Abstract
1,5,7-Triazabicyclo[4.4.0]dec-5-ene (TBD) polymerizes rac-lactide (rac-LA) to form highly isotactic polylactide (PLA) with a Pm = 0.88, while meso-LA yields heterotactic PLA (Pm ~ 0.8) at −75 °C. The stereocontrol of the cryogenic-based ring-opening polymerization comes from a perfect imbrication of both chiral LA and the propagating chiral end-group interacting with the achiral TBD catalyst.
1. Introduction
Polylactide (PLA) is a versatile bioplastic that can be derived from annually-renewable resources. While PLA can be synthesized via polycondensation of lactic acid, the ring-opening polymerization (ROP) of lactide (LA) is by far preferred, on account of the more facile access to higher molecular weight polymers with control over the molecular parameters and end groups [1]. LA possesses two stereocenters, conferring it three distinct isomers: R,R-LA (D-LA), S,S-LA (L-LA) and R,S-LA (meso-LA). In addition to the three diastereomers, a commercially available racemate of D- and L-LA also exists as rac-LA. Because the chemical and physical properties of PLA are drastically affected by its tacticity [2], the nature of the enantiopure monomer involved in the ROP process, or the application of stereocontrolled polymerizations in which stereoregular polymers result from diastereomeric mixtures of LA, are of high importance.
During the past decades, there has been significant emphasis placed on the preparation of catalysts that are able to produce isotactic PLA directly from rac-LA [3,4,5,6,7]. The isoselective ROP of rac-LA is by far dominated by metal salts and coordination complexes that operate by a coordination-insertion mechanism. Generally, the significant difference in the rates of insertion of both D- and L-LA is created by the association of the ligand system and the growing polymer chain end. While excellent stereocontrol is achieved by those systems, they are commonly complex and costly to prepare, which in part motivated the development of simple, metal-free, organo-catalytic systems [8].
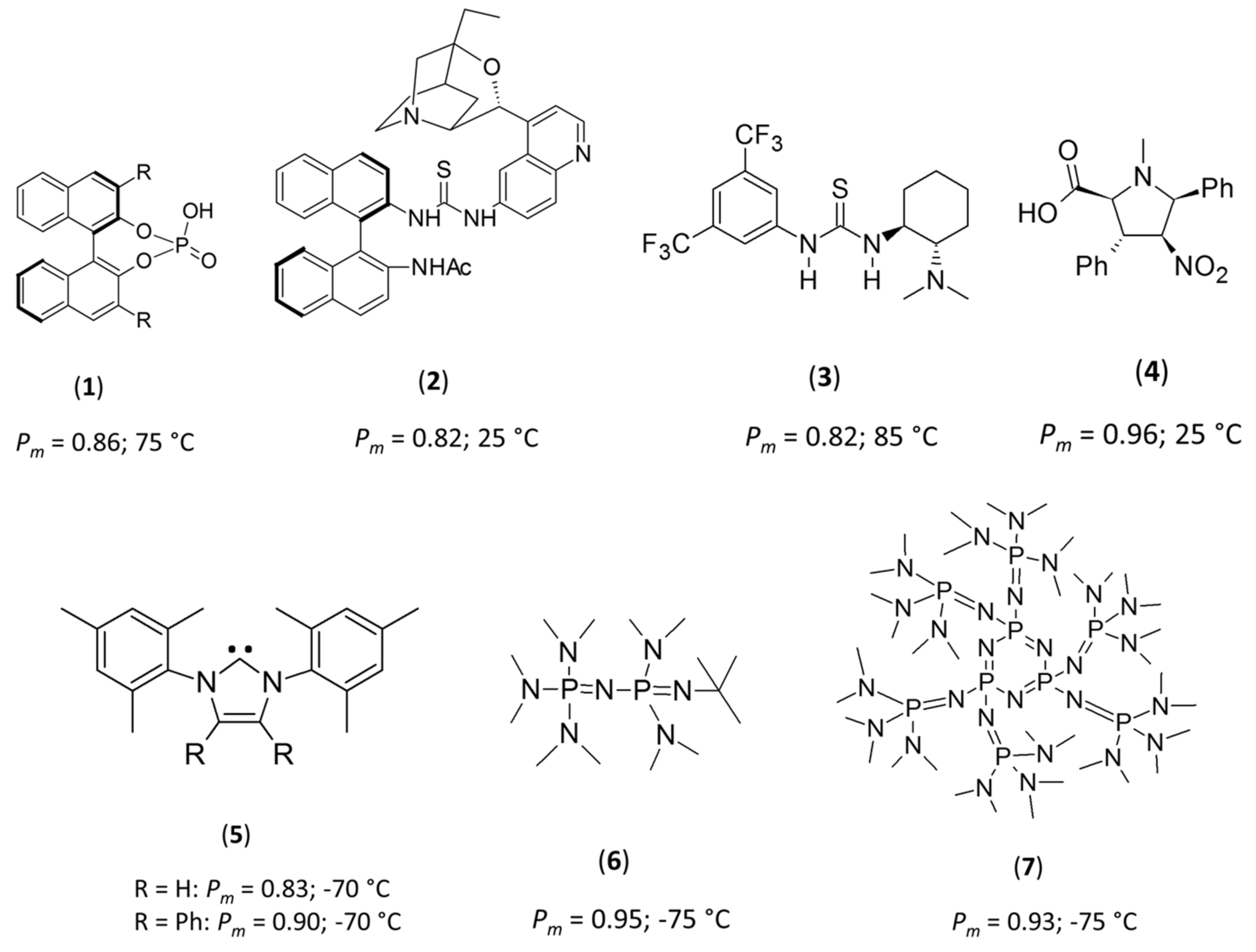
To date, only a handful of studies of the stereo-controlled ROPs of rac-LA, using either chiral or achiral organo-based catalysts have been reported [9,10,11,12,13,14,15,16,17,18,19]. Chiral organo-based catalysts, including binaphthol-type phosphoric acids (1), β-isocupreidine/thiourea/chiral binaphthylamines (2), Takemoto’s catalyst (3), densely substituted proline-type amino acids (4) and rotaxanes, are known to produce isotactic PLA, presenting a probability of isotactic dyad (Pm) of 0.82–0.96, for ROP reactions performed at 25 to 85 °C (Figure 1) [13,14,15,16,17,18,19].
