Synthesis and Characterization of N-Doped SiC Powder with Enhanced Photocatalytic and Photoelectrochemical Performance
Abstract
1. Introduction
2. Results and Discussion
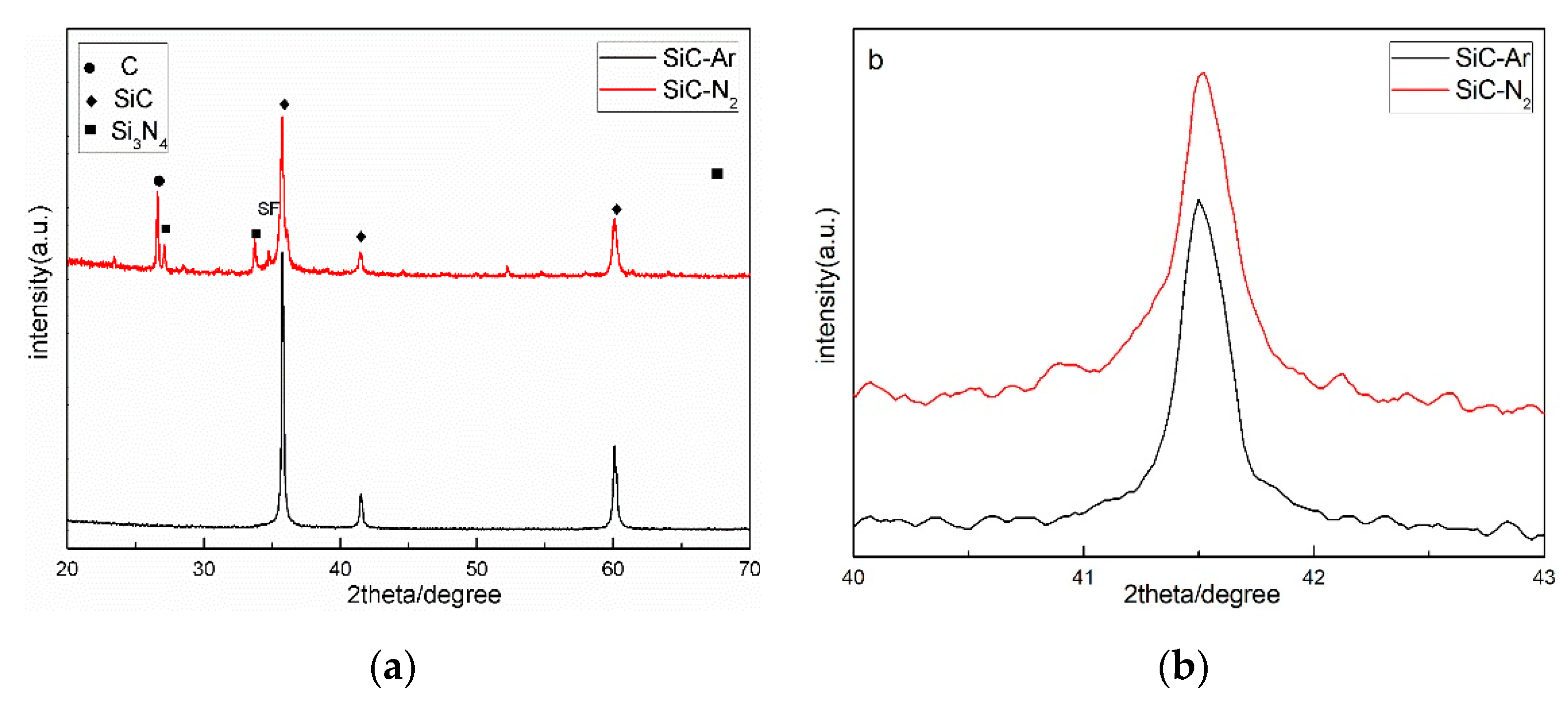
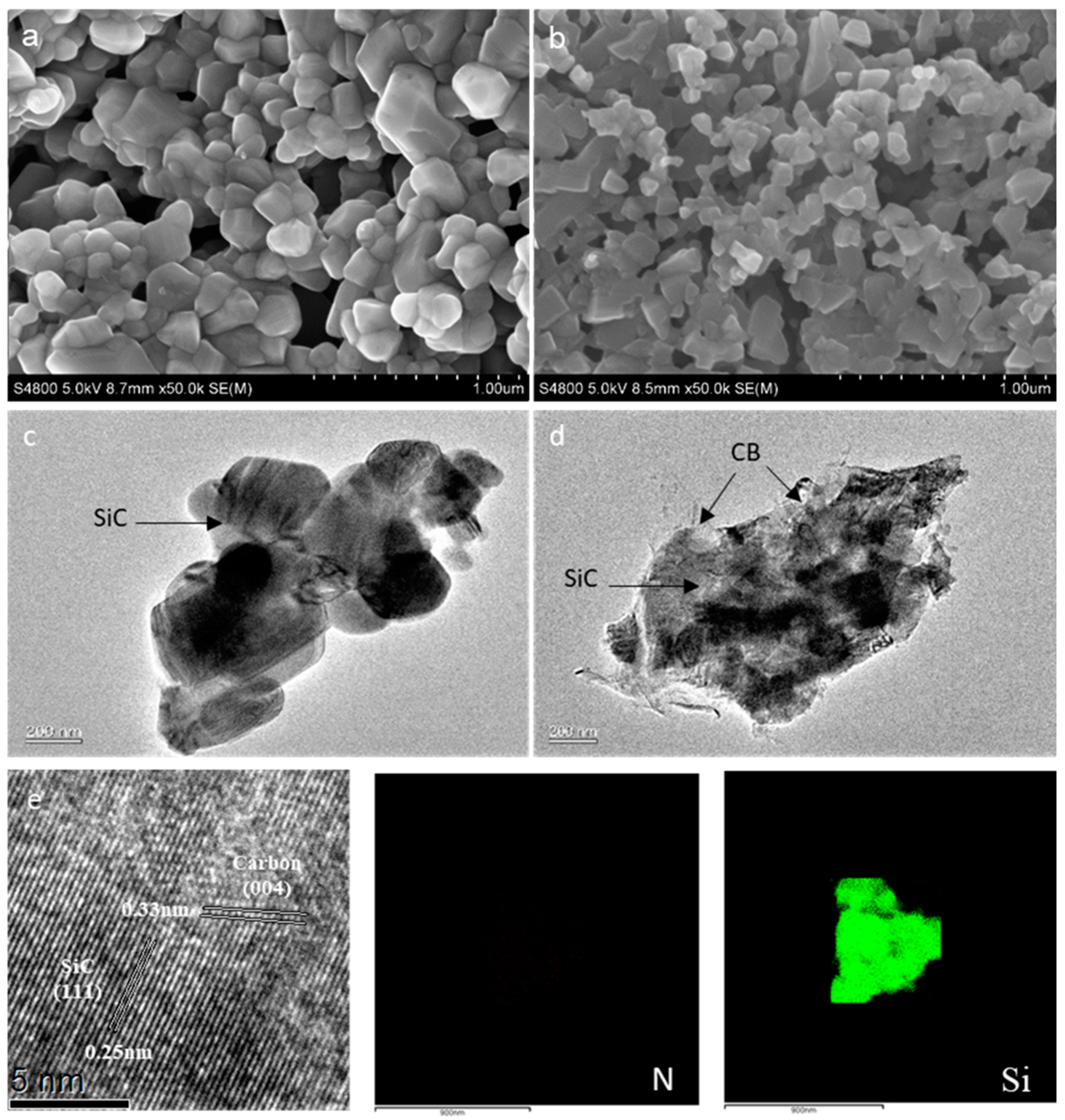
2.1. Synthesis and Characterization
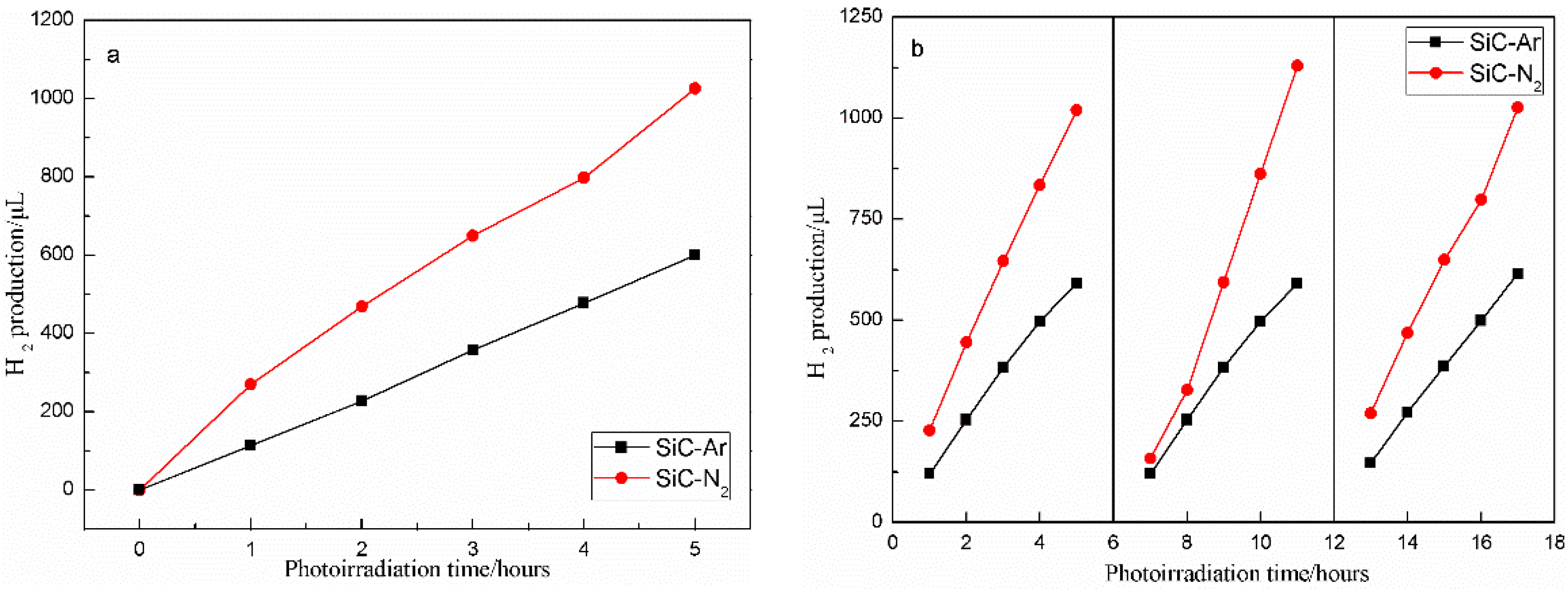
2.2. Photocatalytic, Kinetic, and Photoelectrochemical Properties
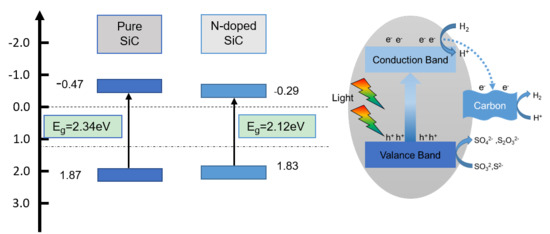
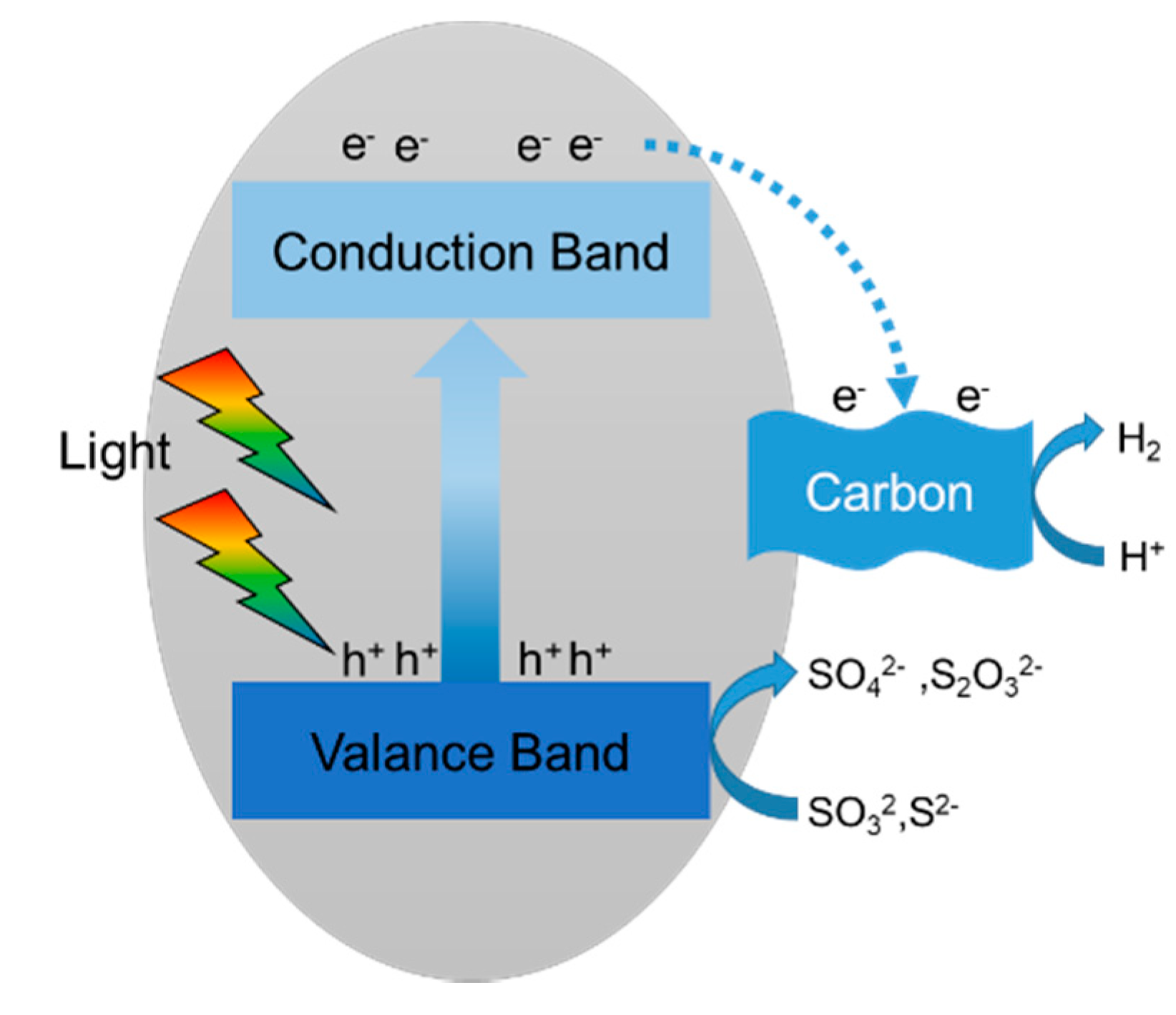
2.3. Reaction Mechanisms
3. Experimental
3.1. Chemicals and Materials
3.2. Material Synthesis
3.3. Material Characterizations
3.4. Photocatalytic Tests
3.5. Photocurrent Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schloegl, R. Energy: Fuel for thought. Nat. Mater. 2008, 7, 772–774. [Google Scholar] [CrossRef] [PubMed]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev 2009, 38, 253. [Google Scholar] [CrossRef]
- Getoff, N. Photoelectrochemical and photocatalytic methods of hydrogen production: A short review. Int. J. Hydrogen. Energy 1990, 15, 407–417. [Google Scholar] [CrossRef]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Kudo, A. Recent progress in the development of visible light-driven powdered photocatalysts for water splitting. Int. J. Hydrogen Energy 2007, 32, 2673–2678. [Google Scholar] [CrossRef]
