Reduced Graphene Oxide/ZnIn2S4 Nanocomposite Photocatalyst with Enhanced Photocatalytic Performance for the Degradation of Naproxen under Visible Light Irradiation
Abstract
1. Introduction
2. Results and Discussion
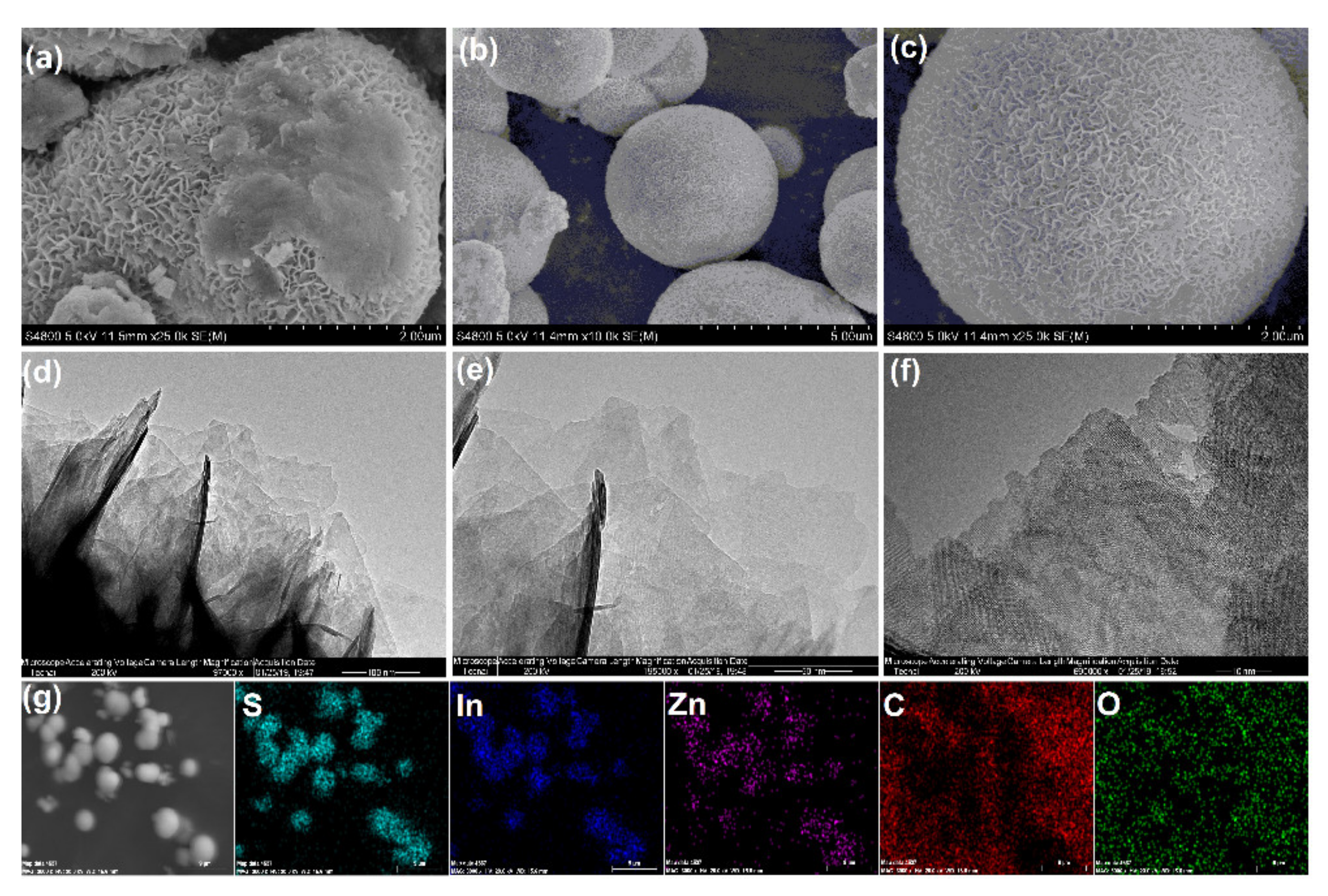
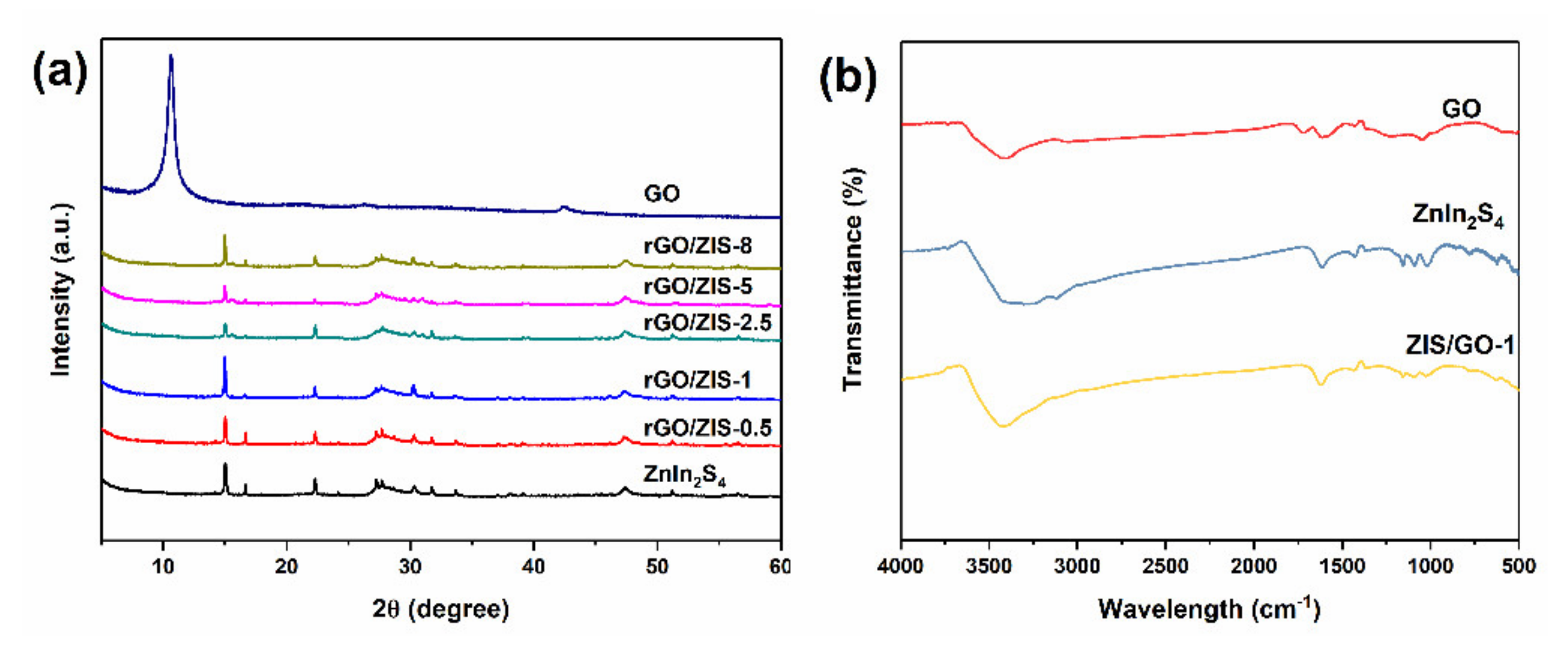
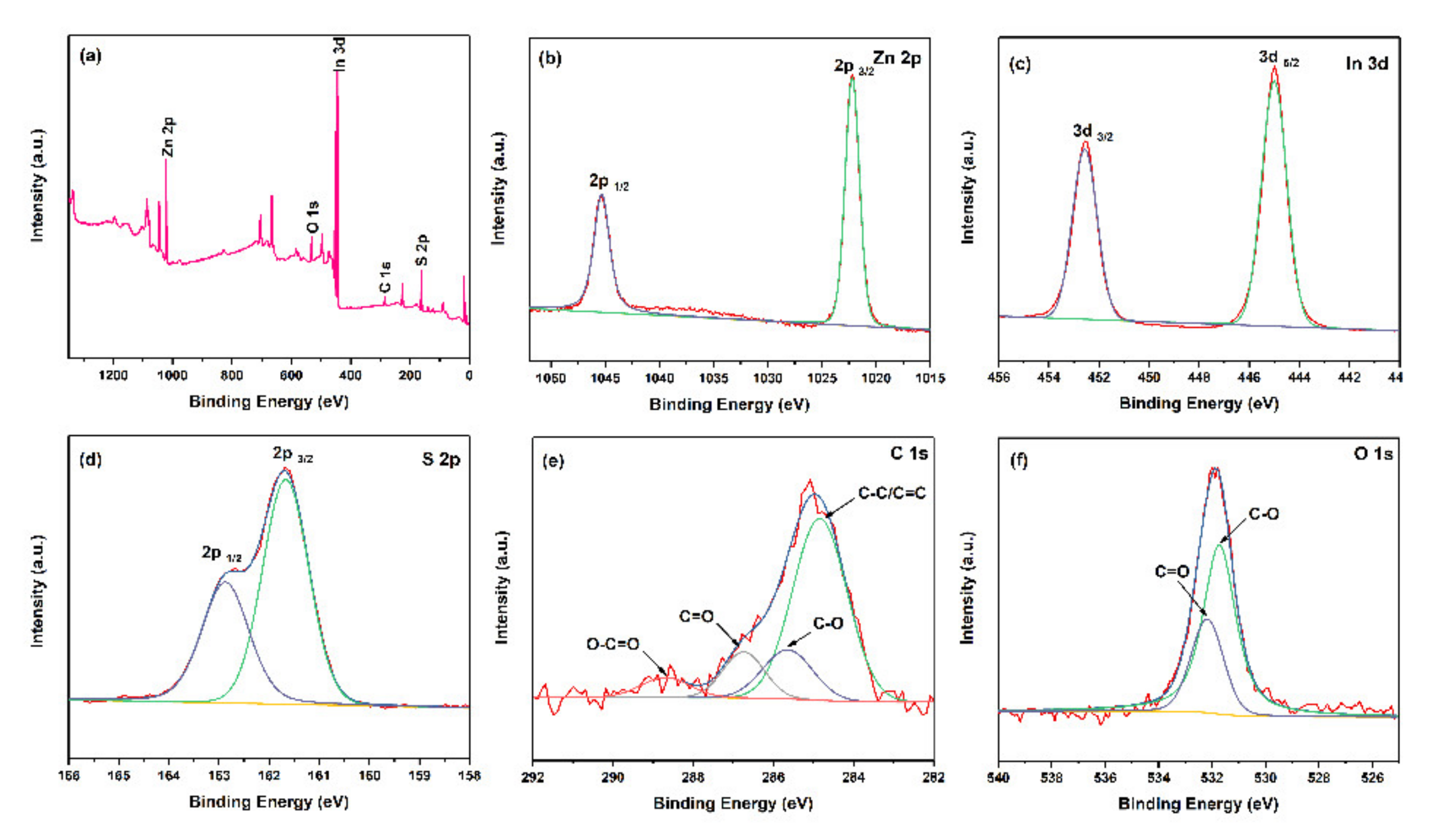
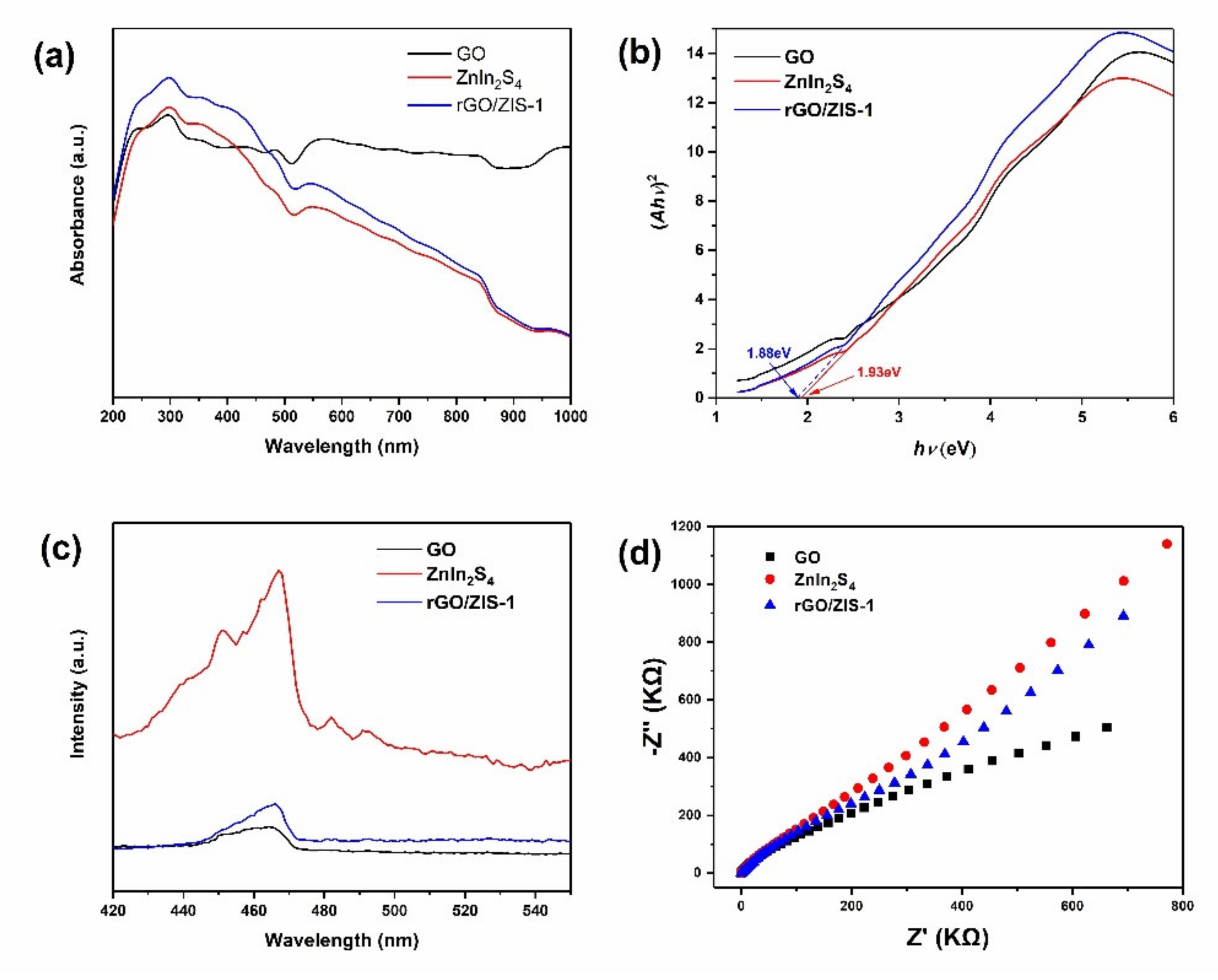
2.1. Characterization
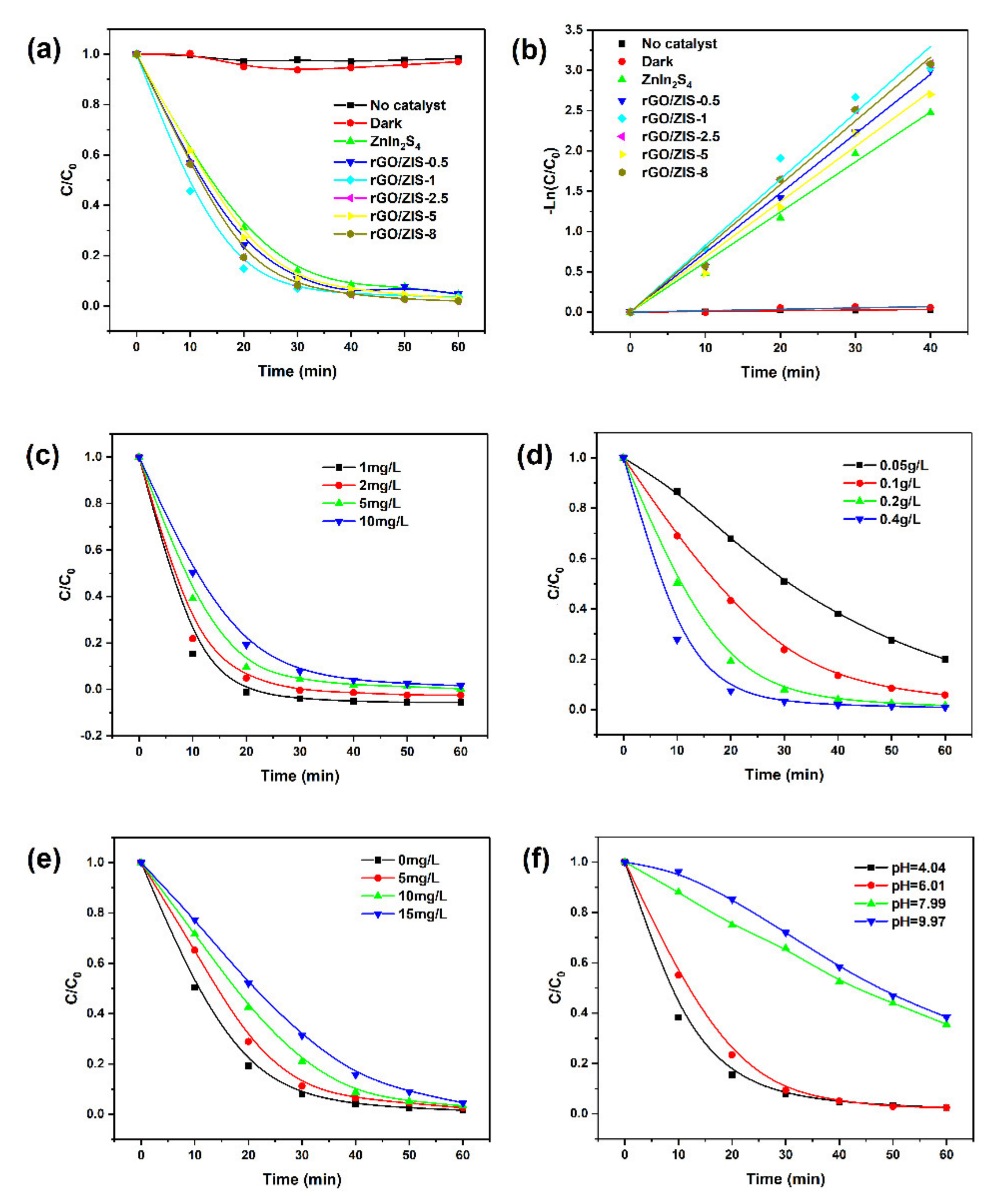
2.2. Photocatalytic Performance
2.3. Photocatalytic Mechanism
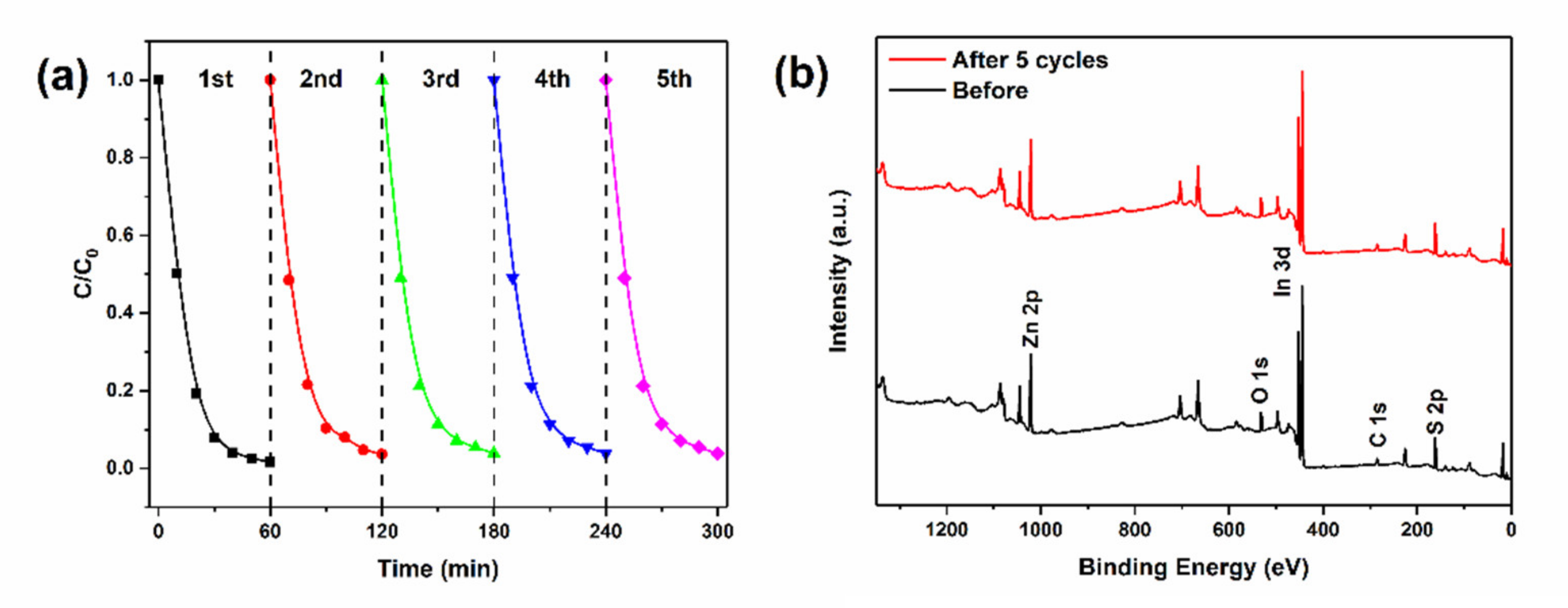
2.4. Reusability and Stability
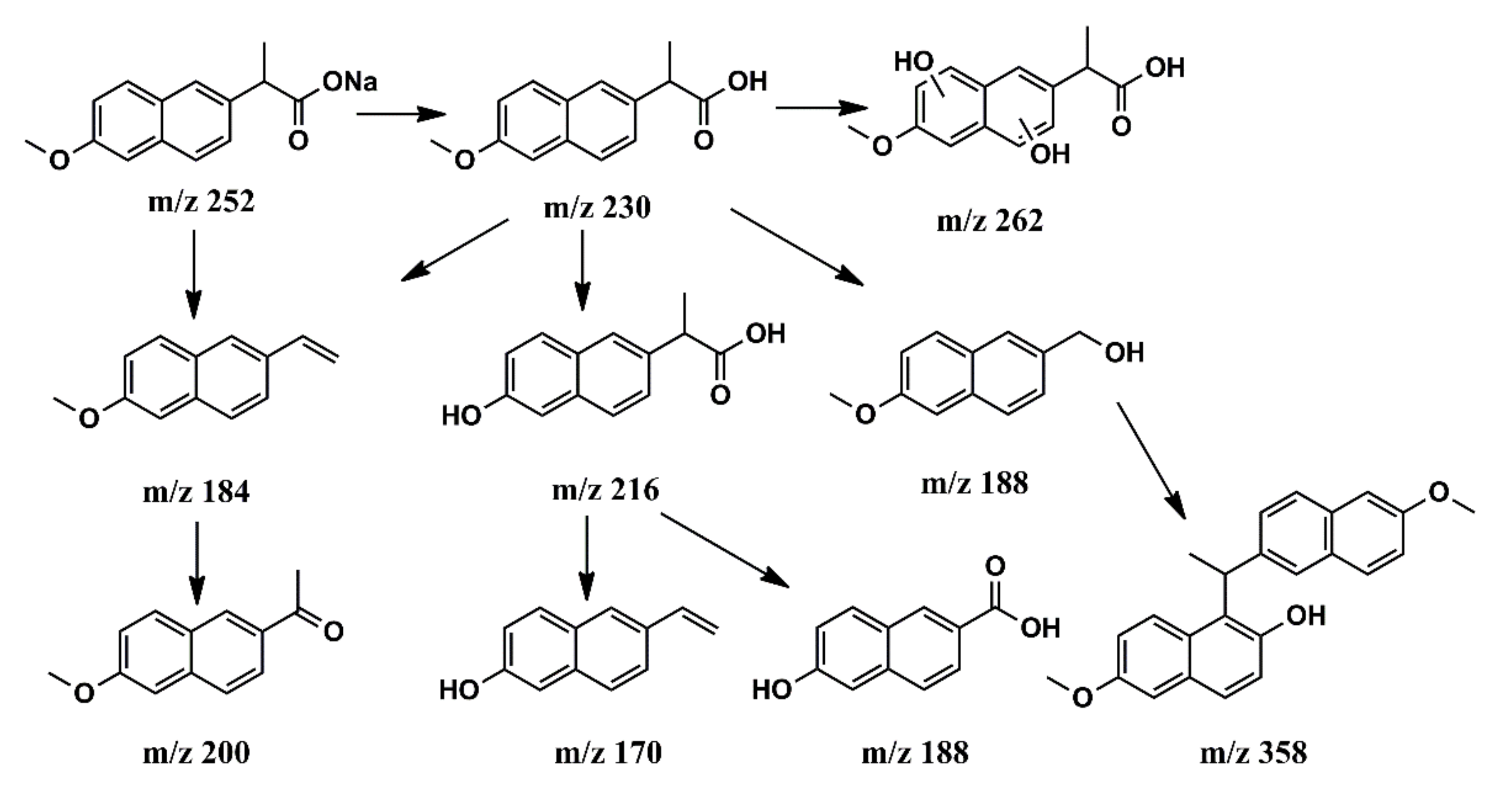
2.5. Photodegradation Intermediates and Suggested Pathways
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Photocatalysts
3.3. Characterization of Photocatalysts
3.4. Photocatalytic Degradation Experiments
3.5. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liang, R.; Luo, S.; Jing, F.; Shen, L.; Qin, N.; Wu, L. A simple strategy for fabrication of Pd@MIL-100(Fe) nanocomposite as a visible-light-driven photocatalyst for the treatment of pharmaceuticals and personal care products (PPCPs). Appl. Catal. B 2015, 176–177, 240–248. [Google Scholar] [CrossRef]
- Kumar, A.; Khan, M.; Fang, L.; Lo, I.M.C. Visible-light-driven N-TiO2@SiO2@Fe3O4 magnetic nanophotocatalysts: Synthesis, characterization, and photocatalytic degradation of PPCPs. J. Hazard Mater. 