Zeolites and Related Materials as Catalyst Supports for Hydrocarbon Oxidation Reactions
Abstract
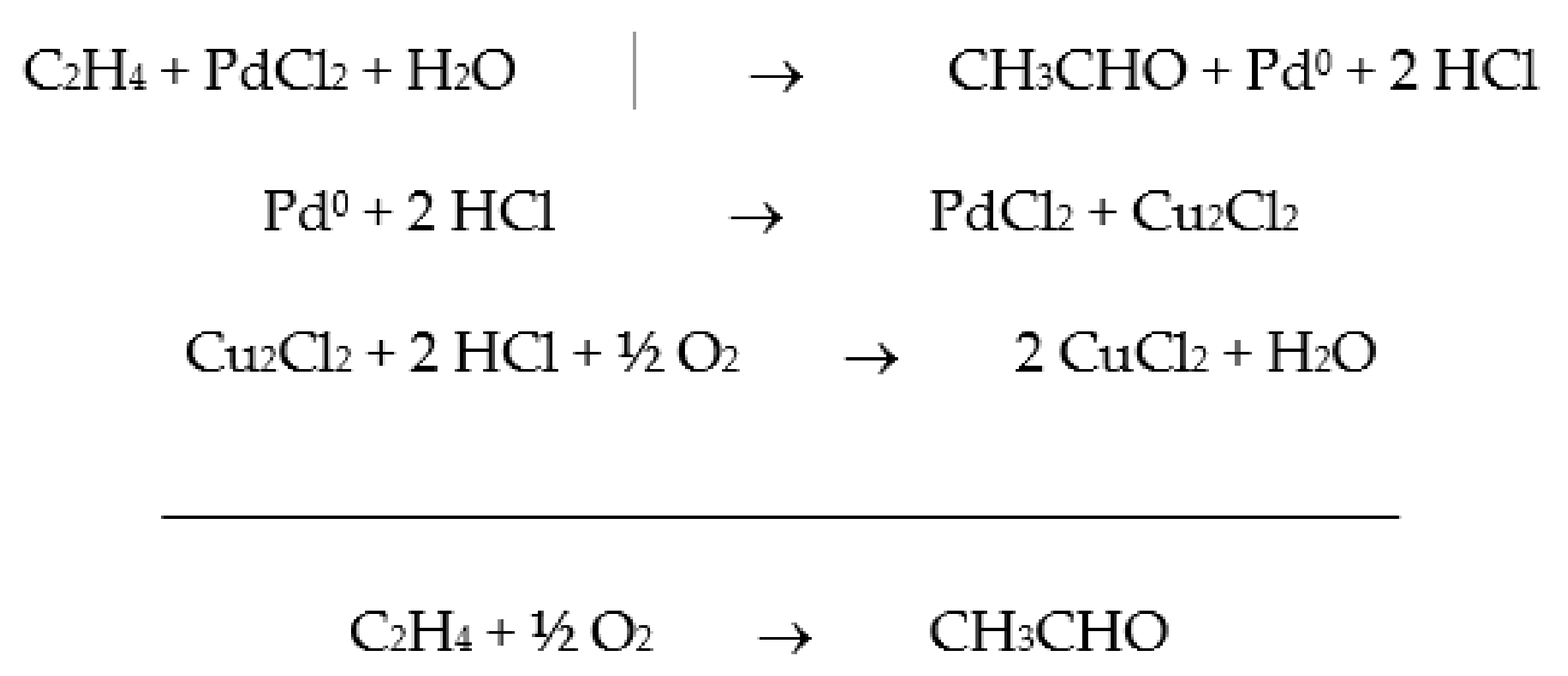
1. Industrial Hydrocarbon Oxidation Reactions
2. From Homogeneous to Heterogenized Catalysts
3. Zeolites and Related Materials as Support for the Heterogenization of the Catalysts
3.1. Hierarchical Zeolites
3.1.1. Bottom-Up Strategies
Hard Templating
Soft Templating
3.1.2. Top-Down Strategies
Dealumination
Desilication
Surfactant-Templated Zeolites
Mechanochemistry
Delamination and Pillaring
3.2. Mesoporous Silicas and Composite Hierarchical Materials
4. Immobilization Methodologies
4.1. Complexes
4.2. Metal Particles
5. Catalytic Applications
5.1. Oxidation of Alkanes
5.2. Oxidation of Alkenes
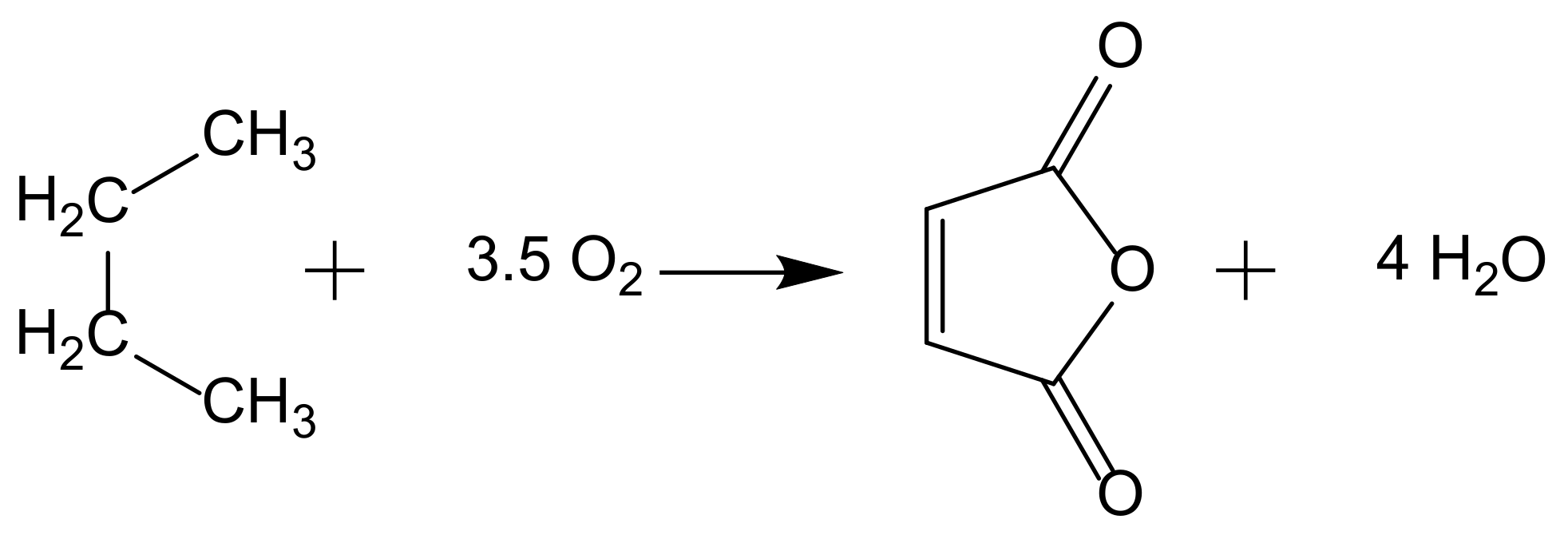
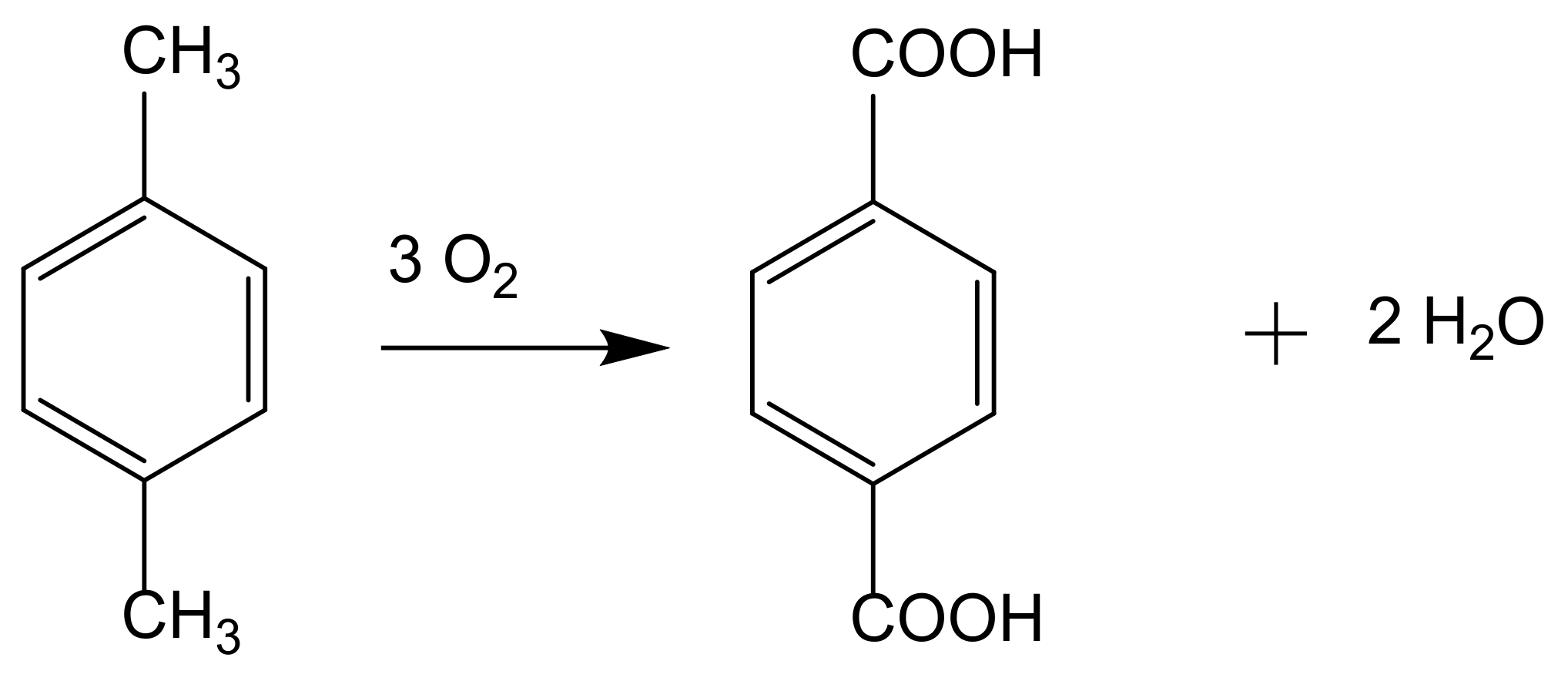
5.3. Oxidation of Aromatics
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rios, J.; Lebeau, J.; Yang, T.; Li, S.; Lynch, M.D. A critical review on the progress and challenges to a more sustainable, cost competitive synthesis of adipic acid. Green Chem. 2021, 23, 3172–3190. [Google Scholar] [CrossRef]
- Hodnett, B.K. Heterogeneous Catalytic Oxidation. Fundametal and Technological Aspects of the Selective Oxidation of Organic Compounds; Wiley: New York, NY, USA, 2000; ISBN 978-0-471-48994-8. [Google Scholar]
- Ullmann’s Encyclopedia of Industrial Chemistry; Wiley, VCH: Weinheim, Germany, 2002. [CrossRef]
- Clerici, M.G.; Ricci, M.S.G. Formation of C-O bonds by oxidation. In Metal-Catalysis in Industrial Organic Processes; Gian Paolo Chiusoli, P.M.M., Ed.; Royal Society of Chemistry: London, UK, 2006; pp. 23–78. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S. Catalytic oxidation of alkanes to high-added value products: The role of C-Scorpionate metal complexes. In Alkanes, Properties, Production and Applications; Martins, L.M.D.R.S., Ed.; Nova Science Publishers: New York, NY, USA, 2019; pp. 69–92. [Google Scholar]
- Li, Y.; Yu, J. New stories of zeolite structures: Their descriptions, determinations, predictions, and evaluations. Chem. Rev. 2014, 114, 7268–7316. [Google Scholar] [CrossRef]
- Barrer, R.M. Zeolites and their synthesis. Zeolites 1981, 1, 130–140. [Google Scholar] [CrossRef]
- Ribeiro, F.R.; Guisnet, M. Les Zeolithes: Un Nanomonde au Service de la Catalyse; EDP Science: Les Ullis, France, 2006. [Google Scholar]
- International Zeolite Association International Zeolite Association Structure Commission, (n.d.). Available online: http://iza-online.org/ (accessed on 29 December 2021).
