ADMET Polymerization of Dimeric Cinchona Squaramides for the Preparation of a Highly Enantioselective Polymeric Organocatalyst
Abstract
1. Introduction
2. Results and Discussion
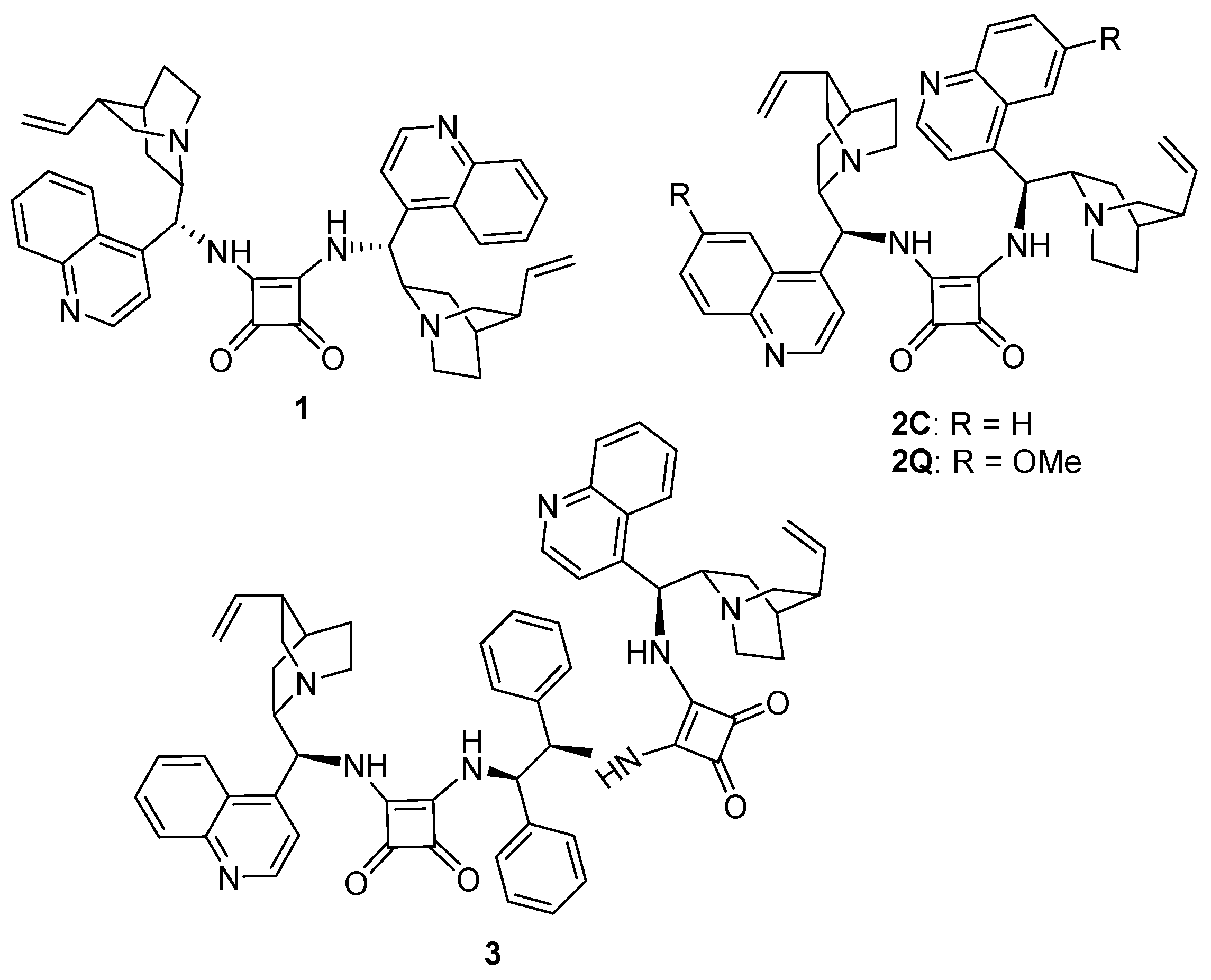
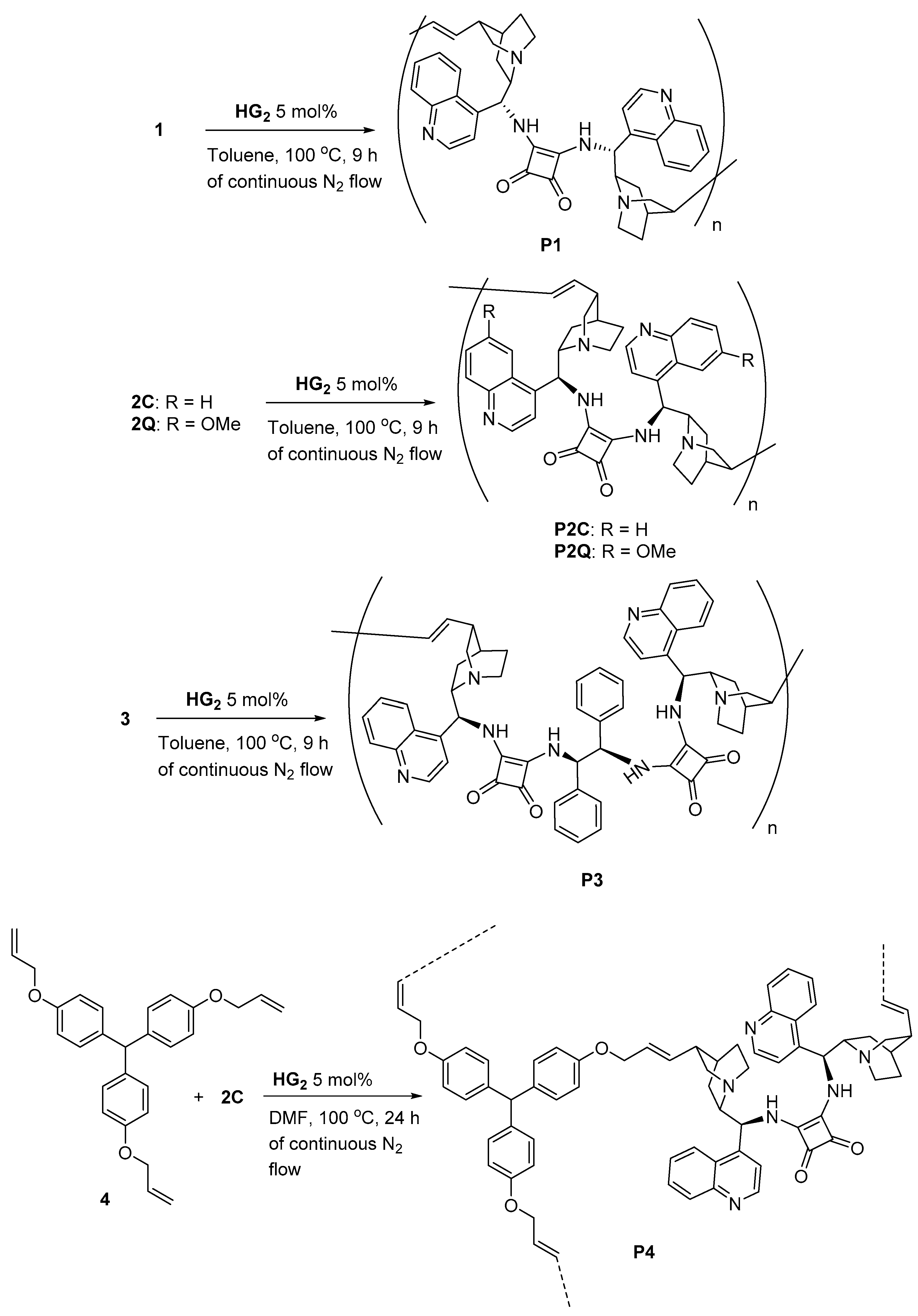
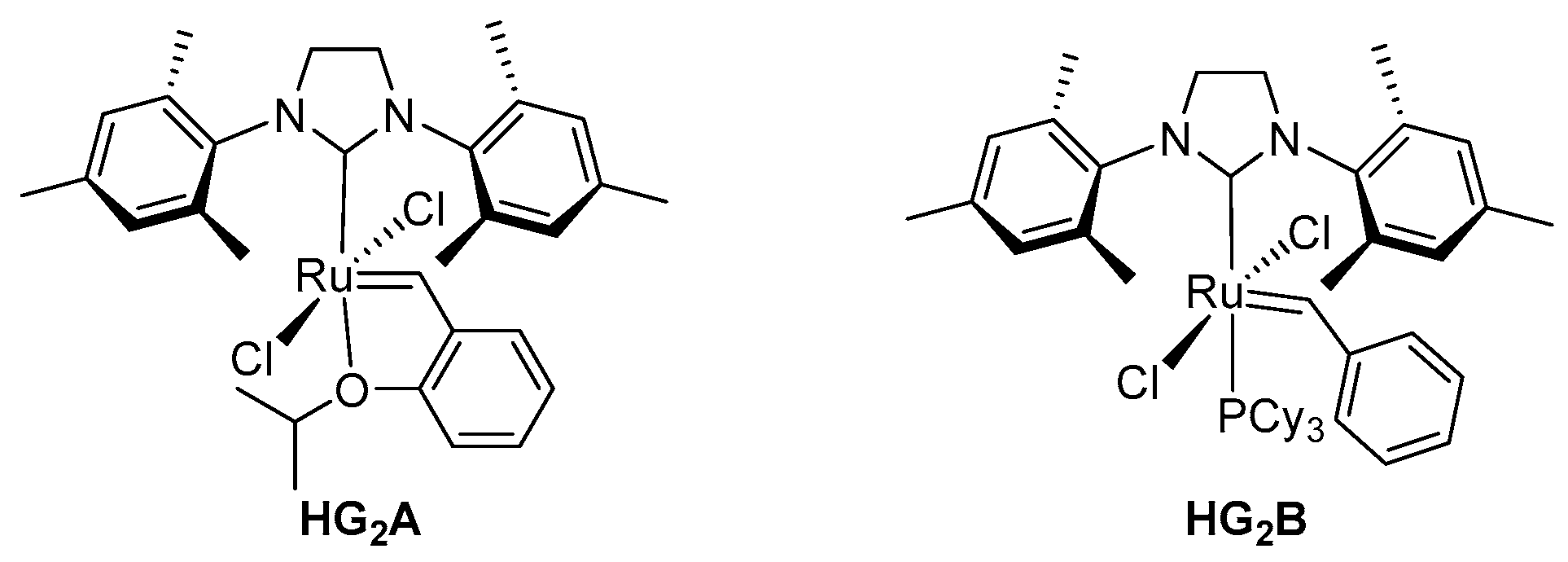
Synthesis of Dimeric Cinchona Squaramides and Their Corresponding Polymers by ADMET Polymerization
3. Materials and Methods
3.1. Materials and General Considerations
3.2. Synthesis of Cinchona-Based Squaramide Containing the Chiral ADMET Polymer
3.2.1. Polymer P1
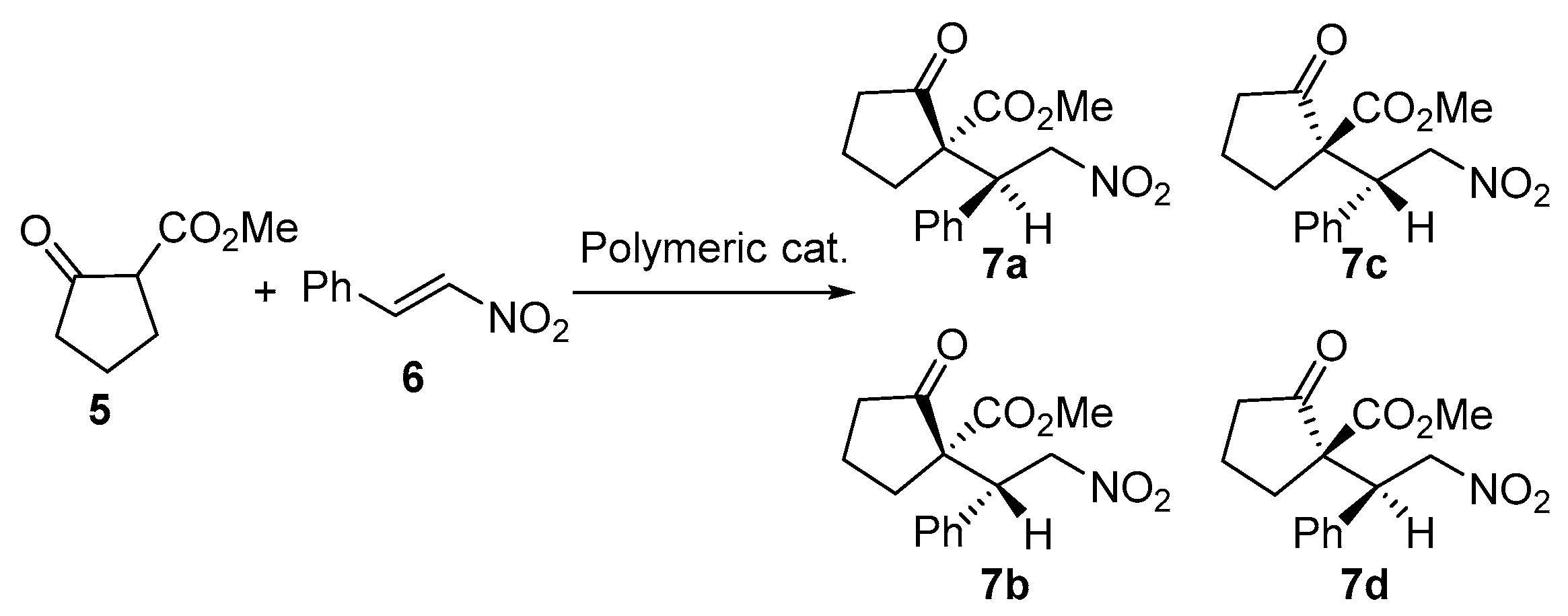
3.2.2. Representative Procedure for the Asymmetric Michael Reaction between β-Ketoesters and Trans-β-Nitrostyrene to Yield Nitroolefins Using P1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Itsuno, S.; Haraguchi, N. Catalyst Immobilization, Methods and Applications, Chapter 2; Benaglia, M., Puglisi, A., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 23–75. [Google Scholar]
- Itsuno, S.; Ullah, M.S. Flow Chemistry in Organic Synthesis, Science of Synthesis; Jamison, T.F., Koch, G., Eds.; Thieme: New York, NY, USA, 2018; pp. 347–380. [Google Scholar]
- Itsuno, S.; Paul, D.K.; Ishimoto, M.; Haraguchi, N. Designing Chiral Quaternary Ammonium Polymers: Novel Type of Polymeric Catalyst for Asymmetric Alkylation Reaction. Chem. Lett. 2010, 39, 86–87. [Google Scholar] [CrossRef]
- Itsuno, S.; Paul, D.K.; Salam, M.A.; Haraguchi, N. Main-Chain Ionic Chiral Polymers: Synthesis of Optically Active Quaternary Ammonium Sulfonate Polymers and Their Application in Asymmetric Catalysis. J. Am. Chem. Soc. 2010, 132, 2864–2865. [Google Scholar] [CrossRef]