- Abe, R. Recent progress on photocatalytic and photoelectrochemical water splitting under visible light irradiation. J. Photochem. Photobio. C Photochem. Rev. 2010, 11, 179–209. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Low, J.; Fang, Y.; Xiao, J.; Chen, X. Engineering heterogeneous semiconductors for solar water splitting. J. Mat. Chem. A 2015, 3, 2485–2534. [Google Scholar] [CrossRef]
- Steenackers, M.; Sharp, I.D.; Larsson, K.; Hutter, N.A.; Stutzmann, M.; Jordan, R. Structured Polymer Brushes on Silicon Carbide. Chem. Mat 2010, 22, 272–278. [Google Scholar] [CrossRef]
- Li, X.; Zhou, X.; Gao, Q.; Yuan, J.; Wen, J.; Fang, Y.; Liu, W.; Zhang, S.; Liu, Y. Metal-free carbon nanotube–SiC nanowire heterostructures with enhanced photocatalytic H2 evolution under visible light irradiation. Catal. Sci. Technol. 2015, 5, 2798–2806. [Google Scholar]
- Yang, J.; Zeng, X.; Chen, L.; Yuan, W. Photocatalytic water splitting to hydrogen production of reduced graphene oxide/SiC under visible light. Appl. Phys. Lett. 2013, 102, 83101. [Google Scholar] [CrossRef]
- Wu, X.L.; Xiong, S.J.; Zhu, J.; Wang, J.; Shen, J.C.; Chu, P.K. Identification of surface structures on 3C-SiC nanocrystals with hydrogen and hydroxyl bonding by photoluminescence. Nano Lett. 2009, 9, 4053. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Yan, L.; Wang, Y.; Zhang, Y. SiC nanowires: A photocatalytic nanomaterial. Appl. Phys. Lett. 2006, 89, 37. [Google Scholar] [CrossRef]
