Degradation of Carbamazepine by Photo(electro)catalysis on Nanostructured TiO2 Meshes: Transformation Products and Reaction Pathways
Abstract
1. Introduction
2. Results and Discussion
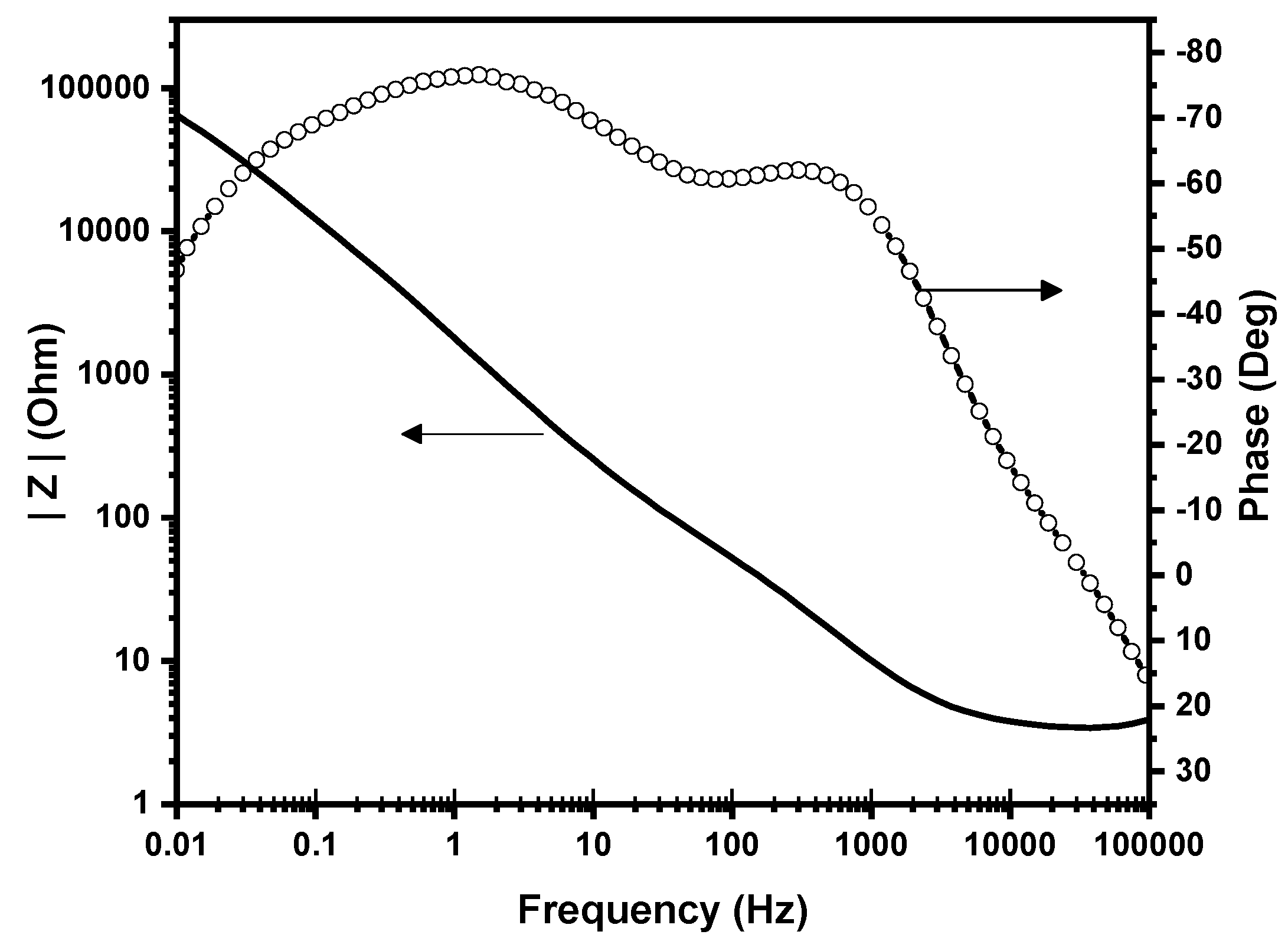
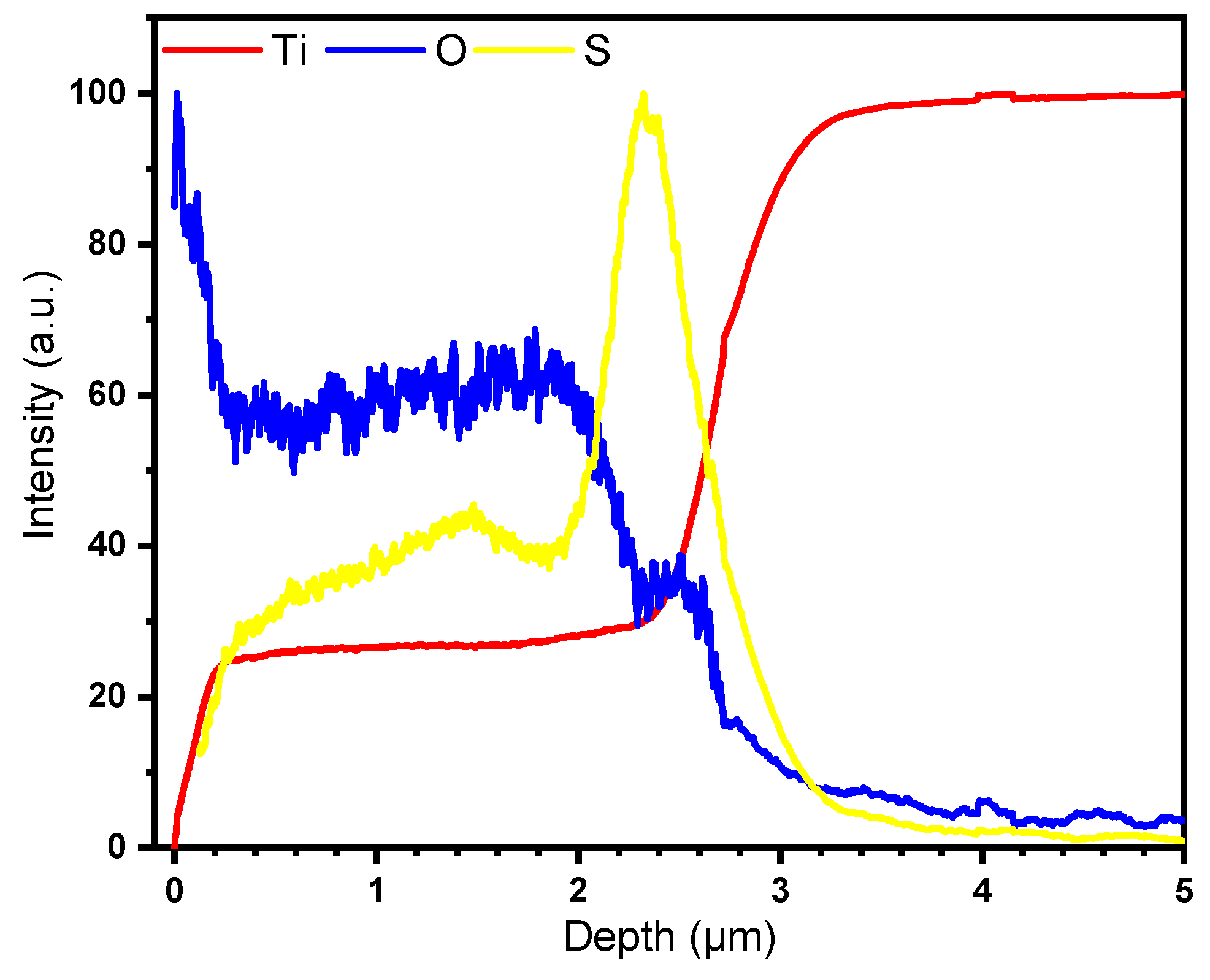
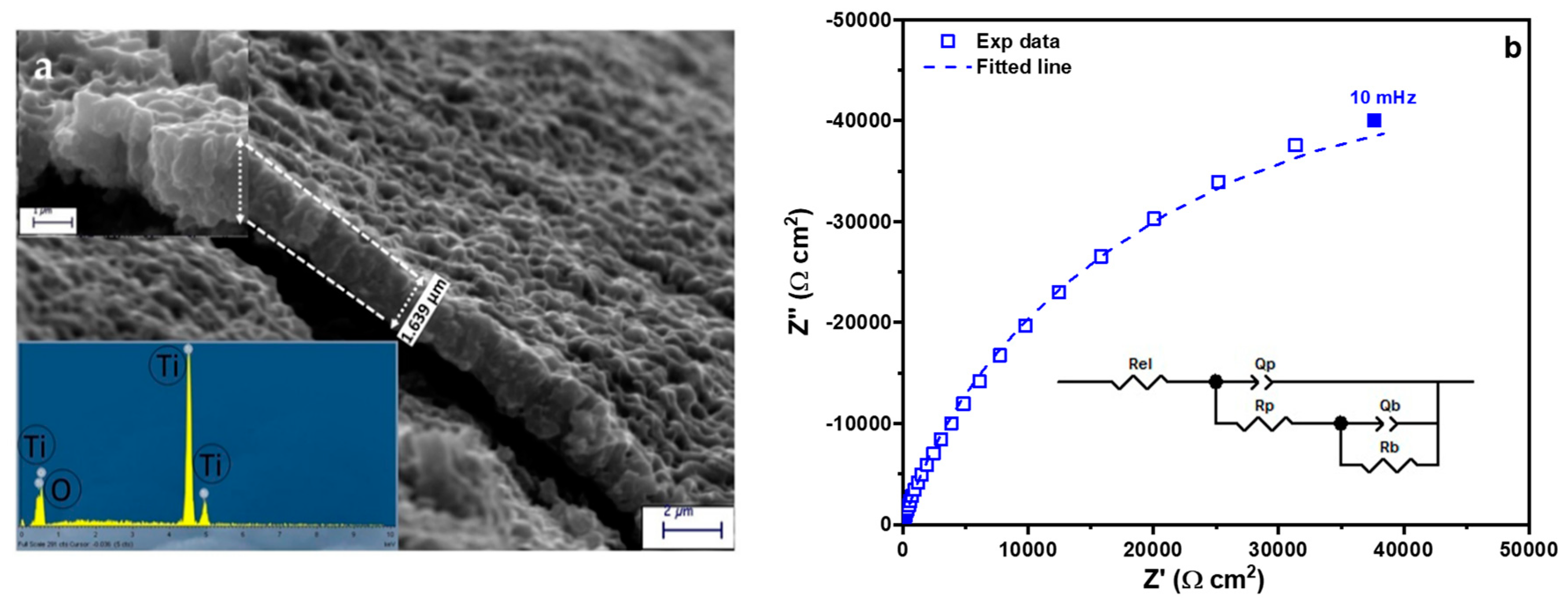
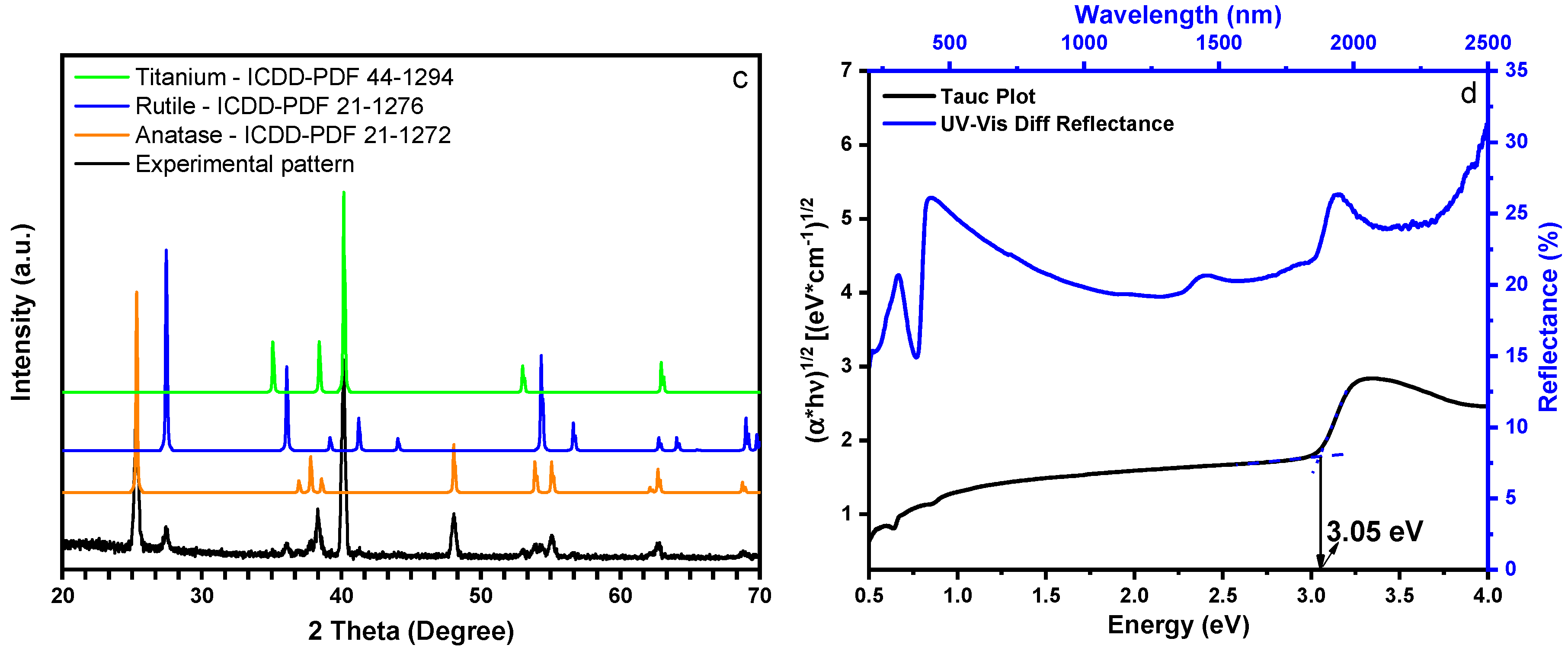
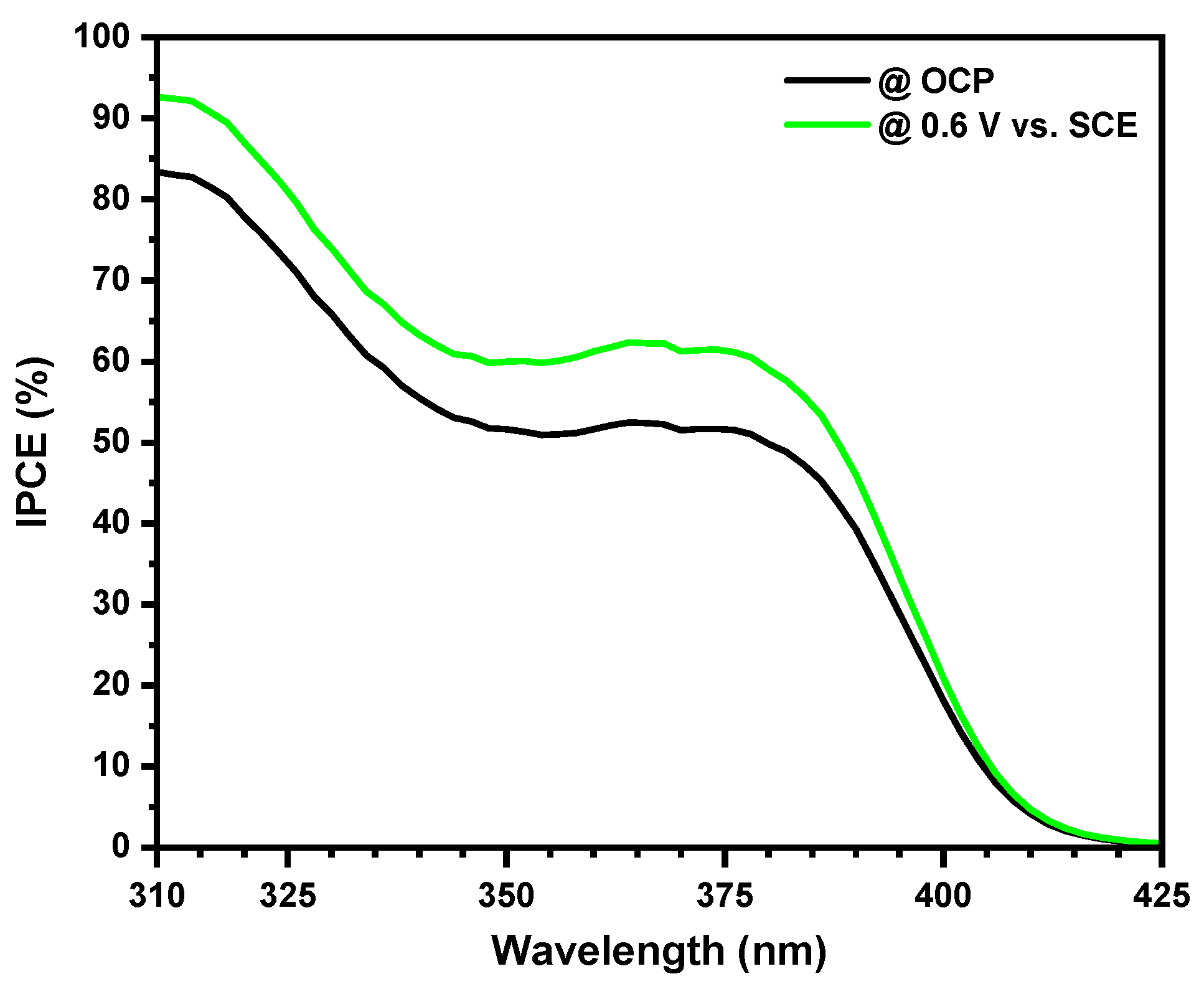
2.1. Characterization of the Nanostructured Catalyst
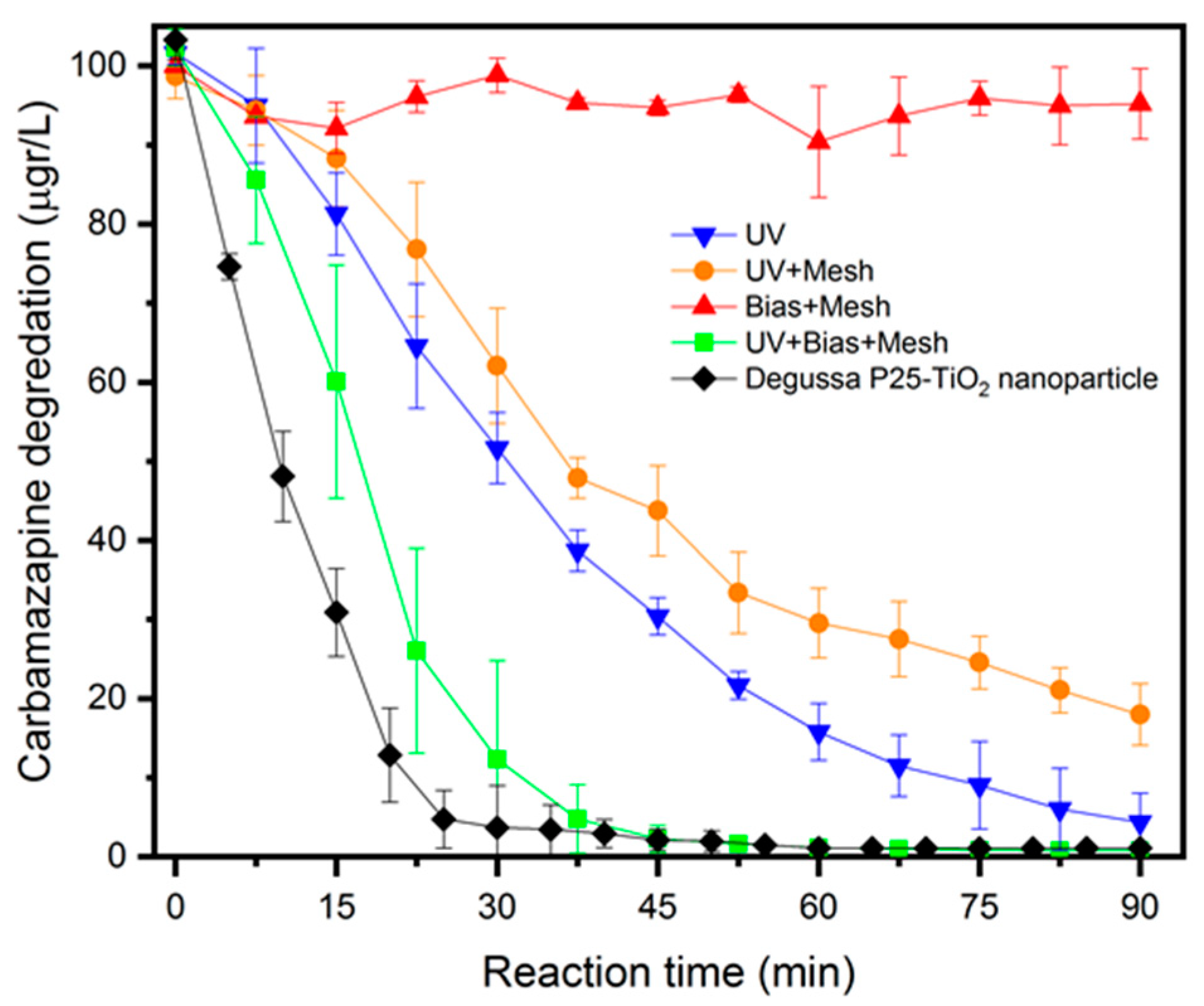