- Parvez, M.M.; Haraguchi, N.; Itsuno, S. Molecular design of chiral quaternary ammonium polymers for asymmetric catalysis applications. Org. Biomol. Chem. 2012, 10, 2870–2877. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, N.; Kiyono, H.; Takemura, Y.; Itsuno, S. Design of main-chain polymers of chiral imidazolidinone for asymmetric organocatalysis application. Chem. Commun. 2012, 48, 4011–4013. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, N.; Takenaka, N.; Najwa, A.; Takahara, Y.; Mun, M.K.; Itsuno, S. Synthesis of Main-Chain Ionic Polymers of Chiral Imidazolidinone Organocatalysts and Their Application to Asymmetric Diels–Alder Reactions. Adv. Synth. Catal. 2018, 360, 112–123. [Google Scholar] [CrossRef]
- Ullah, M.S.; Itsuno, S. Synthesis of cinchona alkaloid squaramide polymers as bifunctional chiral organocatalysts for the enantioselective Michael addition of β-ketoesters to nitroolefins. Mol. Catal. 2017, 438, 239–244. [Google Scholar] [CrossRef]
- Parvez, M.M.; Haraguchi, N.; Itsuno, S. Synthesis of Cinchona Alkaloid-Derived Chiral Polymers by Mizoroki–Heck Polymerization and Their Application to Asymmetric Catalysis. Macromolecules 2014, 47, 1922–1928. [Google Scholar] [CrossRef]
- Ullah, M.S.; Itsuno, S. Cinchona Squaramide-Based Chiral Polymers as Highly Efficient Catalysts in Asymmetric Michael Addition Reaction. ACS Omega 2018, 4573–4582. [Google Scholar] [CrossRef]
- Boratynski, P.J. Dimeric Cinchona alkaloids. Mol. Divers. 2015, 19, 385–422. [Google Scholar] [CrossRef]
- Lindmarkhamberg, M.; Wagener, K.B. Acyclic metathesis polymerization: The olefin metathesis reaction of 1,5-hexadiene and 1,9-decadiene. Macromolecules 1987, 20, 2949–2951. [Google Scholar] [CrossRef]
