Hepatoprotective Effects of Pleurotus ostreatus Protein Hydrolysates Yielded by Pepsin Hydrolysis
Abstract
1. Introduction
2. Results
2.1. Degree of Hydrolysis
2.2. Amino Acid Composition
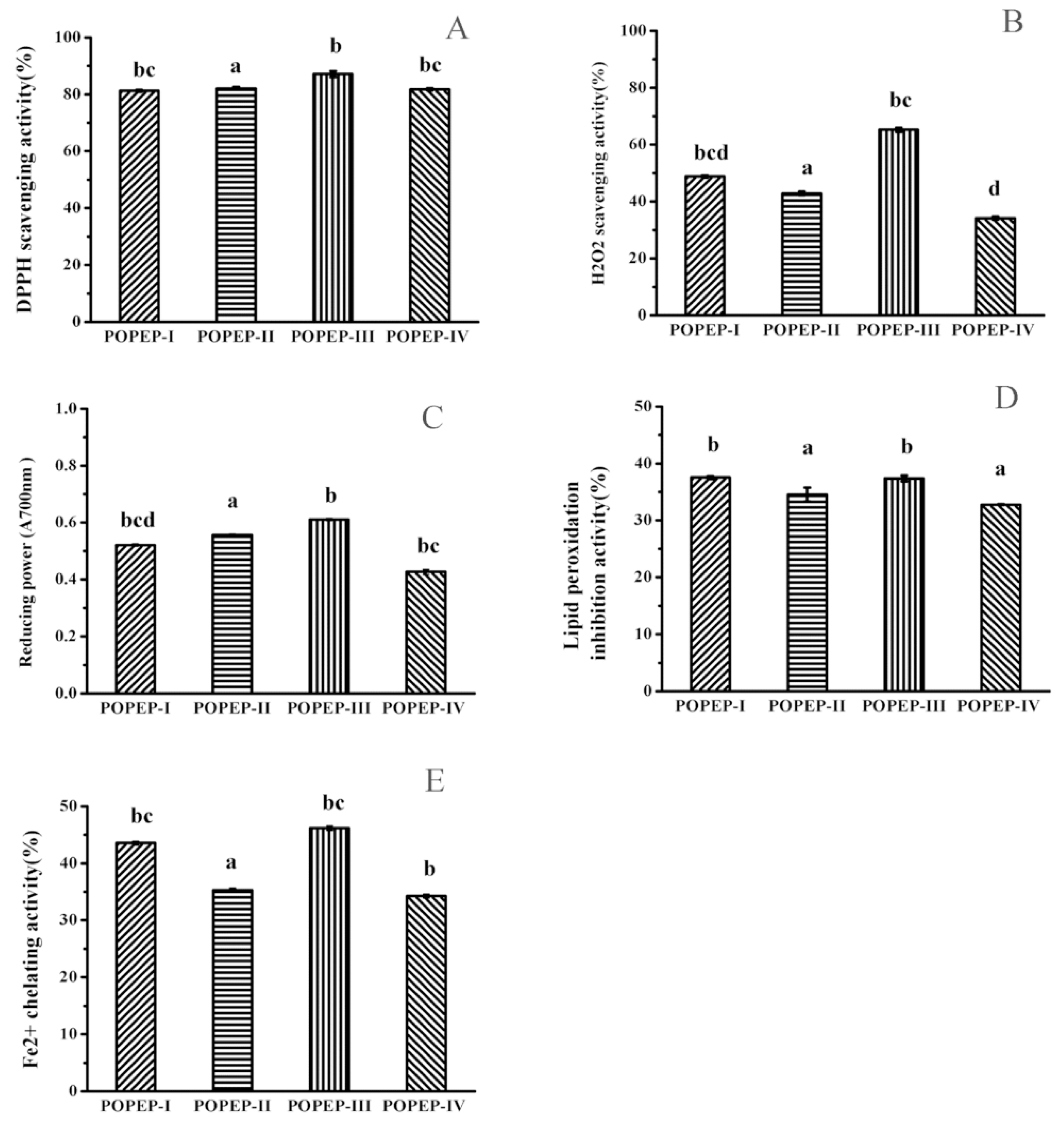
2.3. Preparation of Protein Hydrolysatess and Antioxidant Activity
2.4. Effects of POPEP-III on Liver Weight, HI and Body Weight in Mice
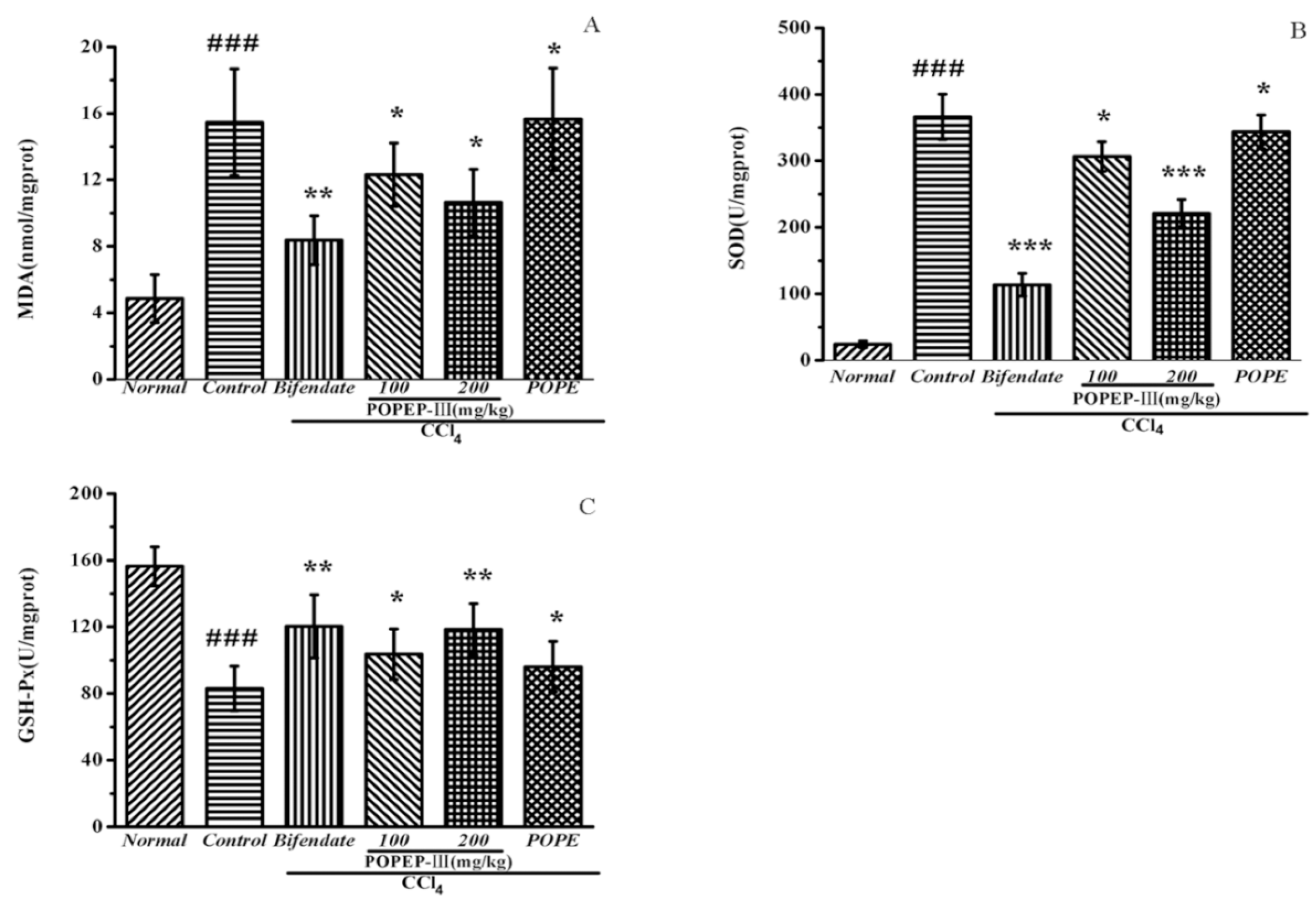
2.5. Effects of POPEP-III on AST, ALT, MDA, GSH-Px, and SOD Activities
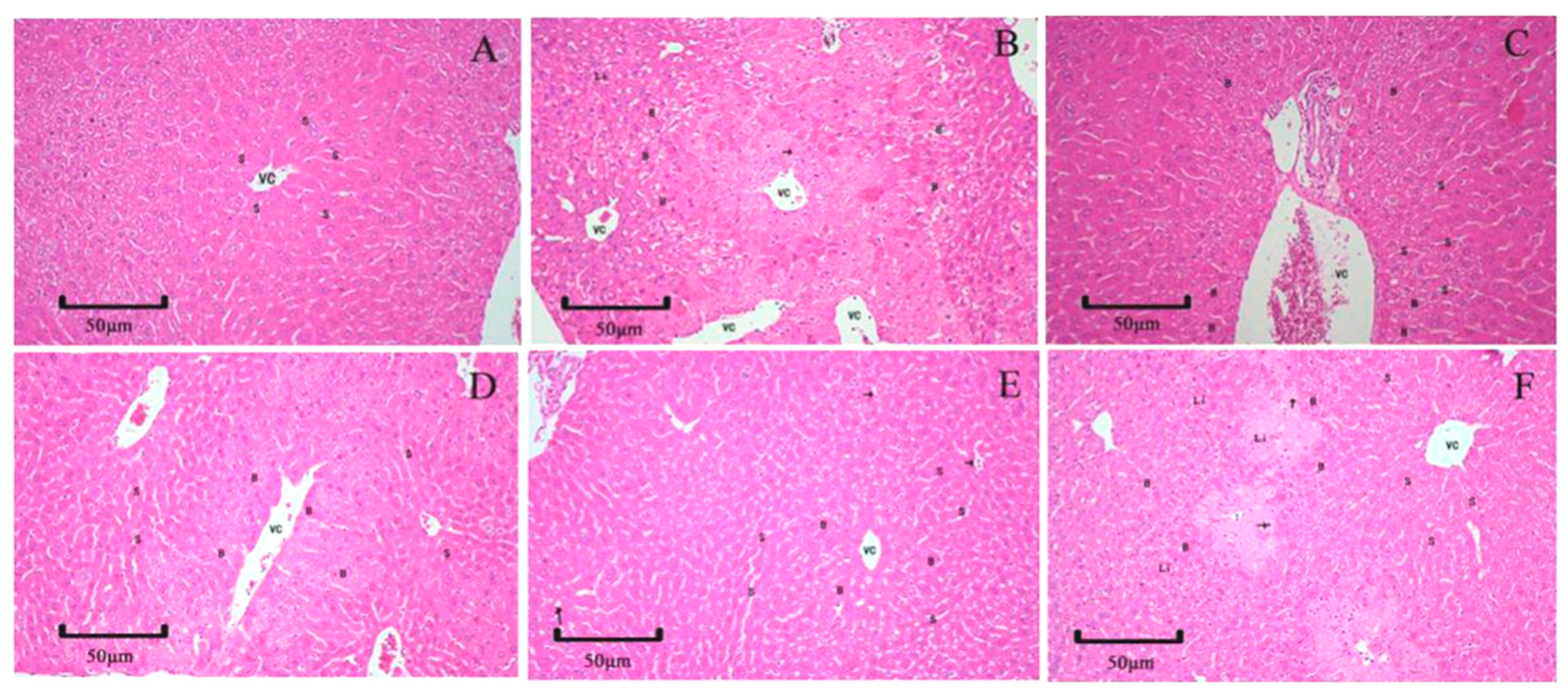
2.6. Histopathology Examination
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of Crude P. ostreatus Protein
3.3. Enzymatic Hydrolysis of POPE
3.4. Ultrafiltration
3.5. Degree of Hydrolysis (DH)
3.6. Analysis of Amino Acid Composition and Molecular Weight Distribution of POPEs
3.7. Analysis of Antioxidant Activities In Vitro
3.7.1. Free Radical Scavenging Activity
3.7.2. Hydrogen Peroxide Scavenging Activity
3.7.3. Reducing Power
3.7.4. Inhibitory Effect on Lipid Peroxidation
3.7.5. Ferrous Ion Chelating Activity
3.8. Animal Experiments
3.9. Biochemical Assays
3.10. Histopathological Study on the Liver
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| MDA | malondialdehyde |
| SOD | superoxide dismutase |
| GSH-Px | glutathione peroxidase |
| ROS | reactive oxygen species |
| HI | hepatosomatic index |
| THAA | total hydrophobic amino acid |
| BSA | bovine serum albumin |
References
- Gao, B.; Jeong, W.; Tian, Z.G. Liver: An organ with predominant innate immunity. Hepatology 2008, 47, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Taub, R.A. Liver regeneration: From myth to mechanism. Nat. Rev. Mol. Cell Biol. 2004, 5, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Stoyanovsky, D.A.; Cederbaum, A.I. Thiol oxidation and cytochrome P450-dependent metabolism of CCl4 triggers Ca2+ release from liver microsome. Biochemistry 1996, 35, 15839–15845. [Google Scholar] [CrossRef] [PubMed]
