Recent Understanding of Low-Temperature Copper Dynamics in Cu-Chabazite NH3-SCR Catalysts
Abstract
1. Introduction
2. Theoretical Prediction
2.1. Nature of Dynamic Cu Species
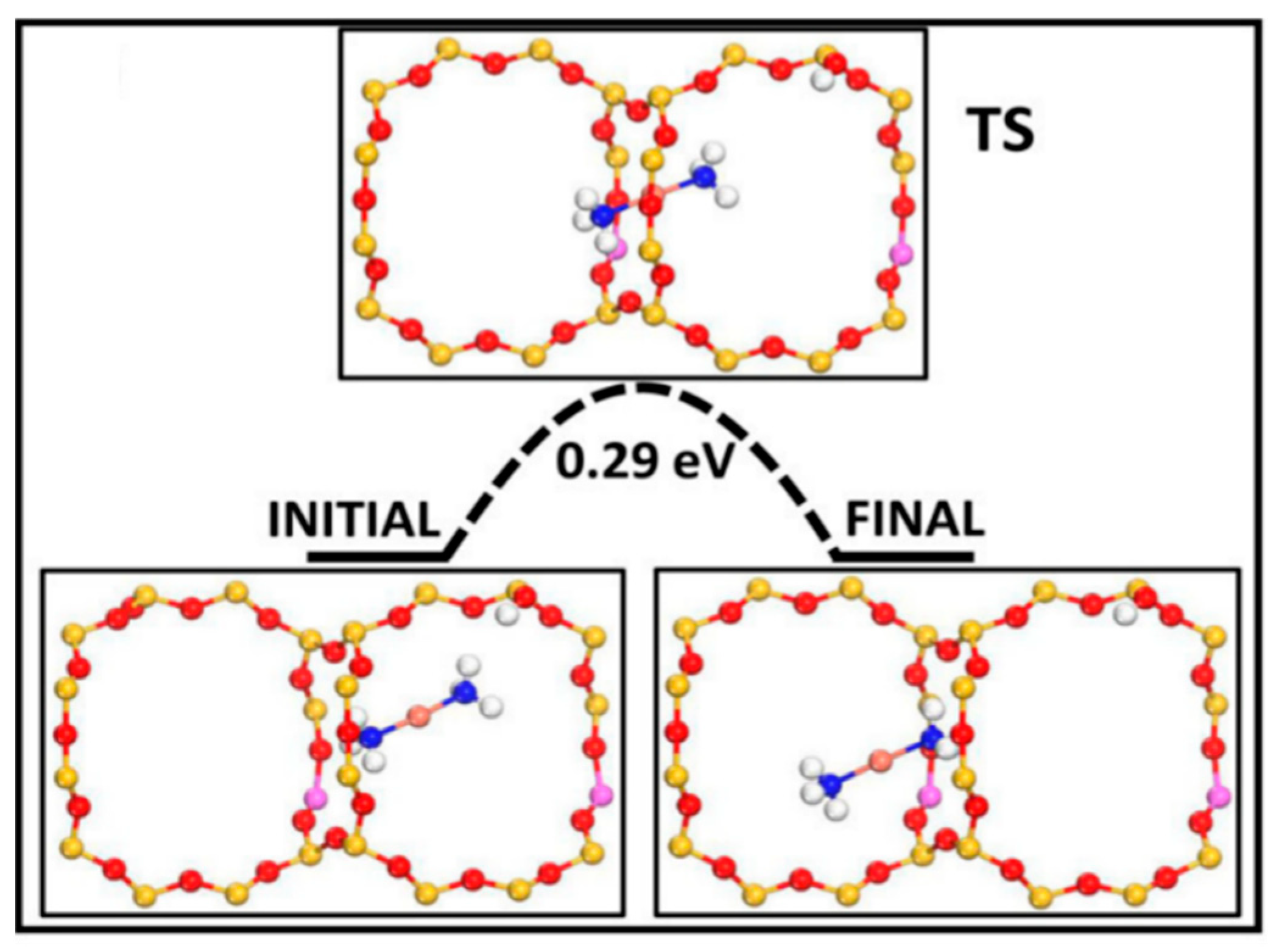
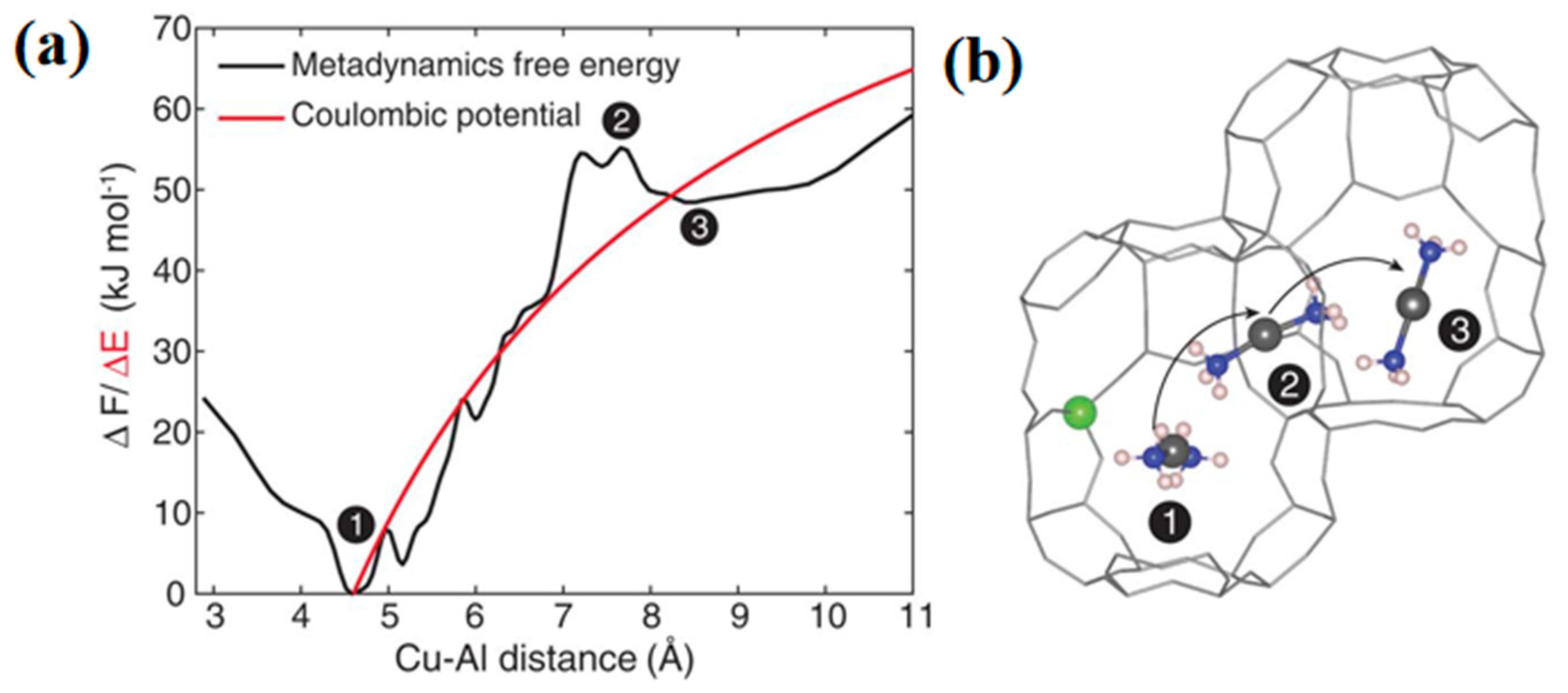
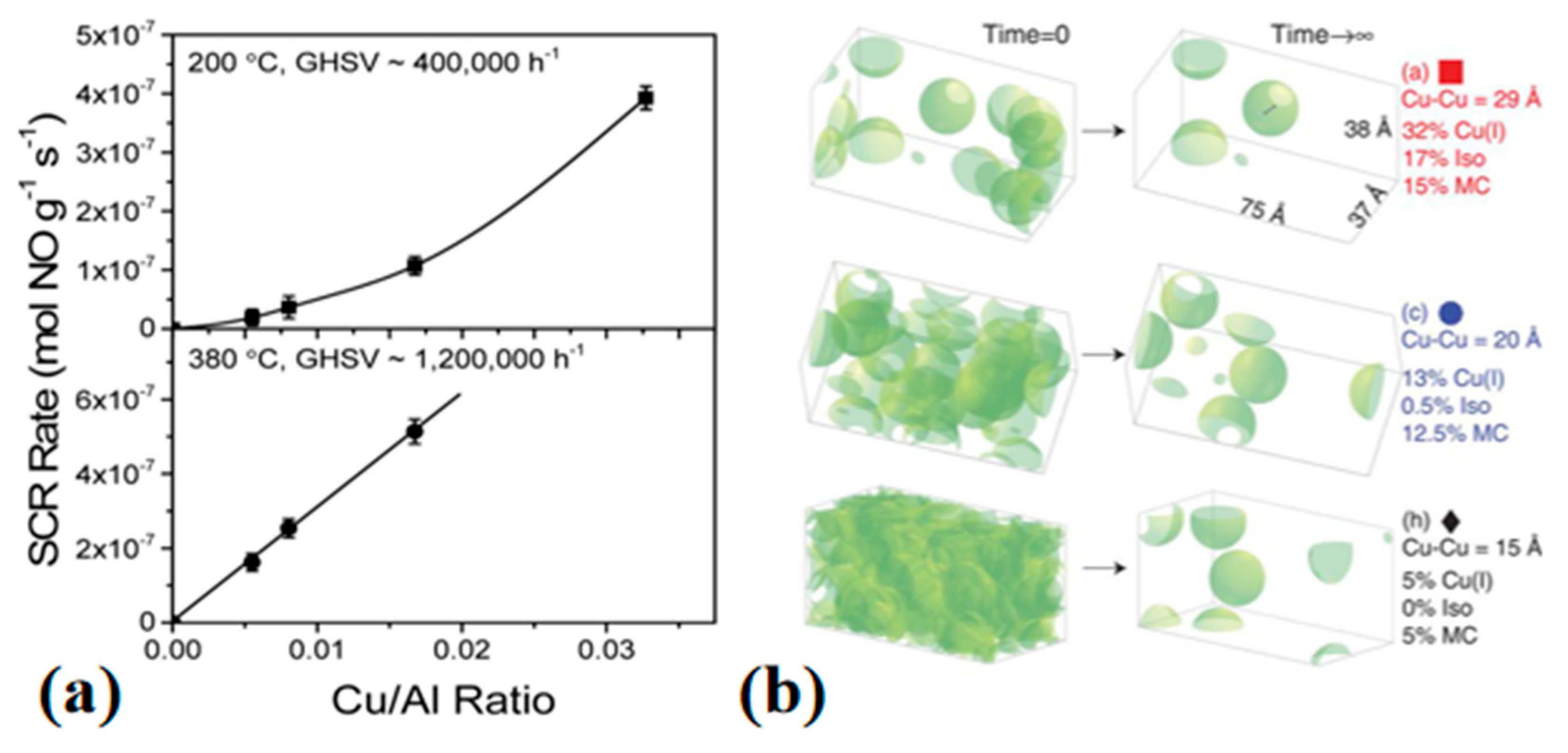
2.2. Inter-Cage Motion of Cu Sites
3. Experimental Detection
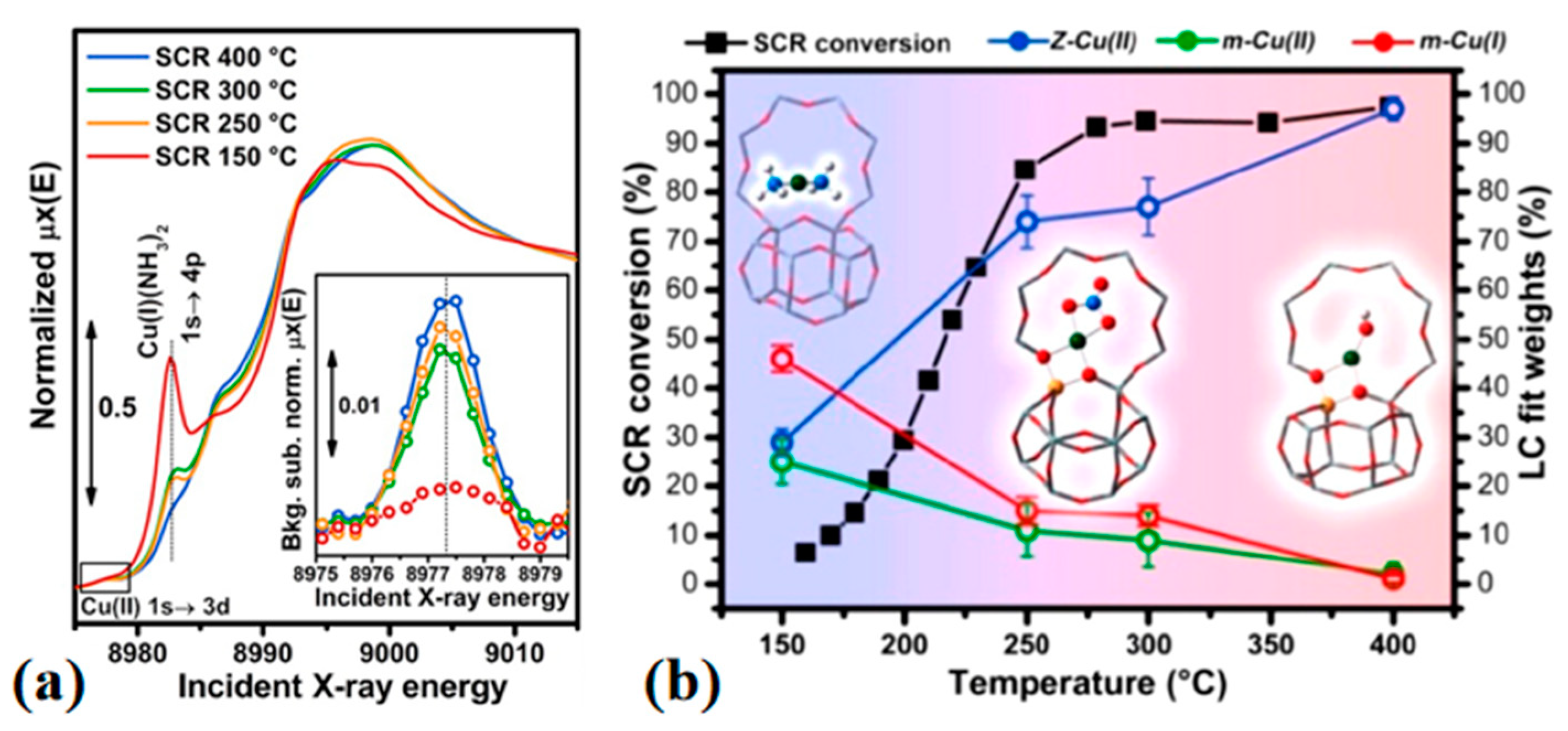
3.1. X-ray Absorption Spectroscopy Based Techniques
3.2. Optical Spectroscopy Techniques
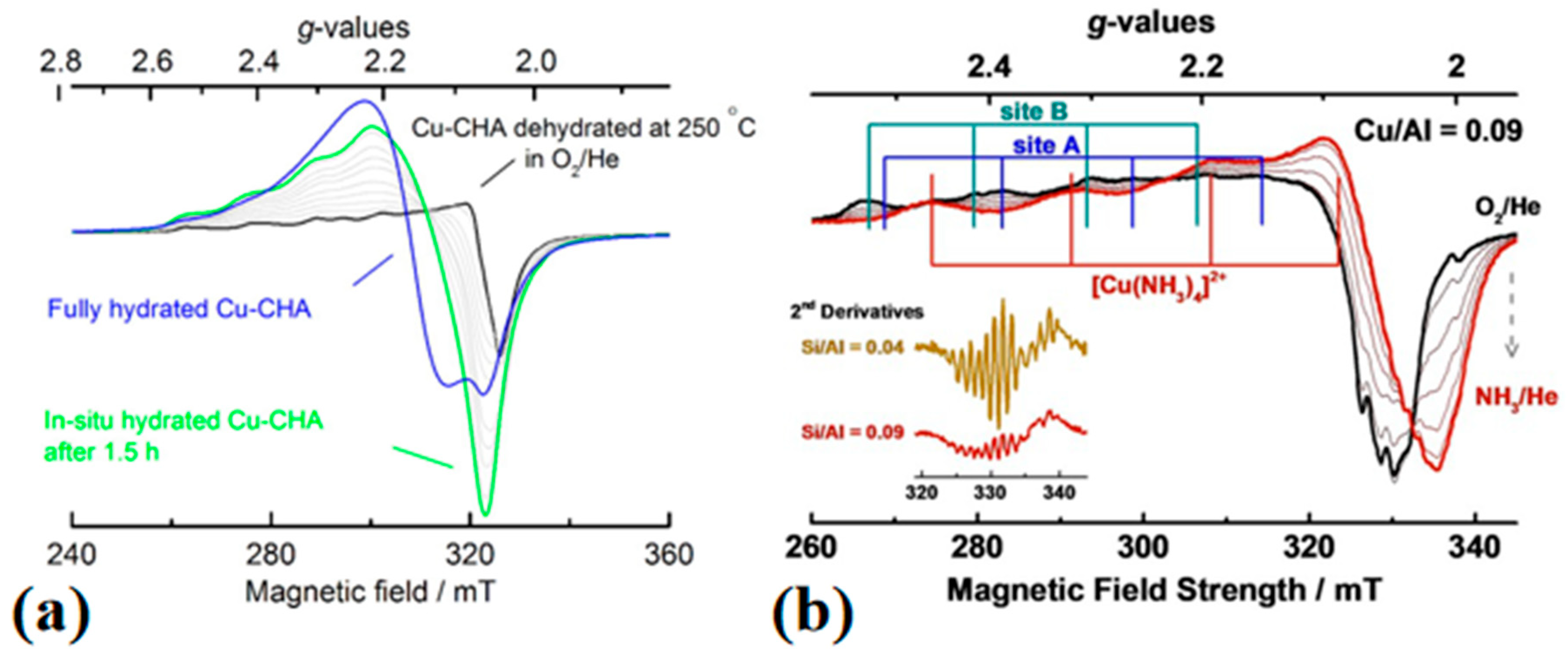
3.3. Electron Paramagnetic Resonance (EPR)
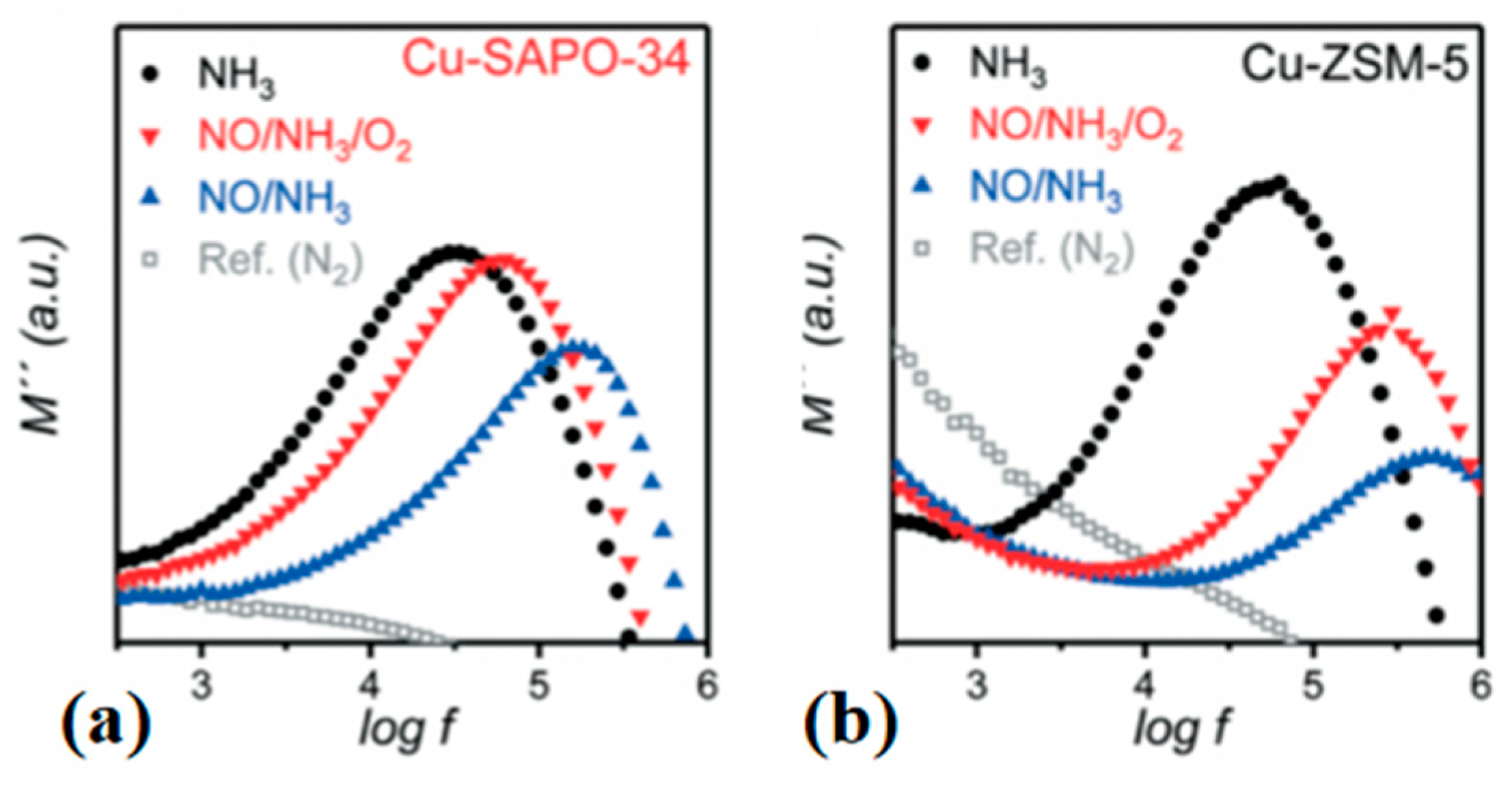
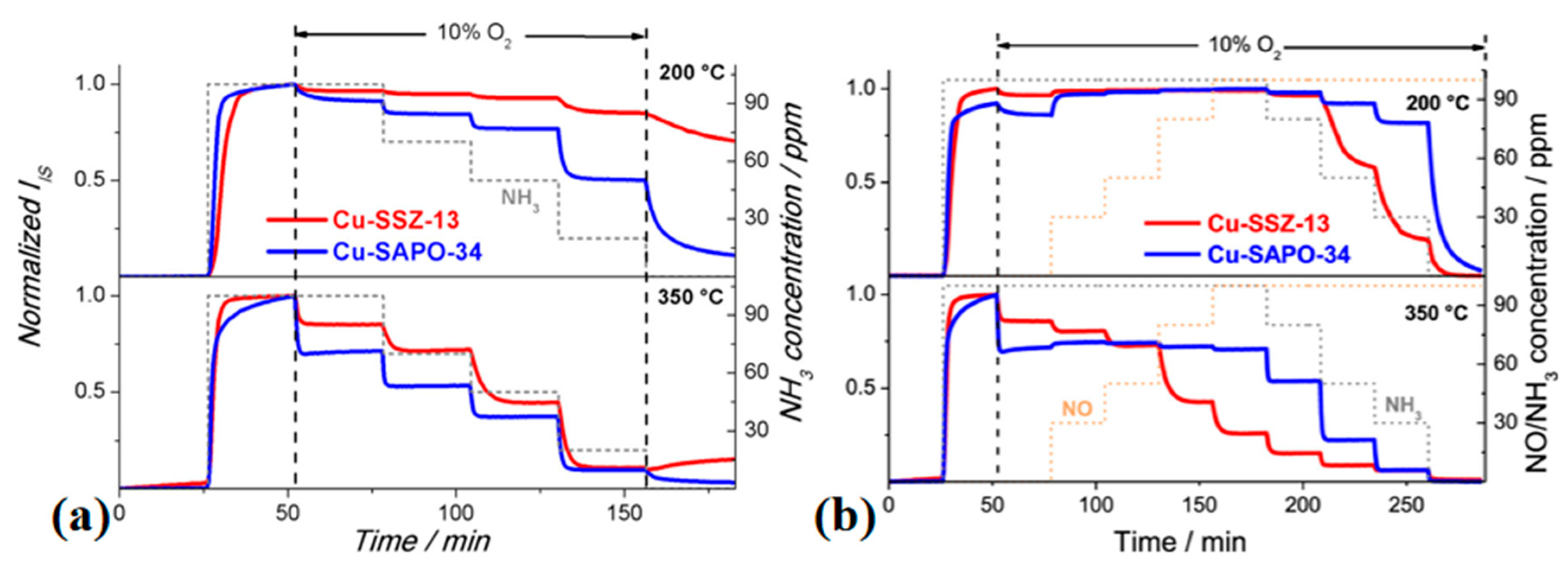
3.4. Impedance Spectroscopy
4. Role of Cu Mobility in NH3-SCR Catalysis
4.1. Regulating Low-Temperature Cu Redox
4.2. Promoting Solid-State Cu Ion Exchange
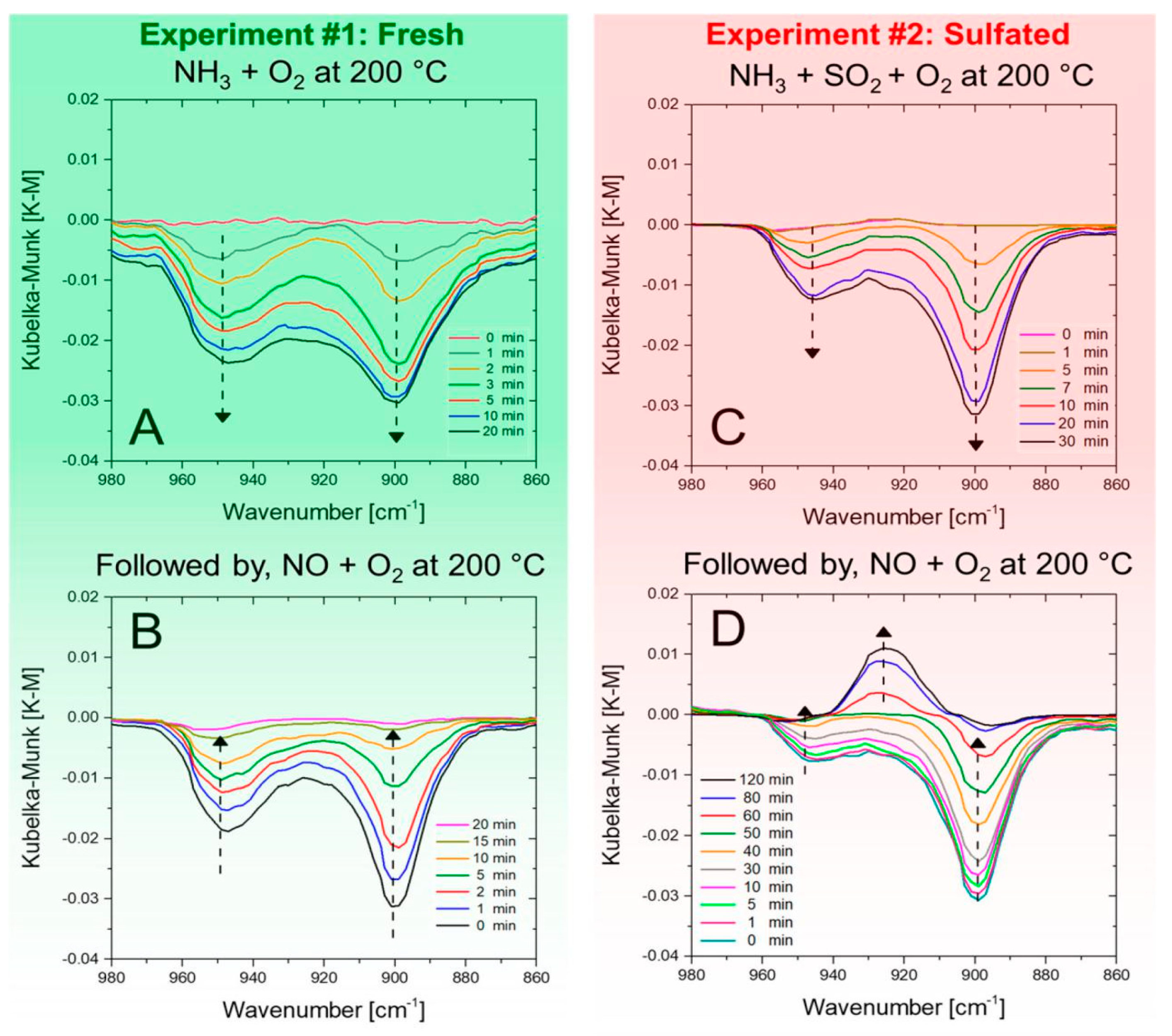
4.3. Monitoring NH3 Storage and Conversion
5. Summary and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Unger, N.; Zheng, Y.; Yue, X.; Harper, K.L. Mitigation of ozone damage to the world’s land ecosystems by source sector. Nat. Clim. Chang. 2020, 10, 134–137. [Google Scholar] [CrossRef]
- Yu, T.; Hao, T.; Fan, D.; Wang, J.; Shen, M.; Li, W. Recent NH3-SCR Mechanism Research over Cu/SAPO-34 Catalyst. J. Phys. Chem. C 2014, 118, 6565–6575. [Google Scholar] [CrossRef]
- Li, Y.; Deng, J.; Song, W.; Liu, J.; Zhao, Z.; Gao, M.; Wei, Y.; Zhao, L. The Nature of Cu Species in Cu-SAPO-18 Catalyst for NH3-SCR: Combination of Experiments and DFT Calculations. J. Phys. Chem. C 2016, 120, 14669–14680. [Google Scholar] [CrossRef]
- Lezcano-Gonzalez, I.; Deka, U.; Arstad, B.; Deyne, Y.D.; Hemelsoet, K.; Waroquier, M.; Speybroeck, V.V.; Weckhuysen, B.M.; Beale, A.M. Determining the storage, availability and reactivity of NH3 within Cu-Chabazite-based Ammonia Selective Catalytic Reduction systems. Phys. Chem. Chem. Phys. 2013, 16, 1639–1650. [Google Scholar] [CrossRef] [PubMed]
