Clarifying the Role of the Reducers-to-Oxidizers Ratio in the Solution Combustion Synthesis of Ba0.5Sr0.5Co0.8Fe0.2O3-δ Oxygen Electrocatalysts
Abstract
1. Introduction
2. Results
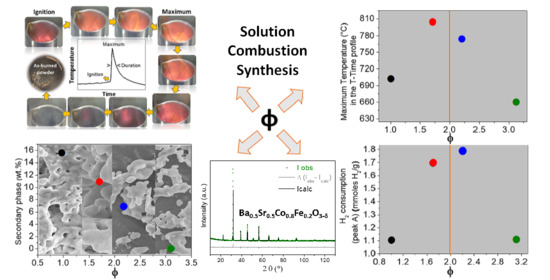
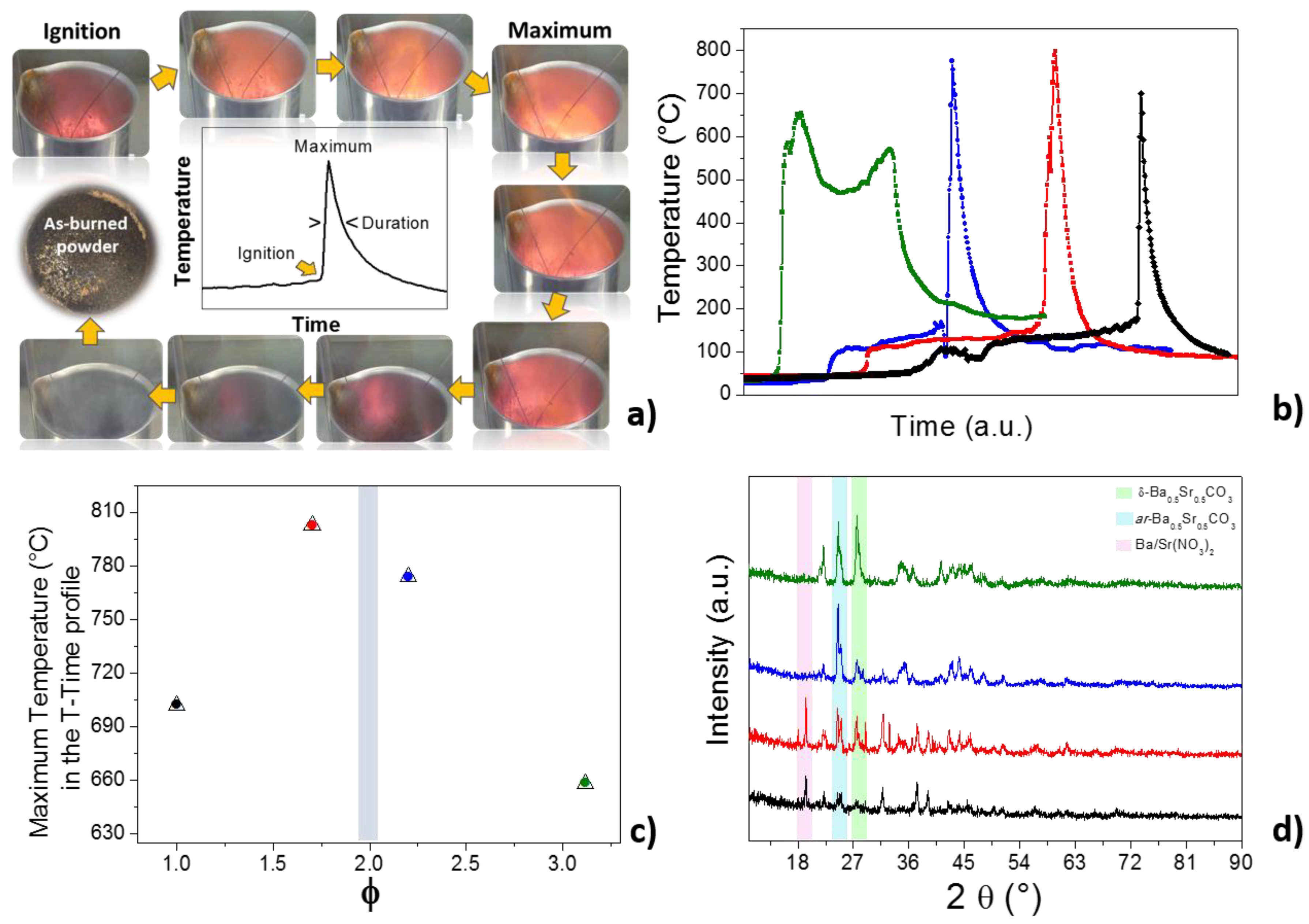
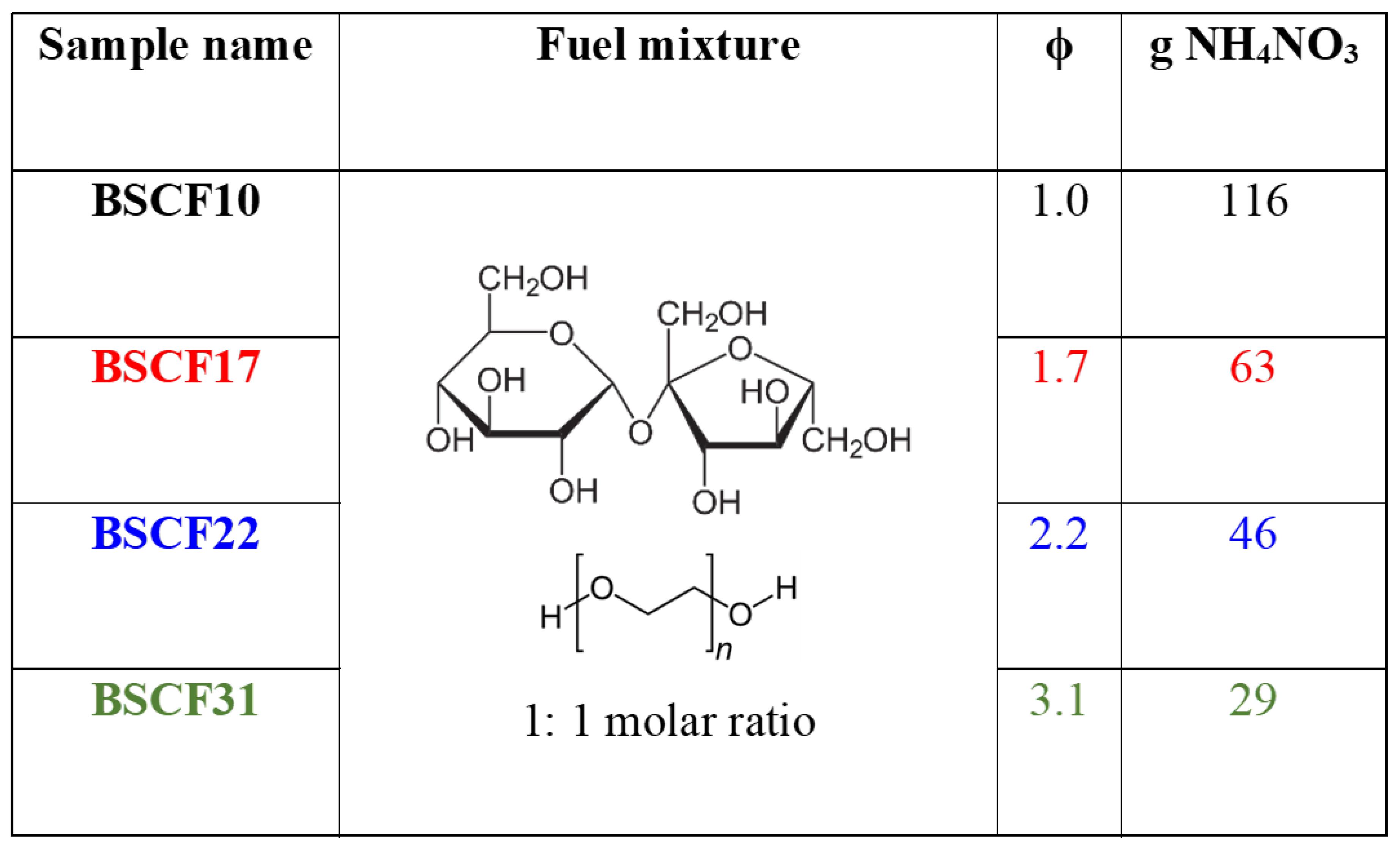
2.1. The Combustion Process and the As-Burned Powders
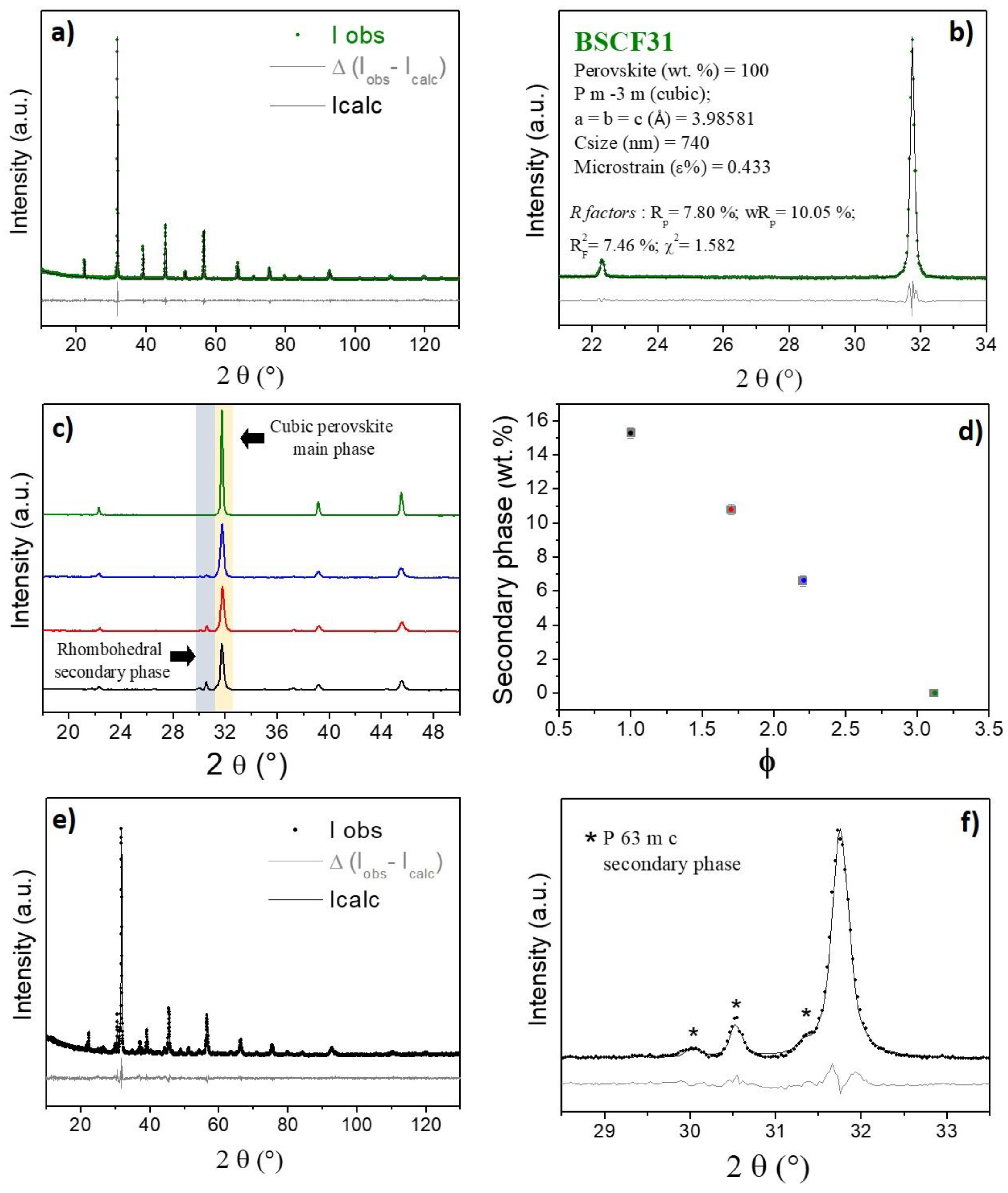
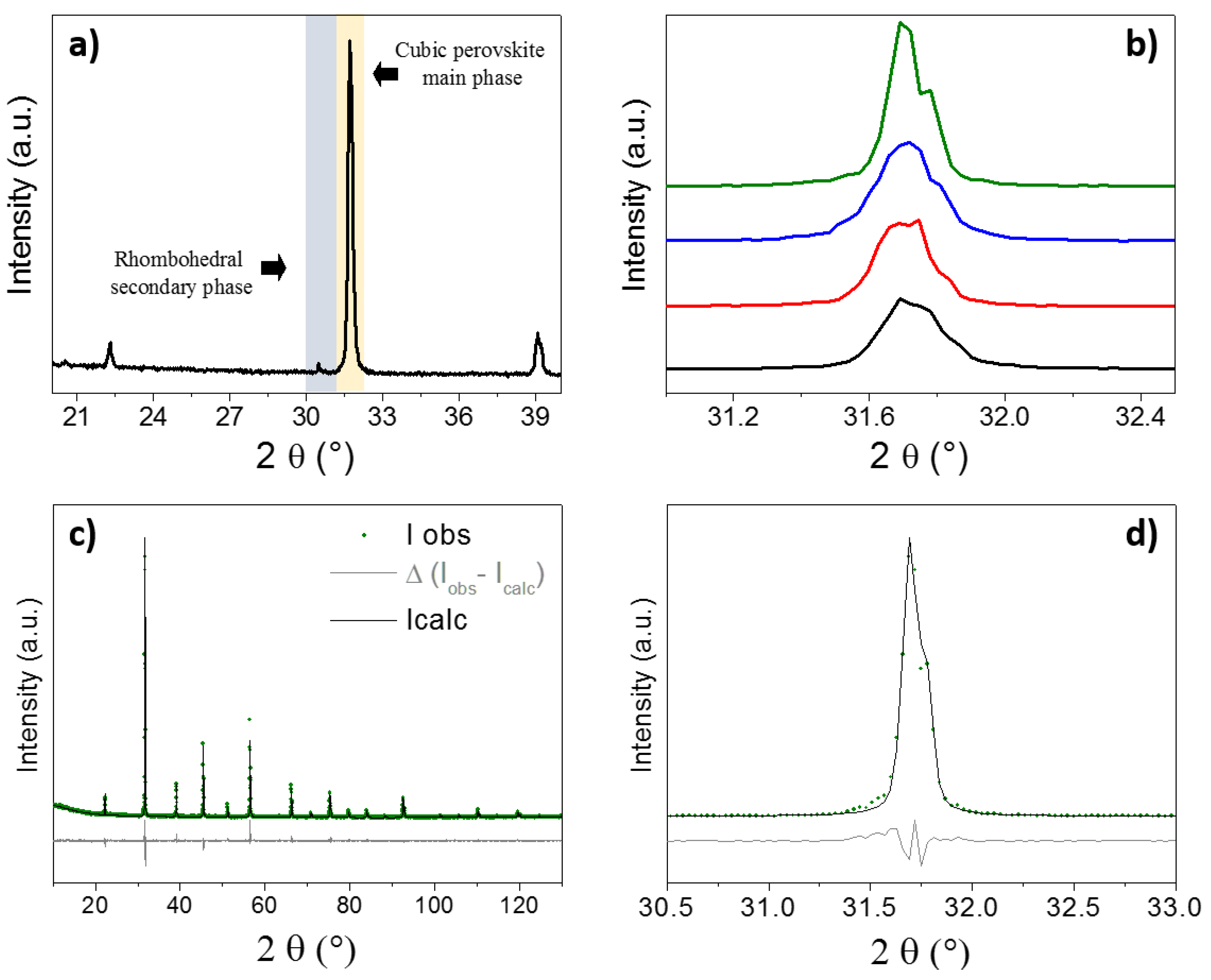
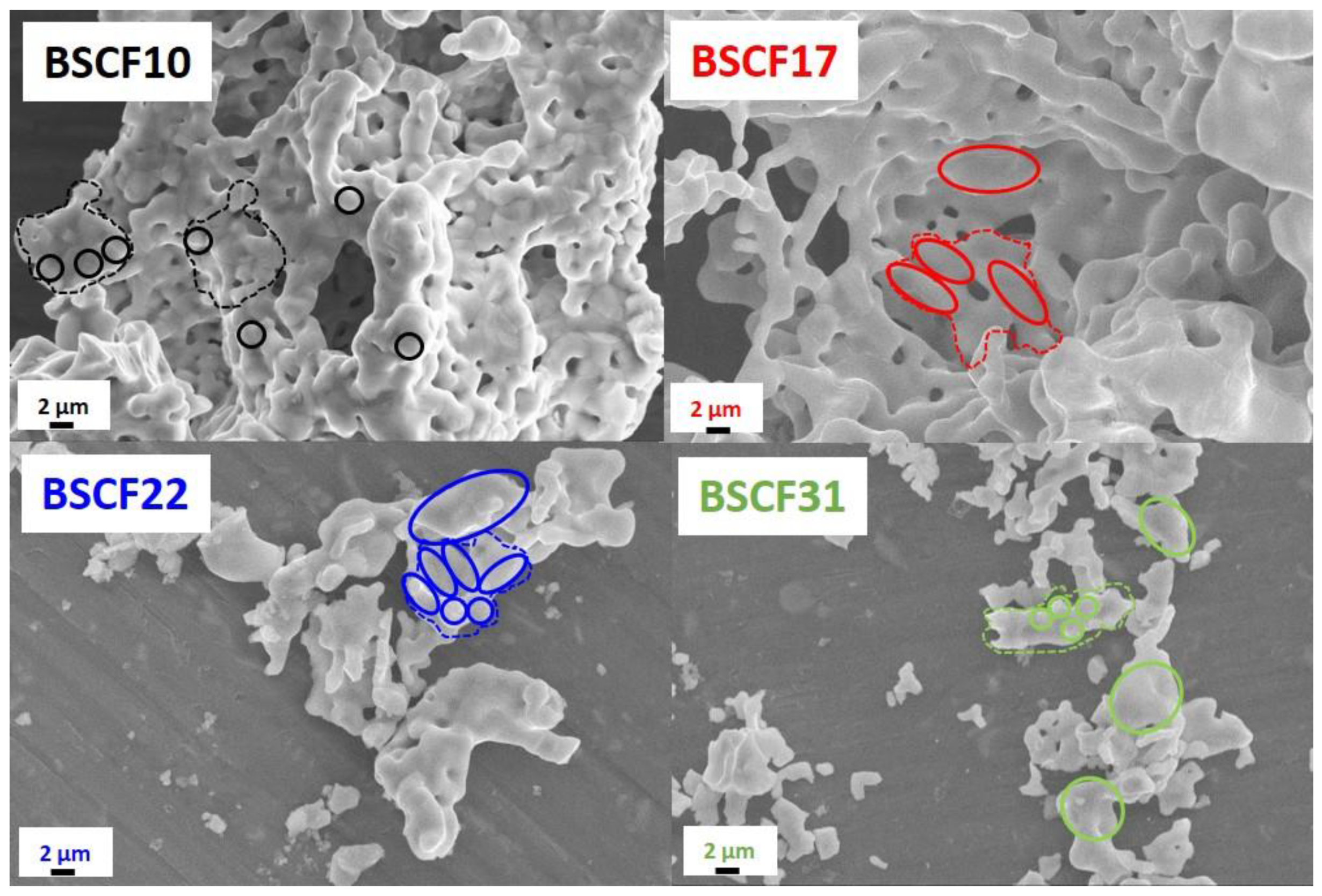
2.2. Structure, Microstructure and Texture after Further Thermal Treatments
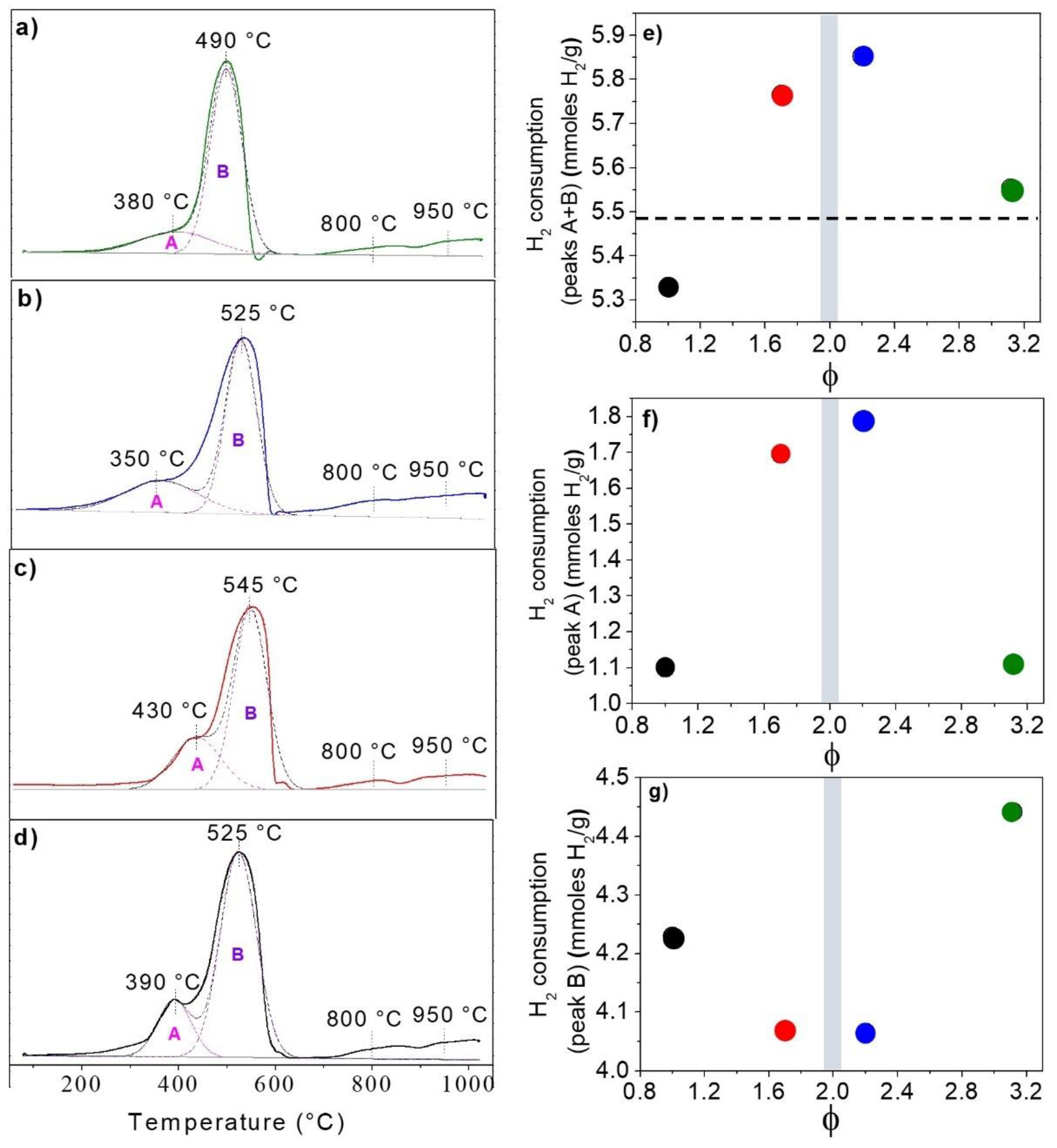
2.3. Redox Properties
2.4. Electrocatalytic Activity
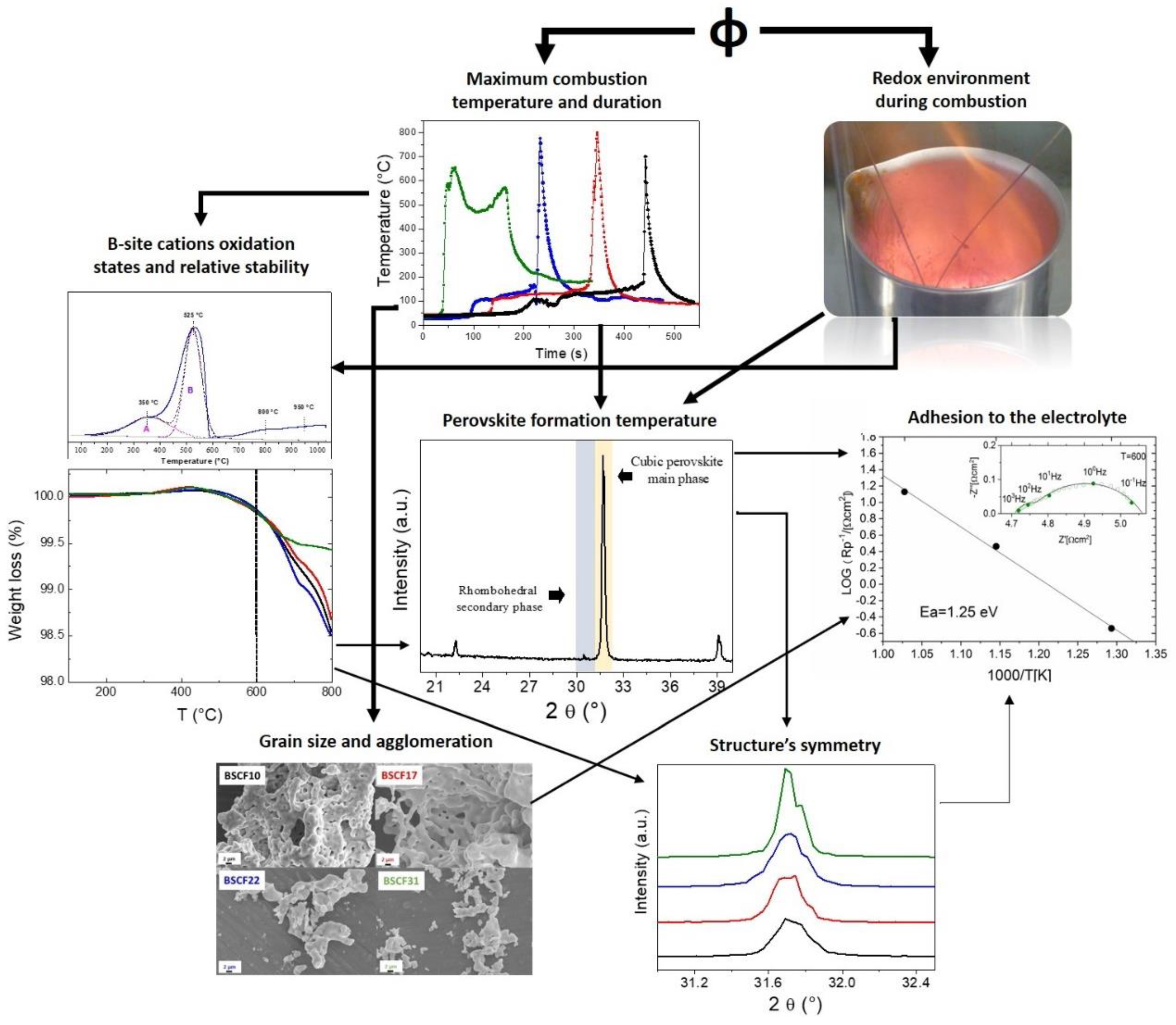
3. Discussion
4. Materials and Methods
4.1. Powders Preparation
4.2. Powder Characterization
4.2.1. Temperature/Time Profiles
4.2.2. X-Ray Powder Diffraction
4.2.3. Thermogravimetric Analysis
4.2.4. Temperature Programmed Reductions
4.2.5. N2 Adsorption Measurements
4.2.6. Scanning Electron Microscopy
4.3. Half-Cell Preparation
4.4. Electrochemical Characterization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arul, N.S.; Nithya, V.D. (Eds.) Revolution of Perovskite; Materials Horizons: From Nature to Nanomaterials; Springer: Singapore, 2020; ISBN 978-981-15-1266-7. [Google Scholar]
- Abdalla, A.M.; Hossain, S.; Azad, A.T.; Petra, P.M.I.; Begum, F.; Eriksson, S.G.; Azad, A.K. Nanomaterials for solid oxide fuel cells: A review. Renew. Sustain. Energy Rev. 2018, 82, 353–368. [Google Scholar] [CrossRef]
- Zhou, W.; Ran, R.; Shao, Z. Progress in understanding and development of Ba0.5Sr0.5Co0.8Fe0.2O3−δ-based cathodes for intermediate-temperature solid-oxide fuel cells: A review. J. Power Sources 2009, 192, 231–246. [Google Scholar] [CrossRef]
- Adler, S.B. Factors governing oxygen reduction in solid oxide fuel cell cathodes. Chem. Rev. 2004, 104, 4791–4843. [Google Scholar] [CrossRef]
- Yang, G.; Su, C.; Shi, H.; Zhu, Y.; Song, Y.; Zhou, W.; Shao, Z. Toward Reducing the Operation Temperature of Solid Oxide Fuel Cells: Our Past 15 Years of Efforts in Cathode Development. Energy Fuels 2020, in press. [Google Scholar] [CrossRef]
- Mcintosh, S.; Vente, J.; Haije, W.; Blank, D.; Bouwmeester, H. Structure and oxygen stoichiometry of SrCo0.8Fe0.2O3−δ and Ba0.5Sr0.5Co0.8Fe0.2O3−δ. Solid State Ionics 2006, 177, 1737–1742. [Google Scholar] [CrossRef]
- Jun, A.; Kim, J.; Shin, J.; Kim, G. Perovskite as a Cathode Material: A Review of its Role in Solid-Oxide Fuel Cell Technology. ChemElectroChem 2016, 3, 511–530. [Google Scholar] [CrossRef]
- Shao, Z. Investigation of the permeation behavior and stability of a Ba0.5Sr0.5Co0.8Fe0.2O3−δ oxygen membrane. J. Membr. Sci. 2000, 172, 177–188. [Google Scholar] [CrossRef]
