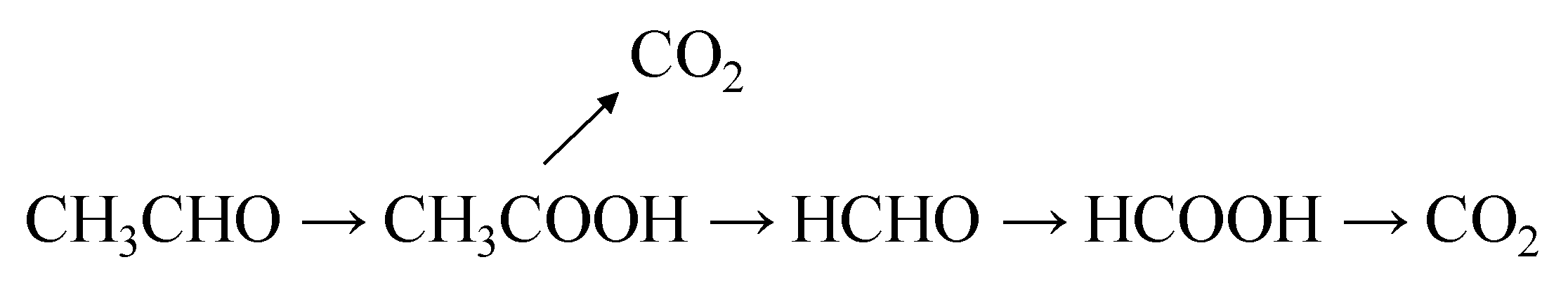1. Introduction
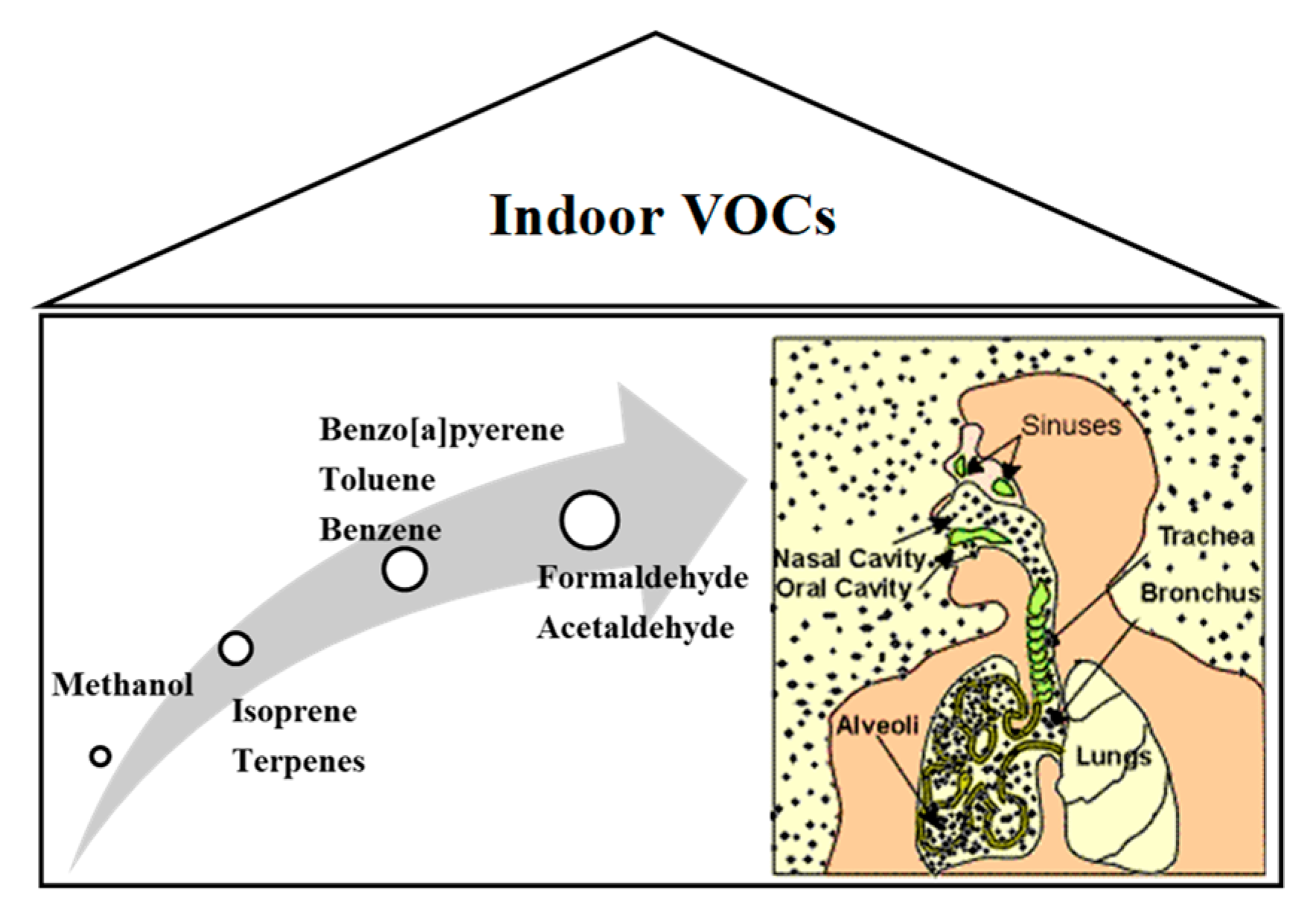
The assurance of good-quality conditions in the indoor air has started to be a subject of many discussions and a wide area of the research studies. Recently, people spend much time inside rooms, and they are exposed to some of the pollutants (mostly VOCs—Volatile Organic Compounds), which are generated by the biological activity of human/animal beings but are also released from the building materials. These VOCs are suspected to induce so-called Sick Building Syndrome, which causes numerous health problems, such as headache and eye, nose, and throat irritation [
1]. The effect of the polluted indoor air on human health is schematically illustrated in
Figure 1.
According to the classification provided by companies to ECHA (European Chemicals Agency) in REACH (Registration, Evaluation and Authorization of Chemicals) registrations, acetaldehyde was identified as a substance which is suspected of causing cancer. Formaldehyde was also classified as a cancerogenic substance and its concentration in wooden products is regulated under the TSCA (Toxic Substances Control Act) program in the United States. According to the European Standard, for woodgrain boards in E1 class used for indoor application, the maximum concentration of formaldehyde should not be higher than 0.1 ppm (0.125 mg/m
3). The concentration of VOCs in the indoor air is not high, however it can be dangerous for human health. Therefore, some of air cleaning processes can be utilized to solve this problem [
2,
3]. Recently, one of the most explored methods of VOCs degradation is photocatalysis, with application of TiO
2 as the photocatalyst [
4,
5]. TiO
2 is commonly used as an additive to the different building materials or can just simply be used as a coating for many building constructions [
6,
7]. A lot of patents on TiO
2 preparation and utilization have been developed and the interest of the business companies in this matter has greatly increased [
8]. In 2010, the technical committee in Europe elaborated some ISO (International Organization for Standarization) standards for testing methods of photocatalytic activity of semiconductor photocatalysts [
9]. In total, eight tests under ISO standards were proposed, for performance in water, air and for self-cleaning surfaces. Acetaldehyde, together with toluene and NOx-group gases, was proposed as a model compound for evaluation of the photocatalytic activity of tested materials in air. Therefore, acetaldehyde is commonly used in the research studies for determination of the photocatalytic activity of different semiconductor materials.
Application of TiO
2 for decomposition of low-concentration VOCs was firstly proposed by the group of Fujishima [
10]. They reported that even an ordinary room light may be sufficient to help to purify the air or to keep the walls clean in the indoor environment, because the amounts of pollutants are typically small. They assumed that the intensity of UV light of 1 µW/cm
2 was sufficient to decompose a hydrocarbon layer of approximately 1 µm thick every hour [
10]. Moreover, they demonstrated that the maximum quantum yield (QY) for decomposition of 2-propanol on TiO
2—28%, was achieved for the lowest light intensity and for the highest concentration of 2-propanol (1000 ppmv) [
10]. The explanation of these results was that adsorbed organic molecules helped to inhibit recombination. It was also found that the measured QY values, that could be attributed to a reaction involving hydroxyl radicals, were several orders of magnitude smaller than those that could be attributed to reactions involving holes [
10]. However, the maximum QY depends on the intrinsic quality of TiO
2 and its ability of rapid bulk recombination. Therefore, suppressing of electron/hole pairs recombination in TiO
2 can significantly improve its photocatalytic activity. In the proposed mechanism of acetaldehyde decomposition, the radical-initiated chain reactions with oxygen consumption were illustrated [
10]. The schematic diagram of processes occurring during photocatalytic oxidation of acetaldehyde on an illuminated TiO
2 particle is illustrated in
Figure 2 [
10].
In general, the first oxidation product of acetaldehyde conversion is acetic acid, which can be mineralized to CO
2. The remaining methyl radical is transformed into formaldehyde, which can be further oxidized to formic acid and eventually to CO
2. The scheme of the acetaldehyde transformation on the titania surface upon UV illumination has already been reported elsewhere [
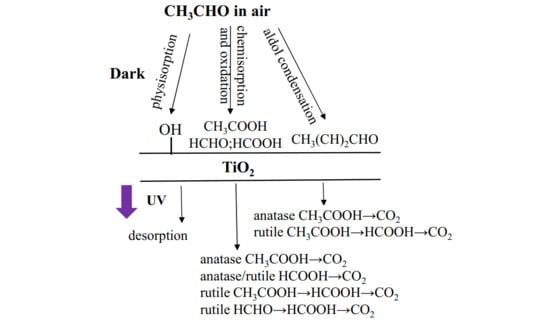
11]. Below, in
Figure 3, there is an outline of the path of acetaldehyde conversion by TiO
2 under weak UV illumination.
Acetaldehyde can be easily decomposed to CO
2 on TiO
2 film, but some harmful byproducts such as formaldehyde are formed upon acetaldehyde conversion and can be desorbed from TiO
2 surface without further mineralization [
12,
13]. Although concentration of formaldehyde was observed to be not very high in the outlet gas by comparison with acetaldehyde, it was increasing with both the initial concentration of acetaldehyde and humidity of the inlet gas [
12]. Higher humidity of air caused adsorption of water molecules on the titania surface in place of acetaldehyde, and then photogenerated holes were utilized in reaction with OH species rather than in oxidation of acetaldehyde. The photocatalytic conversion of acetaldehyde on TiO
2-based materials depends on their structural parameters, phase composition and a way of chemical bonding to the surface [
13,
14,
15,
16,
17]. Acetaldehyde is transformed through the rapid aldol condensation on the surface of anatase-type TiO
2 [
18,
19,
20], whereas on the oxidized rutile (110) surface, O-acetaldehyde complexes form, which can easily undergo irreversible transformation to a highly stable surface acetate [
14]. It can be a reason why the TiO
2 P25, produced by Evonik Company, which consists of both anatase (80%) and rutile (20%) phases, undergoes deactivation with time, that is contrary to anatase-type TiO
2 nanotubes [
16].
The scheme of aldol condensation of acetaldehyde on titania surface is illustrated in
Figure 4 [
19].
Some of the researchers proved that when acetaldehyde was brought in contact with TiO
2, it firstly adsorbed on the surface, and then an aldol condensation took place. This aldol condensation led to the formation of crotonaldehyde. At the same time, a minor fraction of acetate species was formed by oxidation processes [
19].
