Transition Metal Sulfides- and Noble Metal-Based Catalysts for N-Hexadecane Hydroisomerization: A Study of Poisons Tolerance
Abstract
1. Introduction
2. Results
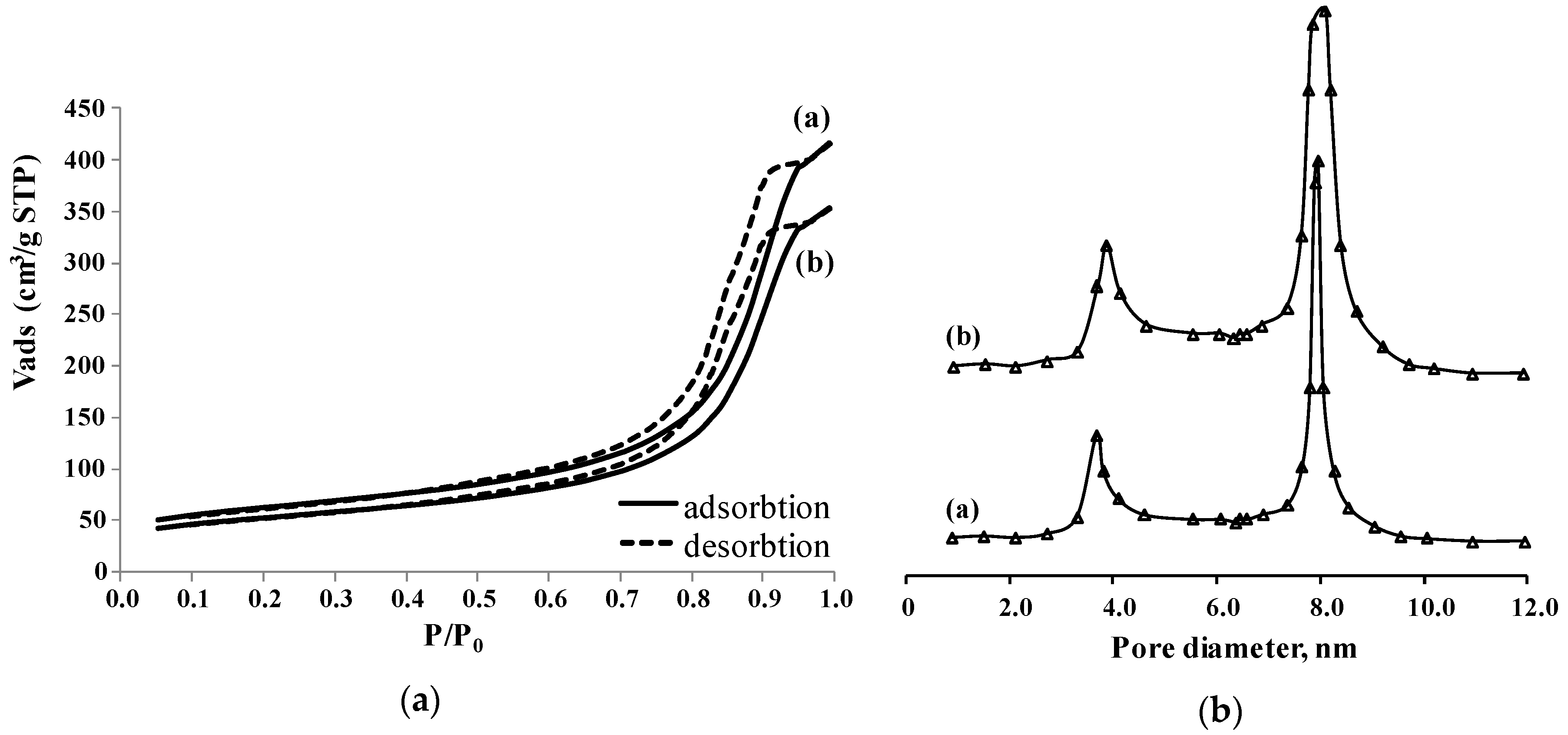
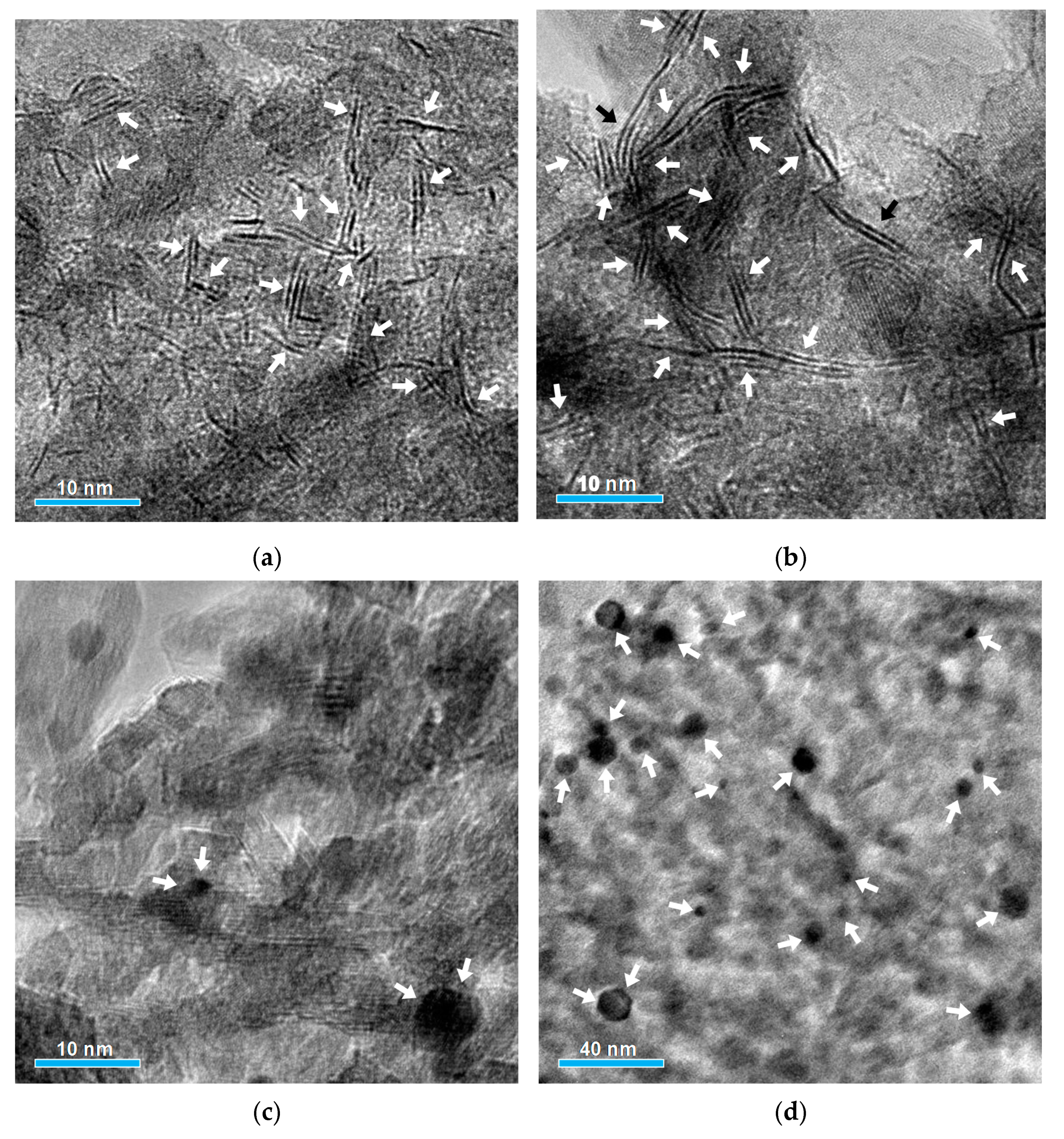
2.1. Physical-Chemical Properties of the Solids
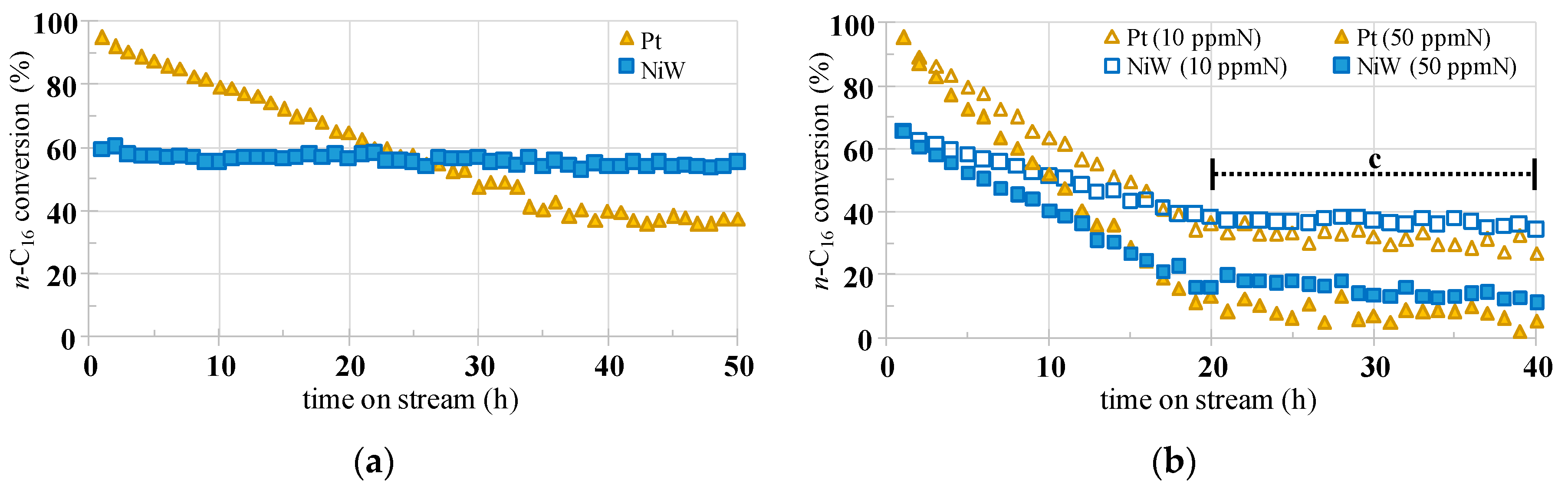
2.2. Catalytic Properties Examination
3. Materials and Methods
3.1. Preparation of the Solids
3.2. Characterization of Supports and Catalysts
3.3. Catalytic Activity Examination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mäki-Arvela, P.; Kaka khel, T.; Azkaar, M.; Engblom, S.; Murzin, D. Catalytic Hydroisomerization of Long-Chain Hydrocarbons for the Production of Fuels. Catalysts 2018, 8, 534. [Google Scholar] [CrossRef]
- Len, C.; Luisi, R. Catalytic Methods in Flow Chemistry. Catalysts 2019, 9, 663. [Google Scholar] [CrossRef]
- Lázaro, N.; Franco, A.; Ouyang, W.; Balu, A.; Romero, A.; Luque, R.; Pineda, A. Continuous-Flow Hydrogenation of Methyl Levulinate Promoted by Zr-Based Mesoporous Materials. Catalysts 2019, 9, 142. [Google Scholar] [CrossRef]
- An, K.; Zhang, Q.; Alayoglu, S.; Musselwhite, N.; Shin, J.-Y.; Somorjai, G.A. High-Temperature Catalytic Reforming of n -Hexane over Supported and Core–Shell Pt Nanoparticle Catalysts: Role of Oxide–Metal Interface and Thermal Stability. Nano Lett. 2014, 14, 4907–4912. [Google Scholar] [CrossRef] [PubMed]
- Beltramini, J.; Trimm, D.L. Catalytic reforming of n-heptane on platinum, tin and platinum-tin supported on alumina. Appl. Catal. 1987, 31, 113–118. [Google Scholar] [CrossRef]
- Weyda, H.; Köhler, E. Modern refining concepts—An update on naphtha-isomerization to modern gasoline manufacture. Catal. Today 2003, 81, 51–55. [Google Scholar] [CrossRef]
- Akhmedov, V.M.; Al-Khowaiter, S.H. Recent Advances and Future Aspects in the Selective Isomerization of High n-Alkanes. Catal. Rev. 2007, 49, 33–139. [Google Scholar] [CrossRef]
- Guisnet, M.; Gilson, J.-P. Zeolites for Cleaner Technologies Catalytic Science Series; Hutchings, G.J., Ed.; Imperial College Press: London, UK, 2002; Volume 3, ISBN 1-86094-329-2. [Google Scholar]
- Martens, J.A.; Verboekend, D.; Thomas, K.; Vanbutsele, G.; Gilson, J.-P.; Pérez-Ramírez, J. Hydroisomerization of Emerging Renewable Hydrocarbons using Hierarchical Pt/H-ZSM-22 Catalyst. ChemSusChem 2013, 6, 421–425. [Google Scholar] [CrossRef]