- Brandenberger, S.; Kröcher, O.; Wokaun, A.; Tissler, A. The role of Brønsted acidity in the selective catalytic reduction of NO with ammonia over Fe-ZSM-5. J. Catal. 2009, 268, 297–306. [Google Scholar] [CrossRef]
- Maier, S.M.; Jentys, A.; Janousch, M.; Bokhoven, J.A.V.; Lercher, J.A. Unique dynamic changes of Fe cationic species under NH3-SCR conditions. J. Phys. Chem. C 2012, 116, 5846–5856. [Google Scholar] [CrossRef]
- Fickel, D.W.; D’Addio, E.; Lauterbach, J.A.; Lobo, R.F. The ammonia selective catalytic reduction activity of copper-exchanged small-pore zeolites. Appl. Catal. B Environ. 2011, 102, 441–448. [Google Scholar] [CrossRef]
- Kumar, A.; Smith, M.A.; Kamasamudram, K.; Currier, N.W.; Yezerets, A. Chemical deSOx: An effective way to recover Cu-zeolite SCR catalysts from sulfur poisoning. Catal. Today 2016, 267, 10–16. [Google Scholar] [CrossRef]
- Olsson, L.; Wijayanti, K.; Leistner, K.; Kumar, A.; Joshi, S.Y.; Kamasamudram, K.; Currier, N.W.; Yezerets, A. A kinetic model for sulfur poisoning and regeneration of Cu/SSZ-13 used for NH3-SCR. Appl. Catal. B Environ. 2016, 183, 394–406. [Google Scholar] [CrossRef]
- Wijayanti, K.; Leistner, K.; Chand, S.; Kumar, A.; Kamasamudram, K.; Currier, N.W.; Yezerets, A.; Olsson, L. Deactivation of Cu-SSZ-13 by SO2 exposure under SCR conditions. Catal. Sci. Technol. 2016, 6, 2565–2579. [Google Scholar] [CrossRef]
- Zheng, Y.; Harold, M.P.; Luss, D. Effects of CO, H2 and C3H6 on Cu-SSZ-13 catalyzed NH3-SCR. Catal. Today 2016, 264, 44–54. [Google Scholar] [CrossRef]
- Schmidt, J.E.; Oord, R.; Guo, W.; Poplawsky, J.D.; Weckhuysen, B.M. Nanoscale tomography reveals the deactivation of automotive copper-exchanged zeolite catalysts. Nat. Commun. 2017, 8, 1666. [Google Scholar] [CrossRef] [PubMed]