- Liang, J.; Liang, Z.; Zou, R.; Zhao, Y. Heterogeneous Catalysis in Zeolites, Mesoporous Silica, and Metal–Organic Frameworks. Adv. Mater. 2017, 29, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Marques, J.C. Feasibiity of Eco-friendly binary and ternary blended binders made of a fly-ash and oil-refinery spent catalyst in ready-mixed concrete production. Sustainability 2018, 10, 3136. [Google Scholar] [CrossRef]
- Abubakar, A.; Waba, I.E.; Yunusa, S.; Gano, Z.S. Use of Reactivated Spent FCC Catalyst as Adsorbent for Lead (II) Ions from Refinery-based Simulated Wastewater. Aceh Int. J. Sci. Technol. 2021, 10, 100–116. [Google Scholar] [CrossRef]
- Li, D.; Chen, Y.; Hu, J.; Deng, B.; Cheng, X.; Zhang, Y. Synthesis of Hierarchical Chabazite Zeolite via Interzeolite Transformation of Coke-containing Spent MFI. Appl. Catal. B Environ. 2020, 270, 118881. [Google Scholar] [CrossRef]
- Kumar Kundu, B.; Chhabra, V.; Malviya, N.; Ganguly, R.; Mishra, G.S.; Mukhopadhyay, S. Zeolite encapsulated host-guest Cu(II) Schiff base complexes: Superior activity towards oxidation reactions over homogenous catalytic systems. Microporous Mesoporous Mater. 2018, 271, 100–117. [Google Scholar] [CrossRef]
- Parmar, D.K.; Butani, P.M.; Thumar, N.J.; Jasani, P.M.; Padaliya, R.V.; Sandhiya, P.R.; Nakum, H.D.; Khan, M.N.; Makwana, D. Oxy-functionalization of olefins with neat and heterogenized binuclear V(IV)O and Fe(II) complexes: Effect of steric hindrance on product selectivity and output in homogeneous and heterogeneous phase. Mol. Catal. 2019, 474, 110424. [Google Scholar] [CrossRef]
- Desai, N.C.; Chudasama, J.A.; Karkar, T.J.; Patel, B.Y.; Jadeja, K.A.; Godhani, D.R.; Mehta, J.P. Studies of styrene oxidation by catalyst based on zeolite-Y nanohybrid materials. J. Mol. Catal. A Chem. 2016, 424, 203–219. [Google Scholar] [CrossRef]
- Godhani, D.R.; Nakum, H.D.; Parmar, D.K.; Mehta, J.P.; Desai, N.C. Zeolite-Y immobilized Metallo-ligand complexes: A novel heterogenous catalysts for selective oxidation. Inorg. Chem. Commun. 2016, 72, 105–116. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Miyake, Y.; Takiguchi, K.; Ihara, D.; Yahiro, H. Oxidation of cyclic hydrocarbons with hydrogen peroxide over iron complexes encapsulated in cation-exchanged zeolite. Catal. Today 2018, 303, 249–255. [Google Scholar] [CrossRef]
- Xu, D.; Lv, H.; Liu, B. Encapsulation of metal nanoparticle catalysts within mesoporous zeolites and their enhanced catalytic performances: A review. Front. Chem. 2018, 6, 550. [Google Scholar] [CrossRef]
- Farrusseng, D.; Tuel, A. Perspectives on zeolite-encapsulated metal nanoparticles and their applications in catalysis. New J. Chem. 2016, 40, 3933–3949. [Google Scholar] [CrossRef]
- Wang, L.; Xu, S.; He, S.; Xiao, F.S. Rational construction of metal nanoparticles fixed in zeolite crystals as highly efficient heterogeneous catalysts. Nano Today 2018, 20, 74–83. [Google Scholar] [CrossRef]
- Jampana, S.R.; Jia, L.; Ramarao, B.V.; Kumar, D. Experimental investigation of the adsorption and desorption of cellulase enzymes on zeolite-β for enzyme recycling applications. Bioprocess Biosyst. Eng. 2021, 44, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jiang, Z.; Xia, Q.; Zhou, D. Progress and perspective of enzyme immobilization on zeolite crystal materials. Biochem. Eng. J. 2021, 172, 108033. [Google Scholar] [CrossRef]
- Chen, N.Y.; Garwood, W.E.; Dwyer, F.G. Shape Selective Catalysis in Industrial Applications, 2nd ed.; Dekker, M., Ed.; CRC Press: New York, NY, USA, 1996. [Google Scholar]
- Schwieger, W.; Machoke, A.G.; Weissenberger, T.; Inayat, A.; Selvam, T.; Klumpp, M.; Inayat, A. Hierarchy concepts: Classification and preparation strategies for zeolite containing materials with hierarchical porosity. Chem. Soc. Rev. 2016, 45, 3353–3376. [Google Scholar] [CrossRef]
- Madsen, C.; Jacobsen, C.J.H. Nanosized zeolite crystals—Convenient control of crystal size distribution by confined space synthesis. Chem. Commun. 1999, 673–674. [Google Scholar] [CrossRef]
- Jacobsen, C.J.H.; Madsen, C.; Houzvicka, J.; Schmidt, I.; Carlsson, A. Mesoporous zeolite single crystals. J. Am. Chem. Soc. 2000, 122, 7116–7117. [Google Scholar] [CrossRef]
- Kustova, M.Y.; Hasselriis, P.; Christensen, C.H. Mesoporous MEL—Type zeolite single crystal catalysts. Catal. Lett. 2004, 96, 205–211. [Google Scholar] [CrossRef]
- Koekkoek, A.J.J.; Xin, H.; Yang, Q.; Li, C.; Hensen, E.J.M. Microporous and Mesoporous Materials Hierarchically structured Fe/ZSM-5 as catalysts for the oxidation of benzene to phenol. Microporous Mesoporous Mater. 2011, 145, 172–181. [Google Scholar] [CrossRef]
- Bértolo, R.; Silva, J.M.; Ribeiro, F.; Maldonado-Hódar, F.J.; Fernandes, A.; Martins, A. Effects of oxidant acid treatments on carbon-templated hierarchical SAPO-11 materials: Synthesis, characterization and catalytic evaluation in n-decane hydroisomerization. Appl. Catal. A Gen. 2014, 485, 230–237. [Google Scholar] [CrossRef]
- García-Martínez, J.; Cazorla-Amorós, D.; Linares-Solano, A.; Lin, Y.S. Synthesis and characterisation of MFI-type zeolites supported on carbon materials. Microporous Mesoporous Mater. 2001, 42, 255–268. [Google Scholar] [CrossRef]
- Sakthivel, A.; Huang, S.J.; Chen, W.H.; Lan, Z.H.; Chen, K.H.; Kim, T.W.; Ryoo, R.; Chiang, A.S.T.; Liu, S. Bin Replication of mesoporous aluminosilicate molecular sieves (RMMs) with zeolite framework from mesoporous carbons (CMKs). Chem. Mater. 2004, 16, 3168–3175. [Google Scholar] [CrossRef]
- Tao, Y.; Kanoh, H.; Kaneko, K. ZSM-5 monolith of uniform mesoporous channels. J. Am. Chem. Soc. 2003, 125, 6044–6045. [Google Scholar] [CrossRef]
- Khoshbin, R.; Oruji, S.; Karimzadeh, R. Catalytic cracking of light naphtha over hierarchical ZSM-5 using rice husk ash as silica source in presence of ultrasound energy: Effect of carbon nanotube content. Adv. Powder Technol. 2018, 29, 2176–2187. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X.; Pan, M.; Yang, X.; Liu, Y.; Sun, J.; Wang, Q.; Zheng, J.; Wang, Y.; Ma, J.; et al. Interfacial effects between carbon nanotube templates and precursors on fabricating a wall-crystallized hierarchical pore system in zeolite crystals. J. Mater. Sci. 2020, 55, 10412–10426. [Google Scholar] [CrossRef]
- Tao, Y.; Kanoh, H.; Kaneko, K. Uniform mesopore-donated zeolite Y using carbon aerogel templating. J. Phys. Chem. B 2003, 107, 10974–10976. [Google Scholar] [CrossRef]
- Naydenov, V.; Tosheva, L.; Antzutkin, O.N.; Sterte, J. Meso/macroporous AlPO-5 spherical macrostructures tailored by resin templating. Microporous Mesoporous Mater. 2005, 78, 181–188. [Google Scholar] [CrossRef]
- Holland, B.T.; Abrams, L.; Stein, A. Dual templating of macroporous silicates with zeolitic microporous frameworks. J. Am. Chem. Soc. 1999, 121, 4308–4309. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, Z.; Wang, Y.; Kong, D.; Yuan, X.; Xie, Z. Nanosized CaCO3 as hard template for creation of intracrystal pores within silicalite-1 crystal. Chem. Mater. 2008, 20, 1134–1139. [Google Scholar] [CrossRef]
- Zhang, B.; Davis, S.A.; Mann, S. Starch gel templating of spongelike macroporous silicalite monoliths and mesoporous films. Chem. Mater. 2002, 14, 1369–1375. [Google Scholar] [CrossRef]
- Dong, A.; Wang, Y.; Tang, Y.; Ren, N.; Zhang, Y.; Yue, Y.; Gao, Z. Zeolitic tissue through wood cell templating. Adv. Mater. 2002, 14, 926–929. [Google Scholar] [CrossRef]
- Valtchev, V.P.; Smaihi, M.; Faust, A.C.; Vidal, L. Equisetum arvense Templating of Zeolite Beta Macrostructures with Hierarchical Porosity. Chem. Mater. 2004, 16, 1350–1355. [Google Scholar] [CrossRef]
- Wang, H.; Pinnavaia, T.J. MFI zeolite with small and uniform intracrystal mesopores. Angew. Chem.-Int. Ed. 2006, 45, 7603–7606. [Google Scholar] [CrossRef]
- Tao, Y.; Kanoh, H.; Kaneko, K. Synthesis of Mesoporous Zeolite A by Resorcinol—Formaldehyde Aerogel Templating. Langmuir 2005, 21, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Groen, J.C.; Zhu, W.; Brouwer, S.; Huynink, S.J.; Kapteijn, F.; Moulijn, J.A.; Pérez-Ramírez, J. Direct demonstration of enhanced diffusion in mesoporous ZSM-5 zeolite obtained via controlled desilication. J. Am. Chem. Soc. 2007, 129, 355–360. [Google Scholar] [CrossRef]
- Choi, M.; Srivastava, R.; Ryoo, R. Organosilane surfactant-directed synthesis of mesoporous aluminophosphates constructed with crystalline microporous frameworks. Chem. Commun. 2006, 4380–4382. [Google Scholar] [CrossRef]
- Wang, B.; Guo, T.; Peng, X.; Chen, F.; Lin, M.; Xia, C.; Zhu, B.; Liao, W.; Luo, Y.; Shu, X. Molybdenum-Confined Hierarchical Titanium Silicalite-1: The Synthesis, Characterization, and Catalytic Activity in Alkene Oxidation. Ind. Eng. Chem. Res. 2020, 59, 1093–1100. [Google Scholar] [CrossRef]
- Li, P.; Li, A.; Ruan, R.; Guo, Y.; He, Q.; Zou, W.; Hou, L. Asymmetrical Gemini Surfactants Directed Synthesis Of Hierarchical ZSM-5 Zeolites and Their Immobilization of Molybdenum Complex for the Catalytic Epoxidation of Alkenes. ChemCatChem 2021, 13, 4442–4452. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, S.; Chen, J.; Wei, Y.; Li, J.; Fan, D.; Yu, Z.; Qi, Y.; He, Y.; Xu, S.; et al. Synthesis of mesoporous ZSM-5 catalysts using different mesogenous templates and the application in methanol conversion for enhanced catalyst lifespan. RSC Adv. 2014, 41, 21479. [Google Scholar] [CrossRef]
- Hoang, P.H.; Van Don, B.; Chung, N.H. Cleavage of double bond using metal-loaded ZSM-5 zeolite catalysts for renewable biochemical application. Can. J. Chem. Eng. 2019, 97, 1086–1091. [Google Scholar] [CrossRef]
- Inayat, A.; Knoke, I.; Spiecker, E.; Schwieger, W. Assemblies of mesoporous FAU-type zeolite nanosheets. Angew. Chem.-Int. Ed. 2012, 51, 1962–1965. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hua, Z.; Zhou, J.; Wang, L.; Zhao, J.; Gong, Y.; Wu, W.; Ruan, M.; Shi, J. Hierarchical mesoporous zeolites: Direct self-assembly synthesis in a conventional surfactant solution by kinetic control over the zeolite seed formation. Chem.-A Eur. J. 2011, 17, 14618–14627. [Google Scholar] [CrossRef]
- Lin, J.C.; Yates, M.Z. Altering the crystal morphology of silicalite-1 through microemulsion-based synthesis. Langmuir 2005, 21, 2117–2120. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Shantz, D.F. Zeolite growth in nonionic microemulsions: Synthesis of hierarchically structured zeolite particles. Chem. Mater. 2005, 17, 409–417. [Google Scholar] [CrossRef]