- Vente, J.F.; McIntosh, S.; Haije, W.G.; Bouwmeester, H.J.M. Properties and performance of BaxSr1−xCo0.8Fe0.2O3−δ materials for oxygen transport membranes. J. Solid State Electrochem. 2006, 10, 581–588. [Google Scholar] [CrossRef]
- Shao, Z.; Haile, S.M. A high-performance cathode for the next generation of solid-oxide fuel cells. In Materials for Sustainable Energy; Macmillan Publishers Ltd. (Irading as Nature Publishing Group): London, UK; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2010; Volume 3, pp. 255–258. ISBN 9789814317665. [Google Scholar]
- Chen, F.; Xue, L.; Shang, Z.; Zhang, Z.; Chen, D. An enhanced non-noble perovskite-based oxygen electrocatalyst for efficient oxygen reduction and evolution reactions. J. Solid State Chem. 2020, 282, 121119. [Google Scholar] [CrossRef]
- Dai, Y.; Yu, J.; Cheng, C.; Tan, P.; Ni, M. Mini-review of perovskite oxides as oxygen electrocatalysts for rechargeable zinc–air batteries. Chem. Eng. J. 2020, 397, 125516. [Google Scholar] [CrossRef]
- Fabbri, E.; Nachtegaal, M.; Cheng, X.; Schmidt, T.J. Superior Bifunctional Electrocatalytic Activity of Ba0.5Sr0.5Co0.8Fe0.2O3–δ/Carbon Composite Electrodes: Insight into the Local Electronic Structure. Adv. Energy Mater. 2015, 5, 1402033. [Google Scholar] [CrossRef]
- Carpanese, M.P.; Clematis, D.; Viviani, M.; Presto, S.; Cerisola, G.; Panizza, M.; Delucchi, M. A Comprehensive Approach to Improve Performance and Stability of State-of-the-Art Air Electrodes for Intermediate Temperature Reversible Cells: An Impedance Spectroscopy Analysis. Bulg. Chem. Commun. 2018, 50, 39–47. [Google Scholar]
- Kannan, R.; Singh, K.; Gill, S.; Fürstenhaupt, T.; Thangadurai, V. Chemically Stable Proton Conducting Doped BaCeO3 -No More Fear to SOFC Wastes. Sci. Rep. 2013, 3, 2138. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Kannan, R.; Thangadurai, V. Perspective of perovskite-type oxides for proton conducting solid oxide fuel cells. Solid State Ionics 2019, 339, 114951. [Google Scholar] [CrossRef]
- Xu, X.; Wang, H.; Fronzi, M.; Wang, X.; Bi, L.; Traversa, E. Tailoring cations in a perovskite cathode for proton-conducting solid oxide fuel cells with high performance. J. Mater. Chem. A 2019, 7, 20624–20632. [Google Scholar] [CrossRef]
- Müller, P.; Störmer, H.; Meffert, M.; Dieterle, L.; Niedrig, C.; Wagner, S.F.; Ivers-Tiffée, E.; Gerthsen, D. Secondary Phase Formation in Ba0.5Sr0.5Co0.8Fe0.2O3–d Studied by Electron Microscopy. Chem. Mater. 2013, 25, 564–573. [Google Scholar] [CrossRef]
- Giuliano, A.; Carpanese, M.P.; Clematis, D.; Boaro, M.; Pappacena, A.; Deganello, F.; Liotta, L.F.; Barbucci, A. Infiltration, Overpotential and Ageing Effects on Cathodes for Solid Oxide Fuel Cells: La0.6Sr0.4Co0.2Fe0.8O3-δ versus Ba0.5Sr0.5Co0.8Fe0.2O3-δ. J. Electrochem. Soc. 2017, 164, F3114–F3122. [Google Scholar] [CrossRef]