- Liu, H.; She, G.; Mu, L.; Shi, W. Porous SiC nanowire arrays as stable photocatalyst for water splitting under UV irradiation. Mat. Res. Bull. 2012, 47, 917–920. [Google Scholar] [CrossRef]
- Hao, J.Y.; Wang, Y.Y.; Tong, X.L.; Jin, G.Q.; Guo, X.Y. SiC nanomaterials with different morphologies for photocatalytic hydrogen production under visible light irradiation. Catal. Today 2013, 212, 220–224. [Google Scholar] [CrossRef]
- Yang, T.; Chang, X.; Chen, J.; Chou, K.C.; Hou, X. B-doped 3C-SiC nanowires with a finned microstructure for efficient visible light-driven photocatalytic hydrogen production. Nanoscale 2015, 7, 8955–8961. [Google Scholar] [CrossRef]
- Dong, L.L.; Wang, Y.Y.; Tong, X.L.; Jin, G.Q.; Guo, X.Y. Synthesis and Characterization of Boron-Doped SiC for Visible Light Driven Hydrogen Production. Acta Phys.-Chim. Sin. 2014, 30, 135–140. [Google Scholar]
- Dang, H.; Li, B.; Li, C.; Zang, Y.; Xu, P.; Zhao, X.; Fan, H.; Qiu, Y. One-dimensional Au/SiC heterojunction nanocomposites with enhanced photocatalytic and photoelectrochemical performances: Kinetics and mechanism insights. Electrochim. Acta 2018, 267, 24–33. [Google Scholar] [CrossRef]