- Byass, P. The global burden of liver disease: A challenge for methods and for public health. BMC Med. 2014, 12, 159. [Google Scholar] [CrossRef] [PubMed]
- Ronchi, J.A.; Vercesi, A.E.; Castilho, R.F. Reactive oxygen species and permeability transition pore in rat liver and kidney mitoplasts. J. Bioenerg. Biomembr. 2011, 43, 709–715. [Google Scholar] [CrossRef]
- Fernandez-Checa, J.C.; Kaplowitz, N. Hepatic mitochondrial glutathione: Transport and role in disease and toxicity. Toxicol. Appl. Pharm. 2005, 204, 263–273. [Google Scholar] [CrossRef]
- Paik, Y.H.; Kim, J.; Aoyama, T.; Minicis, S.D.; Bataller, R.; Brenner, D.A. Role of NADPH oxidases in liver fibrosis. Antioxid. Redox Sign. 2014, 20, 2854–2872. [Google Scholar] [CrossRef]
- Choi, J.; James Ou, J.H. Mechanisms of Liver Injury. III. Oxidative stress in the pathogenesis of hepatitis C virus. Am. J. Physiol.-Gastrointest. Liver Physiol. 2006, 290, 847–851. [Google Scholar] [CrossRef]
- Akanitapichat, P.; Phraibung, K.; Nuchklang, K.; Prompitakkul, S. Antioxidant and hepatoprotective activities of five eggplant varieties. Food Chem. Toxicol. 2010, 48, 3017–3021. [Google Scholar] [CrossRef]
- Panda, V.; Ashar, H.; Srinath, S. Antioxidant and hepatoprotective effect of Garcinia indica fruit rind in ethanol induced hepatic damage in rodents. Interdiscip. Toxicol. 2012, 5, 207–213. [Google Scholar] [CrossRef]
- Zarezade, V.; Moludi, J.; Mostafazadeh, M.; Mohammadi, M.; Veisi, A. Antioxidant and hepatoprotective effects of Artemisia dracunculus against CCl4-induced hepatotoxicity in rats. Avicenna J. Phytomed. 2018, 8, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Bak, M.J.; Jun, M.; Jeong, W.S. Antioxidant and hepatoprotective effects of the red ginseng essential oil in H2O2-treated HepG2 cells and CCl4-treated mice. Int. J. Mol. Sci. 2012, 13, 2314–2330. [Google Scholar] [CrossRef] [PubMed]