- Koekkoek, A.J.J.; Tempelman, C.H.L.; Degirmenci, V.; Guo, M.; Feng, Z.; Li, C.; Hensen, E.J.M. Hierarchical zeolites prepared by organosilane templating: A study of the synthesis mechanism and catalytic activity. Catal. Today 2011, 168, 96–111. [Google Scholar] [CrossRef]
- Narayanan, S.; Vijaya, J.J.; Sivasanker, S.; Kennedy, L.J.; Kathirgamanathan, P.; Azhagu Raj, R. Synthesis of hierarchical ZSM-5 hexagonal cubes and their catalytic activity in the solvent-free selective oxidation of toluene. J. Porous Mater. 2015, 22, 907–918. [Google Scholar] [CrossRef]
- Li, L.; Meng, Q.; Wen, J.; Wang, J.; Tu, G.; Xu, C.; Zhang, F.; Zhong, Y.; Zhu, W.; Xiao, Q. Improved performance of hierarchical Fe-ZSM-5 in the direct oxidation of benzene to phenol by N2O. Microporous Mesoporous Mater. 2016, 227, 252–257. [Google Scholar] [CrossRef]
- Koekkoek, A.J.J.; Kim, W.; Degirmenci, V.; Xin, H.; Ryoo, R.; Hensen, E.J.M. Catalytic performance of sheet-like Fe/ZSM-5 zeolites for the selective oxidation of benzene with nitrous oxide. J. Catal. 2013, 299, 81–89. [Google Scholar] [CrossRef]
- Serrano, D.P.; Aguado, J.; Escola, J.M.; Rodríguez, J.M.; Peral, A. Hierarchical zeolites with enhanced textural and catalytic properties synthesized from organofunctionalized seeds. Chem. Mater. 2006, 18, 2462–2464. [Google Scholar] [CrossRef]
- Tompsett, G.A.; Conner, C.; Yngvesson, K.S. Microwave Synthesis of Nanoporous Materials. ChemPhysChem 2006, 7, 296–319. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T. Adsorption characteristics of polyvinil alcohols on modified zeolites. Colloid Polym. Sci. 2014, 292, 533–538. [Google Scholar] [CrossRef]
- Janssen, A.H.; Koster, A.J.; De Jong, K.P.; De Jong, K.P. On the shape of the mesopores in zeolite Y: A three-dimensional transmission electron microscopy study combined with texture analysis. J. Phys. Chem. B 2002, 106, 11905–11909. [Google Scholar] [CrossRef]
- Xue, H.; Huang, X.; Zhan, E.; Ma, M.; Shen, W. Selective dealumination of mordenite for enhancing its stability in dimethyl ether carbonylation. Catal. Commun. 2013, 37, 75–79. [Google Scholar] [CrossRef]
- De Baerdemaeker, T.; Yilmaz, B.; Müller, U.; Feyen, M.; Xiao, F.S.; Zhang, W.; Tatsumi, T.; Gies, H.; Bao, X.; De Vos, D. Catalytic applications of OSDA-free Beta zeolite. J. Catal. 2013, 308, 73–81. [Google Scholar] [CrossRef]
- Mériaudeau, P.; Tuan, V.A.; Nghiem, V.T.; Lefevbre, F.; Ha, V.T. Characterization and catalytic properties of hydrothermally dealuminated MCM-22. J. Catal. 1999, 185, 378–385. [Google Scholar] [CrossRef]
- You, Q.; Wang, X.; Wu, Y.; Bi, C.; Yang, X.; Sun, M.; Zhang, J.; Hao, Q.; Chen, H.; Ma, X. Hierarchical Ti-beta with a three-dimensional ordered mesoporosity for catalytic epoxidation of bulky cyclic olefins. New J. Chem. 2021, 45, 10303–10314. [Google Scholar] [CrossRef]
- Shahid, A.; Reddy, V.; Hartmann, M.; Schwieger, W. Direct oxidation of benzene to phenol over hierarchical ZSM-5 zeolites prepared by sequential post synthesis modi fi cation. Microporous Mesoporous Mater. 2017, 237, 151–159. [Google Scholar] [CrossRef]
- Groen, J.C.; Hamminga, G.M.; Moulijn, A.; Pe, J. In situ monitoring of desilication of MFI-type zeolites in alkaline medium. Phys. Chem. Chem. Phys. 2007, 9, 4822–4830. [Google Scholar] [CrossRef] [PubMed]
- Verboekend, D.; Mitchell, S.; Milina, M.; Groen, J.C.; Javier, P. Full Compositional Flexibility in the Preparation of Mesoporous MFI Zeolites by Desilication. J. Phys. Chem 2011, 115, 14193–14203. [Google Scholar] [CrossRef]
- Paixão, V.; Carvalho, A.P.; Rocha, J.; Fernandes, A.; Martins, A. Modification of MOR by desilication treatments: Structural, textural and acidic characterization. Microporous Mesoporous Mater. 2010, 131, 350–357. [Google Scholar] [CrossRef]
- Holm, M.S.; Hansen, K.; Hviid, C. “ One-Pot ” Ion-Exchange and Mesopore Formation During Desilication. Eur. J. Inorg. Chem. 2009, 9, 1194–1198. [Google Scholar] [CrossRef]
- Machado, V.; Rocha, J.; Carvalho, A.P.; Martins, A. Modification of MCM-22 zeolite through sequential post-synthesis treatments. Implications on the acidic and catalytic behaviour. Appl. Catal. A Gen. 2012, 445–446, 329–338. [Google Scholar] [CrossRef]
- Aleixo, R.; Elvas-leitão, R.; Martins, F.; Carvalho, A.P.A.P.; Brigas, A.; Nunes, R.; Fernandes, A.; Rocha, J.; Martins, A.; Nunes, N. Zooming in with QSPR on Friedel-Crafts acylation reactions over modified BEA zeolites. Mol. Catal. 2019, 476, 110495. [Google Scholar] [CrossRef]
- Andrade, M.A.; Ansari, L.M.S.; Pombeiro, A.J.L.; Carvalho, A.P.; Martins, A.; Martins, L.M.D.R.S. Fe@hierarchical bea zeolite catalyst for mw-assisted alcohol oxidation reaction: A greener approach. Catalysts 2020, 10, 1029. [Google Scholar] [CrossRef]
- Paixão, V.; Monteiro, R.; Andrade, M.; Fernandes, A.; Rocha, J.; Carvalho, A.P.P.; Martins, A. Desilication of MOR zeolite: Conventional versus microwave assisted heating. Appl. Catal. A Gen. 2011, 402, 59–68. [Google Scholar] [CrossRef]
- Barrer, R.M.; Makki, M.B. Molecular Sieve Sorbents From Clinoptilolite. Can. J. Chem. 1964, 42, 1481–1487. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Nunes, N.; Martins, A. Hierarchical Zeolites: Preparation, Properties and Catalytic Applications. In Comprehensive Guide for Mesoporous Materials, Vol. 3: Properties and Development; Aliofkhazraei, M., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2015; pp. 147–211. ISBN 978-1-63463-318-5. [Google Scholar]
- Serrano, D.P.; Escola, J.M.; Pizarro, P. Synthesis strategies in the search for hierarchical zeolites. Chem. Soc. Rev. 2013, 42, 4004–4035. [Google Scholar] [CrossRef]
- Bai, R.; Song, Y.; Li, Y.; Yu, J. Creating Hierarchical Pores in Zeolite Catalysts. Trends Chem. 2019, 1, 601–611. [Google Scholar] [CrossRef]
- Kerstens, D.; Smeyers, B.; Van Waeyenberg, J.; Zhang, Q.; Yu, J.; Sels, B.F. State of the Art and Perspectives of Hierarchical Zeolites: Practical Overview of Synthesis Methods and Use in Catalysis. Adv. Mater. 2020, 32, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Triantafillidis, C.S.; Vlessidis, A.G.; Evmiridis, N.P. Dealuminated H-Y zeolites: Influence of the degree and the type of dealumination method on the structural and acidic characteristics of H-Y zeolites. Ind. Eng. Chem. Res. 2000, 39, 307–319. [Google Scholar] [CrossRef]
- Valtchev, V.; Majano, G.; Mintova, S.; Pérez-Ramírez, J. Tailored crystalline microporous materials by post-synthesis modification. Chem. Soc. Rev. 2013, 42, 263–290. [Google Scholar] [CrossRef]
- Dessau, R.M.; Valyocsik, E.W.; Goeke, N.H. Aluminum zoning in ZSM-5 as revealed by selective silica removal. Zeolites 1992, 12, 776–779. [Google Scholar] [CrossRef]
- Lietz, G.; Schnabel, K.H.; Peuker, C.; Gross, T.; Storek, W.; Völter, J. Modifications of H-ZSM-5 catalysts by NaOH treatment. J. Catal. 1994, 148, 562–568. [Google Scholar] [CrossRef]
- Ogura, M.; Shinomiya, S.Y.; Tateno, J.; Nara, Y.; Kikuchi, E.; Matsukata, M. Formation of uniform mesopores in ZSM-5 zeolite through treatment in alkaline solution. Chem. Lett. 2000, 8, 882–883. [Google Scholar] [CrossRef]
- Christensen, C.H.; Egeblad, K.; Christensen, C.H.; Groen, J.C. Hierarchical zeolites: Enhanced utilisation of microporous crystals in catalysis by advances in materials design. Chem. Soc. Rev. 2008, 37, 2530–2542. [Google Scholar] [CrossRef]
- Groen, J.C.; Moulijn, J.A.; Pe, J.; Pérez-Ramírez, J. Alkaline posttreatment of MFI zeolites. From accelerated screening to scale-up. Ind. Eng. Chem. Res. 2007, 46, 4193–4201. [Google Scholar] [CrossRef]
- Groen, J.C.; Peffer, L.A.A.; Moulijn, J.A. Mesoporosity development in ZSM-5 zeolite upon optimized desilication conditions in alkaline medium. Colloids Surf. A Physicochem. Eng. Asp. 2004, 241, 53–58. [Google Scholar] [CrossRef]
- Groen, J.C.; Jansen, J.C.; Moulijn, J.A.; Pe, J. Optimal Aluminum-Assisted Mesoporosity Development in MFI Zeolites by Desilication. J. Phys. Chem. B 2004, 108, 13062–13065. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Martins, A.; Alegria, E.C.B.A.; Carvalho, A.P.; Pombeiro, A.J.L. Efficient cyclohexane oxidation with hydrogen peroxide catalysed by a C-scorpionate iron(II) complex immobilized on desilicated MOR zeolite. Appl. Catal. A Gen. 2013, 464–465, 43–50. [Google Scholar] [CrossRef]
- Verboekend, D.; Vilé, G.; Pérez-Ramírez, J. Mesopore Formation in USY and Beta Zeolites by Base Leaching: Selection Criteria and Optimization of Pore-Directing Agents. Cryst. Growth Des. 2012, 12, 3123–3132. [Google Scholar] [CrossRef]
- Verboekend, D.; Pérez-Ramírez, J. Desilication mechanism revisited: Highly mesoporous all-silica zeolites enabled through pore-directing agents. Chem.-A Eur. J. 2011, 17, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Abelló, S.; Pérez-Ramírez, J. Accelerated generation of intracrystalline mesoporosity in zeolites by microwave-mediated desilication. PCCP 2009, 11, 2959–2963. [Google Scholar] [CrossRef]
- García-Martínez, J.; Johnson, M.; Valla, J.; Li, K.; Ying, J.Y. Mesostructured zeolite y—High hydrothermal stability and superior FCC catalytic performance. Catal. Sci. Technol. 2012, 2, 987–994. [Google Scholar] [CrossRef]
- Garcia-Martinez, J. Mesostructured Zeolitic Materials, and Methods of Making and Using the Same. U.S. Patent 8524624 B2, 14 November 2013. [Google Scholar]
- García-Martínez, J.; Li, K. Surfactant-templated mesostructuring of zeolites: From discovery to commercialization. In Mesoporous Zeolites: Preparation, Characterization and Applications; García-Martínez, J., Li, K., Eds.; Wiley, VCH: Weinheim, Germany, 2015; pp. 321–345. ISBN 9783527673957. [Google Scholar]
- Mendoza-castro, M.J.; Serrano, E.; Linares, N.; García-Martínez, J. Surfactant-Templated Zeolites: From Thermodynamics to Direct Observation. Adv. Mater. Interfaces 2020, 8, 1–23. [Google Scholar] [CrossRef]
- Ivanova, I.I.; Kuznetsov, A.S.; Yuschenko, V.V.; Knyazeva, E.E. Design of composite micro/mesoporous molecular sieve catalysts. Pure Appl. Chem. 2004, 76, 1647–1658. [Google Scholar] [CrossRef]
- Wang, S.; Dou, T.; Li, Y.; Zhang, Y.; Li, X.; Yan, Z. A novel method for the preparation of MOR/MCM-41 composite molecular sieve. Catal. Commun. 2005, 6, 87–91. [Google Scholar] [CrossRef]