We have proven that chemically bonded acetaldehyde with anatase surface underwent faster through photocatalytic conversion, compared to that which was weakly bonded by H-bond with OH groups [
13]. Therefore, highly hydroxylated titania surface will be detrimental for the photocatalytic decomposition of acetaldehyde [
13,
21,
22]. It was proven that adsorption of acetaldehyde in the conditions of high humidity was going through the weak physical bounding with OH groups and it was completely reversible [
20]. Under UV irradiation, hydrophilicity of titania surface increases and water molecules compete with acetaldehyde to the adsorption sites of TiO
2 [
23]. Therefore, photocatalytic decomposition of acetaldehyde on TiO
2 surface in the conditions of high humidity is usually lower than in dry air [
11,
12]. In
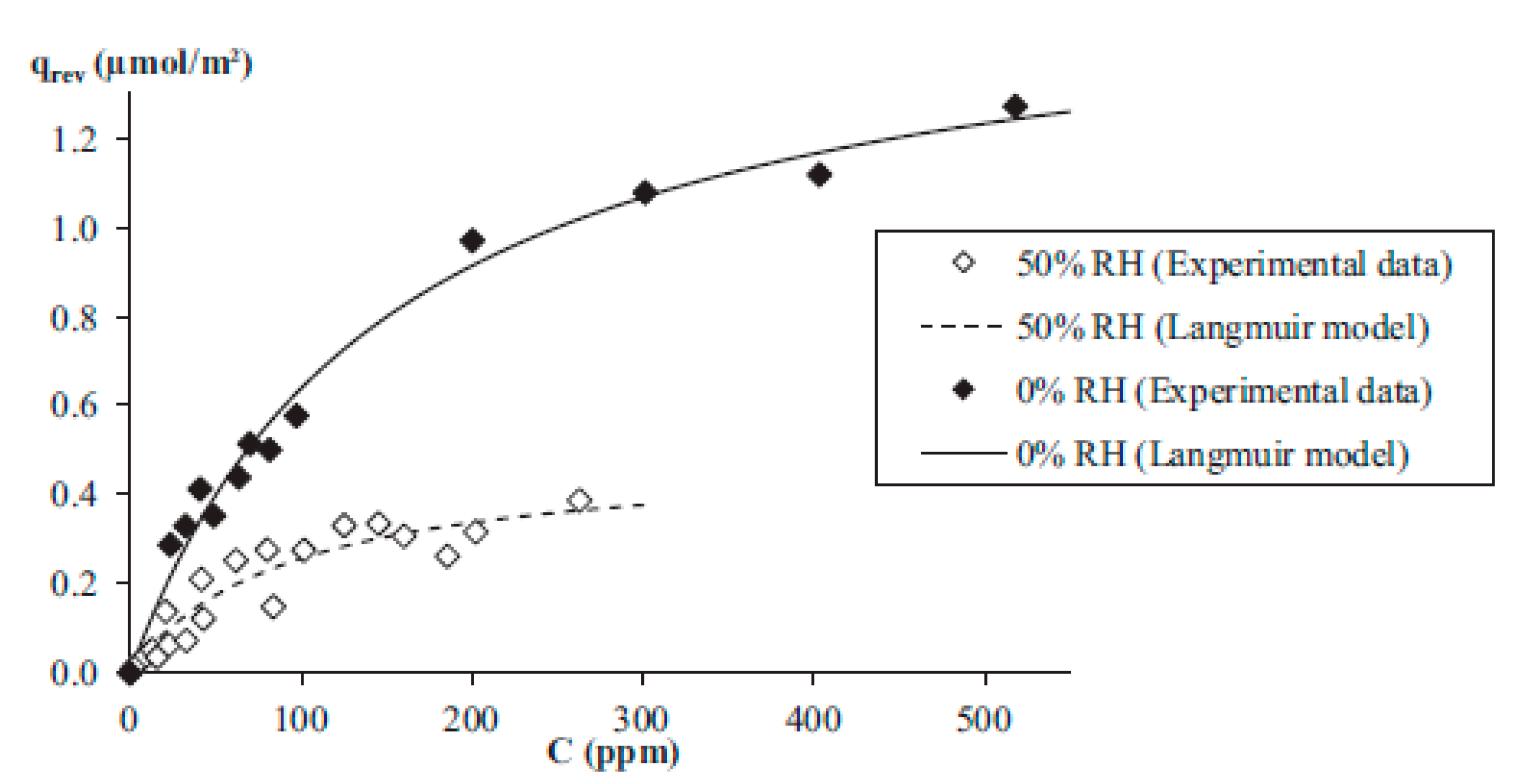
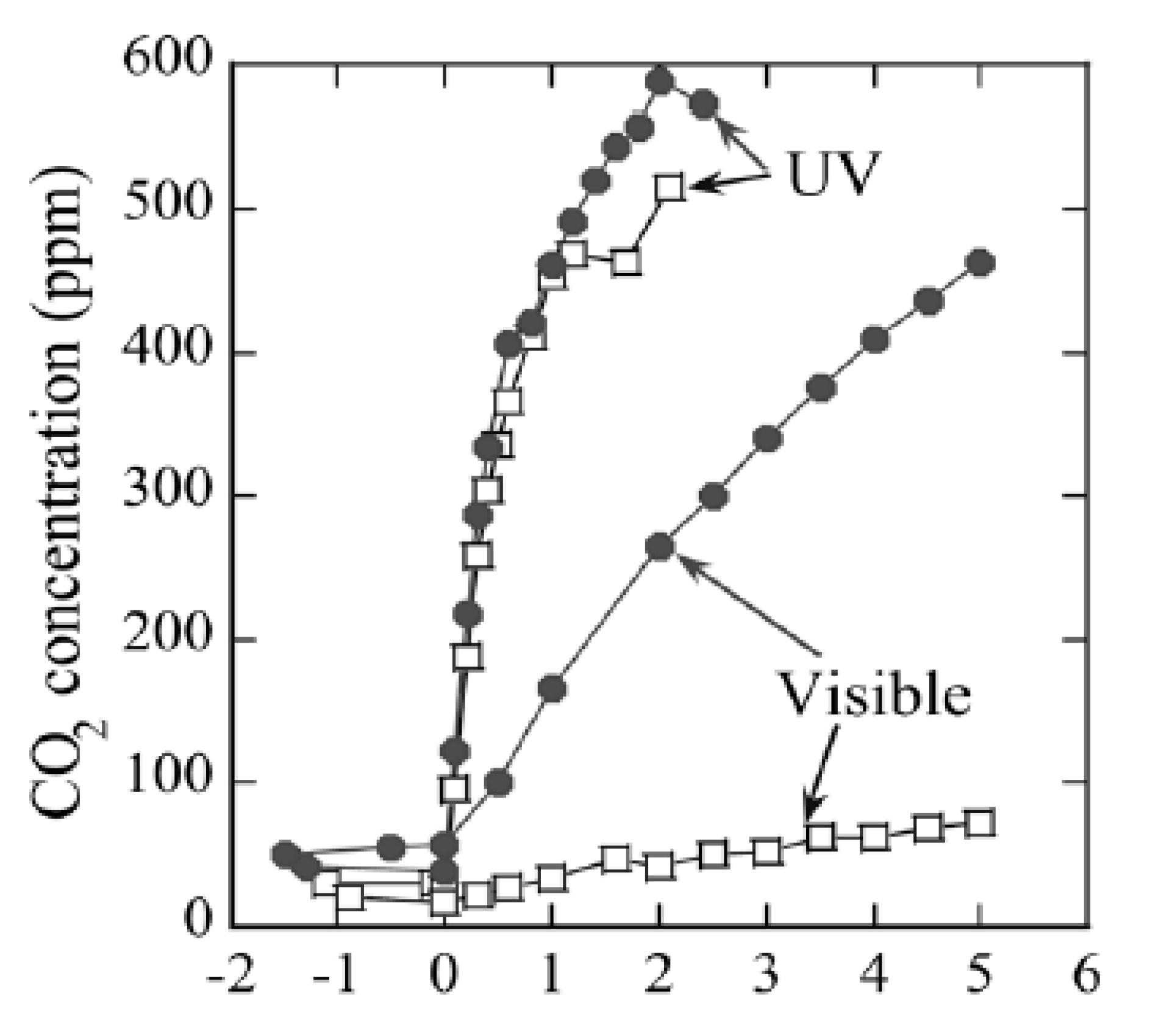
Figure 5, the reversible adsorption of acetaldehyde in the conditions of dry air and high humidity (50%) is shown [
20].
From
Figure 5, it can be observed that the reversible quantity (q
rev) of acetaldehyde in dry air was around 3 times higher than in the conditions of high relative humidity (RH = 50%).
In this paper, there is an overview of the numerous achievements related to the application of TiO2-based materials for acetaldehyde decomposition under different experimental conditions and various sources of light, used for the photocatalyst activation. Some of the new data were added, which complement this review in the context of the way of interaction between acetaldehyde molecules and different structures of TiO2 surface.