- Mortier, R.M.; Fox, M.F.; Malcolm, F.; Orszulik, S.T. Chemistry and Technology of Lubricants; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 9781402086625. [Google Scholar]
- Klein, A.; Keisers, K.; Palkovits, R. Formation of 1,3-butadiene from ethanol in a two-step process using modified zeolite-β catalysts. Appl. Catal. A Gen. 2016, 514, 192–202. [Google Scholar] [CrossRef]
- Mendes, P.S.F.; Mota, F.M.; Silva, J.M.; Ribeiro, M.F.; Daudin, A.; Bouchy, C. A systematic study on mixtures of Pt/zeolite as hydroisomerization catalysts. Catal. Sci. Technol. 2017, 7, 1095–1107. [Google Scholar] [CrossRef]
- Guisnet, M. “Ideal” bifunctional catalysis over Pt-acid zeolites. Catal. Today 2013, 218–219, 123–134. [Google Scholar] [CrossRef]
- Alvarez, F.; Ribeiro, F.R.; Perot, G.; Thomazeau, C.; Guisnet, M. Hydroisomerization and Hydrocracking of Alkanes: 7. Influence of the Balance between Acid and Hydrogenating Functions on the Transformation ofn-Decane on PtHY Catalysts. J. Catal. 1996, 162, 179–189. [Google Scholar] [CrossRef]
- Glotov, A.P.; Artemova, M.I.; Demikhova, N.R.; Smirnova, E.M.; Ivanov, E.V.; Gushchin, P.A.; Egazar’yants, S.V.; Vinokurov, V.A. A Study of Platinum Catalysts Based on Ordered Al–MCM-41 Aluminosilicate and Natural Halloysite Nanotubes in Xylene Isomerization. Pet. Chem. 2019, 59, 1226–1234. [Google Scholar] [CrossRef]
- Radlik, M.; Śrębowata, A.; Juszczyk, W.; Matus, K.; Małolepszy, A.; Karpiński, Z. n-Hexane conversion on γ-alumina supported palladium–platinum catalysts. Adsorption 2019, 25, 843–853. [Google Scholar] [CrossRef]
- van de Runstraat, A.; Kamp, J.A.; Stobbelaar, P.J.; van Grondelle, J.; Krijnen, S.; van Santen, R.A. Kinetics of Hydro-isomerization ofn-Hexane over Platinum Containing Zeolites. J. Catal. 1997, 171, 77–84. [Google Scholar] [CrossRef]
- Suárez París, R.; L’Abbate, M.E.; Liotta, L.F.; Montes, V.; Barrientos, J.; Regali, F.; Aho, A.; Boutonnet, M.; Järås, S. Hydroconversion of paraffinic wax over platinum and palladium catalysts supported on silica–alumina. Catal. Today 2016, 275, 141–148. [Google Scholar] [CrossRef]
