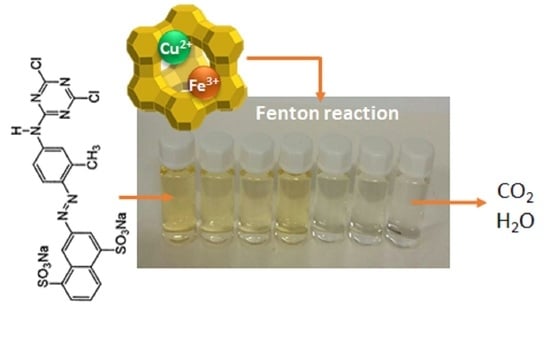
Fenton-Type Bimetallic Catalysts for Degradation of Dyes in Aqueous Solutions
Abstract
1. Introduction
2. Results and Discussion
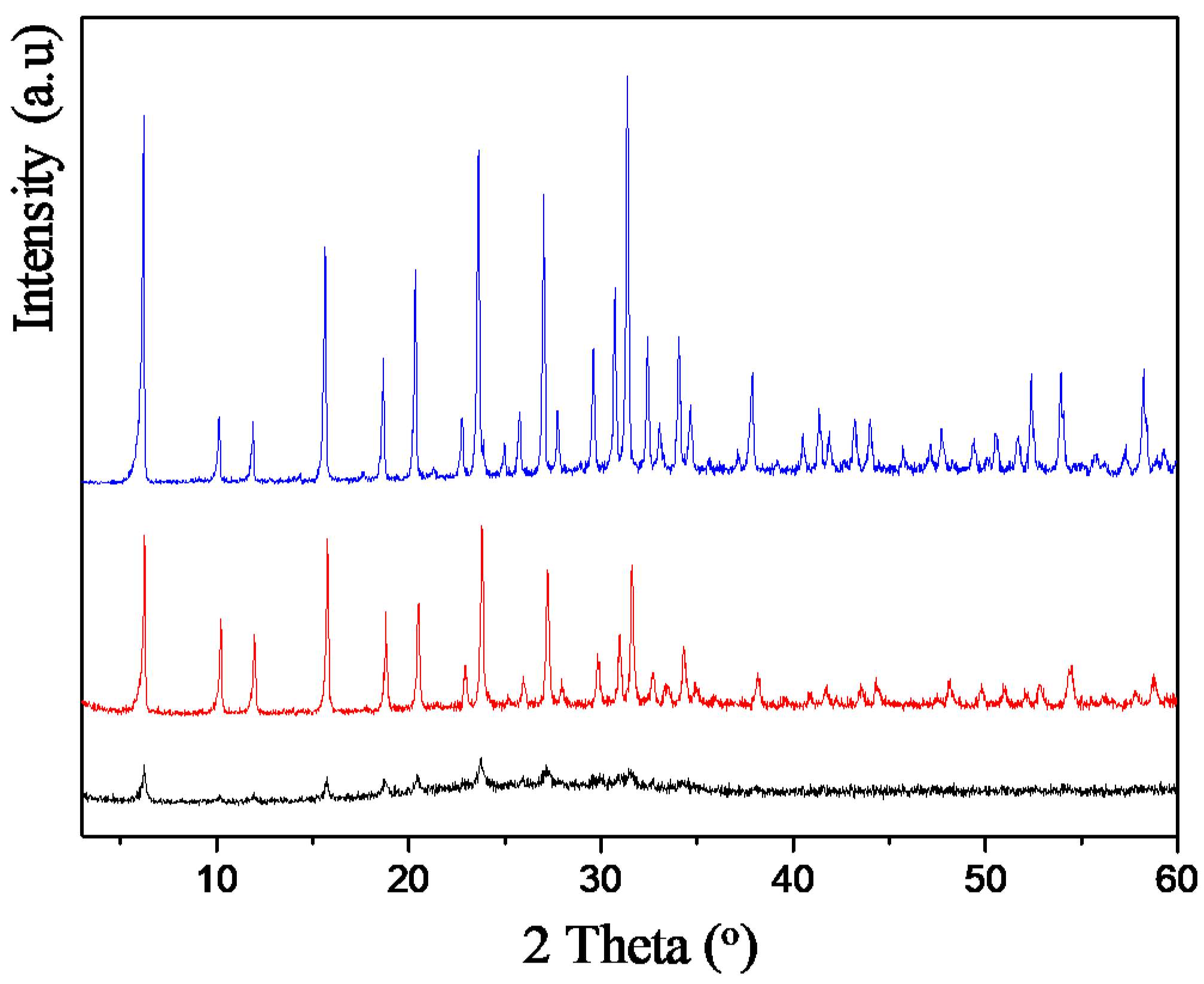
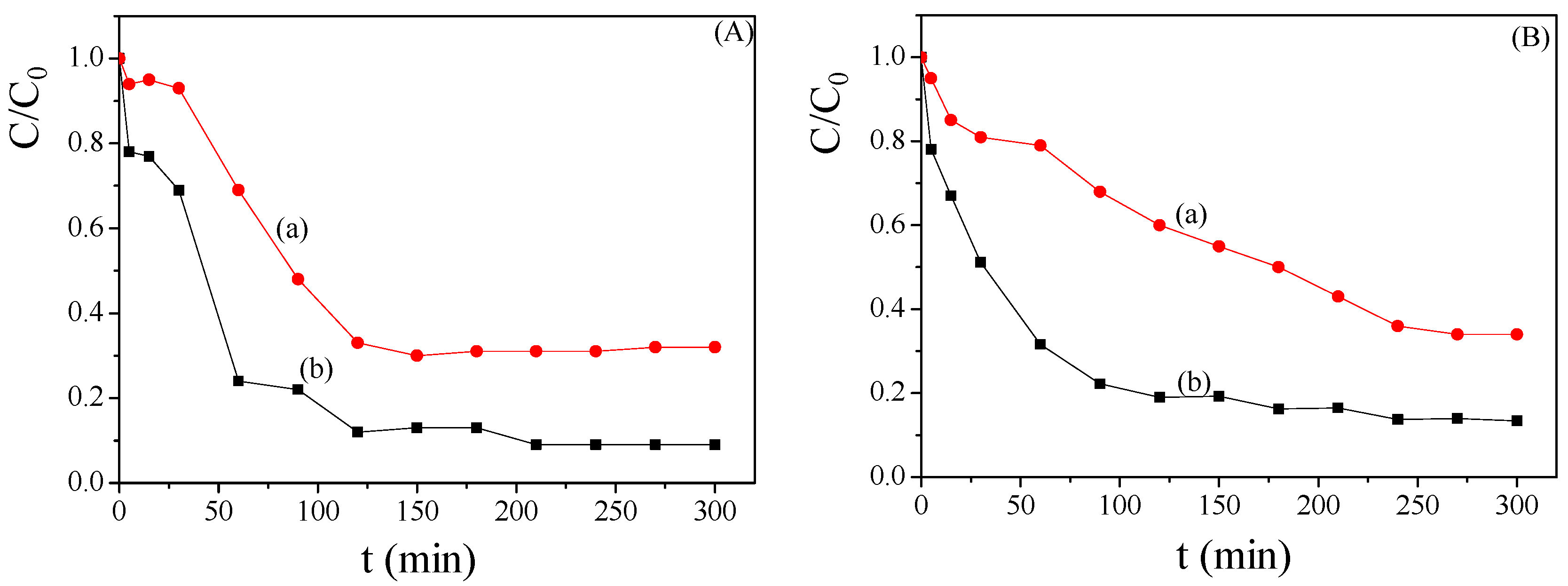
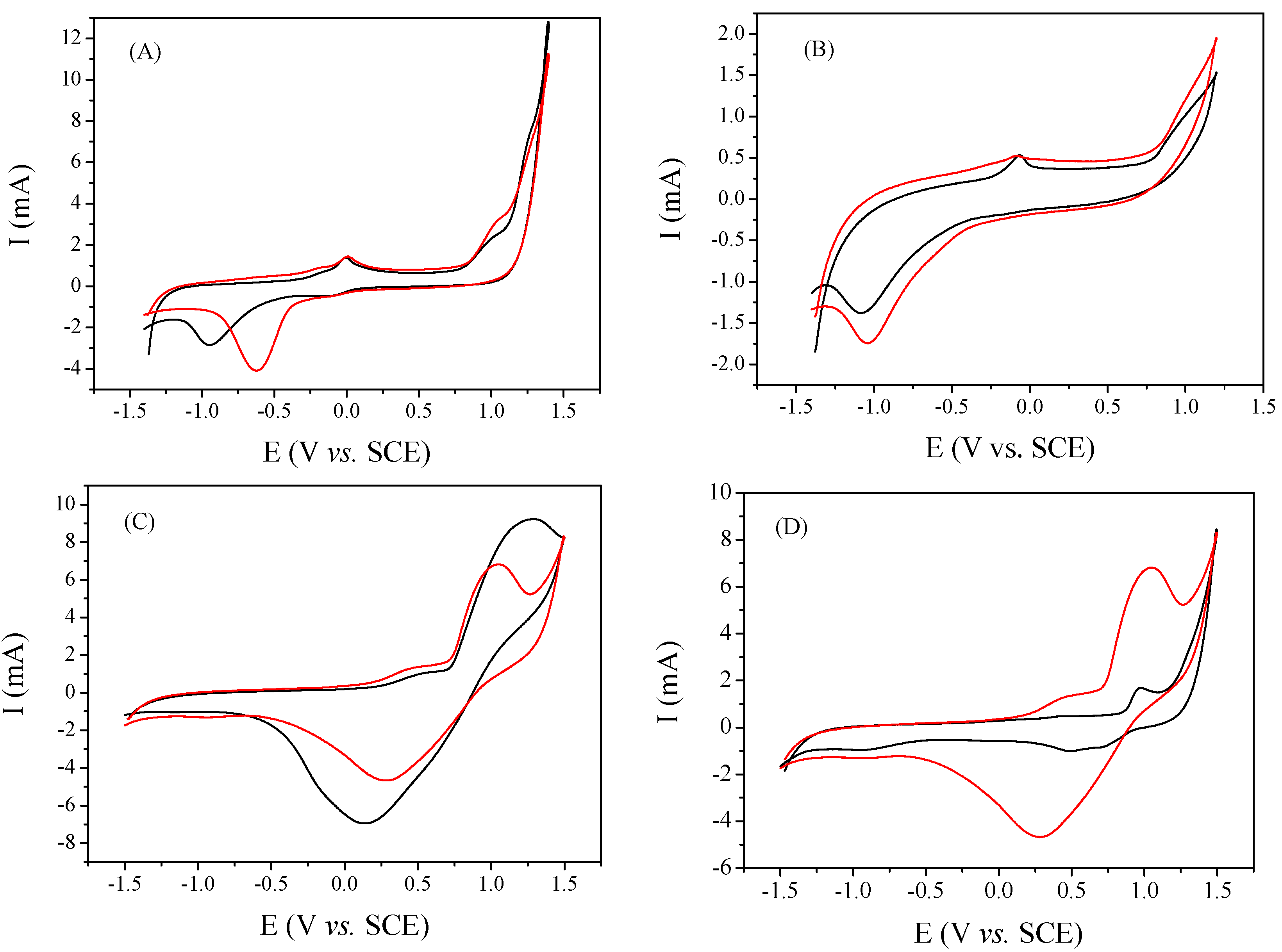
2.1. Selection of the Parent Zeolite Support
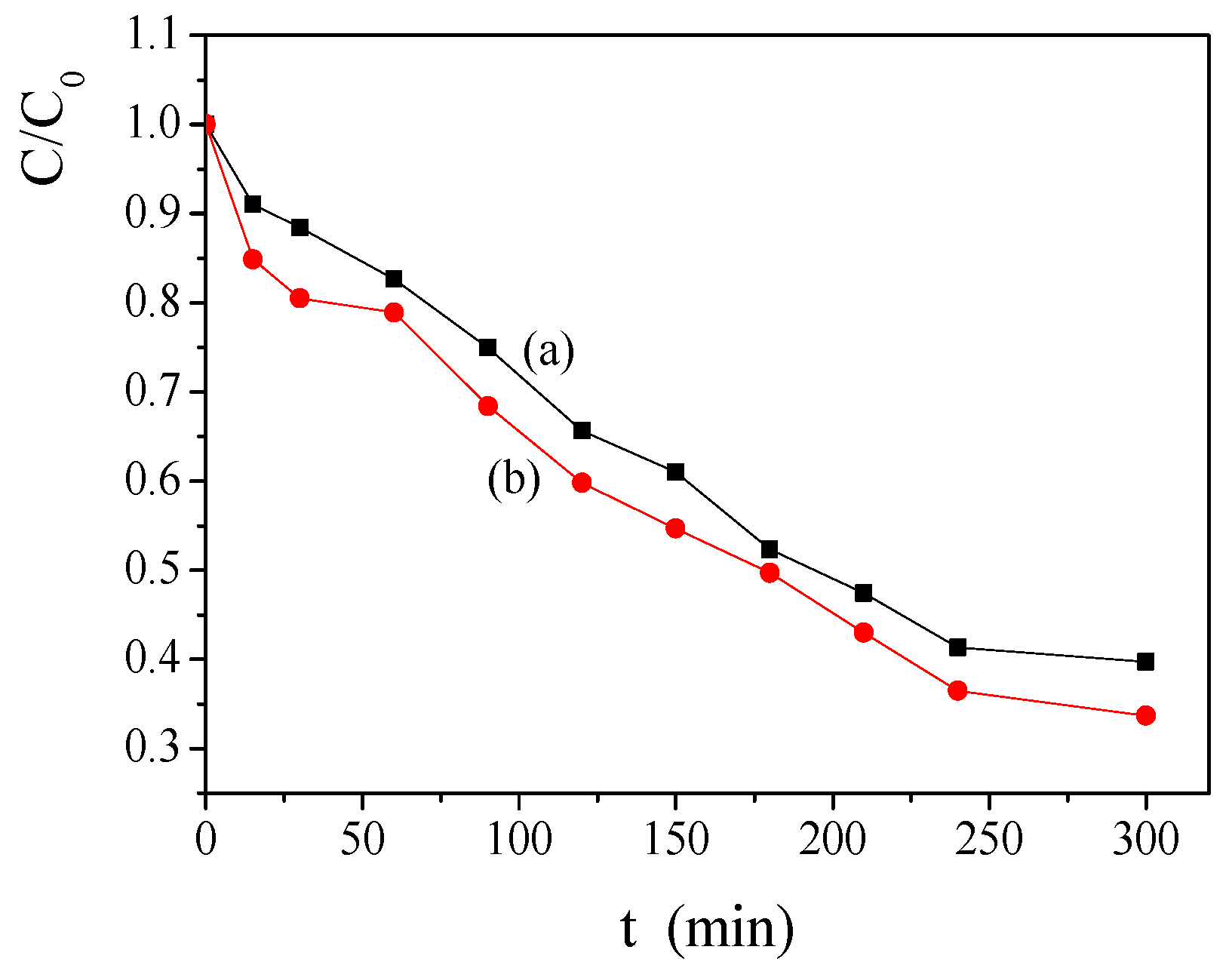
2.2. Effect of the Temperature and the Concentration of H2O2
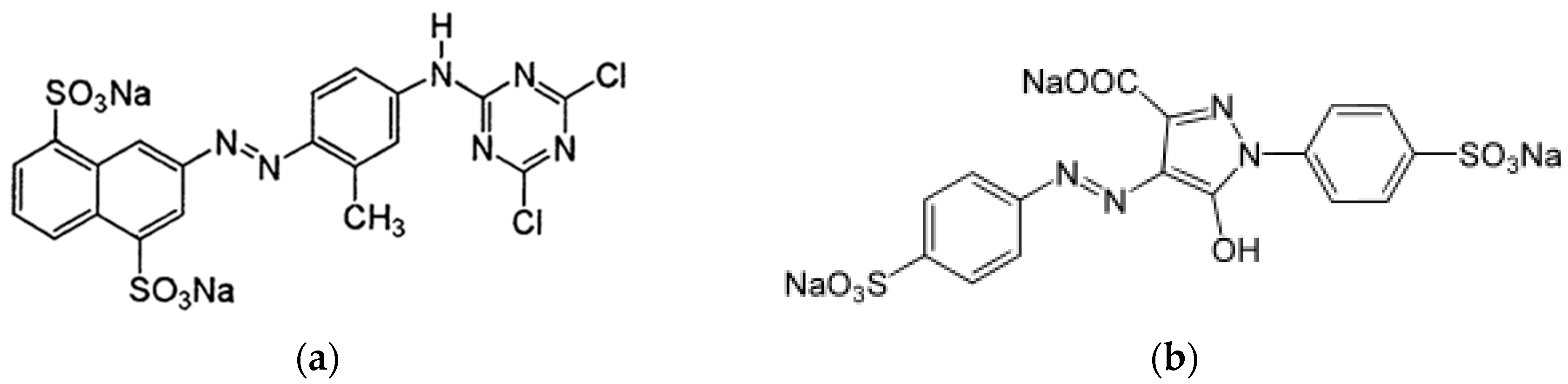
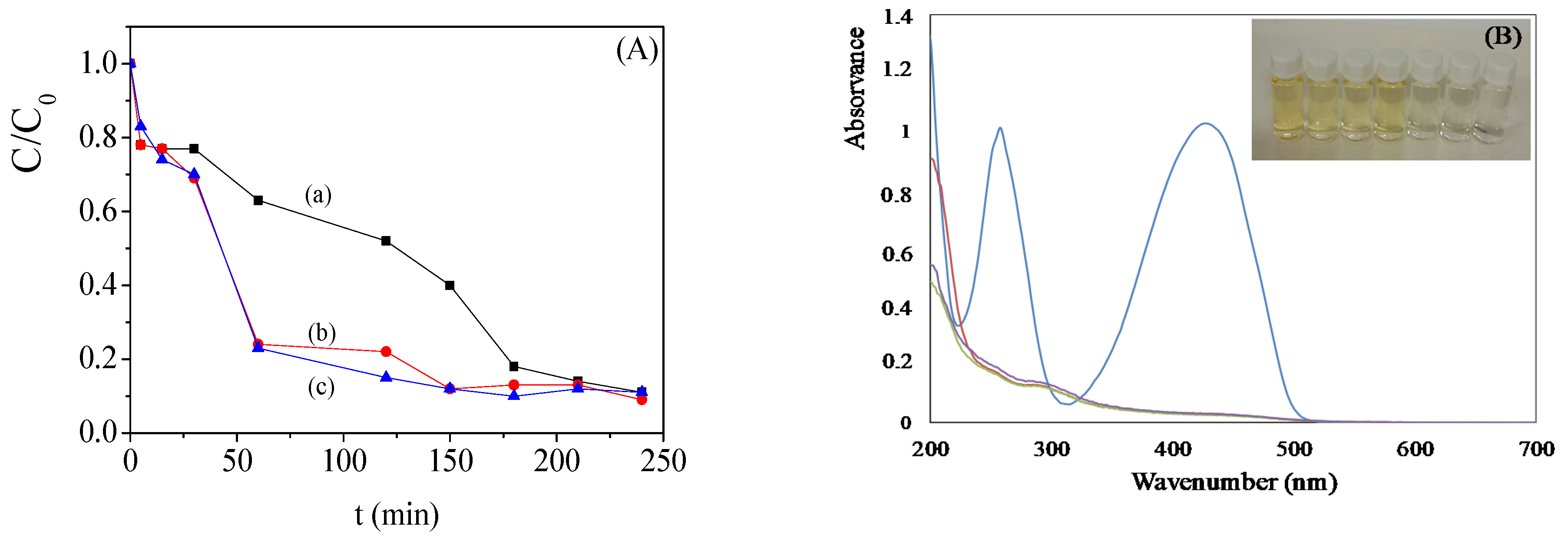
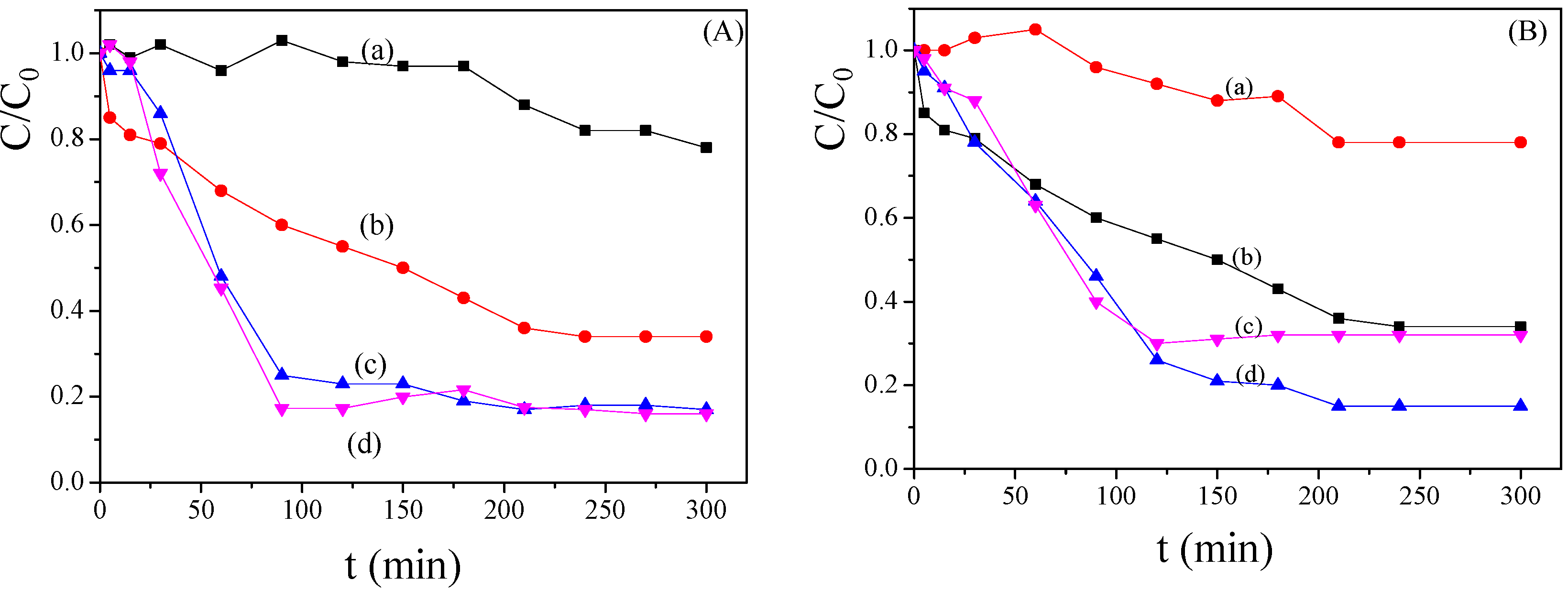
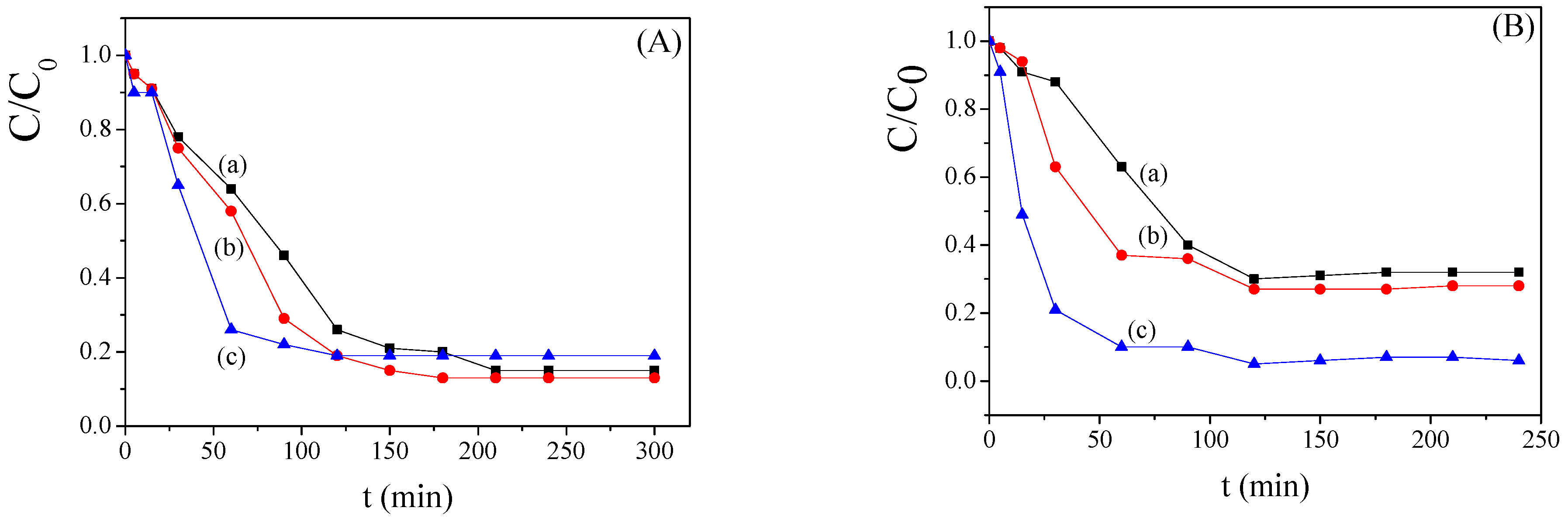
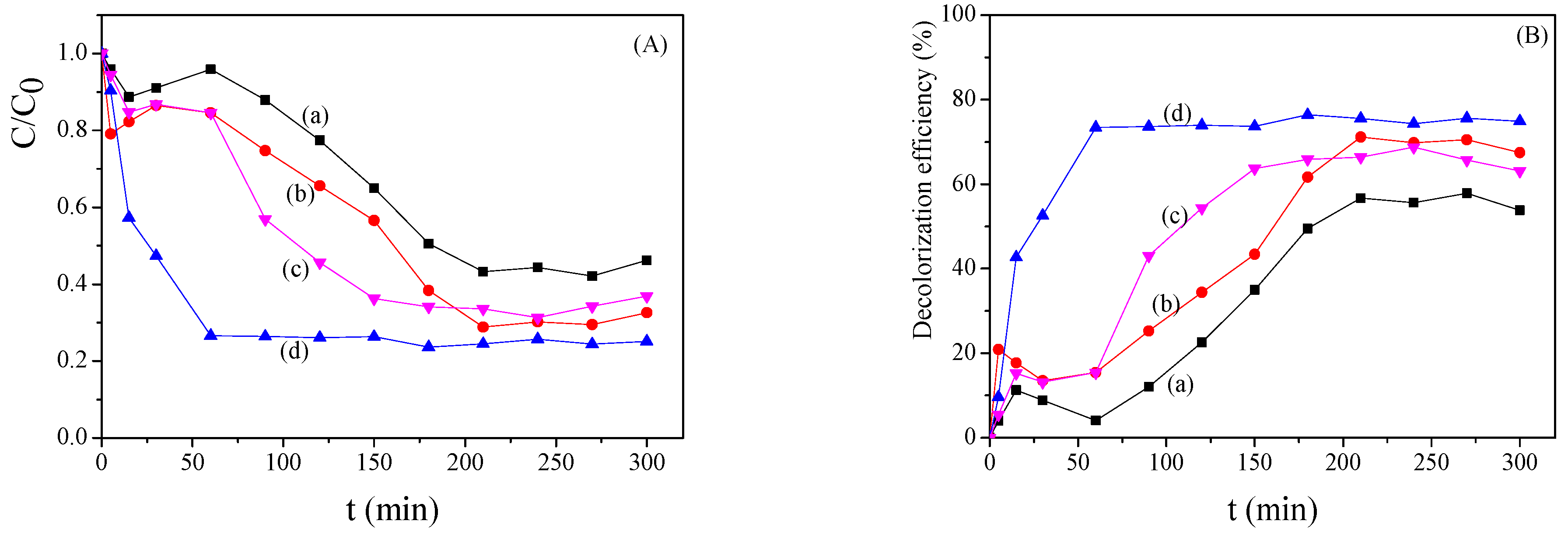
2.3. Bimetallic Zeolite Catalysts in the Degradation of Dye Compounds
2.4. Kinetic Model of the Degradation of Dye Compounds by the Bimetallic Catalysts
2.5. Reutilization of the Bimetallic Catalysts
3. Materials and Methods
3.1. Materials and Reagents
3.2. Catalysts Preparation
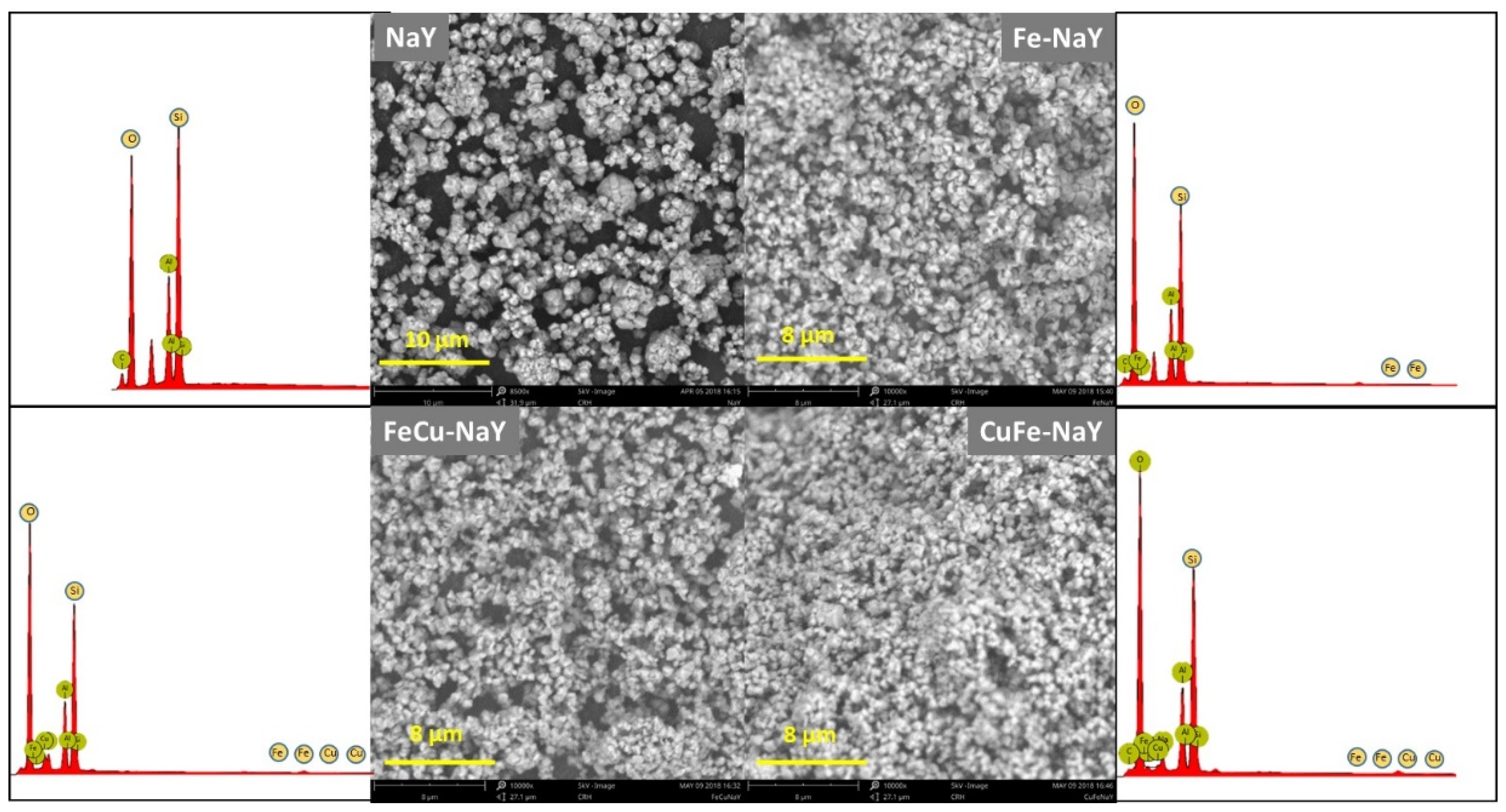
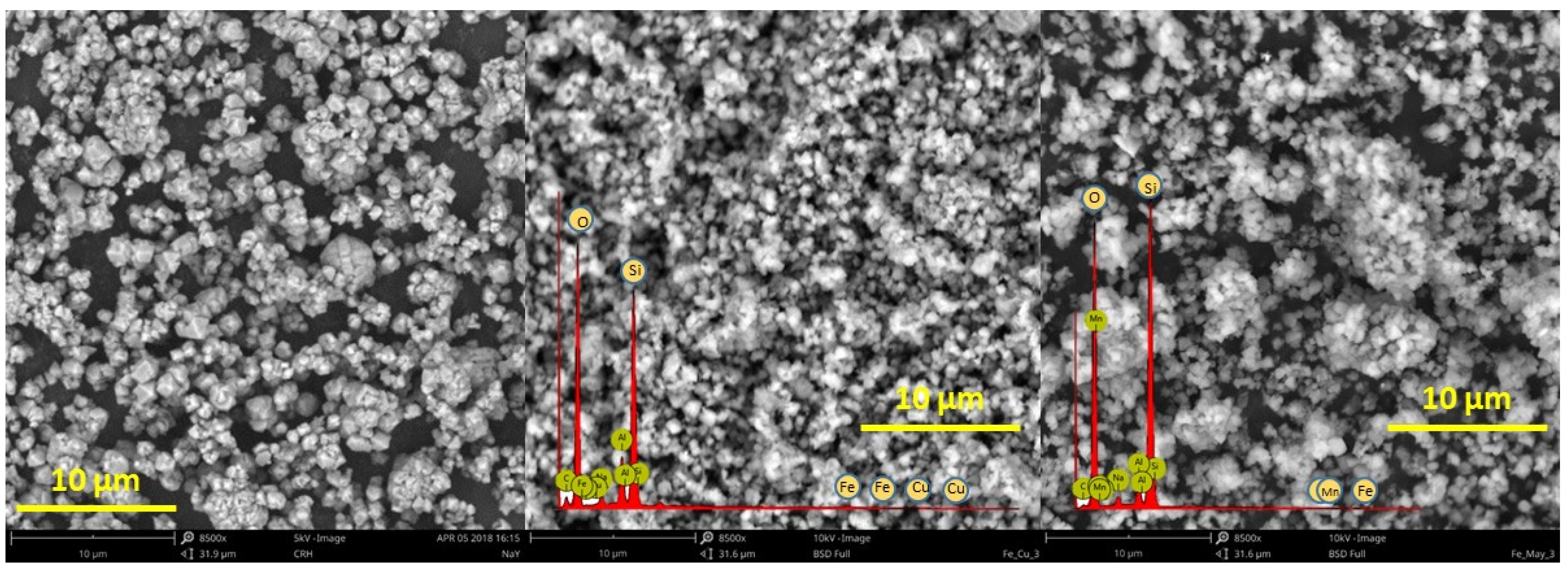
3.3. Catalysts Characterization
3.4. Catalysts Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Georgi, A.; Kopinke, F.-D. Interaction of adsorption and catalytic reactions in water decontamination processes Part I: Oxidation of organic contaminants with hydrogen peroxide catalyzed by activated carbon. Appl. Catal. B Environ. 2005, 58, 9–18. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A review of classic Fenton’s peroxidation as an advanced oxidation technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef]
