Shape-Selective Mesoscale Nanoarchitectures: Preparation and Photocatalytic Performance
Abstract
1. Introduction
2. Results and Discussion
2.1. Designing UV Active Nanophotocatalysts—A Top-Down Approach
2.2. Designing UV-Vis Active Nanophotocatalysts–A Combination of both Bottom-up and Top-down Approaches
2.2.1. Preparation and Characterization of Gold Nanorods - A Bottom up Approach
2.2.2. Self-Assembling of Au Nanorods on the Mesoscale TiO2 and SiO2-TiO2 Pillars
2.3. Photodegradation Studies under UV and Vis Illumination
2.3.1. Photodegradation Studies of TiO2 and SiO2-TiO2 Mesoscale Nanoarchitectures under UV Illumination
2.3.2. Photodegradation Studies of Au-TiO2 and Au-SiO2-TiO2 Mesoscale Nanoarchitectures under UV and Vis Illumination
2.4. Proposed Mechanism
3. Materials and Methods
3.1. Nanoarray Fabrication
3.2. Preparation of Gold Nanorods (AuNRs)
3.3. Functionalization and Characterization
3.3.1. SiO2-TiO2-Coated Cr Arrays
3.3.2. AuNR-Functionalized Post Arrays
3.4. Photodegradation of Methyl Orange
4. Conclusions
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Conflicts of Interest
References
- Isaacoff, B.P.; Brown, K.A. Progress in top-down control of bottom-up assembly. Nano Lett. 2017, 17, 6508–6510. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Bayer, I.S.; Biris, A.S.; Wang, T.; Dervishi, E.; Faupel, F. Advances in top–down and bottom–up surface nanofabrication: Techniques, applications & future prospects. Adv. Colloid Interface Sci. 2012, 170, 2–27. [Google Scholar] [PubMed]
- Tao, C.G.; Cullen, W.G.; Williams, E.D.; Hunyadi, S.E.; Murphy, C.J. Surface morphology and step fluctuations on silver nanowires. Surf. Sci. 2007, 601, 4939–4943. [Google Scholar] [CrossRef]
- Hunyadi Murph, S.E.; Larsen, G.; Lascola, R. Multifunctional hybrid Fe2O3-Au nanoparticles for efficient plasmonic heating. J. Vis. Exp. (JOVE) 2016, 108, e53598. [Google Scholar]
- Hunyadi Murph, S.E.; Jacobs, S.; Siegfried, M.; Hu, T.; Serkiz, S.; Hudson, J. Manganese-doped gold nanoparticles as positive contrast agents for Magnetic Resonance Imaging (MRI). J. Nanopart. Res. 2012, 14, 658–659. [Google Scholar] [CrossRef]
- Hunyadi Murph, S.E.; Lawrence, K.; Sessions, H.; Brown, M.; Larsen, G. Controlled Release of Hydrogen from Hydride-Magnetic Nanomaterials. ACS Appl. Mater. Interfaces 2020, 12, 9478–9488. [Google Scholar] [CrossRef]
