Ni-Based Complexes in Selective Ethylene Oligomerization Processes
Abstract
1. Introduction
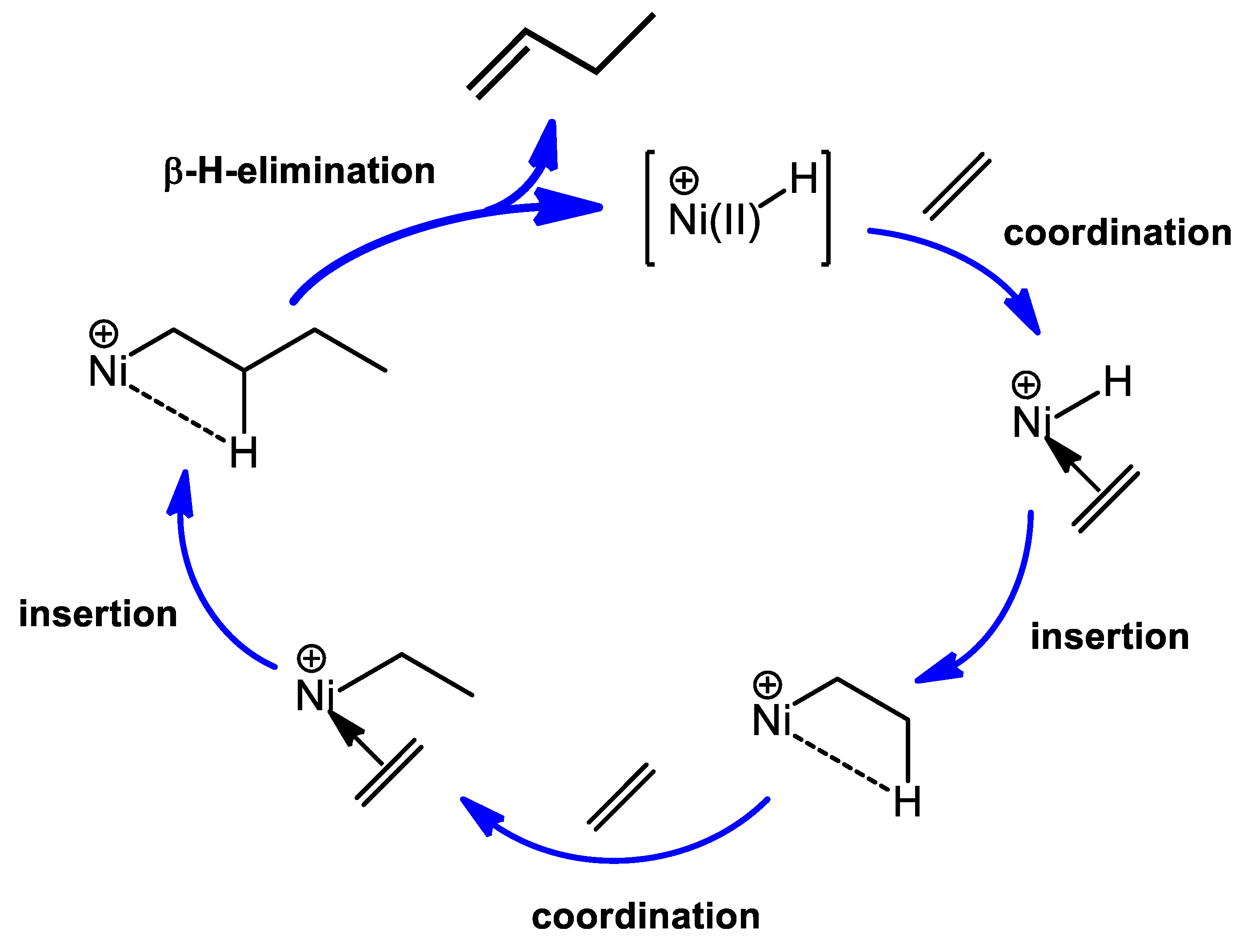
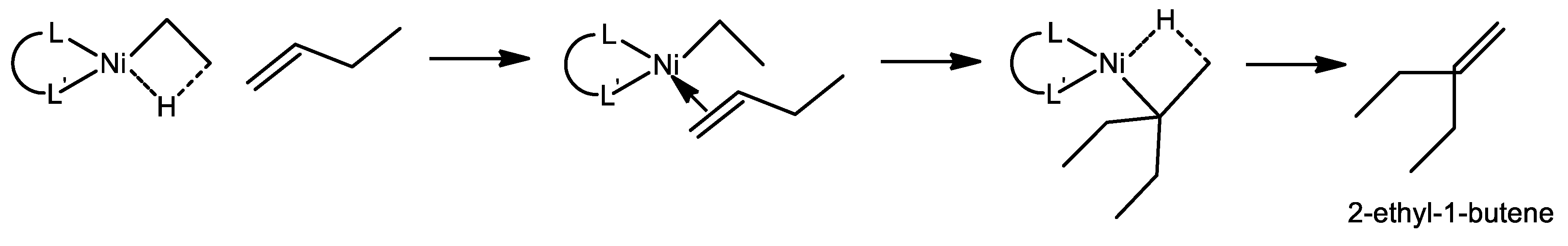
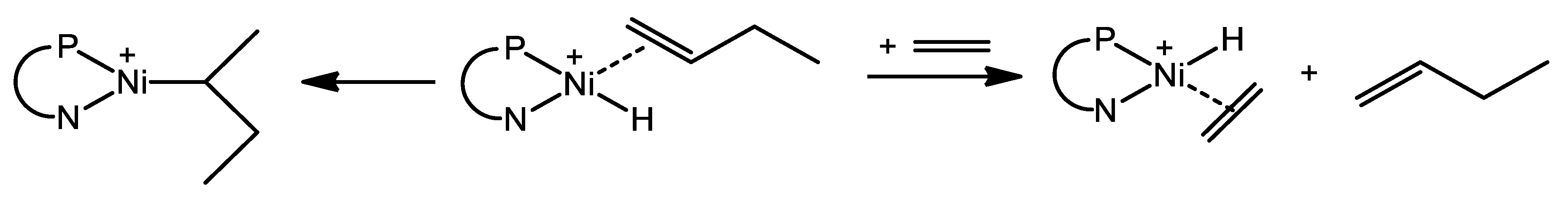
2. The Mechanism of Ethylene Oligomerization at the Ni-Sites
3. Nickel Complexes Bearing Imine Ligands
3.1. Nickel Complexes Bearing Bidentate Ligands
3.1.1. Nickel Complexes Bearing N,N-Ligands
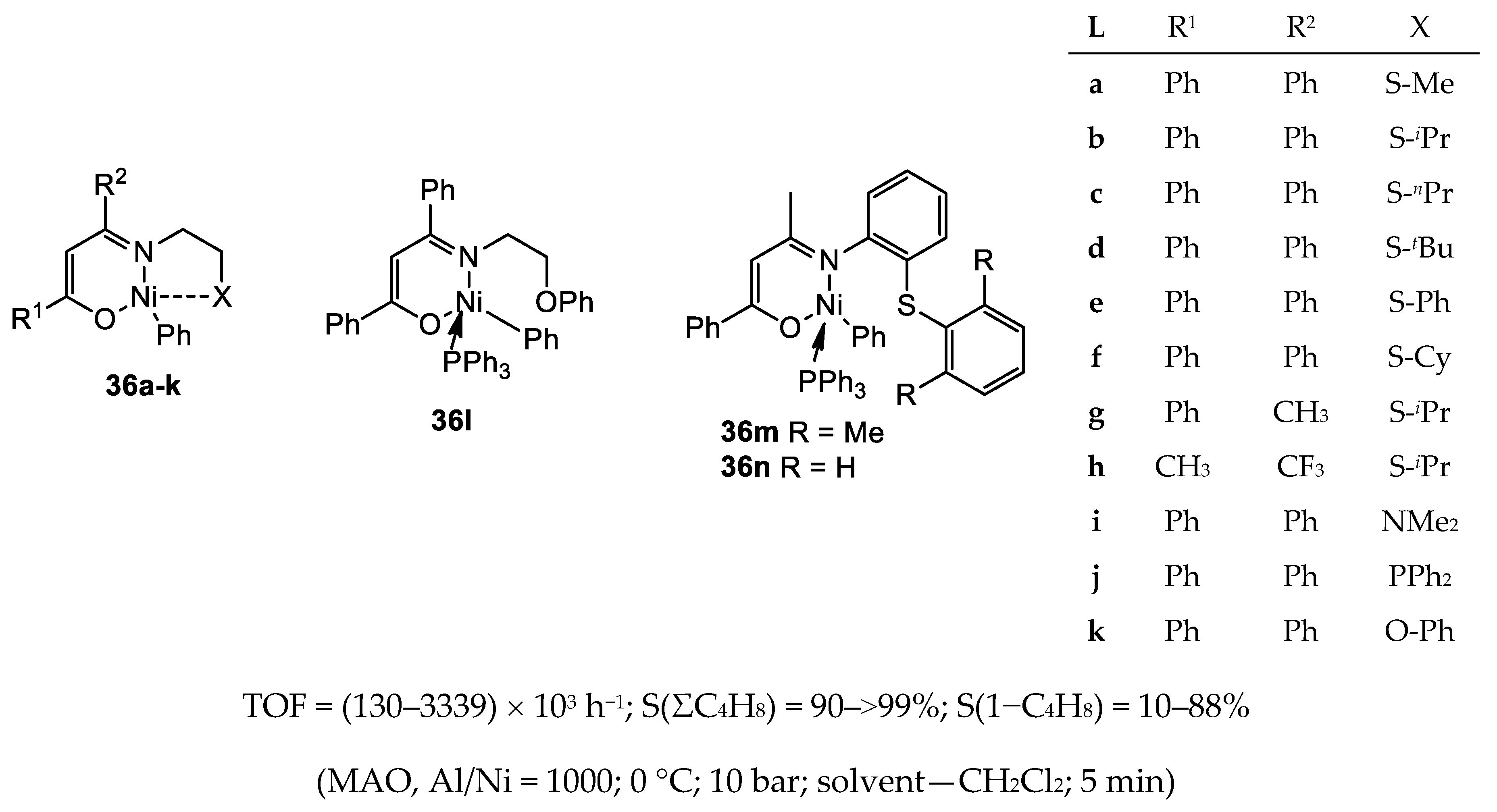
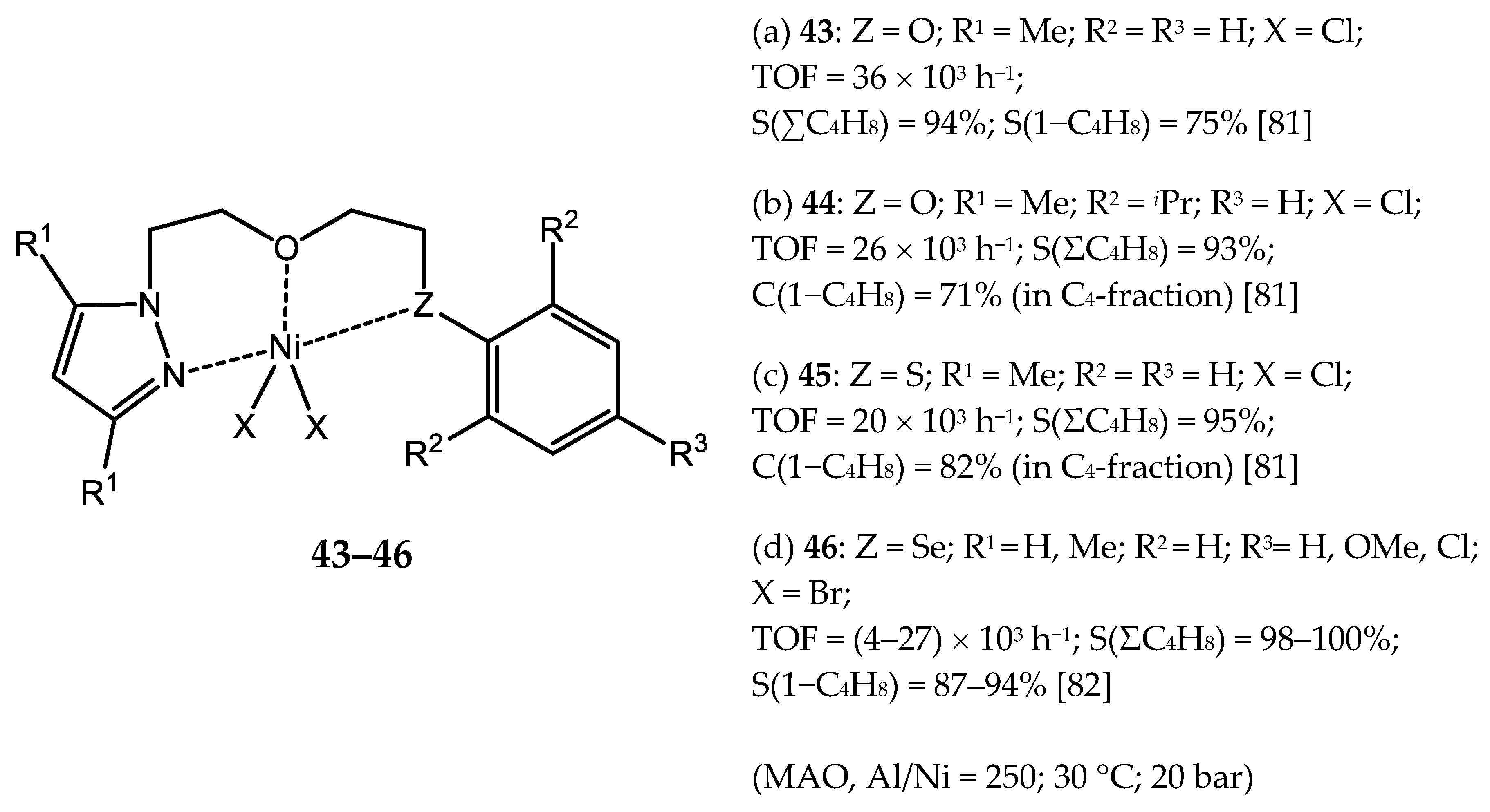
3.1.2. Nickel Complexes Bearing N,O- and N,S-Ligands
3.2. Nickel Complexes Bearing Tridentate Imine Ligands
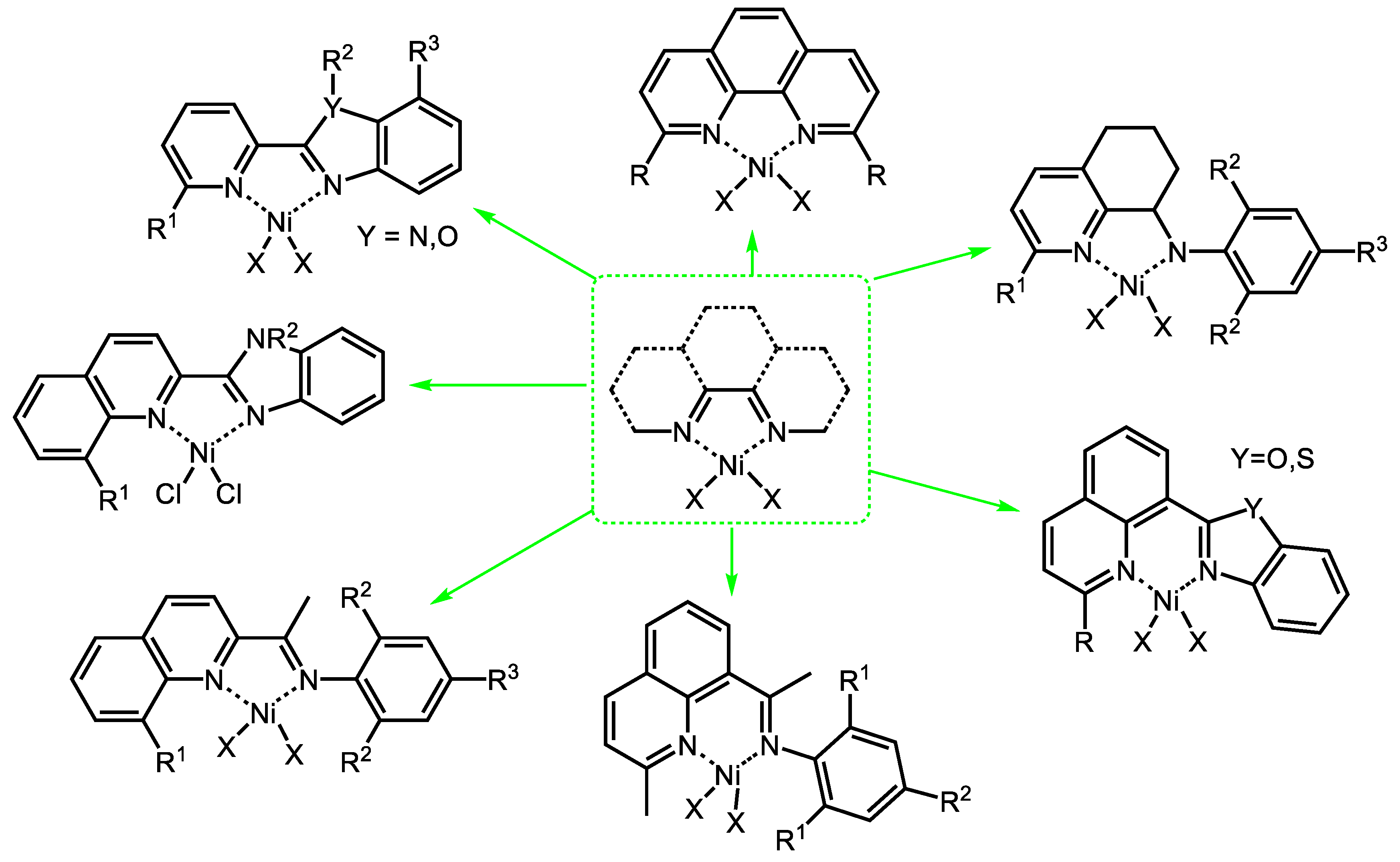
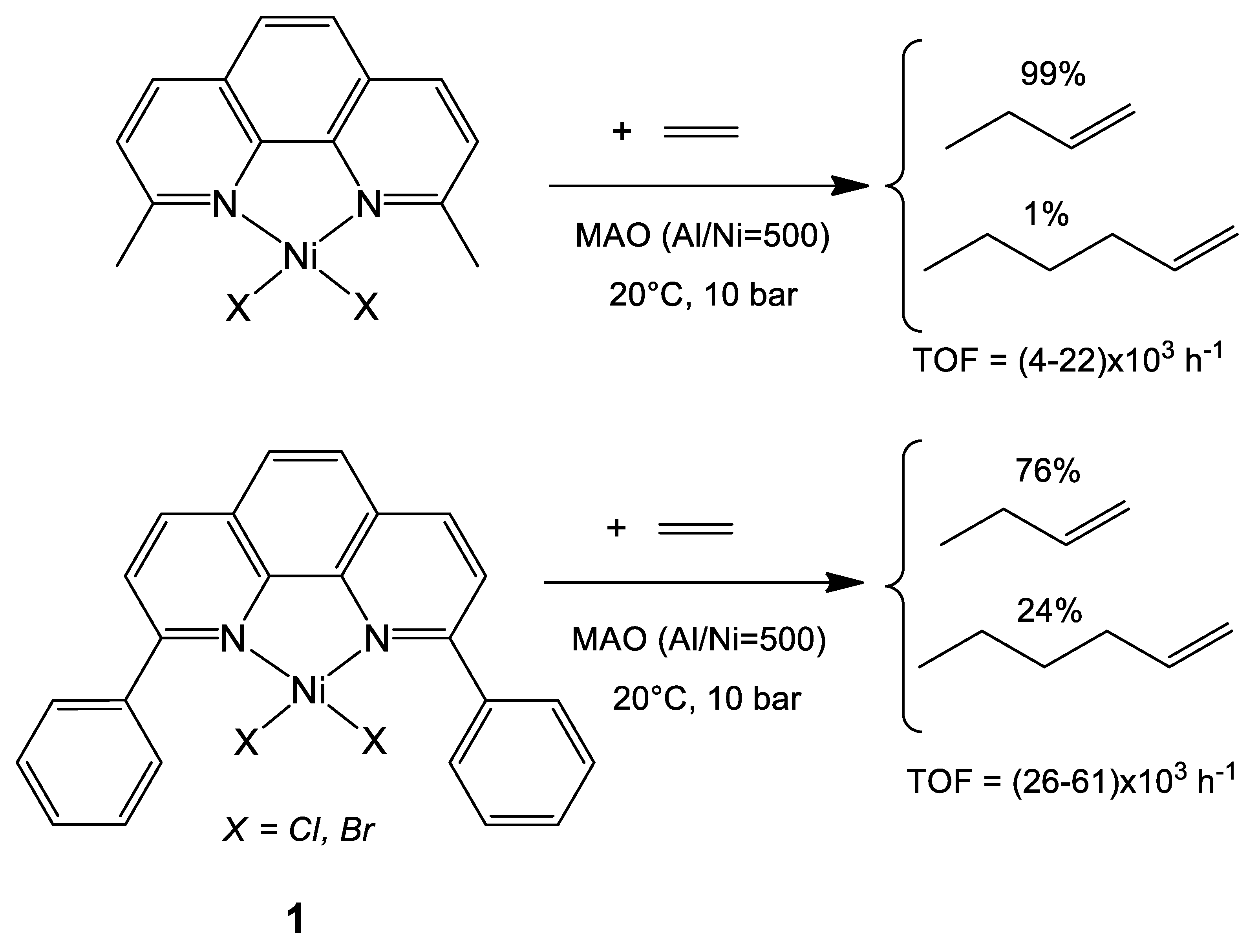
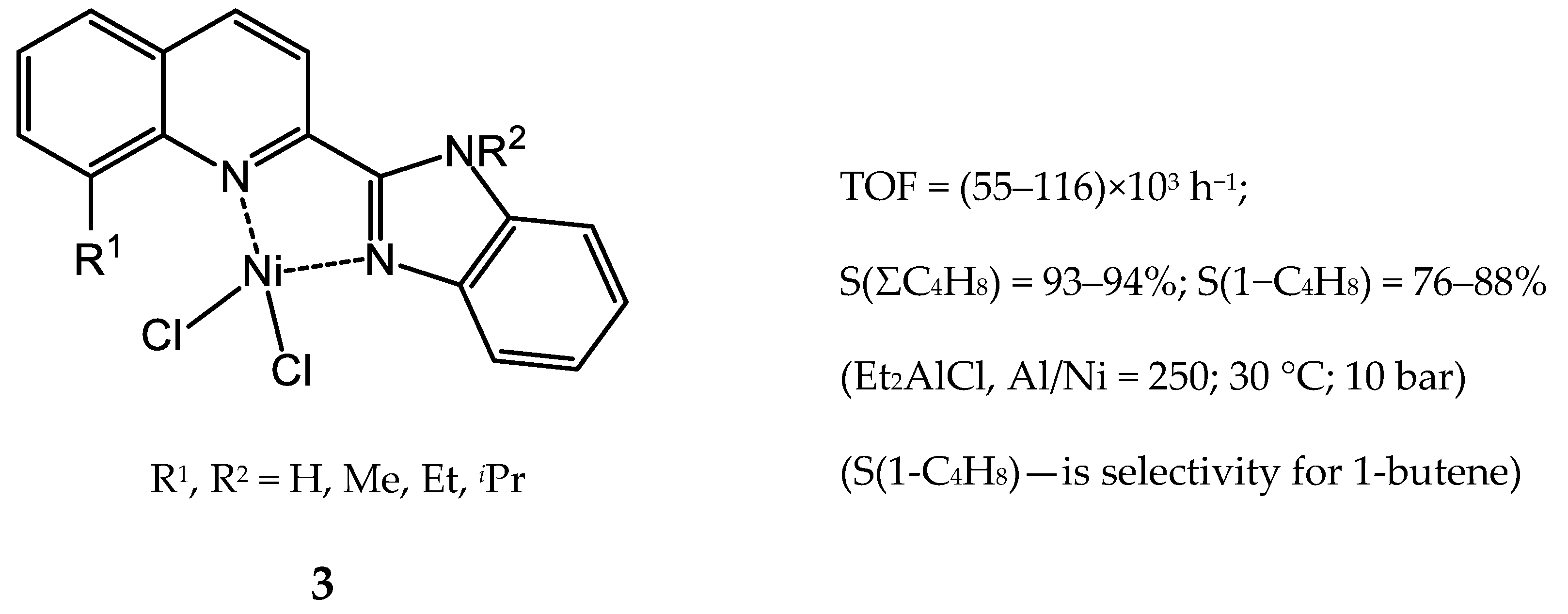
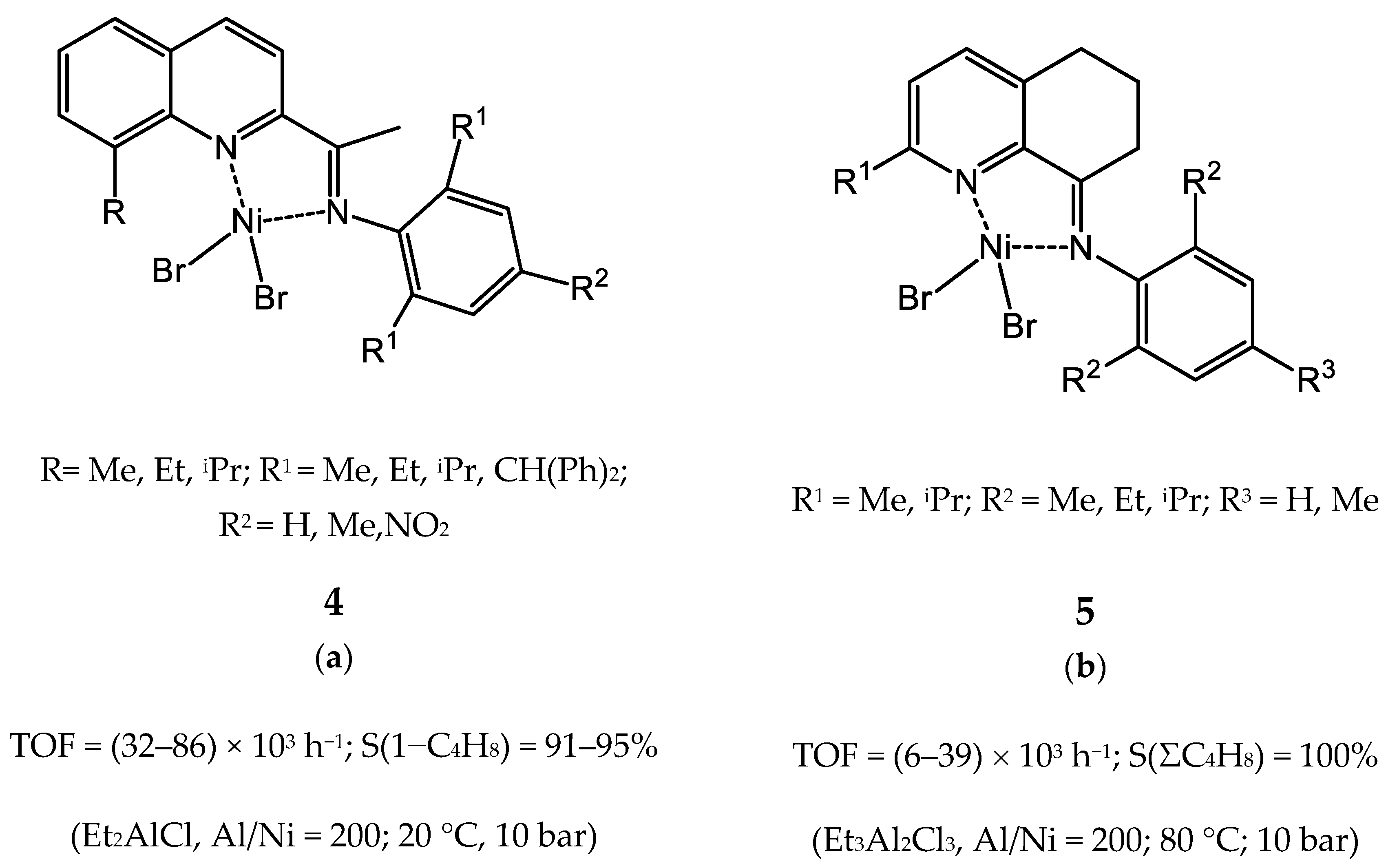
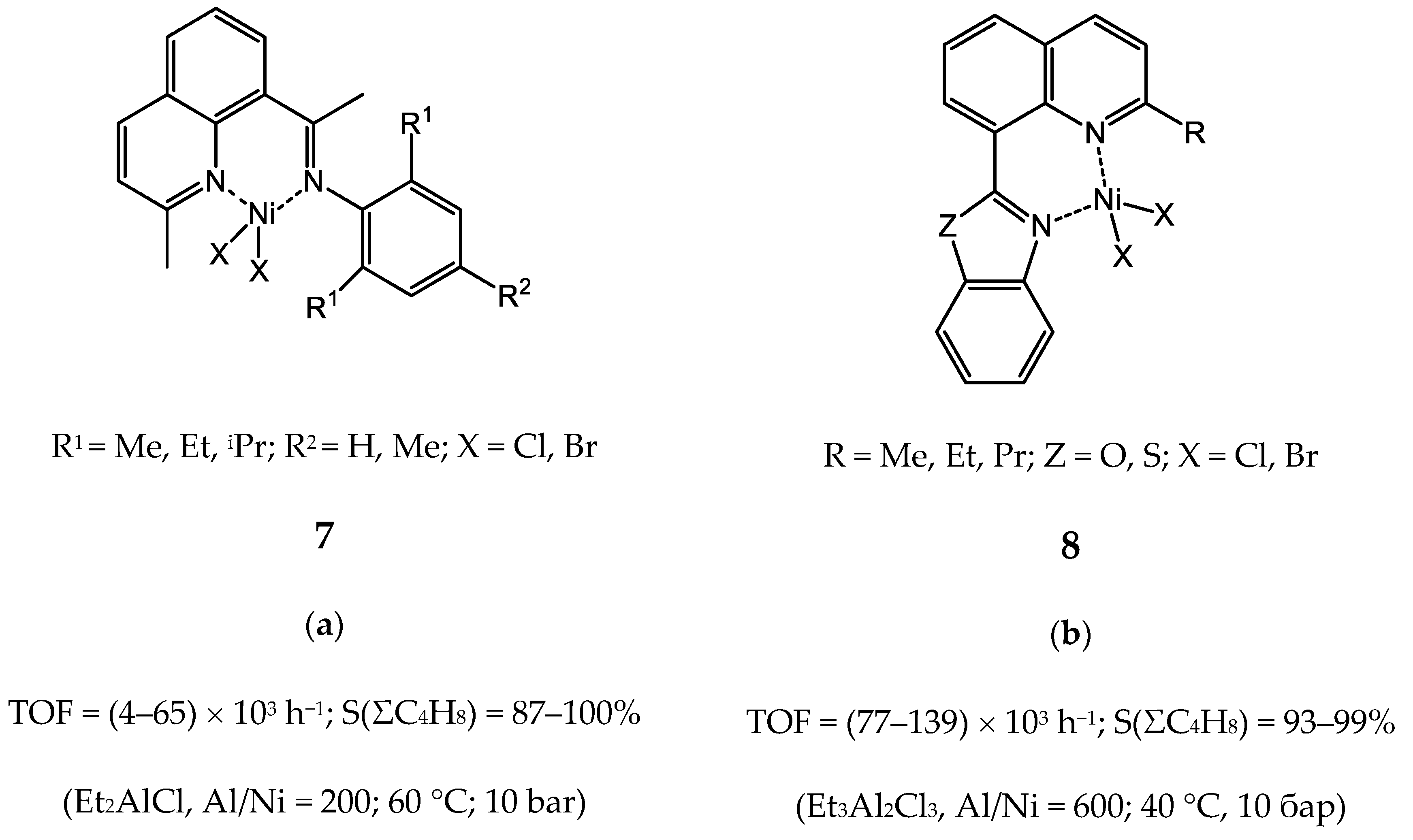
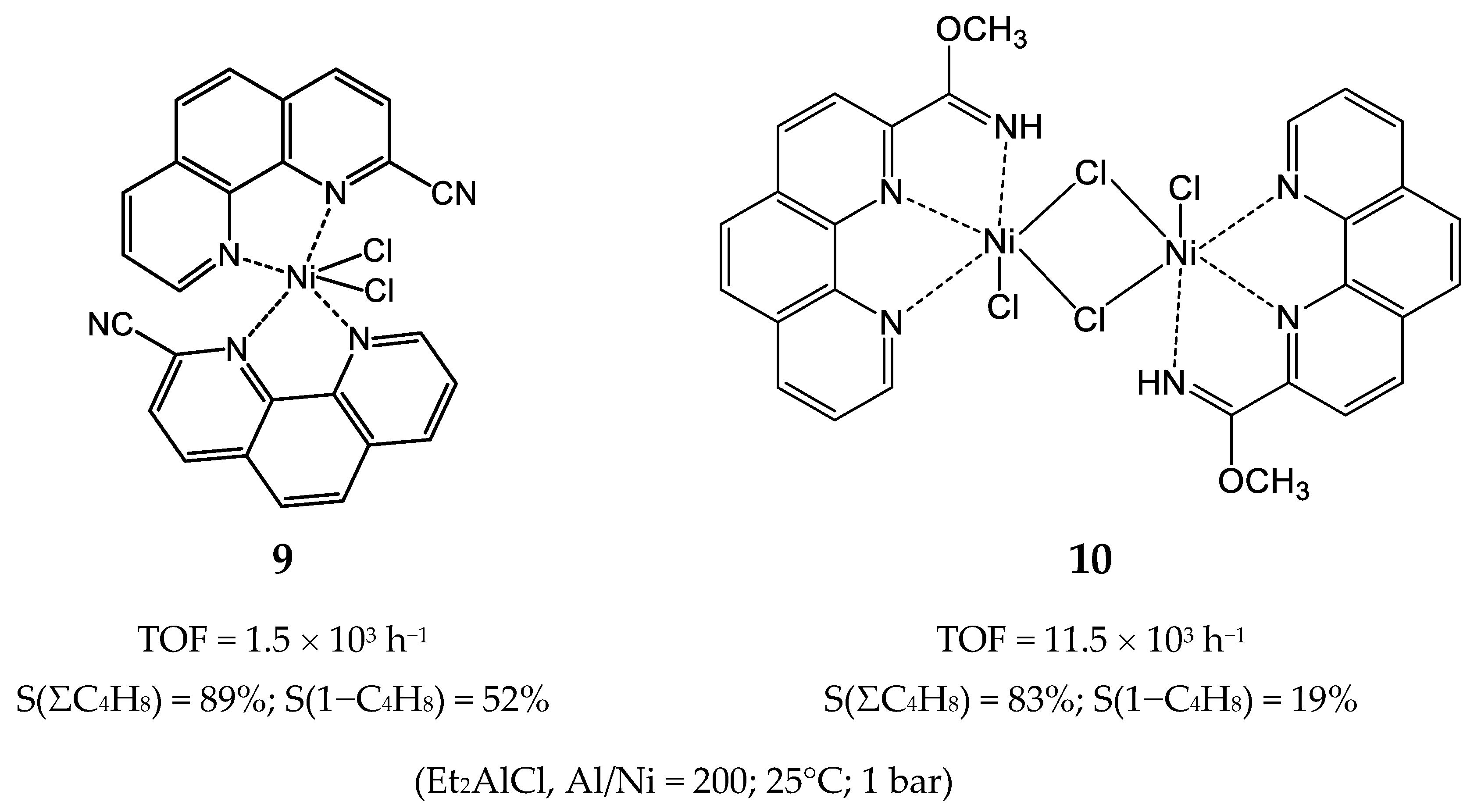
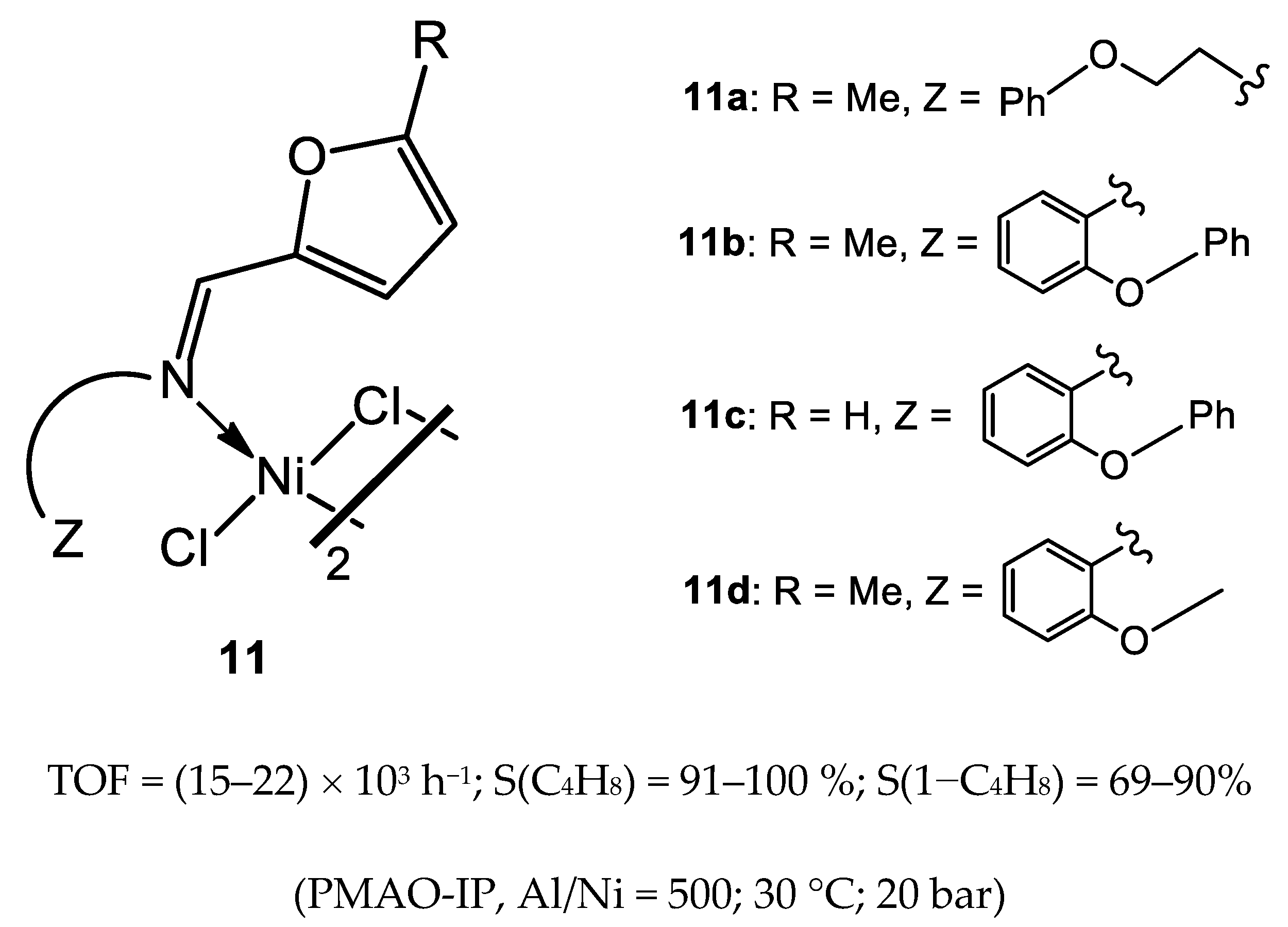
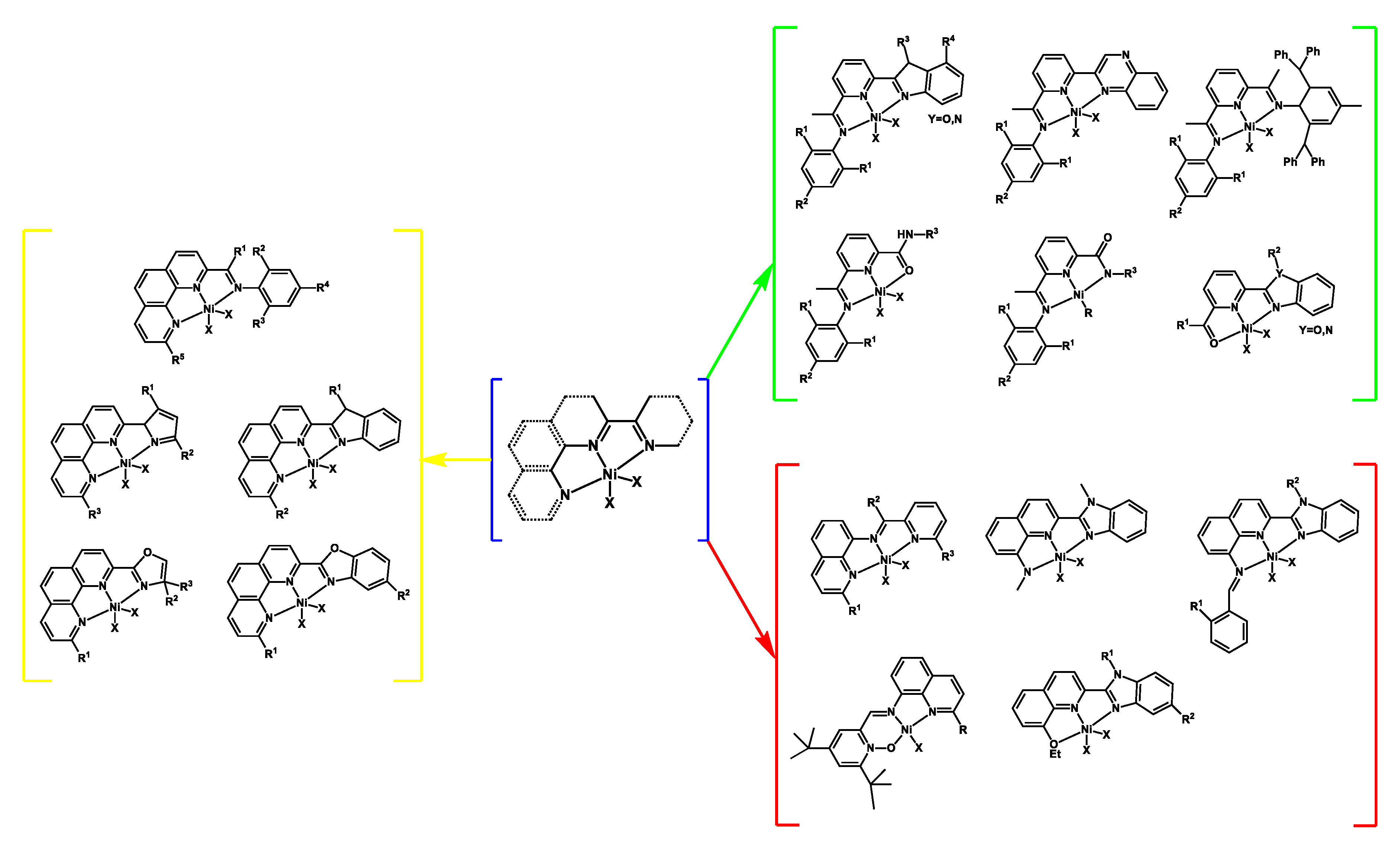
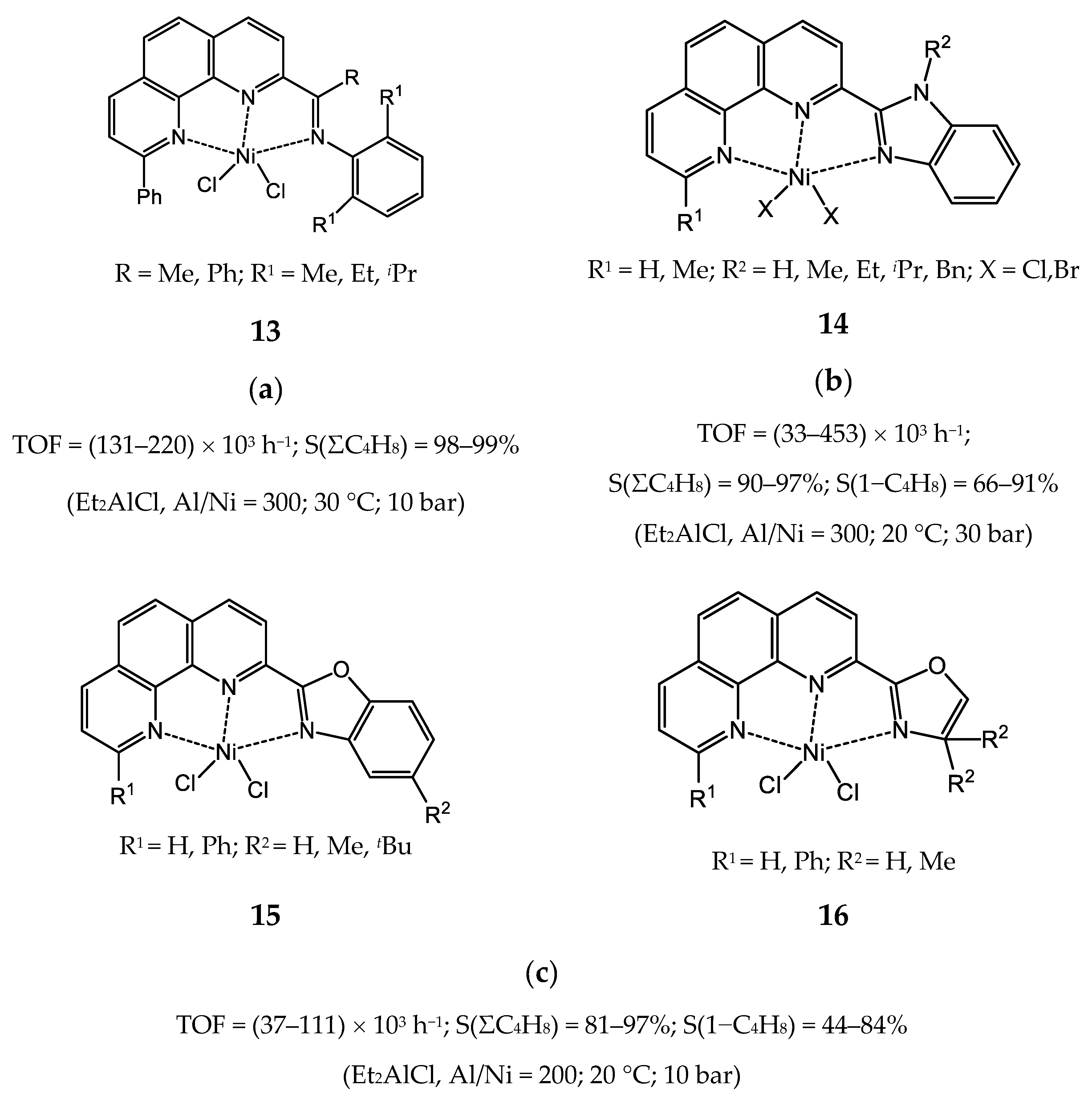
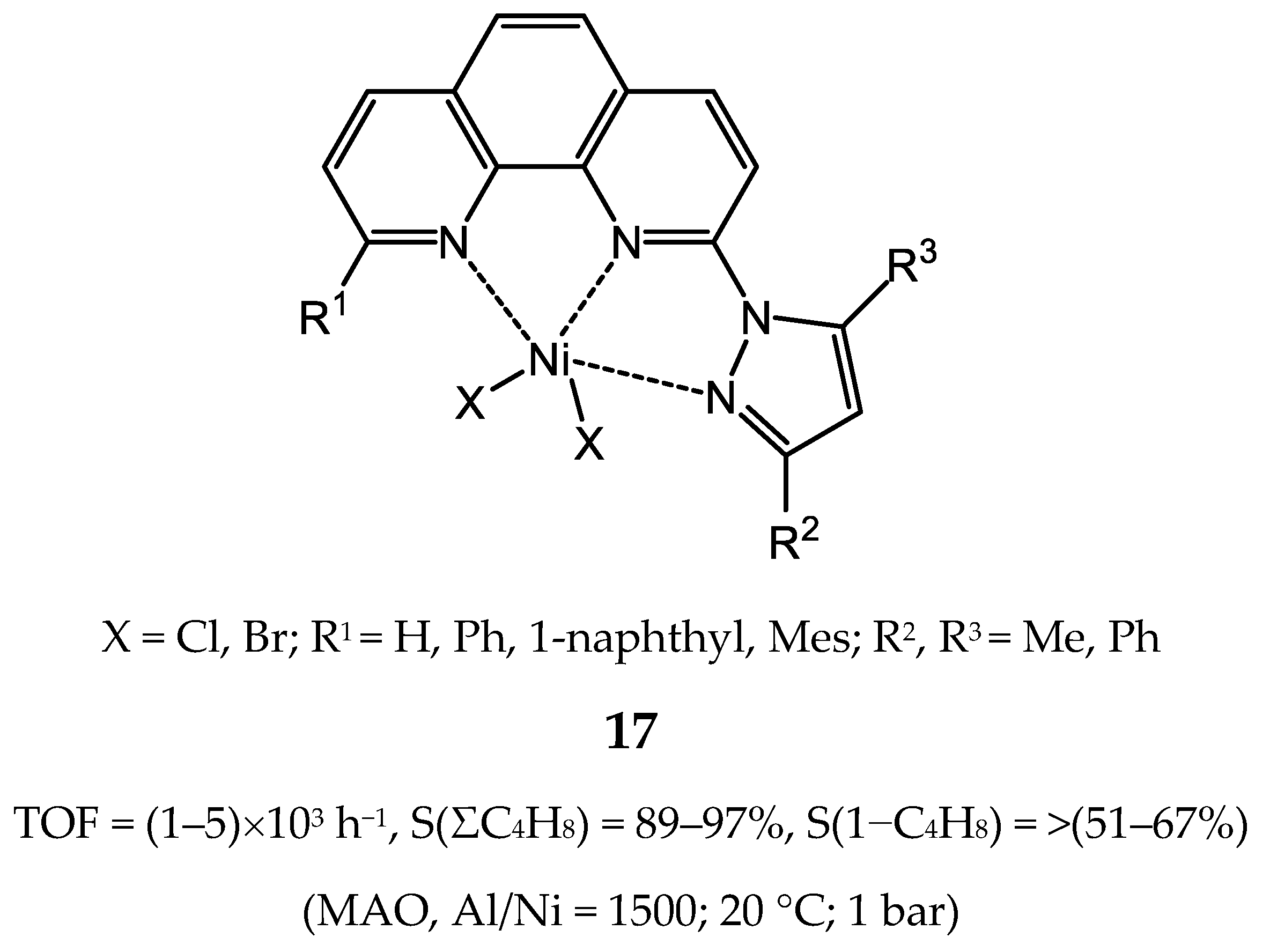
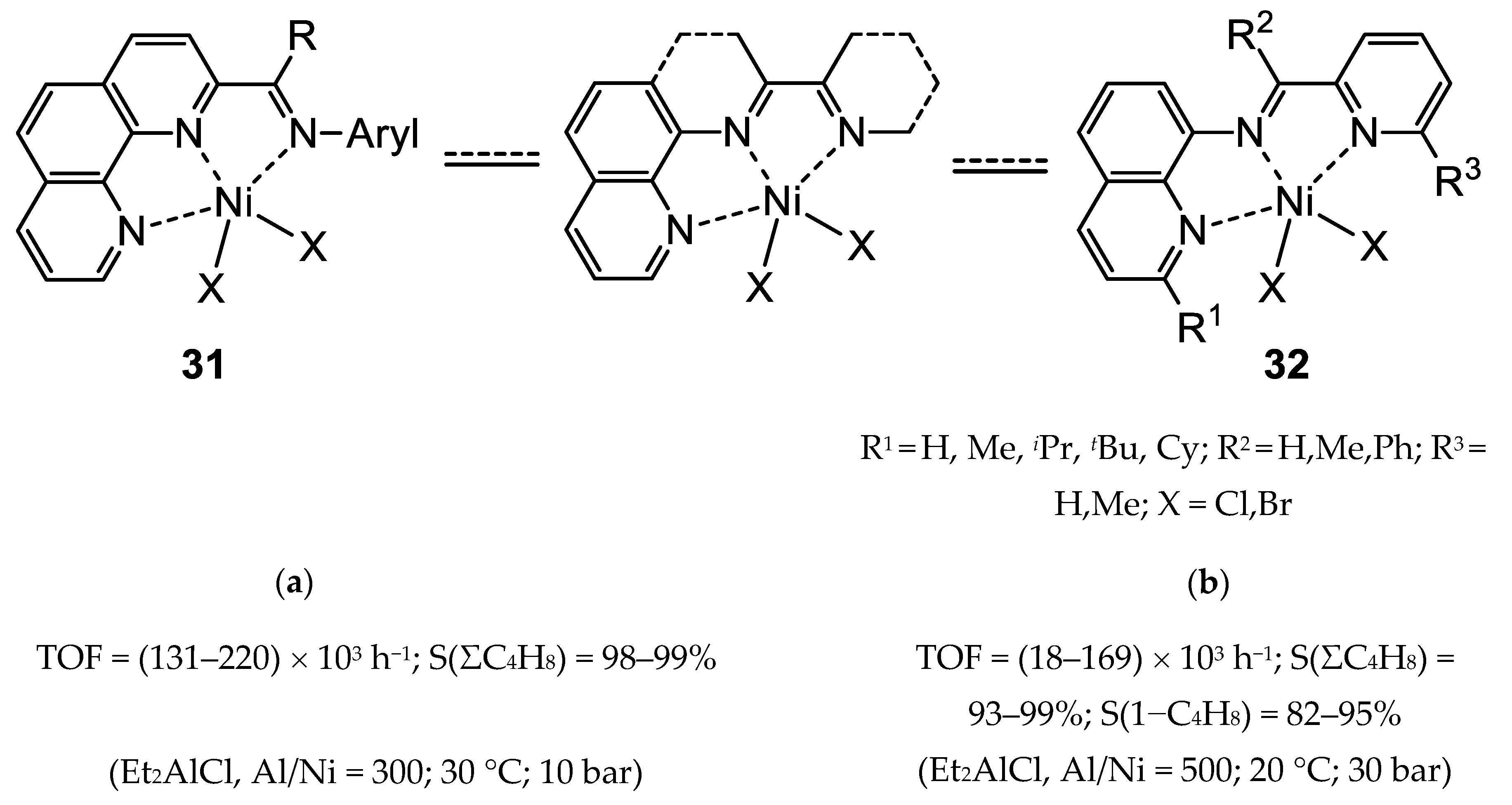
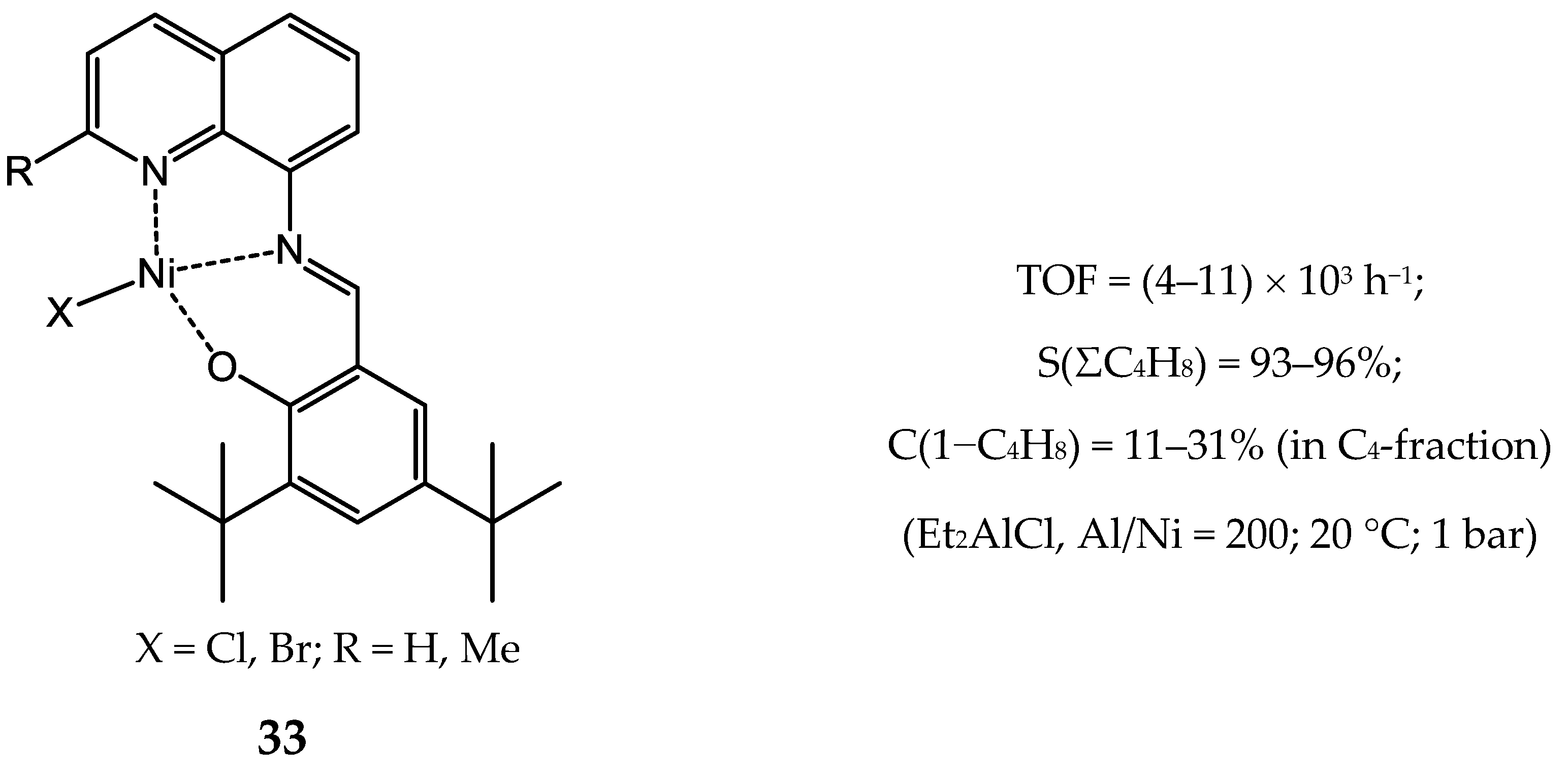
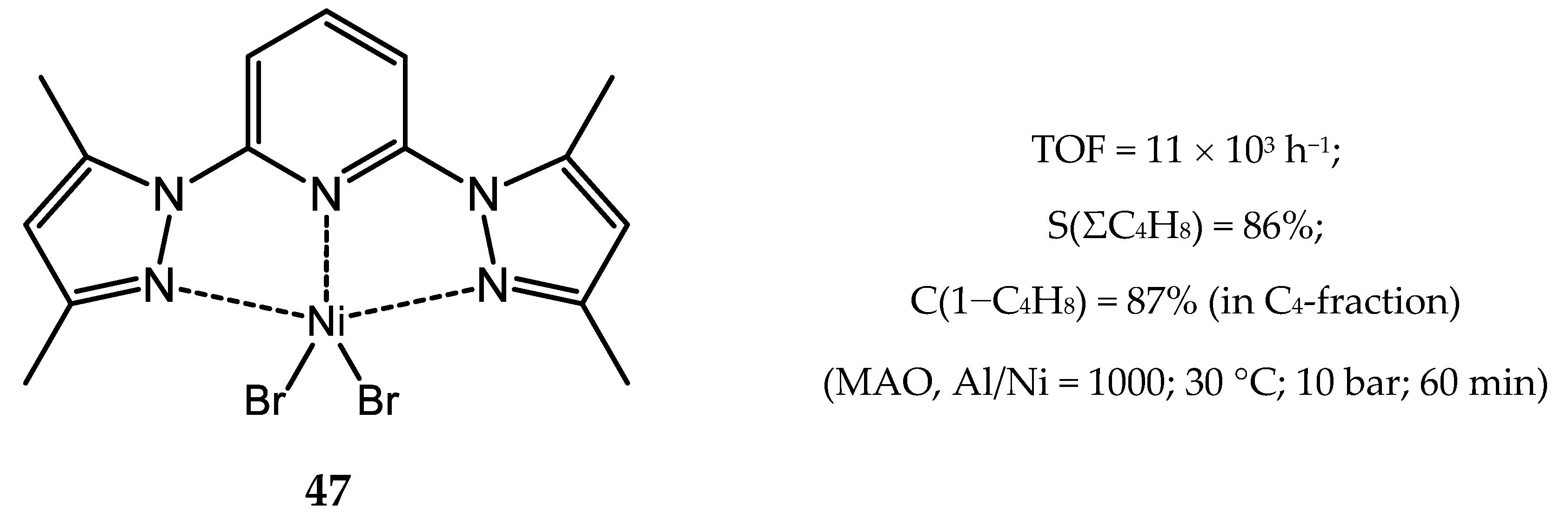
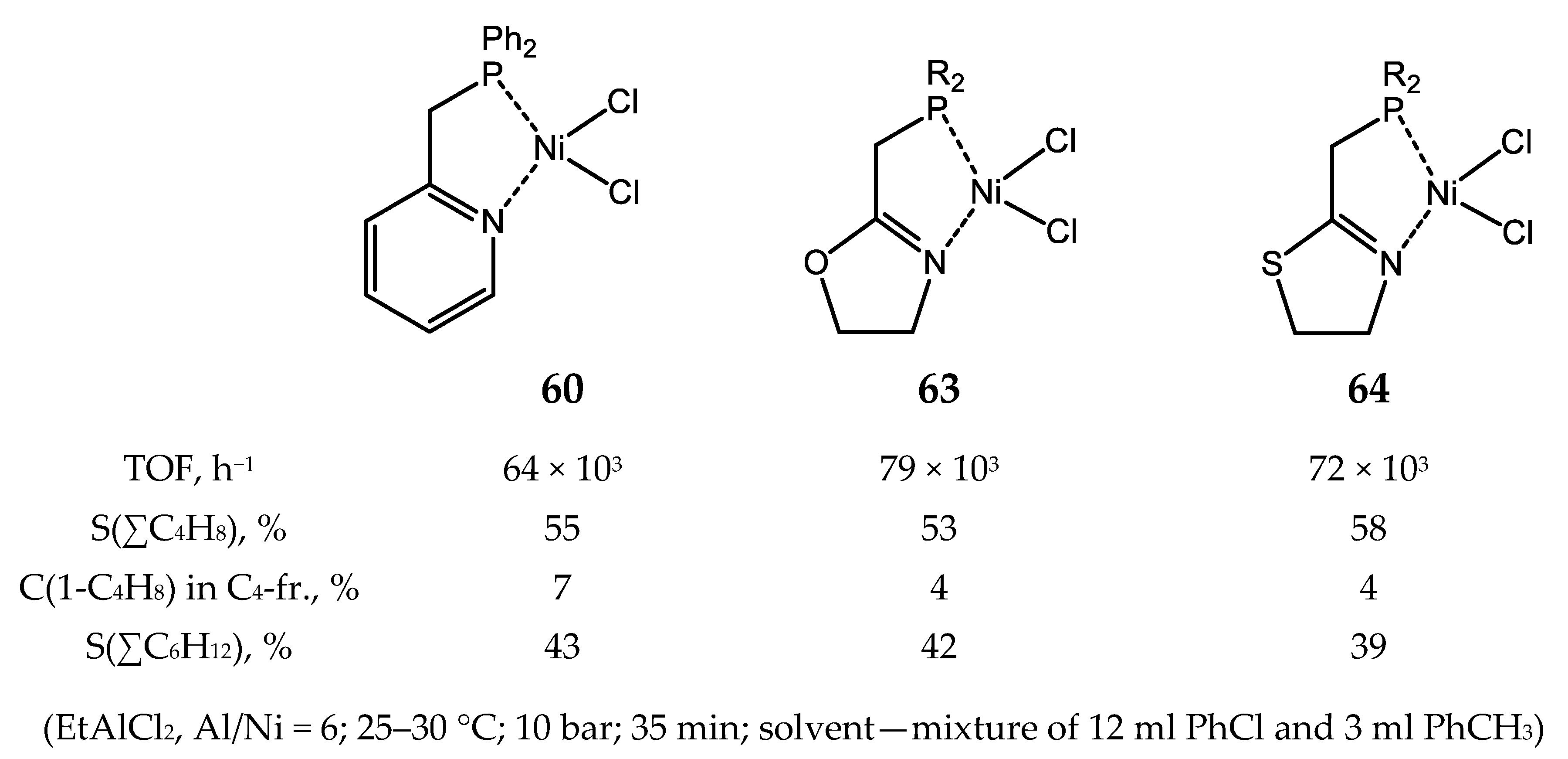
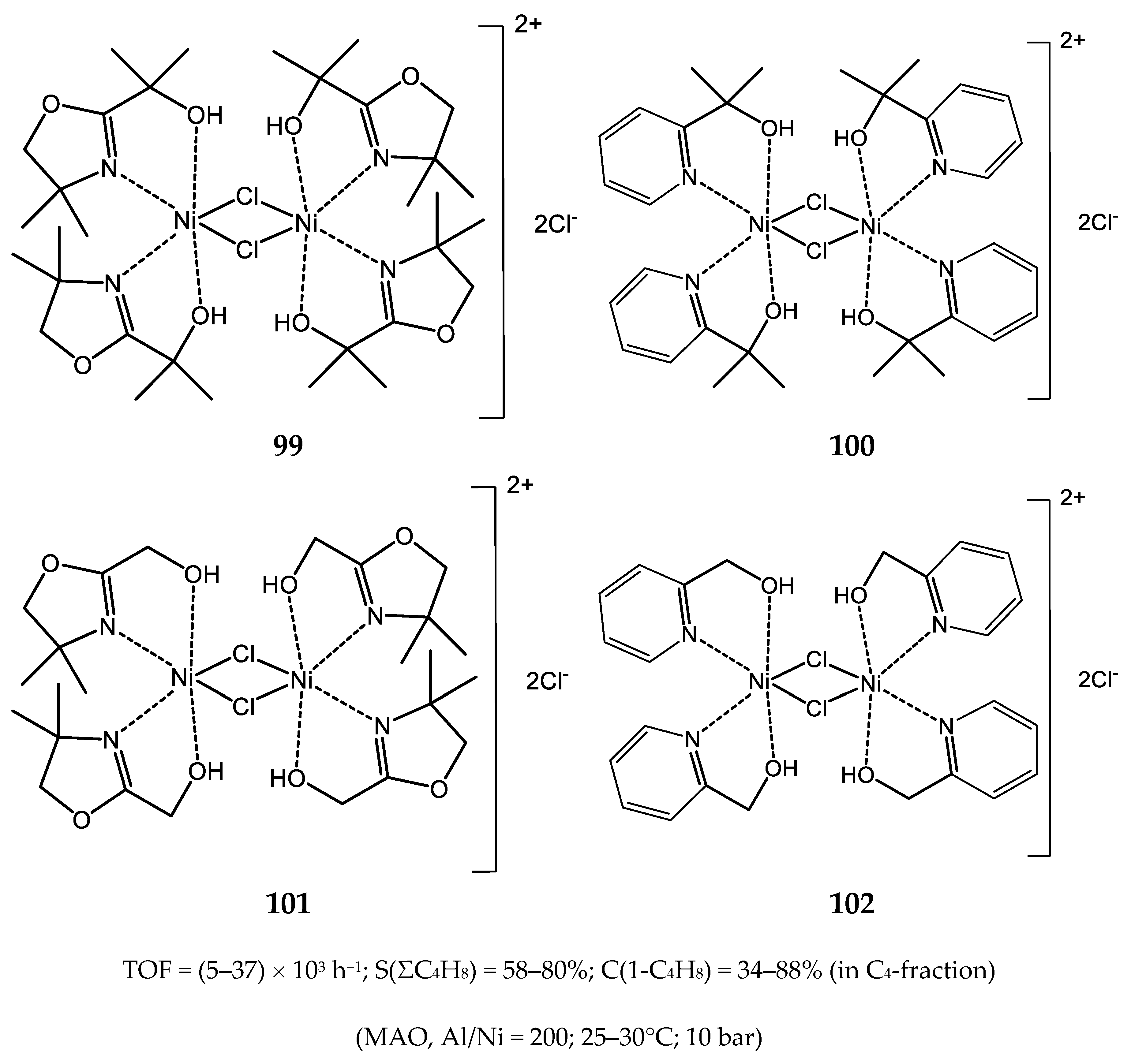
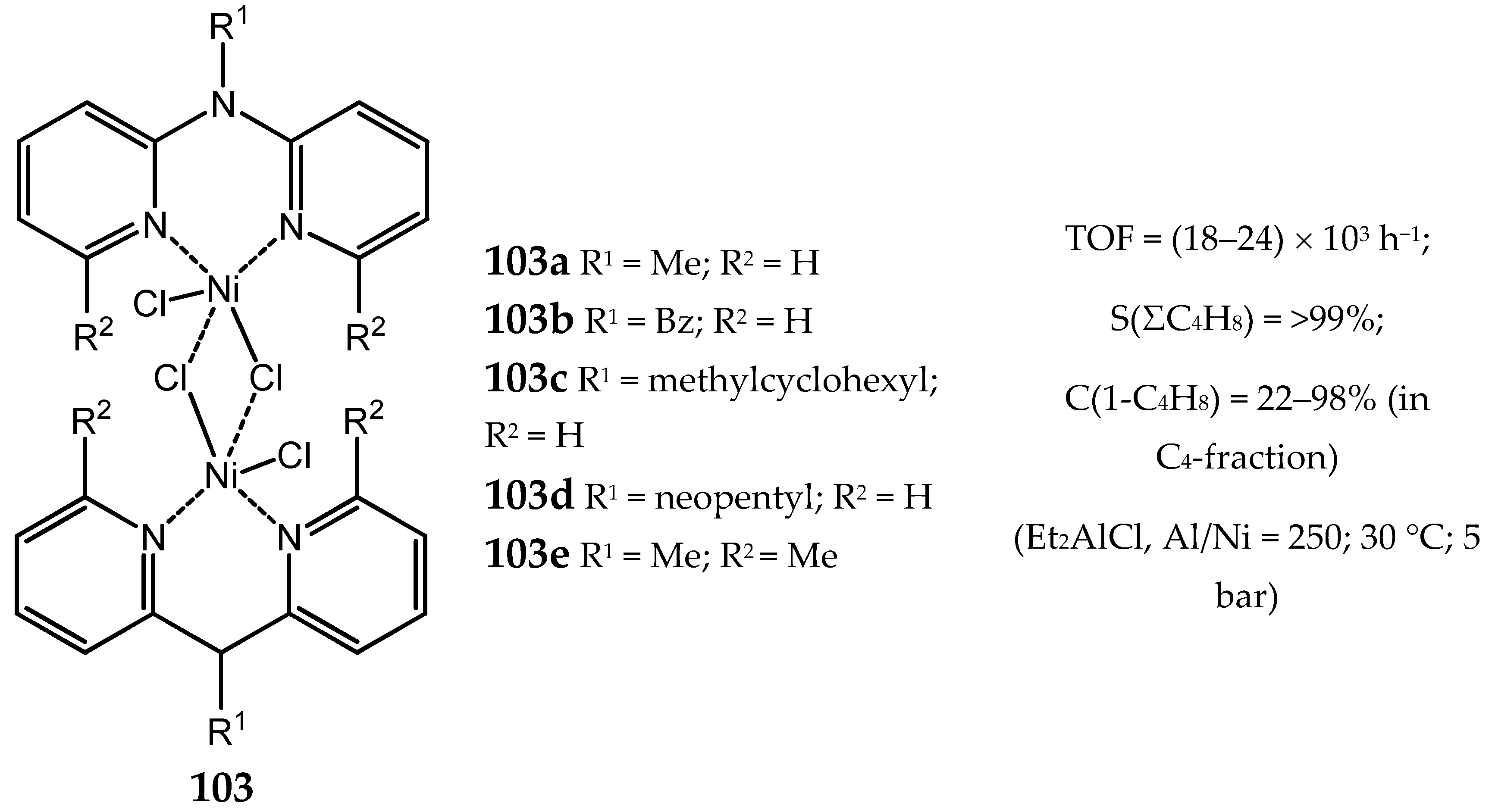
3.2.1. Nickel Complexes Bearing 1,10-Phenanthroline Ligands
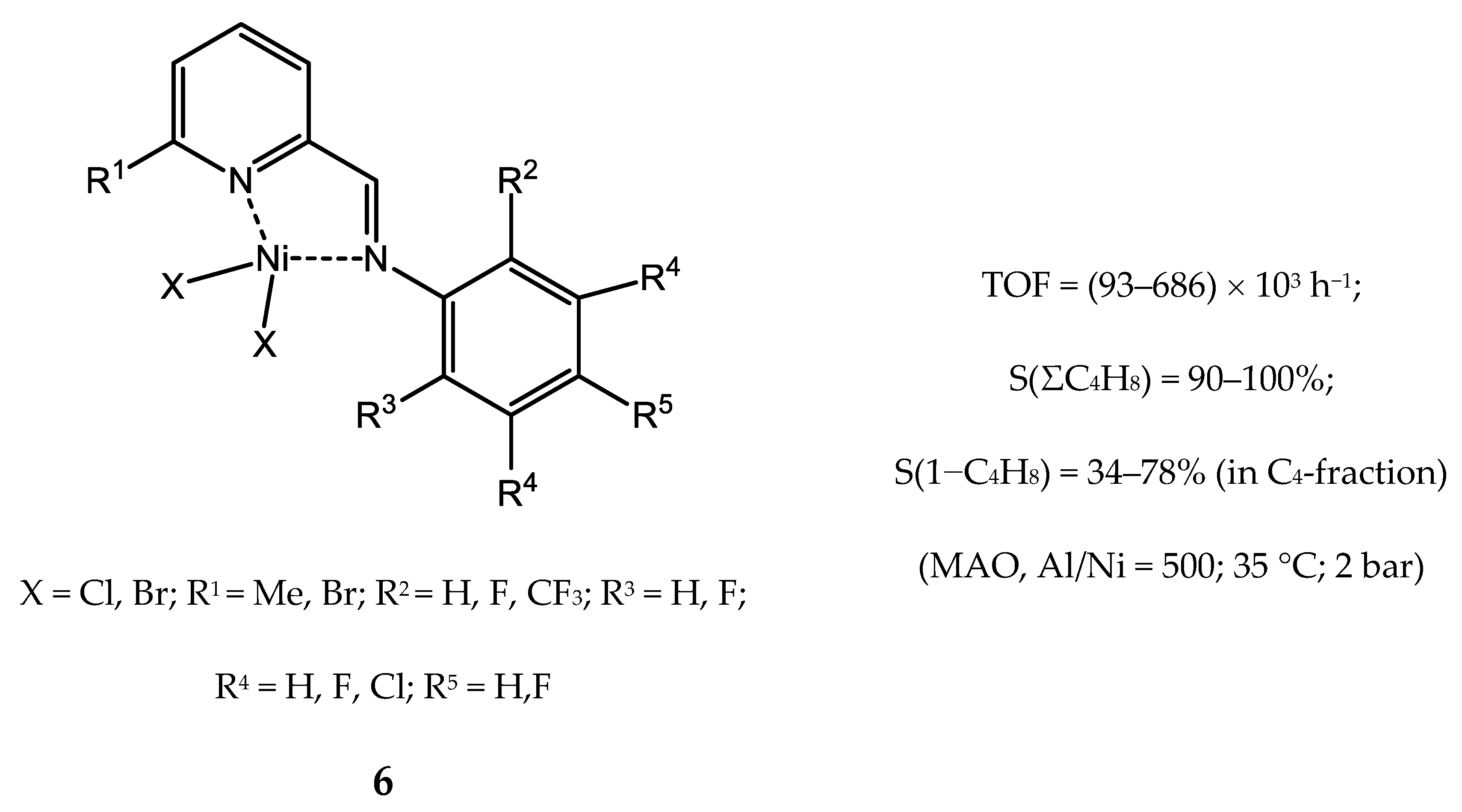
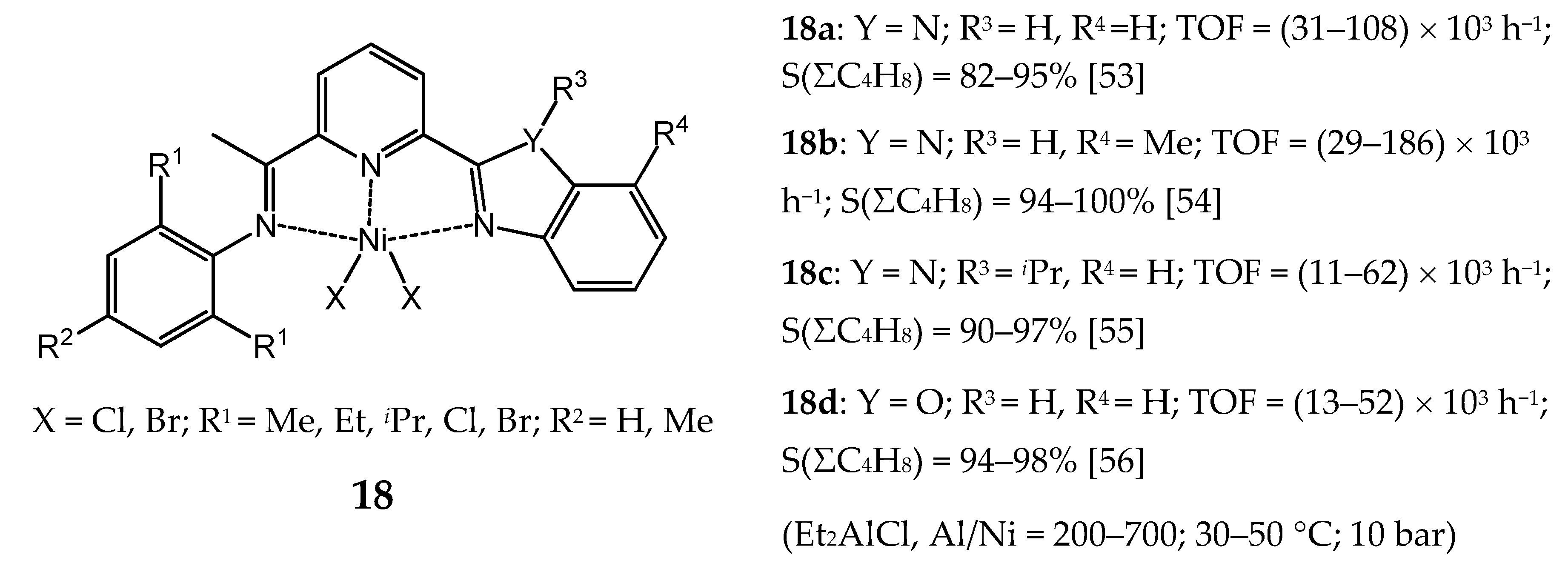
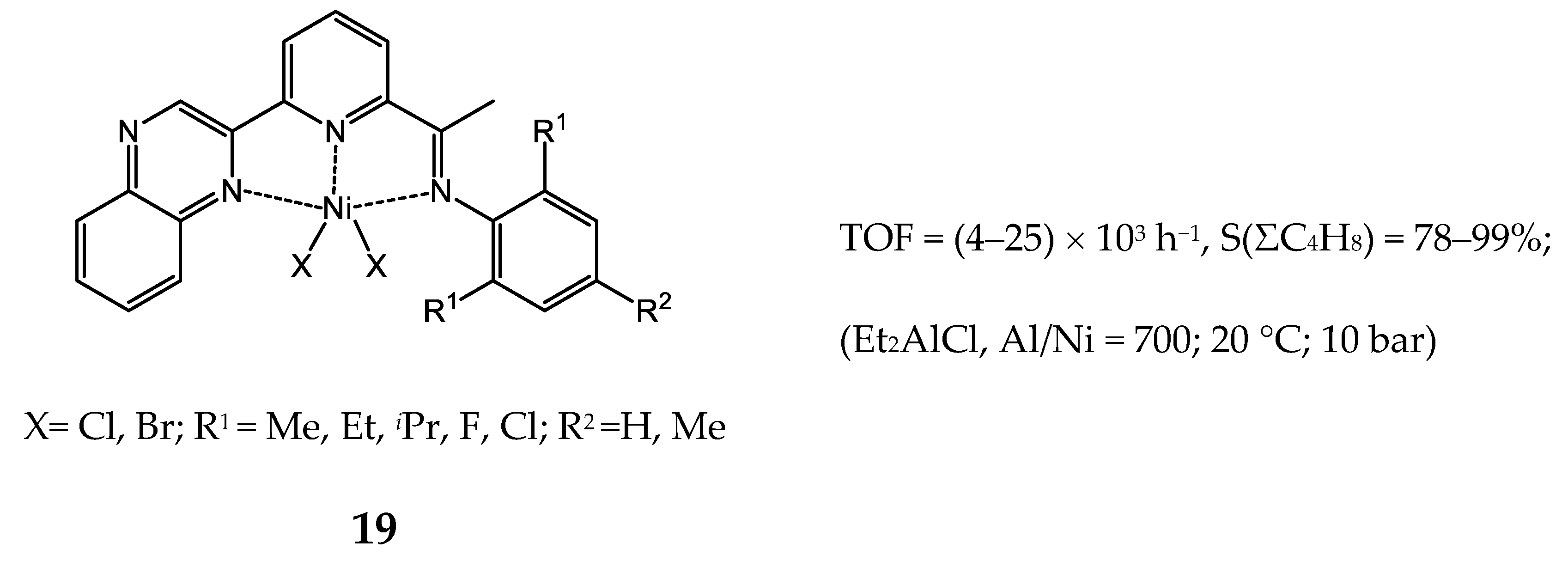
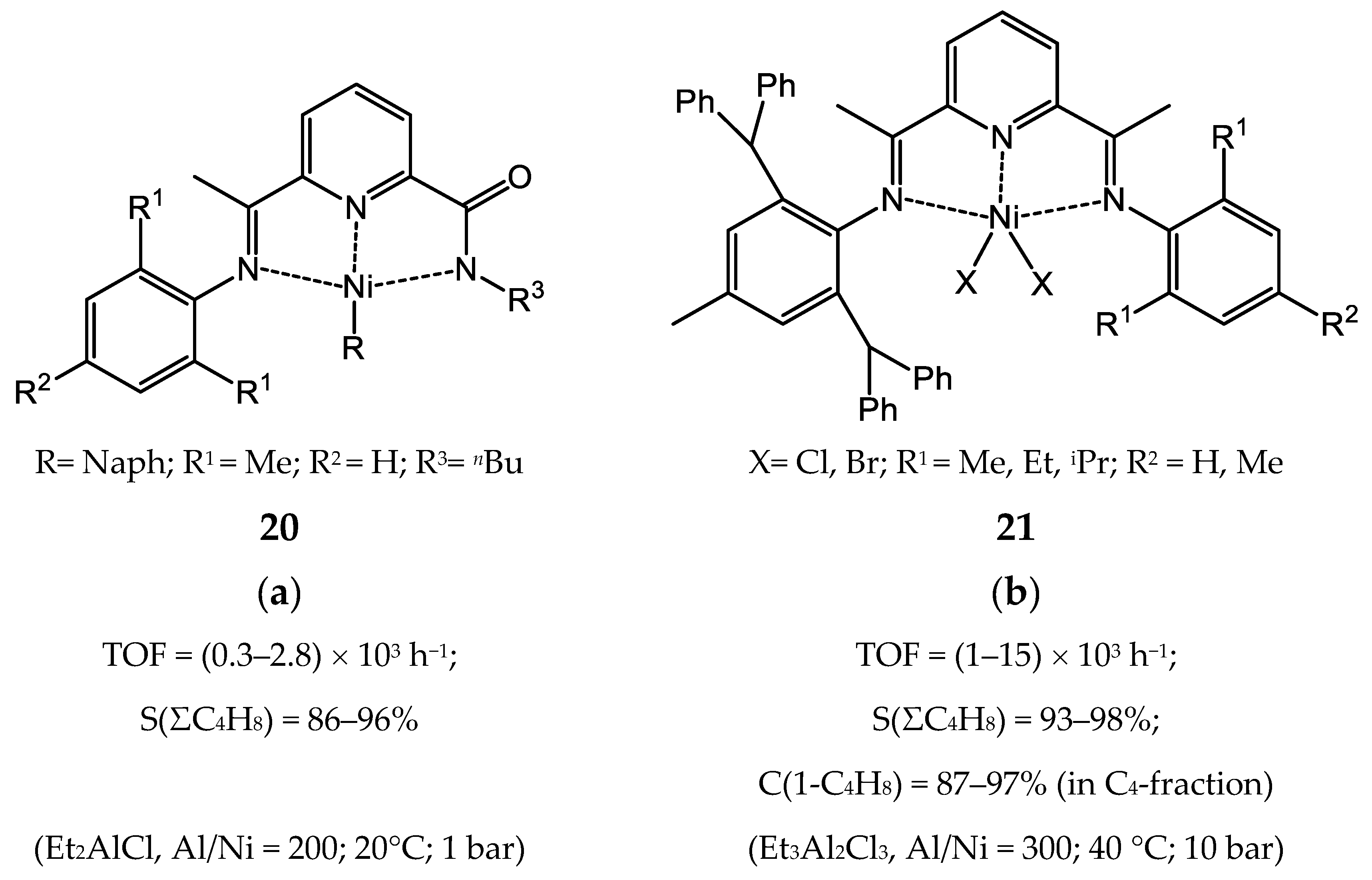
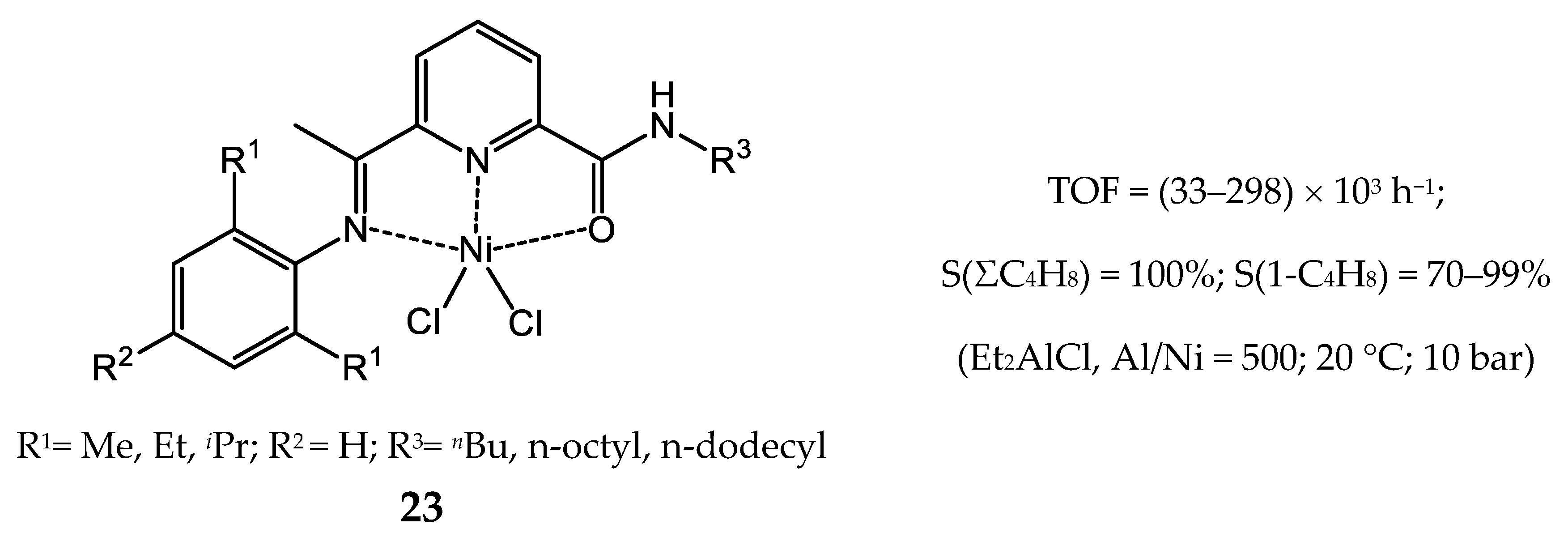
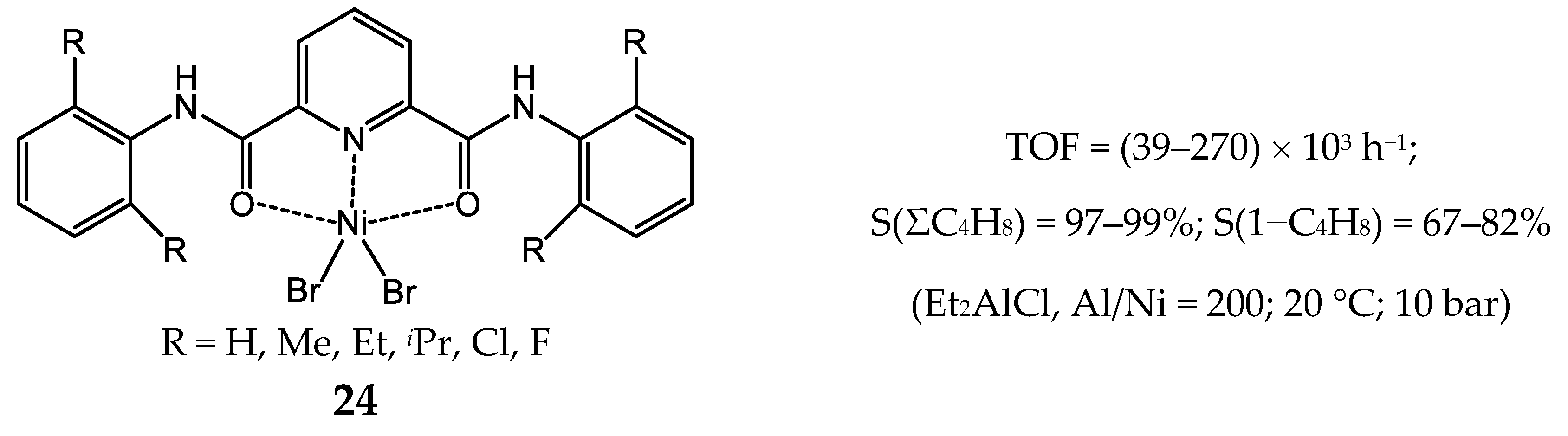
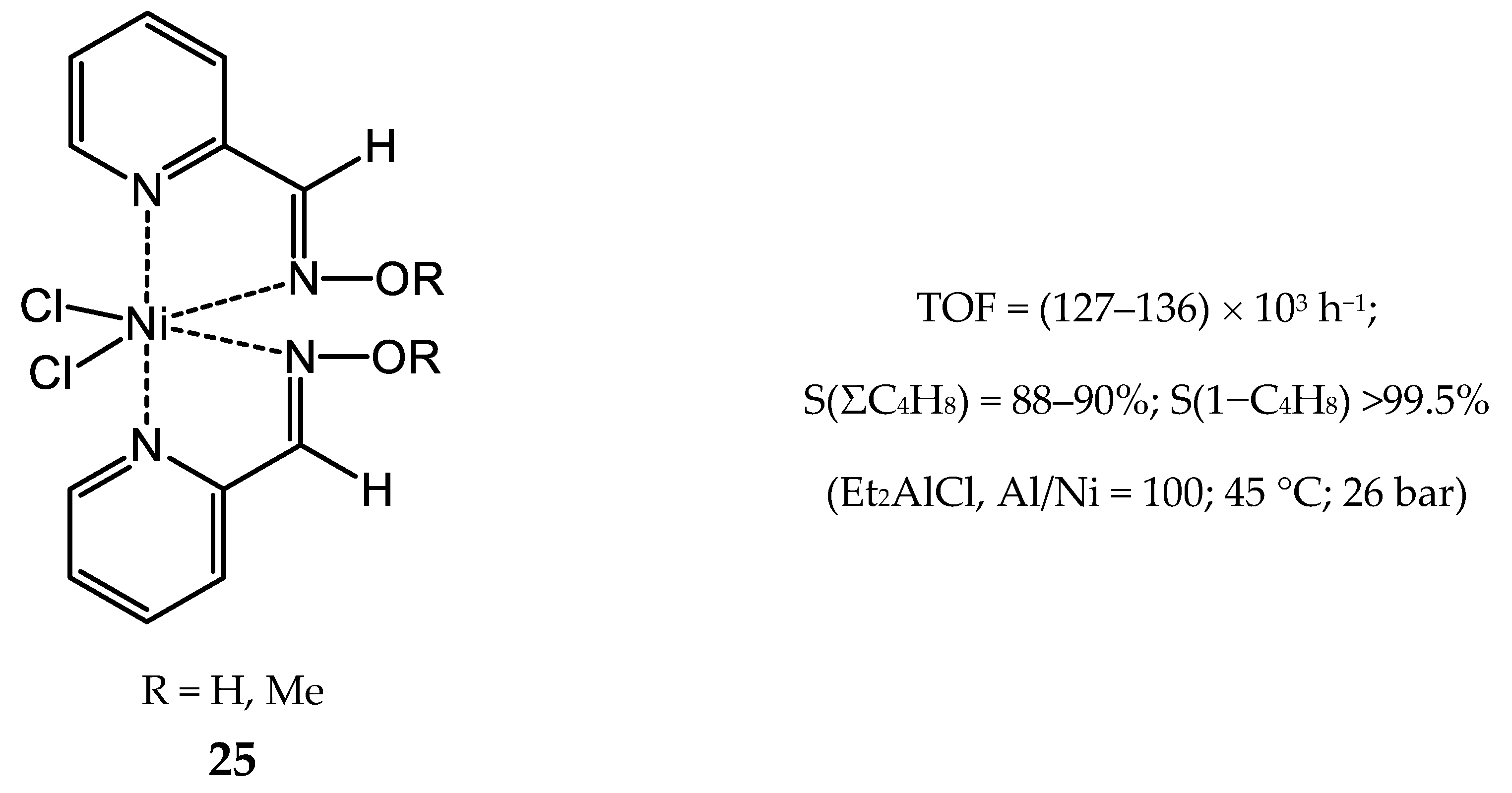
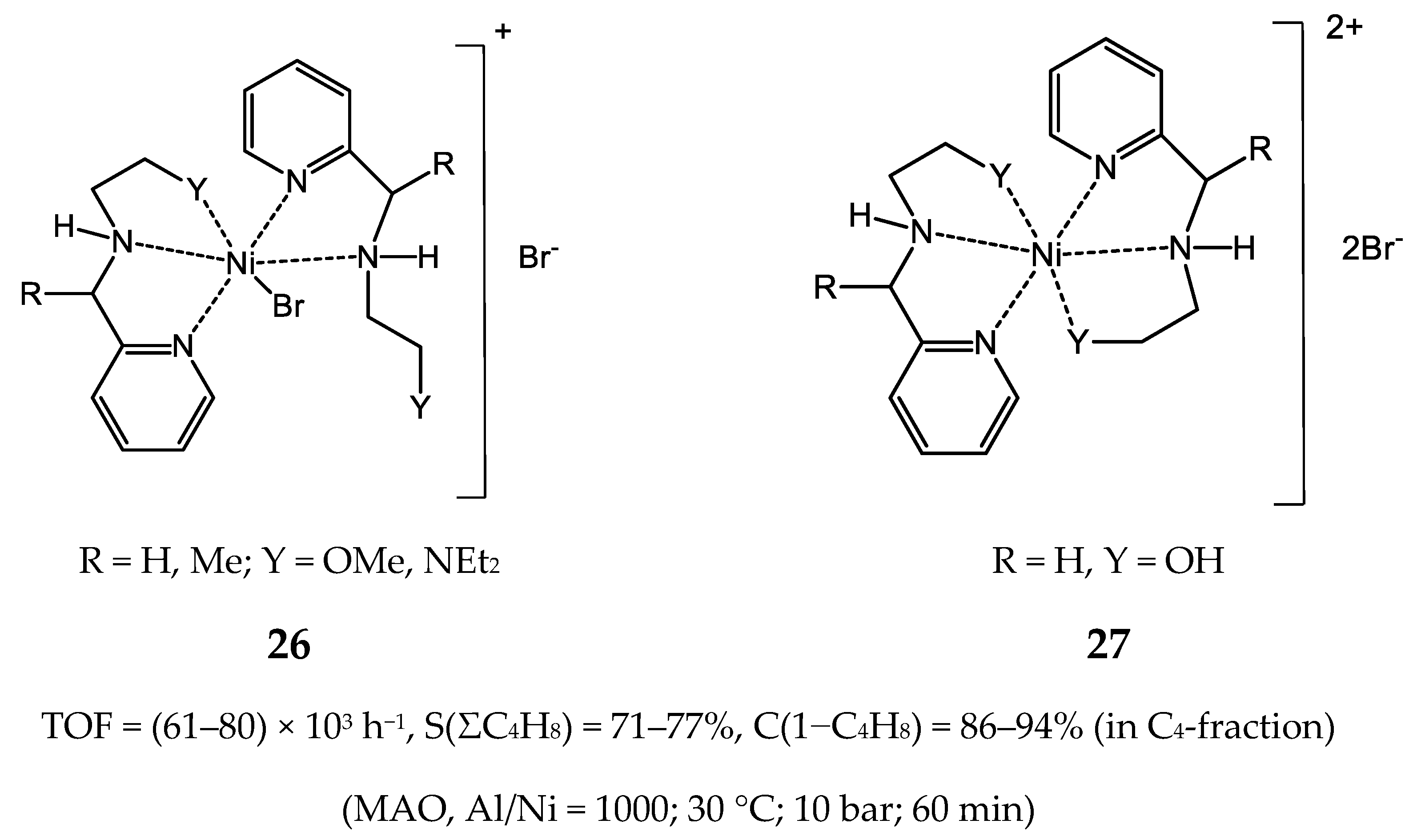
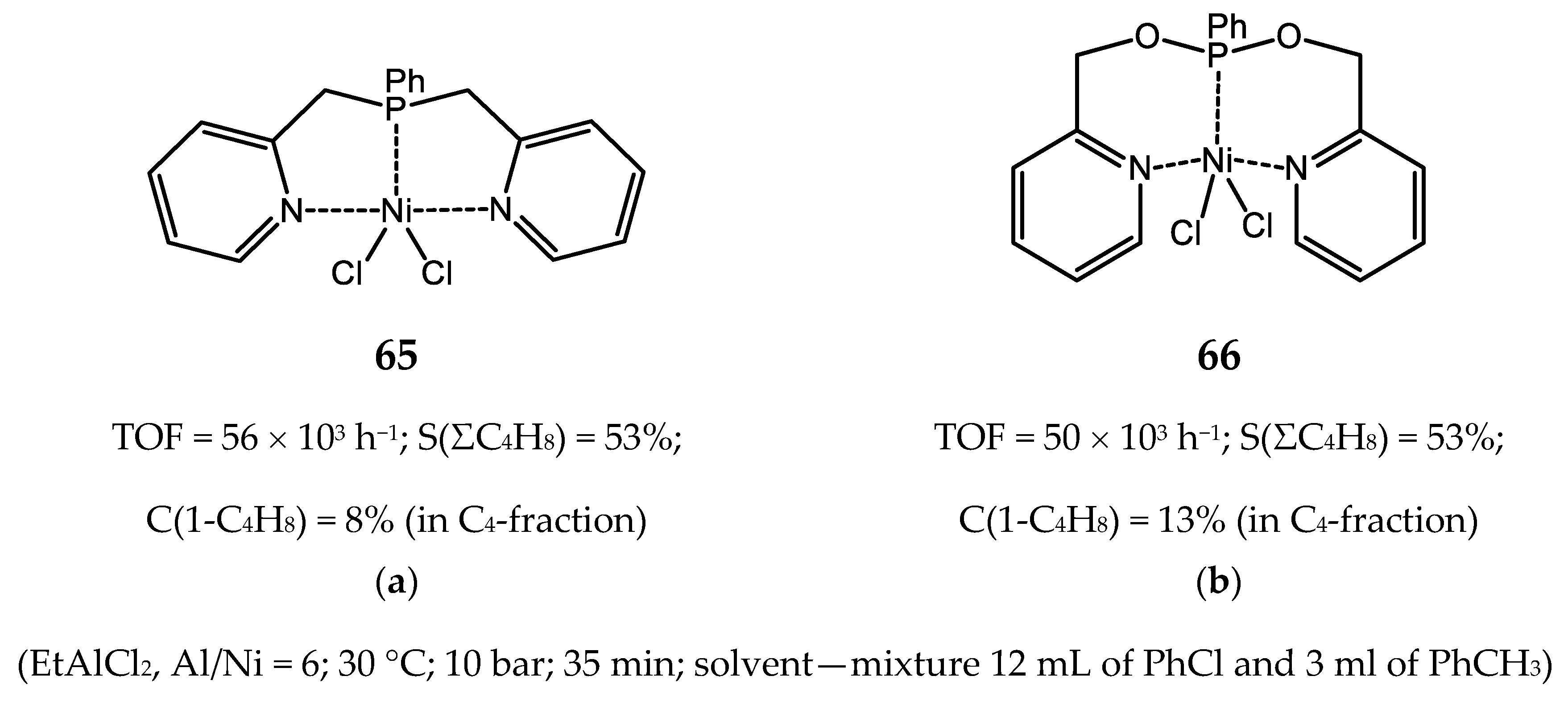
3.2.2. Nickel Complexes Bearing Pyridine Ligands
3.2.3. Nickel Complexes Bearing Quinoline Ligands
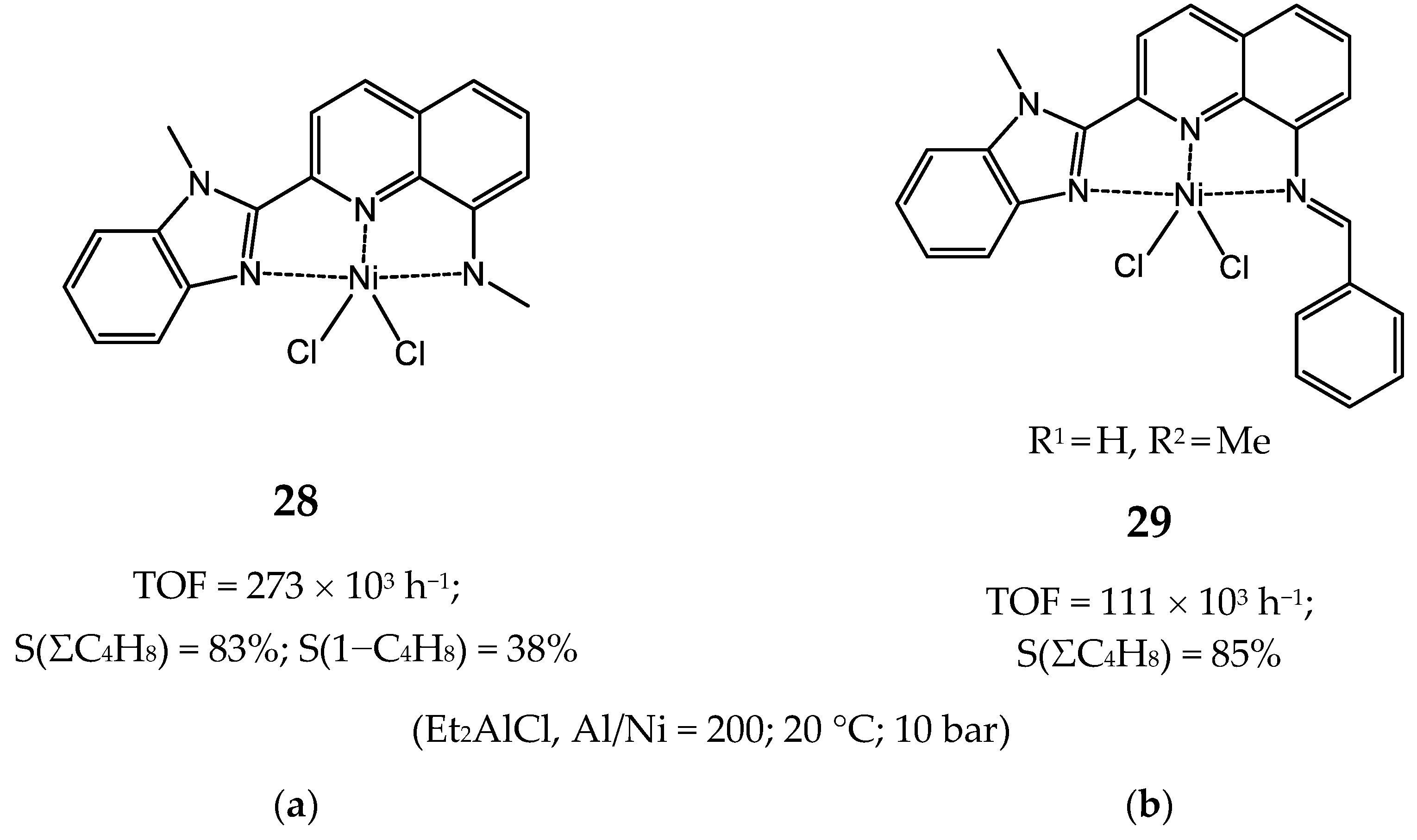
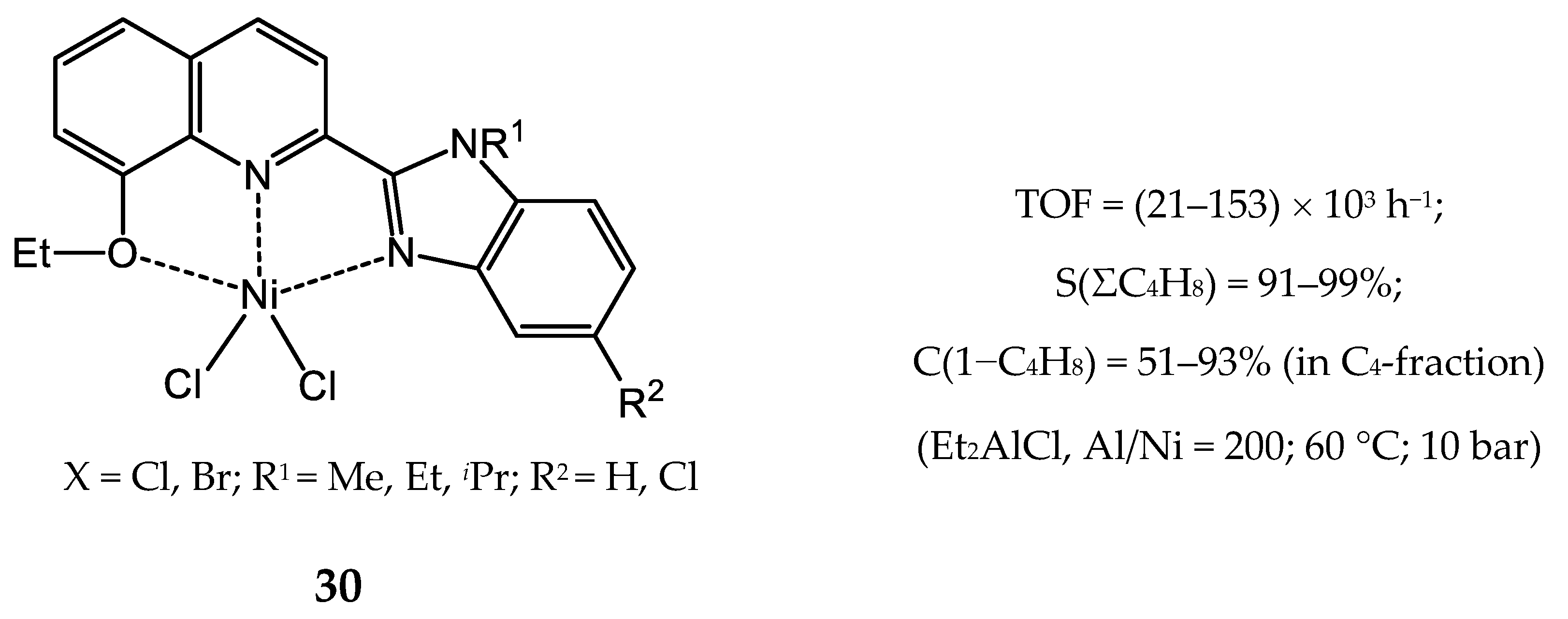
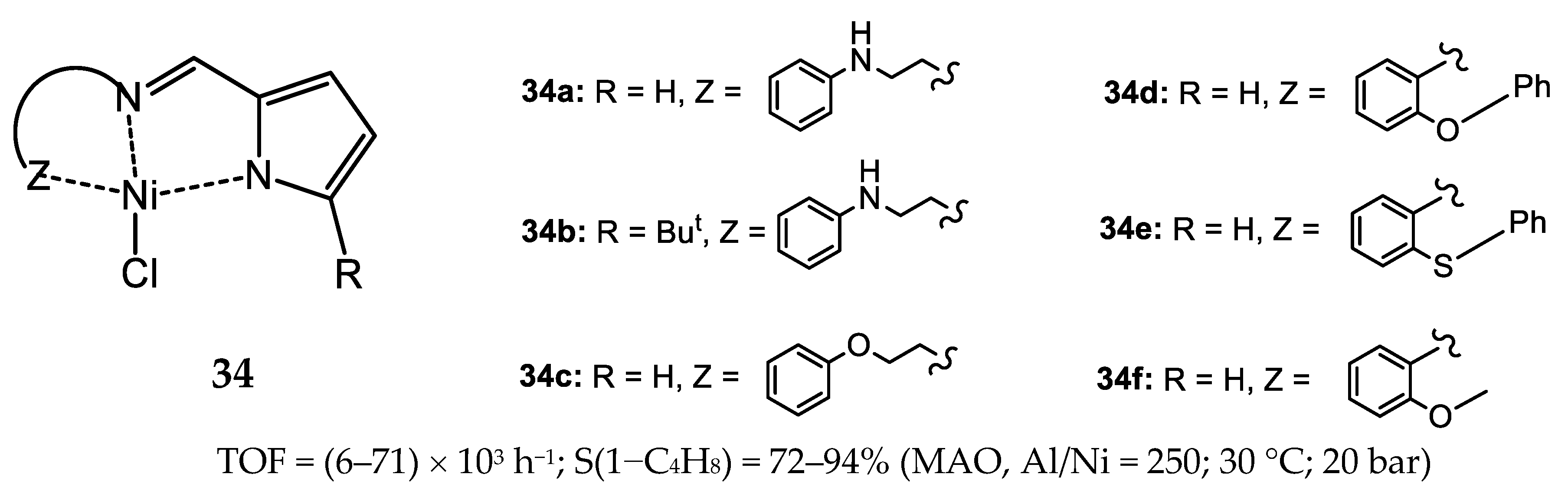
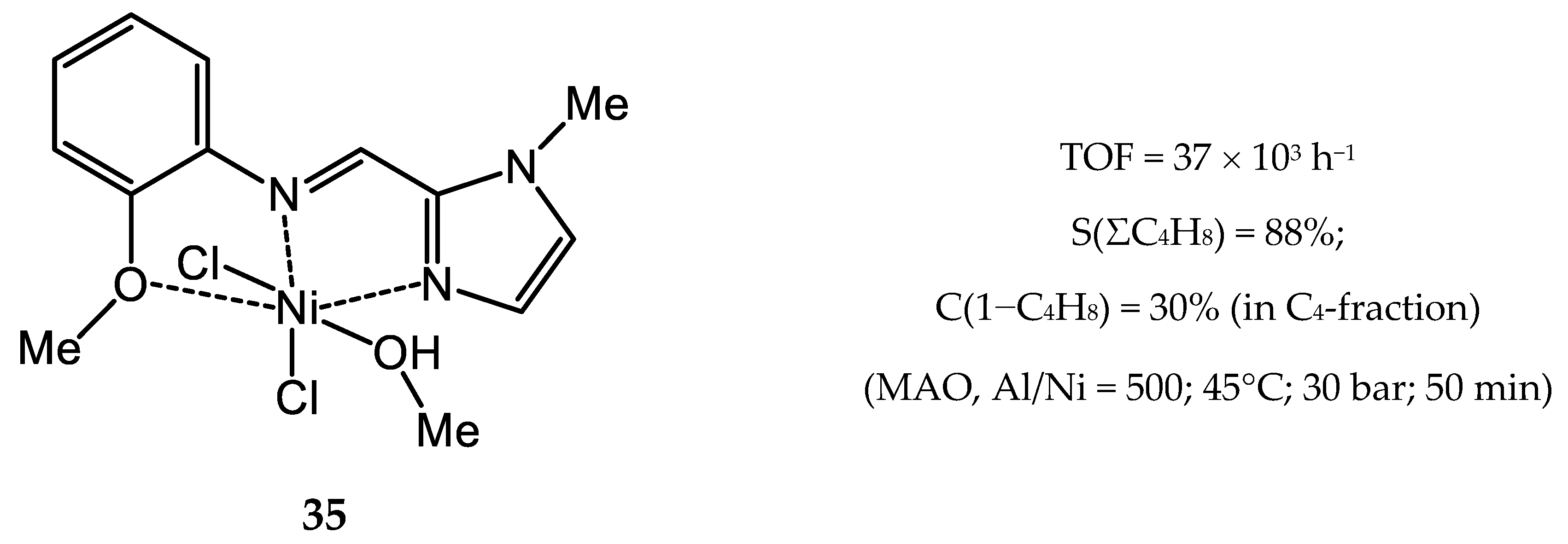
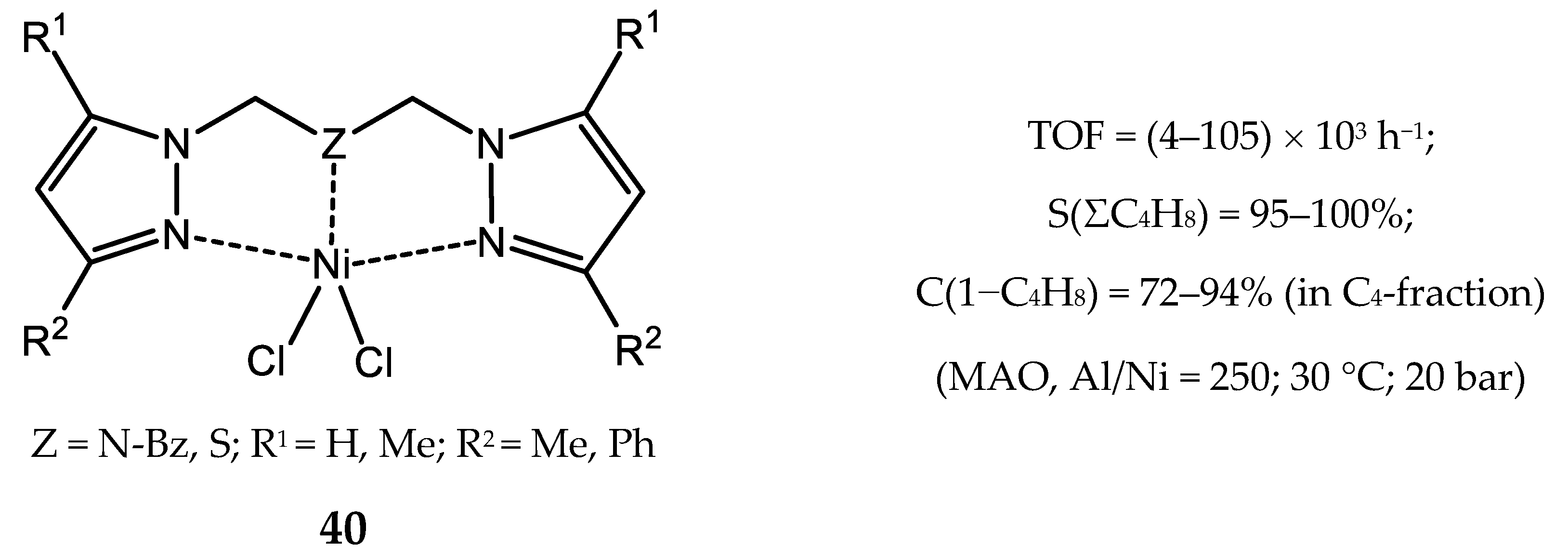
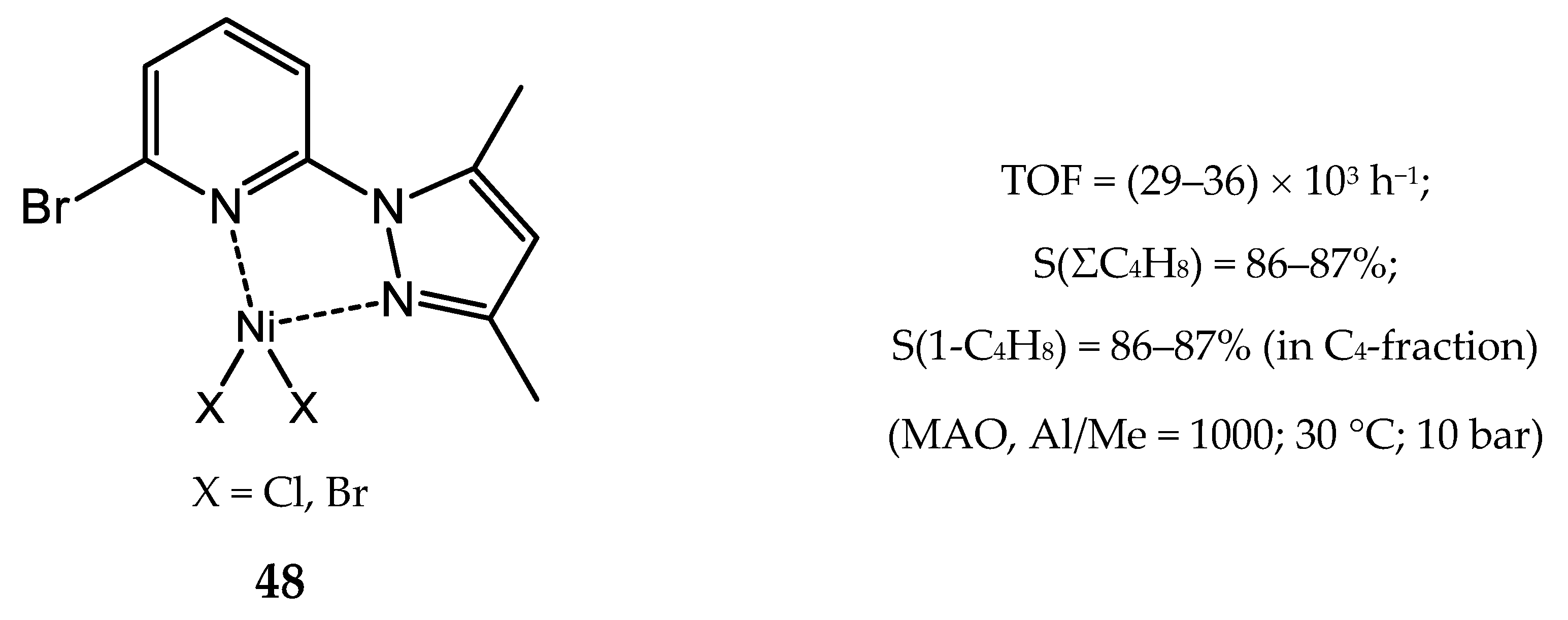
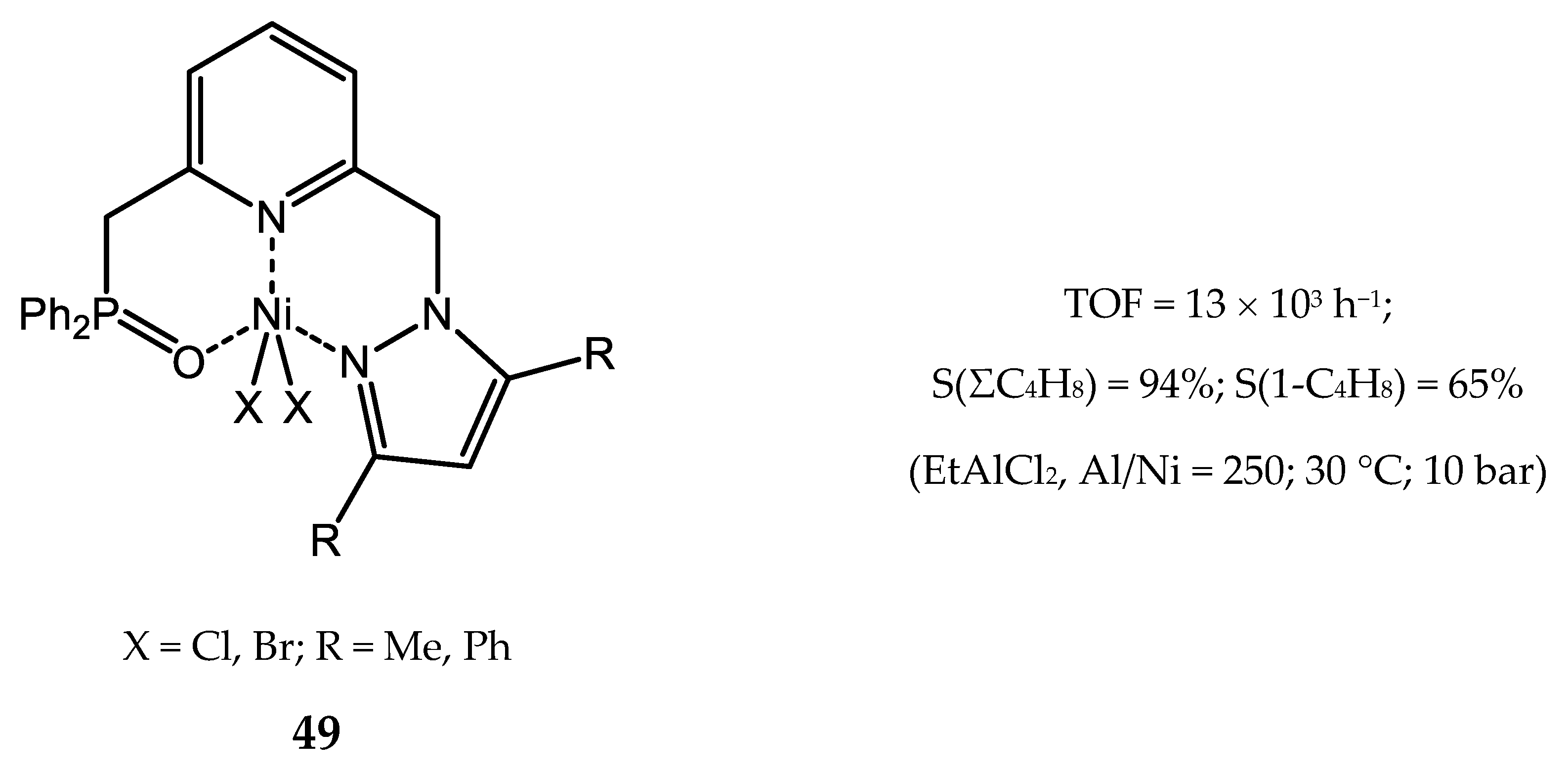
3.2.4. Nickel Complexes Bearing Pyrrole- and Imidazole-Imine Ligands
3.2.5. β-Ketoimine Nickel(II) Complexes
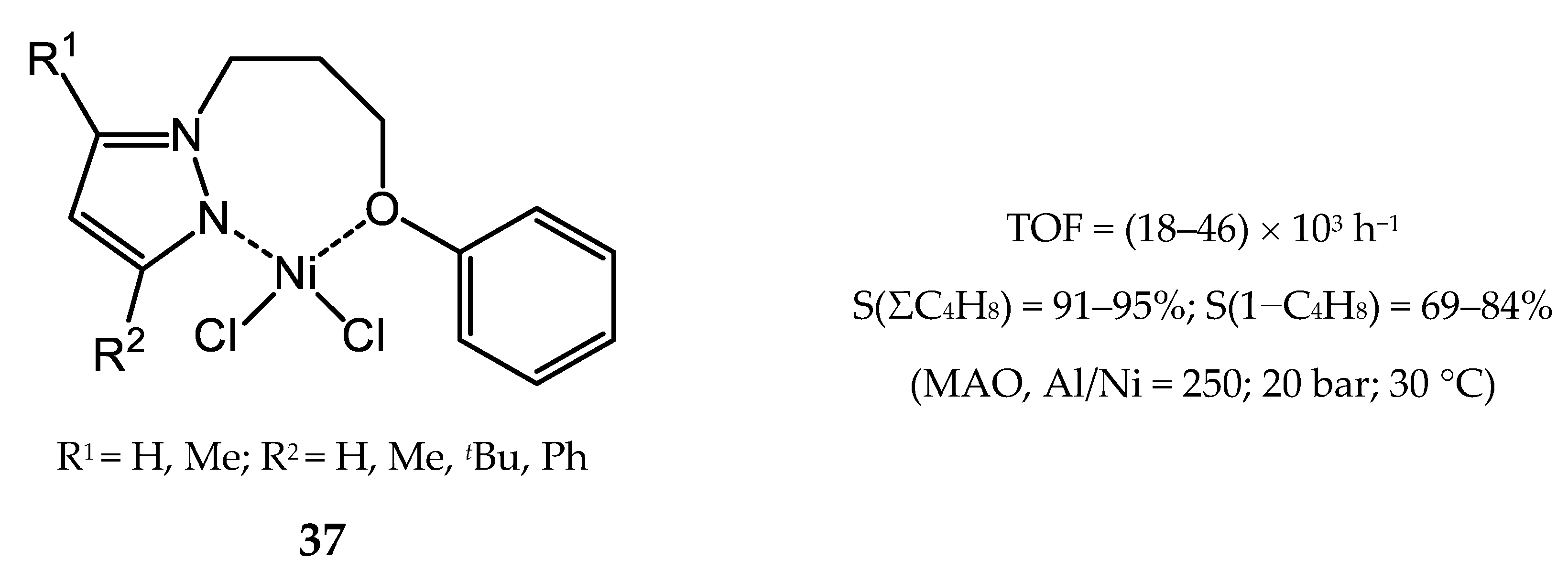
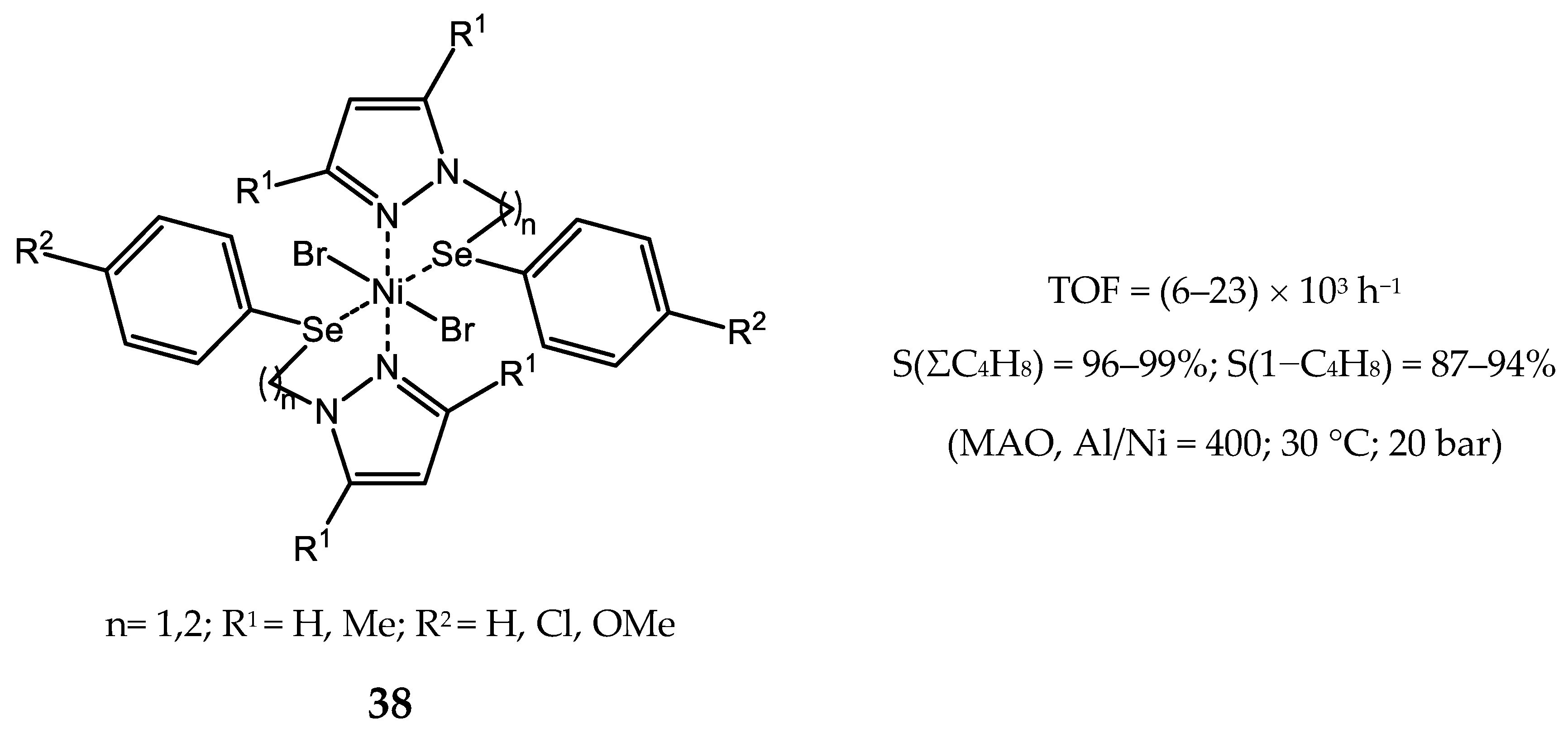
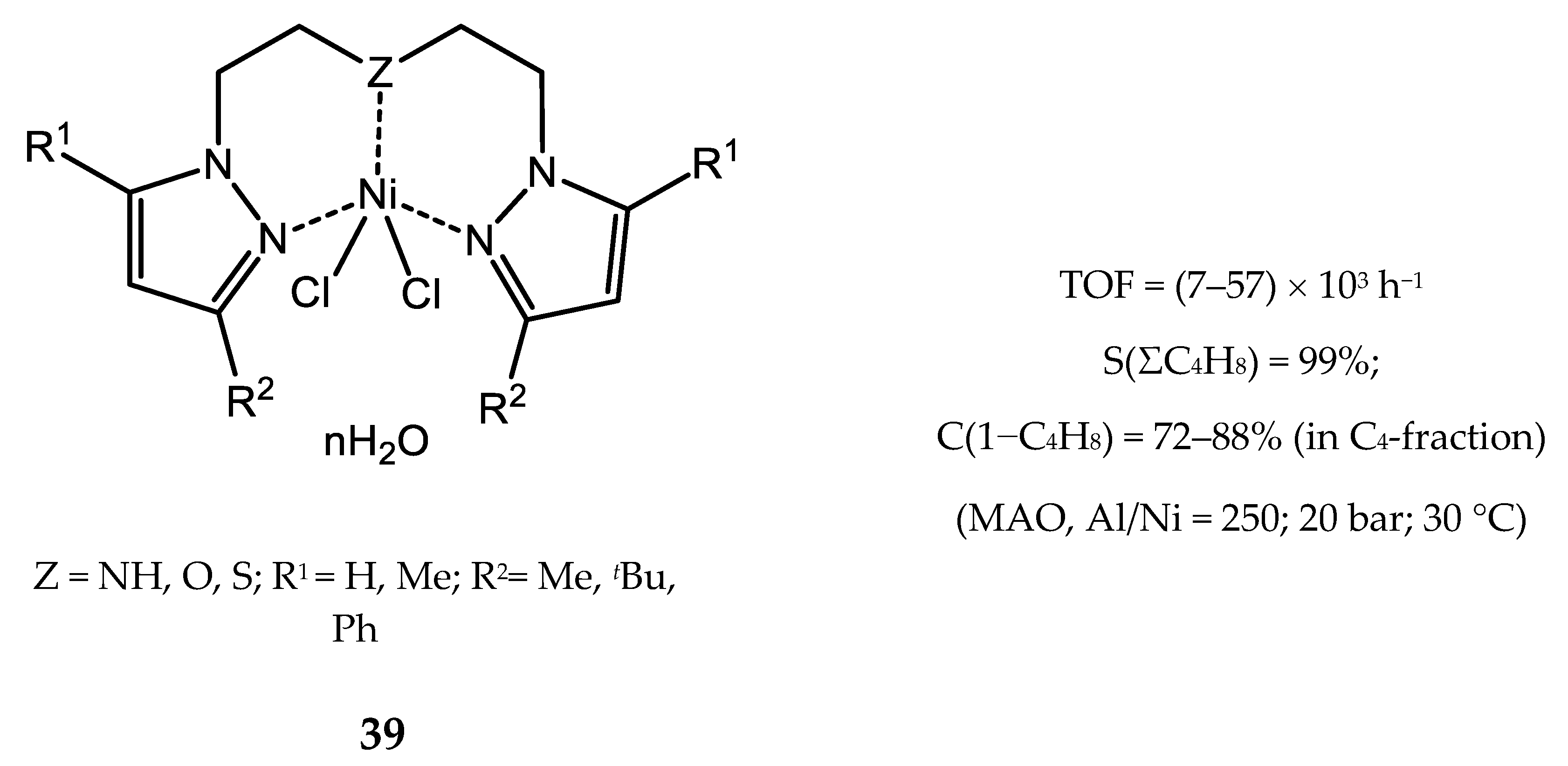
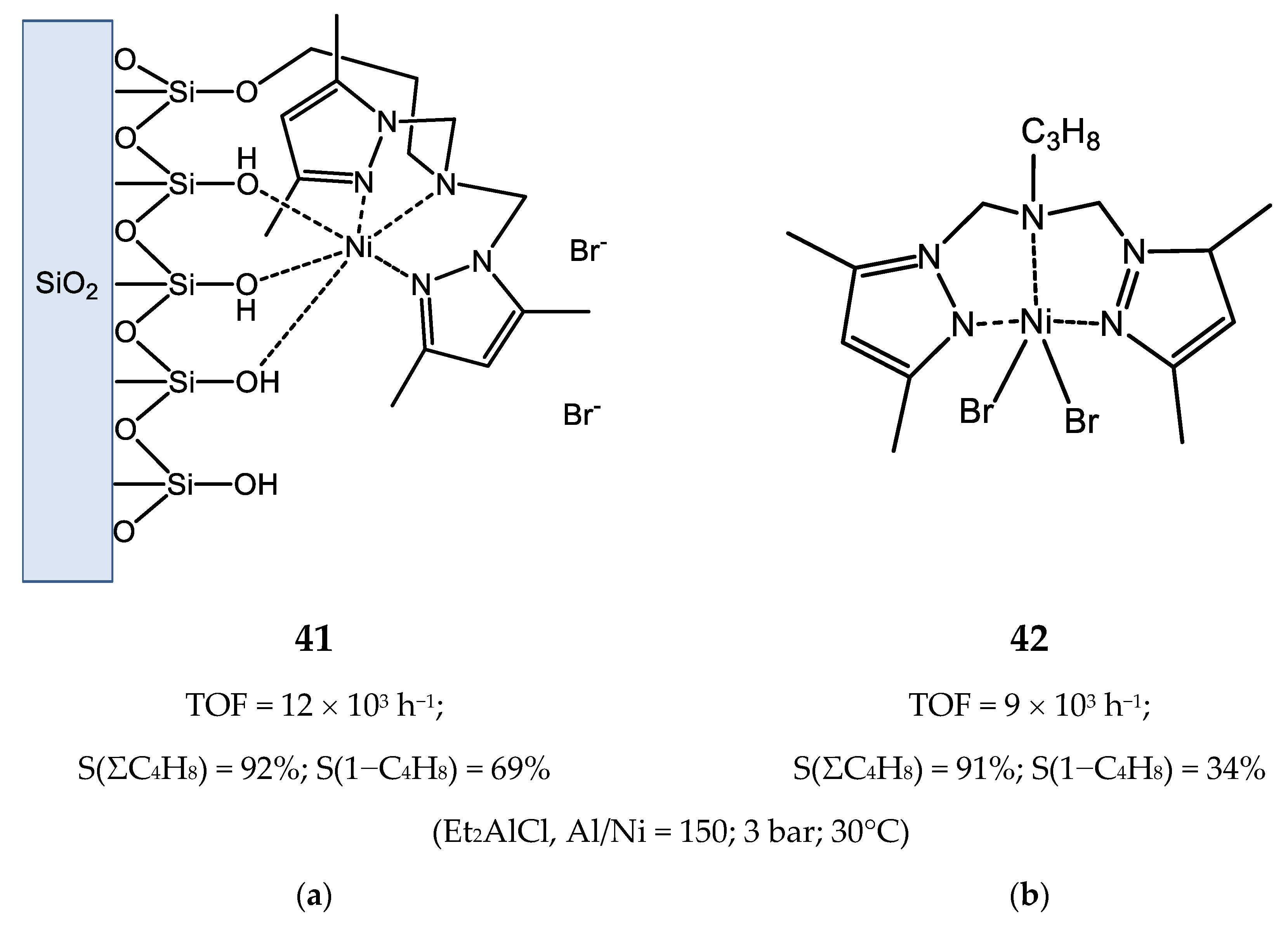
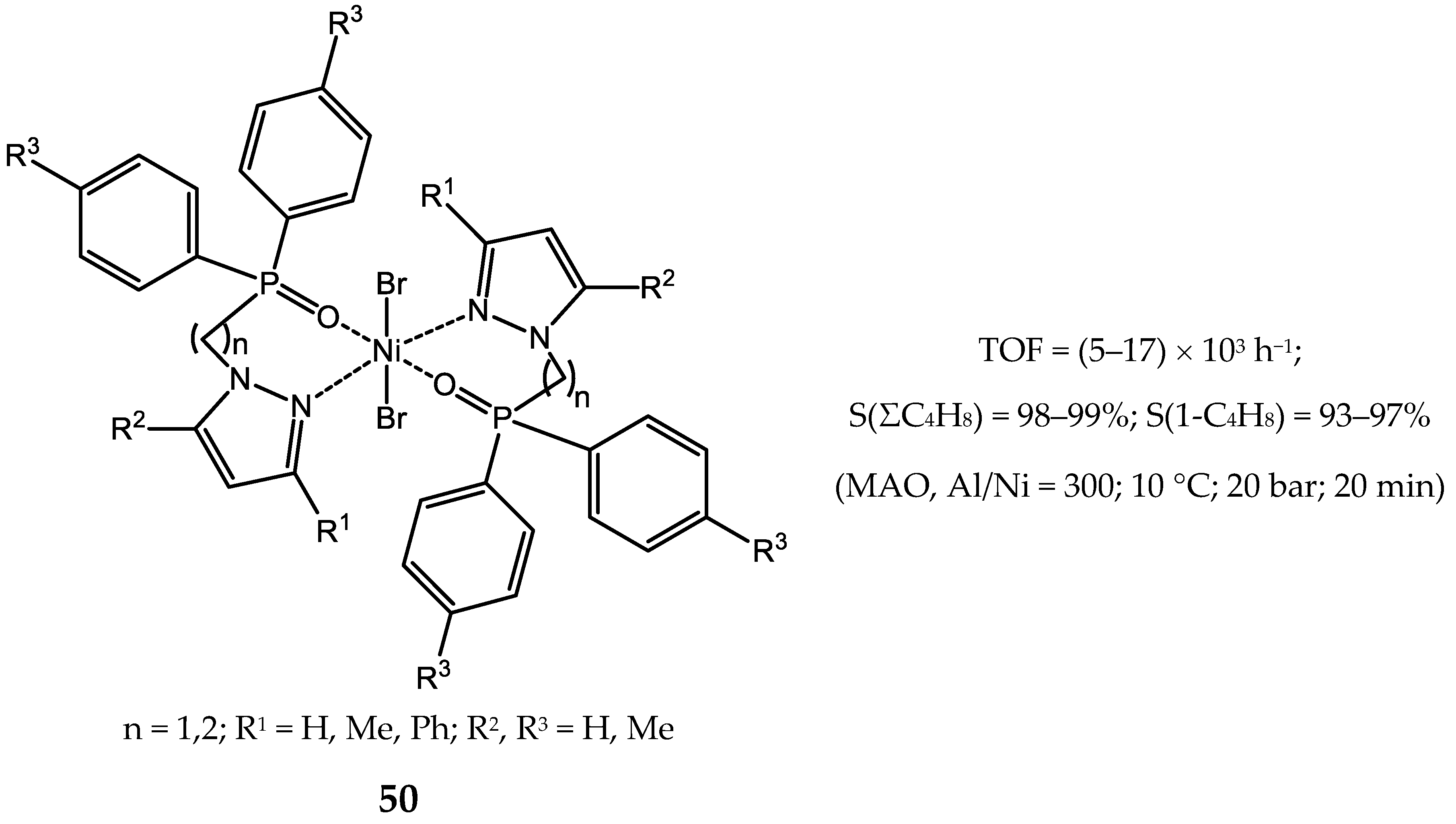
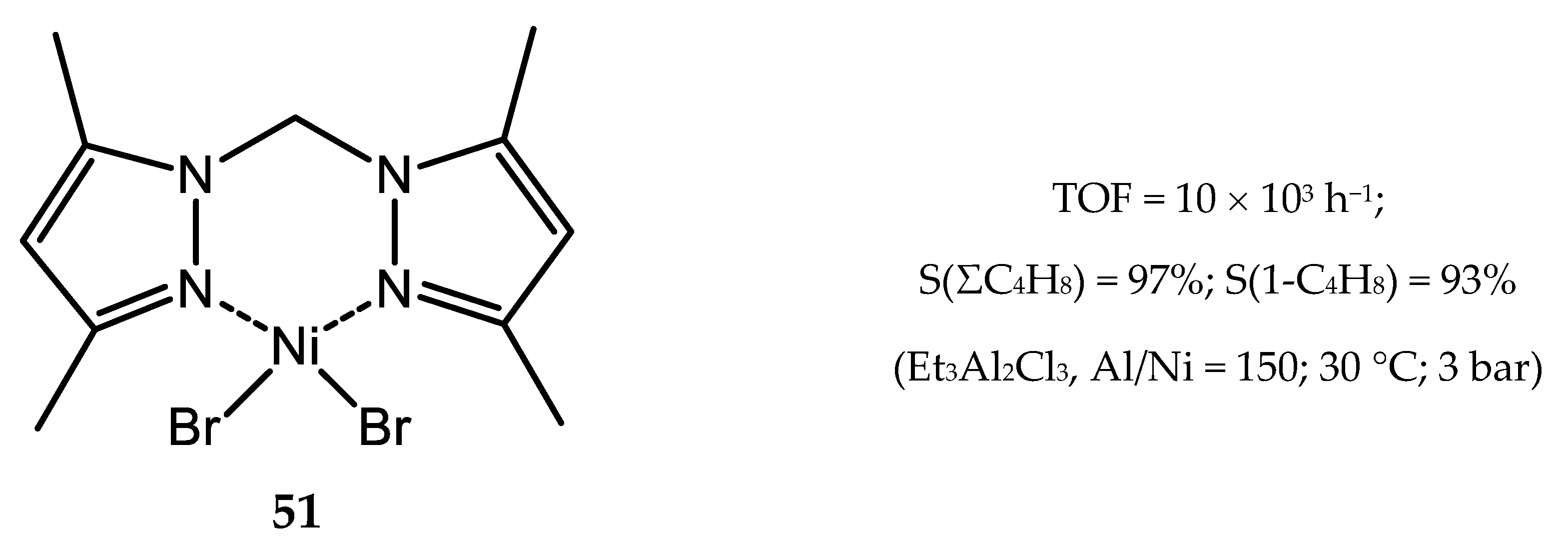
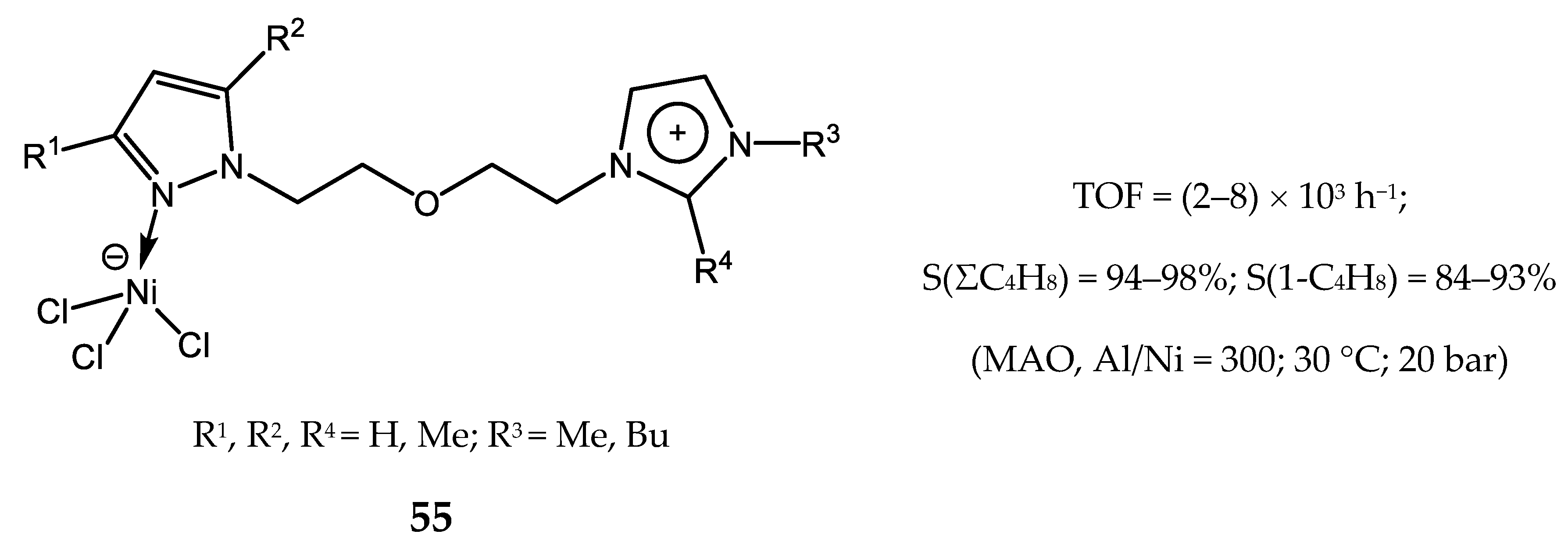
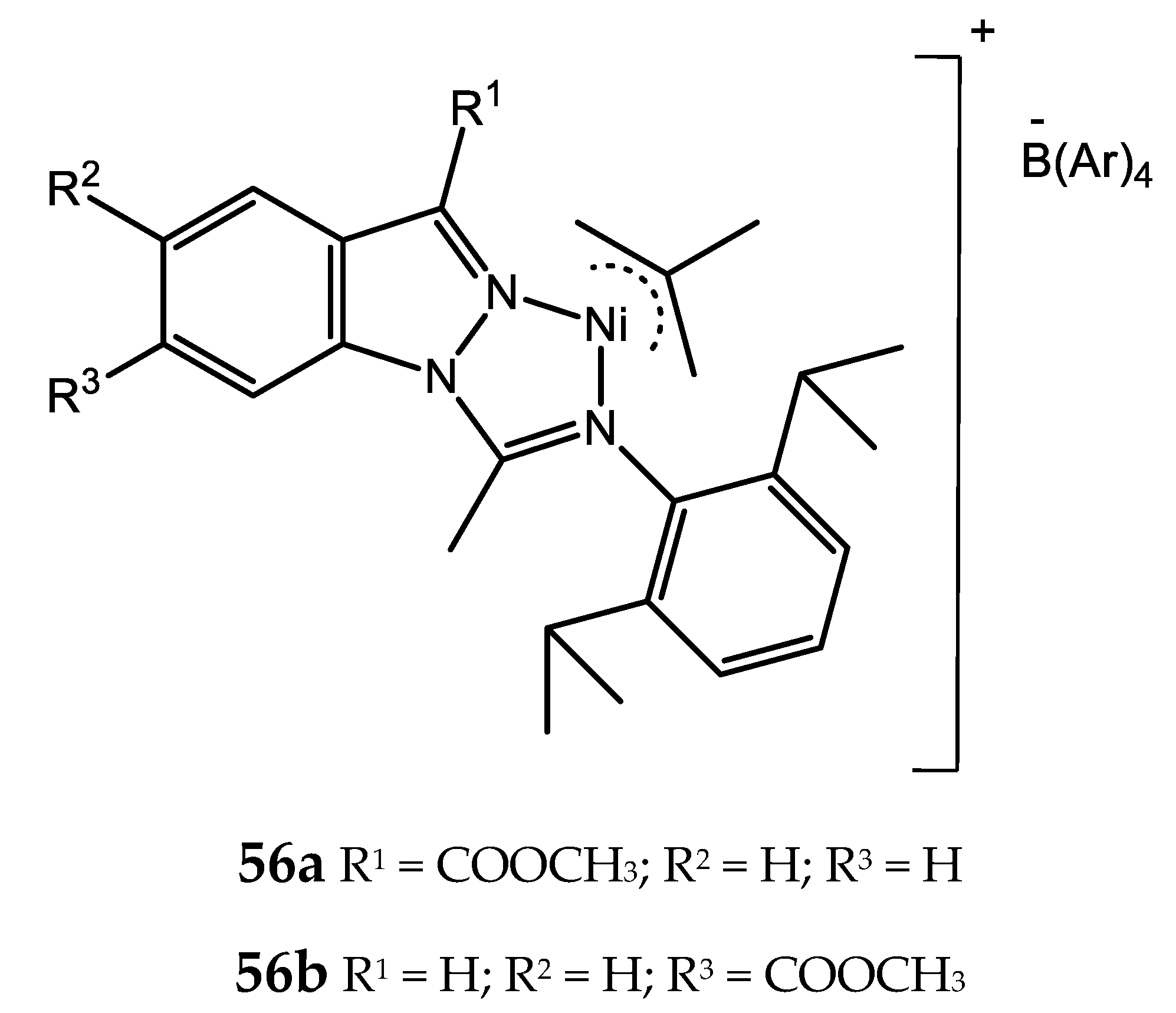
4. Nickel Complexes Bearing Pyrazolyl Ligands
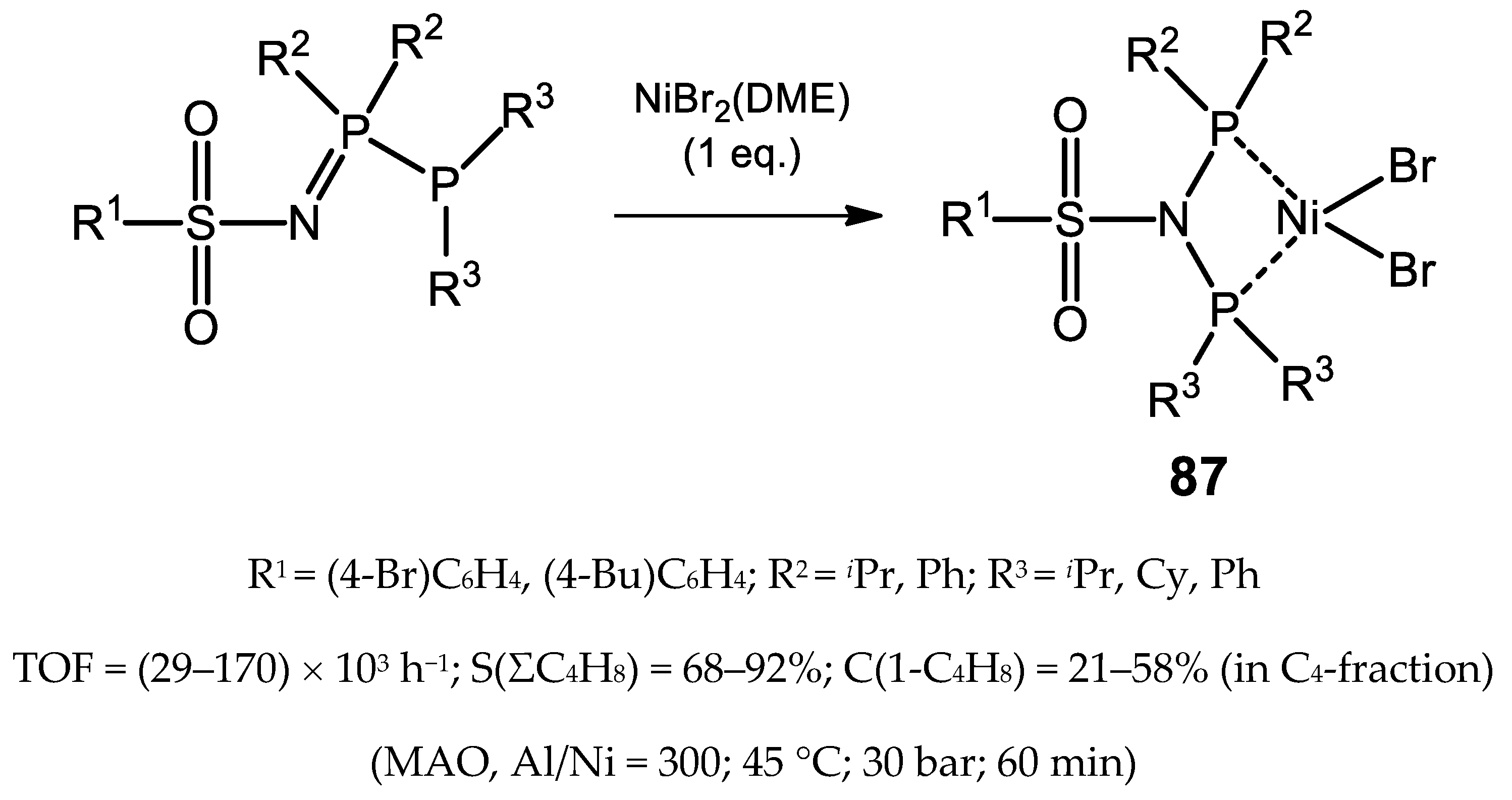
5. Nickel Complexes Bearing Hybrid Phosphorus and Nitrogen Containing Ligands
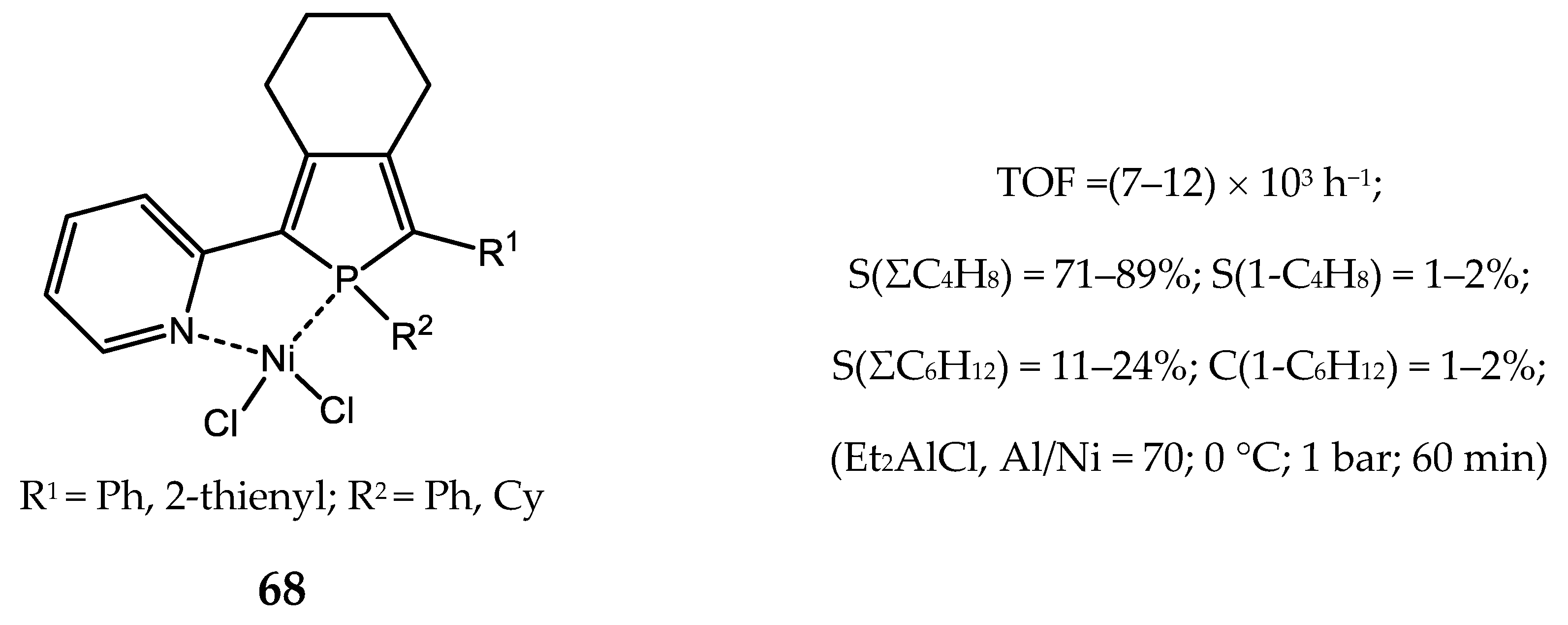
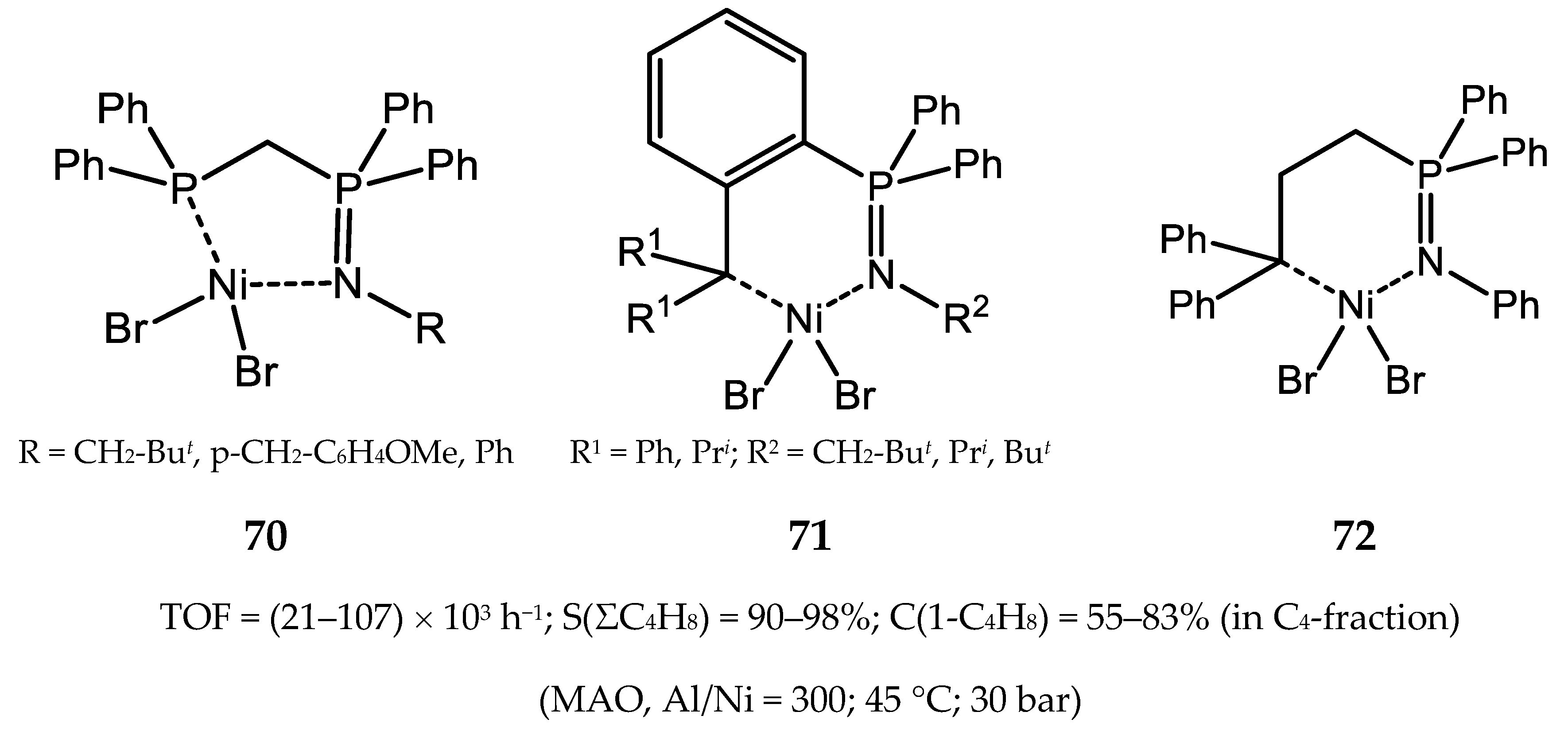
5.1. Nickel(II) Complexes Bearing Phosphine and Phosphinite Ligands
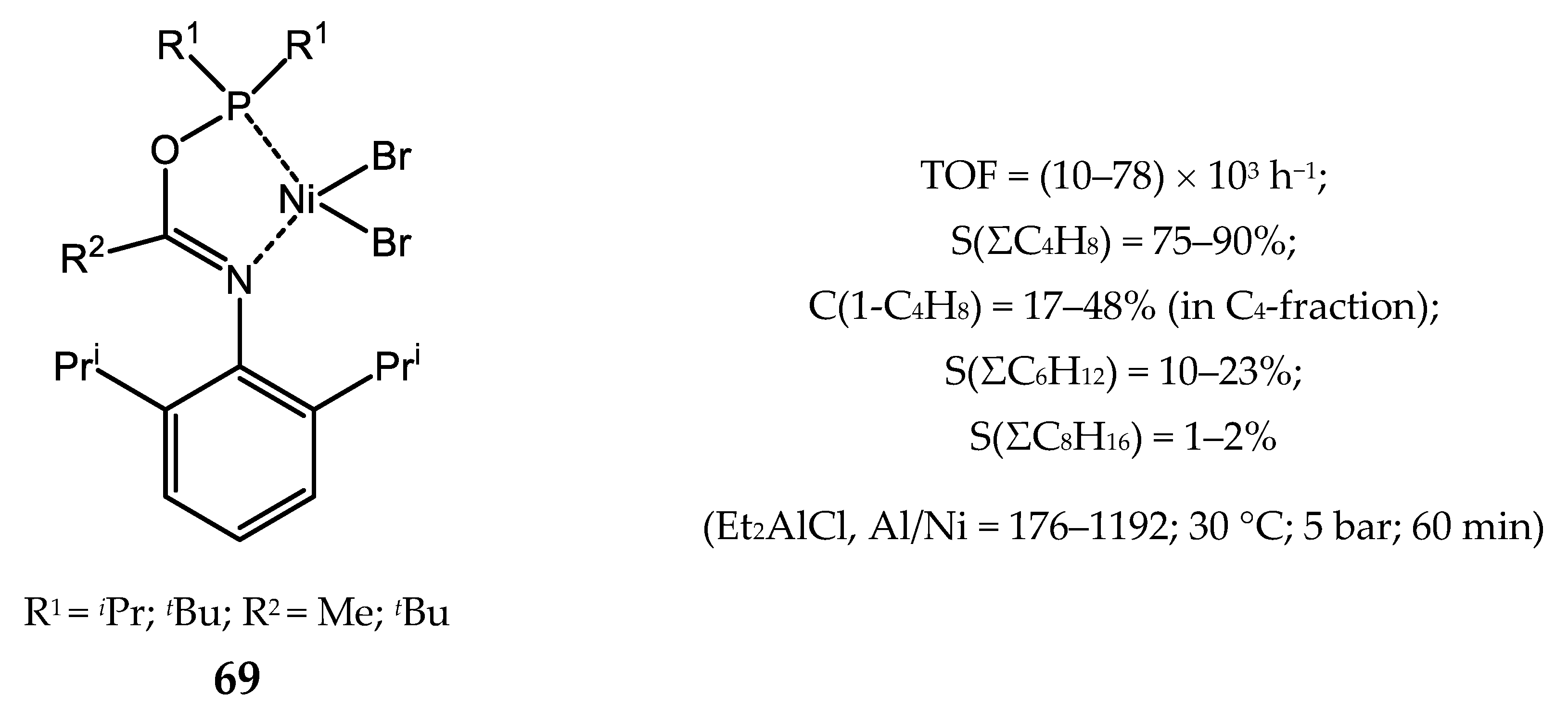
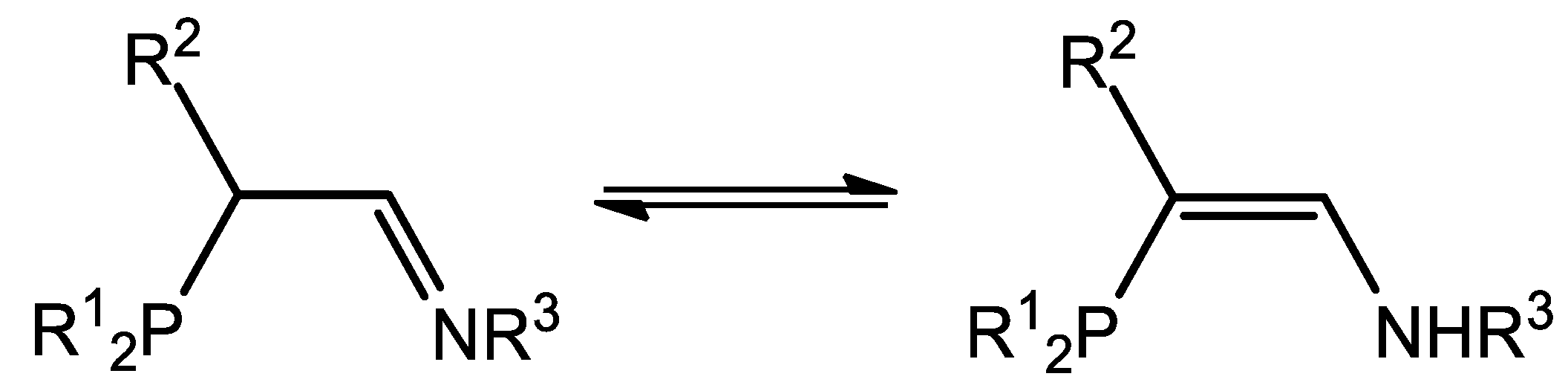
5.2. Nickel Complexes with Phosphinito-Imine Ligands
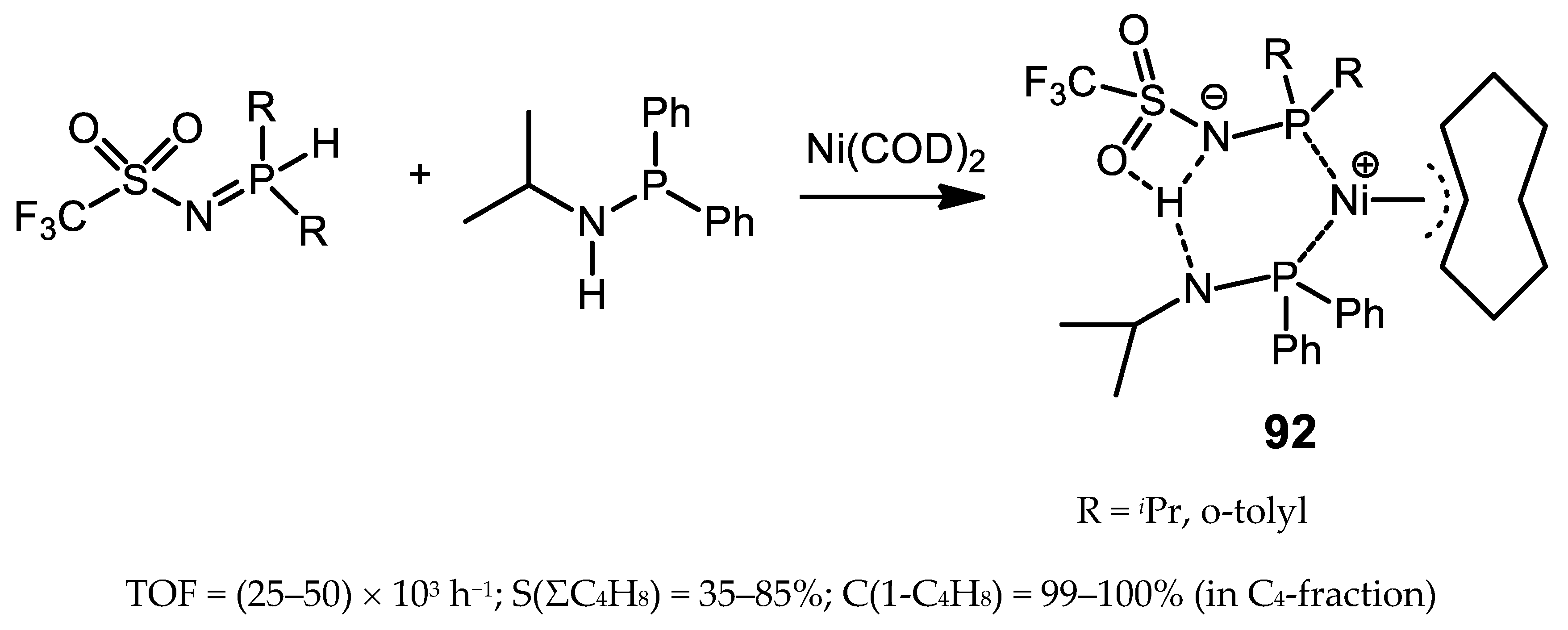
5.3. Nickel Complexes with Phosphinoiminophosphorane Ligands
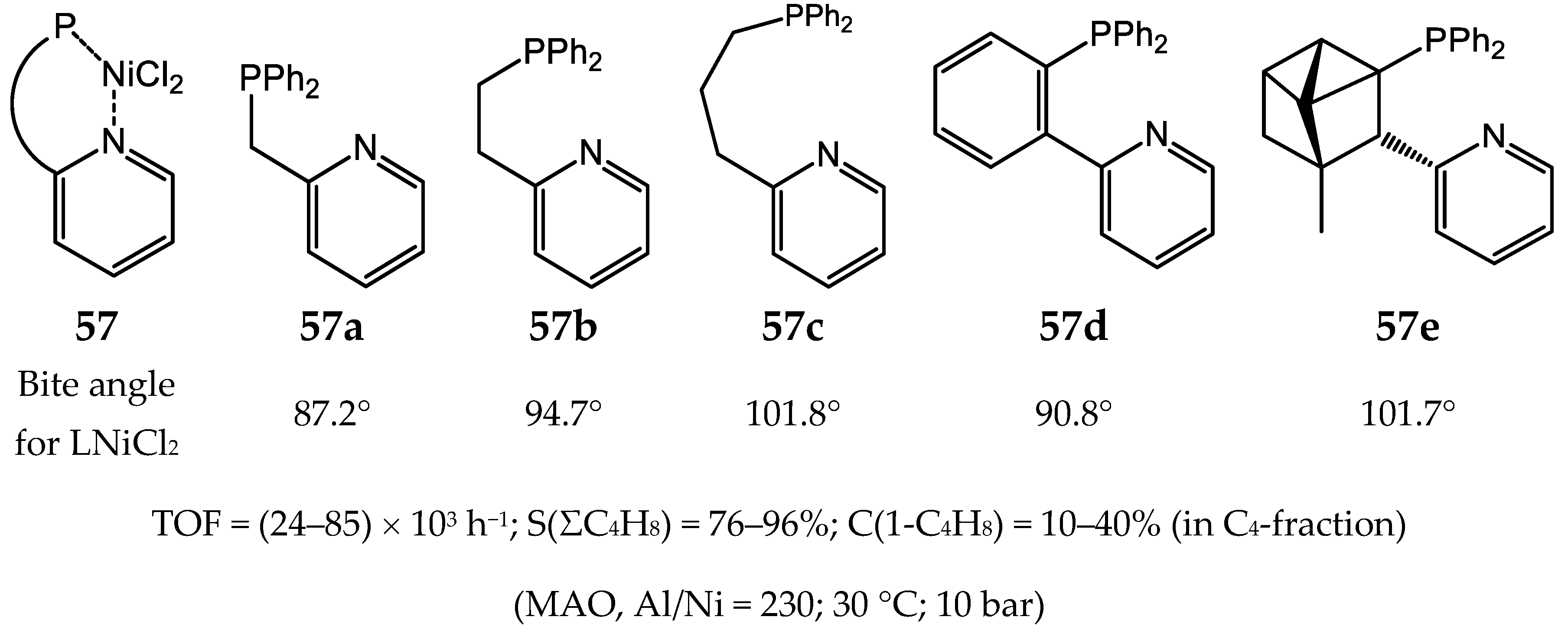
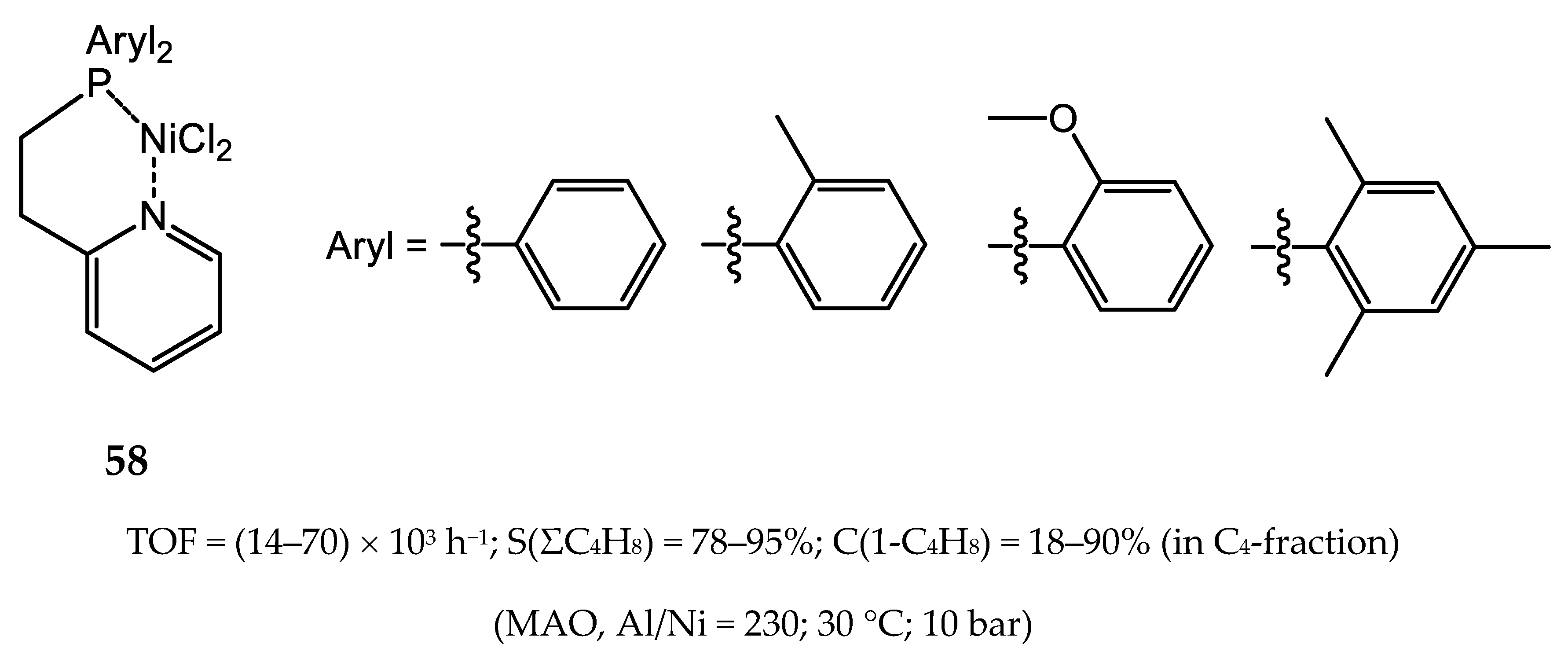
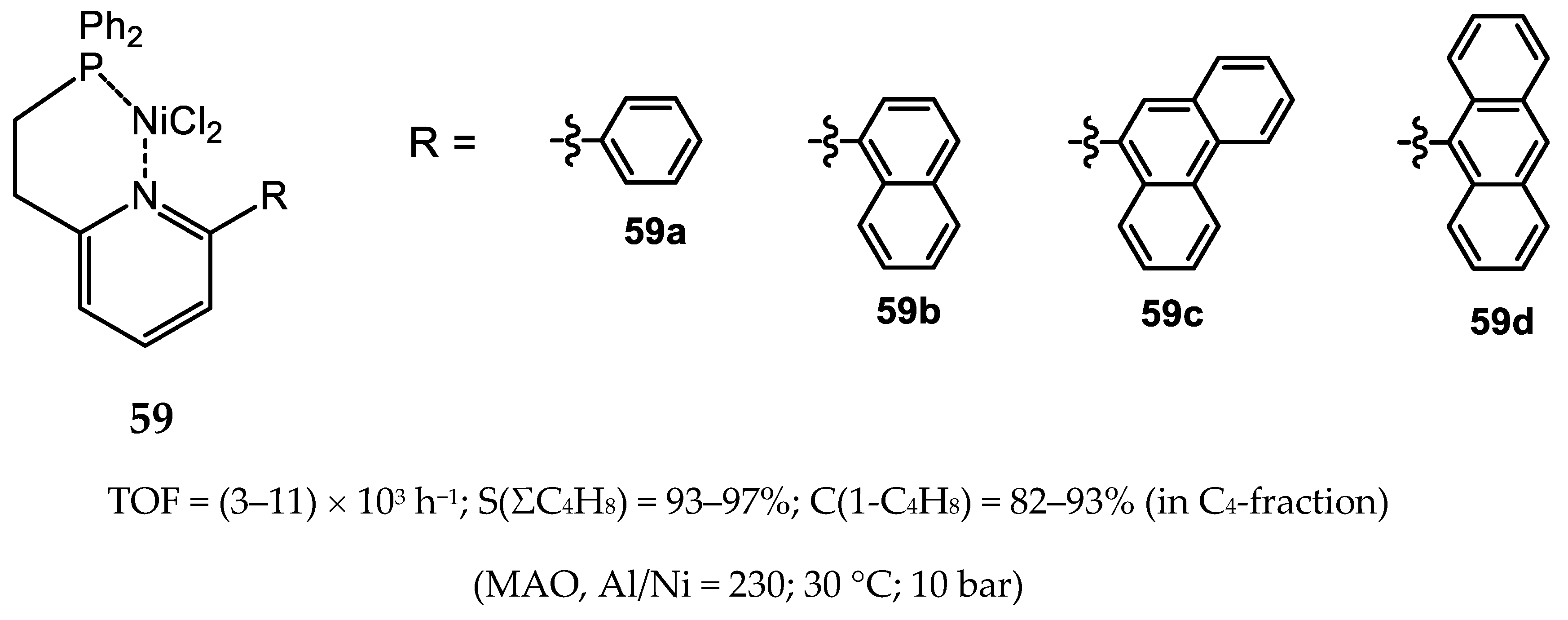
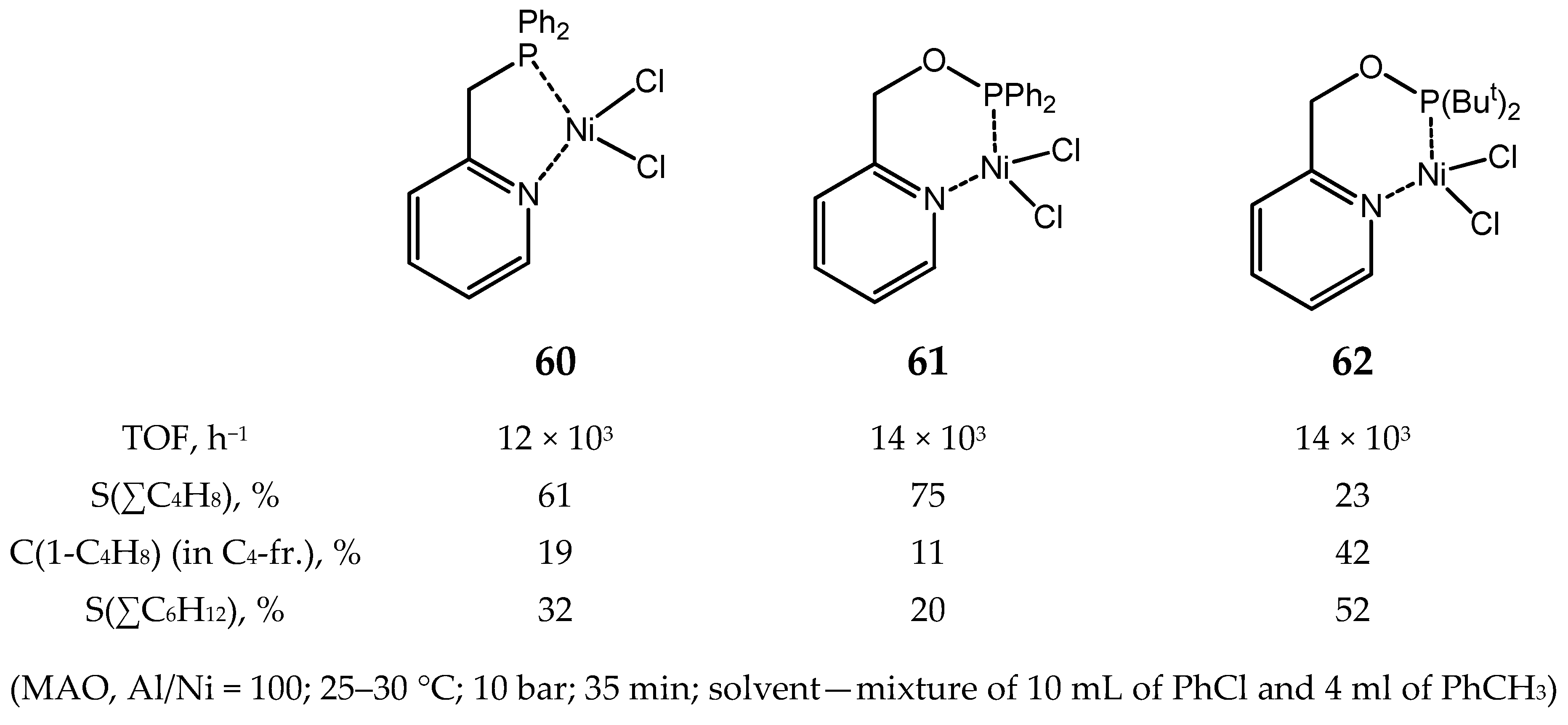
6. Diphosphine Nickel Complexes
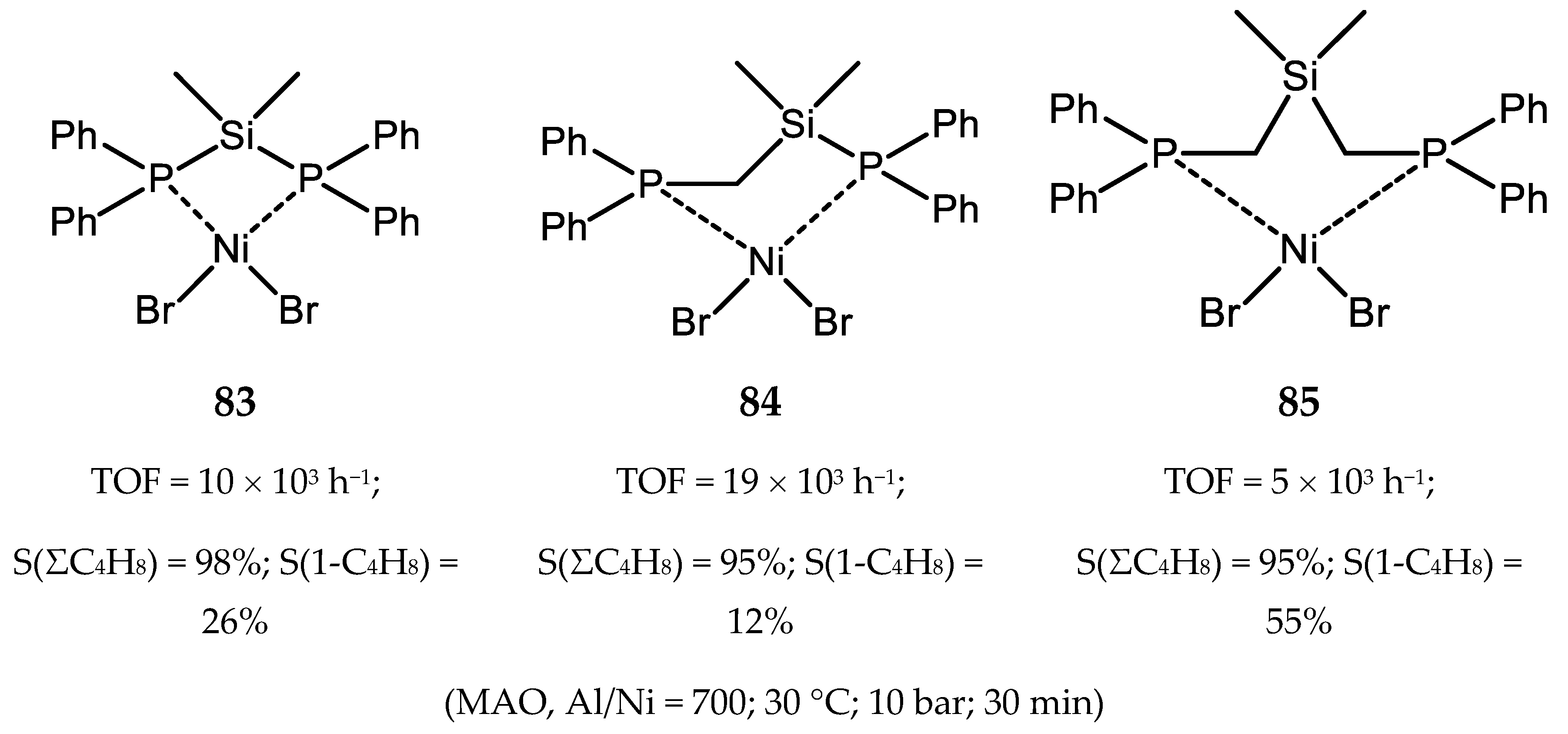
6.1. Nickel(II) Complexes with N- and Si-Bridged Diphosphine Ligands
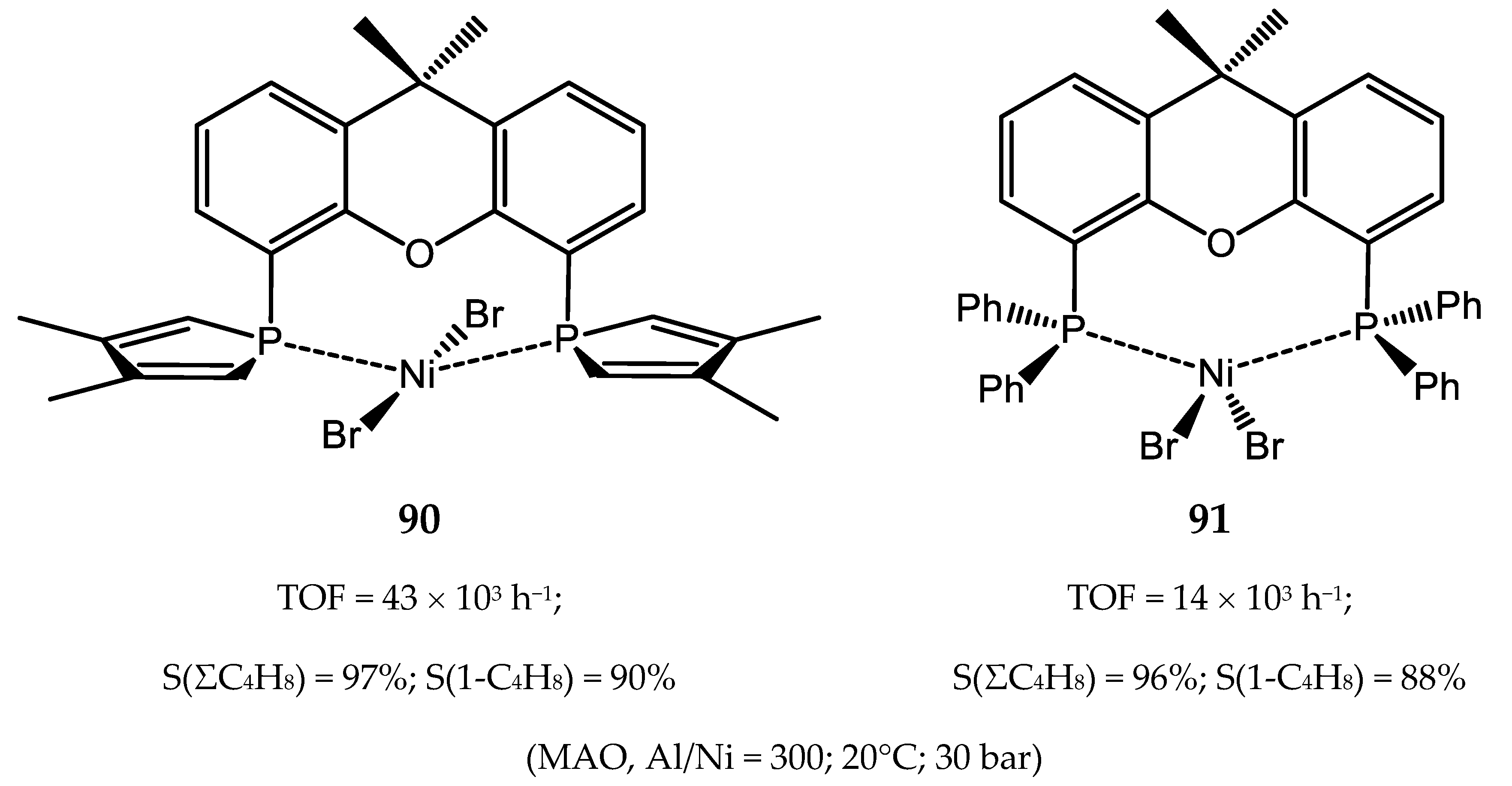
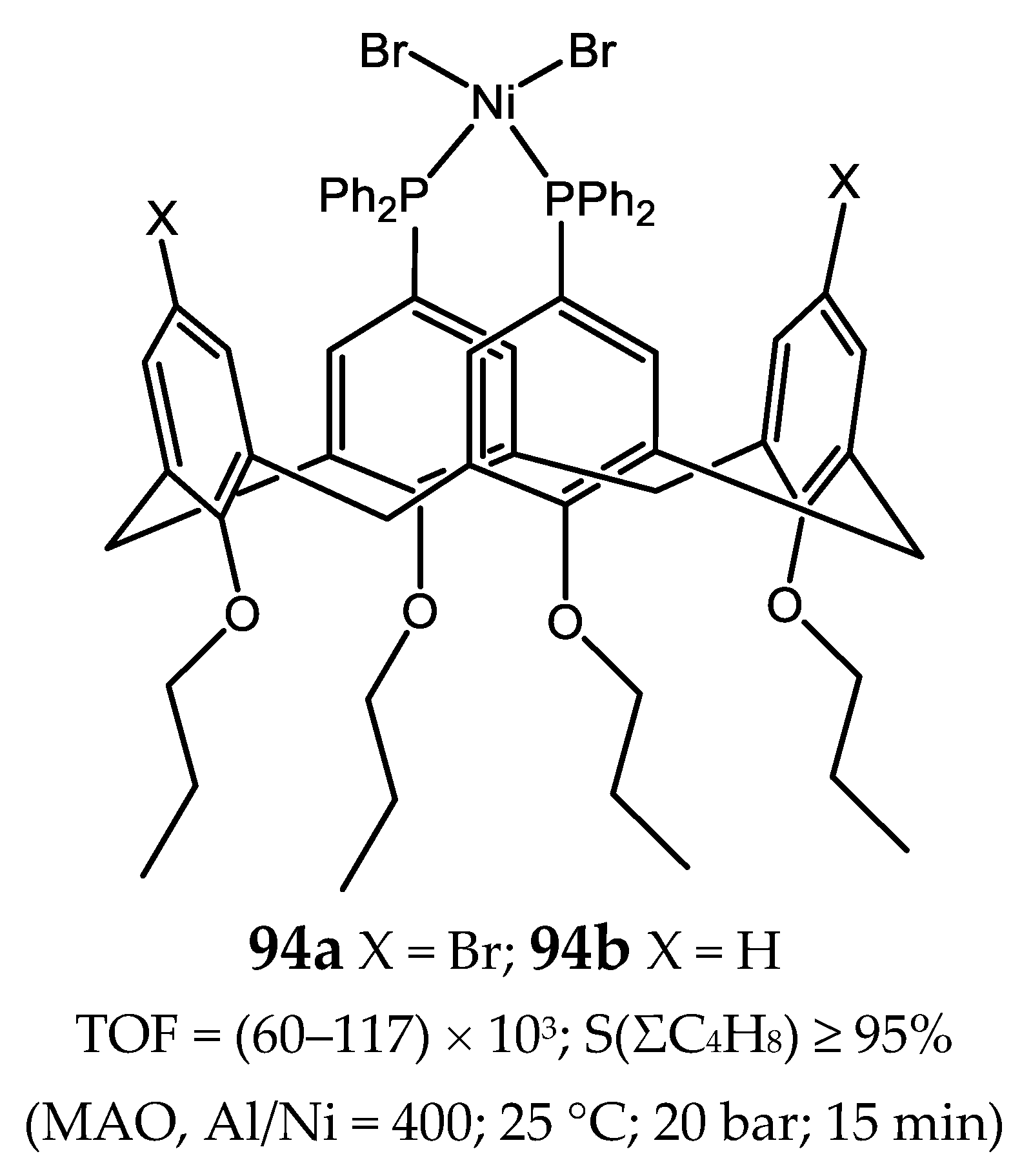
6.2. Nickel(II) Complexes with Xantphos-Type Ligands
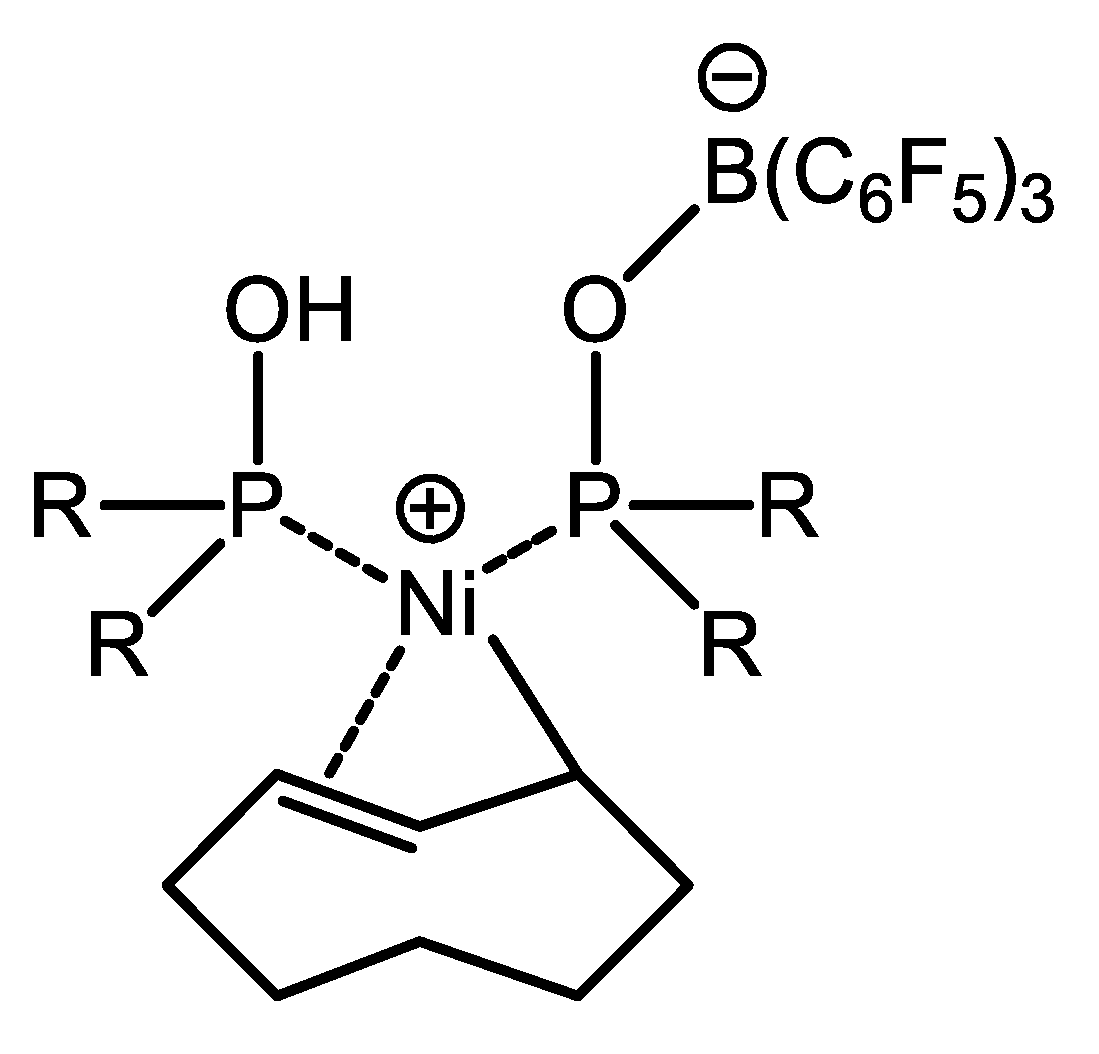
6.3. Nickel Complexes with Supramolecular Diphosphine Ligands
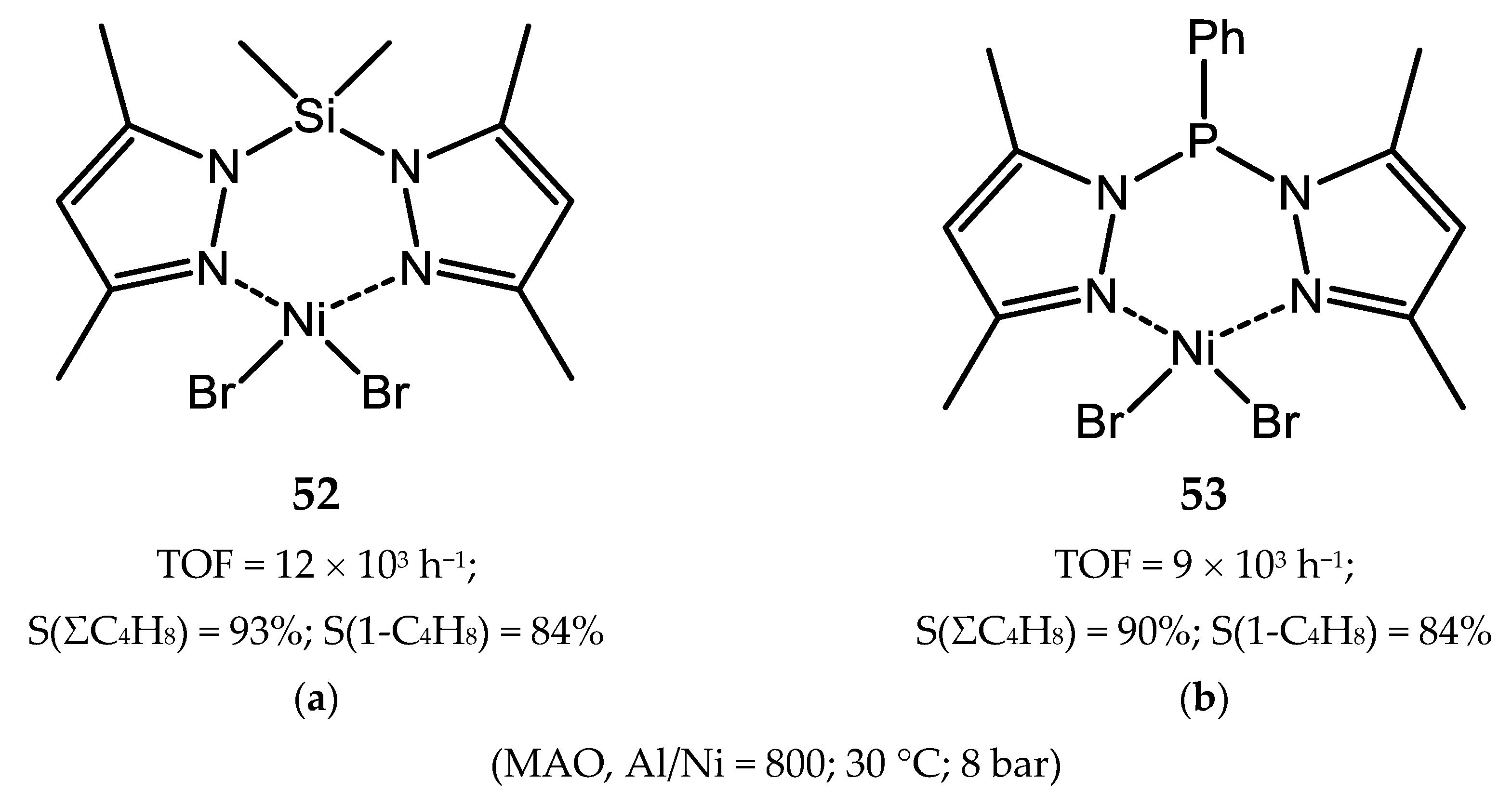
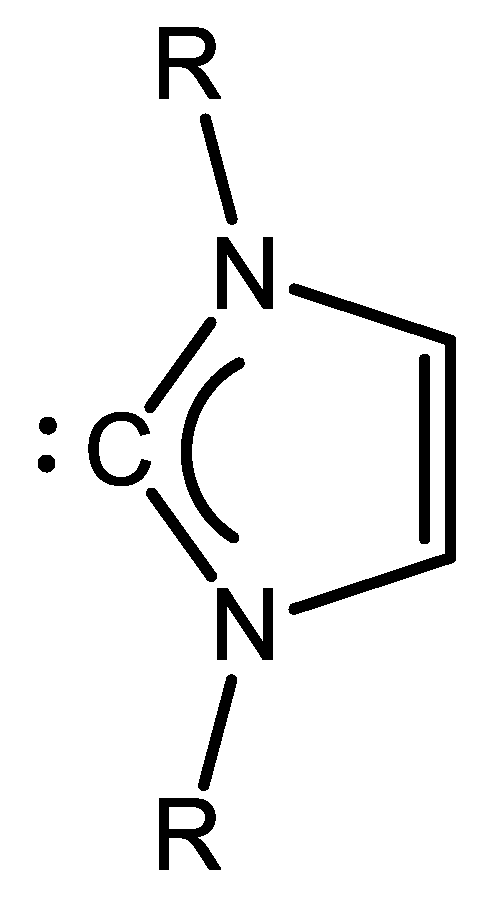
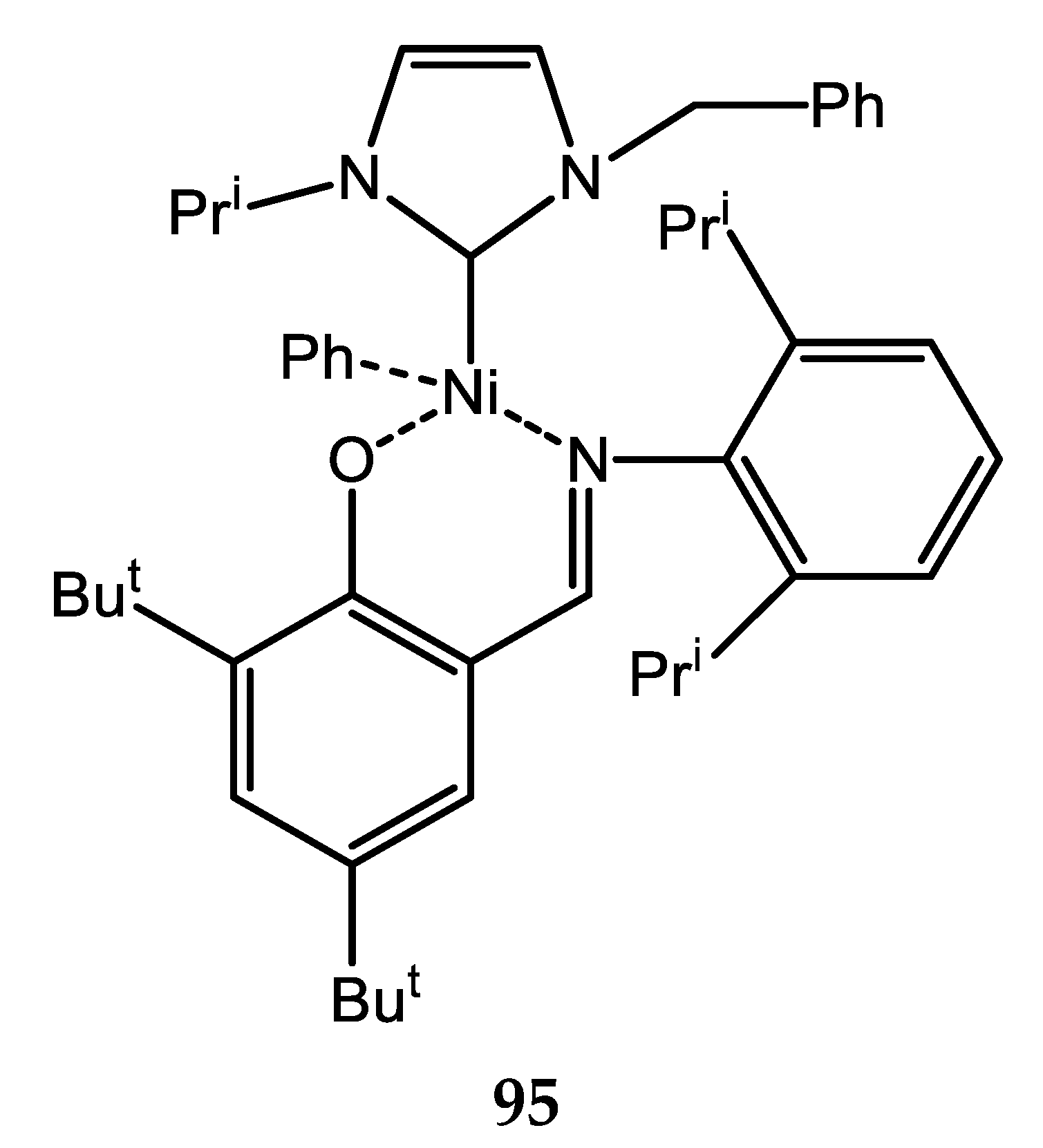
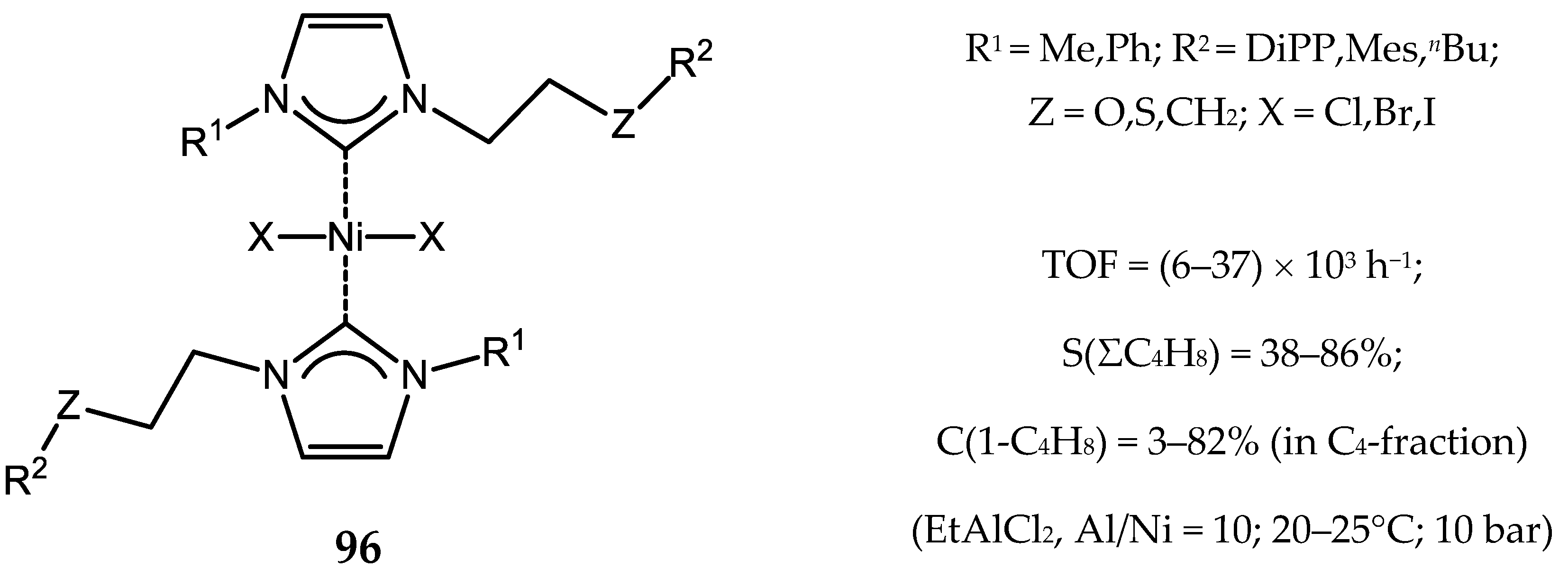
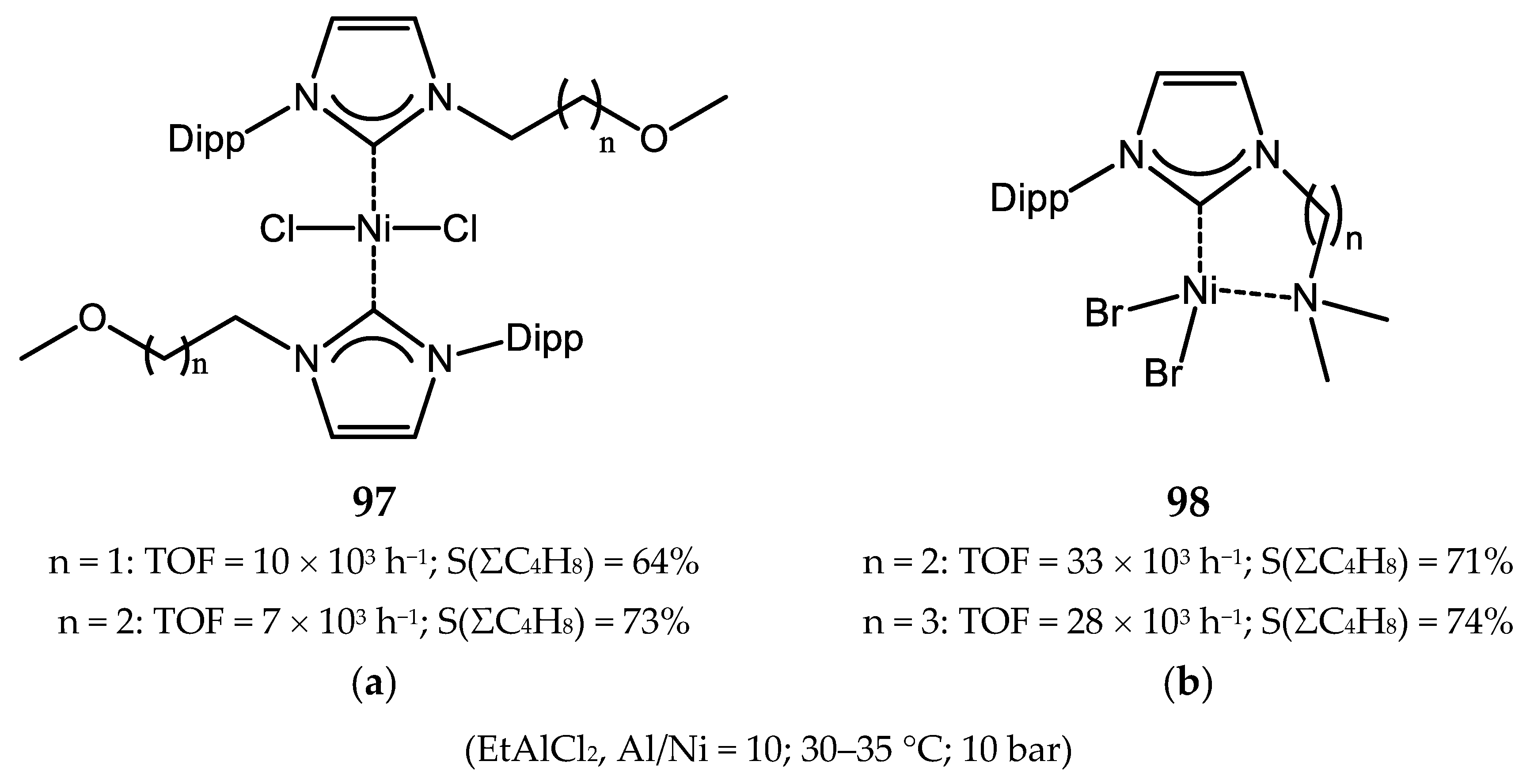
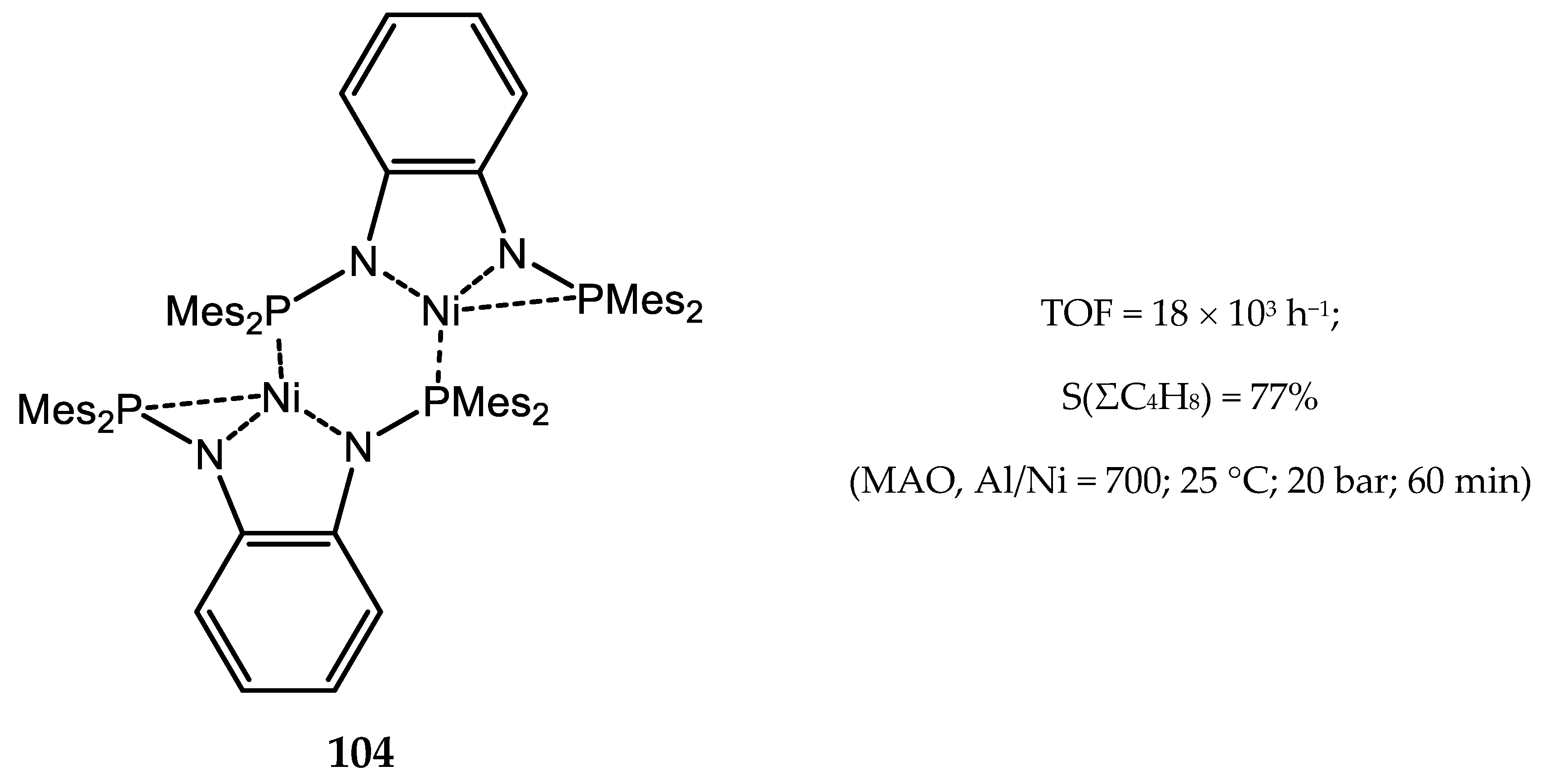
7. Nickel Complexes with N-Heterocyclic Carbene Ligands
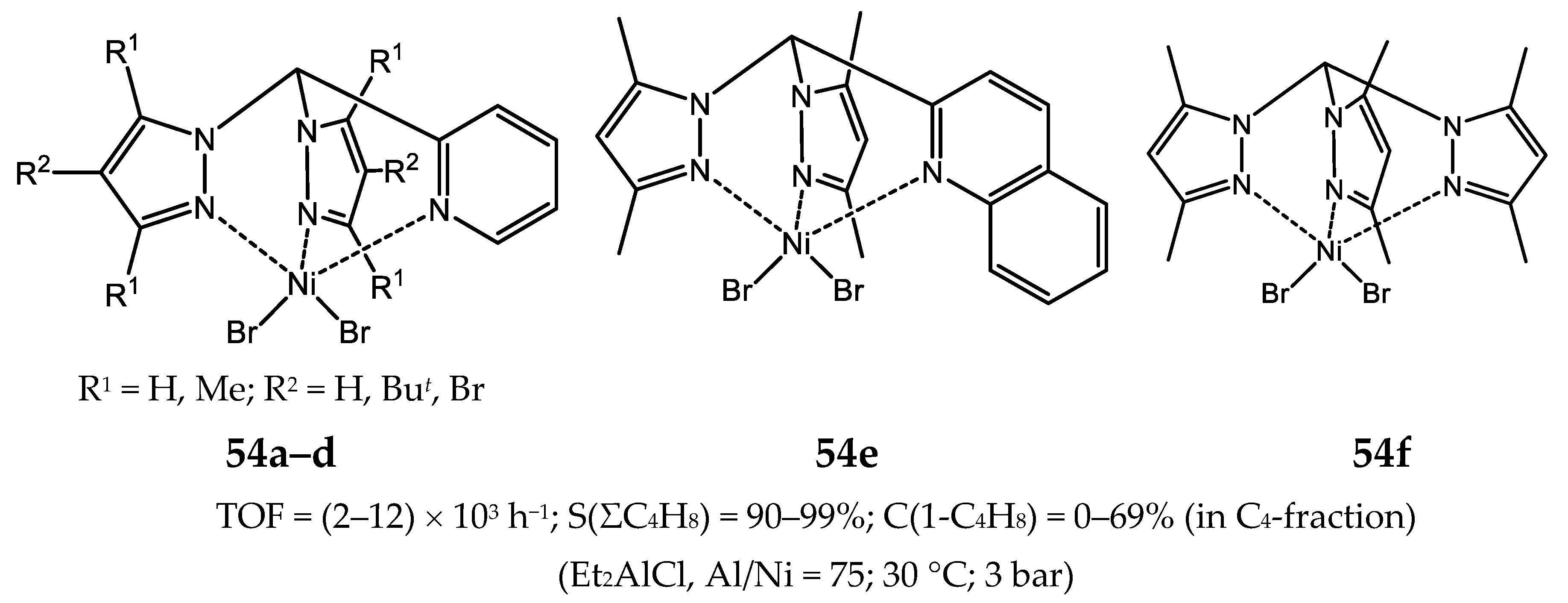
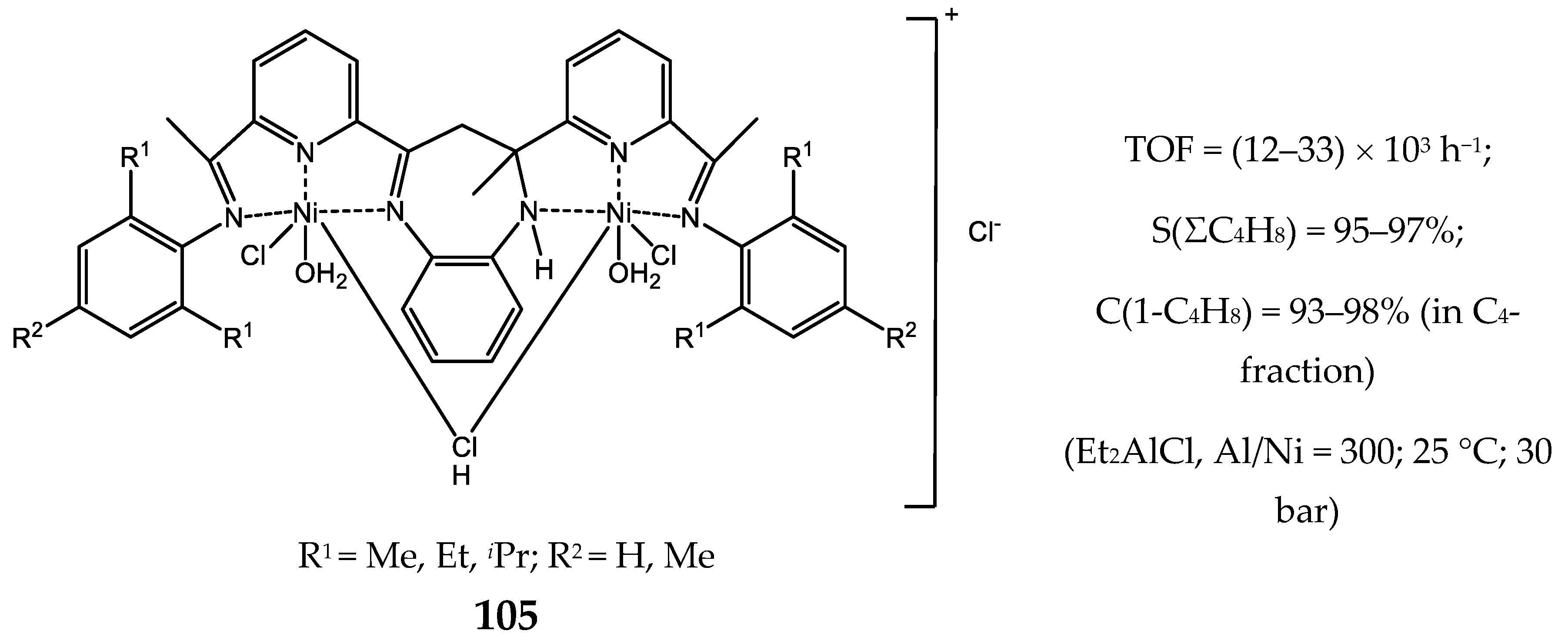
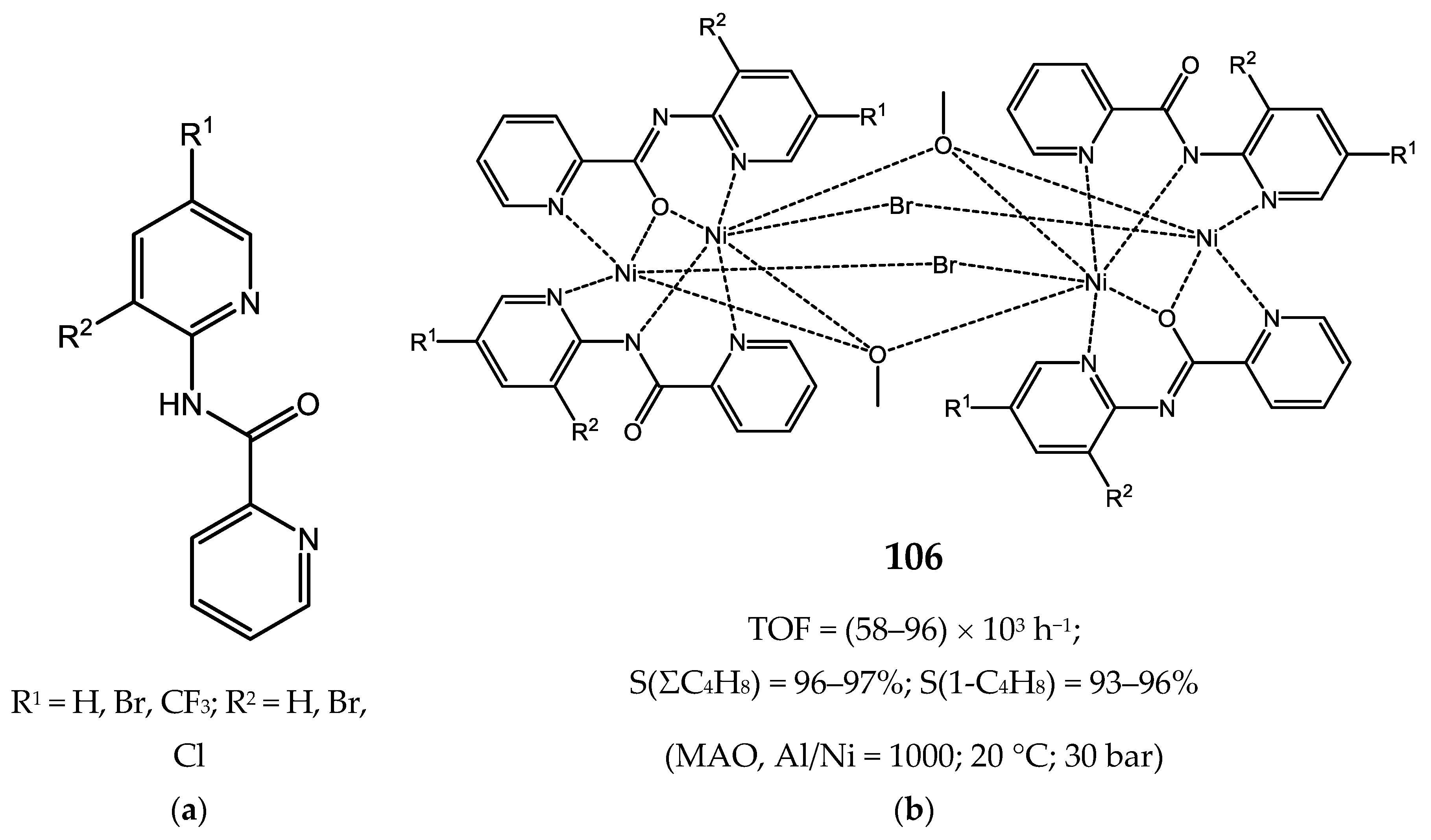
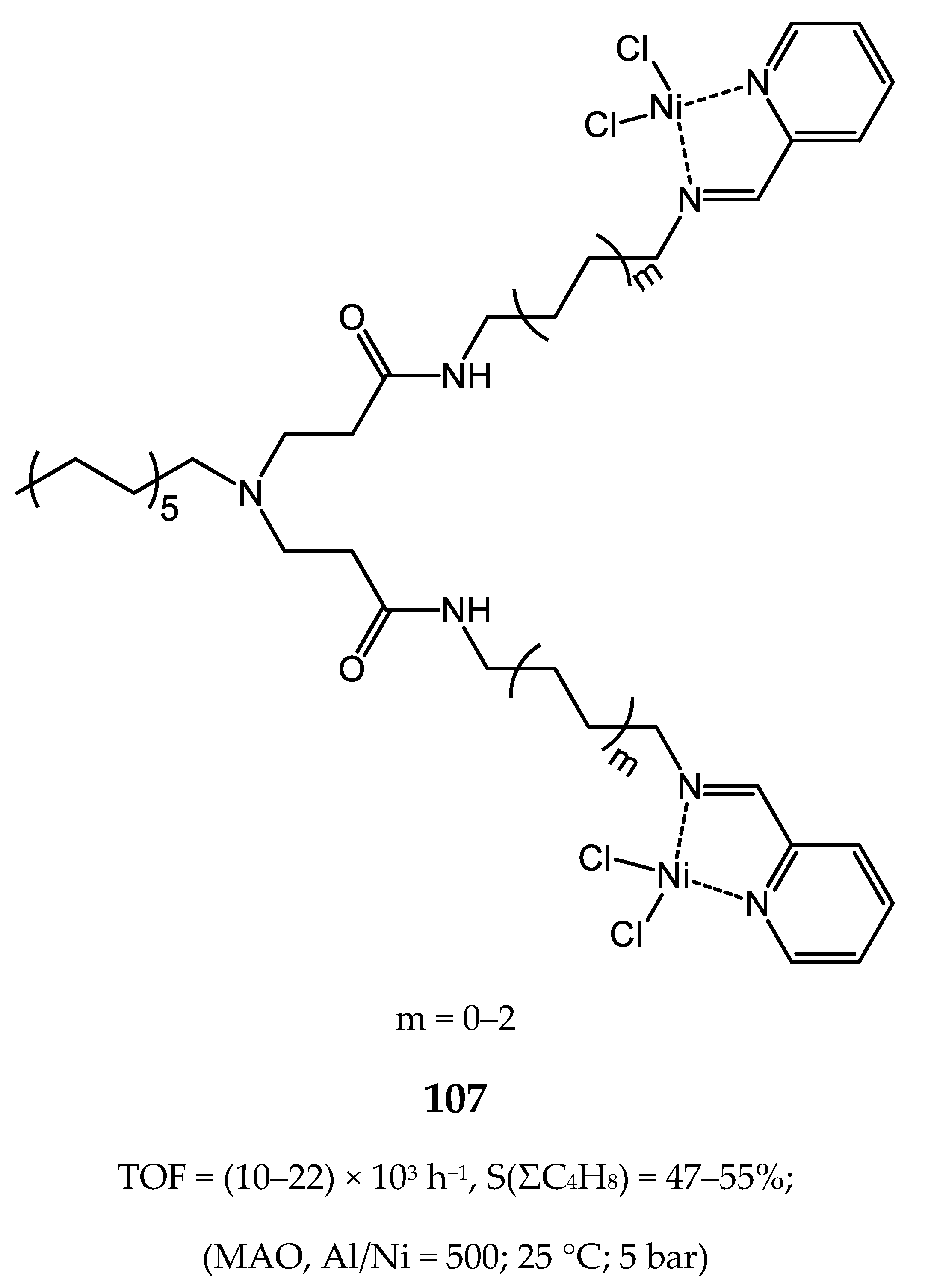
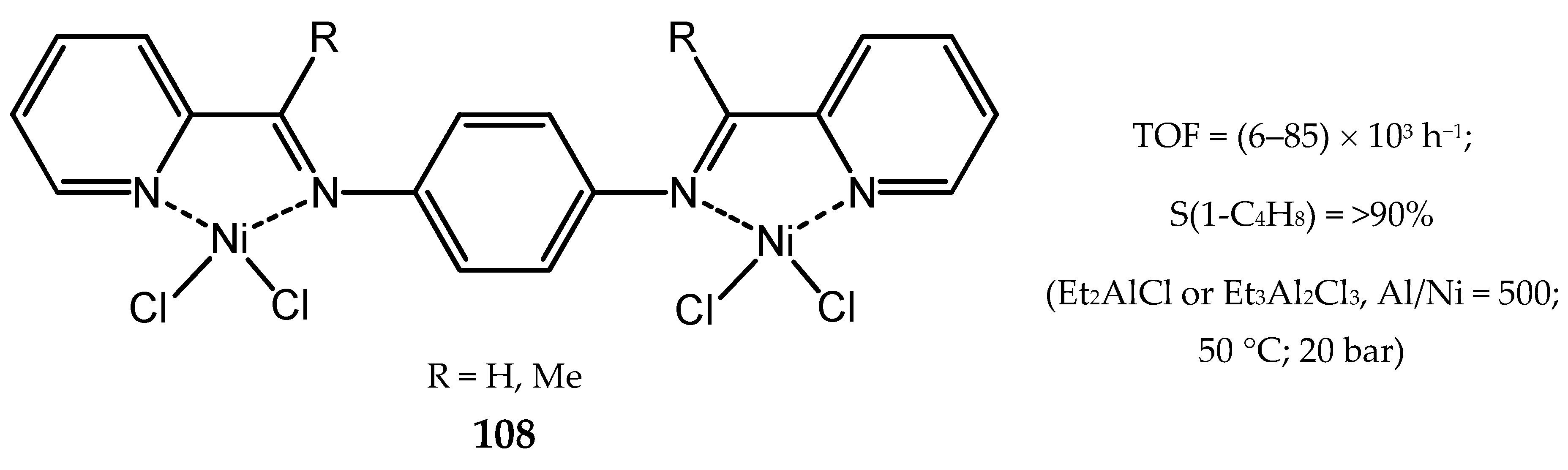
8. Bi- and Multinuclear Nickel Complexes
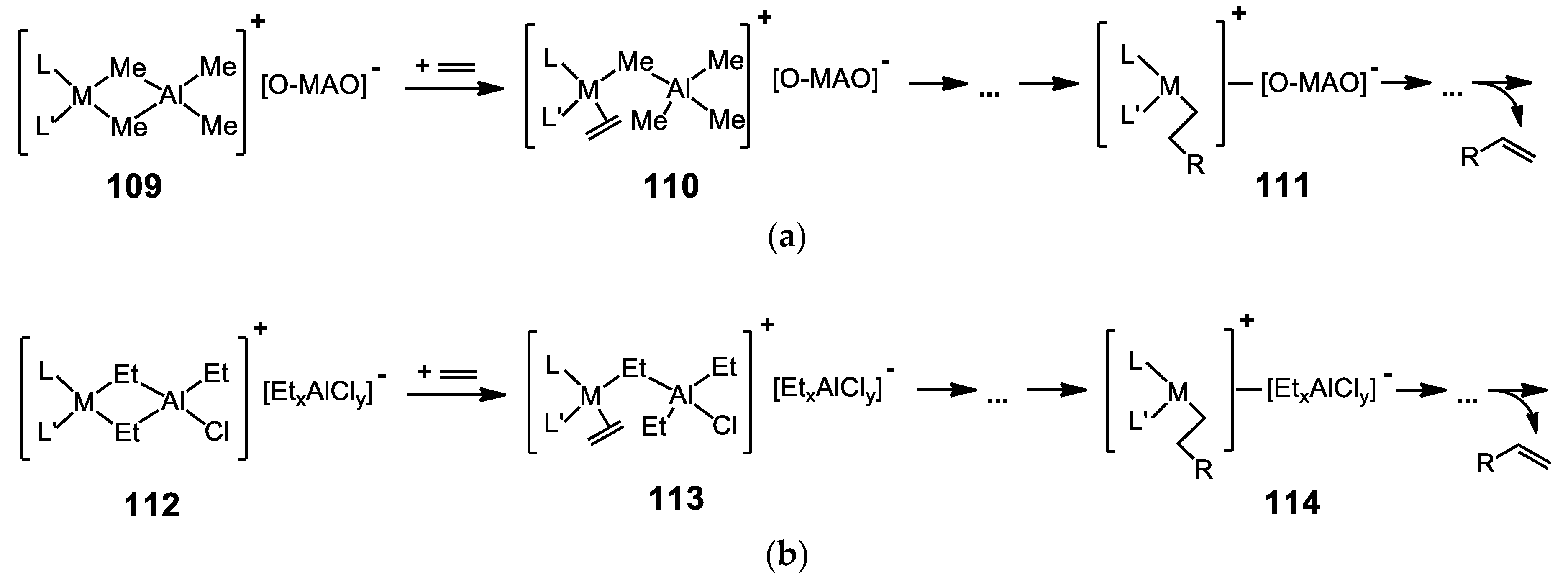
9. Effect of the co-Catalyst on the Catalytic Properties
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Camara Greiner, E.O.; Inoguchi, Y. Chemical Economics Handbook. Linear Alpha-Olefins (681.5030); SRI Consulting: Menlo Park, CA, USA, 2010; pp. 1–78. [Google Scholar]
- Fischer, K.; Jonas, K.; Misbach, P.; Stabba, R.; Wilke, G. The “Nickel Effect”. Angew. Chem. Int. Ed. Engl. 1973, 12, 943–953. [Google Scholar] [CrossRef]
- Keim, W. Nickel: An element with wide application in industrial homogeneous ctalysis. Angew. Chem. Int. Ed. Engl. 1990, 29, 235–244. [Google Scholar] [CrossRef]
- Keim, W. Oligomerization of ethylene to α-olefins: Discovery and development of the Shell Higher Olefin Process (SHOP). Angew Chem. Int. Ed. 2013, 52, 12492–12496. [Google Scholar] [CrossRef]
- Breuil, P.-A.R.; Magna, L.; Olivier-Bourbigou, H. Role of homogeneous catalysis in oligomerization of olefins: Focus on selected examples based on group 4 to group 10 transition metal complexes. Catal. Lett. 2015, 145, 173–192. [Google Scholar] [CrossRef]