- Wang, F.; Nakamura, T.; Yashiro, K.; Mizusaki, J.; Amezawa, K. The crystal structure, oxygen nonstoichiometry and chemical stability of Ba0.5Sr0.5Co0.8Fe0.2O3−δ (BSCF). Phys. Chem. Chem. Phys. 2014, 16, 7307. [Google Scholar] [CrossRef]
- Švarcová, S.; Wiik, K.; Tolchard, J.; Bouwmeester, H.J.M.; Grande, T. Structural instability of cubic perovskite BaxSr1−xCo1−yFeyO3−δ. Solid State Ionics 2008, 178, 1787–1791. [Google Scholar] [CrossRef]
- Zeljkovic, S.; Ivas, T.; Vaucher, S.; Jelic, D.; Gauckler, L. The changes of Ba0.5Sr0.5Co0.8Fe0.2O3-δ perovskite oxide on heating in oxygen and carbon dioxide atmospheres. J. Serbian Chem. Soc. 2014, 79, 1141–1154. [Google Scholar] [CrossRef]
- Deganello, F.; Liotta, L.F.; Marcì, G.; Fabbri, E.; Traversa, E. Strontium and iron-doped barium cobaltite prepared by solution combustion synthesis: Exploring a mixed-fuel approach for tailored intermediate temperature solid oxide fuel cell cathode materials. Mater. Renew. Sustain. Energy 2013, 2, 8. [Google Scholar] [CrossRef][Green Version]
- Zhao, Z.; Liu, L.; Zhang, X.; Tu, B.; Ou, D.; Cheng, M. Carbonates formed during BSCF preparation and their effects on performance of SOFCs with BSCF cathode. Int. J. Hydrog. Energy 2012, 37, 19036–19044. [Google Scholar] [CrossRef]
- Unger, L.-S.; Ruhl, R.; Meffert, M.; Niedrig, C.; Menesklou, W.; Wagner, S.F.; Gerthsen, D.; Bouwmeester, H.J.M.; Ivers-Tiffée, E. Yttrium doping of Ba0.5Sr0.5Co0.8Fe0.2O3–δ part II: Influence on oxygen transport and phase stability. J. Eur. Ceram. Soc. 2018, 38, 2388–2395. [Google Scholar] [CrossRef]
- Kim, J.; Choi, S.; Jun, A.; Jeong, H.Y.; Shin, J.; Kim, G. Chemically Stable Perovskites as Cathode Materials for Solid Oxide Fuel Cells: La-Doped Ba0.5Sr0.5Co0.8Fe0.2O3−δ. ChemSusChem 2014, 7, 1669–1675. [Google Scholar] [CrossRef]
- Abubaker, O.A.; Singh, K.; Thangadurai, V. Investigating the effect of Cu-doping on the electrochemical properties of perovskite-type Ba0.5Sr0.5Fe1-xCuxO3-δ (0 ≤ x ≤ 0.20) cathodes. J. Power Sources 2020, 451, 227777. [Google Scholar] [CrossRef]
- Clematis, D.; Barbucci, A.; Presto, S.; Viviani, M.; Carpanese, M.P. Electrocatalytic activity of perovskite-based cathodes for solid oxide fuel cells. Int. J. Hydrog. Energy 2019, 44, 6212–6222. [Google Scholar] [CrossRef]
- Giuliano, A.; Carpanese, M.P.; Panizza, M.; Cerisola, G.; Clematis, D.; Barbucci, A. Characterisation of La0.6Sr0.4Co0.2Fe0.8O3-δ—Ba0.5Sr0.5Co0.8Fe0.2O3-δ composite as cathode for solid oxide fuel cells. Electrochim. Acta 2017, 240, 258–266. [Google Scholar] [CrossRef]
- Bertei, A.; Barbucci, A.; Carpanese, M.P.; Viviani, M.; Nicolella, C. Morphological and electrochemical modeling of SOFC composite cathodes with distributed porosity. Chem. Eng. J. 2012, 207–208, 167–174. [Google Scholar] [CrossRef]