- Hunyadi Murph, S.E.; Coopersmith, K. Fabrication of silver-rhodium nanomaterials for chemical sensing applications. In Nanocomposites VI: Nanoscience and Nanotechnology in Advanced Composites; The Minerals, Metals & Materials Series; Srivatsan, T., Gupta, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 95–104. [Google Scholar]
- Hunyadi Murph, S.E. Anisotropic metallic and metallic oxide nanostructures-Correlation between their shape and properties. In Anisotropic and Shape-Selective Nanomaterials: Structure-Property Relationships; Nanostructure Science and Technology series; Springer Publisher: Berlin/Heidelberg, Germany, 2017; pp. 105–151. [Google Scholar]
- Hunyadi Murph, S.E.; Larsen, G.; Coopersmith, K. Anisotropic and Shape-Selective Nanomaterials: Structure-Property Relationships; Nanostructure Science and Technology series; Springer Publisher: Berlin/Heidelberg, Germany, 2017; pp. 1–470. [Google Scholar]
- Mahmoud, M.A. Tunable plasmonic neutral density filters and chromatic polarizers: Highly packed 2D arrays of plasmonic nanoparticle on elastomer substrate. J. Phys. Chem. C 2016, 120, 18249–18258. [Google Scholar] [CrossRef]
- Pang, J.; Xiong, S.; Jaeckel, F.; Sun, Z.; Dunphy, D.; Brinker, C.J. Free-standing, patternable nanoparticle polymer monolayer arrays formed by evaporation induced self-assembly at a fluid interface. J. Am. Chem. Soc. 2008, 130, 3284–3285. [Google Scholar] [CrossRef]
- Johnston-Peck, A.C.; Wang, J.; Tracy, J.B. Formation and grain analysis of spin-cast magnetic nanoparticle monolayers. Langmuir 2011, 27, 5040–5046. [Google Scholar] [CrossRef]
- Hunyadi Murph, S.E.; Serkiz, S.; Fox, E.; Colon-Mercado, H.; Sexton, L.; Siegfried, M. Synthesis, functionalization, characterization and application of controlled shape nanoparticles in energy production, Fluorine-Related Nanoscience with Energy Applications. ACS Symp. Ser. 2011, 1064, 127–163. [Google Scholar]
- Namdari, N.; Mohammadian, B.; Jafari, P.; Sojoudi, H.; Ghasemi, H.; Rizvi, R. Advanced functional surfaces through controlled damage and instabilities. Mater. Horiz. 2020, 7, 366–396. [Google Scholar] [CrossRef]
- Nebel, R.; Minhová Macounová, K.; Tarábková, H.; Kavan, L.; Krtil, P.J. Selectivity of Photoelectrochemical Water Splitting on TiO2 Anatase Single Crystals. Phys. Chem. C 2019, 123, 10857–10867. [Google Scholar]
