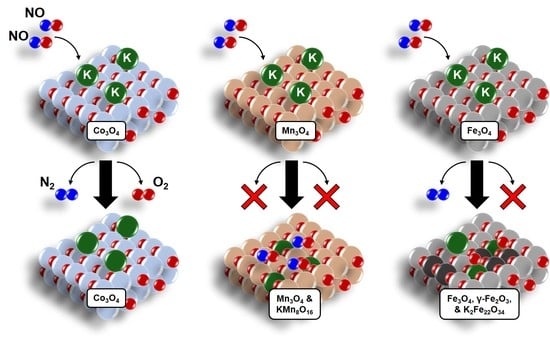
Contrasting Effects of Potassium Addition on M3O4 (M = Co, Fe, and Mn) Oxides during Direct NO Decomposition Catalysis
Abstract
1. Introduction
2. Results and Discussion
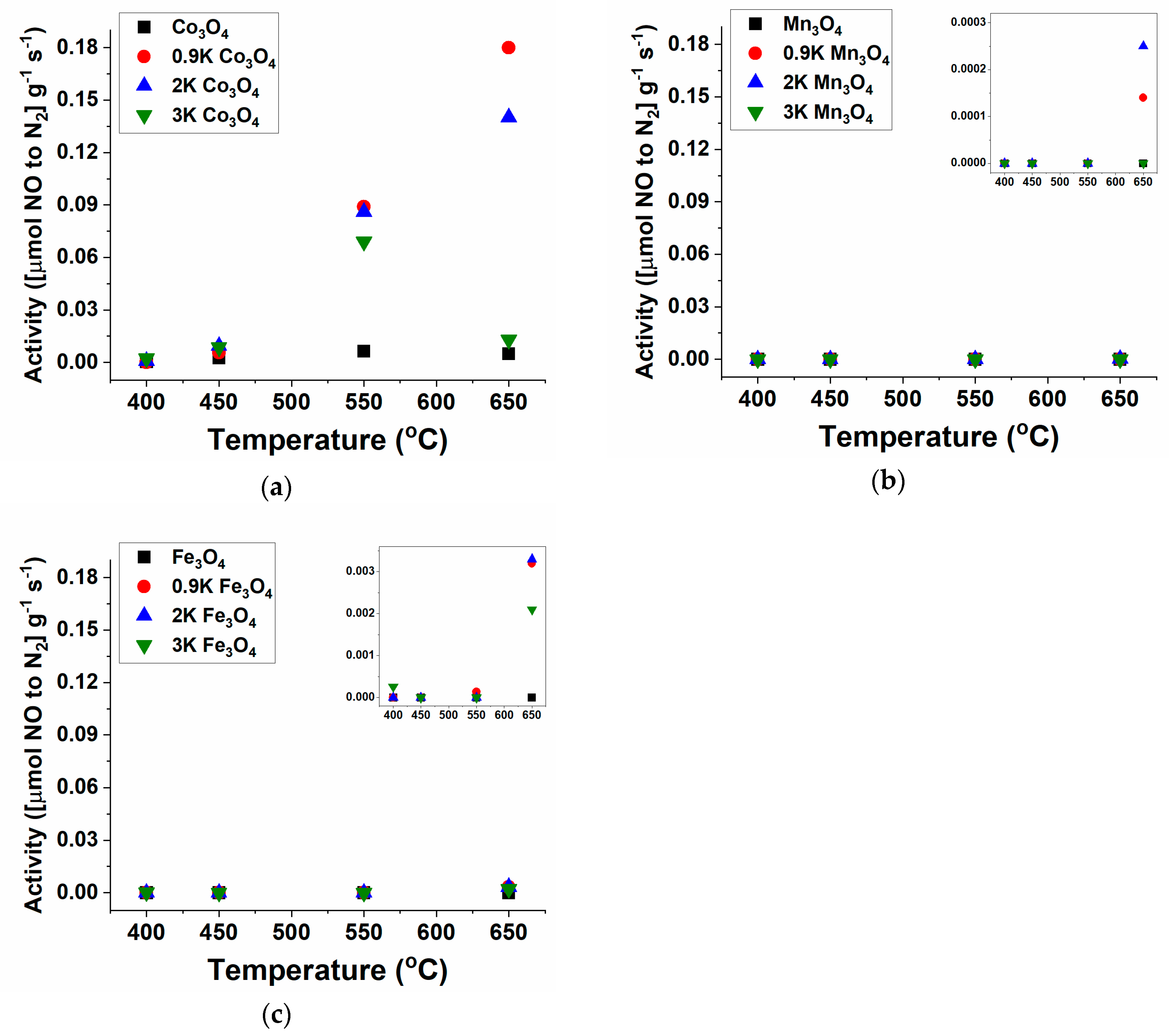
2.1. NO Decomposition Catalytic Performance
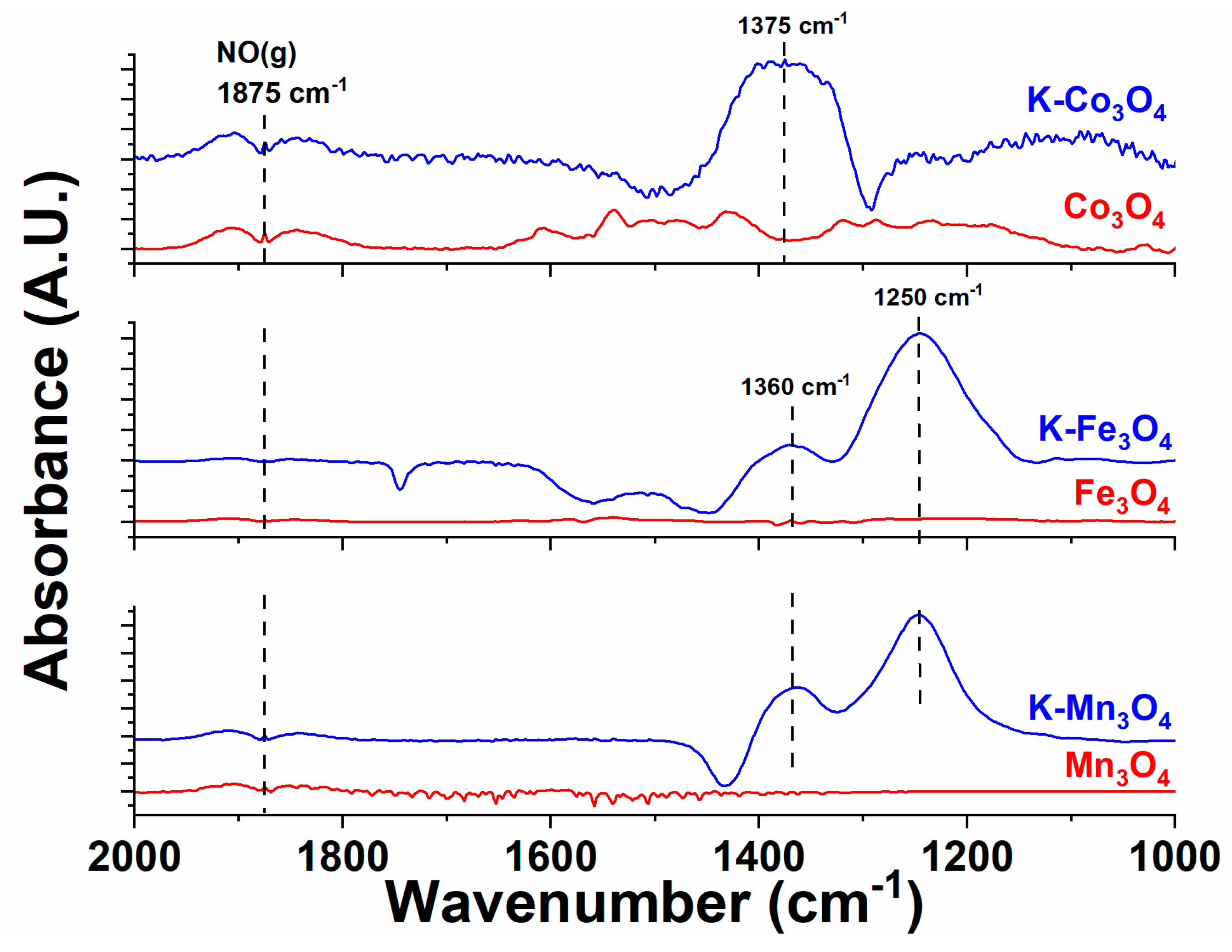
2.2. Comparison of Adsorbed Intermediates
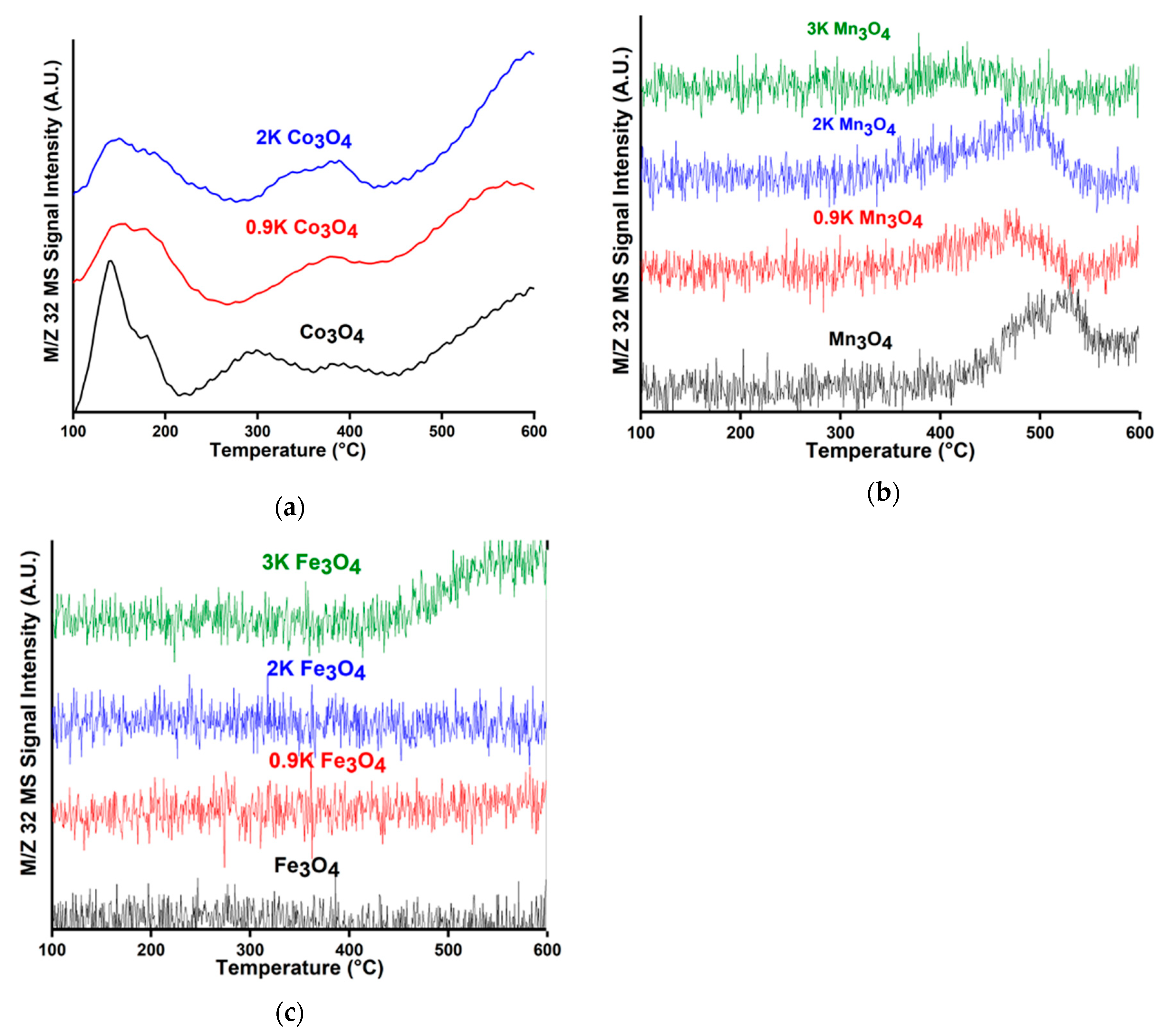
2.3. O2 Release and NO Decomposition and the Effect of K Loading
2.4. Catalyst Structure before and after Reaction—Influence of K Loading
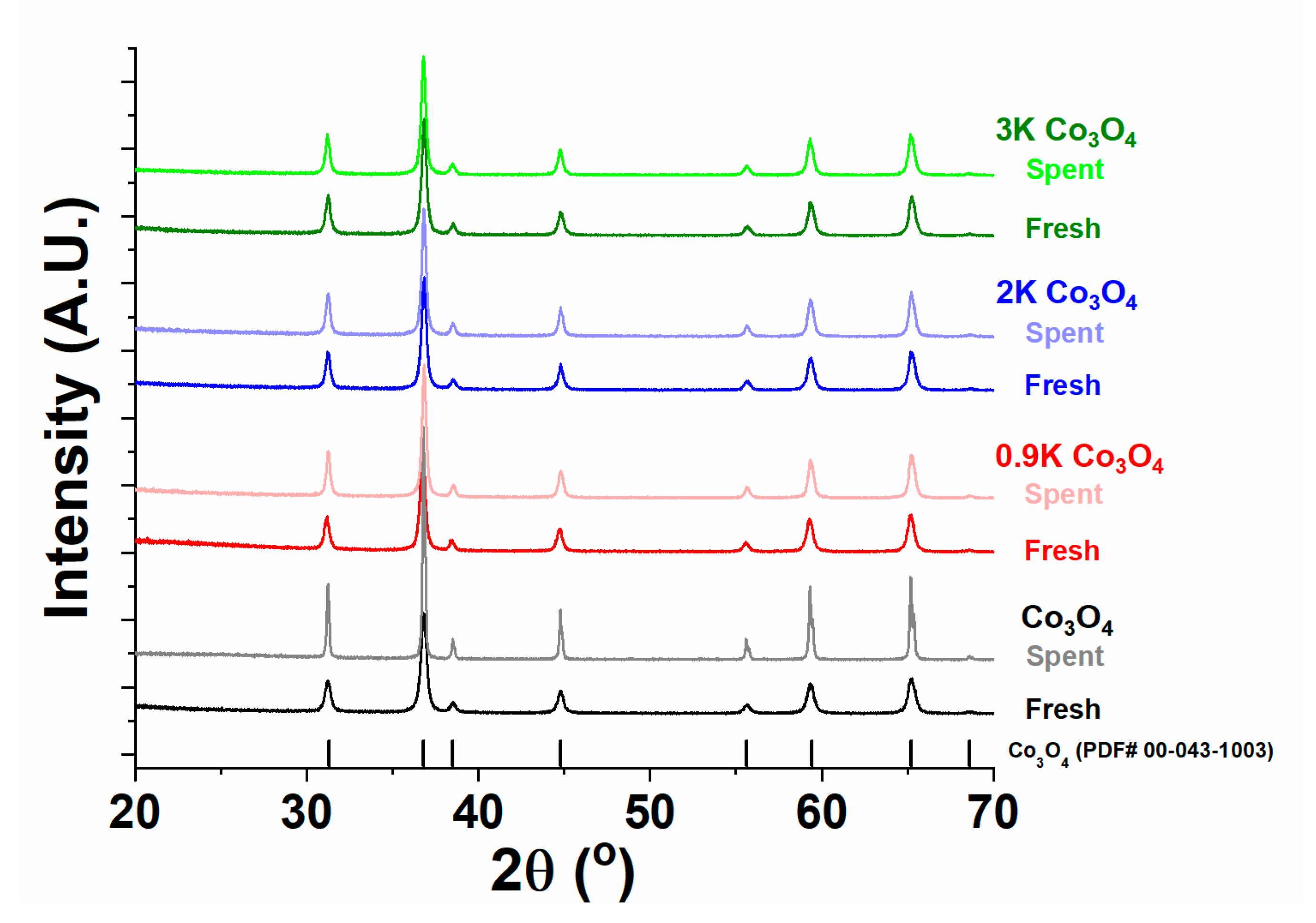
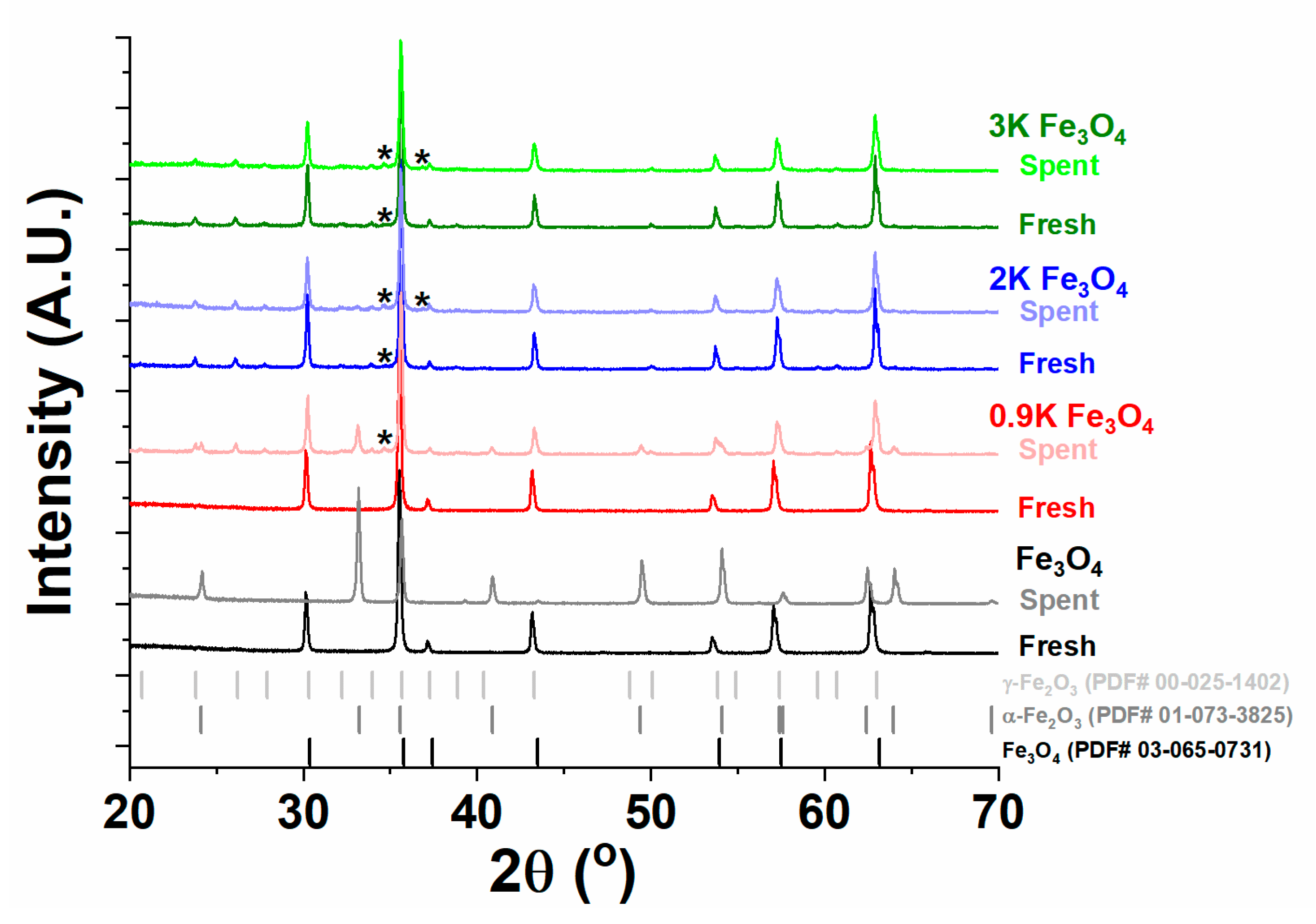
2.4.1. X-ray Diffraction
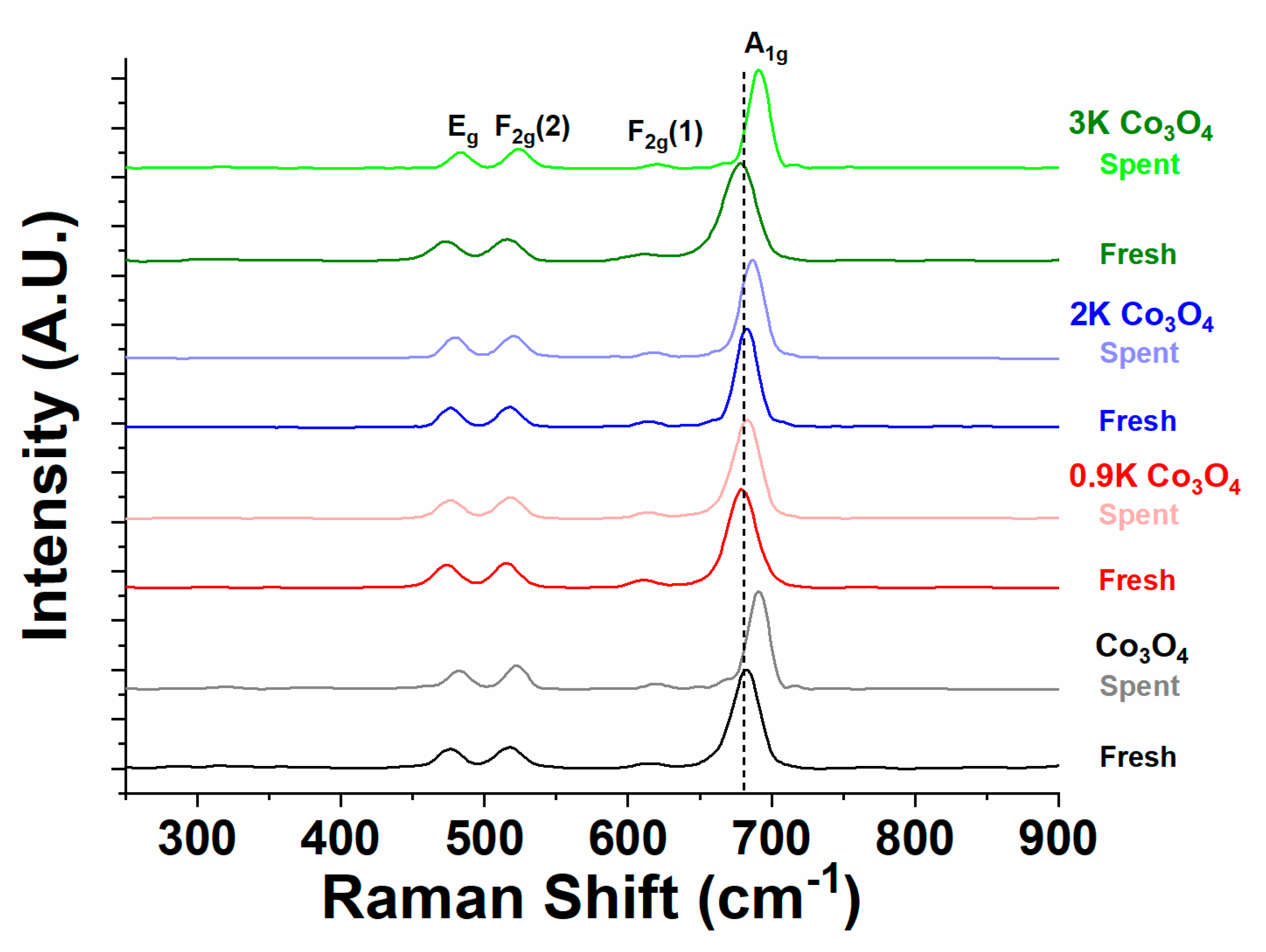
2.4.2. Raman Spectroscopy
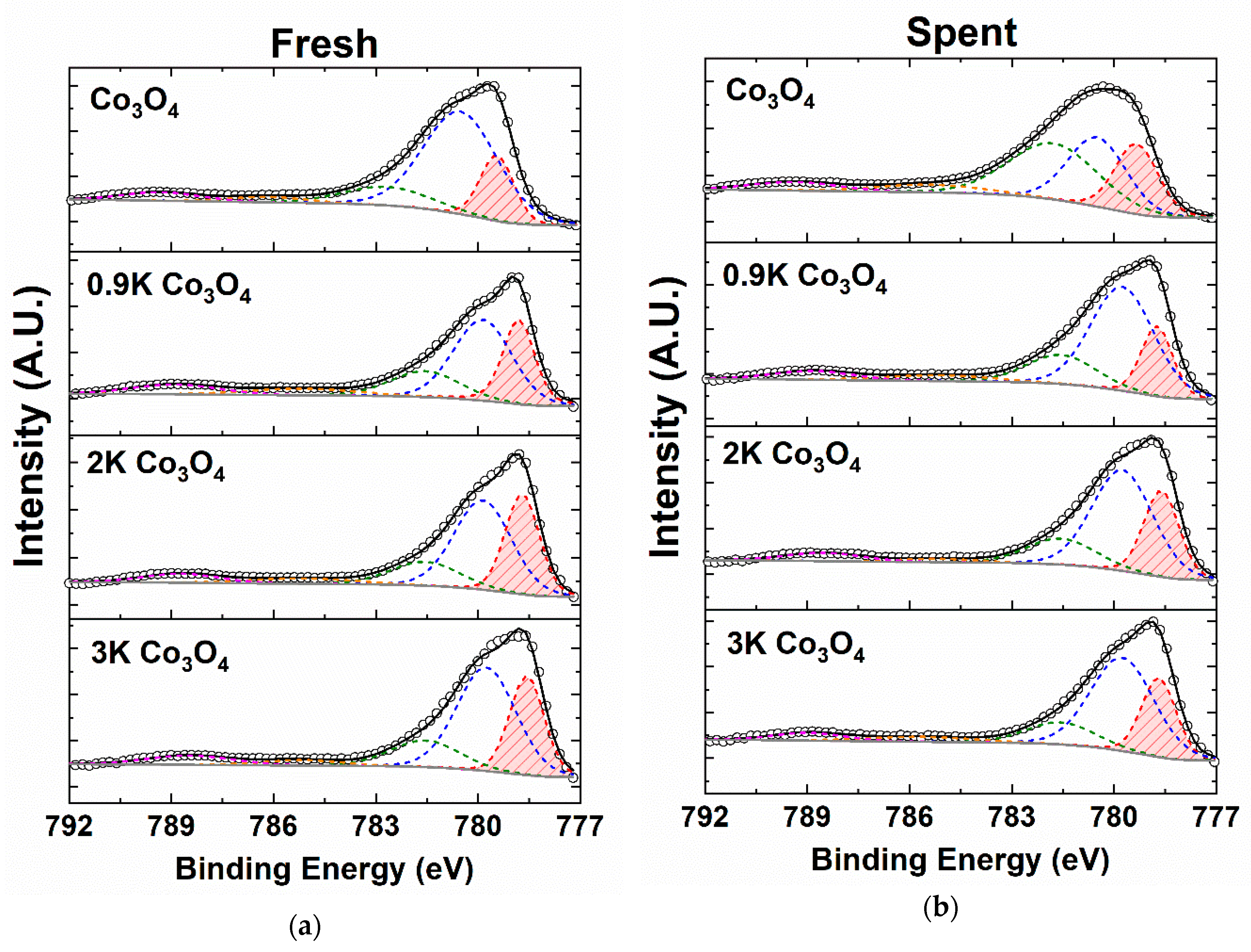
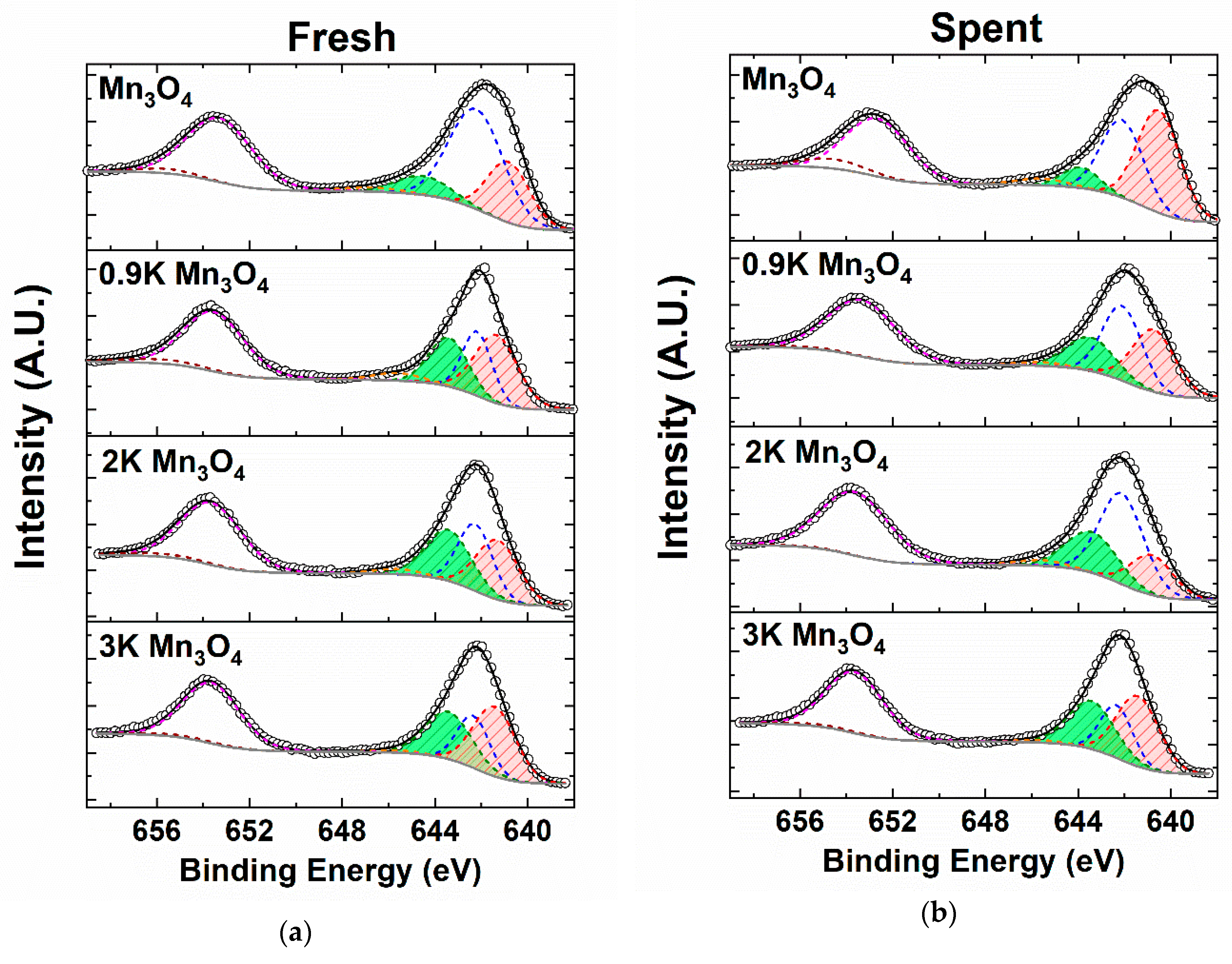
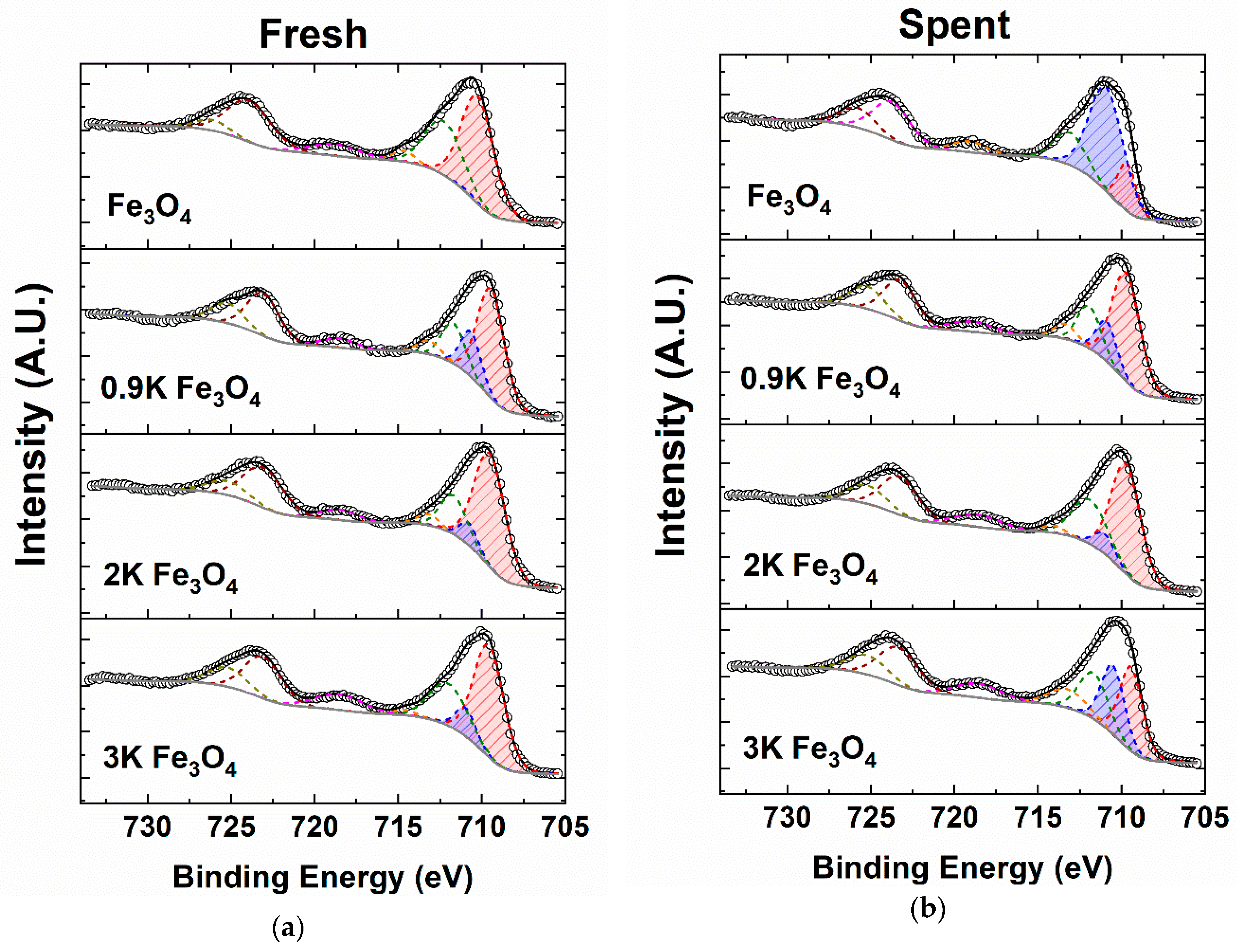
2.4.3. X-ray Photoelectron Spectroscopy
3. Materials and Methods
3.1. Catalyst Synthesis
3.2. Catalytic Evaluation
3.3. In Situ FTIR
