Dehydration of Biomass-Derived Butanediols over Rare Earth Zirconate Catalysts
Abstract
1. Introduction
2. Results
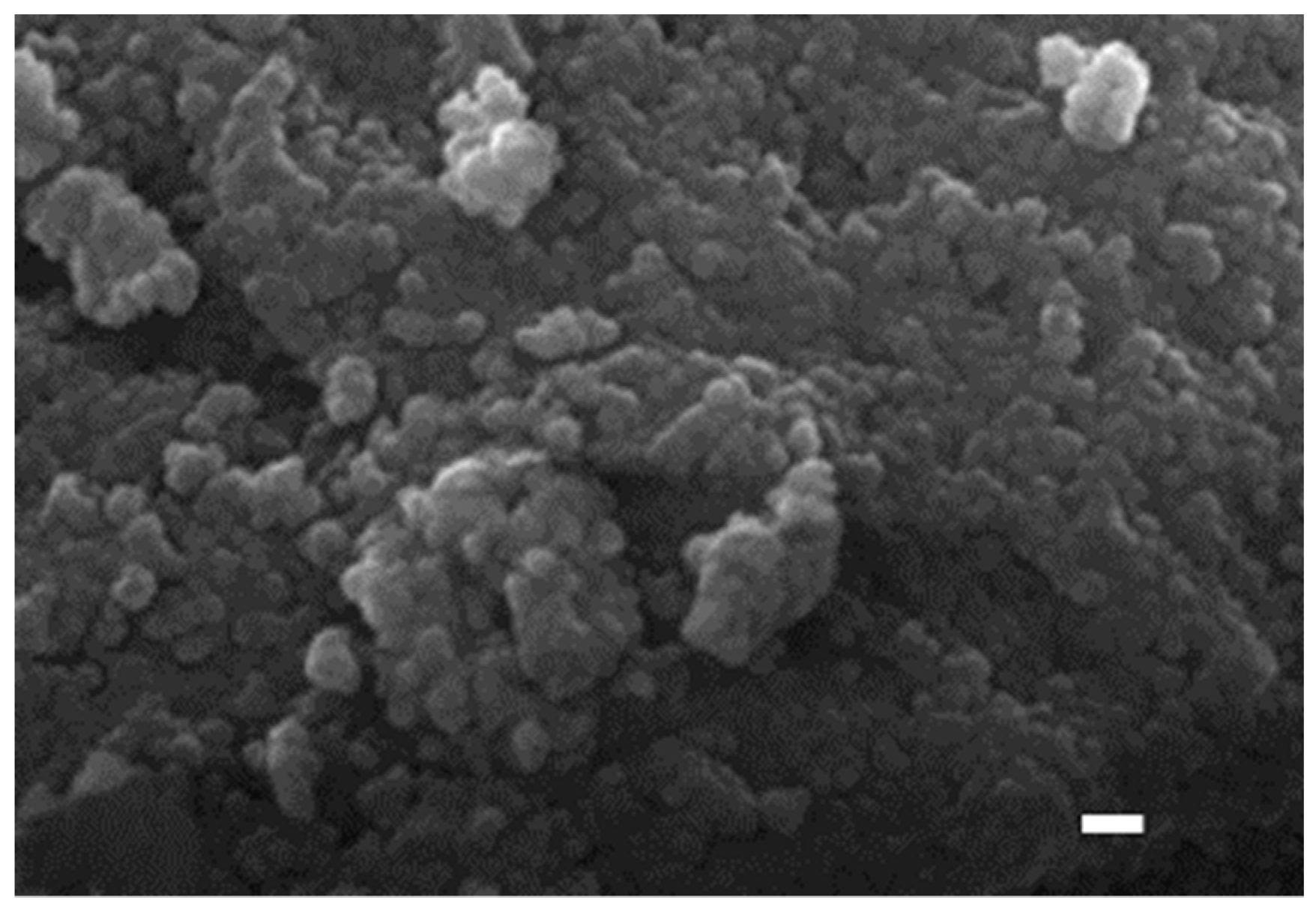
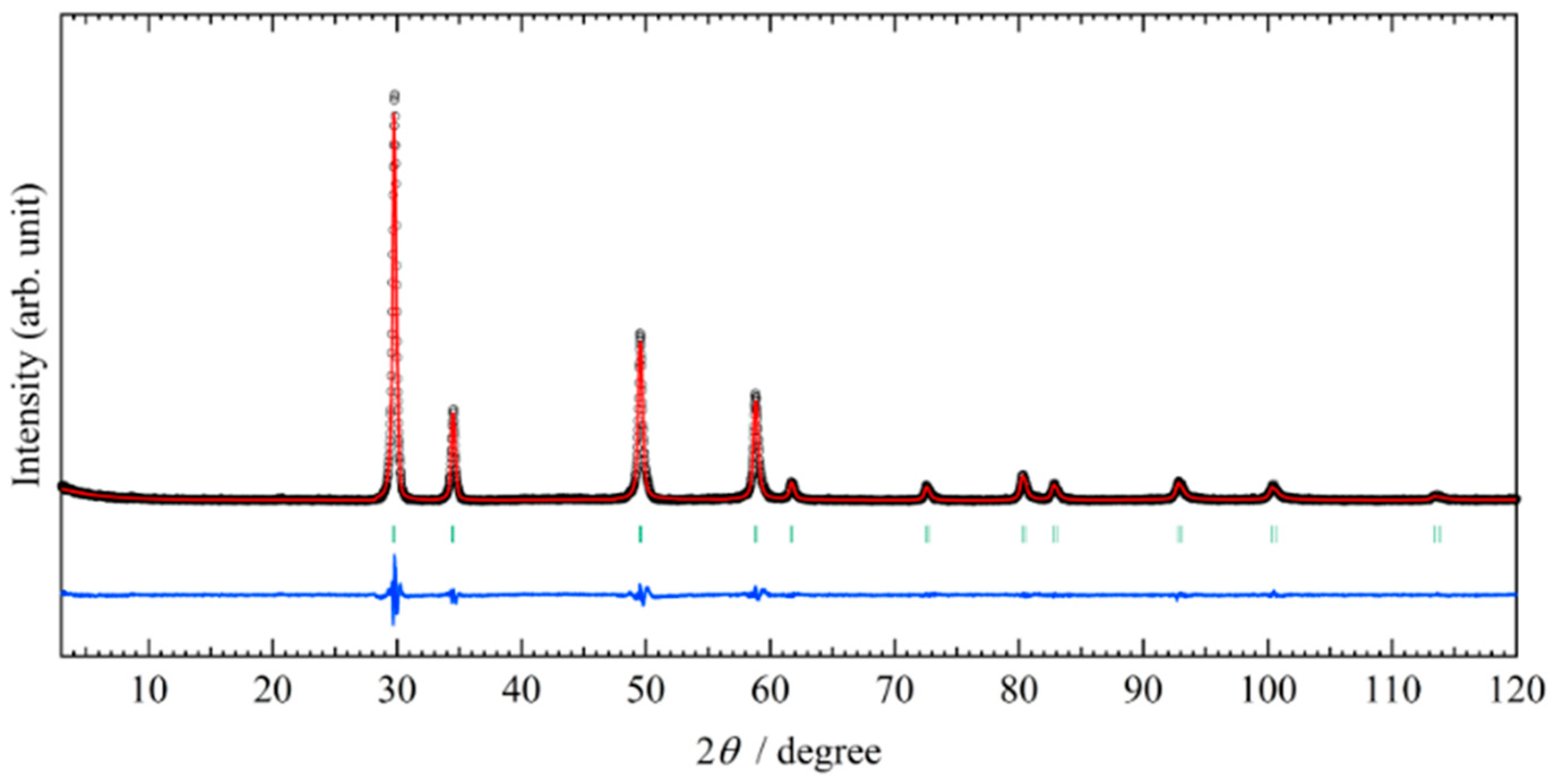
2.1. Structural Property of REZrO Catalysts
2.2. Dehydration of Different BDOs over Various REZrO Catalysts
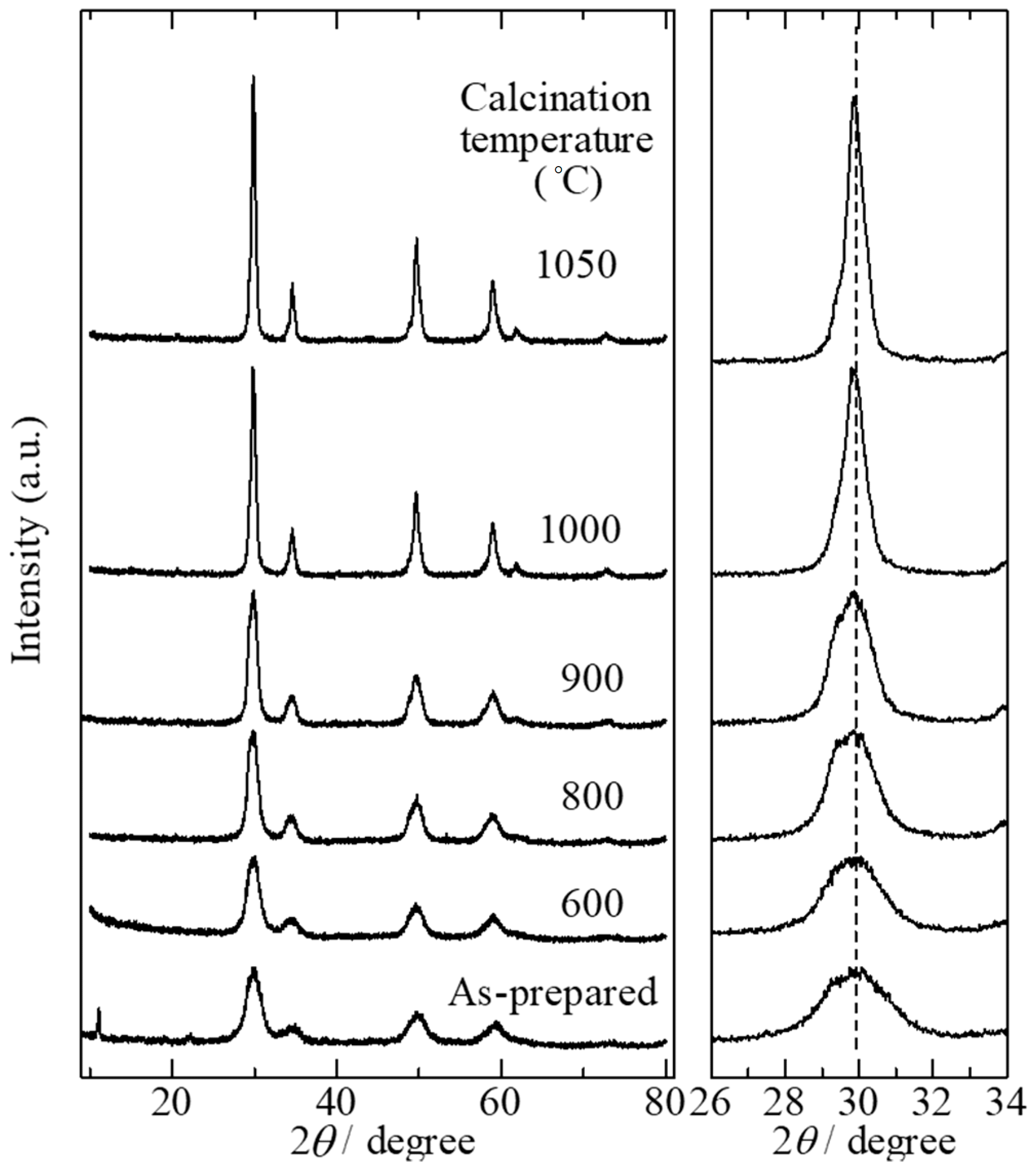
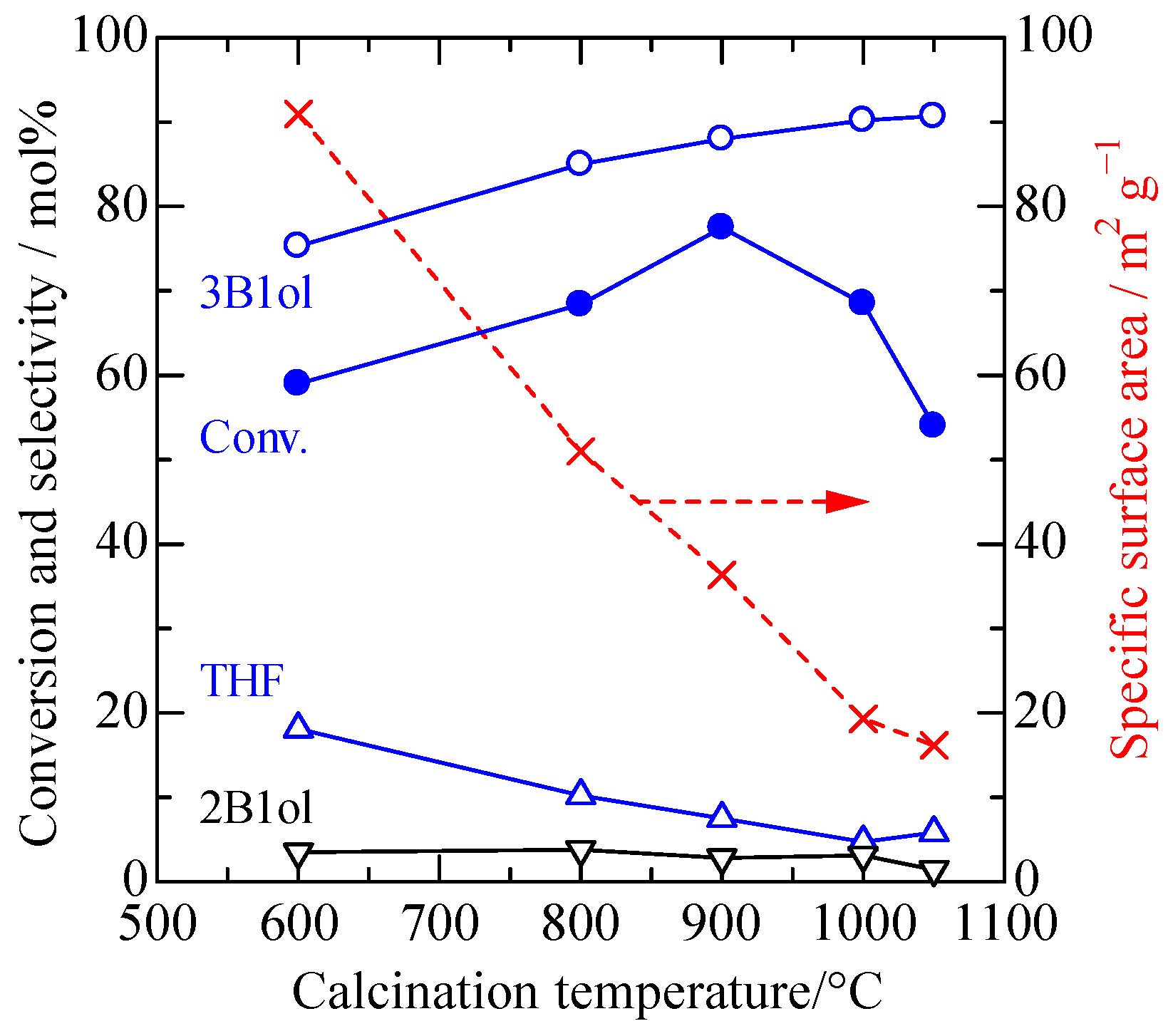
2.3. Effect of Calcination Temperature on the Structure of YZrO Catalysts and Catalytic Activity in the Dehydration of 1,4-BDO
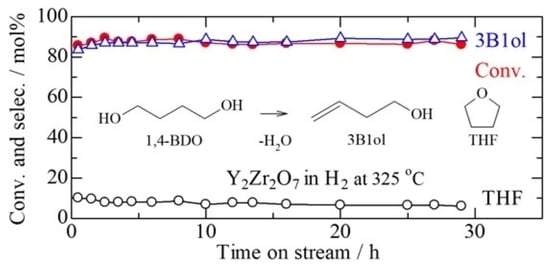
2.4. Catalytic Dehydration of 1,4-BDO over YZrO Calcined at 900 °C under Different Reaction Conditions
2.5. Effect of Carrier Gas on the Catalytic Dehydration of 1,4-BDO over YZrO Calcined at 900 °C
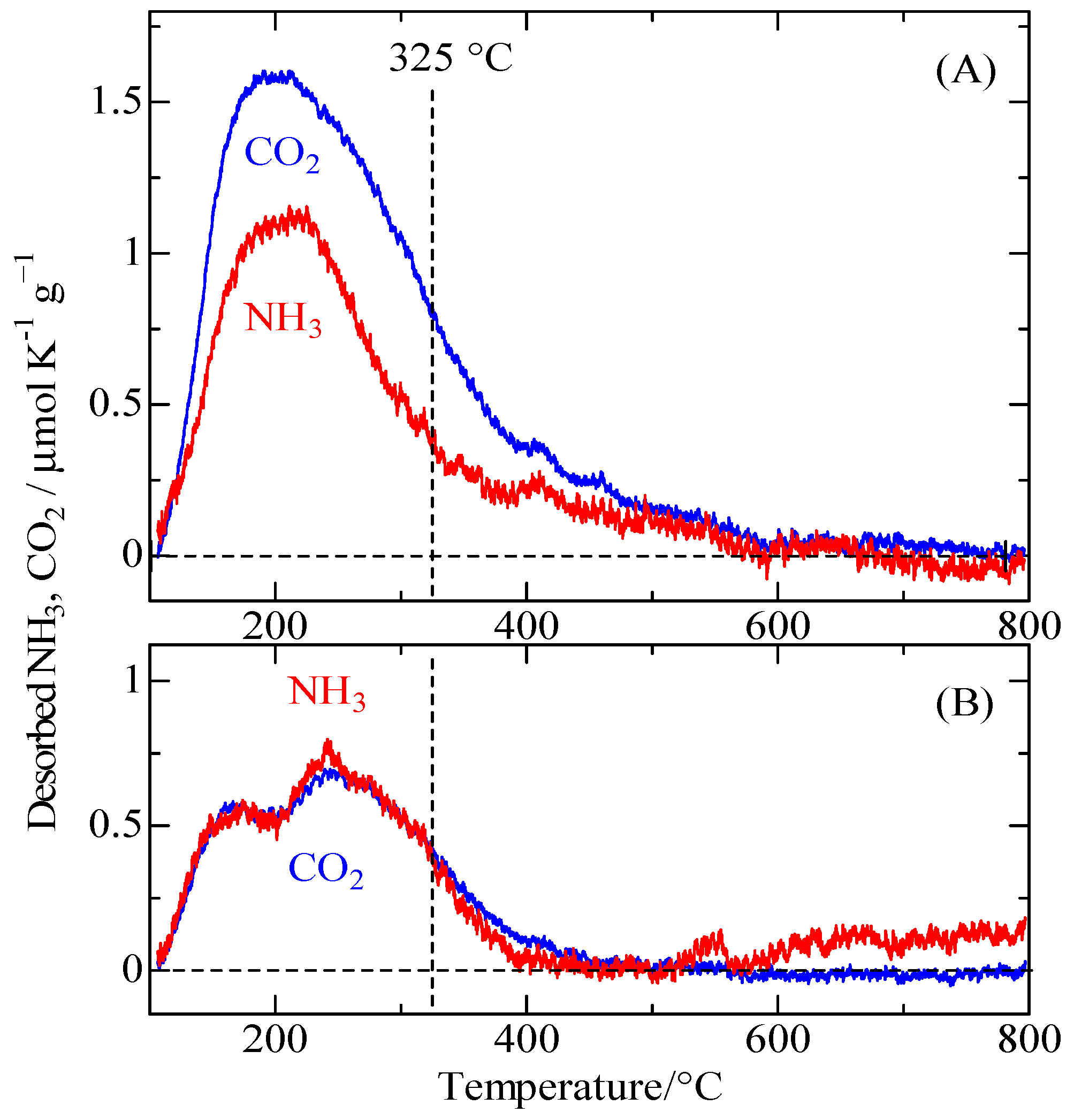
2.6. Acid-base Properties of YZrO Catalyst Calcined at 600 and 900 °C
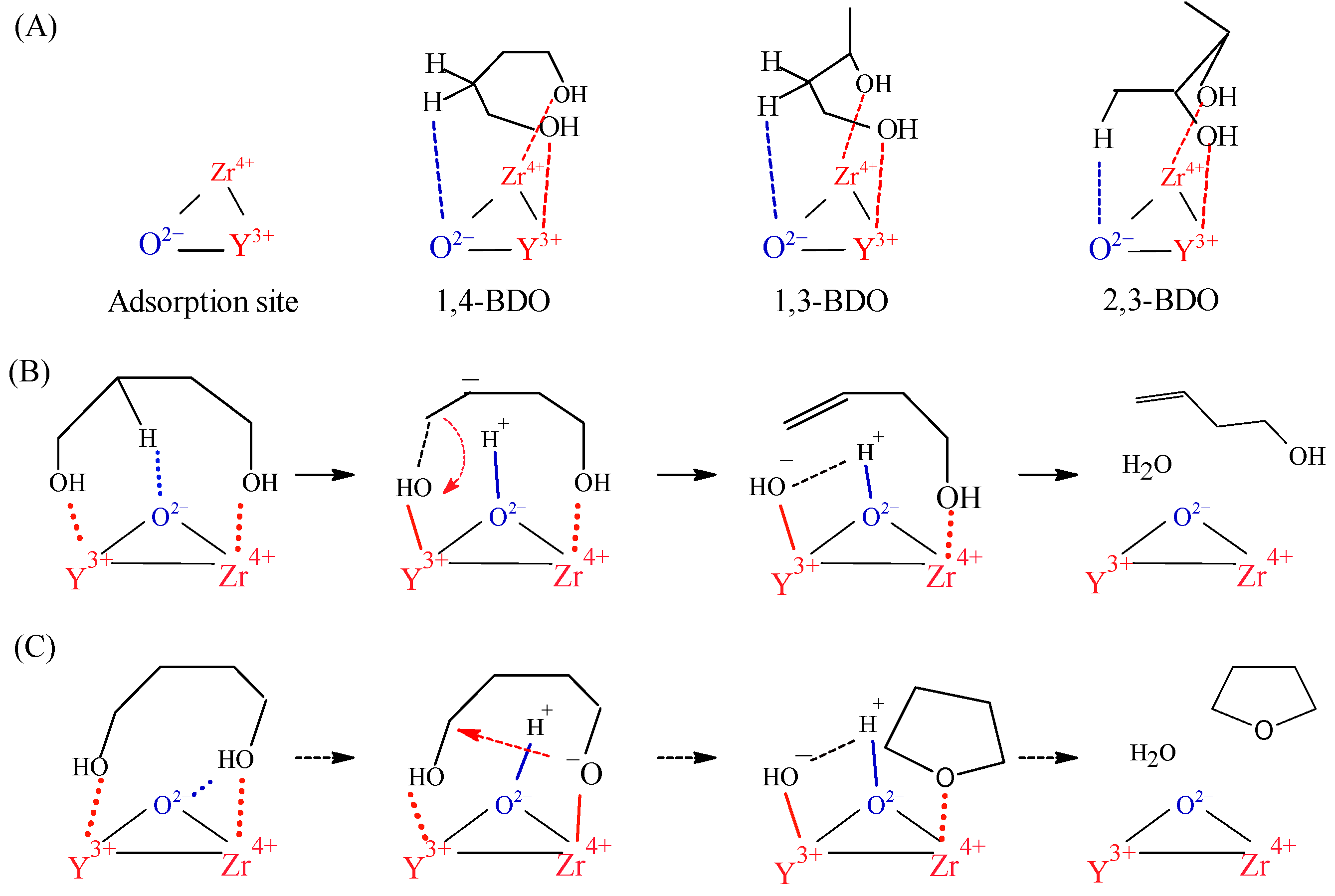
3. Discussion
3.1. Structure of REZrO as an Effective Catalyst for the Dehydration of BDOs
3.2. Dehydration of Different BDOs over Various REZrO Catalysts
3.3. Significant Parameters on the Catalytic Activity of YZrO
3.4. Mechanistic Considerations on the Dehydration of 1,4-BDO over YZrO Catalyst
4. Materials and Methods
4.1. Materials
4.2. Characterization of Catalysts
4.3. Catalytic Reaction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ji, X.J.; Huang, H.; Ouyang, P.K. Microbial 2,3-butanediol production: A state-of-the-art review. Biotechnol. Adv. 2011, 29, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Zeng, A.P.; Sabra, W. Microbial production of diols as platform chemicals: Recent progresses. Curr. Opin. Biotechnol. 2011, 22, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.S.; Kim, B.; Shin, J.H.; Choi, Y.J.; Choi, S.; Song, C.W.; Lee, J.; Park, H.G.; Lee, S.Y. Bio-based production of C2–C6 platform chemicals. Biotechnol. Bioeng. 2012, 109, 2437–2459. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Liu, W.; Zou, H.; Cheng, T.; Tian, N.; Xian, M. Microbial production of short chain diols. Microb. Cell Fact. 2014, 13, 165. [Google Scholar] [CrossRef]
- Sabra, W.; Groeger, C.; Zeng, A.P. Microbial cell factories for diol production. Adv. Biochem. Eng. Biotechnol. 2016, 155, 165–197. [Google Scholar]
- Yang, T.; Rao, Z.; Zhang, X.; Xu, M.; Xu, Z.; Yang, S.T. Metabolic engineering strategies for acetoin and 2,3-butanediol production: Advances and prospects. Critic. Rev. Biotechnol. 2017, 37, 990–1005. [Google Scholar] [CrossRef]