- Van-Dúnem, V.; Carvalho, A.P.; Martins, L.M.D.R.S.; Martins, A. Improved Cyclohexane Oxidation Catalyzed by a Heterogenized Iron (II) Complex on Hierarchical Y Zeolite through Surfactant Mediated Technology. ChemCatChem 2018, 10, 4058–4066. [Google Scholar] [CrossRef]
- Al-Ani, A.; Haslam, J.J.C.; Mordvinova, N.E.; Lebedev, O.I.; Vicente, A.; Fernandez, C.; Zholobenko, V. Synthesis of nanostructured catalysts by surfactantlating of large-pore zeolites. Nanoscale Adv. 2019, 1, 2029–2039. [Google Scholar] [CrossRef]
- Martins, A.; Neves, V.; Moutinho, J.; Nunes, N.; Carvalho, A.P. Friedel-Crafts acylation reaction over hierarchical Y zeo-lite modified through surfactant mediated technology. Microporous Mesoporous Mater. 2021, 323, 111167. [Google Scholar] [CrossRef]
- Ottaviani, D.; Van-Dúnem, V.; Carvalho, A.P.; Martins, A.; Martins, L. Eco-friendly cyclohexane oxidation by a V-scorpionate complex immobilized at hierarchical MOR zeolite. Catal. Today 2020, 348, 37–44. [Google Scholar] [CrossRef]
- Li, K.; Valla, J.; Garcia-Martinez, J. Realizing the commercial potential of hierarchical zeolites: New opportunities in catalytic cracking. ChemCatChem 2014, 6, 46–66. [Google Scholar] [CrossRef]
- Linares, N.; Cirujano, F.G.; De Vos, D.E.; Garcia-Martinez, J. Surfactant-templated zeolites for the production of active pharmaceutical intermediates. Chem. Commun. 2019, 55, 12869–12872. [Google Scholar] [CrossRef]
- Majano, G.; Borchardt, L.; Mitchell, S.; Valtchev, V.; Pérez-Ramírez, J. Rediscovering zeolite mechanochemistry—A pathway beyond current synthesis and modification boundaries. Microporous Mesoporous Mater. 2014, 194, 106–114. [Google Scholar] [CrossRef]
- Akcay, K.; Sirkecioğlu, A.; Tatlıer, M.; Savaşçı, Ö.T.; Erdem-Şenatalar, A. Wet ball milling of zeolite HY. Powder Technol. 2004, 142, 121–128. [Google Scholar] [CrossRef]
- Hernandez-Ramírez, O.; Al-nasri, S.K.; Holmes, S.M. Hierarchical structures based on natural carbons and zeolites. J. Mater. Chem. 2011, 21, 16529–16534. [Google Scholar] [CrossRef]
- Ferreira, L.; Ribeiro, F.; Fernandes, A.; Martins, A. Ball Milling Modified SAPO-11 Based Catalysts for n-Decane Hydroisomerization. ChemistrySelect 2019, 4, 6713–6718. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Castanheira, C.; Cardoso, B.; Pires, J.; Silva, A.R.; Freire, C.; De Castro, B.; De Carvalho, M.B. Simultaneous aluminium oxide pillaring and copper(ii) Schiff base complexes encapsulation in a montmorillonite. J. Mater. Chem. 2004, 14, 374–379. [Google Scholar] [CrossRef]
- Figueiredo, H.; Silva, B.; Kuźniarska-Biernacka, I.; Fonseca, A.M.; Medina, R.; Rasmussen, S.; Bañares, M.A.; Neves, I.C.; Tavares, T. Oxidation of cyclohexanol and cyclohexene with triazenido complexes of chromium immobilized in biosorption FAU supports. Chem. Eng. J. 2014, 247, 134–141. [Google Scholar] [CrossRef]
- Kresge, C.T.; Roth, W.J.; Simmons, K.G.; Vartuli, J.C. Acid Oxide with Micro and Mesoporous Characteristics: MCM-36. World Patent WO92/11934, 23 July 1992. [Google Scholar]
- Roth, W.J.; Kresge, C.T.; Vartuli, J.C.; Leonowicz, M.E.; Fung, A.S.; McCullen, S.B. MCM-36: The first pillared molecular sieve with zeoliteproperties. Stud. Surf. Sci. Catal. 1995, 94, 301–308. [Google Scholar] [CrossRef]
- Liu, G.; Jiang, J.G.; Yang, B.; Fang, X.; Xu, H.; Peng, H.; Xu, L.; Liu, Y.; Wu, P. Hydrothermal synthesis of MWW-type stannosilicate and its post-structural transformation to MCM-56 analogue. Microporous Mesoporous Mater. 2013, 165, 210–218. [Google Scholar] [CrossRef]
- Na, K.; Chol, M.; Park, W.; Sakamoto, Y.; Terasakl, O.; Ryoo, R. Pillared MFI zeolite nanosheets of a single-unit-cell thickness. J. Am. Chem. Soc. 2010, 132, 4169–4177. [Google Scholar] [CrossRef] [PubMed]
- Xiaoling, L.I.U.; Yan, W.; Xujin, W.; Yafei, Z.; Yanjun, G.; Qinghu, X.U.; Jun, X.U. Characterization and Catalytic Performance in n -Hexane Cracking of HEU-1 Zeolites Dealuminated Using Hydrochloric Acid and Hydrothermal Treatments. Chin. J. Catal. 2012, 33, 1889–1900. [Google Scholar] [CrossRef]
- Corma, A.; Fornés, V.; Pergher, S.B.C. Zeolite ITQ-2. World Patent WO9717290A1, 5 May 1997. [Google Scholar]
- Corma, A.; Diaz, U.; Domine, M.E.; Fornés, V. AIITQ-6 and TiITQ-6: Synthesis, characterization, and catalytic activity. Angew. Chem.-Int. Ed. 2000, 39, 1499–1501. [Google Scholar] [CrossRef]
- Corma, A.; Fornés, V.; Díaz, U. ITQ-18 a new delaminated stable zeolite. Chem. Commun. 2001, 1, 2642–2643. [Google Scholar] [CrossRef]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; et al. A New Family of Mesoporous Molecular Sieves Prepared with Liquid Crystal Templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Trong On, D.; Desplantier-Giscard, D.; Danumah, C.; Kaliaguine, S. Perspectives in catalytic applications of mesostructured materials. Appl. Catal. A Gen. 2001, 222, 299–357. [Google Scholar] [CrossRef]
- Mandal, S.; Sinhamahapatra, A.; Rakesh, B.; Kumar, R.; Panda, A.; Chowdhury, B. Synthesis, characterization of Ga-TUD-1 catalyst and its activity towards styrene epoxidation reaction. Catal. Commun. 2011, 12, 734–738. [Google Scholar] [CrossRef]
- Meoto, S.; Kent, N.; Nigra, M.M.; Coppens, M.O. Effect of stirring rate on the morphology of FDU-12 mesoporous silica particles. Microporous Mesoporous Mater. 2017, 249, 61–66. [Google Scholar] [CrossRef]
- Kishor, R.; Ghoshal, A.K. Understanding the hydrothermal, thermal, mechanical and hydrolytic stability of mesoporous KIT-6: A comprehensive study. Microporous Mesoporous Mater. 2017, 242, 127–135. [Google Scholar] [CrossRef]
- Patcas, F.C. The methanol-to-olefins conversion over zeolite-coated ceramic foams. J. Catal. 2005, 231, 194–200. [Google Scholar] [CrossRef]
- Mitra, B.; Kunzru, D. Washcoating of different zeolites on cordierite monoliths. J. Am. Ceram. Soc. 2008, 91, 64–70. [Google Scholar] [CrossRef]
- Ivanova, S.; Louis, B.; Madani, B.; Tessonnier, J.P.; Ledoux, M.J.; Pham-Huu, C. ZSM-5 coatings on β-SiC monoliths: Possible new structured catalyst for the methanol-to-olefins process. J. Phys. Chem. C 2007, 111, 4368–4374. [Google Scholar] [CrossRef]
- Louis, B.; Ocampo, F.; Yun, H.S.; Tessonnier, J.P.; Pereira, M.M. Hierarchical pore ZSM-5 zeolite structures: From micro- to macro-engineering of structured catalysts. Chem. Eng. J. 2010, 161, 397–402. [Google Scholar] [CrossRef]
- Barg, S.; Soltmann, C.; Schwab, A.; Koch, D.; Schwieger, W.; Grathwohl, G. Novel open cell aluminum foams and their use as reactive support for zeolite crystallization. J. Porous Mater. 2011, 18, 89–98. [Google Scholar] [CrossRef]
- Qian, X.; Du, J.; Li, B.; Si, M.; Yang, Y.; Hu, Y.; Niu, G.; Zhang, Y.; Xu, H.; Tu, B.; et al. Controllable fabrication of uniform core-shell structured zeolite@SBA-15 composites. Chem. Sci. 2011, 2, 2006–2016. [Google Scholar] [CrossRef]
- Qian, X.; Che, R.; Asiri, A.M. Exploring Meso-/Microporous Composite Molecular Sieves with Core-Shell Exploring Meso-/Microporous Composite Molecular Sieves with Core—Shell. Chem. Eur. J. 2012, 18, 931–939. [Google Scholar] [CrossRef]
- Lv, Y.; Qian, X.; Tu, B.; Zhao, D. Generalized synthesis of core-shell structured nano-zeolite@ordered mesoporous silica composites. Catal. Today 2013, 204, 2–7. [Google Scholar] [CrossRef]
- Xu, L.; Peng, H.G.; Zhang, K.; Wu, H.; Chen, L.; Liu, Y.; Wu, P. Core-shell-structured titanosilicate as a robust catalyst for cyclohexanone ammoximation. ACS Catal. 2013, 3, 103–110. [Google Scholar] [CrossRef]
- Jarrais, B.; Pereira, C.; Silva, A.R.; Carvalho, A.P.; Pires, J.; Freire, C. Grafting of vanadyl acetylacetonate onto organo-hexagonal mesoporous silica and catalytic activity in the allylic epoxidation of geraniol. Polyhedron 2009, 28, 994–1000. [Google Scholar] [CrossRef]
- Pinto, V.H.A.; Rebouças, J.S.; Ucoski, G.M.; de Faria, E.H.; Ferreira, B.F.; Silva San Gil, R.A.; Nakagaki, S. Mn porphyrins immobilized on non-modified and chloropropyl-functionalized mesoporous silica SBA-15 as catalysts for cyclohexane oxidation. Appl. Catal. A Gen. 2016, 526, 9–20. [Google Scholar] [CrossRef]
- Machado, K.; Tavares, P.B.; Mishra, G.S. Synthesis and application of FeIII, NiII and Mn II complexes anchored to HMS as efficient catalysts for cycloalkane oxyfunctionalization. J. Mol. Catal. A Chem. 2014, 383–384, 159–166. [Google Scholar] [CrossRef]
- Dorbes, S.; Pereira, C.; Andrade, M.; Barros, D.; Pereira, A.M.; Rebelo, S.L.H.; Araújo, J.P.; Pires, J.; Carvalho, A.P.; Freire, C. Oxidovanadium(IV) acetylacetonate immobilized onto CMK-3 for heterogeneous epoxidation of geraniol. Microporous Mesoporous Mater. 2012, 160, 67–74. [Google Scholar] [CrossRef]
- Gaspar, H.; Andrade, M.; Pereira, C.; Pereira, A.M.; Rebelo, S.L.H.; Araújo, J.P.; Pires, J.; Carvalho, A.P.; Freire, C. Alkene epoxidation by manganese(III) complexes immobilized onto nanostructured carbon CMK-3. Catal. Today 2013, 203, 103–110. [Google Scholar] [CrossRef]
- Kuźniarska-Biernacka, I.; Pereira, C.; Carvalho, A.P.; Pires, J.; Freire, C. Epoxidation of olefins catalyzed by manga-nese(III) salen complexes grafted to porous heterostructured clays. Appl. Clay Sci. 2011, 53, 195–203. [Google Scholar] [CrossRef]
- Kuźniarska-Biernacka, I.; Silva, A.R.; Carvalho, A.P.; Pires, J.; Freire, C. Anchoring of chiral manganese(III) salen com-plex onto organo clay and porous clay heterostructure and catalytic activity in alkene epoxidation. Catal. Lett. 2010, 134, 63–71. [Google Scholar] [CrossRef]
- Martins, A.; Silva, J.M.M.; Ribeiro, F.R.R.; Guisnet, M.; Ribeiro, M.F.F. n-Hexane hydroisomerisation over bifunctional Pt/MCM-22 catalysts. Influence of the mode of Pt introduction. Stud. Surf. Sci. Catal. 2008, 174, 1135–1138. [Google Scholar] [CrossRef]
- Azzolina Jury, F.; Polaert, I.; Pierella, L.B.; Estel, L. Optimized benzaldehyde production over a new Co-ZSM-11 catalyst: Reaction parameters effects and kinetics. Catal. Commun. 2014, 46, 6–10. [Google Scholar] [CrossRef]
- Martins, A.; Silva, J.M.M.; Ribeiro, F.R.R.; Ribeiro, M.F.F. Hydroisomerization of n-hexane over Pt-Ni/HBEA using catalysts prepared by different methods. Catal. Lett. 2006, 109, 83–87. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Jian, P.; Jian, R. Highly selective oxidation of styrene to benzaldehyde over a tailor-made cobalt oxide encapsulated zeolite catalyst. J. Colloid Interface Sci. 2018, 517, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Bértolo, R.; Fernandes, A.; Ribeiro, F.; Silva, J.M.; Martins, A.; Ribeiro, F.R. Hydroisomerization of n-decane over SAPO-11 catalysts synthesized with methylamine as co-template. React. Kinet. Mech. Catal. 2010, 99, 183–191. [Google Scholar] [CrossRef][Green Version]