2019, 370, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.K.; Dhakal, D.; Lee, S.W. Rapid degradation of naproxen by AgBr-α-NiMoO4 composite photocatalyst in visible light: Mechanism and pathways. Chem. Eng. J. 2018, 347, 836–848. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.; Feng, Y.; Zeng, Y.; Xie, Z.; Zhang, Q.; Su, Y.; Chen, P.; Liu, Y.; Yao, K.; et al. Novel ternary photocatalyst of single atom-dispersed silver and carbon quantum dots co-loaded with ultrathin g-C3N4 for broad spectrum photocatalytic degradation of naproxen. Appl. Catal. B 2018, 221, 510–520. [Google Scholar] [CrossRef]
- Begum, S.; Ahmaruzzaman, M. Biogenic synthesis of SnO2/activated carbon nanocomposite and its application as photocatalyst in the degradation of naproxen. Appl. Surf. Sci. 2018, 449, 780–789. [Google Scholar] [CrossRef]
- Chi, H.; He, X.; Zhang, J.; Wang, D.; Zhai, X.; Ma, J. Hydroxylamine enhanced degradation of naproxen in Cu2+ activated peroxymonosulfate system at acidic condition: Efficiency, mechanisms and pathway. Chem. Eng. J. 2019, 361, 764–772. [Google Scholar] [CrossRef]
- Chi, H.; Wang, Z.; He, X.; Zhang, J.; Wang, D.; Ma, J. Activation of peroxymonosulfate system by copper-based catalyst for degradation of naproxen: Mechanisms and pathways. Chemosphere 2019, 228, 54–64. [Google Scholar] [CrossRef]
