Preparation of PP/2D-Nanosheet Composites Using MoS2/MgCl2- and BN/MgCl2-Bisupported Ziegler–Natta Catalysts
Abstract
1. Introduction
2. Results and Discussion
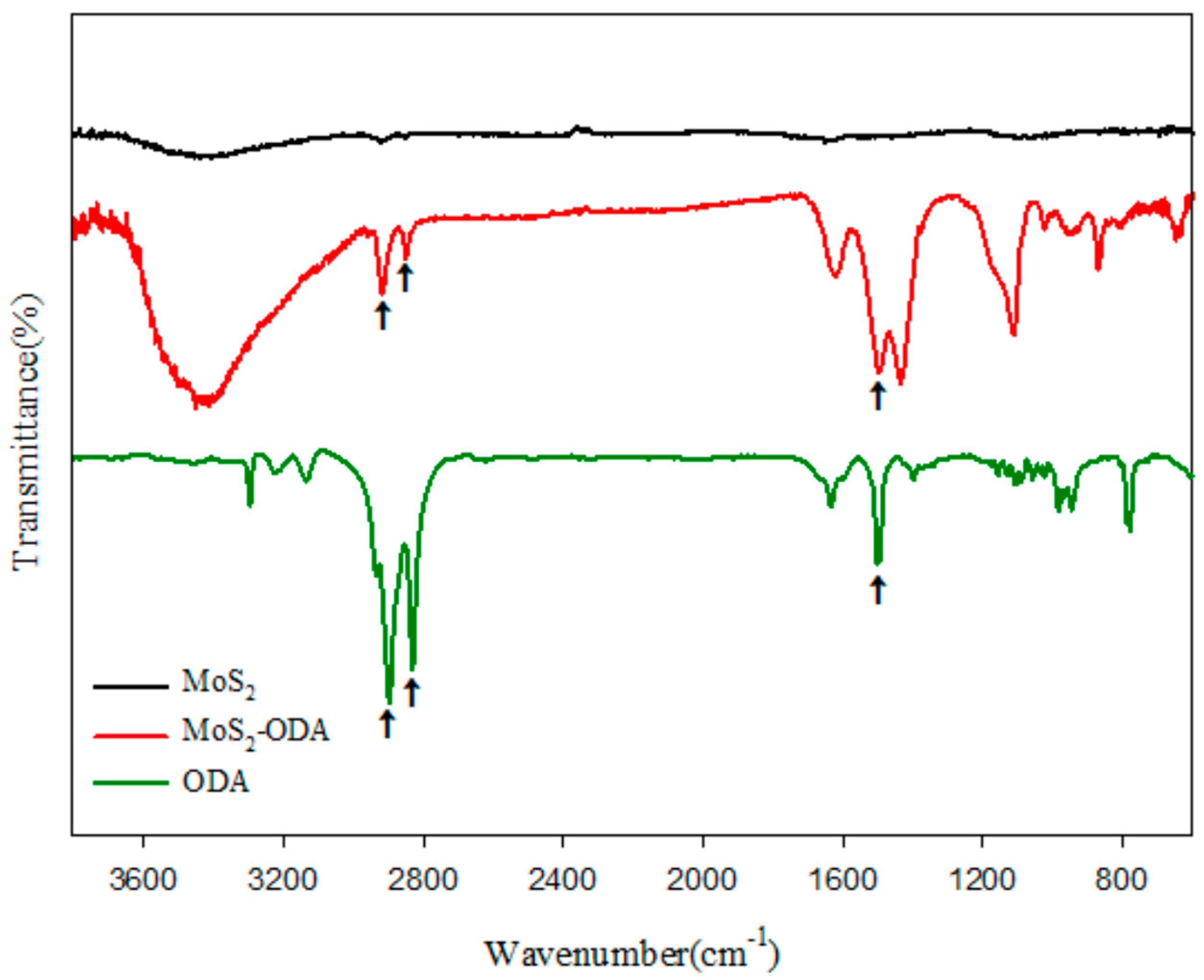
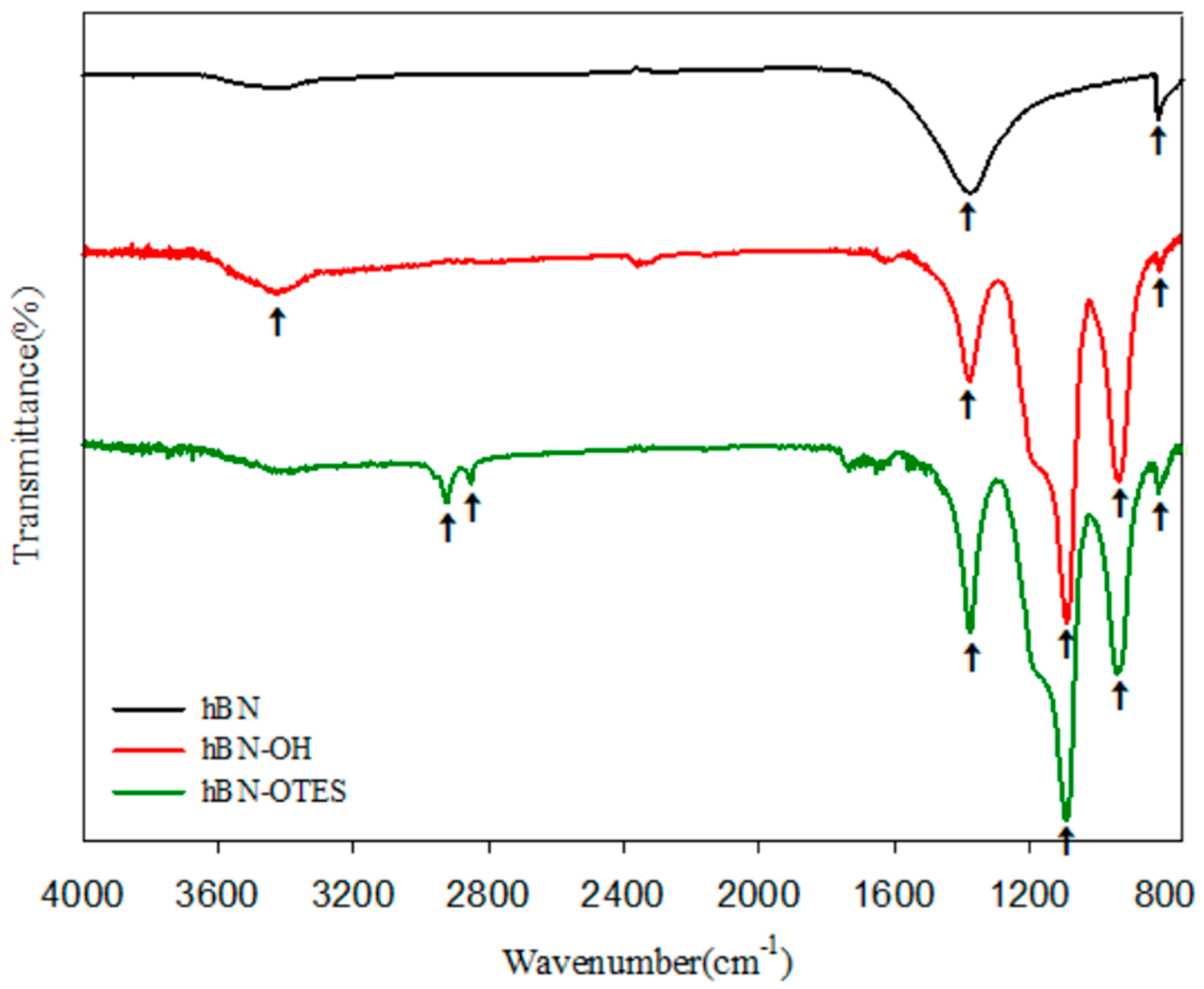
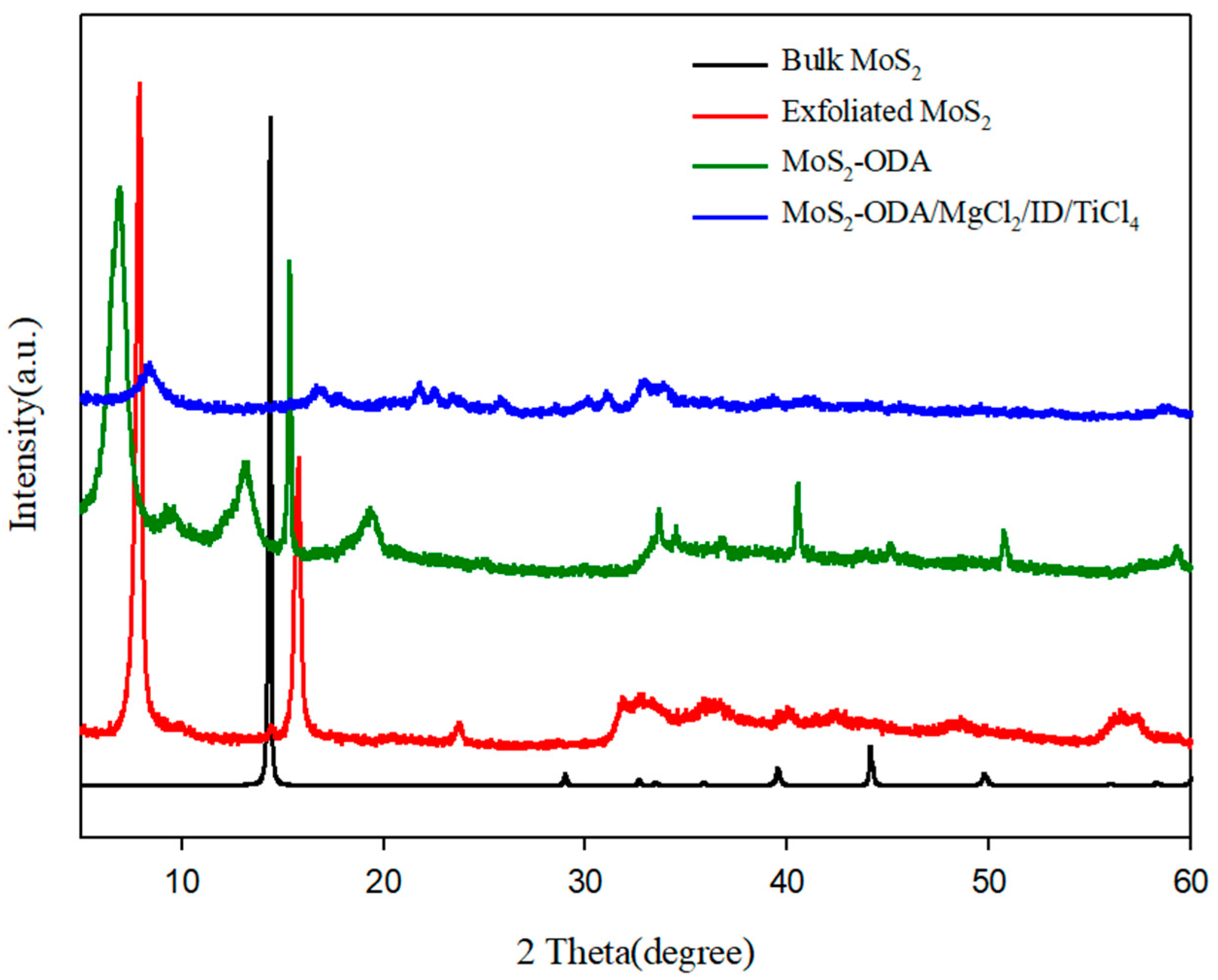
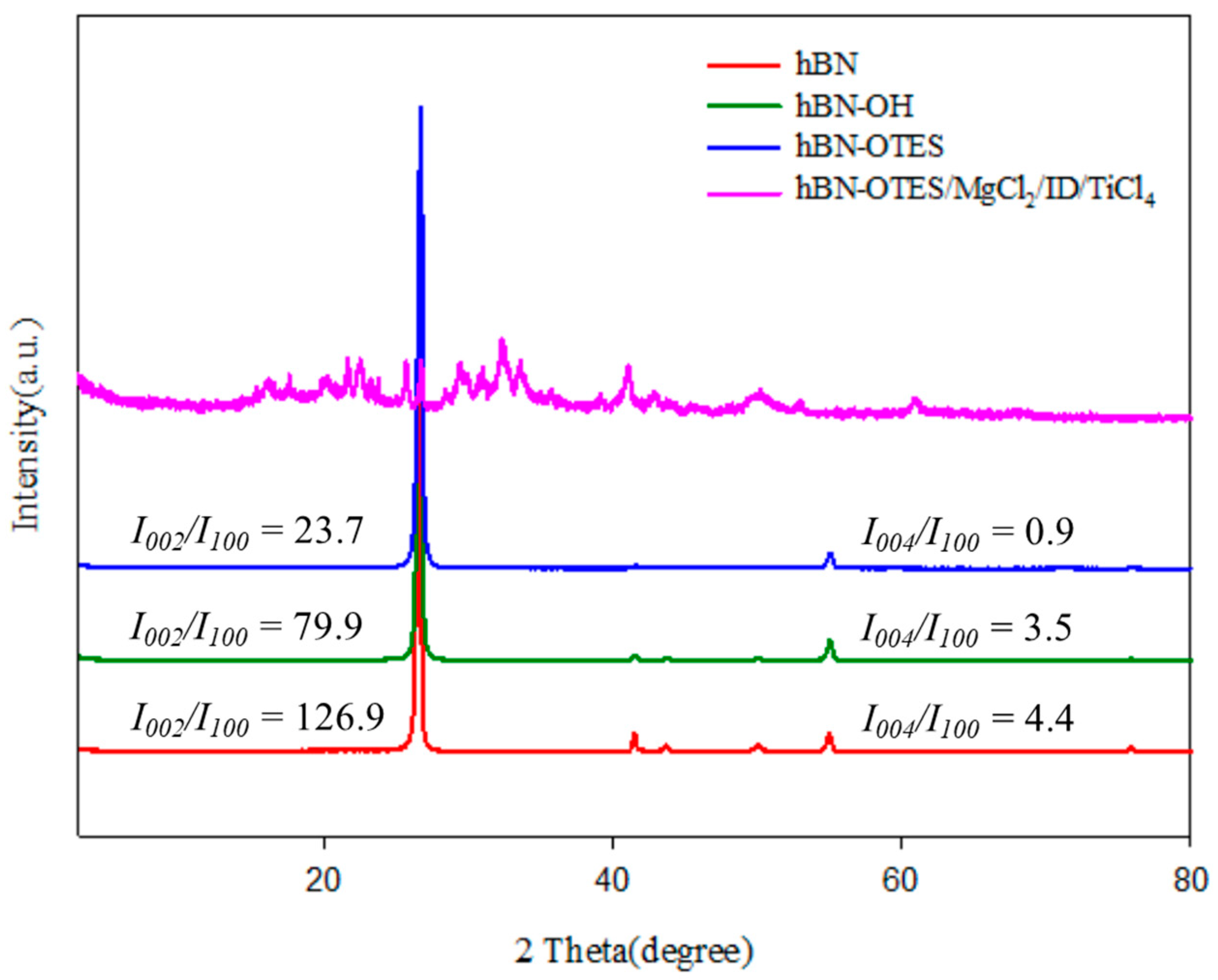
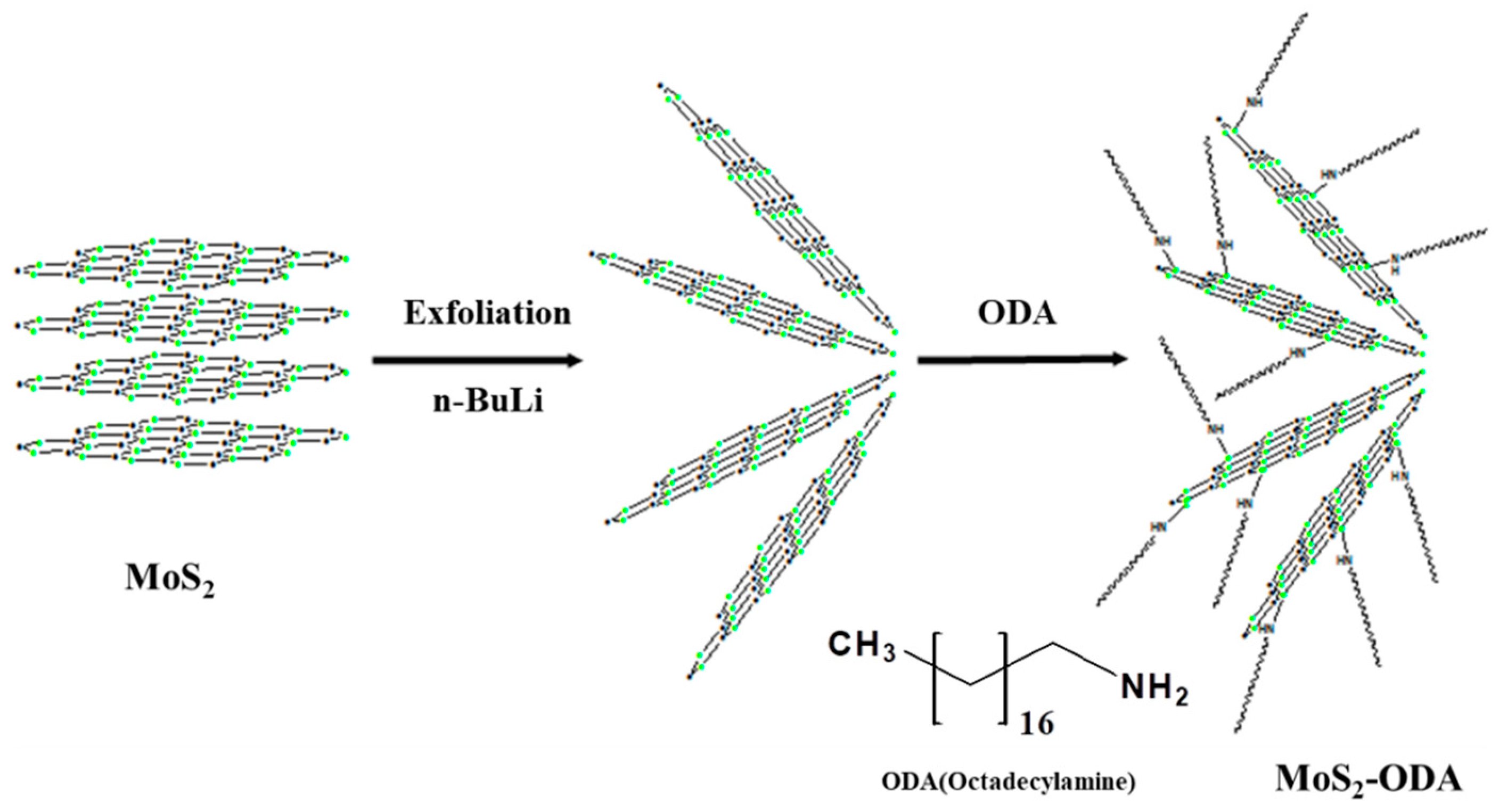
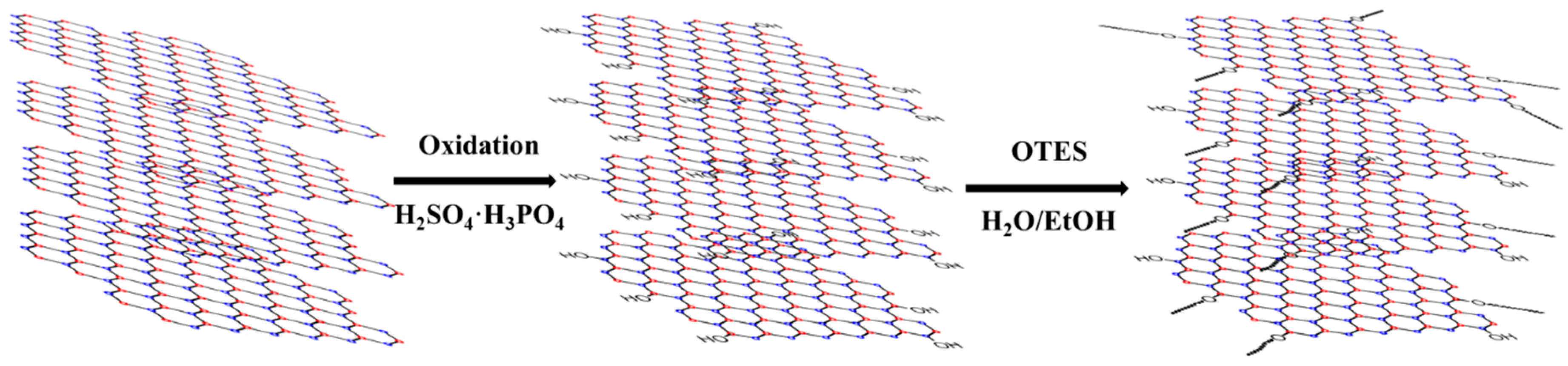
2.1. Characterization of Modified 2D-Nanosheets and Catalysts
2.2. Preparation of PP/2D-Nanosheet Nanocomposites
2.3. Thermal Properties and Stabilities
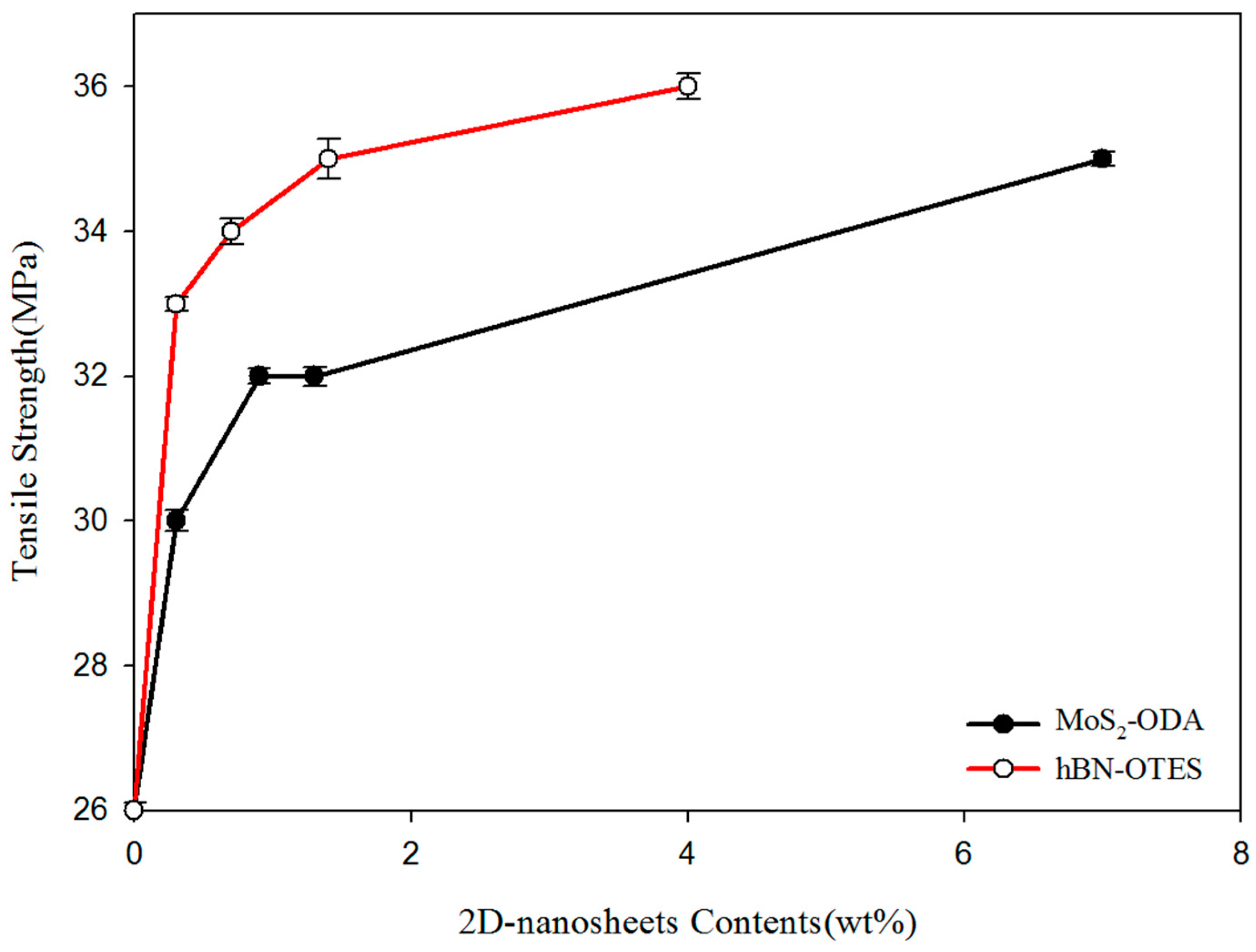
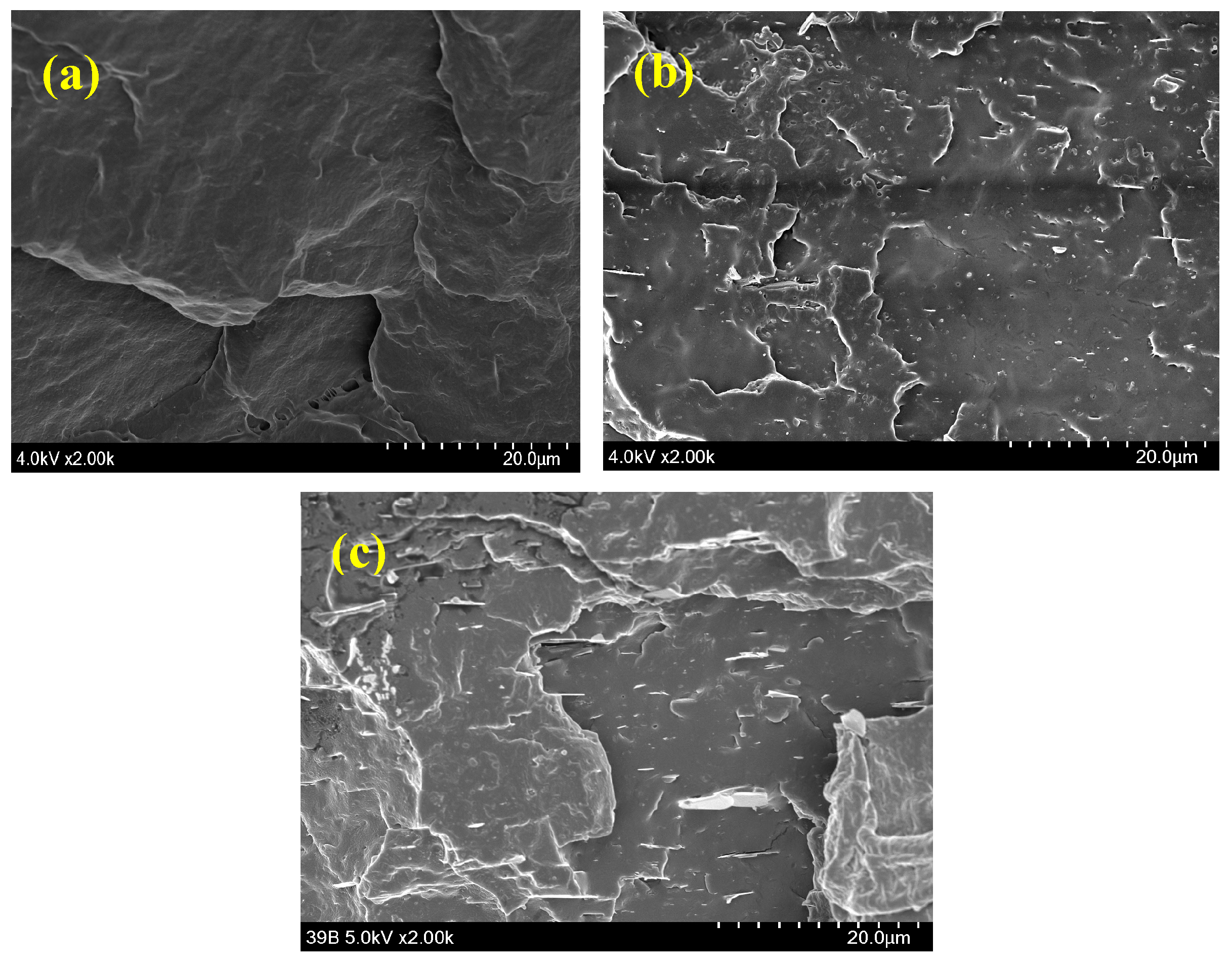
2.4. Mechanical Properties
3. Experimental Section
3.1. Materials
3.2. Synthesis of MoS2-ODA and hBN-OTES
3.3. Synthesis of 2D-Nanosheets/MgCl2-Bisupported Ziegler–Natta Catalyst
3.4. Preparation of the PP/2D-Nanosheet Nanocomposites via In-Situ Polymerization
3.5. Characterizations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Raji, M.; Mekhzoum, M.E.M.; Rodrigue, D.; Qaiss, A.E.K.; Bouhfid, R. Effect of silane functionalization on properties of polypropylene/clay nanocomposites. Compos. Part B Eng. 2018, 146, 106–115. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.J.; Weng, G.J.; Su, Y. The effects of temperature and alignment state of nanofillers on the thermal conductivity of both metal and nonmetal based graphene nanocomposites. Acta Mater. 2020, 185, 461–473. [Google Scholar] [CrossRef]
- Kim, J.; Cha, J.; Chung, B.; Ryu, S.; Hong, S.H. Fabrication and mechanical properties of carbon fiber/epoxy nanocomposites containing high loadings of noncovalently functionalized graphene nanoplatelets. Compos. Sci. Technol. 2020, 192, 108101. [Google Scholar] [CrossRef]