- Biz, A.; Proulx, S.; Xu, Z.; Siddartha, K.; Indrayanti, A.M.; Mahadevan, R. Systems biology based metabolic engineering for non-natural chemicals. Biotechnol. Adv. 2019, 37, 107379. [Google Scholar] [CrossRef]
- Yim, H.; Haselbeck, R.; Niu, W.; Pujol-Baxley, C.; Burgard, A.; Boldt, J.; Khandurina, J.; Trawick, J.D.; Osterhout, R.E.; Stephen, R.; et al. Metabolic engineering of Escherichia coli for direct production of 1,4-butanediol. Nat. Chem. Biol. 2011, 7, 445–452. [Google Scholar] [CrossRef]
- Liu, H.; Lu, T. Autonomous production of 1,4-butanediol via a de novo biosynthesis pathway in engineered Escherichia coli. Metab. Eng. 2015, 29, 135–141. [Google Scholar] [CrossRef]
- Wang, J.; Jain, R.; Shen, X.; Sun, X.; Cheng, M.; Liao, J.C.; Yuan, Q.; Yan, Y. Rational engineering of diol dehydratase enables 1,4-butanediol biosynthesis from xylose. Metab. Eng. 2017, 40, 148–156. [Google Scholar] [CrossRef]
- Tai, C.; Shen, Y.; Guo, Y.; Tao, F. Biosynthesis of 1,4-butanediol from erythritol using whole-cell catalysis. Biocatal. Biotrans. 2019, 37, 92–96. [Google Scholar]
- Ly, B.K.; Minh, D.P.; Pinel, C.; Besson, M.; Tapin, B.; Epron, F.; Especel, C. Effect of addition mode of Re in bimetallic Pd-Re/TiO2 catalysts upon the selective aqueous-phase hydrogenation of succinic acid to 1,4-butanediol. Top. Catal. 2012, 55, 466–473. [Google Scholar] [CrossRef]
- Kataoka, N.; Vangnai, A.S.; Tajima, T.; Nakashimada, Y.; Kato, J. Improvement of (R)-1,3-butanediol production by engineered Escherichia coli. J. Biosci. Bioeng. 2013, 115, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Nemr, K.; Müller, J.E.N.; Joo, J.C.; Gawand, P.; Choudhary, R.; Mendonca, B.; Lu, S.; Yu, X.; Yakunin, A.F.; Mahadevan, R. Engineering a short, aldolase-based pathway for (R)-1,3-butanediol production in Escherichia coli. Metab. Eng. 2018, 48, 13–24. [Google Scholar] [CrossRef]
- Matsuyama, A.; Yamamoto, H.; Kawada, N.; Kobayashi, Y. Industrial production of (R)-1,3-butanediol by new biocatalysts. J. Mol. Catal. B Enzym. 2001, 11, 513–521. [Google Scholar] [CrossRef]
- Itoh, N.; Nakamura, M.; Inoue, K.; Makino, Y. Continuous production of chiral 1,3-butanediol using immobilized biocatalysts in a packed bed reactor: Promising biocatalysis method with an asymmetric hydrogen-transfer bioreduction. Appl. Microbiol. Biotechnol. 2007, 75, 1249–1256. [Google Scholar] [CrossRef]
- Sato, S.; Sato, F.; Gotoh, H.; Yamada, Y. Selective dehydration of alkanediols into unsaturated alcohols over rare earth oxide catalysts. ACS Catal. 2013, 3, 721–734. [Google Scholar] [CrossRef]
