Abstract
A series of yeast strains was tested in order to evaluate their catalytic potential in biocatalysis of one-pot indolizine’s synthesis. Yeast cultivation was performed in a submerged system at 28 °C for 72 h at 180 rpm. An assessment of the reagents’ toxicity on yeast viability and metabolic functionality concluded that the growth potential of three Yarrowia lipolytica strains were least affected by the reactants compared to the other yeast strains. Further, crude fermentation products (biomass and cell-free supernatant)—obtained by submerged cultivation of these yeasts—were used in multistep cascade reactions for the production of fluorescent indolizine compounds with important biologic activities. A whole–cell catalyzed multicomponent reaction of activated alkynes, α-bromo-carbonyl reagents and 4,4′-bipyridine, at room temperature in buffer solution led to the efficient synthesis of bis-indolizines 4a, 4b and 4c, in good-to-excellent yields (47%–77%). The metabolites of the selected Y. lipolytica strains can be considered effective biocatalysts in cycloaddition reactions and the high purity and bioconversion yields of the synthesized indolizines indicates a great potential of this type of “green” catalysts. Seeds of Triticum estivum L. were used to investigate the impact of the final products on the germination and seedling growth. The most sensitive physiological parameters suggest that indolizines, at the concentrations tested, have non-toxic effect on germination and seedling growth of wheat, fact also confirmed by confocal laser scanning microscopy images.
1. Introduction
The application of whole microbial-cell biocatalysis methods is steadily increasing as an environment-friendly alternative to dangerous solvents and catalysts [1,2,3,4]. The use of enzymes in synthetic transformations has many advantages, such as mild and eco-friendly conditions, high regio- and stereoselectivities [5,6,7,8,9], but it also has drawbacks in the preparation of enzymes that require the cultivation of microorganisms, purification and crystallization of enzymes [2,10]. Therefore, the use of whole-cell systems for conducting chemical reactions offers some great advantages such as decreased reaction time, considerable decrease of costs, fewer steps, elimination of toxic solvents, mild conditions, high purity and yields of the obtained compounds and ecofriendly transformations [10,11,12,13].
The Yarrowia lipolytica yeast species is widespread in nature. It considered a non-pathogenic microorganism, the U.S. Food and Drug Administration has given its metabolites the GRAS status (“generally recognized as safe”) [14] and they are isolated from dairy products, dry fermented sausages or mediums rich in fats or hydrocarbons [15]. The potential applications of this yeast are related to its specific ability to use hydrophobic substrates effectively and the high biosynthesis capacity of numerous enzymes, such as lipases/esterases (LIP genes), cytochromes P450 (ALK genes) and peroxisomal acyl-CoA oxidases (POX genes), its ability to produce surfactants [16,17] and was successfully used in biocatalysis [14,15,18,19].
The indolizine core is present in many biologically active compounds and can be considered an important scaffold for the preparation of new pharmaceuticals [20,21]. These class of compounds holds valuable biologic activities [21], such as antimicrobial [22,23], antioxidant [24,25], anticancer [26,27,28], enzyme inhibitors [29,30], anti-inflammatory [21] and anti-HIV-1 [31]. Consequently, different pathways were described in the literature for their synthesis [32,33,34]. Therefore, the development of novel and efficacious methods for the synthesis of these valuable compounds are of significant concern.
This work describes the ability of yeast crude products (whole cells and cultural liquid) to biocatalyze the synthesis of the indolizine ring, in a cascade transformation which involves the “one pot” reaction of three components, a pyridine compound, a phenacyl bromide and an activated alkyne, without the isolation and purification of the intermediate, at room temperature in buffer solution. Literature data pointed out the necessity to assess various culture conditions to detect sustainable, selective and efficient biocatalysts, therefore herein we describe the screening of nine microorganism strains. The selection was based on their physiological functionality in the presence of reagents by toxicity evaluation and possible functionality as biocatalyst for in situ chemical conditions, in reactions involving cycloaddition processes in aqueous media. The high level of lipase activity exhibited by Yarrowia lipolytica during submerged cultivation on nutrient broth supplemented with the reagents suggests that the three reactants do not exhibit a negative effect on the metabolites’ biosynthesis of this yeast. In this context, these microbial enzymes were used in biocatalysis aims due to their broad specificity, high stability under different reaction conditions, high regio- and enantioselectivity. The lipase catalytic promiscuity is well known [35], playing a role not only in hydrolysis reactions, but also in other type of chemical synthesis such as condensation [36,37,38] and chemoenzymatic synthesis of polymeric materials [39]. This methodology is an environmentally-friendly regioselective manner to obtain the indolizine core structure as we previously accomplished by using commercial lipases [5]. The one-pot reaction using the crude multienzyme biocatalyst is described in Figure 1. The final products impact on wheat (Triticum estivum L.) germination and seedling growth is also analyzed, in order to assess the harmful effects to the environment of the biosynthesized indolizine compounds if they will be used as scaffold for pharmaceutical industry. Toxicity studies were performed on plant seed germination as early studies, taking into account the potential hazard of releasing these synthetic chemicals into the environment during production, transport, use or most importantly during disposal. For this reason, it is very important to predict this type of adverse effects directly on the environment or indirectly on humans, through crops or food, before the substance is released into the pharmaceutical industry.
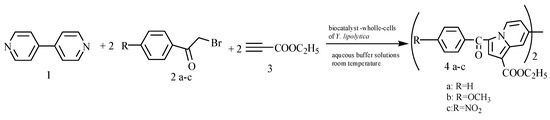
Figure 1.
Indolizine synthesis in one-pot bioformation reactions using Yarrowia lipolytica crude biocatalysts.
2. Results and Discussion
2.1. Evaluation of the Toxicity of Reactants on the Yeasts’ Physiological Activity
An evaluation of the chemicals’ influence on the yeast cells metabolism was performed in order to select the most resistant strains. The selected strains were subsequently used as biocatalysts in indolizines synthesis. In a medium supplemented with 0.1%, 0.2% and 0.3% of each stock solution of all three reactants, 4,4′-bipyridine, 2-bromoacetophenones and ethyl propiolate, (compounds 1, 2, 3 from Figure 1), it was observed that Yarrowia lipolytica tested strains survived in a high rate, compared to other yeasts, developing the largest colonies, with diameters up to 18 mm (Figure 2).
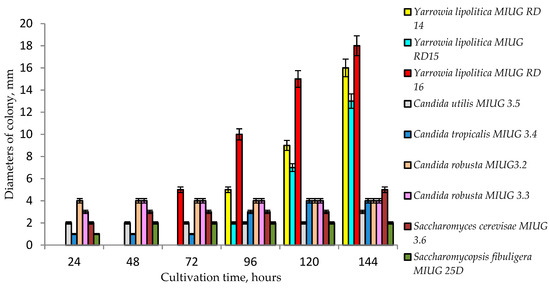
Figure 2.
Impact of the 0.1% reactants on the growth of different strains of yeast.
By increasing the concentrations of all the three chemicals, in the culture medium, three strains of Yarrowia lipolytica MIUG RD 14, MIUG RD 15 and MIUG RD 16 proved to be the most resistant (Figure 3a,b) developing the largest colonies, while other strains such as Candida robusta MIUG 3.3, Saccharomycopsis figuligera MIUG 25D or Saccharomyces cerevisiae MIUG 3.6 have grown very slowly or have not developed any visible colonies. Consequently, because of their high survival rates only the Yarrowia lipolytica strains were selected to be used in indolizine synthesis.