- Wagener, K.B.; Boncella, J.M.; Nel, J.G.; Duttweiler, R.P.; Hillmyer, M.A. The key to successful acyclic diene metathesis polymerization chemistry. Macromol. Chem. 1990, 191, 365–374. [Google Scholar] [CrossRef]
- Wagener, K.B.; Nel, J.G.; Konzelman, J.; Boncella, J.M. Acyclic diene metathesis copolymerization of 1,5-hexadiene and 1, 9-decadiene. Macromolecules 1990, 23, 5155–5157. [Google Scholar] [CrossRef]
- Wagener, K.B.; Boncella, J.M.; Nel, J.G. Acyclic diene metathesis copolymerization of 1,5-hexadiene and 1,9-decadiene. Macromolecules 1991, 24, 2649–2657. [Google Scholar] [CrossRef]
- Wagener, K.B.; Smith, D.W. Acyclic diene metathesis polymerization: Synthesis and characterization of unsaturated poly[carbo(dimethyl)silanes]. Macromolecules 1991, 24, 6073–6078. [Google Scholar] [CrossRef]
- Wagener, K.B.; Brzezinska, K. Acyclic diene metathesis (ADMET) polymerization: Synthesis of unsaturated polyethers. Macromolecules 1991, 24, 5273–5277. [Google Scholar] [CrossRef]
- Wagener, K.B.; Patton, J.T. Acyclic diene metathesis (ADMET) polymerization. Synthesis of unsaturated polycarbonates. Macromolecules 1993, 26, 249–253. [Google Scholar] [CrossRef]
- Portmess, J.D.; Wagener, K.B. Acyclic diene metathesis (ADMET) polymerization: The synthesis of unsaturated polyamines. J. Polym. Sci. Pol. Chem. 1996, 34, 1353–1357. [Google Scholar] [CrossRef]
- Wolfe, P.S.; Wagener, K.B. An ADMET route to unsaturated polyacetals. Macromol. Rapid Commun. 1998, 19, 305–308. [Google Scholar] [CrossRef]
- Wolfe, P.S.; Wagener, K.B. Investigation of Organoboronates in Metathesis Polymerization. Macromolecules 1999, 32, 7961–7967. [Google Scholar] [CrossRef]