- Guo, F.; Cai, Y.; Guan, W.; Huang, H.; Liu, Y. Graphite carbon nitride/ZnIn2S4 heterojunction photocatalyst with enhanced photocatalytic performance for degradation of tetracycline under visible light irradiation. J. Phys. Chem. Solids 2017, 110, 370–378. [Google Scholar] [CrossRef]
- Wang, S.; Guan, B.Y.; Wang, X.; Lou, X.W.D. Formation of Hierarchical Co9S8@ZnIn2S4 Heterostructured Cages as an Efficient Photocatalyst for Hydrogen Evolution. J. Am. Chem. Soc. 2018, 140, 15145–15148. [Google Scholar] [CrossRef]
- Gou, X.L.; Cheng, F.Y.; Shi, Y.H.; Zhang, L.; Peng, S.J.; Chen, J.; Shen, P.W. Shape-controlled synthesis of ternary chalcogenide ZnIn2S4 and CuIn(S,Se)2 nano-/microstructures via facile solution route. J. Am. Chem. Soc. 2006, 128, 7222–7229. [Google Scholar] [CrossRef]
- Tian, Q.; Wu, W.; Liu, J.; Wu, Z.; Yao, W.; Ding, J.; Jiang, C. Dimensional heterostructures of 1D CdS/2D ZnIn2S4 composited with 2D graphene: Designed synthesis and superior photocatalytic performance. Dalton Trans. 2017, 46, 2770–2777. [Google Scholar] [CrossRef]
- Qiu, P.; Yao, J.; Chen, H.; Jiang, F.; Xie, X. Enhanced visible-light photocatalytic decomposition of 2,4-dichlorophenoxyacetic acid over ZnIn2S4/g-C3N4 photocatalyst. J. Hazard Mater. 2016, 317, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, L.; Liu, C.; Luo, J.; Crittenden, J.; Liu, X.; Cai, T.; Yuan, J.; Pei, Y.; Liu, Y. Photocatalytic wastewater purification with simultaneous hydrogen production using MoS2 QD-decorated hierarchical assembly of ZnIn2S4 on reduced graphene oxide