A DFT Investigation on the Origins of Solvent-Dependent Polysulfide Reduction Mechanism in Rechargeable Li-S Batteries
Abstract
1. Introduction
2. Results and Discussions
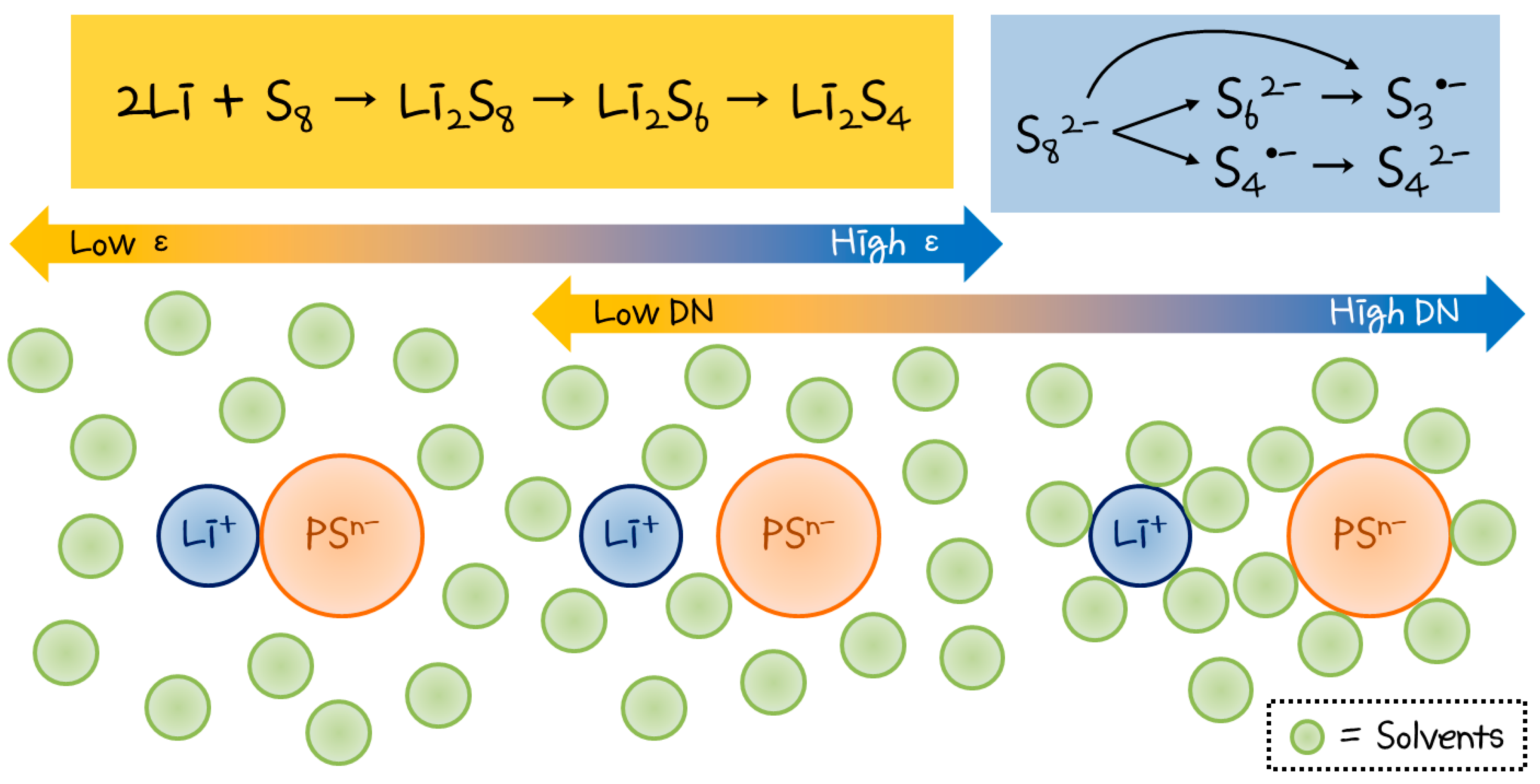
2.1. Solvent-Dependent PS Reaction Pathways
2.2. The Effect of Solvent Donor Number (DN)
3. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yin, Y.-X.; Xin, S.; Guo, Y.-G.; Wan, L.-J. Lithium–sulfur batteries: Electrochemistry, materials, and prospects. Angew. Chem. Int. Ed. 2013, 52, 13186–13200. [Google Scholar] [CrossRef] [PubMed]
- Ould Ely, T.; Kamzabek, D.; Chakraborty, D.; Doherty, M.F. Lithium-sulfur batteries: State of the art and future directions. ACS Appl. Energy Mater. 2018, 1, 1783–1814. [Google Scholar] [CrossRef]
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Deng, N.; Ju, J.; Li, Q.; Wu, D.; Ma, X.; Li, L.; Naebe, M.; Cheng, B. A review of recent developments in rechargeable lithium–sulfur batteries. Nanoscale 2016, 8, 16541–16588. [Google Scholar] [CrossRef] [PubMed]
- Wild, M.; O’Neill, L.; Zhang, T.; Purkayastha, R.; Minton, G.; Marinescu, M.; Offer, G.J. Lithium sulfur batteries, a mechanistic review. Energy Environ. Sci. 2015, 8, 3477–3494. [Google Scholar] [CrossRef]
- Eroglu, D.; Zavadil, K.R.; Gallagher, K.G. Critical link between materials chemistry and cell-level design for high energy density and low cost lithium-sulfur transportation battery. J. Electrochem. Soc. 2015, 162, A982. [Google Scholar] [CrossRef]
- Hagen, M.; Hanselmann, D.; Ahlbrecht, K.; Maca, R.; Gerber, D.; Tubke, J. Lithium-sulfur cells: The gap between the state-of-the-art and the requirements for high energy battery cells. Adv. Energy Mater. 2015, 5, 1401986. [Google Scholar] [CrossRef]
- Salama, M.; Rosy Attias, R.; Yemini, R.; Gofer, Y.; Aurbach, D.; Noked, M. Metal–sulfur batteries: Overview and research methods. ACS Energy Lett. 2019, 4, 436–446. [Google Scholar] [CrossRef]
- Fang, R.; Zhao, S.; Sun, Z.; Wang, D.-W.; Cheng, H.-M.; Li, F. More reliable lithium-sulfur batteries: Status, solutions and prospects. Adv. Mater. 2017, 29, 1606823. [Google Scholar] [CrossRef]