- Wang, M.; Chen, J.; Liao, X.; Liu, Z.; Zhang, J.; Gao, L.; Li, Y. Highly efficient photocatalytic hydrogen production of platinum nanoparticle-decorated SiC nanowires under simulated sunlight irradiation. Int. J. Hydrog. Energy 2014, 39, 14581–14587. [Google Scholar] [CrossRef]
- Wang, D.; Liu, N.; Guo, Z.; Wang, W.; Guo, L.; Yuan, W.; Chen, X. Hexagonal SiC with spatially separated active sites on polar and nonpolar facets achieving enhanced hydrogen production from photocatalytic water reduction. Phys. Chem. Chem. Phys. 2018, 20, 4787–4792. [Google Scholar] [CrossRef]
- Mishra, G.; Parida, K.M.; Singh, S.K. Facile fabrication of S-TiO2/β-SiC nanocomposite photocatalyst for hydrogen evolution under visible light irradiation. ACS Sustain. Chem. Eng. 2015, 3, 245–253. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; Li, X.; Gao, Q.; Liu, X.; Fang, Y. Topological morphology conversion towards SnO2/SiC hollow sphere nanochains with efficient photocatalytic hydrogen evolution. Chem. Commun. 2014, 50, 1070–1073. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Tong, X.; Wang, Y.; Chen, C.; Jin, G.; Guo, X.Y. High photoelectrocatalytic performance of a MoS2-SiC hybrid structure for hydrogen evolution reaction. J. Mat. Chem. A 2013, 1, 4657–4661. [Google Scholar] [CrossRef]
- Peng, Y.; Guo, Z.; Yang, J.; Wang, D.; Yuan, W. Enhanced photocatalytic H2 evolution over micro-SiC by coupling with CdS under visible light irradiation. J. Mat. Chem. A 2014, 2, 6296–6300. [Google Scholar] [CrossRef]