- De Lucas, A.; Sánchez, P.; Fúnez, A.; Ramos, M.J.; Valverde, J.L. Influence of clay binder on the liquid phase hydroisomerization of n-octane over palladium-containing zeolite catalysts. J. Mol. Catal. A Chem. 2006, 259, 259–266. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, Z.; Wu, B.; Xu, J.; Huo, C.; Li, K.; Chen, H.; Yang, Y.; Li, Y. Effect of metal precursors on the performance of Pt/ZSM-22 catalysts for n -hexadecane hydroisomerization. J. Catal. 2015, 322, 1–13. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, S.; Zhang, H.; Lü, E.; Ren, J. Investigation of synthesis and hydroisomerization performance of SAPO-11/Beta composite molecular sieve. Chin. J. Catal. 2014, 35, 1676–1686. [Google Scholar] [CrossRef]
- Karakhanov, E.A.; Glotov, A.P.; Nikiforova, A.G.; Vutolkina, A.V.; Ivanov, A.O.; Kardashev, S.V.; Maksimov, A.L.; Lysenko, S.V. Catalytic cracking additives based on mesoporous MCM-41 for sulfur removal. Fuel Process. Technol. 2016, 153, 50–57. [Google Scholar] [CrossRef]
- Karakhanov, E.; Maximov, A.; Zolotukhina, A.; Vinokurov, V.; Ivanov, E.; Glotov, A. Manganese and Cobalt Doped Hierarchical Mesoporous Halloysite-Based Catalysts for Selective Oxidation of p-Xylene to Terephthalic Acid. Catalysts 2019, 10, 7. [Google Scholar] [CrossRef]
- Glotov, A.; Stytsenko, V.; Artemova, M.; Kotelev, M.; Ivanov, E.; Gushchin, P.; Vinokurov, V. Hydroconversion of Aromatic Hydrocarbons over Bimetallic Catalysts. Catalysts 2019, 9, 384. [Google Scholar] [CrossRef]
- Kramer, G.M.; Schriesheim, A. Heptane Isomerization Mechanism. J. Phys. Chem. 1961, 65, 1283–1286. [Google Scholar] [CrossRef]
- Weitkamp, J. Hydrocracking, Cracking and Isomerization of Hydrocarbons.|Hydrocracken, cracken und isomerisieren von kohlenwasserstoffen. Erdoel Kohle-Erdgas-Petrochem 1978, 31, 13–22. [Google Scholar]
- Krummenacher, J. Catalytic partial oxidation of higher hydrocarbons at millisecond contact times: Decane, hexadecane, and diesel fuel. J. Catal. 2003, 215, 332–343. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Y.; Sun, Q.; Dai, Z.; Gies, H.; Wu, Q.; Pan, S.; Bian, C.; Tian, Z.; Meng, X.; et al. Design and preparation of efficient hydroisomerization catalysts by the formation of stable SAPO-11 molecular sieve nanosheets with 10–20 nm thickness and partially blocked acidic sites. Chem. Commun. 2017, 53, 4942–4945. [Google Scholar] [CrossRef]