- Belov, G.P.; Matkovsky, P.E. Processes for the production of higher linear α-olefins. Pet. Chem. 2010, 50, 283–289. [Google Scholar] [CrossRef]
- Forestière, A.; Olivier-Bourbigou, H.; Saussine, L. Oligomerization of monoolefins by homogeneous catalysts. Oil Gas. Sci. Technol. Rev. IFP 2009, 64, 649–667. [Google Scholar] [CrossRef]
- Nomura, K.; Itagaki, M.; Ishino, M.; Yamamoto, M.; Suzukamo, G. Efficient dimerization of propylene affording 2,3-dimethylbutenes by fluorinated alcohol modified nickel-phosphine catalyst system containing strong sulfonic acid and/or dialkyl sulfates. Catal. Lett. 1997, 47, 47–49. [Google Scholar] [CrossRef]
- Volkova, A.V. The Market for Large-tonnage Polymers. Part I. Polyethylene; HSE University: St. Petersburg, Russia, 2016. [Google Scholar]
- Agapie, T. Selective ethylene oligomerization: Recent advances in chromium catalysis and mechanistic investigations. Coord. Chem. Rev. 2011, 255, 861–880. [Google Scholar] [CrossRef]
- Bryliakov, K.P.; Antonov, A.A. Recent progress of transition metal based catalysts for the selective dimerization of ethylene. J. Organomet. Chem. 2018, 867, 55–61. [Google Scholar] [CrossRef]
- Keim, W. Nickel Hydrides: Catalysis in oligomerization and polymerization reactions of olefins. Ann. N.Y. Acad. Sci. 1983, 415, 191–200. [Google Scholar] [CrossRef]
- Cossee, P. Ziegler-Natta catalysis I. Mechanism of polymerization of α-olefins with Ziegler-Natta catalysts. J. Catal. 1964, 3, 80–88. [Google Scholar] [CrossRef]
- Arlman, E.; Cossee, P. Ziegler-Natta catalysis III. Stereospecific polymerization of propene with the catalyst system TiCl3-AlEt3. J. Catal. 1964, 3, 99–104. [Google Scholar] [CrossRef]
- Skupinska, J. Oligomerization of α-olefins to higher oligomers. Chem. Rev. 1991, 91, 613–648. [Google Scholar] [CrossRef]
- Forget, S.; Olivier-Bourbigou, H.; Delcroix, D. Homogeneous and heterogeneous nickel-catalyzed olefin oligomerization: Experimental investigation for a common mechanistic proposition and catalyst optimization. ChemCatChem 2017, 9, 2408–2417. [Google Scholar] [CrossRef]
- Johnson, L.K.; Killian, C.M.; Brookhart, M. New Pd(II)- and Ni(II)-based catalysts for polymerization of ethylene and α-olefins. J. Am. Chem. Soc. 1995, 117, 6414–6415. [Google Scholar] [CrossRef]
- Britovsek, G.J.; Baugh, S.P.; Hoarau, O.; Gibson, V.C.; Wass, D.F.; White, A.J.; Williams, D.J. The role of bulky substituents in the polymerization of ethylene using late transition metal catalysts: A comparative study of nickel and iron catalyst systems. Inorg. Chim. Acta 2003, 345, 279–291. [Google Scholar] [CrossRef]
- Wang, Z.; Solan, G.A.; Zhang, W.; Sun, W.-H. Carbocyclic-fused N,N,N-pincer ligands as ring-strain adjustable supports for iron and cobalt catalysts in ethylene oligo-/polymerization. Coord. Chem. Rev. 2018, 363, 92–108. [Google Scholar] [CrossRef]
- Bryliakov, K.P.; Talsi, E.P. Frontiers of mechanistic studies of coordination polymerization and oligomerization of α-olefins. Coord. Chem. Rev. 2012, 256, 2994–3007. [Google Scholar] [CrossRef]
- Budagumpi, S.; Keri, R.S.; Biffis, A.; Patil, S.A. Olefin poly/oligomerizations by metal precatalysts bearingnon–heterocyclic N–donor ligands. Appl. Catal. A 2017, 535, 32–60. [Google Scholar] [CrossRef]
- Bianchini, C.; Giambastiani, G.; Mantovani, G.; Meli, A.; Mimeau, D. Oligomerisation of ethylene to linear α-olefins by tetrahedral cobalt(II) precursors stabilised by benzo[b]thiophen-2-yl-substituted (imino)pyridine ligands. J. Organomet. Chem. 2004, 689, 1356–1361. [Google Scholar] [CrossRef]
- Bianchini, C.; Giambastiani, G.; Guerrero Rios, I.; Mantovani, G.; Meli, A.; Segarra, A.M. Ethylene oligomerization, homopolymerization and copolymerization by iron and cobalt catalysts with 2,6-(bis-organylimino)pyridyl ligands. Coord. Chem. Rev. 2006, 250, 1391–1418. [Google Scholar] [CrossRef]
- Bianchini, C.; Gatteschi, D.; Giambastiani, G.; Guerrero Rios, I.; Ienco, A.; Laschi, F.; Mealli, C.; Meli, A.; Sorace, L.; Toti, A.; et al. Electronic influence of the thienyl sulfur atom on the oligomerization of ethylene by cobalt(II) 6-(thienyl)-2-(imino)pyridine catalysis. Organometallics 2007, 26, 726–739. [Google Scholar] [CrossRef]
- Antonov, A.A.; Semikolenova, N.V.; Talsi, E.P.; Matsko, M.A.; Zakharov, V.A.; Bryliakov, K.P. 2-Iminopyridine nickel(II) complexes bearing electron-withdrawing groups in the ligand core: Synthesis, characterization, ethylene oligo- and polymerization behaviour. J. Organomet. Chem. 2016, 822, 241–249. [Google Scholar] [CrossRef]
- Yakhvarov, D.G.; Tazeev, D.I.; Sinyashin, O.G.; Giambastiani, G.; Bianchini, C.; Segarra, A.M.; Lönnecke, P.; Hey-Hawkins, E. Electrochemical synthesis of the σ-aryl complex [NiBr(Mes)(bpy)] and its use as catalyst precursor for the oligomerization of ethylene (Mes = 2,4,6-trimethylphenyl, bpy = 2,20-bipyridine). Polyhedron 2006, 25, 1607–1612. [Google Scholar] [CrossRef]
- Yakhvarov, D.G.; Trofimova, E.A.; Rizvanov, I.K.; Fomina, O.S.; Sinyashin, O.G. Electrochemical synthesis and catalytic activity of organonickel sigma-complexes. Russ. J. Electrochem. 2011, 47, 1100–1110. [Google Scholar] [CrossRef]
