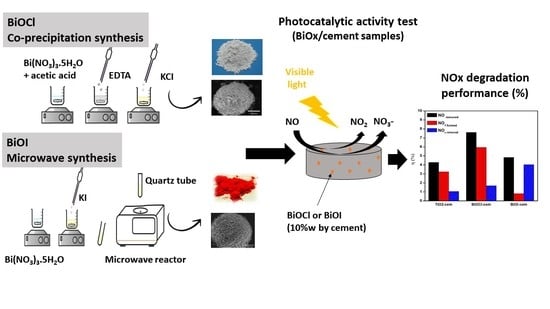
Photocatalytic BiOX Mortars under Visible Light Irradiation: Compatibility, NOx Efficiency and Nitrate Selectivity
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Characterization
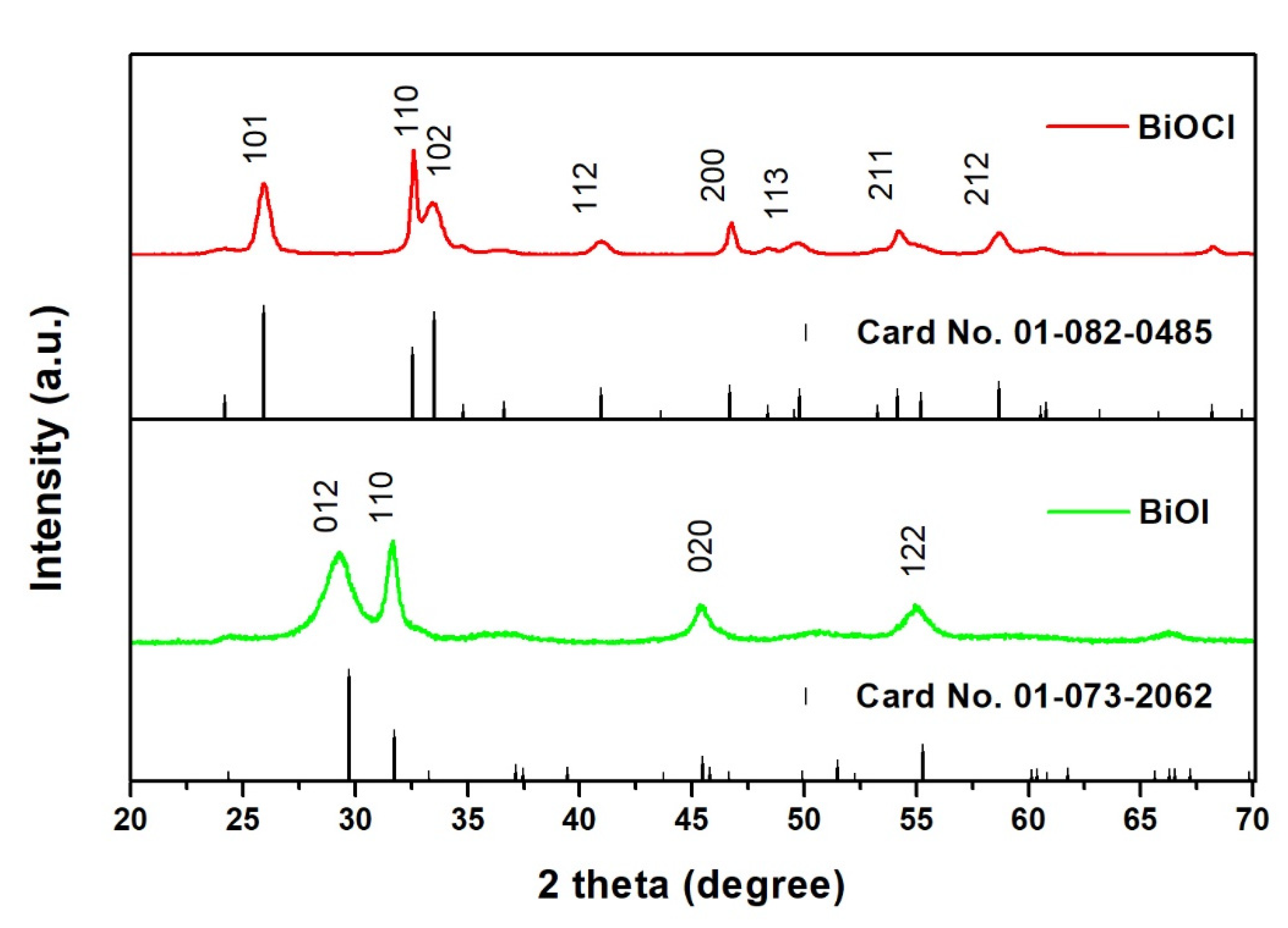
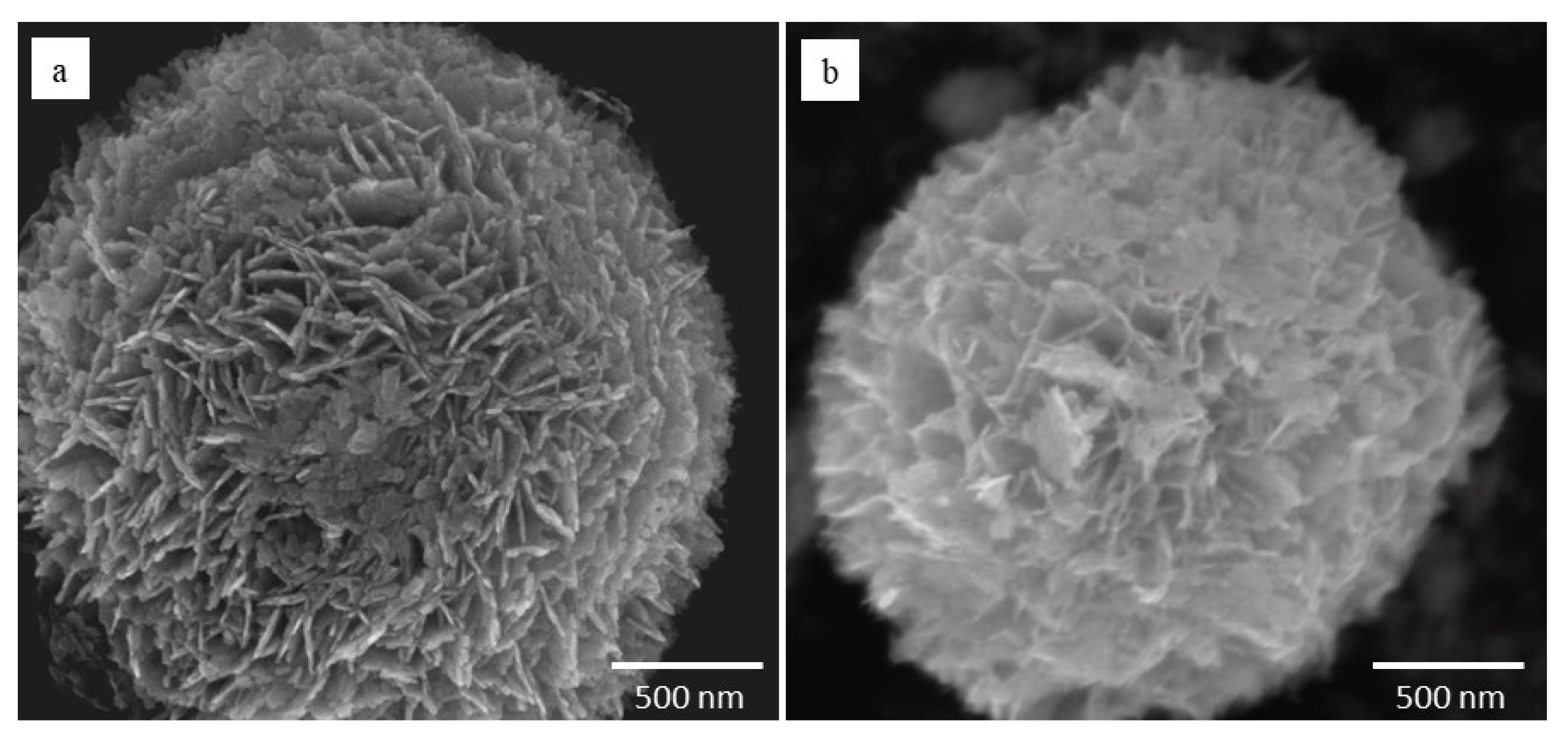