- Gogate, P.R.; Pandit, A.B. A review of imperative technologies for wastewater treatment I: Oxidation technologies at ambient conditions. Adv. Environ. Res. 2004, 8, 501–551. [Google Scholar] [CrossRef]
- Bauer, R.; Fallmann, H. The photo-fenton oxidation—a cheap and efficient wastewater treatment method. Res. Chem. Intermed. 1997, 23, 341–354. [Google Scholar] [CrossRef]
- Alderete, B.L.; Silva, J.; Godoi, R.; Silva, F.R.; Taffarel, S.R.; Silva, L.P.; Garcia, A.L.H.; Júnior, H.M.; Amorim, H.L.N.; Picada, J.N. Evaluation of toxicity and mutagenicity of a synthetic effluent containing azo dye after advanced oxidation process treatment. Chemosphere 2020, 263, 128291. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.R.; Silva, A.M.T. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Olmos, R.; Holzer, F.; Kopinke, F.-D.; Georgi, A. Indications of the reactive species in a heterogeneous fenton-like reaction using Fe-containing zeolites. Appl. Catal. A Gen. 2011, 398, 44–53. [Google Scholar] [CrossRef]
- Cihanoglu, A.; Gündüz, G.; Dükkancı, M. Degradation of acetic acid by heterogeneous Fenton-like oxidation over iron-containing ZSM-5 zeolites. Appl. Catal. B Environ. 2015, 165, 687–699. [Google Scholar] [CrossRef]
