Recent Progress on Improving Low-Temperature Activity of Vanadia-Based Catalysts for the Selective Catalytic Reduction of NOx with Ammonia
Abstract
1. Introduction
2. Performance Improvement at Low Temperatures
2.1. The Modification of Vanadia-Based Catalysts
2.2. The Effect of Different Supports
2.3. The Effect of Preparation Method
2.4. Metal Vanadates
2.5. Specific Structures
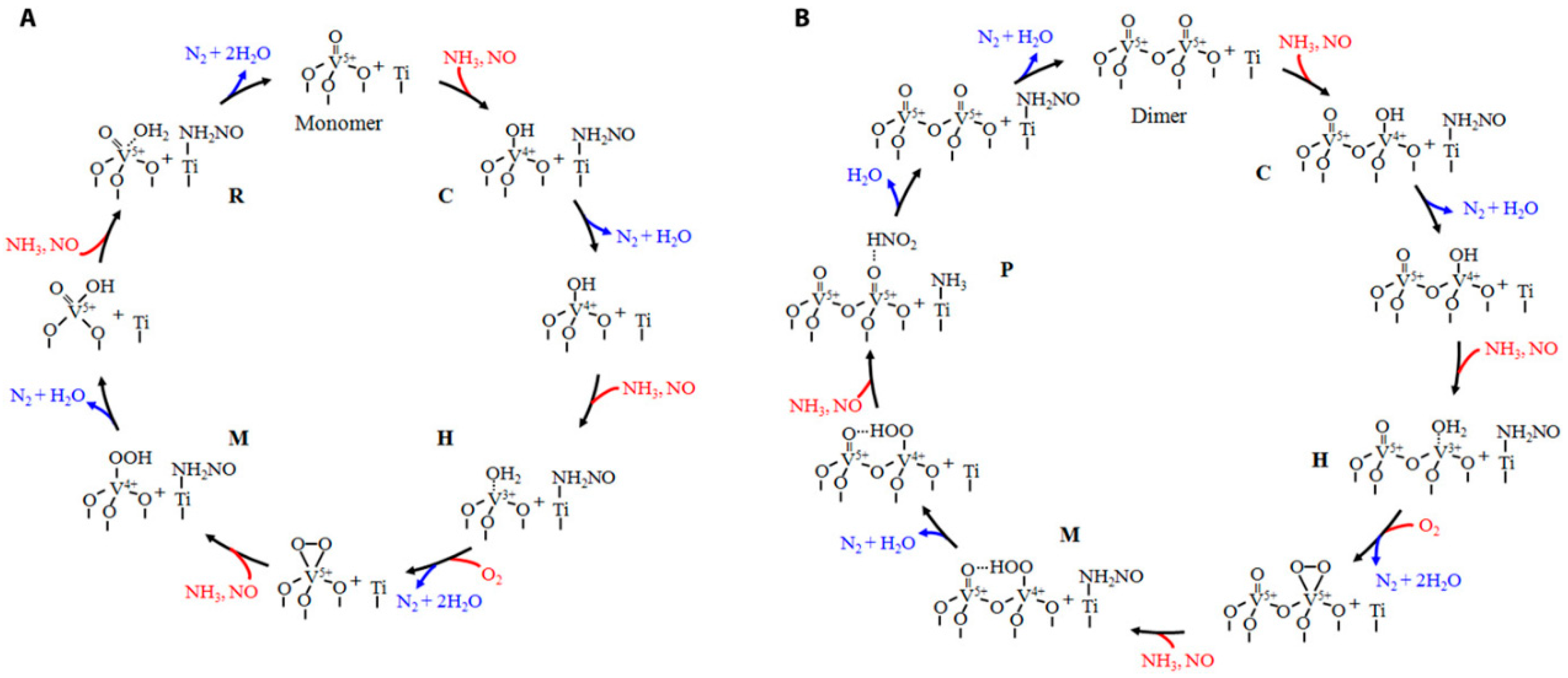
3. Reaction Mechanism at Low Temperatures
4. Conclusions and Perspective
Funding
Conflicts of Interest
References
- Liu, F.; He, H.; Lian, Z.; Shan, W.; Xie, L.; Asakura, K.; Yang, W.; Deng, H. Highly dispersed iron vanadate catalyst supported on TiO2 for the selective catalytic reduction of NOx with NH3. J. Catal. 2013, 307, 340–351. [Google Scholar] [CrossRef]
- Baiker, A.; Dollenmeier, P.; Glinski, M.; Reller, A. Selective catalytic reduction of nitric oxide with ammonia: II. Monolayers of vanadia immobilized on titania-silica mixed gels. Appl. Catal. 1987, 35, 365–380. [Google Scholar] [CrossRef]
- Baiker, A.; Dollenmeier, P.; Glinski, M.; Reller, A. Selective catalytic reduction of nitric oxide with ammonia: I. Monolayer and multilayers of vanadia supported on titania. Appl. Catal. 1987, 35, 351–364. [Google Scholar] [CrossRef]
- Rosenberg, H.S.; Curran, L.M.; Slack, A.V.; Ando, J.; Oxley, J.H.J.P.i.E.; Science, C. Post combustion methods for control of NOx emissions. Prog. Energy Combust. Sci. 1980, 6, 287–302. [Google Scholar] [CrossRef]
- Busca, G.; Lietti, L.; Ramis, G.; Berti, F. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: A review. Appl. Catal. B 1998, 18, 1–36. [Google Scholar] [CrossRef]
- Lai, J.K.; Wachs, I.E. A perspective on the selective catalytic reduction (SCR) of NO with NH3 by supported V2O5-WO3/TiO2 catalysts. ACS Catal. 2018, 8, 6537–6551. [Google Scholar] [CrossRef]
- Han, L.; Cai, S.; Gao, M.; Hasegawa, J.Y.; Wang, P.; Zhang, J.; Shi, L.; Zhang, D. Selective catalytic reduction of NOx with NH3 by using novel catalysts: State of the art and future prospects. Chem. Rev. 2019, 119, 10916–10976. [Google Scholar] [CrossRef]
- Xu, J.; Chen, G.; Guo, F.; Xie, J. Development of wide-temperature vanadium-based catalysts for selective catalytic reducing of NOx with ammonia: Review. Chem. Eng. J. 2018, 353, 507–518. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kuma, R.; Masaki, S.; Sugishima, N. TiO2-SiO2 and V2O5/TiO2-SiO2 catalyst: Physico-chemical characteristics and catalytic behavior in selective catalytic reduction of NO by NH3. Appl. Catal. B 2005, 60, 173–179. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, L.; Li, H.; Hu, H.; Han, J.; Shi, L.; Zhang, D. Highly dispersed V2O5/TiO2 modified with transition metals (Cu, Fe, Mn, Co) as efficient catalysts for the selective reduction of NO with NH3. Chin. J. Catal. 2015, 36, 1886–1899. [Google Scholar] [CrossRef]
- Shen, M.; Li, C.; Wang, J.; Xu, L.; Wang, W.; Wang, J. New insight into the promotion effect of Cu doped V2O5/WO3-TiO2 for low temperature NH3-SCR performance. RSC Adv. 2015, 5, 35155–35165. [Google Scholar] [CrossRef]
- Wang, H.; Wang, B.; Zhou, J.; Li, G.; Zhang, D.; Ma, Z.; Xiong, R.; Sun, Q.; Xu, W.Q. CuO modified vanadium-based SCR catalysts for Hg0 oxidation and NO reduction. J. Environ. Manag. 2019, 239, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Miao, J.; Su, Q.; Yu, Y.; Chen, Y.; Chen, J.; Wang, J. Improvement in alkali metal resistance of commercial V2O5-WO3/TiO2 SCR catalysts modified by Ce and Cu. J. Mater. Sci. 2019, 54, 14707–14719. [Google Scholar] [CrossRef]
- Li, C.; Shen, M.; Wang, J.; Wang, J.; Zhai, Y. New insights into the promotional mechanism of ceria for activity and ammonium bisulfate resistance over V/WTi catalyst for selective catalytic reduction of NO with NH3. Appl. Catal. A 2018, 560, 153–164. [Google Scholar] [CrossRef]
