Ordered Mesoporous Carbon as a Support of Pd Catalysts for CO2 Electrochemical Reduction
Abstract
1. Introduction
2. Results and Discussion
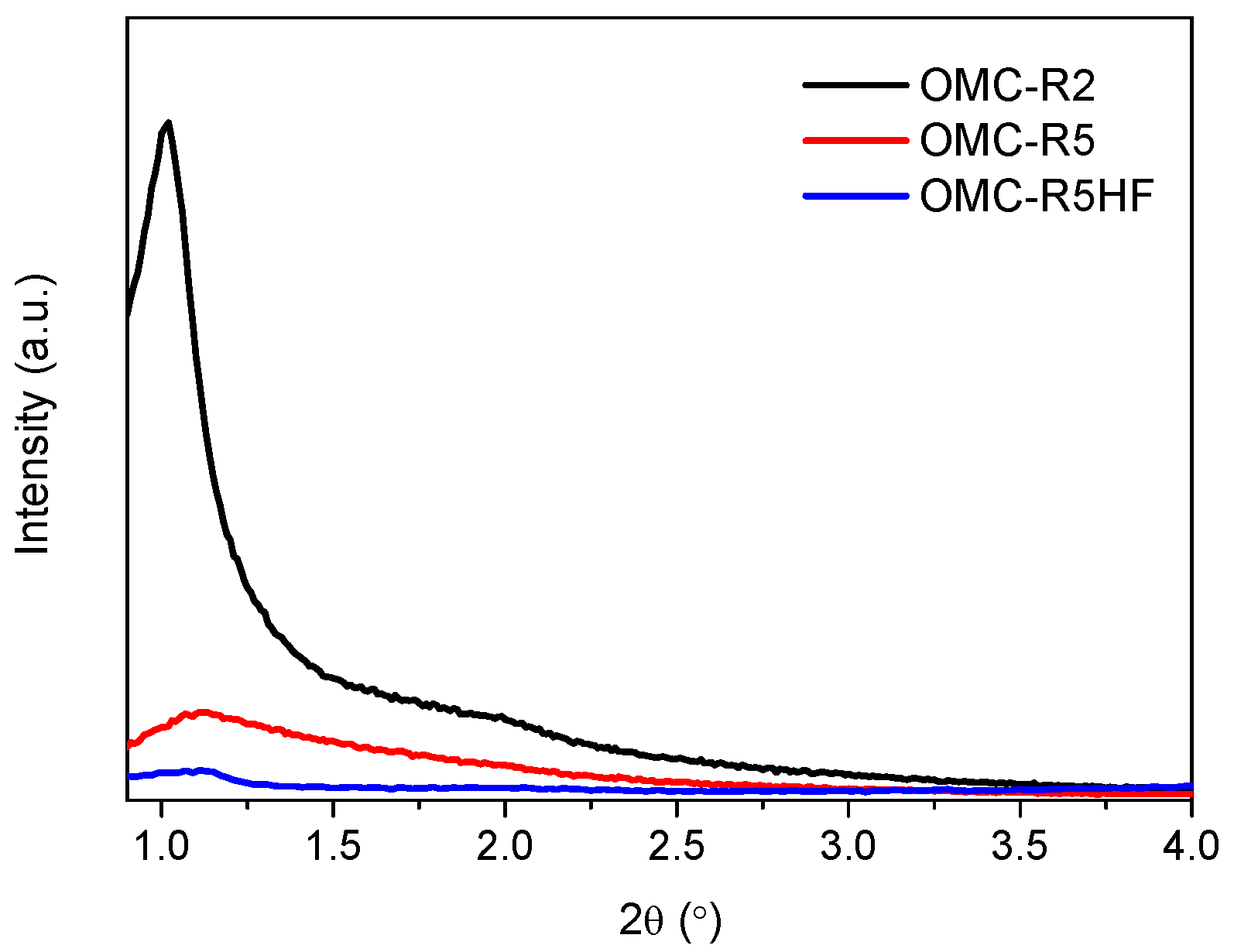
2.1. Physicochemical Characterization of OMC Supports
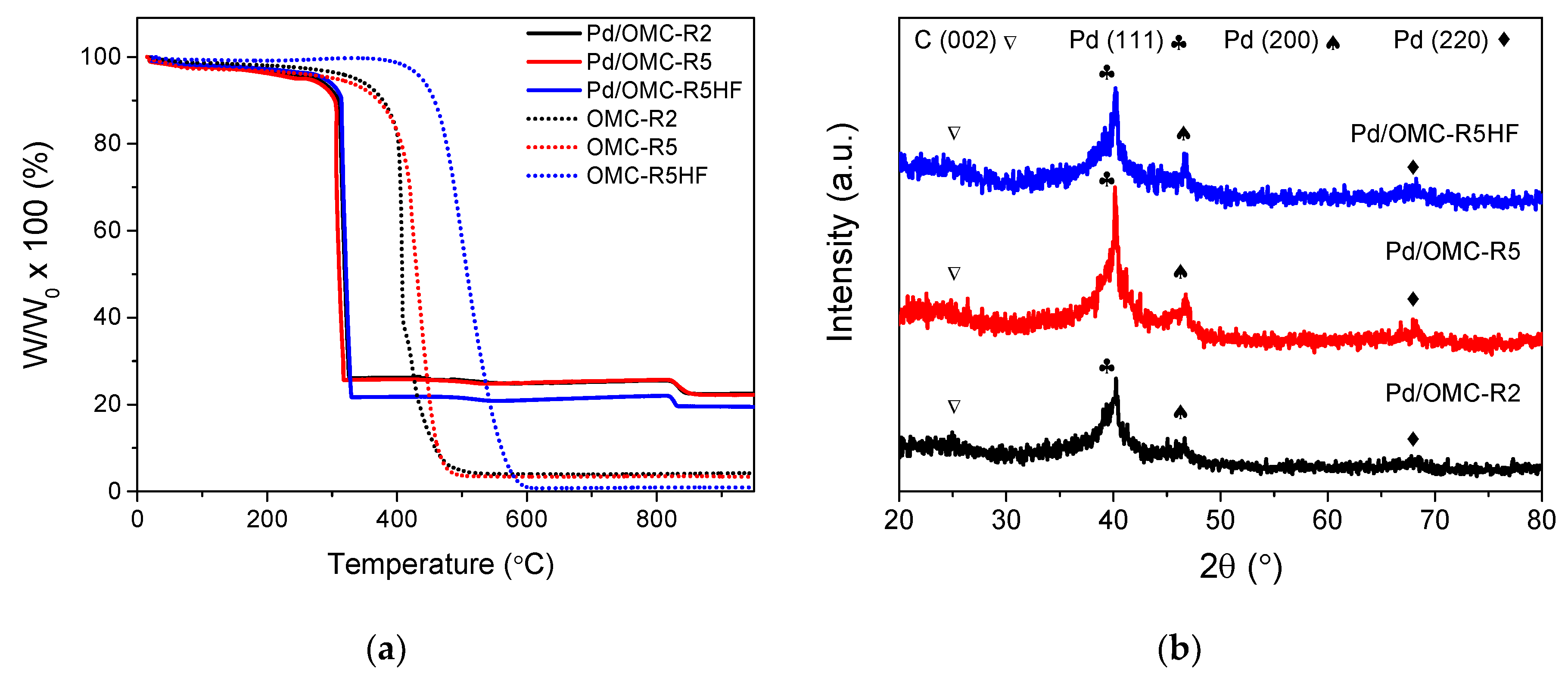
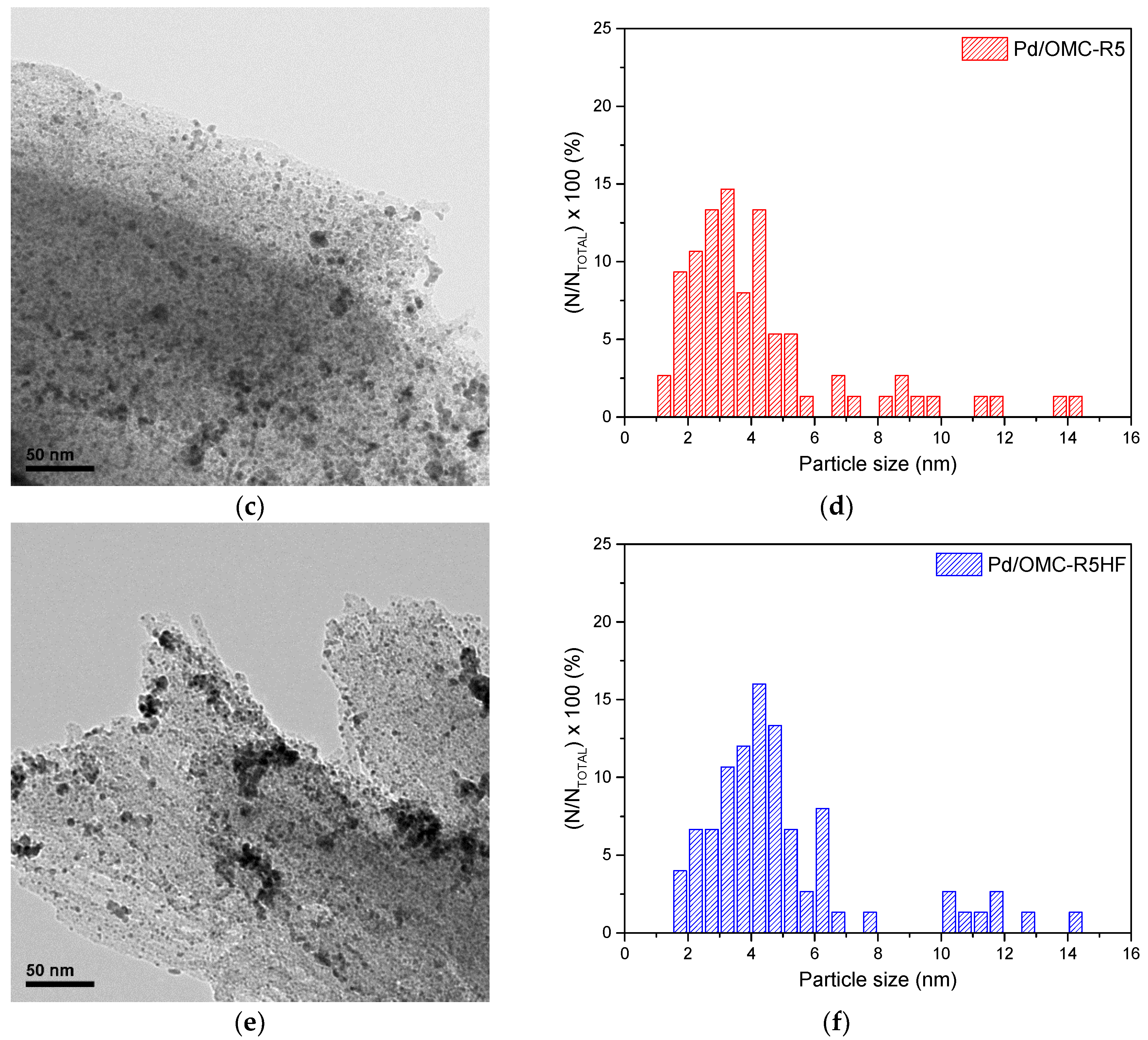
2.2. Physicochemical Characterization of the Pd/OMC Catalysts
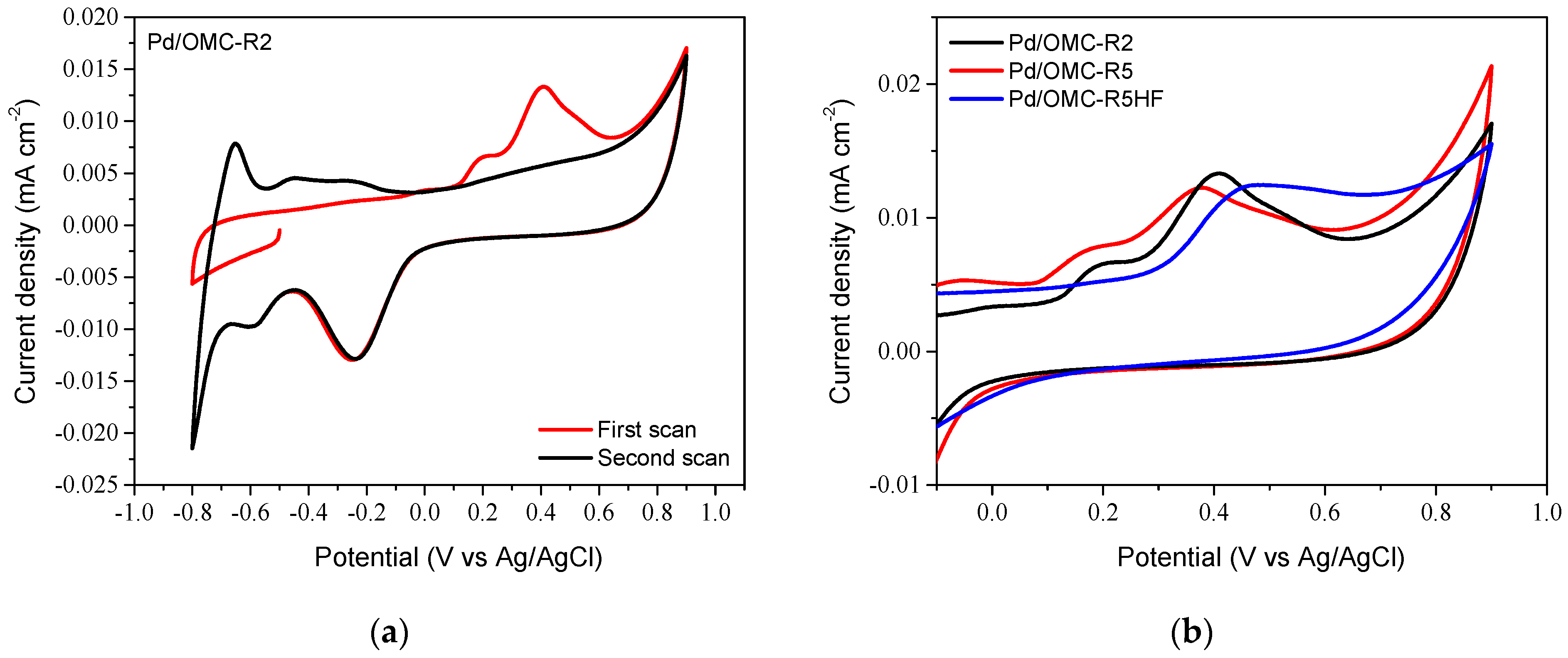
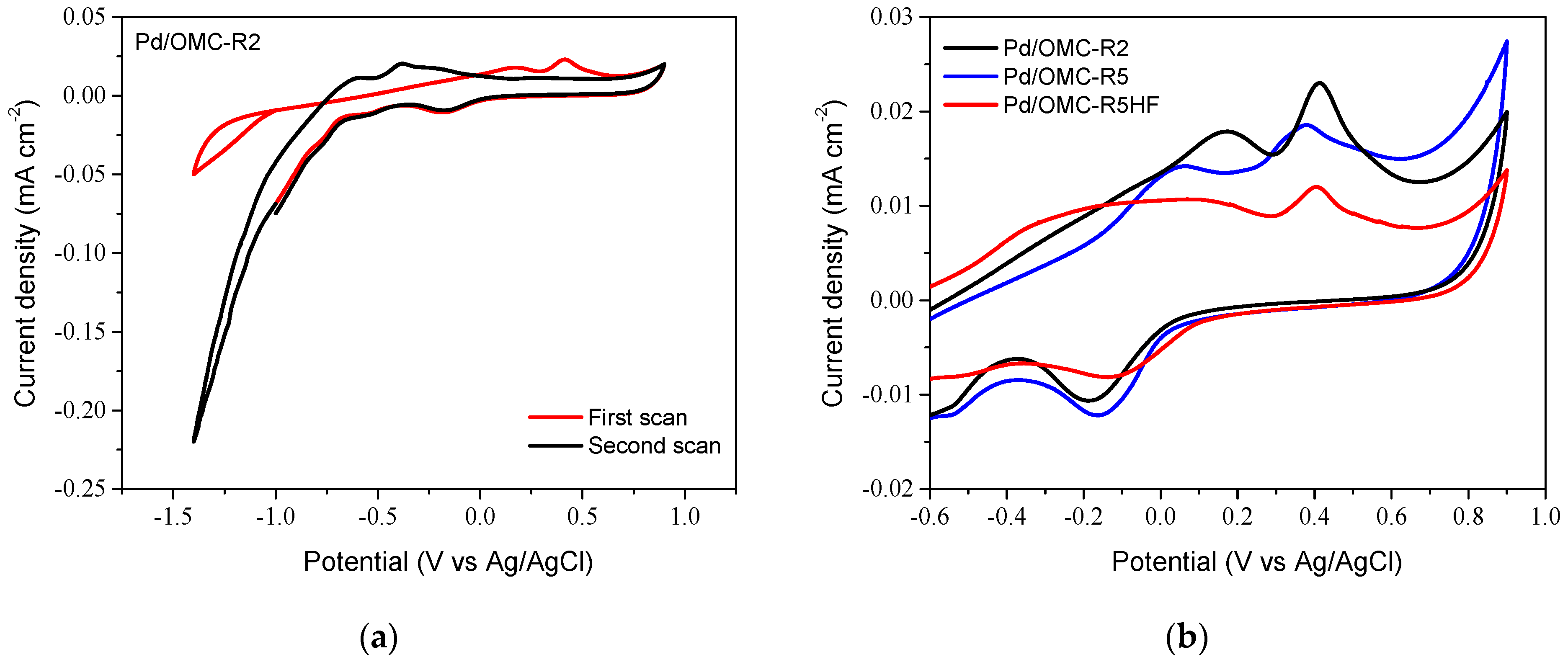
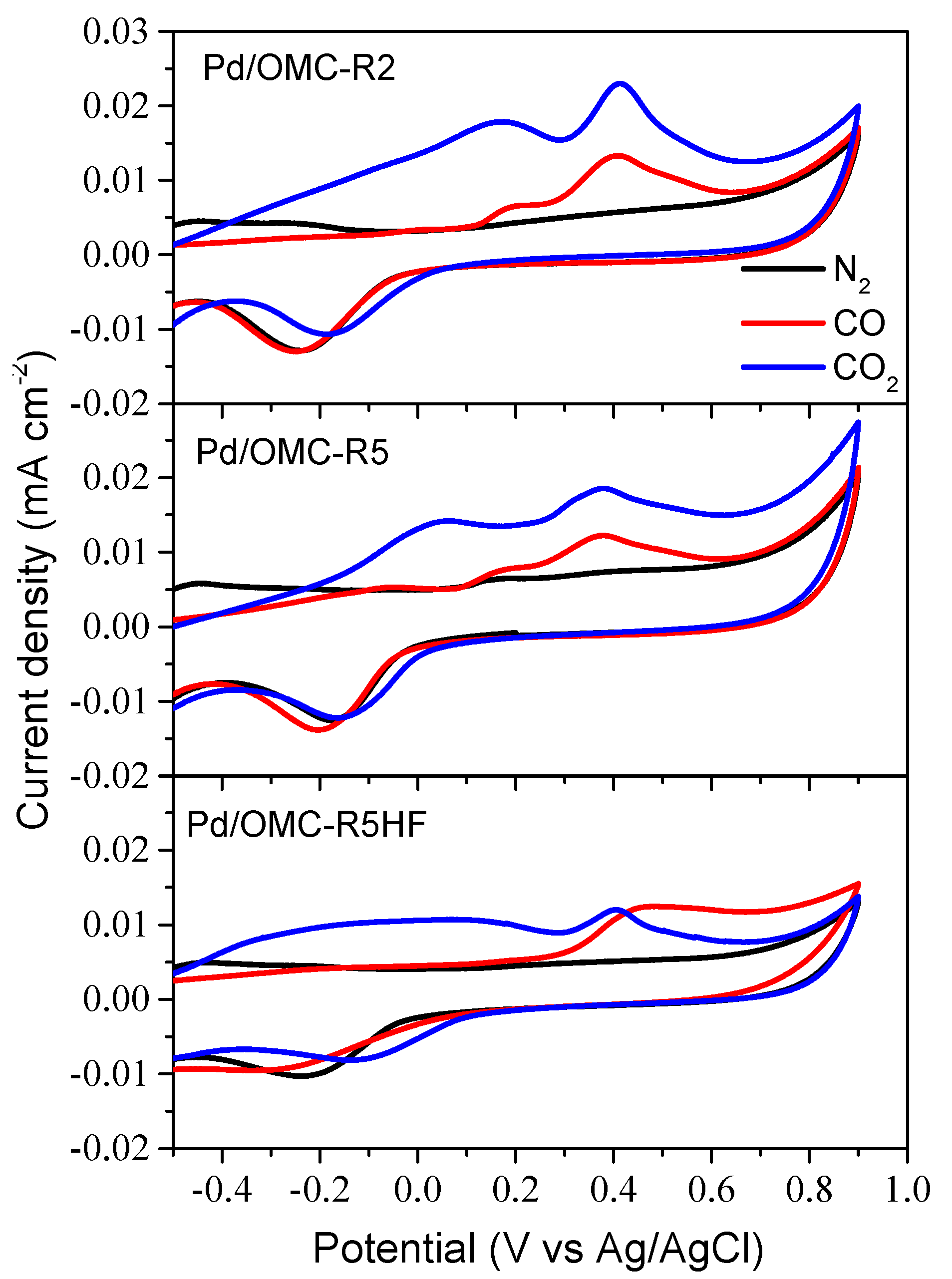
2.3. Electrochemical Characterization of Pd/OMC Catalysts
3. Materials and Methods
3.1. Synthesis of OMC Supports and Pd/OMC Catalysts
3.2. Physicochemical Characterization
3.3. Electrochemical Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hori, Y.; Wakebe, H.; Tsukamoto, T.; Koga, O. Electrocatalytic process of CO selectivity in electrochemical reduction of CO2 at metal electrodes in aqueous media. Electrochim. Acta 1994, 39, 1833–1839. [Google Scholar] [CrossRef]
