Effect of Synthesis Conditions of Nitrogen and Platinum Co-Doped Titania Films on the Photocatalytic Performance under Simulated Solar Light
Abstract
1. Introduction
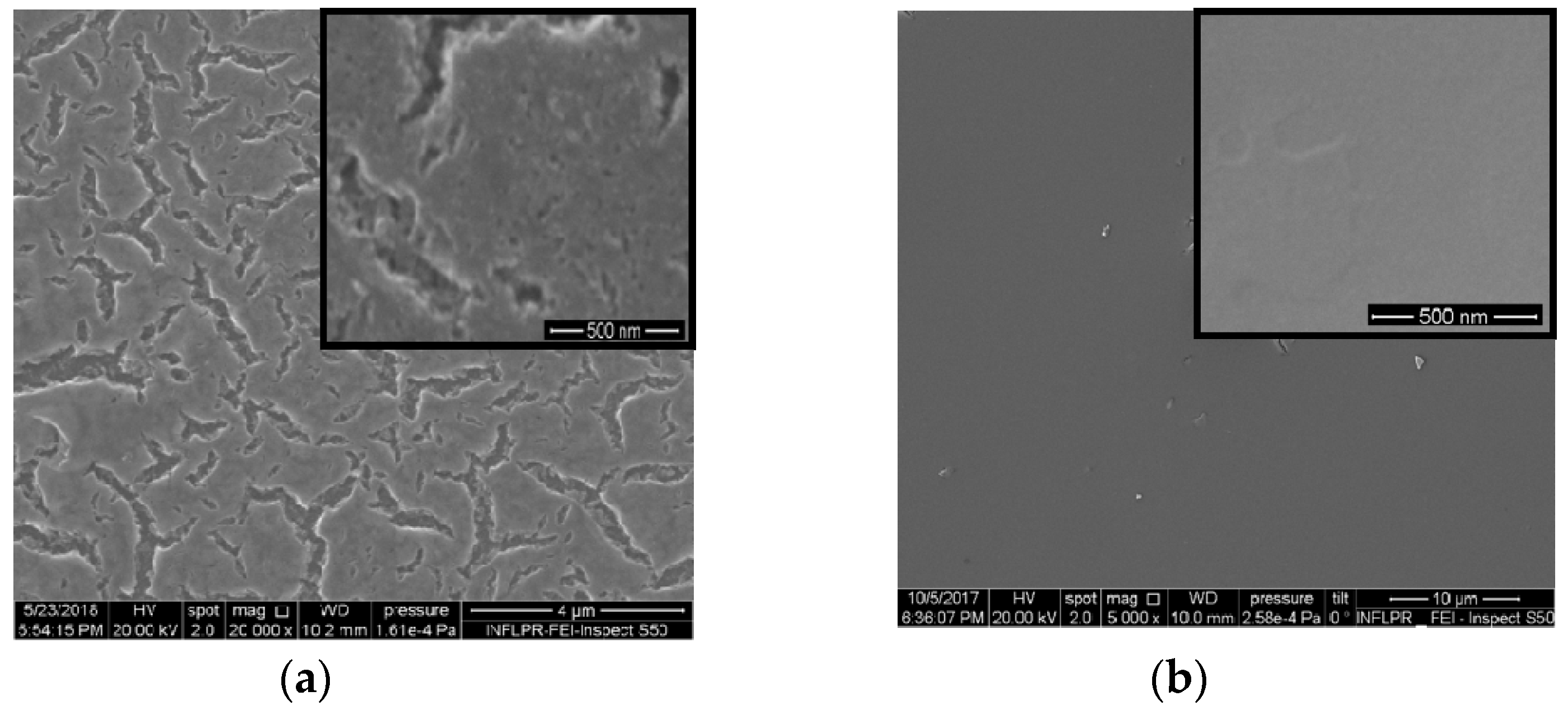
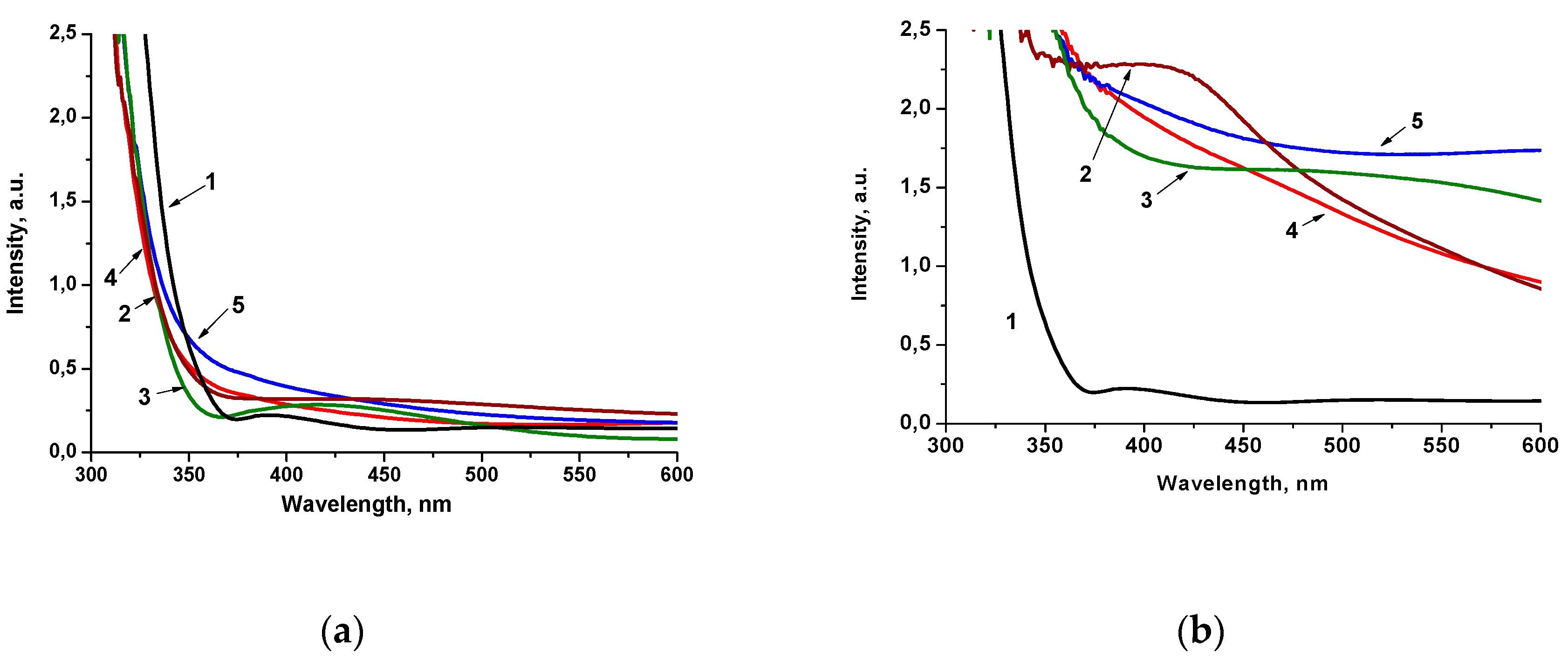
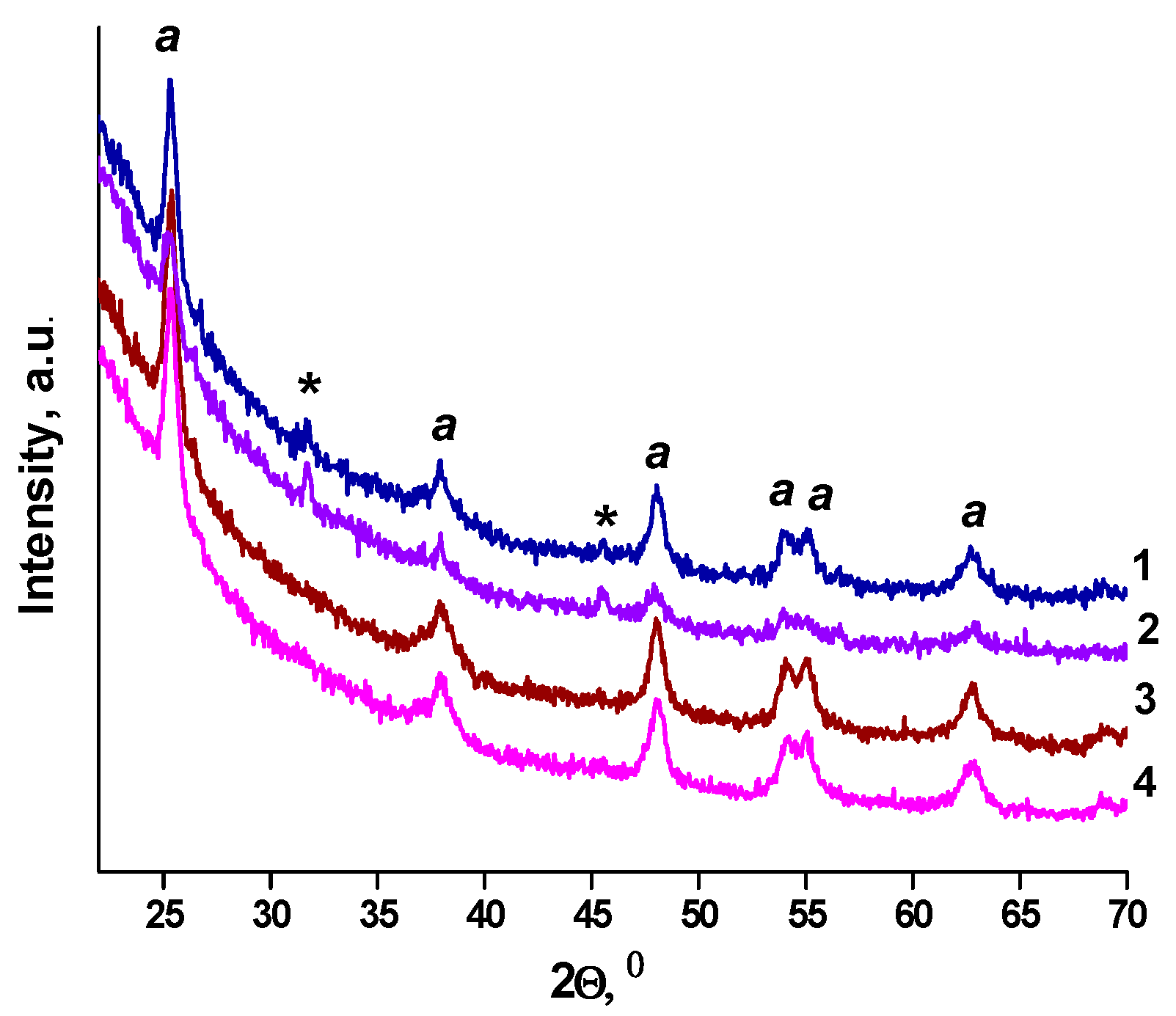
2. Results
3. Discussion
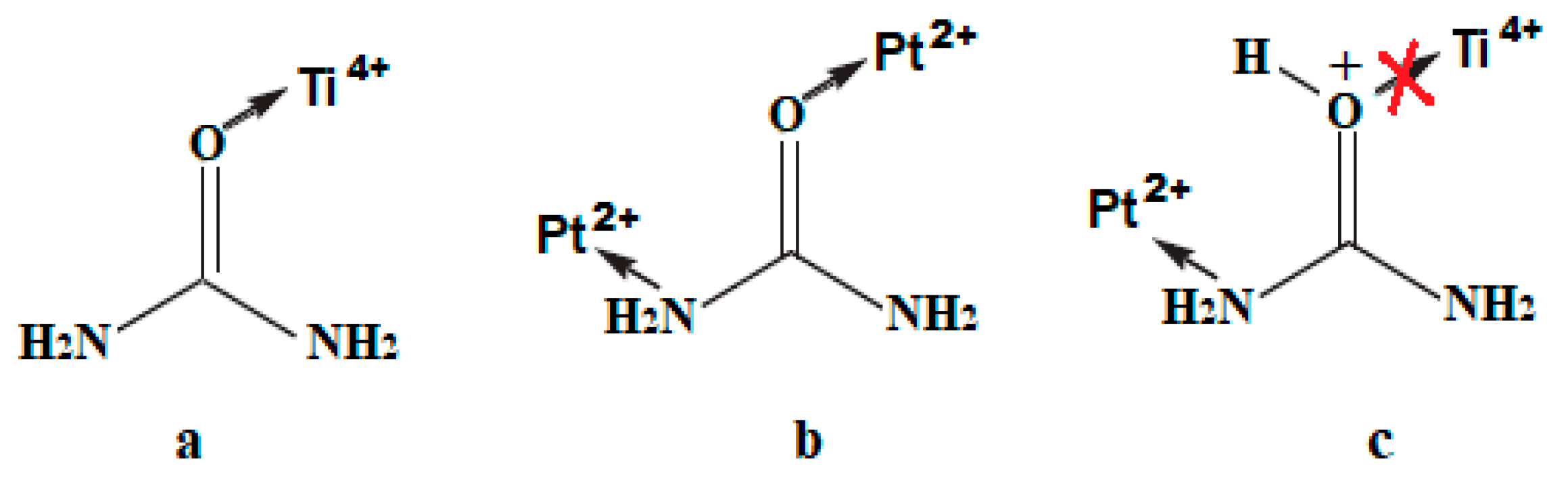
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kutsenko, V.Y.; Lopatina, Y.Y.; Bossard-Giannesini, L.; Marchenko, O.A.; Pluchery, O.; Snegir, S.V. Alkylthiol self-assembled monolayers on Au(111) with tailored tail groups for attaching gold nanoparticles. Nanotechnology 2017, 28, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ischenko, E.V.; Yatsimirsky, V.K.; Dyachenko, A.G.; Borysenko, M.V. Cu-Co-Fe oxide catalysts supported on carbon nanotubes in the reaction of CO oxidation. Pol. J. Chem. 2008, 82, 291–297. [Google Scholar]
- Laguta, I.; Stavinskaya, O.; Kazakova, O.; Fesenko, T.; Brychka, S. Green synthesis of silver nanoparticles using Stevia leaves extracts. Appl. Nanosci. 2019, 9, 755–765. [Google Scholar] [CrossRef]
- Vareda, J.P.; Valente, A.J.M.; Duraes, L. Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Mitoraj, D.; Kisch, H. The nature of nitrogen-modified titanium dioxide Photocatalysts active in visible light. Angew. Chem. Int. Ed. 2008, 47, 9975–9978. [Google Scholar] [CrossRef]
- Surówka, M.; Kobielusz, M.; Trochowski, M.; Buchalska, M.; Kruczała, K.; Broś, P.; Macyk, W. Iron and other metal species as phase-composition controllers influencing the photocatalytic activity of TiO2 materials. Appl. Catal. B: Environ. 2019, 247, 173–181. [Google Scholar] [CrossRef]
- Linnik, O.; Chorna, N.; Smirnova, N. Nonporous iron titanate thin films doped with nitrogen: Optical, structural and photocatalytic properties. Nanoscale Res. Lett. 2017, 12, 249–258. [Google Scholar] [CrossRef]
- Dolat, D.; Mozia, S.; Ohtani, B.; Morawski, A.W. Nitrogen, iron-single modified (N-TiO2, Fe-TiO2) and co-modified (Fe,N-TiO2) rutile titanium dioxide as visible-light active photocatalysts. Chem. Eng. J. 2013, 225, 358–364. [Google Scholar] [CrossRef]