- Kun, Y.; Basnet, P.; Sessions, H.; Larsen, G.; Hunyadi Murph, S.E.; Zhao, Y. Fe2O3/TiO2 Core-Shell Nanorod Array for Visible Light Photocatalysis. Catal. Today Spec. Issue C1 Catal. Chem. 2016, 270, 51–58. [Google Scholar]
- Hunyadi Murph, S.E.; Larsen, G.K.; Korinko, P.; Coopersmith, K.J.; Summer, A.J.; Lewis, R. Nanoparticle Treated Stainless Steel Filters for Metal Vapor Sequestration. JOM 2017, 69, 162–172. [Google Scholar] [CrossRef]
- Hunyadi Murph, S.E.; Heroux, K.; Turick, C.; Thomas, D. Metallic and Hybrid Nanostructures: Fundamentals and Applications. In Applications of Nanomaterials 2012, Series ISBN: 1-62699-000-X, Nanomaterials and Nanostructures, Volume (4); Govil, J.N., Ed.; Studium Press LLC: Houston, TX, USA, 2012; ISBN 1-62699-004-2. [Google Scholar]
- Sułek, A.; Pucelik, B.; Kuncewicz, J.; Dubin, G.; Janusz, M. Dąbrowski Sensitization of TiO2 by halogenated porphyrin derivatives for visible light biomedical and environmental photocatalysis. Catal. Today 2019, 335, 538–549. [Google Scholar] [CrossRef]
- Tian, C.; Li, W.; Pan, K.; Zhang, Q.; Tian, G.; Zhou, W.; Fu, H. One pot synthesis of Ag nanoparticle modified ZnO microspheres in ethylene glycol medium and their enhanced photocatalytic performance. J. Solid State Chem. 2010, 183, 2720–2725. [Google Scholar] [CrossRef]
- Ramesha, G.K.; Brennecke, J.F.; Kamat, P.V. Origin of Catalytic Effect in the Reduction of CO2 at Nanostructured TiO2 Films. ACS Catal. 2014, 4, 3249–3254. [Google Scholar] [CrossRef]
- Ji, S.M.; Jun, H.; Jang, J.S.; Son, H.C.; Borse, P.H.; Lee, J.S. Photocatalytic hydrogen production from natural seawater. J. Photochem. Photobiol. A Chem. 2007, 189, 141–144. [Google Scholar] [CrossRef]
- Justicia, I.; Garcia, G.; Battiston, G.A.; Gerbasi, R.; Ager, F.; Guerra, M.; Caixach, J.; Pardo, J.A.; Riverad, J.; Figueras, A. Photocatalysis in the visible range of sub-stoichiometric anatase films prepared by MOCVD. Electrochim. Acta 2005, 50, 4605–4608. [Google Scholar] [CrossRef]
- Hunyadi Murph, S.E. One-Dimensional Plasmonic Nano-photocatalysts: Synthesis, Characterization and Photocatalytic Activity Solar Hydrogen and Nanotechnology VI, edited by Yasuhiro Tachibana. Proc. SPIE 2011, 8109, 1–11. [Google Scholar]
