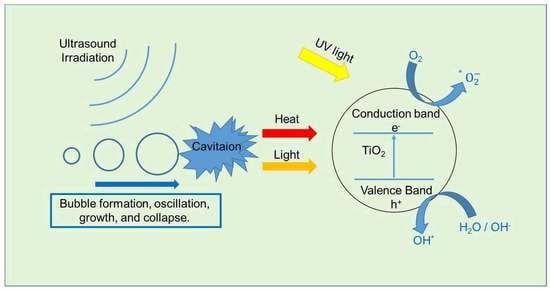
Investigation of the Synergistic Effect of Sonolysis and Photocatalysis of Titanium Dioxide for Organic Dye Degradation
Abstract
1. Introduction
2. Results and Discussion
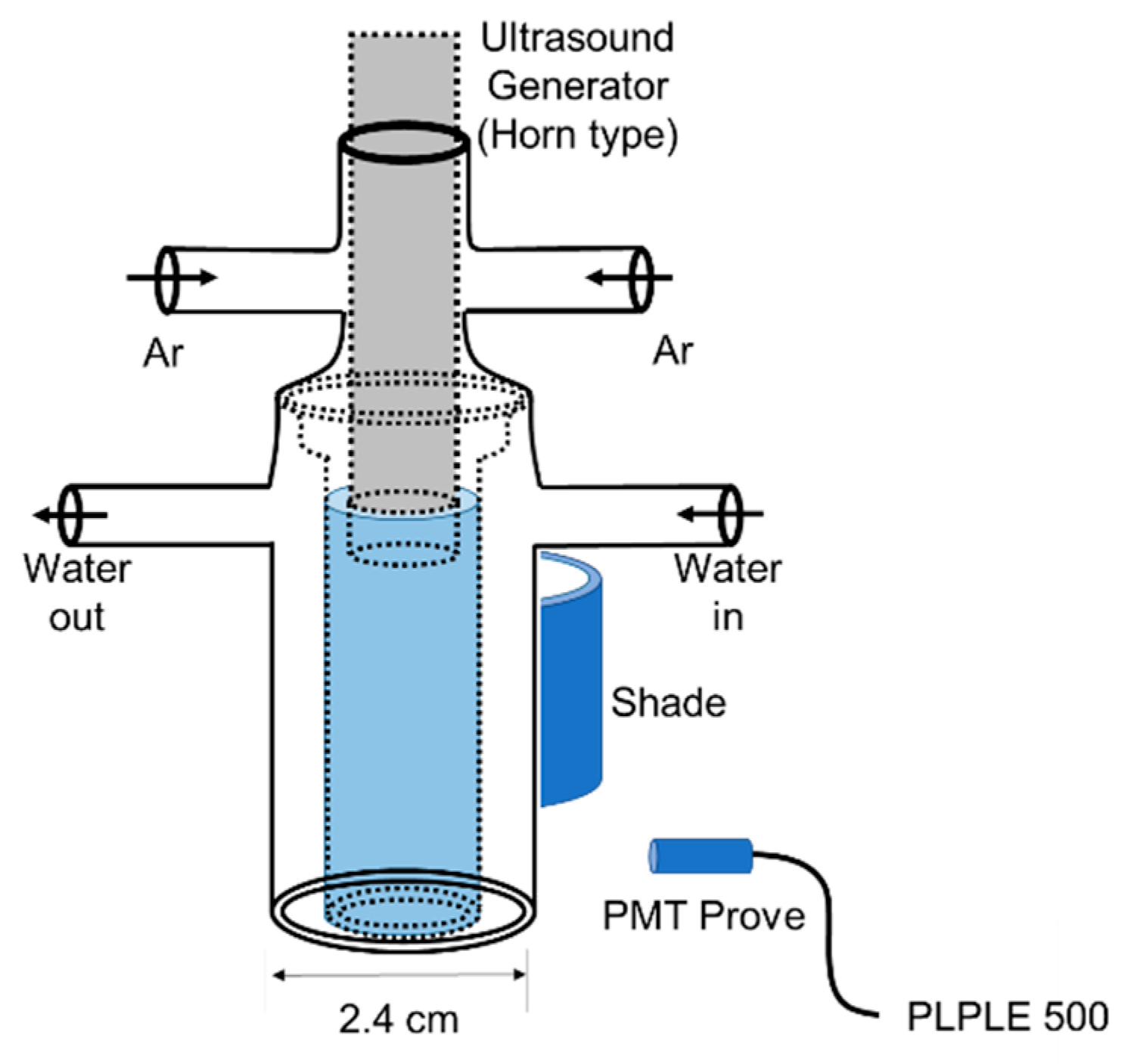
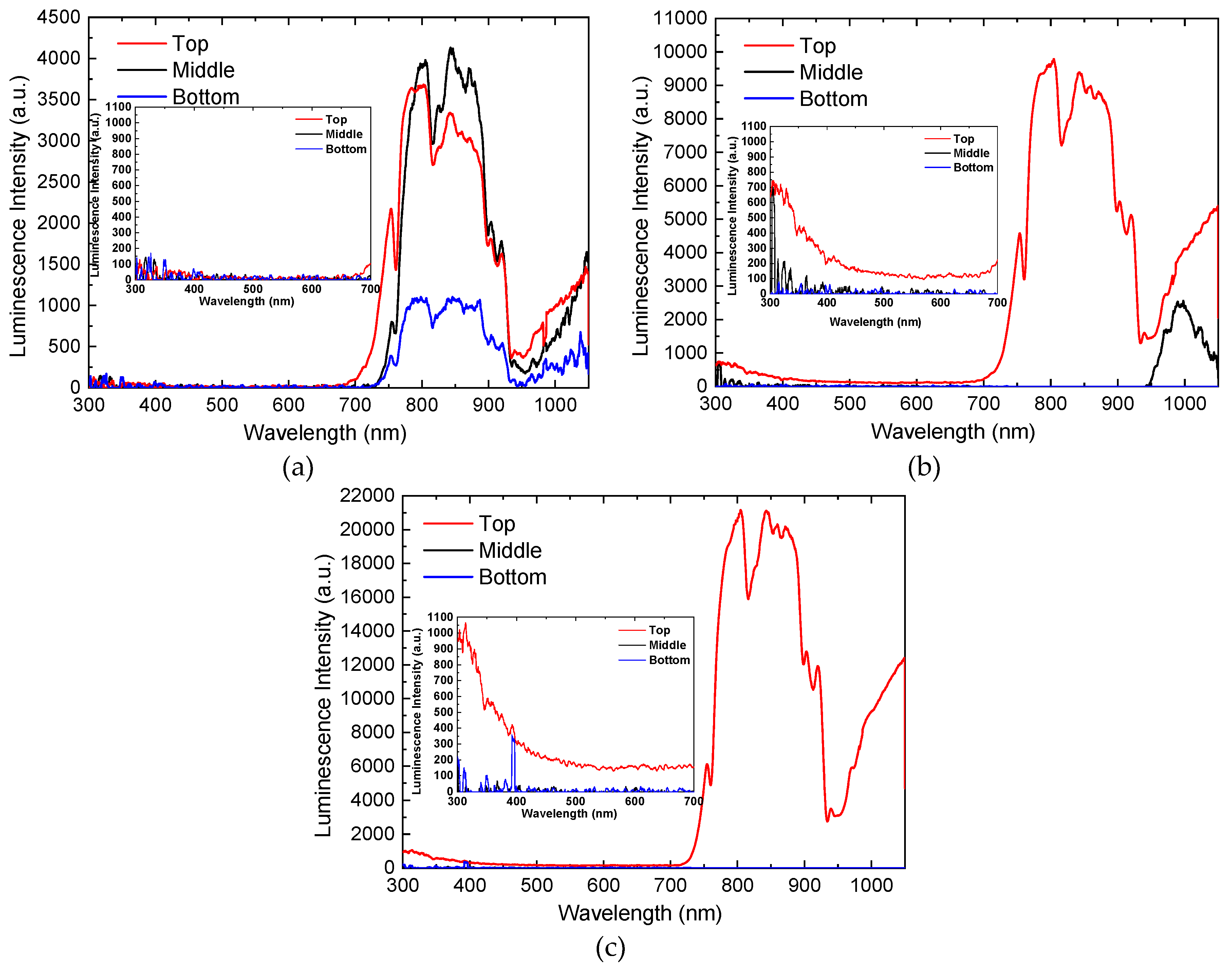
2.1. Analysis of Sonoluminescence
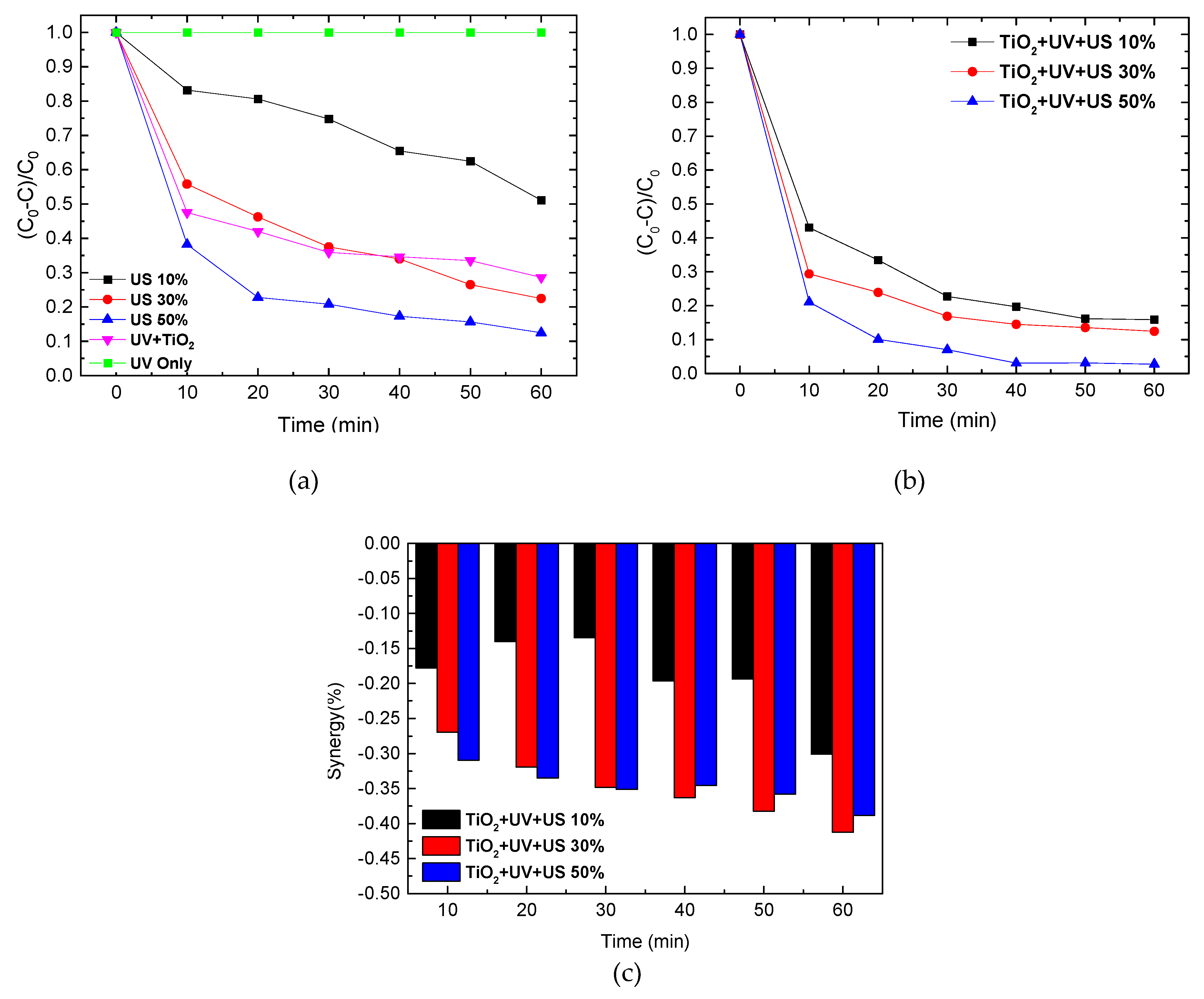
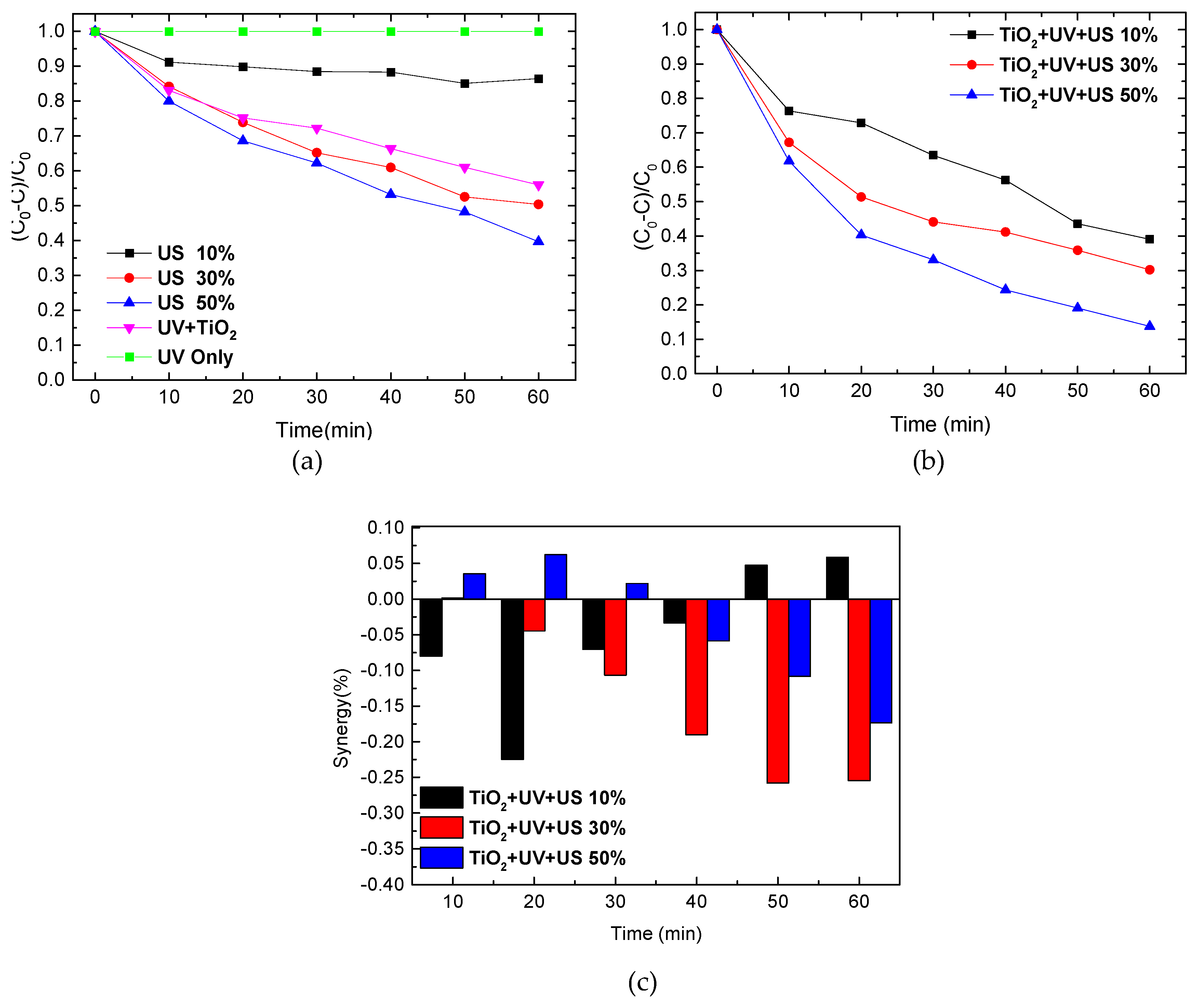
2.2. Sonolysis of Eosin B
2.3. Influence of Sonolysis on Photocatalytic Activity of TiO2
3. Methods
3.1. Chemicals and Instruments
3.2. Sonoluminescence
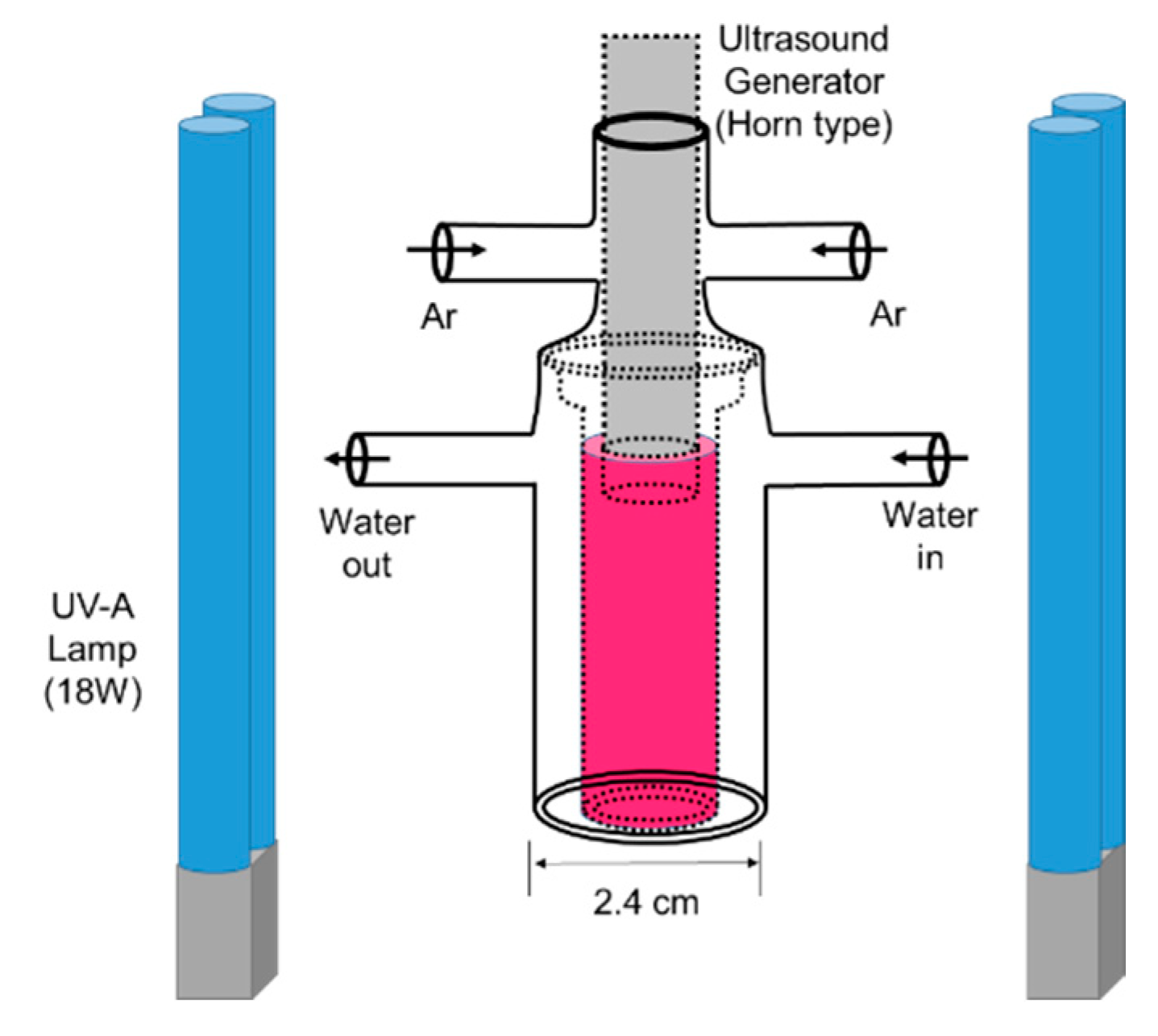
3.3. Sonolysis, Photocatalysis and Sonophotocatalysis of Eosin B
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Oturan, M.A.; Aaron, J.J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Adewuyi, Y.G. Sonochemistry in environmental remediation. 