- Guo, L.; Fan, Y.; Bao, X.; Shi, G.; Liu, H. Two-stage surfactant-assisted crystallization for enhancing SAPO-11 acidity to improve n-octane di-branched isomerization. J. Catal. 2013, 301, 162–173. [Google Scholar] [CrossRef]
- Deldari, H. Suitable catalysts for hydroisomerization of long-chain normal paraffins. Appl. Catal. A Gen. 2005, 293, 1–10. [Google Scholar] [CrossRef]
- Bouchy, C.; Hastoy, G.; Guillon, E.; Martens, J.A. Fischer-Tropsch Waxes Upgrading via Hydrocracking and Selective Hydroisomerization. Oil Gas Sci. Technol. Rev. l’IFP 2009, 64, 91–112. [Google Scholar] [CrossRef]
- Lakhapatri, S.L.; Abraham, M.A. Deactivation due to sulfur poisoning and carbon deposition on Rh-Ni/Al2O3 catalyst during steam reforming of sulfur-doped n-hexadecane. Appl. Catal. A Gen. 2009, 364, 113–121. [Google Scholar] [CrossRef]
- Galperin, L.B. Hydroisomerization of N-decane in the presence of sulfur and nitrogen compounds. Appl. Catal. A Gen. 2001, 209, 257–268. [Google Scholar] [CrossRef]
- Parmar, S.; Pant, K.K.; John, M.; Kumar, K.; Pai, S.M.; Newalkar, B.L. Hydroisomerization of n-hexadecane over Pt/ZSM-22 framework: Effect of divalent cation exchange. J. Mol. Catal. A Chem. 2015, 404–405, 47–56. [Google Scholar] [CrossRef]
- Höchtl, M.; Jentys, A.; Vinek, H. Hydroisomerization of Heptane Isomers over Pd/SAPO Molecular Sieves: Influence of the Acid and Metal Site Concentration and the Transport Properties on the Activity and Selectivity. J. Catal. 2000, 190, 419–432. [Google Scholar] [CrossRef]
- Geng, C.-H.; Zhang, F.; Gao, Z.-X.; Zhao, L.-F.; Zhou, J.-L. Hydroisomerization of n-tetradecane over Pt/SAPO-11 catalyst. Catal. Today 2004, 93–95, 485–491. [Google Scholar] [CrossRef]
- Martens, J.A.; Verboekend, D.; Thomas, K.; Vanbutsele, G.; Pérez-Ramírez, J.; Gilson, J.-P. Hydroisomerization and hydrocracking of linear and multibranched long model alkanes on hierarchical Pt/ZSM-22 zeolite. Catal. Today 2013, 218–219, 135–142. [Google Scholar] [CrossRef]
- Corma, A.; Martínez, A.; Martínez-Soria, V. Hydrogenation of Aromatics in Diesel Fuels on Pt/MCM-41 Catalysts. J. Catal. 1997, 169, 480–489. [Google Scholar] [CrossRef]
- Yasuda, H.; Yoshimura, Y. Hydrogenation of tetralin over zeolite-supported Pd-Pt catalysts in the presence of dibenzothiophene. Catal. Lett. 1997, 46, 43–48. [Google Scholar] [CrossRef]