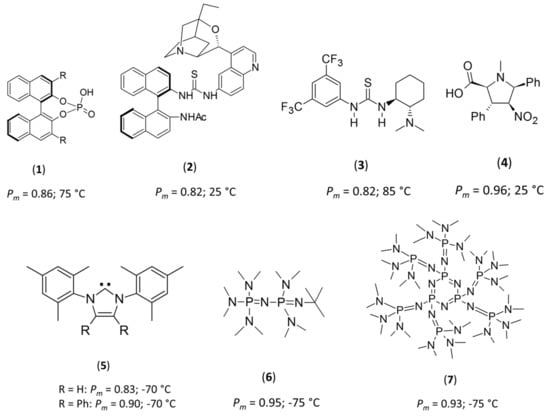
Figure 1.
Structures of chiral and achiral catalysts employed for the stereoselective ring-opening polymerization (ROP) of rac-LA.
Comparatively, achiral catalysts, including N-heterocyclic carbenes (5) [9,10] and phosphazenes (6 and 7) [12,17], necessitate cryogenic conditions to increase the stereoselectivity for ROP of rac-LA. In these systems, ROP at low temperatures (−75 °C) resulted in a marked increase in isotacticity, with Pm values between 0.83 and 0.95 (Figure 1). In all of these cases, the authors postulated that such stereo-control is the result of a steric encumberment at the propagating chain end that resulted in stereo-control by a chain-end control (CEC) mechanism—although no mechanistic investigations were performed to confirm that hypothesis. While several of these species are indeed very sterically-demanding achiral bases, the high level of stereo-control at cryogenic temperatures observed with 1-tert-butyl-2,2,4,4,4-pentakis(dimethylamino)-2Λ5,4Λ5-catenadi(phosphazene), 6, led us to question if this was the primary reason for such high levels of stereocontrol, and led us to question more fundamentally the reasons for such selectivity. Intriguingly, most of the stereoselective ROPs performed on rac-LA from achiral catalysts were realized in toluene, in which LA is poorly soluble (see Supporting Information, Figures S1–S3). Extending these concepts, we postulated that other non-sterically encumbered and achiral bases could also mediate the stereoselective ROP of rac-LA at cryogenic temperatures in toluene. To test this hypothesis, we selected 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD) as our catalyst, as it fits the criteria, and is highly active for the ROP of LA [20,21]. Note also that the 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) catalyst was also studied, but did not lead to any polymer production in the conditions presented here after.