- Ma, Z.; Wu, X.; Feng, Y.; Si, Z.; Weng, D.; Shi, L. Low-temperature SCR activity and SO2 deactivation mechanism of Ce-modified V2O5-WO3/TiO2 catalyst. Prog. Nat. Sci. Mater. Int. 2015, 25, 342–352. [Google Scholar] [CrossRef]
- Arfaoui, J.; Ghorbel, A.; Petitto, C.; Delahay, G. Novel V2O5-CeO2-TiO2-SO42- nanostructured aerogel catalyst for the low temperature selective catalytic reduction of NO by NH3 in excess O2. Appl. Catal. B 2018, 224, 264–275. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, Y.; Liu, S.; Zheng, C.; Gao, X.; Nova, I.; Tronconi, E. Improvement in activity and alkali resistance of a novel V-Ce(SO4)2/Ti catalyst for selective catalytic reduction of NO with NH3. Appl. Catal. B 2017, 206, 449–460. [Google Scholar] [CrossRef]
- Liang, Q.; Li, J.; He, H.; Liang, W.; Zhang, T.; Fan, X. Effects of SO2 on the low temperature selective catalytic reduction of NO by NH3 over CeO2-V2O5-WO3/TiO2 catalysts. Front. Environ. Sci. Eng. 2017, 11, 4. [Google Scholar] [CrossRef]
- Li, C.; Shen, M.; Wang, J.; Wang, J.; Zhai, Y. New insights into the role of WO3 in improved activity and ammonium bisulfate resistance for NO reduction with NH3 over V-W/Ce/Ti catalyst. Ind. Eng. Chem. Res. 2018, 57, 8424–8435. [Google Scholar] [CrossRef]
- Zhao, L.; Li, C.; Zhang, J.; Zhang, X.; Zhan, F.; Ma, J.; Xie, Y.; Zeng, G. Promotional effect of CeO2 modified support on V2O5-WO3/TiO2 catalyst for elemental mercury oxidation in simulated coal-fired flue gas. Fuel 2015, 153, 361–369. [Google Scholar] [CrossRef]
- Wu, S.; Yao, X.; Zhang, L.; Cao, Y.; Zou, W.; Li, L.; Ma, K.; Tang, C.; Gao, F.; Dong, L. Improved low temperature NH3-SCR performance of FeMnTiOx mixed oxide with CTAB-assisted synthesis. Chem. Commun. 2015, 51, 3470–3473. [Google Scholar] [CrossRef] [PubMed]
- Chi, G.; Shen, B.; Yu, R.; He, C.; Zhang, X. Simultaneous removal of NO and Hg0 over Ce-Cu modified V2O5/TiO2 based commercial SCR catalysts. J. Hazard. Mater. 2017, 330, 83–92. [Google Scholar] [CrossRef] [PubMed]
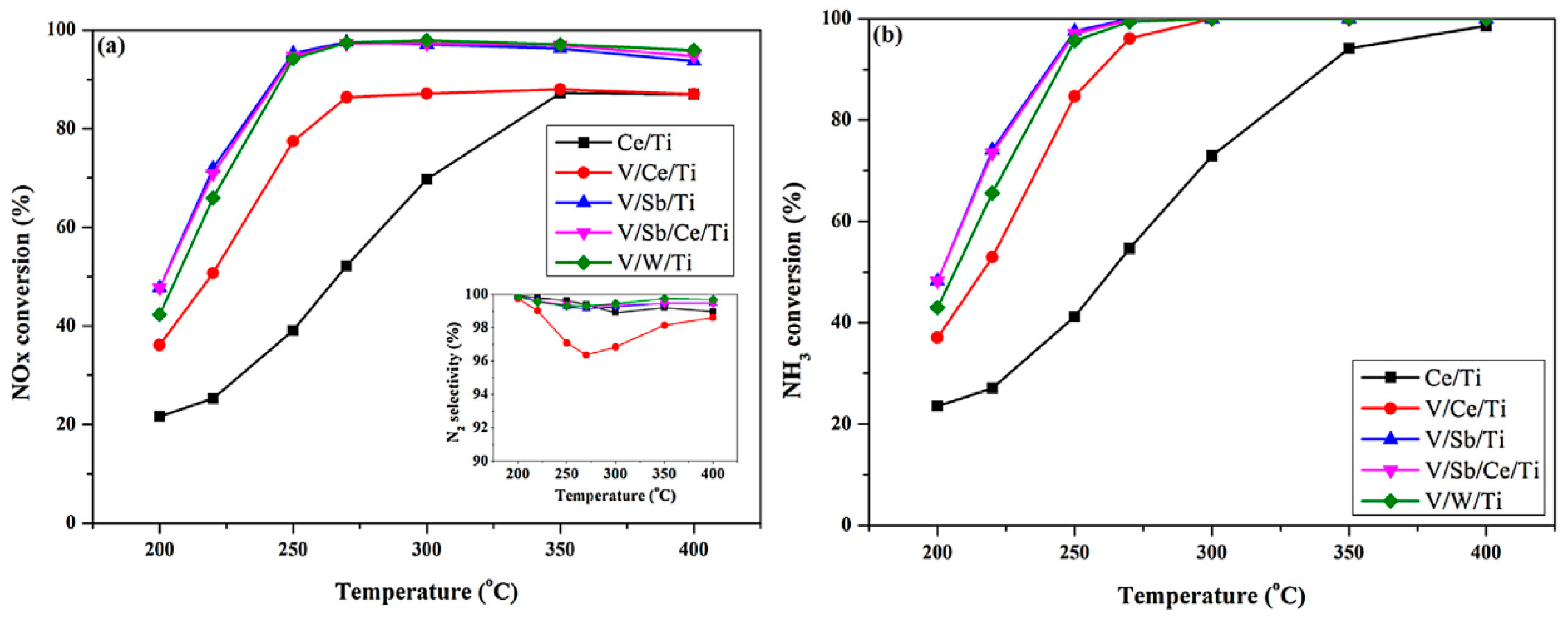
- Ku, C.; Liu, J.; Zhao, Z.; Yu, F.; Cheng, K.; Wei, Y.; Duan, A.; Jiang, G. NH3-SCR denitration catalyst performance over vanadium-titanium with the addition of Ce and Sb. J. Environ. Sci. 2015, 31, 74–80. [Google Scholar]
- Kwon, D.W.; Nam, K.B.; Hong, S.C. The role of ceria on the activity and SO2 resistance of catalysts for the selective catalytic reduction of NOx by NH3. Appl. Catal. B 2015, 166, 37–44. [Google Scholar] [CrossRef]
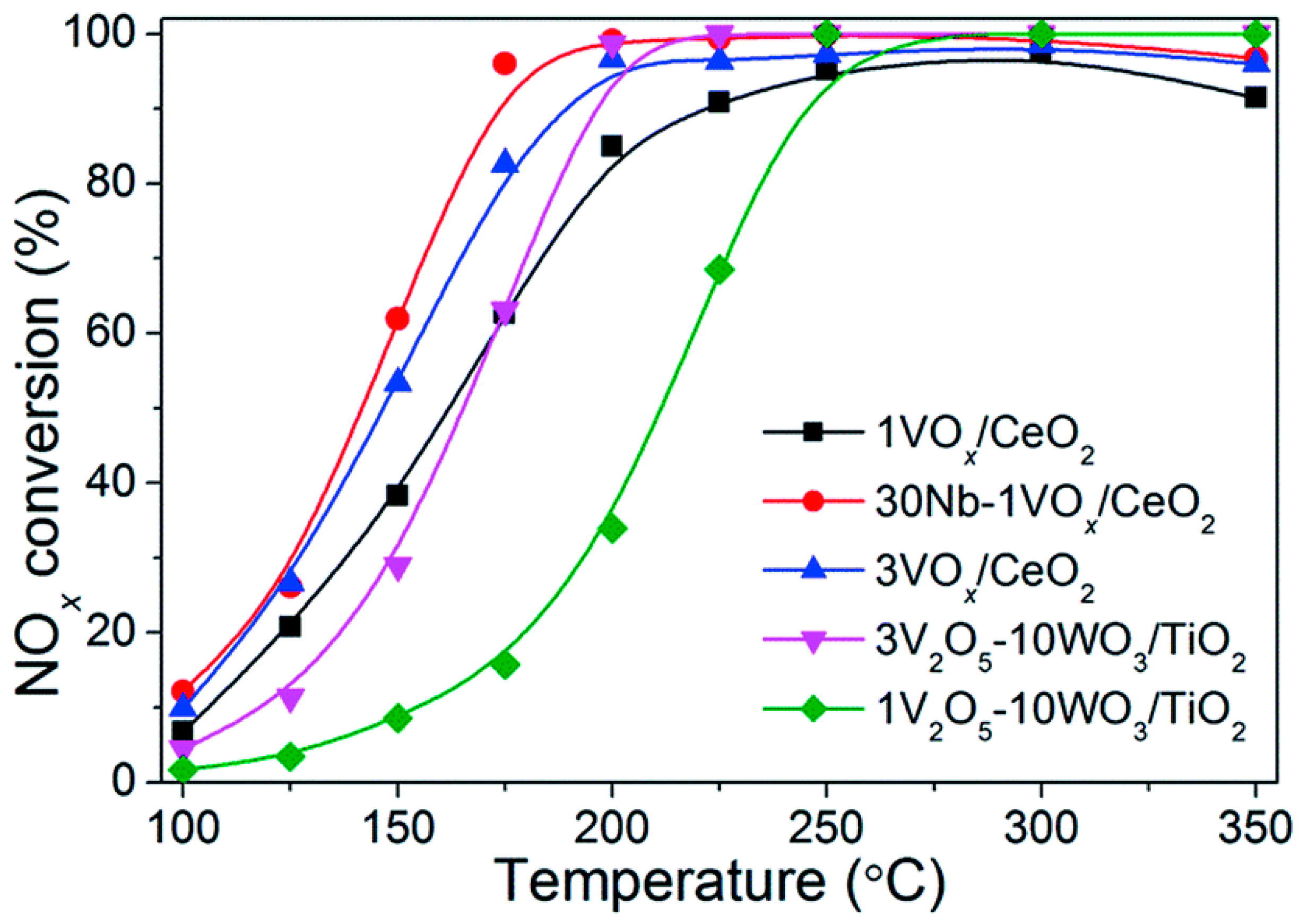
- Wu, X.; Yu, X.; Huang, Z.; Shen, H.; Jing, G. MnOx-decorated VOx/CeO2 catalysts with preferentially exposed {110} facets for selective catalytic reduction of NOx by NH3. Appl. Catal. B 2020, 268, 118419. [Google Scholar] [CrossRef]
- Zhang, D.; Ma, Z.; Wang, B.; Sun, Q.; Xu, W.; Zhu, T. Effects of MOx (M=Mn, Cu, Sb, La) on V-Mo-Ce/Ti selective catalytic reduction catalysts. J. Rare Earths 2020, 38, 157–166. [Google Scholar] [CrossRef]
- Kwon, D.W.; Park, K.H.; Ha, H.P.; Hong, S.C. The role of molybdenum on the enhanced performance and SO2 resistance of V/Mo-Ti catalysts for NH3-SCR. Appl. Surf. Sci. 2019, 481, 1167–1177. [Google Scholar] [CrossRef]
- Zhu, L.; Zhong, Z.; Yang, H.; Wang, C. Effect of MoO3 on vanadium based catalysts for the selective catalytic reduction of NOx with NH3 at low temperature. J. Environ. Sci. 2017, 56, 169–179. [Google Scholar] [CrossRef]
- Yang, R.; Huang, H.F.; Chen, Y.J.; Zhang, X.X.; Lu, H.F. Performance of Cr-doped vanadia/titania catalysts for low-temperature selective catalytic reduction of NOx with NH3. Chin. J. Catal. 2015, 36, 1256–1262. [Google Scholar] [CrossRef]
- Zhu, L.; Zhong, Z.; Xue, J.; Xu, Y.; Wang, C.; Wang, L. NH3-SCR performance and the resistance to SO2 for Nb doped vanadium based catalyst at low temperatures. J. Environ. Sci. 2018, 65, 306–316. [Google Scholar] [CrossRef]