- Fukuchi, S.; Nishimoto, R.; Fukushima, Q.; Zhu, M. Effects of reducing agents on the degradation of 2,4,6-tribromophenol in a heterogeneous Fenton-like system with an iron-loaded natural zeolite. Appl. Catal. B Environ. 2014, 147, 411–419. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Pachamuthu, M.P.; Isaacs, M.A.; Kumar, S.; Lee, A.F.; Sekaran, G. Cu and Fe oxides dispersed on SBA-15: A Fenton type bimetallic catalyst for N, N-diethyl-p-phenyl diamine degradation. Appl. Catal. B Environ. 2016, 199, 323–330. [Google Scholar] [CrossRef]
- Rache, M.L.; García, A.R.; Zea, H.R.; Silva, A.M.T.; Madeira, L.M.; Ramírez, J.H. Azo-dye orange II degradation by the heterogeneous Fenton-like process using a zeolite Y-Fe catalyst-Kinetics with a model based on the Fermi’s equation. Appl. Catal. B Environ. 2014, 146, 192–200. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Li, J.; Luo, R.; Hu, X.; Sun, X.; Shen, J.; Han, W.; Wang, L. In-situ incorporation of iron-copper bimetallic particles in electrospun carbon nanofibers as an efficient Fenton catalyst. Appl. Catal. B Environ. 2017, 207, 316–325. [Google Scholar] [CrossRef]
- Fernández, C.; Larrechi, M.S.; Callao, M.P. An analytical overview of processes for removing organic dyes from wastewater effluents. TrAC Trends Anal. Chem. 2010, 29, 1202–1211. [Google Scholar] [CrossRef]