- Li, D.; Seaman, J.; Hunyadi Murph, S.E.; Kaplan, D.; Taylor-Pashow, T.; Feng, R.; Chang, H.; Tandukar, M. Porous iron material for TcO4- and ReO4- sequestration from groundwater under ambient oxic conditions. J. Hazard. Mater. 2019, 374, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Zallen, R.; Moret, M.P. The optical absorption edge of brookite TiO2. Solid State Commun. 2006, 137, 154–157. [Google Scholar] [CrossRef]
- Liu, G.; Wang, L.; Yang, H.G.; Cheng, H.M.; Lu, G.Q. Titania-based photocatalysts - crystal growth, doping and heterostructuring. J. Mater. Chem. 2010, 20, 831–843. [Google Scholar] [CrossRef]
- Jogi, I.; Pars, M.; Aarik, J.; Aidla, A.; Laan, M.; Sundqvist, J.; Oberbeck, L.; Heitmann, J.; Kukli, K. Conformity and structure of titanium oxide films grown by atomic layer deposition on silicon substrates. Thin Solid Films 2008, 516, 4855–4862. [Google Scholar] [CrossRef]
- Matthews, A. The crystallization of anatase and rutile from amorphous titanium dioxide under hydrothermal conditions. Am. Mineral. 1976, 61, 419–424. [Google Scholar]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Hurum, D.C.; Agrios, A.G.; Crist, S.E.; Gray, K.A.; Rajh, T.; Thurauer, M.C. Probing reaction mechanisms in mixed phase TiO2 by EPR. J. Electron. Spectrosc. 2006, 150, 155–163. [Google Scholar] [CrossRef]
- Wu, N.; Wang, J.; Tafen, D.N.; Wang, H.; Zheng, J.-G.; Lewis, J.P.; Liu, X.; Leonard, S.S.; Manivannan, A. Shape-Enhanced Photocatalytic Activity of Single-Crystalline Anatase TiO2 (101) Nanobelts. J. Am. Chem. Soc. 2010, 132, 6679–6685. [Google Scholar] [CrossRef]
- Hotsenpiller, P.A.M.; Bolt, J.D.; Farneth, W.E.; Lowekamp, J.B.; Rohrer, G.S. Orientation Dependence of Photochemical Reactions on TiO2 Surfaces. J. Phys. Chem. B 1998, 102, 3216–3226. [Google Scholar] [CrossRef]
- Paramasivam, I.; Macak, J.M.; Schmuki, P. Photocatalytic activity of TiO2 nanotube layers loaded with Ag and Au nanoparticles. Electrochem. Commun. 2008, 10, 71–75. [Google Scholar] [CrossRef]
- Fauzi, A.A.; Jalil, A.A.; Mohamed, M.; Triwahyono, S.; Jusoh, N.W.C.; Rahman, A.F.A.; Aziz, F.F.A.; Hassan, N.S.; Khusnun, N.F.; Tanaka, H. Altering fiber density of cockscomb-like fibrous silica-titania catalysts for enhanced photodegradation of ibuprofen. J. Envirom. Manag. 2018, 227, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Sirimahachai, U.; Ndiege, N.; Chandrasekharan, R.; Wongnawa, S.; Shannon, M.A. Nanosized TiO2 particles decorated on SiO2 spheres (TiO2/SiO2): Synthesis and photocatalytic activities. J. Sol-gel Sci. Technol. 2010, 56, 53–60. [Google Scholar] [CrossRef]