- Peng, H.; Wang, D.; Fu, S. Tannic acid-assisted green exfoliation and functionalization of MoS2 nanosheets: Significantly improve the mechanical and flame-retardant properties of polyacrylonitrile composite fibers. Chem. Eng. J. 2020, 384, 123288. [Google Scholar] [CrossRef]
- Pang, W.; Ni, Z.; Chen, G.; Huang, G.; Zhao, Y. Mechanical and thermal properties of graphene oxide/ultrahigh molecular weight polyethylene nanocomposites. RSC Adv. 2015, 5, 63063–63072. [Google Scholar] [CrossRef]
- Chen, Y.; Qi, Y.; Tai, Z.; Yan, X.; Zhu, F.; Xue, Q. Preparation, mechanical properties and biocompatibility of graphene oxide/ultrahigh molecular weight polyethylene composites. Eur. Polym. J. 2012, 48, 1026–1033. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, C. Polyethylene/graphite oxide nanocomposites obtained by in situ polymerization using modified graphite oxide–supported metallocene catalysts. J. Polym. Res. 2012, 20, 39. [Google Scholar] [CrossRef]
- Benavente, E. Intercalation chemistry of molybdenum disulfide. Co-ord. Chem. Rev. 2002, 224, 87–109. [Google Scholar] [CrossRef]
- Joensen, P.; Frindt, R.; Morrison, S. Single-layer MoS2. Mater. Res. Bull. 1986, 21, 457–461. [Google Scholar] [CrossRef]
- Fan, X.; Xu, P.; Zhou, D.; Sun, Y.; Li, Y.C.; Nguyen, M.A.T.; Terrones, M.; Mallouk, T.E. Fast and Efficient Preparation of Exfoliated 2H MoS2 Nanosheets by Sonication-Assisted Lithium Intercalation and Infrared Laser-Induced 1T to 2H Phase Reversion. Nano Lett. 2015, 15, 5956–5960. [Google Scholar] [CrossRef]
