Hydrogenation and Dehydrogenation of Tetralin and Naphthalene to Explore Heavy Oil Upgrading Using NiMo/Al2O3 and CoMo/Al2O3 Catalysts Heated with Steel Balls via Induction
Abstract
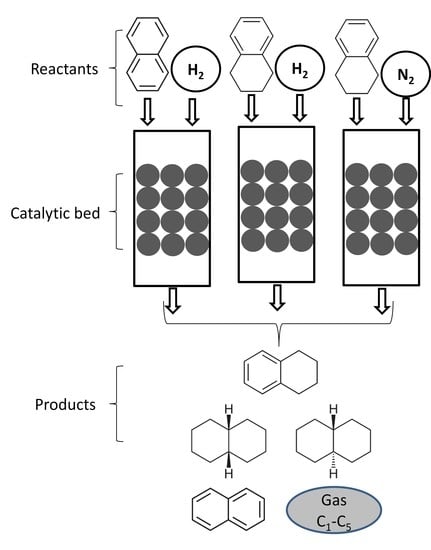
1. Introduction
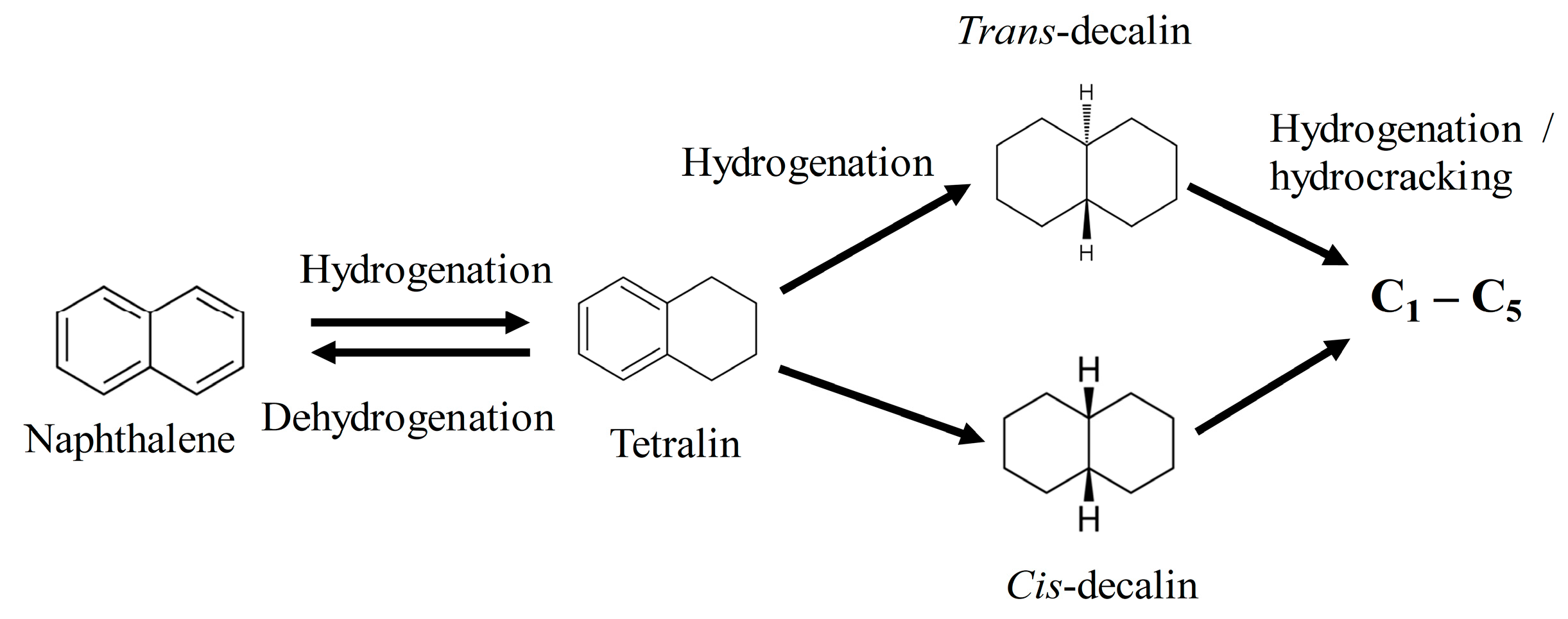
2. Results and Discussion
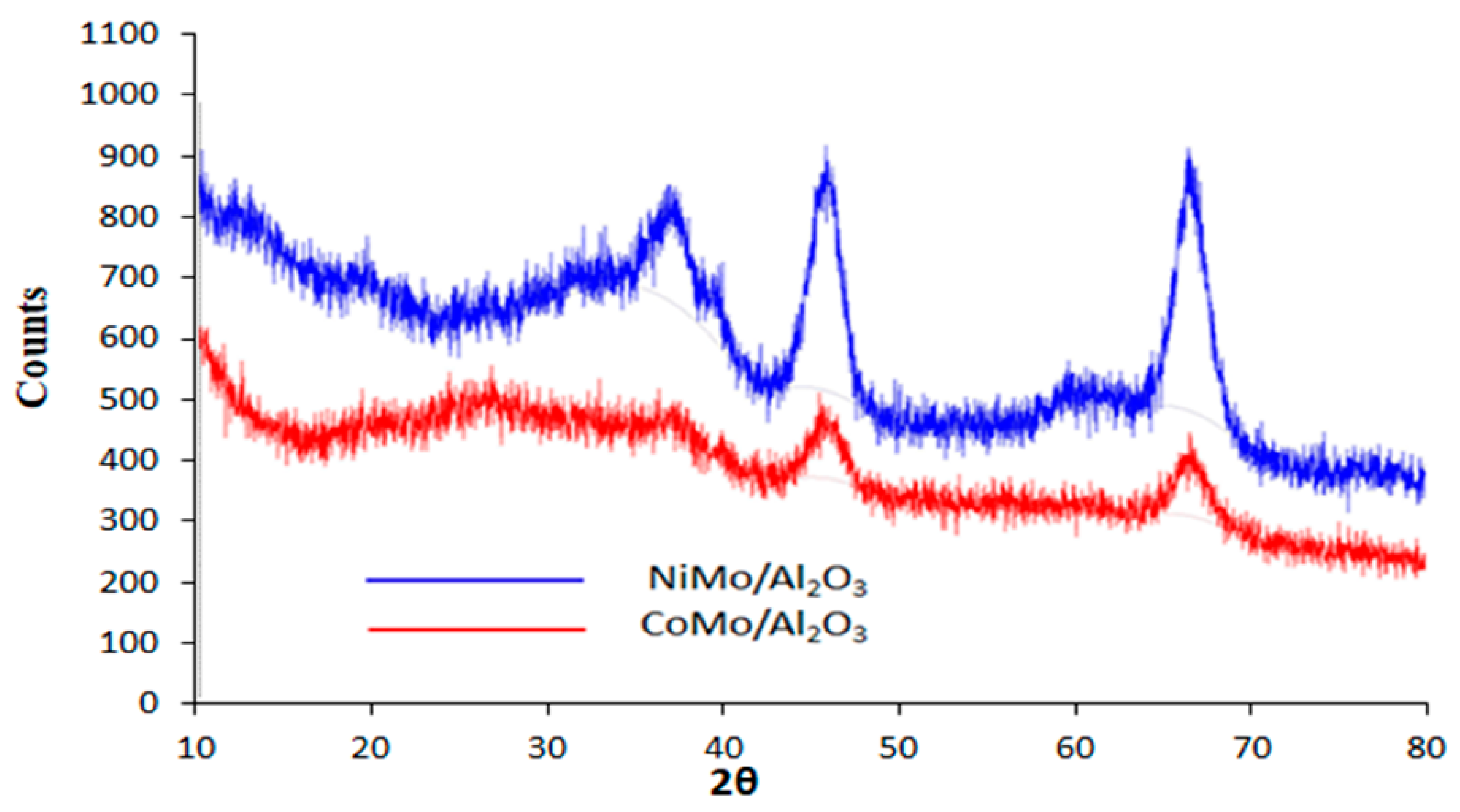
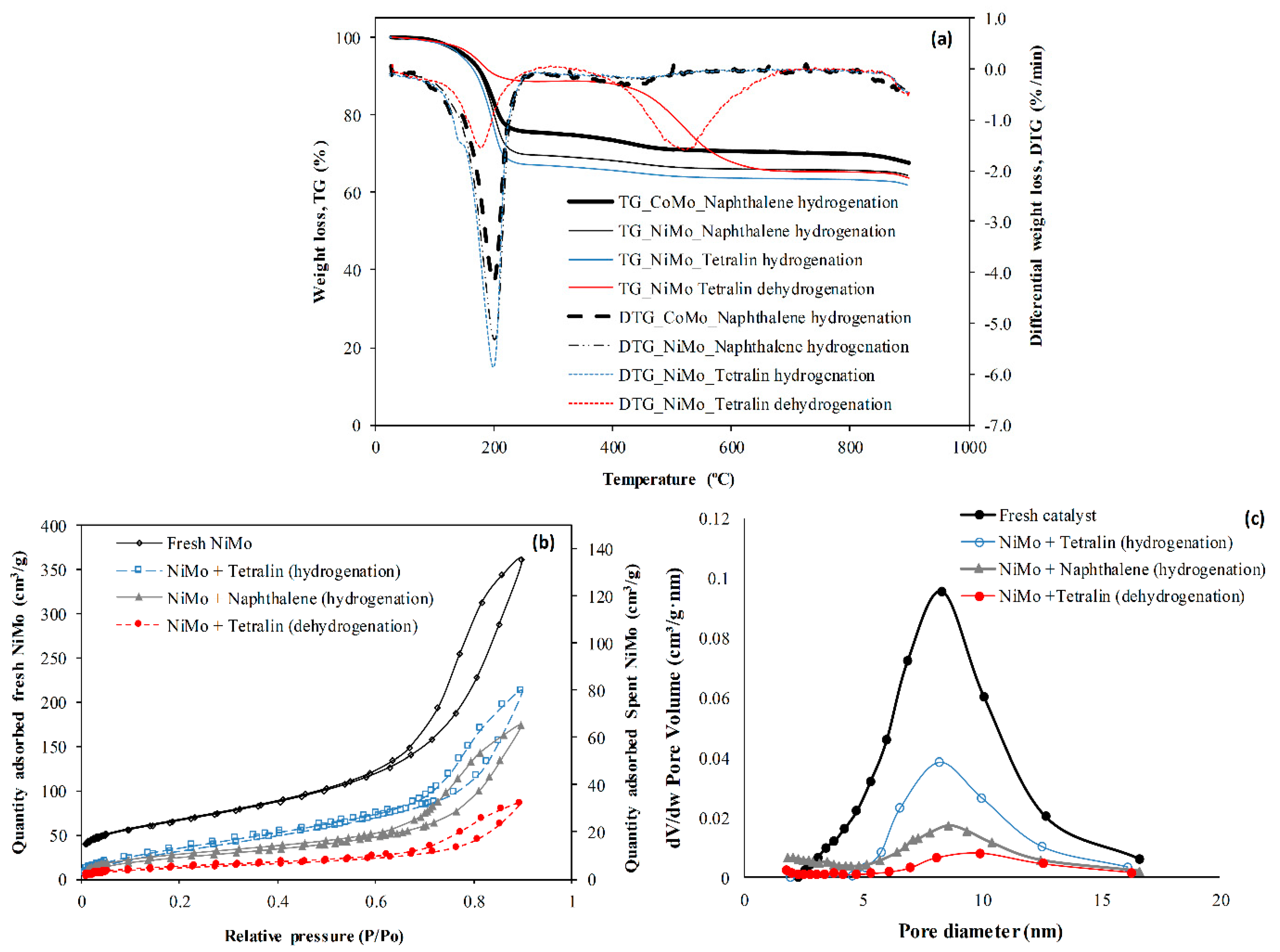
2.1. Catalyst Characterization
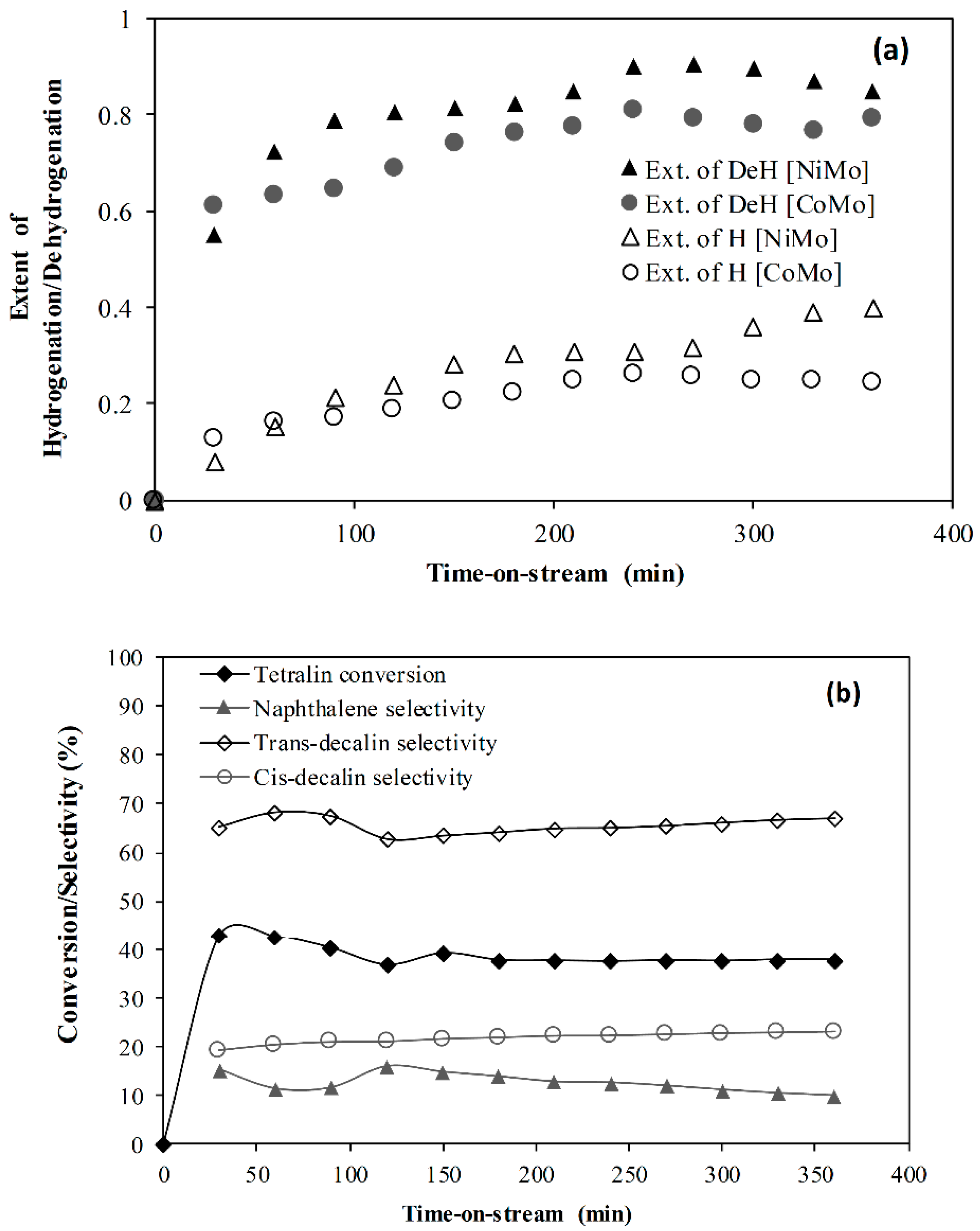
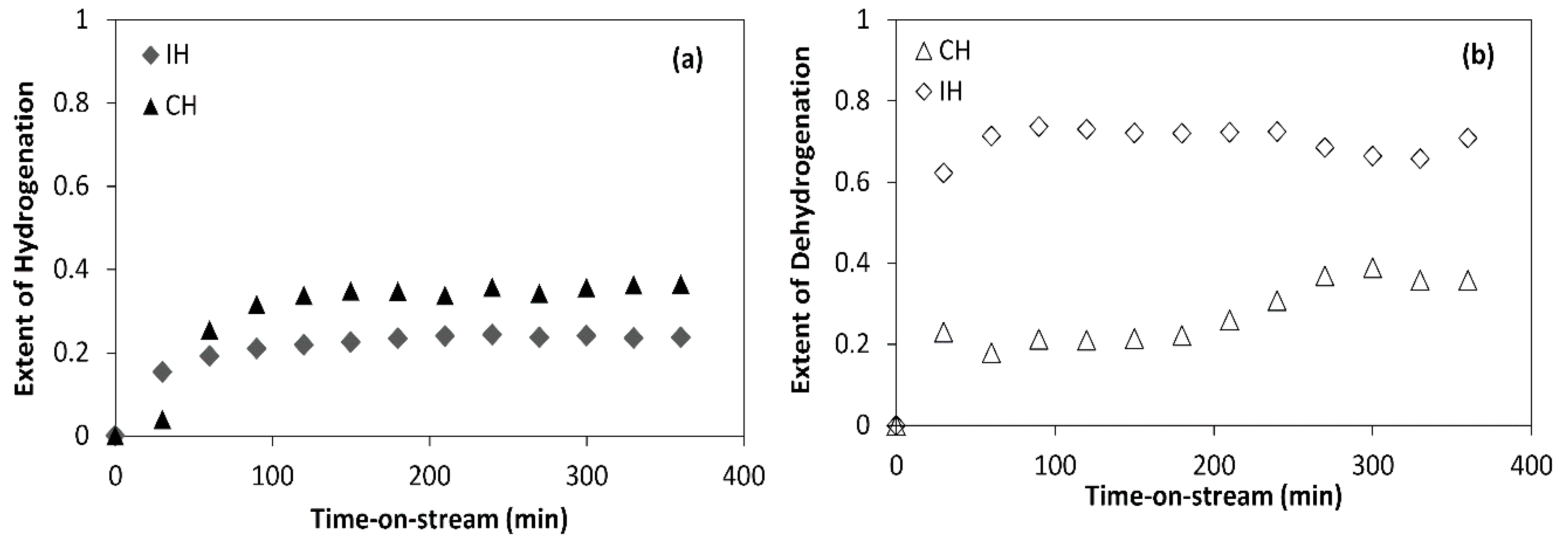
2.2. CoMo Versus NiMo Catalyst