- Brzezinska, K.R.; Schitter, R.; Wagener, K.B. Carbosilane/carbosiloxane-based ADMET homopolymers and copolymers possessing latent reactivity. J. Polym. Sci. Pol. Chem 2000, 38, 1544–1550. [Google Scholar] [CrossRef]
- Cummings, S.K.; Smith, D.W.; Wagener, K.B. Environmental degradation of biodegradable polyesters 1. Poly(ε-caprolactone), poly[(R)-3-hydroxybutyrate], and poly(L-lactide) films in controlled static seawater. Macromol. Rapid Commun. 1995, 16, 347–355. [Google Scholar] [CrossRef]
- Patton, J.T.; Boncella, J.M.; Wagener, K.B. Acyclic diene metathesis (ADMET) polymerization: The synthesis of unsaturated polyesters. Macromolecules 1992, 25, 3862–3867. [Google Scholar] [CrossRef]
- Hopkins, T.E.; Pawlow, J.H.; Koren, D.L.; Deters, K.S.; Solivan, S.M.; Davis, J.A.; Gomez, F.J.; Wagener, K.B. Chiral Polyolefins Bearing Amino Acids. Macromology 2001, 34, 7920–7922. [Google Scholar] [CrossRef]
- Ullah, M.S.; Itsuno, S. Synthesis of Cinchona Alkaloid Derived Chiral Squaramide Polymers by ADMET Polymerization and Their Application to Asymmetric Catalysis. Chem. Lett. 2018, 47, 1220–1223. [Google Scholar] [CrossRef]
- Song, C.E. Cinchona Alkaloids in Synthesis and Catalysis; Wiley: Weinheim, Germany, 2009. [Google Scholar]
- Jianga, L.; Chen, Y.C. Recent advances in asymmetric catalysis with cinchona alkaloid-based primary amines. Catal. Sci. Technol. 2011, 1, 354–365. [Google Scholar] [CrossRef]
- Yeboah, E.M.O.; Yeboah, S.O.; Singh, G.S. Recent applications of Cinchona alkaloids and their derivatives as catalysts in metal-free asymmetric synthesis. Tetrahedron 2011, 67, 1725–1762. [Google Scholar] [CrossRef]
- Marcelli, T. Organocatalysis: Cinchona catalysts. WIREs Comput. Mol. Sci. 2011, 1, 142–152. [Google Scholar] [CrossRef]