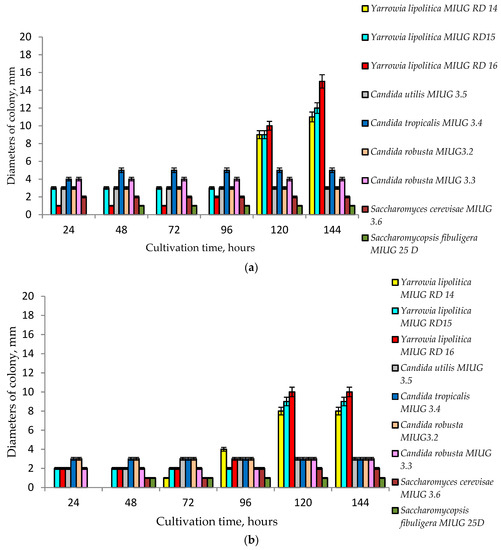
Figure 3.
(a) Impact of 0.2% reactants on the growth of different strains of yeast; (b) impact of 0.3% reactants on the growth of different strains of yeast.
2.2. Growth and Bioconversion Potential of Yarrowia lipolytica in Aerobe Submerged Cultivation Conditions in the Presence of Chemicals
Some reports concerning the lipolytic activity of Yarrowia lipolytica are present in literature. Parfene et al. (2011) [40] studied in situ lipase production during solid state cultivation of selected strains on media supplemented with vegetable fat. Destain et al. (1997) [41] selected a Yarrowia lipolytica mutant obtained by chemical mutagenesis which produced a lipase with a 35-fold increase in activity, compared to the wild type of enzyme. Pignede et al. (2000) [42] described the LIP2 gene responsible for extracellular lipases produced by Yarrowia lipolytica W29, which Fickers et al. (2005) [43] amplified in a Yarrowia lipolytica mutant and obtained high lipase activities of 26.450 U mL−1 in batch operation and 158.246 U mL−1 in fed-batch.
Our previous studies on selected lipase-producing Yarrowia lipolytica strains, using vegetable fats (palm, coconut and shea) as substrate, showed that these strains are active producers of extracellular lipases. Strain Yarrowia lipolytica MIUG RD 14 was more active than the strains Yarrowia lipolytica MIUG RD15 and Yarrowia lipolytica MIUG RD16 [40]. The lipase activity potential of these strains was demonstrated by in situ cultivation of whole biomass and also by extracellular enzyme activity evaluation of cell-free crude supernatants.
Dynamics of culture multiplication and extracellular lipase production of Yarrowia lipolytica selected strains are shown in Figure 4. The high yield of extracellular lipase production was detected after 72 h of submerged cultivation for all three strains. Thus, the extracellular lipase from Yarrowia lipolytica MIUG RD 15 has shown an activity of 375 U mL−1. This biosynthesis yield was similar with the one previously obtained (390 U mL−1) with this strain under normal cultivation conditions [40]. The high level of lipase activity exhibited by the strain Yarrowia lipolytica MIUG RD 14 during submerged cultivation (180 rpm at 28 °C) on nutrient broth supplemented with the reactants suggests that the three compounds do not have a toxic effect on the metabolites biosynthesis of this yeast strains.
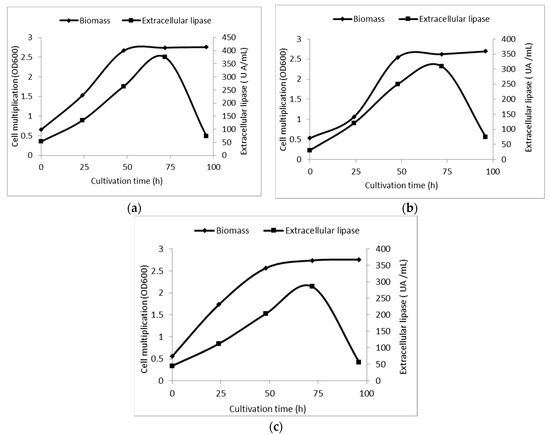
Figure 4.
Kinetics of biomass accumulation and extracellular lipase production by Yarrowia lipolytica strains, coded MIUG RD 14 (a), MIUG RD 15; (b) MIUG RD 16; (c) during submerged cultivation (180 rpm at 28 °C) on nutrient broth supplemented with chemicals.
2.3. Indolizine Synthesis by Bioconversion
The formation of indolizines compounds in some of the samples was qualitatively assessed through the appearance of yellow-brown fluorescent color in the reaction media, observed under UV light irradiation. In the reactions with no color change, the desired reaction products were not formed.
A major drawback to the application of whole cell biomass in biotransformation reactions is the permeability barrier exhibited by the cell membrane to enzymes or reaction substrates. The presence of water is necessary for lipase interfacial activation involving lid opening at oil–water interface [44]. Nevertheless, Yarrowia lipolytica is a nonconventional yeast with the capacity of transforming both polar and nonpolar substrates [45].
As represented in Figure 5 and Figure 6 all the crude fermentation products from the three selected Yarrowia lipolytica strains used as biocatalysts lead to the bioformation of the desired reaction product, with a maximum yield achieved after 144 h of reaction.
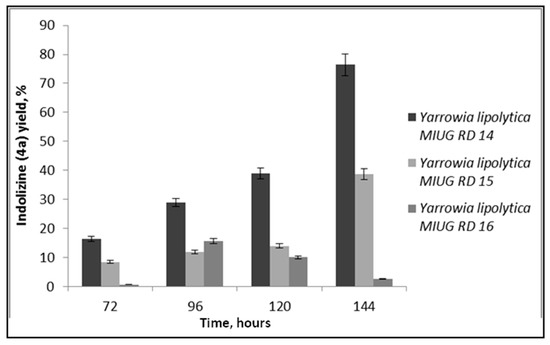
Figure 5.
Yield of indolizine 4a, obtained using Yarrowia lipolytica cell-free supernatant as biocatalyst.
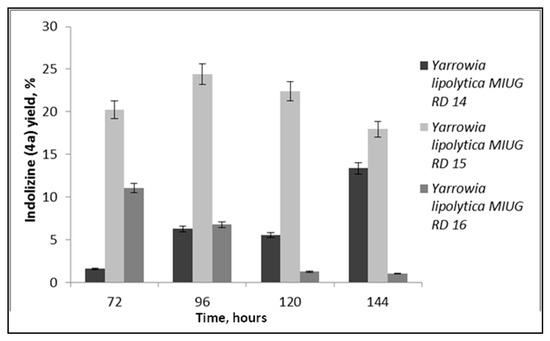
Figure 6.
Yield of indolizine 4a, obtained using Yarrowia lipolytica biomass as biocatalysts.
The best bioconversion yield was observed in the presence of the cell-free supernatant obtained after biomass separation by centrifugation from 144 h of submerged cultivation of Yarrowia lipolytica MIUG RD 14, recommending this strain for further research. This crude biocatalyst led to a bioconversion percentage of almost 80% for indolizine 4a, after 4 days of reaction time in an aqueous medium (Figure 5). These results are correlated with metabolites production potential of these strains and confirm the possible implication of them in bioconversion.