3.4. Oxygen Temperature Programmed Desorption
3.5. X-ray Diffraction
3.6. Raman Spectroscopy
3.7. X-ray Photoelectron Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Howard, C.S.; Daniels, F. The Stability of Nitric Oxide Over a Long Time Interval. J. Phys. Chem. 1958, 62, 320–321. [Google Scholar] [CrossRef]
- Boreskov, G.K. Forms of oxygen bonds on the surface of oxidation catalysts. Discuss. Faraday Soc. 1966, 41, 263. [Google Scholar] [CrossRef]
- Amirnazmi, A. Oxygen inhibition in the decomposition of NO on metal oxides and platinum. J. Catal. 1973, 30, 55–65. [Google Scholar] [CrossRef]
- Cónsul, J.M.D.; Peralta, C.A.; Benvenutti, E.V.; Ruiz, J.A.; Pastore, H.O.; Baibich, I. Direct decomposition of nitric oxide on alumina-modified amorphous and mesoporous silica-supported palladium catalysts. J. Mol. Catal. A: Chem. 2006, 246, 33–38. [Google Scholar] [CrossRef]
- Reddy, G.K.; Ling, C.; Peck, T.; Jia, H. Understanding the chemical state of palladium during the direct NO decomposition—Influence of pretreatment environment and reaction temperature. RSC Adv. 2017, 7, 19645–19655. [Google Scholar] [CrossRef]
- Li, Y.; Hall, W. Catalytic decomposition of nitric oxide over Cu-zeolites. J. Catal. 1991, 129, 202–215. [Google Scholar] [CrossRef]
- Modén, B.; Da Costa, P.; Fonfé, B.; Lee, D.K.; Iglesia, E. Kinetics and Mechanism of Steady-State Catalytic NO Decomposition Reactions on Cu–ZSM5. J. Catal. 2002, 209, 75–86. [Google Scholar] [CrossRef]
- Groothaert, M.H.; Lievens, K.; Leeman, H.; Weckhuysen, B.M.; Schoonheydt, R.A. An operando optical fiber UV–vis spectroscopic study of the catalytic decomposition of NO and N2O over Cu-ZSM-5. J. Catal. 2003, 220, 500–512. [Google Scholar] [CrossRef]
- Tofan, C.; Klvana, D.; Kirchnerova, J. Direct decomposition of nitric oxide over perovskite-type catalystsPart I. Activity when no oxygen is added to the feed. Appl. Catal. A: Gen. 2002, 223, 275–286. [Google Scholar] [CrossRef]