- Falin, A.; Cai, Q.; Santos, E.J.G.; Scullion, D.; Qian, N.; Zhang, R.; Yang, Z.; Huang, S.; Watanabe, K.; Taniguchi, T.; et al. Mechanical properties of atomically thin boron nitride and the role of interlayer interactions. Nat. Commun. 2017, 8, 15815. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, F.; Sun, M. Graphene, hexagonal boron nitride, and their heterostructures: Properties and applications. RSC Adv. 2017, 7, 16801–16822. [Google Scholar] [CrossRef]
- Beckert, F.; Bodendorfer, S.; Zhang, W.; Thomann, R.; Mülhaupt, R. Mechanochemical Route to Graphene-Supported Iron Catalysts for Olefin Polymerization and in Situ Formation of Carbon/Polyolefin Nanocomposites. Macromolecules 2014, 47, 7036–7042. [Google Scholar] [CrossRef]
- Guerrero-Contreras, J.; Caballero-Briones, F. Graphene oxide powders with different oxidation degree, prepared by synthesis variations of the Hummers method. Mater. Chem. Phys. 2015, 153, 209–220. [Google Scholar] [CrossRef]
- Jeffery, A.A.; Nethravathi, C.; Rajamathi, M. Scalable large nanosheets of transition metal disulphides through exfoliation of amine intercalated MS 2 [M = Mo, W] in organic solvents. RSC Adv. 2015, 5, 51176–51182. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, W.; Gao, Y.; Liang, G.; Gu, A.; Yuan, L. Surface functionalization of hexagonal boron nitride and its effect on the structure and performance of composites. Appl. Surf. Sci. 2013, 270, 561–571. [Google Scholar] [CrossRef]