- Chorna, N.; Smirnova, N.; Vorobets, V.; Kolbasov, G.; Linnik, O. Nitrogen doped iron titanate films: Photoelectrochemical, electrocatalytic, photocatalytic and structural features. Appl. Surf. Sci. 2019, 473, 343–351. [Google Scholar] [CrossRef]
- Kisch, H. Semiconductor Photocatalysis Principles and Application; Wiley-VCH Verlag GmbH&Co: Weinheim, Germany, 2015; pp. 55–68. [Google Scholar] [CrossRef]
- Sato, S. Photocatalytic activity of NOx-doped in the visible light region. Chem. Phys. Lett. 1986, 123, 126–128. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Socol, G.; Gnatyuk, Y.; Stefan, N.; Smirnova, N.; Djokić, V.; Sutan, C.; Malinovschi, V.; Stanculescu, A.; Korduban, O.; Mihailescu, I.N. Photocatalytic activity of pulsed laser deposited TiO2 thin films in N2, O2 and CH4. Thin Solid Films 2010, 518, 4648–4653. [Google Scholar] [CrossRef]
- Sakthivel, S.; Janczarek, M.; Kisch, H. Visible light activity and photoelectrochemical properties of nitrogen-doped TiO2. J. Phys. Chem. B 2004, 108, 19384–19387. [Google Scholar] [CrossRef]
- Tryba, B.; Wozniak, M.; Zolnierkiewicz, G.; Guskos, N.; Morawski, M.; Colbeau-Justin, C.; Wrobel, R.; Nitta, A.; Ohtani, B. Influence of an electronic structure of N-TiO2 on its photocatalytic activity towards decomposition of acetaldehyde under UV and fluorescent lamps irradiation. Catalysts 2018, 8, 85. [Google Scholar] [CrossRef]
- Sirivallop, A.; Areerob, T.; Chiarakorn, S. Enhanced visible light photocatalytic activity of N and Ag doped and co-doped TiO2 synthesized by using an in-situ solvothermal method for gas phase ammonia removal. Catalysts 2020, 10, 251. [Google Scholar] [CrossRef]
- Somekawa, S.; Kusumoto, Y.; Ikeda, M.; Ahmmad, B.; Horie, Y. Fabrication of N-doped TiO2 thin films by laser ablation method: Mechanism of N-doping and evaluation of the thin films. Catal. Commun. 2008, 9, 437–440. [Google Scholar] [CrossRef]
- Pandian, R.; Natarajan, G.; Dhaipule, K.N.G.; Prasad, A.K.; Kamruddin, M.; Tyagi, A.K. Types of nitrogen incorporation in reactively sputtered titania thin films: Influence on UV–visible, photocatalytic and photoconduction properties. Thin Solid Films 2016, 616, 466–476. [Google Scholar] [CrossRef]