2. Results and Discussion
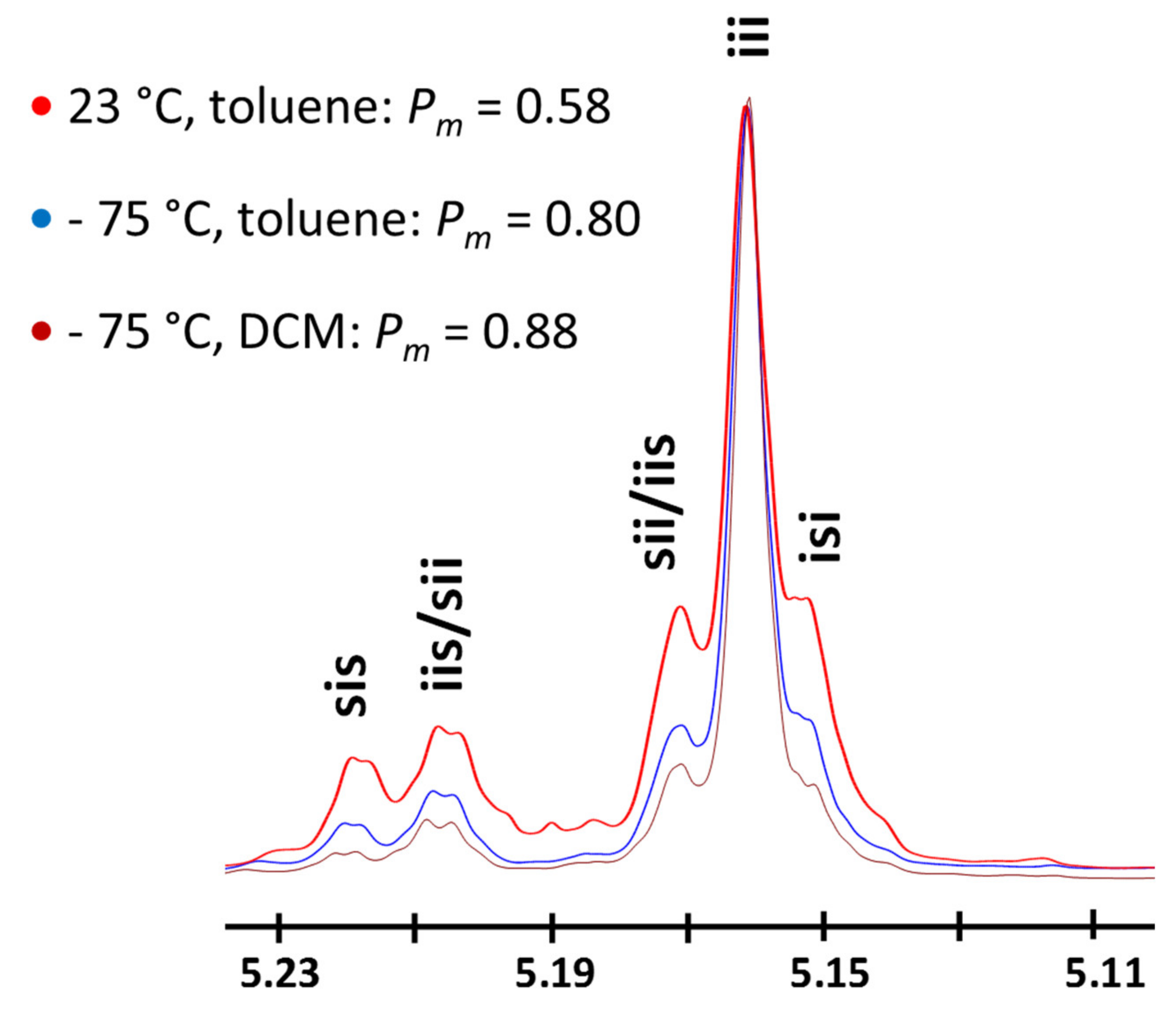
Despite being studied at room temperature, in which a slight preference for isotacticity was reported (Pm = 0.58, confirmed through control experiments—Figure 2), the ROP of LA using TBD as the catalyst has never been previously reported at cryogenic temperatures, presumably due to the assumption that the low steric hindrance around the active site would not lead to high levels of stereo-control in the polymerization.
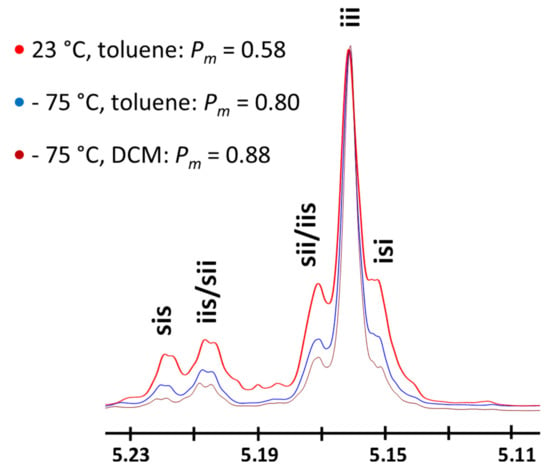
Figure 2.
Methine region of homonuclear decoupled 1H-NMR spectra of poly(rac-LA) obtained at different polymerization temperatures and solvents using TBD as a catalyst (X-axis in ppm). Peak attributions from [4].
At the outset, the ROP of rac-LA was studied in toluene at −75 °C for an initial benzyl alcohol (BnOH)-to-TBD molar ratio of 10 and an initial rac-LA concentration of 0.08 M ([rac-LA]0/[BnOH]0 = 100, Table 1, Entries 1–2). The ROP of rac-LA in such conditions is slow, requiring 2 h to reach an overall monomer conversion of 30% (Table 1, Entry 2). However, the resulting polymerization displays high levels of control with experimental number-average molecular weights (Mn, SEC) consistent with calculated monomer conversions. The resulting PLAs also display dispersity values (ÐM = Mw/Mn) less than 1.1. Homonuclear decoupled 1H NMR spectroscopy of the resulting materials revealed the formation of isotactic PLAs, presenting Pm values as high as 0.80–0.84. Consistent with the formation of semi-crystalline isotactic PLA, the polymers showed a melting transition at ca. 155–157 °C on their differential scanning calorimetry (DSC) thermograms (See Supporting Information, Figures S4–S5). In combination with a higher TBD initial content ([BnOH]0/[TBD]0 = 1), the use of a [rac-LA]0 of 0.22 M was effective to mediate a faster stereoselective ROP, resulting in PLAs of high isotacticity with Pm values of 0.85 (Entries 3–4, Table 1).

Table 1.