- Hou, J.; Li, G.; Yang, N.; Qin, L.; Grami, M.E.; Zhang, Q.; Wang, N.; Qu, X. Preparation and characterization of surface modified boron nitride epoxy composites with enhanced thermal conductivity. RSC Adv. 2014, 4, 44282–44290. [Google Scholar] [CrossRef]
- Fu, S.-Y.; Sun, Z.; Huang, P.; Li, Y.-Q.; Hu, N. Some basic aspects of polymer nanocomposites: A critical review. Nano Mater. Sci. 2019, 1, 2–30. [Google Scholar] [CrossRef]
- Abedi, S.; Abdouss, M. A review of clay-supported Ziegler–Natta catalysts for production of polyolefin/clay nanocomposites through in situ polymerization. Appl. Catal. A Gen. 2014, 475, 386–409. [Google Scholar] [CrossRef]
- Kheradmand, A.; Ramazani, S.A.; Khorasheh, F.; Baghalha, M.; Bahrami, H. Effects of nano graphene oxide as support on the product properties and performance of Ziegler-Natta catalyst in production of UHMWPE. Polym. Adv. Technol. 2015, 26, 315–321. [Google Scholar] [CrossRef]
- Zhang, H.X.; Bae, M.G.; Park, J.H.; Ko, E.B.; Lee, D.H.; Zhang, X.Q.; Yoon, K.-B. Effects of GO oxidation degree on GO/BuMgCl-suppoeted Ti-based Ziegler-Natta catalyst performance and nanocomposite properties. RSC Adv. 2016, 6, 20734–20740. [Google Scholar] [CrossRef]
- Zhang, H.X.; Moon, Y.K.; Zhang, X.Q.; Zhang, H.Z.; Yoon, K.-B. In situ polymerization approach to functionalized MoS2/polyethylene nanocomposites with enhanced thermal stability and mechanical properties. RSC Adv. 2016, 6, 112429–112434. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, D.; Zou, X.; Mao, L.; Shi, L. The exfoliation and functionalization of boron nitride nanosheets and their utilization in silicone composites with improved thermal conductivity. J. Mater. Sci. Mater. Electron. 2017, 28, 12984–12994. [Google Scholar] [CrossRef]