- Saha, N.C.; Tompkins, H.G. Titanium nitride oxidation chemistry: An x-ray photoelectron spectroscopy study. J. Appl. Phys. 1992, 72, 3072–3079. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T. Nitrogen complex species and its chemical nature in TiO2 for visible-light sensitized photocatalysis. Chem. Phys. 2007, 339, 57–63. [Google Scholar] [CrossRef]
- Di Valentin, C.; Pacchioni, G.; Selloni, A.; Livraghi, S.; Giamello, E. Characterization of paramagnetic species in N-doped TiO2 powders by EPR spectroscopy and DFT calculations. J. Phys. Chem. B 2005, 109, 11414–11419. [Google Scholar] [CrossRef] [PubMed]
- Napoli, F.; Chiesa, M.; Livraghi, S.; Giamello, E.; Agnoli, S.; Granozzi, G.; Pacchioni, G.; Di Valentin, C. The nitrogen photoactive centre in N-doped titanium dioxide formed via interaction of N atoms with the solid. Nature and energy level of the species. Chem. Phys. Lett. 2009, 477, 135–138. [Google Scholar] [CrossRef]
- Wu, M.; Hiltunen, J.; Sápi, A.; Avila, A.; Larsson, W.; Liao, H.; Huuhtanen, M.; Tóth, G.; Shchukarev, A.; Laufer, N.; et al. Nitrogen-doped anatase nanofibers decorated with noble metal nanoparticles for photocatalytic production of hydrogen. ACS Nano 2011, 5, 5025–5030. [Google Scholar] [CrossRef]
- Wang, S.; Ding, Z.; Chang, X.; Xu, J.; Wang, D.-H. Modified nano-TiO2 based composites for environmental photocatalytic applications. Catalysts 2020, 10, 759. [Google Scholar] [CrossRef]
- Linnik, O.; Smirnova, N.; Korduban, O.; Eremenko, A. Gold nanoparticles into Ti1-xZnxO2 films: Synthesis, structure and application. Mater. Chem. Phys. 2013, 142, 318–324. [Google Scholar] [CrossRef]
- Ahmed, L.M.; Ivanova, I.; Hussein, F.H.; Bahnemann, D.W. Role of platinum deposited on TiO2 in photocatalytic methanol oxidation and dehydrogenation reactions. Int. J. Photoenergy 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Kim, S.; Hwang, S.-J.; Choi, W. Visible light active platinum-ion-doped TiO2 photocatalyst. J. Phys. Chem. B 2005, 109, 24260–24267. [Google Scholar] [CrossRef]
- Guayaquil-Sosa, J.F.; Calzada, A.; Serrano, B.; Escobedo, S.; de Lasa, H. Hydrogen production via water dissociation using Pt–TiO2 photocatalysts: An oxidation–reduction network. Catalysts 2017, 7, 324. [Google Scholar] [CrossRef]