- Bértolo, R.; Silva, J.M.; Ribeiro, M.F.; Martins, A.; Fernandes, A. Microwave synthesis of SAPO-11 materials for long chain n-alkanes hydroisomerization: Effect of physical parameters and chemical gel composition. Appl. Catal. A Gen. 2017, 542, 28–37. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Xiao, F.S. Metal@zeolite hybrid materials for catalysis. ACS Cent. Sci. 2020, 6, 1685–1697. [Google Scholar] [CrossRef]
- Wang, G.; Xu, S.; Wang, L.; Liu, Z.; Dong, X.; Wang, L.; Zheng, A.; Meng, X.; Xiao, F. traps of zeolite crystals for sinter-resistant catalysts. Chem. Commun. 2018, 54, 3274–3277. [Google Scholar] [CrossRef]
- Zhang, J.; Rao, C.; Peng, H.; Peng, C.; Zhang, L.; Xu, X.; Liu, W.; Wang, Z.; Zhang, N.; Wang, X. Enhanced toluene combustion performance over Pt loaded hierarchical porous MOR zeolite. Chem. Eng. J. 2018, 334, 10–18. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Shakouri-Arani, M.; Davar, F. Flexible ligand synthesis, characterization and catalytic oxidation of cyclohexane with host (nanocavity of zeolite-Y)/guest (Mn(II), Co(II), Ni(II) and Cu(II) complexes of tetrahydro-salophen) nanocomposite materials. Microporous Mesoporous Mater. 2008, 116, 77–85. [Google Scholar] [CrossRef]
- Modi, C.K.; Trivedi, P.M.; Chudasama, J.A.; Nakum, H.D.; Parmar, D.K.; Gupta, S.K.; Jha, P.K. Zeolite-Y entrapped bivalent transition metal complexes as hybrid nanocatalysts: Density functional theory investigation and catalytic aspects. Green Chem. Lett. Rev. 2014, 7, 278–287. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, H.; Cui, X.; Hua, Z.; Chen, Y.; Zhu, Y.; Song, Y.; Gong, Y.; Shi, J. A facile one-pot synthesis of hierarchically porous Cu(I)-ZSM-5 for radicals-involved oxidation of cyclohexane. Appl. Catal. A Gen. 2013, 451, 112–119. [Google Scholar] [CrossRef]
- Alavi, S.; Hosseini-Monfared, H.; Siczek, M. A new manganese(III) complex anchored onto SBA-15 as efficient catalyst for selective oxidation of cycloalkanes and cyclohexene with hydrogen peroxide. J. Mol. Catal. A Chem. 2013, 377, 16–28. [Google Scholar] [CrossRef]
- Niu, X.R.; Li, J.; Zhang, L.; Lei, Z.T.; Zhao, X.L.; Yang, C.H. ZSM-5 functionalized in situ with manganese ions for the catalytic oxidation of cyclohexane. RSC Adv. 2017, 7, 50619–50625. [Google Scholar] [CrossRef]
- Fu, Y.; Zhan, W.; Guo, Y.; Wang, Y.; Liu, X.; Guo, Y.; Wang, Y.; Lu, G. Effect of surface functionalization of cerium-doped MCM-48 on its catalytic performance for liquid-phase free-solvent oxidation of cyclohexane with molecular oxygen. Microporous Mesoporous Mater. 2015, 214, 101–107. [Google Scholar] [CrossRef]
- Rezaei, M.; Chermahini, A.N.; Dabbagh, H.A. Green and selective oxidation of cyclohexane over vanadium pyrophosphate supported on mesoporous KIT-6. Chem. Eng. J. 2017, 314, 515–525. [Google Scholar] [CrossRef]
- Unnarkat, A.P.; Sridhar, T.; Wang, H.; Mahajani, S.M. Study of cobalt molybdenum oxide supported on mesoporous silica for liquid phase cyclohexane oxidation. Catal. Today 2018, 310, 116–129. [Google Scholar] [CrossRef]
- Alshehri, A.A.; Alhanash, A.M.; Eissa, M.; Hamdy, M.S. New catalysts with dual-functionality for cyclohexane selective oxidation. Appl. Catal. A Gen. 2018, 554, 71–79. [Google Scholar] [CrossRef]
- Ghorbanloo, M.; Rahmani, S.; Yahiro, H. Encapsulation of a binuclear manganese(II) complex with an amino acid-based ligand in zeolite y and its catalytic epoxidation of cyclohexene. Transit. Met. Chem. 2013, 38, 725–732. [Google Scholar] [CrossRef]
- Von Willingh, G.; Abbo, H.S.; Titinchi, S.J.J. Selective oxidation reactions over tri- and tetradentate oxovanadium(IV) complexes encapsulated in zeolite-Y. Catal. Today 2014, 227, 96–104. [Google Scholar] [CrossRef]
- Rayati, S.; Rafiee, N.; Nejabat, F. CHEMISTRY Oxidation of Alkenes with tert-Butyl Hydroperoxide Catalyzed by Mn (II), Cu (II) and VO (IV) Schiff Base Complexes Encapsulated in the Zeolite-Y: A Comparative Study. Inorg. Chem. Res. 2020, 4, 86–93. [Google Scholar] [CrossRef]
- Modi, C.K.; Vithalani, R.S.; Patel, D.S.; Som, N.N.; Jha, P.K. Zeolite-Y entrapped metallo-pyrazolone complexes as heterogeneous catalysts: Synthesis, catalytic aptitude and computational investigation. Microporous Mesoporous Mater. 2018, 261, 275–285. [Google Scholar] [CrossRef]
- Narayanan, S.; Vijaya, J.J.; Sivasanker, S.; Ragupathi, C.; Sankaranarayanan, T.M.; Kennedy, L.J. Hierarchical ZSM-5 catalytic performance evaluated in the selective oxidation of styrene to benzaldehyde using TBHP. J. Porous Mater. 2016, 23, 741–752. [Google Scholar] [CrossRef]
- Thao, N.T.; Thi, L.; Huyen, K. Catalytic oxidation of styrene over Cu-doped hydrotalcites. Chem. Eng. J. 2015, 279, 840–850. [Google Scholar] [CrossRef]
- Wang, X.; Wu, S.; Li, Z.; Yang, X.; Su, H.; Hu, J.; Huo, Q.; Guan, J.; Kan, Q. Cu (II), Co (II), Fe (III) or VO (II) Schiff base complexes immobilized onto CMK-3 for styrene epoxidation. Microporous Mesoporous Mater. 2016, 221, 58–66. [Google Scholar] [CrossRef]
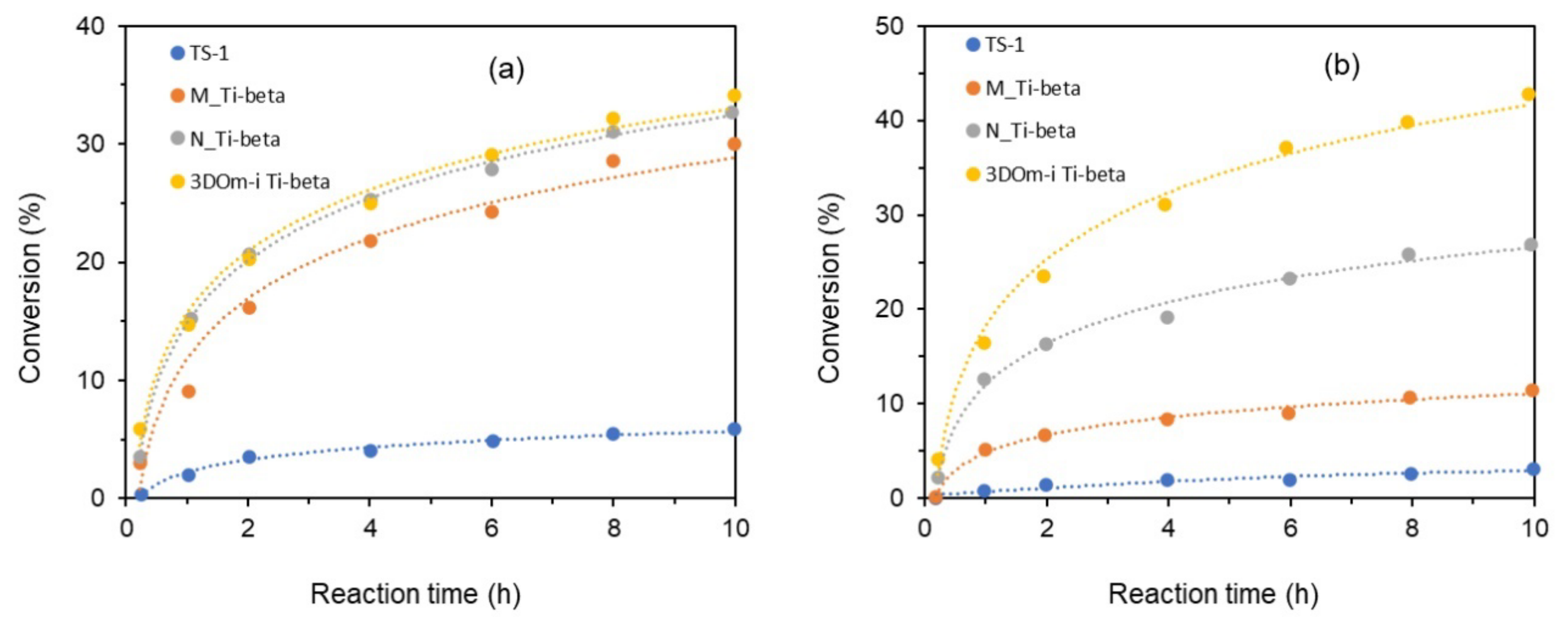
- Yang, Y.; Zhang, T.; Zhou, D.; Liu, X.; Yang, S.; Lu, X.; Xia, Q. Hierarchical Ti-containing hollownest-structured zeolite synthesized by seed-assisted method for catalytic epoxidation of alkenes efficiently. Mater. Chem. Phys. 2019, 236, 121754. [Google Scholar] [CrossRef]
- Azzolina Jury, F.; Polaert, I.; Estel, L.; Pierella, L.B. Synthesis and characterization of MEL and FAU zeolites doped with transition metals for their application to the fine chemistry under microwave irradiation. Appl. Catal. A Gen. 2013, 453, 92–101. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Ohnishi, T.; Miyake, Y.; Hidenori, Y. Effect of Water Added into Acetonitrile Solvent on Oxidation of Benzene with Hydrogen Peroxide over Iron Complexes Encapsulated in Zeolite. Chem. Lett. 2015, 44, 1287–1288. [Google Scholar] [CrossRef]
- Akinlolu, K.; Omolara, B.; Kehinde, O.; Shailendra, T.; Kumar, M. Synthesis and characterization of Cu(II) and Co(II) encapsulated metal complexes in zeolite-Y for the oxidation of phenol and benzene. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Semarang, Indonesia, 7–8 September 2018; IOP Publishing: Bristol, UK, 2019; Volume 509. [Google Scholar]
- Li, X.; Guo, L.; He, P.; Yuan, X.; Jiao, F. Co-SBA-15-Immobilized NDHPI as a New Composite Catalyst for Toluene Aerobic Oxidation. Catal. Lett. 2017, 147, 856–864. [Google Scholar] [CrossRef]
- Nandi, M.; Talukdar, A.K. Ceria—Zirconia solid solution loaded hierarchical MFI zeolite: An efficient catalyst for solvent free oxidation of ethyl benzene. Arab. J. Chem. 2019, 12, 3753–3763. [Google Scholar] [CrossRef]
- Yang, R.; Han, P.; Fan, Y.; Guo, Z.; Zhao, Q. The performance and reaction pathway of d-MnO2/USY for catalytic oxidation of toluene in the presence of ozone at room temperature. Chemosphere 2020, 247, 125864. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.; Huang, H.; Zeng, K.; Wang, Z.; Jia, H. Insights into the size and structural effects of zeolitic supports on gaseous toluene oxidation over MnOx/HZSM-5 catalysts. Appl. Surf. Sci. 2019, 486, 108–120. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, H.; Li, G.; Wang, L.; Song, L.; Li, X. Zeolitic acidity as a promoter for the catalytic oxidation of toluene over MnO x /HZSM-5 catalysts. Catal. Today 2019, 327, 374–381. [Google Scholar] [CrossRef]
- Mochizuki, H.; Yokoi, T.; Imai, H.; Watanabe, R.; Namba, S.; Kondo, J.N.; Tatsumi, T. Facile control of crystallite size of ZSM-5 catalyst for cracking of hexane. Microporous Mesoporous Mater. 2011, 145, 165–171. [Google Scholar] [CrossRef]
- Nemeth, L.; Moscoso, J.; Erdman, N.; Bare, S.R.; Oroskar, A.; Kelly, S.D.; Corma, A.; Valencia, S.; Renz, M. Synthesis and characterization of Sn-Beta as a selective oxidation catalyst. In Studies in Surface Science and Catalysis; van Steen, L.H.E., Callanan, M.C., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2004; Volume 154, pp. 2626–2631. [Google Scholar]
- Wilde, N.; Přech, J.; Pelz, M.; Kubů, M.; Čejka, J.; Gläser, R. Accessibility enhancement of TS-1-based catalysts for improving the epoxidation of plant oil-derived substrates. Catal. Sci. Technol. 2016, 6, 7280–7288. [Google Scholar] [CrossRef]
- Shen, X.; Wang, J.; Liu, M.; Li, M.; Lu, J. Preparation of the Hierarchical Ti-Rich TS-1 via TritonX-100-Assisted Synthetic Strategy for the Direct Oxidation of Benzene. Catal. Lett. 2019, 149, 2586–2596. [Google Scholar] [CrossRef]




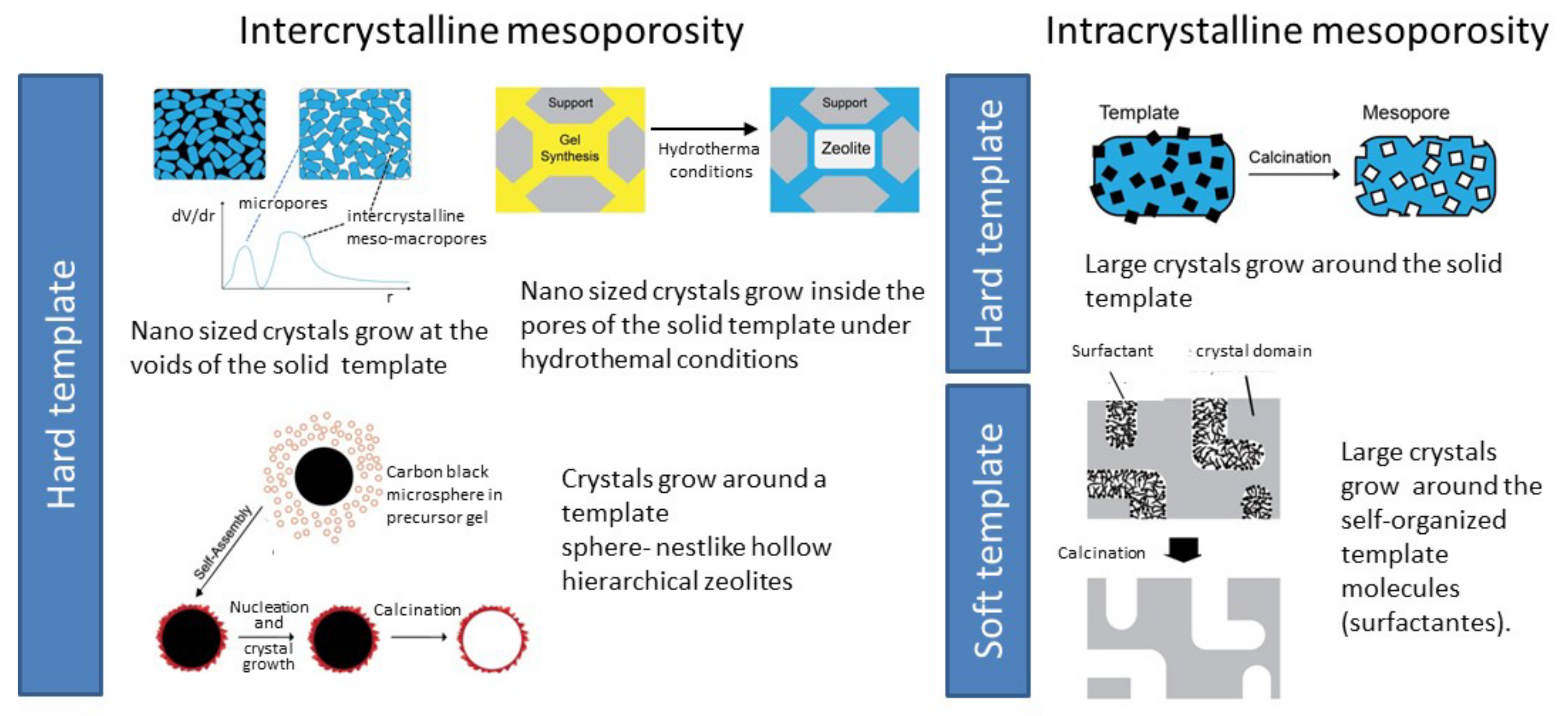

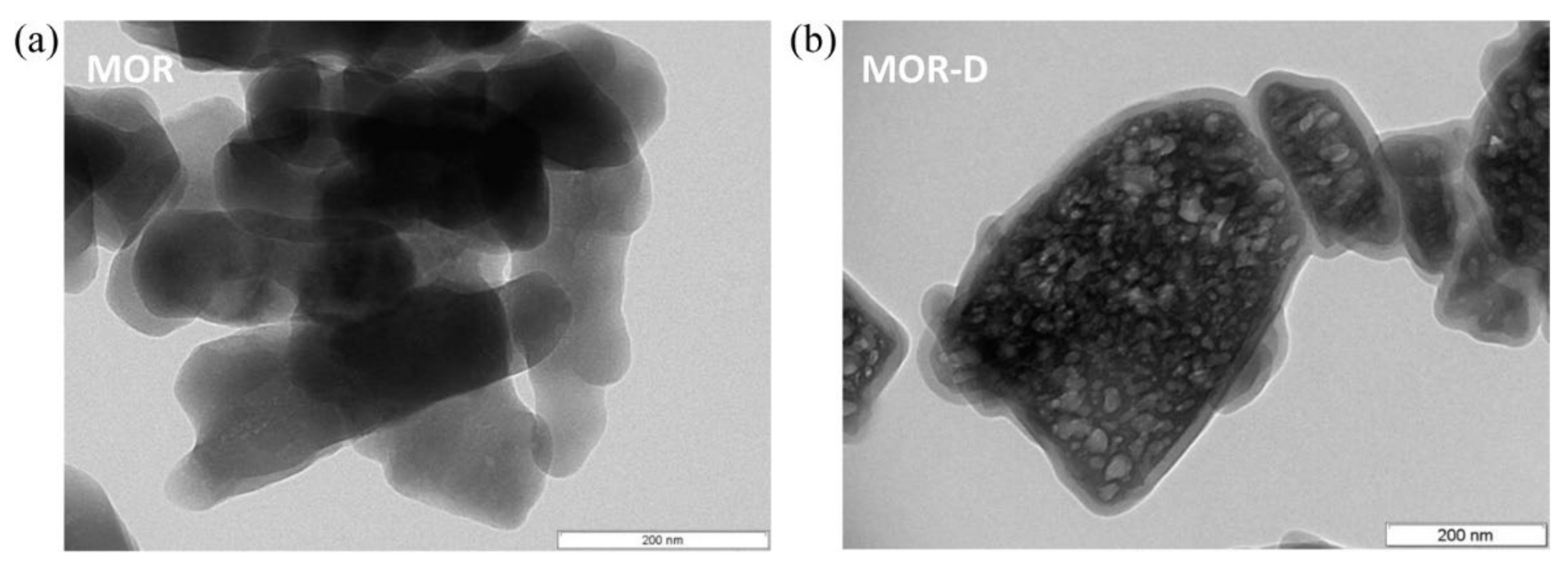




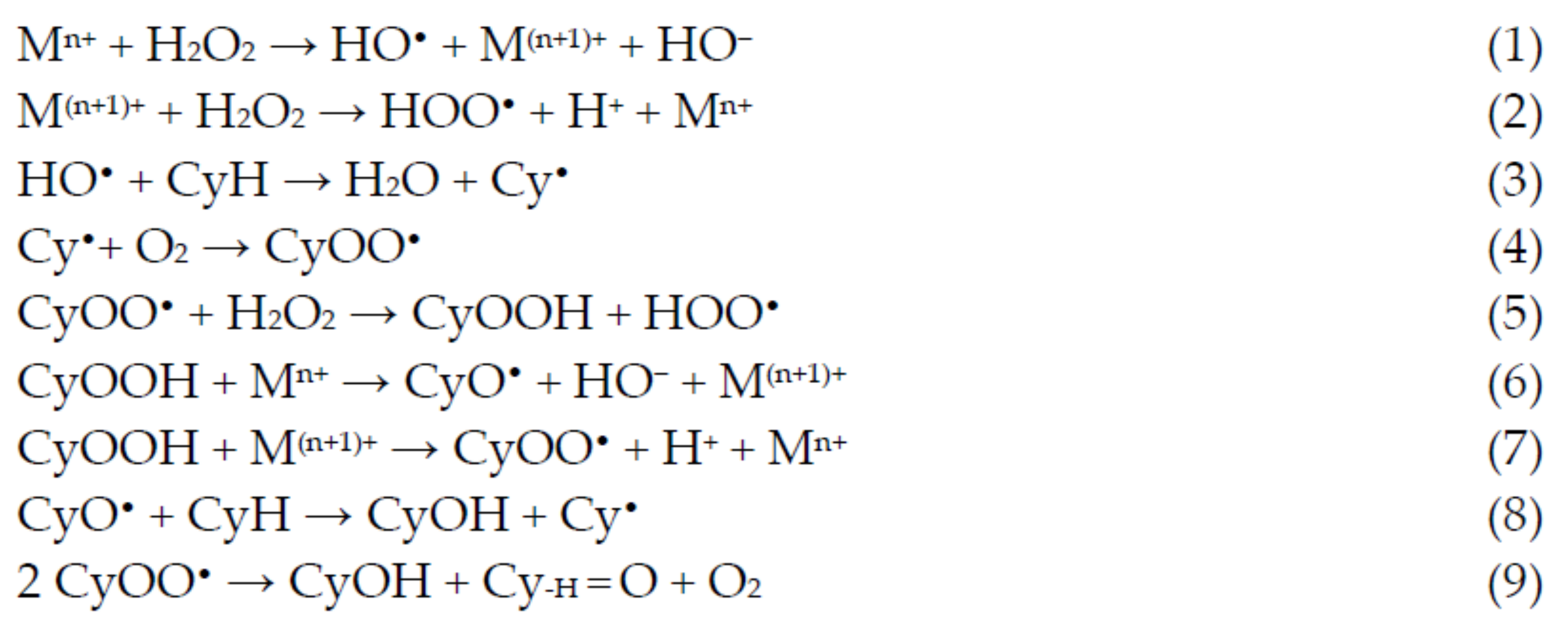













| Zeolite Structure | Template | Observations | Ref. |
|---|---|---|---|
| Hard templating | |||
| MFI | Carbon black | Nanosized crystals with intercrystalline mesoporosity. | [26] |
| MFI | Carbon black | Crystals with intracrystalline mesoporosity. | [27] |
| MEL | Carbon particles | Zeolite single crystals with intracrystalline mesopore volumes between 0.31 and 0.44 cm3 g−1 were isolated. | [28] |
| MFI | Carbon black | Zeolite crystals with a large mesopore volume. Uniform and narrow mesopore size distribution centered at around 20 nm, due to the uniform size of the carbon black particles of about 18 nm. | [29] |
| AEL | Commercial carbon | Hierarchical SAPO-11 materials with both micro- and meso-pores were obtained with irregular cavities in shape and size. | [30] |
| MFI | Carbon fibers, carbon cloths, and monoliths | The combustion of zeolite/carbon composites produced, in the case of carbon fibers and carbon cloths, microtubes of pure zeolite. | [31] |
| RMMs | Carbon mesoporous molecular sieves | Aluminosilicate mesoporous materials with zeolite secondary building units were obtained The novel RMMs possess acidity and high hydrothermal, mechanical, and steam stability. | [32] |
| MFI | Carbon aerogel | Meso-ZSM-5 monolith was synthesized with the crystalline form of ZSM-5 (micropores + mesopores). | [33] |
| MFI | Carbon nanotubes | The use of carbon nanotubes led to creating new mesopores in the ZSM-5 structure. However, increasing the carbon nanotubes content led to destruction of micropores along with some acidity decreasing. | [34] |