- Escobar, J.; Núñez, S.; Montesinos-Castellanos, A.; de los Reyes, J.A.; Rodríguez, Y.; González, O.A. Dibenzothiophene hydrodesulfurization over PdPt/Al2O3–TiO2. Influence of Ti-addition on hydrogenating properties. Mater. Chem. Phys. 2016, 171, 185–194. [Google Scholar] [CrossRef]
- Xiong, J.; Ma, Y. Catalytic Hydrodechlorination of Chlorophenols in a Continuous Flow Pd/CNT-Ni Foam Micro Reactor Using Formic Acid as a Hydrogen Source. Catalysts 2019, 9, 77. [Google Scholar] [CrossRef]
- Flego, C.; Galasso, L.; Vidotto, S.; Faraci, G. Effects of H2S on Bifunctional Catalysts; Elsevier: Amsterdam, The Netherlands, 1997; pp. 479–486. [Google Scholar]
- Marafi, M.; Stanislaus, A.; Furimsky, E. Catalyst Deactivation. In Handbook of Spent Hydroprocessing Catalysts; Elsevier: Amsterdam, The Netherlands, 2017; pp. 67–140. [Google Scholar]
- Topsøe, H.; Clausen, B.S.; Massoth, F.E. Hydrotreating Catalysis; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Xing, G.; Liu, S.; Guan, Q.; Li, W. Investigation on hydroisomerization and hydrocracking of C 15 –C 18 n -alkanes utilizing a hollow tubular Ni-Mo/SAPO-11 catalyst with high selectivity of jet fuel. Catal. Today 2019, 330, 109–116. [Google Scholar]
- Liu, P.; Wu, M.-Y.; Wang, J.; Zhang, W.-H.; Li, Y.-X. Hydroisomerization of n-heptane over MoP/Hβ catalyst doped with metal additive. Fuel Process. Technol. 2015, 131, 311–316. [Google Scholar] [CrossRef]
- Karakoulia, S.A.; Heracleous, E.; Lappas, A.A. Mild hydroisomerization of heavy naphtha on mono- and bi-metallic Pt and Ni catalysts supported on Beta zeolite. Catal. Today 2019. [Google Scholar] [CrossRef]
- Stanislaus, A.; Marafi, A.; Rana, M.S. Recent advances in the science and technology of ultra low sulfur diesel (ULSD) production. Catal. Today 2010, 153, 1–68. [Google Scholar] [CrossRef]
- van Veen, J.A.R. What’s new? On the development of sulphidic HT catalysts before the molecular aspects. Catal. Today 2017, 292, 2–25. [Google Scholar] [CrossRef]
- Vutolkina, A.V.; Makhmutov, D.F.; Zanina, A.V.; Maximov, A.L.; Glotov, A.P.; Sinikova, N.A.; Karakhanov, E.A. Hydrogenation of Aromatic Substrates over Dispersed Ni–Mo Sulfide Catalysts in System H2O/CO. Pet. Chem. 2018, 58, 528–534. [Google Scholar] [CrossRef]
- Vutolkina, A.V.; Makhmutov, D.F.; Zanina, A.V.; Maximov, A.L.; Kopitsin, D.S.; Glotov, A.P.; Egazar’yants, S.V.; Karakhanov, E.A. Hydroconversion of Thiophene Derivatives over Dispersed Ni–Mo Sulfide Catalysts. Pet. Chem. 2018, 58, 1227–1232. [Google Scholar] [CrossRef]