- Higashimoto, S.; Katsuura, K.; Yamamoto, M.; Takahashi, M. Photocatalytic activity for decomposition of volatile organic compound on Pt-WO3 enhanced by simple physical mixing with TiO2. Catal. Commun. 2020, 133, 105831. [Google Scholar] [CrossRef]
- Tossi, C.; Hällström, L.; Selin, J.; Vaelma, M.; See, E.; Lahtinen, J.; Tittonen, I. Size- and density-controlled photodeposition of metallic platinum nanoparticles on titanium dioxide for photocatalytic applications. J. Mater. Chem. A 2019, 7, 14519–14525. [Google Scholar] [CrossRef]
- See, E.M.; Tossi, C.; Hällström, L.; Tittonen, I. Photodeposition of RuOx nanostructures on TiO2 films with a controllable morphology. ACS Omega 2020, 5, 10671–10679. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Choi, W. Photocatalytic reactivity of surface platinized TiO2: Substrate specificity and the effect of Pt oxidation state. J. Phys. Chem. B 2005, 109, 7399–7406. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhou, G.; Liu, S.; Ang, H.M.; Tadé, M.O.; Wang, S. Visible light responsive titania photocatalysts codoped by nitrogen and metal (Fe, Ni, Ag, or Pt) for remediation of aqueous pollutants. Chem. Eng. J. 2013, 231, 18–25. [Google Scholar] [CrossRef]
- Gaponenko, N.V.; Kortov, V.S.; Smirnova, N.P.; Orekhovskaya, T.I.; Nikolaenko, I.A.; Pustovarov, V.A.; Zvonarev, S.V.; Slesarev, A.I.; Linnik, O.P.; Zhukovskii, M.A.; et al. Sol–gel derived structures for optical design and photocatalytic application. Microelectron. Eng. 2012, 90, 131–137. [Google Scholar] [CrossRef]
- Kang, T.D.; Yoon, J.-G. Optical characterization of surface plasmon resonance of Pt nanoparticles in TiO2-SiO2 nanocomposite films. J. Appl. Phys. 2017, 122, 134302. [Google Scholar] [CrossRef]
- Beranek, R.; Kisch, H. Tuning the optical and photoelectrochemical properties of surface-modified TiO2. Photochem. Photobiol. Sci. 2008, 7, 40–48. [Google Scholar] [CrossRef]
- Mitoraj, D.; Kisch, H. On the mechanism of urea-induced titania modification. Chem. A Eur. J. 2010, 16, 261–269. [Google Scholar] [CrossRef]
- Linnik, O.; Khoroshko, L. Non-porous nitrogen and ruthenium co-doped titania films for photocatalysis. Int. J. Nanosci. 2019, 18, 1940043. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Long, J.; Gu, Q.; Ding, Z.; Fu, X. Nitrogen-doped titanium dioxide visible light photocatalyst: Spectroscopic identification of photoactive centers. J. Catal. 2010, 276, 201–214. [Google Scholar] [CrossRef]