- Du, M.; Wu, Y.; Hao, X. A facile chemical exfoliation method to obtain large size boron nitride nanosheets. CrystEngComm 2013, 15, 1782. [Google Scholar] [CrossRef]
- Sun, W.; Meng, Y.; Fu, Q.; Wang, F.; Wang, G.; Gao, W.; Huang, X.-C.; Lu, F. High-Yield Production of Boron Nitride Nanosheets and Its Uses as a Catalyst Support for Hydrogenation of Nitroaromatics. ACS Appl. Mater. Interfaces 2016, 8, 9881–9888. [Google Scholar] [CrossRef]
- Cecchin, G.; Marchetti, E.; Baruzzi, G. On the mechanism of polypropylene growth over MgCl2/TiCl4 catalyst systems. Macromol. Chem. Phys. 2001, 202, 1987–1994. [Google Scholar] [CrossRef]
- Naffakh, M.; Marco, C.; Gómez-FatouM, A. Isothermal Crystallization Kinetics of Novel Isotactic Polypropylene/MoS2Inorganic Nanotube Nanocomposites. J. Phys. Chem. B 2011, 115, 2248–2255. [Google Scholar] [CrossRef]
- Yang, N.; Xu, C.; Hou, J.; Yao, Y.; Zhang, Q.; Grami, M.E.; He, L.; Wang, N.; Qu, X. Preparation and properties of thermally conductive polyimide/boron nitride composites. RSC Adv. 2016, 6, 18279–18287. [Google Scholar] [CrossRef]
- Yang, M.; Gao, Y.; Li, H.; Adronov, A. Functionalization of multiwalled carbon nanotubes with polyamide 6 by anionic ring-opening polymerization. Carbon 2007, 45, 2327–2333. [Google Scholar] [CrossRef]
- Siddiqui, G.U.; Rehman, M.M.; Yang, Y.-J.; Choi, K.-H. A two-dimensional hexagonal boron nitride/polymer nanocomposite for flexible resistive switching devices. J. Mater. Chem. C 2017, 5, 862–871. [Google Scholar] [CrossRef]