- Duan, H.; Yamada, Y.; Sato, S. Future prospect of the production of 1,3-butadiene from butanediols. Chem. Lett. 2016, 45, 1036–1047. [Google Scholar] [CrossRef]
- Zeng, F.; Hohn, K. Catalytic conversion of biomass-derived compounds to C4 chemicals. Catalysis 2019, 31, 1–36. [Google Scholar]
- Sun, D.; Li, Y.; Yang, C.; Su, Y.; Yamada, Y.; Sato, S. Production of 1,3-butadiene from biomass-derived C4 alcohols. Fuel Proc. Technol. 2020, 197, 106193. [Google Scholar] [CrossRef]
- Kim, W.; Shin, W.; Lee, K.J.; Song, H.; Kim, H.S.; Seung, D.; Filimonov, I.N. 2,3-Butanediol dehydration catalyzed by silica-supported sodiumphosphates. Appl. Catal. A Gen. 2016, 511, 156–167. [Google Scholar] [CrossRef]
- Tsukamoto, D.; Sakami, S.; Ito, M.; Yamada, K.; Yonehara, T. Production of bio-based 1,3-butadiene by highly selective dehydration of 2,3-butanediol over SiO2-supported cesium dihydrogen phosphate catalyst. Chem. Lett. 2016, 45, 831–833. [Google Scholar] [CrossRef]
- Jing, F.; Katryniok, B.; Araque, M.; Wojcieszak, R.; Capron, M.; Paul, S.; Daturi, M.; Clacens, J.M.; De Campo, F.; Liebens, A.; et al. Direct dehydration of 1,3-butanediol into butadiene over aluminosilicate catalysts. Catal. Sci. Technol. 2016, 6, 5830–5840. [Google Scholar] [CrossRef]
- Jing, F.; Katryniok, B.; Paul, S.; Fang, L.; Liebens, A.; Shen, M.; Hu, B.; Dumeignil, F.; Pera-Titus, M. Al-doped SBA-15 catalysts for low-temperature dehydration of 1,3-butanediol into butadiene. ChemCatChem 2017, 9, 258–262. [Google Scholar] [CrossRef]
- Fang, L.; Jing, F.; Lu, J.; Hu, B.; Pera-Titus, M. Nano-flowered Ce@MOR hybrids with modulated acid properties for the vapor-phase dehydration of 1,3-butanediol into butadiene. Green Chem. 2017, 19, 4610–4621. [Google Scholar] [CrossRef]
- Lee, J.H.; Hong, S.B. Dehydration of 1,3-butanediol to butadiene over medium-pore zeolites: Another example of reaction intermediate shape selectivity. Appl. Catal. B Environ. 2021, 280, 119446. [Google Scholar] [CrossRef]
- Yamamoto, N.; Sato, S.; Takahashi, R.; Inui, K. Synthesis of homoallyl alcohol from 1,4-butanediol over ZrO2 catalyst. Catal. Commun. 2005, 6, 480–484. [Google Scholar] [CrossRef]
- Yamamoto, N.; Sato, F.; Takahashi, R.; Inui, K. Synthesis of 3-buten-1-ol from 1,4-butanediol over ZrO2 catalyst. J. Mol. Catal. A Chem. 2006, 243, 52–59. [Google Scholar] [CrossRef]