ROP of rac-LA in toluene at −75 °C.
At this stage, we sought to identify the reasons for the remarkable selectivity observed with TBD at −75 °C. The importance of the solvent on the observed selectivity during the rac-LA ROP under cryogenic conditions was first addressed. To check whether solubility is ruling the stereo-control over the polymerization process, toluene was substituted by DCM in which rac-LA is highly soluble. With a ratio of 100:1:0.1 for [rac-LA]0/[BnOH]0/[TBD]0 ([rac-LA]0 = 0.08M), the monomer was almost quantitatively converted to polymer within 1 h at -75 °C (conv. ~0.9). The produced PLA possessed a Mn, SEC of 12,000 g.mol−1 with a narrow dispersity (1.09). As in the polymerizations realized in toluene, a high level of isotacticity was confirmed by homonuclear decoupled 1H NMR spectroscopy (Pm = 0.88, Figure 2) and DSC analysis (Tg = 58.6 °C, Tm = 158.5 °C, ΔHm = 29.3 J.g−1). Such results indicate that the selectivity observed by using TBD as the catalyst under cryogenic conditions is not related to the nature of the solvent, and hence, we hypothesize that the unexpected stereoselectivity must arise from intermolecular interactions between the catalyst, chain end and monomer.
The difference in reactivity of TBD towards either the L- or the D-stereogenic center was then evaluated. To this end, a cryogenic polymerization of meso-lactide in toluene was performed ([meso-lactide]0/[BnOH]0/[TBD]0 = 100/1/1). As expected, the polymer obtained (Mn, SEC = 17,200 g.mol−1; ÐM = 1.24) was heterotactic (Pm = 0.80), demonstrating that the propagating alcohol has an important impact on the polymerization propagating step. Considering the size and geometry of the achiral TBD molecule, along with its mode of action, proceeding through concurrent activation of both monomer and initiating/propagating alcohol [23], we postulated that the stereocontrol of the cryogenic-based ROP must come from a perfect imbrication of both chiral LA and propagating chiral end-group both in interaction with the TBD catalyst, which is facilitated at low temperatures. To confirm this hypothesis, the reaction profiles corresponding to the first step of the ROP process were simulated with a quantum-chemical approach, since earlier works [23,24], and a recent review devoted to organocatalytic ring-opening polymerization of cyclic esters [25], have pointed out that it is the rate-determining step. This step corresponds to the attack of the alcohol on the lactide activated by the TBD catalyst [23].
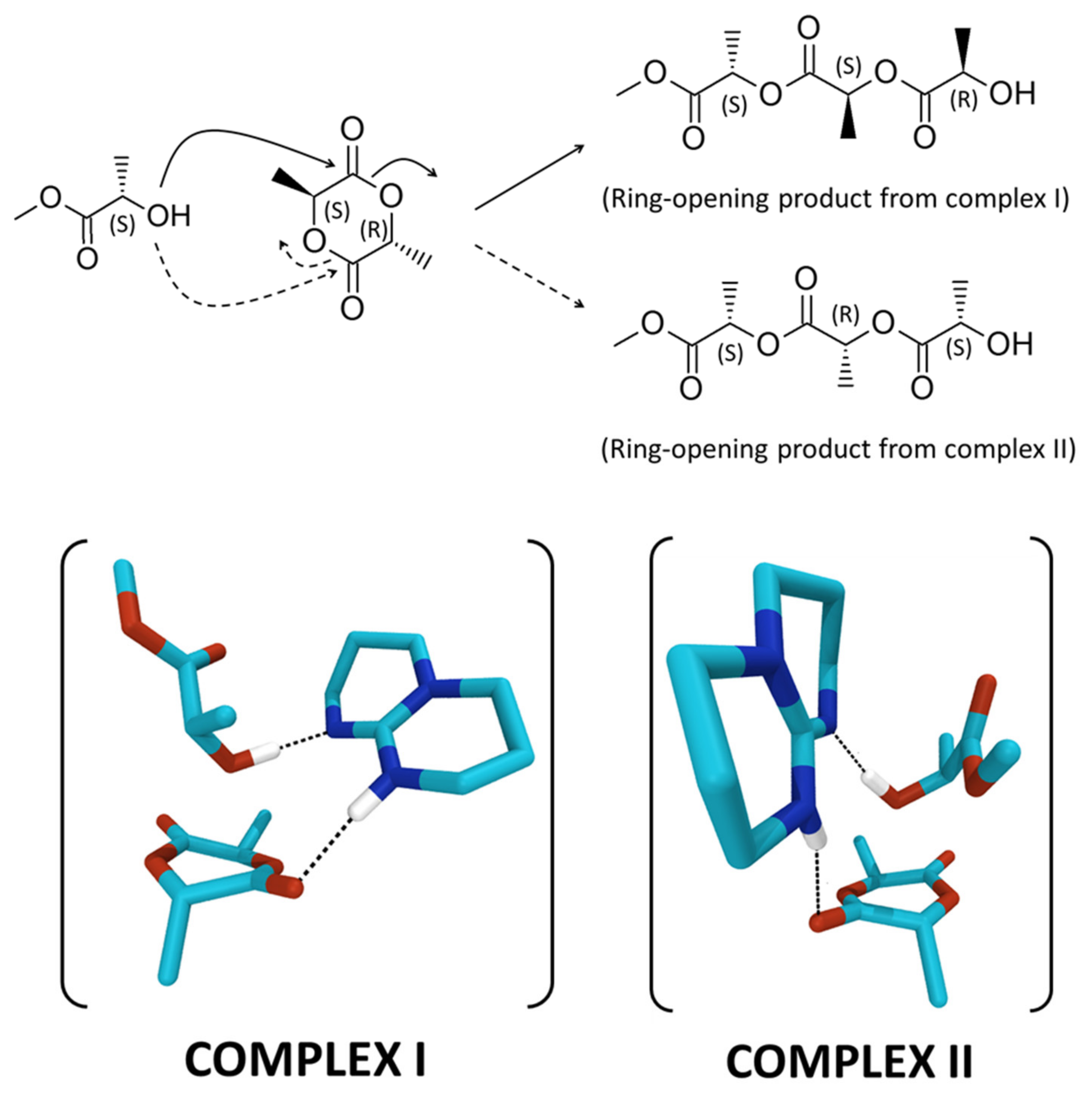