- Marberger, A.; Ferri, D.; Rentsch, D.; Krumeich, F.; Elsener, M.; Krocher, O. Effect of SiO2 on co-impregnated V2O5/WO3/TiO2 catalysts for the selective catalytic reduction of NO with NH3. Catal. Today 2019, 320, 123–132. [Google Scholar] [CrossRef]
- Zhao, W.; Dou, S.; Zhang, K.; Wu, L.; Wang, Q.; Shang, D.; Zhong, Q. Promotion effect of S and N co-addition on the catalytic performance of V2O5/TiO2 for NH3-SCR of NOx. Chem. Eng. J. 2019, 364, 401–409. [Google Scholar] [CrossRef]
- Zhao, W.; Zhong, Q.; Pan, Y.; Zhang, R. Systematic effects of S-doping on the activity of V2O5/TiO2 catalyst for low-temperature NH3-SCR. Chem. Eng. J. 2013, 228, 815–823. [Google Scholar] [CrossRef]
- Zhang, S.; Zhong, Q.; Zhao, W.; Li, Y. Surface characterization studies on F-doped V2O5/TiO2 catalyst for NO reduction with NH3 at low-temperature. Chem. Eng. J. 2014, 253, 207–216. [Google Scholar] [CrossRef]
- Liang, Q.; Li, J.; He, H.; Yue, T.; Tong, L. Effects of SO2 and H2O on low-temperature NO conversion over F-V2O5-WO3/TiO2 catalysts. J. Environ. Sci. 2020, 90, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Liu, Q.; Wang, S.; Xu, G.; Wu, M.; Chen, J.; Li, J. Promoter rather than inhibitor: Phosphorus incorporation accelerates the activity of V2O5-WO3/TiO2 catalyst for selective catalytic reduction of NOx by NH3. ACS Catal. 2020, 10, 2747–2753. [Google Scholar] [CrossRef]
- Nam, K.B.; Yeo, J.H.; Hong, S.C. Study of the phosphorus deactivation effect and resistance of vanadium-based catalysts. Ind. Eng. Chem. Res. 2019, 58, 18930–18941. [Google Scholar] [CrossRef]
- Putluru, S.S.R.; Schill, L.; Godiksen, A.; Poreddy, R.; Mossin, S.; Jensen, A.D.; Fehrmann, R. Promoted V2O5/TiO2 catalysts for selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. B 2016, 183, 282–290. [Google Scholar] [CrossRef]
- Bosch, F.J.H. Formation and control of nitrogen oxides. Catal. Today 1988, 2, 369–379. [Google Scholar]
- Amiridis, M.D.; Duevel, R.V.; Wachs, I.E. The effect of metal oxide additives on the activity of V2O5/TiO2 catalysts for the selective catalytic reduction of nitric oxide by ammonia. Appl. Catal. B 1999, 20, 111–122. [Google Scholar] [CrossRef]
- Roy, S.; Hegde, M.S.; Madras, G.J.A.E. Catalysis for NOx abatement. Appl. Energy 2009, 86, 2283–2297. [Google Scholar] [CrossRef]
- Song, I.; Youn, S.; Lee, H.; Lee, S.G.; Cho, S.J.; Kim, D.H. Effects of microporous TiO2 support on the catalytic and structural properties of V2O5/microporous TiO2 for the selective catalytic reduction of NO by NH3. Appl. Catal. B 2017, 210, 421–431. [Google Scholar] [CrossRef]
- Youn, S.; Song, I.; Lee, H.; Cho, S.J.; Kim, D.H. Effect of pore structure of TiO2 on the SO2 poisoning over V2O5/TiO2 catalysts for selective catalytic reduction of NOx with NH3. Catal. Today 2018, 303, 19–24. [Google Scholar] [CrossRef]
- Liu, X.; Li, J.; Li, X.; Peng, Y.; Wang, H.; Jiang, X.; Wang, L. NH3 selective catalytic reduction of NO: A large surface TiO2 support and its promotion of V2O5 dispersion on the prepared catalyst. Chin. J. Catal. 2016, 37, 878–887. [Google Scholar] [CrossRef]
- Tran, T.; Yu, J.; Gan, L.; Guo, F.; Phan, D.; Xu, G. Upgrading V2O5-WO3/TiO2 deNOx catalyst with TiO2-SiO2 support prepared from Ti-bearing blast furnace slag. Catalysts 2016, 6, 56. [Google Scholar] [CrossRef]
- Ferjani, W.; Boudali, L.K.; Delahay, G.; Petitto, C. Reduction of nitrogen oxide by ammonia over vanadium supported on mixed tungsten-titanium-pillared clays. Chem. Lett. 2016, 45, 872–874. [Google Scholar] [CrossRef]
- Li, X.; Wu, Z.; Zeng, Y.; Han, J.; Zhang, S.; Zhong, Q. Reduced TiO2 inducing highly active V2O5 species for selective catalytic reduction of NO by NH3. Chem. Phys. Lett. 2020, 750, 137494. [Google Scholar] [CrossRef]