- Yakhvarov, D.G.; Khusnuriyalova, A.F.; Sinyashin, O.G. Electrochemical synthesis and properties of organonickel σ-complexes. Organometallics. 2014, 33, 4574–4589. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Q.; Solan, G.A.; Sun, W.-H. Recent advances in Ni-mediated ethylene chain growth: Nimine-donor ligand effects on catalytic activity, thermal stability and oligo-/polymer structure. Coord. Chem. Rev. 2017, 350, 68–83. [Google Scholar] [CrossRef]
- Wang, S.; Sun, W.-H.; Redshaw, C. Recent progress on nickel-based systems for ethylene oligo-/polymerization catalysis. J. Organomet. Chem. 2014, 751, 717–741. [Google Scholar] [CrossRef]
- Bianchini, C.; Giambastiani, G.; Luconi, L.; Meli, A. Olefin oligomerization, homopolymerization and copolymerization by late transition metals supported by (imino)pyridine ligands. Coord. Chem. Rev. 2010, 254, 431–455. [Google Scholar] [CrossRef]
- Killian, C.M.; Johnson, L.K.; Brookhart, M. Preparation of linear α-olefins using cationic nickel(II) α-diimine catalysts. Organometallics 1997, 16, 2005–2007. [Google Scholar] [CrossRef]
- Song, S.; Xiao, T.; Liang, T.; Wang, F.; Redshaw, C.; Sun, W.H. Synthesis, characterization and ethylene oligomerization behaviour of 8-(1-aryliminoethylidene)quinaldinylnickel dihalides. Catal. Sci. Technol. 2011, 1, 69–75. [Google Scholar] [CrossRef]
- Sun, W.-H.; Wang, K.; Wedeking, K.; Zhang, D.; Zhang, S.; Cai, J.; Li, Y. Synthesis, characterization, and ethylene oligomerization of nickel complexes bearing N-((pyridin-2-yl)methylene)quinolin-8-amine derivatives. Organometallics 2007, 26, 4781–4790. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, S.; Deng, Y.; Jie, S.; Li, L.; Lu, X.; Sun, W.-H. 2,9-disubstituted 1,10-phenanthroline nickel complexes: Syntheses, characterization, and their ethylene oligomerization. Kinet. Catal. 2007, 48, 664–668. [Google Scholar] [CrossRef]
- Gao, R.; Xiao, L.; Hao, X.; Sun, W.-H.; Wang, F. Synthesis of benzoxazolylpyridine nickel complexes and their efficient dimerization of ethylene to α-butene. Dalton Trans. 2008, 5645–5651. [Google Scholar] [CrossRef] [PubMed]
- Hao, P.; Zhang, S.; Sun, W.-H.; Shi, Q.; Adewuyi, S.; Lu, X.; Li, P. Synthesis, characterization and ethylene oligomerization studies of nickel complexes bearing 2-benzimidazolylpyridine derivatives. Organometallics 2007, 26, 2439–2446. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, S.; Hao, P.; Jie, S.; Sun, W.-H.; Li, P.; Lu, X. Nickel complexes bearing 2-(benzimidazol-2-yl)-1,10-phenanthrolines: Synthesis, characterization and their catalytic behavior toward ethylene oligomerization. Eur. J. Inorg. Chem. 2007, 2007, 3816–3826. [Google Scholar] [CrossRef]
- Wei, X.; Jiang, Y.; Cui, X.; Li, Y.; Wang, H.; Qi, X. 2-Benzoimidazol-8-alkylquinolinylnickel(II) complexes as efficient catalysts for ethylene oligomerization and vinyl polymerization of norbornene. J. Coord. Chem. 2015, 68, 3825–3838. [Google Scholar] [CrossRef]
- Song, S.; Li, Y.; Redshaw, C.; Wang, F.; Sun, W.-H. 2-(1-Aryliminoethylidene)quinolylnickel(II) dibromides: Synthesis, characterization and ethylene dimerization capability. J. Organomet. Chem. 2011, 696, 3772–3778. [Google Scholar] [CrossRef]
- Chai, W.; Yu, J.; Wang, L.; Hu, X.; Redshaw, C.; Sun, W.-H. Synthesis, characterization and ethylene oligomerization behavior of N-(2-alkyl-5,6,7-trihydroquinolin-8-ylidene)arylaminonickel(II) dichlorides. Inorg. Chim. Acta. 2012, 385, 21–26. [Google Scholar] [CrossRef]
- Antonov, A.A.; Semikolenova, N.V.; Soshnikov, I.E.; Talsi, E.P.; Bryliakov, K.P. Selective ethylene dimerization into 2-butenes using homogeneous and supported nickel(II) 2-iminopyridine catalysts. Top. Catal. 2020, 63, 222–228. [Google Scholar] [CrossRef]
- Hao, P.; Song, S.; Xiao, T.; Li, Y.; Redshaw, C.; Sun, W.-H. Highly active 8-benzoxazolyl- or 8-benzothiazolyl-2-alkylquinolinylnickel(II) complexes for ethylene dimerization and vinyl polymerization of norbornene. Polyhedron 2013, 52, 1138–1144. [Google Scholar] [CrossRef]
- Xiao, L.; Jie, S.; Song, Y.; Cao, X.; Sun, W.-H. Transformation of 2-alkoxyimidate-1,10-phenanthroline metal (Mn2+, Co2+ and Ni2+) chlorides from bis(2-cyano-1,10-phenanthroline) metal chlorides: Syntheses, characterizations and their catalytic behavior toward ethylene oligomerization. J. Organomet. Chem. 2008, 693, 3858–3866. [Google Scholar] [CrossRef]
- Milani, J.L.S.; Casagrande, A.C.A.; Livotto, P.R.; Casagrande, O.L. Synthesis and characterization of ether-imine-furfural [ONO] nickel(II) complexes and their application in oligomerization of ethylene. Appl. Catal. A 2016, 523, 247–254. [Google Scholar] [CrossRef]
- Fernando de Souza, R. XAS study of the nickel(α-diimine) catalyst for olefin polymerization. J. Catal. 2003, 214, 165–168. [Google Scholar] [CrossRef]
- Corker, J.M.; Evans, J. EXAFS studies of the activation of homogeneous nickel catalysts for propene dimerisation by aluminium reagents. J. Chem. Soc. Chem. Commun. 1991, 1104–1106. [Google Scholar] [CrossRef]
- Cornils, B.; Herrmann, W.A.; Beller, M.; Paciello, R. Oligomerization, cyclooligomerization, dimerization. In Applied Homogeneous Catalysis with Organometallic Compounds: A Comprehensive Handbook in Four Volumes; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2018; pp. 307–409. [Google Scholar]