photocatalyst. Water Res. 2017, 121, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Ye, X.; Zhang, Q.; Hui, Z.; Wang, X.; Chen, S. Efficient utilization of photogenerated electrons and holes for photocatalytic redox reactions using visible light-driven Au/ZnIn2S4 hybrid. J. Hazard Mater. 2019, 367, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.; Shi, X.; Huang, F.; Fujitsuka, M.; Majima, T. Efficient photocatalytic H2 evolution using NiS/ZnIn2S4 heterostructures with enhanced charge separation and interfacial charge transfer. Appl. Catal. B 2019, 250, 163–170. [Google Scholar] [CrossRef]
- Lim, W.Y.; Hong, M.; Ho, G.W. In situ photo-assisted deposition and photocatalysis of ZnIn2S4/transition metal chalcogenides for enhanced degradation and hydrogen evolution under visible light. Dalton Trans. 2016, 45, 552–560. [Google Scholar] [CrossRef]
- Jiao, X.; Chen, Z.; Li, X.; Sun, Y.; Gao, S.; Yan, W.; Wang, C.; Zhang, Q.; Lin, Y.; Luo, Y.; et al. Defect-Mediated Electron-Hole Separation in One-Unit-Cell ZnIn2S4 Layers for Boosted Solar-Driven CO2 Reduction. J. Am. Chem. Soc. 2017, 139, 7586–7594. [Google Scholar] [CrossRef]
- Ye, L.; Fu, J.; Xu, Z.; Yuan, R.; Li, Z. Facile one-pot solvothermal method to synthesize sheet-on-sheet reduced graphene oxide (RGO)/ZnIn2S4 nanocomposites with superior photocatalytic performance. ACS Appl. Mater. Interfaces 2014, 6, 3483–3490. [Google Scholar] [CrossRef]