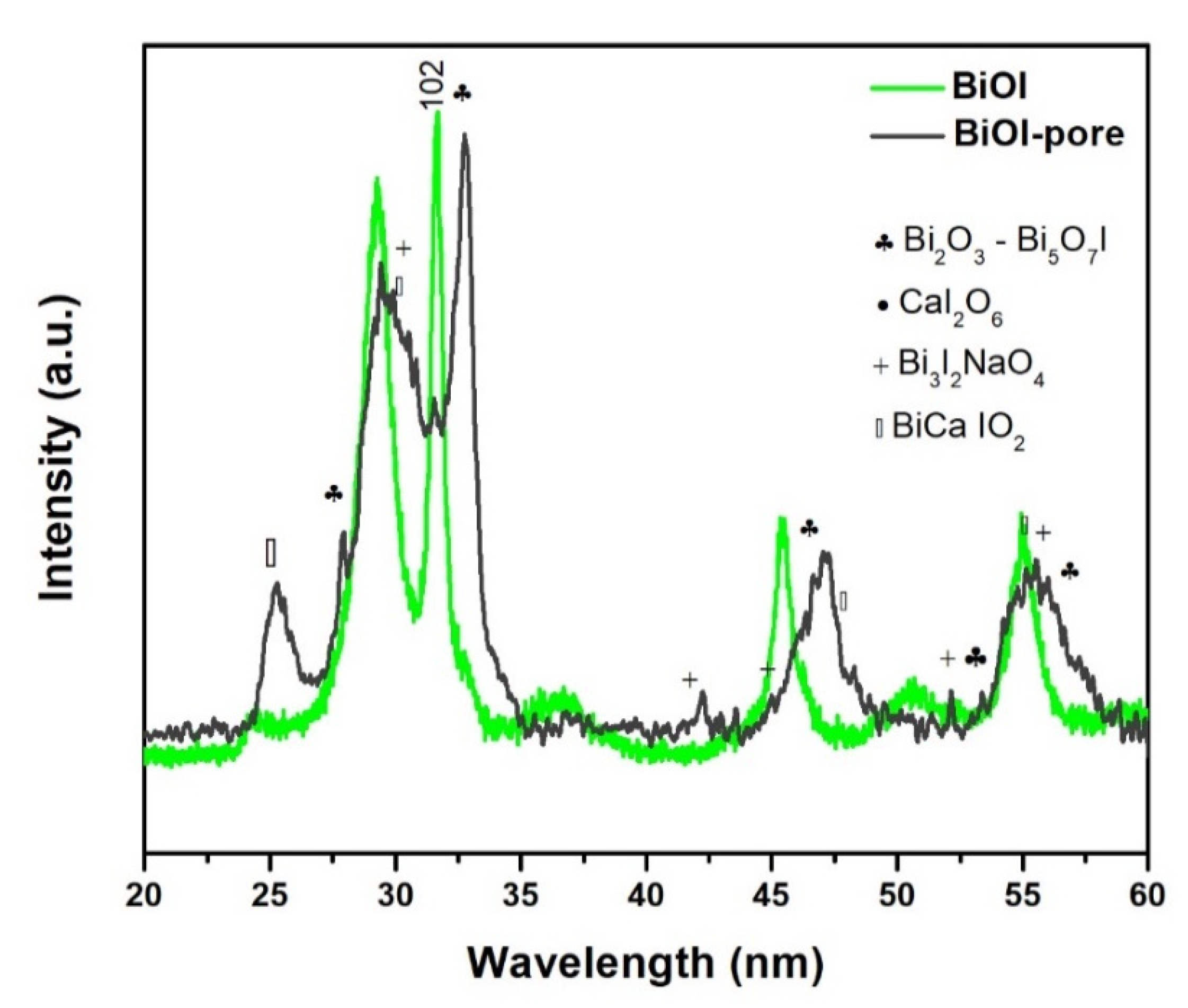
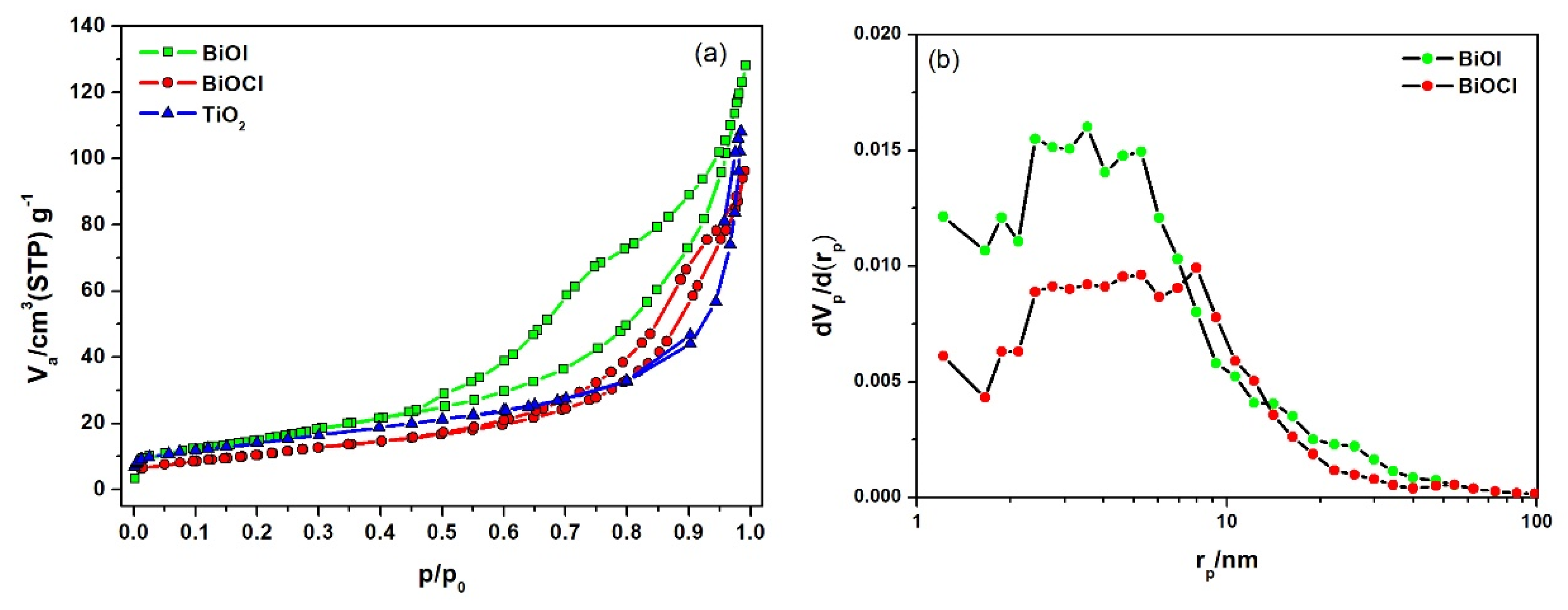
2.1.1. Photocatalysts
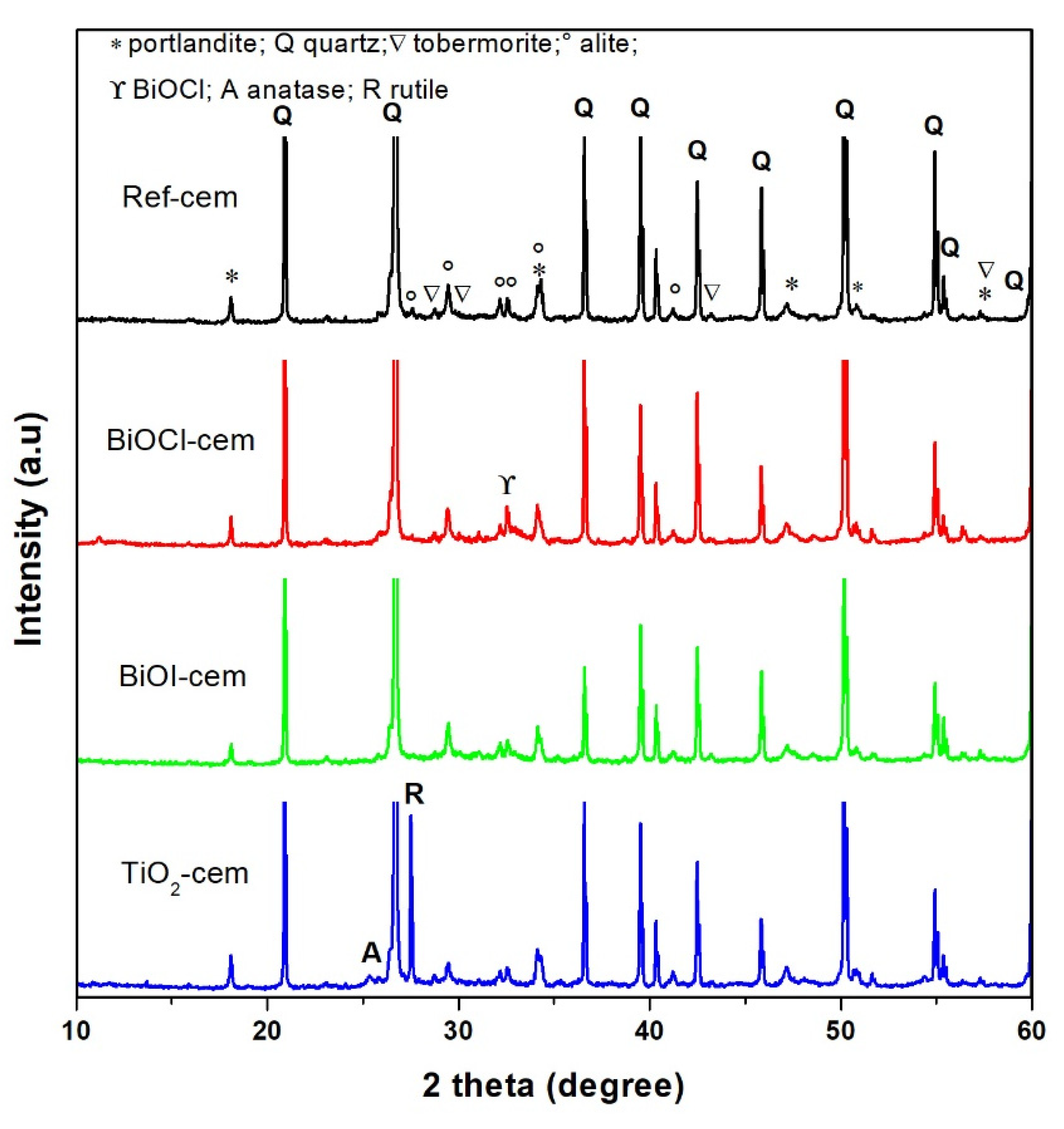
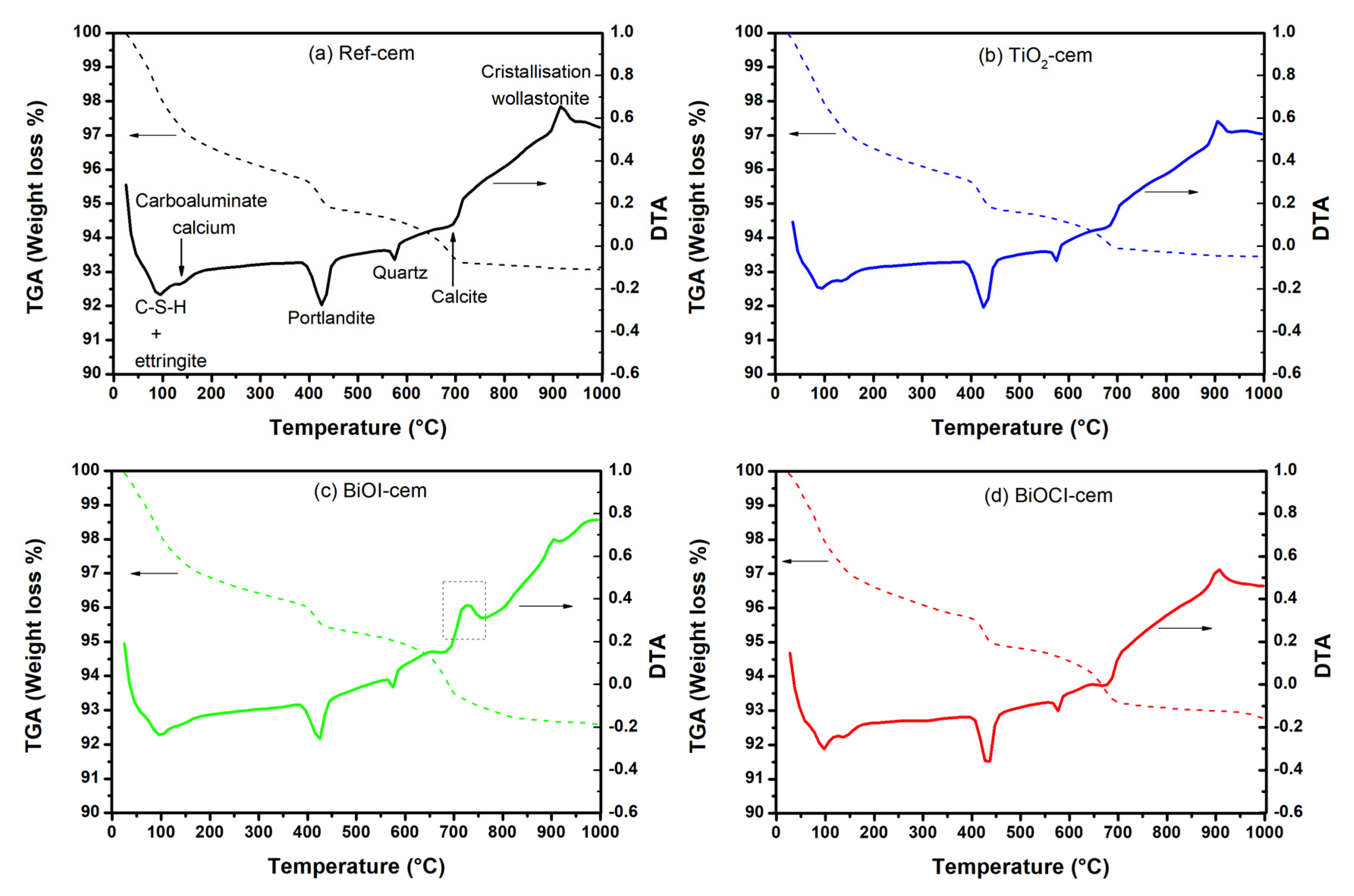
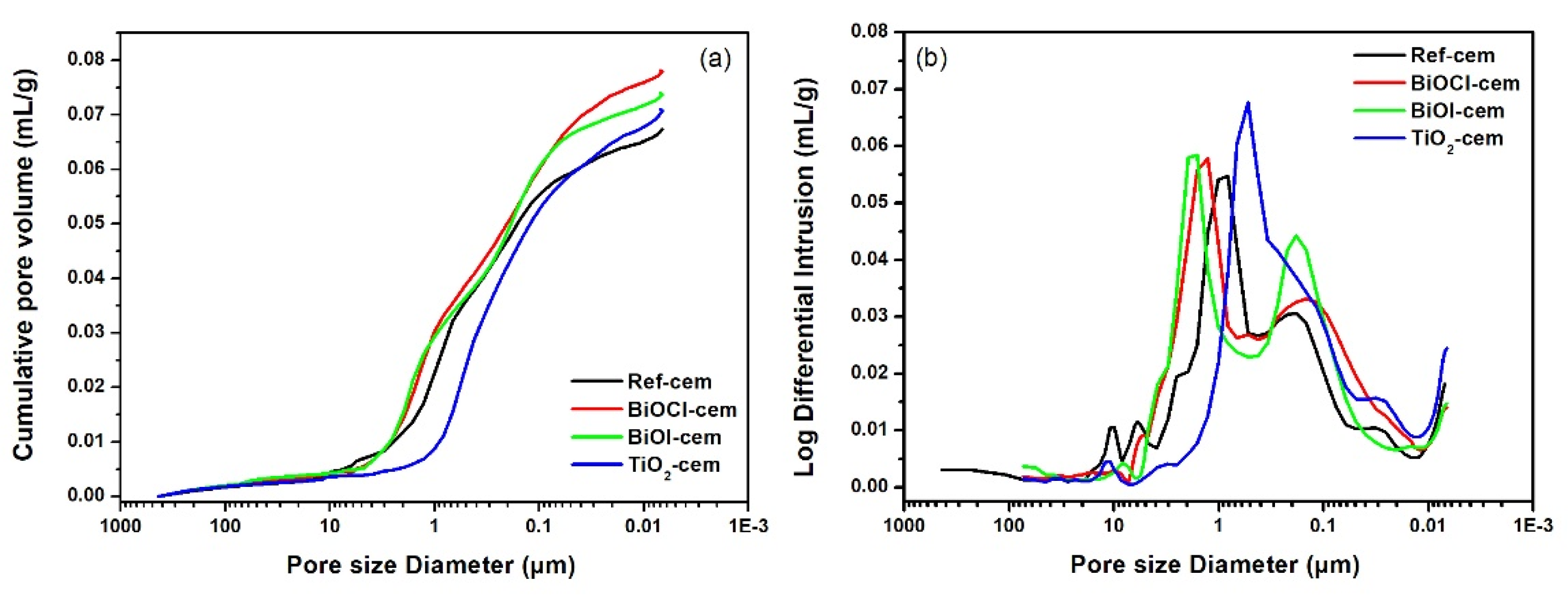
2.1.2. Cement-Based Materials