2. Preparation of TiO2 and Impact of Its Structural Properties on Acetaldehyde Decomposition
TiO
2 is industrially produced, mainly as a white pigment, for application as an additive in paints, varnishes, plastics, sunscreens, papers, toothpastes or other materials. For large production of TiO
2, both sulphate and chloride technologies are mainly used for the final product of rutile. However, for the photocatalytic processes, nanocrystalline anatase is required, therefore the other methods are applied. The commonly used photocatalytic TiO
2, Aeroxide P25, is produced by Evonik Company by a flame method. This TiO
2 reveals relatively high BET (Brunauer–Emmett–Teller) surface area and consists of mixed phases, anatase (80%) and rutile (20%). For production of nanosized TiO
2, several other methods were developed, including: hydrothermal [
24], sol-gel [
25], chemical vapor deposition (CVD) [
26], solvothermal [
27], flame spray pyrolysis [
28], electrochemical [
29], microemulsion [
30], micelle and inverse micelle methods [
31], sonochemical reactions [
32] and plasma evaporation [
33]. Among all the mentioned methods, the most used are sol-gel and hydrothermal routes. The advantage of these methods relies on their flexibility to control morphology, particle size and crystallinity of the products. In the photocatalytic degradation of acetaldehyde, the mixture of anatase and brookite appeared to be advantageous [
33,
34,
35]. Titania with mixed anatase and brookite was prepared by a sol-gel method from titanium isopropoxide solution and addition of alcohol with subsequent calcination at low temperatures, 200 or 450 °C [
34,
35]. It was proven that the presence of mixed phases anatase and brookite enhanced the density of electron traps due to the photoinduced interphase electron transfer and thereby improved photocatalytic performance of TiO
2 [
35]. In
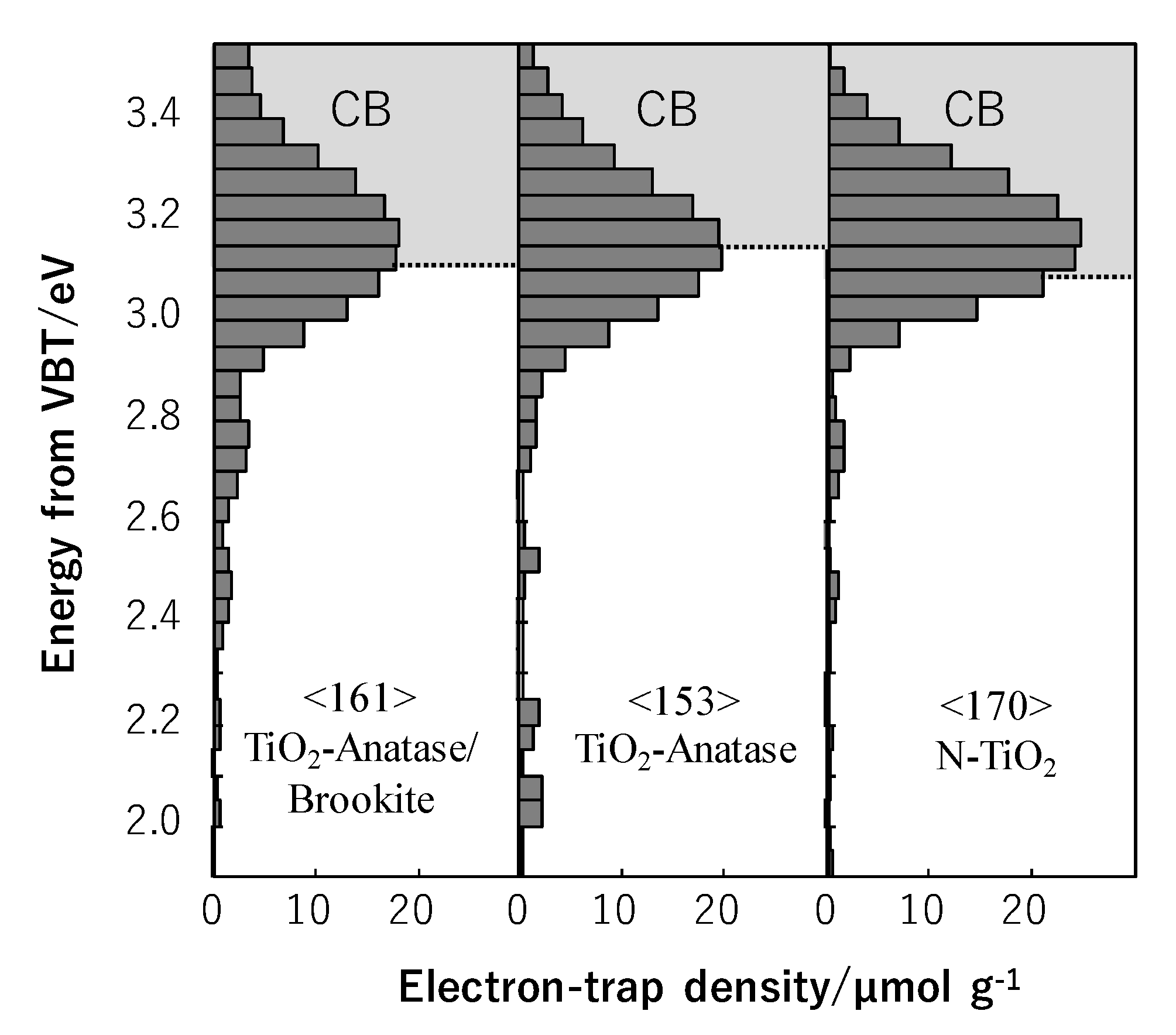
Figure 6, decomposition of acetaldehyde with initial concentration around 1800 ppm in the circulated flow reactor under UV is illustrated. The samples were prepared by the sol-gel method from tetraisopropoxide solution with addition of ethanol at the different pH: 3, 10 and neutral. For acid and alkalic pH, the acetic acid and ammonia solution were used, respectively. The samples were calcinated at 400 °C. At the presence of the ammonia solution, N-TiO
2 was formed, whereas at the neutral pH, TiO
2 of mixed phases, anatase and brookite, was obtained [
35].
Table 1 shows the physicochemical properties of samples [
35].
It was evidenced that in case of both titania, anatase/brookite and that doped with nitrogen, the electron trap densities were higher in comparison with that of single anatase phase, as it was shown in
Figure 7.
N-TiO
2 revealed higher density of electron traps than TiO
2 consisting of mixed phases of anatase and brookite, however its photocatalytic activity towards acetaldehyde decomposition was lower. The reason for that was formation of Ti
3+ centers and oxygen vacancies in N-TiO
2, which were detrimental for acetaldehyde decomposition [
35]. The most probable at the presence of oxygen vacancies, adsorption of acetaldehyde molecules on titania surface is quite different than in case of stoichiometric TiO
2 and its photocatalytic transformation towards complete mineralization is slower.
The brookite phase can also be obtained by the hydrothermal method, anodization of titanium foil or long-time grinding of anatase sample, which was previously heated at 600 °C for a few hours [
36]. The solution plasma treatment of anatase can also lead to phase transformation of anatase to brookite [
33]. Moreover, solution plasma treatment of TiO
2 resulted in the formation of some oxygen surface defects, however Ti
3+ centers were not formed [
33]. Both oxygen surface defects and mixture of anatase and brookite improved charge separation in the titania sample [
33,
34,
35]. Prepared in such a way, TiO
2 showed high activity towards acetaldehyde decomposition under both UV and visible light. Visible light activity of this TiO
2 was even higher than that prepared by nitrogen doping [
33].
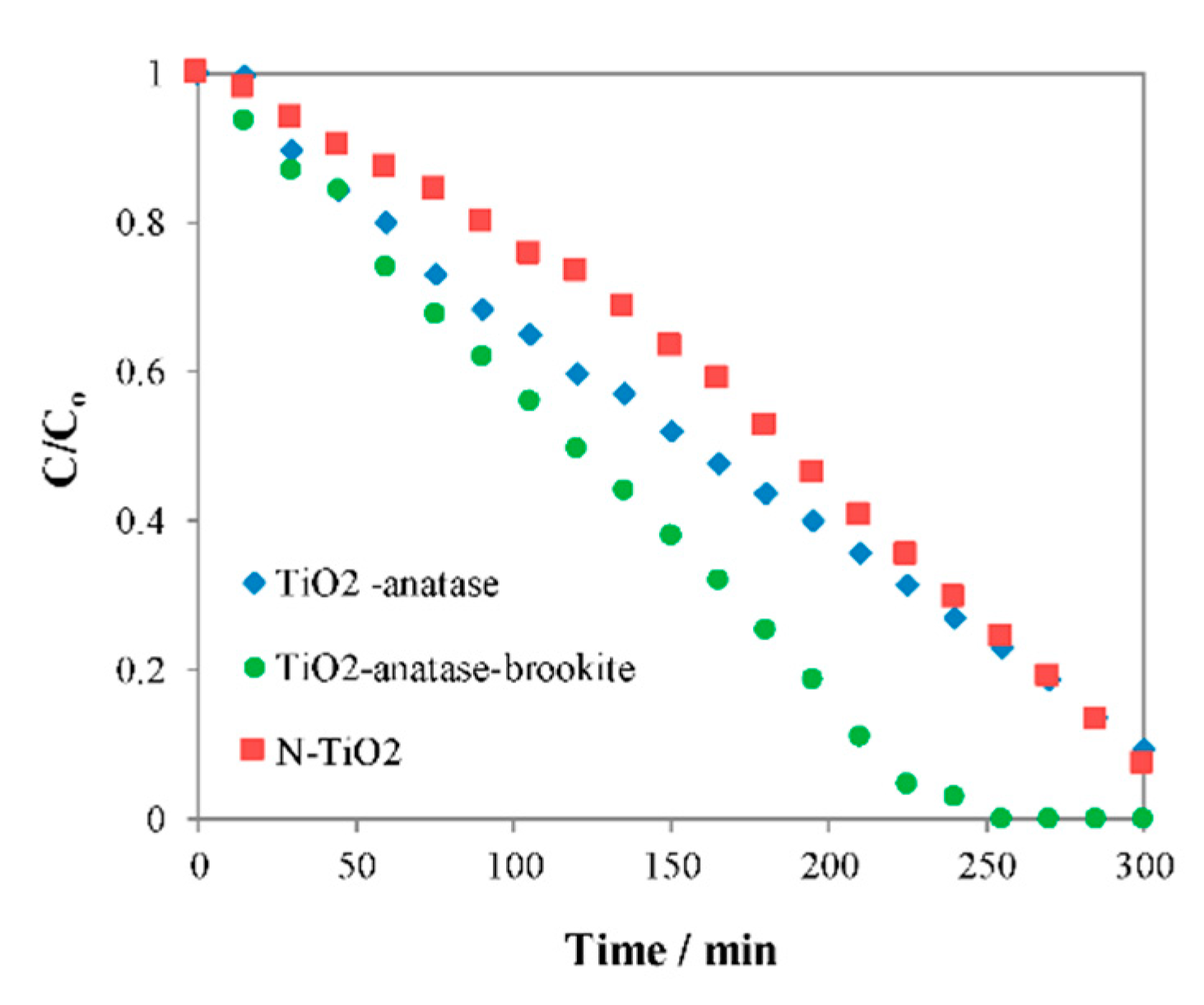
Figure 8 shows conversion of acetaldehyde towards CO
2 on the different TiO
2 samples before and after the solution plasma process (SPP), and N-TiO
2 was added for comparison [
33].
The other researchers reported that Ti
3+ defects, which were formed upon doping of carbon quantum dots, could improve separation of free charges and enhance photocatalytic decomposition of acetaldehyde [
37]. However, the observed effect of enhanced photocatalytic activity was caused by the increased yield in formation of superoxide radicals rather than quantity of Ti
3+ species. Moreover, formation of Ti
3+ was caused not by the reducing titania itself but through the interaction of carbon quantum dots (CQDs) with TiO
2 surface. It was reported that in the CQDs/TiO
2 composite, the CQDs were grafted onto TiO
2 via Ti-O-C bond, and some delocalized electrons of CQDs migrated to TiO
2 due to the work function difference, resulting in the generation of Ti
3+ defects in the TiO
2 matrix and positively charged surface environment of CQDs. The CQDs could promote the composites to adsorb organic compounds, improving the contact with target gas and further benefitting the photo-degradation process [
37].