- Wang, H.; Sa, N.; He, M.; Liang, X.; Nazar, L.F.; Balasubramanian, M.; Gallagher, K.G.; Key, B. In situ NMR observation of the temporal speciation of lithium sulfur batteries during electrochemical cycling. J. Phys. Chem. C 2017, 121, 6011–6017. [Google Scholar] [CrossRef]
- Busche, M.R.; Adelhelm, P.; Sommer, H.; Schneider, H.; Leitner, K.; Janek, J. Systematical electrochemical study on the parasitic shuttle-effect in lithium-sulfur-cells at different temperatures and different rates. J. Power Sources 2014, 259, 289–299. [Google Scholar] [CrossRef]
- Xu, N.; Qian, T.; Liu, X.; Liu, J.; Chen, Y.; Yan, C. Greatly suppressed shuttle effect for improved lithium sulfur battery performance through short chain intermediates. Nano Lett. 2017, 17, 538–543. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Y.; Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 2017, 12, 194. [Google Scholar] [PubMed]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.-M. Li–O2 and Li–S batteries with high energy storage. Nat. Mater. 2012, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Carbone, L.; Coneglian, T.; Gobet, M.; Munoz, S.; Devany, M.; Greenbaum, S.; Hassoun, J. A simple approach for making a viable, safe, and high-performances lithium-sulfur battery. J. Power Sources 2018, 377, 26–35. [Google Scholar] [CrossRef]
- Angulakshmi, N.; Stephan, A.M. Efficient electrolytes for lithium–sulfur batteries. Front. Energy Res. 2015, 3, 17. [Google Scholar] [CrossRef]
- Younesi, R.; Veith, G.M.; Johansson, P.; Edstrom, K.; Vegge, T. Lithium salts for advanced lithium batteries: Li–metal, Li–O 2, and Li–S. Energy Environ. Sci. 2015, 8, 1905–1922. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, W.; Fan, H.; Cheng, F.; Su, D.; Wang, G. Promoting lithium polysulfide/sulfide redox kinetics by the catalyzing of zinc sulfide for high performance lithium-sulfur battery. Nano Energy 2018, 51, 73–82. [Google Scholar] [CrossRef]
- Li, X.; Cao, Y.; Qi, W.; Saraf, L.V.; Xiao, J.; Nie, Z.; Mietek, J.; Zhang, J.-G.; Stchwenzer, B.; Liu, J. Optimization of mesoporous carbon structures for lithium–sulfur battery applications. J. Mater. Chem. 2011, 21, 16603–16610. [Google Scholar] [CrossRef]
- Ghosh, A.; Garapati, M.S.; Saroja, A.P.V.K.; Sundara, R. Polar Bilayer Cathode for Advanced Lithium–Sulfur Battery: Synergy Between Polysulfide Conversion and Confinement. J. Phys. Chem. C 2019, 123, 10777–10787. [Google Scholar] [CrossRef]