- da Cunha, B.N.; Goncalves, A.M.; da Silveira, R.G.; Urquieta-Gonzalez, E.A.; Nunes, L.M. The influence of a silica pillar in lamellar tetratitanate for selective catalytic reduction of NOx using NH3. Mater. Res. Bull. 2015, 61, 124–129. [Google Scholar] [CrossRef]
- Bukowski, A.; Schill, L.; Nietsen, D.; Mossin, S.; Riisager, A.; Albert, J. NH3-SCR of NO with novel active, supported vanadium-containing Keggin-type heteropolyacid catalysts. React. Chem. Eng. 2020, 5, 935–948. [Google Scholar] [CrossRef]
- Mejia-Centeno, I.; Castillo, S.; Camposeco, R.; Marin, J.; Garcia, L.A.; Fuentes, G.A. Activity and selectivity of V2O5/H2Ti3O7, V2O5-WO3/H2Ti3O7 and Al2O3/H2Ti3O7 model catalysts during the SCR-NO with NH3. Chem. Eng. J. 2015, 264, 873–885. [Google Scholar] [CrossRef]
- Cheng, J.; Song, Y.; Ye, Q.; Cheng, S.; Kang, T.; Dai, H. A mechanistic investigation on the selective catalytic reduction of NO with ammonia over the V-Ce/Ti-PILC catalysts. Mol. Catal. 2018, 445, 111–123. [Google Scholar] [CrossRef]
- Vuong, T.H.; Radnik, J.; Rabeah, J.; Bentrup, U.; Schneider, M.; Atia, H.; Armbruster, U.; Grünert, W.; Brückner, A. Efficient VOx/Ce1−xTixO2 catalysts for low-temperature NH3-SCR: Reaction mechanism and active sites assessed by in situ/operando spectroscopy. ACS Catal. 2017, 7, 1693–1705. [Google Scholar] [CrossRef]
- Lian, Z.; Liu, F.; He, H. Effect of preparation methods on the activity of VOx/CeO2 catalysts for the selective catalytic reduction of NOx with NH3. Catal. Sci. Technol. 2015, 5, 389–396. [Google Scholar] [CrossRef]
- Lian, Z.; Liu, F.; He, H. Enhanced activity of Ti-modified V2O5/CeO2 catalyst for the selective catalytic reduction of NOx with NH3. Ind. Eng. Chem. Res. 2014, 53, 19506–19511. [Google Scholar] [CrossRef]
- Lian, Z.; Liu, F.; He, H.; Liu, K. Nb-doped VOx/CeO2 catalyst for NH3-SCR of NOx at low temperatures. RSC Adv. 2015, 5, 37675–37681. [Google Scholar] [CrossRef]
- Lian, Z.; Liu, F.; Shan, W.; He, H. Improvement of Nb doping on SO2 resistance of VOx/CeO2 catalyst for the selective catalytic reduction of NOx with NH3. J. Phys. Chem. C 2017, 121, 7803–7809. [Google Scholar] [CrossRef]
- Liu, S.; Wang, H.; Wei, Y.; Zhang, R.; Royer, S. Morphology-Oriented ZrO2-Supported Vanadium Oxide for the NH3-SCR Process: Importance of Structural and Textural Properties. ACS Appl. Mater. Interfaces 2019, 11, 22240–22254. [Google Scholar] [CrossRef]
- Thanh Huyen, V.; Radnik, J.; Kondratenko, E.; Schneider, M.; Armbruster, U.; Brueckner, A. Structure-reactivity relationships in VOx/CexZr1−xO2 catalysts used for low-temperature NH3-SCR of NO. Appl. Catal. B 2016, 197, 159–167. [Google Scholar]
- Zhao, L.K.; Li, C.T.; Li, S.H.; Wang, Y.; Zhang, J.Y.; Wang, T.; Zeng, G.M. Simultaneous removal of elemental mercury and NO in simulated flue gas over V2O5/ZrO2-CeO2 catalyst. Appl. Catal. B 2016, 198, 420–430. [Google Scholar] [CrossRef]
- Zhao, L.; Li, C.; Du, X.; Zeng, G.; Gao, L.; Zhai, Y.; Wang, T.; Zhang, J. Effect of Co addition on the performance and structure of V/ZrCe catalyst for simultaneous removal of NO and Hg0 in simulated flue gas. Appl. Surf. Sci. 2018, 437, 390–399. [Google Scholar] [CrossRef]
- Niu, C.; Wang, Y.; Ren, D.; Xiao, L.; Duan, R.; Wang, B.; Wang, X.; Xu, Y.; Li, Z.; Shi, J.W. The deposition of VWOx on the CuCeOy microflower for the selective catalytic reduction of NOx with NH3 at low temperatures. J. Colloid Interface Sci. 2020, 561, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.; Agostini, G.; Antoni, H.; Kreyenschulte, C.R.; Atia, H.; Rabeah, J.; Bentrup, U.; Brueckner, A. The effect of iron and vanadium in VOy/Ce1−xFexO2-delta catalysts in low-temperature selective catalytic reduction of NOx by ammonia. ChemCatChem 2020, 12, 2440–2451. [Google Scholar] [CrossRef]