- Ingemann, S.; Hiemstra, H. Comprehensive Enantioselective Organocatalysis; Dalko, P.I., Ed.; Wiley: Weinheim, Germany, 2013; pp. 119–160. [Google Scholar]
- Itsuno, S.; Parvez, M.M.; Haraguchi, N. Polymeric chiral organocatalysts. Polym. Chem. 2011, 2, 1942–1949. [Google Scholar] [CrossRef]
- Itsuno, S.; Hassan, M.M. Polymer-immobilized chiral catalysts. RSC Adv. 2014, 4, 52023–52043. [Google Scholar] [CrossRef]
- Haraguchi, N.; Itsuno, S. Polymeric Chiral Catalyst Design and Chiral Polymer Synthesis; Itsuno, S., Ed.; Wiley: Hoboken, NJ, USA, 2011; Volume 2, pp. 17–61. [Google Scholar]
- Malerich, J.P.; Hagihara, K.; Rawal, V.H. Chiral Squaramide Derivatives are Excellent Hydrogen Bond Donor Catalysts. J. Am. Chem. Soc. 2008, 130, 14416–14417. [Google Scholar] [CrossRef]
- Tsakos, M.; Kokotos, C.G. Primary and secondary amine-(thio)ureas and squaramides and their applications in asymmetric organocatalysis. Tetrahedron 2013, 69, 10199–10222. [Google Scholar] [CrossRef]
- Zhao, B.L.; Du, D.M. Chiral Squaramide-Catalyzed Michael/Alkylation Cascade Reaction for the Asymmetric Synthesis of Nitro-Spirocyclopropanes. Eur. J. Org. Chem. 2015, 2015, 5350–5359. [Google Scholar] [CrossRef]
- Lee, J.W.; Ryu, T.H.; Oh, J.S.; Bae, H.Y.; Jang, H.B.; Song, C.E. Self-association-free dimeric cinchona alkaloid organocatalysts: Unprecedented catalytic activity, enantioselectivity and catalyst recyclability in dynamic kinetic resolution of racemic azlactones. Chem. Commun. 2009, 46, 7224–7226. [Google Scholar] [CrossRef]
- Rao, K.S.; Ramesh, P.; Chowhan, L.R.; Trivedi, R. Asymmetric Mannich reaction: Highly enantioselective synthesis of 3-amino-oxindoles via chiral squaramide based H-bond donor catalysis. RSC Adv. 2016, 6, 84242–84247. [Google Scholar] [CrossRef]