- Che, W.; Luo, Y.; Deng, F.; Zhang, A.; Zhao, L.; Luo, X.; Ruan, Q. Facile solvothermal fabrication of cubic-like reduced graphene oxide/AgIn5S8 nanocomposites with anti-photocorrosion and high visible-light photocatalytic performance for highly-efficient treatment of nitrophenols and real pharmaceutical wastewater. Appl. Catal. A Gen. 2018, 565, 170–180. [Google Scholar] [CrossRef]
- Wang, J.; Tsuzuki, T.; Tang, B.; Hou, X.; Sun, L.; Wang, X. Reduced graphene oxide/ZnO composite: Reusable adsorbent for pollutant management. ACS Appl. Mater. Interfaces 2012, 4, 3084–3090. [Google Scholar] [CrossRef]
- Xie, C.; Lu, X.; Deng, F.; Luo, X.; Gao, J.; Dionysiou, D.D. Unique surface structure of nano-sized CuInS2 anchored on rGO thin film and its superior photocatalytic activity in real wastewater treatment. Chem. Eng. J. 2018, 338, 591–598. [Google Scholar] [CrossRef]
- Li, H.; Yu, H.; Chen, S.; Zhao, H.; Zhang, Y.; Quan, X. Fabrication of graphene wrapped ZnIn2S4 microspheres heterojunction with enhanced interfacial contact and its improved photocatalytic performance. Dalton Trans. 2014, 43, 2888–2894. [Google Scholar] [CrossRef] [PubMed]
- Jeevitha, G.; Abhinayaa, R.; Mangalaraj, D.; Ponpandian, N. Tungsten oxide-graphene oxide (WO3-GO) nanocomposite as an efficient photocatalyst, antibacterial and anticancer agent. J. Phys. Chem. Solids 2018, 116, 137–147. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, M.; He, X.; Du, T.; Wang, Y.; Li, Y.; Hao, T. Facile prepared ball-like TiO2 at GO composites for oxytetracycline removal under solar and visible lights. Water Res. 2019, 160, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Khazaee, Z.; Mahjoub, A.R.; Cheshme Khavar, A.H.; Srivastava, V.; Sillanpää, M. Synthesis of layered perovskite Ag,F-Bi2MoO6/rGO: A surface plasmon resonance and oxygen vacancy promoted nanocomposite as a visible-light photocatalyst. J. Photochem. Photobiol. A Chem. 2019, 379, 130–143. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Liu, C.; Luo, S.; Wang, L.; Cai, T.; Zeng, Y.; Yuan, J.; Dong, W.; Pei, Y.; et al. MoS2 Quantum Dot Growth Induced by S Vacancies in a ZnIn2S4 Monolayer: Atomic-Level Heterostructure for Photocatalytic Hydrogen Production. ACS Nano 2018, 12, 751–758. [Google Scholar] [CrossRef]
- Tien, H.N.; Luan, V.H.; Hoa, L.T.; Khoa, N.T.; Hahn, S.H.; Chung, J.S.; Shin, E.W.; Hur, S.H. One-pot synthesis of a reduced graphene oxide–zinc oxide sphere composite and its use as a visible light photocatalyst. Chem. Eng. J. 2013, 229, 126–133. [Google Scholar] [CrossRef]
- Yan, T.; Yan, Q.; Wang, X.; Liu, H.; Li, M.; Lu, S.; Xu, W.; Sun, M. Facile fabrication of heterostructured g-C3N4/Bi2MoO6 microspheres with highly efficient activity under visible light irradiation. Dalton Trans. 2015, 44, 1601–1611. [Google Scholar] [CrossRef]