When biomass was used as biocatalyst, it was observed that Yarrowia lipolytica MIUG RD 15 strain had higher biotransformation yield, suggesting the capacity of viable cells to release the enzyme in stationary conditions, during the bioconversion process (Figure 6). The bioconversion yields are lower than in the case when the cell-free supernatant was used, according to the extracellular enzyme production yield and also due to the partial biosorbtion of the bioderivates on the cell surface, demonstrated by fluorescence analysis, which cannot be fully extracted with the method employed. The reaction products, the bis-indolizines, after 144 h were obtained in moderate to good yields of 77% (4a), 47% (4b) and 48% (4c) in biocatalysis. The one-pot bioconversion products and their purity were further confirmed by HPLC, FTIR, NMR and elemental analysis.
The structures of all compounds were confirmed by UV-Vis absorption spectra, FTIR, NMR, MS and elemental analysis. The synthesized bis-indolizines have the expected structure and purity, the results of the performed analyses being in concordance with those of the same bis-indolizines formerly synthesized through classical chemical synthesis and characterized [46]. The HPLC chromatograms of the products are shown in Figures S1–S3 of the Supplementary Material. Characteristics of the bioconversion products, from the Supplementary Materials (Figures S4–S9) are as follows:
1,1′-Diethyldicarboxylate-3,3′-benzoyl-7,7′-bis(indolizine) (4a), yellow powder, mp 273–275 °C; IR (ATR, cm−1): 1071 (s, C-N); 1167, 1216 (s, C-O-C); 1341 (s, COester); 1427, 1459, 1479, 1512, 1524 (s, C=N, C=Carom); 1645 (s, C=O); 1700 (s, C=Oester); 2979 (l, CHalif); 3139 (l, CHarom.); 1H-NMR (400 MHz, CDCl3, TMS) δ/ppm: 1.43 (t, J = 7.2, 6H); 4.4 (q, J = 7.2, 4H); 7.42–7.73 [m, 8H: 4H, 2H, 2H (7.52, J = 7.5, J = 1.2)]; 7.78–7.88 [m, 6H: 4H, 2H (7.82)]; 8.77(d, J = 1.2, 2H); 9.98 (d, J = 7.5, 2H); 13C-NMR (400 MHz, CDCl3, TMS δ/ppm ): 14.59 (CH3), 60.37 (CH2), 107.55 (C), 113.53 (CH), 116.96 (C), 122.90 (C), 128.52 (CH), 129.05 (CH), 129.33 (CH), 129.53 (CH), 131.74 (CH), 136.61 (C), 139.70 (C), 139.78 (C), 163.97 (C=Oester), 185.67 (C=O); MS (APCI+): m/z: 585 [M + H+]; anal. C 73.72; H 5.14; N 4.76%, calcd. for C36H28N2O6, C 73.96, H 4.83, N 4.79%.
1,1′-Diethyldicarboxylate-3,3′-bis(p-methoxy-benzoyl)-7,7′-bis(indolizine) (4b), yellow powder, mp 290–291 °C; IR (ATR, cm−1): 1077 (s, C-N); 1249, 1220, 1167 (s, C-O-C); 1339 (s, COester); 1463, 1508, 1597 (s, C=N, C=Carom); 1665 (s, C=O); 1700 (s, C=Oester); 2977 (l, CHalif); 3140 (l, CHarom.); 1H-NMR (400 MHz, CDCl3, TMS) δ/ppm: 1.43 (t, J = 7.2, 6H); 3.93 (s, 6H); 4.43 (q, J = 7.2, 4H); 7.03 (d, J = 8.8, 4H); 7.33 (d, J = 8.8, 4H); 7.76 (s, 2H); 7.79 (d, J = 7.5, 2H); 8.80 (d, J = 1.2, 2H); 9.98 (d, J = 7.5, 2H); 13C-NMR (400 MHz, CDCl3, TMS δ/ppm ): 14.73 (CH3), 56.03 (OCH3), 61.09 (CH2), 107 (C), 113.98 (CH), 114 (CH), 117.72 (CH), 122 (CH), 122.2 (CH), 129.04 (CH), 131.81 (CH), 132.42 (C), 136.57 (CH), 138.57 (C), 163.71 (C), 165.28 (C=Oester), 186.06 (C=O); MS (APCI+): m/z: 645 [M + H+]; anal. C 70.92; H 4.88; N 4.28%, calcd. for C38H32N2O8, C 70.80, H 5.00, N 4.35%.
1,1′-Diethyldicarboxylate-3,3′-bis(p-nitro-benzoyl)-7,7′-bis(indolizine) (4c), dark yellow powder, mp 271–272 °C; IR (ATR, cm−1): 1077 (s, C-N);1169, 1208 (s, C-O-C); 1338, 1521 (s, NO2); 1428, 1464 (s, C=N, C=Carom); 1656 (s, C=O);1700 (s, C=Oester); 2978 (l, CHalif); 3359 (l, CHarom.); 1H-NMR (400 MHz, CDCl3, TMS) δ/ppm: 1.43 (t, J = 7.2, 6H); 4.4 (q, J = 7.2, 4H); 7.42 (s, 2H); 7.48 (d, J = 7.5, 2H); 7.8 (d, J = 8.8, 4H); 8.03 (d, J = 8.8, 4H); 8.99 (d, J = 1.2, 2H); 10.13 (d, J = 7.5, 2H); 13C-NMR (400 MHz, CDCl3, TMS δ/ppm): 14.29 (CH3), 60.53 (CH2); 108.16 (C); 113.07 (CH); 118.28 (CH); 122.74 (C); 123.52 (CH); 127.46 (CH); 129.14 (CH); 129.86 (CH); 132.60 (C); 138.14 (C);143.06 (C); 150.16 (C), 164.52 (C=Oester), 184.15 (C=O); MS (APCI+): m/z: 675 [M + H+]; anal. C 64.15; H 4.11; N 8.26%, calcd. for C36H26N4O10, C 64.09, H 3.88, N 8.31%.
2.4. Evaluation of the Toxicity of Indolizines on the Germination of Triticum estivum L.
Seed germination has gained much attention in recent years due to their simplicity, time effectiveness and low cost, as an environmental exposure model to measure the toxicity of organic compounds at different concentrations [47,48,49]. Herein, we evaluated the potential of synthetic indolizines to cause particular forms of toxicity in case of their use as pharmaceuticals by plants seeds germination test. The evaluation of germination most sensitive indicators was performed according to our previous method [50] with slight modifications. The test was made in accordance with the Organization for Economic Cooperation and Development (OECD) Guideline for testing of chemicals [51]. The seed germination (SG) values, very similar to those of the control samples, but also relative radicle growth (RRG%) and relative seed germination (RSG%) values greater than 100% for the indolizines treated samples, according to the literature data, means that the presence of the assessed compounds did not exert a harmful influence on seed germination [48,52]. The germination index (GI) values greater than those of the control samples (Table 1), reveals that the tested indolizines have no phytotoxicity [53]. The application of the indolizine 4a at both concentrations tested, had some positive influence by increasing the average number of roots in wheat germination process (3.7 ± 0.92; 3.6 ± 0.78), in comparison to the control (3.1 ± 0.42). In conclusion, the germination physiological indexes showed that the tested compounds at different concentrations, have no ecotoxicological effects on wheat seedlings (Table 1), compared to control.