- Usui, T.; Liu, Z.; Ibe, S.; Zhu, J.; Anand, C.; Igarashi, H.; Onaya, N.; Sasaki, Y.; Shiramata, Y.; Kusamoto, T.; et al. Improve the Hydrothermal Stability of Cu-SSZ-13 Zeolite Catalyst by Loading a Small Amount of Ce. ACS Catal. 2018, 8, 9165–9173. [Google Scholar] [CrossRef]
- Usui, T.; Liu, Z.; Igarashi, H.; Sasaki, Y.; Shiramata, Y.; Yamada, H.; Ohara, K.; Kusamoto, T.; Wakihara, T. Identifying the Factors Governing the Early-Stage Degradation of Cu-Chabazite Zeolite for NH3-SCR. ACS Omega 2019, 4, 3653–3659. [Google Scholar] [CrossRef] [PubMed]
- Selleri, T.; Nova, I.; Tronconi, E.; Schmeisser, V.; Seher, S. The impact of light and heavy hydrocarbons on the NH3-SCR activity of commercial Cu- and Fe-zeolite catalysts. Catal. Today 2019, 320, 100–111. [Google Scholar] [CrossRef]
- Ruggeri, M.P.; Nova, I.; Tronconi, E.; Collier, J.E.; York, A.P.E. Structure-Activity Relationship of Different Cu-Zeolite Catalysts for NH3-SCR. Top. Catal. 2016, 59, 875–881. [Google Scholar] [CrossRef]
- Beale, A.M.; Gao, F.; Lezcano-Gonzalez, I.; Peden, C.H.F.; Szanyi, J. Recent advances in automotive catalysis for NOx emission control by small-pore microporous materials. Chem. Soc. Rev. 2015, 44, 7371–7405. [Google Scholar] [CrossRef]
- Gao, F.; Wang, Y.; Washton, N.M.; Kollar, M.; Szanyi, J. Effects of Alkali and Alkaline Earth Cocations on the Activity and Hydrothermal Stability of Cu/SSZ-13 NH3–SCR Catalysts. ACS Catal. 2015, 5, 6780–6791. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Haller, G.; Li, Y. Recent advances in the selective catalytic reduction of NOx with NH3 on Cu-Chabazite catalysts. Appl. Catal. B Environ. 2017, 202, 346–354. [Google Scholar] [CrossRef]
- Xie, L.; Liu, F.; Ren, L.; Shi, X.; Shou, F. Excellent Performance of One-Pot Synthesized Cu-SSZ-13 Catalyst for the Selective Catalytic Reduction of NOx with NH3. Environ. Sci. Technol. 2014, 48, 566–572. [Google Scholar] [CrossRef]