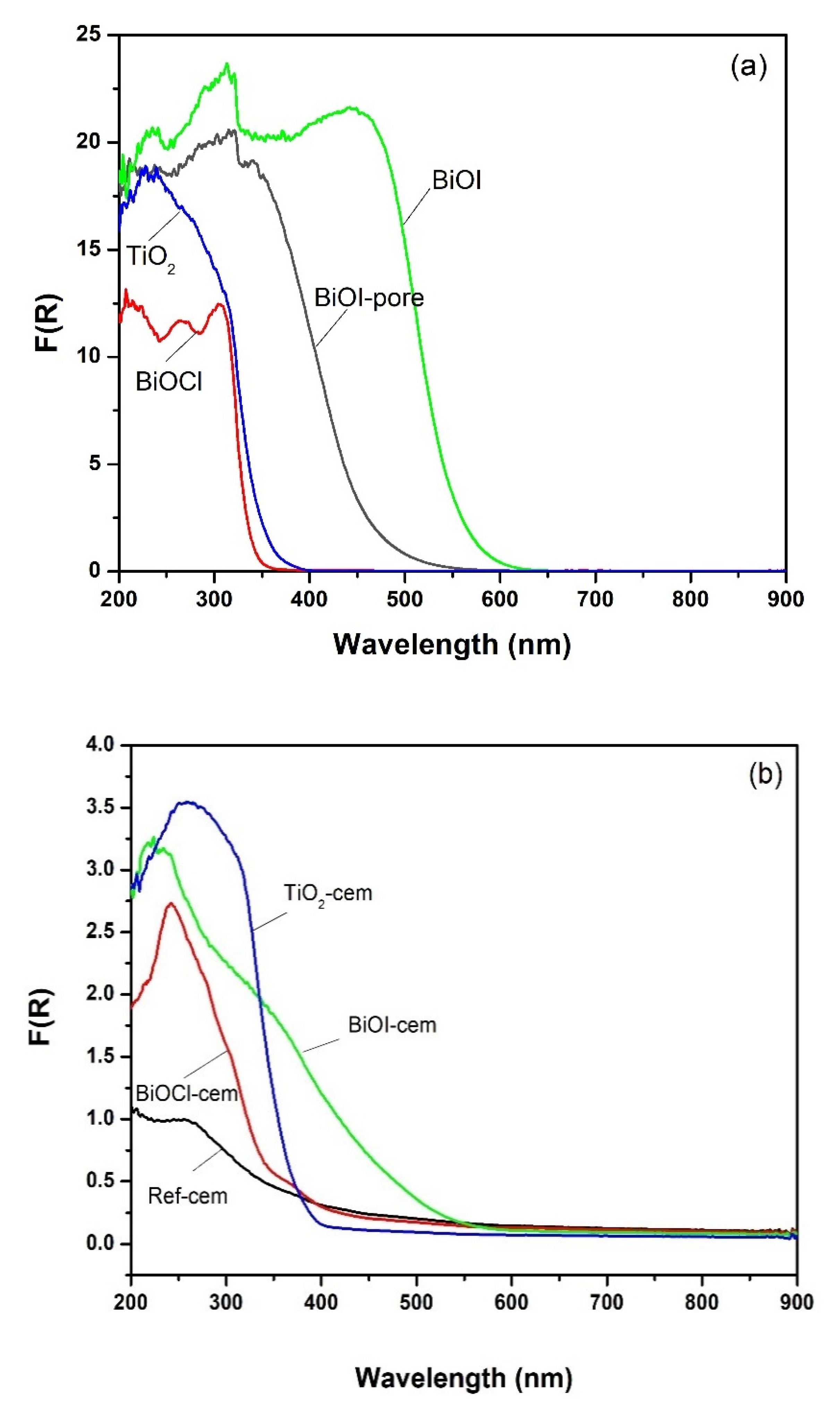
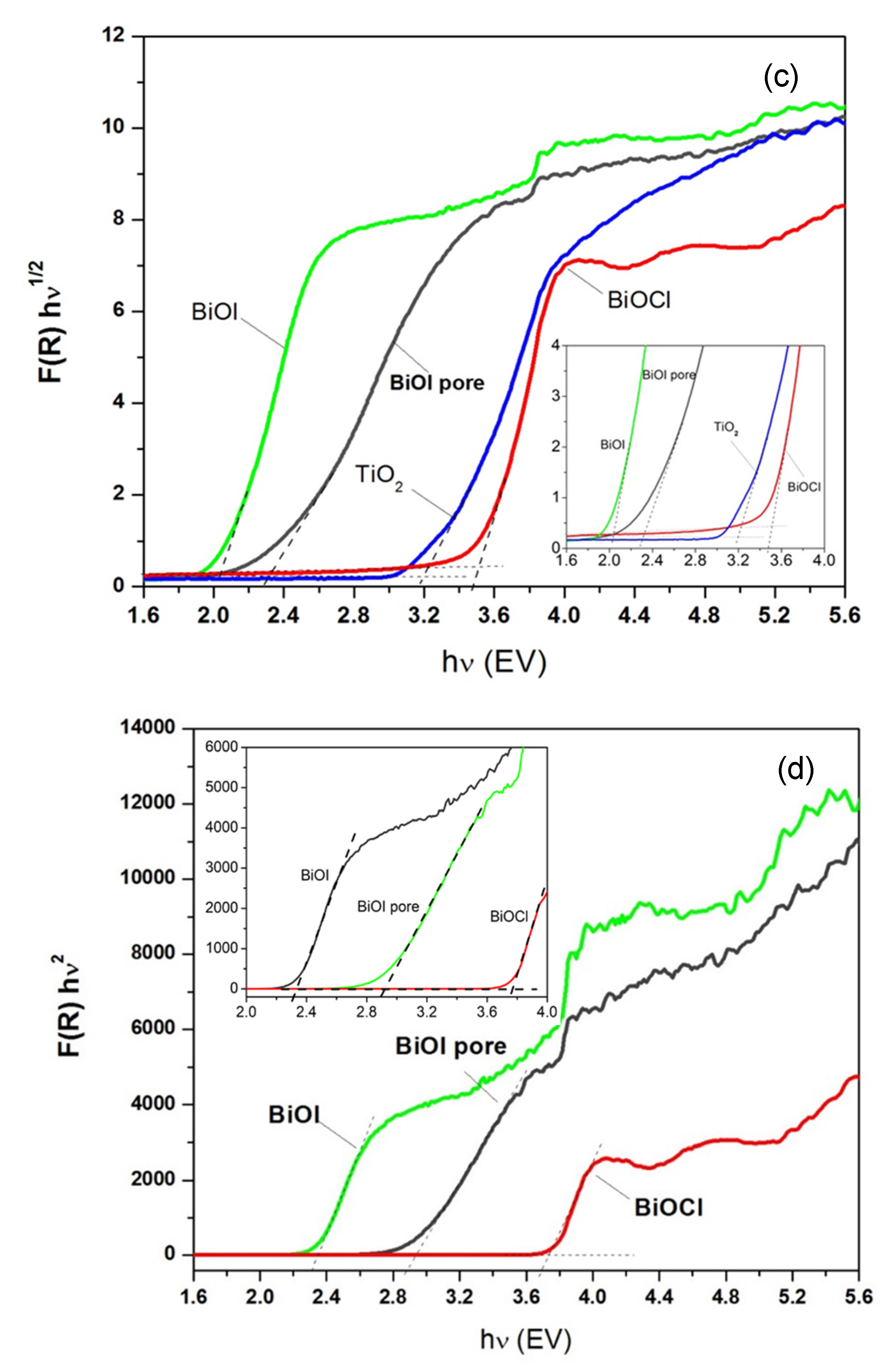
2.2. Optical Properties
2.3. Photocatalytic Activity
3. Experimental
3.1. Synthesis of BiOX (X = Cl and I) Powders
3.2. Preparation of Cement-Based Samples
3.3. Physicochemical Characterization
3.3.1. Photocatalysts Powders
3.3.2. Cement-Based Materials
3.4. Optical Properties
3.5. Photocatalytic Performance
4. Conclusions
- BiOCl and BiOI are particles of spherical shape with a flower-like morphology and micrometric size, which reduces the concerns about the possible unwanted effects of the use of nanoparticles.