- Polyak, S.J.; Morishima, C.; Lohmann, V.; Pal, S.; Lee, D.Y.W.; Liu, Y.Z.; Graf, T.N.; Oberlies, N.H. Identification of hepatoprotective flavonolignans from silymarin. Proc. Natl. Acad. Sci. USA 2010, 107, 5995–5999. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhu, M.J.; Geng, X.R.; Wang, H.X.; Ng, T.B. Characterization of polysaccharides with antioxidant and hepatoprotective activities from the edible mushroom Oudemansiella radicata. Molecules 2017, 22, 234. [Google Scholar] [CrossRef]
- Liu, Q.; Tian, G.T.; Yan, H.; Geng, X.R.; Cao, Q.P.; Wang, H.X.; Ng, T.B. Characterization of polysaccharides with antioxidant and hepatoprotective activities from the wild edible mushroom Russula vinosa Lindblad. J. Agric. Food Chem. 2014, 62, 8858–8866. [Google Scholar] [CrossRef]
- Zhao, H.J.; Zhang, J.J.; Liu, X.C.; Yang, Q.H.; Dong, Y.H.; Jia, L. The antioxidant activities of alkalicextractable polysaccharides from Coprinus comatus on alcohol induced liver injury in mice. Sci. Rep. 2018, 8, 11695. [Google Scholar] [CrossRef]
- Sun, J.; He, H.; Xie, B.J. Novel antioxidant peptides from fermented mushroom Ganoderma lucidum. J. Agric. Food Chem. 2004, 52, 6646–6652. [Google Scholar] [CrossRef]
- Zhu, B.; Li, Y.Z.; Hu, T.; Zhang, Y. The hepatoprotective effect of polysaccharides from Pleurotus ostreatus on carbon tetrachloride-induced acute liver injury rats. Int. J. Biol. Macromol. 2019, 131, 1–9. [Google Scholar] [CrossRef]
- Xia, F.G.; Fan, J.H.; Zhu, M.; Tong, H.B. Antioxidant effects of a water-soluble proteoglycan isolated from the fruiting bodies of Pleurotus ostreatus. J. Taiwan Inst. Chem. E. 2011, 42, 402–407. [Google Scholar] [CrossRef]
- Thanaswkaran, J.; Muniyan, S.; Thomas, P.A.; Geraldine, P.L. Pleurotus ostreatus, an oyster mushroom, decreases the oxidative stress induced by carbon tetrachloride in rat kidneys, heart and brain. Chem.-Biol. Interactions 2008, 176, 108–120. [Google Scholar] [CrossRef]
- Thanaswkaran, J.; Ramesh, E.; Geraldine, P. Antioxidant activity of the oyster mushroom, Pleurotus ostreatus, on CCl4-induced liver injury in rats. Food Chem. Toxicol. 2006, 44, 1989–1996. [Google Scholar] [CrossRef]