The great role of superoxide radicals in acetaldehyde decomposition was revealed in the literature, while the meaning of hydroxyl radicals was discounted [
14,
33,
37]. It was widely reported in the literature, that for the efficient acetaldehyde decomposition, anatase-type TiO
2 is more active than rutile [
38]. Therefore, a lot of methods of TiO
2 preparation were focused on obtaining TiO
2 with anatase structure, having relatively high BET surface area and high crystallinity [
38,
39,
40]. In the sol-gel method, titanium alkoxide is usually used as a precursor and hydrolysis is accelerated by the use of acidic conditions or sonication [
21]. The optimal temperature of calcination is around 400–450 °C [
20,
21,
38,
39]. Above this temperature, the anatase crystallites grow rapidly and BET surface area significantly decreases [
20,
25]. The sol-gel technique is also used for coating TiO
2 on various substrates in the form of a thin film [
41]. It is worth to mention that some glass-coating techniques, such as dip-coating, spray-coating or spin-coating, often require to use the process of post-annealing for the crystallization into anatase structure. Post-annealing at temperatures above 450 °C causes the sodium ion diffusion from window glass into TiO
2 film and results in the decrease of the photocatalytic efficiency [
26]. Therefore, the other techniques, which do not require to use the heat treatment after coating, such as CVD, spray pyrolysis or atomic layer deposition, will be more advantageous [
26,
28,
42,
43]. Some textural properties of TiO
2 can also be designed by using the template technique, solvent evaporation or an addition of a chemical agent for surface modification, such as polyethylene glycol, for example [
39,
43,
44]. It was reported that mesoporous structure of titania, which possessed a homogeneous pore diameter of about 6.0 nm and 11.0 nm crystalline anatase pore wall, as well as large surface area of 117 m
2/g, was the most active for acetaldehyde decomposition among the other prepared samples [
39]. Nanostructural TiO
2 can be obtained by the hydrothermal or electrochemical methods [
15,
29,
45]. Titania nanotubes (TNT) of anatase structure can be obtained by the hydrothermal treatment of titania solution in an alkalic pH with addition of sodium hydroxide [
45,
46]. However, vertically oriented nanotubes are prepared by electrochemical anodization of titanium foil, plate or wires [
15,
29,
47,
48,
49]. The length and the diameter of nanotubes can be controlled by the voltage, current density, anodization time and electrolyte used. In the case of the photocatalytic decomposition of toluene, the most active TNTs were obtained in ethylene glycol-based electrolyte by applying voltage of 40 V, followed by 1 h calcination at 450 °C [
49]. Other authors reported that photocatalytic activity of ordered TNTs towards acetaldehyde decomposition could be enhanced by increasing their length, and they found optimum length of around 17 µm [
16]. They reported that highly ordered TiO
2 nanotube array presents a porous surface and straight channels, which allow free exchange of intermediate molecules with air and/or acetaldehyde molecules. This favorable gas-phase exchange can restrain the accumulation of intermediates on the photocatalyst surface and alleviate the deactivation of nanotube arrays. They also emphasized that TiO
2 nanoparticulate film is disordered and consists of randomly packed and interconnected nanoparticles, which results in tortuous channels for the diffusion of intermediates, air and/or acetaldehyde. Therefore, compared to nanotube arrays, more intermediates can accumulate on the surface of TiO
2 nanoparticles and deactivate this photocatalyst. As a consequence, higher mineralization degree during acetaldehyde decomposition was obtained on ordered TNTs in comparison with titania film of the same thickness. In
Figure 9, the SEM (Scanning Electron Microscope) images of the prepared TNTs by anodization of titanium foil are presented [
16].
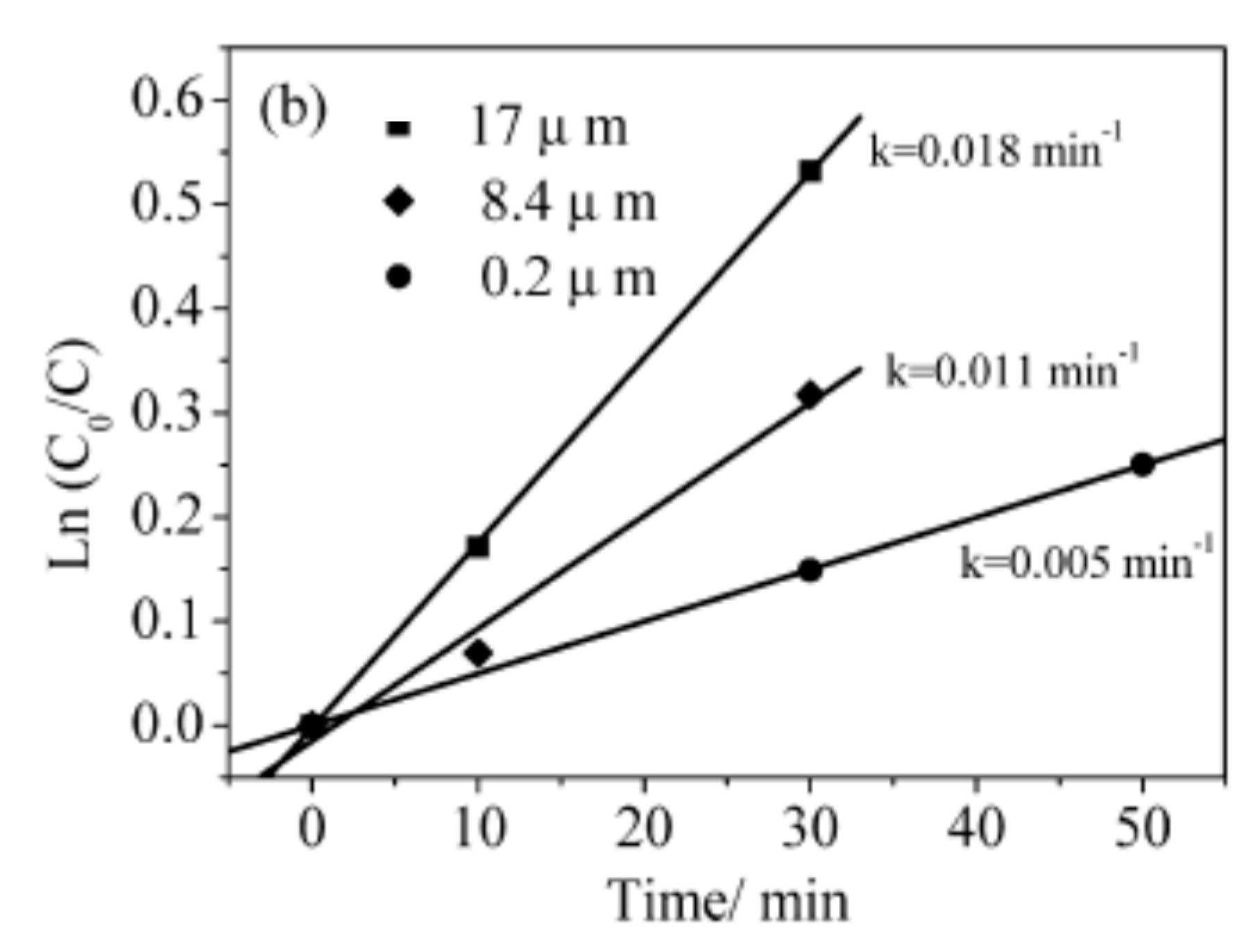
It is worth mentioning that Ti nanotubes’ high length, such as 17 µm, were obtained only in a formamide-based electrolyte. In
Figure 10, the kinetics of the photocatalytic degradation of acetaldehyde by TiO
2 nanotube arrays is illustrated.
Pichat previously reported that the average ratio of the efficacy of titania nanotubes (TNTs) over that of TiO
2 P25 for removal of toluene and acetaldehyde was 1.5 [
50]. He assumed that preparation of TNTs by anodic oxidation could give the photocatalytic material of better performance compared to the TNT of disordered structure [
50]. The other nanostructures of TiO
2 such as nanorods, nanowires or nanofibers can also be prepared by the hydrothermal method, however there are still not many studies referring to their application in the air purification systems [
51,
52,
53]. It was reported that modification of titania nanostructures, such as hydrogenation of nanorods or deposition of Au on titania nanofibers, could enhance their photocatalytic activity during decomposition of acetaldehyde [
52,
53]. There are also some studies performed for acetaldehyde adsorption on titania nanorods of anatase structure [
54]. These studies indicated that adsorption of acetaldehyde depended greatly on the partial pressure of acetaldehyde gas. For low partial pressure values, acetaldehyde was poorly adsorbed on the titania nanorods surface and this adsorption was reversible, whereas above partial pressures of 10
−4 Torr, 96% of surface-bound acetaldehyde was chemisorbed. According to others [
54], chemisorbed acetaldehyde was converted to 3-hydroxybutanal, acetate and crotonaldehyde. Low and reversible adsorption of acetaldehyde on titania nanorods can limit its application in the photocatalytic processes. However efficient decomposition of acetaldehyde was reported over brookite TiO
2 nanorods, which were prepared by the hydrothermal method [
55]. The photocatalytic activity for decomposition of acetaldehyde increased with an increase in the aspect ratio of (2 1 0) to (2 1 2) exposed crystal faces, which were attributed to reduction and oxidation sites, respectively. Site-selective Fe
3+ modification of brookite nanorods enhanced its photocatalytic activity [
55]. Dependence of the exposed anatase crystal faces on the photocatalytic decomposition of acetaldehyde was reported by another research group [
17]. Titania films with a large fraction of exposed anatase (001) facets exhibited higher activity towards acetaldehyde decomposition than those exposing predominantly (101) [
17]. Ohno et al. [
56] prepared TiO
2 nanorods consisting of rutile phase from TiCl
3 solution containing NaCl under hydrothermal conditions [
56]. The obtained rutile fine particles showed high photocatalytic activity for degradation of 2-propanol and acetaldehyde under UV irradiation compared to the activity of other commercial anatase fine particles (ST-01) developed for environmental clean-up [
56]. Their studies showed that the (110) face of rutile provides reductive sites and the (111) face has mainly oxidative sites. They concluded that the crystal faces facilitate the separation of electrons and holes, resulting in improvement of the photocatalytic activity [
56]. The role of the crystal faces of anatase and rutile in the photocatalytic reactions of oxidation and reduction was discussed elsewhere [
57].