Table 1.
Evaluation of the impact on the seed germination of Triticum estivum L. exposed to different concentrations of indolizine compounds. The results represent the mean values ± standard deviation of four replicates.
The confocal microscopic analysis of the cross-cut sections through the roots and stems of the sprouts resulting from the germination of wheat caryopses, under control conditions (ultrapure water) (Figure 7) or in the presence of indolizines 4a (Figure 8) or 4c (Figure 9) with different concentrations, was performed in order to observe possibles morpho-structural changes of plant tissues. The microscopic examinations showed that there were no major differences in the tissue appearance of the sections from the germination of wheat exposed to indolizine compounds compared to control. No lysed or turgid cells were observed in the microscopic analysis. The cells in the cortical parenchyma of roots and stems had intact cell walls (CW), sometimes resulting with a slight tendency to thicken, probably due to the attachment or the adsorption on the surface of wheat seeds of the compounds to which they were exposed during germination. The underlying adsorption mechanism still needs to be analyzed in more detail in further work. For example, in Figure 8a, in the section through the root, it can be seen the annular collenchyma, with uniformly thickened cell walls (3.25–6.28 µm). This collenchyma cells were annexed to the vascular cambium for increasing structural support and integrity. The parenchymal cells of the control sample reached dimensions of 47.24 µm (Figure 7b)—and in the case of samples germinated in the presence of indolizines, the cells had slightly smaller dimensions, on average between 15–25 µm (Figure 8a and Figure 9a,b) and they were more compact, with smaller intercellular spaces. The normal cellular components were visible in both experimental cases: nucleus (N), proplastids/chloroplasts (in orange or red in Figure 8b and Figure 9b) as well as epidermal formations such as stomata (S) type Zea mays, hairs (Figure 8b) or tracheids (T) with spiral thickenings of the secondary wall and with diameters of 12–15 µm (Figure 7a and Figure 9b). The 4a and 4c indolizines at the tested concentrations (10−3 M, 10−4 M) did not have a cytotoxic effect on plant organisms, their adsorption did not affect the structure of plant tissues or the physiological processed such as cell division, growth and cell differentiation. Therefore, the results from the microscopic analysis are in agreement with the most sensitive physiological parameters evaluated in Table 1. Such in vivo assays in plant cell models are particularly useful to validate various subsequent applications of the compounds in the pharmaceutical industry [47,51].
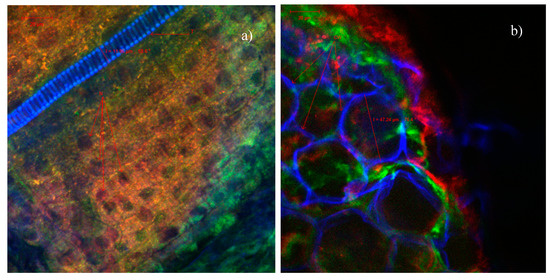
Figure 7.
Confocal laser scanning microscopy (CLSM) images of the Triticum estivum L. sprout sections-control sample (germination in ultrapure water): (a) root, (b) shoot.
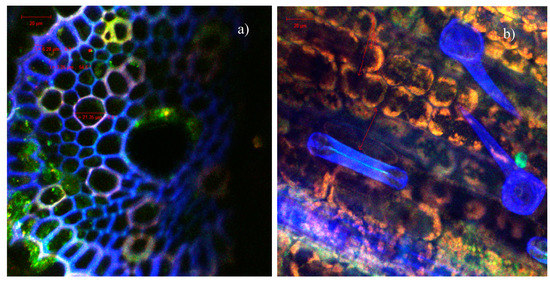
Figure 8.
CLSM images of the wheat sprout sections of the indolizine 4a treated samples at two different concentrations: (a) 10−3 M, (b) 10−4 M.
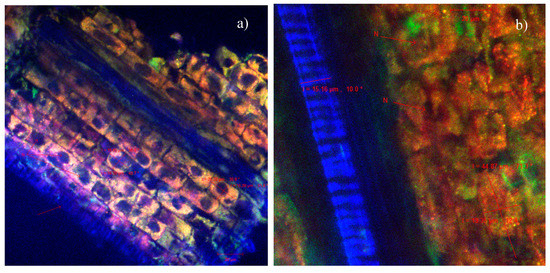
Figure 9.
CLSM images of the Triticum estivum L. sprout sections of the indolizine 4c treated samples at two different concentrations: (a) 10−3 M, (b) 10−4 M.
3. Materials and Methods
3.1. Microorganisms and Chemicals
All the nine yeast strains, Candida robusta MIUG 3.2, Candida robusta MIUG 3.3, Candida tropicalis MIUG 3.4, Candida utilis MIUG 3.5, Sacharomyces cerevisae MIUG 3.6, Yarrowia lipolytica MIUG RD 14, Yarrowia lipolytica MIUG RD 15, Yarrowia lipolytica MIUG RD 16 and Saccharomycopsis fibuligera MIUG 25D, used in this study, were provided from the Microorganims Collection (acronym MIUG) of the Research and Education Platform (Bioaliment) of „Dunărea de Jos” University of Galati, Romania. The pure stock cultures were maintained by cultivation on yeast extract peptone dextrose (YPD) agar medium and preserved at 4 °C.
3.2. Cultivation Conditions
The basic medium for yeasts cultivation was YPD broth containing (g L−1): peptone (20), yeast extract (10), glucose (10), pH = 5.5. YPD with 20 g L−1agar was also used for pure culture preservation and multiplication. All the reagents were purchased from Merck KGaA (Darmstadt, Germany). The inoculum was prepared from fresh biomass, 72 h of incubation. The sterile YPD liquid medium was inoculated (106 CFU mL−1) and the culture was placed on an orbital shaker (Companion Comecta S.A., Spain) at 180 rpm and 28 °C for 72 h.
3.3. Evaluation of the Toxicity of the Reactants on the Microorganisms’ Physiological Functionality
In the first screening step, the toxicity of the reactants which were used for indolizine synthesis (three starting chemicals, 4,4′-bipyridine, 2-bromoacetophenones and ethyl propiolate) upon yeast cells metabolic functionality was tested by stationary cultivation on YPD agar medium supplemented with reagent solutions in different concentrations (0.1%, 0.2%, 0.3%). The reagents ratio in the reaction media was 1:3:3, so this proportion was kept in the culture medium thus obtaining 0.5, 1.0 and 1.5 mmol L−1 of 4,4′-bipyridine and 1.5-, 3.0- and 4.5-mmol L−1 of 2-bromoacetophenones and ethyl propiolate, respectively, in sterile Petri dishes. The yeast cells from pure active culture were seeded by pinching on the surface of the solide medium, using a sterile microbiologic loop. The inoculated plates were incubated at 28 °C for 144 h. The toxicity of the tested chemicals was evaluated by measuring the diameter of the formed colonies, expressed in mm.