- He, Y.; Basnet, P.; Hunyadi Murph, S.E.; Zhao, Y. Ag Nanoparticle Embedded TiO2 Composite Nanorod Arrays Fabricated by Oblique Angle Deposition: Toward Plasmonic Photocatalysis. ACS Appl. Mater. Interfaces 2013, 5, 11818–11827. [Google Scholar] [CrossRef] [PubMed]
- Hunyadi Murph, S.E.; Murphy, C.J. Patchy Silica-Coated Silver Nanowires as SERS Substrates. J. Nanopart. Res. 2013, 15, 1607. [Google Scholar] [CrossRef][Green Version]
- Martin, N.; Rousselot, V.; Rondot, D.; Palmino, F.; Mercier, R. Microstructure modification of amorphous titanium oxide thin films during annealing treatment. Thin Solid Films 1997, 300, 113–121. [Google Scholar] [CrossRef]
- Dai, S.; Wu, S.; Sakai, T.; Du, Z.; Sakai, H.; Abe, M. Preparation of Highly Crystalline TiO2 Nanostructures by Acid-assisted Hydrothermal Treatment of Hexagonal structured Nanocrystalline Titania/Cetyltrimethyammonium Bromide Nanoskeleton. Nanoscale Res. Lett. 2010, 5, 1829–1835. [Google Scholar] [CrossRef]
- Russell, J.L.; Mallouk, T.E. Double Replication of Silica Colloidal Crystal Films. ACS Appl. Mater. Interfaces 2017, 9, 42075–42083. [Google Scholar] [CrossRef]
- Santara, B.; Giri1, P.K.; Imakita, K.; Fujii, M. Microscopic origin of lattice contraction and expansion in undoped rutile TiO2 nanostructures. J. Phys. D Appl. Phys. 2014, 47, 215302. [Google Scholar] [CrossRef]
- Zhang, H.; Chena, B.; Banfield, J.B. The size dependence of the surface free energy of titania nanocrystals Phys. Chem. Chem. Phys. 2009, 11, 2553–2558. [Google Scholar] [CrossRef]
- Tanemura, S.; Miao, L.; Wunderlich, W.; Tanemura, M.; Mori, Y.; Toh, S.; Kaneko, K. Fabrication and characterization of anatase/rutile–TiO2 thin films by magnetron sputtering: A review. Sci. Technol. Adv. Mater. 2005, 6, 11–17. [Google Scholar] [CrossRef]
- Hunyadi Murph, S.E.; Murphy, C.; Colon-Mercado, H.; Torres, R.; Heroux, K.; Fox, E.; Thompson, L.; Haasch, R. Tuning of size and shape of Au-Pt nanocatalyst for direct methanol fuel cells. J. Nanopart. Res. 2011, 13, 6347–6364. [Google Scholar] [CrossRef]