- Yang, W.; Du, D.M. Chiral Squaramide-Catalyzed Highly Enantioselective Michael Addition of 2-Hydroxy-1,4-naphthoquinones to Nitroalkene. Adv. Synth. Catal. 2011, 353, 1241–1246. [Google Scholar] [CrossRef]
- Konishi, H.; Lam, T.Y.; Malerich, J.P.; Rawal, V.H. Enantioselective α-Amination of 1,3-Dicarbonyl Compounds Using Squaramide Derivatives as Hydrogen Bonding Catalysts. Org. Lett. 2012, 12, 2028–2031. [Google Scholar] [CrossRef]
- Yang, W.; Jia, Y.; Du, D.M. Squaramide-catalyzed enantioselective Michael addition of malononitrile to chalcones. Org. Biomol. Chem. 2012, 10, 332–338. [Google Scholar] [CrossRef]
- Yang, W.; Du, D.M. Highly Enantioselective Michael Addition of Nitroalkanes to Chalcones Using Chiral Squaramides as Hydrogen Bonding Organocatalysts. Org. Lett. 2010, 12, 5450–5453. [Google Scholar] [CrossRef]
- Yang, W.; Du, D.M. Cinchona-based squaramide-catalysed cascade aza-Michael–Michael addition: Enantioselective construction of functionalized spirooxindole tetrahydroquinolines. Chem. Commun. 2013, 49, 8842–8844. [Google Scholar] [CrossRef]
- Yang, W.; Du, D.M. Chiral squaramide-catalyzed highly diastereo- and enantioselective direct Michael addition of nitroalkanes to nitroalkenes. Chem. Commun. 2011, 47, 12706–12708. [Google Scholar] [CrossRef]
- Vakulya, B.; Varga, S.; Csampai, A.; Soos, T. Highly Enantioselective Conjugate Addition of Nitromethane to Chalcones Using Bifunctional Cinchona Organocatalysts. Org. Lett. 2005, 7, 1967–1969. [Google Scholar] [CrossRef]
- Tripathi, C.B.; Kayal, S.; Mukherjee, S. Catalytic Asymmetric Synthesis of α,β-Disubstituted α,γ-Diaminophosphonic Acid Precursors by Michael Addition of α-Substituted Nitrophosphonates to Nitroolefins. Org. Lett. 2012, 14, 3296–3299. [Google Scholar] [CrossRef]