- Sato, F.; Yamada, Y.; Sato, S. Preparation of Er2O3 nanorod catalyst without using organic additive and its application to catalytic dehydration of 1,4-butanediol. Chem. Lett. 2012, 41, 593–594. [Google Scholar] [CrossRef]
- Sato, F.; Sato, S.; Yamada, Y.; Nakamura, M.; Shiga, A. Acid–base concerted mechanism in the dehydration of 1,4-butanediol over bixbyite rare earth oxide catalysts. Catal. Today 2014, 226, 124–133. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Li, H.; Gao, C.; Zhao, Y. Heterogeneous CaO-ZrO2 acid–base bifunctional catalysts for vapor-phase selective dehydration of 1,4-butanediol to 3-buten-1-ol. Appl. Catal. A Gen. 2013, 466, 233–239. [Google Scholar] [CrossRef]
- Inoue, H.; Sato, S.; Takahashi, R.; Izawa, Y.; Ohno, H.; Takahashi, K. Dehydration of 1,4-butanediol over supported rare earth oxide catalysts. Appl. Catal. A Gen. 2009, 352, 66–73. [Google Scholar] [CrossRef]
- Duan, H.; Hirota, T.; Yamada, Y.; Sato, S. Vapor-phase catalytic dehydration of 1,4-butanediol to 3-buten-1-ol over modified ZrO2 catalysts. Appl. Catal. A Gen. 2017, 535, 9–16. [Google Scholar] [CrossRef]
- Ohtsuka, S.; Nemoto, T.; Yotsumoto, R.; Yamada, Y.; Sato, F.; Takahashi, R.; Sato, S. Vapor-phase catalytic dehydration of butanediols to unsaturated alcohols over yttria-stabilized zirconia catalysts. Appl. Catal. A Gen. 2019, 575, 48–57. [Google Scholar] [CrossRef]
- Nemoto, T.; Yamada, Y.; Sato, F.; Takahashi, R.; Sato, S. Catalytic dehydration of 1,3-butanediol over oxygen-defected fluorite Yb2Zr2O7. Mol. Catal. 2019, 473, 110399. [Google Scholar] [CrossRef]
- Fang, X.; Xia, L.; Peng, L.; Luo, Y.; Xu, J.; Xu, L.; Xu, X.; Liu, W.; Zheng, R.; Wang, X. Ln2Zr2O7 compounds (Ln = La, Pr, Sm, Y) with varied rare earth A sites for low temperature oxidative coupling of methane. Chin. Chem. Lett. 2019, 30, 1141–1146. [Google Scholar] [CrossRef]
- Shanon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Duan, H.; Sun, D.; Yamada, Y.; Sato, S. Dehydration of 2,3-butanediol into 3-buten-2-ol catalyzed by ZrO2. Catal. Commun. 2014, 48, 1–4. [Google Scholar] [CrossRef]
- Duan, H.; Yamada, Y.; Sato, S. Vapor-phase catalytic dehydration of 2,3-butanediol into 3-buten-2-ol over Sc2O3. Chem. Lett. 2014, 43, 1773–1775. [Google Scholar] [CrossRef]
- Sun, D.; Arai, S.; Duan, H.; Yamada, Y.; Sato, S. Vapor-phase dehydration of C4 unsaturated alcohols to 1,3-butadiene. Appl. Catal. A Gen. 2017, 531, 21–28. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, D.; Yamada, Y.; Sato, S. Selective production of 1,3-butadiene in the dehydration of 1,4-butanediol over rare earth oxides. Appl. Catal. A Gen. 2018, 562, 11–18. [Google Scholar] [CrossRef]