- Nicolella, C.; Bertei, A.; Viviani, M.; Barbucci, A. Morphology and electrochemical activity of SOFC composite cathodes: II. Mathematical modelling. J. Appl. Electrochem. 2009, 39, 503–511. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhang, X.; Wang, W.; Zhang, D.; Jiang, Y.; Zhou, X.; Lin, B. A Zn-Doped Ba0.5Sr0.5Co0.8Fe0.2O3-δ Perovskite Cathode with Enhanced ORR Catalytic Activity for SOFCs. Catalysts 2020, 10, 235. [Google Scholar] [CrossRef]
- Zhu, X.; Xia, H.; Li, Y.; Lü, Z. A (La,Sr)MnO3 nano-film embedded into (Ba,Sr)(Co,Fe)O3 porous cathode for stability enhancement. Mater. Lett. 2015, 161, 549–553. [Google Scholar] [CrossRef]
- Souza, D.F.; Nunes, E.H.M.; Vasconcelos, W.L. Preparation of Ba0.5Sr0.5Co0.8Fe0.2O3−δ asymmetric structures by freeze-casting and dip-coating. Ceram. Int. 2018, 44, 1002–1006. [Google Scholar] [CrossRef]
- Choi, J.; Park, I.; Lee, H.; Shin, D. Effect of enhanced reaction area in double layered Ba0.5Sr0.5Co0.8Fe0.2O3−δ cathode for intermediate temperature solid oxide fuel cells. Solid State Ionics 2012, 216, 54–57. [Google Scholar] [CrossRef]
- Habiballah, A.S.; Osman, N.; Md Jani, A.M. Microstructural investigation of BSCF-based cathode material for enhanced oxygen reduction reaction performance and electrode stability. Ceram. Int. 2020, 46, 23262–23265. [Google Scholar] [CrossRef]
- Liu, J.; Bao, H.; Zhang, B.; Hua, Q.; Shang, M.; Wang, J.; Jiang, L. Geometric Occupancy and Oxidation State Requirements of Cations in Cobalt Oxides for Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2019, 11, 12525–12534. [Google Scholar] [CrossRef] [PubMed]
- Aegerter, D.; Borlaf, M.; Fabbri, E.; Clark, A.H.; Nachtegaal, M.; Graule, T.; Schmidt, T.J. Tuning the Co Oxidation State in Ba0.5Sr0.5Co0.8Fe0.2O3-δ by Flame Spray Synthesis Towards High Oxygen Evolution Reaction Activity. Catalysts 2020, 10, 984. [Google Scholar] [CrossRef]
- Deganello, F.; Tyagi, A.K. Solution combustion synthesis, energy and environment: Best parameters for better materials. Prog. Cryst. Growth Charact. Mater. 2018, 64, 23–61. [Google Scholar] [CrossRef]
- Varma, A.; Mukasyan, A.S.; Rogachev, A.S.; Manukyan, K.V. Solution Combustion Synthesis of Nanoscale Materials. Chem. Rev. 2016, 116, 14493–14586. [Google Scholar] [CrossRef]
- Deganello, F. Nanomaterials for environmental and energy applications prepared by solution combustion based-methodologies: Role of the fuel. Mater. Today Proc. 2017, 4, 5507–5516. [Google Scholar] [CrossRef]
- Ashok, A.; Kumar, A.; Bhosale, R.R.; Almomani, F.; Saleh Saad, M.A.H.; Suslov, S.; Tarlochan, F. Influence of fuel ratio on the performance of combustion synthesized bifunctional cobalt oxide catalysts for fuel cell application. Int. J. Hydrog. Energy 2019, 44, 436–445. [Google Scholar] [CrossRef]