- Llauradó, G.; Morris, H.J.; Lebeque, Y.; Venet, G.; Fong, O.; Marcos, J.; Fontaine, R.; Cos, P.; Bermúdez, R.C. Oral administration of an aqueous extract from the oyster mushroom Pleurotus ostreatus enhances the immunonutritional recovery of malnourished mice. Biomed. Pharmacother. 2016, 83, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Vieira Gomes, D.C.; de Alencar, M.V.O.B.; Dos Reis, A.C.; de Lima, R.M.T.; de Oliveira Santos, J.V.; da Mata, A.M.O.F.; Soares Dias, A.C.; da Costa, J.S.J.; de Medeiros, M.D.G.F.; Paz, M.F.C.J.; et al. Antioxidant, anti-inflammatory and cytotoxic/antitumoral bioactives from the phylum Basidiomycota and their possible mechanisms of action. Biomed. Pharmacother. 2019, 112, 108643. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.J.; Sun, Y.H.; Chen, S.W.; Zhang, J.Y.; Kang, J.J.; Wang, Y.Q.; Wang, H.X.; Xia, G.L.; Liu, Q.H.; Kang, Y.M. Mushroom lectin enhanced immunogenicity of HBV DNA vaccine in C57BL/6 and HBsAg-transgenic mice. Vaccine 2013, 31, 2273–2280. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Gao, J.Q.; Ng, T.B. A new lectin with highly potent antihepatoma and antisarcoma activities from the oyster mushroom Pleurotus ostreatus. Biochem. Bioph. Res. Co. 2000, 275, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, N.; Mendis, E.; Jung, W.K.; Je, J.Y.; Kim, S.K. Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Food Res. Int. 2005, 38, 175–182. [Google Scholar] [CrossRef]
- Sun, Q.; Shen, H.X.; Luo, Y.K. Antioxidant activity of hydrolysates and peptide fractions derived from porcine hemoglobin. J. Food Sci. Tech. 2011, 48, 53–60. [Google Scholar] [CrossRef]
- Xie, Z.J.; Huang, J.R.; Xu, X.M.; Jin, Z.Y. Antioxidant activity of peptides isolated from alfalfa leaf protein hydrolysate. Food Chem. 2008, 111, 370–376. [Google Scholar] [CrossRef]
- Souza, D.; Sbardelotto, A.F.; Ziegler, D.R.; Marczak, L.D.; Tessaro, I.C. Characterization of rice starch and protein obtained by a fast alkaline extraction method. Food Chem. 2016, 191, 36–44. [Google Scholar] [CrossRef]