- Fang, X.; Peng, H. A revolution in electrodes: Recent progress in rechargeable lithium–sulfur batteries. Small 2015, 11, 1488–1511. [Google Scholar] [CrossRef] [PubMed]
- Paolella, A.; Demers, H.; Chevallier, P.; Gagnon, C.; Girard, G.; Delaporte, N.; Zhu, W.; Vigh, A.; Guerfi, A.; Zaghib, K. A platinum nanolayer on lithium metal as an interfacial barrier to shuttle effect in Li-S batteries. J. Power Sources 2019, 427, 201–206. [Google Scholar] [CrossRef]
- Paolella, A.; Laul, D.; Timoshevskii, V.; Zhu, W.; Marras, S.; Bertoni, G.; Wahba, A.S.; Girard, G.; Gagnon, C.; Rodrigue, L.; et al. The role of metal disulfide interlayer in Li–S batteries. J. Phys. Chem. C 2018, 122, 1014–1023. [Google Scholar] [CrossRef]
- Liu, J.; HYuan, L.; Yuan, K.; Li, Z.; Hao, Z.; Xiang, J.; Huang, Y. SnO2 as a high-efficiency polysulfide trap in lithium–sulfur batteries. Nanoscale 2016, 8, 13638–13645. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Ye, Y.; Chen, R.; Qian, J.; Zhao, T.; Li, L.; Li, W. Systematic effect for an ultralong cycle lithium–sulfur battery. Nano Lett. 2015, 15, 7431–7439. [Google Scholar] [CrossRef] [PubMed]
- Lochala, J.; Liu, D.; Wu, B.; Robinson, C.; Xiao, J. Research progress toward the practical applications of lithium–sulfur batteries. ACS Appl. Mater. Inter. 2017, 9, 24407–24421. [Google Scholar] [CrossRef]
- Chen, X.; Hou, T.; Persson, K.A.; Zhang, Q. Combining theory and experiment in lithium–sulfur batteries: Current progress and future perspectives. Mater. Today 2019, 22, 142–158. [Google Scholar] [CrossRef]
- Park, C.; Ronneburg, A.; Risse, S.; Ballauff, M.; Kanduc, M.; Dzubiella, J. Structural and Transport Properties of Li/S Battery Electrolytes: Role of the Polysulfide Species. J. Phys. Chem. C 2019, 123, 10167–10177. [Google Scholar] [CrossRef]
- Kamphaus, E.P.; Balbuena, P.B. Long-chain polysulfide retention at the cathode of Li–S batteries. J. Phys. Chem. C 2016, 120, 4296–4305. [Google Scholar] [CrossRef]
- Park, C.; Kanduc, M.; Chudoba, R.; Ronneburg, A.; Risse, S.; Ballauff, M.; Dzubiella, J. Molecular simulations of electrolyte structure and dynamics in lithium–sulfur battery solvents. J. Power Sources 2018, 373, 70–78. [Google Scholar] [CrossRef]