- Milani, J.L.S. Chromium (III) and Nickel (II) Catalysts Containing Pyrazolyl/ether and Iimine/thioether Ligands for the Selective Production of Linear α-olefins. Ph.D. Thesis, Federal University of Rio Grande do Sul, Rio Grande do Sul, Brazil, 2016. [Google Scholar]
- Jie, S.; Zhang, S.; Sun, W.-H. 2-Arylimino-9-phenyl-1,10-phenanthrolinyl-iron, -cobalt and -nickel complexes: Synthesis, characterization and ethylene oligomerization behavior. Eur. J. Inorg. Chem. 2007, 2007, 5584–5598. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, R.; Hao, X.; Sun, W.-H. 2-Oxazoline/benzoxazole-1,10-phenanthrolinylmetal (iron, cobalt or nickel) dichloride: Synthesis, characterization and their catalytic reactivity for the ethylene oligomerization. J. Organomet. Chem. 2008, 693, 3867–3877. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, P.; Zhang, C.; Li, G.; Yang, X.-J.; Wu, B.; Janiak, C. Synthesis, structure, and catalytic ethylene oligomerization of nickel complexes bearing 2-pyrazolyl substituted 1,10-phenanthroline ligands. J. Mol. Catal. A Chem. 2008, 296, 9–17. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, M.; Gao, R.; Cao, X.; Sun, W.-H. 2-(1H-2-Benzimidazolyl)-6-(1-(arylimino)ethyl)pyridylnickel complexes: Synthesis, characterization, and ethylene oligomerization. Aust. J. Chem. 2010, 63, 109–115. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, L.; Liang, T.; Hao, X.; Sun, W.-H. 2-(7-methyl-1H-benzoimidazol-2-yl)-6-(1-aryliminoethyl)pyridinylnickel complexes: Synthesis, characterization and their ethylene oligomerization. Comptes Rendus Chim. 2010, 13, 1450–1459. [Google Scholar] [CrossRef]
- Chen, Y.; Hao, P.; Zuo, W.; Gao, K.; Sun, W.-H. 2-(1-Isopropyl-2-benzimidazolyl)-6-(1-aryliminoethyl)pyridyl transition metal (Fe, Co, and Ni) dichlorides: Syntheses, characterizations and their catalytic behaviors toward ethylene reactivity. J. Organomet. Chem. 2008, 693, 1829–1840. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, M.; Liang, T.; Wang, F.; Sun, W.-H. Nickel(II) complexes chelated by 2-arylimino-6-benzoxazolylpyridine: Syntheses, characterization, and ethylene oligomerization. Organometallics 2008, 27, 5641–5648. [Google Scholar] [CrossRef]
- Adewuyi, S.; Li, G.; Zhang, S.; Wang, W.; Hao, P.; Sun, W.-H.; Tang, N.; Yi, J. Nickel(II) complexes chelated by 2-quinoxalinyl-6-iminopyridines: Synthesis, crystal structures and ethylene oligomerization. J. Organomet. Chem. 2007, 692, 3532–3541. [Google Scholar] [CrossRef]
- Shen, M.; Hao, P.; Sun, W.-H. Synthesis, Synthesis, characterization and catalytic behaviors of neutral nickel complexes: Arylnickel N-alkyl-6-(1-(arylimino)ethyl)picolinamide. J. Organomet. Chem. 2008, 693, 1683–1695. [Google Scholar] [CrossRef]
- Lai, J.; Hou, X.; Liu, Y.; Redshaw, C.; Sun, W.-H. 2-[1-(2,6-Dibenzhydryl-4-methylphenylimino)ethyl]-6-[1-(arylimino)ethyl]pyridylnickel(II) halides: Synthesis, characterization and ethylene oligomerization behavior. J. Organomet. Chem. 2012, 702, 52–58. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Yu, J.; Redshaw, C.; Hao, X.; Sun, W.-H. 2-(N-Alkylcarboxamide)-6-iminopyridyl palladium and nickel complexes: Coordination chemistry and catalysis. Dalton Trans. 2011, 40, 12856–12865. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, S.; Li, A.; Ye, H.; Li, Z. Nickel(II) complexes chelated by 2,6-pyridinedicarboxamide: Syntheses, characterization, and ethylene oligomerization. New J. Chem. 2016, 40, 7027–7033. [Google Scholar] [CrossRef]
- Tayade, K.N.; Mane, M.V.; Sen, S.; Murthy, C.N.; Tembe, G.L.; Pillai, S.M.; Vanka, K.; Mukherjee, S. A catalytic and DFT study of selective ethylene oligomerization by nickel(II) oxime-based complexes. J. Mol. Catal. A Chem. 2013, 366, 238–246. [Google Scholar] [CrossRef]
- Mukherjee, S.; Patel, B.A.; Bhaduri, S. Selective ethylene oligomerization with nickel oxime complexes. Organometallics 2009, 28, 3074–3078. [Google Scholar] [CrossRef]
- Nyamato, G.S.; Ojwach, S.O.; Akerman, M.P. Ethylene oligomerization studies by nickel(II) complexes chelated by (amino)pyridine ligands: Experimental and density functional theory studies. Dalton Trans. 2016, 45, 3407–3416. [Google Scholar] [CrossRef]
- Wang, K.; Shen, M.; Sun, W.-H. Synthesis, characterization and ethylene oligomerization of nickel complexes bearing N-(2-(1H-benzo[d]imidazol-2-yl)quinolin-8-yl)benzamide derivatives. Dalton Trans. 2009, 4085–4095. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Gao, R.; Hao, X.; Sun, W.-H. Nickel complexes bearing 2-(1H-benzo[d]imidazol-2-yl)-N-benzylidenequinolin-8-amines: Synthesis, structure and catalytic ethylene oligomerization. Catal. Commun. 2009, 10, 1730–1733. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L.; Chen, L.; Redshaw, C.; Li, Y.; Sun, W.-H. Synthesis, characterization and ethylene oligomerization behavior of 2-benzoimidazol-8-ethoxyquinolylnickel dihalides. Dalton Trans. 2011, 40, 2614–2621. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Hao, X.; Redshaw, C.; Sun, W. Halonickel 2,4-di-t-butyl-6-(quinolin-8-yliminomethyl)phenolates: Synthesis, characterization and ethylene reactivity. Chin. J. Polym. Sci. 2013, 31, 769–777. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Virgili, A.H.; Roisnel, T.; Kirillov, E.; Carpentier, J.F.; Casagrande, O.L. Ni(II) complexes bearing pyrrolide-imine ligands with pendant N-, O- and S-donor groups: Synthesis, structural characterization and use in ethylene oligomerization. RSC Adv. 2015, 5, 91524–91531. [Google Scholar] [CrossRef]