- Yang, W.; Wang, N.-N.; Ping, P.; Yuen, A.C.Y.; Li, A.; Zhu, S.-E.; Wang, L.-L.; Wu, J.; Chen, T.B.-Y.; Si, J.-Y.; et al. Novel 3D Network Architectured Hybrid Aerogel Comprising Epoxy, Graphene, and Hydroxylated Boron Nitride Nanosheets. ACS Appl. Mater. Interfaces 2018, 10, 40032–40043. [Google Scholar] [CrossRef] [PubMed]
- Bian, J.; Wang, Z.J.; Lin, H.L.; Zhou, X.; Xiao, W.Q.; Zhao, X.W. Thermal and mechanical properties of polypropylene nanocomposites reinforced with nano-SiO 2 functionalized graphene oxide. Compos. Part A Appl. Sci. Manuf. 2017, 97, 120–127. [Google Scholar] [CrossRef]
- Valdés, S.S.; Zapata-Domínguez, A.; Martinez-Colunga, J.; Mendez-Nonell, J.; De Valle, L.R.; Espinoza-Martinez, A.; Morales-Cepeda, A.B.; Lozano-Ramirez, T.; LaFleur, P.; Ramirez-Vargas, E. Influence of functionalized polypropylene on polypropylene/graphene oxide nanocomposite properties. Polym. Compos. 2016, 39, 1361–1369. [Google Scholar] [CrossRef]
- Jafariyeh-Yazdi, E.; Ravakoli, A.; Abbasi, F.; Parnian, M.J.; Heidari, A. B-supported Ziegler-Natta TiCl4/MCM-41/MgCl2(ethoxide type) catalyst preparation and comprehensice investigation of produced polyethylene characteristics. J. Appl. Polym. Sci. 2020, 137, 48553. [Google Scholar] [CrossRef]
- Hajibeygi, M.; Mousavi, M.; Shabanian, M.; Vahabi, H. The effect of phosphorus based melamine-terephthaldehyde resin and Mg-Al layered double hydroxide on the thermal stability, flame retardancy and mechanical properties of polypropylene MgO composites. Mater. Today Commun. 2020, 23, 100880. [Google Scholar] [CrossRef]


| Catalysts | Catalysts (mg) | [Al]/[Ti] | Time (min) | Isotactic Index (I.I) (%) | Activity (PP kg/g-Ti-h) | 2D-Nanosheets Contents (wt %) | Melt Index (M.I) |