The simulations have been performed by using methyl (L)-lactate as the (S) chiral initiator, and considering two possible complexes formed between the meso-lactide, the TBD and the (S) chiral alcohol (Scheme 1). The complex corresponding to the nucleophilic attack of the (S) alcohol on the carbonyl carbon adjacent to the (S)-stereocenter of the meso-lactide, which will give rise to a (R) chiral alcohol after ring-opening, is referred to as complex I. The complex corresponding to the nucleophilic attack of the (S) alcohol on the carbonyl carbon adjacent to the (R)-stereocenter of the meso-lactide, which will give rise to a (S) chiral alcohol after ring-opening, is referred to as complex II (Scheme 1).
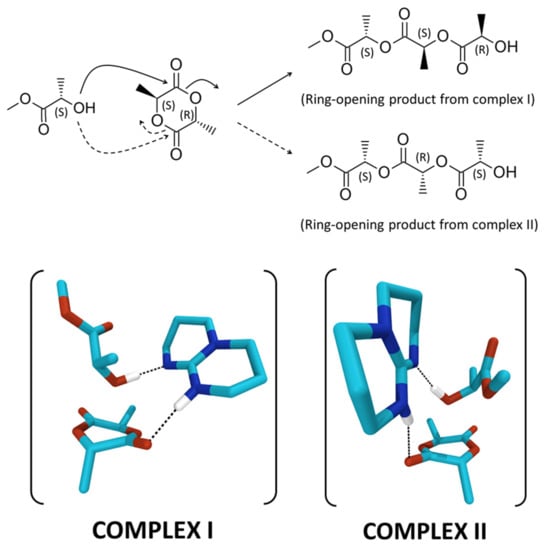
Scheme 1.
Ring-opening products obtained from complexes I and II formed between meso-LA, methyl (L)-lactate and TBD.
For both complexes, we obtain transitions states corresponding to the concomitant attack of the oxygen atom of the alcohol on the carbon atom of the carbonyl group, the transfer of the hydrogen atom of the alcohol to the nitrogen atom of TBD, and the shift of the hydrogen atom on TBD towards the oxygen atom of the carbonyl moiety. This type of arrangement, which favors the ring opening of the lactide, thanks to the concomitant activation of the lactide carbonyl and the alcohol from the catalyst, is similar to that described by Chuma et al. [23]. Moreover, those structures are particularly favorable to the formation of the polymer chain, due to the proximity between the proton transferred to the catalyst and the oxygen atom of the lactide ring (Figure S9). The energy barriers between reactants and transition states have then been estimated by performing internal reaction coordinate (IRC) calculations from the transition states (Figure S9). Interestingly, they differ by 2.61 kcal.mol−1 in favor of complex I. The difference arises mainly from a much shorter distance between the oxygen atom of the alcohol and the carbon atom of the carbonyl group on the lactide for complex I in the reactant geometry (2.62 Å vs. 2.79 Å, Figure S8). Such close contact geometry is therefore more efficient to form a chemical bond and requires less structural deformation.
These results suggest that the nucleophilic attack of a (S) chiral alcohol will lead to a polymer chain bearing a (R) chiral chain end. If we consider the attack of a (R) chiral alcohol on meso-lactide, we expect the opposite result, i.e., the nucleophilic attack of a (R) chiral alcohol would lead preferentially to a new (S) chiral chain end, as observed during the experiments. Altogether, these calculations allow us to rationalize the observed heterotacticity of the polymer (resulting from isoselectivity), and therefore suggest that, when applied to rac-LA, one of the two isomers will be polymerized predominantly over the other one, consistent with our experimental results.