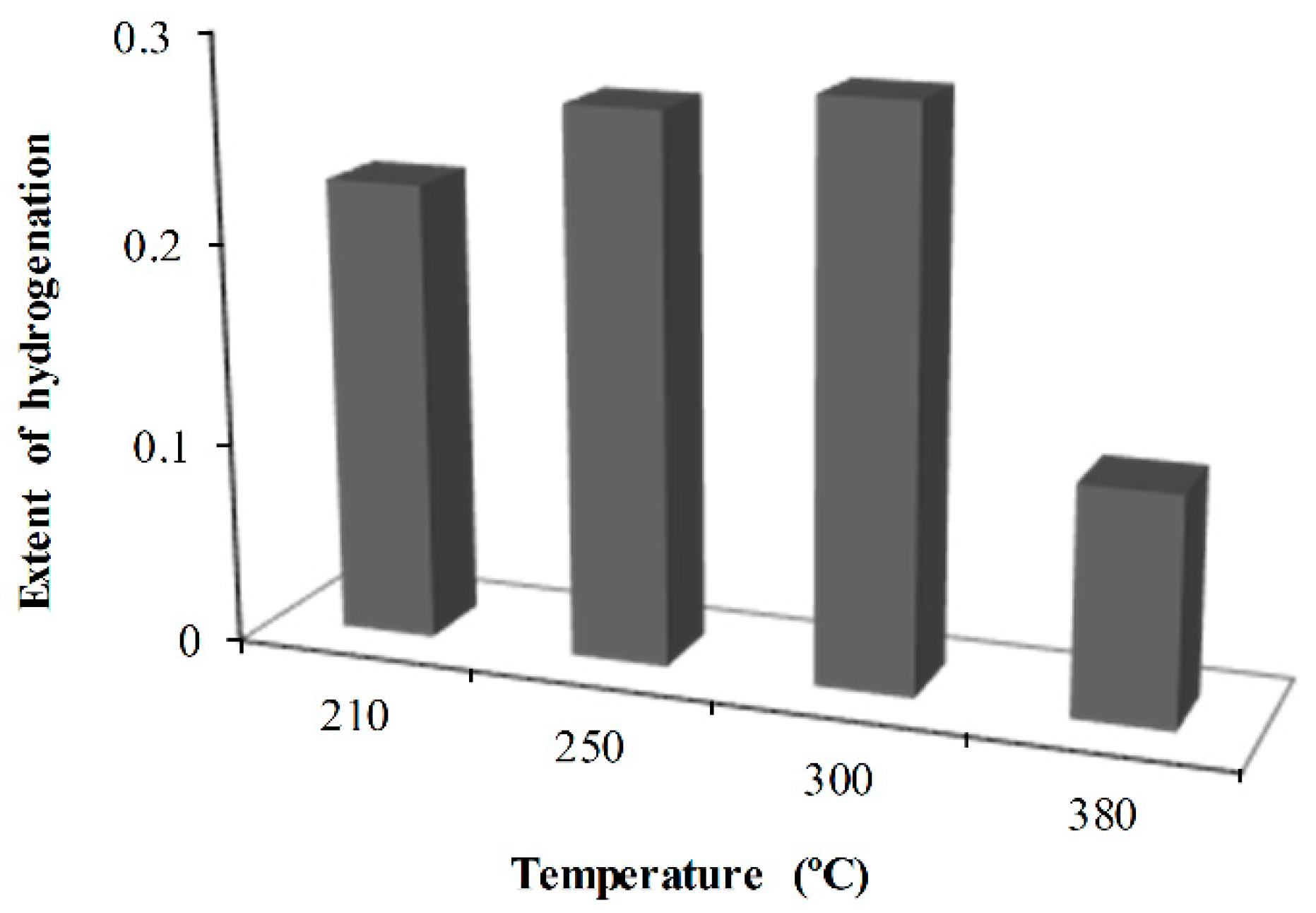
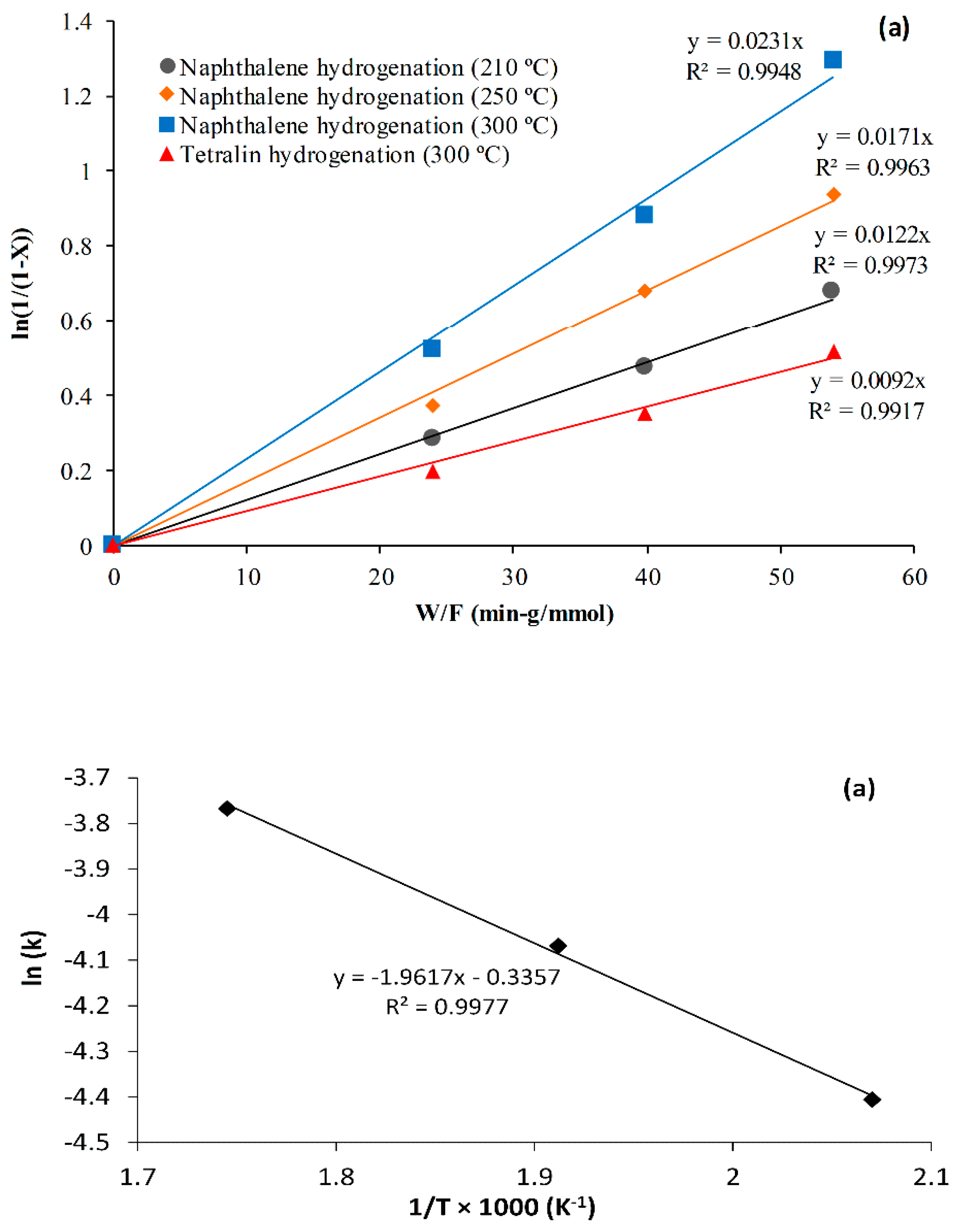
2.3. Effect of Reaction Temperature
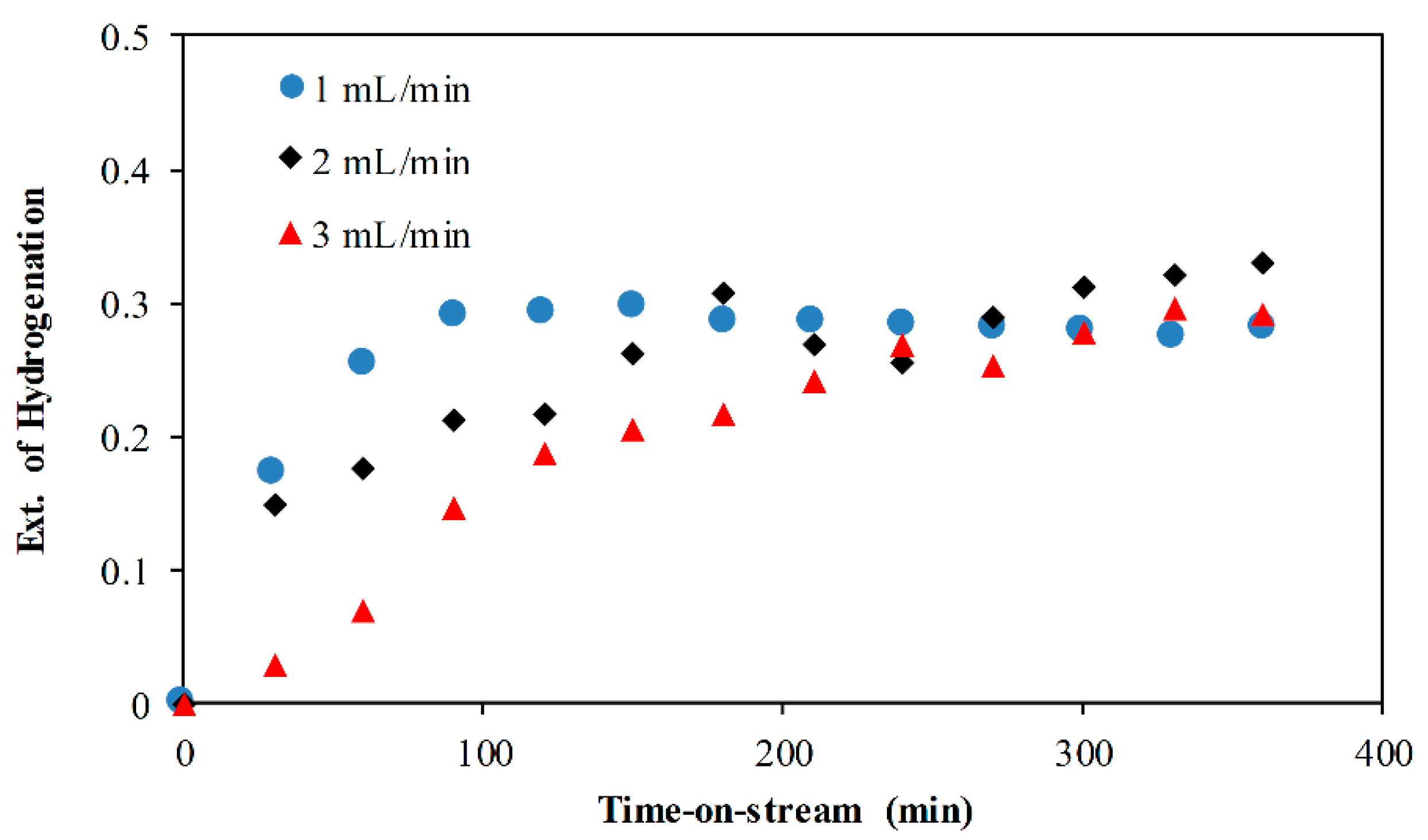
2.4. Effect of Flowrate
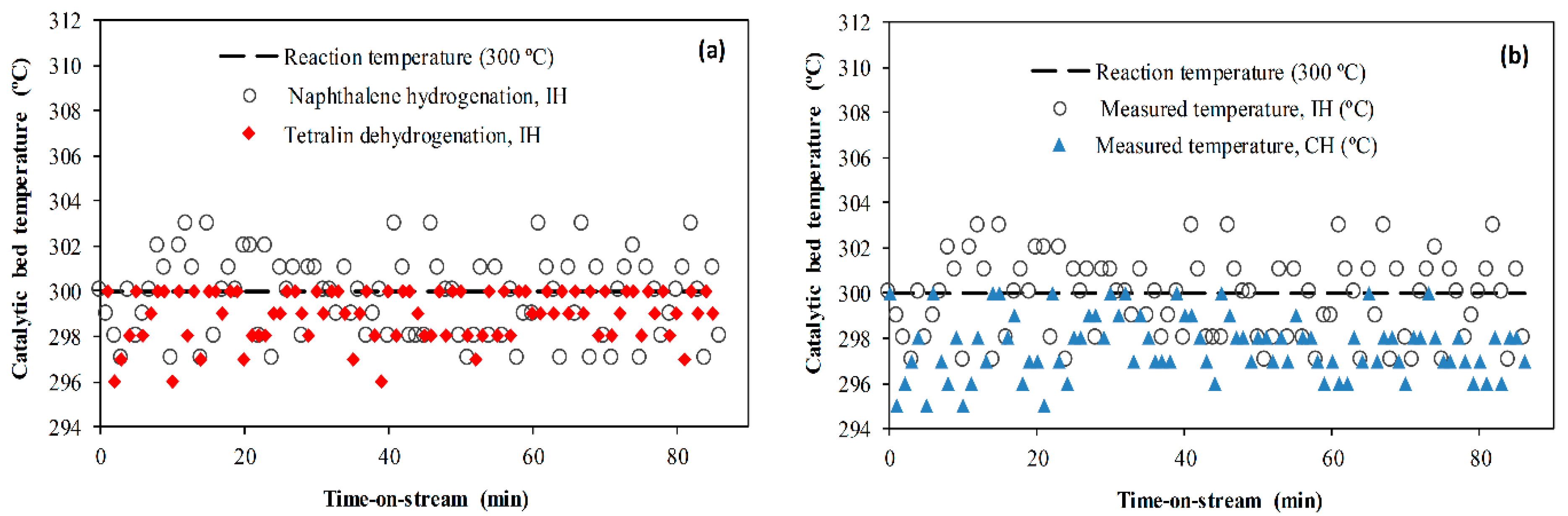
2.5. Effect of Heating Method
2.6. Gaseous Products
2.7. Coke Formation
2.8. The Roles of Hydrogen in Catalytic Upgrading of Heavy Oil
3. Materials and Methods
3.1. Materials
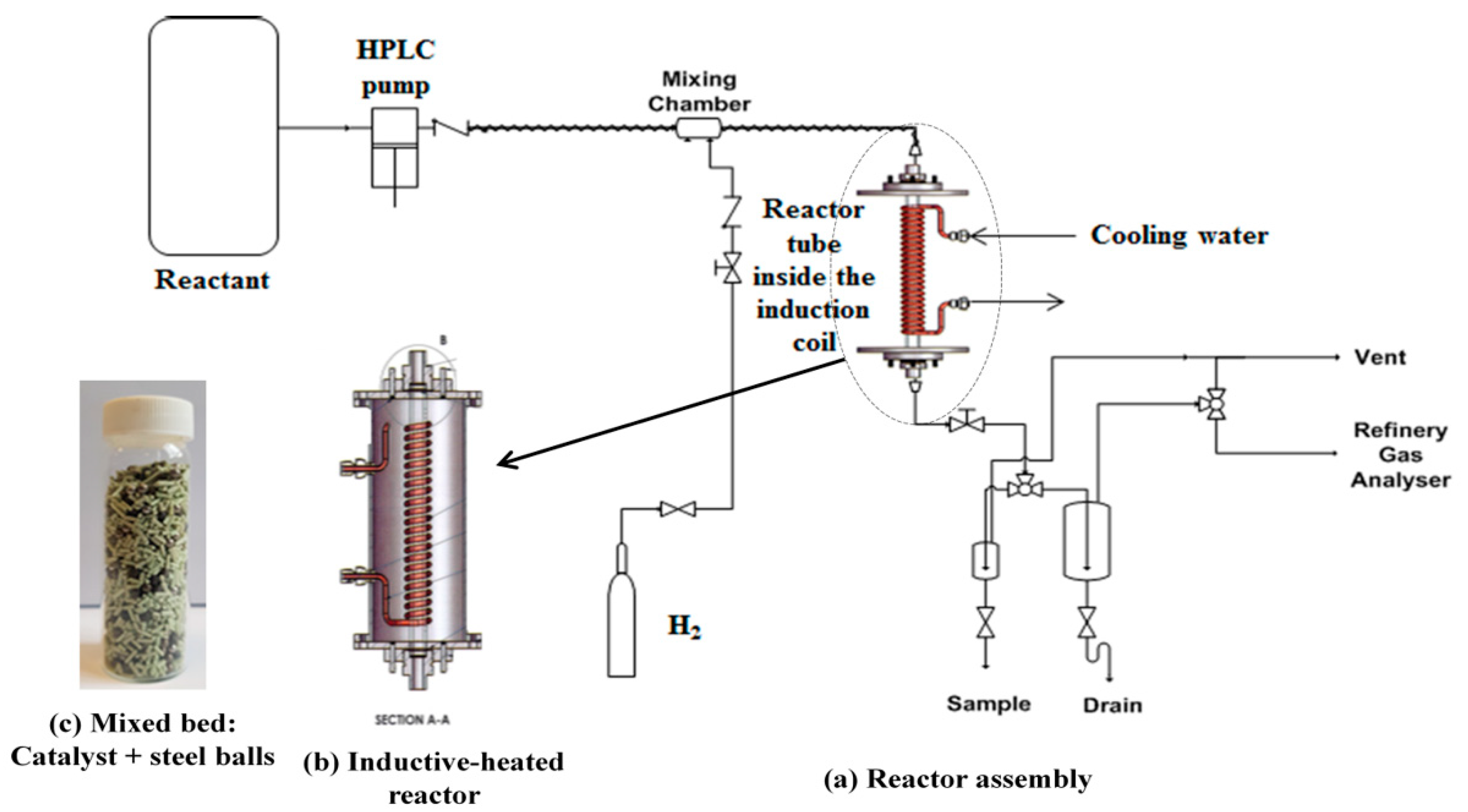
3.2. Lab-Scale Inductive Heated Catalytic Reactor
3.3. Analytical Methods
4. Conclusions