- Martín, A.J.; Larrazábal, G.O.; Pérez-Ramírez, J. Towards sustainable fuels and chemicals through the electrochemical reduction of CO2: Lessons from water electrolysis. Green Chem. 2015, 17, 5114–5130. [Google Scholar] [CrossRef]
- Vasileff, A.; Zheng, Y.; Qiao, S.Z. Carbon Solving Carbon’s Problems: Recent Progress of Nanostructured Carbon-Based Catalysts for the Electrochemical Reduction of CO2. Adv. Energy Mater. 2017, 7, 1700759. [Google Scholar] [CrossRef]
- Nitopi, S.; Bertheussen, E.; Scott, S.B.; Liu, X.; Engstfeld, A.K.; Horch, S.; Seger, B.; Stephens, I.E.L.; Chan, K.; Hahn, C.; et al. Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte. Chem. Rev. 2019, 119, 7610–7672. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodríguez, S.; Rillo, N.; Lázaro, M.J.; Pastor, E. Pd catalysts supported onto nanostructured carbon materials for CO2 valorization by electrochemical reduction. Appl. Catal. B Environ. 2015, 163, 83–95. [Google Scholar] [CrossRef]
- Kolbe, D.; Vielstich, W. Adsorbate formation during the electrochemical reduction of carbon dioxide at palladium—A DEMS study. Electrochim. Acta 1996, 41, 2457–2460. [Google Scholar] [CrossRef]
- Cai, F.; Gao, D.; Zhou, H.; Wang, G.; He, T.; Gong, H.; Miao, S.; Yang, F.; Wang, J.; Bao, X. Electrochemical promotion of catalysis over Pd nanoparticles for CO2 reduction. Chem. Sci. 2017, 8, 2569–2573. [Google Scholar] [CrossRef]
- Umeda, M.; Niitsuma, Y.; Horikawa, T.; Matsuda, S.; Osawa, M. Electrochemical Reduction of CO2 to Methane on Platinum Catalysts without Overpotentials: Strategies for Improving Conversion Efficiency. ACS Appl. Energy Mater. 2020, 3, 1119–1127. [Google Scholar] [CrossRef]