| MFI | Hydroxylated carbon nanotubes | Mesoporous structure with a size of about 10–35 nm, similar to the template diameter of was produced. Catalytic cracking of tri-isopropylbenzene was chosen as a probe reaction due to increased external surfaces. | [35] |
| FAU | Carbon aerogel | The pore size distribution obtained from N2 adsorption data shows the presence of meso and micropores with average pore widths are ca. 10 and 0.75 nm, respectively. | [36] |
| AFI | Cation exchange resin beads | Highly crystalline and mechanically stable AlPO-5 spheres were prepared. The presence of the micropores is due to the presence of AlPO-5, whereas the meso and macropores emanate from the resin removal. | [37] |
| MFI | Polystyrene beads | Silicates with bimodal pore structures of macropores (250 nm average diameter), surrounded by microporous silicalite walls were produced. | [38] |
| MFI | Nanosized CaCO3 | Intracrystalline pores in the range of 50–100 nm were detected, which correspond to the morphology of nanosized CaCO3. | [39] |
| MFI | Starch gel | Silicalite-starch gel monoliths and films, as well as sponge-like starch foams infiltrated with silicalite nanoparticles originated macroporous architectures of the zeolite silicalite with, at least, two levels of hierarchy in pore organization. | [40] |
| MFI | Wood cells | Seeded growth strategy was applied to fabricate self-standing zeolitic tissue that faithfully inherits the initial cellular structure of wood at various hierarchical levels. | [41] |
| BEA | Leaves and stems | BEA zeolite macrostructures with hierarchical porosity were prepared, retaining the morphological features of the vegetal template. | [42] |
| MFI | Silane-functionalized polyethylenimine polymer | Hierarchical MFI with small intracrystalline mesoporosity (average pore size 2.0–3.0 nm) and narrow pore size distributions (ca. 1.0–1.5 nm width at half maximum) was obtained. | [43] |
| LTA | Resorcinol-formaldehyde aerogels | The pore size shows a bimodal distribution with micropores and mesopores. | [44] |
| BEA, MEL | Polyvinyl butyral gel | The materials contained two levels of porosity: well-defined microporosity and irregular mesoporosity. | [45] |
| CHA | Coke containing spent MFI | Robust interzeolite transformation was reported to synthesize the hierarchical CHA with high crystallinity and well-developed mesoporosity (mesopore volume = 0.38 m3 g−1). | [13] |
| Soft templating | |||
| AFI | TPHAC | Direct hydrothermal assembly produced mesoporous aluminophosphates constructed with crystalline microporous frameworks. | [46] |
| MFI | TEOS | Direct hydrothermal sylanization synthesis with molybdenum and titanium species encapsulated inside the zeolite crystals. | [47] |
| MFI | Gemini surfactant | Hierarchical ZSM-5 using Gemini surfactants as template that was directly added to the synthesis gel. Abundant intercrystalline mesoporosity was observed. | [48] |
| MFI | TPOAC, CTAB | The usage of different two mesogenous templates during the synthesis procedure resulted in the difference in the mesopore–micropore interconnectivity. Sample prepared with TPOAC presented excellent interconnectivity between micropores and mesopores and higher relative crystallinity. | [49] |
| MFI | CTAB | Hydrothermal crystallization using CTAB as a mesogenous template during synthesis, with crystal size and shape typical of MFI structure. | [50] |
| FAU | TPHAC | TPHAC surfactant was added prior to the hydrothermal step. Zeolite nanosheets with intracrystalline mesopores (around 7 nm) from which the zeolitic micropores can be accessed. This pore system is constructed of zeolitic nanosheets in a house-of-cards-like assembly with wide macroporous interstices between the nanosheet stacks. | [51] |
| MFI | CTAB | A nearly transparent zeolite seed solution allowed the direct self-assembly synthesis of the hierarchical zeolite using CTAB as the mesoporogen, and ethanol as a self-assembly modulator added prior to the hydrothermal step. Overaging the seed solution resulted in nanocrystals of about 100 nm in size and large aggregates. | [52] |
| MFI | CTAB | The crystal morphology was adjusted through a microemulsion-based hydrothermal synthesis. CTAB with cosurfactant butanol was used to form water-in-oil microemulsions. Small irregular rod-shaped nanoparticles were obtained, smaller, and more uniform than those synthesized without the microemulsion. | [53] |
| MFI | Igepal CO-520, CO-720, and CO-890, | The synthesis of silicalite-1 in nonionic microemulsions showed the ability to manipulate the shape and size of silicalite-1 materials, growing both as spheres and platelets. Large particles are robust aggregates of small submicron particles that were stable to calcination. | [54] |
| MFI | CTAB, TBAOH | Hierarchically macro-/meso-/micro-porous structured ZSM-5 with enhanced hydrothermal stability was synthesized by a simple route using CTAB and TBAOH as the meso- and micro-pore templates. Ethanol was used to generate macropores, probably via a proposed “ethanol-in-water” microemulsion mechanism. | [55] |
| MFI | Triton X-100 | ZSM-5 with hexagonal cubic morphology was successfully synthesized using a nonionic surfactant as mesopore template, added prior to the hydrothermal step. | [56] |
| MFI | GPTMS | Hierarchical Fe-ZSM-5 zeolites with microspherical morphology was synthesized in the presence of GPTMS through a localized crystallization process. | [57] |
| MFI | TPOAC | Hierarchical ZSM-5 was obtained through a two-step synthesis in the presence of organosilane TPOAC as the mesoporogen. The obtained materials are made up from very small microporous zeolite domains with sizes below 50 nm integrated into highly mesoporous particles. | [55] |
| MFI | C16-6-6(Br)2 or C16-6-6(OH)2 | Hierarchical ZSM-5 zeolite was synthesized with a diquaternary ammonium surfactant containing a hydrophobic tail. The materials consist of thin sheets limited in growth in the b-direction (along the straight channels of the MFI network.) The stacking of the zeolite sheets results in the formation of mesopores. | [58] |
| Zeolite Structure | Treatment | Observations | Ref. |
|---|---|---|---|
| Dealumination | |||
| FAU | Steaming + acid treatment | The treated zeolite samples were able to adsorb water-soluble organic molecules, thus improving their hydrophobicity. | [61] |
| FAU | Steaming, steaming twice + acid leaching | High mesoporous volume was obtained, comprising mainly interconnected cylindrical mesopores. | [62] |
| MOR | Steaming | Most of the framework Al species in the 12-membered ring channels of mordenite were removed while those in the 8-membered ring channels were retained. Strong reduction of acid sites located in the 12-membered ring channels. | [63] |
| BEA | Steaming | A significant reduction of acid sites as consequence of steaming dealumination hinders the occurrence of extensive cracking reactions and favors isomerization, as desired. | [64] |
| MWW | Steaming | The number of acid sites was decreased by hydrothermal treatment, but the acid strength was not modified. Aluminum debris located in the porosity reinforced the shape selectivity. | [65] |
| BEA | Acid treatment with HNO3 | As-synthesized Al-BEA zeolite was submitted to acid treatment with HNO3 in a stainless-steel autoclave at 80 °C for 24 h to replace Al by Ti in the framework positions. Highly interconnected three-dimensional ordered mesopores were observed. | [66] |
| MFI | Steaming or acid treatment with oxalic acid and combination of steam+acid+alkali treatment | The steam treatment was performed in a quartz fixed bed tube at 700 °C for 3 h and 0.386 bar. For the acid treatment, 0.1 M oxalic acid for 2 h and 70 °C was used. For the alkaline treatment, 0.2 M NaOH at 80 °C for 2 h was employed. It was observed that the alkaline treatment heals the destructive parts of the zeolite that was produced by the steam and acid treatments. | [67] |
| Desilication | |||
| MFI | NaOH | Alkaline treatment with 0.2 M NaOH at 80 °C for 300 min. Development of mesopores whose size is almost uniform without destruction of microporous structure. | [61] |
| MFI | NaOH | Alkaline treatment with 0.2 M NaOH at 80 °C. Parent zeolite samples with distinct crystal size were submitted to the treatment: commercial sample with small crystallites and laboratory made large crystals. Desilication was proven to be highly efficient to alleviate diffusion limitations in small crystal commercial zeolites by producing hierarchical samples to an extent comparable to model large crystals produced in laboratory scale. | [68] |
| MFI | NaOH followed by acid washing with HCl | Desilication of ZSM-5 with Si/Al ranging from 10 to 1000 using 0.1–1.8 M NaOH. For Si/Al < 20, an additional acid treatment is essential for removing amorphous Al debris from alkaline treated samples, allowing the restoration of the zeolite acidity. | [69] |
| MOR | NaOH | Optimization of the desilication experimental parameters: base concentration, temperature, and time. The optimum desilication conditions (that is, allowing high crystallinity, acidity, and mesoporous volume) were obtained with the sample treated with 0.2 M NaOH, at 85 °C for 2 h. | [70] |
| BEA | TPAOH (tetrabutylammonium hydroxide) | “One-pot” desilication + ion exchange protocol using TPAOH was applied to zeolite BEA. The obtained material was highly mesoporous and acidic; showing that for specific zeolite structures, it is necessary to carefully search for the optimal base that stabilizes sensitive structures. | [71] |
| MWW | NaOH followed by acid treatment with HCl | Mesoporosity was attained using 0.05 M NaOH solution and practically no gain was observed when using 0.1 M NaOH. When desilicated sample with 0.1 M was submitted to acid washing with 0.1 M HCl the extraction of Al from the two internal porous systems promotes their interconnectivity, evolving from a 2-D to a 3-D structure. | [72] |
| BEA | NaOH followed by acid treatment with HCl | BEA zeolites with Si/Al ratio of 12.5 and 32 were submitted to alkaline treatment with 0.1 M NaOH solution followed by acid treatment with 0.1 M HCl. The Si/Al ratio has a determinant role on the properties of the final materials. For Si/Al = 12.5, the loss of acid sites during desilication is reversed upon acid treatment, whereas for Si/Al = 32, a continuous decrease in Brönsted acidity was observed simultaneously with a significant decrease in crystallinity. | [73] |