- Miao, X.; Ji, Z.; Wu, J.; Shen, X.; Wang, J.; Kong, L.; Liu, M.; Song, C. g-C3N4/AgBr nanocomposite decorated with carbon dots as a highly efficient visible-light-driven photocatalyst. J Colloid. Interface Sci. 2017, 502, 24–32. [Google Scholar] [CrossRef]
- Chen, F.; Li, D.; Luo, B.; Chen, M.; Shi, W. Two-dimensional heterojunction photocatalysts constructed by graphite-like C3N4 and Bi2WO6 nanosheets: Enhanced photocatalytic activities for water purification. J. Alloys Compd. 2017, 694, 193–200. [Google Scholar] [CrossRef]
- Dulova, N.; Kattel, E.; Trapido, M. Degradation of naproxen by ferrous ion-activated hydrogen peroxide, persulfate and combined hydrogen peroxide/persulfate processes: The effect of citric acid addition. Chem. Eng. J. 2017, 318, 254–263. [Google Scholar] [CrossRef]
- Arany, E.; Szabo, R.K.; Apati, L.; Alapi, T.; Ilisz, I.; Mazellier, P.; Dombi, A.; Gajda-Schrantz, K. Degradation of naproxen by UV, VUV photolysis and their combination. J. Hazard Mater. 2013, 262, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-q.; Gao, N.-y.; Chu, W.-h.; Yang, Q.-l.; Yin, D.-q. Kinetics and mechanistic investigation into the degradation of naproxen by a UV/chlorine process. RSC Adv. 2017, 7, 33627–33634. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Motti, C.A.; Glass, B.D.; Oelgemoller, M. TiO2 photocatalysis of naproxen: Effect of the water matrix, anions and diclofenac on degradation rates. Chemosphere 2015, 139, 579–588. [Google Scholar] [CrossRef] [PubMed]