3.4. Yeast Multiplication by Submerged Cultivation in the Presence of Chemicals
The Yarrowia lipolytica yeast strains coded MIUG RD 14, MIUG RD 15 and MIUG RD 16 selected as the most resistant to chemicals toxicity were then cultivated in submerged condition in YPD liquid medium supplemented with 0.1% (v/v) of 4,4′-bipyridine (10−2 mol·L−1), 2-bromoacetophenones (3 × 10−2 mol·L−1) and ethyl propiolate (3 × 10−2 mol·L−1). After homogenization, the medium was inoculated with 5% of inoculum (106 CFU mL−1). Control samples were obtained by yeast cultivation in medium without chemicals. The samples were incubated in an orbital shaker (Companion Comecta S.A., Spain) at 180 rpm and 28 °C, for 100 h. Cells multiplication dynamic was evaluated by measurement of the optical density at 600 nm, at every 24 h, during of 100 h of submerged cultivation. The rate of yeast multiplication inhibition was calculated by comparing the growth potential in YPD liquid medium with and without chemicals.
After yeast cultivation, the biomass was separated by centrifugation (Hettich, Tuttlingen, Germany) for 20 min at 6000 rpm. Cell-free supernatants were assayed for crude extracellular lipase activity.
3.5. Lipase Assay
Lipase activity was determined using the titrimetical method of MaJumder et al. (2009) [36], modified. Briefly, 1 mL of culture supernatant was added to the assay substrate, containing 10 mL of 10% (v/v) homogenized palm oil in 10% (w/v) acacia gum, 2.0 mL of 0.6% CaCl2 solution and 5 mL of 250-mmol L−1 phosphate buffer (pH 7.0). All the reagents were purchased from Merck KGaA (Darmstadt, Germany). The biocatalyst and substrate mixture was incubated on orbital shaker with 800 rpm at 30 °C for 20 min. Twenty-five milliliters of ethanol were added to the reaction mixture. The liberated fatty acids were titrated with 0.1-mmol L−1 NaOH using phenolphthalein as an indicator. One lipase unit is defined as the amount of enzyme which releases one micro mole fatty acid per minute under specified assay conditions and expressed as units per mL (U mL−1).
3.6. Preparation of Crude Biocatalysts for Cycloaddition Reactions
50 mL of YPD liquid medium was inoculated with 5% (v/v) of Y. lipolytica inoculum (106 CFU mL−1). Cultivation took place in submerged conditions on an orbital shaker (Companion Comecta S.A., Spain) at 180 rpm and 28 °C, during 144 h. The whole culture was centrifuged for 20 min at 6000 rpm and 4 °C. After centrifugation, the cell-free supernatant was collected in a sterile tube, filtered (Whatman No.1, 0.45 µm) and was used as such as crude enzyme extract. The biomass obtained was washed twice with sterile saline (0.9% NaCl) and used as a whole cell biocatalyst after resuspension in 5 mL 250-mmol L−1 phosphate buffer, pH 7.0.
3.7. Indolizine Bioformation Procedure
Two types of biocatalysts—biomass and cell-free supernatant—obtained after 72, 96, 120 and 144 h of submerged cultivation in aerobe conditions, of the three selected Yarrowia lipolytica strains were tested for use in reactions of cycloadition to obtain fluorescent indolizines. To an Eppendorf containing 1 mL of 250-mmol L−1 phosphate buffer solutions (pH 7.0) and crude enzymes (0.5 mL culture supernatant or biomass 10%, v/v) was added the pyridinium heterocycle compounds, (4,4′-bipyridine) 1 (15.6 mg, 0.1 mmol), halide derivatives 2 (60 mg, 0.3 mmol) and ethyl propiolate, 3 (30.6 µL, 0.3 mmol). The reaction mixture was stirred at room temperature on an orbital shaker (Biosan, Riga, Latvia) for 8 days.
The evolution of each reaction was followed by TLC and HPLC-MS (Thermo Scientific, Waltham, MA, USA) analysis. The composition in relative percentages of indolizines was computed through the normalization method from the HPLC peak areas, and the bioconversion yield was used as a performance indicator.
3.8. Separation and Purification of the Indolizines Bioconversion Products
All the chemicals and the solvents were of analytical grade and commercially available from Merck (Darmstadt, Germany). The biocatalytic reactions were performed in a thermoshaker Biosan TS 100 (Biosan, Riga, Latvia). The biotransformations were analyzed by a Thermo Scientific system, high performance liquid chromatography coupled with a diode array detector. The electrospray ionization (ESI) mass spectra were measured on Thermo Scientific HPLC/MSQ Plus. HPLC analysis was performed with a C18 HPLC column (50 mm × 0.2 mm, film thickness 0.32 μm) using acetonitrile, flow rate 0.3 mL min−1 with UV detector at wavelength λ = 254 nm. 1H NMR and 13C NMR spectra were recorded with a Bruker 400 Ultrashield (400 MHz) spectrometer (Daltonics, Hamburg, Germany) operating at room temperature. Abbreviations for data quoted are m, multiplet; s, singlet; dd, doublet of doublets; d, doublet; t, triplet; q, quartet. CDCl3 was used as solvent. For the NMR data analysis, MestReNova software (version 10.0, Thermo Scientific, Waltham, USA) was used. Melting points were recorded with a Büchi Melting Point B-540 (Essen, Germany). Elemental analyses (C, H, N) were performed with a Fisons Instruments 1108 (Thermo Scientific, Waltham, MA, USA) CHNS-O elemental analyzer. The infrared spectra were collected using a Nicolet iS50 FT-IR spectrometer (Thermo Scientific, Waltham, MA, USA) equipped with a built-in ATR accessory, DTGS detector and KBr beamsplitter. A total of 32 scans were co-added over the range of 4000–400 cm−1 with a resolution of 4 cm−1. After each spectrum, the ATR plate was cleaned with ethanol solution. The FT-IR spectrometer was situated in a room that was air conditioned with controlled temperature (21 °C). For the FT-IR data analysis, Omnic software (version 9.6, Thermo Scientific, Waltham, USA) was used.
The extraction of the bioconversion products was carried out with chloroform (3 × 10 mL). The combined extract was washed with distilled water (3 × 10 mL), in order to remove the main intermediate, a bisquaternary pyridinium salt. The organic phase was dried over anhydrous sodium sulphate and filtered using Whatman No.1 filter paper. The solvent was removed under reduced pressure and the crude product was precipitated with methanol to give pure reaction products the bis-indolizine in moderate to good yields for Y. lipolytica MIUG RD14 strain biocatalysis. The concentrations of obtained products were analyzed by HPLC-MS using as standards the bis-indolizines obtained by classical method [54].