- Peng, Y.; Guo, Z.; Wang, D.; Pan, N.; Yuan, W. Heterogeneous nucleation of CdS to enhance visible-light photocatalytic hydrogen evolution of SiC/CdS composite. Appl. Phys. Lett. 2015, 107, 37. [Google Scholar] [CrossRef]
- Peng, Y.; Han, G.; Wang, D.; Wang, K.; Guo, Z.; Yang, J.; Yuan, W. Improved H2 evolution under visible light in heterostructured SiC/CdS photocatalyst: Effect of lattice match. Int. J. Hydrog. Energy 2017, 42, 14409–14417. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, J.; Huang, F. Enhanced visible light photocatalytic H2 evolution of metal-free g-C3N4 /SiC heterostructured photocatalysts. Appl. Surf. Sci. 2016, 391, 449–456. [Google Scholar] [CrossRef]
- Wang, D.; Guo, Z.; Yuan, P.; Yuan, W. A simple route to significant enhancement of photocatalytic water oxidation on BiVO4 by heterojunction with SiC. Chem. Eng. J. 2015, 281, 102–108. [Google Scholar] [CrossRef]
- Gong, Y.; Jiang, P.G.; Wang, Y.X.; Wu, T.; Lin, J.H. Enhanced photocatalytic performance of chemically bonded SiC-graphene composites for visible-light-driven overall water splitting. Int. J. Hydrog. Energy 2013, 38, 12733–12738. [Google Scholar]
- Zhu, K.; Guo, L.; Lin, J.; Hao, W.; Shang, J.; Jia, Y.; Chen, L.; Jin, S.; Wang, W.; Chen, X. Graphene covered SiC powder as advanced photocatalytic material. Appl. Phys. Lett. 2012, 100, 197. [Google Scholar] [CrossRef]
