles Obtained by Electrochemical/Chemical Method. Mater. Des. 2016, 111, 646–650. [Google Scholar] [CrossRef]
- Mukherjee, K.; Bharti, D.C.; Majumder, S.B. Solution Synthesis and Kinetic Analyses of the Gas Sensing Characteristics of Magnesium Ferrite Particles. Sens. Actuators B Chem. 2010, 146, 91–97. [Google Scholar] [CrossRef]
- Fang, J.; Shama, N.; Tung, L.D.; Shin, E.Y.; O’Connor, C.J.; Stokes, K.L.; Caruntu, G.; Wiley, J.B.; Spinu, L.; Tang, J. Ultrafine NiFe2O4 Powder Fabricated from Reverse Microemulsion Process. J. Appl. Phys. 2003, 93, 7483–7485. [Google Scholar] [CrossRef] [Green Version]
- Holec, P.; Plocek, J.; Nižňanský, D.; Poltierová Vejpravová, J. Preparation of MgFe2O4 Nanoparticles by Microemulsion Method and Their Characterization. J. Sol-Gel Sci. Technol. 2009, 51, 301–305. [Google Scholar] [CrossRef]
- Durrani, S.K.; Naz, S.; Hayat, K. Thermal Analysis and Phase Evolution of Nanocrystalline Perovskite Oxide Materials Synthesized via Hydrothermal and Self-Combustion Methods. J. Therm. Anal. Calorim. 2014, 115, 1371–1380. [Google Scholar] [CrossRef]
- Durranim, S.K.; Naz, S.; Nadeem, M.; Ahmed, E.; Siddique, M. Thermal, Structural Analysis, Mössbauer and Impedance Study of Copper Nickel Ferrite Nanoparticles Synthesized via Tween 80-assisted hydrothermal process. J. Therm. Anal. Calorim. 2015, 119, 253–263. [Google Scholar] [CrossRef]
- Naz, S.; Durrani, S.K.; Mehmood, M.; Nadeem, M. Hydrothermal Synthesis, Structural and Impedance Studies of Nanocrystalline Zinc Chromite Spinel Oxide Material. J. Saudi Chem. Soc. 2016, 20, 585–593. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, H.; Xing, Y.; Song, S.; Yu, S.; Shi, W.; Guo, X.; Yang, J.; Lei, Y.; Cao, F. Studies on the Magnetism of Cobalt Ferrite Nanocrystals Synthesized by Hydrothermal Method. J. Solid State Chem. 2008, 181, 245–252. [Google Scholar] [CrossRef]
- Li, Y.; Zimmerman, A.R.; He, F.; Chen, J.; Han, L.; Chen, H.; Hu, X.; Gao, B. Solvent-Free Synthesis of Magnetic Biochar and Activated Carbon through Ball-Mill Extrusion with Fe3O4 Nanoparticles for Enhancing Adsorption of Methylene Blue. Sci. Total Environ. 2020, 722, 137972–137980. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Gao, B.; He, F.; Ding, C.; Tang, J.; Crittenden, J.C. Ball-Milled Carbon Nanomaterials for Energy and Environmental Applications. ACS Sustain. Chem. Eng. 2017, 5, 9568–9585. [Google Scholar] [CrossRef]
- Gorrasi, G.; Sorrentino, A. Mechanical Milling as a Technology to Produce Structural and Functional Bio-Nanocomposites. Green Chem. 2015, 17, 2610–2625. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Lyu, H.; Zhao, Q.; Jiang, L.; Liu, L. Ball-Milled Biochar for Galaxolide Removal: Sorption Performance and Governing Mechanisms. Sci. Total Environ. 2019, 659, 1537–1545. [Google Scholar] [CrossRef]
- Jang, S.; Kang, S.W.; Chun, D.H.; Lee, H.T.; Yang, J.I.; Jung, H.; Jeong, H.D.; Nam, K.M.; Park, J.C. Robust Iron-Carbide Nanoparticles Supported on Alumina for Sustainable Production of Gasoline-Range Hydrocarbons. New J. Chem. 2017, 41, 2756–2763. [Google Scholar] [CrossRef]
- Kang, S.W.; Kim, K.; Chun, D.H.; Yang, J.I.; Lee, H.T.; Jung, H.; Lim, T.J.; Jang, S.; Kim, C.S.; Lee, C.W.; et al. High-Performance Fe5C2@CMK-3 Nanocatalyst for Selective and High-Yield Production of Gasoline-Range Hydrocarbons. J. Catal. 2017, 349, 66–74. [Google Scholar] [CrossRef]