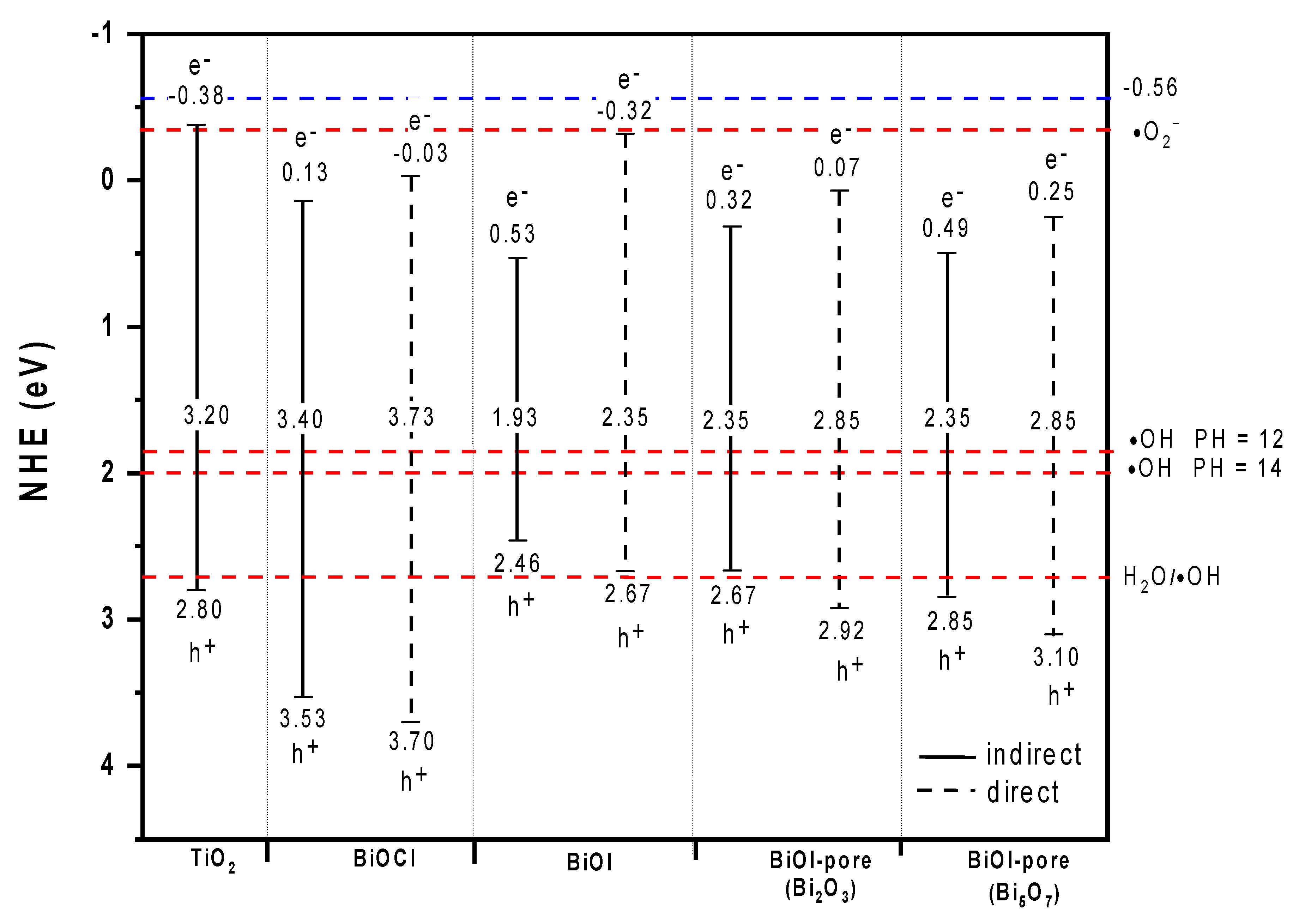
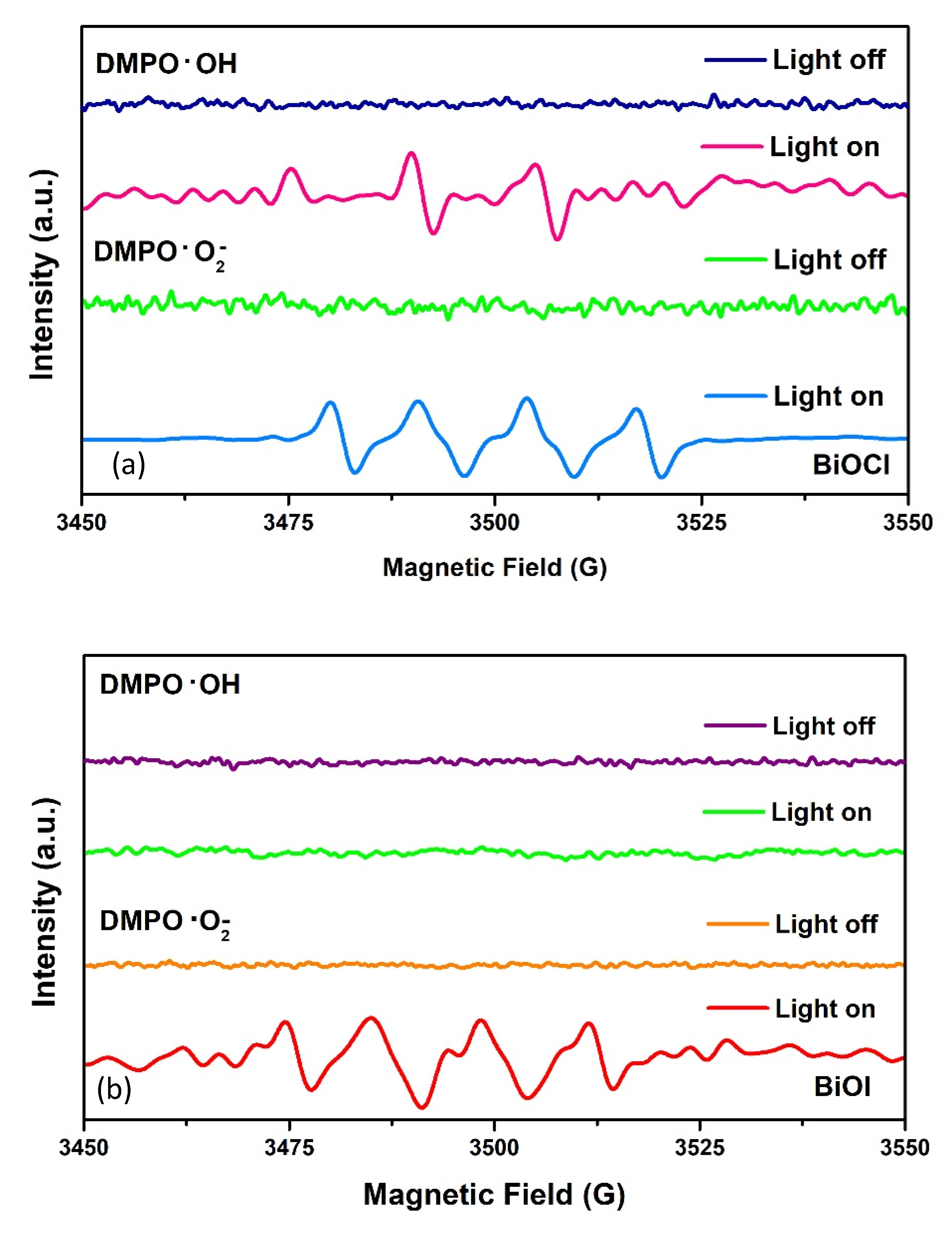
- The presence of the reformed CB with higher reduction potential in the BiOX. Therefore, promoted electrons can reduce oxygen and generate superoxide radicals. The position of the VB of BiOCl allows the oxidation of H2O to form •OH. In the case of BiOI in neutral media, VB has not the potential to oxidize water and therefore, •OH is not detected.