3. Adsorption of Acetaldehyde and Its Photocatalytic Transformation on TiO2
In the photocatalytic gas-phase reactions, contact of the organic molecules with the photocatalyst surface is crucial. It was widely reported that acetaldehyde adsorbed on the anatase surface can undergo an aldol condensation to produce crotonaldehyde [
11,
13,
18,
19,
20,
58,
59,
60]. In situ FTIR (Fourier-transform infrared spectroscopy) experiments of acetaldehyde adsorption showed that acetaldehyde underwent both the aldol condensation and to a minor extent, oxidation [
19]. The aldol condensation resulted in the formation of 3-hydroxybutanal and crotonaldehyde, while the oxidation processes led to formation of bidentate acetate, which was bounded to the titania surface [
19]. It was also revealed that upon illumination of TiO
2 with UV light, the initially formed species were converted into several other intermediates, such as acetic acid, formic acid and formaldehyde [
19]. Crotonaldehyde was photocatalytically converted to acetate and formate species. The formed formate species could be further oxidized to formic acid and eventually to CO
2 [
19]. The other researchers reported that adsorption of acetaldehyde on TiO
2 depended on the exposed anatase faces [
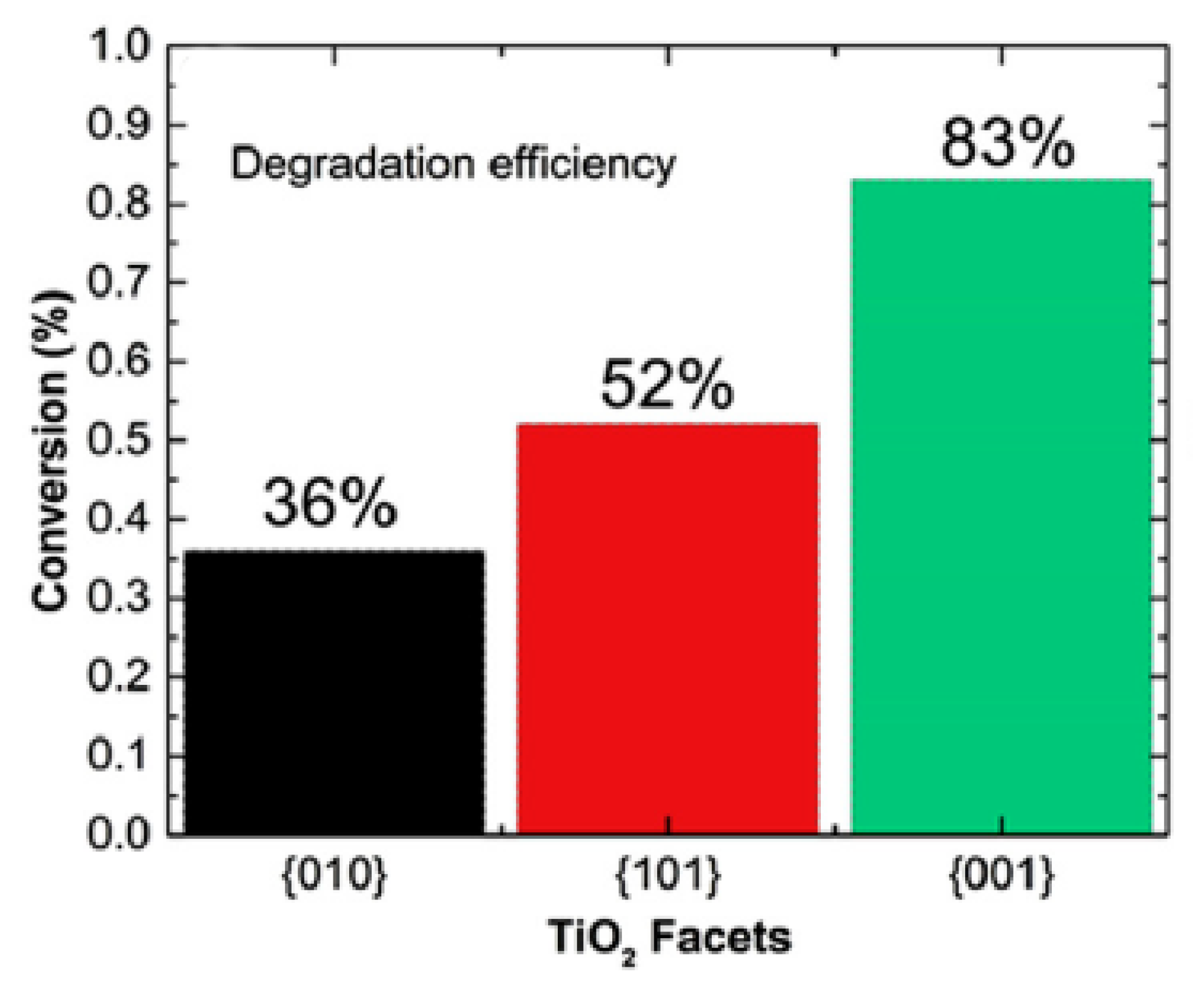
59]. The titania, with dominant anatase face (001), showed higher adsorption and photocatalytic conversion of acetaldehyde than those which had dominant faces of anatase (101) and (010) [
59]. The photocatalytic conversion of flowing acetaldehyde under UV-Vis light in dependence on the dominant face of anatase is shown in
Figure 11 [
59].
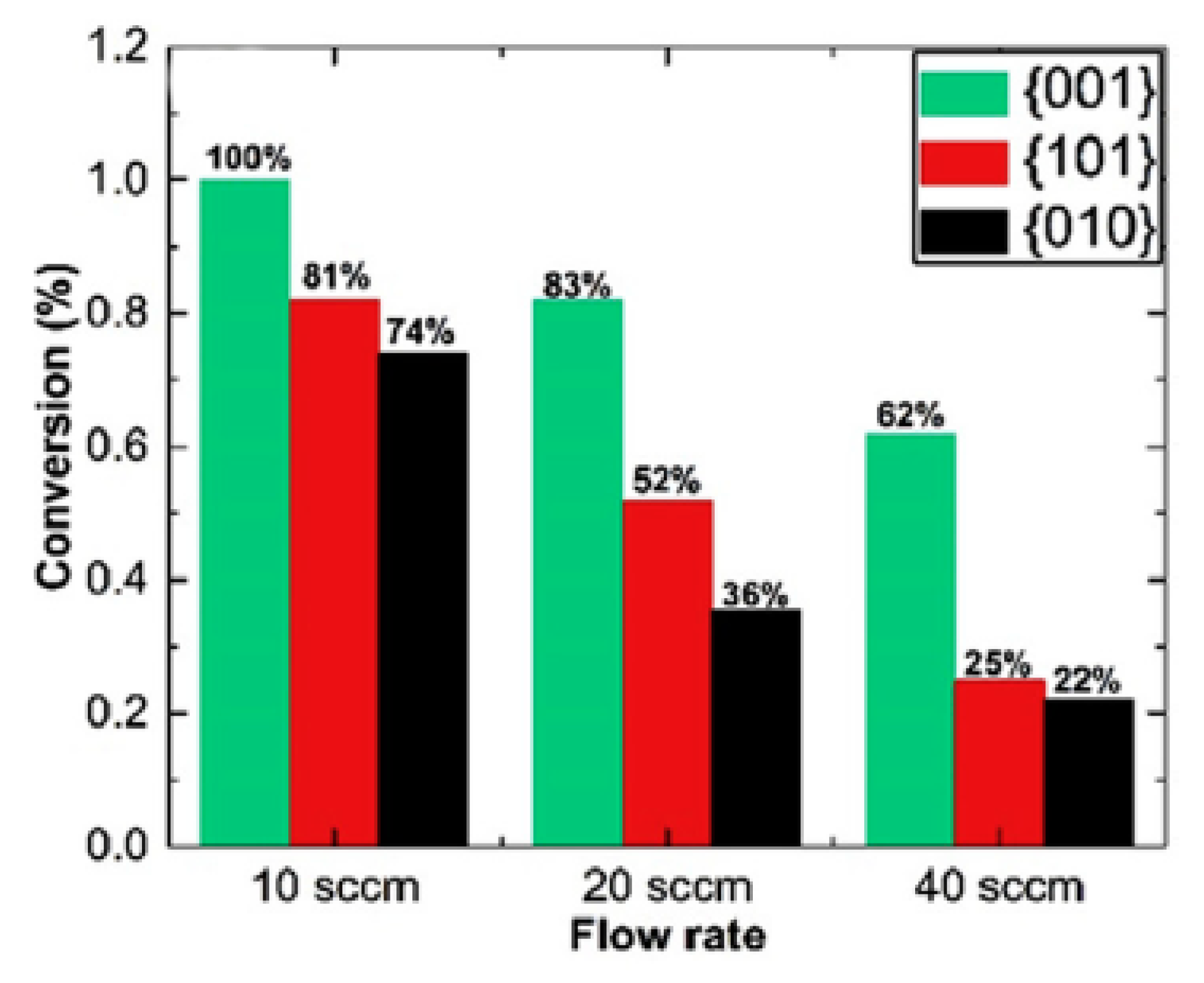
However, a high impact of surface area on the adsorption and decomposition of acetaldehyde was also noticed. Under experimental conditions, the anatase sample with dominant (001) face showed the highest specific surface area among the others [
59]. The increase of the retention time of acetaldehyde over the titania by decreasing the flow rate of gaseous acetaldehyde from 20 to 10 sccm greatly boosted its photocatalytic conversion on samples with dominant anatase faces (101) and (010), as shown in
Figure 12 [
59].
It means that contact time of acetaldehyde molecules with the active surface of photocatalyst is essential. If acetaldehyde is poorly adsorbed on the titania surface, the contact time of molecules with the surface should be long enough to facilitate their diffusion to the surface. Increased adsorption of acetaldehyde on the surface of titania nanorods by increasing the partial pressure of acetaldehyde gas was also reported by others [
54]. Adsorption of acetaldehyde on the titania surface is essential, however, the way acetaldehyde binds with the surface seems to be crucial for its conversion by means of photocatalytic reactions. It was reported that acetaldehyde could be adsorbed either physically or chemically on the titania surface [
11,
13,
20]. Under the humid conditions or on highly hydroxylated titania surface, acetaldehyde is mainly physisorbed with possible reversible desorption [
11,
13,
20]. On the contrary, the chemical binding of acetaldehyde on titania surface takes place on the low hydroxylated surface and then its further conversion occurs [
13]. The stability of formed intermediates on the titania surface influences the efficiency of the acetaldehyde degradation. As it was reported elsewhere [
14,
58], acetaldehyde was adsorbed to a higher extent on the reduced anatase and rutile than on their oxidized forms. It has been found that acetaldehyde molecules undergo adsorption preferentially via the oxygen vacancy sites in the reduced titania, followed by formation of butene complex [
58]. Formation of such species is not a catalytic process. The oxygen vacancy sites are active centers in this reaction, that are consumed irreversibly and are not regenerated during the process [
58]. However, on the stoichiometric and oxidized surface of rutile (110), acetaldehyde can be converted to acetate, which is stabilized on the surface and desorbs at high temperatures [
58]. Such pathway of acetaldehyde conversion on rutile is accompanied by blocking of the active sites on the rutile surface. For that reason, the number of the sites decreases with time and becomes unavailable for the next molecules of acetaldehyde [
58]. The adsorption of acetaldehyde on the photocatalyst surface is also influenced by the formation of active radicals. It was proven that acetaldehyde adsorption on rutile resulted in a decreasing of its efficiency for formation of hydroxyl radicals [
15]. Besides that, photoluminescence spectra showed improved charge separation of free radicals on the rutile surface loaded with adsorbed acetaldehyde molecules. It was caused by the interaction of acetaldehyde molecules with the rutile surface. It was revealed that acetaldehyde acted as a donor molecule when it was adsorbed on the rutile surface [
15]. However, formation of superoxide radicals on rutile with adsorbed acetaldehyde did not differ significantly in comparison with pure rutile [
15]. Nevertheless, the highest decomposition of acetaldehyde on rutile was evidenced in the atmosphere consisted of nitrogen, oxygen and water vapor, but was the lowest in the absence of oxygen. It means that superoxide radicals have a dominant role in the acetaldehyde decomposition over rutile-type TiO
2 [
15]. The aforementioned facts indicate the importance of molecules’ interaction with the photocatalyst surface in terms of the photocatalytic process. Therefore, we have performed new studies using different types of commercial titania samples towards their application for air purification. Adsorption capacities of acetaldehyde were determined together with the interaction of acetaldehyde molecules with titania surface. For experiments, two samples of Kronos International Ltd. (Germany) were used (KRONO
Clean®7000 and KRONO
Clean®7050), which were anatase-type TiO
2 of nanocrystalline structure. KRONO
Clean®7000 was modified by carbon species and was designed as a visible light photocatalyst. Additionally, some samples of Evonik Company (Germany) were also tested, P25 and P90, which consisted of both anatase and rutile structures in different ratios. For comparison, two other commercial samples of rutile structure were studied, nanocrystalline rutile produced by Sachtleben (Germany) and Tytanpol produced by Chemical Factory Azoty (Poland). The brief characteristics of these commercial samples are introduced in
Table 2.