- Saeed, M.; Cheryan, M. Sunflower protein concentrates and isolates low in polyphenols and phytate. J. Food Sci. 1988, 53, 1127–1131. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Cheng, I.C.; Liao, J.X.; Ciou, J.Y.; Huang, L.T.; Chen, Y.W.; Hou, C.Y. Characterization of Protein Hydrolysates from Eel (Anguilla marmorata) and Their Application in Herbal Eel Extracts. Catalysts 2020, 10, 205. [Google Scholar] [CrossRef]
- Wen, T.; Yan, D.D.; Meng, J.; Liu, J.; Xu, H.Y. The enzyme-like property and photocatalytic effect on α, α-diphenyl-β-picrylhydrazyl (DPPH) of CuPt nanocomposite. Catalysts 2019, 9, 813. [Google Scholar] [CrossRef]
- Pushparaj, F.S.; Urooj, A. Antioxidant activity in two pearl millet (Pennisetum typhoideum) cultivars as influenced by processing. Antioxidants 2014, 3, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, S.Y.; Deng, Q.Y.; Li, G.L.; Su, G.C.; Liu, J.W.; Wang, H.M.D. Extraction and characterization of phenolic compounds with antioxidant and antimicrobial activities from pickled radish. Food Chem Toxicol. 2020, 136, 111050–1111055. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.H.; Yang, J.H.; Mau, J.L. Antioxidant properties of polysaccharides from Ganoderma tsugae. Food Chem. 2008, 107, 732–738. [Google Scholar] [CrossRef]
- Chou, H.Y.; Wang, H.M.D.; Kuo, C.H.; Lu, P.H.; Wang, L.; Kang, W.Y.; Sun, C.L. Antioxidant graphene oxide nanoribbon as a novel whiting agent inhibits microphthalmia-associated transcription factor related melanogenesis mechanism. ACS Omega 2020, in press. [Google Scholar] [CrossRef]
- Lu, X.S.; Zhao, Y.; Sun, Y.F.; Yang, S.; Yang, X.X. Characterisation of polysaccharides from green tea of Huangshan Maofeng with antioxidant and hepatoprotective effects. Food Chem. 2013, 141, 3415–3423. [Google Scholar] [CrossRef]