- Bai, S.; Jiang, S.; Li, H.; Guan, Y. Carbon nanotubes loaded with vanadium oxide for reduction NO with NH3 at low temperature. Chin. J. Chem. Eng. 2015, 23, 516–519. [Google Scholar] [CrossRef]
- Bai, S.; Wang, Z.; Li, H.; Xu, X.; Liu, M. SO2 promotion in NH3-SCR reaction over V2O5/SiC catalyst at low temperature. Fuel 2017, 194, 36–41. [Google Scholar] [CrossRef]
- Yang, W.; Liu, F.; Xie, L.; Lian, Z.; He, H. Effect of V2O5 additive on the SO2 resistance of a Fe2O3/AC catalyst for NH3-SCR of NOx at low temperatures. Ind. Eng. Chem. Res. 2016, 55, 2677–2685. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, Q.; Ran, G.; Kong, M.; Ren, S.; Yang, J.; Li, J. V2O5-modified Mn-Ce/AC catalyst with high SO2 tolerance for low-temperature NH3-SCR of NO. Chem. Eng. J. 2019, 370, 810–821. [Google Scholar] [CrossRef]
- He, Y.; Ford, M.E.; Zhu, M.; Liu, Q.; Tumuluri, U.; Wu, Z.; Wachs, I.E. Influence of catalyst synthesis method on selective catalytic reduction (SCR) of NO by NH3 with V2O5-WO3/TiO2 catalysts. Appl. Catal. B 2016, 193, 141–150. [Google Scholar] [CrossRef]
- Shen, M.; Xu, L.; Wang, J.; Li, C.; Wang, W.; Wang, J.; Zhai, Y. Effect of synthesis methods on activity of V2O5/CeO2/WO3-TiO2 catalyst for selective catalytic reduction of NOx with NH3. J. Rare Earths 2016, 34, 259–267. [Google Scholar] [CrossRef]
- Cha, W.; Ehrman, S.H.; Jurng, J. Surface phenomenon of CeO2-added V2O5/TiO2 catalyst based chemical vapor condensation (CVC) for enhanced selective catalytic reduction at low temperatures. Chem. Eng. J. 2016, 304, 72–78. [Google Scholar] [CrossRef]
- Vuong, T.H.; Radnik, J.; Schneider, M.; Atia, H.; Armbruster, U.; Brückner, A. Effect of support synthesis methods on structure and performance of VOx/CeO2 catalysts in low-temperature NH3-SCR of NO. Catal. Commun. 2016, 84, 171–174. [Google Scholar] [CrossRef]
- Cheng, K.; Liu, J.; Zhao, Z.; Wei, Y.C.; Jiang, G.Y.; Duan, A.J. Direct synthesis of V-W-Ti nanoparticle catalysts for selective catalytic reduction of NO with NH3. RSC Adv. 2015, 5, 45172–45183. [Google Scholar] [CrossRef]
- Chen, T.; Lin, H.; Guan, B.; Gong, X.; Li, K.; Huang, Z. Promoting the low temperature activity of Ti-V-O catalysts by premixed flame synthesis. Chem. Eng. J. 2016, 296, 45–55. [Google Scholar] [CrossRef]
- Song, I.; Lee, H.; Kim, D.H. Rotation-assisted hydrothermal synthesis of thermally stable multiwalled titanate nanotubes and their application to selective catalytic reduction of NO with NH3. ACS Appl. Mater. Interfaces 2018, 10, 42249–42257. [Google Scholar] [CrossRef] [PubMed]
- Doronkin, D.E.; Benzi, F.; Zheng, L.; Sharapa, D.I.; Amidani, L.; Studt, F.; Roesky, P.W.; Casapu, M.; Deutschmann, O.; Grunwaldt, J.D. NH3-SCR over V-W/TiO2 investigated by operando X-ray absorption and emission spectroscopy. J. Phys. Chem. C 2019, 123, 14338–14349. [Google Scholar] [CrossRef]
- Gan, L.; Chen, J.; Peng, Y.; Yu, J.; Tran, T.; Li, K.; Wang, D.; Xu, G.; Li, J. NOx removal over V2O5/WO3-TiO2 prepared by a grinding method: Influence of the precursor on vanadium dispersion. Ind. Eng. Chem. Res. 2018, 57, 150–157. [Google Scholar] [CrossRef]
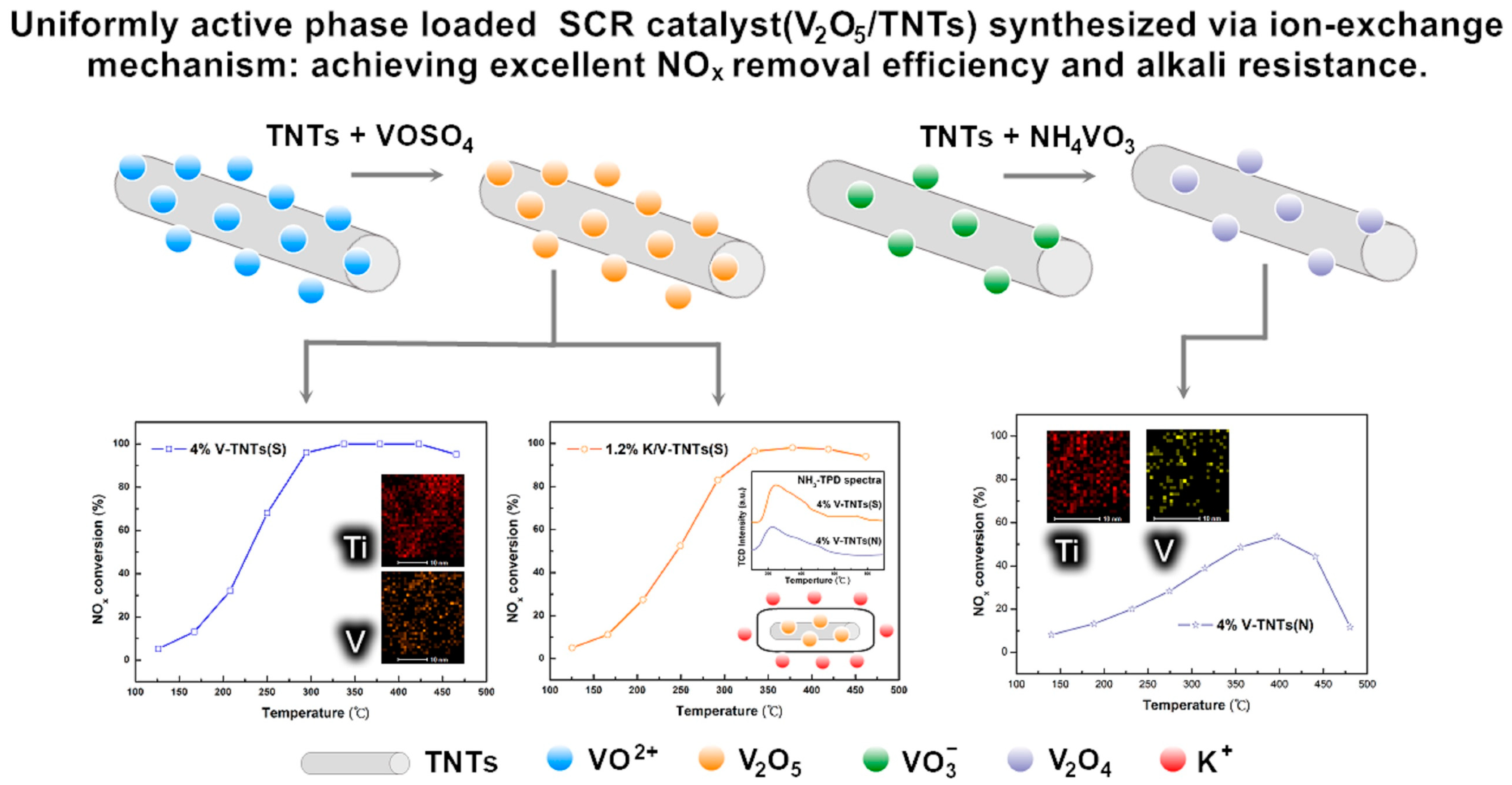
- Wang, H.; Wang, P.; Chen, X.; Wu, Z. Uniformly active phase loaded selective catalytic reduction catalysts (V2O5/TNTs) with superior alkaline resistance performance. J. Hazard. Mater. 2017, 324, 507–515. [Google Scholar] [CrossRef]
- Casanova, M.; Nodari, L.; Sagar, A.; Schermanz, K.; Trovarelli, A. Preparation, characterization and NH3-SCR activity of FeVO4 supported on TiO2-WO3-SiO2. Appl. Catal. B 2015, 176–177, 699–708. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Z.; Ning, R.; Qin, Y.; Zhu, T.; Liu, F. Ce-doped V2O5-WO3/TiO2 with low vanadium loadings as SCR catalysts and the resistance of H2O and SO2. Catal. Lett. 2020, 150, 375–383. [Google Scholar] [CrossRef]