| BEA | NaOH followed by acid treatment with HCl | BEA (Si/Al = 12.5) was treated with 0.2 and 0.4 M NaOH, followed by acid treatment with 0.1 M HCl. Some decrease in microporous volume was observed, especially for the sample treated with 0.4 M NaOH. The development of mesoporosity also occurred with an uptake of 5–7% for the desilicated samples and an increase of 20–22% after the acid treatment. | [74] |
| MOR | NaOH | Desilication treatments with 0.2 M NaOH at 85 °C under conventional (0.5–10 h) and microwave (5–30 min, 300 W) heating. Both heating methods promoted the development of mesoporosity. However, microwave irradiation also results in the partial conversion of the zeolite pristine microporosity into larger micropores. | [75] |
| Support | Catalyst | Reaction Type | Observations | Reference |
|---|---|---|---|---|
| FAU | [M(salophen)] and [M(H4salophen)], (M = Mn(II), Co(II), Ni(II) and Cu(II); salophen = N,N-bis-(salycilidene)-1,2-phenylenediamine; H2[H4salophen] = 2-({2-[(2-hydroxybenzyl)amino]anilino}methyl)phenol | oxidation of cyclohexane with H2O2 | Catalysts encapsulated in the pores of NaY by exchanging the Na by the transition metal M(II), followed by reaction of metal-exchanged Y zeolite (M(II)-NaY) with H2 salophen and H2[H4salophen] in the molten state. The encapsulated catalysts systems [M(H4salophen)]-NaY were more active than the corresponding complexes [M(H4salophen)]. | [150] |
| FAU | [M(SFCH)·xH2O]-Y [M = Mn, Fe, Co, Ni (x = 3) and Cu (x = 1)]; H2SFCH = (E)-N′-(2-hydroxybenzylidene) furan-2-carbohydrazide] | oxidation of cyclohexane with H2O2 | Complexes entrapped in the cages of Y zeolite (suitable in size for the zeolite channels shown by DFT calculations). The initial run showed a conversion of 45.1% which was only slightly reduced after the 2nd reuse of the supported catalysts. | [151] |
| FAU | [M(L)] [M = Co(II) or Cu(II)]; (L = Schiff bases) | oxidation of cyclohexane with H2O2 | Immobilization of the complex inside the zeolite pores using the flexible ligand method. The immobilized complexes afforded a maximum yield of 75.2% and could be recovered and reused without loss of catalytic activity. In addition, active in oxidation of cyclooctane (76.8% yield). | [17] |
| Hierarchical FAU | [FeCl2{κ3-HC(pz)3}] (pz = pyrazol-1-yl) | oxidation of cyclohexanewith H2O2 | Y zeolite was modified through alkaline treatments (NH4OH, NaOH or TPAOH) assisted by CTAB surfactant. Iron complex was immobilized at the support by incipient wetness impregnation method. The reuse of the sample in the catalytic oxidation reaction led only to a small decrease in Fe content, after the 3rd catalytic cycle. | [100] |
| Hierarchical MOR | [VO2{HB(κ3–3,5-Me2pz)3}] (pz = pyrazol-1-yl) | oxidation of cyclohexane with t-BuOOH | Hierarchical MOR zeolite prepared through alkaline treatments (NaOH or TPAOH) assisted by CTAB surfactant. The vanadium complex was immobilized at the support by the incipient wetness impregnation method. Reuse up to four consecutive catalytic cycles with no appreciable leaching | [103] |
| Hierarchical MOR | [FeCl2{κ3-HC(pz)3}] (pz = pyrazol-1-yl) | oxidation of cyclohexane with H2O2 | Hierarchical MOR prepared through desilication using 0.5 M NaOH. Iron complex immobilized at the support by incipient wetness impregnation method. Much higher TON, high yield values, and lower loading of oxidant for the heterogeneous than for the homogeneous one. However, a considerable (46%) loss of activity, mainly due to lixiviation was observed. | [90] |
| Hierarchical MFI | Cu(I)/Cu(II) dispersed in the zeolite framework | oxidation of cyclohexane with H2O2 | Copper species incorporated in one-pot hydrothermal synthesis of hierarchical ZSM-5 zeolite. The excellent catalytic activity was ascribed to the hierarchical porous structure, allowing a fast diffusion of molecules and the highly dispersed framework copper ions (Cu+/Cu2+) as catalytic active sites | [152] |
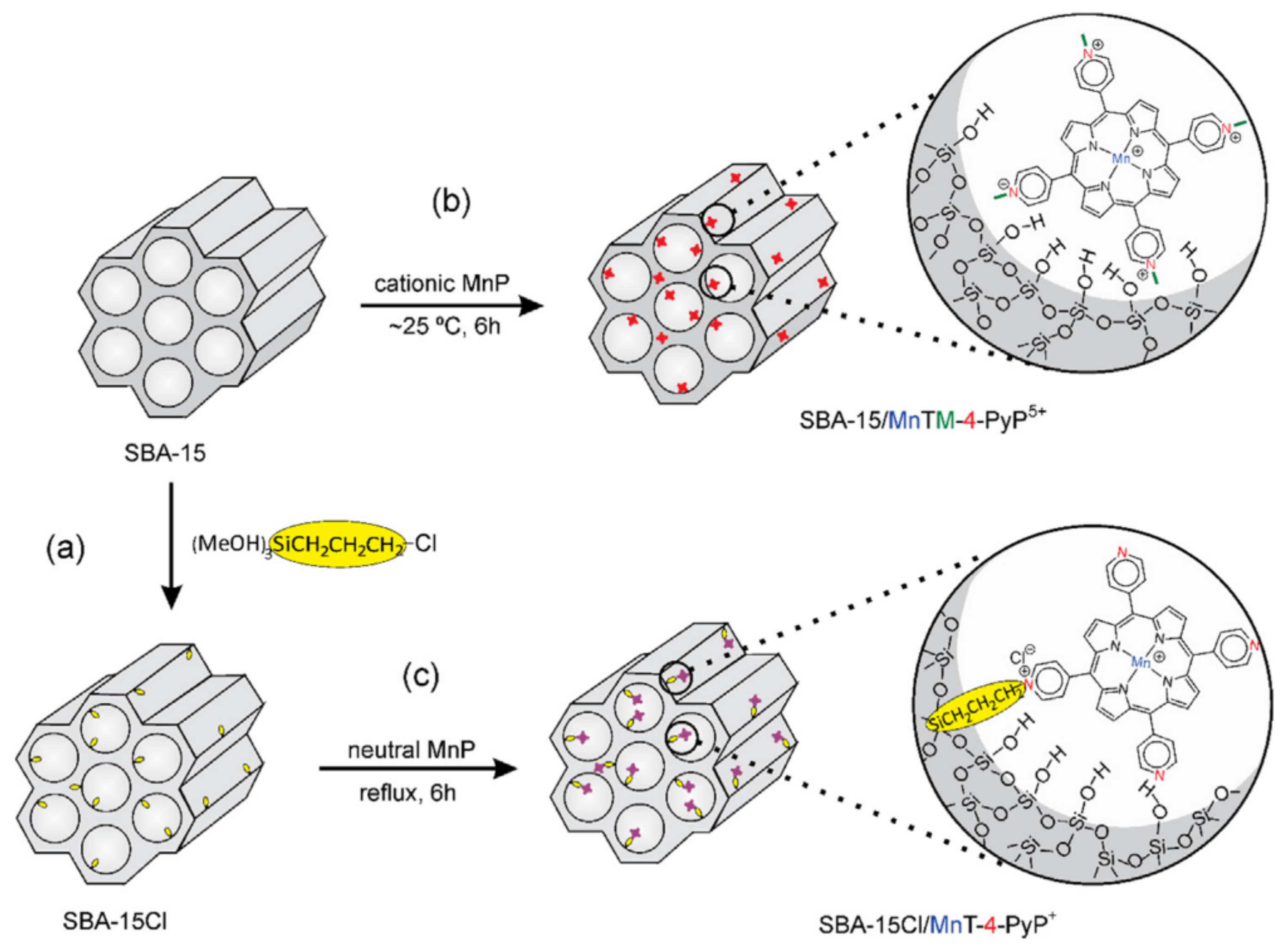
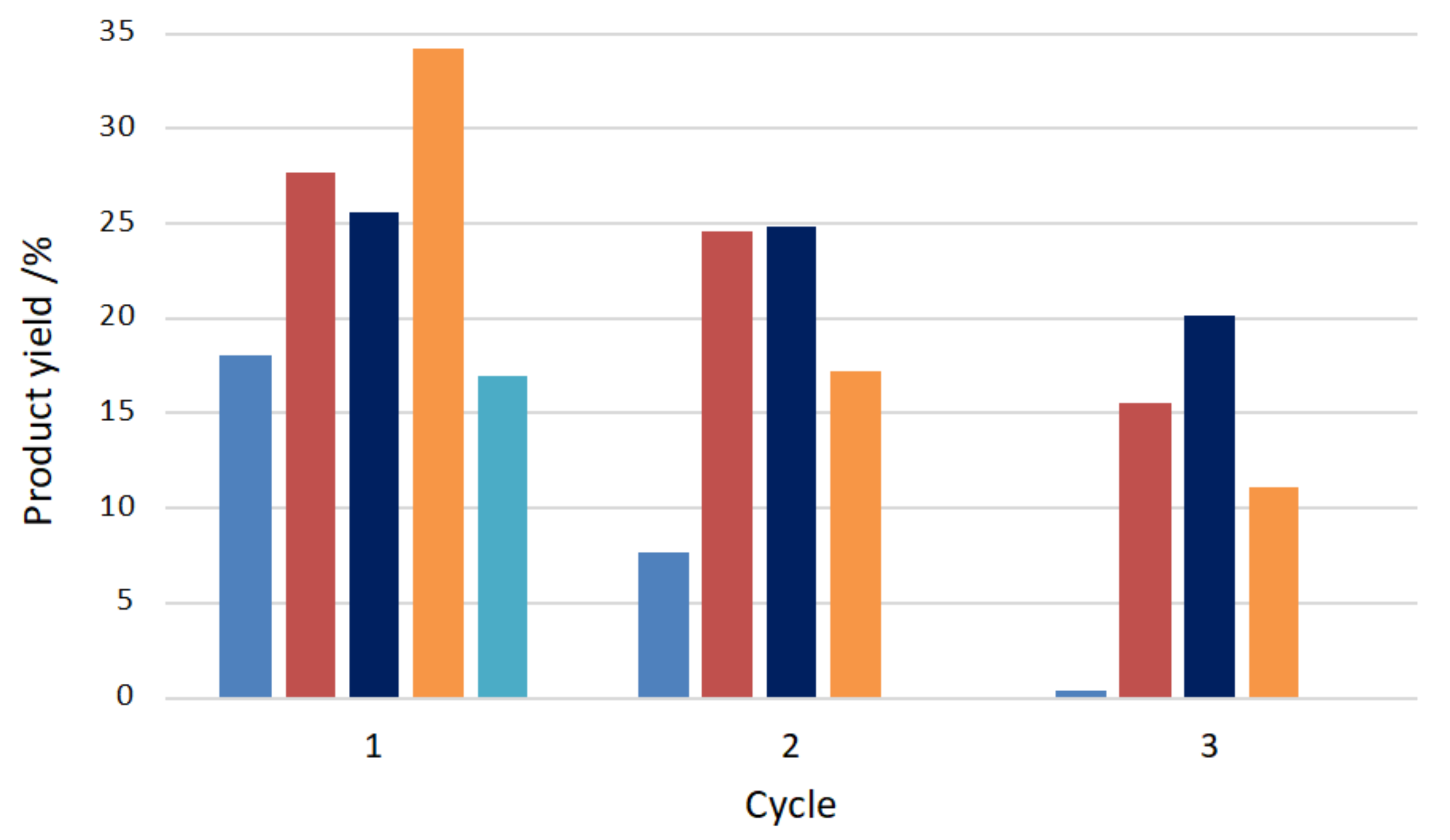
| SBA-15 | Mn(III) N-methylpyridiniumporphyrins (MnTM-X-PyPCl5, X = 2, 3,4) | oxidation of cyclohexane with PhIO | Immobilization of the catalysts by electrostatic interactions or covalent bonding. A low-leaching of manganese porphyrins from the supports was observed in both cases due to strong interaction with SBA-15. | [135] |
| SBA-15 | [Mn(saldien)(N3)] (saldien = N,N’-d bis(salicylidene)diethylenetriamine) | oxidation of cyclohexane with H2O2 | [Mn(saldien)(N3)] was anchored on mesoporous SBA-15. The immobilized complex is stable and recyclable and gives comparable or even higher cyclohexene conversion than homogeneous system. In the oxidation of cyclohexane, products were formed in up to 355 turnovers. | [153] |
| MFI | Manganese Mn(IV) | oxidation of cyclohexane with H2O2 | ZSM-5 zeolite functionalized in situ with Mn was obtained via a one-pot hydrothermal approach. Mn-ZSM-5 materials, especially the 2% Mn4+ doped ZSM-5, shows a high catalytic activity and selectivity. | [154] |
| HMS | [M(Sal)(PMeOSi)DPTA] (M = Fe, Ni, Mn; Sal = salicylaldehyde; DPTA = bis(2,4-pentanedionato)cobalt, bis(aminopropyl)amine) | oxidation of cyclohexane with O2 | Complexes covalently anchored into HMS (2–10 nm pore size) via condensation process. The supported catalysts were stable under the applied temperature (200 °C) and could be recycled. | [136] |
| MCM-48 | Ce(III) and Ce(IV) | oxidation of cyclohexane with O2 | Cerium-doped MCM-48 was prepared hydrothermally, and its surface was modified with organic groups or fluorine. Postfunctionalized Ce-MCM-48 exhibited the higher conversion and selectivity The F-modified catalyst showed excellent reusability, and its catalytic performance has no deterioration upon 5 reuse cycles. | [155] |
| KIT-6 | (VO)2P2O7 | oxidation of cyclohexane with H2O2 | Vanadium catalysts postsynthesis incorporated at KIT-6. The catalyst was reused several times, and no leaching/soluble vanadium phosphate species could be detected in the filtrate. | [156] |
| SBA-15, KIT-6 and FDU-12 | CoMoO4 | oxidation of cyclohexane with O2 | The supports were soaked with the catalyst’s precursors for 3–4 h, dried at 70 °C, and calcined changing the temperature from 350 to 550 °C. The calcination temperature influenced the catalyst activity, being the lowest temperature the most favorable. All suffer deactivation but activity could be restored on recalcination. Catalysts were used in multiple cycles of regeneration and reaction with no decrease in the performance. | [157] |
| TUD-1 | Co(II) and a metal oxide (Cr, Cu, Ti, Mn, Bi, V or Sr) | oxidation of cyclohexane with t-BuOOH | One step synthesis of dual function M-Co on mesoporous TUD-1. The dual-function catalysts exhibited higher activity than either Co-TUD-1 or other M-TUD-1. Mn-Co-TUD-1 and Ti-Co-TUD-1 exhibited an excellent stability during the reaction, and Mn-Co-TUD-1 was successfully recycled up to 4 runs. | [158] |
| Support | Catalyst | Reaction Type | Observations | Reference |
|---|---|---|---|---|
| FAU | Mn(II) complex with a Schiff base ligand derived from L-tyrosine plus salicylaldehyde | oxidation of cyclohexene with H2O2 | Manganese(II) complex encapsulated in the supercages of Y zeolite by the flexible ligand method. The heterogeneous catalyst exhibited higher activity and selectivity than the homogeneous system. Catalytic activity of heterogenized system almost unchanged up to 5 cycles. | [159] |
| FAU | VO(IV), Mn(II), Fe(II), Co(II), Ni(II), Cu(II), and HNIMMPP Schiff base ligands | oxidation of styrene with t-BuOOH | Flexible ligand method used to synthesize Y zeolite imprisoned transition metal complexes. [VO(HNIMMPP)(H2O)]-Y afforded the highest conversion of styrene and selectivity of benzaldehyde. | [16] |
| FAU | VO(IV) complexes of 7-amino-5-aza-4-methyl-hept-3-en-2-one and 4,4 -(ethane-1,2-diyldinitrilo)dipentan-2-one | oxidation of phenol, benzene, styrene, cyclohexene with H2O2 | Complexes immobilized inside the cages of Y zeolite using the flexible ligand method. The homogeneous complexes were found to be more active than the corresponding encapsulated VO(IV) complexes, but the heterogenized complexes showed higher turnover frequency values (TOF) and better selectivity than the corresponding homogeneous catalysts. | [160] |
| FAU | V(IV)O, Mn(II), Co(II) complexes of a Schiff base ligand derived from 2,4-dihydroxyacetophenone and 1,2-diaminocyclohexane | oxidation of cyclooctene, cyclohexene, styrene, α-methyl styrene with t-BuOOH | Complexes encapsulated in the cavities of Y zeolite by the flexible ligand method. The catalytic activity decreased in the order CuL-Y > VOL-Y > MnL-Y. 100% selectivity for epoxide formation was obtained in the case of cyclooctene. | [161] |
| FAU | Binuclear complexes of V(IV)O and Fe(II) | oxidation of cyclohexene, limonene, α-pinenewith H2O2 | Complexes encapsulated in the cavities of Y zeolite by the flexible ligand method. The formation of olefinic oxidation products follows the sequence α-pinene < limonene < cyclohexene due to steric hindrance. The heterogeneous catalyst kept the catalytic activity upon two consecutive runs. | [15] |
| FAU | [VO(sal2bz)]2 and [Fe(sal2bz)(H2O)2]2·2H2O H2sal2bz = (Z)-3-methyl-1-phenyl-4-(2,2,2- trifluoro-1-(2-hydroxyphenyl)imino)ethyl)-1H-pyrazol-5-ol | oxidation of limonene, cyclohexene, styrene, and α-pinene with H2O2 | Complexes entrapped in the supercages of Y zeolite by the flexible ligand method. The effect of solvents, mole ratio of substrate and oxidant, amount of catalyst, and reaction time was tested. [VO(L)·H2O]-Y exhibits exceptional activity by providing superior conversion (>80%) of limonene. | [162] |
| FAU | [CuL1(NO3)]n and [CuL2Cl] (HL1 = 1- [(3-dimethylaminopropyl-imino)methyl]-naphthalen-2-ol and HL2 = 3-[(3-dimethylamino-2,2-dimethyl-propylimino) methyl]-naphthalen-2-ol | oxidation of styrene and cyclohexene with H2O2 | Complexes entrapped in the supercages of NaY in the solvent phase through two-stage process (i) ion exchange of the selected Cu(II)-salt; (ii) encapsulation of Schiff-base ligands (HL1 /HL2) in Cu(II) exchanged zeolite. The heterogeneous catalyst can be reused for several cycles without decay of activity as confirmed by PXRD, cyclic voltammetry, SEM, and FTIR studies. | [14] |