- Al-Megrin, W.A.; Alkhuriji, A.F.; Yousef, A.O.S.; Metwally, D.M.; Habotta, O.A.; Kassab, R.B.; Abdel Moneim, A.E.; El-Khadragy, M.F. Antagonistic efficacy of luteolin against lead acetate exposure-associated with hepatotoxicity is mediated via antioxidant, anti-inflammatory, and anti-apoptotic activities. Antioxidants 2020, 9, 10. [Google Scholar] [CrossRef]


| POPE (mg) | >10 kDa (mg) | 5–10 kDa (mg) | 3–5 kDa (mg) | <3 kDa (mg) | DH (%) | |
|---|---|---|---|---|---|---|
| Pep | 500 | 319.96 ± 3.15 | 45.76 ± 1.15 | 23.15 ± 0.86 | 11.14 ± 0.19 | 16.01 ± 0.12 |
| Try | 500 | 431.77 ± 5.31 | 34.79 ± 2.19 | 19.68 ± 1.17 | 10.32 ± 1.39 | 12.96 ± 0.33 |
| Dis | 500 | 429.63 ± 7.31 | 44.39 ± 0.98 | 17.93 ± 2.87 | 9.68 ± 2.63 | 14.40 ± 0.19 |
| Pap | 500 | 434.75 ± 4.39 | 39.37 ± 2.33 | 20.19 ± 1.75 | 10.17 ± 3.18 | 13.95 ± 0.22 |
| Bro | 500 | 430.07 ± 8.63 | 40.33 ± 1.77 | 19.47 ± 0.96 | 8.65 ± 1.19 | 13.67 ± 0.96 |
| Amino Acid (g/100 g) | POPE | POPEP-I (>10 kDa) | POPEP-II (5–10 kDa) | POPEP-III (3–5 kDa) | POPEP-IV (<3 kDa) | |
|---|---|---|---|---|---|---|
| Hydrophobic | Phe | 8.02 | 7.89 | 5.39 | 14.27 | 7.78 |
| Leu | 8.56 | 8.82 | 5.84 | 7.11 | 11.6 | |
| Ala | 5.67 | 5.83 | 4.39 | 5.13 | 6.13 | |
| Met | 13.8 | 11.04 | 11.95 | 11.52 | 13.02 | |
| Val | 6.52 | 5.57 | 5.08 | 6.05 | 5.99 | |
| Ile | 5.45 | 4.84 | 4.17 | 4.94 | 4.84 | |
| Gly | 3.96 | 3.62 | 4.75 | 4.48 | 3.08 | |
| Pro | 3.53 | 2.57 | 3.51 | 4.02 | 3.19 | |
| Hydrophilic | Thr | 3.09 | 4.40 | 2.89 | 3.53 | 3.48 |
| Ser | 3.64 | 3.84 | 3.94 | 3.23 | 4.27 | |
| Cys | 2.35 | 2.20 | 4.35 | 1.88 | 1.21 | |
| Tyr | 6.74 | 8.20 | 4.91 | 4.66 | 4.6 | |
| Lys | 3.1 | 2.75 | 3.19 | 2.27 | 1.94 | |
| Arg | 4.28 | 4.28 | 3.81 | 3.35 | 4.6 | |
| His | 1.39 | 1.45 | 1.39 | 1.09 | 1 | |
| Asp | 6.74 | 7.22 | 6.71 | 7.21 | 6.18 | |
| Glu | 9.2 | 8.91 | 13.37 | 11.32 | 8.31 | |
| DPPH Scavenging Activity (%) | H2O2 Scavenging Activity (%) | Reducing Power (A700nm) | Lipid Peroxidantion Inhibitionactivity (%) | Ferrous Ion Chelating Activity (%) | |
|---|---|---|---|---|---|
| Pep | 91.32 ± 0.60 *** | 62.52 ± 14.42 * | 0.87 ± 0.008 *** | 63.34 ± 0.34 *** | 84.28 ± 0.92 *** |