- Chen, G.; Xu, J.; Yu, H.; Guo, F.; Xie, J.; Wang, Y. Effect of the non-thermal plasma treatment on the structure and SCR activity of vanadium-based catalysts. Chem. Eng. J. 2020, 380, 122286. [Google Scholar] [CrossRef]
- Lian, Z.; Xin, S.; Zhu, N.; Wang, Q.; Xu, J.; Zhang, Y.; Shan, W.; He, H. Effect of treatment atmosphere on the vanadium species of V/TiO2 catalysts for the selective catalytic reduction of NOx with NH3. Catal. Sci. Technol. 2020, 10, 311–314. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, L.; Li, H.R.; Hu, H.; Hu, X.N.; Shi, L.Y.; Zhang, D.S. Promotional effects of zirconium doped CeVO4 for the low-temperature selective catalytic reduction of NOx with NH3. Appl. Catal. B 2016, 183, 269–281. [Google Scholar] [CrossRef]
- Mu, J.; Li, X.; Sun, W.; Fan, S.; Wang, X.; Wang, L.; Qin, M.; Gan, G.; Yin, Z.; Zhang, D. Inductive Effect Boosting Catalytic Performance of Advanced Fe1−xVxOδ Catalysts in Low-Temperature NH3 Selective Catalytic Reduction: Insight into the Structure, Interaction, and Mechanisms. ACS Catal. 2018, 8, 6760–6774. [Google Scholar] [CrossRef]
- Kim, J.; Kim, D.H.; Kwon, D.W.; Ha, H.P. Rational selection of Fe2V4O13 over FeVO4 as a preferred active site on Sb-promoted TiO2 for catalytic NOx reduction with NH3. Catal. Sci. Technol. 2018, 8, 4774–4787. [Google Scholar] [CrossRef]
- Marberger, A.; Elsener, M.; Ferri, D.; Sagar, A.; Schermanz, K.; Kroecher, O. Generation of NH3 selective catalytic reduction active catalysts from decomposition of supported FeVO4. ACS Catal. 2015, 5, 4180–4188. [Google Scholar] [CrossRef]
- Wu, G.X.; Li, J.; Fang, Z.T.; Lan, L.; Wang, R.; Lin, T.; Gong, M.C.; Chen, Y.Q. Effectively enhance catalytic performance by adjusting pH during the synthesis of active components over FeVO4/TiO2-WO3-SiO2 monolith catalysts. Chem. Eng. J. 2015, 271, 1–13. [Google Scholar] [CrossRef]
- Casanova, M.; Llorca, J.; Sagar, A.; Schermanz, K.; Trovarelli, A. Mixed iron-erbium vanadate NH3-SCR catalysts. Catal. Today 2015, 241, 159–168. [Google Scholar] [CrossRef]
- Gillot, S.; Tricot, G.; Vezin, H.; Dacquin, J.P.; Dujardin, C.; Granger, P. Development of stable and efficient CeVO4 systems for the selective reduction of NOx by ammonia: Structure-activity relationship. Appl. Catal. B 2017, 218, 338–348. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, L.; Namuangruk, S.; Hu, H.; Hu, X.N.; Shi, L.Y.; Zhang, D.S. Morphology-dependent performance of Zr-CeVO4/TiO2 for selective catalytic reduction of NO with NH3. Catal. Sci. Technol. 2016, 6, 5543–5553. [Google Scholar] [CrossRef]
- Gao, S.; Wang, P.; Yu, F.; Wang, H.; Wu, Z. Dual resistance to alkali metals and SO2: Vanadium and cerium supported on sulfated zirconia as an efficient catalyst for NH3-SCR. Catal. Sci. Technol. 2016, 6, 8148–8156. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, G.; Dong, F.; Tang, Z. The remarkable promotional effect of Sn on CeVO4 catalyst for wide temperature NH3-SCR process by citric acid-assisted solvothermal synthesis and post-hydrothermal treatment. Catal. Sci. Technol. 2018, 8, 5604–5615. [Google Scholar] [CrossRef]
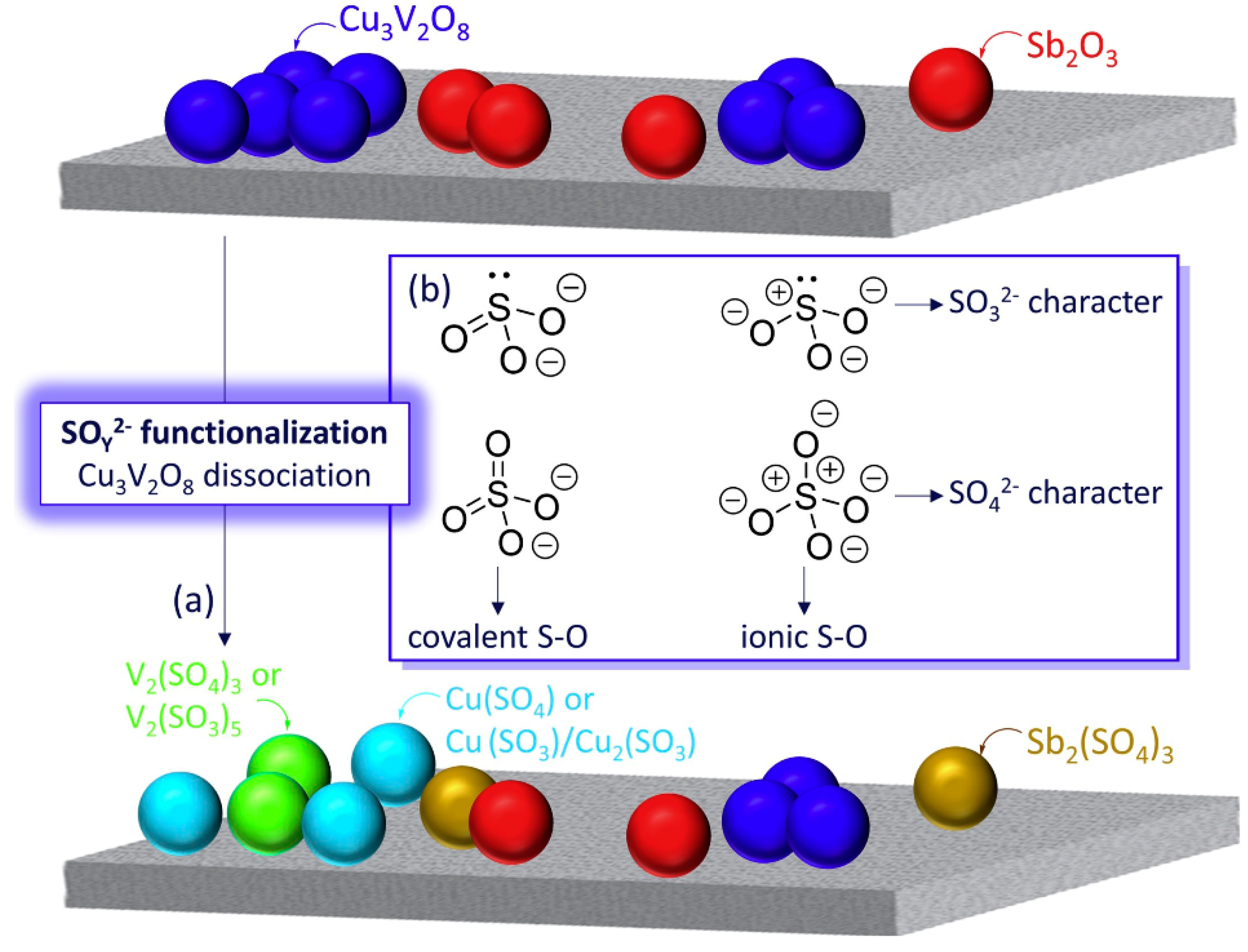
- Kim, J.; Kwon, D.W.; Lee, S.; Ha, H.P. Exploration of surface properties of Sb-promoted copper vanadate catalysts for selective catalytic reduction of NOx by NH3. Appl. Catal. B 2018, 236, 314–325. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.; Kwon, D.W.; Lee, K.Y.; Ha, H.P. SO32-/SO42- functionalization-tailorable catalytic surface features of Sb-promoted Cu3V2O8 on TiO2 for selective catalytic reduction of NOx with NH3. Appl. Catal. A 2019, 570, 355–366. [Google Scholar] [CrossRef]
