Abstract
Enzymatic reaction cascades represent a powerful tool to convert biogenic resources into valuable chemicals for fuel and commodity markets. Sugars and their breakdown products constitute a significant group of possible substrates for such biocatalytic conversion strategies to value-added products. However, one major drawback of sugar cascades is the need for cofactor recycling without using additional enzymes and/or creating unwanted by-products. Here, we describe a novel, multi-enzymatic reaction cascade for the one-pot simultaneous synthesis of L-alanine and L-serine, using the sugar degradation product 2-keto-3-deoxygluconate and ammonium as precursors. To pursue this aim, we used four different, thermostable enzymes, while the necessary cofactor NADH is recycled entirely self-sufficiently. Buffer and pH optimisation in combination with an enzyme titration study yielded an optimised production of 21.3 +/− 1.0 mM L-alanine and 8.9 +/− 0.4 mM L-serine in one pot after 21 h.
1. Introduction
The development of bio-based alternatives to petroleum-based products as well as an urgent demand for a reduction of global warming is a crucial topic of today’s research. To satisfy the prerequisites of green chemistry, novel production processes for fuels and chemicals based on renewable resources will be in even higher demand [1]. To develop such processes, catalysis and especially biocatalysis is a primary key. For biocatalytic conversion strategies, several reaction steps and corresponding enzymes are often needed. Multi-enzyme reactions consist of two or more enzymatic reactions and offer the possibility of using multi-stage reactions, combined with their necessary cofactors in a controlled environment to produce interesting target products without the separate preparation of intermediates [2,3,4,5,6]. With a wise selection of enzymes, their various advantages can be combined in a single pot reaction. By working with thermostable enzymes, for example, protein purification is less time-intensive, and higher temperatures can be used for the reaction conditions, which speeds up the production of the target molecule [7]. Consequently, the research interest and number of published papers on artificial bio-catalysed cascades has increased continuously within the past years [5,6].
Artificial enzyme cascades are often based on existing natural pathways where central intermediates are formed, e.g., by using the non-phosphorylated Entner–Doudoroff pathway which yields pyruvate and 2-keto-3-deoxygluconate from D-glucose. 2-keto-3-deoxygluconate was previously produced in a couple of sugar degradation reaction schemes, for example in an enzymatic cascade, in which D-glucose is converted to D-gluconate by use of a glucose dehydrogenase and further on to 2-keto-3-deoxygluconate by a D-gluconate-dehydratase [8,9,10]. The glucose dehydrogenase requires NAD+ and using this method directly creates a cofactor imbalance with one NADH left for the following reactions. Depending on the subsequent steps of the cascade, this can be a challenge. Sperl et al. developed a compartmented combination of heterogeneous and enzyme catalysis to produce 2-keto-3-deoxygluconate from glucose, where gold-catalysis is used for sugar oxidation instead of the dehydrogenase [11]. Other ways to generate 2-keto-3-deoxygluconate chemically or by enzymatic means are shown by Matsubara et al. [12]. Using a cofactor free synthesis opens the possibility to design the following enzymatic reactions cofactor independently.
Gmelch et al. showed an enzymatic cascade to convert D-glucose via this sugar degradation product 2-keto-3-deoxygluconate to the amino acid L-alanine [9]. Currently, several millions of tons of amino acids are produced per year and used in various parts of our everyday life, for example as an additive in food, feed, cosmetics, and medicine [13,14]. Previously, Li et al. developed a different cascade reaction for the conversion of glycerol, where they showed how to create L-serine, among other products, from the intermediate glycerate [15].
The ingenious design of enzymatic cascades by combining naturally selected enzyme variants is thus a great strategy to develop production pathways for renewable resources. Nevertheless, the simple combination of enzymes in one-pot generally yields only low conversion rates and product titers [6]. To reach good product yields, different strategies for optimisation like varying the cascade parameters are crucial. A common method is to identify the reaction properties, depending on kinetic analyses and inhibitor- and pH-effects of the required enzymes [16]. Liu et al. described an experimental enzyme titration method, where a single enzyme is varied at a time to identify possible bottlenecks of the enzymatic reactions [17].
In this work, we designed a cell-free enzymatic cascade, starting with 2-keto-3-deoxygluconate and using of ammonium sulphate for the simultaneous production of L-alanine and L-serine. Hence, a sugar degradation product can be converted into value-added amino acids. Aside from the implementation of this cascade, we optimised the yields of L-alanine and L-serine. For this, the used enzymes were evaluated regarding their kinetic parameters and the specific activities depending on different buffers and pH values were measured. Furthermore, the cascade was tested in a titration study and based on this, an optimised form is shown.
2. Results
2.1. Cascade Design
The cofactor-balanced production of amino acids from renewable starting materials via enzymatic cascades has been described previously [9,15]. Starting from either D-glucose or glycerol completely cofactor neutral cascades yielded either L-alanine (breakdown of D-glucose) or L-serine (conversion of glycerol). Aiming at a bioeconomy future featuring biorefineries that allow the conversion of molecules of varying oxidation state, we envisioned a cascade reaction starting at 2-keto-3-deoxy-gluconate (KDG). KDG was previously produced by converting D-glucose to ethanol, but also using an NAD+-independent method, by coupling the Au-catalyzed oxidation of sugars by molecular oxygen to a dihydroxyacid dehydratase catalysed enzymatic dehydration step [8,11].