- Larsen, G.; Farr, W.; Hunyadi Murph, S.E. Multifunctional Fe2O3-Au Nanoparticles with Different Shapes: Enhanced Catalysis, Photothermal Effects, and Magnetic Recyclability. J. Phys. Chem. C 2016, 120, 15162–15172. [Google Scholar] [CrossRef]
- Mazinani, B.; Masrom, A.K.; Beitollahi, A.; Luque, R. Photocatalytic activity, surface area and phase modification of mesoporous SiO2–TiO2 prepared by a one-step hydrothermal procedure. Ceram. Int. 2014, 40, 11525–11532. [Google Scholar] [CrossRef]
- Chen, H.S.; Huang, S.H.; Perng, T.P. Preparation and Characterization of Molecularly Homogeneous Silica–Titania Film by Sol–Gel Process with Different Synthetic Strategies. ACS Appl. Mater. Interfaces 2012, 4, 5188–5195. [Google Scholar] [CrossRef] [PubMed]
- Hunyadi, S.E.; Murphy, C.J. Tunable One-Dimensional Silver-Silica Nanopeapod Architectures. J. Phys. Chem. B 2006, 110, 7226–7231. [Google Scholar] [CrossRef]
- Jitputti, J.; Suzuki, Y.; Yoshikawa, S. Synthesis of TiO2 nanowires and their photocatalytic activity for hydrogen evolution. Catal. Commun. 2008, 9, 1265–1271. [Google Scholar] [CrossRef]
- Al-Ekabi, H.; Serpone, N. Kinetic Studies in Heterogeneous Photocatalysis. 1. Photocatalytic Degradation of Chlorinated Phenols in Aerated Aqueous Solutions over TiO2 Supported on a Glass Matrix. J. Phys. Chem. 1998, 92, 5726. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C. Herrmann, Photocatalytic degradation pathway of methylene blue in water. J. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Davis, R.J.; Liu, Z. Titania-Silica: A Model Binary Oxide Catalyst System. Chem. Mater. 1997, 9, 2311–2324. [Google Scholar] [CrossRef]
- Dohshi, S.; Takeuchi, M.; Anpo, M. Effect of the local structure of Ti-oxide species on the photocatalytic reactivity and photo-induced super-hydrophilic properties of Ti/Si and Ti/B binary oxide thin films. Catal. Today 2003, 85, 199–206. [Google Scholar] [CrossRef]
- Nilchia, A.; Janitabar-Darzia, S.; Mahjoubb, A.R.; Rasouli-Garmarodia, S. New TiO2/SiO2 nanocomposites - Phase transformations and photocatalytic studies. Colloids Surf. A Physicochem. Eng. Asp. 2010, 361, 25–30. [Google Scholar] [CrossRef]
- Sang, Y.; Liu, H.; Umar, A. Photocatalysis from UV/Vis to near-infrared light: Towards full solar-light spectrum activity. Chem. Cat. Chem. 2015, 7, 559–573. [Google Scholar]