- Camacho-Forero, L.E.; Smith, T.W.; Bertolini, S.; Balbuena, P.B. Reactivity at the lithium–metal anode surface of lithium–sulfur batteries. J. Phys. Chem. C 2015, 119, 26828–26839. [Google Scholar] [CrossRef]
- Liu, Z.; Bertolini, S.; Balbuena, P.B.; Mukherjee, P.P. Li2S film formation on lithium anode surface of Li–S batteries. ACS Appl. Mater. Interfaces 2016, 8, 4700–4708. [Google Scholar] [CrossRef] [PubMed]
- Pascal, T.A.; Wujcik, K.H.; Velasco-Velez, J.; Wu, C.; Teran, A.A.; Kapilashrami, M.; Cabana, J.; Guo, J.; Salmeron, M.; Balsara, N.; et al. X-ray absorption spectra of dissolved polysulfides in lithium–sulfur batteries from first-principles. J. Phys. Chem. Lett. 2014, 5, 1547–1551. [Google Scholar] [CrossRef] [PubMed]
- Kawase, A.; Shirai, S.; Yamoto, Y.; Arakawa, R.; Takata, T. Electrochemical reactions of lithium–sulfur batteries: An analytical study using the organic conversion technique. Phys. Chem. Chem. Phys. 2014, 16, 9344–9350. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-C.; He, Q.; Gasteiger, H.A. Probing the lithium–sulfur redox reactions: A rotating-ring disk electrode study. J. Phys. Chem. C 2014, 118, 5733–5741. [Google Scholar] [CrossRef]
- Assary, R.S.; Curtiss, L.A.; Moore, J.S. Toward a molecular understanding of energetics in Li–S batteries using nonaqueous electrolytes: A high-level quantum chemical study. J. Phys. Chem. C 2014, 118, 11545–11558. [Google Scholar] [CrossRef]
- See, K.A.; Wu, H.-L.; Lau, K.C.; Shin, M.; Cheng, L.; Balasubramanian, M.; Gallagher, K.G.; Curtiss, L.A.; Gewirth, A.A. Effect of hydrofluoroether cosolvent addition on Li solvation in acetonitrile-based solvate electrolytes and its influence on S reduction in a Li–S battery. ACS Appl. Mater. Interfaces 2016, 8, 34360–34371. [Google Scholar] [CrossRef]
- Zou, Q.; Lu, Y.-C. Solvent-dictated lithium sulfur redox reactions: An operando UV–vis spectroscopic study. J Phys. Chem. Lett. 2016, 7, 1518–1525. [Google Scholar] [CrossRef]
- Pascal, T.A.; Wujcik, K.H.; Wang, D.R.; Balsara, N.P.; Prendergast, D. Thermodynamic origins of the solvent-dependent stability of lithium polysulfides from first principles. Phys. Chem. Chem. Phys. 2017, 19, 1441–1448. [Google Scholar] [CrossRef]