We further designed our cascade by cleaving KDG into pyruvate and D-glyceraldehyde by action of the 2-keto-3-deoxygluconate aldolase from Picrophilus torridus (PtKDGA). Pyruvate and ammonium are then converted to L-alanine by the reductive amination activity of the L-alanine dehydrogenase from Archaeoglobus fulgidus (AfAlaDH). The NADH demand for this reaction is covered by the simultaneous transformation of D-glyceraldehyde into D-glycerate, whereby NAD+ is reduced to NADH by the aldehyde dehydrogenase of Methanocaldococcus jannaschii (MjAlDH). Converting the produced D-glycerate to a second pyruvate molecule for subsequent reductive amination to L-alanine, described by Gmelch et al., was not possible in our case, as we started our reaction schema at KDG, which represents a singly oxidised sugar component [9]. We thus decided to choose another path to yield L-serine, which was previously described by Li et al. [15]. Accordingly, D-glycerate was first converted to hydroxypyruvate, catalysed by the glyoxylate reductase from Thermococcus litorialis (TlGR) and then further converted to L-serine in a two-step hydrogen borrowing reaction. The latter reaction was again catalysed by the AfAlaDH and required ammonium for the reductive activity required to produce L-serine (Figure 1).
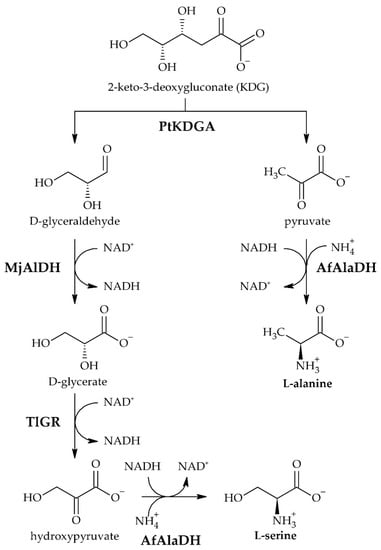
Figure 1.
Enzyme cascade reactions for the synthesis of L-alanine and L-serine out of the sugar degradation product KDG. Legend: KDG (2-keto-3-deoxygluconate), NAD+/NADH (Nicotinamide adenine nucleotide). Biocatalysts: PtKDGA (2-keto-3-desoxygluconate aldolase from Picrophilus torridus), MjAlDH (aldehyde dehydrogenase from Methanocaldococcus jannaschii), TlGR (glyoxylate reductase from Thermococcus litorialis), AfAlaDH (alanine dehydrogenase from Archaeoglobus fulgidus).
2.2. Kinetic Parameters of the Enzymes
Developing a multi-enzyme system requires a deep understanding of every single biotransformation to set up a concerted and viable reaction. To obtain feasible product yields from the beginning, we assessed each employed enzyme regarding its preferred temperature, pH-value and buffer. Using these assessments, we aimed for “best for all” reaction condition for the system. For this, the starting point of this work was evaluating the enzymes involved in the designed cascade reaction scheme by analysing the kinetic parameters of single enzyme catalysed reactions (Table 1).

Table 1.
Overview of the kinetic data of the used enzymes. Data are taken from the literature and were supplemented with own measurements [9,18].
PtKDGA is triggering the first cascade reaction with the highest substrate Km of all chosen enzymes of the cascade. Since it is the starting enzyme, PtKDGA works most of the time at saturating substrate concentrations and therefore shows reaction velocities near vmax. MjAlDH and AfAlaDH show a Km well below 1 mM for their substrates. The exact concentrations of the intermediate products are unknown during the reaction, but since for the most part the resulting product is used immediately as an educt by the subsequent enzyme, it can be assumed that the concentrations of the intermediates are low. Therefore, the low Km of the enzymes are advantageous for a smooth run of the cascade.
Compared to pyruvate, AfAlaDH has a significantly lower vmax for hydroxypyruvate conversion. This fact, in combination with the much longer and complex reaction path, leads to the assumption that the formation of L-serine will be slower than that of L-alanine.
For the TlGR, the equilibrium of its oxidoreductase reaction between hydroxypyruvate and D-glycerate lies almost completely on the D-glycerate side. Because of this, suitable kinetic and pH-dependency studies starting with D-glycerate as substrate were not possible.
Biocatalysts are not only highly selective natural reagents, but they are compatible with each other within specific ranges of operating conditions [5,6]. This allows for the integration of several biocatalytic transformations in one-pot cascades for the synthesis of complex molecules [19]. For the design of enzymatic networks in a one-pot system, the enzymes have to be matched up in terms of operating conditions, because the enzymatic activity is strongly influenced by the milieu that is provided by the buffer used [20]. Evaluating the effect of buffers and pH on the single enzymes, the specific activity for TRIS-HCl, MOPS, HEPES and KPi were investigated, depending on the pKs values of the different buffers [21]. It allowed us to cover a broad range of pH 6.5–8.5 with the four commonly used buffers.
For an exact comparison, the overall results for different buffers and pH values related to the specific enzyme-substrate activities are shown in Table 2. The activity is calculated relative to the highest activity shown.

Table 2.
Measured activity for the enzymes PtKDGA, MjAlDH and AfAlaDH dependent on different buffers and pH. The percentage depends on the maximum measured activity of each respective enzyme-substrate combination. 0–50% is shown in red, 51–75% in yellow and 76–100% in green. Exact reaction conditions and values for every enzyme can be seen in the Supplementary Figures S1–S4.