- Lu, W.; Wang, D.; Guo, L.; Jia, Y.; Ye, M.; Huang, J.; Li, Z.; Peng, Y.; Yuan, W.; Chen, X. Bipolar Carrier Transfer Channels in Epitaxial Graphene/SiC Core-Shell Heterojunction for Efficient Photocatalytic Hydrogen Evolution. Adv. Mat. 2015, 27, 7986–7991. [Google Scholar] [CrossRef]
- Zhou, X.; Gao, Q.; Li, X.; Liu, Y.; Zhang, S.; Fang, Y.; Li, J. Ultra-thin SiC Layers Covered Graphene Nanosheets as Advanced Photocatalysts for Hydrogen Evolution. J. Mat. Chem. A 2015, 3, 10999–11005. [Google Scholar] [CrossRef]
- Cui, Y.; Hu, X.; Yang, K.; Yang, X.; Xie, X.; Xiao, L.; Xu, X. Influence of Nitrogen Concentrations on the Lattice Constants and Resistivities of n-Type 4H-SiC Single Crystals. Cryst. Growth Des. 2015, 15, 3131–3136. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, W.; Luo, F.; Huang, Y.; Li, G.; Su, X. Improving the dielectric properties of SiC powder through nitrogen doping. Mat. Sci. Eng. B 2011, 176, 942–944. [Google Scholar] [CrossRef]
- Wang, M.J.; Wada, H. Synthesis and characterization of silicon nitride whiskers. J. Mat. Sci. 1990, 25, 1690–1698. [Google Scholar] [CrossRef]
- Choi, J.Y.; Chong, H.K.; Kim, D.K. Carbothermic Synthesis of Monodispersed Spherical Si3N4/SiC Nanocomposite Powder. J. Am. Ceram. Soc. 1999, 82, 2665–2671. [Google Scholar] [CrossRef]