- It was found that the Ni metal promoter enhanced the hydrogenating activity of Mo supported on an alumina catalyst more than Co. Hence, a lower coke deposit was observed with the NiMo/Al2O3 catalyst than with the CoMo/Al2O3 counterpart.
- The effect of reaction temperature showed that naphthalene hydrogenation increased as the temperature increased from 210 to 300 °C and decreased when the temperature was further increased to 380 °C. On the other hand, as the flowrate increased from 1 to 3 mL/min, naphthalene hydrogenation decreased with 1 and 2 mL/min, giving approximately the same extent of hydrogenation.
- The kinetic data from the fixed-bed inductive heated catalytic with steel balls were evaluated with a first-order reaction rate on the assumption of plug flow. The experimental data were fitted 99% by the first-order rate equation, and 16.3 kJ/mol apparent activation energy was obtained for naphthalene hydrogenation using NiMo/Al2O3, a temperature range of 210–300 °C, pressure 18 bar, flowrate 1 mL/min, and catalyst/steel balls 70% v/v.
- With nitrogen gas environment, the naphthenic and aliphatic hydrocarbons lose hydrogen to form olefins and aromatic compounds. As a consequence, higher coking was observed in tetralin dehydrogenation under nitrogen atmosphere than tetralin and naphthalene hydrogenation using a NiMo/Al2O3 catalyst.
- A smaller temperature gradient was found when the NiMo/Al2O3 catalyst was heated with steel balls (catalyst/steel balls, 70% v/v) through induction compared to conventional heating via a furnace. Hence, it was thermodynamically favorable to endothermic tetralin dehydrogenation than exothermic tetralin and naphthalene hydrogenation reaction.
- Lower coke formation was observed in the NiMo/Al2O3 catalyst heated inductively with steel balls than conventional heating for naphthalene hydrogenation. This is because the smaller temperature gradient between the catalyst and surrounding fluid, as well as the non-thermal effect of magnetic field, enhanced the desorption and transport properties of the naphthalene and its hydrogenated products (i.e., tetralin and decalins) when heated inductively.