The PtKDGA displayed a general improvement of the specific activity with lower pH values. For example, the activity in the presence of HEPES buffer at pH 6.5 was 3.3 times higher than in HEPES buffer at pH 8.5. Furthermore, the best buffer at higher pH values seemed to be KPi, and at lower pH values KPi and HEPES showed the highest activity. The highest specific activity was reached in HEPES buffer pH 6.5. TRIS-HCl buffer led to the lowest specific activities of MjAlDH, probably because of the reaction of the substrate D-glyceraldehyde with TRIS-HCl [22]. HEPES and KPi seemed to be the best choice for higher pH values, while the maximal activity was achieved in HEPES buffer pH 8.0. As opposed to the other two enzymes, TRIS-HCl led to the highest specific activities of both substrates of AfAlaDH.
Contrary to PtKDGA, more basic pH values are better for the AfAlaDH, as previously described by Li et al. [15]. For hydroxypyruvate, the best buffer is TRIS-HCl, while for pyruvate, the buffer must be alkaline. Summing up, TRIS-HCL seemed to be the best buffer for the AfAlaDH. In contrast to that, it was the worst buffer for PtKDGA and MjAlDH. Concerning the different pH values, MjAlDH and AfAlaDH showed increased activity with higher pH values, while PtKDGA preferred lower pH values.
Every buffer-pH combination showed drawbacks for the cascade to some extent. Since PtKDGA occurs as the first enzyme in the bio-catalysed pathway, the enzyme is saturated with the substrate. Therefore, it realises its maximal specific activity and is less depending on steady-state concentrations. Since the PtKDGA also has one of the highest specific activities of the enzymes used, a reduction in the vmax for this enzyme is acceptable. Considering Table 2, KPi buffer pH 8.0 seems to be the best for the combination of all enzymes in one cascade, while it is the only combination where all enzymes show an activity higher than 50% of the maximum measured.
2.3. Enzyme Titration Study
In the above sections, the designed reaction cascade and the properties of individual enzymes were examined. Based on these results, KPi pH 8.0 was selected as a suitable buffer condition for the complete cascade. With this knowledge, it was possible to combine all enzymes into the planned cascade. For a first attempt, the enzymes were used with the same amount of activity determined under substrate saturation conditions. As shown in in Figure 2 (reference), L-alanine showed almost full conversion with 23.3 +/− 0.4 mM, while the yield of L-serine turned out much lower with 5.3 +/− 0.3 mM. Therefore, the next aim was to increase the yield of L-serine without lowering the L-alanine concentration.
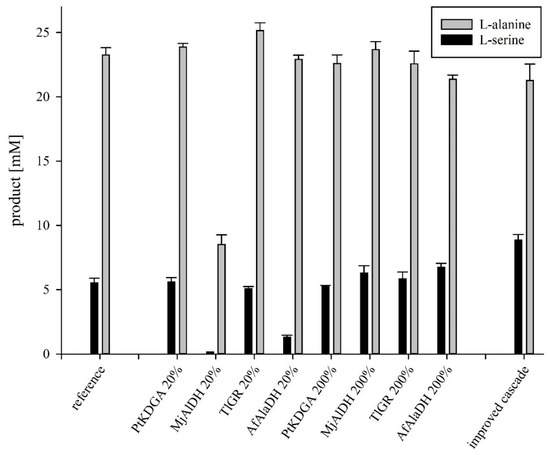
Figure 2.
Overview of differently composed cascade variants as well as the resulting, improved cascade. Reaction mixtures contained enzymes, 150 mM ammonium sulfate, 5 mM NAD+, 25 mM KDG and 100 mM KPi buffer pH 8.0 at 50 °C for 21 h. 0.5 U of each enzyme was used as a reference, based on the activity of the respective enzymes in the buffer KPi pH 8.0. As an approximation, the TIGR was quantified using the reverse reaction. Percentages relate to the enzyme specified in each case, based on the value of the reference. For the improved cascade, PtKDGA stayed at the value of the reference while the enzymes AfAlaDH, TlGR and MjAlDH were increased to 200%.
A well-matched stoichiometry of enzymes within the cascade is an essential part of cascade optimisation. Because of this, we conducted a titration study whereby the ratio of one applied biocatalyst was varied down to 20% (0.1 U) or up to 200% (1.0 U) and the concentrations of the others were maintained at 0.5 U.
All tested enzyme ratios, except one with a decreased MjAlDH amount, achieved a high L-alanine yield of more than 85%. Due to that independence of the variation of the enzymes to produce L-alanine, the L-serine yield was the primary focus for further analysis. Compared with the 1:1 ratio, reducing AfAlaDH or MjAlDH lead to a significant decrease in L-serine titers down to having no L-serine production. This indicates that both enzymes are limiting factors while lowering the amount of TlGR and PtKDGA did not have a significant effect on the yield and indicated that both are sufficiently incorporated. However, the individual increase of AfAlaDH or MjAlDH only leads to a modestly higher L-serine yield of around 120% of the reference. With this knowledge, buffers only promoting suboptimal activities for PtKDGA while showing similar activities for AfAlaDH and MjAlDH, e.g., HEPES pH 8.0, might perform equal to the phosphate buffer regarding product yields of the overall cascade. Nevertheless, as the activities of the limiting enzymes AfAlaDH and MjAlDH were not significantly higher in HEPES buffer, we decided to continue our yield optimisation approach without analysing the full cascade with other buffers.