3. Materials and Methods
3.1. Materials
Benzyl alcohol (BnOH, from Sigma-Aldrich, Pegasuslaan 5, 1831, Diegem, Belgium, 97%) was dried over calcium hydride for 48 h at room temperature and distilled under reduced pressure and stored in a MBraun LABM-1973 glove box. L-, D- and meso-lactides (L-LA, D-LA and meso-LA, Corbion Purac) were recrystallized three times from dried toluene and stored in a glove box under dry nitrogen atmosphere before use. 1,5,7-Triazabicyclo[4.4.0] dec-5-ene (TBD, Acros) was dried under vacuum at 80 °C for 12 h and stored in a glove box. Toluene and CH2Cl2 (DCM) solvents were dried using a MBraun Solvent Purification System (model MB-SPS 800) equipped with alumina drying columns.
3.2. Characterization Techniques
1H NMR and homonuclear decoupled 1H NMR measurements were performed at room temperature on a Bruker Avance instrument at 400 MHz. CDCl3 was used as an internal reference (δ = 7.26), and the relaxation time was set to 2 s. For homonuclear decoupled 1H NMR analysis, the relaxation time was measured and set to 2.04 s. Samples were obtained in CDCl3 solutions with the decoupling pulse based on the methyl region (~1.6 ppm). In the case of good resolution of the methine region (~5.00–5.20 ppm), the global spectral deconvolution technique was implemented by an attribution of five mesodyads. Size-exclusion chromatography (SEC) was performed at room temperature using tetrahydrofuran (THF) as the eluent at a flow rate of 1.0 mL/min. A 5 mg/mL sample was used for each analysis. The calibration was performed with polystyrene standard (PS). A differential refractive index (RI) detector was used. The Mn values that were obtained by SEC are multiplied by a 0.58 correlation factor for PLA analyzed with PS standards, corresponding to the Mark Houwink equation. Tg, Tm and ΔHm values were determined by differential scanning calorimetry (DSC) using Q200 TA instruments. Polymeric samples were first heated from 25 °C to 200 °C at 10 °C/min. All thermal data were obtained from the first scan.
3.3. Protocol of Rac-LA Polymerization
In a glove box (H2O < 1 ppm, O2 < 0.1 ppm), a first dried vial was charged by BnOH (7.5 mg, 0.069 mmol), TBD (9.6 mg, 0.069 mmol), 1 ml of dried toluene ([BnOH]0 = 0.069 M) and a magnetic bar. In a second dried vial, L- and D-LA (50 mg each, 34.7 mmol each) were solubilized in 3 mL of dried toluene ([rac-LA]0 = 0.22 M) at r.t., in the presence of a magnetic bar (Figure S1). In a cold room (~5 °C), both vials were immersed in a cryogenic bath thermostatized at −75 °C. After stirring for 10 min, 100 mL of the alcohol solution (6.9 × 10−6 mol) was quickly injected into the insoluble monomer medium (Figure S2, [rac-LA]0/[BnOH]0/[TBD]0 = 100/1/1). Over time, the initially insoluble medium becomes more and more soluble at −75 °C, attesting to the good formation of the polymer (Figure S3). After an appropriate reaction time, the medium is quenched by an addition of cold heptane, allowing both monomer and polymer to precipitate. The conversion is then determined by SEC analysis on the crude medium while the Pm, Tg and Tm values are obtained after precipitation of the crude medium in cold methanol.
3.4. Theoretical Investigations
All the geometries of the reactants, transition states and products have been optimized at the Density Functional Theory (DFT) level (ωB97XD functional and a 6-31G** basis set) using Gaussian16 (Revision A03) (https://gaussian.com/citation/). Frequency calculations were performed on each optimized structure to confirm that (i) reactants and products correspond to minima on the potential energy surface and (ii) transition states only have one imaginary frequency. From the transition states, we performed intrinsic reaction coordinates (IRC) calculations to confirm the expected connection to the reactants and products on the potential energy surface, and the geometry of those transition states was then optimized.
4. Conclusions
In summary, we show that an achiral catalyst as simple and readily available as TBD can efficiently induce the stereoselective ring-opening polymerization of rac- and meso-lactides, achieving a highly iso- and heterotactic polylactide, respectively. The explanation for such performance, as proposed from combined experimental and computational results, lies in significantly different activation energies originating from subtle differences in intermolecular interactions within the complex formed by the reactants.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/10/6/620/s1, Figure S1: Mixture of L- and D-LA (1/1 molar ratio) at 23 °C ([rac-LA]0 = 0.22 M), Figure S2: Mixture of L- and D-LA (1/1 molar ratio) after 10 min at −75 °C ([rac-LA]0 = 0.22 M), Figure S3: Mixture of L- and D-LA (1/1 molar ratio) after 20 (A) and 30 min (B) of polymerization at –75 °C (Table 1, Entries 2 and 3), Figure S4: Differential Scanning Calorimetry Thermogram of poly(rac-LA) obtained by rac-LA ROP in toluene at –75 °C, initiated by BnOH and catalyzed by TBD ([LA]0/[BnOH]0/[TBD]0 = 100/1/0.1; [rac-LA]0 = 0.08 M) – Entry 1, Table 1, Figure S5: Differential Scanning Calorimetry Thermogram of poly(rac-LA) obtained by rac-LA ROP in toluene at −75 °C, initiated by BnOH and catalyzed by TBD ([LA]0/[BnOH]0/[TBD]0 = 100/1/0.1; [rac-LA]0 = 0.08 M) – Entry 2, Table 1, Figure S6: Differential Scanning Calorimetry Thermogram of poly(rac-LA) obtained by rac-LA ROP in toluene at −75 °C, initiated by BnOH and catalyzed by TBD ([LA]0/[BnOH]0/[TBD]0 = 100/1/1; [rac-LA]0 = 0.22 M) – Entry 3, Table 1, Figure S7: Differential Scanning Calorimetry Thermogram of poly(rac-LA) obtained by rac-LA ROP in toluene at −75 °C, initiated by BnOH and catalyzed by TBD ([LA]0/[BnOH]0/[TBD]0 = 100/1/1; [rac-LA]0 = 0.22 M) – Entry 4, Table 1, Figure S8. DFT-optimized (ωB97XD/6-31G**) reactants from IRC calculations of complex I (left) and complex II (right). The distances between the alcohol oxygen and the carbon of the carbonyl are highlighted in green and is much shorter in complex I (2.62 Å vs. 2.79 Å). Hydrogens have been omitted for clarity except those involved in hydrogen bonds Figure S9. DFT-calculated (ωB97XD/6-31G**) energy profiles corresponding to the nucleophilic attack of the oxygen of the alcohol on the carbonyl carbon for complexes I (blue) and II (red). The energy barriers between reactants and transition states are relative to its reactants. The optimized reactants (left), transition states (center) and products (right) geometries of all the complexes are also displayed. Hydrogens have been omitted for clarity, except those involved in hydrogen bonds.