- Chen, D.; Xu, R. Hydrothermal synthesis and characterization of La2M2O7 (M = Ti, Zr) powders. Mater. Res. Bull. 1998, 33, 409–417. [Google Scholar] [CrossRef]
- Liu, Z.-G.; Ouyang, J.-H.; Zhou, Y.; Li, J.; Xia, X.-L. Densification, structure, and thermophysical properties of ytterbium-gadolinium zirconate ceramics. Int. J. Appl. Ceram. Technol. 2009, 6, 485–491. [Google Scholar] [CrossRef]
- Walker, J.D.S.; Hayes, J.R.; Gaultois, M.W.; Aluri, E.R.; Grosvenor, A.P. A case for oxygen deficiency in Gd2Ti2−xZrxO7 pyrochlore-type oxides. J. Alloys Compd. 2013, 565, 44–49. [Google Scholar] [CrossRef]
- Gotoh, H.; Yamada, Y.; Sato, S. Dehydration of 1,3-butanediol over rare earth oxides. Appl. Catal. A Gen. 2010, 377, 92–98. [Google Scholar] [CrossRef]
- Sato, S.; Takahashi, R.; Kobune, M.; Inoue, H.; Izawa, Y.; Ohno, H.; Takahashi, K. Dehydration of 1,4-butanediol over rare earth oxides. Appl. Catal. A Gen. 2009, 356, 64–71. [Google Scholar] [CrossRef]
- Kobune, M.; Sato, S.; Takahashi, R. Surface-structure sensitivity of CeO2 for several catalytic reactions. J. Mol. Catal. A Chem. 2008, 279, 10–19. [Google Scholar] [CrossRef]
- Sharma, S.; Hilaire, S.; Vohs, J.M.; Gorte, R.J.; Jen, H.-W. Evidence for oxidation of ceria by CO2. J. Catal. 2000, 190, 199–204. [Google Scholar] [CrossRef]
- Moulijn, J.A.; Tarfaoui, A.; Kapteijn, F. General aspects of catalyst testing. Catal. Today 1991, 11, 1–12. [Google Scholar] [CrossRef]
- Sato, S.; Takahashi, R.; Sodesawa, T.; Yamamoto, N. Dehydration of 1,4-butanediol into 3-buten-1-ol catalyzed by ceria. Catal. Commun. 2004, 5, 397–400. [Google Scholar] [CrossRef]
- Ichikawa, N.; Sato, S.; Takahashi, R.; Sodesawa, T. Catalytic reaction of 1,3-butanediol over solid acids. J. Mol. Catal. A Chem. 2006, 256, 106–112. [Google Scholar] [CrossRef]
- Duan, H.; Yamada, Y.; Sato, S. Selective dehydration of 2,3-butanediol to 3-buten-2-ol over ZrO2 modified with CaO. Appl. Catal. A Gen. 2014, 487, 226–233. [Google Scholar] [CrossRef]
- Izumi, F.; Momma, K. Three-dimensional visualization in powder diffraction. Solid State Phenom. 2007, 130, 15–20. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 82th ed.; CRC Press: London, UK, 2001; pp. 4-95–4-96. [Google Scholar]
- Takahashi, R.; Yamada, I.; Iwata, A.; Kurahashi, N.; Yoshida, S.; Sato, S. Synthesis of 3-buten-1-ol from 1,4-butanediol over indium oxide. Appl. Catal. A Gen. 2010, 383, 134–140. [Google Scholar] [CrossRef]