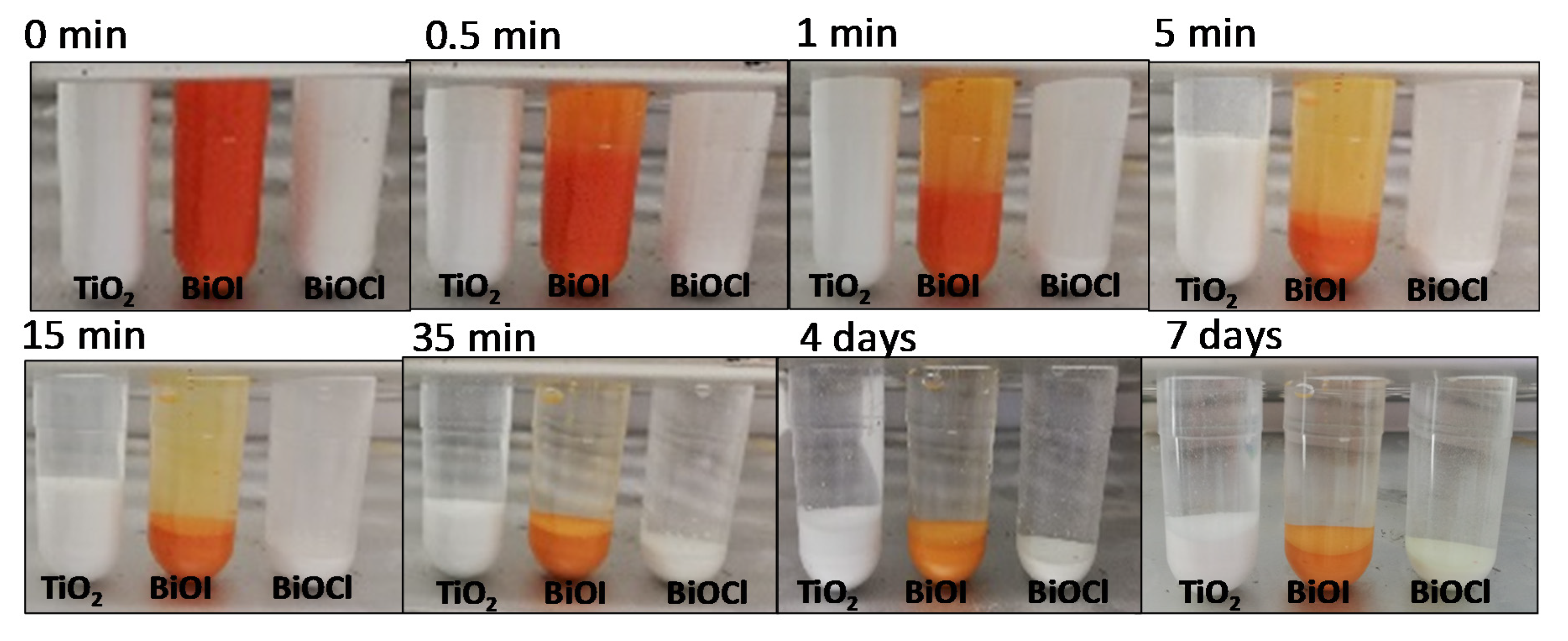
- In alkaline media, TiO2 forms flocculated sediment. Meanwhile, BiOI presents intermediate behavior and for BiOCl no flocculation occurred. The differences can be attributed to particle size.
- There is a chemical interaction between the BiOI and the alkaline aqueous phase of the cement, which leads to the formation, among others, of Bi2O3 and Bi5O7I. These compounds show a lower visible light absorption capacity than BiOI.
- The interaction between BiOX photocatalysts and cement matrix does not appear to adversely affect the inherent properties of cement-based materials. TiO2 results in drier samples, while BiOX induces more fluid behavior. Only TiO2 acts as a filler to refine the cement pore structure.
- The BiOCl-cem sample showed higher photocatalytic activity than the other samples in terms of NO removal. BiOI-cem showed a nitrate selectivity of ~83%, which is much higher than TiO2-cem (~24%) and BiOCl-cem (~22%). The lower amount of NO2 produced by BiOI-cem results in the highest NOx removal efficiency. These results make its use a safer and environmentally sustainable option.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, J.; Poon, C. Photocatalytic construction and building materials: From fundamentals to applications. Build. Environ. 2009, 44, 1899–1906. [Google Scholar] [CrossRef]
- Jimenez-Relinque, E.; Rodriguez-Garcia, J.R.; Castillo, A.; Castellote, M. Characteristics and efficiency of photocatalytic cementitious materials: Type of binder, roughness and microstructure. Cem. Concr. Res. 2015, 71, 124–131. [Google Scholar] [CrossRef]
- Guo, S.; Wu, Z.; Zhao, W. TiO2-based building materials: Above and beyond traditional applications. Chin. Sci. Bull. 2009, 54, 1137–1142. [Google Scholar] [CrossRef]
- Laplaza, A.; Jimenez-Relinque, E.; Campos, J.; Castellote, M. Photocatalytic behavior of colored mortars containing TiO2 and iron oxide based pigments. Constr. Build. Mater. 2017, 144, 300–310. [Google Scholar] [CrossRef]
- Sapiña, M.; Jimenez-Relinque, E.; Castellote, M. Controlling the levels of airborne pollen: Can heterogeneous photocatalysis help? Environ. Sci. Technol. 2013, 47, 11711–11716. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Relinque, E.; Sapiña, M.; Nevshupa, R.; Roman, E.; Castellote, M. Photocatalytic decomposition of pollen allergenic extracts of Cupressus arizonica and Platanus hybrida. Chem. Eng. J. 2016, 286, 560–570. [Google Scholar] [CrossRef]
- Jiménez-Relinque, E.; Hingorani, R.; Rubiano, F.; Grande, M.; Castillo, Á.; Castellote, M. In situ evaluation of the NOx removal efficiency of photocatalytic pavements: Statistical analysis of the relevance of exposure time and environmental variables. Environ. Sci. Pollut. Res. 2019, 1–8. [Google Scholar] [CrossRef]
- Bengtsson, N.; Castellote, M. Heterogeneous photocatalysis on construction materials: Effect of catalyst properties on the efficiency for degrading NOx and self cleaning. Mater. Constr. 2014, 64, 1–17. [Google Scholar] [CrossRef]
- Ai, Z.; Ho, W.; Lee, S.; Zhang, L. Efficient photocatalytic removal of NO in indoor air with hierarchical bismuth oxybromide nanoplate microspheres under visible light. Environ. Sci. Technol. 2009, 43, 4143–4150. [Google Scholar] [CrossRef]
- Dong, F.; Zhao, Z.; Sun, Y.; Zhang, Y.; Yan, S.; Wu, Z. An Advanced Semimetal-Organic Bi Spheres-g-C3N4 Nanohybrid with SPR-Enhanced Visible-Light Photocatalytic Performance for NO Purification. Environ. Sci. Technol. 2015, 49, 12432–12440. [Google Scholar] [CrossRef]
- Doustkhah, E.; Ide, Y. Microporous Layered Silicates: Old but New Microporous Materials. New J. Chem. 2020, 1–30. [Google Scholar] [CrossRef]
- Yang, X.; Yang, D.; Zhu, H.; Liu, J.; Martins, W.N.; Frost, R.; Daniel, L.; Shen, Y. Mesoporous structure with size controllable anatase attached on silicate layers for efficient photocatalysis. J. Phys. Chem. C 2009, 113, 8243–8248. [Google Scholar] [CrossRef]
- Xiao, F.X.; Miao, J.; Liu, B. Layer-by-layer self-assembly of CdS quantum dots/graphene nanosheets hybrid films for photoelectrochemical and photocatalytic applications. J. Am. Chem. Soc. 2014, 136, 1559–1569. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.; Chen, S.; Quan, X.; Yu, H.; Zhao, H. Graphene oxide modified g-C3N4 hybrid with enhanced photocatalytic capability under visible light irradiation. J. Mater. Chem. 2012, 22, 2721–2726. [Google Scholar] [CrossRef]
- Niu, P.; Zhang, L.; Liu, G.; Cheng, H.M. Graphene-like carbon nitride nanosheets for improved photocatalytic activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- De la Peña-Benítez, P.R.; García-Santos, A.; Santonja, R.; Sapiña, M.; Jiménez-Relinque, E.; Castellote, M.; Sánchez-Cifuentes, M. Evaluación ambiental de pinturas al agua para exteriores de los edificios modificadas con óxido de grafeno. Superficies y vacío 2016, 29, 105–111. Available online: http://journal.smctsm.org.mx/index.php/SyV/article/viewFile/85/55 (accessed on 16 January 2020).