| Try | 56.27 ± 2.30 * | 43.47 ± 3.09 *** | 0.51± 0.02 * | 47.56 ± 0.88 *** | 91.06 ± 0.36 *** |
| Dis | 76.51 ± 0.68 *** | 26.37 ± 2.28 *** | 0.90 ± 0.04 * | 70.89 ± 0.78 *** | 84.98 ± 0.19 *** |
| Pap | 89.19 ± 0.66 *** | 54.93 ± 0.42 *** | 0.68 ± 0.02 * | 32.17 ± 0.37 ** | 49.37 ± 1.44 *** |
| Bro | 49.46 ± 1.39 * | 50.34 ± 2.67 ** | 0.84 ± 0.02 *** | 49.26 ± 0.10 *** | 68.22 ± 0.42 *** |
| POPE | 52.64 ± 0.62 | 10.01 ± 1.84 | 0.49 ± 0.004 | 36.89 ± 0.66 | 18.05 ± 0.81 |
| DPPH Scavenging Activity (%) | H2O2 Scavenging Activity (%) | Reducing Power (A700nm) | Lipid Peroxidantion Inhibitionactivity (%) | Ferrous Ion Chelating Activity (%) | |
|---|---|---|---|---|---|
| POPEP-I | 81.26 ± 0.40 b,c | 48.79 ± 0.34 b,c,d | 0.52 ± 0.004 b,c,d | 37.53 ± 0.24 b | 43.56 ± 0.31 b,c |
| POPEP-II | 82.1 ± 1.00 a | 42.87 ± 0.60 a | 0.56 ± 0.04 a | 34.56 ± 1.21 a | 35.28 ± 0.35 b,c |
| POPEP-III | 87.15 ± 0.55 b | 65.22 ± 0.78 b,c | 0.61 ± 0.004 b | 37.38 ± 0.50 b | 46.19 ± 0.39 b,c |
| POPEP-IV | 81.73 ± 0.37 b,c | 34.18 ± 0.50 d | 0.43 ± 0.08 b,c | 32.77 ± 0.09 a | 34.25 ± 0.35 b |
| Treatments | Dose (mg/kg) | Body Weight (g) | Liver Weight (g) | HI (%) |
|---|---|---|---|---|
| Normal | - | 34.32 ± 2.98 a | 1.21 ± 0.14 b | 35.1 ± 2.27 c |
| CCl4 only | - | 33.35 ± 2.15 a | 1.43 ± 0.27 a | 42.49 ± 5.29 a |
| CCl4 + Bifendate | 200 | 34.32 ± 2.35 a | 1.24 ± 0.1 b | 36.1 ± 1.86 c |
| CCl4 + POPEP-III | 100 | 34.02 ± 2.28 a | 1.26 ± 0.13 a | 36.36 ± 4.45 c |
| CCl4 + POPEP-III | 200 | 34.02 ± 2.28 a | 1.26 ± 0.13 a | 36.97 ± 2.54 c |
| CCl4 + POPE | 200 | 32.83 ± 2.41 a | 1.26 ± 0.16 a | 38.21 ± 2.61 b |
| ALT (IU/L) | AST (IU/L) | |
|---|---|---|
| Normal | 24.68 ± 5.64 | 44.62 ± 5.25 |
| Control | 365.44 ± 36.87 ### | 352.52 ± 13.44 ### |
| Bifendate | 114.18 ± 18.36 ** | 144.57 ± 21.81 *** |
| POPEP-III 100 | 312.53 ± 24.04 * | 238.83 ± 25.17 *** |
| POPEP-III 200 | 220.23 ± 22.27 ** | 206.75 ± 17.26 *** |
| POPE | 341.93 ± 27.56 * | 333.40 ± 19.04 * |
| MDA (nmol/mg prot) | SOD (U/mg prot) | GSH-Px (U/mg prot) | |