- Wu, H.-L.; Huff, L.A.; Gewirth, A.A. In situ Raman spectroscopy of sulfur speciation in lithium–sulfur batteries. ACS Appl. Mater. Interfaces 2015, 7, 1709–1719. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Govind, N.; Walter, E.; Burton, S.D.; Shukla, A.; Devaraj, A.; Xiao, J.; Liu, J.; Wang, C.; Karim, A.; et al. Molecular structure and stability of dissolved lithium polysulfide species. Phys. Chem. Chem. Phys. 2014, 16, 10923–10932. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Curtiss, L.A.; Zavadil, K.R.; Gewirth, A.A.; Shao, Y.; Gallagher, K.G. Sparingly solvating electrolytes for high energy density lithium–sulfur batteries. ACS Energy Lett. 2016, 1, 503–509. [Google Scholar] [CrossRef]
- Wujcik, K.H.; Wang, D.R.; Raghunathan, A.; Drake, M.; Pascal, T.A.; Prendergast, D.; Balsara, N.P. Lithium polysulfide radical anions in ether-based solvents. J. Phys. Chem. C 2016, 120, 18403–18410. [Google Scholar] [CrossRef]
- Steudel, R.; Chivers, T. The role of polysulfide dianions and radical anions in the chemical, physical and biological sciences, including sulfur-based batteries. Chem. Soc. Rev. 2019, 48, 3279–3319. [Google Scholar] [CrossRef] [PubMed]
- Cuisinier, M.; Hart, C.; Balasubramanian, M.; Garsuch, A.; Nazar, L.F. Radical or not radical: Revisiting lithium–sulfur electrochemistry in nonaqueous electrolytes. Adv. Energy Mater. 2015, 5, 1401801. [Google Scholar] [CrossRef]
- Zou, Q.; Liang, Z.; Du, G.-Y.; Liu, C.-Y.; Li, E.Y.; Lu, Y.-C. Cation-directed selective polysulfide stabilization in alkali metal–sulfur batteries. J. Am. Chem. Soc. 2018, 140, 10740–10748. [Google Scholar] [CrossRef]
- Li, G.; Li, Z.; Zhang, B.; Lin, Z. Developments of electrolyte systems for lithium–sulfur batteries: A review. Front. Energy Res. 2015, 3, 5. [Google Scholar] [CrossRef]
- Wujcik, K.H.; Pascal, T.A.; Pemmaraju, C.D.; Devaux, D.; Stolte, W.C.; Balsara, N.P.; Prendergast, D. Characterization of Polysulfide Radicals Present in an Ether-Based Electrolyte of a Lithium–Sulfur Battery During Initial Discharge Using In Situ X-Ray Absorption Spectroscopy Experiments and First-Principles Calculations. Adv. Energy Mater 2015, 5, 1500285. [Google Scholar] [CrossRef]