- The rate of naphthalene hydrogenation is higher than that of tetralin. However, the dehydrogenation of tetralin caused more coking and catalyst pore plugging than the hydrogenation of tetralin and naphthalene. This is because of the deposition of strongly adsorbed condense arenes naphthalene and other polymerization reactions due to the presence of olefins and aromatic compounds.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zadražil, A.; Štěpánek, F. Remote control of reaction rate by radiofrequency heating of composite catalyst pellets. Chem. Eng. Sci. 2015, 134, 721–726. [Google Scholar] [CrossRef]
- Varsano, F.; Bellusci, M.; Provini, A.; Petrecca, M. NiCo as catalyst for magnetically induced dry reforming of methane. IOP Conf. Ser. Mater. Sci. Eng. 2018, 323, 012005. [Google Scholar] [CrossRef]
- Idakiev, V.V.; Marx, S.; Roßau, A.; Bück, A.; Tsotsas, E.; Mörl, L. Inductive heating of fluidized beds: Influence on fluidization behavior. Powder Technol. 2015, 286, 90–97. [Google Scholar] [CrossRef]
- Vinum, M.G.; Almind, M.R.; Engbaek, J.S.; Vendelbo, S.B.; Hansen, M.F.; Frandsen, C.; Bendix, J.; Mortensen, P.M. Dual-Function Cobalt–Nickel Nanoparticles Tailored for High-Temperature Induction-Heated Steam Methane Reforming. Angew. Chem. Int. Ed. 2018, 57, 10569–10573. [Google Scholar] [CrossRef]
- How Much Crude Oil Has the World Really Consumed? Available online: https://oilprice.com/Energy/Crude-Oil/How-Much-Crude-Oil-Has-The-World-Really-Consumed.html, (accessed on 25 August 2019).
- Hart, A.; Shah, A.; Leeke, G.; Greaves, M.; Wood, J. Optimization of the CAPRI Process for Heavy Oil Upgrading: Effect of Hydrogen and Guard Bed. Ind. Eng. Chem. Res. 2013, 52, 15394–15406. [Google Scholar] [CrossRef]
- Upreti, S.R.; Lohi, A.; Kapadia, R.A.; El-Haj, R. Vapor Extraction of Heavy Oil and Bitumen: A Review. Energy Fuels 2007, 21, 1562–1574. [Google Scholar] [CrossRef]
- Shah, A.; Fishwick, R.P.; Leeke, G.A.; Wood, J.; Rigby, S.P.; Greaves, M. Experimental optimisation of catalytic process in situ for heavy-oil and bitumen upgrading. J. Can. Petrol. Technol. 2011, 50, 33–47. [Google Scholar] [CrossRef]
- Mazyar, O.A. In-situ hydrogenation of aromatic compounds for heavy oil upgrading. US Patent, No. 9,169,448 B2, 27 October 2015. [Google Scholar]
- Hart, A.; Wood, J. In Situ Catalytic Upgrading of Heavy Crude with CAPRI: Influence of Hydrogen on Catalyst Pore Plugging and Deactivation due to Coke. Energies 2018, 11, 636. [Google Scholar] [CrossRef]
- Galadima, A.; Muraza, O. Ring opening of hydrocarbons for diesel and aromatics production: Design of heterogeneous catalytic systems. Fuel 2016, 181, 618–629. [Google Scholar] [CrossRef]
- Monteiro-Gezork, A.C.A.; Natividad, R.; Winterbottom, J.M. Hydrogenation of naphthalene on NiMo- Ni- and Ru/Al2O3 catalysts: Langmuir–Hinshelwood kinetic modelling. Catal. Today 2008, 130, 471–485. [Google Scholar] [CrossRef]
- Hart, A.; Wood, J.; Greaves, M. In situ catalytic upgrading of heavy oil using a pelletized Ni-Mo/Al2O3 catalyst in the THAI process. J. Pet. Sci. Eng. 2017, 156, 958–965. [Google Scholar] [CrossRef]
- Petrobank Energy and Resources Ltd. Strength in Heavy Oil: Growing THAITM Globally. 2018. Available online: https://www.knotia.ca/kstore/productinfo/fric08/PDFs/Petrobank%20Energy%20and%20Resources%20Ltd.%20AR_2007.pdf (accessed on 29 February 2018).
- Hu, L.; Li, H.; Babadagli, T.; Ahmadloo, M. Experimental investigation of combined electromagnetic heating and solvent-assisted gravity drainage for heavy oil recovery. J. Pet. Sci. Eng. 2017, 154, 589–601. [Google Scholar] [CrossRef]
- Sadeghi, A.; Hassanzadeh, H.; Harding, T.G. Modeling of desiccated zone development during electromagnetic heating of oil sands. J. Pet. Sci. Eng. 2017, 154, 163–171. [Google Scholar] [CrossRef]
- Jha, A.C.K.N. Heavy-Oil Recovery from Thin Pay Zones by Electromagnetic Heating. Energy Sources 1999, 21, 63–73. [Google Scholar] [CrossRef]
- Pizarro, J.; Trevisan, O. Electrical Heating of Oil Reservoirs: Numerical Simulation and Field Test Results. J. Pet. Technol. 1990, 42, 1320–1326. [Google Scholar] [CrossRef]
- Abu-Laban, M.; Muley, P.D.; Hayes, D.J.; Boldor, D. Ex-situ up-conversion of biomass pyrolysis bio-oil vapors using Pt/Al2O3 nanostructured catalyst synergistically heated with steel balls via induction. Catal. Today 2017, 291, 3–12. [Google Scholar] [CrossRef]
- Hart, A.; Adam, M.; Robinson, J.P.; Rigby, S.P.; Wood, J. Tetralin and Decalin H-Donor Effect on Catalytic Upgrading of Heavy Oil Inductively Heated with Steel Balls. Catalysts 2020, 10, 393. [Google Scholar] [CrossRef]
- Liu, F.; Xu, S.; Cao, L.; Chi, Y.; Zhang, T.; Xue, D. A Comparison of NiMo/Al2O3Catalysts Prepared by Impregnation and Coprecipitation Methods for Hydrodesulfurization of Dibenzothiophene. J. Phys. Chem. C 2007, 111, 7396–7402. [Google Scholar] [CrossRef]