- Chhanda, S.A.; Itsuno, S. Design and synthesis of chiral hyperbranched polymers containing cinchona squaramide moieties and their catalytic activity in the asymmetric Michael addition reaction. J. Catal. 2019, 377, 543–549. [Google Scholar] [CrossRef]
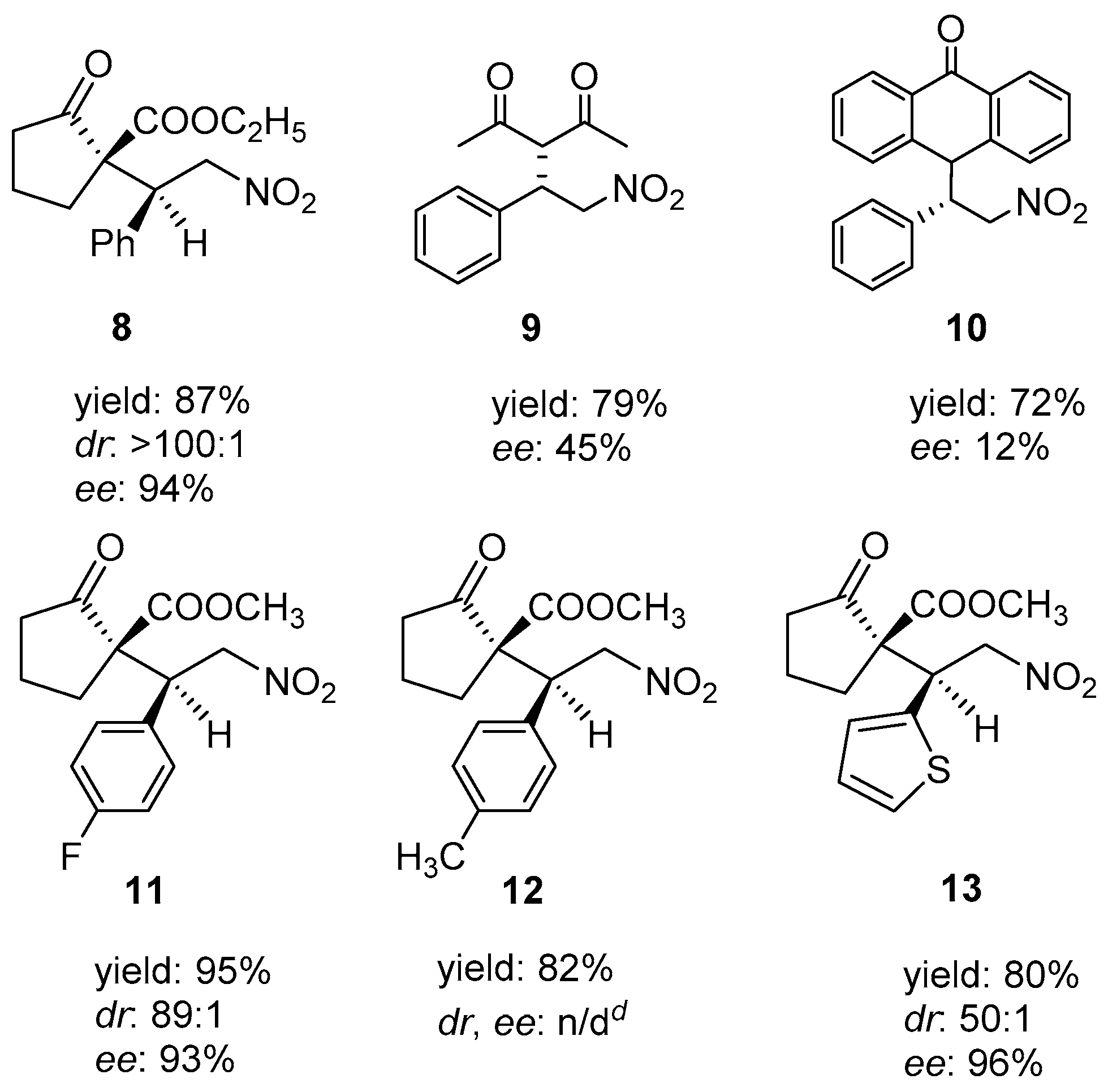
| Entry | Polymer | Diene | Solvent | Time (h) | Yield (%) | Mnb | Mwb | Mw/Mnb |
|---|---|---|---|---|---|---|---|---|
| 1 d | P2Q | 2Q | DMF | 24 | 81 | 49,000 | 58,000 | 1.18 |
| 2 c,d | P2Q | 2Q | DMF | 24 | 70 | 44,000 | 52,000 | 1.18 |
| 3 c,d,e | P2Q | 2Q | DMF | 24 | 58 | 46,000 | 53,000 | 1.15 |
| 4 d,f | P2Q | 2Q | DMF | 24 | 74 | 41,000 | 47,000 | 1.14 |
| 5 | P2Q | 2Q | o-Dichloro benzene | 24 | 77 | 56,000 | 74,000 | 1.32 |
| 6 | P2Q | 2Q | Toluene | 9 | 92 | 42,000 | 46,000 | 1.10 |
| 7 g | P2Q | 2Q | Toluene | 25 | 52 | 49,000 | 56,000 | 1.14 |
| 8 | P1 | 1 | Toluene | 9 | 77 | 47,000 | 49,000 | 1.04 |
| 9 | P2C | 2C | Toluene | 9 | 93 | 54,000 | 55,000 | 1.02 |
| 10 | P3 | 3 | Toluene | 9 | 86 | 74,000 | 75,000 | 1.01 |
| 11 | P4 | 2C + 4 | DMF | 24 | 70 | 9000 | 19,000 | 2.19 |
| Entry | Catalyst | Solvent | Temp (°C) | Reaction Time (h) | Yield (%) b | dr c | % ee c |
|---|---|---|---|---|---|---|---|
| 1 | 1 | MeOH | 25 | 6 | 91 | 1:14 | 93 |
| 2 | P1 | MeOH | 25 | 6 | 77 | 1:12 | 87 |
| 3 | P1 | DCM | 25 | 30 | 52 | 1:81 | 97 |
| 4 | P1 | THF | 25 | 7 | 89 | 1:80 | 99 |
| 5 | P1 | EtOAc | 25 | 27 | 34 | 1:62 | 92 |
| 6 | P1 | ether | 25 | 20 | 87 | 1:45 | 97 |
| 7 | P1 | Toluene | 25 | 24 | 29 | 1:3 | 90 |
| 8 | P1 | Hexane | 25 | 24 | 84 | 1:38 | 96 |
| 9 | P1 | Acetonitrile | 25 | 9 | 97 | 1:>100 | 95 |