- Wattanasiriwech, D.; Wattanasiriwech, S. Effects of fuel contents and surface modification on the sol-gel combustion Ce0.9 Gd0.1O1.95 nanopowder. Energy Procedia 2013, 34, 524–533. [Google Scholar] [CrossRef][Green Version]
- Yavetskiy, R.P.; Kosyanov, D.Y.; Baumer, V.N.; Doroshenko, A.G.; Fedorov, A.I.; Matveevskaya, N.A.; Tolmachev, A.V.; Vovk, O.M. Low-agglomerated yttria nanopowders via decomposition of sulfate-doped precursor with transient morphology. J. Rare Earth 2014, 32, 320–325. [Google Scholar] [CrossRef]
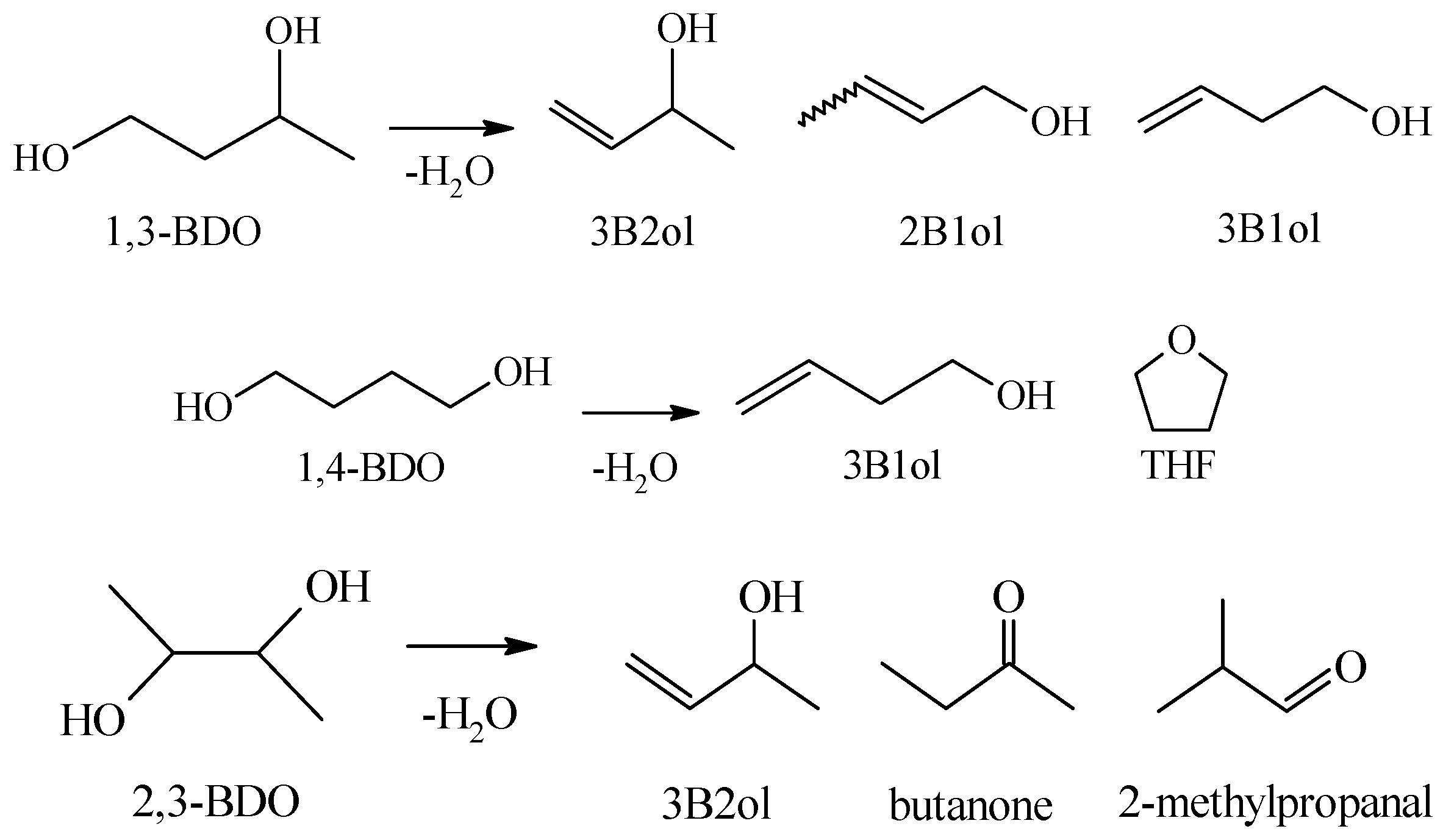
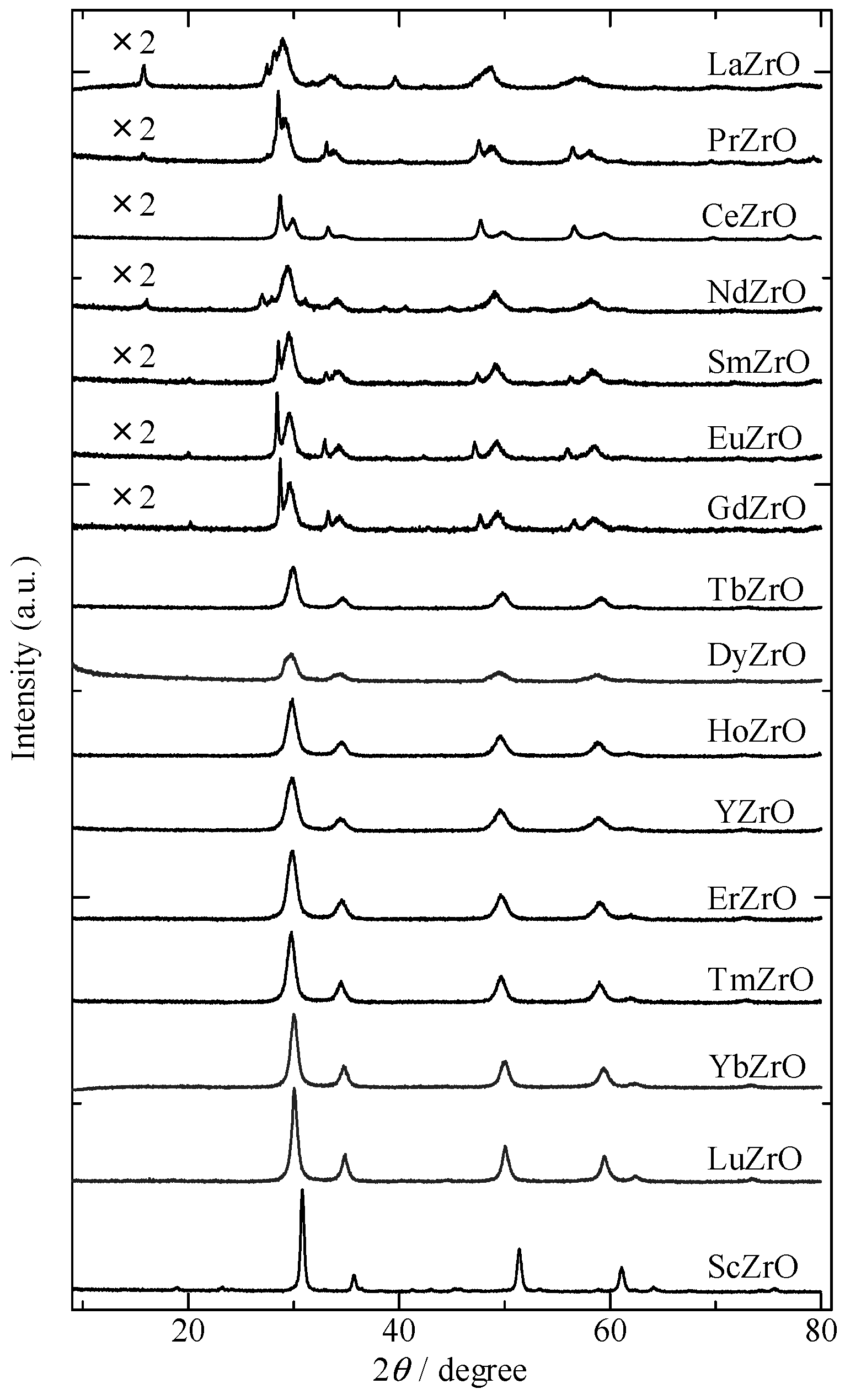



| Catalyst | Ionic Radius of Rare Earth Cation a (nm) | Average Ionic Radius, R b (nm) | SAc (m2 g−1) | Molar Ratio of RE/Zr/Hf d | Ratio of RE/Zr d |
|---|---|---|---|---|---|
| LaZrO | 0.1032 | 0.0936 | 16 | 43.3/56.1/0.6 | 0.77 |
| PrZrO | 0.0990 | 0.0915 | 29 | 45.1/54.0/0.9 | 0.84 |
| NdZrO | 0.0983 | 0.09115 | 25 | 52.8/46.6/0.6 | 1.13 |
| CeZrO | 0.0970 | 0.0905 | 30 | 47.1/52.3.0.6 | 0.90 |
| SmZrO | 0.0958 | 0.0899 | 32 | 52.9/46.7/0.4 | 1.13 |
| EuZrO | 0.0947 | 0.08935 | 35 | 51.0/48.4/0.6 | 1.05 |
| GdZrO | 0.0938 | 0.0889 | 35 | 52.2/47.1/0.7 | 1.11 |
| TbZrO | 0.0923 | 0.08815 | 34 | 51.8/47.6/0.6 | 1.09 |
| DyZrO | 0.0912 | 0.0876 | 32 | 53.2/46.2/0.6 | 1.15 |
| HoZrO | 0.0901 | 0.08705 | 30 | 53.5/45.9/0.6 | 1.17 |
| YZrO | 0.0900 | 0.0870 | 36 | 51.2/48.0/0.8 | 1.07 |
| ErZrO | 0.0890 | 0.0865 | 30 | 54.1/45.5/0.4 | 1.19 |
| TmZrO | 0.0880 | 0.0860 | 39 | 53.4/46.2/0.4 | 1.16 |
| YbZrO | 0.0868 | 0.0854 | 27 | 55.0/44.6/0.4 | 1.23 |
| LuZrO | 0.0861 | 0.08505 | 26 | 52.8/47.0/0.2 | 1.12 |
| ScZrO | 0.0745 | 0.07925 | 23 | 27.2/71.8/1.0 | 0.38 |
| Catalyst | Conversion | Selectivity (mol%) | ||||||
|---|---|---|---|---|---|---|---|---|
| (%) | 3B1ol | 2B1ol | UOLs | BD | THF | GBL | Others | |
| LaZrO | 29.1 | 6.1 | 0.5 | 6.6 | 0.0 | 88.4 | 3.1 | 1.9 |
| PrZrO | 25.2 | 61.7 | 1.5 | 63.2 | 0.0 | 32.7 | 2.5 | 1.6 |
| NdZrO | 37.7 | 32.4 | 0.8 | 33.2 | 0.0 | 63.7 | 0.5 | 2.6 |
| CeZrO | 24.7 | 60.8 | 5.6 | 66.4 | 0.0 | 24.9 | 5.6 | 3.1 |
| SmZrO | 37.3 | 65.8 | 1.3 | 67.1 | 0.1 | 30.8 | 0.9 | 1.1 |
| EuZrO | 52.8 | 82.6 | 2.8 | 85.4 | 0.8 | 10.8 | 2.2 | 0.8 |
| GdZrO | 68.5 | 81.5 | 1.9 | 83.4 | 0.5 | 15.3 | 0.4 | 0.4 |
| TbZrO | 52.4 | 76.1 | 1.1 | 77.2 | 0.6 | 21.0 | 0.9 | 0.3 |
| DyZrO | 69.4 | 86.5 | 2.7 | 89.2 | 1.6 | 7.9 | 0.4 | 0.9 |