- Kermani, F.; Mollazadeh, S.; Vahdati Khaki, J. A simple thermodynamics model for estimation and comparison the concentration of oxygen vacancies generated in oxide powders synthesized via the solution combustion method. Ceram. Int. 2019, 45, 13496–13501. [Google Scholar] [CrossRef]
- Xu, X.; Pan, Y.; Zhou, W.; Chen, Y.; Zhang, Z.; Shao, Z. Toward Enhanced Oxygen Evolution on Perovskite Oxides Synthesized from Different Approaches: A Case Study of Ba0.5Sr0.5Co0.8Fe0.2O3−δ. Electrochim. Acta 2016, 219, 553–559. [Google Scholar] [CrossRef]
- Nuernberg, R.B.; Morelli, M.R. Synthesis of BSCF perovskites using a microwave-assisted combustion method. Ceram. Int. 2016, 42, 4204–4211. [Google Scholar] [CrossRef]
- Ail, S.S.; Benedetti, V.; Baratieri, M.; Dasappa, S. Fuel-Rich Combustion Synthesized Co/Al2O3 Catalysts for Wax and Liquid Fuel Production via Fischer-Tropsch Reaction. Ind. Eng. Chem. Res. 2018, 57, 3833–3843. [Google Scholar] [CrossRef]
- Afshari, M.; Rouhani Isfahani, A.; Hasani, S.; Davar, F.; Jahanbani Ardakani, K. Effect of apple cider vinegar agent on the microstructure, phase evolution, and magnetic properties of CoFe2O4 magnetic nanoparticles. Int. J. Appl. Ceram. Technol. 2019, 16, 1612–1621. [Google Scholar] [CrossRef]
- Abu-Zied, B.M. Controlled synthesis of praseodymium oxide nanoparticles obtained by combustion route: Effect of calcination temperature and fuel to oxidizer ratio. Appl. Surf. Sci. 2019, 471, 246–255. [Google Scholar] [CrossRef]
- Ashok, A.; Kumar, A.; Bhosale, R.R.; Almomani, F.; Malik, S.S.; Suslov, S.; Tarlochan, F. Combustion synthesis of bifunctional LaMO3 (M = Cr, Mn, Fe, Co, Ni) perovskites for oxygen reduction and oxygen evolution reaction in alkaline media. J. Electroanal. Chem. 2018, 809, 22–30. [Google Scholar] [CrossRef]
- Shimna, T.; Thomas, J.; Joseph, T.; Thomas, T.; Thomas, N. Porosity tuned NiO nanocrystallites by solution combustion synthesis for the development of a voltammetric sensor for dopamine. J. Porous Mater. 2019, 26, 1455–1463. [Google Scholar] [CrossRef]
- da Conceição, L.; Ribeiro, N.F.P.; Furtado, J.G.M.; Souza, M.M.V.M. Effect of propellant on the combustion synthesized Sr-doped LaMnO3 powders. Ceram. Int. 2009, 35, 1683–1687. [Google Scholar] [CrossRef]
- Jamale, A.P.; Shanmugam, S.; Bhosale, C.H.; Jadhav, L.D. Physiochemical properties of combustion synthesized La0.6Sr0.4Co0.8Fe0.2O3−δ perovskite: A role of fuel to oxidant ratio. Mater. Sci. Semicond. Process. 2015, 40, 855–860. [Google Scholar] [CrossRef]
- Aali, H.; Mollazadeh, S.; Khaki, J.V. Quantitative evaluation of ambient O2 interference during solution combustion synthesis process:considering iron nitrate—Fuel system. Ceram. Int. 2019, 45, 17775–17783. [Google Scholar] [CrossRef]