2.2. Degradation of Carbamazepine
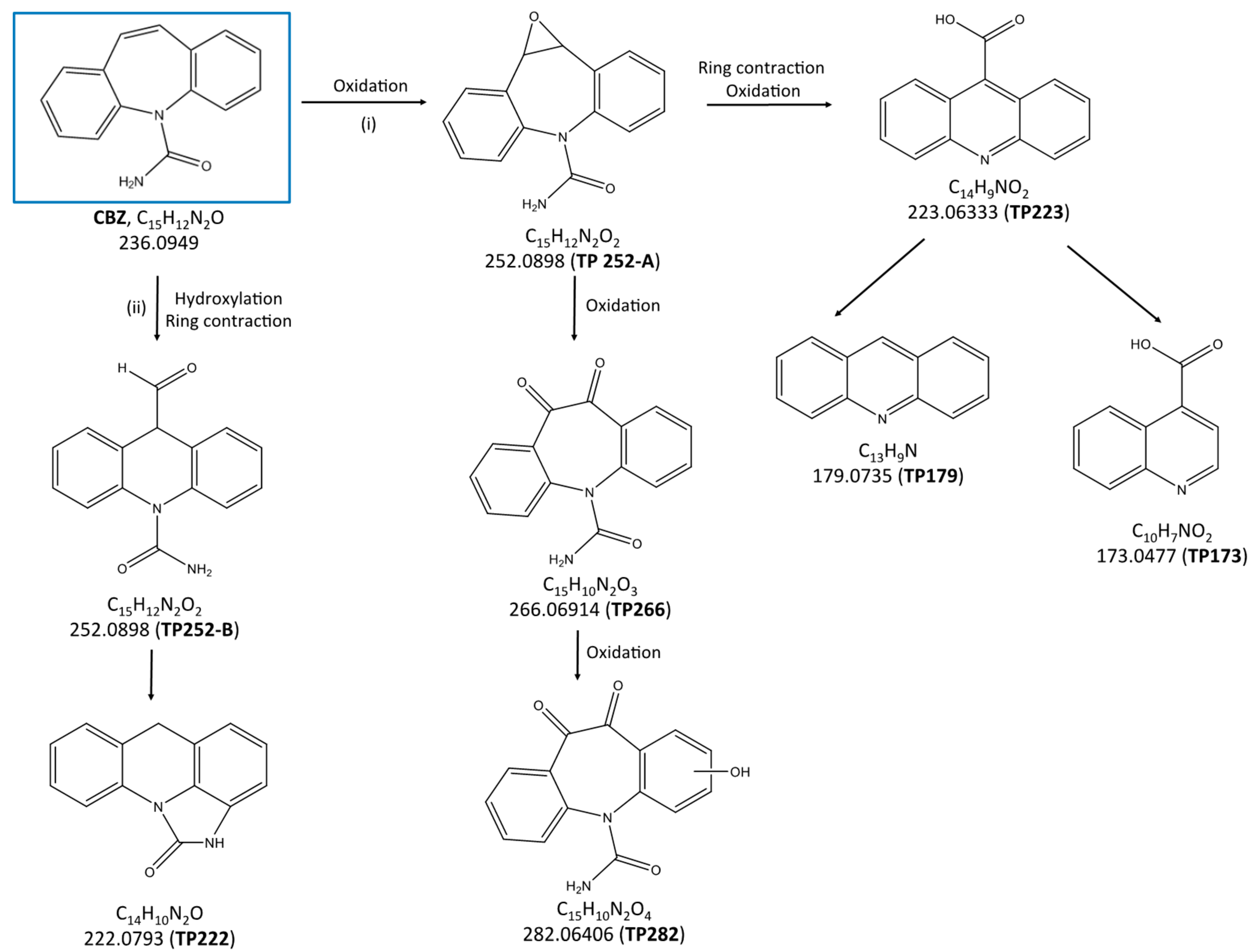
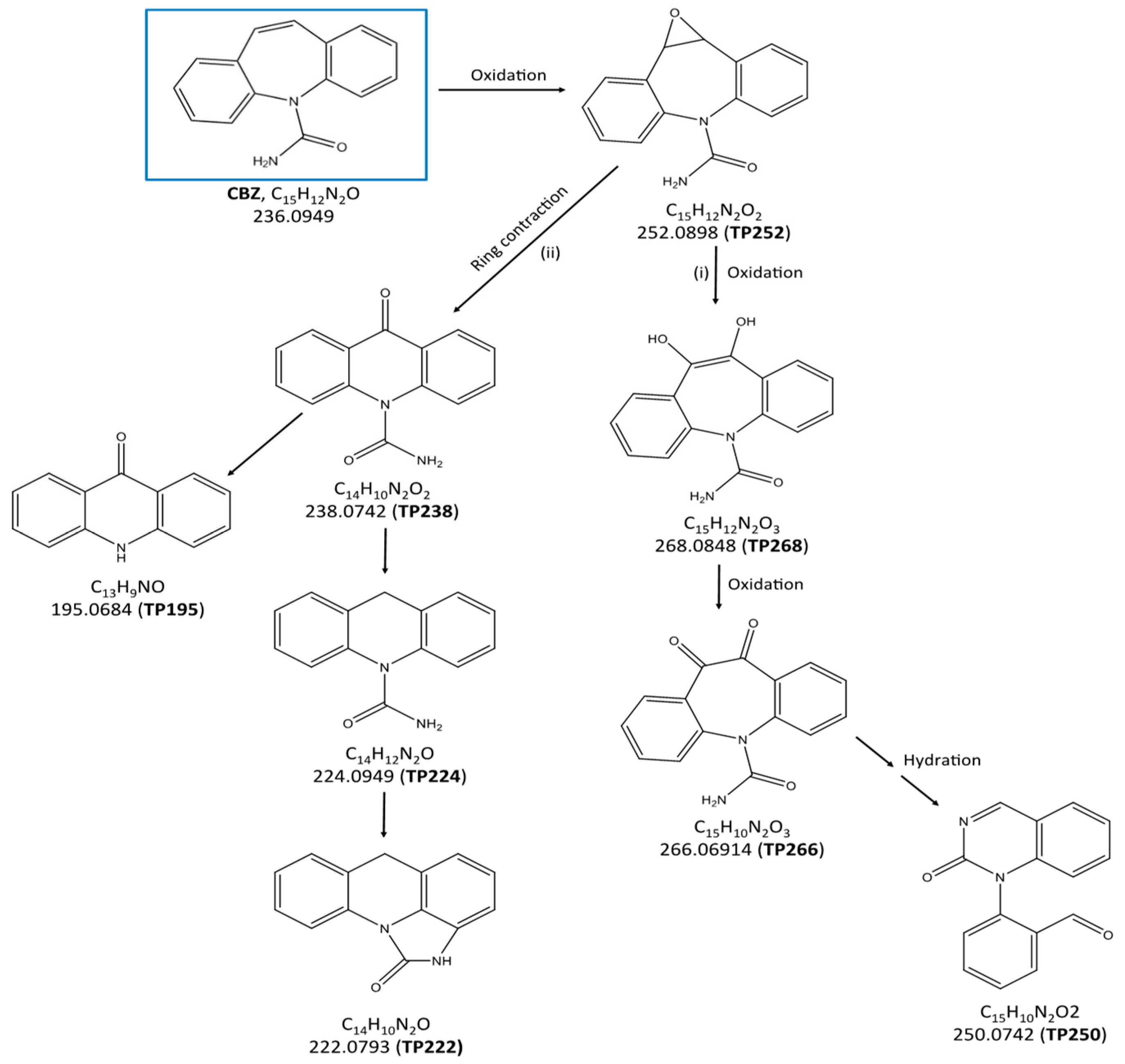
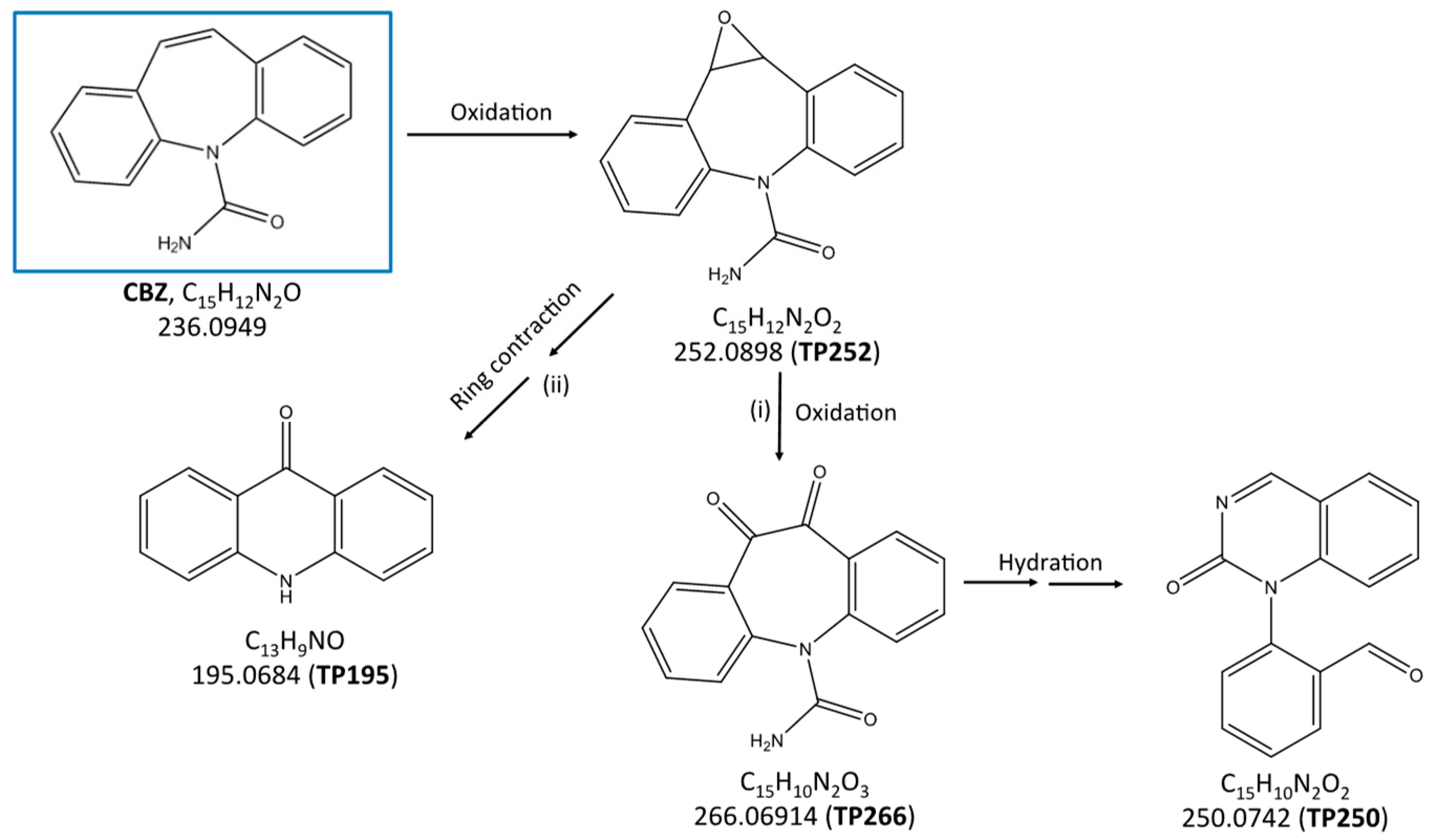
2.3. Identification of the Transformation Products and Degradation Pathways of Carbamazepine
3. Materials and Methods
3.1. Syntehsis and Characterization of the Nanostructured TiO2 Catalyst
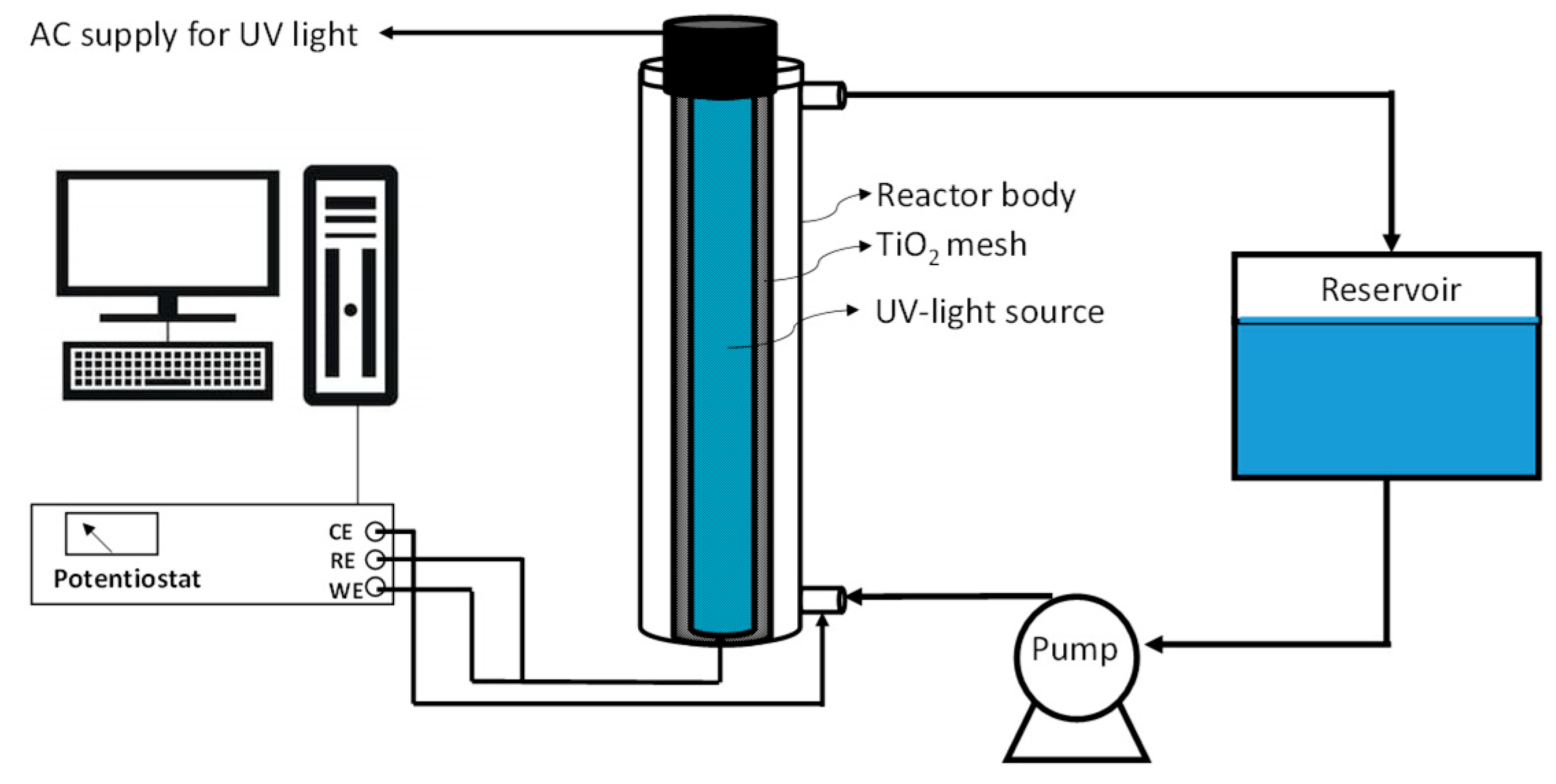
3.2. Bench-Scale Experiments
3.3. Analytical Set-Up and Data Processing
- the AB-Sciex software namely SciexOS, PeakView and MasterView were employed to screen samples for a list of known TPs (collected from data reported in the literature or from prediction models) based on the mass exact, isotopic cluster, fragmentation MS/MS spectrum and estimated chromatographic retention time (suspect target screening);
- for each acquired file, a list of precursor ions with a specific retention time and peak intensity was generated by an open source software (i.e., enviMass); the list of detected ions was successively reduced by replicate sample intersection, isotope grouping and adduct grouping. Moreover, the list of detected ions was reduced by removing ions which have also been detected in blank samples (non-target screening);
- the reduced peak list was processed by SciexOS software using both the formula finder algorithm (which tries to predict the possible chemical formula based on the MS and MS/MS spectrum using the precursor ion’s mass accuracy, isotopic pattern and MS/MS fragmentation pattern) and the library searching capabilities (LibraryView). Structure identification was carried out based on high resolution MS/MS data [17]. For a more confident identification, the detected compound were linked to ChemSpider and Metlin [58].
- Finally, in order to obtain additional information about the occurrence of possible TPs the final peak list was processed using a linkage analysis script in R statistical environment [47].