For the improved cascade reaction, the applied units of MjAlDH, TlGR and AfAlaDH were thus increased by a factor of two up to 1.0 U, whereas the amount of PtKDGA was maintained at 0.5 U. MjAlDH and AfAlaDH were shown to be a bottleneck while the increasing of TlGR was performed since this is the central enzyme in the production of L-serine and we did not want to create a new weak point in this part of the cascade. Comparing the improved variant with the reverence variant shows an increased yield of L-serine by a factor of 1.6 from 5.5 +/− 0.3 mM up to 8.9 +/− 0.4 mM. The L-alanine yield differs only slightly with 23.3 +/− 0.4 mM before and 21.3 +/− 1.0 mM after the optimisation.
For this improved cascade variant, we also had a closer look at the product formation over time to get an overview of the speed of formation of the two products (Figure 3). Furthermore, this analysis could also give us hints for possible bottlenecks of the cascade. The increase of L-serine was almost linear during the observed period of 21 h, which shows that the enzymes are stable at 50 °C and catalyse their reactions at maximum speed under the developed steady-state conditions. As expected, the formation of L-alanine is faster and almost stoichiometric conversion was already reached after two hours with a yield of 98.0% +/− 1.3%. This also means, that PtKDGA had already transformed 25 mM KDG to pyruvate and D-glyceraldehyde by that time. This fits to the results of our titration study, where we could show that the influence of PtKDGA is low of the total yield after 21 h.
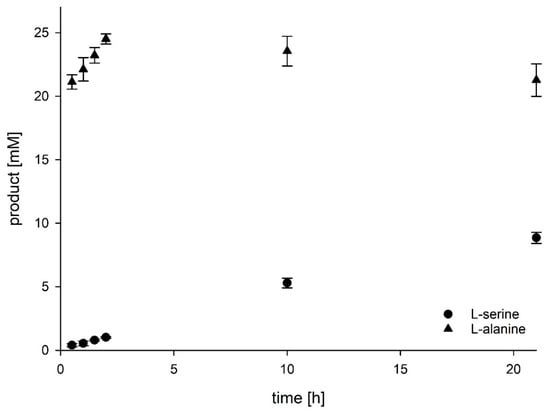
Figure 3.
Time-dependent formation of L-alanine and L-serine by the improved cascade reaction. Reaction mixtures contained enzymes, 150 mM ammonium sulfate, 5 mM NAD+, 25 mM KDG and 100 mM KPi buffer pH 8.0 at 50 °C. 1 U of AfAlaDH, TlGR and MjAlDH and 0.5 U of PtKDGA were used, based on the activity of the respective enzymes in the buffer KPi pH 8.0. As an approximation, the TIGR was quantified using the reverse reaction.
Furthermore, the conversion of D-glyceraldehyde into D-glycerate was also largely accomplished after 2 h, since this reaction delivers the reduced cofactor NADH, which is needed for the L-alanine formation. Afterwards, the detected L-alanine concentration decreased again. The equilibrium between hydroxypyruvate, pyruvate, L-alanine, and L-serine, which was described by Sallach, could have been responsible for that [23]. Hence, L-alanine might have reacted back to pyruvate to produce NADH, which is needed for the conversion of hydroxypyruvate to L-serine. Within the first two hours, this effect was not observed, what might be attributed to the low L-serine amount generated during that time. Due to the balance in the reaction of TlGR being strongly shifted to D-glycerate, it can also be assumed that almost no hydroxypyruvate existed in the reaction mixtures. Summing up, large amounts of D-glycerate and L-alanine might have existed in the reaction mixtures after 2 h. Following the conversion of D-glycerate, the cofactor dependent equilibrium, outlined above, was established, which accompanied a slight decrease in L-alanine yield.
3. Discussion
2-keto-3-deoxy-sugar acids are compounds of bacterial polysaccharides, lipopolysaccharides, and cell wall components. As a central intermediate in pathways like the Entner-Doudoroff, the Dahms or the Weimberg, they occur in all three types of cells, bacteria, archaea and eukarya [24,25,26]. For this reason, these sugar degradation products are interesting to use as source material for the green production of biofuels or bio-based chemicals. One example for these sugar acids is KDG, which can be generated out of glucose among others by chemo-enzymatic catalysis with a gold-catalyst and the enzyme SsDHAD [11].
Herein, we designed an in vitro biosystem for the biotransformation of KDG into value-added amino acids. KDG is cleaved by PtKDGA and in combination with ammonia can be converted into L-alanine and L-serine by an artificial enzymatic reaction cascade composed of MjAlDH, TlGR, and AfAlaDH. The designed pathway was consolidated to use only NADH as an electron carrier. Since the production of KDG can be cofactor free, our cascade is furthermore able to recycle the needed NAD+/NADH completely self-sufficient [11]. The use of thermostable enzymes enables an increased reaction temperature.
For an initial understanding of the performance of the planned enzymes, their kinetic data were determined. We found that most enzymes have an activity of greater 1 U/mg and a low substrate and cofactor Km of less than 1 mM. Also, AfAlaDH shows only a minor activity for hydroxypyruvate, which will slow down the last amination to L-serine.