- Taguchi, S.; Aramata, A.; Enyo, M. Reduced CO2 on polycrystalline Pd and Pt electrodes in neutral solution: Electrochemical and in situ Fourier transform IR studies. J. Electroanal. Chem. 1994, 372, 161–169. [Google Scholar] [CrossRef]
- Gao, D.; Zhou, H.; Cai, F.; Wang, J.; Wang, G.; Bao, X. Pd-Containing Nanostructures for Electrochemical CO2 Reduction Reaction. ACS Catal. 2018, 8, 1510–1519. [Google Scholar] [CrossRef]
- Arévalo, M.C.; Gomis-Bas, C.; Hahn, F.; Beden, B.; Arévalo, A.; Arvia, A.J. A contribution to the mechanism of “reduced” CO2 adsorbates electro-oxidation from combined spectroelectrochemical and voltammetric data. Electrochim. Acta 1994, 39, 793–799. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, S.; Barreras, F.; Pastor, E.; Lázaro, M.J. Electrochemical reactors for CO2 reduction: From acid media to gas phase. Int. J. Hydrog. Energy 2016, 41, 19756–19765. [Google Scholar] [CrossRef]
- Azuma, M.; Hashimoto, K.; Watanabe, M.; Sakata, T. Electrochemical reduction of carbon dioxide to higher hydrocarbons in a KHCO3 aqueous solution. J. Electroanal. Chem. Interfacial Electrochem. 1990, 294, 299–303. [Google Scholar] [CrossRef]
- Bezerra, C.W.B.; Zhang, L.; Liu, H.; Lee, K.; Marques, A.L.B.; Marques, E.P.; Wang, H.; Zhang, J. A review of heat-treatment effects on activity and stability of PEM fuel cell catalysts for oxygen reduction reaction. J. Power Sources 2007, 173, 891–908. [Google Scholar] [CrossRef]
- Antolini, E. Carbon supports for low-temperature fuel cell catalysts. Appl. Catal. B Environ. 2009, 88, 1–24. [Google Scholar] [CrossRef]
- Sharma, S.; Pollet, B.G. Support materials for PEMFC and DMFC electrocatalysts—A review. J. Power Sources 2012, 208, 96–119. [Google Scholar] [CrossRef]
- Ma, T.-Y.; Liu, L.; Yuan, Z.-Y. Direct synthesis of ordered mesoporous carbons. Chem. Soc. Rev. 2013, 42, 3977–4003. [Google Scholar] [CrossRef]