- Gillot, S.; Tricot, G.; Vezin, H.; Dacquin, J.P.; Dujardin, C.; Granger, P. Induced effect of tungsten incorporation on the catalytic properties of CeVO4 systems for the selective reduction of NOx by ammonia. Appl. Catal. B 2018, 234, 318–328. [Google Scholar] [CrossRef]
- Marberger, A.; Ferri, D.; Elsener, M.; Sagar, A.; Artner, C.; Schermanz, K.; Krocher, O. Relationship between structures and activities of supported metal vanadates for the selective catalytic reduction of NO by NH3. Appl. Catal. B 2017, 218, 731–742. [Google Scholar] [CrossRef]
- Wu, G.; Li, J.; Fang, Z.; Lan, L.; Wang, R.; Gong, M.; Chen, Y. FeVO4 nanorods supported TiO2 as a superior catalyst for NH3-SCR reaction in a broad temperature range. Catal. Commun. 2015, 64, 75–79. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, G.; Tang, Z. Facile fabrication of Ce/V-modified multi-channel TiO2 nanotubes and their enhanced selective catalytic reduction performance. Chem. Asian J. 2020, 15, 371–379. [Google Scholar] [CrossRef]
- Lee, S.G.; Lee, H.J.; Song, I.; Youn, S.; Kim, D.H.; Cho, S.J. Suppressed N2O formation during NH3 selective catalytic reduction using vanadium on zeolitic microporous TiO2. Sci. Rep. 2015, 5, 12702. [Google Scholar] [CrossRef]
- Zhang, T.; Chang, H.; Li, K.; Peng, Y.; Li, X.; Li, J. Different exposed facets VOx/CeO2 catalysts for the selective catalytic reduction of NO with NH3. Chem. Eng. J. 2018, 349, 184–191. [Google Scholar] [CrossRef]
- Song, L.; Zhang, R.; Zang, S.; He, H.; Su, Y.; Qiu, W.; Sun, X. Activity of selective catalytic reduction of NO over V2O5/TiO2 catalysts preferentially exposed anatase {001} and {101} facets. Catal. Lett. 2017, 147, 934–945. [Google Scholar] [CrossRef]
- Boningari, T.; Pappas, D.K.; Smirniotis, P.G. Metal oxide-confined interweaved titania nanotubes M/TNT (M = Mn, Cu, Ce, Fe, V, Cr, and Co) for the selective catalytic reduction of NOx in the presence of excess oxygen. J. Catal. 2018, 365, 320–333. [Google Scholar] [CrossRef]
- Shan, W.; Song, H. Catalysts for the selective catalytic reduction of NOx with NH3 at low temperature. Catal. Sci. Technol. 2015, 5, 4280–4288. [Google Scholar] [CrossRef]
- Forzatti, P. Present status and perspectives in de-NOx SCR catalysis. Appl. Catal. A 2001, 222, 221–236. [Google Scholar] [CrossRef]
- Shan, W.; Yu, Y.; Zhang, Y.; He, G.; Peng, Y.; Li, J.; He, H. Theory and practice of metal oxide catalyst design for the selective catalytic reduction of NO with NH3. Catal. Today 2020, in press. [Google Scholar]
- Zhu, M.; Lai, J.K.; Tumuluri, U.; Wu, Z.; Wachs, I.E. Nature of active sites and surface intermediates during SCR of NO with NH3 by supported V2O5-WO3/TiO2 catalysts. J. Am. Chem. Soc. 2017, 139, 15624–15627. [Google Scholar] [CrossRef]
- Marberger, A.; Ferri, D.; Elsener, M.; Krocher, O. The significance of Lewis acid sites for the selective catalytic reduction of nitric oxide on vanadium-based catalysts. Angew. Chem. Int. Ed. 2016, 55, 11989–11994. [Google Scholar] [CrossRef]
- Song, I.; Lee, H.; Jeon, S.W.; Kim, D.H. Understanding the dynamic behavior of acid sites on TiO2-supported vanadia catalysts via operando DRIFTS under SCR-relevant conditions. J. Catal. 2020, 382, 269–279. [Google Scholar] [CrossRef]
- Liu, H.; You, C.; Wang, H. Time-resolved in-situ IR and DFT study: NH3 adsorption and redox cycle of acid site on vanadium-based catalysts for NO abatement via selective catalytic reduction. Chem. Eng. J. 2020, 382, 122756. [Google Scholar] [CrossRef]
- Kong, M.; Liu, Q.; Jiang, L.; Tong, W.; Yang, J.; Ren, S.; Li, J.; Tian, Y. K+ deactivation of V2O5-WO3/TiO2 catalyst during selective catalytic reduction of NO with NH3: Effect of vanadium content. Chem. Eng. J. 2019, 370, 518–526. [Google Scholar] [CrossRef]
- He, G.; Lian, Z.; Yu, Y.; Yang, Y.; Liu, K.; Shi, X.; Yan, Z.; Shan, W.; He, H. Polymeric vanadyl species determine the low-temperature activity of V-based catalysts for the SCR of NOx with NH3. Sci. Adv. 2018, 4, eaau4637. [Google Scholar] [CrossRef]