- Park, J.C.; Jang, S.; Rhim, G.B.; Lee, J.H.; Choi, H.; Jeong, H.D.; Youn, M.H.; Lee, D.W.; Koo, K.Y.; Kang, S.W.; et al. A Durable Nanocatalyst of Potassium-Doped Iron-Carbide/Alumina for Significant Production of Linear Alpha Olefins via Fischer-Tropsch Synthesis. Appl. Catal. A Gen. 2018, 564, 190–198. [Google Scholar] [CrossRef]
- Kweon, H.; Jang, S.; Bereketova, A.; Park, J.C.; Park, K.H. Highly Dispersed Ni Nanoparticles on Mesoporous Silica Nanospheres by Melt infiltration for Transfer Hydrogenation of Aryl Ketones. RSC Adv. 2019, 9, 14154–14159. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Wang, L.; Yang, S.; Schott, J.A.; Liu, X.; Mahurin, S.M.; Huang, C.; Zhang, Y.; Fulvio, P.F.; Chisholm, M.F.; et al. Solid-state synthesis of ordered mesoporous carbon catalysts via a mechanochemical assembly through coordination cross-linking. Nat. Commun. 2017, 8, 15020–15029. [Google Scholar] [CrossRef] [PubMed]
- Stummann, M.Z.; Hansen, A.B.; Hansen, L.P.; Davidsen, B.; Rasmussen, S.B.; Wiwel, P.; Gabrielsen, J.; Jensen, P.A.; Jensen, A.D.; Høj, M. Catalytic Hydropyrolysis of Biomass Using Molybdenum Sulfide Based Catalyst. Effect of Promoters. Energy Fuels 2019, 33, 1302–1313. [Google Scholar] [CrossRef] [Green Version]
- Woolfolk, L.G.; Geantet, C.; Massin, L.; Laurenti, D.; Reyes, J.A.D.L. Solvent Effect over the Promoter Addition for a Supported NiWS Hydrotreating Catalyst. Appl. Catal. B Environ. 2017, 201, 331–338. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Atia, H.; Ibrahim, A.A.; Fakeeha, A.H.; Singh, S.K.; Labhsetwar, N.K.; Shaikh, H.; Qasim, S.O. CO2 Reforming of CH4: Effect of Gd as Promoter for Ni Supported over MCM-41 as Catalyst. Renew. Energy 2019, 140, 658–667. [Google Scholar] [CrossRef]
- Pastor-Pérez, L.; Shah, M.; le Saché, E.; Reina, T.R. Improving Fe/Al2O3 Catalysts for the Reverse Water-Gas Shift Reaction: On the Effect of Cs as Activity/Selectivity Promoter. Catalysts 2018, 8, 608. [Google Scholar] [CrossRef] [Green Version]
- Tian, Z.; Wang, C.; Yue, J.; Zhang, X.; Ma, L. Effect of a Potassium Promoter on the Fischer–Tropsch Synthesis of Light Olefins over Iron Carbide Catalysts Encapsulated in Graphene-like Carbon. Catal. Sci. Technol. 2019, 9, 2728–2741. [Google Scholar] [CrossRef]
- Desai, S.P.; Ye, J.; Zheng, J.; Ferrandon, M.S.; Webber, T.E.; Platero-prats, A.E.; Duan, J.; Garcia-Holley, P.; Camaioni, D.M.; Chapman, K.W.; et al. Well-Defined Rhodium−Gallium Catalytic Sites in a Metal−Organic Framework: Promoter-Controlled Selectivity in Alkyne Semihydrogenation to E-Alkenes. J. Am. Chem. Soc. 2018, 140, 15309–15318. [Google Scholar] [CrossRef] [PubMed]
- Zasada, F.; Stelmachowski, P.; Maniak, G.; Paul, J.F.; Kotarba, A.; Sojka, Z. Potassium Promotion of Cobalt Spinel Catalyst for N2O Decomposition-Accounted by Work Function Measurements and DFT Modelling. Catal. Lett. 2009, 127, 126–131. [Google Scholar] [CrossRef]
- Park, J.C.; Yeo, S.C.; Chun, D.H.; Lim, J.T.; Yang, J.I.; Lee, H.T.; Hong, S.; Lee, H.M.; Kim, C.S.; Jung, H. Highly Activated K-Doped Iron Carbide Nanocatalysts Designed by Computational Simulation for Fischer–Tropsch Synthesis. J. Mater. Chem. A 2014, 2, 14371–14379. [Google Scholar] [CrossRef]
- Park, J.C.; Chun, D.H.; Yang, J.I.; Lee, H.T.; Hong, S.; Rhim, G.B.; Jang, S.; Jung, H. Cs promoted Fe5C2/charcoal nanocatalysts for sustainable liquid fuel production. RSC Adv. 2015, 5, 44211–44217. [Google Scholar] [CrossRef]