| FAU | Triazenido Cr complexes recovered from biosorption studies | oxidation of cyclohexene with t-BuOOH | Chromium containing FAU zeolite, in sodium form (NaY) and in proton form (HY), was recovered from biosorption studies and reused as a support for the preparation of heterogeneous catalysts by the flexible ligand method, using 1,3-diphenyltriazene derivatives. Cr–FAU supports lost some of the Cr into the reaction medium, whereas immobilization of Cr-complexes reduced the referred leaching. | [111] |
| Hierarchical MFI | Native active sites of the zeolite with Si/Al ratio modified during synthesis. | oxidation of styrene with t-BuOOH | Hierarchical ZSM-5 samples were prepared with Si/Al = 20, 60 and 100 using TPAOH as structure directing agent. Sample with Si/Al = 60 showed significantly higher yield of benzaldehyde. The catalyst was recovered and recycled three times without a significant loss in activity and selectivity. | [163] |
| MFI | Co3O4 | oxidation of styrene with H2O2 | The precursor Co3O4/SiO2 with 10 wt.% Co loading was prepared via impregnating the SiO2 support with an aqueous solution containing the required amount of Co(NO3)2.6H2O. Under the optimized reaction condition, the yield of benzaldehyde can achieve 78.9% with 96.8% styrene conversion and 81.5% benzaldehyde selectivity. Such an excellent catalytic performance can be attributed to the synergistic effect between the Co3O4 encapsulated zeolite structure with a confined reaction environment and the acidity. | [144] |
| Hydrotalcites | Cu(II) | oxidation of styrene with t-BuOOH or H2O2 | Substitution of Mg2+ by Cu2+ in the brucite sheets. The selectivity to products (80–90%) was dependent on the copper ions in the catalysts and on the nature of the oxidant. | [164] |
| CMK-3 | Cu(II), Co(II), Fe(III) or V(II)O Schiff base complexes | oxidation of styrene with air | CMK-3 support was prepared using as solid template SBA-15 and sucrose as carbon precursor. High conversion of styrene (94.1%) and selectivity to styrene oxide (73.9%) can be achieved over the heterogeneous Co(II) catalyst with isobutyraldehyde as co-reductant. Functionalized CMK-3 catalyst was quite stable and could be recycled at least three times. | [165] |
| Mesoporous MFI | Cr and W-Cr | oxidation of styrene in the presence of H2O2 | Mesoporous ZSM-5 was produced by adding CTAB soft template during synthesis. Chromium and tungsten were introduced in the zeolite through incipient impregnation method. W-Cr-ZSM-5 sample showed higher catalytic activity than that of Cr-ZSM-5. | [50] |
| MEL | Co | oxidation of styrene in the presence of H2O2 | Microwave-assisted introduction of cobalt in the zeolite by ionic exchange. Co-ZSM-11 catalyst presented a reaction rate about 30% higher than the found in the literature and a higher benzaldehyde selectivity (ca. 80%) under optimal reaction conditions. | [142] |
| Hierarchical MFI | Mo and Ti | oxidation of 1-octene and cyclohexene with H2O2 or t-BuOOH | Molybdenum confined hierarchical materials prepared via a silanization procedure. Most of Mo and titanium are encapsulated inside the zeolite pores giving high stability to the catalysts. | [47] |
| Hollownest-structured zeolite (Ti-HSZ) | Ti | oxidation of cyclooctene, cyclopentene with t-BuOOH | Seed-assisted synthesis of Ti-containing hollownest-structured zeolite (Ti-HSZ). About 85% cyclopentene and 87% cyclooctene can be converted into corresponding epoxides (selectivity for epoxides of nearly 100%). The hierarchical porous structure increases the diffusion and mass transfer of various reaction molecules (alkene, oxidant, and solvent). | [166] |
| Hierarchical Ti-BEA | Ti | oxidation of cyclohexene and cyclooctene with H2O2 | Hierarchical titanosilicate beta zeolite was produced through successive postsynthetic dealumination and titanation. The highly interconnected intracrystalline meso-/micro-porosity enhanced diffusion and improved the accessibility of active sites to the bulky substrates. The catalyst exhibited not only a superior stability but also a facile recyclability by simple calcination. | [66] |
| Hierarchical MFI | Mo | cyclooctene, cyclohexene, norbornene, and styrene with t-BuOOH | Gemini surfactants were synthesized to act as templates for the synthesis of hierarchical ZSM-5, with high intercrystalline mesoporosity and OH rich surface, used to immobilize molybdenum compound by the stable Mo-O bond. The supported catalysts exhibited high yield and selectivity for the epoxidation of several alkenes and the ability to be reused more than 5 cycles. | [48] |
| Support | Catalyst | Reaction Type | Observations | Reference |
|---|---|---|---|---|
| FAU | [Fe(bpy)3]2+(bpy = bipyridine) | oxidation of benzene with H2O2 | Fe-bipyridine complex was encapsulated into cation-exchanged Y-type zeolites (M-Na-Y: M = K+, Cs+, Mg2+, Ca2+, NH4+, tetramethylammonium (TMA+), or tetrabutylammonium (TBA+). The catalytic activity towards phenol production in different solvents is dependent on the accessibility of benzene to the iron site in [Fe(bpy)3]@M-Na-Y, controlled by the hydrated ionic radius of the cation (M) introduced. | [18] |
| Efficient selective formation of phenol in acetonitrile–water mixed solvents over [Fe(bpy)3]2+ encapsulated in Y zeolite. The catalytic activity improved by increasing the amount of water added to acetonitrile. | [168] | |||
| FAU | Cu(II) and Co(II) complexes of phthalocynamine | oxidation of benzene with H2O2 | Metal complexes encapsulated into the supercages of Y zeolite by the flexible ligand method. The prepared catalysts were reused three times with almost no change in catalytic activity. Cobalt encapsulated metal complex afforded the best catalytic activity. | [169] |
| SBA-15 | N,N-dihydroxypyromellitimide (NDHPI) and Co | oxidation of toluene with O2 | Composite catalyst prepared by immobilizing NDHPI on Co-doped mesoporous sieve SBA-15 doped with Co, using 3-glycidoxypropyltrimethoxysilane (GPTMS) the silylation agent. The presence of N–OH active sites was confirmed, as well as the preservation of the mesoporous structure of the catalyst. The catalyst kept the activity, and no Co was lost from SBA-15 after 3 reaction runs. | [170] |
| Hierarchical MFI | Fe | oxidation of benzene with N2O | Hierarchical Fe-HZSM-5 prepared through different synthetic routes: carbon black as hard template, organosilane as soft template, and postsynthesis desilication. The best catalytic performance was achieved by organosilane templated zeolites. | [29] |
| Hierarchical Fe-ZSM-5 catalyst directly synthesized in the presence of the silane coupling agent (GPTMS). Internal mass transfer limitations of the hierarchical Fe-ZSM-5 significantly improved. | [57] | |||
| Crystallization of hierarchical ZSM-5 in the presence of the organosilane octadecyl-dimethyl-(3-trimethoxysilyl-propyl)-ammonium chloride as the mesoporogen. Iron precursor introduced during synthesis. Fe-containing zeolites are excellent catalysts since hierarchical pore structure leads to higher reaction rates due to increased mass transfer and increased catalyst longevity despite more substantial coke formation. | [55] | |||
| Hierarchical MFI | Fe | oxidation of benzene with N2O | Parent zeolites containing small amount of iron as impurities (~0.04 wt.%) modified by post synthesis treatments of commercial ZSM-5: alkali, acid or steam treatment, combination of acid-alkali treatments, and combination of steam-acid-alkali treatments. Such modifications led to remarkable catalytic performance in the oxidation of benzene to phenol. | [67] |
| Hierarchical Fe/ZSM-5 zeolites synthesized with a diquaternary ammonium surfactant containing a hydrophobic tail. The sheet-like zeolites deactivate much slower than bulk Fe/ZSM-5, which was attributed to the much lower probability of secondary reactions of phenol in the short straight channels of the sheets. | [58] | |||
| Hierarchical MFI | ceria–zirconia | oxidation of ethyl benzene with t-BuOOH | Hierarchical MFI supported ceria–zirconia solid solution was synthesized by deposition–precipitation method. The catalyst was resistant to leaching and retained its activity even after the fifth run. | [171] |
| FAU | δ-MnO2 | oxidation of toluene with O3 | δ-MnO2/USY with different contents of δ-MnO2 was synthesized using hydrothermal method. 3.0 wt.% δ-MnO2/USY displayed the best performance. | [172] |
| Hollow structured MFI | MnOx | oxidation of toluene with O2 | HZSM-5 zeolites with micro-/nano-crystallites and hollow structure were, respectively, synthesized for the preparation of supported MnOx catalysts. The active MnOx nanoparticles on MnOx/H-ZSM-5 surface significantly accelerated the oxidative destruction of toluene and other organics, reducing the coke formation. | [173] |
| MFI | native active sites of zeolite | oxidation of toluene with t-BuOOH | The smaller crystallite size, higher surface area, and total pore volume of the sample synthesized in the presence of a surfactant as mesopore template produced an effective catalyst that could be reused for a minimum of four runs without significant loss of in conversion and selectivity. | [56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, A.; Nunes, N.; Carvalho, A.P.; Martins, L.M.D.R.S. Zeolites and Related Materials as Catalyst Supports for Hydrocarbon Oxidation Reactions. Catalysts 2022, 12, 154. https://doi.org/10.3390/catal12020154
Martins A, Nunes N, Carvalho AP, Martins LMDRS. Zeolites and Related Materials as Catalyst Supports for Hydrocarbon Oxidation Reactions. Catalysts. 2022; 12(2):154. https://doi.org/10.3390/catal12020154
Chicago/Turabian StyleMartins, Angela, Nelson Nunes, Ana P. Carvalho, and Luísa M. D. R. S. Martins. 2022. "Zeolites and Related Materials as Catalyst Supports for Hydrocarbon Oxidation Reactions" Catalysts 12, no. 2: 154. https://doi.org/10.3390/catal12020154
APA StyleMartins, A., Nunes, N., Carvalho, A. P., & Martins, L. M. D. R. S. (2022). Zeolites and Related Materials as Catalyst Supports for Hydrocarbon Oxidation Reactions. Catalysts, 12(2), 154. https://doi.org/10.3390/catal12020154