- Damma, D.; Ettireddy, P.R.; Reddy, B.M.; Smirniotis, P.G. A Review of Low Temperature NH3-SCR for Removal of NOx. Catalysts 2019, 9, 349. [Google Scholar] [CrossRef]
- Shan, W.; Song, H. Catalysts for the selective catalytic reduction of NOx with NH3 at low temperature. Catal. Sci. Technol. 2015, 5, 4280–4288. [Google Scholar] [CrossRef]
- Colombo, M.; Nova, I.; Tronconi, E.; Schmeisser, V.; Weibel, M. Mathematical modelling of cold start effects over zeolite SCR catalysts for exhaust gas aftertreatment. Catal. Today 2014, 231, 99–104. [Google Scholar] [CrossRef]
- Su, W.; Chang, H.; Peng, Y.; Zhang, C.; Wi, J. Reaction Pathway Investigation on the Selective Catalytic Reduction of NO with NH3 over Cu/SSZ-13 at Low Temperatures. Environ. Sci. Technol. 2015, 49, 467–473. [Google Scholar] [CrossRef] [PubMed]
- De Prins, M.; Verheyen, E.; Hoffmann, A.; Vanbutsele, G.; Sree, S.P.; Kerkhofs, S.; Van Tendeloo, L.; Schuetze, F.-W.; Martens, J. Structural parameters governing low temperature activity of small pore copper zeolites in NH3-SCR. J. Catal. 2020, 390, 224–236. [Google Scholar] [CrossRef]
- Fan, C.; Ming, S.; Chen, Z.; Pang, L.; Guo, W.; Dong, C.; Liu, P.; Li, T. Cold Start Wetting Effect on the Catalytic Property and Hydrothermal Stability of a Cu-SSZ-13 Catalyst for NH3-SCR. Ind. Eng. Chem. Res. 2020, 59, 12304–12312. [Google Scholar] [CrossRef]
- Liu, Z.; Wakihara, T.; Oshima, K.; Nishioka, D.; Hotta, Y. Widening Synthesis Bottlenecks: Realization of Ultrafast and Continuous-Flow Synthesis of High-Silica Zeolite SSZ-13 for NOx Removal. Angew. Chem. Int. Ed. 2015, 54, 5683–5687. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B. Database of Zeolite Structures. Available online: http://www.iza-structure.org/databases/ (accessed on 25 November 2020).