- Liu, H.S.; Fang, X.Y.; Song, W.L.; Hou, Z.L.; Cao, M.S. Modification of Band Gap of beta-SiC by N-Doping. Chin. Phys. Lett. 2009, 26, 237–240. [Google Scholar]
- Ténégal, F.; de la Rocque, A.G.; Dufour, G.; Sénémaud, C.; Doucey, B.; Bahloul-Hourlier, D.; Goursat, P.; Mayne, M.; Cauchetier, M. Structural determination of sintered Si3N4/SiC nanocomposite using the XPS differential charge effect. J. Electron. Spectrosc. Relat. Phenom. 2000, 109, 241–248. [Google Scholar] [CrossRef]
- Ito, S.; Murata, T.; Hasegawa, M.; Bito, Y.; Toyoguchi, Y. Anode Materials C xN with Graphite-like Structure for Secondary Lithium Batteries. Denki Kagaku 1996, 64, 1180–1184. [Google Scholar] [CrossRef]
- Smirnova, T.P.; Badalian, A.M.; Yakovkina, L.V.; Kaichev, V.V.; Bukhtiyarov, V.I.; Shmakov, A.N.; Asanov, I.P.; Rachlin, V.I.; Fomina, A.N. SiCN alloys obtained by remote plasma chemical vapour deposition from novel precursors. Thin Solid Films 2003, 429, 144–151. [Google Scholar] [CrossRef]
- Cho, W.S.; Oh, Y.S.; Kim, C.S.; Osada, M.; Kakihana, M.; Lim, D.S.; Cheong, D.S. Characterization of Si3N4/SiC nanocomposite by Raman scattering and XPS. J. Alloy. Compd. 1999, 285, 255–259. [Google Scholar] [CrossRef]
- Oh, Y.S.; Cho, W.S.; Kim, C.S.; Lim, D.S.; Cheong, D.S. XPS Investigation of Si3N4/SiC Nanocomposites Prepared Using a Commercial Polymer. J. Am. Ceram. Soc. 1999, 82, 927–932. [Google Scholar] [CrossRef]