|---|---|---|---|---|---|---|---|
| MgCl2/DIBP/TiCl4–TEA/DCPDMS | 200 200 | 5 10 | 60 30 | 93 93 | 0.68 0.95 | - | 0.53 0.62 |
| MoS2-ODA/ MgCl2/DIBP/TiCl4–TEA/DCPDMS | 210 | 10 | 30 | 92 | 0.63 | 0.3 | 0.49 |
| 250 | 10 | 60 | 93 | 0.90 | 0.9 | 0.45 | |
| 250 | 10 | 30 | 91 | 1.28 | 1.3 | 0.50 | |
| 400 | 30 | 30 | 92 | 0.96 | 7.0 | 0.52 | |
| hBN-OTES/ MgCl2/DIBP/TiCl4–TEA/DCPDMS | 210 | 10 | 60 | 93 | 0.59 | 0.3 | 0.48 |
| 250 | 10 | 60 | 94 | 1.13 | 0.7 | 0.45 | |
| 250 | 7 | 15 | 92 | 2.52 | 1.4 | 0.45 | |
| 400 | 10 | 45 | 91 | 1.12 | 4.0 | 0.49 |
| Catalysts | 2D-Nanosheets Contents (wt %) | Tc (°C) | Tm (°C) | Xc (%) |
|---|---|---|---|---|
| MgCl2/DIBP/TiCl4 | - | 116.7 | 163.6 | 38.6 |
| MoS2-ODA/ MgCl2/DIBP/TiCl4 | 0.3 | 119.0 | 164.6 | 43.4 |
| 0.9 | 119.0 | 161.0 | 45.8 | |
| 1.3 | 122.0 | 163.0 | 55.5 | |
| 7.0 | 123.0 | 163.0 | 57.9 | |
| hBN-OTES/ MgCl2/DIBP/TiCl4 | 0.3 | 123.8 | 164.0 | 45.4 |
| 0.7 | 124.1 | 162.2 | 48.3 | |
| 1.4 | 124.9 | 163.8 | 53.6 | |
| 4.0 | 125.6 | 162.6 | 58.4 |
| Samples | Td5% (°C) | Td10% (°C) | Tdmax (°C) | Char Yield (wt %) |
|---|---|---|---|---|
| Neat PP | 313 | 332 | 404 | 0.8 |
| PP/MoS2-ODA (0.3 wt %) | 318 | 338 | 409 | 0.8 |
| PP/MoS2-ODA (0.9 wt %) | 327 | 345 | 412 | 0.9 |
| PP/MoS2-ODA (1.3 wt %) | 331 | 349 | 426 | 1.4 |
| PP/MoS2-ODA (7.0 wt %) | 380 | 395 | 441 | 3.6 |
| PP/hBN-OTES (0.3 wt %) | 325 | 345 | 416 | 0.6 |
| PP/hBN-OTES (0.7 wt %) | 331 | 349 | 414 | 1.0 |
| PP/hBN-OTES (1.4 wt %) | 319 | 338 | 411 | 1.4 |
| PP/hBN-OTES (4.0 wt %) | 325 | 346 | 419 | 3.2 |