- Linnik, O.; Petrik, I.; Smirnova, N.; Kandyba, V.; Korduban, O.; Eremenko, A.; Socol, G.; Stefan, N.; Ristoscu, C.; Mihailescu, I.N.; et al. TiO2/ZrO2 thin films synthesized by PLD in low pressure N-, C- and/or O-containing gases: Structural, optical and photocatalytic properties. Dig. J. Nanomater. Biostruct. 2012, 7, 1343–1352. [Google Scholar]
- Linnik, O.; Popescu-Pelin, G.; Stefan, N.; Chorna, N.; Smirnova, N.; Mihailescu, C.N.; Ristoscu, C.; Mihailescu, I.N. Investigation of iron doped TiO2 films synthesized in N2/CH4 via pulsed laser deposition technique. Appl. Nanosci. 2020. [Google Scholar] [CrossRef]
- Sakatani, Y.; Ando, H.; Okusako, K.; Koike, H. Metal ion and N co-doped TiO2 as a visible-light photocatalyst. J. Mater. Res. 2004, 19, 2100–2108. [Google Scholar] [CrossRef]
- Hao, H.; Zhang, J. The study of Iron (III) and Nitrogen co-doped mesoporous TiO2 photocatalysts: Synthesis, characterization and activity. Microporous Mesoporous Mater. 2009, 121, 52–57. [Google Scholar] [CrossRef]
- Bear, J.C.; Gomez, V.; Kefallinos, N.S.; Mcgettrick, J.D.; Barron, A.R.; Dunnill, C.W. Anatase/rutile bi-phasic titanium dioxide nanoparticles for photocatalytic applications enhanced by nitrogen doping and platinum. J. Colloid Interface Sci. 2015, 460, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Batalovic, K.; Bundaleski, N.; Radaković, J.; Abazović, N.; Mitrić, M.; Silva, R.A.; Savić, M.; Belošević-Čavor, J.; Rakočević, Z.; Rangel, C.M. Modification of N-doped TiO2 photocatalysts using noble Metals (Pt, Pd )—A combined XPS and DFT study. Phys. Chem. Chem. Phys. 2017, 19, 7062–7071. [Google Scholar] [CrossRef]
- Theophanides, T.; Harvey, P.D. Structural and spectroscopic of metal-urea complexes. Coord. Chem. Rev. 1987, 76, 237–264. [Google Scholar] [CrossRef]
- Woon, T.C.; Wickramasinghe, W.A.; Fairlie, D.P. Oxygen versus nitrogen coordination of a urea to (diethylenetriamine)platinum(II). Inorg. Chem. 1993, 32, 2190–2194. [Google Scholar] [CrossRef]
- Li, J.; Yang, X.; Ishigaki, T. Urea coordinated titanium trichloride Ti III [OC(NH)2]6Cl3: A single molecular precursor yielding highly visible light responsive TiO2 nanocrystallites. J. Phys. Chem. B 2006, 110, 14611–14618. [Google Scholar] [CrossRef]