- Boudier, A.; Breuil, P.-A.R.; Magna, L.; Olivier-Bourbigou, H.; Braunstein, P. Nickel(II) complexes with imino-imidazole chelating ligands bearing pendant donor groups (SR, OR, NR2, PR2) as precatalysts in ethylene oligomerization. J. Organomet. Chem. 2012, 718, 31–37. [Google Scholar] [CrossRef]
- Xu, C.; Shen, Q.; Sun, X.; Tang, Y. Synthesis, characterization, and highly selective ethylene dimerization to 1-butene of [O−NX]Ni(II) complexes. Chin. J. Chem. 2012, 30, 1105–1113. [Google Scholar] [CrossRef]
- Ojwach, S.O.; Darkwa, J. Pyrazole and (pyrazol-1-yl)metal complexes as carbon–carbon coupling catalysts. Inorg. Chim. Acta 2010, 363, 1947–1964. [Google Scholar] [CrossRef]
- Gafurov, Z.N.; Bekmukhamedov, G.E.; Kagilev, A.A.; Kantyukov, A.O.; Sakhapov, I.F.; Mikhailov, I.K.; Khayarov, K.R.; Zaripov, R.B.; Islamov, D.R.; Usachev, K.S.; et al. Unsymmetrical pyrazole-based PCN pincer NiII halides: Reactivity and catalytic activity in ethylene oligomerization. J. Organomet. Chem. 2020, 912, 121163. [Google Scholar] [CrossRef]
- Ainooson, M.K.; Guzei, I.A.; Spencer, L.C.; Darkwa, J. Pyrazolylimine iron and cobalt, and pyrazolylamine nickel complexes: Synthesis and evaluation of nickel complexes as ethylene oligomerization catalysts. Polyhedron. 2013, 53, 295–303. [Google Scholar] [CrossRef]
- Ulbrich, A.H.D.P.S.; Campedelli, R.R.; Milani, J.L.S.; dos Santos, J.H.Z.; Casagrande, O.D.L. Nickel catalysts based on phenyl ether-pyrazol ligands: Synthesis, XPS study, and use in ethylene oligomerization. Appl. Catal. A 2013, 453, 280–286. [Google Scholar] [CrossRef]
- Dresch, L.C.; de Araújo, B.B.; Casagrande, O.D.L.; Stieler, R. A novel class of nickel(II) complexes containing selenium-based bidentate ligands applied in ethylene oligomerization. RSC Adv. 2016, 6, 104338–104344. [Google Scholar] [CrossRef]
- Ajellal, N.; Kuhn, M.C.A.; Boff, A.D.G.; Hörner, M.; Thomas, C.M.; Carpentier, J.-F.; Casagrande, O.L. Nickel complexes based on tridentate pyrazolyl ligands for highly efficient dimerization of ethylene to 1-butene. Organometallics 2006, 25, 1213–1216. [Google Scholar] [CrossRef]
- de Oliveira, L.; Campedelli, R.; Bergamo, A.; Santos, A.; Casagrande, O. Substituted tridentate pyrazolyl ligands for chromium and nickel-catalyzed ethylene oligomerization reactions. Effect of auxiliary ligand on activity and selectivity. J. Braz. Chem. Soc. 2010, 21, 1318–1328. [Google Scholar] [CrossRef]
- de Oliveira, L.L.; Campedelli, R.R.; Kuhn, M.C.A.; Carpentier, J.-F.; Casagrande, O.L. Highly selective nickel catalysts for ethylene oligomerization based on tridentate pyrazolyl ligands. J. Mol. Catal. A Chem. 2008, 288, 58–62. [Google Scholar] [CrossRef]
- Tuskaev, V.A.; Zubkevic, S.V.; Saracheno, D.; Gagieva, S.C.; Dorovatovskii, P.V.; Kononova, E.G.; Khrustalev, V.N.; Zarubin, D.N.; Bulychev, B.M.; Kissin, Y.V. Nickel(II) complexes with tripodal NNN ligands as homogenous and supported catalysts for ethylene oligomerization. Mol. Catal. 2019, 464, 29–38. [Google Scholar] [CrossRef]
- Ulbrich, A.H.D.P.S.; Bergamo, A.L.; Casagrande, O.D.L. Oligomerization of ethylene using tridentate nickel catalysts bearing ether-pyrazol ligands with pendant O- and S-donor groups. Catal. Commun. 2011, 16, 245–249. [Google Scholar] [CrossRef]
- Dresch, L.C.; Junges, C.H.; Casagrande, O.D.L.; Stieler, R. Nickel complexes supported by selenium-based tridentate ligands and their use as effective catalyst systems for ethylene dimerisation. J. Organomet. Chem. 2018, 856, 34–40. [Google Scholar] [CrossRef]
- Nyamato, G.S.; Alam, M.G.; Ojwach, S.O.; Akerman, M.P. Nickel(II) complexes bearing pyrazolylpyridines: Synthesis, structures and ethylene oligomerization reactions. Appl. Organomet. Chem. 2016, 30, 89–94. [Google Scholar] [CrossRef]
- Nyamato, G.S.; Alam, M.G.; Ojwach, S.O.; Akerman, M.P. (Pyrazolyl)-(phosphinoyl)pyridine iron(II), cobalt(II) and nickel(II) complexes: Synthesis, characterization and ethylene oligomerization studies. J. Organomet. Chem. 2015, 783, 64–72. [Google Scholar] [CrossRef]
- Junges, C.H.; Dresch, L.C.; da Costa, M.T.; Tirloni, B.; Casagrande, O.L., Jr.; Stieler, R. Pyrazolyl-phosphinoyl nickel (II) complexes: Synthesis, characterization and ethylene dimerization studies. Appl. Organomet. Chem. 2019, 33, e4887. [Google Scholar] [CrossRef]
- Zubkevich, S.V.; Tuskaev, V.A.; Gagieva, S.C.; Pavlov, A.A.; Khrustalev, V.N.; Zarubin, D.N.; Kurmaev, D.A.; Kolosov, N.A.; Bulychev, B.M. Molecular structure, magnetic properties and catalytic activity in selective ethylene dimerization of nickel (II) complexes with bis(3,5-dimethylpyrazol-1-yl)methane. J. Mol. Struct. 2020, 1206, 127692. [Google Scholar] [CrossRef]
- Wang, T.; Dong, B.; Chen, Y.-H.; Mao, G.-L.; Jiang, T. Nickel complexes incorporating pyrazole-based ligands for ethylene dimerization to 1-butylene. J. Organomet. Chem. 2015, 798, 388–392. [Google Scholar] [CrossRef]