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A


References
- Sopilniak, A.; Elkayam, R.; Rossin, A.V.; Lev, O. Emerging organic pollutants in the vadose zone of a soil aquifer treatment system: Pore water extraction using positive displacement. Chemosphere 2018, 190, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Tototzintle, M.; Ferreira, I.J.; da Silva Duque, S.; Guimarães Barrocas, P.R.; Saggioro, E.M. Removal of contaminants of emerging concern (CECs) and antibiotic resistant bacteria in urban wastewater using UVA/TiO2/H2O2 photocatalysis. Chemosphere 2018, 210, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Suhogusoff, A.V.; Hirata, R.; Ferrari, L.C.K.M. Water quality and risk assessment of dug wells: A case study for a poor community in the city of São Paulo, Brazil. Environ. Earth Sci. 2013, 68, 899–910. [Google Scholar] [CrossRef]
- The European Commission. Commission Implementing Decision (EU) 2015/495 of 20 March 2015 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council. Off. J. Eur. Union 2015, 78, 40–42. [Google Scholar]
- The European Commission. Commission Implementing Decision (EU) 2018/840 of 5 June 2018 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council and repealing Commission Implementing Decision (EU) 2015/495. Off. J. Eur. Union 2018, 141, 9–12. [Google Scholar]
- Zwiener, C.; Seeger, S.; Glauner, T.; Frimmel, F. Metabolites from the biodegradation of pharmaceutical residues of ibuprofen in biofilm reactors and batch experiments. Anal. Bioanal. Chem. 2002, 372, 569–575. [Google Scholar] [CrossRef]
- Kümmerer, K.; Steger-Hartmann, T.; Meyer, M. Biodegradability of the anti-tumour agent ifosfamide and its occurrence in hospital effluents and communal sewage. Water Res. 1997, 31, 2705–2710. [Google Scholar] [CrossRef]
- Ternes, T.A. Occurrence of drugs in German sewage treatment plants and rivers. Water Res. 1998, 32, 3245–3260. [Google Scholar] [CrossRef]
- Balest, L.; Mascolo, G.; Di Iaconi, C.; Lopez, A. Removal of endocrine disrupter compounds from municipal wastewater by an innovative biological technology. Water Sci. Technol. 2008, 58, 953–956. [Google Scholar] [CrossRef]
- Baghdadi, M.; Ghaffari, E.; Aminzadeh, B. Removal of carbamazepine from municipal wastewater effluent using optimally synthesized magnetic activated carbon: Adsorption and sedimentation kinetic studies. J. Environ. Chem. Eng. 2016, 4, 3309–3321. [Google Scholar] [CrossRef]
- De Laurentiis, E.; Chiron, S.; Kouras-Hadef, S.; Richard, C.; Minella, M.; Maurino, V.; Minero, C.; Vione, D. Photochemical fate of carbamazepine in surface freshwaters: Laboratory measures and modeling. Environ. Sci. Technol. 2012, 46, 8164–8173. [Google Scholar] [CrossRef] [PubMed]
- Kosjek, T.; Andersen, H.R.; Kompare, B.; Ledin, A.; Heath, E. Fate of carbamazepine during water treatment. Environ. Sci. Technol. 2009, 43, 6256–6261. [Google Scholar] [CrossRef] [PubMed]
- Haroune, L.; Salaun, M.; Ménard, A.; Legault, C.Y.; Bellenger, J.-P. Photocatalytic degradation of carbamazepine and three derivatives using TiO2 and ZnO: Effect of pH, ionic strength, and natural organic matter. Sci. Total Environ. 2014, 475, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Mascolo, G.; Tiravanti, G.; Passino, R. Degradation of herbicides (ametryn and isoproturon) during water disinfection by means of two oxidants (hypochlorine and chlorine dioxide). Water Sci. Technol. 1997, 35, 129–136. [Google Scholar] [CrossRef]
- Raja, P.; Bozzi, A.; Jardim, W.F.; Mascolo, G.; Renganathan, R.; Kiwi, J. Reductive/oxidative treatment with superior performance relative to oxidative treatment during the degradation of 4-chlorophenol. Appl. Catal. B Environ. 2005, 59, 249–257. [Google Scholar] [CrossRef]
- Rimoldi, L.; Meroni, D.; Falletta, E.; Pifferi, V.; Falciola, L.; Cappelletti, G.; Ardizzone, S. Emerging pollutant mixture mineralization by TiO2 photocatalysts. The role of the water medium. Photochem. Photobiol. Sci. 2017, 16, 60–66. [Google Scholar] [CrossRef]
- Detomaso, A.; Mascolo, G.; Lopez, A. Characterization of carbofuran photodegradation by-products by liquid chromatography/hybrid quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 2193–2202. [Google Scholar] [CrossRef]
- Malato, S. Removal of emerging contaminants in waste-water treatment: Removal by photo-catalytic processes. In Emerging Contaminants from Industrial and Municipal Waste; Springer: Berlin/Heidelberg, Germany, 2008; pp. 177–197. [Google Scholar]
- Rimoldi, L.; Ambrosi, C.; Di Liberto, G.; Lo Presti, L.; Ceotto, M.; Oliva, C.; Meroni, D.; Cappelli, S.; Cappelletti, G.; Soliveri, G.; et al. Impregnation versus bulk synthesis: How the synthetic route affects the photocatalytic efficiency of Nb/Ta:N codoped TiO2 nanomaterials. J. Phys. Chem. C 2015, 119, 24104–24115. [Google Scholar] [CrossRef]
- Antonello, A.; Soliveri, G.; Meroni, D.; Cappelletti, G.; Ardizzone, S. Photocatalytic remediation of indoor pollution by transparent TiO2 films. Catal. Today 2014, 230, 35–40. [Google Scholar] [CrossRef]
- Luster, E.; Avisar, D.; Horovitz, I.; Lozzi, L.; Baker, M.A.; Grilli, R.; Mamane, H. N-doped TiO2-coated ceramic membrane for carbamazepine degradation in different water qualities. Nanomaterials 2017, 7, 206. [Google Scholar] [CrossRef]
- Ghosh, M.; Lohrasbi, M.; Chuang, S.S.C.; Jana, S.C. Mesoporous titanium dioxide nanofibers with a significantly enhanced photocatalytic activity. ChemCatChem 2016, 8, 2525–2535. [Google Scholar] [CrossRef]
- Noorjahan, M.; Pratap Reddy, M.; Durga Kumari, V.; Lavédrine, B.; Boule, P.; Subrahmanyam, M. Photocatalytic degradation of H-acid over a novel TiO2 thin film fixed bed reactor and in aqueous suspensions. J. Photochem. Photobiol. A Chem. 2003, 156, 179–187. [Google Scholar] [CrossRef]
- Zertal, A.; Molnár-Gábor, D.; Malouki, M.A.; Sehili, T.; Boule, P. Photocatalytic transformation of 4-chloro-2-methylphenoxyacetic acid (MCPA) on several kinds of TiO2. Appl. Catal. B Environ. 2004, 49, 83–89. [Google Scholar] [CrossRef]