- Neamtu, M.; Catrinescu, C.; Kettrup, A. Effect of dealumination of iron(III)-exchanged Y zeolites on oxidation of reactive yellow 84 azo dye in the presence of hydrogen peroxide. Appl. Catal. B Environ. 2004, 51, 149–157. [Google Scholar] [CrossRef]
- Gonçalves, M.S.T.; Pinto, E.M.S.; Nkeonye, P.; Oliveira-Campos, A.M.F. Degradation of C.I. reactive orange 4 and its simulated dye bath wastewater by heterogeneous photocatalysis. Dye. Pigment. 2005, 64, 135–139. [Google Scholar] [CrossRef]
- Thiam, A.; Zhou, M.; Brillas, E.; Sirés, I. Two-step mineralization of tartrazine solutions: Study of parameters and by-products during the coupling of electrocoagulation with electrochemical advanced oxidation processes. Appl. Catal. B Environ. 2014, 150, 116–125. [Google Scholar] [CrossRef]
- Ramirez, J.H.; Costa, C.A.; Madeira, L.M.; Mata, G.; Vicente, M.A.; Rojas-Cervantes, M.L.; López-Peinado, A.J.; Martín-Aranda, R.M. Fenton-like oxidation of orange II solutions using heterogeneous catalysts based on saponite clay. Appl. Catal. B Environ. 2007, 71, 44–56. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Fonseca, A.M.; Parpot, P.; Órfão, J.J.M.; Pereira, M.F.R.; Neves, I.C. Oxidation of volatile organic compounds by highly efficient metal zeolite catalysts. ChemCatChem 2018, 10, 3754–3760. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Marques, L.; Freitas, C.M.A.S.; Fonseca, A.M.; Parpot, P.; Órfão, J.J.M.; Pereira, M.F.R.; Neves, I.C. Mono and bimetallic NaY catalysts with high performance in nitrate reduction in water. Chem. Eng. J. 2015, 281, 411–417. [Google Scholar] [CrossRef]