The test of different buffers and pH values shows how different the activity of enzymes in different conditions can be, and how this especially crucial for the collaboration of several enzymes. Only one combination is possible to reach more of 50% of the maximal activity of every enzyme. While other combinations could be suitable with some cutbacks, others are impossible due to the inactivity of at least one of the used enzymes. Therefore, we finally decided on a buffer of KPi pH 8.0, which shows a good result for all enzymes tested.
Even without optimising the cascade, the L-alanine yield is close to full conversion. As expected, the production of L-serine is lower, mainly because of the unsuitable equilibrium of the TlGR for glycerate to hydroxypyruvate and the greater complexity of this part of the cascade. The used titration study was able to show that an increase of just one enzyme alone does not increase the L-serine yield significantly. However, lowering the amount of single enzymes led to a severe reduction of cascade product yield. The enzymes are dependent on each other via the cofactor dependence and increasing the amount of a single enzyme shows no effect while the NAD+/NADH or the sideproduct balance may be disturbed. Thus, on addition to AfAlaDH and MjAlDH, which proved to be limiting factors, the amount of TlGR was increased equally to produce more L-serine.
With our optimised cascade, we converted 25 mM KDG into 30.2 mM amino acid products in total after 21 h, 34.8% +/− 1.4% stoichiometric conversion for L-serine and 85.2% +/− 4.2% for L-alanine. Regarding L-alanine, Gmelch et al. could generate up to >95% of product out of 25 mM glucose with a precisely optimised enzyme ratio for their four-enzyme cascade [9]. We could reach full conversion of 25 mM KDG to L-alanine within just two hours with only two enzymes. In the cascade of Gmelch et al., KDG is likewise split into pyruvate and D-glyceraldehyde [9], the latter then being converted into a second L-alanine with the result of an additional conversion to L-alanine of 8% after 12 h. In comparison, in our designed way with L-serine we achieved a 2.5 times higher usage of the D-glyceraldehyde with 21.2% +/− 1.2% conversion after 10 h.
Li et al. designed an enzymatic cascade reaction for the conversion of glycerol to produce L-serine. They gained an L-serine yield of 71.3% within 18 h out of 10 mM substrate and three used enzymes [15]. In comparison to our approach with an L-serine yield of 34.8% +/− 1.4% from 25 mM KDG after 21 h, they applied much higher enzyme amounts with 7 U respectively 30 U for the comparable step from glycerate to L-serine instead of only 1 U for both enzymes and used a condensed pathway starting from glycerol [15]. Comparing the concentration of the product, we created 8.7 mM +/− 0.4 mM L-serine in 21 h, what is comparable to the yield of 7.13 mM in 18 h of Li et al. [15]. An advantage of our cascade is the steady rise of the L-serine concentration during the 21 h run, while at Li et al. the product increase slows down sharply after 9 h.
The yields and production rates of the system developed in this study still need to be improved to facilitate a possible industrial application. L-alanine yields up to 13.4 g/(lh) can be reached by transforming fossil resource-based fumarate with immobilised cells of E. coli and P. dacunhae, while the maximal rate of our enzymatic cascade for L-alanine is 1.1 g/(lh) [27]. Especially regarding the L-serine yield, our cascade needs to be improved. Here, we could gain a space-time yield of 57 mg/(lh), while fermentative processes, which are not used in industry now, can produce 270 mg/(lh) [28,29]. Improvements are envisioned by enzyme engineering and further reaction engineering. The slow formation of L-serine is partially due to the suboptimal conversion of glycerate by TIGR, which has glyoxylate as natural substrate. To support the reaction engineering an additional analytic procedure for the detection of the concentration of all intermediates without interference by present substance should be implemented. Hence, it would be possible to ascertain the reaction’s bottleneck precisely and gain more insight into the interactions between intermediates, cofactors, and enzymes. Thus, assumptions about the cofactor-depending balance and associated yield formation could be validated and specific enzymes engineering regarding Km or vmax can be applied to achieve an increased efficiency of the cascade that would enabled a preparative scale production. Summing up, a cascade reaction for amino acid production with high yield was implemented and optimised building a reasonable basis for further developments.
4. Materials and Methods
4.1. Chemicals and Enzymes
All chemicals were of analytical grade and purchased from Sigma-Aldrich (Saint Louis, MO, USA), Carl Roth GmbH (Karlsruhe, Germany), Serva Electrophoresis GmbH (Heidelberg, germany), Merck KGaA (Darmstadt, Germany) and Thermo Fisher Scientific GmbH (Darmstadt, Germany). 2-keto-3-deoxy D-gluconate (KDG) was synthesised using 200 mM D-gluconate and the dihydroxyacid dehydratase from Sulfolobus solfataricus (SsDHAD) as described previously [30]. Lactate dehydrogenase from the rabbit muscle was supplied by Carl Roth GmbH (Karlsruhe, Germany). The HPLC-method for determining amino acids was provided by Dr. Maisch GmbH (Ammerbuch-Entringen, Germany). AA-Standard was from Sigma Aldrich (Saint Louis, MO, USA).