- Han, W.; Nie, H.; Long, X.; Li, M.; Yang, Q.; Li, D. Effects of the support BrØnsted acidity on the hydrodesulfurization and hydrodenitrogention activity of sulfided NiMo/Al2O3catalysts. Catal. Today 2017, 292, 58–66. [Google Scholar]
- Yu, Q.; Zhang, L.; Guo, R.; Sun, J.; Fu, W.; Tang, T.; Tang, T. Catalytic performance of CoMo catalysts supported on mesoporous ZSM-5 zeolite-alumina composites in the hydrodesulfurization of 4,6-dimethyldibenzothiophene. Fuel Process. Technol. 2017, 159, 76–87. [Google Scholar] [CrossRef]
- Wang, X.; Mei, J.; Zhao, Z.; Zheng, P.; Chen, Z.; Gao, D.; Fu, J.; Fan, J.; Duan, A.; Xu, C. Self-Assembly of Hierarchically Porous ZSM-5/SBA-16 with Different Morphologies and Its High Isomerization Performance for Hydrodesulfurization of Dibenzothiophene and 4,6-Dimethyldibenzothiophene. ACS Catal. 2018, 8, 1891–1902. [Google Scholar] [CrossRef]
- Mériaudeau, P.; Tuan, V.A.; Sapaly, G.; Nghiem, V.T.; Naccache, C. Pore size and crystal size effects on the selective hydroisomerisation of C8 paraffins over Pt–Pd/SAPO-11, Pt–Pd/SAPO-41 bifunctional catalysts. Catal. Today 1999, 49, 285–292. [Google Scholar] [CrossRef]
- Yadav, R.; Sakthivel, A. Silicoaluminophosphate molecular sieves as potential catalysts for hydroisomerization of alkanes and alkenes. Appl. Catal. A Gen. 2014, 481, 143–160. [Google Scholar] [CrossRef]
- Lee, E.; Yun, S.; Park, Y.-K.; Jeong, S.-Y.; Han, J.; Jeon, J.-K. Selective hydroisomerization of n-dodecane over platinum supported on SAPO-11. J. Ind. Eng. Chem. 2014, 20, 775–780. [Google Scholar] [CrossRef]
- Kim, M.Y.; Lee, K.; Choi, M. Cooperative effects of secondary mesoporosity and acid site location in Pt/SAPO-11 on n-dodecane hydroisomerization selectivity. J. Catal. 2014, 319, 232–238. [Google Scholar] [CrossRef]
- Pimerzin, A.A.; Roganov, A.A.; Verevkin, S.P.; Konnova, M.E.; Pilshchikov, V.A.; Pimerzin, A.A. Bifunctional catalysts with noble metals on composite Al2O3-SAPO-11 carrier and their comparison with CoMoS one in n-hexadecane hydroisomerization. Catal. Today 2019, 329, 71–81. [Google Scholar] [CrossRef]