- Zhan, X.; Guin, J.A. High-Pressure Hydrogenation of Naphthalene Using a Reduced Iron Catalyst. Energy Fuels 1994, 8, 1384–1393. [Google Scholar] [CrossRef]
- Hassan, F.; Al-Duri, B.; Wood, J. Effect of supercritical conditions upon catalyst deactivation in the hydrogenation of naphthalene. Chem. Eng. J. 2012, 207, 133–141. [Google Scholar] [CrossRef]
- Usman, M.; Li, D.; Razzaq, R.; Yaseen, M.; Li, C.; Zhang, S.-J. Novel MoP/HY catalyst for the selective conversion of naphthalene to tetralin. J. Ind. Eng. Chem. 2015, 23, 21–26. [Google Scholar] [CrossRef]
- Kirumakki, S.; Shpeizer, B.; Sagar, G.; Chary, K.; Clearfield, A. Hydrogenation of Naphthalene over NiO/SiO2–Al2O3 catalysts: Structure–activity correlation. J. Catal. 2006, 242, 319–331. [Google Scholar] [CrossRef]
- Huang, T.-C.; Kang, B.-C. Kinetic Study of Naphthalene Hydrogenation over Pt/Al2O3 Catalyst. Ind. Eng. Chem. Res. 1995, 34, 1140–1148. [Google Scholar] [CrossRef]
- Williams, M.F.; Fonfe, B.; Woltz, C.; Jentys, A.; Van Veen, J.A.R.; Lercher, J.A. Hydrogenation of tetralin on silica-alumina-supported Pt catalysts II. Influence of the support on catalytic activity. J. Catal. 2007, 251, 497–506. [Google Scholar] [CrossRef]
- Hart, A.; Adam, M.; Robinson, J.P.; Rigby, S.P.; Wood, J. Inductive Heating Assisted-Catalytic Dehydrogenation of Tetralin as a Hydrogen Source for Downhole Catalytic Upgrading of Heavy Oil. Top. Catal. 2019, 1–13. [Google Scholar] [CrossRef]
- Li, L.; Hou, Y.; Wu, W.; Liang, S.; Ren, S. Behaviors of tetralin and 9,10-dihydroanthracene as hydrogen donor solvents in the hydrogenolysis of coal-related model compounds. Fuel Process. Technol. 2019, 191, 202–210. [Google Scholar] [CrossRef]
- Gao, L.; Wang, C.; Li, R.; Chen, Q.-W. The Effect of External Magnetic Fields on the Catalytic Activity of Pd Nanoparticles in Suzuki Cross-Coupling Reactions. Nanoscale 2016, 8, 8355–8362. [Google Scholar] [CrossRef]
- Suttisawat, Y.; Sakai, H.; Abe, M.; Rangsunvigit, P.; Horikoshi, S. Microwave effect in the dehydrogenation of tetralin and decalin with a fixed-bed reactor. Int. J. Hydrog. Energy 2012, 37, 3242–3250. [Google Scholar] [CrossRef]
- Hart, A.; Wood, J.; Greaves, M. Laboratory investigation of CAPRI catalytic THAI-add-on process for heavy oil production and in situ upgrading. J. Anal. Appl. Pyrolysis 2017, 128, 18–26. [Google Scholar] [CrossRef]
- Varma, Y.B.G.; Khan, A.A. Flow regime identification and pressure drop in cocurrent gas-liquid upflow through packed beds. Bioprocess Biosyst. Eng. 1997, 16, 355. [Google Scholar] [CrossRef]

| Parameter | CoMo/Al2O3 | NiMo/Al2O3 |
|---|---|---|
| Surface area (m2/g) | 217.6 | 210.4 |
| Pore volume (cm3/g) | 0.46 | 0.62 |
| Pore diameter (nm) | 6.3 | 10.2 |
| Co (wt %) | 3.8 | NA |
| Ni (wt %) | NA | 5.2 |
| Mo (wt %) | 11.2 | 11.5 |
| Gas | Dehydrogenation (%) | Hydrogenation (%) |
|---|---|---|
| Methane | 0.1986 | 0.3944 |
| Ethane | 0.1405 | 0.2224 |
| Ethene | 0.0074 | - |
| Propane | 0.0692 | 0.1971 |
| Propene | 0.0238 | - |
| Iso-butane | 0.0039 | 0.0087 |
| n-butane | 0.0315 | 0.1530 |
| trans-2-butene | - | - |
| 1-butene | 0.0100 | - |
| Cis-2-butene | 0.0039 | - |
| Isopentane | 0.0057 | 0.0020 |
| n-pentane | - | 0.0121 |
| Hydrogen | 20.2000 | - |
| Catalyst | Surface Area(m2/g) | Pore Volume (cm3/g) |
|---|---|---|
| Fresh NiMo | 210.4 | 0.62 |
| NiMo + Naphthalene + H2 | 56.2 | 0.13 |
| NiMo + Tetralin + H2 | 71.6 | 0.22 |
| NiMo + Tetralin + N2 | 23.8 | 0.06 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hart, A.; Adam, M.; Robinson, J.P.; Rigby, S.P.; Wood, J. Hydrogenation and Dehydrogenation of Tetralin and Naphthalene to Explore Heavy Oil Upgrading Using NiMo/Al2O3 and CoMo/Al2O3 Catalysts Heated with Steel Balls via Induction. Catalysts 2020, 10, 497. https://doi.org/10.3390/catal10050497
Hart A, Adam M, Robinson JP, Rigby SP, Wood J. Hydrogenation and Dehydrogenation of Tetralin and Naphthalene to Explore Heavy Oil Upgrading Using NiMo/Al2O3 and CoMo/Al2O3 Catalysts Heated with Steel Balls via Induction. Catalysts. 2020; 10(5):497. https://doi.org/10.3390/catal10050497
Chicago/Turabian StyleHart, Abarasi, Mohamed Adam, John P. Robinson, Sean P. Rigby, and Joseph Wood. 2020. "Hydrogenation and Dehydrogenation of Tetralin and Naphthalene to Explore Heavy Oil Upgrading Using NiMo/Al2O3 and CoMo/Al2O3 Catalysts Heated with Steel Balls via Induction" Catalysts 10, no. 5: 497. https://doi.org/10.3390/catal10050497
APA StyleHart, A., Adam, M., Robinson, J. P., Rigby, S. P., & Wood, J. (2020). Hydrogenation and Dehydrogenation of Tetralin and Naphthalene to Explore Heavy Oil Upgrading Using NiMo/Al2O3 and CoMo/Al2O3 Catalysts Heated with Steel Balls via Induction. Catalysts, 10(5), 497. https://doi.org/10.3390/catal10050497