4.2. Strains and Plasmids
The sequence of glyoxylate reductase from Thermococcus litorialis (EC 1.1.1.29, TlGR) was synthesised codon-optimised for E. coli from Thermo Fisher Scientific GENEART GmbH (Regensburg, Germany) and cloned into pET24a(+) vectors via NdeI/ XhoI restriction sites. The plasmids coding for 2-keto 3-deoxygluconate aldolase from Picrophilus torridus (EC 4.1.2.55, PtKDGA), aldehyde dehydrogenase from Methanocaldococcus jannaschii (EC 1.2.1.22, MjAlDH) and alanine dehydrogenase from Archaeoglobus fulgidus (EC 1.4.1.1, AfAlaDH) have been prepared previously in our lab [9].
4.3. Enzyme Expression
Enzyme expression was performed in E. coli BL21(DE3) as host strain in shaking flask cultures using ZYP-5052 autoinduction media [31], supplemented with 100 μg/mL kanamycin. After 1%-inoculation of the autoinduction media with an overnight grown culture of E. coli BL21(DE3) harbouring the corresponding plasmid, expressions were carried out overnight at 20 °C and 100 rpm. Cell pellets were harvest by centrifugation for 30 min at 4000× g and stored at −20 °C.
4.4. Enzyme Purification
Cells were resuspended in 100 mM HEPES buffer pH 7.5 and disrupted by ultrasonication on ice. After clarifying the lysates via centrifugation, the enzymes were loaded onto a His GraviTrap column (GE Healthcare, Freiburg, Germany) equilibrated with 100 mM Tris pH 7.5 containing 20 mM imidazole and 500 mM NaCl following the manufacturer’s recommendations. The elution buffer contained 500 mM imidazole and subsequently, the eluted proteins were desalted using a HiPrep 26/10 column (GE Healthcare, Freiburg, Germany) equilibrated with 100 mM HEPES pH 7.5. Purified proteins were flash-frozen in liquid nitrogen, stored at −80 °C and thawed freshly every day.
For changing the buffer of enzyme solutions, a PD-10 desalting hand-column (GE Healthcare, Freiburg, Germany)) was used. For higher volumetric activities, enzyme solutions were concentrated by using a VIVASPIN 10 kDa filter (Satorius AG, Göttingen, Germany) following the manufacturer’s recommendations.
Protein concentration was measured by a Bradford protein assay using the Roti-Nanoquant reagent (Carl Roth GmbH, Karlsruhe, Germany) according to the manufacturer’s recommendations with bovine serum albumin as standard.
4.5. Activity Assays
All enzymes were analysed photometrically following NADH consumption or production at 340 nm (εNADH = 6.22 mM−1·cm−1) with an Epoch 2 Microplate Spectrophotometer (BioTek GmbH, Sursee, Switzerland) at 50 °C. If not mentioned otherwise, reactions were carried out in triplicates using 96 well plates (Greiner Bio-OneTM PP 96-Well F-Bottom Microplates, Kremsmünster, Austria) containing a final volume of 200 μL.
Michaelis-Menten constants were determined for all enzymes by measuring twelve different substrate concentrations, plotting initial velocities vs. substrate concentration, and fitting the parameters with Sigma Plot (version 13.0, Systat Software GmbH, Erkrath, Germany). One unit (1 U) is defined as the amount of enzyme that consumes 1 μmol of substrate per minute. Determination of enzyme activity in different buffers of AfAlaDH contained 0.2 mM NADH, 150 mM ammonium sulfate, 5 mM hydroxypyruvate or 10 mM pyruvate with the respective buffer 100 mM and for MjAlDH 1 mM NAD+, 150 mM ammonium sulfate, 2 mM D-/L-glyceraldehyde with the respective buffer 100 mM. Reaction mixtures for determining the activity of PtKDGA containing 0.5 mM NADH, 150 mM ammonium sulfate, 2.5 mM KDG, 5 U LDH and the respective buffer 100 mM.
4.6. Cascade Reactions
Mixtures for initial cascade reactions were prepared to contain 5 mM NAD+, 25 mM KDG, 150 mM ammonium sulfate, 100 mM KPi buffer pH 8.0 at 50 °C and each of the enzymes PtKDGA, TlGR, MjAlDH, and AfAlaDH with 0.5 U, calculated by the buffer measurements. The reactions proceeded at 50 °C and 300 rpm for 21 h.
Enzyme titration studies and the improved cascade were performed identically, but different amounts of enzymes were added. Titration studies mixtures were prepared where the amount of one enzyme was either decreased to 0.1 U or increased to 1.0 U. For the improved variant, PtKDGA was maintained at 0.5 U, while the other enzymes were increased up to 1.0 U. The sampling was done after 0.5 h, 1 h, 1.5 h, 2 h, 10 h, and 21 h.