| Samples | Tensile Strength (MPa) | Modulus (MPa) | Elogation at Break |
|---|---|---|---|
| Neat PP | 26.0 ± 1 | 850 ± 20 | ≥500 |
| PP/MoS2-ODA (0.3 wt %) | 30.0 ± 1 | 900 ± 30 | ≥500 |
| PP/MoS2-ODA (0.9 wt %) | 32.0 ± 1 | 1250 ± 40 | ≥500 |
| PP/MoS2-ODA (1.3 wt %) | 32.0 ± 1 | 1350 ± 40 | ≥500 |
| PP/MoS2-ODA (7.0 wt %) | 35.0 ± 1 | 1550 ± 30 | ≥500 |
| PP/hBN-OTES (0.3 wt %) | 33.0 ± 1 | 900 ± 40 | ≥500 |
| PP/hBN-OTES (0.7 wt %) | 34.0 ± 1 | 1000 ± 30 | ≥500 |
| PP/hBN-OTES (1.4 wt %) | 35.0 ± 1 | 1250 ± 20 | ≥500 |
| PP/hBN-OTES (4.0 wt %) | 36.0 ± 1 | 1400 ± 30 | ≥500 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.-x.; Shin, B.-G.; Lee, D.-E.; Yoon, K.-B. Preparation of PP/2D-Nanosheet Composites Using MoS2/MgCl2- and BN/MgCl2-Bisupported Ziegler–Natta Catalysts. Catalysts 2020, 10, 596. https://doi.org/10.3390/catal10060596
Zhang H-x, Shin B-G, Lee D-E, Yoon K-B. Preparation of PP/2D-Nanosheet Composites Using MoS2/MgCl2- and BN/MgCl2-Bisupported Ziegler–Natta Catalysts. Catalysts. 2020; 10(6):596. https://doi.org/10.3390/catal10060596
Chicago/Turabian StyleZhang, He-xin, Byeong-Gwang Shin, Dong-Eun Lee, and Keun-Byoung Yoon. 2020. "Preparation of PP/2D-Nanosheet Composites Using MoS2/MgCl2- and BN/MgCl2-Bisupported Ziegler–Natta Catalysts" Catalysts 10, no. 6: 596. https://doi.org/10.3390/catal10060596
APA StyleZhang, H.-x., Shin, B.-G., Lee, D.-E., & Yoon, K.-B. (2020). Preparation of PP/2D-Nanosheet Composites Using MoS2/MgCl2- and BN/MgCl2-Bisupported Ziegler–Natta Catalysts. Catalysts, 10(6), 596. https://doi.org/10.3390/catal10060596