- Xu, L.; Wanga, J. Fenton-like degradation of 2, 4-dichlorophenol using Fe3O4 magnetic nanoparticles. Appl. Catal. B Environ. 2012, 123, 117–1266. [Google Scholar] [CrossRef]
- Fonseca, A.M.; Gonçalves, S.; Parpot, P.; Neves, I.C. Host–guest chemistry of the (N, N0-diarylacetamidine) rhodium (III) complex in zeolite Y. Phys. Chem. Chem. Phys. 2009, 11, 6308–6314. [Google Scholar] [CrossRef] [PubMed]
- Biernacka, I.K.; Parpot, P.; Oliveira, C.; Silva, A.R.; Alves, M.J.; Fonseca, A.M.; Neves, I.C. Norbornene oxidation by chiral Complexes encapsulated in NaY zeolite. J. Phys. Chem. C 2014, 118, 19042–19050. [Google Scholar] [CrossRef]
- Rouquerol, J.; Llewellyn, P.; Rouquerol, F. “Is the BET Equation Applicable to Microporous Adsorbents?”, In Characterization of Porous Solids VII; Llewellyn, P., Rodriguez-Reinoso, F., Rouquerol, J., Seaton, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 160, pp. 49–56. [Google Scholar]
- Vilaça, N.; Gallo, J.; Fernandes, R.; Figueiredo, F.; Fonseca, A.M.; Baltazar, F.; Neves, I.C.; Bañobre-López, M. Synthesis, characterization and in vitro validation of a magnetic zeolite nanocomposite with T2-MRI properties towards theranostic applications. J. Mater. Chem. B 2019, 7, 3351–3361. [Google Scholar] [CrossRef]