1. Combinative and hybrid sonophotochemical oxidation processes for the treatment of pollutants in water. Environ. Sci. Technol. 2005, 39, 3409–3420. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, J.; Kumar, P.S.S.; Anandan, S.; Zhou, M.; Grieser, F.; Ashokkumar, M. Ultrasound assisted photocatalytic degradation of diclofenac in an aqueous environment. Chemosphere 2010, 80, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Chakma, S.; Moholkar, V.S. Physical mechanism of sono-Fenton process. AIChE J. 2013, 59, 4303–4313. [Google Scholar] [CrossRef]
- Asghar, A.; Raman, A.A.A.; Daud, W.M.A.W. Advanced oxidation processes for in-situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: A review. J. Clean. Prod. 2015, 87, 826–838. [Google Scholar] [CrossRef]
- Reuter, F.; Mettin, R. Mechanisms of single bubble cleaning. Ultrason. Sonochem. 2016, 29, 550–562. [Google Scholar] [CrossRef]
- Suslick, K.S.; Didenko, Y.; Fang, M.M.; Hyeon, T.; Kolbeck, K.J.; McNamara, W.B.; Mdleleni, M.M.; Wong, M. Acoustic cavitation and its chemical consequences. Philos. Trans. R. Soc. A 1999, 357, 335–353. [Google Scholar] [CrossRef]
- Skorb, E.V.; Fix, D.; Shchukin, D.G.; Möhwald, H.; Sviridov, D.V.; Mousa, R.; Wanderka, N.; Schäferhans, J.; Pazos-Pérez, N.; Fery, A. Sonochemical formation of metal sponges. Nanoscale 2011, 3, 985–993. [Google Scholar] [CrossRef]
- Cravotto, G.; Gaudino, E.C.; Cintas, P. On the mechanochemical activation by ultrasound. Chem. Soc. Rev. 2013, 42, 7521–7534. [Google Scholar] [CrossRef]
- Qiu, P.; Park, B.; Choi, J.; Thokchom, B.; Pandit, A.B.; Khim, J. A review on heterogeneous sonocatalyst for treatment of organic pollutants in aqueous phase based on catalytic mechanism. Ultrason. Sonochem. 2018, 45, 29–49. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Mahamuni, N.N.; Adewuyi, Y.G. Advanced oxidation processes (AOPs) involving ultrasound for waste water treatment: A review with emphasis on cost estimation. Ultrason. Sonochem. 2010, 17, 990–1003. [Google Scholar] [CrossRef] [PubMed]
- Adewuyi, Y.G. Sonochemistry in environmental remediation. 2. Heterogeneous sonophotocatalytic oxidation processes for the treatment of pollutants in water. Environ. Sci. Technol. 2005, 39, 8557–8570. [Google Scholar] [CrossRef] [PubMed]
- Lops, C.; Ancona, A.; Di Cesare, K.; Dumontel, B.; Garino, N.; Canavese, G.; Hérnandez, S.; Cauda, V. Sonophotocatalytic degradation mechanisms of Rhodamine B dye via radicals generation by micro-and nano-particles of ZnO. Appl. Catal. B Environ. 2019, 243, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Sun, M.; Yuan, X.; Zhu, Y.; Lin, X.; Anandan, S. One-step hydrothermal synthesis of N/Ti3+ co-doping multiphasic TiO2/BiOBr heterojunctions towards enhanced sonocatalytic performance. Ultrason. Sonochem. 2018, 49, 69–78. [Google Scholar] [CrossRef]
- Soltani, R.D.C.; Jorfi, S.; Ramezani, H.; Purfadakari, S. Ultrasonically induced ZnO-biosilica nanocomposite for degradation of a textile dye in aqueous phase. Ultrason. Sonochem. 2016, 28, 69–78. [Google Scholar] [CrossRef]
- Balaji, C.; Moholkar, V.S.; Pandit, A.B.; Ashokkumar, M. Mechanistic investigations on sonophotocatalytic degradation of textile dyes with surface active solutes. Ind. Eng. Chem. Res. 2011, 50, 11485–11494. [Google Scholar] [CrossRef]
- Chakma, S.; Moholkar, V.S. Investigation in mechanistic issues of sonocatalysis and sonophotocatalysis using pure and doped photocatalysts. Ultrason. Sonochem. 2015, 22, 287–299. [Google Scholar] [CrossRef]
- Gogate, P.R.; Pandit, A.B. Sonophotocatalytic reactors for wastewater treatment: A critical review. AIChE J. 2004, 50, 1051–1079. [Google Scholar] [CrossRef]
- Mahdavi, R.; Talesh, S.S.A. Enhancement of ultrasound-assisted degradation of Eosin B in the presence of nanoparticles of ZnO as sonocatalyst. Ultrason. Sonochem. 2019, 51, 230–240. [Google Scholar] [CrossRef]
- Rooze, J.; Rebrov, E.V.; Schouten, J.C.; Keurentjes, J.T. Dissolved gas and ultrasonic cavitation—A review. Ultrason. Sonochemistry 2013, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Didenko, Y.T.; Pugach, S.P. Spectra of Water Sonoluminescence. J. Phys. Chem. 1994, 98, 9742–9749. [Google Scholar] [CrossRef]
- Ji, R.; Pflieger, R.; Virot, M.; Nikitenko, S.I. Multibubble Sonochemistry and Sonoluminescence at 100 kHz: The Missing Link between Low- and High-Frequency Ultrasound. J. Phys. Chem. B 2018, 122, 6989–6994. [Google Scholar] [CrossRef] [PubMed]
- Luminol. Available online: https://en.wikipedia.org/wiki/Luminol (accessed on 8 January 2020).