- Pradhan, S.K.; Mao, Y.; Wong, S.S.; Chupas, P.; Petkov, V. Atomic-Scale Structure of Nanosized Titania and Titanate: Particles, Wires, and Tubes. Chem. Mater. 2007, 19, 6180–6186. [Google Scholar] [CrossRef]
- Wu, T.; Liu, G.; Zhao, J.; Hidaka, H.; Serpone, N. Photoassisted degradation of dye pollutants. V. Self-photosensitized oxidative transformation of Rhodamine B under visible light irradiation in aqueous TiO2 dispersions. J. Phys. Chem. B 1998, 102, 5845–5851. [Google Scholar] [CrossRef]
- Chatterjee, D.; Patnam, V.R.; Sikdar, A.; Joshi, P.; Misra, R.; Rao, N.N. Kinetics of the decoloration of reactive dyes over visible light-irradiated TiO2 semiconductor photocatalyst. J. Hazard. Mater. 2008, 156, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Hunyadi Murph, S.E.; Coopersmith, K.; Larsen, G. Nanoscale Materials: Fundamentals and Emergent Properties. In Anisotropic and Shape-Selective Nanomaterials: Structure-Property Relationships; Nanostructure Science and Technology series; Springer Publisher: Berlin/Heidelberg, Germany, 2017; pp. 7–28. [Google Scholar]


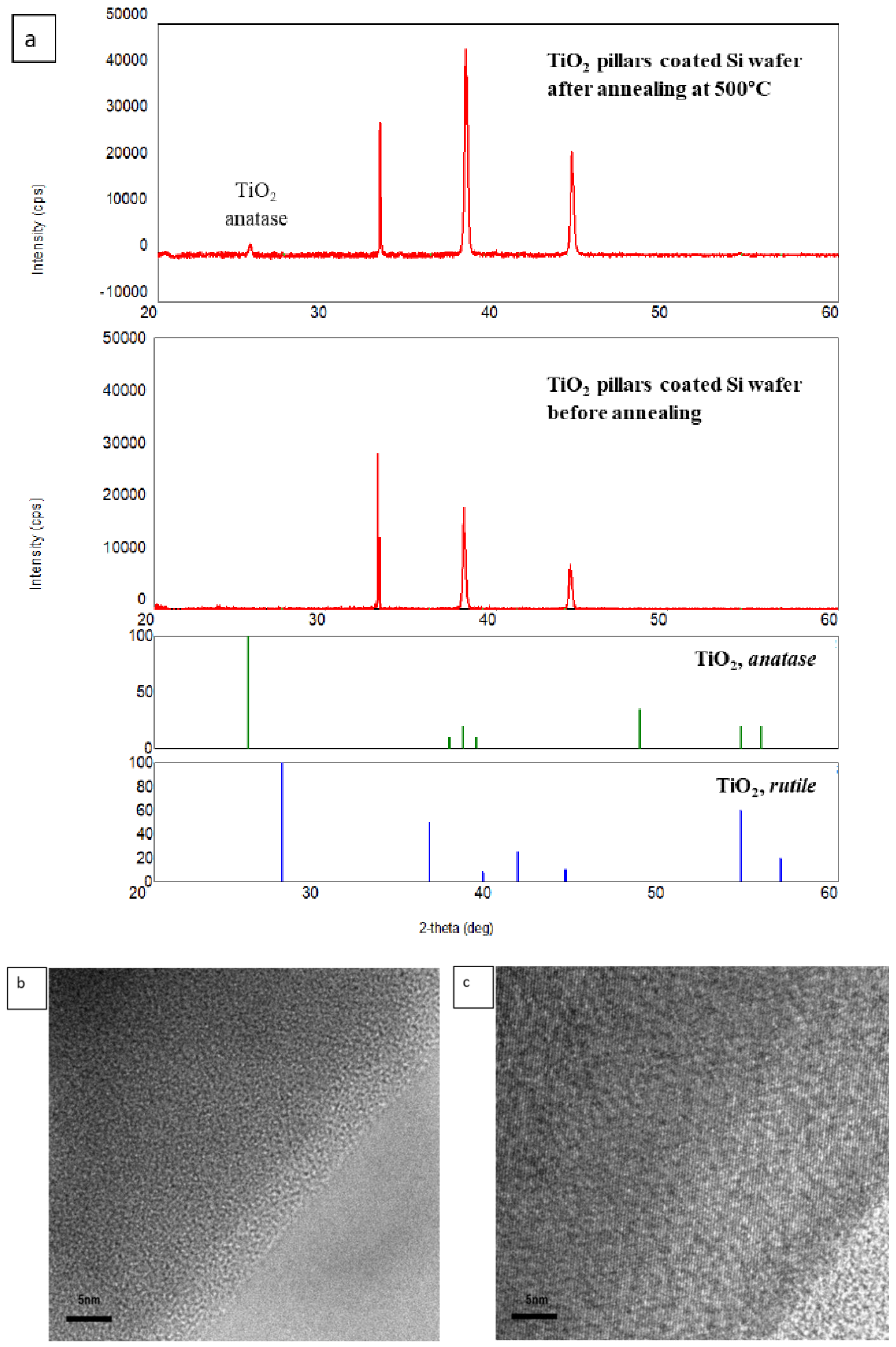

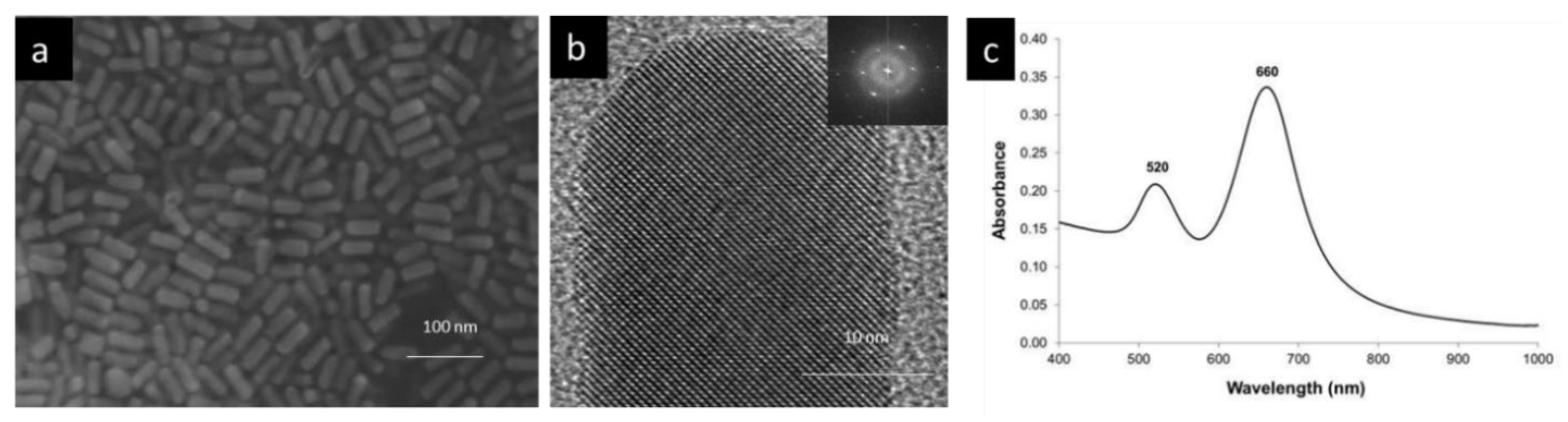





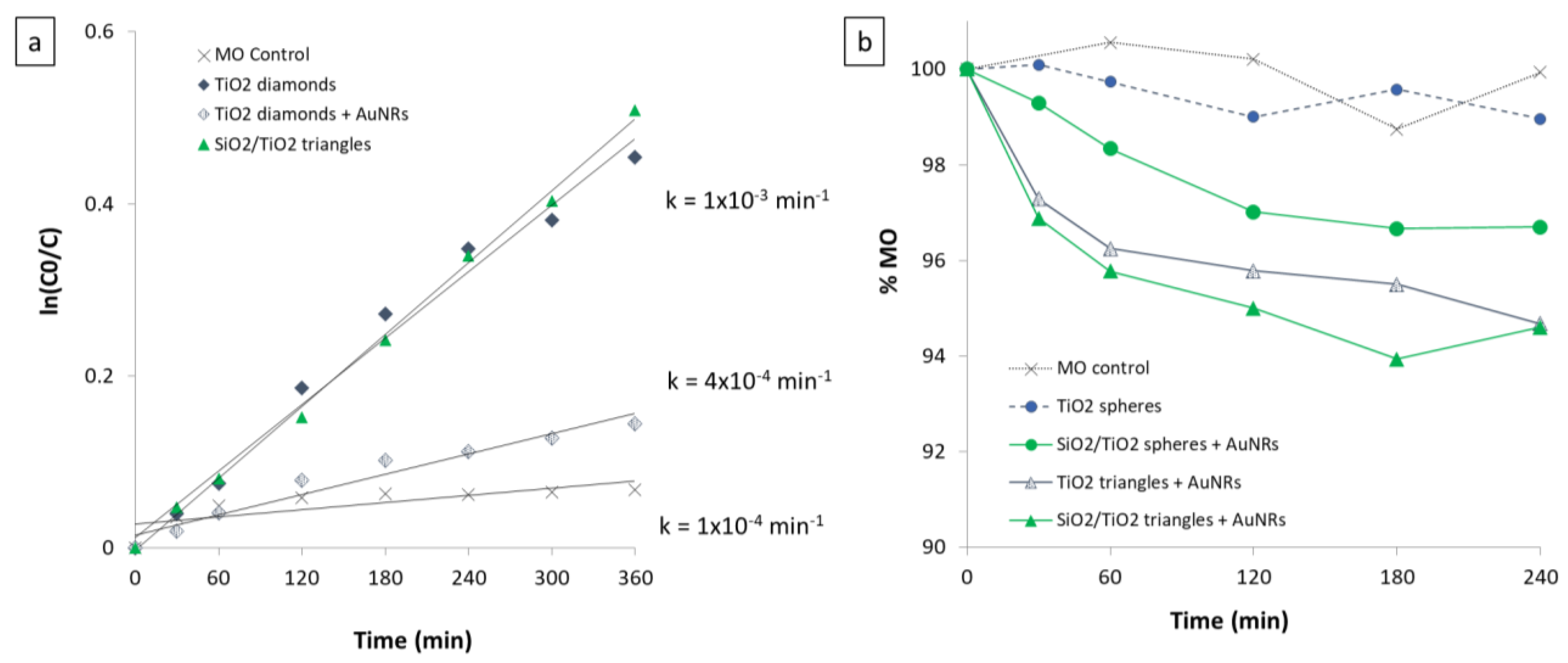
| - | TiO2-Coated | SiO2-TiO2-Coated |
|---|---|---|
| Spheres | Diameter: 329 (± 11) nm | Diameter: 325 (± 3) nm |
| Diamonds | L: 284 (± 7) nm Diagonal: 306 (± 3) nm | L: 272 (± 6) nm Diagonal: 325 (± 8) nm |
| Squares | L: 272 (± 7) nm | L: 289 (± 5) nm |
| Triangles | L: 237 (± 5) nm | L: 246 (± 5) nm |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hunyadi Murph, S.E.; Heruox, K. Shape-Selective Mesoscale Nanoarchitectures: Preparation and Photocatalytic Performance. Catalysts 2020, 10, 532. https://doi.org/10.3390/catal10050532
Hunyadi Murph SE, Heruox K. Shape-Selective Mesoscale Nanoarchitectures: Preparation and Photocatalytic Performance. Catalysts. 2020; 10(5):532. https://doi.org/10.3390/catal10050532
Chicago/Turabian StyleHunyadi Murph, Simona E., and Katie Heruox. 2020. "Shape-Selective Mesoscale Nanoarchitectures: Preparation and Photocatalytic Performance" Catalysts 10, no. 5: 532. https://doi.org/10.3390/catal10050532
APA StyleHunyadi Murph, S. E., & Heruox, K. (2020). Shape-Selective Mesoscale Nanoarchitectures: Preparation and Photocatalytic Performance. Catalysts, 10(5), 532. https://doi.org/10.3390/catal10050532