The adsorption capacity of titania samples with dominant anatase structure towards acetaldehyde was well-correlated with their specific surface area, whereas in the case of rutile-type samples, it was somewhat lower than on anatase, but was also dependent on the materials’ porosity. Photocatalytic decomposition of acetaldehyde under fluorescent lamp irradiation was performed according to the procedure described elsewhere [
61]. The studies of acetaldehyde decomposition (300 ppm) under the flow rate of 20 ml/min were carried out. The performed experiments showed that acetaldehyde decomposition was not dependent on the surface area of titania samples, and results are listed in
Table 3. This process was conducted under continuous flow of gaseous acetaldehyde. Acetaldehyde decomposition after 10 h was listed to compare it with the CO
2 formation. After 1 h of light illumination, part of formed CO
2 was a result of decontamination of titania samples from some of the carbonaceous impurities.
The highest photocatalytic decomposition of acetaldehyde was noted for P90 and KRONOClean®7050 samples, whereas rutile-type TiO2 were poorly active, regardless of their porosity. The measurements of CO2 formation upon irradiation time showed that mineralization degree of acetaldehyde was decreasing over time for P25, KRONOClean®7000 and rutile-type samples.
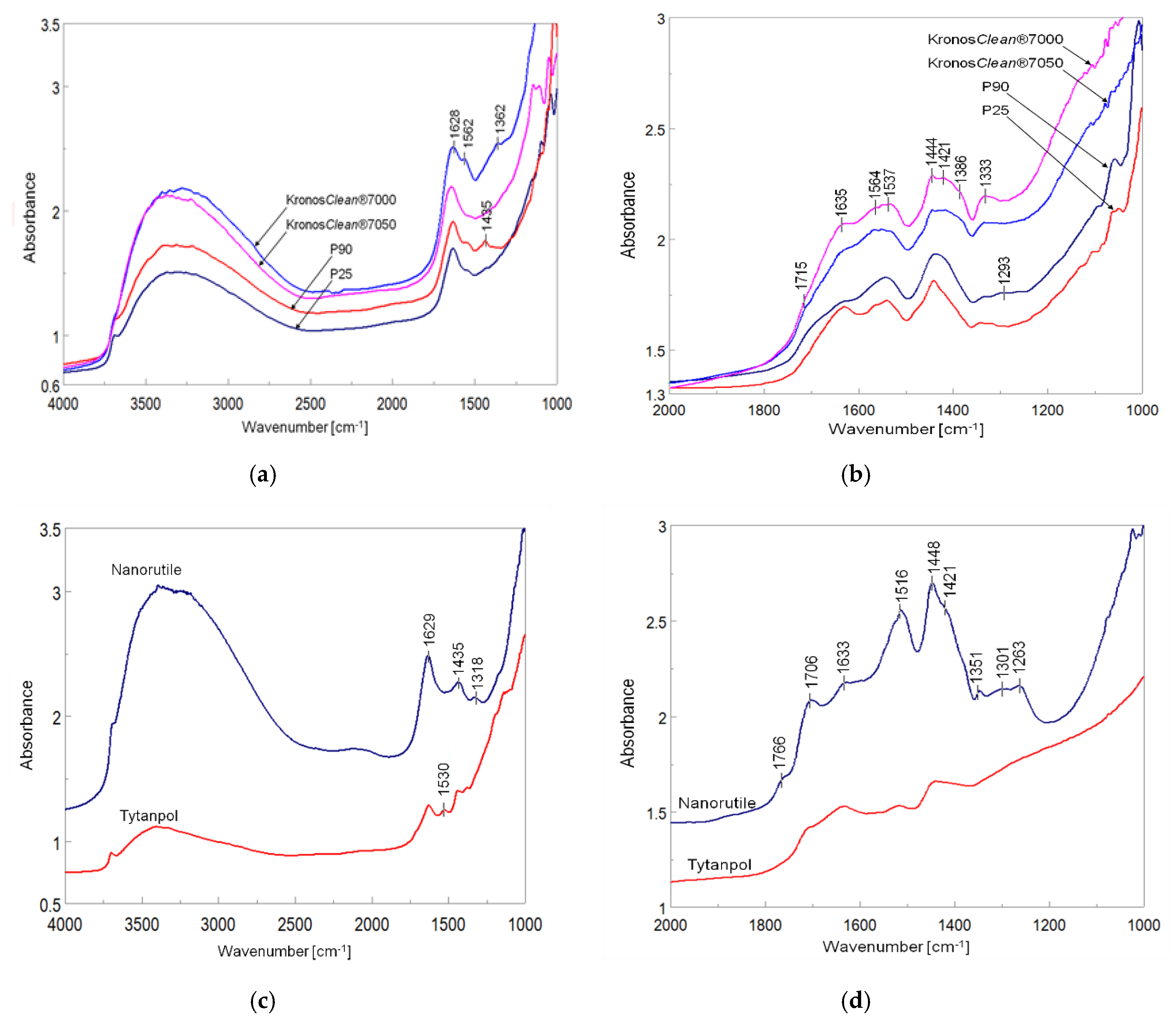
To understand the mechanism of acetaldehyde conversion via the photocatalytic process, FTIR spectra of samples after adsorption and following photocatalytic decomposition were performed and illustrated in
Figure 13. The samples produced by Kronos Company had higher hydroxylated surface in comparison to these obtained by Evonik. Nanorutile also has more hydroxylated surface than Tytanpol. P25, P90, KRONO
Clean® 7000 and nanorutile samples showed some bounds of carbon species on FTIR spectra. After adsorption of acetaldehyde on titania samples, new bonds appeared, such as C=O, COO, C=C, C-H, C-C and CH
3, which were assigned to adsorbed acetaldehyde, crotonaldehyde and acetate species. The list of FTIR bands with their identification is introduced in
Table 4,
Table 5,
Table 6,
Table 7 and
Table 8. The adsorption of acetaldehyde on TiO
2 surface differed between anatase and rutile. The bands at 1444 and 1537 cm
−1 assigned to COO species from CH
3COO
− [
19] were more intensive on nanorutile and TiO
2 samples containing rutile structure. However, the bands of CH
3 species at 1333 and 1421 cm
−1 assigned to adsorbed CH
3COO
− [
19] were the most intensive on Kronos samples. Most likely, the bonding of acetate species with anatase surface is different than that with rutile. The C=O bands at 1706, 1715 and 1766 cm
−1, assigned to HCOOH species [
19], were observed after acetaldehyde adsorption on all the titania surfaces, however on rutile, they were more intensive. The band at 1351 cm
−1 observed on nanorutile after adsorption of acetaldehyde can be assigned to C-O species in HCOOH [
62]. The band at 1633−1635 cm
−1 assigned to C=C vibrations in crotonaldehyde was the most intensive in titania Kronos and P90 samples, it could be connected with high adsorption of acetaldehyde on these samples and occurring aldol condensation on anatase structure. These FTIR measurements showed that acetaldehyde reacted with TiO
2 surface through the formation of crotonaldehyde and was partly oxidized to acetic and formic acids. Oxidation of acetaldehyde to acetate and formate species was carried out more efficiently on rutile than anatase, but an aldol condensation to crotonaldehyde was more efficient on anatase surface. In
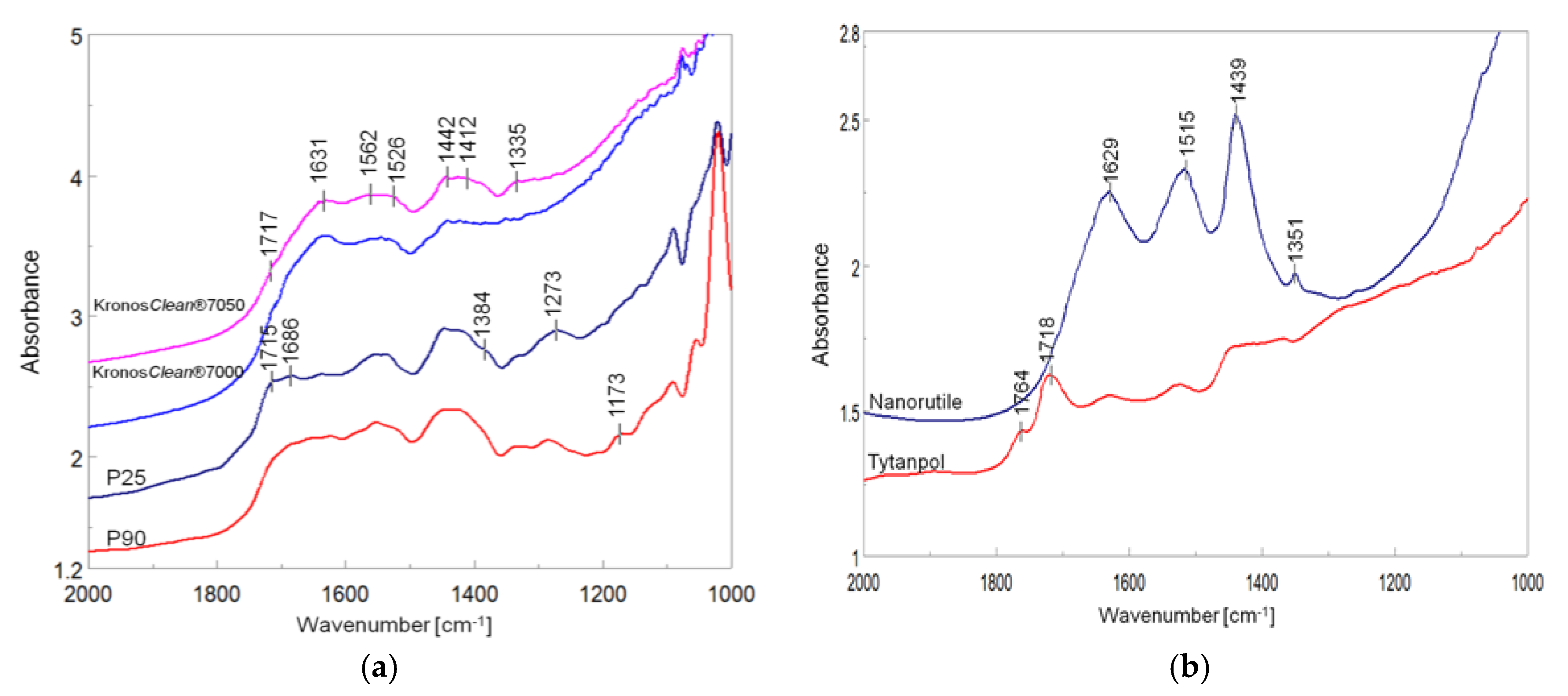
Figure 14, the FTIR spectra of titania samples after the photocatalytic process are shown. P25 and P90 revealed a lower intensity band at 1631 cm
−1 assigned to adsorbed water molecules than Kronos samples and higher intensity bands at 1412 and 1442 cm
−1 assigned to CH
3 species in the acetic acid and crotonaldehyde, respectively [
19,
62]. The bands of C=O at 1686 and 1715 cm
−1 assigned to CH
3COOH and HCOOH respectively, were more intensive on TiO
2 samples, which contained rutile. The highly intensive band at 1439 cm
−1 on nanorutile was assigned to COO vibrations in the acetic acid [
11]. Tytanpol rutile showed highly intensive bands at 1718 and 1764 cm
−1 assigned to C=O groups in formate species. These measurements revealed that the interaction of acetaldehyde with anatase surface is quite different than that with rutile. Acetaldehyde is more favorable adsorbed on anatase, and undergoes an aldol condensation in the dark, whereas on rutile, the oxidation of acetaldehyde to acetic and formate species takes place. Under illumination of a fluorescent light, both acetic and formic acids are deposited on rutile and slow down the process of acetaldehyde decomposition. A similar effect was noted by other researchers [
62], who observed that deposition of formic acid on TiO
2 during acetaldehyde decomposition deactivated its surface. After the photocatalytic process, the amount of both the acetic and formic acids adsorbed on titania surface was higher on rutile than anatase-type samples, most probably, they were poorly mineralized on rutile. It can be concluded that rutile oxidizes acetaldehyde at the presence of oxygen easier than anatase. The products of acetaldehyde oxidation are deposited on TiO
2 surface and slow down the process of its further decomposition. The scheme illustrating transformation of acetaldehyde on anatase and rutile surfaces under dark conditions and after illumination with weak UV light is shown in
Figure 15. Other researchers investigated the photocatalytic properties of nanorutile, and they proved that rutile was a material of more reductive properties than anatase and was able to adsorb more oxygen [
63]. It was also proven that oxygen could react with adsorbed acetaldehyde at low temperature and oxidize it to the acetic acid [
64]. Such pathway of acetaldehyde conversion on TiO
2 surface appeared to be less efficient, because acetic acid deposited on TiO
2 was stable and its further decomposition was limited [
15]. It is worth mentioning that during acetaldehyde decomposition, acetic acid was detected in the outlet stream of the acetaldehyde gas. The concentration of acetic acid was increasing with time during adsorption in the dark for all types of TiO
2, but after the lamp was switched on, this concentration was decreasing for anatase-type samples and increasing for rutile. It means that proceeding photocatalytic reactions on anatase could mineralize acetic acid more efficiently than those occurring on rutile.