- Zubkevich, S.V.; Gagieva, S.C.; Tuskaev, V.A.; Dorovatovskii, P.V.; Khrustalev, V.N.; Sizov, A.I.; Bulychev, B.M. Synthesis and reactivity in ethylene oligomerization by heteroscorpionate dibromonickel(II) complexes. Inorg. Chim. Acta 2017, 458, 58–67. [Google Scholar] [CrossRef]
- Ulbrich, A.H.D.P.S.; Milani, J.L.S.; Roisnel, T.; Carpentier, J.-F.; Casagrande, O.L. Zwitterionic Ni(II) complexes bearing pyrazolyl-ether-imidazolium ligands: Synthesis, structural characterization and use in ethylene oligomerization. New J. Chem. 2015, 39, 7234–7242. [Google Scholar] [CrossRef]
- Cabrera, A.R.; Martinez, I.; Daniliuc, C.G.; Galland, G.B.; Salas, C.O.; Rojas, R.S. New air stable cationic methallyl Ni complexes bearing imidoyl-indazole carboxylate ligand: Synthesis, characterization and their reactivity towards ethylene. J. Mol. Catal. A Chem. 2016, 414, 19–26. [Google Scholar] [CrossRef]
- Espinet, P.; Soulantica, K. Phosphine-pyridyl and related ligands in synthesis and catalysis. Coord. Chem. Rev. 1999, 193–195, 499–556. [Google Scholar] [CrossRef]
- Braunstein, P.; Naud, F. Hemilability of hybrid ligands and the coordination chemistry of oxazoline-based systems. Angew Chem. Int. Ed. 2001, 40, 680–699. [Google Scholar] [CrossRef]
- Flapper, J.; Kooijman, H.; Lutz, M.; Spek, A.L.; van Leeuwen, P.W.N.M.; Elsevier, C.J.; Kamer, P.C.J. Nickel and palladium complexes of pyridine-phosphine ligands as ethene oligomerization catalysts. Organometallics 2009, 28, 1180–1192. [Google Scholar] [CrossRef]
- Freixa, Z.; van Leeuwen, P.W.N.M. Bite angle effects in diphosphine metal catalysts: Steric or electronic? Dalton Trans. 2003, 10, 1890–1901. [Google Scholar] [CrossRef]
- van Leeuwen, P.W.N.M.; Kamer, P.C.J.; Reek, J.N.H.; Dierkes, P. Ligand bite angle effects in metal-catalyzed C−C bond formation. Chem. Rev. 2000, 100, 2741–2770. [Google Scholar] [CrossRef] [PubMed]
- Flapper, J.; Kooijman, H.; Lutz, M.; Spek, A.L.; van Leeuwen, P.W.N.M.; Elsevier, C.J.; Kamer, P.C.J. Nickel and palladium complexes of new pyridine-phosphine ligands and their use in ethene oligomerization. Organometallics 2009, 28, 3272–3281. [Google Scholar] [CrossRef]
- Kermagoret, A.; Braunstein, P. Mono- and dinuclear nickel complexes with phosphino-, phosphinito-, and phosphonitopyridine ligands: Synthesis, structures, and catalytic oligomerization of ethylene. Organometallics 2008, 27, 88–99. [Google Scholar] [CrossRef]
- Flapper, J.; van Leeuwen, P.W.N.M.; Elsevier, C.J.; Kamer, P.C.J. Nickel and palladium complexes of pyridine-phosphine ligands bearing aromatic substituents and their behavior as catalysts in ethene oligomerization. Organometallics 2009, 28, 3264–3271. [Google Scholar] [CrossRef]
- Yang, Q.-Z.; Kermagoret, A.; Agostinho, M.; Siri, O.; Braunstein, P. Nickel complexes with functional zwitterionic N,O-benzoquinonemonoimine-type ligands: Syntheses, structures, and catalytic oligomerization of ethylene. Organometallics 2006, 25, 5518–5527. [Google Scholar] [CrossRef]
- Chavez, P.; Rios, I.G.; Kermagoret, A.; Pattacini, R.; Meli, A.; Bianchini, C.; Giambastiani, G.; Braunstein, P. Nickel complexes with phosphinito-oxazoline ligands: Temperature-controlled formation of mono- or dinuclear complexes and catalytic oligomerization of ethylene and propylene. Organometallics 2009, 28, 1776–1784. [Google Scholar] [CrossRef]
- Kermagoret, A.; Tomicki, F.; Braunstein, P. Nickel and iron complexes with N,P,N-type ligands: Synthesis, structure and catalytic oligomerization of ethylene. Dalton Trans. 2008, 2945–2955. [Google Scholar] [CrossRef]
- Dyer, P.W.; Fawcett, J.; Hanton, M.J. Rigid N-phosphino guanidine P,N ligands and their use in nickel-catalyzed ethylene oligomerization. Organometallics 2008, 27, 5082–5087. [Google Scholar] [CrossRef]
- de Souza, R.; Bernardo-Gusmão, K.; Cunha, G.A.; Loup, C.; Leca, F.; Réau, R. Ethylene dimerization into 1-butene using 2-pyridylphosphole nickel catalysts. J. Catal. 2004, 226, 235–239. [Google Scholar] [CrossRef]
- de la Tabla, L.O.; Matas, I.; Palma, P.; Álvarez, E.; Cámpora, J. Nickel and palladium complexes with new phosphinito-imine ligands and their application as ethylene oligomerization catalysts. Organometallics 2012, 31, 1006–1016. [Google Scholar] [CrossRef]
- Daugulis, O.; Brookhart, M. Polymerization of ethylene with cationic palladium and nickel catalysts containing bulky nonenolizable imine-phosphine ligands. Organometallics 2002, 21, 5926–5934. [Google Scholar] [CrossRef]
- Buchard, A.; Auffrant, A.; Klemps, C.; Vu-Do, L.; Boubekeur, L.; Goff, X.F.L.; Floch, P.L. Highly efficient P–N nickel(II) complexes for the dimerisation of ethylene. Chem. Commun. 2007, 1502–1504. [Google Scholar] [CrossRef] [PubMed]
- Cheisson, T.; Cao, T.-P.-A.; Le Goff, X.F.; Auffrant, A. Nickel complexes featuring iminophosphorane–phenoxide ligands for catalytic ethylene dimerization. Organometallics. 2014, 33, 6193–6199. [Google Scholar] [CrossRef]
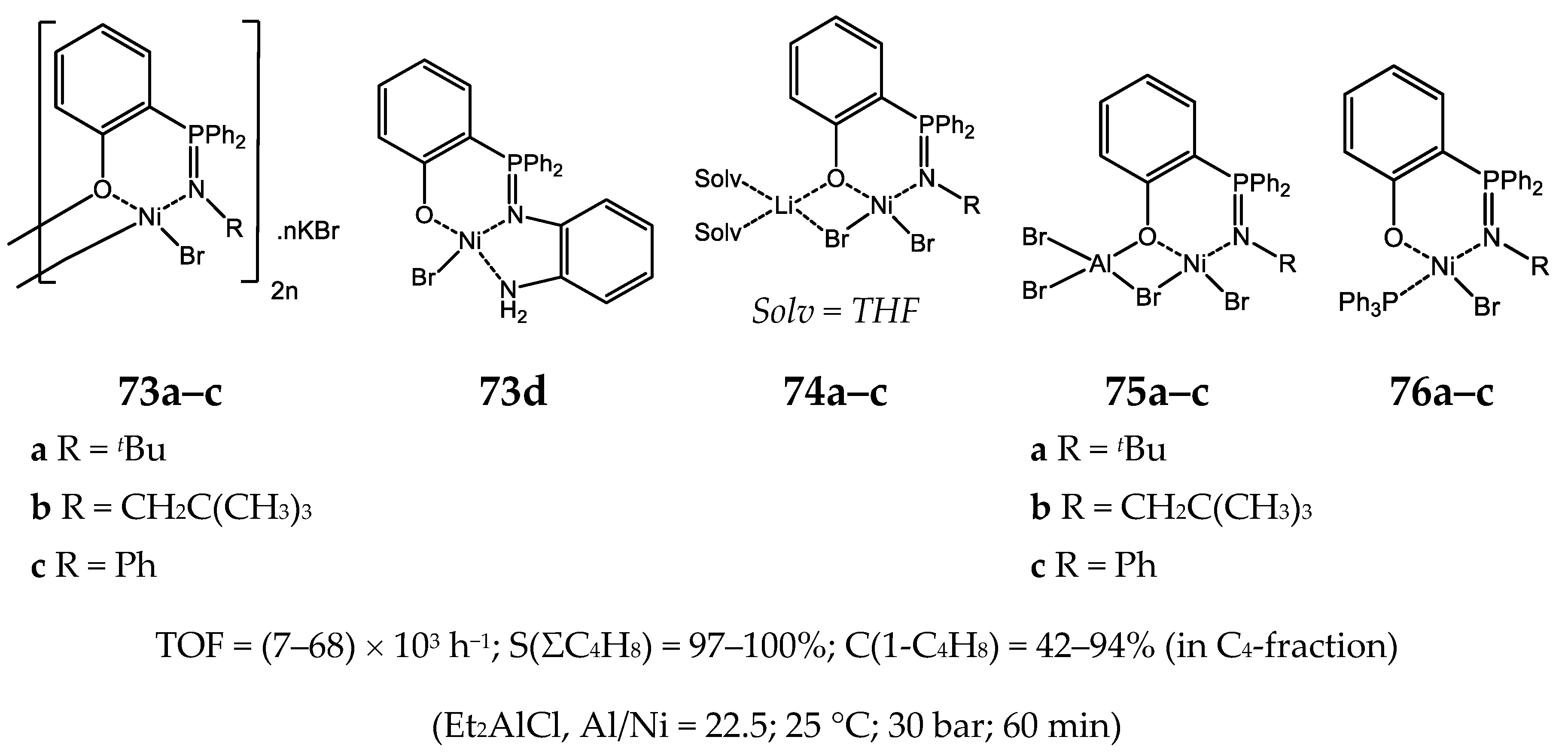
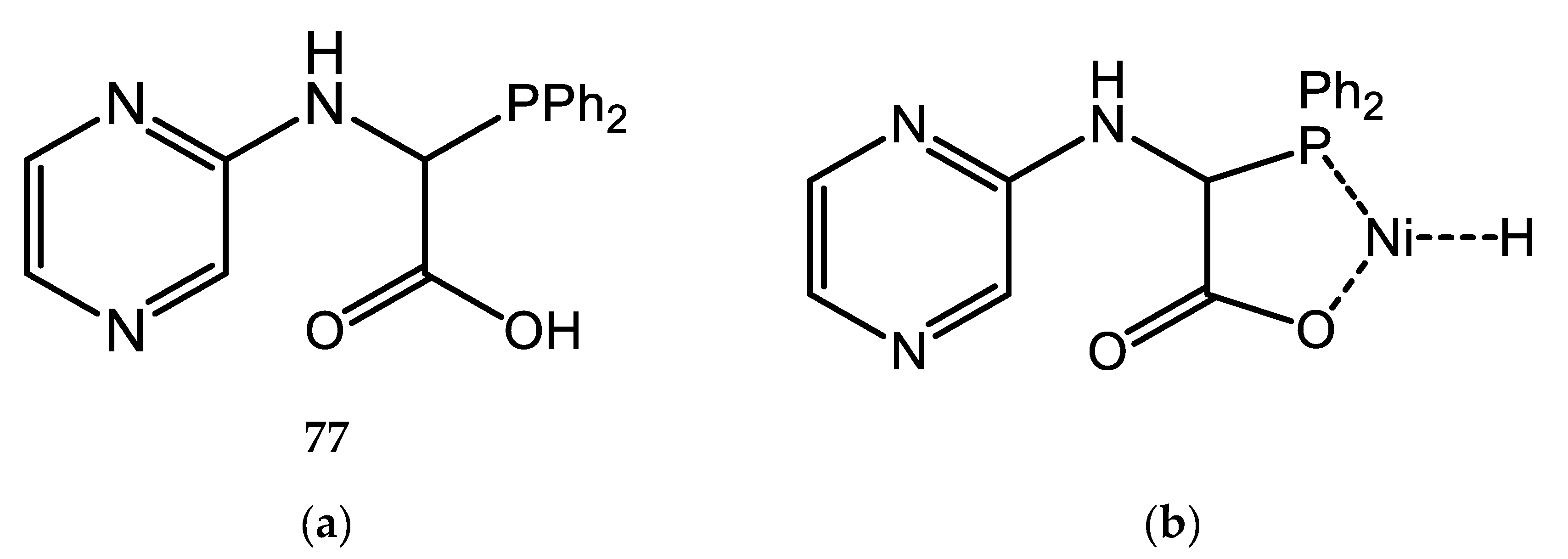
- Peulecke, N.; Yakhvarov, D.G.; Heinicke, J.W. Chemistry of α-phosphanyl α-amino acids. Eur. J. Inorg. Chem. 2019, 1507–1518. [Google Scholar] [CrossRef]
- Ghalib, M.; Lach, J.; Fomina, O.S.; Yakhvarov, D.G.; Jones, P.G.; Heinicke, J. Benzazaphospholine-2-carboxylic acids: Synthesis, structure and properties of heterocyclic phosphanyl amino acids. Polyhedron 2014, 77, 10–16. [Google Scholar] [CrossRef]