| 10 | P1 | Acetonitrile | 0 | 24 | 92 | 1:>100 | 96 |
| 11 | P1 | Acetonitrile | −10 | 24 | 99 | 1:89 | 99 |
| 12 | P1 | Acetonitrile | 80 | 6 | 78 | 1:16 | 95 |
| 13 e | 2C | DCM | 25 | 4 | 95 | >100:1 | 98 |
| 14 | P2C | Acetonitrile | 25 | 9 | 75 | 68:1 | 91 |
| 15 d | 2Q | DCM | 25 | 2 | 95 | 79:1 | 91 |
| 16 d | P2Q | DCM | 25 | 48 | 45 | 59:1 | 96 |
| 17 | P3 | acetonitrile | 25 | 9 | 67 | 95:1 | 97 |
| 18 | P4 | acetonitrile | 25 | 20 | 85 | 24:1 | 99 |
| Cycle | Yield (%) b | drc | % ee c |
|---|---|---|---|
| original | 97 | 1:>100 | 95 |
| 1 | 73 | 1:54 | 97 |
| 2 | 93 | 1:59 | 97 |
| 3 | 90 | 1:39 | 94 |
| 4 | 82 | 1:46 | 93 |
| 5 | 72 | 1:51 | 95 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, M.S.; Chhanda, S.A.; Itsuno, S. ADMET Polymerization of Dimeric Cinchona Squaramides for the Preparation of a Highly Enantioselective Polymeric Organocatalyst. Catalysts 2020, 10, 591. https://doi.org/10.3390/catal10050591
Ullah MS, Chhanda SA, Itsuno S. ADMET Polymerization of Dimeric Cinchona Squaramides for the Preparation of a Highly Enantioselective Polymeric Organocatalyst. Catalysts. 2020; 10(5):591. https://doi.org/10.3390/catal10050591
Chicago/Turabian StyleUllah, Mohammad Shahid, Sadia Afrin Chhanda, and Shinichi Itsuno. 2020. "ADMET Polymerization of Dimeric Cinchona Squaramides for the Preparation of a Highly Enantioselective Polymeric Organocatalyst" Catalysts 10, no. 5: 591. https://doi.org/10.3390/catal10050591
APA StyleUllah, M. S., Chhanda, S. A., & Itsuno, S. (2020). ADMET Polymerization of Dimeric Cinchona Squaramides for the Preparation of a Highly Enantioselective Polymeric Organocatalyst. Catalysts, 10(5), 591. https://doi.org/10.3390/catal10050591