- Ferreira, M.; Pinto, M.F.; Soares, O.S.G.P.; Pereira, M.F.R.; Órfão, J.J.M.; Figueiredo, J.L.; Neves, I.C.; Fonseca, A.M.; Parpot, P. Electrocatalytic oxidation of oxalic and oxamic acids in aqueous media at carbon nanotube modified electrodes. Electrochim. Acta 2012, 60, 278–286. [Google Scholar] [CrossRef]
- Ramirez, J.H.; Lampinen, M.; Vicente, M.A.; Costa, C.A.; Madeira, L.M. Experimental design to optimize the oxidation of orange II dye solution using a clay-based fenton-like catalyst. Ind. Eng. Chem. Res. 2008, 47, 284–294. [Google Scholar] [CrossRef]


| Samples | M1 (wt%) | M2 (wt%) | ||||
|---|---|---|---|---|---|---|
| Fe | Mn | Cu | Fe | Mn | Cu | |
| Fe-NaY | 1.13 | - | - | - | - | - |
| Cu-NaY | - | - | 1.39 | - | - | - |
| Mn-NaY | - | 1.09 | - | - | - | - |
| FeCu-NaY | 1.06 | - | - | - | - | 3.43 |
| CuFe-NaY | - | - | 0.85 | 3.49 | - | - |
| MnFe-NaY | - | 0.46 | - | 3.84 | - | - |
| FeMn-NaY | 1.11 | - | - | - | 2.77 | - |
| Samples | SBET (m2/g)1 | Vtotal (cm3/g)2 | Sext (m2/g)3 | Vmicro (cm3/g)3 | Vmeso (cm3/g)4 | SDR (m2/g)5 | VDR,mic (cm3/g)5 | VHK,total (cm3/g)6 | VHK,mic (cm3/g)6 |
|---|---|---|---|---|---|---|---|---|---|
| NaY | 907 | 0.35 | 102 | 0.29 | 0.06 | 938 | 0.33 | 0.33 | 0.33 |
| Fe-NaY | 828 | 0.33 | 38 | 0.29 | 0.04 | 874 | 0.31 | 0.33 | 0.31 |
| Cu-NaY | 913 | 0.32 | 26 | 0.33 | - | 957 | 0.34 | 0.34 | 0.34 |
| Mn-NaY | 910 | 0.35 | 54 | 0.32 | 0.03 | 955 | 0.34 | 0.35 | 0.34 |