3.9. Evaluation of the Toxicity of Indolizines on the Germination of Triticum azestivum L.
In this study, healthy and mature wheat seeds were pretreated with sodium hypochlorite (1%) two times for 10 min in order to sterilize them, after which they were rinsed five times with ultrapure water. Germination boxes with 24 germination compartments were used, and in each germination compartment were inserted two layers of filter papers and 25 seeds in the presence and in the absence of two concentrations of biologic interest of indolizines compounds (10−3-M and 10−4 M). Control seeds were treated with distilled water, and four replicates were analyzed for each condition. The seeds were incubated in a dark chamber with relative humity and temperature control (70% ± 3% and 23 ± 2 °C), for 5 days and the appropriate water per day was added (Figure 10). Seeds were regarded to have germinated when the radicle was over 2.0 mm in length. The parameters evaluated were the seed germination (SG), the seed germination index (GI), the relative radicle growth (RRG), the relative seed germination (RSG) and the vigor index (VI%) [48,49,50] as follows:
SG = (Number of germinated seeds after 5 days/Number of total seeds) × 100%
RSG = (Number of germinated seeds(sample)/Number of germinated seeds (control)) × 100%
RRG = (Total radicle length of germinated seeds (sample)/Total radicle length of germinated seeds (control)) × 100%
GI = (% SG) × (% RRG)/100%
VI = (% SG) * mean of radicle length/mean of shoot length
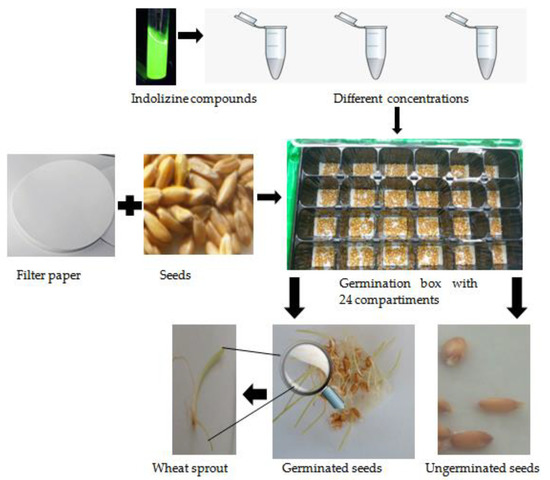
Figure 10.
Phytotoxic analysis of the germination of wheat seeds exposed to indolizines compounds at different concentrations.
Confocal laser scanning microscopy (CLSM) using a Zeiss system (LSM 710) (Carl Zeiss Microscopy, GmbH, Jena, Germany) equipped with a diode laser (405 nm), Ar laser (458, 488, 514 nm), DPSS laser (diode pumped solid state e 561 nm) and He–Ne laser (633 nm) was used to analyze the germination of the wheat seeds. The microscopic glass slides with sections through shoot and root of samples were observed using a Zeiss Axio Observer Z1 inverted microscope equipped with a 40×apochromatic objective (numerical aperture 1.4) and the FS49, FS38 and FS15 filters. The parameters used for image acquisition were line scan mode, mean method, average number 8, speed 6, 12-bit-depth. The images were analyzed with the ZEN 2012 SP1 software (Black Edition, Carl Zeiss Microscopy, GmbH, Jena, Germany).
3.10. Statistical Analysis
The data presented here represents the mean ± standard deviation of three or four replicates. Data were subJected to statistical analysis in Microsoft Excel program. Data related to the indolizine biosynthesis was subJected to analysis of variance (one-way ANOVA) in Duncan multiple range test using SPSS (version 10, IBM, Armonk, NY, USA) statistical software. The differences with p < 0.05 were considered significant.
4. Conclusions
In summary, we managed to synthesize indolizine compounds with important biologic properties in good yields through a new method by using whole cells biocatalysis. The high purity and yields of the synthesized bis-indolizines denotes a remarkable potential of selected Y. lipolytica strains to biosynthesis a multi-enzymatic catalytic system, consisting of oxidases, lipases and cytochromes P450, responsible for the bioconversion. These effective biocatalticproperties were exhibited in cycloaddition reactions. This multicomponent reaction that takes place under environmentally friendly conditions has provided a novel and interesting route to construct indolizine nucleus—important scaffolds for the preparation of pharmaceuticals. Furthermore, this “one-pot” reaction highlights relevant features such as mild conditions, selectivity and high efficiency as compared to classical methods that take place in steps with the formation of intermediate compounds, where toxic, expensive and hard-to-recover catalysts are usually used. These indolizine compounds do not exhibit toxic action on plants, evaluated by the effect on seed germination of wheat. The effect of indolizine compounds on mammalian cells and various microorganisms and underlying molecular mechanisms will be explored in our following studies. The present work highlights the role of yeasts species in novel products discovery and drug development.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/10/6/629/s1. Figure S1: HPLC chromatogram of bis-indolizine 4a, Figure S2. HPLC chromatogram of bis-indolizine 4b, Figure S3. HPLC chromatogram of bis-indolizine 4c, Figure S4. FTIR spectrum of compound 4a, Figure S5. FTIR spectrum of compound 4b, Figure S6. FTIR spectrum of compound 4c, Figure S7. 1H NMR spectrum of bis-indolizine 4a, Figure S8. 1H NMR spectrum of bis-indolizine 4b, Figure S9. 1H NMR spectrum of bis-indolizine 4c.
Author Contributions
Conceptualization, A.V.B., B.F.; and R.-M.D.; methodology, B.F., R.-M.D., I.O.G., G.H., F.B.; software, G.H.; formal analysis I.O.G., V.B., G.H., L.F., A.V.B.; resources, R.-M.D., G.-E.B.; writing—original draft preparation, A.V.B., I.O.G., G.H., L.F., R.-M.D.; writing—review and editing, All; supervision, R.-M.D., B.F., L.F., G.B.; proJect administration, R.-M.D., B.F.; funding acquisition, R.-M.D., G.-E.B. Investigation, A.V.B., V.B., F.B.; All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant of the Romanian National Authority for Scientific Research, CNCS-UEFISCDI, project number PN-II-ID-PCE-2011-3-0226.
Acknowledgments
Authors are indebted to the University of “Dunarea de Jos” of Galati, Romania. M.C. thanks the project “Excellence, performance and competitiveness in the Research, Development and Innovation activities at “Dunarea de Jos” University of Galati”, acronym “EXPERT”, financed by the Romanian Ministry of Research and Innovation in the framework of program 1—Development of the national research and development system, Sub-program 1.2—Institutional Performance—Projects for financing excellence in Research, Development andInnovation, Contract no. 14PFE/17.10.2018. The authors are grateful for the technical support offered by Center MoRAS developed through Grant POSCCE ID 1815, cod SMIS 48,745 (www.moras.ugal.ro).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhao, Q.; Ansorge-Schumacher, M.B.; Haag, R.; Wu, C. Living whole-cell catalysis in compartmentalized emulsion. Bioresour. Technol. 2020, 295, 122221. [Google Scholar] [CrossRef]