| HoZrO | 51.2 | 82.6 | 1.7 | 84.3 | 0.8 | 13.3 | 0.7 | 0.9 |
| YZrO | 77.6 | 87.7 | 2.8 | 90.5 | 1.2 | 7.5 | 0.6 | 0.2 |
| ErZrO | 64.4 | 85.8 | 4.0 | 89.8 | 1.9 | 7.4 | 0.3 | 0.6 |
| TmZrO | 55.9 | 82.8 | 1.3 | 84.1 | 1.1 | 13.3 | 0.3 | 1.2 |
| YbZrO | 61.3 | 84.8 | 2.2 | 87.0 | 0.8 | 11.5 | 0.3 | 0.4 |
| LuZrO | 51.2 | 84.3 | 2.0 | 86.3 | 2.6 | 9.0 | 0.3 | 1.8 |
| ScZrO | 35.5 | 30.0 | 0.1 | 30.1 | 0.0 | 68.9 | 0.3 | 0.7 |
| Catalyst | Reactant | Conv. | Selectivity (mol%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | 3B2ol | 3B1ol | 2B1ol | UOLs | BD | MEK | THF | 3H2BO | Others | ||
| LaZrO | 1,3-BDO | 40.2 | 44.9 | 1.2 | 35.1 | 81.2 | 1.7 | 6.8 | - | - | 10.3 a |
| 1,4-BDO | 29.1 | - | 6.1 | 0.5 | 6.6 | 0 | - | 88.4 | - | 5.0 b | |
| 2,3-BDO | 4.8 | 28.3 | - | - | 28.3 | 0 | 16.1 | - | 37.1 | 18.5 c | |
| CeZrO | 1,3-BDO | 64.7 | 55.8 | 1.0 | 35.1 | 91.9 | 2.8 | 0.7 | - | - | 4.6 a |
| 1,4-BDO | 24.7 | - | 60.8 | 5.6 | 66.4 | 0 | - | 24.9 | - | 8.7 b | |
| 2,3-BDO | 6.6 | 15.4 | - | - | 15.4 | 0 | 29.4 | - | 33.4 | 21.8 c | |
| YZrO | 1,3-BDO | 93.9 | 52.1 | 1.3 | 36.8 | 90.2 | 7.2 | 0.2 | - | - | 2.4 a |
| 1,4-BDO | 77.6 | - | 87.7 | 2.8 | 90.5 | 1.2 | - | 7.5 | - | 0.8 b | |
| 2,3-BDO | 17.0 | 30.3 | - | - | 30.3 | 0 | 10.9 | - | 26.8 | 32.0 c | |
| YbZrO | 1,3-BDO | 89.8 | 53.9 | 1.4 | 40.9 | 96.2 | 1.0 | 1.0 | - | - | 1.8 a |
| 1,4-BDO | 61.3 | - | 84.8 | 2.2 | 87.0 | 0.8 | - | 11.5 | - | 0.7 b | |
| 2,3-BDO | 12.2 | 46.9 | - | - | 46.9 | 0 | 7.3 | - | 28.4 | 17.4 c | |
| LuZrO | 1,3-BDO | 83.0 | 50.8 | 1.7 | 42.9 | 95.4 | 2.0 | 0.9 | - | - | 1.7 a |
| 1,4-BDO | 51.2 | - | 84.3 | 2.0 | 86.3 | 2.6 | - | 9.0 | - | 2.1 b | |
| 2,3-BDO | 11.3 | 50.8 | - | - | 50.8 | 0 | 10.1 | - | 24.2 | 14.9 c | |
| ScZrO | 1,3-BDO | 20.6 | 43.1 | 12.7 | 39.7 | 95.5 | 0 | 1.4 | - | - | 3.1 a |
| 1,4-BDO | 35.5 | - | 30.0 | 0.1 | 30.1 | 0 | - | 68.9 | - | 1.0 b | |
| 2,3-BDO | 18.4 | 47.4 | - | - | 47.4 | 0 | 18.7 | - | 16.0 | 17.9 c | |
| Calcination | SA | FWHM at 2θ = 29.9° | Particle Size, | Crystallite Size, |
|---|---|---|---|---|
| (°C) | (m2 g−1) | (degree) | DBET (nm) | DXRD (nm) |
| 600 | 91 | 1.792 | 12 | 4.6 |
| 800 | 51 | 1.439 | 22 | 5.7 |
| 900 | 36 | 1.242 | 31 | 6.6 |
| 1000 | 19 | 0.670 | 58 | 12 |
| 1050 | 16 | 0.543 | 70 | 15 |
| Sample | Y2Zr2O7 | ||
|---|---|---|---|
| Space Group | Fm−3ma | ||
| a (Å) | 5.19878 | B (Y) | 1.81615 |
| α (degree) | 90.0000 | B (Zr) | 1.77582 |
| β (degree) | 90.0000 | B (O) | 3.46843 |