- Moreno-Gonzalez, M.; Millan, R.; Concepcion, P.; Blasco, T.; Boronat, M. Spectroscopic Evidence and Density Functional Theory (DFT) Analysis of Low-Temperature Oxidation of Cu+ to Cu2+NOx in Cu-CHA Catalysts: Implications for the SCR-NOx Reaction Mechanism. ACS Catal. 2019, 9, 2725–2738. [Google Scholar] [CrossRef]
- Astala, R.; Auerbach, S.M.; Monson, P.A. Density functional theory study of silica zeolite structures: Stabilities and mechanical properties of SOD, LTA, CHA, MOR, and MFI. J. Phys. Chem. B 2004, 108, 9208–9215. [Google Scholar] [CrossRef]
- Burton, A.W.; Lee, G.S.; Zones, S.I. Phase selectivity in the syntheses of cage-based zeolite structures: An investigation of thermodynamic interactions between zeolite hosts and structure directing agents by molecular modeling. Microporous Mesoporous Mater. 2006, 90, 129–144. [Google Scholar] [CrossRef]
- Nakatsuka, A.; Okada, H.; Fujiwara, K.; Nakayama, N.; Mizota, T. Crystallographic configurations of water molecules and exchangeable cations in a hydrated natural CHA-zeolite (chabazite). Microporous Mesoporous Mater. 2007, 102, 188–195. [Google Scholar] [CrossRef]
- Liang, J.; Su, J.; Wang, Y.; Lin, Z.; Mu, W.; Zheng, H.; Zou, R.; Liao, F.; Lin, J. CHA-type zeolites with high boron content: Synthesis, structure and selective adsorption properties. Microporous Mesoporous Mater. 2014, 194, 97–105. [Google Scholar] [CrossRef]
- Lok, B.M.; Messina, C.A.; Patton, R.L.; Gajek, R.T.; Cannan, T.R.; Flanigen, E.M. Silicoaluminophosphate molecular sieves: Another new class of microporous crystalline inorganic solids. Chem. Inf. 1985, 106, 6092–6093. [Google Scholar] [CrossRef]
- Chen, Z.; Fan, C.; Pang, L.; Ming, S.; Guo, W.; Liu, P.; Chen, H.; Li, T. One-pot synthesis of high performance Cu-SAPO-18 catalyst for NO reduction by NH3-SCR: Influence of silicon content on the catalytic properties of Cu-SAPO-18. Chem. Eng. J. 2018, 348, 608–617. [Google Scholar] [CrossRef]
- Paolucci, C.; Di Iorio, J.R.; Ribeiro, F.H.; Gounder, R.; Schneider, W.F. Catalysis Science of NOx Selective Catalytic Reduction With Ammonia Over Cu-SSZ-13 and Cu-SAPO-34. Adv. Catal. 2016, 59, 1–107. [Google Scholar]
- Fan, C.; Chen, Z.; Pang, L.; Ming, S.; Zhang, X.; Albert, K.B.; Liu, P.; Chen, H.; Li, T. The influence of Si/Al ratio on the catalytic property and hydrothermal stability of Cu-SSZ-13 catalysts for NH3-SCR. Appl. Catal. A Gen. 2018, 550, 256–265. [Google Scholar] [CrossRef]
- Paolucci, C.; Parekh, A.A.; Khurana, I.; Di Iorio, J.R.; Li, H.; Albarracin Caballero, J.D.; Shih, A.J.; Anggara, T.; Delgass, W.N.; Miller, J.T. Catalysis in a cage: Condition-dependent speciation and dynamics of exchanged Cu cations in SSZ-13 zeolites. J. Am. Chem. Soc. 2016, 138, 6028–6048. [Google Scholar] [CrossRef]
- Gao, F.; Washton, N.M.; Wang, Y.; Kollar, M.; Szanyi, J.; Peden, C.H.F. Effects of Si/Al ratio on Cu/SSZ-13 NH3-SCR catalysts: Implications for the active Cu species and the roles of Bronsted acidity. J. Catal. 2015, 331, 25–38. [Google Scholar] [CrossRef]
- Kwak, J.H.; Zhu, H.; Lee, J.H.; Peden, C.H.; Szanyi, J. Two different cationic positions in Cu-SSZ-13? Chem. Commun. 2012, 48, 4758–4760. [Google Scholar] [CrossRef]
- Andersen, C.W.; Bremholm, M.; Vennestrøm, P.N.R.; Blichfeld, A.B.; Lundegaard, L.F.; Iversen, B.B. Location of Cu2+ in CHA zeolite investigated by X-ray diffraction using the Rietveld/maximum entropy method. IUCrJ 2014, 1, 382–386. [Google Scholar] [CrossRef]
- Borfecchia, E.; Beato, P.; Svelle, S.; Olsbye, U.; Lamberti, C.; Bordiga, S. Cu-CHA—A model system for applied selective redox catalysis. Chem. Soc. Rev. 2018, 47, 8097–8133. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, S.T.; Fickel, D.W.; Lobo, R.F.; Weckhuysen, B.M.; Beale, A.M. Isolated Cu2+ ions: Active sites for selective catalytic reduction of NO. Chem. Commun. 2011, 47, 800–802. [Google Scholar] [CrossRef] [PubMed]
- Deka, U.; Juhin, A.; Eilertsen, E.A.; Emerich, H.; Green, M.A.; Korhonen, S.T.; Weckhuysen, B.M.; Beale, A.M. Confirmation of isolated Cu2+ ions in SSZ-13 zeolite as active sites in NH3-selective catalytic reduction. J. Phys. Chem. C 2012, 116, 4809–4818. [Google Scholar] [CrossRef]
- Bates, S.A.; Verma, A.A.; Paolucci, C.; Parekh, A.A.; Anggara, T.; Yezerets, A.; Schneider, W.F.; Miller, J.T.; Delgass, W.N.; Ribeiro, F.H. Identification of the active Cu site in standard selective catalytic reduction with ammonia on Cu-SSZ-13. J. Catal. 2014, 312, 87–97. [Google Scholar] [CrossRef]
- Leistner, K.; Xie, K.; Kumar, A.; Kamasamudram, K.; Olsson, L. Ammonia Desorption Peaks Can Be Assigned to Different Copper Sites in Cu/SSZ-13. Catal. Lett. 2017, 147, 1882–1890. [Google Scholar] [CrossRef]
- Concepcion, P.; Boronat, M.; Millan, R.; Moliner, M.; Corma, A. Identification of Distinct Copper Species in Cu-CHA Samples Using NO as Probe Molecule. A Combined IR Spectroscopic and DFT Study. Top. Catal. 2017, 60, 1653–1663. [Google Scholar] [CrossRef]
- Martini, A.; Alladio, E.; Borfecchia, E. Determining Cu-Speciation in the Cu-CHA Zeolite Catalyst: The Potential of Multivariate Curve Resolution Analysis of In Situ XAS Data. Top. Catal. 2018, 61, 1396–1407. [Google Scholar] [CrossRef]
- Janssens, T.V.W.; Falsig, H.; Lundegaard, L.F.; Vennestrom, P.N.R.; Rasmussen, S.B.; Moses, P.G.; Giordanino, F.; Borfecchia, E.; Lomachenko, K.A.; Lamberti, C.; et al. A Consistent Reaction Scheme for the Selective Catalytic Reduction of Nitrogen Oxides with Ammonia. ACS Catal. 2015, 5, 2832–2845. [Google Scholar] [CrossRef]
- Marberger, A.; Petrov, A.W.; Steiger, P.; Elsener, M.; Krocher, O.; Nachtegaal, M.; Ferri, D. Time-resolved copper speciation during selective catalytic reduction of NO on Cu-SSZ-13. Nat. Catal. 2018, 1, 221–227. [Google Scholar] [CrossRef]
- Zhang, T.; Qiu, F.; Chang, H.; Li, X.; Li, J. Identification of active sites and reaction mechanism on low-temperature SCR activity over Cu-SSZ-13 catalysts prepared by different methods. Catal. Sci. Technol. 2016, 6, 6294–6304. [Google Scholar] [CrossRef]
- Gao, F.; Peden, C.H.F. Recent Progress in Atomic-Level Understanding of Cu/SSZ-13 Selective Catalytic Reduction Catalysts. Catalysts 2018, 8, 140. [Google Scholar] [CrossRef]