- Takata, T.; Furumi, Y.; Shinohara, K.; Tanaka, A.; Hara, M.; Kondo, J.N.; Domen, K. Photocatalytic Decomposition of Water on Spontaneously Hydrated Layered Perovskites. Chem. Mater. 1997, 9, 1063–1064. [Google Scholar] [CrossRef]
- Sano, T.; Tsutsui, S.; Koike, K.; Hirakawa, T.; Teramoto, Y.; Negishi, N.; Takeuchi, K. Activation of graphitic carbon nitride (g-C3N4) by alkaline hydrothermal treatment for photocatalytic NO oxidation in gas phase. J. Mater. Chem. A 2013, 1, 6489–6496. [Google Scholar] [CrossRef]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric Photocatalysts Based on Graphitic Carbon Nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef]
- Mohapatra, L.; Parida, K. A review on the recent progress, challenges and perspective of layered double hydroxides as promising photocatalysts. J. Mater. Chem. A 2016, 4, 10744–10766. [Google Scholar] [CrossRef]
- Ye, L.; Su, Y.; Jin, X.; Xie, H.; Zhang, C. Recent advances in BiOX (X = Cl, Br and I) photocatalysts: Synthesis, modification, facet effects and mechanisms. Environ. Sci. Nano 2014, 1, 90–112. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, C.; Lai, C.; Zeng, G.; Huang, D.; Cheng, M.; Wang, J.; Chen, F.; Zhou, C.; Xiong, W. BiOX (X = Cl, Br, I) photocatalytic nanomaterials: Applications for fuels and environmental management. Adv. Colloid Interface Sci. 2018, 254, 76–93. [Google Scholar] [CrossRef]
- Ou, M.; Dong, F.; Zhang, W.; Wu, Z. Efficient visible light photocatalytic oxidation of NO in air with band-gap tailored (BiO)2CO3-BiOI solid solutions. Chem. Eng. J. 2014, 255, 650–658. [Google Scholar] [CrossRef]
- Lv, J.; Hu, Q.; Cao, C.; Zhao, Y. Modulation of valence band maximum edge and photocatalytic activity of BiOX by incorporation of halides. Chemosphere 2018, 191, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Sun, Y.; Fu, M.; Wu, Z.; Lee, S.C. Room temperature synthesis and highly enhanced visible light photocatalytic activity of porous BiOI/BiOCl composites nanoplates microflowers. J. Hazard. Mater. 2012, 219, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, K.; Zhang, G.; Yin, S. Facile preparation of BiOX (X = Cl, Br, I) nanoparticles and up-conversion phosphors/BiOBr composites for efficient degradation of NO gas: Oxygen vacancy effect and near infrared light responsive mechanism. Chem. Eng. J. 2017, 325, 59–70. [Google Scholar] [CrossRef]
- Jiang, G.; Li, X.; Lan, M.; Shen, T.; Lv, X.; Dong, F.; Zhang, S. Monodisperse bismuth nanoparticles decorated graphitic carbon nitride: Enhanced visible-light-response photocatalytic NO removal and reaction pathway. Appl. Catal. B Environ. 2017, 205, 532–540. [Google Scholar] [CrossRef]
- Mera, A.C.; la Cruz, A.M.; Pérez-Tijerina, E.; Meléndrez, M.F.; Valdés, H. Nanostructured BiOI for air pollution control: Microwave-assisted synthesis, characterization and photocatalytic activity toward NO transformation under visible light irradiation. Mater. Sci. Semicond. Process. 2018, 88, 20–27. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Q.; Dong, F. Visible-light photocatalytic removal of NO in air over BiOX (X = Cl, Br, I) single-crystal nanoplates prepared at room temperature. Ind. Eng. Chem. Res. 2013, 52, 6740–6746. [Google Scholar] [CrossRef]
- Jimenez-Relinque, E.; Castellote, M. Quantification of hydroxyl radicals on cementitious materials by fluorescence spectrophotometry as a method to assess the photocatalytic activity. Cem. Concr. Res. 2015, 74, 108–115. [Google Scholar] [CrossRef]
- Yang, L.; Hakki, A.; Wang, F.; Macphee, D.E. Different Roles of Water in Photocatalytic DeNOx Mechanisms on TiO2: Basis for Engineering Nitrate Selectivity? ACS Appl. Mater. Interfaces 2017, 9, 17034–17041. [Google Scholar] [CrossRef] [PubMed]
- Ohko, Y.; Nakamura, Y.; Fakuda, A.; Matsuzawa, S.; Takeuchi, K. Photocatalytic oxidation of nitrogen monoxide using TiO2 thin films under continuous UV light illumination. J. Photochem. Photobiol. A Chem. 2008, 112, 10502–10508. [Google Scholar] [CrossRef]
- Wang, D.; Hou, P.; Yang, P.; Cheng, X. BiOBr@SiO2 flower-like nanospheres chemically-bonded on cement-based materials for photocatalysis. Appl. Surf. Sci. 2018, 430, 539–548. [Google Scholar] [CrossRef]
- Tallapally, V.; Nakagawara, T.A.; Demchenko, D.O.; Özgür, Ü.; Arachchige, I.U. Ge1-:XSnx alloy quantum dots with composition-tunable energy gaps and near-infrared photoluminescence. Nanoscale 2018, 10, 20296–20305. [Google Scholar] [CrossRef] [PubMed]
- Borchert, H.; Shevchenko, E.V.; Robert, A.; Mekis, I.; Kornowski, A.; Grübel, G.; Weller, H. Determination of nanocrystal sizes: A comparison of TEM, SAXS, and XRD studies of highly monodisperse CoPt3 particles. Langmuir 2005, 21, 1931–1936. [Google Scholar] [CrossRef] [PubMed]
- Henle, J.; Simon, P.; Frenzel, A.; Scholz, S.; Kaskel, S. Nanosized BiOX (X = Cl, Br, I) particles synthesized in reverse microemulsions. Chem. Mater. 2007, 19, 366–373. [Google Scholar] [CrossRef]
- Henle, J.; Kaskel, S. Preparation of photochromic transparent BiOX (X = Cl, I)/PLA nanocomposite materials via microemulsion polymerization. J. Mater. Chem. 2007, 17, 4964–4971. [Google Scholar] [CrossRef]
- Folli, A.; Pochard, I.; Nonat, A.; Jakobsen, U.H.; Shepherd, A.M.; Macphee, D.E. Engineering Photocatalytic Cements: Understanding TiO2 Surface Chemistry to Control and Modulate Photocatalytic Performances. J. Am. Ceram. Soc. 2010, 93, 3360–3369. [Google Scholar] [CrossRef]
- Shaw, D.J. Introduction to Colloid and Surface Chemistry; Butterworths: Waltham, MA, USA, 1968; p. 212. [Google Scholar]
- Sun, S.; Wang, W.; Zhang, L.; Zhou, L.; Yin, W.; Meng, S. Bi5O7Br and its structural relation to α-Bi5O7I. Environ. Sci. Technol. 2009, 43, 2005–2010. [Google Scholar] [CrossRef]
- Wu, G.; Zhao, Y.; Li, Y.; Ma, H.; Zhao, J. pH-dependent synthesis of iodine-deficient bismuth oxyiodide microstructures: Visible-light photocatalytic activity. J. Colloid Interface Sci. 2018, 510, 228–236. [Google Scholar] [CrossRef]
- Long, M.; Hu, P.; Wu, H.; Chen, Y.; Tan, B.; Cai, W. Understanding the composition and electronic structure dependent photocatalytic performance of bismuth oxyiodides. J. Mater. Chem. A 2015, 3, 5592–5598. [Google Scholar] [CrossRef]
- Cote, P.; Gilliam, M. Environmental Aspects of Stabilization and Solidification of Hazardous and Radioactive Wastes; ASTM International: West Conshohocken, PA, USA, 1989; p. 1033. [Google Scholar]
- Yang, J.; Xu, L.; Liu, C.; Xie, T. Preparation and photocatalytic activity of porous Bi5O7I nanosheets. Appl. Surf. Sci. 2014, 319, 265–271. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, J.; Zhang, Y.; Li, Q.; Gong, J.R. Visible light photocatalytic H2-production activity of CuS/ZnS porous nanosheets based on photoinduced interfacial charge transfer. Nano Lett. 2011, 11, 4774–4779. [Google Scholar] [CrossRef]