- Schaber, P.M.; Colson, J.; Higgins, S.; Thielen, D.; Anspach, B.; Brauer, J. Thermal decomposition (pyrolysis) of urea in an open reaction vessel. Thermochim. Acta 2004, 424, 131–142. [Google Scholar] [CrossRef]
- Saeid, S.; Kråkström, M.; Tolvanen, P.; Kumar, N.; Eränen, K.; Mikkola, J.-P.; Kronberg, L.; Eklund, P.; Peurla, M.; Aho, A.; et al. Advanced oxidation process for degradation of carbamazepine from aqueous solution: Influence of metal modified microporous, mesoporous catalysts on the ozonation process. Catalysts 2020, 10, 90. [Google Scholar] [CrossRef]
- Garbarino, S.; Pereira, A.; Hamel, C.; Irissou, E.; Chaker, M.; Guay, D. Effect of size on the electrochemical stability of Pt nanoparticles deposited on gold substrate. J. Phys. Chem. C 2010, 114, 2980–2988. [Google Scholar] [CrossRef]
- Mosquera, A.; Horwat, D.; Vazquez, L.; Gutiérrez, A. Thermal decomposition and fractal properties of sputter-deposited platinum oxide thin films. J. Mater. Res. 2012, 27, 829–836. [Google Scholar] [CrossRef]
- Womes, M.; Lynch, J.; Bazin, D.; Le Peltier, F.; Morin, S.; Didillon, B. Interaction between Pt(acac)2 and alumina surfaces studied by XAS. Catal. Lett. 2003, 85, 25–31. [Google Scholar] [CrossRef]
- Filez, M.; Redekop, E.A.; Poelman, H.; Galvita, V.V.; Ramachandran, R.K.; Dendooven, J.; Detavernier, C.; Marin, G.B. Unravelling the formation of Pt−Ga alloyed nanoparticles on calcined Ga-modified hydrotalcites by in situ XAS. Chem. Mater. 2014, 26, 5936–5949. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV−Vis spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]


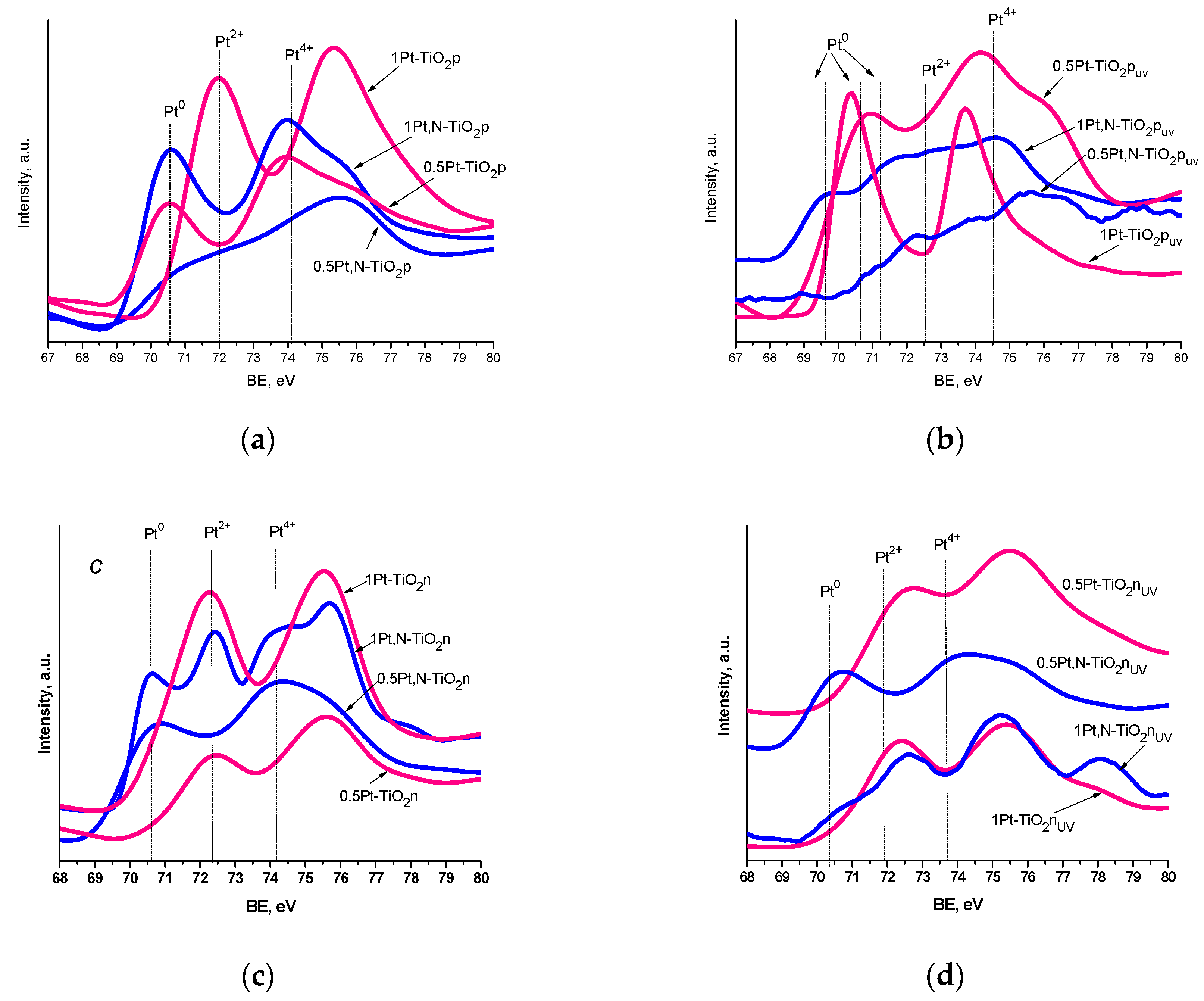

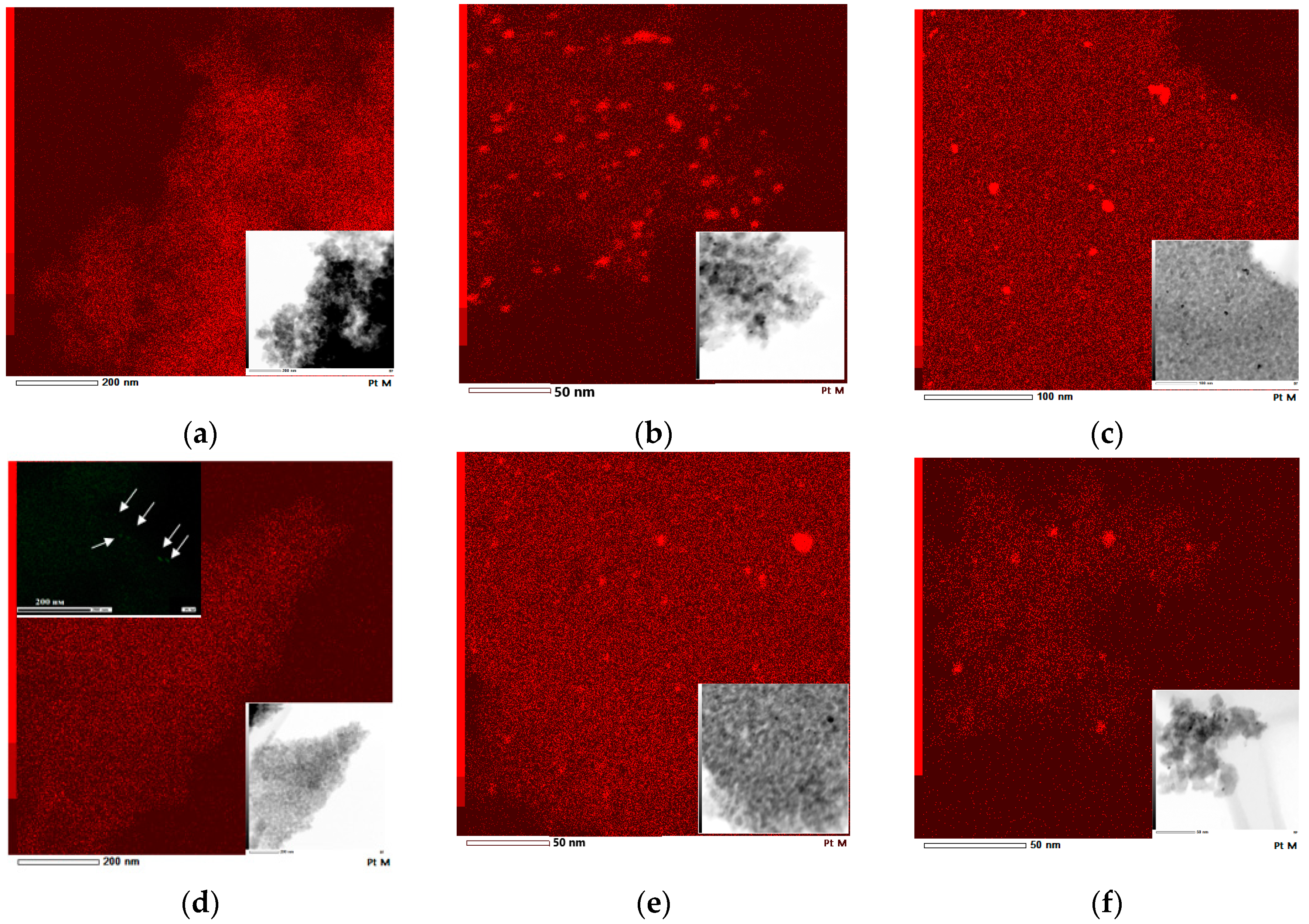

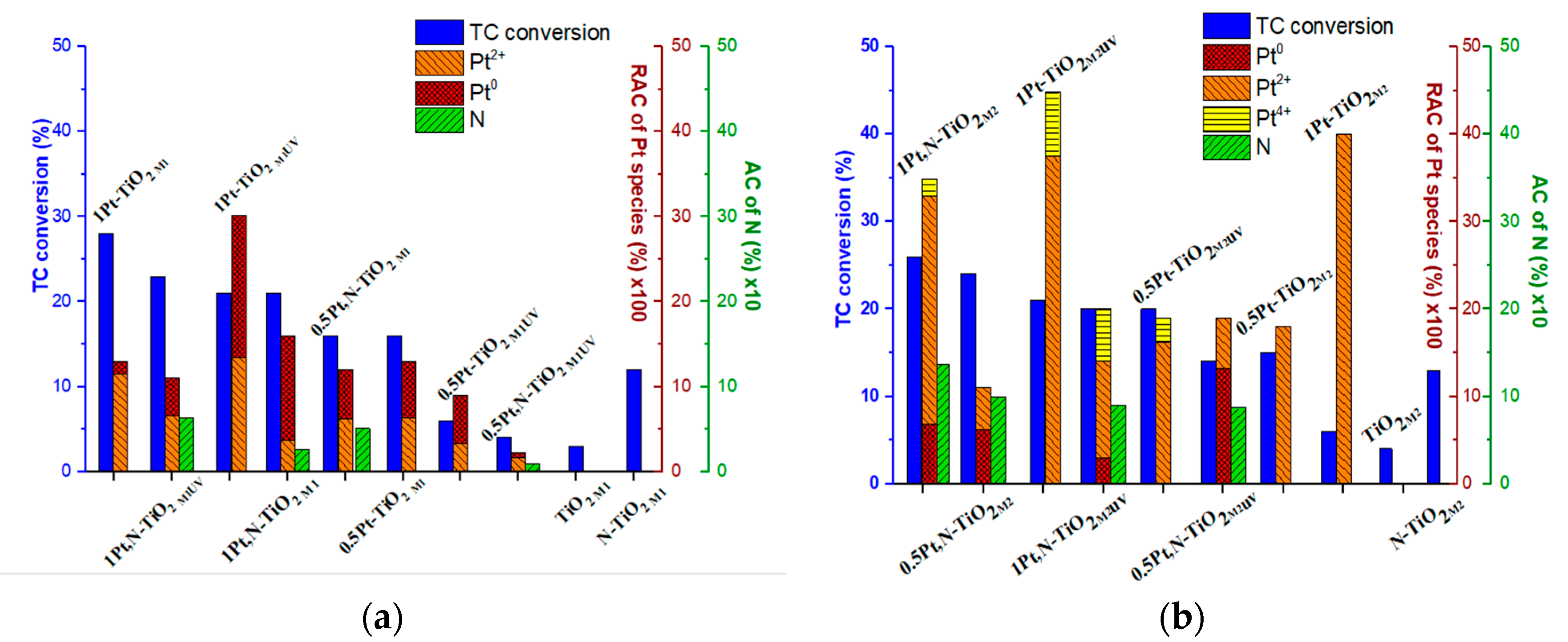
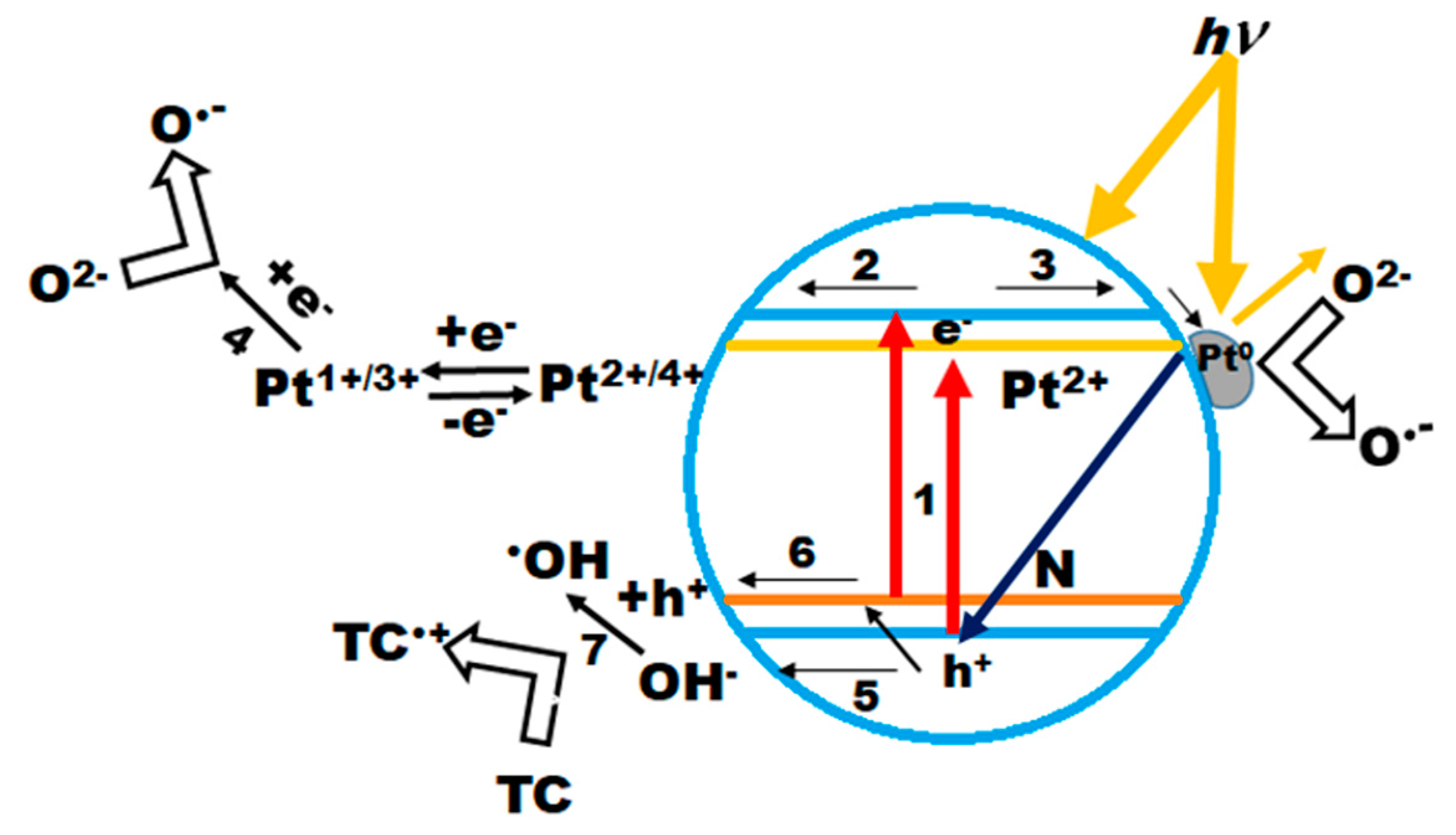
| Film | Method 1 | Method 2 |
|---|---|---|
| Ebg, eV | ||
| TiO2 | 3.7 | 3.6 |
| N-TiO2 | 3.8 | 3.6 |
| 0.5Pt-TiO2 | 3.5 | 3.6 |
| 0.5Pt-TiO2UV | 3.7 | 3.4 |
| 0.5Pt,N-TiO2 | 3.5 | 3.4 |
| 0.5Pt,N-TiO2UV | 3.5 | 3.4 |
| 1Pt-TiO2 | 3.5 | 3.5 |
| 1Pt-TiO2UV | 3.5 | 3.3 |
| 1Pt,N-TiO2 | 3.6 | 3.3 |
| 1Pt,N-TiO2UV | 3.6 | 3.2 |