Author Contributions
Conceptualization, O.C.; methodology, O.C.; software, S.H., V.L. and R.L.; validation, O.C, D.T., A.P.D. and R.L.; formal analysis, S.M., B.O., K.D.C. and O.C.; investigation, S.M. and S.H.; resources, O.C. and R.L.; data curation, O.C, A.P.D. and V.L.; writing—original draft preparation, O.C., A.P.D., V.L. and R.L.; writing—review and editing, O.C., A.P.D. and R.L.; visualization, O.C.; supervision, O.C.; project administration, O.C. and R.L.; funding acquisition, O.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Commission and Region Wallonne FEDER program (BIORGEL project), and by the FNRS-FRFC (Consortium des Équipements de Calcul Intensif—CÉCI). The bioprofiling platform was supported by the European Regional Development Fund and the Walloon Region, Belgium.
Acknowledgments
O.C. is research associate for the F.R.S.-FNRS. S.H. is grateful to the FRIA for financial support. Corbion and Purac are gratefully thanked for their gift of lactide isomers.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Okada, M. Chemical Synthesis of Biodegradable Polymers. Prog. Polym. Sci. 2002, 27, 87–133. [Google Scholar] [CrossRef]
- Thomas, C.M. Stereocontrolled Ring-Opening Polymerization of Cyclic Esters: Synthesis of New Polyester Microstructures. Chem. Soc. Rev. 2010, 39, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Spassky, N.; Pluta, C.; Simic, V.; Thiam, M.; Wisniewski, M. Stereochemical Aspects of the Controlled Ring-Opening Polymerization of Chiral Cyclic Esters. Macromol. Symp. 1998, 128, 39–51. [Google Scholar] [CrossRef]
- Ovitt, T.M.; Coates, G.W. Stereochemistry of Lactide Polymerization with Chiral Catalysts: New Opportunities for Stereocontrol Using Polymer Exchange Mechanisms. J. Am. Chem. Soc. 2002, 124, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Worch, J.C.; Prydderch, H.; Jimaja, S.; Bexis, P.; Becker, M.L.; Dove, A.P. Stereochemical enhancement of polymer properties. Nat. Rev. Chem. 2019, 3, 514–535. [Google Scholar] [CrossRef]
- Stanford, M.J.; Dove, A.P. Stereocontrolled Ring-Opening Polymerisation of Lactide. Chem. Soc. Rev. 2010, 39, 486–494. [Google Scholar] [CrossRef]
- Dijkstra, P.J.; Du, H.; Feijen, J. Single site catalysts for stereoselective ring-opening polymerization of lactide. Polym. Chem. 2011, 2, 520–527. [Google Scholar] [CrossRef]
- Ottou, W.N.; Sardon, H.; Mecerreyes, D.; Vignolle, J.; Taton, D. Update and challenges in organo-mediated polymerization reactions. Prog. Polym. Sci. 2016, 56, 64–115. [Google Scholar] [CrossRef]
- Jensen, T.R.; Breyfogle, L.E.; Hillmyer, M.A.; Tolman, W.B. Stereoselective polymerization of D,L-lactide using N-heterocyclic carbene based compounds. Chem. Comm. 2004, 10, 2504–2505. [Google Scholar] [CrossRef]
- Dove, A.P.; Li, H.; Pratt, R.C.; Lohmeijer, B.G.G.; Culkin, D.A.; Waymouth, R.M.; Hedrick, J.L. Stereoselective Polymerization of Rac- and Meso-Lactide Catalyzed by Sterically Encumbered N-Heterocyclic Carbenes. Chem. Commun. 2006, 27, 2881. [Google Scholar] [CrossRef]
- Pratt, R.C.; Lohmeijer, B.G.G.; Long, D.A.; Lundberg, P.N.P.; Dove, A.P.; Li, H.; Wade, C.G.; Waymouth, R.M.; Hedrick, J.L. Exploration, Optimization, and Application of Supramolecular Thiourea- Amine Catalysts for the Synthesis of Lactide (Co)Polymers. Macromolecules 2006, 39, 7863–7871. [Google Scholar] [CrossRef]
- Zhang, L.; Nederberg, F.; Messman, J.M.; Pratt, R.C.; Hedrick, J.L.; Wade, C.G. Organocatalytic Stereoselective Ring-Opening Polymerization of Lactide with Dimeric Phosphazene Bases. J. Am. Chem. Soc. 2007, 129, 12610–12611. [Google Scholar] [CrossRef] [PubMed]