- Xu, C.; Manukyan, K.V.; Adams, R.A.; Pol, V.G.; Chen, P.; Varma, A. One-step solution combustion synthesis of CuO/Cu2O/C anode for long cycle life Li-ion batteries. Carbon N. Y. 2019, 142, 51–59. [Google Scholar] [CrossRef]
- Singh, G.; Tiwari, V.S.; Tiwari, P.; Srivastava, A.K.; Gupta, P.K. Effect of oxidant-to-fuel ratios on phase formation of PLZT powder; prepared by gel-combustion. J. Alloys Compd. 2011, 509, 4127–4131. [Google Scholar] [CrossRef]
- Ehi-Eromosele, C.O.; Ita, B.I.; Iweala, E.E.J.; Ogunniran, K.O.; Adekoya, J.A.; Ehi-Eromosele, F.E. Structural and magnetic characterization of La0.7Sr0.3MnO3 nanoparticles obtained by the citrate-gel combustion method: Effect of fuel to oxidizer ratio. Ceram. Int. 2016, 42, 636–643. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Y. Ba0.5Sr0.5Co0.8Fe0.2O3 nanopowders prepared by glycine–nitrate process for solid oxide fuel cell cathode. J. Alloys Compd. 2008, 453, 418–422. [Google Scholar] [CrossRef]
- Mukasyan, A.S.; Dinka, P. Novel approaches to solution-combustion synthesis of nanomaterials. Int. J. Self Propag. High Temp. Synth. 2007, 16, 23–35. [Google Scholar] [CrossRef]
- Yusoff, F.; Aziz, A.; Mohamed, N.; Ab Ghani, S. Synthesis and characterizations of BSCF at different ph as future cathode materials for fuel cell. Int. J. Electrochem. Sci. 2013, 8, 10672–10687. [Google Scholar]
- Kim, Y.-M.; Kim-Lohsoontorn, P.; Bae, J.-M. Characterization and Electrochemical Performance of Composite BSCF Cathode for Intermediate-temperature Solid Oxide Fuel Cell. J. Electrochem. Sci. Technol. 2011, 2, 32–38. [Google Scholar] [CrossRef]
- Yaremchenko, A.A.; Patrakeev, M.V.; Naumovich, E.N.; Khalyavin, D.D. The p (O2)–T stability domain of cubic perovskite Ba0.5Sr0.5Co0.8Fe0.2O3−δ. Phys. Chem. Chem. Phys. 2018, 20, 4442–4454. [Google Scholar] [CrossRef]
- Sahini, M.G.; Tolchard, J.R.; Wiik, K.; Grande, T. High temperature X-ray diffraction and thermo-gravimetrical analysis of the cubic perovskite Ba0.5Sr0.5Co0.8Fe0.2O3−δ under different atmospheres. Dalton Trans. 2015, 44, 10875–10881. [Google Scholar] [CrossRef]
- Deganello, F.; Marcì, G.; Deganello, G. Citrate–nitrate auto-combustion synthesis of perovskite-type nanopowders: A systematic approach. J. Eur. Ceram. Soc. 2009, 29, 439–450. [Google Scholar] [CrossRef]
- Tummino, M.L.; Liotta, L.F.; Magnacca, G.; Lo Faro, M.; Trocino, S.; Campagna Zignani, S.; Aricò, A.S.; Deganello, F. Sucrose-Assisted Solution Combustion Synthesis of Doped Strontium Ferrate Perovskite-Type Electrocatalysts: Primary Role of the Secondary Fuel. Catalysts 2020, 10, 134. [Google Scholar] [CrossRef]