| FeCu-NaY | 814 | 0.35 | 96 | 0.27 | 0.08 | 879 | 0.31 | 0.34 | 0.31 |
| CuFe-NaY | 648 | 0.28 | 111 | 0.21 | 0.07 | 700 | 0.25 | 0.28 | 0.25 |
| MnFe-NaY | 613 | 0.24 | 77 | 0.20 | 0.04 | 664 | 0.24 | 0.24 | 0.23 |
| FeMn-NaY | 815 | 0.33 | 64 | 0.28 | 0.05 | 878 | 0.31 | 0.33 | 0.31 |
| Samples | T (°C) | t50 (min) 1 | (C/C0)final (%) 2 | Decolorization Efficiency (%) 3 | TOCfinal (ppm) 2,4 | Mineralization Efficiency (%) 5 |
|---|---|---|---|---|---|---|
| FeCu-NaY | 40 | 80 | 15 | 85 | 9.4 | 19.7 |
| 45 | 65 | 13 | 87 | 11.5 | 1.7 | |
| 50 | 45 | 19 | 81 | 11.3 | 3.4 | |
| CuFe-NaY | 40 | 70 | 32 | 68 | 8.9 | 24.2 |
| 45 | 45 | 28 | 72 | 10.8 | 7.7 | |
| 50 | 15 | 7 | 93 | 10.4 | 11.1 | |
| MnFe-NaY | 40 | 50 | 16 | 84 | 10.2 | 12.8 |
| FeMn-NaY | 40 | 60 | 17 | 83 | 11.0 | 6.0 |
| Samples | T (°C) | t50 (min) 1 | (C/C0)final (%) 2 | Decolorization Efficiency (%) 3 | TOCfinal (ppm) 2,4 | Mineralization Efficiency (%) 5 |
|---|---|---|---|---|---|---|
| FeCu-NaY | 40 | 150 | 30 | 70 | 9.9 | 8.3 |
| CuFe-NaY | 40 | 180 | 45 | 55 | 10.2 | 6.0 |
| MnFe-NaY | 40 | 30 | 25 | 75 | 9.1 | 15.7 |
| FeMn-NaY | 40 | 90 | 35 | 65 | 10.6 | 1.8 |
| Samples | PY | Tar | ||||
|---|---|---|---|---|---|---|
| Equation | R2 | k, (10−5 L·mg−1·min−1) | Equation | R2 | k, (10−5 L·mg−1·min−1) | |
| FeCu-NaY | y = −0.0049x + 0.9528 | 0.9272 | 1.06 | y = −0.0023x + 0.9081 | 0.9055 | 1.15 |