- Wang, B.; Alhassan, S.M.; Pantelides, S.T. Formation of large polysulfide complexes during the lithium-sulfur battery discharge. Phys. Rev. Appl. 2014, 2, 034004. [Google Scholar] [CrossRef]
- Xin, S.; Gu, L.; Zhao, N.-H.; Yin, Y.-X.; Zhou, L.-J.; Guo, Y.-G.; Wan, L.-J. Smaller sulfur molecules promise better lithium–sulfur batteries. J. Am. Chem. Soc. 2012, 134, 18510–18513. [Google Scholar] [CrossRef]
- Steudel, R.; Steudel, Y. Polysulfide Chemistry in Sodium-Sulfur Batteries and Related Systems–A Computational Study by G3X (MP2) and PCM Calculations. Chem.: Eur. J. 2013, 19, 3162–3176. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.U.M.; Dominko, R. Application of in operando UV/vis spectroscopy in lithium–sulfur batteries. ChemSusChem 2014, 7, 2167–2175. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.U.M.; Demir-Cakan, R.; Morcrette, M.; Tarascon, J.-M.; Gaberscek, M.; Dominko, R. Li-S Battery Analyzed by UV/Vis in Operando Mode. ChemSusChem 2013, 6, 1177–1181. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, W.R. Acidity and basicity scales for polar solvents. J. Phys. Chem. 1993, 97, 9540–9546. [Google Scholar] [CrossRef]
- He, Q.; Gorlin, Y.; Patel, M.U.; Gasteiger, H.A.; Lu, Y.-C. Unraveling the correlation between solvent properties and sulfur redox behavior in lithium-sulfur batteries. J. Electrochem. Soc. 2018, 165, A4027. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Peverati, R.; Truhlar, D.G. Improving the accuracy of hybrid meta-GGA density functionals by range separation. J. Phys. Chem. Lett. 2011, 2, 2810–2817. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Sriana, T.; Leggesse, E.G.; Jiang, J.C. Novel benzimidazole salts for lithium ion battery electrolytes: Effects of substituents. Phys. Chem. Chem. Phys. 2015, 17, 16462–16468. [Google Scholar] [CrossRef]
- Marenich, A.V.; Ho, J.; Coote, M.L.; Cramer, C.J.; Truhlar, D.G. Computational electrochemistry: Prediction of liquid-phase reduction potentials. Phys. Chem. Chem. Phys. 2014, 16, 15068–15106. [Google Scholar] [CrossRef]
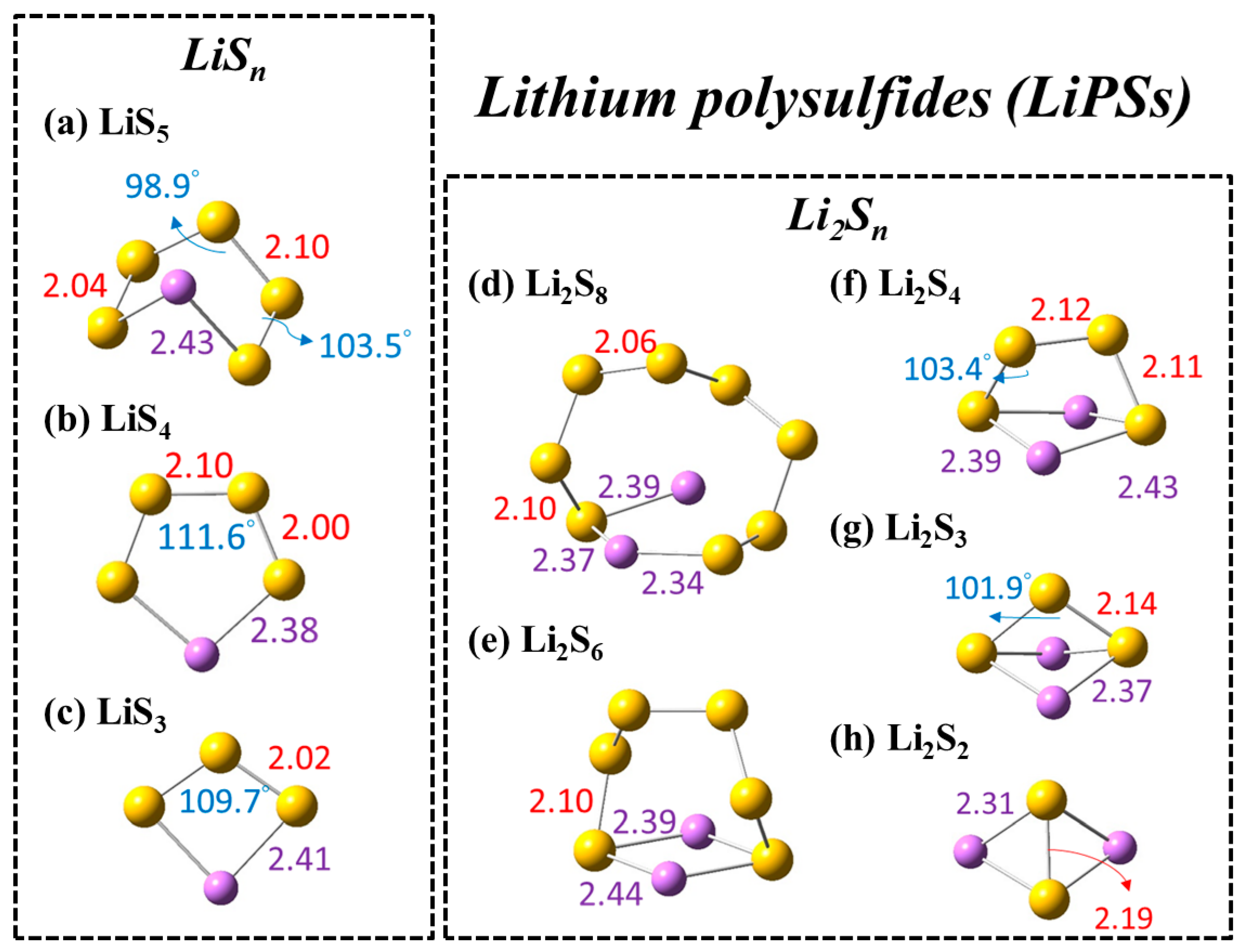
| # | Reactions | DIOX (2.21) | THF (7.43) | Acetone (20.49) | ACN (35.69) | DMSO (46.83) |
|---|---|---|---|---|---|---|
| 1 | S82−→S62− + S2 | 0.47 | 0.38 | 0.31 | 0.32 | 0.32 |