- Puleo, F.; Liotta, L.F.; Parola, V.; La Banerjee, D.; Martorana, A.; Longo, A. Palladium local structure of La1−xSrxCo1−yFey−0.03Pd0.03O3−δ perovskites synthesized using a one pot citrate method. Phys. Chem. Chem. Phys. 2014, 16, 22677–22686. [Google Scholar] [CrossRef] [PubMed]
- Fino, D.; Russo, N.; Saracco, G.; Specchia, V. The role of suprafacial oxygen in some perovskites for the catalytic combustion of soot. J. Catal. 2003, 217, 367–375. [Google Scholar] [CrossRef]
- Liotta, L.F.; Ousmane, M.; Di Carlo, G.; Pantaleo, G.; Deganello, G.; Marcì, G.; Retailleau, L.; Giroir-Fendler, A. Total oxidation of propene at low temperature over Co3O4–CeO2 mixed oxides: Role of surface oxygen vacancies and bulk oxygen mobility in the catalytic activity. Appl. Catal. A Gen. 2008, 347, 81–88. [Google Scholar] [CrossRef]
- Aliotta, C.; Liotta, L.F.; La Parola, V.; Martorana, A.; Muccillo, E.N.; Muccillo, R.; Deganello, F. Ceria-based electrolytes prepared by solution combustion synthesis: The role of fuel on the materials properties. Appl. Catal. B Environ. 2016, 197, 14–22. [Google Scholar] [CrossRef]
- Lee, S.; Lim, Y.; Lee, E.A.; Hwang, H.J.; Moon, J.-W. Ba0.5Sr0.5Co0.8Fe0.2O3−δ (BSCF) and La0.6Ba0.4Co0.2Fe0.8O3−δ (LBCF) cathodes prepared by combined citrate-EDTA method for IT-SOFCs. J. Power Sources 2006, 157, 848–854. [Google Scholar] [CrossRef]
- Chen, C.-H.; Chang, C.-L.; Hwang, B.-H. Electrochemical and microstructure characteristics of Ba0.5Sr0.5Co0.8Fe0.2O3−δ (BSCF) cathodes prepared by citrate precursor method for SOFCs. Mater. Chem. Phys. 2009, 115, 478–482. [Google Scholar] [CrossRef]
- Tafaroji, S.; Farbod, M.; Kazeminezhad, I.; Kheirmand, M. Effect of pre-sintering temperature and ball-milling on the conductivity of Ba0.5Sr0.5Co0.8Fe0.2O3−δ as a cathode for solid oxide fuel cells prepared by sol-gel thermolysis method. Mater. Res. Express 2019, 6, 095522. [Google Scholar] [CrossRef]
- Hieu, N.T.; Park, J.; Tae, B. Synthesis and characterization of nanofiber-structured Ba0.5Sr0.5Co0.8Fe0.2O3−δ perovskite oxide used as a cathode material for low-temperature solid oxide fuel cells. Mater. Sci. Eng. B 2012, 177, 205–209. [Google Scholar] [CrossRef]
- Almar, L.; Störmer, H.; Meffert, M.; Szász, J.; Wankmüller, F.; Gerthsen, D.; Ivers-Tiffée, E. Improved Phase Stability and CO2 Poisoning Robustness of Y-Doped Ba0.5Sr0.5Co0.8Fe0.2O3−δ SOFC Cathodes at Intermediate Temperatures. ACS Appl. Energy Mater. 2018, 1, 1316–1327. [Google Scholar] [CrossRef]
- Clematis, D.; Presto, S.; Carpanese, M.P.; Barbucci, A.; Deganello, F.; Liotta, L.F.; Aliotta, C.; Viviani, M. Distribution of Relaxation Times and Equivalent Circuits Analysis of Ba0.5Sr0.5Co0.8Fe0.2O3−δ. Catalysts 2019, 9, 441. [Google Scholar] [CrossRef]