4.7. Determination of Amino Acids via HPLC
For analysis, the samples were filtrated with a 10 kDa centrifuge filter (10 kDa MWCO, modified PES, VWR, Germany) and the analysis was performed by HPLC (Ultimate-300 HPLC-system, Dionex Softron GmbH, Germaring, Germany). For the quantification, derivation with OPA/3-MPA reagent was used, following the manufacturer’s recommendations of Dr. Maisch GmbH (Ammerbuch-Entringen, Germany). As column, a GromSil OPA-1, 3 µM, 100 mm × 4.6 mm from Dr. Maisch GmbH (Ammerbuch-Entringen, Germany) was used. For the quantification, the standard containing the reaction matrix with 150 mM ammonium sulfate, 5 mM NAD+ and 100 mM KPi buffer pH 8.0. Samples were prepared by diluting them 1:25 and adding 0.2 parts borate buffer 1 M pH 10.7. The derivatisation was performed by automatic mixing of 3parts OPA/3-MPA reagent and 7 parts of the sample for 90 sec at room temperature. The system runs with 1.1 mL/min of a mixture of the two buffers NPi A (sodium phosphate buffer 12.5 mM pH 7.2 with 3% (v/v) acetonitrile) and NPi B (sodium phosphate buffer 12.5 mM pH 7.2 with 50% (v/v) acetonitrile), whereby the ration of buffer NPi B increased during the first 8 min from 0% to 20%, to 16 min up to 55%, and till 20 min with a decrease to 0%. The peaks were detected by absorption at 330 nm.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/11/1/31/s1, Figure S1: Specific activities of PtKDGA for the conversion of KDG to D-glyceraldehyde and pyruvate; Figure S2: Specific activities of MjAlDH for the conversion of D-glyceraldehyde to D-glycerate; Figure S3: Specific activities of AfAlaDH for the conversion of hydroxypyruvate to L-serine; Figure S4: Specific activities of AfAlaDH for the conversion of pyruvate to L-alanine.
Author Contributions
Conceptualisation, B.B. and J.S.; experimental work, A.H. and B.B.; data analysis, A.H. and B.B.; writing—original draft preparation, B.B.; writing—review and editing, J.S.; supervision, J.S and V.S. All authors have read and agreed to the published version of the manuscript.
Funding
Supported by Deutsche Forschungsgemeinschaft (DFG) through TUM International Graduate School of Science and Engineering (IGSSE), GSC 81. Publishing was supported by the Technical University of Munich within the founding program Open Access Publishing.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank Alexander Benson for critical reading of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AA | Amino acids |
| AfAlaDH | alanine dehydrogenase from Archaeoglobus fulgidus |
| SsDHAD | dihydroxyacid dehydratase from Sulfolobus solfataricus |
| E. coli | Escherichia coli |
| KDG | 2-keto-3-deoxygluconate |
| KPi | Potassium phosphate buffer |
| LDH | Lactate dehydrogenase |
| MjAlDH | aldehyde dehydrogenase from Methanocaldococcus jannaschii |
| NAD+, NADH | Nicotinamide adenine dinucleotide |
| NPi | Sodium phosphate buffer |
| OPA/3-MPA | o-phthaldialdehyde /3-mercaptopropionic acid |
| PtKDGA | 2-keto-3-desoxygluconate from Picrophilus torridus |
| TlGR | glyoxylate reductase from Thermococcus litorialis |
| U | Enzyme unit |
| +/− | Indicates the standard deviation of the determined values |
References
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998; ISBN 0198502346. [Google Scholar]
- France, S.P.; Hepworth, L.J.; Turner, N.J.; Flitsch, S.L. Constructing Biocatalytic Cascades: In Vitro and in Vivo Approaches to de Novo Multi-Enzyme Pathways. ACS Catal. 2017, 7, 710–724. [Google Scholar] [CrossRef]
- Hodgman, C.E.; Jewett, M.C. Cell-free synthetic biology: Thinking outside the cell. Metab. Eng. 2012, 14, 261–269. [Google Scholar] [CrossRef]
- Ricca, E.; Brucher, B.; Schrittwieser, J.H. Multi-Enzymatic Cascade Reactions: Overview and Perspectives. Advanced Synthesis & Catalysis. Adv. Synth. Catal. 2011, 353, 2239–2262. [Google Scholar] [CrossRef]
- Schrittwieser, J.H.; Velikogne, S.; Hall, M.; Kroutil, W. Artificial Biocatalytic Linear Cascades for Preparation of Organic Molecules. Chem. Rev. 2018, 118, 270–348. [Google Scholar] [CrossRef]
- Sperl, J.M.; Sieber, V. Multienzyme Cascade Reactions—Status and Recent Advances. ACS Catal. 2018, 8, 2385–2396. [Google Scholar] [CrossRef]
- You, C.; Percival Zhang, Y.-H. Biomanufacturing by in vitro biosystems containing complex enzyme mixtures. Process Biochem. 2017, 52, 106–114. [Google Scholar] [CrossRef]
- Guterl, J.-K.; Garbe, D.; Carsten, J.; Steffler, F.; Sommer, B.; Reiße, S.; Philipp, A.; Haack, M.; Rühmann, B.; Koltermann, A.; et al. Cell-free metabolic engineering: Production of chemicals by minimized reaction cascades. ChemSusChem 2012, 5, 2165–2172. [Google Scholar] [CrossRef]