- Eftekhari, A.; Fan, Z. Ordered mesoporous carbon and its applications for electrochemical energy storage and conversion. Mater. Chem. Front. 2017, 1, 1001–1027. [Google Scholar] [CrossRef]
- Guo, Z.; Xiao, N.; Li, H.; Wang, Y.; Li, C.; Liu, C.; Xiao, J.; Bai, J.; Zhao, S.; Qiu, J. Achieving efficient electroreduction CO2 to CO in a wide potential range over pitch-derived ordered mesoporous carbon with engineered Ni-N sites. J. CO2 Util. 2020, 38, 212–219. [Google Scholar] [CrossRef]
- Zhou, S.; Xu, H.; Yuan, Q.; Shen, H.; Zhu, X.; Liu, Y.; Gan, W. N-Doped Ordered Mesoporous Carbon Originated from a Green Biological Dye for Electrochemical Sensing and High-Pressure CO2 Storage. ACS Appl. Mater. Interfaces 2016, 8, 918–926. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S.; Winè, G.; Gangeri, M. Electrocatalytic conversion of CO2 to long carbon-chain hydrocarbons. Green Chem. 2007, 9, 671–678. [Google Scholar] [CrossRef]
- Li, W.; Herkt, B.; Seredych, M.; Bandosz, T.J. Pyridinic-N groups and ultramicropore nanoreactors enhance CO2 electrochemical reduction on porous carbon catalysts. Appl. Catal. B Environ. 2017, 207, 195–206. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, S.; Sebastián, D.; Lázaro, M.J. Insights on the Electrochemical Oxidation of Ordered Mesoporous Carbons. J. Electrochem. Soc. 2020, 167, 024511. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, S.; Sebastián, D.; Lázaro, M.J. Electrochemical oxidation of ordered mesoporous carbons and the influence of graphitization. Electrochim. Acta 2019, 303, 167–175. [Google Scholar] [CrossRef]
- Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores | Science. Available online: https://science.sciencemag.org/content/279/5350/548.full (accessed on 16 July 2020).