- Avila, J.; Sacedon, J.L. Reactivity at the Al/Si3N4 interfaces. Appl. Phys. Lett. 1995, 66, 757–759. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]


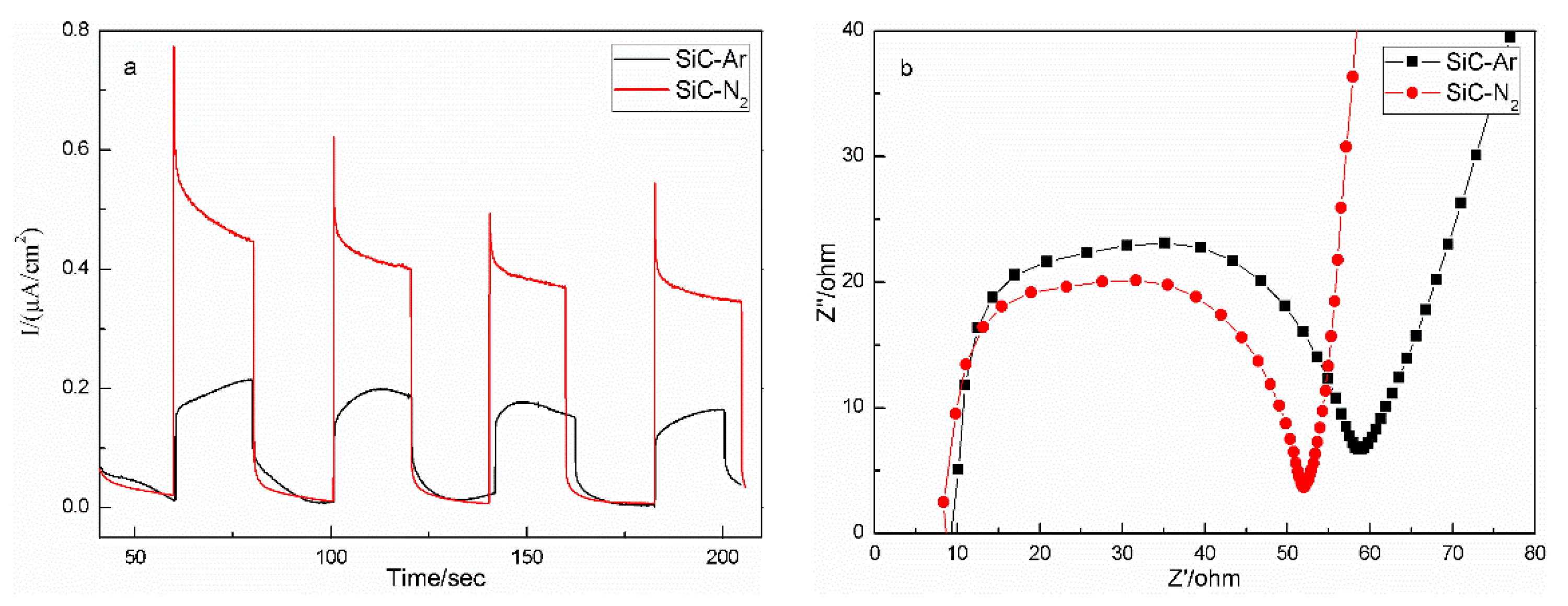
| Sample | A/nm | Eg/eV | Efb/V |
|---|---|---|---|
| SiC–Ar | 0.436 | 2.34 | −0.47 |
| SiC–N2 | 0.420 | 2.17 | −0.29 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Li, Q.; Yang, X.; Chen, X.; Xu, X. Synthesis and Characterization of N-Doped SiC Powder with Enhanced Photocatalytic and Photoelectrochemical Performance. Catalysts 2020, 10, 769. https://doi.org/10.3390/catal10070769
Liu W, Li Q, Yang X, Chen X, Xu X. Synthesis and Characterization of N-Doped SiC Powder with Enhanced Photocatalytic and Photoelectrochemical Performance. Catalysts. 2020; 10(7):769. https://doi.org/10.3390/catal10070769
Chicago/Turabian StyleLiu, Wanli, Qi Li, Xianglong Yang, Xiufang Chen, and Xiangang Xu. 2020. "Synthesis and Characterization of N-Doped SiC Powder with Enhanced Photocatalytic and Photoelectrochemical Performance" Catalysts 10, no. 7: 769. https://doi.org/10.3390/catal10070769
APA StyleLiu, W., Li, Q., Yang, X., Chen, X., & Xu, X. (2020). Synthesis and Characterization of N-Doped SiC Powder with Enhanced Photocatalytic and Photoelectrochemical Performance. Catalysts, 10(7), 769. https://doi.org/10.3390/catal10070769