| 2 | S82-→S5− + S3− | −0.87 | −0.08 | 0.16 | 0.23 | 0.26 |
| 3 | S82−→S42− + S4 | 1.63 | 1.25 | 1.12 | 1.11 | 1.11 |
| 4 | S82−→2S4− | −0.76 | 0.04 | 0.23 | 0.29 | 0.31 |
| 5 | S62−→2S3− | −1.30 | −0.45 | −0.19 | −0.12 | −0.10 |
| 6 | S62−→S42− + S2 | 0.98 | 0.70 | 0.63 | 0.62 | 0.62 |
| 7 | 2S42−→2S3− + S22− | −0.27 | 0.39 | 0.60 | 0.66 | 0.69 |
| 8 | 2S42−→S62− + S22− | 1.03 | 0.84 | 0.80 | 0.78 | 0.78 |
| # | Reactions | DIOX (2.21) | THF (7.43) | Acetone (20.49) | ACN (35.69) | DMSO (46.83) |
|---|---|---|---|---|---|---|
| 9 | Li2S8→Li2S6 + S2 | 0.20 | 0.19 | 0.17 | 0.18 | 0.19 |
| 10 | Li2S8→LiS5 + LiS3 | 1.13 | 0.95 | 0.85 | 0.80 | 0.91 |
| 11 | Li2S8→Li2S4 + S4 | 0.85 | 0.81 | 0.79 | 0.79 | 0.81 |
| 12 | Li2S8→2LiS4 | 1.15 | 0.97 | 0.89 | 0.87 | 0.87 |
| 13 | Li2S6→2LiS3 | 0.88 | 0.67 | 0.57 | 0.48 | 0.70 |
| 14 | Li2S6→Li2S4 + S2 | 0.47 | 0.45 | 0.44 | 0.45 | 0.46 |
| 15 | 2Li2S4→2LiS3 + Li2S2 | 2.11 | 1.71 | 1.37 | 1.21 | 1.12 |
| 16 | 2Li2S4→Li2S6 + Li2S2 | 0.97 | 0.82 | 0.70 | 0.65 | 0.64 |
| Solvent | DN | ε | np | ||||
|---|---|---|---|---|---|---|---|
| n = 1 | n = 2 | n = 3 | n = 4 | ||||
| ACN | 14.1 | 35.69 | 2 | −0.40 | −0.35 | −0.29 | −0.22 |
| Acetone | 17.0 | 20.49 | 3 | −0.51 | −0.45 | −0.31 | −0.25 |
| DMSO | 29.8 | 46.83 | 4 | −0.77 | −0.67 | −0.52 | −0.40 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, G.-Y.; Liu, C.-Y.; Li, E.Y. A DFT Investigation on the Origins of Solvent-Dependent Polysulfide Reduction Mechanism in Rechargeable Li-S Batteries. Catalysts 2020, 10, 911. https://doi.org/10.3390/catal10080911
Du G-Y, Liu C-Y, Li EY. A DFT Investigation on the Origins of Solvent-Dependent Polysulfide Reduction Mechanism in Rechargeable Li-S Batteries. Catalysts. 2020; 10(8):911. https://doi.org/10.3390/catal10080911
Chicago/Turabian StyleDu, Guan-Ying, Chi-You Liu, and Elise Y. Li. 2020. "A DFT Investigation on the Origins of Solvent-Dependent Polysulfide Reduction Mechanism in Rechargeable Li-S Batteries" Catalysts 10, no. 8: 911. https://doi.org/10.3390/catal10080911
APA StyleDu, G.-Y., Liu, C.-Y., & Li, E. Y. (2020). A DFT Investigation on the Origins of Solvent-Dependent Polysulfide Reduction Mechanism in Rechargeable Li-S Batteries. Catalysts, 10(8), 911. https://doi.org/10.3390/catal10080911