- Stoyanova, D.D.; Petrovic, S.P.; Georgieva, P.Z.; Tarlecki-Baricevic, A.; Mehandjiev, D.R. Direct Decomposition of Nitric Oxide on Perovskite Type Catalysts. J. Chem. Technol. Metall. 2017, 52, 75–80. [Google Scholar]
- Zhu, Y.; Wang, N.; Yuan, F.; Zhang, G.; Fu, H. Direct NO decomposition over La2−xBaxNiO4 catalysts containing BaCO3 phase. Appl. Catal. B: Environ. 2008, 82, 255–263. [Google Scholar] [CrossRef]
- Tsujimoto, S.; Nishimura, C.; Masui, T.; Imanaka, N. Direct decomposition of nitrogen monoxide on (Ho, Zr, Pr)2O3+δ Catalysts. Catal. Commun. 2014, 43, 84–87. [Google Scholar] [CrossRef]
- Masui, T.; Uejima, S.; Tsujimoto, S.; Nagai, R.; Imanaka, N. Direct NO decomposition over C-type cubic Y2O3–Pr6O11–Eu2O3 solid solutions. Catal. Today 2015, 242, 338–342. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, Z.; Wang, D.; Hong, Z.; Zhou, M.-D.; Li, X. A review on the catalytic decomposition of NO to N2 and O2: Catalysts and processes. Catal. Sci. Technol. 2018, 8, 4563–4575. [Google Scholar] [CrossRef]