| Film | Pt | N | Ti: | O | Pt | N | Ti | O |
|---|---|---|---|---|---|---|---|---|
| Method 1 | Method 2 | |||||||
| 0.5Pt-TiO2 | 0.004 | - | 1.000 | 2.285 | 0.006 | - | 1.000 | 2.248 |
| 0.5Pt,N-TiO2 | 0.004 | 0.017 | 1.000 | 2.288 | 0.004 | 0.032 | 1.000 | 2.163 |
| 0.5Pt-TiO2UV | 0.003 | - | 1.000 | 2.868 | 0.006 | - | 1.000 | 2.463 |
| 0.5Pt,N-TiO2UV | 0.001 | 0.004 | 1.000 | 3.264 | 0.006 | 0.028 | 1.000 | 2.207 |
| 1Pt-TiO2 (EDS) | 0.004 (0.016) | - - | 1.000 (1.000) | 2.401 (1.645) | 0.013 (0.011) | - - | 1.000 (1.000) | 2.149 (2.116) |
| 1Pt,N-TiO2 (EDS) | 0.005 (0.011) | 0.009 - | 1.000 (1.000) | 2.357 (1.932) | 0.011 (0.014) | 0.043 - | 1.000 (1.000) | 2.085 (1.871) |
| 1Pt-TiO2UV | 0.010 | - | 1.000 | 2.321 | 0.015 | - | 1.000 | 2.230 |
| 1Pt,N-TiO2UV | 0.005 | 0.027 | 1.000 | 3.239 | 0.010 | 0.12 | 1.000 | 2.680 |
| BE, eV Film | 395.8 ± 0.2 | 397.1 | 398.0 ± 0.3 | 399.8 ± 0.2 | 400.5–404.1 |
|---|---|---|---|---|---|
| Irel, % | |||||
| 0.5Pt,N-TiO2M1 | - | - | 4.8 | 81.7 | 13.5 |
| 0.5Pt,N-TiO2M1UV | - | - | - | 85.3 | 14.7 |
| 1Pt,N-TiO2M1 | 3.5 | - | - | 74.2 | 22.3 |
| 1Pt,N-TiO2M1UV | - | 8.1 | - | 81.8 | 9.4 |
| 0.5Pt,N-TiO2M2 | - | - | 3.5 | 76.8 | 19.7 |
| 0.5Pt,N-TiO2M2UV | - | - | - | 67.6 | 32.4 |
| 1Pt,N-TiO2M2 | 1.9 | - | 6.1 | 84.9 | 7.0 |
| 1Pt,N-TiO2M2UV | - | - | 8.3 | 66.2 | 25.5 |
| BE, еV | Pt0 | Pt2+ | Pt0 | Pt2+ | Pt4+ | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pt4f7/2 | Іrel | Pt4f7/2 | Іrel | Pt4f7/2 | Іrel | Pt4f7/2 | Іrel | Pt4f7/2 | Іrel | |
| Film | Method 1 | Method 2 | ||||||||
| 0.5Pt-TiO2 | 70.2 | 51.2 | 71.0 72.4 | 36.3 12.5 | - | - | 72.3 | 100.0 | ||
| 0.5Pt,N-TiO2 | 70.8 | 48.3 | 72.4 | 51.7 | 70.6 | 56.3 | 72.3 | 43.7 | - | - |
| 0.5Pt-TiO2UV | 70.7 | 62.3 | 72.5 | 37.7 | - | - | 72.4 | 85.3 | 74.2 | 14.7 |
| 0.5Pt,N-TiO2UV | 71.0 | 32.5 | 72.1 | 67.5 | 70.6 | 69.5 | 72.3 | 30.5 | - | - |
| 1Pt-TiO2 | 71.4 | 11.6 | 72.1 | 88.4 | - | - | 72.0 | 100.0 | - | - |
| 1Pt,N-TiO2 | 70.4 | 76.6 | 72.0 | 23.4 | 70.5 | 19.5 | 71.2 72.5 | 29.2 45.5 | 74.3 | 5.8 |
| 1Pt-TiO2UV | 70.3 | 55.5 | 71.1 | 44.5 | - | - | 72.3 | 83.8 | 74.3 | 16.2 |
| 1Pt,N-TiO2UV | 69.6 | 40.2 | 71.4 | 59.8 | 70.8 | 15.2 | 72.5 | 55.9 | 74.6 | 28.9 |
| Sample | Mean Particle Size, nm | d-Spacing, nm | |