- Nevagi, R.J.; Dighe, S.N.; Dighe, S.N. Biological and Medicinal Significance of Benzofuran. Eur. J. Med. Chem. 2015, 97, 561–581. [Google Scholar] [CrossRef]
- Kawasaki, K.; Masubuchi, M.; Morikami, K.; Sogabe, S.; Aoyama, T.; Ebiike, H.; Niizuma, S.; Hayase, M.; Fujii, T.; Sakata, K.; et al. Design and Synthesis of Novel Benzofurans as a New Class of Antifungal Agents Targeting Fungal N-Myristoyltransferase. Part 3. Bioorg. Med. Chem. Lett. 2003, 13, 87–91. [Google Scholar] [CrossRef]
- Ahmad, G.; Mishra, P.K.; Gupta, P.; Yadav, P.P.; Tiwari, P.; Tamrakar, A.K.; Srivastava, A.K.; Maurya, R. Synthesis of Novel Benzofuran Isoxazolines as Protein Tyrosine Phosphatase 1B Inhibitors. Bioorg. Med. Chem. Lett. 2006, 16, 2139–2143. [Google Scholar] [CrossRef] [PubMed]
- Logoglu, E.; Yilmaz, M.; Katircioglu, H.; Yakut, M.; Mercan, S. Synthesis and Biological Activity Studies of Furan Derivatives. Med. Chem. Res. 2010, 19, 490–497. [Google Scholar] [CrossRef]
- Jin, M.; Jeong, H.; Kim, T.; So, K.P.; Cui, Y.; Yu, W.J.; Ra, E.J.; Lee, Y.H. Synthesis and Systematic Characterization of Functionalized Graphene Sheets Generated by Thermal Exfoliation at Low Temperature. J. Phys. D Appl. Phys. 2010, 43, 275402–275406. [Google Scholar] [CrossRef]
- Brun, M.; Berthet, A.; Bertolini, J.C. XPS, AES and Auger Parameter of Pd and PdO. J. Electron Spectrosc. Relat. Phenom. 1999, 104, 55–60. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; St, R.; Smart, C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]

| Entry | Amount of catalyst (mol%) | Base | t (h) | Conv. (%) 1 | Sel. (%) |
|---|---|---|---|---|---|
| 1 | 1.3 | NaOAc | 5 | 90 | 43 |
| 2 | 1.3 | NaOAc | 3 | 82 | 43 |
| 3 | 1.3 | NaOAc | 1 | 84 | 55 |
| 4 | 1.3 | Cs2CO3 | 1 | n.d. | n.d. |
| 5 | 1.3 | Na2CO3 | 1 | 8 | <10 |
| 6 | 1.3 | LiOAc | 1 | 72 | 49 |
| 7 | 1.3 | KOH | 1 | 73 | 87 |
| 8 | 1.3 | NaOH | 1 | 63 | 71 |
| 9 | 4.0 | KOH | 1 | 88 | 82 |
| 10 | 6.6 | KOH | 1 | >99 | 90 |
| Entry | Catalyst | Conv. (%) 1 | Sel. (%) |
|---|---|---|---|
| 1 | Pd/C | >99 | 45 |
| 2 | Cu2O | n.d. | n.d. |
| 3 | CuO | n.d. | n.d. |
| 4 | PdCu-Pd-Cu@C | >99 | 76 |
| 5 | K-PdCu-Pd-Cu@C | >99 | 90 |
| 6 | Na-PdCu-Pd-Cu@C | >99 | 82 |
| 7 | Cs-PdCu-Pd-Cu@C | >99 | 78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, S.; Annas, D.; Song, S.; Bae, J.-S.; Park, S.; Park, K.H. Non-Solvent Synthesis of a Robust Potassium-Doped PdCu-Pd-Cu@C Nanocatalyst for High Selectively Tandem Reactions. Catalysts 2021, 11, 1191. https://doi.org/10.3390/catal11101191
Jang S, Annas D, Song S, Bae J-S, Park S, Park KH. Non-Solvent Synthesis of a Robust Potassium-Doped PdCu-Pd-Cu@C Nanocatalyst for High Selectively Tandem Reactions. Catalysts. 2021; 11(10):1191. https://doi.org/10.3390/catal11101191
Chicago/Turabian StyleJang, Sanha, Dicky Annas, Sehwan Song, Jong-Seong Bae, Sungkyun Park, and Kang Hyun Park. 2021. "Non-Solvent Synthesis of a Robust Potassium-Doped PdCu-Pd-Cu@C Nanocatalyst for High Selectively Tandem Reactions" Catalysts 11, no. 10: 1191. https://doi.org/10.3390/catal11101191
APA StyleJang, S., Annas, D., Song, S., Bae, J.-S., Park, S., & Park, K. H. (2021). Non-Solvent Synthesis of a Robust Potassium-Doped PdCu-Pd-Cu@C Nanocatalyst for High Selectively Tandem Reactions. Catalysts, 11(10), 1191. https://doi.org/10.3390/catal11101191