- Topsøe, H.; Clausen, B.S.; Topsøe, N.-Y.; Zeuthen, P. Progress in the Design of Hydrotreating Catalysts Based on Fundamental Molecular Insight. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1989; Volume 53, pp. 77–102. ISBN 9780444882110. [Google Scholar]
- Topsøe, H. The role of Co–Mo–S type structures in hydrotreating catalysts. Appl. Catal. A Gen. 2007, 322, 3–8. [Google Scholar] [CrossRef]
- Pimerzin, A.A.; Savinov, A.A.; Ishutenko, D.I.; Verevkin, S.P.; Pimerzin, A.A. Isomerization of Linear Paraffin Hydrocarbons in the Presence of Sulfide CoMo and NiW Catalysts on Al2O3—SAPO-11 Support. Russ. J. Appl. Chem. 2019, 92, 1772–1779. [Google Scholar] [CrossRef]
- Mériaudeau, P.; Tuan, V.A.; Nghiem, V.T.; Lai, S.Y.; Hung, L.N.; Naccache, C. SAPO-11, SAPO-31, and SAPO-41 Molecular Sieves: Synthesis, Characterization, and Catalytic Properties in n-Octane Hydroisomerization. J. Catal. 1997, 169, 55–66. [Google Scholar] [CrossRef]
- Kasztelan, S.; Toulhoat, H.; Grimblot, J.; Bonnelle, J.P. A geometrical model of the active phase of hydrotreating catalysts. Appl. Catal. 1984, 13, 127–159. [Google Scholar] [CrossRef]
- Hensen, E.J.; Kooyman, P.; van der Meer, Y.; van der Kraan, A.; de Beer, V.H.; van Veen, J.A.; van Santen, R. The Relation between Morphology and Hydrotreating Activity for Supported MoS2 Particles. J. Catal. 2001, 199, 224–235. [Google Scholar] [CrossRef]
- Pimerzin, A.A.; Nikulshin, P.A.; Mozhaev, A.V.; Pimerzin, A.A.; Lyashenko, A.I. Investigation of spillover effect in hydrotreating catalysts based on Co₂Mo₁₀-heteropolyanion and cobalt sulphide species. Appl. Catal. B Environ. 2015, 168–169, 396–407. [Google Scholar] [CrossRef]

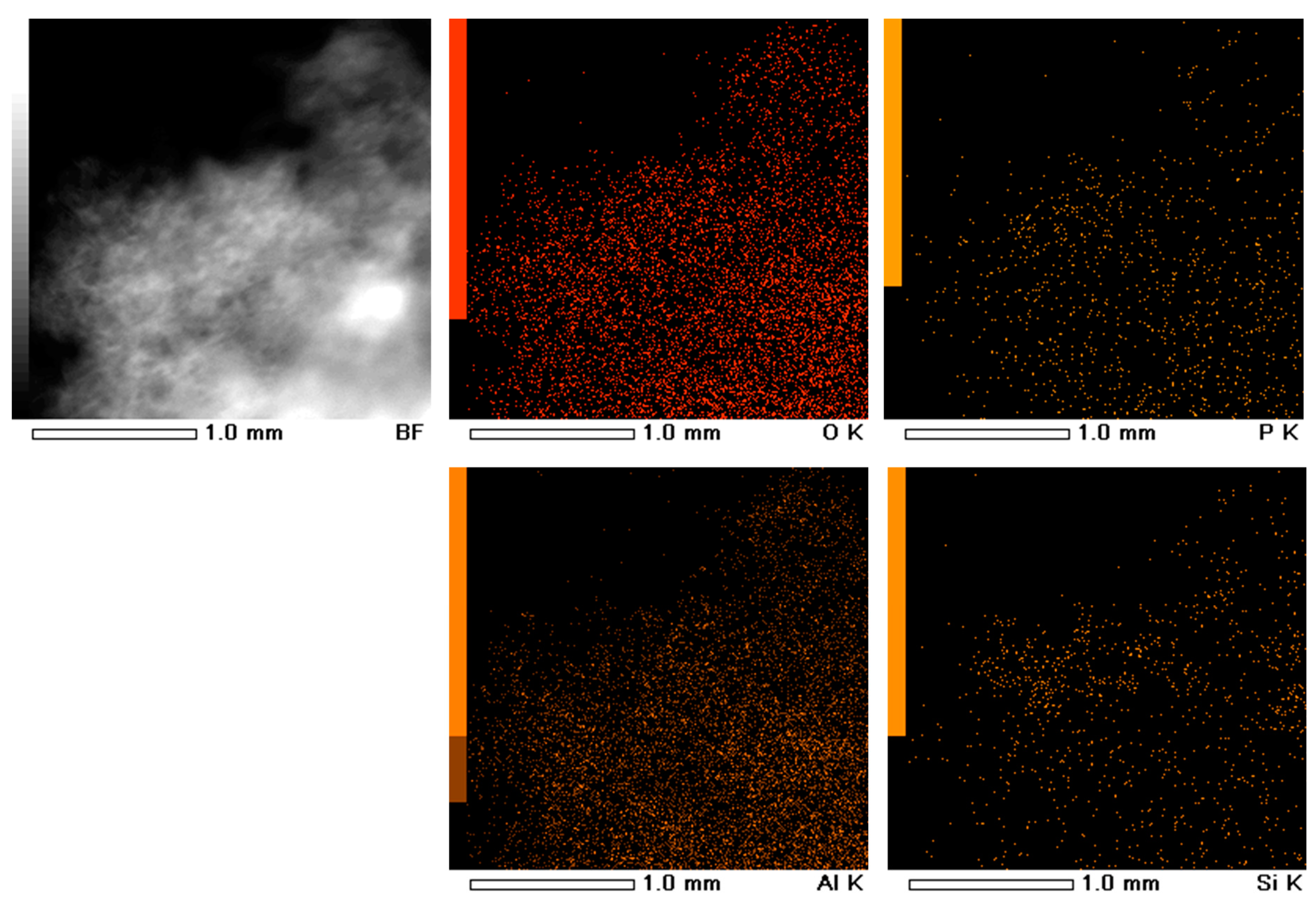

| Catalyst (Support) | Brunauer–Emmett–Teller (BET) | t-Plot & DFT | ||||
|---|---|---|---|---|---|---|
| SBET | Dp avr. | Vpore | Smicro | Dmicro | Vmicro | |
| (m2/g) | (nm) | (cm3/g) | (m2/g) | (nm) | (cm3/g) | |
| Al2O3-SAPO-11 (support) | 268 | <4.0 & 8.1 * | 0.50 | 69 | 3.8 | 0.06 |
| CoMoS/Al2O3-SAPO-11 | 204 | <4.0 & 8.0 * | 0.49 | 66 | 3.7 | 0.05 |
| NiWS/Al2O3-SAPO-11 | 198 | <4.0 & 7.9 * | 0.47 | 64 | 3.8 | 0.05 |
| Pt/Al2O3-SAPO-11 | 259 | <4.0 & 8.1 * | 0.51 | 59 | 3.7 | 0.04 |
| Parameter | Catalyst | ||
|---|---|---|---|
| CoMoS/ Al2O3-SAPO-11 | NiWS/ Al2O3-SAPO-11 | Pt/ Al2O3-SAPO-11 | |
| Active metals loading (wt %) | |||
| 10.6 | 18.5 | 1.0 |
| 3.1 | 3.0 | - |