- Miyake, G.M.; Chen, E.Y.X. Cinchona Alkaloids as Stereoselective Organocatalysts for the Partial Kinetic Resolution Polymerization of Rac-Lactide. Macromolecules 2011, 44, 4116–4124. [Google Scholar] [CrossRef]
- Makiguchi, K.; Yamanaka, T.; Kakuchi, T.; Terada, M.; Satoh, T. Binaphthol-Derived Phosphoric Acids as Efficient Chiral Organocatalysts for the Enantiomer-Selective Polymerization of Rac-Lactide. Chem. Commun. 2014, 50, 2883–2885. [Google Scholar] [CrossRef]
- Zhu, J.B.; Chen, E.Y.X. From Meso-Lactide to Isotactic Polylactide: Epimerization by B/N Lewis Pairs and Kinetic Resolution by Organic Catalysts. J. Am. Chem. Soc. 2015, 137, 12506–12509. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, A.; Rivilla, I.; Agirre, M.; Basterretxea, A.; Etxeberria, A.; Veloso, A.; Sardon, H.; Mecerreyes, D.; Cossío, F.P. Enantioselective Ring-Opening Polymerization of Rac-Lactide Dictated by Densely Substituted Amino Acids. J. Am. Chem. Soc. 2017, 139, 4805–4814. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Zhao, N.; Li, Z. Stereoselective Ring-Opening Polymerization of Rac -Lactide Using Organocatalytic Cyclic Trimeric Phosphazene Base. ACS Macro Lett. 2018, 7, 624–628. [Google Scholar] [CrossRef]
- Orhan, B.; Tschan, M.J.-L.; Wirotius, A.-L.; Dove, A.P.; Coulembier, O.; Taton, D. Isoselective Ring-Opening Polymerization of rac-Lactide from Chiral Takemoto’s Organocatalysts: Elucidation of Stereocontrol. ACS Macro Lett. 2018, 7, 1413–1419. [Google Scholar] [CrossRef]
- Lim, J.Y.C.; Yuntawattana, N.; Beer, P.D.; Williams, C.K. Isoselective Lactide Ring Opening Polymerisation using [2]Rotaxane Catalysts. Angew. Chem. Int. Ed. 2019, 58, 6007–6011. [Google Scholar] [CrossRef]
- Lohmeijer, B.G.G.; Pratt, R.C.; Leibfarth, F.; Logan, J.W.; Long, D.A.; Dove, A.P.; Nederberg, F.; Choi, J.; Wade, C.; Waymouth, R.M.; et al. Guanidine and Amidine Organocatalysts for Ring-Opening Polymerization of Cyclic Esters. Macromolecules 2006, 39, 8574–8583. [Google Scholar] [CrossRef]
- Pratt, R.C.; Lohmeijer, B.G.G.; Long, D.A.; Waymouth, R.M.; Hedrick, J.L. Triazabicyclodecene: A Simple Bifunctional Organocatalyst for Acyl Transfer and Ring-Opening Polymerization of Cyclic Esters. J. Am. Chem. Soc. 2006, 128, 4556–4557. [Google Scholar] [CrossRef] [PubMed]
- Baran, J.; Duda, A.; Kowalski, A.; Szymanski, R.; Penczek, S. Intermolecular chain transfer to polymer with chain scission: General treatment and determination of kp/ktr in L,L-lactide polymerization. Macromol. Rapid Commun. 1997, 18, 325–333. [Google Scholar] [CrossRef]
- Chuma, A.; Horn, H.W.; Swope, W.C.; Pratt, R.C.; Zhang, L.; Lohmeijer, B.G.G.; Wade, C.G.; Waymouth, R.M.; Hedrick, J.L.; Rice, L.E. The Reaction Mechanism for the Organocatalytic Ring-Opening Polymerization of l-Lactide Using a Guanidine-Based Catalyst: Hydrogen-Bonded or Covalently Bound? J. Am. Chem. Soc. 2008, 130, 6749–6754. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.; Goodman, J.M. The Mechanism of TBD-Catalyzed Ring-Opening Polymerization of Cyclic Esters. J. Org. Chem. 2007, 72, 9656–9662. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Ivchenko, P. DFT Modeling of Organocatalytic Ring-Opening Polymerization of Cyclic Esters: A Crucial Role of Proton Exchange and Hydrogen Bonding. Polymers 2019, 11, 2078. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).