- Basvani, K.R.; Fomina, O.S.; Yakhvarov, D.G.; Heinicke, J. Synthesis and properties of zwitterionic phosphonioglycolates. Polyhedron 2014, 67, 306–313. [Google Scholar] [CrossRef]
- Soficheva, O.S.; Bekmukhamedov, G.E.; Dobrynin, A.B.; Heinicke, J.W.; Sinyashin, O.G.; Yakhvarov, D.G. α-Diphenylphosphino-N-(pyrazin-2-yl)glycine as a ligand in Ni-catalyzed ethylene oligomerization. Mendeleev Commun. 2019, 29, 575–577. [Google Scholar] [CrossRef]
- Appleby, T.; Woollins, J.D. Inorganic backbone phosphines. Coord. Chem. Rev. 2002, 235, 121–140. [Google Scholar] [CrossRef]
- Alferov, K.A.; Belov, G.P.; Meng, Y. Chromium catalysts for selective ethylene oligomerization to 1-hexene and 1-octene: Recent results. Appl. Catal. A 2017, 542, 71–124. [Google Scholar] [CrossRef]
- Song, K.; Gao, H.; Liu, F.; Pan, J.; Guo, L.; Zai, S.; Wu, Q. Syntheses, structures, and catalytic ethylene oligomerization behaviors of bis(phosphanyl)aminenickel(II) complexes containing N-functionalized pendant groups. Eur. J. Inorg. Chem. 2009, 2009, 3016–3024. [Google Scholar] [CrossRef]
- Ghisolfi, A.; Fliedel, C.; Rosa, V.; Monakhov, K.Y.; Braunstein, P. Combined experimental and theoretical study of bis(diphenylphosphino)(N-thioether)amine-type ligands in nickel(II) complexes for catalytic ethylene oligomerization. Organometallics 2014, 33, 2523–2534. [Google Scholar] [CrossRef]
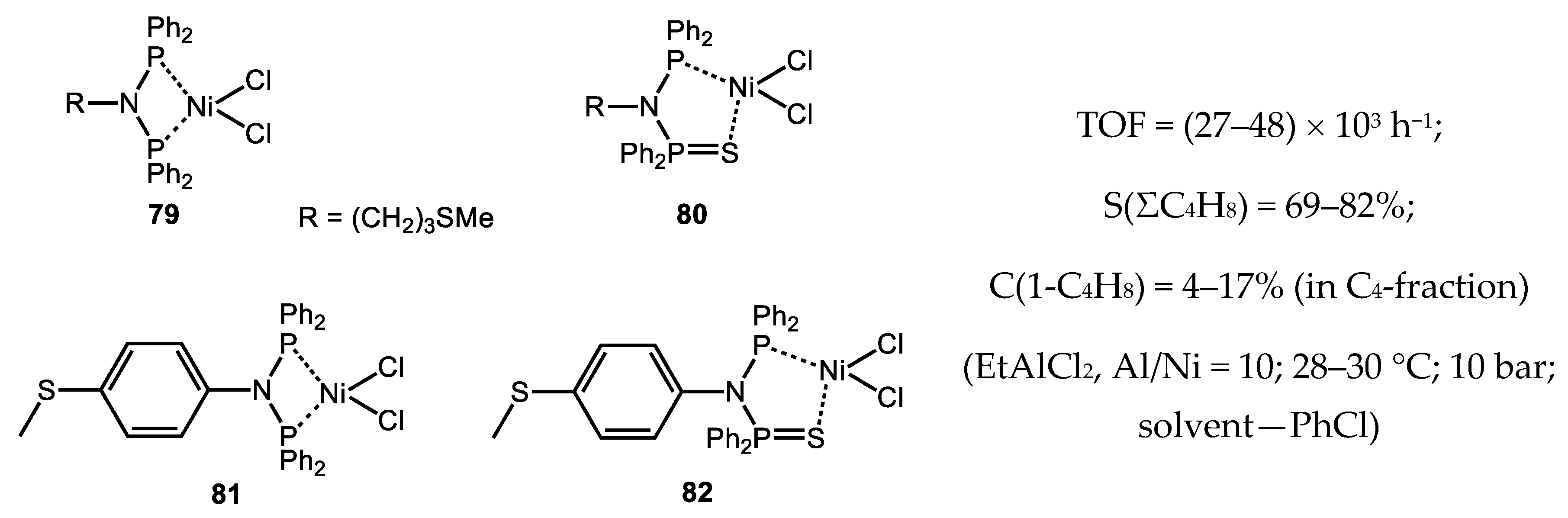
- Meng, X.; Zhang, L.; Chen, Y.; Jiang, T. Silane-bridged diphosphine ligands for nickel-catalyzed ethylene oligomerization. React. Kinet. Mech. Catal. 2016, 119, 481–490. [Google Scholar] [CrossRef]
- Wei, W.; Yu, B.; Alam, F.; Huang, Y.; Cheng, S.; Jiang, T. Ethylene oligomerization promoted by nickel-based catalysts with silicon-bridged diphosphine amine ligands. Transition Met. Chem. 2019, 44, 125–133. [Google Scholar] [CrossRef]
- Bekmukhamedov, G.E.; Sukhov, A.V.; Kuchkaev, A.M.; Khayarov, K.R.; Gerasimov, A.V.; Vasilenko, I.V.; Kostjuk, S.V.; Yakhvarov, D.G. Electrochemical synthesis of zirconium pre-catalysts for homogeneous ethylene oligomerization. Catalysts 2019, 9, 1059. [Google Scholar] [CrossRef]
- Boulens, P.; Lutz, M.; Jeanneau, E.; Olivier-Bourbigou, H.; Reek, J.N.H.; Breuil, P.-A.R. Iminobisphosphines to (non-)symmetrical diphosphinoamine ligands: Metal-induced synthesis of diphosphorus nickel complexes and application in ethylene oligomerisation reactions. Eur. J. Inorg. Chem. 2014, 2014, 3754–3762. [Google Scholar] [CrossRef]
- Dierkes, P.; van Leeuwen, P.W.N.M. The bite angle makes the difference: A practical ligand parameter for diphosphine ligands. J. Chem. Soc. Dalton Trans. 1999, 1519–1530. [Google Scholar] [CrossRef]
- Mora, G.; van Zutphen, S.; Klemps, C.; Ricard, L.; Jean, Y.; Le Floch, P. Synthesis, X-ray, and electronic structures of a new nickel dibromide complex. Activity in the regioselective catalyzed dimerization of ethylene into 1-butene. Inorg. Chem. 2007, 46, 10365–10371. [Google Scholar] [CrossRef]
- Boulens, P.; Pellier, E.; Jeanneau, E.; Reek, J.N.H.; Olivier-Bourbigou, H.; Breuil, P.-A.R. Self-assembled organometallic nickel complexes as catalysts for selective dimerization of ethylene into 1-butene. Organometallics 2015, 34, 1139–1142. [Google Scholar] [CrossRef]
- Lhermet, R.; Moser, E.; Jeanneau, E.; Olivier-Bourbigou, H.; Breuil, P.-A.R. Outer-sphere reactivity shift of secondary phosphine oxide-based nickel complexes: From ethylene hydrophosphinylation to oligomerization. Chem. Eur. J. 2017, 23, 7433–7437. [Google Scholar] [CrossRef]
- Lejeune, M.; Sémeril, D.; Jeunesse, C.; Matt, D.; Peruch, F.; Lutz, P.J.; Ricard, L. Diphosphines with expandable bite angles: Highly active ethylene dimerisation catalysts based on upper rim, distally diphosphinated calix[4]arenes. Chem. Eur. J. 2004, 10, 5354–5360. [Google Scholar] [CrossRef]
- Liddle, S.T.; Edworthy, I.S.; Arnold, P.L. Anionic tethered N-heterocyclic carbene chemistry. Chem. Soc. Rev. 2007, 36, 1732–1744. [Google Scholar] [CrossRef] [PubMed]
- Cavell, K.J.; McGuinness, D.S. Redox processes involving hydrocarbylmetal (N-heterocyclic carbene) complexes and associated imidazolium salts: Ramifications for catalysis. Coord. Chem. Rev. 2004, 248, 671–681. [Google Scholar] [CrossRef]
- McGuinness, D.S.; Mueller, W.; Wasserscheid, P.; Cavell, K.J.; Skelton, B.W.; White, A.H.; Englert, U. Nickel(II) heterocyclic carbene complexes as catalysts for olefin dimerization in an imidazolium chloroaluminate ionic liquid. Organometallics 2002, 21, 175–181. [Google Scholar] [CrossRef]
- Prakasham, A.P.; Ghosh, P. Nickel N-heterocyclic carbene complexes and their utility in homogeneous catalysis. Inorg. Chim. Acta 2014, 431, 61–100. [Google Scholar] [CrossRef]
- Li, W.-F.; Sun, H.-M.; Chen, M.-Z.; Shen, Q.; Zhang, Y. Synthesis and catalytic activity of neutral salicylaldiminato nickel(II) complexes bearing a single N-heterocyclic carbene ligand. J. Organomet. Chem. 2008, 693, 2047–2051. [Google Scholar] [CrossRef]
- McGuinness, D.S.; Cavell, K.J.; Skelton, B.W.; White, A.H. Zerovalent palladium and nickel complexes of heterocyclic carbenes: Oxidative addition of organic halides, carbon−carbon coupling processes, and the Heck reaction. Organometallics 1999, 18, 1596–1605. [Google Scholar] [CrossRef]