- Mcewen, J.S.; Anggara, T.; Schneider, W.F.; Kispersky, V.F.; Miller, J.T.; Delgass, W.N.; Ribeiro, F.H.J.C.T. Integrated operando X-ray absorption and DFT characterization of Cu–SSZ-13 exchange sites during the selective catalytic reduction of NOx with NH3. Catal. Today 2012, 184, 129–144. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Zhao, H.; Liu, M.; Ma, Y.; Yong, X.; Chen, H.; Li, Y. The Cu migration of Cu-SAPO-34 catalyst for ammonia selective catalytic reduction of NOx during high temperature hydrothermal aging treatment. Catal. Today 2019, 327, 126–133. [Google Scholar] [CrossRef]
- Kwak, J.H.; Varga, T.; Peden, C.H.F.; Gao, F.; Hanson, J.C.; Szanyi, J. Following the movement of Cu ions in a SSZ-13 zeolite during dehydration, reduction and adsorption: A combined in situ TP-XRD, XANES/DRIFTS study. J. Catal. 2014, 314, 83–93. [Google Scholar] [CrossRef]
- Paolucci, C.; Khurana, I.; Parekh, A.A.; Li, S.; Shih, A.J.; Li, H.; Di Iorio, J.R.; Albarracin-Caballero, J.D.; Yezerets, A.; Miller, J.T.; et al. Dynamic multinuclear sites formed by mobilized copper ions in NOx selective catalytic reduction. Science 2017, 357, 898. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Mei, D.; Wang, Y.; Szanyi, J.; Peden, C.H.F. Selective Catalytic Reduction over Cu/SSZ-13: Linking Homo- and Heterogeneous Catalysis. J. Am. Chem. Soc. 2017, 139, 4935–4942. [Google Scholar] [CrossRef]
- Beale, A.M.; Lezcano-Gonzalez, I.; Slawinski, W.A.; Wragg, D.S. Correlation between Cu ion migration behaviour and deNO(x) activity in Cu-SSZ-13 for the standard NH3-SCR reaction. Chem. Commun. 2016, 55, 1667. [Google Scholar] [CrossRef]
- Andersen, C.W.; Borfecchia, E.; Bremholm, M.; Jorgensen, M.R.V.; Vennestrom, P.N.R.; Lamberti, C.; Lundegaard, L.F.; Iversen, B.B. Redox-Driven Migration of Copper Ions in the Cu-CHA Zeolite as Shown by the In Situ PXRD/XANES Technique. Angew. Chem. Int. Ed. 2017, 56, 10367–10372. [Google Scholar] [CrossRef]
- Chen, P.; Jablonska, M.; Weide, P.; Caumanns, T.; Weirich, T.; Muhler, M.; Moos, R.; Palkovits, R.; Simon, U. Formation and Effect of NH4+ Intermediates in NH3-SCR over Fe-ZSM-5 Zeolite Catalysts. ACS Catal. 2016, 6, 7696–7700. [Google Scholar] [CrossRef]
- Chen, P.; Schönebaum, S.; Simons, T.; Rauch, D.; Moos, R.; Simon, U. In situ monitoring of DeNOx-SCR on zeolite catalysts by means of simultaneous impedance and DRIFT spectroscopy. Procedia Eng. 2015, 120, 257–260. [Google Scholar] [CrossRef]
- Chen, P.; Schoenebaum, S.; Simons, T.; Rauch, D.; Dietrich, M.; Moos, R.; Simon, U. Correlating the Integral Sensing Properties of Zeolites with Molecular Processes by Combining Broadband Impedance and DRIFT Spectroscopy-A New Approach for Bridging the Scales. Sensors 2015, 15, 28915–28941. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Simboeck, J.; Schoenebaum, S.; Rauch, D.; Simons, T.; Palkovits, R.; Moos, R.; Simon, U. Monitoring NH3 storage and conversion in Cu-ZSM-5 and Cu-SAPO-34 catalysts for NH3-SCR by simultaneous impedance and DRIFT spectroscopy. Sens. Actuators B Chem. 2016, 236, 1075–1082. [Google Scholar] [CrossRef]
- Simons, T.; Chen, P.; Rauch, D.; Moos, R.; Simon, U. Sensing catalytic conversion: Simultaneous DRIFT and impedance spectroscopy for in situ monitoring of NH3-SCR on zeolites. Sens. Actuators B Chem. 2016, 224, 492–499. [Google Scholar] [CrossRef]
- Chen, P.; Rauch, D.; Weide, P.; Schoenebaum, S.; Simons, T.; Muhler, M.; Moos, R.; Simon, U. The effect of Cu and Fe cations on NH3-supported proton transport in DeNO(x)-SCR zeolite catalysts. Catal. Sci. Technol. 2016, 6, 3362–3366. [Google Scholar] [CrossRef]
- Simons, T.; Simon, U. Zeolites as nanoporous, gas-sensitive materials for in situ monitoring of DeNO(x)-SCR. Beilstein J. Nanotechnol. 2012, 3, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Moos, R.; Simon, U. Metal Loading Affects the Proton Transport Properties and the Reaction Monitoring Performance of Fe-ZSM-5 and Cu-ZSM-5 in NH3-SCR. J. Phys. Chem. C 2016, 120, 25361–25370. [Google Scholar] [CrossRef]
- Rizzotto, V.; Chen, D.; Tabak, B.M.; Yang, J.-Y.; Ye, D.; Simon, U.; Chen, P. Spectroscopic identification and catalytic relevance of NH4+ intermediates in selective NOx reduction over Cu-SSZ-13 zeolites. Chemosphere 2020, 250, 126272. [Google Scholar] [CrossRef]
- Paolucci, C.; Verma, A.A.; Bates, S.A.; Kispersky, V.F.; Miller, J.T.; Gounder, R.; Delgass, W.N.; Ribeiro, F.H.; Schneider, W.F. Isolation of the Copper Redox Steps in the Standard Selective Catalytic Reduction on Cu-SSZ-13. Angew. Chem. Int. Ed. 2014, 53, 11828–11833. [Google Scholar] [CrossRef]
- Paolucci, C.; Di Iorio, J.R.; Schneider, W.F.; Gounder, R. Solvation and Mobilization of Copper Active Sites in Zeolites by Ammonia: Consequences for the Catalytic Reduction of Nitrogen Oxides. Acc. Chem. Res. 2020, 53, 1881–1892. [Google Scholar] [CrossRef]
- Liu, K.; Yan, Z.; Shan, W.; Shan, Y.; Shi, X.; He, H. Quantitative determination of the Cu species, acid sites and NH3-SCR mechanism on Cu-SSZ-13 and H-SSZ-13 at low temperatures. Catal. Sci. Technol. 2020, 10, 1135–1150. [Google Scholar] [CrossRef]
- Luo, J.; Gao, F.; Kamasamudram, K.; Currier, N.; Peden, C.H.F.; Yezerets, A. New insights into Cu/SSZ-13 SCR catalyst acidity. Part I: Nature of acidic sites probed by NH3 titration. J. Catal. 2017, 348, 291–299. [Google Scholar] [CrossRef]
- Chen, L.; Janssens, T.V.W.; Skoglundh, M.; Gronbeck, H. Interpretation of NH3-TPD Profiles from Cu-CHA Using First-Principles Calculations. Top. Catal. 2019, 62, 93–99. [Google Scholar] [CrossRef]
- Chen, P.; Khetan, A.; Jablonska, M.; Simboeck, J.; Muhler, M.; Palkovits, R.; Pitsch, H.; Simon, U. Local dynamics of copper active sites in zeolite catalysts for selective catalytic reduction of NOx with NH3. Appl. Catal. B Environ. 2018, 237, 263–272. [Google Scholar] [CrossRef]
- Xu, L.; Wang, C.; Chang, H.; Wu, Q.; Zhang, T.; Li, J. New Insight into SO2 Poisoning and Regeneration of CeO2-WO3/TiO2 and V2O5-WO3/TiO2 Catalysts for Low-Temperature NH3-SCR. Environ. Sci. Technol. 2018, 52, 7064–7071. [Google Scholar] [CrossRef]
- Moreno-González, M.; Hueso, B.; Boronat, M.; Blasco, T.; Corma, A. Ammonia-containing species formed in Cu-Chabazite as per in situ EPR, solid-state NMR, and DFT calculations. J. Phys. Chem. Lett. 2015, 6, 1011–1017. [Google Scholar] [CrossRef]
- Wu, Z.; Ran, R.; Ma, Y.; Wu, X.; Si, Z.; Weng, D. Quantitative control and identification of copper species in Cu-SAPO-34: A combined UV-vis spectroscopic and H-2-TPR analysis. Res. Chem. Intermed. 2019, 45, 1309–1325. [Google Scholar] [CrossRef]