| CuFe-NaY | y = −0.0053x + 0.9854 | 0.9504 | 0.98 | y = −0.0021x + 0.9859 | 0.9087 | 1.05 |
| MnFe-NaY | y = −0.0067x + 0.9754 | 0.9110 | 1.34 | y = −0.0118x + 0.9026 | 0.8731 | 5.90 |
| FeMn-NaY | y = −0.0051x + 0.9671 | 0.9373 | 1.02 | y = −0.0038x + 0.9684 | 0.9495 | 1.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, B.L.C.; Parpot, P.; Soares, O.S.G.P.; Pereira, M.F.R.; Rombi, E.; Fonseca, A.M.; Correia Neves, I. Fenton-Type Bimetallic Catalysts for Degradation of Dyes in Aqueous Solutions. Catalysts 2021, 11, 32. https://doi.org/10.3390/catal11010032
Santos BLC, Parpot P, Soares OSGP, Pereira MFR, Rombi E, Fonseca AM, Correia Neves I. Fenton-Type Bimetallic Catalysts for Degradation of Dyes in Aqueous Solutions. Catalysts. 2021; 11(1):32. https://doi.org/10.3390/catal11010032
Chicago/Turabian StyleSantos, Bebiana L. C., Pier Parpot, Olívia S. G. P. Soares, Manuel F. R. Pereira, Elisabetta Rombi, António M. Fonseca, and Isabel Correia Neves. 2021. "Fenton-Type Bimetallic Catalysts for Degradation of Dyes in Aqueous Solutions" Catalysts 11, no. 1: 32. https://doi.org/10.3390/catal11010032
APA StyleSantos, B. L. C., Parpot, P., Soares, O. S. G. P., Pereira, M. F. R., Rombi, E., Fonseca, A. M., & Correia Neves, I. (2021). Fenton-Type Bimetallic Catalysts for Degradation of Dyes in Aqueous Solutions. Catalysts, 11(1), 32. https://doi.org/10.3390/catal11010032