- Gmelch, T.J.; Sperl, J.M.; Sieber, V. Optimization of a reduced enzymatic reaction cascade for the production of L-alanine. Sci. Rep. 2019, 9, 11754. [Google Scholar] [CrossRef]
- Sutiono, S.; Teshima, M.; Beer, B.; Schenk, G.; Sieber, V. Enabling the Direct Enzymatic Dehydration of d -Glycerate to Pyruvate as the Key Step in Synthetic Enzyme Cascades Used in the Cell-Free Production of Fine Chemicals. ACS Catal. 2020, 10, 3110–3118. [Google Scholar] [CrossRef]
- Sperl, J.M.; Carsten, J.M.; Guterl, J.-K.; Lommes, P.; Sieber, V. Reaction Design for the Compartmented Combination of Heterogeneous and Enzyme Catalysis. ACS Catal. 2016, 6, 6329–6334. [Google Scholar] [CrossRef]
- Matsubara, K.; Köhling, R.; Schönenberger, B.; Kouril, T.; Esser, D.; Bräsen, C.; Siebers, B.; Wohlgemuth, R. One-step synthesis of 2-keto-3-deoxy-d-gluconate by biocatalytic dehydration of d-gluconate. J. Biotechnol. 2014, 191, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Hermann, T. Industrial production of amino acids by coryneform bacteria. J. Biotechnol. 2003, 104, 155–172. [Google Scholar] [CrossRef]
- Izumi, Y.; Chibata, I.; Itoh, T. Herstellung und Verwendung von Aminosäuren. Angew. Chem. 1978, 90, 187–194. [Google Scholar] [CrossRef]
- Li, Z.; Yan, J.; Sun, J.; Xu, P.; Ma, C.; Gao, C. Production of value-added chemicals from glycerol using in vitro enzymatic cascades. Commun. Chem. 2018, 1, 1–7. [Google Scholar] [CrossRef]
- Yu, X.; Liu, T.; Zhu, F.; Khosla, C. In vitro reconstitution and steady-state analysis of the fatty acid synthase from Escherichia coli. Proc. Natl. Acad. Sci. USA 2011, 108, 18643–18648. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Y.; Jia, X.; Hu, M.; Deng, Z.; Xu, Y.; Liu, T. In Vitro Reconstitution and Optimization of the Entire Pathway to Convert Glucose into Fatty Acid. ACS Synth. Biol. 2017, 6, 701–709. [Google Scholar] [CrossRef]
- Ohshima, T.; Nunoura-Kominato, N.; Kudome, T.; Sakuraba, H. A novel hyperthermophilic archaeal glyoxylate reductase from Thermococcus litoralis. Characterization, gene cloning, nucleotide sequence and expression in Escherichia coli. Eur. J. Biochem. 2001, 268, 4740–4747. [Google Scholar] [CrossRef]
- Claaßen, C.; Gerlach, T.; Rother, D. Stimulus-Responsive Regulation of Enzyme Activity for One-Step and Multi-Step Syntheses. Adv. Synth. Catal. 2019, 361, 2387–2401. [Google Scholar] [CrossRef]
- Lorillière, M.; Guérard-Hélaine, C.; Gefflaut, T.; Fessner, W.-D.; Clapés, P.; Charmantray, F.; Hecquet, L. Convergent in situ Generation of Both Transketolase Substrates via Transaminase and Aldolase Reactions for Sequential One-Pot, Three-Step Cascade Synthesis of Ketoses. ChemCatChem 2020, 12, 812–817. [Google Scholar] [CrossRef]
- Dawson, R.M.C. Data for Biochemical Research, 3rd ed.; Clarendon Press: Oxford, UK, 1993; ISBN 0198553587. [Google Scholar]
- Bubb, W.A.; Berthon, H.A.; Kuchel, P.W. Tris Buffer Reactivity with Low-Molecular-Weight Aldehydes: NMR Characterization of the Reactions of Glyceraldehyde-3-Phosphate. Biorgan. Chem. 1995, 23, 119–130. [Google Scholar] [CrossRef]
- Sallach, H.J. Formation of serine from hydroxypyruvate and L-alanine. J. Biol. Chem. 1956, 223, 1101–1108. [Google Scholar] [PubMed]
- Entner, N.; Doudoroff, M. Glucose and gluconic acid oxidation of Pseudomonas saccharophila. J. Biol. Chem. 1952, 196, 853–862. [Google Scholar]
- Weimberg, R.; Doudoroff, M. The oxidation of L-arabinose by Pseudomonas saccharophila. J. Biol. Chem. 1955, 217, 607–624. [Google Scholar] [PubMed]
- Dahms, A.; Anderson, R.L. 2-Keto-3-deoxy-L-arabonate aldolase and its role in a new pathway of L-arabinose degradation. Biochem. Biophys. Res. Commun. 1969, 36, 809–814. [Google Scholar] [CrossRef]
- Takamatsu, S.; Umemura, I.; Yamamoto, K.; Sato, T.; Tosa, T.; Chibata, I. Production of l-alanine from ammonium fumarate using two immobilized microorganisms. Appl. Microbiol. Biotechnol. 1982, 15, 147–152. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, X.; Luo, Y.; Guo, W.; Xu, G.; Shi, J.; Xu, Z. L-Serine overproduction with minimization of by-product synthesis by engineered Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2015, 99, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, G.; Shi, J.; Koffas, M.A.G.; Xu, Z. Microbial Production of l-Serine from Renewable Feedstocks. Trends Biotechnol. 2018, 36, 700–712. [Google Scholar] [CrossRef]
- Carsten, J.M.; Schmidt, A.; Sieber, V. Characterization of recombinantly expressed dihydroxy-acid dehydratase from Sulfobus solfataricus-A key enzyme for the conversion of carbohydrates into chemicals. J. Biotechnol. 2015, 211, 31–41. [Google Scholar] [CrossRef]
- Studier, F.W. Protein production by auto-induction in high density shaking cultures. Protein Expr. Purif. 2005, 41, 207–234. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).