- DellaGreca, M.; Brigante, M.; Isidori, M.; Nardelli, A.; Previtera, L.; Rubino, M.; Temussi, F. Phototransformation and ecotoxicity of the drug Naproxen-Na. Environ. Chem. Lett. 2003, 1, 237–241. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, K.; Pan, Y.; Ding, C.; Shi, J.; Deng, H. Reduced Graphene Oxide/ZnIn2S4 Nanocomposite Photocatalyst with Enhanced Photocatalytic Performance for the Degradation of Naproxen under Visible Light Irradiation. Catalysts 2020, 10, 710. https://doi.org/10.3390/catal10060710
Fu K, Pan Y, Ding C, Shi J, Deng H. Reduced Graphene Oxide/ZnIn2S4 Nanocomposite Photocatalyst with Enhanced Photocatalytic Performance for the Degradation of Naproxen under Visible Light Irradiation. Catalysts. 2020; 10(6):710. https://doi.org/10.3390/catal10060710
Chicago/Turabian StyleFu, Kun, Yishuai Pan, Chao Ding, Jun Shi, and Huiping Deng. 2020. "Reduced Graphene Oxide/ZnIn2S4 Nanocomposite Photocatalyst with Enhanced Photocatalytic Performance for the Degradation of Naproxen under Visible Light Irradiation" Catalysts 10, no. 6: 710. https://doi.org/10.3390/catal10060710
APA StyleFu, K., Pan, Y., Ding, C., Shi, J., & Deng, H. (2020). Reduced Graphene Oxide/ZnIn2S4 Nanocomposite Photocatalyst with Enhanced Photocatalytic Performance for the Degradation of Naproxen under Visible Light Irradiation. Catalysts, 10(6), 710. https://doi.org/10.3390/catal10060710