| γ (degree) | 90.0000 | Rwp (%) | 12.229% |
| g (Y) b | 0.5000 | RB (%) | 2.039% |
| g (Zr) b | 0.5000 | RF (%) | 1.593% |
| g (O) b | 0.8750 | S | 1.3891 |
| Temperature | Conv. | Selectivity (mol%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| (°C) | (%) | 3B1ol | 2B1ol | UOLs | BD | Propylene | THF | GBL | Others |
| 300 | 25.9 | 84.0 | 1.1 | 85.1 | 0.4 | 0.0 | 12.4 | 0.6 | 1.5 |
| 325 | 77.6 | 87.7 | 2.8 | 90.5 | 1.2 | 0.0 | 7.5 | 0.6 | 0.2 |
| 350 | 99.8 | 70.2 | 7.1 | 77.3 | 11.5 | 0.0 | 9.9 | 0.2 | 1.1 |
| 360 | 100.0 | 53.0 | 10.1 | 63.1 | 22.2 | 0.0 | 11.5 | 0.1 | 3.1 |
| 375 | 100.0 | 23.8 | 4.4 | 28.2 | 58.8 | 5.8 | 3.7 | 0.1 | 3.4 a |
| Carrier Gas | Conversion | Selectivity (mol%) | Productivity of 3B1ol | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (%) | 3B1ol | 2B1ol | UOLs | BD | THF | GBL | Others | mol h−1 kg−1 | |
| T = 325 °C | |||||||||
| N2 | 77.6 | 87.7 | 2.8 | 90.5 | 1.2 | 7.5 | 0.6 | 0.2 | 24.2 |
| CO2 | 74.6 | 64.6 | 1.5 | 66.1 | 0.3 | 32.3 | 0.8 | 0.5 | 17.1 |
| NH3 | 81.9 | 85.5 | 6.5 | 92.0 | 0.0 | 6.8 | 0.1 | 1.1 | 24.9 |
| H2 | 87.8 | 86.7 | 3.3 | 90.0 | 1.0 | 8.4 | 0.4 | 0.2 | 27.0 |
| T = 300 °C | |||||||||
| N2 | 25.9 | 84.0 | 1.1 | 85.1 | 0.4 | 12.4 | 0.6 | 1.5 | 7.7 |
| CO2 | 19.2 | 60.8 | 1.5 | 62.3 | 0.0 | 34.0 | 1.6 | 2.1 | 4.1 |
| NH3 | 32.3 | 87.6 | 1.5 | 89.1 | 0.0 | 9.9 | 0.2 | 0.7 | 10.1 |
| N2 (NH3) a | 16.0 | 76.6 | 10.3 | 86.9 | 0.0 | 7.8 | 1.5 | 3.8 | 4.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuda, A.; Matsumura, Y.; Nakazono, K.; Sato, F.; Takahashi, R.; Yamada, Y.; Sato, S. Dehydration of Biomass-Derived Butanediols over Rare Earth Zirconate Catalysts. Catalysts 2020, 10, 1392. https://doi.org/10.3390/catal10121392
Matsuda A, Matsumura Y, Nakazono K, Sato F, Takahashi R, Yamada Y, Sato S. Dehydration of Biomass-Derived Butanediols over Rare Earth Zirconate Catalysts. Catalysts. 2020; 10(12):1392. https://doi.org/10.3390/catal10121392
Chicago/Turabian StyleMatsuda, Asami, Yoshitaka Matsumura, Kazuki Nakazono, Fumiya Sato, Ryoji Takahashi, Yasuhiro Yamada, and Satoshi Sato. 2020. "Dehydration of Biomass-Derived Butanediols over Rare Earth Zirconate Catalysts" Catalysts 10, no. 12: 1392. https://doi.org/10.3390/catal10121392
APA StyleMatsuda, A., Matsumura, Y., Nakazono, K., Sato, F., Takahashi, R., Yamada, Y., & Sato, S. (2020). Dehydration of Biomass-Derived Butanediols over Rare Earth Zirconate Catalysts. Catalysts, 10(12), 1392. https://doi.org/10.3390/catal10121392