- Jimenez-Relinque, E.; Llorente, I.; Castellote, M. TiO2 cement-based materials: Understanding optical properties and electronic band structure of complex matrices. Catal. Today 2017, 287, 203–209. [Google Scholar] [CrossRef]
- Galan, I.; Andrade, C.; Castellote, M. Thermogravimetrical analysis for monitoring carbonation of cementitious materials: Uptake of CO2 and deepening in C-S-H knowledge. J. Anal. Calorim. 2012, 110, 309–319. [Google Scholar] [CrossRef]
- Yu, C.; Fan, C.; Yu, J.C.; Zhou, W.; Yang, K. Preparation of bismuth oxyiodides and oxides and their photooxidation characteristic under visible/UV light irradiation. Mater. Res. Bull. 2011, 46, 140–146. [Google Scholar] [CrossRef]
- Tallapally, V.; Esteves, R.J.A.; Nahar, L.; Arachchige, I.U. Multivariate Synthesis of Tin Phosphide Nanoparticles: Temperature, Time, and Ligand Control of Size, Shape, and Crystal Structure. Chem. Mater. 2016, 28, 5406–5414. [Google Scholar] [CrossRef]
- Nadtochenko, V.; Denisov, N.; Gorenberg, A.; Kozlov, Y.; Chubukov, P.; Rengifo, J.A.; Pulgarin, C.; Kiwi, J. Correlations for photocatalytic activity and spectral features of the absorption band edge of TiO2 modified by thiourea. Appl. Catal. B Environ. 2009, 91, 460–469. [Google Scholar] [CrossRef]
- Singla, P.; Pandey, O.P.; Singh, K. Study of photocatalytic degradation of environmentally harmful phthalate esters using Ni-doped TiO2 nanoparticles. Int. J. Environ. Sci. Technol. 2015, 13, 849–856. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Yao, H.; Dang, L.; Li, Z. Chemical etching preparation of BiOI/Bi2O3 heterostructures with enhanced photocatalytic activities. Catal. Commun. 2011, 12, 660–664. [Google Scholar] [CrossRef]
- Yi, S.; Yue, X.; Xu, D.; Liu, Z.; Zhao, F.; Wang, D.; Lin, Y. Study on photogenerated charge transfer properties and enhanced visible-light photocatalytic activity of p-type Bi2O3/n-type ZnO heterojunctions. New J. Chem. 2015, 39, 2917–2924. [Google Scholar] [CrossRef]
- Huang, H.; Han, X.; Li, X.; Wang, S.; Chu, P.K.; Zhang, Y. Fabrication of multiple heterojunctions with tunable visible-light-active photocatalytic reactivity in BiOBr-BiOI full-range composites based on microstructure modulation and band structures. ACS Appl. Mater. Interfaces 2015, 7, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.L.; Liu, C.M.; Huang, F.Q.; Zheng, C.; Wang, W.D. Study of the electronic structure and photocatalytic activity of the BiOCl photocatalyst. Appl. Catal. B Environ. 2006, 68, 125–129. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A. Oxygen defect dependent variation of band gap, Urbach energy and luminescence property of anatase, anatase-rutile mixed phase and of rutile phases of TiO2 nanoparticles. Phys. E Low-Dimens. Syst. Nanostruct. 2014, 56, 364–371. [Google Scholar] [CrossRef]
- Smith, A.M.; Nie, S. Semiconductor nanocrystals: Structure, properties, and band gap engineering. Acc. Chem. Res. 2010, 43, 190–200. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, X.; Sun, P.; Zhang, L. ZnO/BiOI heterostructures: Photoinduced charge-transfer property and enhanced visible-light photocatalytic activity. J. Phys. Chem. C 2011, 115, 20555–20564. [Google Scholar] [CrossRef]
- Bloh, J.Z.; Dillert, R.; Bahnemann, D.W. Ruthenium-modified zinc oxide, a highly active vis-photocatalyst: The nature and reactivity of photoactive centres. Phys. Chem. Chem. Phys. 2014, 16, 5833–5845. [Google Scholar] [CrossRef]
- Houst, Y.F.; Bowen, P.; Perche, F.; Kauppi, A.; Borget, P.; Galmiche, L.; le Meins, J.; Lafuma, F.; Flatt, R.J.; Schober, I.; et al. Design and function of novel superplasticizers for more durable high performance concrete (superplast project). Cem. Concr. Res. 2008, 38, 1197–1209. [Google Scholar] [CrossRef]
- Castellote, M.; Andrade, C.; Alonso, M.C. Standardization, to a Reference of 25 °C, of Electrical Resistivity for Mortars and Concretes in Saturated or Isolated Conditions. ACI Mater. J. 2003, 99, 119–128. [Google Scholar]


| Samples | Workability Diameter Slump | Density in Fresh State | Compressive Strength (28 days) | Resistivity (28 days) |
|---|---|---|---|---|
| (mm) | (Kg/m3) | (MPa) | (KΩ.m) | |
| Ref-cem | 138 ± 2 | 2110 | 38.42 ± 0.61 | 0.10 ± 0.00 |
| BiOCl-cem | 180 ± 3 | 2130 | 37.54 ± 1.00 | 0.07 ± 0.00 |
| BiOI-cem | 176 ± 6 | 2150 | 38.89 ± 0.30 | 0.11 ± 0.00 |
| TiO2-cem | 117 ± 0 | 2120 | 39.01 ± 0.66 | 0.11 ± 0.01 |
| Ref-cem | TiO2-cem | BiOCl-cem | BiOI-cem | |
|---|---|---|---|---|
| Free water | 2.14 | 1.96 | 1.97 | 2.03 |
| Bound water | 2.61 | 2.74 | 2.81 | 2.47 |
| Portlandite | 2.55 | 2.71 | 2.10 | 1.60 |
| Calcite | 3.02 | 2.14 | 3.28 | 3.86 |
| BiOI | - | - | - | 8.32 |
| Samples | SBET (m2 g−1) | Bulk Density * (g/mL) | Total Porosity * (% vol) |
|---|---|---|---|
| Ref-cem | 4.7 | 2.12 | 14.3 |
| BiOCl-cem | 12.7 | 2.08 | 16.2 |
| BiOI-cem | 10.1 | 2.09 | 15.4 |
| TiO2-cem | 7.39 | 2.11 | 14.9 |
| Sample | Χ (eV) | Eg (eV) | ECB (eV) | EVB (eV) | Eg (eV) | ECB (eV) | EVB (eV) |
|---|---|---|---|---|---|---|---|
| Indirect | Direct | ||||||
| BiOCl | 6.33 a | 3.40 | 0.13 | 3.53 | 3.73 | −0.03 | 3.70 |
| BiOI | 5.99 a | 1.93 | 0.53 | 2.46 | 2.35 | −0.32 | 2.67 |
| BiOI-pore (Bi2O3) | 5.99 b | 2.35 | 0.32 | 2.67 | 2.85 | 0.07 | 2.92 |
| BiOI-pore (Bi5O7I) | 6.17 c | 2.35 | 0.49 | 2.85 | 2.85 | 0.25 | 3.10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nava-Núñez, M.Y.; Jimenez-Relinque, E.; Grande, M.; Martínez-de la Cruz, A.; Castellote, M. Photocatalytic BiOX Mortars under Visible Light Irradiation: Compatibility, NOx Efficiency and Nitrate Selectivity. Catalysts 2020, 10, 226. https://doi.org/10.3390/catal10020226
Nava-Núñez MY, Jimenez-Relinque E, Grande M, Martínez-de la Cruz A, Castellote M. Photocatalytic BiOX Mortars under Visible Light Irradiation: Compatibility, NOx Efficiency and Nitrate Selectivity. Catalysts. 2020; 10(2):226. https://doi.org/10.3390/catal10020226
Chicago/Turabian StyleNava-Núñez, Magaly Y., Eva Jimenez-Relinque, Maria Grande, Azael Martínez-de la Cruz, and Marta Castellote. 2020. "Photocatalytic BiOX Mortars under Visible Light Irradiation: Compatibility, NOx Efficiency and Nitrate Selectivity" Catalysts 10, no. 2: 226. https://doi.org/10.3390/catal10020226
APA StyleNava-Núñez, M. Y., Jimenez-Relinque, E., Grande, M., Martínez-de la Cruz, A., & Castellote, M. (2020). Photocatalytic BiOX Mortars under Visible Light Irradiation: Compatibility, NOx Efficiency and Nitrate Selectivity. Catalysts, 10(2), 226. https://doi.org/10.3390/catal10020226