- Yamada, I.; Odake, T.; Asai, K.; Oka, K.; Kawaguchi, S.; Wada, K.; Yagi, S. High-pressure synthesis of highly oxidized Ba0.5Sr0.5Co0.8Fe0.2O3−δ cubic perovskite. Mater. Chem. Front. 2019, 3, 1209–1217. [Google Scholar] [CrossRef]
- Meffert, M.; Unger, L.; Störmer, H.; Sigloch, F.; Wagner, S.F.; Ivers-Tiffée, E.; Gerthsen, D. The effect of B-site Y substitution on cubic phase stabilization in (Ba0.5Sr0.5) (Co0.8Fe0.2) O3−δ. J. Am. Ceram. Soc. 2019, 102, 4929–4942. [Google Scholar] [CrossRef]
- Lee, S.; Lee, K.; Kang, S.; Kang, J.; Song, S.; Bae, J. Investigation of electrospun Ba0.5Sr0.5Co0.8Fe0.2O3−δ-Gd0.1Ce0.9O1.95 cathodes for enhanced interfacial adhesion. Int. J. Hydrog. Energy 2018, 43, 21535–21546. [Google Scholar] [CrossRef]
- Patra, H.; Rout, S.K.; Pratihar, S.K.; Bhattacharya, S. Effect of process parameters on combined EDTA–citrate synthesis of Ba0.5Sr0.5Co0.8Fe0.2O3−δ perovskite. Powder Technol. 2011, 209, 98–104. [Google Scholar] [CrossRef]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Presto, S.; Artini, C.; Pani, M.; Carnasciali, M.M.; Massardo, S.; Viviani, M. Ionic conductivity and local structural features in Ce1−xSmxO2−x/2. Phys. Chem. Chem. Phys. 2018, 20, 28338–28345. [Google Scholar] [CrossRef]
- Raikova, G.; Carpanese, P.; Stoynov, Z.; Vladikova, D.; Viviani, M.; Barbucci, A. Inductance correction in impedance studies of solid oxide fuel cells. Bulg. Chem. Commun. 2009, 41, 199–206. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deganello, F.; Liotta, L.F.; Aliotta, C.; Barbucci, A.; Viviani, M.; Clematis, D.; Carpanese, M.P.; Presto, S. Clarifying the Role of the Reducers-to-Oxidizers Ratio in the Solution Combustion Synthesis of Ba0.5Sr0.5Co0.8Fe0.2O3-δ Oxygen Electrocatalysts. Catalysts 2020, 10, 1465. https://doi.org/10.3390/catal10121465
Deganello F, Liotta LF, Aliotta C, Barbucci A, Viviani M, Clematis D, Carpanese MP, Presto S. Clarifying the Role of the Reducers-to-Oxidizers Ratio in the Solution Combustion Synthesis of Ba0.5Sr0.5Co0.8Fe0.2O3-δ Oxygen Electrocatalysts. Catalysts. 2020; 10(12):1465. https://doi.org/10.3390/catal10121465
Chicago/Turabian StyleDeganello, Francesca, Leonarda F. Liotta, Chiara Aliotta, Antonio Barbucci, Massimo Viviani, Davide Clematis, Maria Paola Carpanese, and Sabrina Presto. 2020. "Clarifying the Role of the Reducers-to-Oxidizers Ratio in the Solution Combustion Synthesis of Ba0.5Sr0.5Co0.8Fe0.2O3-δ Oxygen Electrocatalysts" Catalysts 10, no. 12: 1465. https://doi.org/10.3390/catal10121465
APA StyleDeganello, F., Liotta, L. F., Aliotta, C., Barbucci, A., Viviani, M., Clematis, D., Carpanese, M. P., & Presto, S. (2020). Clarifying the Role of the Reducers-to-Oxidizers Ratio in the Solution Combustion Synthesis of Ba0.5Sr0.5Co0.8Fe0.2O3-δ Oxygen Electrocatalysts. Catalysts, 10(12), 1465. https://doi.org/10.3390/catal10121465