- Borges, M.; García, D.; Hernández, T.; Ruiz-Morales, J.; Esparza, P. Supported photocatalyst for removal of emerging contaminants from wastewater in a continuous packed-bed photoreactor configuration. Catalysts 2015, 5, 77–87. [Google Scholar] [CrossRef]
- Murgolo, S.; Yargeau, V.; Gerbasi, R.; Visentin, F.; El Habra, N.; Ricco, G.; Lacchetti, I.; Carere, M.; Curri, M.L.; Mascolo, G. A new supported TiO2 film deposited on stainless steel for the photocatalytic degradation of contaminants of emerging concern. Chem. Eng. J. 2017, 318, 103–111. [Google Scholar] [CrossRef]
- Petronella, F.; Fanizza, E.; Mascolo, G.; Locaputo, V.; Bertinetti, L.; Martra, G.; Coluccia, S.; Agostiano, A.; Curri, M.L.; Comparelli, R. Photocatalytic activity of nanocomposite catalyst films based on nanocrystalline metal/semiconductors. J. Phys. Chem. C 2011, 115, 12033–12040. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Dimboukou-Mpira, A.; El Khakani, M.A. Photoelectrocatalytic degradation of carbamazepine using Ti/TiO2 nanostructured electrodes deposited by means of a pulsed laser deposition process. Chemosphere 2013, 93, 2756–2766. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Robinson, B.W.; Tighe, C.J.; Gruar, R.I.; Mills, A.; Parkin, I.P.; Tabecki, A.K.; De Villiers Lovelock, H.L.; Darr, J.A. Suspension plasma sprayed coatings using dilute hydrothermally produced titania feedstocks for photocatalytic applications. J. Mater. Chem. A 2015, 3, 12680–12689. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Dozzi, M.V.; Selli, E. TiO2-based materials for photocatalytic hydrogen production. J. Energy Chem. 2017, 26, 250–258. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Tealdi, C.; Mustarelli, P.; Selli, E. Fabrication of Pt/Ti/TiO2 photoelectrodes by RF-magnetron sputtering for separate hydrogen and oxygen production. Materials 2016, 9, 279. [Google Scholar] [CrossRef] [PubMed]
- Bestetti, M.; Franz, S.; Cuzzolin, M.; Arosio, P.; Cavallotti, P.L. Structure of nanotubular titanium oxide templates prepared by electrochemical anodization in H2SO4/HF solutions. Thin Solid Films 2007, 505, 5253–5258. [Google Scholar] [CrossRef]
- Eskandarloo, H.; Hashempour, M.; Vicenzo, A.; Franz, S.; Badiei, A.; Behnajady, M.A.; Bestetti, M. High-temperature stable anatase-type TiO2 nanotube arrays: A study of the structure-activity relationship. Appl. Catal. B Environ. 2016, 185, 119–132. [Google Scholar] [CrossRef]
- Zlamal, M.; Macak, J.M.; Schmuki, P.; Krýsa, J. Electrochemically assisted photocatalysis on self-organized TiO2 nanotubes. Electrochem. Commun. 2007, 9, 2822–2826. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S.J. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Teh, T.H.; Berkani, A.; Mato, S.; Skeldon, P.; Thompson, G.E.; Habazaki, H.; Shimizu, K. Initial stages of plasma electrolytic oxidation of titanium. Corros. Sci. 2003, 45, 2757–2768. [Google Scholar] [CrossRef]
- Franz, S.; Perego, D.; Marchese, O.; Lucotti, A.; Bestetti, M. Photoactive TiO2 coatings obtained by plasma electrolytic oxidation in refrigerated electrolytes. Appl. Surf. Sci. 2016, 385, 498–505. [Google Scholar] [CrossRef]
- Franz, S.; Perego, D.; Marchese, O.; Bestetti, M. Photoelectrochemical advanced oxidation processes on nanostructured TiO2 catalysts: Decolorization of a textile azo-dye. J. Water Chem. Technol. 2015, 37, 108–115. [Google Scholar] [CrossRef]
- Murgolo, S.; Franz, S.; Arab, H.; Bestetti, M.; Falletta, E.; Mascolo, G. Degradation of emerging organic pollutants in wastewater effluents by electrochemical photocatalysis on nanostructured TiO2 meshes. Water Res. 2019, 164, 114920. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Zuliani, A.; Ceresoli, D.; Martinazzo, R.; Selli, E. Exploiting the photonic crystal properties of TiO2 nanotube arrays to enhance photocatalytic hydrogen production. ACS Catal. 2016, 6, 1345–1353. [Google Scholar] [CrossRef]
- Pascual, J.; Camassel, J.; Mathieu, H. Fine structure in the intrinsic absorption edge of TiO2. Phys. Rev. B 1978, 18, 5606–5614. [Google Scholar] [CrossRef]
- Amtout, A.; Leonelli, R. Optical properties of rutile near its fundamental band gap. Phys. Rev. B Condens. Matter. 1995, 15, 6842–6851. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Lévy, F.; Berger, H.; Schmid, P.E. Urbach tail of anatase TiO2. Phys. Rev. B Condens. Matter. 1995, 15, 7771–7774. [Google Scholar] [CrossRef] [PubMed]
- Schollée, J.E.; Bourgin, M.; von Gunten, U.; McArdell, C.S.; Hollender, J. Non-target screening to trace ozonation transformation products in a wastewater treatment train including different post-treatments. Water Res. 2018, 142, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Liu, N.; Lei, Z.D.; Wang, T.; Wang, J.J.; Zhang, X.D.; Xu, G.; Tang, L. Radiolysis of carbamazepine aqueous solution using electron beam irradiation combining with hydrogen peroxide: Efficiency and mechanism. Chem. Eng. J. 2016, 295, 484–493. [Google Scholar] [CrossRef]
- Ghasemian, S.; Nasuhoglu, D.; Omanovic, S.; Yargeau, V. Photoelectrocatalytic degradation of pharmaceutical carbamazepine using Sb-doped Sn80%-W20%-oxide electrodes. Sep. Purif. Technol. 2017, 188, 52–59. [Google Scholar] [CrossRef]
- Martínez, C.; Canle, M.L.; Fernández, M.I.; Santaballa, J.A.; Faria, J. Kinetics and mechanism of aqueous degradation of carbamazepine by heterogeneous photocatalysis using nanocrystalline TiO2, ZnO and multi-walled carbon nanotubes-anatase composites. Appl. Catal. B Environ. 2011, 102, 563–571. [Google Scholar] [CrossRef]
- Bessa, V.S.; Moreira, I.S.; Murgolo, S.; Mascolo, G.; Castro, P.M.L. Carbamazepine is degraded by the bacterial strain Labrys portucalensis F11. Sci. Total Environ. 2019, 690, 739–747. [Google Scholar] [CrossRef]
- Calza, P.; Medana, C.; Padovano, E.; Giancotti, V.; Baiocchi, C. Identification of the unknown transformation products derived from clarithromycin and carbamazepine using liquid chromatography/high-resolution mass spectrometry. Rapid Commun. Mass Spectrom. 2012, 26, 1687–1704. [Google Scholar] [CrossRef]