|---|---|---|---|
| Normal | 5.28 ± 1.55 | 237.65 ± 36.06 | 152.11 ± 12.58 |
| Control | 15.28 ± 3.47 ### | 187.49 ± 19.81 ### | 84.01 ± 14.54 ### |
| Bifendate | 8.9 ± 1.58 ** | 240.11 ± 34.22 *** | 118.12 ± 20.44 ** |
| POPEP-III 100 | 12.07 ± 2.16 * | 205.65 ± 24.31 * | 100.86 ± 16.37 * |
| POPEP-III 200 | 10.04 ± 2.06 * | 233.35 ± 34.23 *** | 115.9 ± 16.57 ** |
| POPEP | 15.01 ±3.32 * | 213.45 ± 21.45 * | 86.61 ± 16.35 * |
| Protease | Activity (U/g) | Optimal Conditions | |||
|---|---|---|---|---|---|
| Temp. (°C) | pH | Time (h) | E/S (w/w) | ||
| Pepsin (Porcine stomach mucosa) | 30,000 | 37 | 2.0 | 3 | 0.5% |
| Trypsin (Porcine pancreas) | 25,000 | 45 | 8.0 | 5 | 0.5% |
| Dispase (Bacillus subtilis) | >60,000 | 50 | 7.0 | 5 | 0.5% |
| Papain (Carica papaya) | 800,000 | 45 | 6.2 | 5 | 0.5% |
| Bromelin (Pineapple) | 600,000 | 45 | 6.0 | 5 | 0.5% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Lu, Y.; Feng, X.; Liu, Q.; Li, Y.; Hao, J.; Wang, Y.; Dong, Y.; Wang, H.D. Hepatoprotective Effects of Pleurotus ostreatus Protein Hydrolysates Yielded by Pepsin Hydrolysis. Catalysts 2020, 10, 595. https://doi.org/10.3390/catal10060595
Zhang L, Lu Y, Feng X, Liu Q, Li Y, Hao J, Wang Y, Dong Y, Wang HD. Hepatoprotective Effects of Pleurotus ostreatus Protein Hydrolysates Yielded by Pepsin Hydrolysis. Catalysts. 2020; 10(6):595. https://doi.org/10.3390/catal10060595
Chicago/Turabian StyleZhang, Liwei, Yuxiao Lu, Xiaobin Feng, Qinghong Liu, Yuanhui Li, Jiamin Hao, Yanqiong Wang, Yongqiang Dong, and Huimin David Wang. 2020. "Hepatoprotective Effects of Pleurotus ostreatus Protein Hydrolysates Yielded by Pepsin Hydrolysis" Catalysts 10, no. 6: 595. https://doi.org/10.3390/catal10060595
APA StyleZhang, L., Lu, Y., Feng, X., Liu, Q., Li, Y., Hao, J., Wang, Y., Dong, Y., & Wang, H. D. (2020). Hepatoprotective Effects of Pleurotus ostreatus Protein Hydrolysates Yielded by Pepsin Hydrolysis. Catalysts, 10(6), 595. https://doi.org/10.3390/catal10060595