- Feng, G.; Wu, H.; Li, X.; Lai, F.; Zhao, G.; Yang, M.; Liu, H. A new, efficient and highly-regioselective approach to synthesis of 6-O-propionyl-d-glucose by using whole-cell biocatalysts. Biochem. Eng. J. 2015, 95, 56–61. [Google Scholar] [CrossRef]
- Anteneh, Y.S.; Franco, C.M.M. Whole cell actinobacteria as biocatalysts. Front. Microbiol. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Garzón-Posse, F.; Becerra-Figueroa, L.; Hernández-Arias, J.; Gamba-Sánchez, D. Whole cells as biocatalysts in organic transformations. Molecules 2018, 23, 1265. [Google Scholar] [CrossRef]
- Dinica, R.M.; Furdui, B.; Ghinea, I.O.; Bahrim, G.; Bonte, S.; Demeunynck, M. Novel one-pot green synthesis of indolizines biocatalysed by Candida antarctica lipases. Mar. Drugs 2013, 11, 431–439. [Google Scholar] [CrossRef]
- Matsuda, T.; Yamanaka, R.; Nakamura, K. Recent progress in biocatalysis for asymmetric oxidation and reduction. Tetrahedron Asymmetry 2009, 20, 513–557. [Google Scholar] [CrossRef]
- Kudalkar, G.P.; Tiwari, V.K.; Lee, J.D.; Berkowitz, D.B. A Hammett Study of Clostridium acetobutylicum Alcohol Dehydrogenase (CaADH): An enzyme with remarkable substrate promiscuity and utility for organic synthesis. Synlett 2020, 31, 237–247. [Google Scholar] [CrossRef]
- Antonopoulou, I.; Dilokpimol, A.; Iancu, L.; Mäkelä, M.R.; Varriale, S.; Cerullo, G.; Hüttner, S.; Uthoff, S.; Jütten, P.; Piechot, A.; et al. The synthetic potential of fungal feruloyl esterases: A correlation with current classification systems and predicted structural properties. Catalysts 2018, 8, 242. [Google Scholar] [CrossRef]
- Yin, D.H.; Liu, W.; Wang, Z.X.; Huang, X.; Zhang, J.; Huang, D.C. Enzyme-catalyzed direct three-component aza-Diels–Alder reaction using lipase from Candida sp. 99–125. Chin. Chem. Lett. 2017, 28, 153–158. [Google Scholar] [CrossRef]
- Carballeira, J.D.; Quezada, M.A.; Hoyos, P.; Simeó, Y.; Hernaiz, M.J.; Alcantara, A.R.; Sinisterra, J.V. Microbial cells as catalysts for stereoselective red-ox reactions. Biotechnol. Adv. 2009, 27, 686–714. [Google Scholar] [CrossRef]
- Ratnayake, N.D.; Theisen, C.; Walter, T.; Walker, K.D. Whole-cell biocatalytic production of variously substituted β-aryl- and β-heteroaryl-β-amino acids. J. Biotechnol. 2016, 217, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Birolli, W.G.; Ferreira, I.M.; Alvarenga, N.; De Santos, D.A.; De Matos, I.L.; Comasseto, J.V.; Porto, A.L.M. Biocatalysis and biotransformation in Brazil: An overview. Biotechnol. Adv. 2015, 33, 481–510. [Google Scholar] [CrossRef]
- Wachtmeister, J.; Rother, D. Recent advances in whole cell biocatalysis techniques bridging from investigative to industrial scale. Curr. Opin. Biotechnol. 2016, 42, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Rywińska, A.; Juszczyk, P.; Wojtatowicz, M.; Robak, M.; Lazar, Z.; Tomaszewska, L.; Rymowicz, W. Glycerol as a promising substrate for Yarrowia lipolytica biotechnological applications. Biomass Bioenergy 2013, 48, 148–166. [Google Scholar] [CrossRef]
- Zieniuk, B.; Fabiszewska, A. Yarrowia lipolytica: A beneficious yeast in biotechnology as a rare opportunistic fungal pathogen: A minireview. World J. Microbiol. Biotechnol. 2019, 35, 1–8. [Google Scholar] [CrossRef]
- Fickers, P.; Benetti, P.H.; Waché, Y.; Marty, A.; Mauersberger, S.; Smit, M.S.; Nicaud, J.M. Hydrophobic substrate utilisation by the yeast Yarrowia lipolytica, and its potential applications. FEMS Yeast Res. 2005, 5, 527–543. [Google Scholar] [CrossRef]
- Gonçalves, F.A.G.; Colen, G.; Takahashi, J.A. Yarrowia lipolytica and its multiple applications in the biotechnological industry. Sci. World J. 2014, 2014, 1–14. [Google Scholar] [CrossRef]
- Casas-Godoy, L.; Marty, A.; Sandoval, G.; Ferreira-Dias, S. Optimization of medium chain length fatty acid incorporation into olive oil catalyzed by immobilized Lip2 from Yarrowia lipolytica. Biochem. Eng. J. 2013, 77, 20–27. [Google Scholar] [CrossRef]
- Facin, B.R.; Melchiors, M.S.; Valério, A.; Oliveira, J.V.; De Oliveira, D. Driving immobilized lipases as biocatalysts: 10 years state of the art and future prospects. Ind. Eng. Chem. Res. 2019, 58, 5358–5378. [Google Scholar] [CrossRef]
- Arvin-Berod, M.; Desroches-Castan, A.; Bonte, S.; Brugière, S.; Couté, Y.; Guyon, L.; Feige, J.J.; Baussanne, I.; Demeunynck, M. Indolizine-based scaffolds as efficient and versatile tools: Application to the synthesis of biotin-tagged antiangiogenic drugs. ACS Omega 2017, 2, 9221–9230. [Google Scholar] [CrossRef]
- Sharma, V.; Kumar, V. Indolizine: A biologically active moiety. Med. Chem. Res. 2014, 23, 3593–3606. [Google Scholar] [CrossRef]
- Narayanaswamy, K.; Id, V.; Chandrashekharappa, S.; Pillay, M.; Abdallah, H.H.; Mahomoodally, F.M.; Bhandary, S.; Chopra, D.; Attimarad, M.; Aldhubiab, B.E.; et al. Computational, crystallographic studies, cytotoxicity and anti-tubercular activity of substituted 7-methoxy-indolizine analogues. PLoS ONE 2019, 14, e0217270. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Tratrat, C.; Chandrashekharappa, S. Anti-tubercular potency and computationally assessed drug-likeness and toxicology of diversely substituted indolizines. Indian J. Pharm. Educ. 2019, 53. [Google Scholar] [CrossRef]
- Uppar, V.; Chandrashekharappa, S.; Basarikatti, A.I.; Banuprakash, G.; Mahendra, K.; Chougala, M.; Mudnakudu-nagaraju, K.K.; Ningegowda, R.; Padmashali, B. Synthesis, antibacterial, and antioxidant studies of 7-amino-3-(4-fluorobenzoyl)indolizine-1-carboxylate derivatives. J. Appl. Pharm. Sci. 2020, 10, 77–85. [Google Scholar]
- Narajji, C.; Karvekar, M.D.; Das, A.K. Synthesis and antioxidant activity of 3,3'-diselanediylbis (N,N-disubstituted indolizine-1-carboxamide) and derivatives. S. Afr. J. Chem. 2008, 61, 53–55. [Google Scholar]
- Moon, S.H.; Jung, Y.; Kim, S.H.; Kim, I. Synthesis, characterization and biological evaluation of anti-cancer indolizine derivatives via inhibiting β-catenin activity and activating p53. Bioorganic Med. Chem. Lett. 2016, 26, 110–113. [Google Scholar] [CrossRef]
- Belal, A.; Gouda, A.M.; Ahmed, A.S.; Abdel Gawad, N.M. Synthesis of novel indolizine, diazepinoindolizine and Pyrimidoindolizine derivatives as potent and selective anticancer agents. Res. Chem. Intermed. 2015, 41, 9687–9701. [Google Scholar] [CrossRef]