- Jaegers, N.R.; Lai, J.K.; He, Y.; Walter, E.; Dixon, D.A.; Vasiliu, M.; Chen, Y.; Wang, C.; Hu, M.Y.; Mueller, K.T.; et al. Mechanism by which tungsten oxide promotes the activity of supported V2O5/TiO2 catalysts for NOx abatement: Structural effects revealed by V-51 MAS NMR spectroscopy. Angew. Chem. Int. Ed. 2019, 58, 12609–12616. [Google Scholar] [CrossRef]
- Liu, K.; Yan, Z.; He, H.; Feng, Q.; Shan, W. The effects of H2O on a vanadium-based catalyst for NH3-SCR at low temperatures: A quantitative study of the reaction pathway and active sites. Catal. Sci. Technol. 2019, 9, 5593–5604. [Google Scholar] [CrossRef]



| Catalysts | Preparation Methods | Reaction Conditions | NOx (or NO) Conversion (Temperature Range) | GHSV | Source |
|---|---|---|---|---|---|
| Cu-V/TiO2 | Impregnation | 0.05% NO, 0.05% NH3, 5 vol% O2 | >90% (225–375 °C) | 26,000 h−1 | 10 |
| CuV/WTi | Impregnation | 0.05% NO, 0.05% NH3, 5 vol% O2 | >84% (250–400 °C) | 10,000 h−1 | 11 |
| VWCeCuTi | Impregnation | 0.05% NO, 0.05% NH3, 5 vol% O2 | >80% (250–375 °C) | 60,000 h−1 | 13 |
| V/Ce/WTi | Precipitation | 0.05% NO, 0.05% NH3, 5 vol% O2, 5vol% H2O | 95% (250–450 °C) | 18,000 h−1 | 14 |
| VCeTiSO42- | Sol−gel | 0.1% NO, 0.1% NH3, 8 vol% O2, 3.5vol%H2O | >80% (275–450 °C) | 120,000 h−1 | 16 |
| V-Ce(SO4)2/Ti | Impregnation | 0.08% NO, 0.08% NH3, 5 vol% O2, 0.05%SO2, 5vol%H2O | 100% (300–450 °C) | 150,000 mL/g·h | 17 |
| 7%Ce-1%CuV/Ti | Impregnation | 0.05% NO, 0.05% NH3, 5 vol% O2 | >97% (200–400 °C) | 45,000 h−1 | 22 |
| V5Ce35Sb2/TiO2 | Impregnation | 0.1% NO, 0.1% NH3, 3 vol% O2 | >90% (225–400 °C) | 45,000 h−1 | 23 |
| V-Mn/Ce | Hydrothermal | 0.05% NO, 0.05% NH3, 5 vol% O2 | >80% (200–350 °C) | 160,000 h−1 | 25 |
| V3Mo5/WTi | Impregnation | 0.05% NO, 0.05% NH3, 3 vol% O2 | >80% (200–300 °C) | 60,000 h−1 | 28 |
| 10%Cr0.2-V0.8/TiO2 | Impregnation | 0.05% NO, 0.05% NH3, 3 vol% O2 | >85% (160–280 °C) | 60,000 mL/g·h | 29 |
| 6%Nb-3% V/W-Ti | Impregnation | 0.05% NO, 0.05% NH3, 3 vol% O2 | >90% (225–400 °C) | 60,000 h−1 | 30 |
| 4%Si2%V/10%W/Ti | Co-impregnation | 0.05% NO, 0.06% NH3, 10 vol% O2 | >80% (300–500 °C) | 50,000 h−1 | 31 |
| S3N1V/Ti100 | Sol−gel | 0.05% NO, 0.05% NH3, 5 vol% O2 | 100% (240–450 °C) | 27,549 h−1 | 32 |
| VTiF-(NH4)2TiF6 | Sol−gel | 0.05% NO, 0.06% NH3, 5 vol% O2 | 78.5% (240 °C) | 38,900 h−1 | 34 |
| 0.2%F-VW/Ti | Impregnation | 0.07% NO, 0.07% NH3, 5 vol% O2 | >95% (160–360 °C) | 30,000 h−1 | 35 |
| 1P-VWTi | Impregnation | 0.05% NO, 0.05% NH3, 5 vol% O2 | 96% (250 °C) | 70,000 h−1 | 36 |
| 5%V/15%TPA/Ti | Impregnation | 0.1% NO, 0.1% NH3, 4 vol% O2 2.3 vol% H2O | 100% (300 °C) | 180,000 h−1 | 38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lian, Z.; Li, Y.; Shan, W.; He, H. Recent Progress on Improving Low-Temperature Activity of Vanadia-Based Catalysts for the Selective Catalytic Reduction of NOx with Ammonia. Catalysts 2020, 10, 1421. https://doi.org/10.3390/catal10121421
Lian Z, Li Y, Shan W, He H. Recent Progress on Improving Low-Temperature Activity of Vanadia-Based Catalysts for the Selective Catalytic Reduction of NOx with Ammonia. Catalysts. 2020; 10(12):1421. https://doi.org/10.3390/catal10121421
Chicago/Turabian StyleLian, Zhihua, Yingjie Li, Wenpo Shan, and Hong He. 2020. "Recent Progress on Improving Low-Temperature Activity of Vanadia-Based Catalysts for the Selective Catalytic Reduction of NOx with Ammonia" Catalysts 10, no. 12: 1421. https://doi.org/10.3390/catal10121421
APA StyleLian, Z., Li, Y., Shan, W., & He, H. (2020). Recent Progress on Improving Low-Temperature Activity of Vanadia-Based Catalysts for the Selective Catalytic Reduction of NOx with Ammonia. Catalysts, 10(12), 1421. https://doi.org/10.3390/catal10121421