To summarize the impact of acetaldehyde adsorption on titania surface for its further conversion during photocatalytic process, it can be concluded that the chemical bonding of acetaldehyde with TiO
2 surface is essential and depends on its crystalline structure. Low hydroxylated anatase surface can easily adsorb acetaldehyde by the chemical way through the aldol condensation to crotonaldehyde. Such adsorbed molecules can undergo further transformation via the photocatalytic process, leading to formation of acetate, formaldehyde, formate species and CO
2. Conversion of acetaldehyde on rutile-type surface conducts fast oxidation of acetaldehyde to stable-surface acetate species, which limits its further decomposition. Geometry of the crystalline structure of anatase and rutile influences the quantity of acetaldehyde adsorption. Anatase with dominant (001) face and both reduced anatase (001) and reduced rutile with dominant (110) face showed enhanced adsorption of acetaldehyde [
14,
17,
58]. However, adsorption of acetaldehyde on the reduced defect sites of TiO
2 conducts irreversible utilization of these active sites, so it can improve the adsorption abilities of TiO
2 up to saturation of oxygen vacancies. Nevertheless, exposition of reduced titania surface to acetaldehyde gas for a long period of time can partly deactivate its surface.
4. The Role of Reactive Oxygen Radicals in the Photocatalytic Conversion of Acetaldehyde
Various potential pathways for the photocatalytic degradation by TiO
2 have been proposed, including action of reactive species such as: hydroxyl radicals, carbonyl radicals, superoxide radicals and hydrogen peroxide [
33].
Other studies concerned a general mechanistic way of acetaldehyde decomposition through its conversion to acetate, and then to formaldehyde, followed by transformation of the latter to formates and finally to CO
2 [
11]. However, both interaction of acetaldehyde with the reactive surface and formed oxygen radicals may reveal an impact on the reaction pathway. Transformation of acetaldehyde to crotonaldehyde and its partial oxidation to acetic acid on anatase surface was confirmed by many researchers [
11,
19,
20]. Impact of oxygen on the oxidation and decomposition of acetaldehyde was also recognized and reported [
10,
11]. The mechanism of acetaldehyde decomposition via radical-initiated chain reactions consuming oxygen was also proposed [
10]. It was evidenced [
15] that acetaldehyde adsorbed on rutile-type TiO
2 significantly limited the extent of hydroxyl radicals’ formation, but at the same time, was recognized as neutral for the formation of oxygen superoxide species. In addition, a great role of superoxide radicals in the photocatalytic decomposition of acetaldehyde on rutile was reported [
15]. Other researchers also postulated that efficient decomposition of acetaldehyde could be achieved in the presence of formed O
2−• species [
33]. The authors reported that oxygen vacancies formed upon the solution plasma process and surface defects below the conduction band in TiO
2, were capable to accelerate formation of reactive species [
33]. These defects attracted atmospheric oxygen, and converted it to O
2−•, as illustrated by reaction scheme (1).
Photogenerated electrons take part in the following reaction:
Whereas formed holes (h
+) mediate in the reaction of carbonyl radicals’ formation:
The carbonyl radicals (CH
3CO
•) are capable to react with O
2, which mediates the chain reactions of acetaldehyde oxidation. In these multiple reactions, acetic acid is the main intermediate in the transformation of acetaldehyde to CO
2, according to the reaction [
33]:
Therefore, the improved separation of free radicals formed in TiO
2 can conduct enhanced photocatalytic conversion of acetaldehyde. The improvement in charge separation can be achieved by mixing of two different TiO
2 phases—either brookite with anatase or anatase with rutile [
34,
35], or by the synthesis of TiO
2 consisting of the proper ratio of the oxidative and reductive centers [
12,
17,
55,
56,
57,
59]. This effect can be realized for example by increasing the share of anatase (001) face of in the synthesized titania [
17].
Formation of both types of radicals, O
2−• and
•OH, is demanded for complete decomposition of acetaldehyde. The main route of
•OH formation includes action of the photogenerated holes:
Other options of hydroxyl radicals’ generation are illustrated by reaction schemes (5) and (6):
According to Zeng et al. [
15], high yield of acetaldehyde decomposition on rutile was observed under atmosphere of nitrogen, oxygen and water vapor [
15]. However, due to the competition between acetaldehyde and water to the adsorption sites of TiO
2, the presence of water may suppress acetaldehyde decomposition on TiO
2 [
11,
20]. Compared to anatase, the ability of rutile to form hydroxyl radicals is poor. Nevertheless, the formation of a high quantity of
•OH radicals on rutile was confirmed in the presence of H
2O
2. It was proven [
64] that O
2−• radicals formed on the surface of nanosized rutile reduced H
2O
2 to
•OH, according to the reaction (6).
The pathways of H
2O
2 formation during photocatalytic processes occurring on TiO
2 are illustrated by reaction schemes (7)–(10):
It is assumed that poor photocatalytic activity of rutile in comparison with anatase could be caused by high transformation of acetaldehyde to both acetic and formic acids, and by the high stability of the acetate species on rutile surface. On the contrary, adsorption of acetaldehyde on anatase TiO
2 was proceeded greatly through an aldol condensation to crotonaldehyde. Under UV irradiation, crotonaldehyde was either oxidized to acetate and formate species or directly mineralized to CO
2. Such pathway of acetaldehyde decomposition on anatase was more efficient in the formation of CO
2 than that on rutile. Undoubtedly, oxygen takes part in the photocatalytic oxidation of acetaldehyde and it is consumed during photocatalytic chain reactions. Interestingly, it was reported that in the absence of gaseous oxygen, surface-adsorbed oxygen was consumed during the formation of the acetate and the formate. However, if the adsorbed O
2 was consumed in the process, only minor amounts of acetaldehyde were decomposed into the formate [
11]. After the photocatalytic process of acetaldehyde decomposition, the rutile type of TiO
2 indicated some content of formate species adsorbed on the surface. The amount of formate species formed was much higher on rutile than on anatase surface samples (
Figure 14). This implies that oxygen adsorbs on rutile surface more efficiently than on anatase, and this adsorbed oxygen takes part in the oxidation of acetate species. Nevertheless, rutile is less efficient in mineralization of acetic acid than anatase-type TiO
2.
5. The Impact of Light Wavelength Region on the Photocatalytic Decomposition of Acetaldehyde
The efficiency of the photocatalytic reactions depends on the energy of the incident photons. However, even a few photons of the required energy can induce the photocatalytic process. As reported by Fujishima et al. [
10], in a well-lit room with the total light intensity of 10 µW/cm
2 (1 µW/cm
2 of UV needed to excite TiO
2), the energy of photons would be sufficient enough to decompose a hydrocarbon layer of approximately 1 µm thick every hour [
10]. Therefore, they recommended to use a fluorescent light as a source capable to induce photocatalytic decomposition of VOCs on TiO
2. The studies, conducted by different teams, indicated that acetaldehyde was successfully undergoing photocatalytic decomposition on TiO
2 surface under light emitted by the fluorescent lamps. However, efficiency of this process was lower than under UV-A light generated by a typical UV lamp [
12,
33,
61]. To increase utilization of solar light, a lot of recent studies have been focused on the preparation and examination of visible-light active photocatalysts. One of the first works on the activity of nitrogen-doped TiO
2 towards acetaldehyde decomposition under visible light was reported by Asahi et al. in
Science Journal [
65]. They prepared TiO
2-x N
x films by sputtering the TiO
2 target in an N
2 (40%)/Ar gas mixture followed by annealing at 550 °C in N
2 gas. The obtained nitrogen-doped TiO
2 film showed absorption of visible light of a wavelength below 500 nm and the photocatalytic degradation of acetaldehyde for λ = 435 nm. The activities of both TiO
2 films, doped with nitrogen and undoped, under UV light were comparable and higher than under visible light irradiation. The results are illustrated in
Figure 16 [
65].