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Órfão, J.J.M. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Langford, J.I.; Wilson, A.J.C. Scherrer after sixty years: A survey and some new results in the determination of crystallite size. J. Appl. Crystallogr. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Sarkar, S.; Peter, S.C. An overview on Pd-based electrocatalysts for the hydrogen evolution reaction. Inorg. Chem. Front. 2018, 5, 2060–2080. [Google Scholar] [CrossRef]
- Zheng, J.; Zhou, S.; Gu, S.; Xu, B.; Yan, Y. Size-Dependent Hydrogen Oxidation and Evolution Activities on Supported Palladium Nanoparticles in Acid and Base. J. Electrochem. Soc. 2016, 163, F499. [Google Scholar] [CrossRef]
- Azuma, M.; Hashimoto, K.; Hiramoto, M.; Watanabe, M.; Sakata, T. Electrochemical Reduction of Carbon Dioxide on Various Metal Electrodes in Low-Temperature Aqueous KHCO3 Media. J. Electrochem. Soc. 1990, 137, 1772. [Google Scholar] [CrossRef]
- Juárez-Marmolejo, L.; Pérez-Rodríguez, S.; Montes de Oca-Yemha, M.G.; Palomar-Pardavé, M.; Romero-Romo, M.; Ezeta-Mejía, A.; Morales-Gil, P.; Martínez-Huerta, M.V.; Lázaro, M.J. Carbon supported PdM (M = Fe, Co) electrocatalysts for formic acid oxidation. Influence of the Fe and Co precursors. Int. J. Hydrog. Energy 2019, 44, 1640–1649. [Google Scholar] [CrossRef]
- Miyake, H.; Okada, T.; Samjeské, G.; Osawa, M. Formic acid electrooxidation on Pd in acidic solutions studied by surface-enhanced infrared absorption spectroscopy. Phys. Chem. Chem. Phys. 2008, 10, 3662–3669. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Qin, C.; Wang, R.; Ma, S.; Wang, Y.; Qi, T. Electro-catalysis of carbon black or titanium sub-oxide supported Pd–Gd towards formic acid electro-oxidation. RSC Adv. 2016, 6, 68989–68996. [Google Scholar] [CrossRef]
- Calvillo, L.; Celorrio, V.; Moliner, R.; Cabot, P.L.; Esparbé, I.; Lázaro, M.J. Control of textural properties of ordered mesoporous materials. Microporous Mesoporous Mater. 2008, 116, 292–298. [Google Scholar] [CrossRef]
- Arévalo, M.C.; Rodríguez, J.L.; Pastor, E. Elucidation of the reaction pathways of allyl alcohol at polycrystalline palladium electrodes. J. Electroanal. Chem. 2001, 505, 62–71. [Google Scholar] [CrossRef]
- Arévalo, M.C.; Rodríguez, J.L.; Pastor, E. Adsorption, oxidation and reduction reactions of propargyl alcohol on palladium as studied by electrochemical mass spectrometry. J. Electroanal. Chem. 1999, 472, 71–82. [Google Scholar] [CrossRef]



| OMC | SBET (m2 g−1) | VT (cm3 g−1) | Dp (nm) |
|---|---|---|---|
| OMC-R2 | 884 | 0.59 | 3.0 |
| OMC-R5 | 812 | 0.55 | 3.5 |
| OMC-R5HF | 1050 | 0.77 | 3.6 |
| OMC | CO (µmol g−1) | CO2 (µmol g−1) | CO/CO2 | O (wt.%) |
|---|---|---|---|---|
| OMC-R2 | 1527 | 841 | 1.8 | 5.1 |
| OMC-R5 | 1475 | 770 | 1.9 | 4.8 |
| OMC-R5HF | 1467 | 276 | 5.3 | 3.2 |
| Catalyst | % Pd (wt.-EDX) | % Pd (wt.-TGA) | d (nm) |
|---|---|---|---|
| Pd/OMC-R2 | 22.4 | 18.2 | 3.0 |
| Pd/OMC-R5 | 19.9 | 18.9 | 3.5 |
| Pd/OMC-R5HF | 21.8 | 18.5 | 4.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Rodríguez, S.; Pastor, E.; Lázaro, M.J. Ordered Mesoporous Carbon as a Support of Pd Catalysts for CO2 Electrochemical Reduction. Catalysts 2020, 10, 912. https://doi.org/10.3390/catal10080912
Pérez-Rodríguez S, Pastor E, Lázaro MJ. Ordered Mesoporous Carbon as a Support of Pd Catalysts for CO2 Electrochemical Reduction. Catalysts. 2020; 10(8):912. https://doi.org/10.3390/catal10080912
Chicago/Turabian StylePérez-Rodríguez, Sara, Elena Pastor, and María Jesús Lázaro. 2020. "Ordered Mesoporous Carbon as a Support of Pd Catalysts for CO2 Electrochemical Reduction" Catalysts 10, no. 8: 912. https://doi.org/10.3390/catal10080912
APA StylePérez-Rodríguez, S., Pastor, E., & Lázaro, M. J. (2020). Ordered Mesoporous Carbon as a Support of Pd Catalysts for CO2 Electrochemical Reduction. Catalysts, 10(8), 912. https://doi.org/10.3390/catal10080912