- Shelef, M.; Otto, K.; Gandhi, H. The heterogeneous decomposition of nitric oxide on supported catalysts. Atmos. Environ. (1967) 1969, 3, 107–122. [Google Scholar] [CrossRef]
- Winter, E. The catalytic decomposition of nitric oxide by metallic oxides. J. Catal. 1971, 22, 158–170. [Google Scholar] [CrossRef]
- Gassan-Zade, G.Z.; Wood, M.Y.; Alkhazov, T.G. Interaction of nitric oxide with NiCr2O4. React. Kinet. Catal. Lett. 1985, 28, 167–171. [Google Scholar] [CrossRef]
- Park, P.; Kil, J.; Kung, H.H.; Kung, M. NO decomposition over sodium-promoted cobalt oxide. Catal. Today 1998, 42, 51–60. [Google Scholar] [CrossRef]
- Haneda, M.; Kintaichi, Y.; Bion, N.; Hamada, H. Alkali metal-doped cobalt oxide catalysts for NO decomposition. Appl. Catal. B: Environ. 2003, 46, 473–482. [Google Scholar] [CrossRef]
- Roberts, C.A.; Paidi, V.K.; Shepit, M.; Peck, T.C.; Stamm-Masias, K.L.; van Lierop, J.; Reddy, G.K. Effect of Cu Substitution on the Structure and Reactivity of CuxCo3-xO4 Spinel Catalysts for Direct NOx Decomposition. Catal. Today. submitted manuscript.
- Peck, T.; Reddy, G.K.; Roberts, C.A. Monolayer supported CuOx/Co3O4 as an active and selective low temperature NOx decomposition catalyst. Catal. Sci. Technol. 2019, 9, 1132–1140. [Google Scholar] [CrossRef]
- Abu-Zied, B.M.; Soliman, S.A.; Abdellah, S.E. Enhanced direct N2O decomposition over CuxCo1−xCo2O4 (0.0 ≤ x ≤ 1.0) spinel-oxide catalysts. J. Ind. Eng. Chem. 2015, 21, 814–821. [Google Scholar] [CrossRef]
- Abu-Zied, B.M.; Soliman, S.A.; Abdellah, S.E. Pure and Ni-substituted Co3O4 spinel catalysts for direct N2O decomposition. Chin. J. Catal. 2014, 35, 1105–1112. [Google Scholar] [CrossRef]
- Wójcik, S.; Grzybek, G.; Stelmachowski, P.; Sojka, Z.; Kotarba, A. Bulk, Surface and Interface Promotion of Co3O4 for the Low-Temperature N2O Decomposition Catalysis. Catalysts 2019, 10, 41. [Google Scholar] [CrossRef]
- Maniak, G.; Stelmachowski, P.; Zasada, F.; Piskorz, W.; Kotarba, A.; Sojka, Z. Guidelines for optimization of catalytic activity of 3d transition metal oxide catalysts in N2O decomposition by potassium promotion. Catal. Today 2011, 176, 369–372. [Google Scholar] [CrossRef]
- Jirátová, K.; Pacultová, K.; Balabánová, J.; Karásková, K.; Klegová, A.; Bílková, T.; Jandová, V.; Kostejn, M.; Martaus, A.; Kotarba, A.; et al. Precipitated K-Promoted Co–Mn–Al Mixed Oxides for Direct NO Decomposition: Preparation and Properties. Catalysts 2019, 9, 592. [Google Scholar] [CrossRef]
- Yamashita, T. NO Decomposition over Mn2O3 and Mn3O4. J. Catal. 1996, 163, 158–168. [Google Scholar] [CrossRef]
- Jones, S. Ceria Based Catalysts for Low Temperature NOx Storage and Release. Theses and Dissertations−Chemistry, University of Kentucky, Lexington, KY, USA, 2016. Available online: https://uknowledge.uky.edu/cgi/viewcontent.cgi?article=1073&context=chemistry_etds (accessed on 29 June 2019).