- Villamaina, R.; Liu, S.; Nova, I.; Tronconi, E.; Ruggeri, M.P.; Collier, J.; York, A.; Thompsett, D. Speciation of Cu Cations in Cu-CHA Catalysts for NH3-SCR: Effects of SiO2/AlO3 Ratio and Cu-Loading Investigated by Transient Response Methods. ACS Catal. 2019, 9, 8916–8927. [Google Scholar] [CrossRef]
- Xie, K.; Woo, J.; Bernin, D.; Kumar, A.; Kamasamudram, K.; Olsson, L. Insights into hydrothermal aging of phosphorus-poisoned Cu-SSZ-13 for NH3-SCR. Appl. Catal. B Environ. 2019, 241, 205–216. [Google Scholar] [CrossRef]
- Xie, K.; Wang, A.; Woo, J.; Kumar, A.; Kamasamudram, K.; Olsson, L. Deactivation of Cu-SSZ-13 SCR catalysts by vapor-phase phosphorus exposure. Appl. Catal. B Environ. 2019, 256. [Google Scholar] [CrossRef]
- Chen, L.; Jansson, J.; Skoglundh, M.; Gronbeck, H. Mechanism for Solid-State Ion Exchange of Cu+ into Zeolites. J. Phys. Chem. C 2016, 120, 29182–29189. [Google Scholar] [CrossRef]
- O’Malley, A.J.; Sarwar, M.; Armstrong, J.; Catlow, C.R.A.; Silverwood, I.P.; York, A.P.E.; Hitchcock, I. Comparing ammonia diffusion in NH3-SCR zeolite catalysts: A quasielastic neutron scattering and molecular dynamics simulation study. Phys. Chem. Chem. Phys. 2018, 20, 11976–11986. [Google Scholar] [CrossRef]
- Guenter, T.; Doronkin, D.E.; Carvalho, H.W.P.; Casapu, M.; Grunwaldt, J.D. HERFD-XANES and XES as complementary operando tools for monitoring the structure of Cu-based zeolite catalysts during NOx-removal by ammonia SCR. J. Phys. Conf. Ser. 2016, 712, 012071. [Google Scholar] [CrossRef]
- Lomachenko, K.A.; Borfecchia, E.; Negri, C.; Berlier, G.; Lamberti, C.; Beato, P.; Falsig, H.; Bordiga, S. The Cu-CHA deNO(x) Catalyst in Action: Temperature-Dependent NH3-Assisted Selective Catalytic Reduction Monitored by Operando XAS and XES. J. Am. Chem. Soc. 2016, 138, 12025–12028. [Google Scholar] [CrossRef]
- Pankin, I.A.; Martini, A.; Lomachenko, K.A.; Soldatov, A.V.; Bordiga, S.; Borfecchia, E. Identifying Cu-oxo species in Cu-zeolites by XAS: A theoretical survey by DFT-assisted XANES simulation and EXAFS wavelet transform. Catal. Today 2020, 345, 125–135. [Google Scholar] [CrossRef]
- Lomachenko, K.A.; Borfecchia, E.; Bordiga, S.; Soldatov, A.V.; Beato, P.; Lamberti, C. Active sites in Cu-SSZ-13 deNO(x) catalyst under reaction conditions: A XAS/XES perspective. J. Phys. Conf. Ser. 2016, 712, 012041. [Google Scholar] [CrossRef]
- Fahami, A.R.; Guenter, T.; Doronkin, D.E.; Casapu, M.; Zengel, D.; Vuong, T.H.; Simon, M.; Breher, F.; Kucherov, A.V.; Brueckner, A.; et al. The dynamic nature of Cu sites in Cu-SSZ-13 and the origin of the seagull NOx conversion profile during NH3-SCR. React. Chem. Eng. 2019, 4, 1000–1018. [Google Scholar] [CrossRef]
- Borfecchia, E.; Negri, C.; Lomachenko, K.A.; Lamberti, C.; Janssens, T.V.W.; Berlier, G. Temperature-dependent dynamics of NH3-derived Cu species in the Cu-CHA SCR catalyst. React. Chem. Eng. 2019, 4, 1067–1080. [Google Scholar] [CrossRef]
- Zhu, H.; Kwak, J.H.; Peden, C.H.F.; Szanyi, J. In situ DRIFTS-MS studies on the oxidation of adsorbed NH3 by NOx over a Cu-SSZ-13 zeolite. Catal. Today 2013, 205, 16–23. [Google Scholar] [CrossRef]
- Jangjou, Y.; Do, Q.; Gu, Y.; Lim, L.-G.; Sun, H.; Wang, D.; Kumar, A.; Li, J.; Grabow, L.C.; Epling, W.S. Nature of Cu Active Centers in Cu-SSZ-13 and Their Responses to SO2 Exposure. ACS Catal. 2018, 8, 1325–1337. [Google Scholar] [CrossRef]
- Negri, C.; Signorile, M.; Porcaro, N.G.; Borfecchia, E.; Berlier, G.; Janssens, T.V.W.; Bordiga, S. Dynamic Cu2+/CuI speciation in Cu-CHA catalysts by in situ Diffuse Reflectance UV–vis-NIR spectroscopy. Appl. Catal. A Gen. 2019, 578, 1–9. [Google Scholar] [CrossRef]
- Goltl, F.; Conrad, S.; Wolf, P.; Muller, P.; Love, A.M.; Burt, S.P.; Wheeler, J.N.; Hamers, R.J.; Hummer, K.; Kresse, G.; et al. UV-Vis and Photoluminescence Spectroscopy to Understand the Coordination of Cu Cations in the Zeolite SSZ-13. Chem. Mater. 2019, 31, 9582–9592. [Google Scholar] [CrossRef]
- Giordanino, F.; Vennestrom, P.N.R.; Lundegaard, L.F.; Stappen, F.N.; Mossin, S.; Beato, P.; Bordiga, S.; Lamberti, C. Characterization of Cu-exchanged SSZ-13: A comparative FTIR, UV-Vis, and EPR study with Cu-ZSM-5 and Cu-beta with similar Si/Al and Cu/Al ratios. Dalton Trans. 2013, 42, 12741–12761. [Google Scholar] [CrossRef] [PubMed]
- Oda, A.; Shionoya, H.; Hotta, Y.; Takewaki, T.; Sawabe, K.; Satsuma, A. Spectroscopic Evidence of Efficient Generation of Dicopper Intermediate in Selective Catalytic Reduction of NO over Cu-Ion-Exchanged Zeolites. ACS Catal. 2020, 10, 12333–12339. [Google Scholar] [CrossRef]
- Liu, L.; Wu, X.; Ma, Y.; Zhang, X.; Ran, R.; Si, Z.; Weng, D. Potassium deactivation of Cu-SSZ-13 catalyst for NH3-SCR: Evolution of salts, zeolite and copper species. Chem. Eng. J. 2020, 383. [Google Scholar] [CrossRef]
- Liu, C.; Kubota, H.; Amada, T.; Kon, K.; Toyao, T.; Maeno, Z.; Ueda, K.; Ohyama, J.; Satsuma, A.; Tanigawa, T.; et al. In Situ Spectroscopic Studies on the Redox Cycle of NH3-SCR over Cu-CHA Zeolites. Chemcatchem 2020, 12, 3050–3059. [Google Scholar] [CrossRef]
- Gao, F.; Walter, E.D.; Karp, E.M.; Luo, J.; Tonkyn, R.G.; Kwak, J.H.; Szanyi, J.; Peden, C.H.F. Structure–activity relationships in NH3-SCR over Cu-SSZ-13 as probed by reaction kinetics and EPR studies. J. Catal. 2013, 300, 20–29. [Google Scholar] [CrossRef]
- Godiksen, A.; Vennestrom, P.N.R.; Rasmussen, S.B.; Mossin, S. Identification and Quantification of Copper Sites in Zeolites by Electron Paramagnetic Resonance Spectroscopy. Top. Catal. 2017, 60, 13–29. [Google Scholar] [CrossRef]
- Godiksen, A.; Stappen, F.N.; Vennestrøm, P.N.R.; Giordanino, F.; Rasmussen, S.B.; Lundegaard, L.F.; Mossin, S. Coordination Environment of Copper Sites in Cu-CHA Zeolite Investigated by Electron Paramagnetic Resonance. J. Phys. Chem. C 2014, 118, 23126–23138. [Google Scholar] [CrossRef]
- Fernandez, E.; Moreno-Gonzalez, M.; Moliner, M.; Blasco, T.; Boronat, M.; Corma, A. Modeling of EPR Parameters for Cu(II): Application to the Selective Reduction of NOx Catalyzed by Cu-Zeolites. Top. Catal. 2018, 61, 810–832. [Google Scholar] [CrossRef]
- Godiksen, A.; Isaksen, O.L.; Rasmussen, S.B.; Vennestrom, P.N.R.; Mossin, S. Site-Specific Reactivity of Copper Chabazite Zeolites with Nitric Oxide, Ammonia, and Oxygen. Chemcatchem 2018, 10, 366–370. [Google Scholar] [CrossRef]