- Ghinet, A.; Abuhaie, C.M.; Gautret, P.; Rigo, B.; Dubois, J.; Farce, A.; Belei, D.; Bîcu, E. Studies on indolizines. Evaluation of their biological properties as microtubule-interacting agents and as melanoma targeting compounds. Eur. J. Med. Chem. 2015, 89, 115–127. [Google Scholar] [CrossRef]
- Weide, T.; Arve, L.; Prinz, H.; Waldmann, H.; Kessler, H. 3-Substituted indolizine-1-carbonitrile derivatives as phosphatase inhibitors. Bioorganic Med. Chem. Lett. 2006, 16, 59–63. [Google Scholar] [CrossRef]
- Sharma, V.; Kumar, V. Pharmacophore mapping studies on indolizine derivatives as 15-LOX inhibitors. Bull. Fac. Pharm. Cairo Univ. 2015, 53, 63–68. [Google Scholar] [CrossRef]
- Huang, W.; Zuo, T.; Luo, X.; Jin, H.; Liu, Z.; Yang, Z.; Yu, X.; Zhang, L.; Zhang, L. Indolizine derivatives as HIV-1 VIF-elonginc interaction inhibitors. Chem. Biol. Drug Des. 2013, 81, 730–741. [Google Scholar] [CrossRef]
- De Souza, C.R.; Gonçalves, A.C.; Amaral, M.F.Z.J.; Dos Santos, A.A.; Clososki, G.C. Recent synthetic developments and reactivity of aromatic indolizines. Targets Heterocycl. Syst. 2016, 20, 365–392. [Google Scholar]
- Sadowski, B.; Klajn, J.; Gryko, D.T. Recent advances in the synthesis of indolizines and their π-expanded analogues. Org. Biomol. Chem. 2016, 14, 7804–7828. [Google Scholar] [CrossRef]
- Ghinea, I.O.; Dinica, R.M. Breakthroughs in indole and indolizine chemistry-new synthetic pathways, new applications. In Scope of Selective Heterocycles from Organic and Pharmaceutical Perspective; Chapter 5; Ravi, V., Ed.; IntechOpen: London, UK, 2016; pp. 115–142. [Google Scholar] [CrossRef]
- Kapoor, M.; Gupta, M.N. Lipase promiscuity and its biochemical applications. Process. Biochem. 2012, 47, 555–569. [Google Scholar] [CrossRef]
- Majumder, A.B.; Ramesh, N.G.; Gupta, M.N. A lipase catalyzed condensation reaction with a tricyclic diketone: Yet another example of biocatalytic promiscuity. Tetrahedron Lett. 2009, 50, 5190–5193. [Google Scholar] [CrossRef]
- Reetz, M.T.; Mondière, R.; Carballeira, J.D. Enzyme promiscuity: First protein-catalyzed Morita-Baylis-Hillman reaction. Tetrahedron Lett. 2007, 48, 1679–1681. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.Y.; Wang, H.R.; Zhang, H.; Su, Y.L.; Ji, T.F.; Wang, L. Lipase-catalyzed Knoevenagel condensation between α,β unsaturated aldehydes and active methylene compounds. Chin. Chem. Lett. 2014, 25, 802–804. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J.; Wu, D.; Xing, Z.; Zhou, Y.; Shi, W.; Li, Q. Chemoenzymatic synthesis of polymeric materials using lipases as catalysts: A review. Biotechnol. Adv. 2014, 32, 642–651. [Google Scholar] [CrossRef]
- Parfene, G.; Horincar, V.B.; Bahrim, G.; Vannini, L.; Gottardi, D.; Maria Elisabetta, G. Lipolytic activity of lipases from different strains of Yarrowia lipolytica in hydrolysed vegetable fats at low temperature and water activity. Rom. Biotechnol. Lett. 2011, 16, 46–52. [Google Scholar]
- Destain, J.; Roblain, D.; Thonart, P. Improvement of lipase production from Yarrowia lipolytica. Biotechnol. Lett. 1997, 19, 105–108. [Google Scholar] [CrossRef]
- Pignede, G.; Wang, H.J.; Fudalej, F.; Seman, M.; Gaillardin, C.; Nicaud, J.M. Autocloning and amplification of LIP2 in Yarrowia lipolytica. Appl. Environ. Microbiol. 2000, 66, 3283–3289. [Google Scholar] [CrossRef] [PubMed]
- Fickers, P.; Fudalej, F.; Nicaud, J.M.; Destain, J.; Thonart, P. Selection of new over-producing derivatives for the improvement of extracellular lipase production by the non-conventional yeast Yarrowia lipolytica. J. Biotechnol. 2005, 115, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Białecka-Florjańczyk, E.; Krzyczkowska, J.; Stolarzewicz, I.; Kapturowska, A. Synthesis of 2-phenylethyl acetate in the presence of Yarrowia lipolytica KKP 379 biomass. J. Mol. Catal. B Enzym. 2012, 74, 241–245. [Google Scholar] [CrossRef]
- Napora, K.; Wrodnigg, T.M.; Kosmus, P.; Thonhofer, M.; Robins, K.; Winkler, M. Yarrowia lipolytica dehydrogenase/reductase: An enzyme tolerant for lipophilic compounds and carbohydrate substrates. Bioorganic Med. Chem. Lett. 2013, 23, 3393–3395. [Google Scholar] [CrossRef] [PubMed]
- Druta, I.; Dinica, R.M.; Bacu, E.; Humelnicu, I. Synthesis of 7, 7’-bisindolizines by the reaction of 4,4’-bipyridinium-ylides with activated alkynes. Tetrahedron 1998, 54, 10811–10818. [Google Scholar] [CrossRef]
- Mazumder, P.; Khwairakpam, M.; Kalamdhad, A.S. Bio-inherent attributes of water hyacinth procured from contaminated water body–effect of its compost on seed germination and radicle growth. J. Environ. Manag. 2020, 257, 109990. [Google Scholar] [CrossRef]
- Li, R.; He, J.; Xie, H.; Wang, W.; Bose, S.K.; Sun, Y.; Hu, J.; Yin, H. Effects of chitosan nanoparticles on seed germination and seedling growth of wheat (Triticum aestivum L.). Int. J. Biol. Macromol. 2019, 126, 91–100. [Google Scholar] [CrossRef]
- Cudalbeanu, M.; Furdui, B.; Cârâc, G.; Barbu, V.; Iancu, A.V.; Campello, M.P.C.; Leitão, J.H.; Sousa, S.A.; Dinica, R.M. Antifungal, Antitumoral and Antioxidant Potential of the Danube Delta Nymphaea alba Extracts. Antibiotion 2019, 9, 7. [Google Scholar] [CrossRef]
- Tabacaru, A.; Botezatu-Dediu, A.-V.; Horincar, G.; Furdui, B.; Dinica, R.M. Green Accelerated Synthesis, Antimicrobial Activity. Molecules 2019, 24, 2424. [Google Scholar] [CrossRef] [PubMed]
- OECD. OECD test guideline 208: Terrestrial plant test—Seedling emergence and seedling growth test. Guidel. Test Chem. Terr. Plant Test Seedl. Emerg. Seedl. Growth Test 2006, 227, 1–21. [Google Scholar]
- Luo, Y.; Liang, J.; Zeng, G.; Chen, M.; Mo, D.; Li, G.; Zhang, D. Seed germination test for toxicity evaluation of compost: Its roles, problems and prospects. Waste Manag. 2018, 71, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Tiquia, S.M.; Tam, N.F.Y.; Hodgkiss, I.J. Effects of composting on phytotoxicity of spent pig-manure sawdust litter. Environ. Pollut. 1996, 93, 249–256. [Google Scholar] [CrossRef]
- Barth, G.; Gaillardin, C. Yarrowia lipolytica. In Nonconventional Yeasts in Biotechnology: A Handbook; Springer: Berlin/Heidelberg, Germany, 1996; pp. 313–388. ISBN 978-3-642-79856-6. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).