After publishing Asahi’s findings [
65], numerous research works were targeted to obtain the visible light active TiO
2 through doping of different anionic species, such as N, C, S and F as single doping or co-doped [
37,
66,
67,
68,
69,
70,
71], also with some metallic species such as Au, Ag, Cu, Co, Pt, Mn, Fe and others [
72,
73,
74,
75,
76,
77,
78,
79]. The composites of TiO
2 with other metal oxides such as WO
3 or CeO
2 were also prepared and showed photocatalytic activity under visible light irradiation towards acetaldehyde decomposition [
80,
81]. An activity of doped TiO
2 under visible light was mainly obtained due to either the narrowing of the band gap in TiO
2 [
65,
66,
69] or by the introduction of an impurity level of dopant inside the titania structure and formation of intra-band-gap energy levels [
77]. Formation of surface defects in TiO
2 was also reported [
33] as resulting in increasing the activity of titania under visible light. Nitrogen doped to TiO
2 can be localized at the interstitial or substituted position. Asahi et al. [
65] reported that in the anatase-type TiO
2, oxygen atoms are substituted by nitrogen ones, causing mixing of O2p and N2p states and narrowing of the titania band gap. However, the other researchers stated that by substitutional doping of nitrogen to TiO
2, some localized impurity states are formed just above the valence band of TiO
2 without mixing of N2p and O2p states [
82]. Moreover, they claimed that nitrogen builds into the titania lattice in the interstitial position and forms localized energy levels in the form of NO bonds [
82]. The authors did not confirm the narrowing of the band gap, but such interstitial doped nitrogen caused activity of TiO
2 in the range of visible light [
82]. Such observation was also confirmed by Meroni et al. [
66] and Sakatani et al. [
83], who reported that the interstitially doped nitrogen and concentration of the paramagnetic centers in nitrogen-doped TiO
2 seemed to control the absorption of visible light and the activity of titania samples. Similar results were reported by our research group [
61]. Generally, it is accepted that nitrogen doped to TiO
2 increases absorption of light to the visible region. However, photocatalytic activity of TiO
2 modified in such a way depends on its electronic structure, rather than the ability for visible light absorption [
33,
61,
66]. Fujishima et al. [
33] reported that although samples of TiO
2 prepared through the solution plasma process indicated absorption of visible light, their photocatalytic activity towards acetaldehyde decomposition was governed mainly by the intensity of UV light, with a wavelength below 400 nm. The solution plasma process caused formation of surface defects in TiO
2, which were most likely responsible for its enhanced photocatalytic activity. Nitrogen doping to TiO
2 also generates some oxygen vacancies and surface defects, however the superiority of TiO
2 modified by solution plasma process over the N-doped TiO
2 in the photocatalytic decomposition of acetaldehyde was demonstrated [
33]. Meroni et al. [
66] also indicated the poor activity of N-doped TiO
2 under visible light towards acetaldehyde decomposition, compared to the effect achieved under UV or UV-Vis. Doping of some species to TiO
2 can retard charge separation and enhance its photocatalytic activity through the formation of different surface defects. Higher density of electron traps was measured in N-doped TiO
2 in comparison with the undoped one, however photocatalytic decomposition of acetaldehyde under irradiation of a fluorescent lamp was more efficient for TiO
2 itself [
61]. The presence of Ti
3+ surface defects in N-TiO
2 appeared to be detrimental in acetaldehyde decomposition [
61]. It is worth mentioning that the solution plasma process did not cause formation of Ti
3+ centers in TiO
2 [
33]. Co-doping of anionic or anionic/metallic species to TiO
2 can increase both the amount and type of active sites, which conducts successful narrowing of the band gap, suppresses the recombination process between photoinduced pairs of h
+/e
− and increases its photocatalytic activity [
69,
72,
73,
74,
79]. As reported elsewhere [
72,
73,
74], different oxidation states of metals are responsible for charge trapping and improved charge separation [
72,
73,
74]. Synergistic effects of co-dopants (nitrogen and platinum species) were reported by Kim et al. [
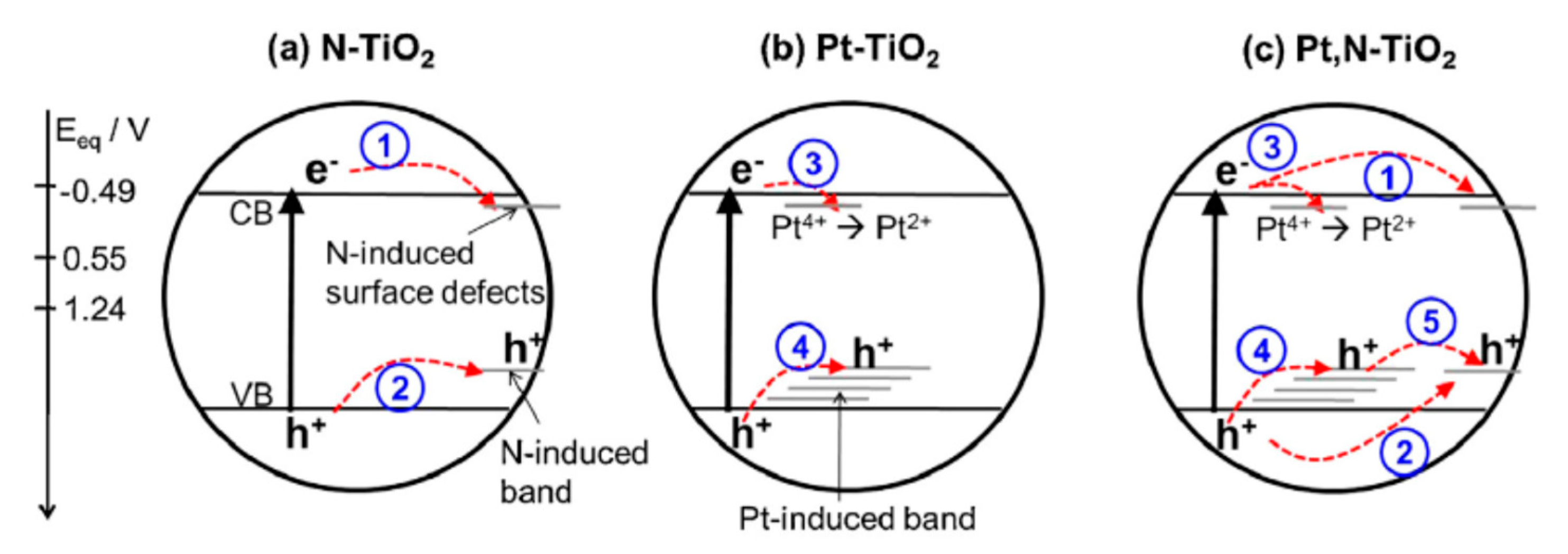
74] and illustrated in
Figure 17.
The superiority of Pt,N-TiO
2 photocatalyst for acetaldehyde oxidation observed by the researchers could be attributed to the presence of the new impurity doping levels created by the co-doped Pt and N species and/or the change in the charge transfer dynamics and kinetics [
74].
Other researchers [
73] observed photocatalytic activity of TiO
2 co-doped with nitrogen and different metals under visible light (λ > 410 nm) for acetaldehyde decomposition. They found that photocatalytic activity over TiO
2-xN
x was markedly enhanced by Cu or Pt loading, while Ni-, Zn- or La-loaded TiO
2-xN
x showed a similar photodegradation rate to the bare TiO
2-xN
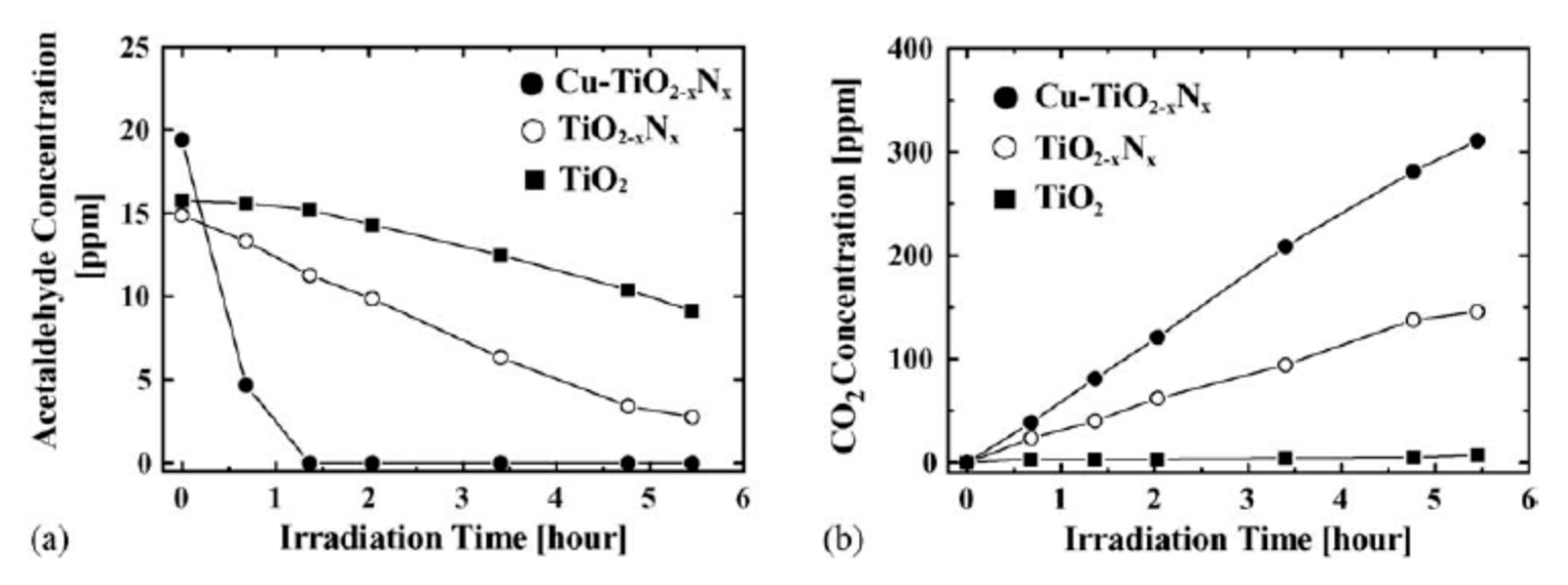
x. Among them, the enhancement effect of Cu ion was found to be the highest. The optimum concentration of Cu was found to be 0.5 wt.% and nitrogen concentration around 0.25 at.%.
Figure 18 shows the formation of CO
2 upon acetaldehyde oxidation under visible light with TiO
2 doped with nitrogen and co-doped with nitrogen and Cu [
73].
Thermo-photocatalytic decomposition was observed for titania samples loaded with platinum nanoparticles [
79,
84]. The extremely high rate of formic acid oxidation over Pt-TiO
2-xN
x was found to be due to a combined effect of both photocatalysis and thermal catalysis at room temperature, facilitated by nanosized (1–2 nm) Pt [
79]. The other researchers noted increased decomposition rate of acetaldehyde by decreasing distance between photocatalyst (Pt-TiO
2/SiO
2) and the source of light used in the process [
84]. They observed that in the presence of platinum, byproducts and coke precursors were easily burned. For that reason, the combination of the catalytic properties of some metals with the photocatalytic activity of TiO
2 seems to be a very interesting and promising solution for the complete decomposition of VOCs compounds. Fujishima et al. reported that during weak UV irradiation used for acetaldehyde decomposition, acetic acid was quantitatively formed as the main byproduct of the photocatalytic process, and its amount was increasing with the decrease of the number of absorbed photons [
85]. The authors noted ca. 150% maximum quantum yield for acetaldehyde conversion to acetic acid, which was a result of a radical chain-type process with a chain length of approximately five [
85]. Although conversion of acetaldehyde during photocatalytic reactions carried out on doped TiO
2 is possible to be higher than on the undoped titania, quantitative formation of byproducts decreases the mineralization degree. For instance, Mn-doped TiO
2 indicated activity under visible light and higher conversion of acetaldehyde under visible light irradiation than TiO
2. On the other hand, formation of CO
2 on the former photocatalyst was confirmed to be much lower than on the latter one [
78]. Other researchers investigated the commercial TiO
2 KRONO
Clean®7000 for acetaldehyde decomposition under irradiation with light emitted by a fluorescent lamp, and they observed formaldehyde as the main byproduct of acetaldehyde conversion [
12]. Some metal species doped to TiO
2 can act as a catalyst and thus support the complete oxidation of acetaldehyde. Efficient decomposition of acetaldehyde under visible light irradiation by TiO
2 co-doped with Cu and Co was reported [
76]. The main products of acetaldehyde conversion were H
2O and CO
2 [
76]. Doping some noble metals like Ag, Au, Pt or Pd to TiO
2 results in obtaining materials revealing enhanced photocatalytic activity under visible light. The observed enhancement is due to efficient transport of electrons generated over the doped nanoparticles by local surface plasmon resonance (LSPR), across the metal/semiconductor interface via the defect states of TiO
2 [
75]. Another way of increasing activity of TiO
2 under visible light is preparation of composites of titania with another semiconductor, revealing lower energy of the band gap being able to absorb the photons induced by the visible light [
80,
81]. In such modified titania, photoinduced transport of electrons occurs between both semiconductors and improvement of the charge separation process takes place.