- Hadjiivanov, K.I. Identification of Neutral and Charged N x O y Surface Species by IR Spectroscopy. Catal. Rev. 2000, 42, 71–144. [Google Scholar] [CrossRef]
- Legutko, P.; Pęza, J.; Rossi, A.V.; Marzec, M.; Jakubek, T.; Kozieł, M.; Adamski, A. Elucidation of Unexpectedly Weak Catalytic Effect of Doping with Cobalt of the Cryptomelane and Birnessite Systems Active in Soot Combustion. Top. Catal. 2019, 62, 599–610. [Google Scholar] [CrossRef]
- Parkinson, G.S. Iron Oxide Surfaces. Surf. Sci. 2016, 71, 272–365. [Google Scholar] [CrossRef]
- Hadjiev, V.G.; Iliev, M.; Vergilov, I.V. The Raman spectra of Co3O4. J. Phys. C: Solid State Phys. 1988, 21, L199–L201. [Google Scholar] [CrossRef]
- Gawali, S.R.; Gandhi, A.C.; Gaikwad, S.; Pant, J.; Chan, T.-S.; Cheng, C.-L.; Ma, Y.-R.; Wu, S.Y. Role of cobalt cations in short range antiferromagnetic Co3O4 nanoparticles: A thermal treatment approach to affecting phonon and magnetic properties. Sci. Rep. 2018, 8, 249. [Google Scholar] [CrossRef] [PubMed]
- Gwag, J.-S.; Sohn, Y. Interfacial Natures and Controlling Morphology of Co Oxide Nanocrystal Structures by Adding Spectator Ni Ions. Bull. Korean Chem. Soc. 2012, 33, 505–510. [Google Scholar] [CrossRef]
- Larbi, T.; Doll, K.; Manoubi, T. Density functional theory study of ferromagnetically and ferrimagnetically ordered spinel oxide Mn3O4. A quantum mechanical simulation of their IR and Raman spectra. J. Alloy Compd. 2016, 688, 692–698. [Google Scholar] [CrossRef]
- Sada, K.; Senthilkumar, B.; Barpanda, P. Cryptomelane K1.33Mn8O16 as a cathode for rechargeable aqueous zinc-ion batteries. J. Mater. Chem. A 2019, 7, 23981–23988. [Google Scholar] [CrossRef]
- Sepúlveda, M.; Gutiérrez, S.; Vallette, M.C.; Standen, V.G.; Arriaza, B.T.; Cárcamo, J. Micro-Raman spectral identification of manganese oxides black pigments in an archaeological context in Northern Chile. Heritage Sci. 2015, 3, 3447. [Google Scholar] [CrossRef]
- Akkopru-Akgun, B.; Trolier-McKinstry, S.; Lanagan, M.T. MnO2 Thin Film Electrodes for Enhanced Reliability of Thin Glass Capacitors. J. Am. Ceram. Soc. 2015, 98, 3270–3279. [Google Scholar] [CrossRef]
- Freitas, R.M.; Perilli, T.A.G.; Ladeira, A.C.Q. Oxidative Precipitation of Manganese from Acid Mine Drainage by Potassium Permanganate. J. Chem. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Hanesch, M. Raman spectroscopy of iron oxides and (oxy)hydroxides at low laser power and possible applications in environmental magnetic studies. Geophys. J. Int. 2009, 177, 941–948. [Google Scholar] [CrossRef]
- De smit, E.; Weckhuysen, B.M. The renaissance of Iron-based Fischer-Tropsch Synthesis: On the multifaceted catalyst deactivation behavior. Chem. Soc. Rev. 2008, 37, 2758–2781. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.; Gerson, A.R.; Smart, R.S. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peck, T.C.; Roberts, C.A.; Reddy, G.K. Contrasting Effects of Potassium Addition on M3O4 (M = Co, Fe, and Mn) Oxides during Direct NO Decomposition Catalysis. Catalysts 2020, 10, 561. https://doi.org/10.3390/catal10050561
Peck TC, Roberts CA, Reddy GK. Contrasting Effects of Potassium Addition on M3O4 (M = Co, Fe, and Mn) Oxides during Direct NO Decomposition Catalysis. Catalysts. 2020; 10(5):561. https://doi.org/10.3390/catal10050561
Chicago/Turabian StylePeck, Torin C., Charles A. Roberts, and Gunugunuri K. Reddy. 2020. "Contrasting Effects of Potassium Addition on M3O4 (M = Co, Fe, and Mn) Oxides during Direct NO Decomposition Catalysis" Catalysts 10, no. 5: 561. https://doi.org/10.3390/catal10050561
APA StylePeck, T. C., Roberts, C. A., & Reddy, G. K. (2020). Contrasting Effects of Potassium Addition on M3O4 (M = Co, Fe, and Mn) Oxides during Direct NO Decomposition Catalysis. Catalysts, 10(5), 561. https://doi.org/10.3390/catal10050561