- Barsukov, Y.; Macdonald, J. Impedance Spectroscopy: Theory, Experiment, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Lvovich, V.F. Impedance Spectroscopy: Applications to Electrochemical and Dielectric Phenomena; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Beattie, I.R. The electrical conductivity of analcite and ion-exchanged forms of analcite and chabazite. Trans. Faraday Soc. 1954, 50, 581–587. [Google Scholar] [CrossRef]
- Freeman, D.; Stamires, D. Electrical Conductivity of Synthetic Crystalline Zeolites. J. Chem. Phys. 1961, 35, 799–806. [Google Scholar] [CrossRef]
- Simon, U.; Flesch, U. Cation-cation interaction in dehydrated zeolites X and Y monitored by modulus spectroscopy. J. Porous Mater. 1999, 6, 33–40. [Google Scholar] [CrossRef]
- Franke, M.E.; Simon, U. Characteristics of proton hopping in zeolite H-ZSM5. Phys. Status Solidi B Basic Res. 2000, 218, 287–290. [Google Scholar] [CrossRef]
- Rizzotto, V.; Chen, P.; Simon, U. Mobility of NH3-Solvated Cu-II Ions in Cu-SSZ-13 and Cu-ZSM-5 NH3-SCR Catalysts: A Comparative Impedance Spectroscopy Study. Catalysts 2018, 8, 162. [Google Scholar] [CrossRef]
- Chen, P.; Rizzotto, V.; Khetan, A.; Xie, K.; Moos, R.; Pitsch, H.; Ye, D.; Simon, U. Mechanistic Understanding of Cu-CHA Catalyst as Sensor for Direct NH3-SCR Monitoring: The Role of Cu Mobility. ACS Appl. Mater. Interfaces 2019, 11, 8097–8105. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Rizzotto, V.; Xie, K.; Simon, U. Tracking mobile active sites and intermediates in NH3-SCR over zeolite catalysts by impedance-based in situ spectroscopy. React. Chem. Eng. 2019, 4, 986–994. [Google Scholar] [CrossRef]
- Chen, L.; Falsig, H.; Janssens, T.V.W.; Grönbeck, H. Activation of oxygen on (NH3-Cu-NH3)+ in NH3-SCR over Cu-CHA. J. Catal. 2018, 358, 179–186. [Google Scholar] [CrossRef]
- Wallin, M.; Karlsson, C.J.; Skoglundh, M.; Palmqvist, A. Selective catalytic reduction of NOx with NH3 over zeolite H-ZSM-5: Influence of transient ammonia supply. J. Catal. 2003, 218, 354–364. [Google Scholar] [CrossRef]
- Chen, L.; Falsig, H.; Janssens, T.V.W.; Jansson, J.; Skoglundh, M.; Gronbeck, H. Effect of Al-distribution on oxygen activation over Cu-CHA. Catal. Sci. Technol. 2018, 8, 2131–2136. [Google Scholar] [CrossRef]
- Liu, C.; Kubota, H.; Toyao, T.; Maeno, Z.; Shimizu, K.-i. Mechanistic insights into the oxidation of copper(i) species during NH3-SCR over Cu-CHA zeolites: A DFT study. Catal. Sci. Technol. 2020, 10, 3586–3593. [Google Scholar] [CrossRef]
- Negri, C.; Selleri, T.; Borfecchia, E.; Martini, A.; Lomachenko, K.A.; Janssens, T.V.W.; Cutini, M.; Bordiga, S.; Berlier, G. Structure and Reactivity of Oxygen-Bridged Diamino Dicopper(II) Complexes in Cu-Ion-Exchanged Chabazite Catalyst for NH3-Mediated Selective Catalytic Reduction. J. Am. Chem. Soc. 2020, 142, 15884–15896. [Google Scholar] [CrossRef]
- Clemens, A.K.S.; Shishkin, A.; Carlsson, P.A.; Skoglundh, M.; Martinez-Casado, F.J.; Matej, Z.; Balmes, O.; Harelind, H. Reaction-driven Ion Exchange of Copper into Zeolite SSZ-13. ACS Catal. 2015, 5, 6209–6218. [Google Scholar] [CrossRef]
- Vennestrøm, P.N.R.; Janssens, T.V.W.; Kustov, A.; Grill, M.; Puig-Molina, A.; Lundegaard, L.F.; Tiruvalam, R.R.; Concepción, P.; Corma, A. Influence of lattice stability on hydrothermal deactivation of Cu-ZSM-5 and Cu-IM-5 zeolites for selective catalytic reduction of NOx by NH3. J. Catal. 2014, 309, 477–490. [Google Scholar] [CrossRef]
- Shwan, S.; Skoglundh, M.; Lundegaard, L.F.; Tiruvalam, R.R.; Janssens, T.V.W.; Carlsson, A.; Vennestrom, P.N.R. Solid-State Ion-Exchange of Copper into Zeolites Facilitated by Ammonia at Low Temperature. ACS Catal. 2014, 5, 16–19. [Google Scholar] [CrossRef]
- Vennestrom, P.N.R.; Lundegaard, L.F.; Tyrsted, C.; Bokarev, D.A.; Mytareva, A.I.; Baeva, G.N.; Stakheev, A.Y.; Janssens, T.V.W. The Role of Protons and Formation Cu(NH3)2+ During Ammonia-Assisted Solid-State Ion Exchange of Copper(I) Oxide into Zeolites. Top. Catal. 2019, 62, 100–107. [Google Scholar] [CrossRef]
- Rauch, D.; Kubinski, D.; Simon, U.; Moos, R. Detection of the ammonia loading of a Cu Chabazite SCR catalyst by a radio frequency-based method. Sens. Actuators B Chem. 2014, 205, 88–93. [Google Scholar] [CrossRef]
- Wales, D.J.; Grand, J.; Ting, V.P.; Burke, R.D.; Edler, K.J.; Bowen, C.R.; Mintova, S.; Burrows, A.D. Gas sensing using porous materials for automotive applications. Chem. Soc. Rev. 2015, 44, 4290–4321. [Google Scholar] [CrossRef]
- Moos, R. Catalysts as sensors—A promising novel approach in automotive exhaust gas aftertreatment. Sensors 2010, 10, 6773–6787. [Google Scholar] [CrossRef]
- Li, S.; Zheng, Y.; Gao, F.; Szanyi, J.; Schneider, W.F. Experimental and computational interrogation of fast SCR mechanism and active sites on H-form SSZ-13. ACS Catal. 2017, 7, 5087–5096. [Google Scholar] [CrossRef]
- Mao, Y.; Wang, Z.; Wang, H.-F.; Hu, P. Understanding catalytic reactions over zeolites: A density functional theory study of selective catalytic reduction of NOx by NH3 over Cu-SAPO-34. ACS Catal. 2016, 6, 7882–7891. [Google Scholar] [CrossRef]
- Narsimhan, K.; Iyoki, K.; Dinh, K.; Roman-Leshkov, Y. Catalytic oxidation of methane into methanol over copper-exchanged zeolites with oxygen at low temperature. ACS Cent. Sci. 2016, 2, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Brogaard, R.Y.; Kømurcu, M.; Dyballa, M.M.; Botan, A.; Van Speybroeck, V.; Olsbye, U.; De Wispelaere, K. Ethene dimerization on zeolite-hosted Ni ions: Reversible mobilization of the active site. ACS Catal. 2019, 9, 5645–5650. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, H.; Rizzotto, V.; Guo, A.; Ye, D.; Simon, U.; Chen, P. Recent Understanding of Low-Temperature Copper Dynamics in Cu-Chabazite NH3-SCR Catalysts. Catalysts 2021, 11, 52. https://doi.org/10.3390/catal11010052
Lei H, Rizzotto V, Guo A, Ye D, Simon U, Chen P. Recent Understanding of Low-Temperature Copper Dynamics in Cu-Chabazite NH3-SCR Catalysts. Catalysts. 2021; 11(1):52. https://doi.org/10.3390/catal11010052
Chicago/Turabian StyleLei, Huarong, Valentina Rizzotto, Anqi Guo, Daiqi Ye, Ulrich Simon, and Peirong Chen. 2021. "Recent Understanding of Low-Temperature Copper Dynamics in Cu-Chabazite NH3-SCR Catalysts" Catalysts 11, no. 1: 52. https://doi.org/10.3390/catal11010052
APA StyleLei, H., Rizzotto, V., Guo, A., Ye, D., Simon, U., & Chen, P. (2021). Recent Understanding of Low-Temperature Copper Dynamics in Cu-Chabazite NH3-SCR Catalysts. Catalysts, 11(1), 52. https://doi.org/10.3390/catal11010052