|---|---|---|---|
| Pt | TiO2 | ||
| 1% Pt-TiO2M2 | - | 8.7 ± 2.6 | 0.347 |
| 1% Pt,N-TiO2M2 | 3.2 ± 1.5 | 11.6 ± 1.4 | 0.351 |
| 1% Pt-TiO2M1 | 3.2 ± 0.8 | 12.6 ± 1.9 | 0.350 |
| 1% Pt,N-TiO2M1 | 3.3 ± 0.4 | 8.7 ± 1.2 | 0.350 |
| 1% Pt-TiO2M1UV | 3.2 ± 1.7 | 11.5 ± 2.1 | 0.361 |
| 1% Pt,N-TiO2M1UV | 4.1 ± 2.7 | 14.1 ± 3.1 | 0.357 |
| Film | N | Pt | Pt0 | Pt2+ | N | Pt | Pt0 | Pt2+ | Pt4+ |
|---|---|---|---|---|---|---|---|---|---|
| AC, % | RAC⋅102, % | AC, % | RAC⋅102, % | ||||||
| Method 1 | Method 2 | ||||||||
| 0.5Pt-TiO2 | - | 0.13 | 6.7 | 6.3 | - | 0.18 | - | 18.0 | - |
| 0.5Pt,N-TiO2 | 0.51 | 0.12 | 5.8 | 6.2 | 1.00 | 0.11 | 6.2 | 4.8 | - |
| 0.5Pt-TiO2UV | - | 0.09 | 5.6 | 3.4 | - | 0.19 | - | 16.2 | 2.8 |
| 0.5Pt,N-TiO2UV | 0.09 | 0.02 | 0.7 | 1.6 | 0.87 | 0.19 | 13.2 | 5.8 | - |
| 1Pt-TiO2 | - | 0.13 | 1.5 | 11.5 | - | 0.40 | - | 40.0 | - |
| 1Pt,N-TiO2 | 0.26 | 0.16 | 12.3 | 3.7 | 1.37 | 0.35 | 6.8 | 26.1 | 2.0 |
| 1Pt-TiO2UV | - | 0.30 | 16.7 | 13.4 | - | 0.45 | - | 37.5 | 7.3 |
| 1Pt,N-TiO2UV | 0.64 | 0.11 | 4.4 | 6.6 | 0.90 | 0.20 | 3.0 | 11.0 | 6.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ihnatiuk, D.; Tossi, C.; Tittonen, I.; Linnik, O. Effect of Synthesis Conditions of Nitrogen and Platinum Co-Doped Titania Films on the Photocatalytic Performance under Simulated Solar Light. Catalysts 2020, 10, 1074. https://doi.org/10.3390/catal10091074
Ihnatiuk D, Tossi C, Tittonen I, Linnik O. Effect of Synthesis Conditions of Nitrogen and Platinum Co-Doped Titania Films on the Photocatalytic Performance under Simulated Solar Light. Catalysts. 2020; 10(9):1074. https://doi.org/10.3390/catal10091074
Chicago/Turabian StyleIhnatiuk, Daryna, Camilla Tossi, Ilkka Tittonen, and Oksana Linnik. 2020. "Effect of Synthesis Conditions of Nitrogen and Platinum Co-Doped Titania Films on the Photocatalytic Performance under Simulated Solar Light" Catalysts 10, no. 9: 1074. https://doi.org/10.3390/catal10091074
APA StyleIhnatiuk, D., Tossi, C., Tittonen, I., & Linnik, O. (2020). Effect of Synthesis Conditions of Nitrogen and Platinum Co-Doped Titania Films on the Photocatalytic Performance under Simulated Solar Light. Catalysts, 10(9), 1074. https://doi.org/10.3390/catal10091074