- Hameury, S.; de Frémont, P.; Breuil, P.-A.R.; Olivier-Bourbigou, H.; Braunstein, P. Bis(ether-functionalized NHC) nickel(II) complexes, trans to cis isomerization triggered by water coordination, and catalytic ethylene oligomerization. Organometallics 2014, 34, 2183–2201. [Google Scholar] [CrossRef]
- Ren, X.; Wesolek, M.; Bailly, C.; Karmazin, L.; Braunstein, P. Silver(I) and nickel(II) complexes with oxygen- or nitrogen functionalized NHC ditopic ligands and catalytic ethylene oligomerization. Eur. J. Inorg. Chem. 2020, 1073–1087. [Google Scholar] [CrossRef]
- Kermagoret, A.; Braunstein, P. Synthesis of nickel complexes with bidentate N,O-type ligands and application in the catalytic oligomerization of ethylene. Dalton Trans. 2008, 1564–1573. [Google Scholar] [CrossRef]
- Speiser, F.; Braunstein, P.; Saussine, L. Dinuclear nickel complexes with bidentate N,O ligands: Synthesis, structure, and catalytic oligomerization of ethylene. Inorg. Chem. 2004, 43, 4234–4240. [Google Scholar] [CrossRef]
- Swarts, A.J.; Mapolie, S.F. The synthesis and application of novel Ni(II) N-alkyl dipyridylaldiminato complexes as selective ethylene oligomerisation catalysts. Dalton Trans. 2014, 43, 9892–9900. [Google Scholar] [CrossRef] [PubMed]
- Majoumo-Mbe, F.; Lönnecke, P.; Volkis, V.; Sharma, M.; Eisen, M.S.; Hey-Hawkins, E. Oligomerization of α-olefins by the dimeric nickel bisamido complex [Ni{1-N(PMes2)-2-N(μ-PMes2)C6H4-k3N,N′,P,-k1P′}]2 activated by methylalumoxane (MAO). J. Organomet. Chem. 2008, 693, 2603–2609. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, W.-H.; Kuang, X.; Vystorop, I.; Yi, J. Unsymmetric bimetal(II) complexes: Synthesis, structures and catalytic behaviors toward ethylene. J. Organomet. Chem. 2007, 692, 5307–5316. [Google Scholar] [CrossRef]
- Wang, K.; Shen, M.; Sun, W.-H. N-(Pyridin-2-yl)picolinamide tetranickel clusters: Synthesis, structure and ethylene oligomerization. Polyhedron 2010, 29, 564–568. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, J.; Huo, H.; Chen, L.; Shi, W.; Li, C.; Wang, J. Iron, cobalt and nickel complexes bearing hyperbranched iminopyridyl ligands: Synthesis, characterization and evaluation as ethylene oligomerization catalysts. Inorg. Chim. Acta. 2018, 469, 209–216. [Google Scholar] [CrossRef]
- Chen, L.; Huo, H.; Wang, L.; Kuang, Q.; Shi, W.; Zhang, N.; Li, Z.; Wang, J. Ethylene oligomerization studies utilizing nickel complexes bearing pyridine-imine ligands. Inorg. Chim. Acta 2019, 491, 67–75. [Google Scholar] [CrossRef]
- Chen, L.; Ma, L.; Jiang, Y.; Liu, J.; Li, C.; Zhang, N.; Wang, J. Synthesis and characterization of iron, cobalt and nickel complexes bearing para-phenylene-linked pyridine imine ligand and their catalytic properties for ethyleneoligomerization. Polym. Bull. 2020. [Google Scholar] [CrossRef]
- Velthoen, M.E.Z.; Boereboom, J.M.; Bulo, R.E.; Weckhuysen, B.M. Insights into the activation of silica-supported metallocene olefin polymerization catalysts by methylaluminoxane. Catal. Today. 2019, 334, 223–230. [Google Scholar] [CrossRef]
- Velthoen, M.E.Z.; Muñoz-Murillo, A.; Bouhmadi, A.; Cecius, M.; Diefenbach, S.; Weckhuysen, B.M. The multifaceted role of methylaluminoxane in metallocene-based olefin polymerization catalysis. Macromolecules 2018, 51, 343–355. [Google Scholar] [CrossRef]
- Hirvi, J.T.; Bochmann, M.; Severn, J.R.; Linnolahti, M. Formation of octameric methylaluminoxanes by hydrolysis of trimethylaluminum and the mechanisms of catalyst activation in single-site α-olefin polymerization catalysis. ChemPhysChem 2014, 15, 2732–2742. [Google Scholar] [CrossRef]
- McGuinness, D.S. Olefin oligomerization via metallacycles: Dimerization, trimerization, tetramerization, and beyond. Chem. Rev. 2011, 111, 2321–2341. [Google Scholar] [CrossRef] [PubMed]
- Shmidt, R. Catalytic Compositions for Dimerization of Ethylene. Russian Patent 2,647,726, 3 December 2014. [Google Scholar]
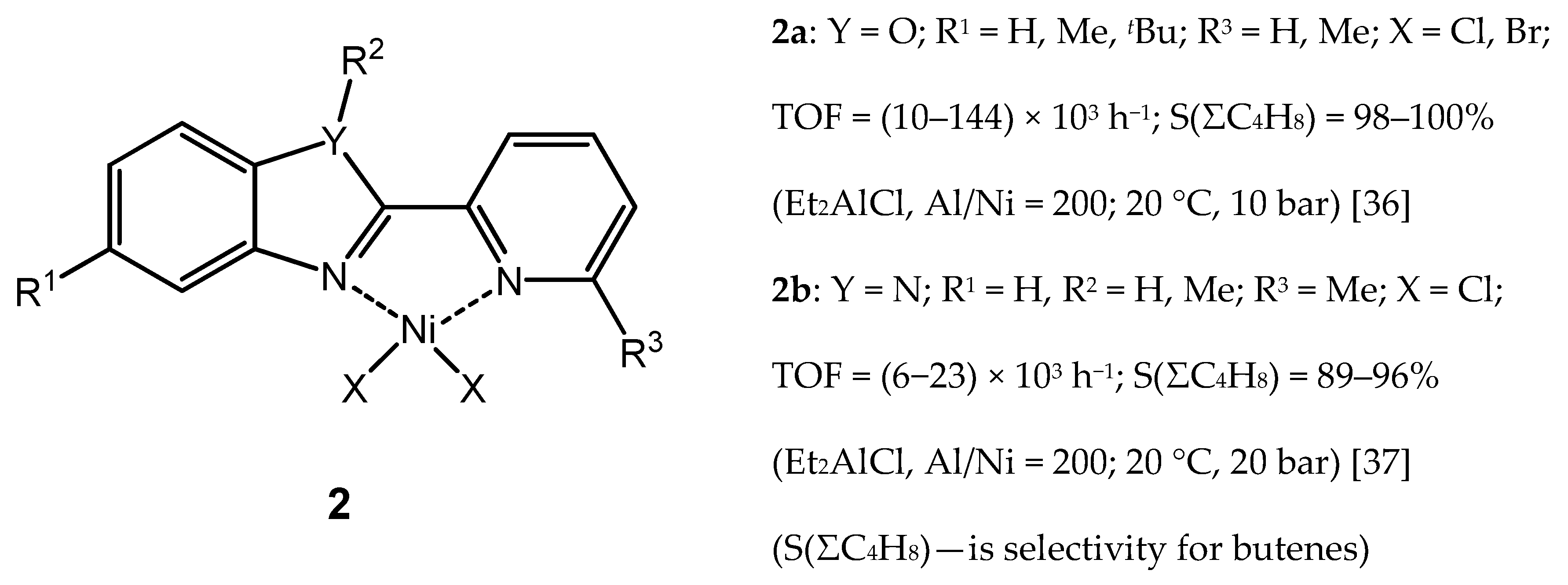
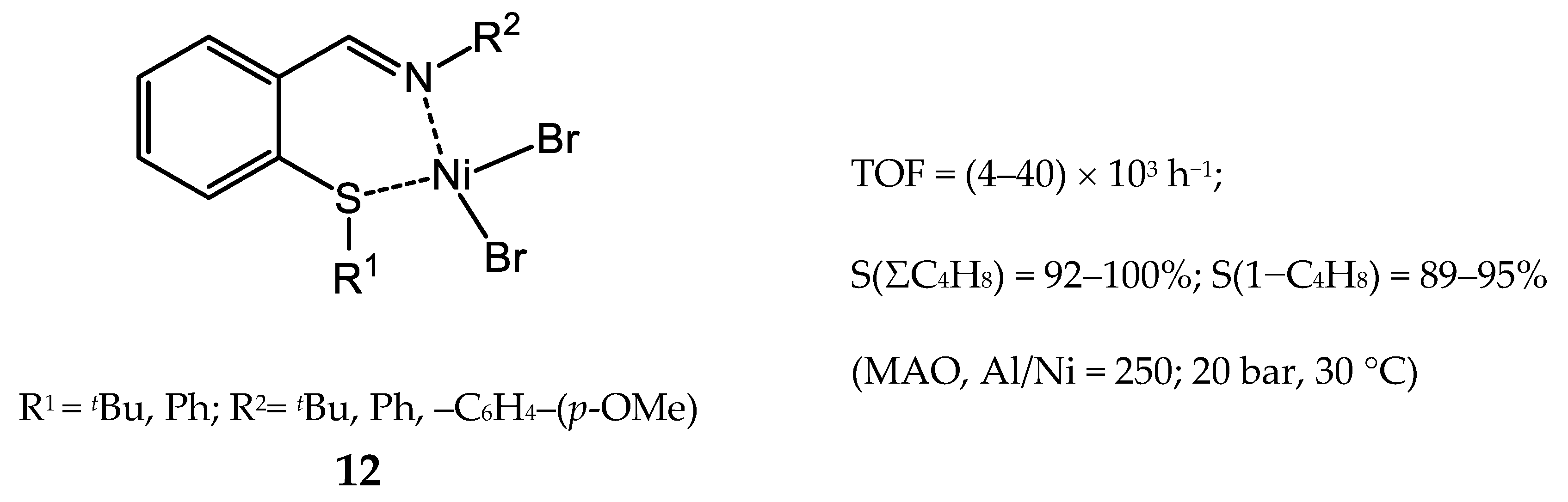
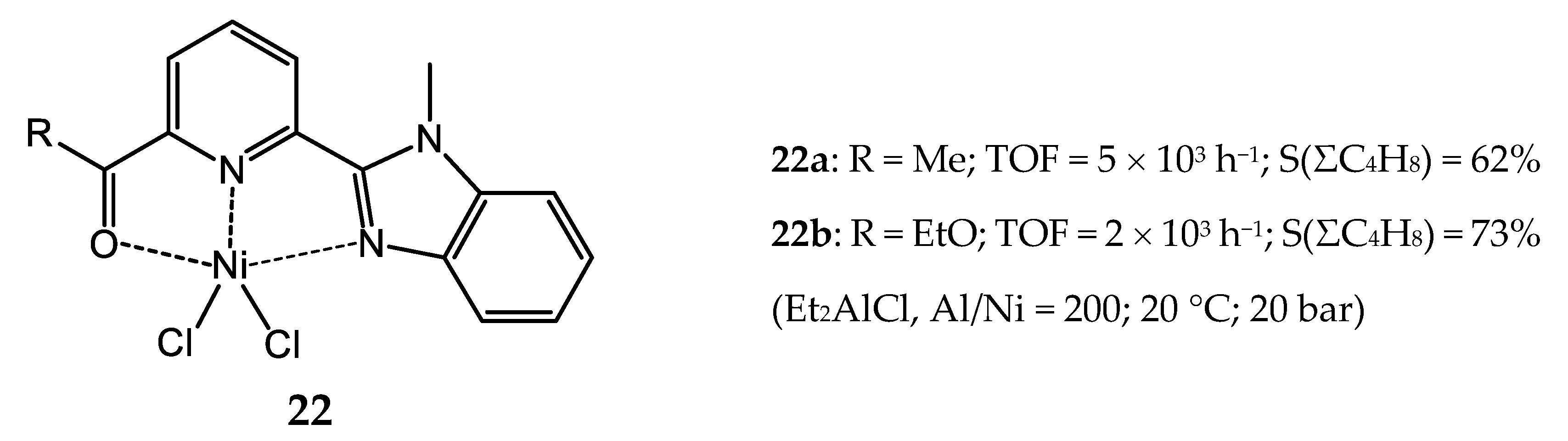

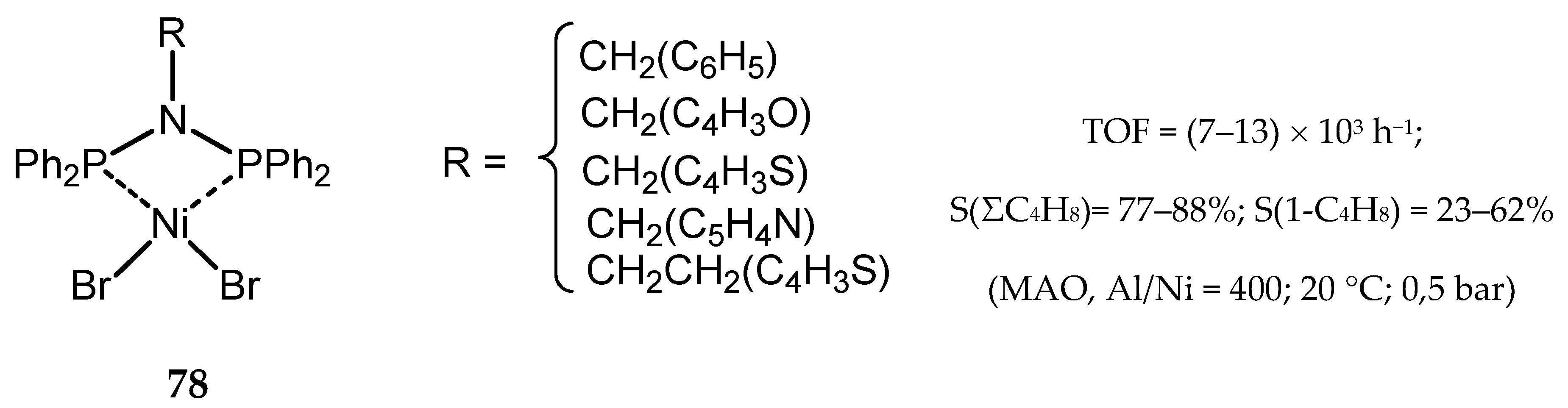
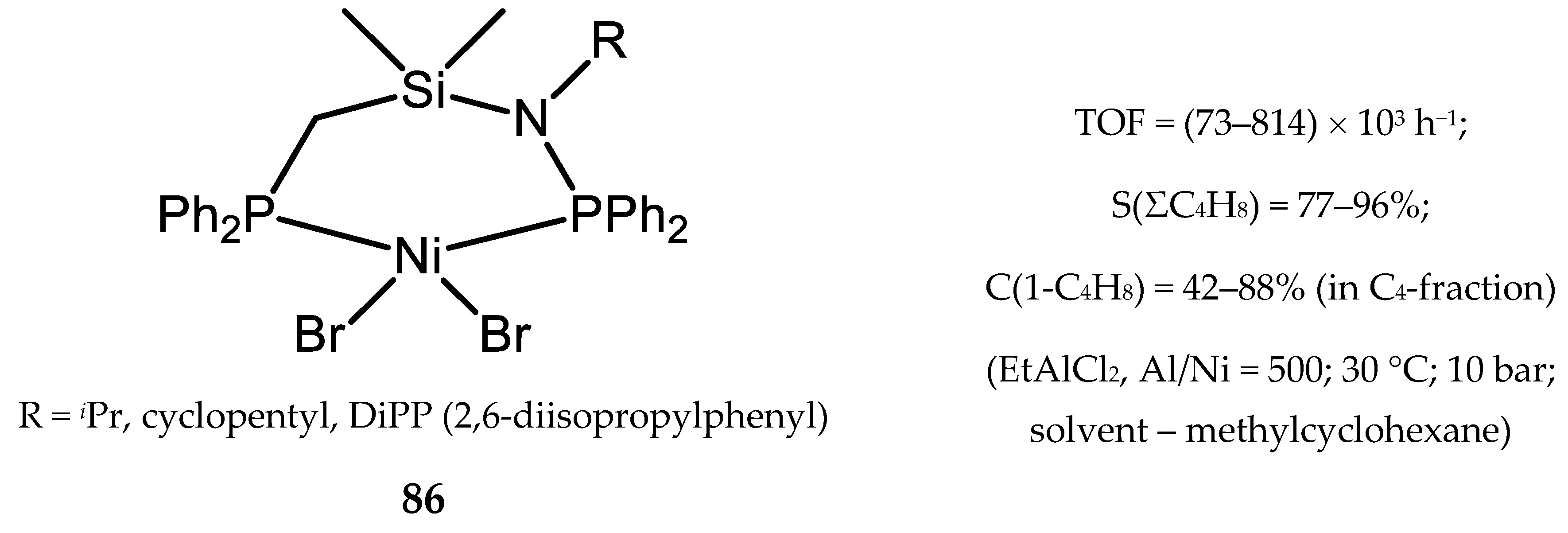

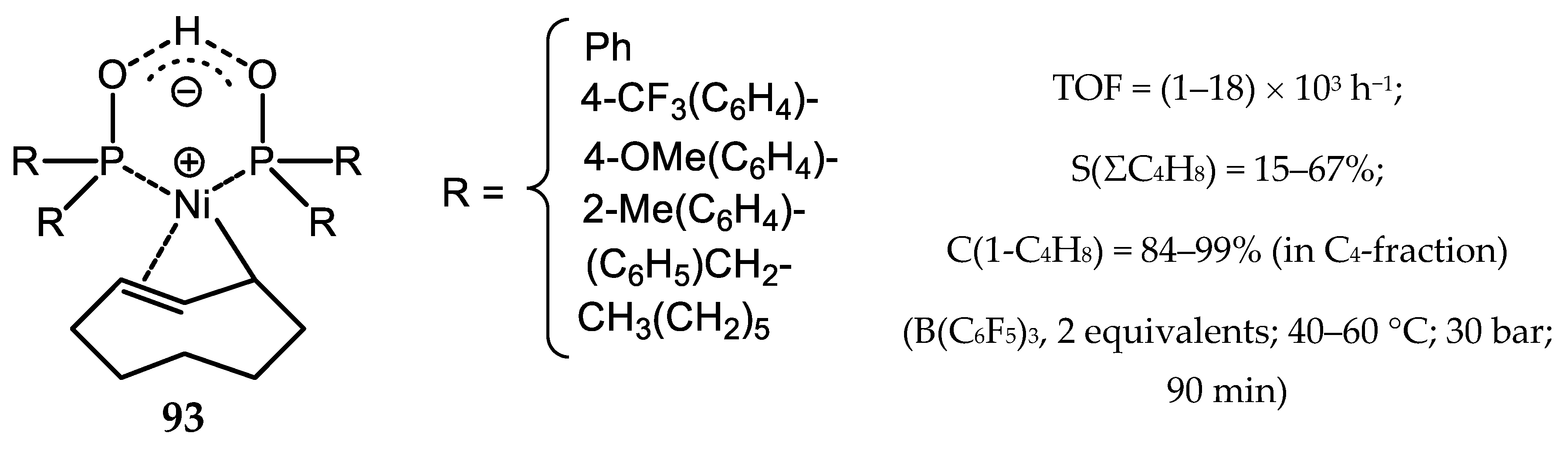
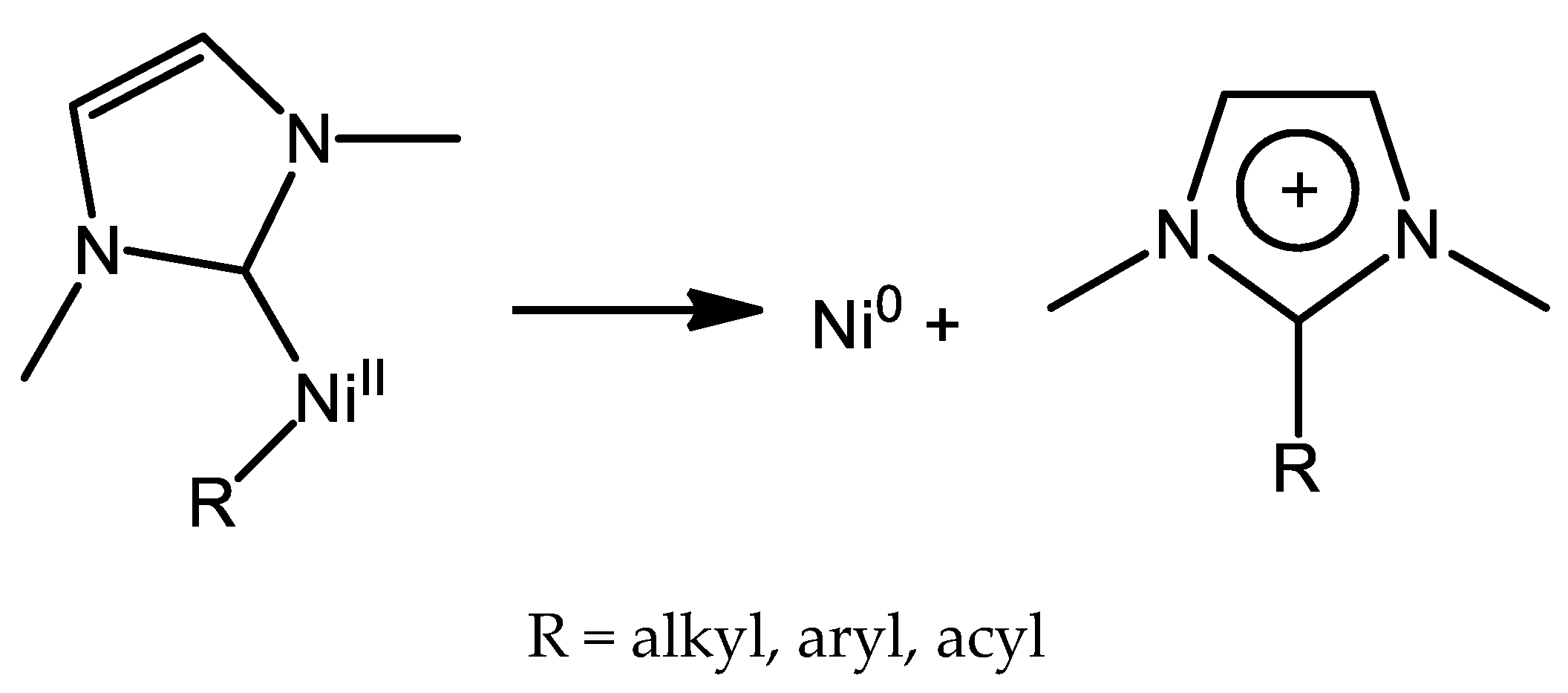
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekmukhamedov, G.E.; Sukhov, A.V.; Kuchkaev, A.M.; Yakhvarov, D.G. Ni-Based Complexes in Selective Ethylene Oligomerization Processes. Catalysts 2020, 10, 498. https://doi.org/10.3390/catal10050498
Bekmukhamedov GE, Sukhov AV, Kuchkaev AM, Yakhvarov DG. Ni-Based Complexes in Selective Ethylene Oligomerization Processes. Catalysts. 2020; 10(5):498. https://doi.org/10.3390/catal10050498
Chicago/Turabian StyleBekmukhamedov, Giyjaz E., Aleksandr V. Sukhov, Aidar M. Kuchkaev, and Dmitry G. Yakhvarov. 2020. "Ni-Based Complexes in Selective Ethylene Oligomerization Processes" Catalysts 10, no. 5: 498. https://doi.org/10.3390/catal10050498
APA StyleBekmukhamedov, G. E., Sukhov, A. V., Kuchkaev, A. M., & Yakhvarov, D. G. (2020). Ni-Based Complexes in Selective Ethylene Oligomerization Processes. Catalysts, 10(5), 498. https://doi.org/10.3390/catal10050498