- McMurray, H.N.; Wilson, B.P. Mechanistic and spatial study of ultrasonically induced luminol chemiluminescence. J. Phys. Chem. A 1999, 103, 3955–3962. [Google Scholar] [CrossRef]
- Crum, L.A.; Mason, T.J.; Reisse, J., Suslick. Sonochemistry and Sonoluminescence; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1999; pp. 225–246. [Google Scholar]
- Janzen, E.G.; Kotake, Y.; Hinton, R.D. Stabilities of Hydroxyl Radical Spin Adducts of Pbn-Type Spin Traps. Free Radic. Biol. Med. 1992, 12, 169–173. [Google Scholar] [CrossRef]
- Khan, P.; Idrees, D.; Moxley, M.A.; Corbett, J.A.; Ahmad, F.; von Figura, G.; Sly, W.S.; Waheed, A.; Hassan, M.I. Luminol-based chemiluminescent signals: Clinical and non-clinical application and future uses. Appl. Biochem. Biotechnol. 2014, 173, 333–355. [Google Scholar] [CrossRef]
- Electromagnetic Absorption by Water. Available online: https://en.wikipedia.org/wiki/Electromagnetic_absorption_by_water (accessed on 23 February 2020).
- Ashokkumar, M.; Grieser, F. The effect of surface active solutes on bubbles in an acoustic field. Phys. Chem. Chem. Phys. 2007, 9, 5631–5643. [Google Scholar] [CrossRef]
- Rayaroth, M.P.; Aravind, U.K.; Aravindakumar, C.T. Sonochemical degradation of Coomassie Brilliant Blue: Effect of frequency, power density, pH and various additives. Chemosphere 2015, 119, 848–855. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.; Lee, D.; Hong, S.; Khan, S.; Darya, B.; Lee, J.-Y.; Chung, J.; Cho, S.-H. Investigation of the Synergistic Effect of Sonolysis and Photocatalysis of Titanium Dioxide for Organic Dye Degradation. Catalysts 2020, 10, 500. https://doi.org/10.3390/catal10050500
Choi Y, Lee D, Hong S, Khan S, Darya B, Lee J-Y, Chung J, Cho S-H. Investigation of the Synergistic Effect of Sonolysis and Photocatalysis of Titanium Dioxide for Organic Dye Degradation. Catalysts. 2020; 10(5):500. https://doi.org/10.3390/catal10050500
Chicago/Turabian StyleChoi, Yunseok, Daein Lee, Sungje Hong, Sovann Khan, Burak Darya, Jae-Young Lee, Jaewon Chung, and So-Hye Cho. 2020. "Investigation of the Synergistic Effect of Sonolysis and Photocatalysis of Titanium Dioxide for Organic Dye Degradation" Catalysts 10, no. 5: 500. https://doi.org/10.3390/catal10050500
APA StyleChoi, Y., Lee, D., Hong, S., Khan, S., Darya, B., Lee, J.-Y., Chung, J., & Cho, S.-H. (2020). Investigation of the Synergistic Effect of Sonolysis and Photocatalysis of Titanium Dioxide for Organic Dye Degradation. Catalysts, 10(5), 500. https://doi.org/10.3390/catal10050500