- Rayaroth, M.P.; Prasanthkumar, K.P.; Kang, Y.-G.; Lee, C.-S.; Chang, Y.-S. Degradation of carbamazepine by singlet oxygen from sulfidized nanoscale zero-valent iron–citric acid system. Chem. Eng. J. 2020, 382, 122828. [Google Scholar] [CrossRef]
- Mascolo, G.; Lopez, A.; Detomaso, A.; Lovecchio, G. Ion chromatography–electrospray mass spectrometry for the identification of low-molecular-weight organic acids during the 2,4-dichlorophenol degradation. J. Chromatogr. A 2005, 1067, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Spurr, R.A.; Myers, H. Quantitative analysis of anatase-rutile mixtures with an X-ray diffractometer. Anal. Chem. 1957, 29, 760–762. [Google Scholar] [CrossRef]
- Trasatti, S.; Petrii, O.A. Real surface area measurements in electrochemistry. J. Electroanal. Chem. 1992, 327, 353–376. [Google Scholar] [CrossRef]
- Khazri, H.; Hassine, S.B.; Ghorbel-Abid, I.; Kalfat, R.; Trabelsi-Ayadi, M. Presence of carbamazepine, naproxen, and ibuprofen in wastewater from northern Tunisia. J. Environ. Forensics 2019, 20, 121–128. [Google Scholar] [CrossRef]
- Zhang, Y.; Geißen, S.-U.; Gal, C. Carbamazepine and diclofenac: Removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 2008, 73, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Guijas, C.; Montenegro-Burke, J.R.; Domingo-Almenara, X.; Palermo, A.; Warth, B.; Hermann, G.; Koellensperger, G.; Huan, T.; Uritboonthai, W.; Aisporna, A.E.; et al. METLIN: A technology platform for identifying knowns and unknowns. Anal. Chem. 2018, 90, 3156–3164. [Google Scholar] [CrossRef]

| Reactor Configuration | k (min−1) | h.l.t (min) | EEO (KWh/m3) |
|---|---|---|---|
| UV + Bias + Mesh | 0.076 ± 0.002 | 17 | 7.58 |
| UV + Mesh | 0.018 ± 0.007 | 36 | 31.98 |
| Mesh + Bias | 0.0008 ± 0.0002 | ∞ | 719.55 |
| UV | 0.028 ± 0.001 | 31 | 20.55 |
| UV + Degussa P25 | 0.174 ± 0.018 × 10−15 | 4.5 | 3.30 |
| Product Code | m/z [M + H]+ | Elemental Composition | Structure | MS Error (ppm) | Trend | Refs |
|---|---|---|---|---|---|---|
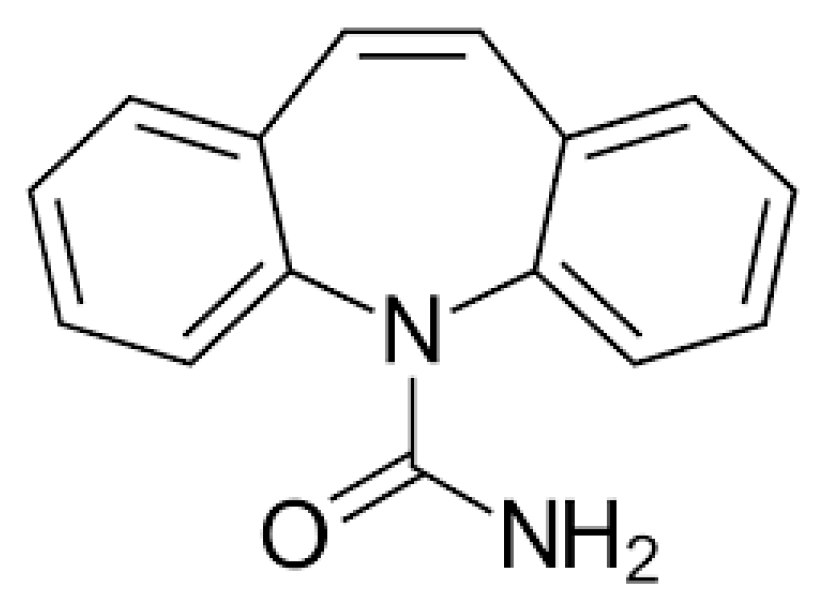
| CBZ | 237.1016 | C15H12N2O | 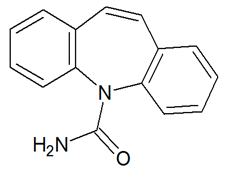 | 5.0 | descending | |
| TP129 | 130.9648 | - | - | 6.5 | bell-shape | - |
| TP223 | 224.0695 | C14H9NO2 | 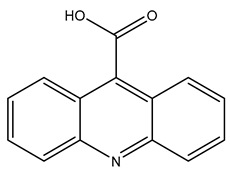 | 7.4 | bell-shape | [40,47] |
| TP173 | 174.0538 | C10H7NO2 | 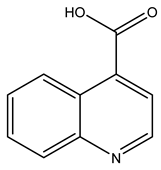 | 12.0 | bell-shape | - |
| TP119 | 120.0547 | C6H5N3 | 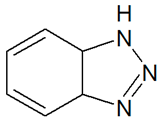 | 12.3 | increasing | - |
| TP179 | 180.0794 | C13H9N |  | 10.7 | bell-shape | [40,47,48,49] |
| TP266 | 267.0758 | C15H10N2O3 | 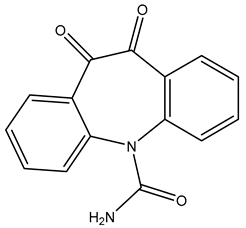 | 4.4 | bell-shape | [40,48,50] |
| TP252-A | 253.0966 | C15H12N2O2 | 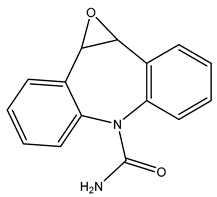 | 4.4 | bell-shape | [40,47,48,49,50] |
| TP252-B | 253.0964 | C15H12N2O2 | 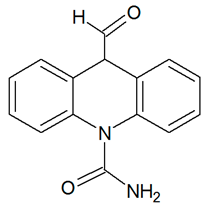 | 5.1 | bell-shape | [51] |
| TP282 | 283.0708 | C15H10N2O4 |  | 3.8 | bell-shape | [50] |
| TP222 | 223.086 | C14H10N2O |  | 5.1 | bell-shape | [51] |
| Product Code | m/z [M + H]+ | Elemental Composition | Structure | MS Error (ppm) | Trend | Refs |
|---|---|---|---|---|---|---|
| CBZ | 237.1016 | C15H12N2O | 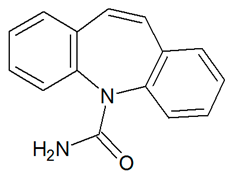 | 5.0 | descending | |
| TP252 | 253.0966 | C15H12N2O2 | 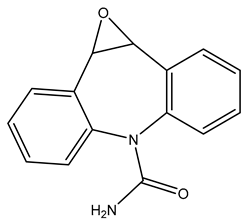 | 4.4 | bell-shape | [40,47,48,49,50] |
| TP229 | 230.0807 | C13H11NO3 | 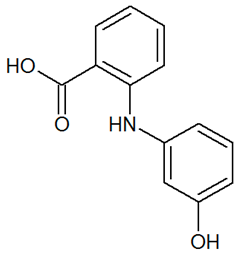 | 4.4 | increasing | - |
| TP173 | 174.0534 | C10H7NO2 | 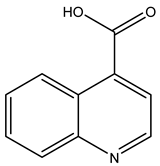 | 12.0 | bell-shape | - |
| TP238 | 239.0805 | C14H10N2O2 | 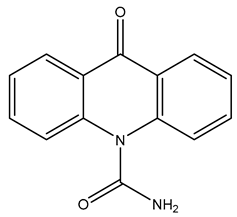 | 6.5 | bell-shape | - |
| TP266 | 267.0766 | C15H10N2O3 | 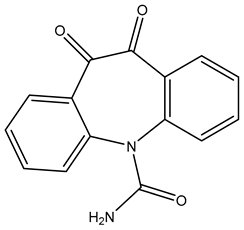 | 1.4 | bell-shape | [40,48,50] |
| TP250 | 251.0804 | C15H10N2O2 | 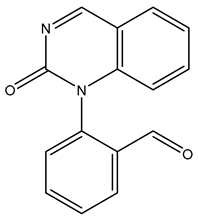 | 6.6 | bell-shape | [40,48] |
| TP268 | 269.0913 | C15H12N2O3 | 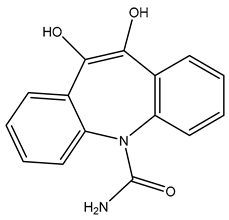 | 4.9 | bell-shape | [48] |
| TP195 | 196.0744 | C13H9NO | 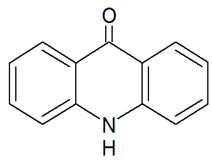 | 9.4 | bell-shape | [47,48,50] |
| TP222 | 223.0857 | C14H10N2O |  | 5.1 | bell-shape | [51] |
| TP224 | 225.1022 | C14H12N2O | 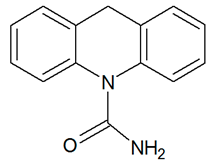 | 2.6 | bell-shape | - |
| Product Code | m/z [M + H]+ | Elemental Composition | Structure | MS Error (ppm) | Trend | Refs |
|---|---|---|---|---|---|---|
| CBZ | 237.1017 | C15H12N2O |  | 4.6 | descending | - |
| TP217 | 218.0816 | C12H11NO3 | 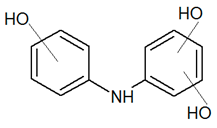 | 0.5 | increasing | - |
| TP252 | 253.0965 | C15H12N2O2 | 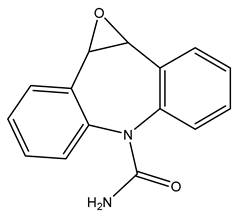 | 4.8 | bell-shape | [40,47,48,49,50] |
| TP266 | 267.0766 | C15H10N2O3 | 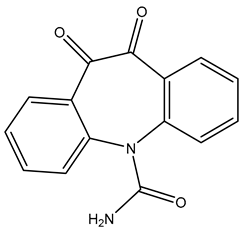 | 1.4 | bell-shape | [40,48,50] |
| TP195 | 196.075 | C13H9NO | 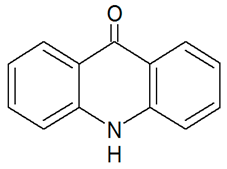 | 6.3 | bell-shape | [47,48,50] |
| TP250 | 251.0804 | C15H10N2O2 | 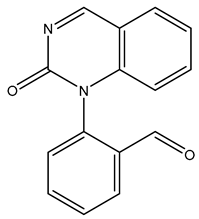 | 6.6 | bell-shape | [40,48] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franz, S.; Falletta, E.; Arab, H.; Murgolo, S.; Bestetti, M.; Mascolo, G. Degradation of Carbamazepine by Photo(electro)catalysis on Nanostructured TiO2 Meshes: Transformation Products and Reaction Pathways. Catalysts 2020, 10, 169. https://doi.org/10.3390/catal10020169
Franz S, Falletta E, Arab H, Murgolo S, Bestetti M, Mascolo G. Degradation of Carbamazepine by Photo(electro)catalysis on Nanostructured TiO2 Meshes: Transformation Products and Reaction Pathways. Catalysts. 2020; 10(2):169. https://doi.org/10.3390/catal10020169
Chicago/Turabian StyleFranz, Silvia, Ermelinda Falletta, Hamed Arab, Sapia Murgolo, Massimiliano Bestetti, and Giuseppe Mascolo. 2020. "Degradation of Carbamazepine by Photo(electro)catalysis on Nanostructured TiO2 Meshes: Transformation Products and Reaction Pathways" Catalysts 10, no. 2: 169. https://doi.org/10.3390/catal10020169
APA StyleFranz, S., Falletta, E., Arab, H., Murgolo, S., Bestetti, M., & Mascolo, G. (2020). Degradation of Carbamazepine by Photo(electro)catalysis on Nanostructured TiO2 Meshes: Transformation Products and Reaction Pathways. Catalysts, 10(2), 169. https://doi.org/10.3390/catal10020169