| Active phase morphology | |||
| 3.6 | 4.6 | 1.5 |
| 2.1 | 1.9 | - |
| 0.33 | 0.27 | 0.91 |
| Acidity—TPD NH3, mmol/g * | |||
| 0.421 | 0.423 | 0.367 |
| 0.193 | 0.204 | 0.261 |
| 0.507 | 0.616 | 0.236 |
| 1.121 | 1.243 | 0.865 |
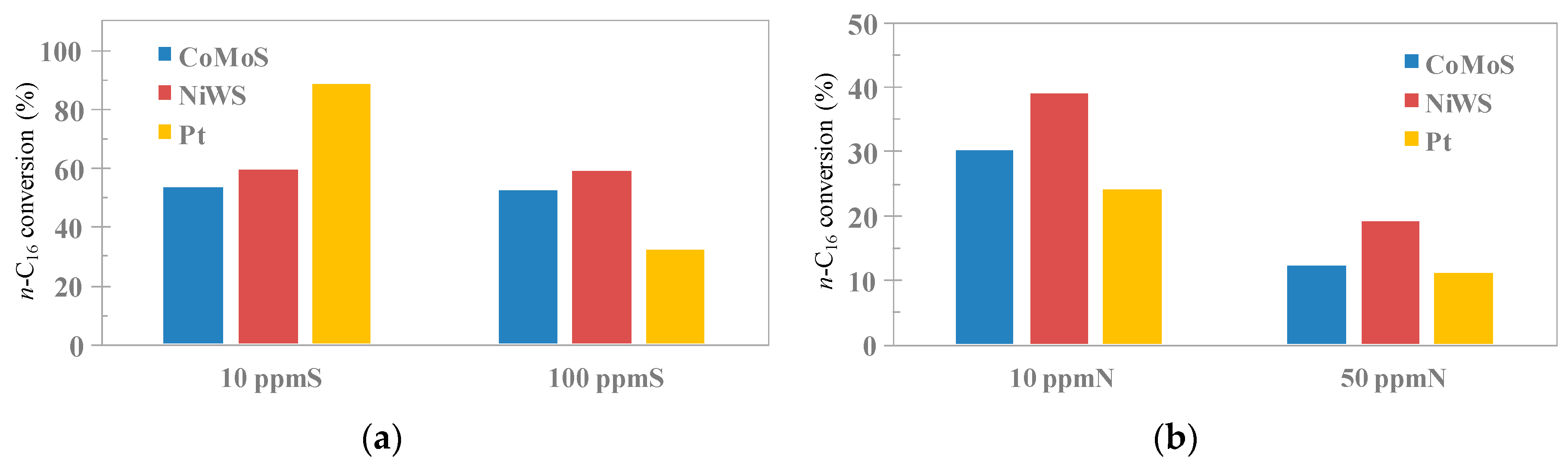
| Catalyst & Reaction Parameter | Reaction Conditions (T = 340 °C, P = 15 bar, H2/Feedstock = 150nL/L) | |||||
|---|---|---|---|---|---|---|
| WHSV (h−1) | Feedstock: N-Hexadecane 2.9 wt % + Catalytic Poison | |||||
| No Poison | 10 ppmS | 100 ppmS | 10 ppmN | 50 ppmN | ||
| CoMoS/Al2O3-SAPO-11 | ||||||
| 1.0 | 52.8 | 53.1 | 52.0 | 29.8 | 12.4 |
| 0.76 ± 0.02 | 0.77 ± 0.02 | 0.74 ± 0.01 | 0.36 ± 0.01 | 0.13 ± 0.003 | |
| - | - | 2% | 52% | 83% | |
| NiWS/Al2O3-SAPO-11 | ||||||
| 1.0 | 59.0 | 59.5 | 58.8 | 38.8 | 18.8 |
| 0.90 ± 0.01 | 0.92 ± 0.02 | 0.90 ± 0.02 | 0.50 ± 0.01 | 0.21 ± 0.005 | |
| - | - | 1% | 45% | 76% | |
| Pt/Al2O3-SAPO-11 | ||||||
| 3.0 | 78.6 | 51.8 | 8.5 | 9.1 | 2.9 |
| 4.75 ± 0.22 | 2.2 ± 0.044 | 0.35 ± 0.006 | 0.29 ± 0.01 | 0.09 ± 0.004 | |
| - | 53% | 77% | 94% | 98% | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pimerzin, A.; Savinov, A.; Vutolkina, A.; Makova, A.; Glotov, A.; Vinokurov, V.; Pimerzin, A. Transition Metal Sulfides- and Noble Metal-Based Catalysts for N-Hexadecane Hydroisomerization: A Study of Poisons Tolerance. Catalysts 2020, 10, 594. https://doi.org/10.3390/catal10060594
Pimerzin A, Savinov A, Vutolkina A, Makova A, Glotov A, Vinokurov V, Pimerzin A. Transition Metal Sulfides- and Noble Metal-Based Catalysts for N-Hexadecane Hydroisomerization: A Study of Poisons Tolerance. Catalysts. 2020; 10(6):594. https://doi.org/10.3390/catal10060594
Chicago/Turabian StylePimerzin, Aleksey, Aleksander Savinov, Anna Vutolkina, Anna Makova, Aleksandr Glotov, Vladimir Vinokurov, and Andrey Pimerzin. 2020. "Transition Metal Sulfides- and Noble Metal-Based Catalysts for N-Hexadecane Hydroisomerization: A Study of Poisons Tolerance" Catalysts 10, no. 6: 594. https://doi.org/10.3390/catal10060594
APA StylePimerzin, A., Savinov, A., Vutolkina, A., Makova, A., Glotov, A., Vinokurov, V., & Pimerzin, A. (2020). Transition Metal Sulfides- and Noble Metal-Based Catalysts for N-Hexadecane Hydroisomerization: A Study of Poisons Tolerance. Catalysts, 10(6), 594. https://doi.org/10.3390/catal10060594





